User login
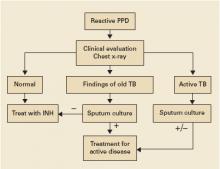
Clinical evaluation and chest x-ray are recommended for asymptomatic patients with a positive purified protein derivative (PPD) test result, to exclude the slight possibility of active tuberculosis (TB). Patients with radiographic evidence of old (healed) TB infection should also undergo sputum testing (strength of recommendation [SOR]: C, expert opinion).
Treatment with isoniazid (INH) monotherapy (300 mg/d) reduces progression of latent tuberculosis to active disease (SOR: A, large randomized controlled trials [RCT]), with 9 months as the optimal treatment length (SOR: B, derivation from RCTs). A 3-month course of combined rifampin (600 mg/d) and INH (300 mg/d) is equivalent in efficacy to INH monotherapy and is associated with similar rates of toxicity (SOR: A, meta-analysis of RCTs), but this regimen is not included in Centers for Disease Control and Prevention recommendations.
Address patient concerns about TB and treatment side effects
Richard Guthmann, MD
University of Illinois at Chicago/Advocate Illinois Masonic Family Medicine Residency, Chicago
Patients’ understanding of tuberculosis—the disease, the treatment, and the outcome—poses an important challenge in the care of an asymptomatic PPD-positive patient. These patients may ask, “Will I get sick? Do I have to take the medicine? Are there side effects? And would you take the medicine?” We need to be prepared to answer these questions.
Most patients with a positive PPD will not get active tuberculosis, but when they do it can be serious and it can spread easily. The medication significantly decreases the risk of developing active tuberculosis. The medication side effects are uncommon but can be severe. These side effects are reversible if the medication is stopped promptly. Under the supervision of my physician, I would take the medicine.
Evidence summary
Clinical evaluation with medical history and physical exam, chest radiography, and selected sputum sampling to exclude active tuberculosis are part of the recommended algorithm for all patients who develop a positive PPD (FIGURE).1-3 These recommendations are derived from expert opinion, and their usefulness has not been evaluated in any population-based study of asymptomatic PPD-positive patients.
A comprehensive review of RCTs from the 1950s and 1960s demonstrated that INH treatment of patients with latent tuberculosis infection is effective in decreasing the progression to active tuberculosis.4 A series of double-blinded RCTs performed by the US Public Health Service included 25,923 patients with latent tuberculosis who were randomized to receive either daily INH or placebo for 1 year with 6- to 10-year follow-up. Groups studied included household contacts of patients with active tuberculosis (rate of progression to active disease in placebo group [baseline rate]=27/1000, relative risk with INH [RR]=0.4, number needed to treat [NNT]=63), patients in mental institutions (baseline rate=12/1000, RR=0.3, NNT=121), and patients with x-ray findings of healed tuberculosis (baseline rate=69/1000, RR=0.4, NNT=23).
The optimal length of treatment for PPD-positive patients without active disease was evaluated through 1 double-blinded RCT enrolling 28,000 patients with 5-year follow-up after 12, 24, or 52 weeks of INH or placebo. Active TB developed in 0.35% (24/6919) after 52 weeks of INH compared with 0.49% (34/6965) after 24 weeks (RR=1.4, NNT=708).5 Incidence in the placebo group was 1.4%. Subgroup analysis determined that maximum efficacy with fewest side effects was achieved at 9 months.6 Nine months of INH is also recommended for HIV-positive patients, based on extrapolations from these and other studies.3
INH monotherapy was compared with combination INH and rifampin in a 2005 meta-analysis of 5 RCTs of variable quality involving 1926 patients.7 This meta-analysis found equivalency in risk of active TB and mortality between INH monotherapy for 6 to 12 months and the combination of rifampin and INH for 3 months (pooled risk difference=0%; 95% confidence interval [CI], –1% to 2%). This study also showed similar rates of adverse events in both groups (pooled risk difference=–1%; 95% CI, –7% to 5%). Short-course combination rifampin and pyrazinamide is no longer recommended after an open-label RCT with 589 patients demonstrated severe hepatoxicity in 7.7% (16/207) on a 2-month course of pyrazinamide and rifampin, compared with 1% (2/204) on 6 months of INH (RR=7.9, number needed to harm=15).8 Rifampin monotherapy has only been studied in patients with silicosis in a RCT enrolling 652 participants with latent tuberculosis. A 12-week course of rifampin (600 mg daily) was as effective as 6 months of INH in preventing development of active TB over the next 5 years.9
FIGURE
Suggested workup of asymptomatic, HIV-negative patients with a positive PPD
Source: Am J Respir Crit Care Med 2000;2 Jasmer et al, N Engl J Med 2002.3
Recommendations from others
Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Disease Society of America guidelines recommend targeted screening of high-risk persons followed by further clinical evaluation of all those with a reactive PPD (FIGURE).2,10 The recommended treatment regimen for latent TB is daily INH for 9 months. Less preferable regimens are daily INH for 6 months, or daily rifampin for 4 months in patients who cannot tolerate INH. A 2-month course of rifampin and pyrazinamide is no longer recommended. The recent meta-analysis supporting a 3-month regimen of combination INH and rifampin has not been incorporated into expert guidelines.7
1. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
2. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med 2002;347:1860-1866.
4. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc 1970;26:28-106.
5. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555-564.
6. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;3:847-850.
7. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005;40:670-676.
8. Jasmer RM, Saukkonen JJ, Blumberg HM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640-647.
9. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41.
10. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. MMWR Recomm Rep 2005;54(RR-12):1-81.
Clinical evaluation and chest x-ray are recommended for asymptomatic patients with a positive purified protein derivative (PPD) test result, to exclude the slight possibility of active tuberculosis (TB). Patients with radiographic evidence of old (healed) TB infection should also undergo sputum testing (strength of recommendation [SOR]: C, expert opinion).
Treatment with isoniazid (INH) monotherapy (300 mg/d) reduces progression of latent tuberculosis to active disease (SOR: A, large randomized controlled trials [RCT]), with 9 months as the optimal treatment length (SOR: B, derivation from RCTs). A 3-month course of combined rifampin (600 mg/d) and INH (300 mg/d) is equivalent in efficacy to INH monotherapy and is associated with similar rates of toxicity (SOR: A, meta-analysis of RCTs), but this regimen is not included in Centers for Disease Control and Prevention recommendations.
Address patient concerns about TB and treatment side effects
Richard Guthmann, MD
University of Illinois at Chicago/Advocate Illinois Masonic Family Medicine Residency, Chicago
Patients’ understanding of tuberculosis—the disease, the treatment, and the outcome—poses an important challenge in the care of an asymptomatic PPD-positive patient. These patients may ask, “Will I get sick? Do I have to take the medicine? Are there side effects? And would you take the medicine?” We need to be prepared to answer these questions.
Most patients with a positive PPD will not get active tuberculosis, but when they do it can be serious and it can spread easily. The medication significantly decreases the risk of developing active tuberculosis. The medication side effects are uncommon but can be severe. These side effects are reversible if the medication is stopped promptly. Under the supervision of my physician, I would take the medicine.
Evidence summary
Clinical evaluation with medical history and physical exam, chest radiography, and selected sputum sampling to exclude active tuberculosis are part of the recommended algorithm for all patients who develop a positive PPD (FIGURE).1-3 These recommendations are derived from expert opinion, and their usefulness has not been evaluated in any population-based study of asymptomatic PPD-positive patients.
A comprehensive review of RCTs from the 1950s and 1960s demonstrated that INH treatment of patients with latent tuberculosis infection is effective in decreasing the progression to active tuberculosis.4 A series of double-blinded RCTs performed by the US Public Health Service included 25,923 patients with latent tuberculosis who were randomized to receive either daily INH or placebo for 1 year with 6- to 10-year follow-up. Groups studied included household contacts of patients with active tuberculosis (rate of progression to active disease in placebo group [baseline rate]=27/1000, relative risk with INH [RR]=0.4, number needed to treat [NNT]=63), patients in mental institutions (baseline rate=12/1000, RR=0.3, NNT=121), and patients with x-ray findings of healed tuberculosis (baseline rate=69/1000, RR=0.4, NNT=23).
