User login
DENVER – The investigational Watchman left atrial appendage closure device for stroke prevention in patients with atrial fibrillation proved markedly superior to warfarin in the 4-year follow-up of the randomized PROTECT AF trial.
Watchman recipients demonstrated a 40% reduction in the primary efficacy endpoint – a composite of stroke, systemic embolism, or cardiovascular death – compared with the warfarin-treated controls (95% confidence interval, 0.38-0.97; P = .03).
The Watchman group also showed a statistically significant 34% reduction in risk of all-cause mortality, compared with the 4.8% incidence in the warfarin arm (95% CI, 0.45-0.98; P = .04).
"This is something that’s really extraordinary. I wasn’t expecting to see a statistically significant decrease in all-cause mortality," Dr. Vivek Reddy said in presenting the new PROTECT AF data at the annual meeting of the Heart Rhythm Society.
The reduced all-cause mortality rate in the Watchman group resulted from fewer deaths due to cardiovascular-, pulmonary disease–, and hemorrhagic stroke–related causes.
In an earlier interim study analysis with 2.3 years and roughly 1,600 person-years of follow-up, the Watchman was merely statistically noninferior to warfarin in terms of efficacy (Circulation 2013;127:720-9). But in the updated analysis, with a mean follow-up of 45 months and 2,621 person-years, the device therapy has evolved in statistical parlance from noninferior to superior.
"The amount of benefit hasn’t changed. It’s been about a 40% difference throughout most of the trial. What’s changed is that with more follow-up the confidence intervals have narrowed, increasing the degree of certainty that the number is really true," according to Dr. Reddy of Mount Sinai School of Medicine, New York.
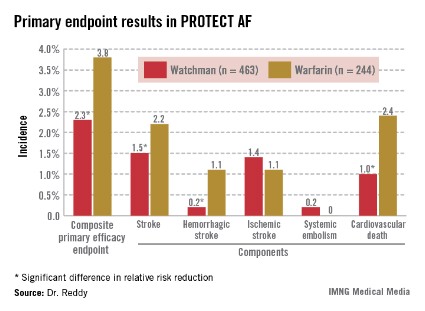
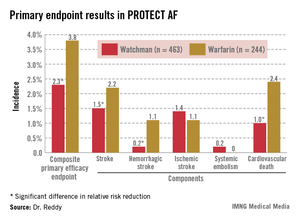
The Watchman’s superiority in terms of the primary efficacy endpoint was driven by an 85% reduction in the risk of hemorrhagic stroke and a 60% decrease in cardiovascular death compared with the warfarin group (see graphic). Importantly, the Watchman’s efficacy advantage was preserved in all prespecified patient subgroups, including the participants at greatest stroke risk: the roughly 20% with a baseline prior stroke or transient ischemic attack (TIA).
The Watchman is designed as a replacement for warfarin, a stroke prophylaxis drug whose numerous shortcomings are well known. The device is implanted in the left atrial appendage in a catheter procedure which entails an atrial puncture. Upon deployment, the Watchman excludes the left atrial appendage from the systemic circulation. The benefits seen in PROTECT AF and other Watchman studies confirm the notion that the left atrial appendage is critical to the pathogenesis of stroke in atrial fibrillation (AF), Dr. Reddy observed.
The 707 PROTECT AF participants at 59 centers were split fairly evenly between those with paroxysmal, persistent, and permanent AF. Subjects were randomized two-to-one to the Watchman or conventional warfarin therapy. Subjects in the warfarin group spent an average of 70% of their time with drug levels in therapeutic range, a considerably higher figure than studies would suggest is the norm in everyday clinical practice.
The composite primary safety endpoint occurred in a similar proportion of Watchman recipients and controls. However, the nature and timing of the adverse events differed in the two groups.
The most common adverse event in the Watchman group was serious pericardial effusion, which occurred in 4.8% of patients. Two-thirds of affected patients were managed via percutaneous intervention; the rest underwent surgery. The incidence of pericardial effusion decreased with greater operator experience: The rate was 6.3% in the first half of the study and 3.7% afterwards. Of note, in more recent studies begun since procedural refinements were introduced, the pericardial effusion rate has been lower than in PROTECT AF.
