User login
Case
A 19-year-old female with a history of sickle cell anemia and hemoglobin SS, presents with a 2-day history of worsening lower back pain and dyspnea. Physical exam reveals oxygen saturation of 87% on room air, a temperature of 39.2° C, respiratory rate of 24 breaths per minute, and right-sided rales. Her hemoglobin is 5.3 g/dL (baseline hemoglobin of 7.8 g/dL). Chest radiograph reveals a right upper lobe pneumonia, and she is diagnosed with acute chest syndrome.
What are indications and complications of acute transfusion in sickle cell anemia?
Background
Chronic hemolytic anemia is a trademark of sickle cell anemia (SCA) or hemoglobin (Hb) SS as is acute anemia during illness or vaso-occlusive crises. Blood transfusions were the first therapy used in sickle cell disease, long before the pathophysiology was understood. Transfusion of red blood cells (RBC) increases the percentage of circulating normal Hb A, thereby decreasing the percentage of abnormal, sickled cells. This increases the oxygen-carrying capacity of the patient’s RBCs, improves organ perfusion, prevents organ damage, and can be life saving. SCA patients are the largest users of the United States rare donor blood bank registry.1
Unfortunately, transfusion comes with many risks including infection, transfusion reactions, alloimmunization, iron overload, hyperviscosity, and volume overload.
As SCA is a low-prevalence disease in a minority population, very few studies have been performed. Currently, the guidance available regarding blood transfusion is primarily based on expert opinion.
What to transfuse
Leukoreduced and intensive phenotypically matched RBC are not possible in many medical centers. Previous studies have noted decreased incidence of febrile nonhemolytic anemia transfusion reactions, cytomegalovirus transmission, and human leukocyte antigen alloimmunization in leukoreduced blood transfusions, however, these studies did not include SCA patients.2
Complications from transfusion
Complications from blood transfusions include febrile nonhemolytic transfusion reaction, acute hemolytic transfusion reaction (ABO incompatibility), transfusion-associated lung injury (TRALI), transfusion-associated circulatory overload (TACO), infections, and anaphylaxis. The National Heart, Lung, and Blood Institute guidelines specifically highlight the complications of delayed hemolytic transfusion reaction, iron overload, and hyperviscosity in SCA.Approximately 30% of SCA patients have alloantibodies.2 SCA patients may also develop autoimmunization, an immune response to their own RBC, particularly if the patient has multiple autoantibodies.
Infection is a risk for all individuals receiving transfusion. Screening for hepatitis B, hepatitis C, HIV, human T-cell lymphotropic virus, syphilis, West Nile virus, Trympanosoma, and bacteria are routinely performed but not 100% conclusive. Other diseases not routinely screened for include Creutzfeldt-Jakob disease, Babesia, human herpesvirus-8, dengue fever, malaria, and newer concerns such as Zika virus. 2,3
Febrile nonhemolytic transfusion reactions present as an increase in body temperature of more than 1° C during or shortly after receiving a blood transfusion in the absence of other pyrexic stimulus. Febrile nonhemolytic transfusion reaction occurs more frequently in patients with a previous history of transfusions. The use of leukoreduced RBCs reduces the occurrence to less than 1%.2
TRALI presents with the acute onset of hypoxemia and noncardiogenic pulmonary edema within 6 hours of a blood transfusion in the absence of other etiologies. The mechanism of TRALI is caused by an inflammatory response causing injury to the alveolar capillary membrane and the development of pulmonary edema.1
TACO presents with cardiogenic pulmonary edema not from another etiology. This is usually seen after transfusion of excessive volumes of blood or after excessively rapid rates of transfusion.1
Delayed hemolytic transfusion reaction (DHTR) may be a life-threatening immune response to donor cell antigens. The reaction is identified by a drop in the patient’s hemoglobin below the pretransfusion level, reticulocytopenia, a positive direct Coombs test, and occasionally jaundice on physical exam.2 Patients may have an unexpectedly high hemoglobin S% after transfusion from the hemolysis of donor cells. The pathognomonic feature is development of a new alloantibody. DHTR occurs more often in individuals who have received recurrent transfusions and has been reported in 4%-11% of transfused SCA patients.3 Donor and native cells hemolyze intra- and extravascularly 5-20 days after receiving a transfusion.2 DHTR is likely underestimated in SCA as it may be confused for a vaso-occlusive crisis.
Iron overload from recurrent transfusions is a slow, chronic process resulting in end organ damage of the heart, liver, and pancreas. It is associated with more frequent hospitalizations and higher mortality in SCA.3 The average person has 4-5 g of iron with no process to remove the excess. One unit of packed red blood cells adds 250-300 mg of iron.2 Ferritin somewhat correlates to iron overload but is not a reliable method because it is an acute-phase reactant. Liver biopsy is the current diagnostic gold standard, however, noninvasive MRI is gaining diagnostic credibility.
