User login
Ms. K, age 35, soon will deliver her second child. She has a 12-year history of bipolar disorder, which was well controlled with lithium, 1,200 mg/d. During her first pregnancy 3 years ago, Ms. K stopped taking lithium because she was concerned about the risk of Ebstein’s anomaly. She experienced a bipolar relapse after her healthy baby was born, and developed postpartum psychosis that was treated by restarting lithium, 1,200 mg/d, and adding olanzapine, 10 mg/d.
Ms. K has continued these medications throughout her current pregnancy. She wants to breast-feed her infant and is concerned about the effects that psychotropics might have on her newborn.
Breast-feeding and medications
The benefits of breast-feeding for mother and infant are well-known. Despite this, some women with bipolar disorder are advised not to breast-feed or, worse, to discontinue their medications in order to breast-feed. Decisions about breast-feeding while taking medications should be based on evidence of benefits and risks to the infant, along with a discussion of the risks of untreated illness, which is high postpartum. The prescribing information for many of the medications used to treat bipolar disorder advise against breast-feeding, although there is little evidence of harm.
Drug dosages and levels in breast milk can be reported a few different ways:
• percentage of maternal dosage measured in the breast milk
• percentage weight-adjusted maternal dosage
• percentage of maternal plasma level, and milk-to-plasma ratio (M:P).
Daily infant dosage can be calculated by multiplying the average concentration of the drug in breast milk (mg/mL) by the average volume of milk the baby ingests in 24 hours (usually 150 mL).1 The relative infant dosage can be calculated as the percentage maternal dosage, which is the daily infant dosage (mg/kg/d) ÷ maternal dosage (mg/kg/d) × 100.1
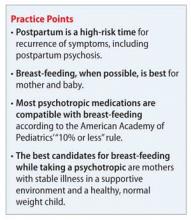
According to the American Academy of Pediatrics, ≤10% of the maternal dosage is compatible with breast-feeding.1 Most psychotropics studied fall below this threshold. Keep in mind that all published research is for breast-feeding a full-term infant; exercise caution with premature or low birth weight infants. Infants born to mothers taking a psychotropic should be monitored for withdrawal symptoms, which might be associated with antidepressants and benzodiazepines, but otherwise are rare.
Lithium
Breast-feeding during lithium treatment has been considered contraindicated based on early reports that lithium was highly excreted in breast milk.2 A 2003 study2 of 11 women found that lithium was excreted in breast milk in amounts between zero and 30% of maternal dosage (mean, 12.2% ± 8.5%; median, 11.2%; 95% CI, 6.3% to 18.0%). Researchers measured serum concentrations in 2 infants and found that 1 received 17% to 20% of the maternal dosage, and the other showed 50%. None of the infants experienced adverse events. In a study of 10 mother-infant pairs, breast milk lithium concentration averaged 0.35 mEq/L (standard deviation [SD] = 0.10, range 0.19 to 0.48 mEq/L), with paired infant serum concentrations of 0.16 mEq/L (SD = 0.06, range 0.09 to 0.25 mEq/L).3 Some transient abnormalities were found in infant serum concentrations of thyroid-stimulating hormone (TSH), blood urea nitrogen, and creatinine; there were no adverse effects on development. The authors recommend monitoring for TSH abnormalities in infants.
Olanzapine
Olanzapine prescribing information cites a study reporting that 1.8% of the maternal dosage is transferred to breast milk.4 Yet the olanzapine prescribing information states, “It is recommended that women receiving olanzapine should not breast-feed.” Olanzapine use during breast-feeding has been studied more than many medications, in part because of a database maintained by the manufacturer. In a study using the manufacturer’s database (N = 102) adverse reactions were reported in 15.6% of the infants, with the most common being somnolence (3.9%), irritability (2%), tremor (2%), and insomnia (2%).5
Other second-generation antipsychotics
Aripiprazole. The only case report of aripiprazole excretion in human breast milk found a concentration of approximately 20% of the maternal plasma level and an M:P ratio of 0.18:0.2.6
Asenapine. According to asenapine prescribing information7 and a literature search, it is not known whether asenapine is excreted in breast milk of humans, although it is found in the milk of lactating rats.