The chief adverse event in the warfarin group was, not surprisingly, major bleeding, which occurred in 4.1% of subjects, compared with a 0.6% rate in Watchman recipients. Unlike adverse events in the Watchman group, which were mostly procedure related, complications in the warfarin group were spread out over the full course of follow-up.
Dr. Reddy considers the PROTECT AF results broadly applicable even in this era of new oral anticoagulants.
"Despite the availability of novel anticoagulants, warfarin is still the most widely prescribed agent for stroke prevention," he noted.
Asked if he thought the Food and Drug Administration is likely to approve the Watchman based upon the new evidence from PROTECT AF as well as other studies, Dr. Reddy said it’s difficult to predict the agency’s actions but he certainly considers the device approvable.
If and when the Watchman is approved, he added, the first population where it is likely to see widespread use is in AF patients for whom warfarin is contraindicated. It would also be logical to consider the device in patients with a relative contraindication to warfarin, such as those on dialysis, at increased risk for falls, and elderly patients on polypharmacy; indeed, those are patient groups in which any anticoagulant is problematic. Head-to-head comparative studies of the Watchman and the novel anticoagulants would be welcome but are unlikely to occur anytime soon, Dr. Reddy said.
A fifth and final year of follow-up is scheduled for the PROTECT AF study.
The trial is sponsored by Boston Scientific. Dr. Reddy reported serving as a consultant to or receiving research grant support from Boston Scientific, Coherex Medical, and St. Jude Medical.
DENVER – The investigational Watchman left atrial appendage closure device for stroke prevention in patients with atrial fibrillation proved markedly superior to warfarin in the 4-year follow-up of the randomized PROTECT AF trial.
Watchman recipients demonstrated a 40% reduction in the primary efficacy endpoint – a composite of stroke, systemic embolism, or cardiovascular death – compared with the warfarin-treated controls (95% confidence interval, 0.38-0.97; P = .03).

The Watchman group also showed a statistically significant 34% reduction in risk of all-cause mortality, compared with the 4.8% incidence in the warfarin arm (95% CI, 0.45-0.98; P = .04).
"This is something that’s really extraordinary. I wasn’t expecting to see a statistically significant decrease in all-cause mortality," Dr. Vivek Reddy said in presenting the new PROTECT AF data at the annual meeting of the Heart Rhythm Society.
The reduced all-cause mortality rate in the Watchman group resulted from fewer deaths due to cardiovascular-, pulmonary disease–, and hemorrhagic stroke–related causes.
In an earlier interim study analysis with 2.3 years and roughly 1,600 person-years of follow-up, the Watchman was merely statistically noninferior to warfarin in terms of efficacy (Circulation 2013;127:720-9). But in the updated analysis, with a mean follow-up of 45 months and 2,621 person-years, the device therapy has evolved in statistical parlance from noninferior to superior.
"The amount of benefit hasn’t changed. It’s been about a 40% difference throughout most of the trial. What’s changed is that with more follow-up the confidence intervals have narrowed, increasing the degree of certainty that the number is really true," according to Dr. Reddy of Mount Sinai School of Medicine, New York.
The Watchman’s superiority in terms of the primary efficacy endpoint was driven by an 85% reduction in the risk of hemorrhagic stroke and a 60% decrease in cardiovascular death compared with the warfarin group (see graphic). Importantly, the Watchman’s efficacy advantage was preserved in all prespecified patient subgroups, including the participants at greatest stroke risk: the roughly 20% with a baseline prior stroke or transient ischemic attack (TIA).
The Watchman is designed as a replacement for warfarin, a stroke prophylaxis drug whose numerous shortcomings are well known. The device is implanted in the left atrial appendage in a catheter procedure which entails an atrial puncture. Upon deployment, the Watchman excludes the left atrial appendage from the systemic circulation. The benefits seen in PROTECT AF and other Watchman studies confirm the notion that the left atrial appendage is critical to the pathogenesis of stroke in atrial fibrillation (AF), Dr. Reddy observed.