Hb SS blood has up to 10 times higher viscosity than does non–sickle cell blood at the same hemoglobin level. RBC transfusion increases the already hyperviscous state of SCA resulting in slow blood flow through vessels. The slow flow through small vessels from hyperviscosity may result in additional sickling and trigger or worsen a vaso-occlusive crisis. Avascular necrosis is theorized to be a result of hyperviscosity as it occurs more commonly in sickle cell patients with higher hemoglobin. It is important not to transfuse to baseline or above a hemoglobin of 10 g/dL to avoid worsening hyperviscosity.2
When to consider transfusion
Unfortunately, there are no strong randomized controlled trials to definitively dictate when simple transfusions or exchange transfusions are indicated. Acute simple transfusions should be considered in certain circumstances including acute chest syndrome, acute stroke, aplastic anemia, preoperative transfusion, splenic sequestration plus severe anemia, acute hepatic sequestration, and severe acute intrahepatic cholestasis.2
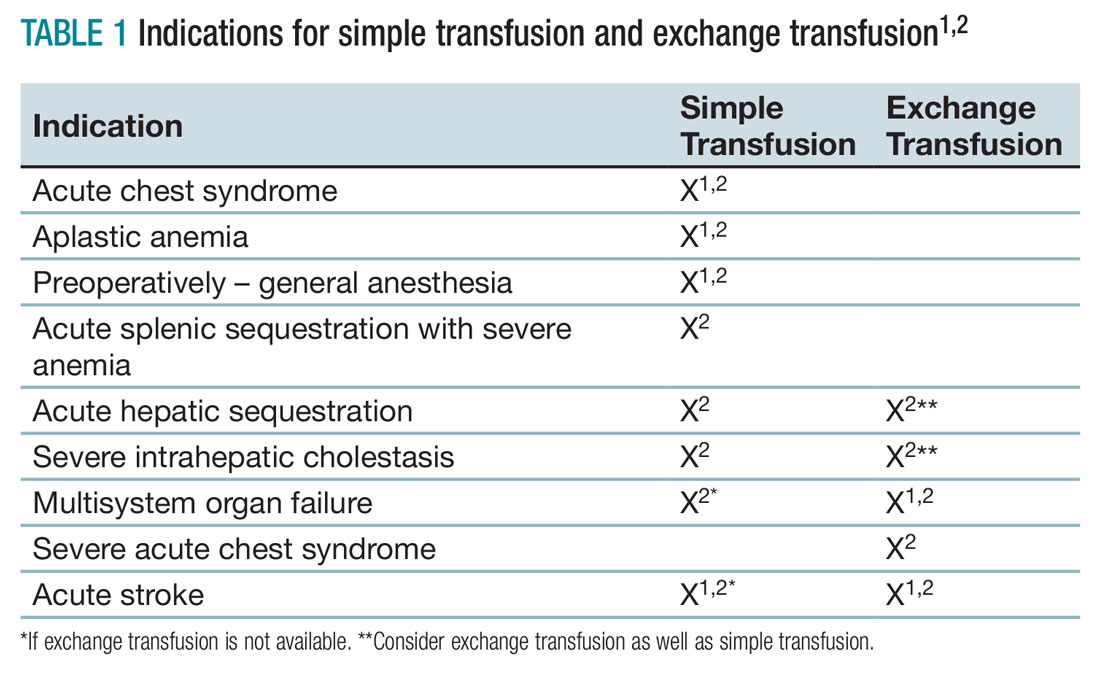
Few studies compare simple transfusion and exchange transfusion.2 The decision to use exchange transfusion over simple transfusion often is based on availability of exchange transfusion, ability of simple transfusion to decrease the percentage of hemoglobin S, and/or the patient’s current hemoglobin to avoid hyperviscosity from simple transfusion.3 Exchange transfusion should be considered for hemoglobin greater than 8-9 g/dL.2
Acute hepatic sequestration (AHS) occurs with the sequestration of RBCs in the liver and is marked by greater than 2 g/dL decrease in hemoglobin and hepatic enlargement, compared with baseline. The stretching of the hepatic capsule results in right upper quadrant pain. AHS often develops over a few hours to a few days with only mild elevation of liver function tests. AHS may be underestimated as two-thirds of SCA patients have hepatomegaly. Unless the hepatomegaly is radiographically monitored it may not be possible to determine an acute increase in liver size.2
Aplastic crisis presents as a gradual onset of fatigue, shortness of breath, and sometimes syncope or fever. Physical examination may reveal tachycardia and occasionally frank heart failure. The hemoglobin is usually far below the patient’s baseline level with an inappropriate, severely low reticulocyte count. Aplastic crisis should be transfused immediately because of the markedly short life expectancy of hemoglobin S RBCs, but does not need to be transfused to baseline.2
Acute splenic sequestration presents as a decrease in hemoglobin by greater than 2 g/dL, elevated reticulocyte count and circulating nucleated red blood cells, thrombocytopenia, and sudden splenomegaly.2 The goal of transfusion is for partial correction because of the risk of hyperviscosity when the spleen releases the sequestered RBCs.
