User login
Too few patients are being screened for abdominal aortic aneurysm (AAA), resulting in severe morbidity and mortality. Many patients with AAA aren’t identified until they present with rupture, leading to mortality as high as 90%.1 Early detection is critical.
Medicare offers one-time free screening to eligible individuals > 65 years of age, and several professional organizations promote screening with published guidelines, which we discuss later in this article.
So who is at risk, who should be screened, and what is the best way to screen your patients?
Risk factors and sex differences
AAA has a prevalence of between 1% and 5% in men > 65 years old,2,3 and it is 4 to 6 times more common in men than women.4 Major risk factors include smoking, older age, family history, and genetic factors, while hypertension, history of coronary artery disease, hyperlipidemia, and peripheral arterial disease have weaker associations.3,4 Exercise and diabetes seem to have protective effects.5
The incidence and mortality of AAA increased between the 1950s and the mid-1990s; however, both indicators have decreased in numerous countries in the 21st century.6 Although the prevalence is much lower in women, they have a higher risk of rupture than men at equivalent lesion diameters.3 The prevalence of AAA in women who smoke and are > 70 years of age is > 1%.3
Silent but deadly
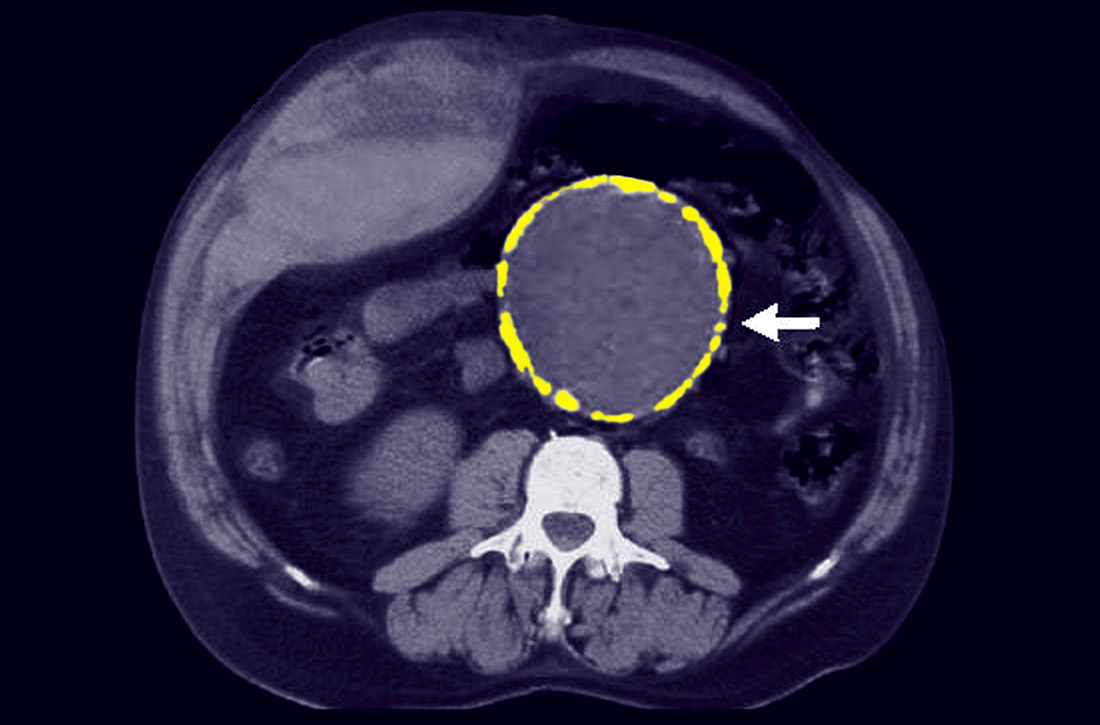
Most patients with AAA are asymptomatic. Their lesions are often detected incidentally on magnetic resonance imaging of the spine obtained for back pain, on an abdominal ultrasound (US) for gallstones, or on a routine computed tomography (CT) scan for the evaluation of abdominal pain. Some patients will experience vague abdominal discomfort from rapid expansion of an aneurysm prior to rupture, necessitating urgent repair. Also, some large aneurysms can erode into the spine and cause chronic back pain prior to rupture. An infrarenal abdominal aortic diameter > 30 mm defines an aneurysm,7 and once the diameter reaches 55 mm, the threat of rupture often justifies operative repair. (See “The preferred approach to repair.”)
SIDEBAR
The preferred approach to repair
Since the introduction of endovascular aneurysm repair (EVAR) in the latter part of the 20th century, it has become the standard of care for the surgical management of aneurysmal disease. Currently, > 80% of patients with an abdominal aortic aneurysm (AAA) who undergo repair are treated with EVAR.23
Typically, the AAA diameter is assessed via ultrasound. If repair is indicated, a computed tomography arteriogram is obtained to define the anatomy and help determine if the AAA is amenable to endografting. The most common contraindications to EVAR are either a short proximal neck (not enough distance below the renal arteries to safely anchor the stent graft) or an iliac artery diameter that is too small to allow delivery of the device. The operation can be performed under local, regional, or general anesthesia, and patients are usually discharged on the first postoperative day. These patients require lifelong surveillance due to the risk of delayed endoleak and reperfusion of the aneurysm sac.24
Ruptured aneurysms will classically manifest with severe abdominal and/or back pain. Often a ruptured aneurysm will be contained in the retroperitoneum, allowing the patient to remain hemodynamically stable for a period of time and thus providing a window of opportunity for emergent repair.
