User login
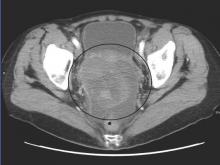
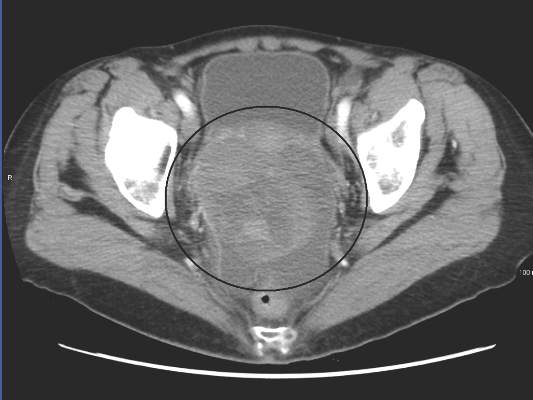
In patients with recurrent platinum-resistant or platinum-refractory ovarian cancer, volasertib demonstrated activity and resulted in manageable adverse effects, which were mostly hematologic, according to phase II trial results.
The 24-week disease control rate with volasertib was 30.6% (95% confidence interval, 18.0-43.2) and with single-agent chemotherapy was 43.1% (95% CI, 29.5-56.7). The median progression-free survival (PFS) for volasertib was 13.1 weeks (interquartile range, 6.6-30.1) and was 20.6 weeks (11.6-30.7) for chemotherapy (hazard ratio, 1.01; 95% CI, 0.66-1.53). In the volasertib arm, six patients (11%) achieved PFS greater than or equal to 1 year, in contrast to no patients in the chemotherapy arm.
“This suggests that volasertib might achieve a durable response in a subpopulation of patients with heavily pretreated resistant/refractory ovarian cancer,” wrote Dr. Eric Pujade-Lauraine of the Centre des Cancers de la Femme et Recherche Clinique, Paris, and head of the Group d’Investigateurs Nationaux pour l’Étude des Cancers Ovariens (GINECO), and his colleagues (J Clin Onc. 2016 Jan 11. doi: 10.1200/JCO.2015.62.1474).
“Further clinical development of volasertib in this setting should [be pursued only] after a biomarker is identified to select for patients with a greater chance of response to monotherapy or combination therapies,” they wrote.
Immunohistochemical analysis of potential biomarkers, including Plk1, Ki-67, Aurora kinases A and B, and pHH3, showed no relationship between biomarker expression and drug response.
The phase II open-label, randomized controlled trial evaluated 109 patients with ovarian cancer who had undergone two or three prior lines of treatment. Patients were randomized 1:1 to receive volasertib, a cell cycle kinase inhibitor selective for Plk, or an investigator’s choice of single-agent, nonplatinum, cytotoxic chemotherapy.
The volasertib arm had a higher rate of hematologic adverse events than did the chemotherapy arm, with 33 patients (61.1%) experiencing grade 3 or greater toxicity. Nonhematologic drug–related toxicity, such as nausea, fatigue, and peripheral neuropathy, occurred more frequently in the chemotherapy arm. The rate of treatment discontinuation because of adverse events was higher in the chemotherapy vs. volasertib arm (27.3% vs. 13.0%, respectively).
In patients with recurrent platinum-resistant or platinum-refractory ovarian cancer, volasertib demonstrated activity and resulted in manageable adverse effects, which were mostly hematologic, according to phase II trial results.
The 24-week disease control rate with volasertib was 30.6% (95% confidence interval, 18.0-43.2) and with single-agent chemotherapy was 43.1% (95% CI, 29.5-56.7). The median progression-free survival (PFS) for volasertib was 13.1 weeks (interquartile range, 6.6-30.1) and was 20.6 weeks (11.6-30.7) for chemotherapy (hazard ratio, 1.01; 95% CI, 0.66-1.53). In the volasertib arm, six patients (11%) achieved PFS greater than or equal to 1 year, in contrast to no patients in the chemotherapy arm.
“This suggests that volasertib might achieve a durable response in a subpopulation of patients with heavily pretreated resistant/refractory ovarian cancer,” wrote Dr. Eric Pujade-Lauraine of the Centre des Cancers de la Femme et Recherche Clinique, Paris, and head of the Group d’Investigateurs Nationaux pour l’Étude des Cancers Ovariens (GINECO), and his colleagues (J Clin Onc. 2016 Jan 11. doi: 10.1200/JCO.2015.62.1474).