The 707 PROTECT AF participants at 59 centers were split fairly evenly between those with paroxysmal, persistent, and permanent AF. Subjects were randomized two-to-one to the Watchman or conventional warfarin therapy. Subjects in the warfarin group spent an average of 70% of their time with drug levels in therapeutic range, a considerably higher figure than studies would suggest is the norm in everyday clinical practice.
The composite primary safety endpoint occurred in a similar proportion of Watchman recipients and controls. However, the nature and timing of the adverse events differed in the two groups.
The most common adverse event in the Watchman group was serious pericardial effusion, which occurred in 4.8% of patients. Two-thirds of affected patients were managed via percutaneous intervention; the rest underwent surgery. The incidence of pericardial effusion decreased with greater operator experience: The rate was 6.3% in the first half of the study and 3.7% afterwards. Of note, in more recent studies begun since procedural refinements were introduced, the pericardial effusion rate has been lower than in PROTECT AF.
The chief adverse event in the warfarin group was, not surprisingly, major bleeding, which occurred in 4.1% of subjects, compared with a 0.6% rate in Watchman recipients. Unlike adverse events in the Watchman group, which were mostly procedure related, complications in the warfarin group were spread out over the full course of follow-up.
Dr. Reddy considers the PROTECT AF results broadly applicable even in this era of new oral anticoagulants.
"Despite the availability of novel anticoagulants, warfarin is still the most widely prescribed agent for stroke prevention," he noted.
Asked if he thought the Food and Drug Administration is likely to approve the Watchman based upon the new evidence from PROTECT AF as well as other studies, Dr. Reddy said it’s difficult to predict the agency’s actions but he certainly considers the device approvable.
If and when the Watchman is approved, he added, the first population where it is likely to see widespread use is in AF patients for whom warfarin is contraindicated. It would also be logical to consider the device in patients with a relative contraindication to warfarin, such as those on dialysis, at increased risk for falls, and elderly patients on polypharmacy; indeed, those are patient groups in which any anticoagulant is problematic. Head-to-head comparative studies of the Watchman and the novel anticoagulants would be welcome but are unlikely to occur anytime soon, Dr. Reddy said.
A fifth and final year of follow-up is scheduled for the PROTECT AF study.
The trial is sponsored by Boston Scientific. Dr. Reddy reported serving as a consultant to or receiving research grant support from Boston Scientific, Coherex Medical, and St. Jude Medical.
DENVER – The investigational Watchman left atrial appendage closure device for stroke prevention in patients with atrial fibrillation proved markedly superior to warfarin in the 4-year follow-up of the randomized PROTECT AF trial.
Watchman recipients demonstrated a 40% reduction in the primary efficacy endpoint – a composite of stroke, systemic embolism, or cardiovascular death – compared with the warfarin-treated controls (95% confidence interval, 0.38-0.97; P = .03).

The Watchman group also showed a statistically significant 34% reduction in risk of all-cause mortality, compared with the 4.8% incidence in the warfarin arm (95% CI, 0.45-0.98; P = .04).
"This is something that’s really extraordinary. I wasn’t expecting to see a statistically significant decrease in all-cause mortality," Dr. Vivek Reddy said in presenting the new PROTECT AF data at the annual meeting of the Heart Rhythm Society.
The reduced all-cause mortality rate in the Watchman group resulted from fewer deaths due to cardiovascular-, pulmonary disease–, and hemorrhagic stroke–related causes.
In an earlier interim study analysis with 2.3 years and roughly 1,600 person-years of follow-up, the Watchman was merely statistically noninferior to warfarin in terms of efficacy (Circulation 2013;127:720-9). But in the updated analysis, with a mean follow-up of 45 months and 2,621 person-years, the device therapy has evolved in statistical parlance from noninferior to superior.
"The amount of benefit hasn’t changed. It’s been about a 40% difference throughout most of the trial. What’s changed is that with more follow-up the confidence intervals have narrowed, increasing the degree of certainty that the number is really true," according to Dr. Reddy of Mount Sinai School of Medicine, New York.