Continue to: What is the evidence that screening is effective?
What is the evidence that screening is effective?
In 1988, researchers in Chichester, England, randomized > 6000 men ages 65 to 80 years to either a control group or a group that was offered a one-time US screen for AAA. After 15 years of follow-up, no significant difference in AAA mortality was seen between the groups, although 26% of those invited for screening declined to participate and accounted for more than half of the AAA-related deaths in the group receiving an invitation for screening.8
The MASS Trial,9 another British study, began in 1997 and screened men ages 65 to 74. More than 67,000 men were randomized, with 1 group invited for AAA screening and the other serving as a control. The final report on this trial was published in 2012. After 13 years of follow-up, there was a 42% reduction in AAA-related deaths in the group invited for screening, a small reduction in all-cause mortality, and a significant reduction in risk of AAA rupture (hazard ratio = 0.57). The researchers noted that 216 patients would have to be invited for screening to prevent 1 death over 13 years. They also reported that 21% of the invited patients that had an AAA-related death had an initial scan that was negative for AAA (aortic diameter < 3 cm). However, despite this finding, screening still appeared to be beneficial.
Lindholdt et al10 randomized > 12,000 Danish men ages 64 to 73 to serve as controls or to be invited for US screening for AAA. After 13 years of follow-up, those invited for screening had a 66% relative risk reduction in AAA-related mortality, with screening considered cost effective. There were no differences between the groups in all-cause mortality. Conversely, the Western Australia Trial studied an older group of men, ages 64 to 83, but was unable to show a benefit of screening in lowering AAA-related mortality.11
Tikagi et al6 performed a meta-analysis on the data from the 4 trials above and reported up to 15 years of follow-up. Patients who attended screening sessions had a reduction in all-cause mortality with an odds ratio (OR) of 0.6, and a marked reduction in AAA-related mortality with an OR of 0.4. The favorable data on screening have prompted the United Kingdom and Sweden to offer screening to all men ≥ 65 years, based on the current estimate of a 1% prevalence of AAA, although screening is felt to remain cost effective down to a prevalence of 0.35%.12
Massachusetts General Hospital also reported13 that the detection rate of AAA increased, with the diagnosis made at smaller aneurysm dimensions, following publication of the US Preventive Services Task Force recommendations (reviewed below).
Continue to: When to screen
When to screen
Several professional organizations, as well as Medicare, have published AAA screening recommendations. While there are notable differences among them, their shared message is crucial: screen. Consider adding applicable reminders to your practice’s electronic medical record system to increase screening rates for eligible patients.
US Preventive Services Task Force 14
- Recommend one-time AAA screening with ultrasonography for men ages 65 to 75 years who have ever smoked (B recommendation).
- Selectively offer AAA screening to men ages 65 to 75 years who have never smoked, rather than routinely screening all men in this group (C recommendation). Individual attributes that could favor screening include a family history of AAA, the presence of other arterial aneurysms, and the number of risk factors for cardiovascular disease.
- Do not routinely screen women for AAA if they have never smoked and have no family history of AAA (D recommendation). For women ages 65 to 75 years who have ever smoked or have a family history of AAA, current evidence is insufficient to assess the balance of benefits and harms of screening for AAA (I statement). (See the related Practice Alert.)
Canadian Task Force on Preventive Health Care4
- Recommend one-time screening for AAA with US for men 65 to 80 years of age (weak level of recommendation; moderate-quality evidence).
- Do not recommend screening for men older than 80 years of age (weak recommendation; low quality of evidence).
- Do not recommend screening for women (strong recommendation; very low quality of evidence).
Society for Vascular Surgery15
- Recommend one-time US screening for AAA for men or women 65 to 75 years of age with a history of tobacco use (strong recommendation with high-quality evidence).
- Recommend one-time screening if there is a history of smoking for men or women > 75 years of age who are in good health and have not previously been screened. (Weak recommendation with low-quality evidence).
- Recommend one-time screening of men or women 65 to 75 years of age who are first-degree relatives of someone with AAA, or in those older than age 75 and in good health (weak recommendation with low-quality evidence).