Lurasidone. According to the lurasidone prescribing information8 and a literature search, it is not known whether lurasidone is excreted in human breast milk, although it is found in the milk of lactating rats.
Quetiapine. An initial study reported that 0.09% to 0.43% of the maternal dosage of quetiapine was excreted in breast milk.9 Further studies found excretion to be 0.09% of maternal dosage, with infant plasma levels reaching 6% of the maternal dosage.10 A case series found that one-third of babies exposed to quetiapine during breast-feeding showed some neurodevelopmental delay, although these mothers also were taking other psychotropics.11
Risperidone. A 2000 study12 of risperidone in lactation reported that 0.84% weight-adjusted maternal risperidone dosage and 3.46% of its metabolite 9-hydroxyrisperidone is transferred to the infant. A later study showed 2.3% to 4.7% of the maternal dosage is transferred, with no adverse events reported in infants.13 A case study reported no adverse events and normal neurodevelopment in a the child of a mother taking risperidone.14
Ziprasidone. According to the ziprasidone prescribing information15 and a literature search, is not known whether ziprasidone is excreted in human breast milk.
See the Table4,6-10,12,13,15 for a summary of the evidence levels of second-generation antipsychotics that are excreted in breast milk.
Other mood stabilizers
Carbamazepine has been measured in breast milk at 3.8% to 5.9% of the maternal dosage.16
Lamotrigine. In a study of 30 lactating women, the breast milk contained an average of 9.2% of the maternal dosage of lamotrigine.17 Mild thrombocytosis was detected in 7 of 8 infants; no other adverse effects were reported. A case study describes a woman who breast-fed while taking lamotrigine, 850 mg/d, and who experienced dizziness and visual disturbances. The infant had apnea episodes followed by a cyanotic crisis, which required resuscitation. The infant’s plasma lamotrigine level was 4.87 μg/mL. Symptoms disappeared when the mother stopped breast-feeding.18 Lamotrigine is considered to be moderately safe in breast-feeding patients with proper monitoring. The drug also has a known safety profile because of its use in children with epilepsy.
Valproic acid. Because of its high plasma protein binding, valproic acid does not pass readily into the breast milk. Newborns receive approximately 1.4% to 1.7% of the maternal dosage.16 Caution is advised, however, because of some reported adverse events. One case reported thrombocytopenic purpura and anemia in an infant.19 Valproic acid is considered to be compatible with breast-feeding with proper monitoring.
Benzodiazepines
Benzodiazepines can be helpful adjunctive medications to aid sleep, which is essential for the mother’s and infant’s health. In a prospectively recruited, retrospectively assessed cohort study that evaluated 124 women taking benzodiazepines while breast-feeding, adverse effects, specifically sedation, were noted in 1.6% of infants.20
Future developments in prescribing information
Under a 2008 FDA recommendation, the “Nursing Mothers” section of prescribing information would be replaced with a section entitled “Lactation.” This new heading would include the sub-headings Risk Summary, Clinical Considerations, and Data.1 It is expected that this new format will be more practical and will help clinicians and patients make informed decisions. The prescribing changes will be in effect on June 30, 2015.21
Related Resources
• Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
• LactMed. http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm.
• MOTHERISK. www.motherisk.org/women/ breastfeeding.jsp.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Quetiapine • Seroquel
Carbamazepine • Tegretol Risperidone • Risperdal
Lamotrigine • Lamictal Valproic acid • Depakene
Lithium • Eskalith, Lithobid Ziprasidone • Geodon
Lurasidone • Latuda
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sach HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3);e796-e809.
2. Moretti ME, Koren G, Verjee Z, et al. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25(3):364-366.
3. Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342-345.
4. Xyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
5. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38.
6. Schlotterbeck P, Leube D, Kircher T, et al. Aripiprazole in human milk. Int J Neuropsychopharmacol. 2007;10(3):433.
7. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2014.
8. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals; 2013.