The Watchman’s superiority in terms of the primary efficacy endpoint was driven by an 85% reduction in the risk of hemorrhagic stroke and a 60% decrease in cardiovascular death compared with the warfarin group (see graphic). Importantly, the Watchman’s efficacy advantage was preserved in all prespecified patient subgroups, including the participants at greatest stroke risk: the roughly 20% with a baseline prior stroke or transient ischemic attack (TIA).
The Watchman is designed as a replacement for warfarin, a stroke prophylaxis drug whose numerous shortcomings are well known. The device is implanted in the left atrial appendage in a catheter procedure which entails an atrial puncture. Upon deployment, the Watchman excludes the left atrial appendage from the systemic circulation. The benefits seen in PROTECT AF and other Watchman studies confirm the notion that the left atrial appendage is critical to the pathogenesis of stroke in atrial fibrillation (AF), Dr. Reddy observed.
The 707 PROTECT AF participants at 59 centers were split fairly evenly between those with paroxysmal, persistent, and permanent AF. Subjects were randomized two-to-one to the Watchman or conventional warfarin therapy. Subjects in the warfarin group spent an average of 70% of their time with drug levels in therapeutic range, a considerably higher figure than studies would suggest is the norm in everyday clinical practice.
The composite primary safety endpoint occurred in a similar proportion of Watchman recipients and controls. However, the nature and timing of the adverse events differed in the two groups.
The most common adverse event in the Watchman group was serious pericardial effusion, which occurred in 4.8% of patients. Two-thirds of affected patients were managed via percutaneous intervention; the rest underwent surgery. The incidence of pericardial effusion decreased with greater operator experience: The rate was 6.3% in the first half of the study and 3.7% afterwards. Of note, in more recent studies begun since procedural refinements were introduced, the pericardial effusion rate has been lower than in PROTECT AF.
The chief adverse event in the warfarin group was, not surprisingly, major bleeding, which occurred in 4.1% of subjects, compared with a 0.6% rate in Watchman recipients. Unlike adverse events in the Watchman group, which were mostly procedure related, complications in the warfarin group were spread out over the full course of follow-up.
Dr. Reddy considers the PROTECT AF results broadly applicable even in this era of new oral anticoagulants.
"Despite the availability of novel anticoagulants, warfarin is still the most widely prescribed agent for stroke prevention," he noted.
Asked if he thought the Food and Drug Administration is likely to approve the Watchman based upon the new evidence from PROTECT AF as well as other studies, Dr. Reddy said it’s difficult to predict the agency’s actions but he certainly considers the device approvable.
If and when the Watchman is approved, he added, the first population where it is likely to see widespread use is in AF patients for whom warfarin is contraindicated. It would also be logical to consider the device in patients with a relative contraindication to warfarin, such as those on dialysis, at increased risk for falls, and elderly patients on polypharmacy; indeed, those are patient groups in which any anticoagulant is problematic. Head-to-head comparative studies of the Watchman and the novel anticoagulants would be welcome but are unlikely to occur anytime soon, Dr. Reddy said.
A fifth and final year of follow-up is scheduled for the PROTECT AF study.
The trial is sponsored by Boston Scientific. Dr. Reddy reported serving as a consultant to or receiving research grant support from Boston Scientific, Coherex Medical, and St. Jude Medical.
AT HEART RHYTHM 2013
Major finding: Patients with atrial fibrillation who received the Watchman left atrial appendage closure device had a 40% lower risk of the combined endpoint of stroke, systemic embolism, or cardiovascular death than those randomized to warfarin. They also had a 34% lower risk of all-cause mortality.
Data source: This analysis of the prospective, multicenter PROTECT AF trial included 707 randomized patients followed for a mean of 45 months.
Disclosures: PROTECT AF is sponsored by Boston Scientific. Dr. Reddy reported serving as a consultant to or receiving research grant support from Boston Scientific, Coherex Medical, and St. Jude Medical.