How to screen
Physical exam. The abdominal aorta is often palpable in the epigastric region, and a thorough abdominal exam should include an attempt to detect it. It is critical that the patient be supine during palpation, to allow compression of the aorta against the lumbar spine. Even with a well-performed exam, however, its sensitivity is just 76% in the detection of AAA ≥ 5 cm.16
Continue to: Imaging
Imaging. US is the preferred imaging procedure when screening for AAA, given its high sensitivity and specificity.17 If US yields poor image quality, noncontrast CT is suggested, with magnetic resonance angiography being another alternative.18 Handheld US has the potential to supplement the physical exam, and has been shown to be a viable method to detect AAA in an outpatient primary care setting at a reasonable cost.19 Typically, a formal aortic US requires a patient to go without food or liquids for 8 hours before the procedure to obtain the best image; however, a good estimate of aortic diameter can be obtained without this restriction.
Despite Medicare coverage and recs, few people are screened
In 2007, Medicare started the SAAVE Program (Screening Aortic Aneurysm Very Effectively), offering a one-time US screening for AAA for eligible patients, as part of the Welcome to Medicare Program. Eligible individuals are men between 65 and 75 years of age with a history of smoking at least 100 cigarettes in their lifetime, and men or women in the same age group with a family history of AAA.2
Despite these recommendations, few patients receive screening. Centers for Medicare & Medicaid Services data show that < 10% of eligible men were screened between 2004 and 2008,20 and Olchanski et al21 report that < 1% of eligible patients were screened from 2005-2009. A simulation model estimates that 131 additional life years could be gained per 1000 patients screened if the utilization rate could be increased to 80%, a seemingly achievable goal.21 Moreover, expanding the screening program to include female smokers could increase 10-year life expectancy by 13%.21 Reasons for underutilization of this Medicare screening benefit may include lack of awareness by physicians and patients, costs of co-pays, and underutilization of the basic Welcome to Medicare exam.21
An additional consequence of low utilization of AAA screening is a high percentage of patients who are identified only late in the course of the disease. Mell et al22 report that 39% of patients undergoing AAA repair were identified < 6 months prior to surgery, a higher percentage than would be expected in a well-screened population. They also determined that slightly more than one-third of patients undergoing surgery for ruptured AAA had diagnostic imaging performed > 6 months prior to surgery, suggesting the possibility that these patients may not have been properly surveilled for aneurysm expansion, although the authors note that other potential explanations include delays in treatment due to comorbidities, and patient-related factors such as refusal of surgery or noncompliance with follow-up.
CORRESPONDENCE
Jeffrey S. Todd, MD, 127 McClanahan Street, Suite 300, Roanoke, VA 24014; jstodd@carilionclinic.org.
1. Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgrad Med J. 2009;85:268-273.
2. SBU—Swedish agency for Health Technology Assessment and Assessment of Social Services. Screening for abdominal aortic aneurysm. 2018. www.sbu.se/en/publications/sbu-assesses/screening-for-abdominal-aortic-aneurysm/. Accessed April 24, 2020.
3. Spanos K, Labropoulos N, Giannoukas A. Abdominal aortic aneurysm screening: do we need to shift toward a targeted strategy? Angiology. 2018;69:192-194.
4. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-1145.
5. Stackelberg O, Wolk, A, Eliasson K, et al. Lifestyle and risk of screening-detected abdominal aortic aneurysm in men. J Am Heart Assoc. 2017;6:e004725.
6. Tikagi H, Ando T, Umemoto T; ALICE (All-Literature Investigation of Cardiovascular Evidence) group. Abdominal aortic aneurysm screening reduces all-cause mortality: make screening great again. Angiology. 2017;69:205-211.
7. Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
8. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701.
9. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
10. Lindholdt JS, Sørenson J, Søgaard R et al. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97:826-834.
11. McCaul KA, Lawrence-Brown M, Dickinson JA, et al. Long-term outcomes of the Western Australian trial of screening for abdominal aortic aneurysms: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2016;176:1761-1766.
12. Earnshaw JJ, Lees T. Update on screening for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2017;54:1-2.
13. Zucker EJ, Misono AS, Prabhakar AM. Abdominal aortic aneurysm screening practices: impact of the 2014 US Preventive Services Task Force recommendations. J Am Coll Radiol. 2017;14:868-874.
14. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement.
15. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.
16. Venkatasubramaniam AK, Mehta T, Chetter IC, et al. The value of abdominal examination in the diagnosis of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2004;24:56-60.
17. Lindholdt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
18. Desjardins B, Dill KE, Flamm SD, et al. ACR Appropriateness Criteria®, pulsatile abdominal mass, suspected abdominal aortic aneurysm. Int J Cardiovasc Imaging. 2013;29:177-183.
19. Sisó-Almirall A, Kostov B, Navarro-González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
20. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462.
21. Olchanski N, Winn A, Cohen JT, et al. Abdominal aortic aneurysm screening: how many life years lost from underuse of the Medicare screening benefit? J Gen Intern Med. 2014;29:1155-1161.
22. Mell MW, Hlatky MA, Shreibati JB, et al. Late diagnosis of abdominal aortic aneurysms substantiates underutilization of abdominal aortic aneurysm screening for Medicare beneficiaries. J Vasc Surg. 2013;57:1519-1523.
23. England A, McWilliams R. Endovascular aortic aneurysm repair (EVAR). Ulster Med J. 2013;82:3-10.
24. Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg. 2006;43:446-451.
