User login
Case: Patient seeks clarification and next steps on her breast density classification
Your patient, a 51-year-old postmenopausal woman (G0P0) in good health, had an annual screening mammogram that showed no evidence of malignancy. She is white and has a mother with a history of breast cancer. She has never had a breast biopsy. Following the mammogram, she received a letter from the imaging center, stating:
She calls your office and asks, “What should I do next?”
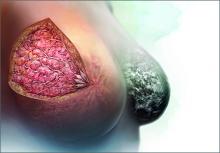
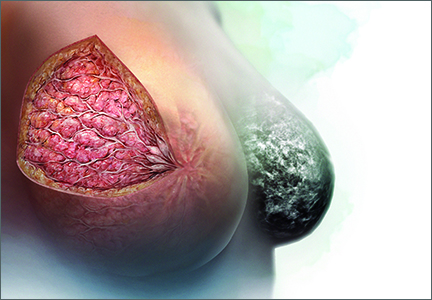
Breasts are composed of fibrous, glandular, and adipose tissue. If the breasts contain a lot of fibrous and glandular tissue, and little adipose tissue, they are considered to be “dense.” Using mammography, the current standard is to report the density of breast tissue using 4 categories:
- almost entirely fatty
- scattered fibroglandular densities
- heterogeneously dense
- extremely dense.
Dense breast tissue is defined to include the 2 categories heterogeneously dense and extremely dense.
Observational studies have reported that dense breast tissue is associated with an increased risk of breast cancer, and dense breast tissue makes it more difficult to detect breast cancer on mammography. According to data from the Breast Cancer Surveillance Consortium, among women aged 50 or older, the relative risk of breast cancer stratified by the 4 categories of breast density is 0.59, 1.00, 1.46, and 1.77, for almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, and extremely dense, respectively.1 In one study, the sensitivity of mammography to detect breast cancer was 82% to 88% for women with nondense breasts and 62% to 69% in women with dense breasts.2 These data have catalyzed investigators to explore the use of supplemental imaging to enhance cancer detection in women with dense breasts.
The link between breast density and breast cancer risk and reduced sensitivity of mammography also has catalyzed activists and legislators to champion breast density notification laws, which have passed in more than 20 states. These laws require facilities that perform mammography to notify women with dense breasts that this finding is associated with an increased risk of breast cancer and that dense breasts reduce the ability of mammography to detect cancer. In some states, the law mandates that women with dense breasts be offered supplemental ultrasound imaging and that insurers must cover the cost of the ultrasound studies. Many of the laws recommend that the patient discuss the situation with the clinician who ordered the mammogram.
When I first saw the recommendation for patients to contact me about how to manage dense breasts, my initial response was, “Who? Me?” I felt ill equipped to provide any useful advice and suspected that many of my patients knew more than I about this issue.
Based on a review of the evidence, my current clinical recommendation is outlined in the 2 options below, including a low-resource utilization option and a high-resource utilization option. For patients, physicians, and health systems that are concerned that excessive breast cancer screening tests might cause more harm than benefit, the identification of dense breasts on mammogram is unlikely to be a trigger to perform any additional testing. In this situation, the pragmatic low-resource option is most relevant.
Alternatively, for patients and physicians who strongly believe in the value of screening mammography (see “Utilize tomosynthesis digital mammography technology for your patients” below), a reasonable strategy is to recommend that women with dense breasts and an increased risk for breast cancer be offered supplemental imaging.
In this editorial I elaborate these 2 approaches to breast cancer screening in women with dense breasts.