Acute chest syndrome (ACS) presents as a pneumonia radiographically consistent with a respiratory tract infection caused by cough, shortness of breath, retractions, and/or rales. ACS is the most common cause of death in SCA. ACS is usually from infection but may be because of fat embolism, intrapulmonary aggregates of sickled cells, atelectasis, or pulmonary edema.2 If ACS has a hemoglobin decrease of greater than 1g/dL, consider transfusion.1,2
Severe acute chest syndrome is distinguished by radiographic evidence of multilobe pneumonia, increased work of breathing, pleural effusions, and oxygen saturation below 95% with supplemental oxygen. Severe ACS may have a decrease in hemoglobin despite receiving transfusion. Exchange transfusion is recommended because of the high mortality in severe ACS.2
Preoperative transfusion is used to decrease the incidence of postoperative vaso-occlusive crisis, acute stroke, or ACS for patients receiving general anesthesia. The goal for transfusion hemoglobin is 10g/dL. In SCA patients with a hemoglobin greater than 9g/dL, exchange transfusion may be considered to avoid hyperviscosity.1,2
Multisystem organ failure (MSOF) is severe and life-threatening lung, liver, and/or kidney failure. MSOF may occur after several days of hospitalization. It is often unanticipated and swift, frequently presenting with fever, a rapid increase in anemia, thrombocytopenia, and altered mental status. Lung failure often presents as ACS. Liver failure is marked by hyperbilirubinemia, elevated transaminases, and coagulopathy. Kidney failure is marked by elevated creatinine, with or without change in urine output and hyperkalemia. Rapid treatment with transfusion or exchange transfusion reduces mortality.
The incidence of acute ischemic stroke in SCA decreases with prophylactic transfusion of patients with elevated transcranial Dopplers. Acute stroke is usually secondary to stenosis or an occlusion of the internal carotid or middle cerebral artery. Acute hemorrhagic stroke may present as severe headache and loss of consciousness. Acute stroke should be confirmed radiographically, then exchange transfusion instituted rapidly.2
When not to transfuse
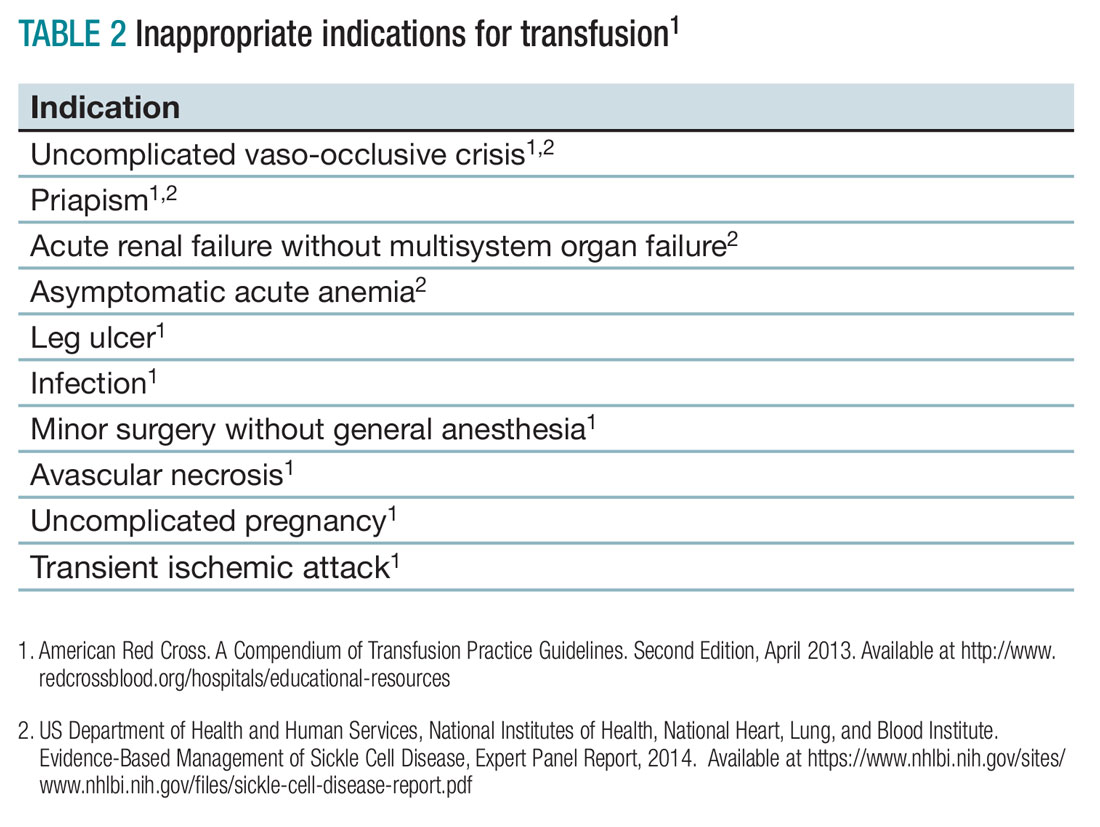
- Do not transfuse for simple vaso-occlusive crisis in the absence of symptoms attributable to acute anemia.1-3
- Do not transfuse for priapism.2
- Do not transfuse for acute renal failure unless there is MSOF.2
Back to the case
The patient was admitted for vaso-occlusive crisis and was started on patient-controlled analgesia with hydromorphone and IV fluids. Azithromycin and ceftriaxone were initiated empirically for community-acquired pneumonia. She was given one unit of phenotypically matched, leukoreduced RBCs for acute chest syndrome. Her hemoglobin increased to 6.1 g/dL. Her fever resolved on day 2, and her dyspnea improved on day 3 of hospitalization. She was weaned off of her patient-controlled analgesia on day 4 and discharged home on day 5 with moxifloxacin to complete 7 days of antibiotics.