9. Lee A, Giesbrecht E, Dunn E, et al. Excretion of quetiapine in breast milk. Am J Psychiatry. 2004;161(9):1715-1716.
10. Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breastfeeding. Ann Pharmacother. 2007;41(4):711-714.
11. Misri S, Corral M, Wardrop AA, et al. Quetiapine augmentation in lactation: a series of case reports. J Clin Psychopharmacol. 2006;26(5):508-511.
12. Hill RC, McIvor RJ, Wojnar-Horton RE, et al. Risperidone distribution and excretion into human milk: case report and estimated infant exposure during breast-feeding. J Clin Psychopharmacol. 2000;20(2):285-286.
13. Ilett KF, Hackett LP, Kristensen JH, et al. Transfer of risperidone and 9-hydroxyrisperidone into human milk. Ann Pharmacother. 2004;38(2):273-276.
14. Aichhorn W, Stuppaek C, Whitworth AB. Risperidone and breast-feeding. J Psychopharmacol. 2005;19(2):211-213.
15. Geodon [package insert]. New York, NY: Pfizer; 2014.
16. Davanzo R, Dal Bo S, Bua J, et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50.
17. Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223-e231.
18. Nordmo E, Aronsen L, Wasland K, et al. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893-1897.
19. Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130(6):1001-1003.
20. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451.
21. U.S. Food and Drug Administration. FDA issues final rule on changes to pregnancy and lactation labeling information for prescription drug and biological products. http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm425317.htm. Published December 3. 2014. Accessed March 4, 2015.
Ms. K, age 35, soon will deliver her second child. She has a 12-year history of bipolar disorder, which was well controlled with lithium, 1,200 mg/d. During her first pregnancy 3 years ago, Ms. K stopped taking lithium because she was concerned about the risk of Ebstein’s anomaly. She experienced a bipolar relapse after her healthy baby was born, and developed postpartum psychosis that was treated by restarting lithium, 1,200 mg/d, and adding olanzapine, 10 mg/d.
Ms. K has continued these medications throughout her current pregnancy. She wants to breast-feed her infant and is concerned about the effects that psychotropics might have on her newborn.
Breast-feeding and medications
The benefits of breast-feeding for mother and infant are well-known. Despite this, some women with bipolar disorder are advised not to breast-feed or, worse, to discontinue their medications in order to breast-feed. Decisions about breast-feeding while taking medications should be based on evidence of benefits and risks to the infant, along with a discussion of the risks of untreated illness, which is high postpartum. The prescribing information for many of the medications used to treat bipolar disorder advise against breast-feeding, although there is little evidence of harm.
Drug dosages and levels in breast milk can be reported a few different ways:
• percentage of maternal dosage measured in the breast milk
• percentage weight-adjusted maternal dosage
• percentage of maternal plasma level, and milk-to-plasma ratio (M:P).
Daily infant dosage can be calculated by multiplying the average concentration of the drug in breast milk (mg/mL) by the average volume of milk the baby ingests in 24 hours (usually 150 mL).1 The relative infant dosage can be calculated as the percentage maternal dosage, which is the daily infant dosage (mg/kg/d) ÷ maternal dosage (mg/kg/d) × 100.1
According to the American Academy of Pediatrics, ≤10% of the maternal dosage is compatible with breast-feeding.1 Most psychotropics studied fall below this threshold. Keep in mind that all published research is for breast-feeding a full-term infant; exercise caution with premature or low birth weight infants. Infants born to mothers taking a psychotropic should be monitored for withdrawal symptoms, which might be associated with antidepressants and benzodiazepines, but otherwise are rare.
Lithium
Breast-feeding during lithium treatment has been considered contraindicated based on early reports that lithium was highly excreted in breast milk.2 A 2003 study2 of 11 women found that lithium was excreted in breast milk in amounts between zero and 30% of maternal dosage (mean, 12.2% ± 8.5%; median, 11.2%; 95% CI, 6.3% to 18.0%). Researchers measured serum concentrations in 2 infants and found that 1 received 17% to 20% of the maternal dosage, and the other showed 50%. None of the infants experienced adverse events. In a study of 10 mother-infant pairs, breast milk lithium concentration averaged 0.35 mEq/L (standard deviation [SD] = 0.10, range 0.19 to 0.48 mEq/L), with paired infant serum concentrations of 0.16 mEq/L (SD = 0.06, range 0.09 to 0.25 mEq/L).3 Some transient abnormalities were found in infant serum concentrations of thyroid-stimulating hormone (TSH), blood urea nitrogen, and creatinine; there were no adverse effects on development. The authors recommend monitoring for TSH abnormalities in infants.