Utilize tomosynthesis digital mammography technology for your patients
Mammograms are the primary modality used for breast cancer screening because screening mammography has been shown to reduce breast cancer deaths by 15% to 30%.1,2 Annual or biennial mammograms are recommended for women aged 40 years or older by many professional organizations, including the American College of Obstetricians and Gynecologists and the American College of Radiology. However, mammography screening programs have been criticized because of false-positive tests resulting in unnecessary biopsies, limited sensitivity, and the theoretical risk of over-diagnosing clinically insignificant cancers.3,4
Mammography technology continues to evolve. Film-based mammography has been replaced by digital mammography. Tomosynthesis digital mammography, also known as 3-D mammography, is now replacing standard digital mammography.5
With tomosynthesis, digital mammography image acquisition is performed using an x-ray source that moves through an arc across the breast with the capture of a series of images from different angles and reconstruction of the data into thin slices approximately 1 mm in width. The presentation of breast images in thin slices permits superior detection of lesions. In addition, the collected images can be reconstructed to present a virtual 2-D image for analysis.
Tomosynthesis has been demonstrated to increase the sensitivity of mammography to detect cancer and reduce false-positive examinations. In a study of 454,850 mammography examinations, investigators found that the invasive cancer detection rate per 1,000 studies increased from 2.9 with standard digital mammography to 4.1 with tomosynthesis.6
Tomosynthesis also reduces the patient recall rate to perform additional views or subsequent ultrasound. In one large study, the recall rate was 12% for standard digital mammography and 8.4% for tomosynthesis.7
The limitations of tomosynthesis include higher costs and higher radiation doses.
If the technology is available, I recommend that women have their mammograms using the best technology, tomosynthesis digital mammography.8
References
1. Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806.
2. Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786.
3. US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendations statement. Ann Intern Med. 2009;151(10):716–726, W-236.
4. Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammograms. JAMA Intern Med. 2014;174(3):448–454.
5. Destounis SV, Morgan R, Areino A. Screening for dense breasts: digital tomosynthesis. AJR Am J Roentgenol. 2015;204(2):261–264.
6. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
7. Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269(3):694–700.
8. Pisano ED, Yaffe MJ. Breast cancer screening: should tomosynthesis replace digital mammography? JAMA. 2014;311(24):2488–2489.
A pragmatic, low-resource utilization screening approach for women with dense breasts
There are no published randomized clinical trials that provide high-quality evidence on what to do if dense breasts are identified on mammography.3 Authors of observational studies have evaluated the potential role of supplemental imaging, including ultrasound and magnetic resonance imaging (MRI), in the management of dense breast tissue (see “Supplemental breast cancer screening modalities” below). Supplemental imaging involves complex trade-offs, balancing the potential benefit of identifying occult early breast cancer lesions not identified by mammography with the risk of subjecting many women without cancer to additional testing and unnecessary biopsies.
A pragmatic, low-resource utilization plan for women with dense breasts involves emphasizing that mammography is the best available screening tool and that annual or biennial mammography is the foundation of all current approaches to breast cancer screening. Supplemental imaging is unnecessary with this approach because there is no evidence that it reduces breast cancer mortality. There is, however, substantial evidence that using supplemental imaging for all women with dense breasts will result in little benefit and great costs, including many unnecessary biopsies.1,4 Women with dense breasts also could consider annual clinical breast examination.
Supplemental breast cancer screening modalities
Ultrasound and magnetic resonance imaging (MRI) are available as supplemental imaging, although ultrasound is the only supplemental imaging test that is specifically approved for women with dense breasts. Among the clinically available imaging modalities, MRI can detect the greatest number of cancers.
Ultrasound
In women with dense breasts, ultrasound can detect another 3 to 4 cancers that were not detected by mammography. However, ultrasound imaging generates many false positive results that lead to additional biopsies. According to one analysis, compared with mammography alone, mammography plus ultrasound would prevent 0.36 breast cancer deaths and cause 354 additional biopsies per 1,000 women with dense breasts screened biennially for 25 years.1
Ultrasound commonly is used to follow up an abnormal mammogram to further evaluate masses and differentiate cysts from solid tumors. Ultrasound is also a useful breast-imaging tool for women who are pregnant. In 2012, the US Food and Drug Administration approved an automated breast ultrasound device to be used for supplemental imaging of asymptomatic women with dense breasts and a mammogram negative for cancer. This device may facilitate the use of ultrasound for supplemental imaging of women with dense breasts on mammography.