Bottom line
Acute simple transfusions and exchange transfusions are indicated for multiple serious and life-threatening complications in SCA. However, transfusion has many serious and life-threatening potential adverse effects. It is essential to conduct a thorough risk-benefit analysis for each individual SCA patient. Whenever possible, intensive phenotypically matched and leukoreduced RBCs should be used. TH
References
1. American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease, Expert Panel Report, 2014.
3. Smith-Whitely, K and Thompson, AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59(2):358-64.
- SCA patients are at risk for serious transfusion complications including iron overload, delayed hemolytic transfusion reaction, and hyperviscosity in addition to the usual transfusion risks.
- Do not transfuse an uncomplicated vaso-occlusive crisis without symptomatic anemia.1-3
- Repeated transfusions create alloimmunization in SCA patients increasing risk for life-threatening transfusion reactions and difficulty locating phenotypically matched RBCs.
- Transfusion should be considered in SCA patients experiencing acute chest syndrome, aplastic anemia, splenic sequestration with acute anemia, acute hepatic sequestration, and severe intrahepatic cholestasis.1,2
- If available, exchange transfusion should be considered for SCA patients experiencing multisystem organ failure, acute stroke, and severe acute chest syndrome.1,2
- American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
Case
A 19-year-old female with a history of sickle cell anemia and hemoglobin SS, presents with a 2-day history of worsening lower back pain and dyspnea. Physical exam reveals oxygen saturation of 87% on room air, a temperature of 39.2° C, respiratory rate of 24 breaths per minute, and right-sided rales. Her hemoglobin is 5.3 g/dL (baseline hemoglobin of 7.8 g/dL). Chest radiograph reveals a right upper lobe pneumonia, and she is diagnosed with acute chest syndrome.
What are indications and complications of acute transfusion in sickle cell anemia?
Background
Chronic hemolytic anemia is a trademark of sickle cell anemia (SCA) or hemoglobin (Hb) SS as is acute anemia during illness or vaso-occlusive crises. Blood transfusions were the first therapy used in sickle cell disease, long before the pathophysiology was understood. Transfusion of red blood cells (RBC) increases the percentage of circulating normal Hb A, thereby decreasing the percentage of abnormal, sickled cells. This increases the oxygen-carrying capacity of the patient’s RBCs, improves organ perfusion, prevents organ damage, and can be life saving. SCA patients are the largest users of the United States rare donor blood bank registry.1
Unfortunately, transfusion comes with many risks including infection, transfusion reactions, alloimmunization, iron overload, hyperviscosity, and volume overload.
As SCA is a low-prevalence disease in a minority population, very few studies have been performed. Currently, the guidance available regarding blood transfusion is primarily based on expert opinion.
What to transfuse
Leukoreduced and intensive phenotypically matched RBC are not possible in many medical centers. Previous studies have noted decreased incidence of febrile nonhemolytic anemia transfusion reactions, cytomegalovirus transmission, and human leukocyte antigen alloimmunization in leukoreduced blood transfusions, however, these studies did not include SCA patients.2
Complications from transfusion
Complications from blood transfusions include febrile nonhemolytic transfusion reaction, acute hemolytic transfusion reaction (ABO incompatibility), transfusion-associated lung injury (TRALI), transfusion-associated circulatory overload (TACO), infections, and anaphylaxis. The National Heart, Lung, and Blood Institute guidelines specifically highlight the complications of delayed hemolytic transfusion reaction, iron overload, and hyperviscosity in SCA.Approximately 30% of SCA patients have alloantibodies.2 SCA patients may also develop autoimmunization, an immune response to their own RBC, particularly if the patient has multiple autoantibodies.