“Further clinical development of volasertib in this setting should [be pursued only] after a biomarker is identified to select for patients with a greater chance of response to monotherapy or combination therapies,” they wrote.
Immunohistochemical analysis of potential biomarkers, including Plk1, Ki-67, Aurora kinases A and B, and pHH3, showed no relationship between biomarker expression and drug response.
The phase II open-label, randomized controlled trial evaluated 109 patients with ovarian cancer who had undergone two or three prior lines of treatment. Patients were randomized 1:1 to receive volasertib, a cell cycle kinase inhibitor selective for Plk, or an investigator’s choice of single-agent, nonplatinum, cytotoxic chemotherapy.
The volasertib arm had a higher rate of hematologic adverse events than did the chemotherapy arm, with 33 patients (61.1%) experiencing grade 3 or greater toxicity. Nonhematologic drug–related toxicity, such as nausea, fatigue, and peripheral neuropathy, occurred more frequently in the chemotherapy arm. The rate of treatment discontinuation because of adverse events was higher in the chemotherapy vs. volasertib arm (27.3% vs. 13.0%, respectively).
In patients with recurrent platinum-resistant or platinum-refractory ovarian cancer, volasertib demonstrated activity and resulted in manageable adverse effects, which were mostly hematologic, according to phase II trial results.
The 24-week disease control rate with volasertib was 30.6% (95% confidence interval, 18.0-43.2) and with single-agent chemotherapy was 43.1% (95% CI, 29.5-56.7). The median progression-free survival (PFS) for volasertib was 13.1 weeks (interquartile range, 6.6-30.1) and was 20.6 weeks (11.6-30.7) for chemotherapy (hazard ratio, 1.01; 95% CI, 0.66-1.53). In the volasertib arm, six patients (11%) achieved PFS greater than or equal to 1 year, in contrast to no patients in the chemotherapy arm.
“This suggests that volasertib might achieve a durable response in a subpopulation of patients with heavily pretreated resistant/refractory ovarian cancer,” wrote Dr. Eric Pujade-Lauraine of the Centre des Cancers de la Femme et Recherche Clinique, Paris, and head of the Group d’Investigateurs Nationaux pour l’Étude des Cancers Ovariens (GINECO), and his colleagues (J Clin Onc. 2016 Jan 11. doi: 10.1200/JCO.2015.62.1474).
“Further clinical development of volasertib in this setting should [be pursued only] after a biomarker is identified to select for patients with a greater chance of response to monotherapy or combination therapies,” they wrote.
Immunohistochemical analysis of potential biomarkers, including Plk1, Ki-67, Aurora kinases A and B, and pHH3, showed no relationship between biomarker expression and drug response.
The phase II open-label, randomized controlled trial evaluated 109 patients with ovarian cancer who had undergone two or three prior lines of treatment. Patients were randomized 1:1 to receive volasertib, a cell cycle kinase inhibitor selective for Plk, or an investigator’s choice of single-agent, nonplatinum, cytotoxic chemotherapy.
The volasertib arm had a higher rate of hematologic adverse events than did the chemotherapy arm, with 33 patients (61.1%) experiencing grade 3 or greater toxicity. Nonhematologic drug–related toxicity, such as nausea, fatigue, and peripheral neuropathy, occurred more frequently in the chemotherapy arm. The rate of treatment discontinuation because of adverse events was higher in the chemotherapy vs. volasertib arm (27.3% vs. 13.0%, respectively).
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Volasertib showed activity against platinum-resistant or -refractory ovarian cancer; adverse effects were mostly hematologic and manageable.
Major finding: The 24-week disease control rate with volasertib was 30.6% (95% confidence interval, 18.0-43.2) and 43.1% (29.5-56.7) with chemotherapy.
Data source: The phase II randomized controlled trial evaluated 109 patients with platinum-resistant or -refractory ovarian cancer who received volasertib or single-agent chemotherapy.
Disclosures: Research was supported by Boehringer Ingelheim. Dr. Pujade-Lauraine reported having no disclosures. Several of his coauthors reported ties to industry.