Too few patients are being screened for abdominal aortic aneurysm (AAA), resulting in severe morbidity and mortality. Many patients with AAA aren’t identified until they present with rupture, leading to mortality as high as 90%.1 Early detection is critical.
Medicare offers one-time free screening to eligible individuals > 65 years of age, and several professional organizations promote screening with published guidelines, which we discuss later in this article.
So who is at risk, who should be screened, and what is the best way to screen your patients?
Risk factors and sex differences
AAA has a prevalence of between 1% and 5% in men > 65 years old,2,3 and it is 4 to 6 times more common in men than women.4 Major risk factors include smoking, older age, family history, and genetic factors, while hypertension, history of coronary artery disease, hyperlipidemia, and peripheral arterial disease have weaker associations.3,4 Exercise and diabetes seem to have protective effects.5
The incidence and mortality of AAA increased between the 1950s and the mid-1990s; however, both indicators have decreased in numerous countries in the 21st century.6 Although the prevalence is much lower in women, they have a higher risk of rupture than men at equivalent lesion diameters.3 The prevalence of AAA in women who smoke and are > 70 years of age is > 1%.3
Silent but deadly
Most patients with AAA are asymptomatic. Their lesions are often detected incidentally on magnetic resonance imaging of the spine obtained for back pain, on an abdominal ultrasound (US) for gallstones, or on a routine computed tomography (CT) scan for the evaluation of abdominal pain. Some patients will experience vague abdominal discomfort from rapid expansion of an aneurysm prior to rupture, necessitating urgent repair. Also, some large aneurysms can erode into the spine and cause chronic back pain prior to rupture. An infrarenal abdominal aortic diameter > 30 mm defines an aneurysm,7 and once the diameter reaches 55 mm, the threat of rupture often justifies operative repair. (See “The preferred approach to repair.”)
SIDEBAR
The preferred approach to repair
Since the introduction of endovascular aneurysm repair (EVAR) in the latter part of the 20th century, it has become the standard of care for the surgical management of aneurysmal disease. Currently, > 80% of patients with an abdominal aortic aneurysm (AAA) who undergo repair are treated with EVAR.23
Typically, the AAA diameter is assessed via ultrasound. If repair is indicated, a computed tomography arteriogram is obtained to define the anatomy and help determine if the AAA is amenable to endografting. The most common contraindications to EVAR are either a short proximal neck (not enough distance below the renal arteries to safely anchor the stent graft) or an iliac artery diameter that is too small to allow delivery of the device. The operation can be performed under local, regional, or general anesthesia, and patients are usually discharged on the first postoperative day. These patients require lifelong surveillance due to the risk of delayed endoleak and reperfusion of the aneurysm sac.24
Ruptured aneurysms will classically manifest with severe abdominal and/or back pain. Often a ruptured aneurysm will be contained in the retroperitoneum, allowing the patient to remain hemodynamically stable for a period of time and thus providing a window of opportunity for emergent repair.
Continue to: What is the evidence that screening is effective?
What is the evidence that screening is effective?
In 1988, researchers in Chichester, England, randomized > 6000 men ages 65 to 80 years to either a control group or a group that was offered a one-time US screen for AAA. After 15 years of follow-up, no significant difference in AAA mortality was seen between the groups, although 26% of those invited for screening declined to participate and accounted for more than half of the AAA-related deaths in the group receiving an invitation for screening.8
The MASS Trial,9 another British study, began in 1997 and screened men ages 65 to 74. More than 67,000 men were randomized, with 1 group invited for AAA screening and the other serving as a control. The final report on this trial was published in 2012. After 13 years of follow-up, there was a 42% reduction in AAA-related deaths in the group invited for screening, a small reduction in all-cause mortality, and a significant reduction in risk of AAA rupture (hazard ratio = 0.57). The researchers noted that 216 patients would have to be invited for screening to prevent 1 death over 13 years. They also reported that 21% of the invited patients that had an AAA-related death had an initial scan that was negative for AAA (aortic diameter < 3 cm). However, despite this finding, screening still appeared to be beneficial.
Lindholdt et al10 randomized > 12,000 Danish men ages 64 to 73 to serve as controls or to be invited for US screening for AAA. After 13 years of follow-up, those invited for screening had a 66% relative risk reduction in AAA-related mortality, with screening considered cost effective. There were no differences between the groups in all-cause mortality. Conversely, the Western Australia Trial studied an older group of men, ages 64 to 83, but was unable to show a benefit of screening in lowering AAA-related mortality.11
Tikagi et al6 performed a meta-analysis on the data from the 4 trials above and reported up to 15 years of follow-up. Patients who attended screening sessions had a reduction in all-cause mortality with an odds ratio (OR) of 0.6, and a marked reduction in AAA-related mortality with an OR of 0.4. The favorable data on screening have prompted the United Kingdom and Sweden to offer screening to all men ≥ 65 years, based on the current estimate of a 1% prevalence of AAA, although screening is felt to remain cost effective down to a prevalence of 0.35%.12
Massachusetts General Hospital also reported13 that the detection rate of AAA increased, with the diagnosis made at smaller aneurysm dimensions, following publication of the US Preventive Services Task Force recommendations (reviewed below).