The optimal length of treatment for PPD-positive patients without active disease was evaluated through 1 double-blinded RCT enrolling 28,000 patients with 5-year follow-up after 12, 24, or 52 weeks of INH or placebo. Active TB developed in 0.35% (24/6919) after 52 weeks of INH compared with 0.49% (34/6965) after 24 weeks (RR=1.4, NNT=708).5 Incidence in the placebo group was 1.4%. Subgroup analysis determined that maximum efficacy with fewest side effects was achieved at 9 months.6 Nine months of INH is also recommended for HIV-positive patients, based on extrapolations from these and other studies.3
INH monotherapy was compared with combination INH and rifampin in a 2005 meta-analysis of 5 RCTs of variable quality involving 1926 patients.7 This meta-analysis found equivalency in risk of active TB and mortality between INH monotherapy for 6 to 12 months and the combination of rifampin and INH for 3 months (pooled risk difference=0%; 95% confidence interval [CI], –1% to 2%). This study also showed similar rates of adverse events in both groups (pooled risk difference=–1%; 95% CI, –7% to 5%). Short-course combination rifampin and pyrazinamide is no longer recommended after an open-label RCT with 589 patients demonstrated severe hepatoxicity in 7.7% (16/207) on a 2-month course of pyrazinamide and rifampin, compared with 1% (2/204) on 6 months of INH (RR=7.9, number needed to harm=15).8 Rifampin monotherapy has only been studied in patients with silicosis in a RCT enrolling 652 participants with latent tuberculosis. A 12-week course of rifampin (600 mg daily) was as effective as 6 months of INH in preventing development of active TB over the next 5 years.9
FIGURE
Suggested workup of asymptomatic, HIV-negative patients with a positive PPD
Source: Am J Respir Crit Care Med 2000;2 Jasmer et al, N Engl J Med 2002.3
Recommendations from others
Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Disease Society of America guidelines recommend targeted screening of high-risk persons followed by further clinical evaluation of all those with a reactive PPD (FIGURE).2,10 The recommended treatment regimen for latent TB is daily INH for 9 months. Less preferable regimens are daily INH for 6 months, or daily rifampin for 4 months in patients who cannot tolerate INH. A 2-month course of rifampin and pyrazinamide is no longer recommended. The recent meta-analysis supporting a 3-month regimen of combination INH and rifampin has not been incorporated into expert guidelines.7
Clinical evaluation and chest x-ray are recommended for asymptomatic patients with a positive purified protein derivative (PPD) test result, to exclude the slight possibility of active tuberculosis (TB). Patients with radiographic evidence of old (healed) TB infection should also undergo sputum testing (strength of recommendation [SOR]: C, expert opinion).
Treatment with isoniazid (INH) monotherapy (300 mg/d) reduces progression of latent tuberculosis to active disease (SOR: A, large randomized controlled trials [RCT]), with 9 months as the optimal treatment length (SOR: B, derivation from RCTs). A 3-month course of combined rifampin (600 mg/d) and INH (300 mg/d) is equivalent in efficacy to INH monotherapy and is associated with similar rates of toxicity (SOR: A, meta-analysis of RCTs), but this regimen is not included in Centers for Disease Control and Prevention recommendations.
Address patient concerns about TB and treatment side effects
Richard Guthmann, MD
University of Illinois at Chicago/Advocate Illinois Masonic Family Medicine Residency, Chicago
Patients’ understanding of tuberculosis—the disease, the treatment, and the outcome—poses an important challenge in the care of an asymptomatic PPD-positive patient. These patients may ask, “Will I get sick? Do I have to take the medicine? Are there side effects? And would you take the medicine?” We need to be prepared to answer these questions.
Most patients with a positive PPD will not get active tuberculosis, but when they do it can be serious and it can spread easily. The medication significantly decreases the risk of developing active tuberculosis. The medication side effects are uncommon but can be severe. These side effects are reversible if the medication is stopped promptly. Under the supervision of my physician, I would take the medicine.