Infection is a risk for all individuals receiving transfusion. Screening for hepatitis B, hepatitis C, HIV, human T-cell lymphotropic virus, syphilis, West Nile virus, Trympanosoma, and bacteria are routinely performed but not 100% conclusive. Other diseases not routinely screened for include Creutzfeldt-Jakob disease, Babesia, human herpesvirus-8, dengue fever, malaria, and newer concerns such as Zika virus. 2,3
Febrile nonhemolytic transfusion reactions present as an increase in body temperature of more than 1° C during or shortly after receiving a blood transfusion in the absence of other pyrexic stimulus. Febrile nonhemolytic transfusion reaction occurs more frequently in patients with a previous history of transfusions. The use of leukoreduced RBCs reduces the occurrence to less than 1%.2
TRALI presents with the acute onset of hypoxemia and noncardiogenic pulmonary edema within 6 hours of a blood transfusion in the absence of other etiologies. The mechanism of TRALI is caused by an inflammatory response causing injury to the alveolar capillary membrane and the development of pulmonary edema.1
TACO presents with cardiogenic pulmonary edema not from another etiology. This is usually seen after transfusion of excessive volumes of blood or after excessively rapid rates of transfusion.1
Delayed hemolytic transfusion reaction (DHTR) may be a life-threatening immune response to donor cell antigens. The reaction is identified by a drop in the patient’s hemoglobin below the pretransfusion level, reticulocytopenia, a positive direct Coombs test, and occasionally jaundice on physical exam.2 Patients may have an unexpectedly high hemoglobin S% after transfusion from the hemolysis of donor cells. The pathognomonic feature is development of a new alloantibody. DHTR occurs more often in individuals who have received recurrent transfusions and has been reported in 4%-11% of transfused SCA patients.3 Donor and native cells hemolyze intra- and extravascularly 5-20 days after receiving a transfusion.2 DHTR is likely underestimated in SCA as it may be confused for a vaso-occlusive crisis.
Iron overload from recurrent transfusions is a slow, chronic process resulting in end organ damage of the heart, liver, and pancreas. It is associated with more frequent hospitalizations and higher mortality in SCA.3 The average person has 4-5 g of iron with no process to remove the excess. One unit of packed red blood cells adds 250-300 mg of iron.2 Ferritin somewhat correlates to iron overload but is not a reliable method because it is an acute-phase reactant. Liver biopsy is the current diagnostic gold standard, however, noninvasive MRI is gaining diagnostic credibility.
Hb SS blood has up to 10 times higher viscosity than does non–sickle cell blood at the same hemoglobin level. RBC transfusion increases the already hyperviscous state of SCA resulting in slow blood flow through vessels. The slow flow through small vessels from hyperviscosity may result in additional sickling and trigger or worsen a vaso-occlusive crisis. Avascular necrosis is theorized to be a result of hyperviscosity as it occurs more commonly in sickle cell patients with higher hemoglobin. It is important not to transfuse to baseline or above a hemoglobin of 10 g/dL to avoid worsening hyperviscosity.2
When to consider transfusion
Unfortunately, there are no strong randomized controlled trials to definitively dictate when simple transfusions or exchange transfusions are indicated. Acute simple transfusions should be considered in certain circumstances including acute chest syndrome, acute stroke, aplastic anemia, preoperative transfusion, splenic sequestration plus severe anemia, acute hepatic sequestration, and severe acute intrahepatic cholestasis.2

Few studies compare simple transfusion and exchange transfusion.2 The decision to use exchange transfusion over simple transfusion often is based on availability of exchange transfusion, ability of simple transfusion to decrease the percentage of hemoglobin S, and/or the patient’s current hemoglobin to avoid hyperviscosity from simple transfusion.3 Exchange transfusion should be considered for hemoglobin greater than 8-9 g/dL.2
Acute hepatic sequestration (AHS) occurs with the sequestration of RBCs in the liver and is marked by greater than 2 g/dL decrease in hemoglobin and hepatic enlargement, compared with baseline. The stretching of the hepatic capsule results in right upper quadrant pain. AHS often develops over a few hours to a few days with only mild elevation of liver function tests. AHS may be underestimated as two-thirds of SCA patients have hepatomegaly. Unless the hepatomegaly is radiographically monitored it may not be possible to determine an acute increase in liver size.2
Aplastic crisis presents as a gradual onset of fatigue, shortness of breath, and sometimes syncope or fever. Physical examination may reveal tachycardia and occasionally frank heart failure. The hemoglobin is usually far below the patient’s baseline level with an inappropriate, severely low reticulocyte count. Aplastic crisis should be transfused immediately because of the markedly short life expectancy of hemoglobin S RBCs, but does not need to be transfused to baseline.2
Acute splenic sequestration presents as a decrease in hemoglobin by greater than 2 g/dL, elevated reticulocyte count and circulating nucleated red blood cells, thrombocytopenia, and sudden splenomegaly.2 The goal of transfusion is for partial correction because of the risk of hyperviscosity when the spleen releases the sequestered RBCs.