Continue to: When to screen
When to screen
Several professional organizations, as well as Medicare, have published AAA screening recommendations. While there are notable differences among them, their shared message is crucial: screen. Consider adding applicable reminders to your practice’s electronic medical record system to increase screening rates for eligible patients.
US Preventive Services Task Force 14
- Recommend one-time AAA screening with ultrasonography for men ages 65 to 75 years who have ever smoked (B recommendation).
- Selectively offer AAA screening to men ages 65 to 75 years who have never smoked, rather than routinely screening all men in this group (C recommendation). Individual attributes that could favor screening include a family history of AAA, the presence of other arterial aneurysms, and the number of risk factors for cardiovascular disease.
- Do not routinely screen women for AAA if they have never smoked and have no family history of AAA (D recommendation). For women ages 65 to 75 years who have ever smoked or have a family history of AAA, current evidence is insufficient to assess the balance of benefits and harms of screening for AAA (I statement). (See the related Practice Alert.)
Canadian Task Force on Preventive Health Care4
- Recommend one-time screening for AAA with US for men 65 to 80 years of age (weak level of recommendation; moderate-quality evidence).
- Do not recommend screening for men older than 80 years of age (weak recommendation; low quality of evidence).
- Do not recommend screening for women (strong recommendation; very low quality of evidence).
Society for Vascular Surgery15
- Recommend one-time US screening for AAA for men or women 65 to 75 years of age with a history of tobacco use (strong recommendation with high-quality evidence).
- Recommend one-time screening if there is a history of smoking for men or women > 75 years of age who are in good health and have not previously been screened. (Weak recommendation with low-quality evidence).
- Recommend one-time screening of men or women 65 to 75 years of age who are first-degree relatives of someone with AAA, or in those older than age 75 and in good health (weak recommendation with low-quality evidence).
How to screen
Physical exam. The abdominal aorta is often palpable in the epigastric region, and a thorough abdominal exam should include an attempt to detect it. It is critical that the patient be supine during palpation, to allow compression of the aorta against the lumbar spine. Even with a well-performed exam, however, its sensitivity is just 76% in the detection of AAA ≥ 5 cm.16
Continue to: Imaging
Imaging. US is the preferred imaging procedure when screening for AAA, given its high sensitivity and specificity.17 If US yields poor image quality, noncontrast CT is suggested, with magnetic resonance angiography being another alternative.18 Handheld US has the potential to supplement the physical exam, and has been shown to be a viable method to detect AAA in an outpatient primary care setting at a reasonable cost.19 Typically, a formal aortic US requires a patient to go without food or liquids for 8 hours before the procedure to obtain the best image; however, a good estimate of aortic diameter can be obtained without this restriction.
Despite Medicare coverage and recs, few people are screened
In 2007, Medicare started the SAAVE Program (Screening Aortic Aneurysm Very Effectively), offering a one-time US screening for AAA for eligible patients, as part of the Welcome to Medicare Program. Eligible individuals are men between 65 and 75 years of age with a history of smoking at least 100 cigarettes in their lifetime, and men or women in the same age group with a family history of AAA.2
Despite these recommendations, few patients receive screening. Centers for Medicare & Medicaid Services data show that < 10% of eligible men were screened between 2004 and 2008,20 and Olchanski et al21 report that < 1% of eligible patients were screened from 2005-2009. A simulation model estimates that 131 additional life years could be gained per 1000 patients screened if the utilization rate could be increased to 80%, a seemingly achievable goal.21 Moreover, expanding the screening program to include female smokers could increase 10-year life expectancy by 13%.21 Reasons for underutilization of this Medicare screening benefit may include lack of awareness by physicians and patients, costs of co-pays, and underutilization of the basic Welcome to Medicare exam.21
An additional consequence of low utilization of AAA screening is a high percentage of patients who are identified only late in the course of the disease. Mell et al22 report that 39% of patients undergoing AAA repair were identified < 6 months prior to surgery, a higher percentage than would be expected in a well-screened population. They also determined that slightly more than one-third of patients undergoing surgery for ruptured AAA had diagnostic imaging performed > 6 months prior to surgery, suggesting the possibility that these patients may not have been properly surveilled for aneurysm expansion, although the authors note that other potential explanations include delays in treatment due to comorbidities, and patient-related factors such as refusal of surgery or noncompliance with follow-up.
CORRESPONDENCE
Jeffrey S. Todd, MD, 127 McClanahan Street, Suite 300, Roanoke, VA 24014; jstodd@carilionclinic.org.
Too few patients are being screened for abdominal aortic aneurysm (AAA), resulting in severe morbidity and mortality. Many patients with AAA aren’t identified until they present with rupture, leading to mortality as high as 90%.1 Early detection is critical.