Olanzapine
Olanzapine prescribing information cites a study reporting that 1.8% of the maternal dosage is transferred to breast milk.4 Yet the olanzapine prescribing information states, “It is recommended that women receiving olanzapine should not breast-feed.” Olanzapine use during breast-feeding has been studied more than many medications, in part because of a database maintained by the manufacturer. In a study using the manufacturer’s database (N = 102) adverse reactions were reported in 15.6% of the infants, with the most common being somnolence (3.9%), irritability (2%), tremor (2%), and insomnia (2%).5
Other second-generation antipsychotics
Aripiprazole. The only case report of aripiprazole excretion in human breast milk found a concentration of approximately 20% of the maternal plasma level and an M:P ratio of 0.18:0.2.6
Asenapine. According to asenapine prescribing information7 and a literature search, it is not known whether asenapine is excreted in breast milk of humans, although it is found in the milk of lactating rats.
Lurasidone. According to the lurasidone prescribing information8 and a literature search, it is not known whether lurasidone is excreted in human breast milk, although it is found in the milk of lactating rats.
Quetiapine. An initial study reported that 0.09% to 0.43% of the maternal dosage of quetiapine was excreted in breast milk.9 Further studies found excretion to be 0.09% of maternal dosage, with infant plasma levels reaching 6% of the maternal dosage.10 A case series found that one-third of babies exposed to quetiapine during breast-feeding showed some neurodevelopmental delay, although these mothers also were taking other psychotropics.11
Risperidone. A 2000 study12 of risperidone in lactation reported that 0.84% weight-adjusted maternal risperidone dosage and 3.46% of its metabolite 9-hydroxyrisperidone is transferred to the infant. A later study showed 2.3% to 4.7% of the maternal dosage is transferred, with no adverse events reported in infants.13 A case study reported no adverse events and normal neurodevelopment in a the child of a mother taking risperidone.14
Ziprasidone. According to the ziprasidone prescribing information15 and a literature search, is not known whether ziprasidone is excreted in human breast milk.
See the Table4,6-10,12,13,15 for a summary of the evidence levels of second-generation antipsychotics that are excreted in breast milk.
Other mood stabilizers
Carbamazepine has been measured in breast milk at 3.8% to 5.9% of the maternal dosage.16
Lamotrigine. In a study of 30 lactating women, the breast milk contained an average of 9.2% of the maternal dosage of lamotrigine.17 Mild thrombocytosis was detected in 7 of 8 infants; no other adverse effects were reported. A case study describes a woman who breast-fed while taking lamotrigine, 850 mg/d, and who experienced dizziness and visual disturbances. The infant had apnea episodes followed by a cyanotic crisis, which required resuscitation. The infant’s plasma lamotrigine level was 4.87 μg/mL. Symptoms disappeared when the mother stopped breast-feeding.18 Lamotrigine is considered to be moderately safe in breast-feeding patients with proper monitoring. The drug also has a known safety profile because of its use in children with epilepsy.
Valproic acid. Because of its high plasma protein binding, valproic acid does not pass readily into the breast milk. Newborns receive approximately 1.4% to 1.7% of the maternal dosage.16 Caution is advised, however, because of some reported adverse events. One case reported thrombocytopenic purpura and anemia in an infant.19 Valproic acid is considered to be compatible with breast-feeding with proper monitoring.