Acute chest syndrome (ACS) presents as a pneumonia radiographically consistent with a respiratory tract infection caused by cough, shortness of breath, retractions, and/or rales. ACS is the most common cause of death in SCA. ACS is usually from infection but may be because of fat embolism, intrapulmonary aggregates of sickled cells, atelectasis, or pulmonary edema.2 If ACS has a hemoglobin decrease of greater than 1g/dL, consider transfusion.1,2
Severe acute chest syndrome is distinguished by radiographic evidence of multilobe pneumonia, increased work of breathing, pleural effusions, and oxygen saturation below 95% with supplemental oxygen. Severe ACS may have a decrease in hemoglobin despite receiving transfusion. Exchange transfusion is recommended because of the high mortality in severe ACS.2
Preoperative transfusion is used to decrease the incidence of postoperative vaso-occlusive crisis, acute stroke, or ACS for patients receiving general anesthesia. The goal for transfusion hemoglobin is 10g/dL. In SCA patients with a hemoglobin greater than 9g/dL, exchange transfusion may be considered to avoid hyperviscosity.1,2
Multisystem organ failure (MSOF) is severe and life-threatening lung, liver, and/or kidney failure. MSOF may occur after several days of hospitalization. It is often unanticipated and swift, frequently presenting with fever, a rapid increase in anemia, thrombocytopenia, and altered mental status. Lung failure often presents as ACS. Liver failure is marked by hyperbilirubinemia, elevated transaminases, and coagulopathy. Kidney failure is marked by elevated creatinine, with or without change in urine output and hyperkalemia. Rapid treatment with transfusion or exchange transfusion reduces mortality.
The incidence of acute ischemic stroke in SCA decreases with prophylactic transfusion of patients with elevated transcranial Dopplers. Acute stroke is usually secondary to stenosis or an occlusion of the internal carotid or middle cerebral artery. Acute hemorrhagic stroke may present as severe headache and loss of consciousness. Acute stroke should be confirmed radiographically, then exchange transfusion instituted rapidly.2
When not to transfuse
- Do not transfuse for simple vaso-occlusive crisis in the absence of symptoms attributable to acute anemia.1-3
- Do not transfuse for priapism.2
- Do not transfuse for acute renal failure unless there is MSOF.2
Back to the case
The patient was admitted for vaso-occlusive crisis and was started on patient-controlled analgesia with hydromorphone and IV fluids. Azithromycin and ceftriaxone were initiated empirically for community-acquired pneumonia. She was given one unit of phenotypically matched, leukoreduced RBCs for acute chest syndrome. Her hemoglobin increased to 6.1 g/dL. Her fever resolved on day 2, and her dyspnea improved on day 3 of hospitalization. She was weaned off of her patient-controlled analgesia on day 4 and discharged home on day 5 with moxifloxacin to complete 7 days of antibiotics.
Bottom line
Acute simple transfusions and exchange transfusions are indicated for multiple serious and life-threatening complications in SCA. However, transfusion has many serious and life-threatening potential adverse effects. It is essential to conduct a thorough risk-benefit analysis for each individual SCA patient. Whenever possible, intensive phenotypically matched and leukoreduced RBCs should be used. TH
References
1. American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease, Expert Panel Report, 2014.
3. Smith-Whitely, K and Thompson, AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59(2):358-64.
- SCA patients are at risk for serious transfusion complications including iron overload, delayed hemolytic transfusion reaction, and hyperviscosity in addition to the usual transfusion risks.
- Do not transfuse an uncomplicated vaso-occlusive crisis without symptomatic anemia.1-3
- Repeated transfusions create alloimmunization in SCA patients increasing risk for life-threatening transfusion reactions and difficulty locating phenotypically matched RBCs.
- Transfusion should be considered in SCA patients experiencing acute chest syndrome, aplastic anemia, splenic sequestration with acute anemia, acute hepatic sequestration, and severe intrahepatic cholestasis.1,2
- If available, exchange transfusion should be considered for SCA patients experiencing multisystem organ failure, acute stroke, and severe acute chest syndrome.1,2
- American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
Case
A 19-year-old female with a history of sickle cell anemia and hemoglobin SS, presents with a 2-day history of worsening lower back pain and dyspnea. Physical exam reveals oxygen saturation of 87% on room air, a temperature of 39.2° C, respiratory rate of 24 breaths per minute, and right-sided rales. Her hemoglobin is 5.3 g/dL (baseline hemoglobin of 7.8 g/dL). Chest radiograph reveals a right upper lobe pneumonia, and she is diagnosed with acute chest syndrome.
What are indications and complications of acute transfusion in sickle cell anemia?
Background
Chronic hemolytic anemia is a trademark of sickle cell anemia (SCA) or hemoglobin (Hb) SS as is acute anemia during illness or vaso-occlusive crises. Blood transfusions were the first therapy used in sickle cell disease, long before the pathophysiology was understood. Transfusion of red blood cells (RBC) increases the percentage of circulating normal Hb A, thereby decreasing the percentage of abnormal, sickled cells. This increases the oxygen-carrying capacity of the patient’s RBCs, improves organ perfusion, prevents organ damage, and can be life saving. SCA patients are the largest users of the United States rare donor blood bank registry.1
Unfortunately, transfusion comes with many risks including infection, transfusion reactions, alloimmunization, iron overload, hyperviscosity, and volume overload.
As SCA is a low-prevalence disease in a minority population, very few studies have been performed. Currently, the guidance available regarding blood transfusion is primarily based on expert opinion.