Medicare offers one-time free screening to eligible individuals > 65 years of age, and several professional organizations promote screening with published guidelines, which we discuss later in this article.
So who is at risk, who should be screened, and what is the best way to screen your patients?
Risk factors and sex differences
AAA has a prevalence of between 1% and 5% in men > 65 years old,2,3 and it is 4 to 6 times more common in men than women.4 Major risk factors include smoking, older age, family history, and genetic factors, while hypertension, history of coronary artery disease, hyperlipidemia, and peripheral arterial disease have weaker associations.3,4 Exercise and diabetes seem to have protective effects.5
The incidence and mortality of AAA increased between the 1950s and the mid-1990s; however, both indicators have decreased in numerous countries in the 21st century.6 Although the prevalence is much lower in women, they have a higher risk of rupture than men at equivalent lesion diameters.3 The prevalence of AAA in women who smoke and are > 70 years of age is > 1%.3
Silent but deadly
Most patients with AAA are asymptomatic. Their lesions are often detected incidentally on magnetic resonance imaging of the spine obtained for back pain, on an abdominal ultrasound (US) for gallstones, or on a routine computed tomography (CT) scan for the evaluation of abdominal pain. Some patients will experience vague abdominal discomfort from rapid expansion of an aneurysm prior to rupture, necessitating urgent repair. Also, some large aneurysms can erode into the spine and cause chronic back pain prior to rupture. An infrarenal abdominal aortic diameter > 30 mm defines an aneurysm,7 and once the diameter reaches 55 mm, the threat of rupture often justifies operative repair. (See “The preferred approach to repair.”)
SIDEBAR
The preferred approach to repair
Since the introduction of endovascular aneurysm repair (EVAR) in the latter part of the 20th century, it has become the standard of care for the surgical management of aneurysmal disease. Currently, > 80% of patients with an abdominal aortic aneurysm (AAA) who undergo repair are treated with EVAR.23
Typically, the AAA diameter is assessed via ultrasound. If repair is indicated, a computed tomography arteriogram is obtained to define the anatomy and help determine if the AAA is amenable to endografting. The most common contraindications to EVAR are either a short proximal neck (not enough distance below the renal arteries to safely anchor the stent graft) or an iliac artery diameter that is too small to allow delivery of the device. The operation can be performed under local, regional, or general anesthesia, and patients are usually discharged on the first postoperative day. These patients require lifelong surveillance due to the risk of delayed endoleak and reperfusion of the aneurysm sac.24
Ruptured aneurysms will classically manifest with severe abdominal and/or back pain. Often a ruptured aneurysm will be contained in the retroperitoneum, allowing the patient to remain hemodynamically stable for a period of time and thus providing a window of opportunity for emergent repair.
Continue to: What is the evidence that screening is effective?
What is the evidence that screening is effective?
In 1988, researchers in Chichester, England, randomized > 6000 men ages 65 to 80 years to either a control group or a group that was offered a one-time US screen for AAA. After 15 years of follow-up, no significant difference in AAA mortality was seen between the groups, although 26% of those invited for screening declined to participate and accounted for more than half of the AAA-related deaths in the group receiving an invitation for screening.8
The MASS Trial,9 another British study, began in 1997 and screened men ages 65 to 74. More than 67,000 men were randomized, with 1 group invited for AAA screening and the other serving as a control. The final report on this trial was published in 2012. After 13 years of follow-up, there was a 42% reduction in AAA-related deaths in the group invited for screening, a small reduction in all-cause mortality, and a significant reduction in risk of AAA rupture (hazard ratio = 0.57). The researchers noted that 216 patients would have to be invited for screening to prevent 1 death over 13 years. They also reported that 21% of the invited patients that had an AAA-related death had an initial scan that was negative for AAA (aortic diameter < 3 cm). However, despite this finding, screening still appeared to be beneficial.
Lindholdt et al10 randomized > 12,000 Danish men ages 64 to 73 to serve as controls or to be invited for US screening for AAA. After 13 years of follow-up, those invited for screening had a 66% relative risk reduction in AAA-related mortality, with screening considered cost effective. There were no differences between the groups in all-cause mortality. Conversely, the Western Australia Trial studied an older group of men, ages 64 to 83, but was unable to show a benefit of screening in lowering AAA-related mortality.11
Tikagi et al6 performed a meta-analysis on the data from the 4 trials above and reported up to 15 years of follow-up. Patients who attended screening sessions had a reduction in all-cause mortality with an odds ratio (OR) of 0.6, and a marked reduction in AAA-related mortality with an OR of 0.4. The favorable data on screening have prompted the United Kingdom and Sweden to offer screening to all men ≥ 65 years, based on the current estimate of a 1% prevalence of AAA, although screening is felt to remain cost effective down to a prevalence of 0.35%.12
Massachusetts General Hospital also reported13 that the detection rate of AAA increased, with the diagnosis made at smaller aneurysm dimensions, following publication of the US Preventive Services Task Force recommendations (reviewed below).