Benzodiazepines
Benzodiazepines can be helpful adjunctive medications to aid sleep, which is essential for the mother’s and infant’s health. In a prospectively recruited, retrospectively assessed cohort study that evaluated 124 women taking benzodiazepines while breast-feeding, adverse effects, specifically sedation, were noted in 1.6% of infants.20
Future developments in prescribing information
Under a 2008 FDA recommendation, the “Nursing Mothers” section of prescribing information would be replaced with a section entitled “Lactation.” This new heading would include the sub-headings Risk Summary, Clinical Considerations, and Data.1 It is expected that this new format will be more practical and will help clinicians and patients make informed decisions. The prescribing changes will be in effect on June 30, 2015.21
Related Resources
• Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
• LactMed. http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm.
• MOTHERISK. www.motherisk.org/women/ breastfeeding.jsp.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Quetiapine • Seroquel
Carbamazepine • Tegretol Risperidone • Risperdal
Lamotrigine • Lamictal Valproic acid • Depakene
Lithium • Eskalith, Lithobid Ziprasidone • Geodon
Lurasidone • Latuda
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. K, age 35, soon will deliver her second child. She has a 12-year history of bipolar disorder, which was well controlled with lithium, 1,200 mg/d. During her first pregnancy 3 years ago, Ms. K stopped taking lithium because she was concerned about the risk of Ebstein’s anomaly. She experienced a bipolar relapse after her healthy baby was born, and developed postpartum psychosis that was treated by restarting lithium, 1,200 mg/d, and adding olanzapine, 10 mg/d.
Ms. K has continued these medications throughout her current pregnancy. She wants to breast-feed her infant and is concerned about the effects that psychotropics might have on her newborn.
Breast-feeding and medications
The benefits of breast-feeding for mother and infant are well-known. Despite this, some women with bipolar disorder are advised not to breast-feed or, worse, to discontinue their medications in order to breast-feed. Decisions about breast-feeding while taking medications should be based on evidence of benefits and risks to the infant, along with a discussion of the risks of untreated illness, which is high postpartum. The prescribing information for many of the medications used to treat bipolar disorder advise against breast-feeding, although there is little evidence of harm.
Drug dosages and levels in breast milk can be reported a few different ways:
• percentage of maternal dosage measured in the breast milk
• percentage weight-adjusted maternal dosage
• percentage of maternal plasma level, and milk-to-plasma ratio (M:P).
Daily infant dosage can be calculated by multiplying the average concentration of the drug in breast milk (mg/mL) by the average volume of milk the baby ingests in 24 hours (usually 150 mL).1 The relative infant dosage can be calculated as the percentage maternal dosage, which is the daily infant dosage (mg/kg/d) ÷ maternal dosage (mg/kg/d) × 100.1
According to the American Academy of Pediatrics, ≤10% of the maternal dosage is compatible with breast-feeding.1 Most psychotropics studied fall below this threshold. Keep in mind that all published research is for breast-feeding a full-term infant; exercise caution with premature or low birth weight infants. Infants born to mothers taking a psychotropic should be monitored for withdrawal symptoms, which might be associated with antidepressants and benzodiazepines, but otherwise are rare.
Lithium
Breast-feeding during lithium treatment has been considered contraindicated based on early reports that lithium was highly excreted in breast milk.2 A 2003 study2 of 11 women found that lithium was excreted in breast milk in amounts between zero and 30% of maternal dosage (mean, 12.2% ± 8.5%; median, 11.2%; 95% CI, 6.3% to 18.0%). Researchers measured serum concentrations in 2 infants and found that 1 received 17% to 20% of the maternal dosage, and the other showed 50%. None of the infants experienced adverse events. In a study of 10 mother-infant pairs, breast milk lithium concentration averaged 0.35 mEq/L (standard deviation [SD] = 0.10, range 0.19 to 0.48 mEq/L), with paired infant serum concentrations of 0.16 mEq/L (SD = 0.06, range 0.09 to 0.25 mEq/L).3 Some transient abnormalities were found in infant serum concentrations of thyroid-stimulating hormone (TSH), blood urea nitrogen, and creatinine; there were no adverse effects on development. The authors recommend monitoring for TSH abnormalities in infants.