What to transfuse
Leukoreduced and intensive phenotypically matched RBC are not possible in many medical centers. Previous studies have noted decreased incidence of febrile nonhemolytic anemia transfusion reactions, cytomegalovirus transmission, and human leukocyte antigen alloimmunization in leukoreduced blood transfusions, however, these studies did not include SCA patients.2
Complications from transfusion
Complications from blood transfusions include febrile nonhemolytic transfusion reaction, acute hemolytic transfusion reaction (ABO incompatibility), transfusion-associated lung injury (TRALI), transfusion-associated circulatory overload (TACO), infections, and anaphylaxis. The National Heart, Lung, and Blood Institute guidelines specifically highlight the complications of delayed hemolytic transfusion reaction, iron overload, and hyperviscosity in SCA.Approximately 30% of SCA patients have alloantibodies.2 SCA patients may also develop autoimmunization, an immune response to their own RBC, particularly if the patient has multiple autoantibodies.
Infection is a risk for all individuals receiving transfusion. Screening for hepatitis B, hepatitis C, HIV, human T-cell lymphotropic virus, syphilis, West Nile virus, Trympanosoma, and bacteria are routinely performed but not 100% conclusive. Other diseases not routinely screened for include Creutzfeldt-Jakob disease, Babesia, human herpesvirus-8, dengue fever, malaria, and newer concerns such as Zika virus. 2,3
Febrile nonhemolytic transfusion reactions present as an increase in body temperature of more than 1° C during or shortly after receiving a blood transfusion in the absence of other pyrexic stimulus. Febrile nonhemolytic transfusion reaction occurs more frequently in patients with a previous history of transfusions. The use of leukoreduced RBCs reduces the occurrence to less than 1%.2
TRALI presents with the acute onset of hypoxemia and noncardiogenic pulmonary edema within 6 hours of a blood transfusion in the absence of other etiologies. The mechanism of TRALI is caused by an inflammatory response causing injury to the alveolar capillary membrane and the development of pulmonary edema.1
TACO presents with cardiogenic pulmonary edema not from another etiology. This is usually seen after transfusion of excessive volumes of blood or after excessively rapid rates of transfusion.1
Delayed hemolytic transfusion reaction (DHTR) may be a life-threatening immune response to donor cell antigens. The reaction is identified by a drop in the patient’s hemoglobin below the pretransfusion level, reticulocytopenia, a positive direct Coombs test, and occasionally jaundice on physical exam.2 Patients may have an unexpectedly high hemoglobin S% after transfusion from the hemolysis of donor cells. The pathognomonic feature is development of a new alloantibody. DHTR occurs more often in individuals who have received recurrent transfusions and has been reported in 4%-11% of transfused SCA patients.3 Donor and native cells hemolyze intra- and extravascularly 5-20 days after receiving a transfusion.2 DHTR is likely underestimated in SCA as it may be confused for a vaso-occlusive crisis.
Iron overload from recurrent transfusions is a slow, chronic process resulting in end organ damage of the heart, liver, and pancreas. It is associated with more frequent hospitalizations and higher mortality in SCA.3 The average person has 4-5 g of iron with no process to remove the excess. One unit of packed red blood cells adds 250-300 mg of iron.2 Ferritin somewhat correlates to iron overload but is not a reliable method because it is an acute-phase reactant. Liver biopsy is the current diagnostic gold standard, however, noninvasive MRI is gaining diagnostic credibility.
Hb SS blood has up to 10 times higher viscosity than does non–sickle cell blood at the same hemoglobin level. RBC transfusion increases the already hyperviscous state of SCA resulting in slow blood flow through vessels. The slow flow through small vessels from hyperviscosity may result in additional sickling and trigger or worsen a vaso-occlusive crisis. Avascular necrosis is theorized to be a result of hyperviscosity as it occurs more commonly in sickle cell patients with higher hemoglobin. It is important not to transfuse to baseline or above a hemoglobin of 10 g/dL to avoid worsening hyperviscosity.2
When to consider transfusion
Unfortunately, there are no strong randomized controlled trials to definitively dictate when simple transfusions or exchange transfusions are indicated. Acute simple transfusions should be considered in certain circumstances including acute chest syndrome, acute stroke, aplastic anemia, preoperative transfusion, splenic sequestration plus severe anemia, acute hepatic sequestration, and severe acute intrahepatic cholestasis.2

Few studies compare simple transfusion and exchange transfusion.2 The decision to use exchange transfusion over simple transfusion often is based on availability of exchange transfusion, ability of simple transfusion to decrease the percentage of hemoglobin S, and/or the patient’s current hemoglobin to avoid hyperviscosity from simple transfusion.3 Exchange transfusion should be considered for hemoglobin greater than 8-9 g/dL.2
Acute hepatic sequestration (AHS) occurs with the sequestration of RBCs in the liver and is marked by greater than 2 g/dL decrease in hemoglobin and hepatic enlargement, compared with baseline. The stretching of the hepatic capsule results in right upper quadrant pain. AHS often develops over a few hours to a few days with only mild elevation of liver function tests. AHS may be underestimated as two-thirds of SCA patients have hepatomegaly. Unless the hepatomegaly is radiographically monitored it may not be possible to determine an acute increase in liver size.2
Aplastic crisis presents as a gradual onset of fatigue, shortness of breath, and sometimes syncope or fever. Physical examination may reveal tachycardia and occasionally frank heart failure. The hemoglobin is usually far below the patient’s baseline level with an inappropriate, severely low reticulocyte count. Aplastic crisis should be transfused immediately because of the markedly short life expectancy of hemoglobin S RBCs, but does not need to be transfused to baseline.2
Acute splenic sequestration presents as a decrease in hemoglobin by greater than 2 g/dL, elevated reticulocyte count and circulating nucleated red blood cells, thrombocytopenia, and sudden splenomegaly.2 The goal of transfusion is for partial correction because of the risk of hyperviscosity when the spleen releases the sequestered RBCs.