Evidence summary
Clinical evaluation with medical history and physical exam, chest radiography, and selected sputum sampling to exclude active tuberculosis are part of the recommended algorithm for all patients who develop a positive PPD (FIGURE).1-3 These recommendations are derived from expert opinion, and their usefulness has not been evaluated in any population-based study of asymptomatic PPD-positive patients.
A comprehensive review of RCTs from the 1950s and 1960s demonstrated that INH treatment of patients with latent tuberculosis infection is effective in decreasing the progression to active tuberculosis.4 A series of double-blinded RCTs performed by the US Public Health Service included 25,923 patients with latent tuberculosis who were randomized to receive either daily INH or placebo for 1 year with 6- to 10-year follow-up. Groups studied included household contacts of patients with active tuberculosis (rate of progression to active disease in placebo group [baseline rate]=27/1000, relative risk with INH [RR]=0.4, number needed to treat [NNT]=63), patients in mental institutions (baseline rate=12/1000, RR=0.3, NNT=121), and patients with x-ray findings of healed tuberculosis (baseline rate=69/1000, RR=0.4, NNT=23).
The optimal length of treatment for PPD-positive patients without active disease was evaluated through 1 double-blinded RCT enrolling 28,000 patients with 5-year follow-up after 12, 24, or 52 weeks of INH or placebo. Active TB developed in 0.35% (24/6919) after 52 weeks of INH compared with 0.49% (34/6965) after 24 weeks (RR=1.4, NNT=708).5 Incidence in the placebo group was 1.4%. Subgroup analysis determined that maximum efficacy with fewest side effects was achieved at 9 months.6 Nine months of INH is also recommended for HIV-positive patients, based on extrapolations from these and other studies.3
INH monotherapy was compared with combination INH and rifampin in a 2005 meta-analysis of 5 RCTs of variable quality involving 1926 patients.7 This meta-analysis found equivalency in risk of active TB and mortality between INH monotherapy for 6 to 12 months and the combination of rifampin and INH for 3 months (pooled risk difference=0%; 95% confidence interval [CI], –1% to 2%). This study also showed similar rates of adverse events in both groups (pooled risk difference=–1%; 95% CI, –7% to 5%). Short-course combination rifampin and pyrazinamide is no longer recommended after an open-label RCT with 589 patients demonstrated severe hepatoxicity in 7.7% (16/207) on a 2-month course of pyrazinamide and rifampin, compared with 1% (2/204) on 6 months of INH (RR=7.9, number needed to harm=15).8 Rifampin monotherapy has only been studied in patients with silicosis in a RCT enrolling 652 participants with latent tuberculosis. A 12-week course of rifampin (600 mg daily) was as effective as 6 months of INH in preventing development of active TB over the next 5 years.9
FIGURE
Suggested workup of asymptomatic, HIV-negative patients with a positive PPD
Source: Am J Respir Crit Care Med 2000;2 Jasmer et al, N Engl J Med 2002.3
Recommendations from others
Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Disease Society of America guidelines recommend targeted screening of high-risk persons followed by further clinical evaluation of all those with a reactive PPD (FIGURE).2,10 The recommended treatment regimen for latent TB is daily INH for 9 months. Less preferable regimens are daily INH for 6 months, or daily rifampin for 4 months in patients who cannot tolerate INH. A 2-month course of rifampin and pyrazinamide is no longer recommended. The recent meta-analysis supporting a 3-month regimen of combination INH and rifampin has not been incorporated into expert guidelines.7
1. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
2. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med 2002;347:1860-1866.
4. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc 1970;26:28-106.
5. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555-564.
6. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;3:847-850.
7. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005;40:670-676.
8. Jasmer RM, Saukkonen JJ, Blumberg HM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640-647.
9. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41.
10. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. MMWR Recomm Rep 2005;54(RR-12):1-81.
1. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
2. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med 2002;347:1860-1866.
4. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc 1970;26:28-106.
5. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555-564.
6. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;3:847-850.
7. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005;40:670-676.
8. Jasmer RM, Saukkonen JJ, Blumberg HM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640-647.
9. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41.
10. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. MMWR Recomm Rep 2005;54(RR-12):1-81.
Evidence-based answers from the Family Physicians Inquiries Network