Magnetic resonance imaging
MRI can detect the greatest number of cancers of any clinically available modality.
It is almost never covered by insurance for women whose only breast cancer risk factor is the identification of dense breasts on mammography. The cost of MRI testing is, however, typically covered for women at very high risk for breast cancer.
Women who are known to be at very high risk for breast cancer should begin annual clinical breast examinations at age 25 years and alternate between screening mammography and screening MRI every 6 months or annually. These women include:
- carriers of clinically significant BRCA1 or BRCA2 mutations
- carriers of other high-risk genetic mutations such as Cowden syndrome (PTEN mutation), Lai-Fraumeni syndrome (TP53 mutation), and Peutz-Jeghers syndrome
- genetically untested women with a first-degree relative with a BRCA mutation.
Women who had thoracic radiation before age 30 also should be considered for this screening protocol beginning 8 to 10 years after the radiation exposure or at age 25 years.2
References
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. CRICO Breast Care Management Algorithm. CRICO; Cambridge, Massachusetts; 2014. https://www.rmf.harvard.edu/~/media/Files/_Global/KC/PDFs/Guidelines/cricormfbca2014_locked.pdf. Accessed July 19, 2015.
A high-resource utilization screening approach
There are no randomized trials to help guide recommendations about how to respond to a finding of dense breasts on mammography. In addition to breast density, many factors influence breast cancer risk, including a patient’s:
- age
- family history
- history of previous breast biopsies
- many reproductive factors, including early age of menarche and late childbearing.
Women with both dense breasts and an increased risk of breast cancer may reap the greatest benefit from supplemental imaging, such as ultrasonography. Therefore, a two-step approach can help.
Step 1: Assess breast cancer risk. This can be accomplished using one of many calculators. Three that are commonly used are the:
- National Cancer Institute (NCI) Breast Cancer Surveillance Consortium (BCSC) calculator5
- NCI Breast Cancer Risk Assessment Tool, Gail model (BRCAT)6
- IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model).7
The BCSC calculator uses age, race/ethnicity, first-degree relatives with breast cancer, a history of a breast biopsy, and breast density to calculate a 5-year risk of developing breast cancer.
The BCRAT tool uses current age, race/ethnicity, age at menarche, age at first live-birth of a child, number of first-degree relatives with breast cancer, a history of breast biopsies, and the identification of atypical hyperplasia to calculate a 5-year risk of breast cancer.
The IBIS model uses many more variables, including a detailed family history to calculate a 10-year and lifetime risk of breast cancer. If a patient has ductal carcinoma in situ, lobular carcinoma in situ, chest irradiation before age 30 years, or known BRCA1 or BRCA2 mutations, she is instructed not to use the risk calculators because they are at very high risk for breast cancer, and they need an individualized intensive plan for monitoring and prevention (see MRI section in “Supplemental breast cancer screening modalities” above).
Step 2: Use breast density and breast cancer risk to develop a screening plan. The NIH Breast Cancer Surveillance Consortium has published data estimating the risk that a woman with a mammogram negative for cancer will develop breast cancer within the next 12 months (based on her age, breast density, and breast cancer risk—calculated with the BCSC tool).8
It reported an increased risk of breast cancer diagnosed within 12 months following a mammogram that was negative for cancer in women with extremely dense breasts and a BCSC 5-year risk of breast cancer of 1.67% or greater and in women with heterogeneously dense breasts and a BCSC 5-year risk of breast cancer of 2.5% or greater.8
Using these cutoffs it is estimated that 24% of all women with heterogeneously or extremely dense breasts would be offered supplemental screening with a modality such as ultrasound, and 76% would be guided not to have supplemental screening because their risk of developing breast cancer in the 12 months following their negative mammogram is low.
