User login
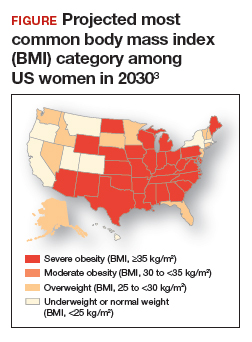
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3
As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