Continue to: When to screen
When to screen
Several professional organizations, as well as Medicare, have published AAA screening recommendations. While there are notable differences among them, their shared message is crucial: screen. Consider adding applicable reminders to your practice’s electronic medical record system to increase screening rates for eligible patients.
US Preventive Services Task Force 14
- Recommend one-time AAA screening with ultrasonography for men ages 65 to 75 years who have ever smoked (B recommendation).
- Selectively offer AAA screening to men ages 65 to 75 years who have never smoked, rather than routinely screening all men in this group (C recommendation). Individual attributes that could favor screening include a family history of AAA, the presence of other arterial aneurysms, and the number of risk factors for cardiovascular disease.
- Do not routinely screen women for AAA if they have never smoked and have no family history of AAA (D recommendation). For women ages 65 to 75 years who have ever smoked or have a family history of AAA, current evidence is insufficient to assess the balance of benefits and harms of screening for AAA (I statement). (See the related Practice Alert.)
Canadian Task Force on Preventive Health Care4
- Recommend one-time screening for AAA with US for men 65 to 80 years of age (weak level of recommendation; moderate-quality evidence).
- Do not recommend screening for men older than 80 years of age (weak recommendation; low quality of evidence).
- Do not recommend screening for women (strong recommendation; very low quality of evidence).
Society for Vascular Surgery15
- Recommend one-time US screening for AAA for men or women 65 to 75 years of age with a history of tobacco use (strong recommendation with high-quality evidence).
- Recommend one-time screening if there is a history of smoking for men or women > 75 years of age who are in good health and have not previously been screened. (Weak recommendation with low-quality evidence).
- Recommend one-time screening of men or women 65 to 75 years of age who are first-degree relatives of someone with AAA, or in those older than age 75 and in good health (weak recommendation with low-quality evidence).
How to screen
Physical exam. The abdominal aorta is often palpable in the epigastric region, and a thorough abdominal exam should include an attempt to detect it. It is critical that the patient be supine during palpation, to allow compression of the aorta against the lumbar spine. Even with a well-performed exam, however, its sensitivity is just 76% in the detection of AAA ≥ 5 cm.16
Continue to: Imaging
Imaging. US is the preferred imaging procedure when screening for AAA, given its high sensitivity and specificity.17 If US yields poor image quality, noncontrast CT is suggested, with magnetic resonance angiography being another alternative.18 Handheld US has the potential to supplement the physical exam, and has been shown to be a viable method to detect AAA in an outpatient primary care setting at a reasonable cost.19 Typically, a formal aortic US requires a patient to go without food or liquids for 8 hours before the procedure to obtain the best image; however, a good estimate of aortic diameter can be obtained without this restriction.
Despite Medicare coverage and recs, few people are screened
In 2007, Medicare started the SAAVE Program (Screening Aortic Aneurysm Very Effectively), offering a one-time US screening for AAA for eligible patients, as part of the Welcome to Medicare Program. Eligible individuals are men between 65 and 75 years of age with a history of smoking at least 100 cigarettes in their lifetime, and men or women in the same age group with a family history of AAA.2
Despite these recommendations, few patients receive screening. Centers for Medicare & Medicaid Services data show that < 10% of eligible men were screened between 2004 and 2008,20 and Olchanski et al21 report that < 1% of eligible patients were screened from 2005-2009. A simulation model estimates that 131 additional life years could be gained per 1000 patients screened if the utilization rate could be increased to 80%, a seemingly achievable goal.21 Moreover, expanding the screening program to include female smokers could increase 10-year life expectancy by 13%.21 Reasons for underutilization of this Medicare screening benefit may include lack of awareness by physicians and patients, costs of co-pays, and underutilization of the basic Welcome to Medicare exam.21
An additional consequence of low utilization of AAA screening is a high percentage of patients who are identified only late in the course of the disease. Mell et al22 report that 39% of patients undergoing AAA repair were identified < 6 months prior to surgery, a higher percentage than would be expected in a well-screened population. They also determined that slightly more than one-third of patients undergoing surgery for ruptured AAA had diagnostic imaging performed > 6 months prior to surgery, suggesting the possibility that these patients may not have been properly surveilled for aneurysm expansion, although the authors note that other potential explanations include delays in treatment due to comorbidities, and patient-related factors such as refusal of surgery or noncompliance with follow-up.
CORRESPONDENCE
Jeffrey S. Todd, MD, 127 McClanahan Street, Suite 300, Roanoke, VA 24014; jstodd@carilionclinic.org.
1. Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgrad Med J. 2009;85:268-273.
2. SBU—Swedish agency for Health Technology Assessment and Assessment of Social Services. Screening for abdominal aortic aneurysm. 2018. www.sbu.se/en/publications/sbu-assesses/screening-for-abdominal-aortic-aneurysm/. Accessed April 24, 2020.
3. Spanos K, Labropoulos N, Giannoukas A. Abdominal aortic aneurysm screening: do we need to shift toward a targeted strategy? Angiology. 2018;69:192-194.
4. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-1145.