If this guidance is followed, it would require 694 supplemental ultrasound studies and many biopsies to detect 1 additional breast cancer, significantly increasing overall health care costs.8 In many states insurers do not cover supplemental ultrasound imaging of the breasts. In most states insurers require preauthorization for supplemental MRI of the breasts. You need to know the insurance practices in the state to help guide decision making about supplemental imaging. The approach described above is consistent with the American College of Obstetricians and Gynecologists recommendation that women with dense breasts, who are asymptomaticand have no additional risk factors for breast cancer, do not need to be offered supplemental imaging.9
Case: Next steps
The BCSC calculator reveals that the 51-year-old woman with a family history of breast cancer and a mammogram showing extremely dense breasts has a 5-year risk of breast cancer of 2.68%. Given that this risk is elevated, this patient could be offered supplemental ultrasound screening and annual breast clinical examination. In addition, she could be further counseled about breast cancer chemoprevention options.10
Women with a strong family history of breast and/or ovarian cancer also could be referred for genetic counseling and BRCA testing.11 The risk of having a BRCA mutation can be calculated using the BRCAPRO tool.12
Most women with dense breast tissue on mammography will never develop breast cancer. Yet the presence of dense breast tissue both increases the risk of breast cancer and decreases the sensitivity of mammography to detect cancer. There are no high-quality data from randomized trials to help guide our recommendations concerning the management of dense breasts identified on mammography. Yet many states have laws that suggest patients ask you to provide advice about breast density.
Patients, clinicians, and health systems vary in their confidence in the clinical value of breast cancer screening programs. Consequently, there is no “right answer” to this vexing problem. The standard of care is to support a range of options tailored to the specific clinical characteristics and needs of each patient.
Many states mandate that patients receive letters from their mammography center that report on breast density. In many states the law requires that the letter contain a statement that dense breasts increase the risk of breast cancer and reduce the ability of mammography to detect breast cancer. Do you believe these letters:
b) are beneficial because they provide the patient important information
c) both a and b
To weigh in and send your Letter to the Editor, visit obgmanagement.com and look for the “Quick Poll” on the right side of the home page.
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175.
3. Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013;4:CD009632.
4. Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs. mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–2163.
5. Breast Cancer Surveillance Consortium risk calculator. BCSC Web site. https://tools.bcsc-scc.org/BC5yearRisk/intro.htm. Updated February 13, 2015. Accessed July 17, 2015.
6. NCI Breast Cancer Risk Assessment Tool (Gail model). National Cancer Institute Web site. http://www.cancer.gov/BCRISKTOOL/. Accessed July 17, 2015.
7. IBIS Breast Cancer Risk Evaluation Tool. http://www.ems-trials.org/riskevaluator/. Updated January 9, 2015. Accessed July 17, 2015.
8. Kerlikowske K, Zhu W, Tosteson AN, et al; Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer. Ann Intern Med. 2015;162(10):673–681.
9. Committee on Gynecologic Practice. Committee Opinion No. 625: Management of women with dense breasts diagnosed by mammography. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;125(3): 750–751.
10. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(34):2942–2962.
11. Profato JL, Arun BK. Genetic risk assessment for breast and gynecological malignancies. Curr Opin Obstet Gynecol. 2015;27(1):1–5.
12. BRCAPRO. BayesMendel Lab. Harvard University Web site. http://bcb.dfci.harvard.edu/bayesmendel/brcapro.php. Accessed July 19, 2015.
Case: Patient seeks clarification and next steps on her breast density classification
Your patient, a 51-year-old postmenopausal woman (G0P0) in good health, had an annual screening mammogram that showed no evidence of malignancy. She is white and has a mother with a history of breast cancer. She has never had a breast biopsy. Following the mammogram, she received a letter from the imaging center, stating:
She calls your office and asks, “What should I do next?”