Acute chest syndrome (ACS) presents as a pneumonia radiographically consistent with a respiratory tract infection caused by cough, shortness of breath, retractions, and/or rales. ACS is the most common cause of death in SCA. ACS is usually from infection but may be because of fat embolism, intrapulmonary aggregates of sickled cells, atelectasis, or pulmonary edema.2 If ACS has a hemoglobin decrease of greater than 1g/dL, consider transfusion.1,2
Severe acute chest syndrome is distinguished by radiographic evidence of multilobe pneumonia, increased work of breathing, pleural effusions, and oxygen saturation below 95% with supplemental oxygen. Severe ACS may have a decrease in hemoglobin despite receiving transfusion. Exchange transfusion is recommended because of the high mortality in severe ACS.2
Preoperative transfusion is used to decrease the incidence of postoperative vaso-occlusive crisis, acute stroke, or ACS for patients receiving general anesthesia. The goal for transfusion hemoglobin is 10g/dL. In SCA patients with a hemoglobin greater than 9g/dL, exchange transfusion may be considered to avoid hyperviscosity.1,2
Multisystem organ failure (MSOF) is severe and life-threatening lung, liver, and/or kidney failure. MSOF may occur after several days of hospitalization. It is often unanticipated and swift, frequently presenting with fever, a rapid increase in anemia, thrombocytopenia, and altered mental status. Lung failure often presents as ACS. Liver failure is marked by hyperbilirubinemia, elevated transaminases, and coagulopathy. Kidney failure is marked by elevated creatinine, with or without change in urine output and hyperkalemia. Rapid treatment with transfusion or exchange transfusion reduces mortality.
The incidence of acute ischemic stroke in SCA decreases with prophylactic transfusion of patients with elevated transcranial Dopplers. Acute stroke is usually secondary to stenosis or an occlusion of the internal carotid or middle cerebral artery. Acute hemorrhagic stroke may present as severe headache and loss of consciousness. Acute stroke should be confirmed radiographically, then exchange transfusion instituted rapidly.2
When not to transfuse
- Do not transfuse for simple vaso-occlusive crisis in the absence of symptoms attributable to acute anemia.1-3
- Do not transfuse for priapism.2
- Do not transfuse for acute renal failure unless there is MSOF.2
Back to the case
The patient was admitted for vaso-occlusive crisis and was started on patient-controlled analgesia with hydromorphone and IV fluids. Azithromycin and ceftriaxone were initiated empirically for community-acquired pneumonia. She was given one unit of phenotypically matched, leukoreduced RBCs for acute chest syndrome. Her hemoglobin increased to 6.1 g/dL. Her fever resolved on day 2, and her dyspnea improved on day 3 of hospitalization. She was weaned off of her patient-controlled analgesia on day 4 and discharged home on day 5 with moxifloxacin to complete 7 days of antibiotics.
Bottom line
Acute simple transfusions and exchange transfusions are indicated for multiple serious and life-threatening complications in SCA. However, transfusion has many serious and life-threatening potential adverse effects. It is essential to conduct a thorough risk-benefit analysis for each individual SCA patient. Whenever possible, intensive phenotypically matched and leukoreduced RBCs should be used. TH
References
1. American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease, Expert Panel Report, 2014.
3. Smith-Whitely, K and Thompson, AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59(2):358-64.
- SCA patients are at risk for serious transfusion complications including iron overload, delayed hemolytic transfusion reaction, and hyperviscosity in addition to the usual transfusion risks.
- Do not transfuse an uncomplicated vaso-occlusive crisis without symptomatic anemia.1-3
- Repeated transfusions create alloimmunization in SCA patients increasing risk for life-threatening transfusion reactions and difficulty locating phenotypically matched RBCs.
- Transfusion should be considered in SCA patients experiencing acute chest syndrome, aplastic anemia, splenic sequestration with acute anemia, acute hepatic sequestration, and severe intrahepatic cholestasis.1,2
- If available, exchange transfusion should be considered for SCA patients experiencing multisystem organ failure, acute stroke, and severe acute chest syndrome.1,2
- American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.