Olanzapine
Olanzapine prescribing information cites a study reporting that 1.8% of the maternal dosage is transferred to breast milk.4 Yet the olanzapine prescribing information states, “It is recommended that women receiving olanzapine should not breast-feed.” Olanzapine use during breast-feeding has been studied more than many medications, in part because of a database maintained by the manufacturer. In a study using the manufacturer’s database (N = 102) adverse reactions were reported in 15.6% of the infants, with the most common being somnolence (3.9%), irritability (2%), tremor (2%), and insomnia (2%).5
Other second-generation antipsychotics
Aripiprazole. The only case report of aripiprazole excretion in human breast milk found a concentration of approximately 20% of the maternal plasma level and an M:P ratio of 0.18:0.2.6
Asenapine. According to asenapine prescribing information7 and a literature search, it is not known whether asenapine is excreted in breast milk of humans, although it is found in the milk of lactating rats.
Lurasidone. According to the lurasidone prescribing information8 and a literature search, it is not known whether lurasidone is excreted in human breast milk, although it is found in the milk of lactating rats.
Quetiapine. An initial study reported that 0.09% to 0.43% of the maternal dosage of quetiapine was excreted in breast milk.9 Further studies found excretion to be 0.09% of maternal dosage, with infant plasma levels reaching 6% of the maternal dosage.10 A case series found that one-third of babies exposed to quetiapine during breast-feeding showed some neurodevelopmental delay, although these mothers also were taking other psychotropics.11
Risperidone. A 2000 study12 of risperidone in lactation reported that 0.84% weight-adjusted maternal risperidone dosage and 3.46% of its metabolite 9-hydroxyrisperidone is transferred to the infant. A later study showed 2.3% to 4.7% of the maternal dosage is transferred, with no adverse events reported in infants.13 A case study reported no adverse events and normal neurodevelopment in a the child of a mother taking risperidone.14
Ziprasidone. According to the ziprasidone prescribing information15 and a literature search, is not known whether ziprasidone is excreted in human breast milk.
See the Table4,6-10,12,13,15 for a summary of the evidence levels of second-generation antipsychotics that are excreted in breast milk.
Other mood stabilizers
Carbamazepine has been measured in breast milk at 3.8% to 5.9% of the maternal dosage.16
Lamotrigine. In a study of 30 lactating women, the breast milk contained an average of 9.2% of the maternal dosage of lamotrigine.17 Mild thrombocytosis was detected in 7 of 8 infants; no other adverse effects were reported. A case study describes a woman who breast-fed while taking lamotrigine, 850 mg/d, and who experienced dizziness and visual disturbances. The infant had apnea episodes followed by a cyanotic crisis, which required resuscitation. The infant’s plasma lamotrigine level was 4.87 μg/mL. Symptoms disappeared when the mother stopped breast-feeding.18 Lamotrigine is considered to be moderately safe in breast-feeding patients with proper monitoring. The drug also has a known safety profile because of its use in children with epilepsy.
Valproic acid. Because of its high plasma protein binding, valproic acid does not pass readily into the breast milk. Newborns receive approximately 1.4% to 1.7% of the maternal dosage.16 Caution is advised, however, because of some reported adverse events. One case reported thrombocytopenic purpura and anemia in an infant.19 Valproic acid is considered to be compatible with breast-feeding with proper monitoring.
Benzodiazepines
Benzodiazepines can be helpful adjunctive medications to aid sleep, which is essential for the mother’s and infant’s health. In a prospectively recruited, retrospectively assessed cohort study that evaluated 124 women taking benzodiazepines while breast-feeding, adverse effects, specifically sedation, were noted in 1.6% of infants.20
Future developments in prescribing information
Under a 2008 FDA recommendation, the “Nursing Mothers” section of prescribing information would be replaced with a section entitled “Lactation.” This new heading would include the sub-headings Risk Summary, Clinical Considerations, and Data.1 It is expected that this new format will be more practical and will help clinicians and patients make informed decisions. The prescribing changes will be in effect on June 30, 2015.21
Related Resources
• Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
• LactMed. http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm.