Breasts are composed of fibrous, glandular, and adipose tissue. If the breasts contain a lot of fibrous and glandular tissue, and little adipose tissue, they are considered to be “dense.” Using mammography, the current standard is to report the density of breast tissue using 4 categories:
- almost entirely fatty
- scattered fibroglandular densities
- heterogeneously dense
- extremely dense.
Dense breast tissue is defined to include the 2 categories heterogeneously dense and extremely dense.
Observational studies have reported that dense breast tissue is associated with an increased risk of breast cancer, and dense breast tissue makes it more difficult to detect breast cancer on mammography. According to data from the Breast Cancer Surveillance Consortium, among women aged 50 or older, the relative risk of breast cancer stratified by the 4 categories of breast density is 0.59, 1.00, 1.46, and 1.77, for almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, and extremely dense, respectively.1 In one study, the sensitivity of mammography to detect breast cancer was 82% to 88% for women with nondense breasts and 62% to 69% in women with dense breasts.2 These data have catalyzed investigators to explore the use of supplemental imaging to enhance cancer detection in women with dense breasts.
The link between breast density and breast cancer risk and reduced sensitivity of mammography also has catalyzed activists and legislators to champion breast density notification laws, which have passed in more than 20 states. These laws require facilities that perform mammography to notify women with dense breasts that this finding is associated with an increased risk of breast cancer and that dense breasts reduce the ability of mammography to detect cancer. In some states, the law mandates that women with dense breasts be offered supplemental ultrasound imaging and that insurers must cover the cost of the ultrasound studies. Many of the laws recommend that the patient discuss the situation with the clinician who ordered the mammogram.
When I first saw the recommendation for patients to contact me about how to manage dense breasts, my initial response was, “Who? Me?” I felt ill equipped to provide any useful advice and suspected that many of my patients knew more than I about this issue.
Based on a review of the evidence, my current clinical recommendation is outlined in the 2 options below, including a low-resource utilization option and a high-resource utilization option. For patients, physicians, and health systems that are concerned that excessive breast cancer screening tests might cause more harm than benefit, the identification of dense breasts on mammogram is unlikely to be a trigger to perform any additional testing. In this situation, the pragmatic low-resource option is most relevant.
Alternatively, for patients and physicians who strongly believe in the value of screening mammography (see “Utilize tomosynthesis digital mammography technology for your patients” below), a reasonable strategy is to recommend that women with dense breasts and an increased risk for breast cancer be offered supplemental imaging.
In this editorial I elaborate these 2 approaches to breast cancer screening in women with dense breasts.
Utilize tomosynthesis digital mammography technology for your patients
Mammograms are the primary modality used for breast cancer screening because screening mammography has been shown to reduce breast cancer deaths by 15% to 30%.1,2 Annual or biennial mammograms are recommended for women aged 40 years or older by many professional organizations, including the American College of Obstetricians and Gynecologists and the American College of Radiology. However, mammography screening programs have been criticized because of false-positive tests resulting in unnecessary biopsies, limited sensitivity, and the theoretical risk of over-diagnosing clinically insignificant cancers.3,4
Mammography technology continues to evolve. Film-based mammography has been replaced by digital mammography. Tomosynthesis digital mammography, also known as 3-D mammography, is now replacing standard digital mammography.5
With tomosynthesis, digital mammography image acquisition is performed using an x-ray source that moves through an arc across the breast with the capture of a series of images from different angles and reconstruction of the data into thin slices approximately 1 mm in width. The presentation of breast images in thin slices permits superior detection of lesions. In addition, the collected images can be reconstructed to present a virtual 2-D image for analysis.
Tomosynthesis has been demonstrated to increase the sensitivity of mammography to detect cancer and reduce false-positive examinations. In a study of 454,850 mammography examinations, investigators found that the invasive cancer detection rate per 1,000 studies increased from 2.9 with standard digital mammography to 4.1 with tomosynthesis.6
Tomosynthesis also reduces the patient recall rate to perform additional views or subsequent ultrasound. In one large study, the recall rate was 12% for standard digital mammography and 8.4% for tomosynthesis.7
The limitations of tomosynthesis include higher costs and higher radiation doses.