5. Stackelberg O, Wolk, A, Eliasson K, et al. Lifestyle and risk of screening-detected abdominal aortic aneurysm in men. J Am Heart Assoc. 2017;6:e004725.
6. Tikagi H, Ando T, Umemoto T; ALICE (All-Literature Investigation of Cardiovascular Evidence) group. Abdominal aortic aneurysm screening reduces all-cause mortality: make screening great again. Angiology. 2017;69:205-211.
7. Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
8. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701.
9. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
10. Lindholdt JS, Sørenson J, Søgaard R et al. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97:826-834.
11. McCaul KA, Lawrence-Brown M, Dickinson JA, et al. Long-term outcomes of the Western Australian trial of screening for abdominal aortic aneurysms: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2016;176:1761-1766.
12. Earnshaw JJ, Lees T. Update on screening for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2017;54:1-2.
13. Zucker EJ, Misono AS, Prabhakar AM. Abdominal aortic aneurysm screening practices: impact of the 2014 US Preventive Services Task Force recommendations. J Am Coll Radiol. 2017;14:868-874.
14. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement.
15. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.
16. Venkatasubramaniam AK, Mehta T, Chetter IC, et al. The value of abdominal examination in the diagnosis of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2004;24:56-60.
17. Lindholdt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
18. Desjardins B, Dill KE, Flamm SD, et al. ACR Appropriateness Criteria®, pulsatile abdominal mass, suspected abdominal aortic aneurysm. Int J Cardiovasc Imaging. 2013;29:177-183.
19. Sisó-Almirall A, Kostov B, Navarro-González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
20. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462.
21. Olchanski N, Winn A, Cohen JT, et al. Abdominal aortic aneurysm screening: how many life years lost from underuse of the Medicare screening benefit? J Gen Intern Med. 2014;29:1155-1161.
22. Mell MW, Hlatky MA, Shreibati JB, et al. Late diagnosis of abdominal aortic aneurysms substantiates underutilization of abdominal aortic aneurysm screening for Medicare beneficiaries. J Vasc Surg. 2013;57:1519-1523.
23. England A, McWilliams R. Endovascular aortic aneurysm repair (EVAR). Ulster Med J. 2013;82:3-10.
24. Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg. 2006;43:446-451.
1. Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgrad Med J. 2009;85:268-273.
2. SBU—Swedish agency for Health Technology Assessment and Assessment of Social Services. Screening for abdominal aortic aneurysm. 2018. www.sbu.se/en/publications/sbu-assesses/screening-for-abdominal-aortic-aneurysm/. Accessed April 24, 2020.
3. Spanos K, Labropoulos N, Giannoukas A. Abdominal aortic aneurysm screening: do we need to shift toward a targeted strategy? Angiology. 2018;69:192-194.
4. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-1145.
5. Stackelberg O, Wolk, A, Eliasson K, et al. Lifestyle and risk of screening-detected abdominal aortic aneurysm in men. J Am Heart Assoc. 2017;6:e004725.
6. Tikagi H, Ando T, Umemoto T; ALICE (All-Literature Investigation of Cardiovascular Evidence) group. Abdominal aortic aneurysm screening reduces all-cause mortality: make screening great again. Angiology. 2017;69:205-211.
7. Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
8. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701.
9. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
10. Lindholdt JS, Sørenson J, Søgaard R et al. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97:826-834.
11. McCaul KA, Lawrence-Brown M, Dickinson JA, et al. Long-term outcomes of the Western Australian trial of screening for abdominal aortic aneurysms: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2016;176:1761-1766.
12. Earnshaw JJ, Lees T. Update on screening for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2017;54:1-2.
13. Zucker EJ, Misono AS, Prabhakar AM. Abdominal aortic aneurysm screening practices: impact of the 2014 US Preventive Services Task Force recommendations. J Am Coll Radiol. 2017;14:868-874.
14. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement.
15. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.
16. Venkatasubramaniam AK, Mehta T, Chetter IC, et al. The value of abdominal examination in the diagnosis of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2004;24:56-60.
17. Lindholdt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
18. Desjardins B, Dill KE, Flamm SD, et al. ACR Appropriateness Criteria®, pulsatile abdominal mass, suspected abdominal aortic aneurysm. Int J Cardiovasc Imaging. 2013;29:177-183.
19. Sisó-Almirall A, Kostov B, Navarro-González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
20. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462.
21. Olchanski N, Winn A, Cohen JT, et al. Abdominal aortic aneurysm screening: how many life years lost from underuse of the Medicare screening benefit? J Gen Intern Med. 2014;29:1155-1161.
22. Mell MW, Hlatky MA, Shreibati JB, et al. Late diagnosis of abdominal aortic aneurysms substantiates underutilization of abdominal aortic aneurysm screening for Medicare beneficiaries. J Vasc Surg. 2013;57:1519-1523.
23. England A, McWilliams R. Endovascular aortic aneurysm repair (EVAR). Ulster Med J. 2013;82:3-10.
24. Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg. 2006;43:446-451.