• MOTHERISK. www.motherisk.org/women/ breastfeeding.jsp.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Quetiapine • Seroquel
Carbamazepine • Tegretol Risperidone • Risperdal
Lamotrigine • Lamictal Valproic acid • Depakene
Lithium • Eskalith, Lithobid Ziprasidone • Geodon
Lurasidone • Latuda
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sach HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3);e796-e809.
2. Moretti ME, Koren G, Verjee Z, et al. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25(3):364-366.
3. Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342-345.
4. Xyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
5. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38.
6. Schlotterbeck P, Leube D, Kircher T, et al. Aripiprazole in human milk. Int J Neuropsychopharmacol. 2007;10(3):433.
7. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2014.
8. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals; 2013.
9. Lee A, Giesbrecht E, Dunn E, et al. Excretion of quetiapine in breast milk. Am J Psychiatry. 2004;161(9):1715-1716.
10. Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breastfeeding. Ann Pharmacother. 2007;41(4):711-714.
11. Misri S, Corral M, Wardrop AA, et al. Quetiapine augmentation in lactation: a series of case reports. J Clin Psychopharmacol. 2006;26(5):508-511.
12. Hill RC, McIvor RJ, Wojnar-Horton RE, et al. Risperidone distribution and excretion into human milk: case report and estimated infant exposure during breast-feeding. J Clin Psychopharmacol. 2000;20(2):285-286.
13. Ilett KF, Hackett LP, Kristensen JH, et al. Transfer of risperidone and 9-hydroxyrisperidone into human milk. Ann Pharmacother. 2004;38(2):273-276.
14. Aichhorn W, Stuppaek C, Whitworth AB. Risperidone and breast-feeding. J Psychopharmacol. 2005;19(2):211-213.
15. Geodon [package insert]. New York, NY: Pfizer; 2014.
16. Davanzo R, Dal Bo S, Bua J, et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50.
17. Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223-e231.
18. Nordmo E, Aronsen L, Wasland K, et al. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893-1897.
19. Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130(6):1001-1003.
20. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451.
21. U.S. Food and Drug Administration. FDA issues final rule on changes to pregnancy and lactation labeling information for prescription drug and biological products. http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm425317.htm. Published December 3. 2014. Accessed March 4, 2015.
1. Sach HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3);e796-e809.
2. Moretti ME, Koren G, Verjee Z, et al. Monitoring lithium in breast milk: an individualized approach for breast-feeding mothers. Ther Drug Monit. 2003;25(3):364-366.
3. Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342-345.
4. Xyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
5. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38.
6. Schlotterbeck P, Leube D, Kircher T, et al. Aripiprazole in human milk. Int J Neuropsychopharmacol. 2007;10(3):433.
7. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2014.
8. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals; 2013.
9. Lee A, Giesbrecht E, Dunn E, et al. Excretion of quetiapine in breast milk. Am J Psychiatry. 2004;161(9):1715-1716.
10. Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breastfeeding. Ann Pharmacother. 2007;41(4):711-714.
11. Misri S, Corral M, Wardrop AA, et al. Quetiapine augmentation in lactation: a series of case reports. J Clin Psychopharmacol. 2006;26(5):508-511.
12. Hill RC, McIvor RJ, Wojnar-Horton RE, et al. Risperidone distribution and excretion into human milk: case report and estimated infant exposure during breast-feeding. J Clin Psychopharmacol. 2000;20(2):285-286.
13. Ilett KF, Hackett LP, Kristensen JH, et al. Transfer of risperidone and 9-hydroxyrisperidone into human milk. Ann Pharmacother. 2004;38(2):273-276.
14. Aichhorn W, Stuppaek C, Whitworth AB. Risperidone and breast-feeding. J Psychopharmacol. 2005;19(2):211-213.
15. Geodon [package insert]. New York, NY: Pfizer; 2014.
16. Davanzo R, Dal Bo S, Bua J, et al. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50.
17. Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223-e231.
18. Nordmo E, Aronsen L, Wasland K, et al. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893-1897.
19. Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130(6):1001-1003.
20. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451.
21. U.S. Food and Drug Administration. FDA issues final rule on changes to pregnancy and lactation labeling information for prescription drug and biological products. http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm425317.htm. Published December 3. 2014. Accessed March 4, 2015.