If the technology is available, I recommend that women have their mammograms using the best technology, tomosynthesis digital mammography.8
References
1. Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806.
2. Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786.
3. US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendations statement. Ann Intern Med. 2009;151(10):716–726, W-236.
4. Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammograms. JAMA Intern Med. 2014;174(3):448–454.
5. Destounis SV, Morgan R, Areino A. Screening for dense breasts: digital tomosynthesis. AJR Am J Roentgenol. 2015;204(2):261–264.
6. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
7. Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269(3):694–700.
8. Pisano ED, Yaffe MJ. Breast cancer screening: should tomosynthesis replace digital mammography? JAMA. 2014;311(24):2488–2489.
A pragmatic, low-resource utilization screening approach for women with dense breasts
There are no published randomized clinical trials that provide high-quality evidence on what to do if dense breasts are identified on mammography.3 Authors of observational studies have evaluated the potential role of supplemental imaging, including ultrasound and magnetic resonance imaging (MRI), in the management of dense breast tissue (see “Supplemental breast cancer screening modalities” below). Supplemental imaging involves complex trade-offs, balancing the potential benefit of identifying occult early breast cancer lesions not identified by mammography with the risk of subjecting many women without cancer to additional testing and unnecessary biopsies.
A pragmatic, low-resource utilization plan for women with dense breasts involves emphasizing that mammography is the best available screening tool and that annual or biennial mammography is the foundation of all current approaches to breast cancer screening. Supplemental imaging is unnecessary with this approach because there is no evidence that it reduces breast cancer mortality. There is, however, substantial evidence that using supplemental imaging for all women with dense breasts will result in little benefit and great costs, including many unnecessary biopsies.1,4 Women with dense breasts also could consider annual clinical breast examination.
Supplemental breast cancer screening modalities
Ultrasound and magnetic resonance imaging (MRI) are available as supplemental imaging, although ultrasound is the only supplemental imaging test that is specifically approved for women with dense breasts. Among the clinically available imaging modalities, MRI can detect the greatest number of cancers.
Ultrasound
In women with dense breasts, ultrasound can detect another 3 to 4 cancers that were not detected by mammography. However, ultrasound imaging generates many false positive results that lead to additional biopsies. According to one analysis, compared with mammography alone, mammography plus ultrasound would prevent 0.36 breast cancer deaths and cause 354 additional biopsies per 1,000 women with dense breasts screened biennially for 25 years.1
Ultrasound commonly is used to follow up an abnormal mammogram to further evaluate masses and differentiate cysts from solid tumors. Ultrasound is also a useful breast-imaging tool for women who are pregnant. In 2012, the US Food and Drug Administration approved an automated breast ultrasound device to be used for supplemental imaging of asymptomatic women with dense breasts and a mammogram negative for cancer. This device may facilitate the use of ultrasound for supplemental imaging of women with dense breasts on mammography.
Magnetic resonance imaging
MRI can detect the greatest number of cancers of any clinically available modality.
It is almost never covered by insurance for women whose only breast cancer risk factor is the identification of dense breasts on mammography. The cost of MRI testing is, however, typically covered for women at very high risk for breast cancer.
Women who are known to be at very high risk for breast cancer should begin annual clinical breast examinations at age 25 years and alternate between screening mammography and screening MRI every 6 months or annually. These women include:
- carriers of clinically significant BRCA1 or BRCA2 mutations
- carriers of other high-risk genetic mutations such as Cowden syndrome (PTEN mutation), Lai-Fraumeni syndrome (TP53 mutation), and Peutz-Jeghers syndrome
- genetically untested women with a first-degree relative with a BRCA mutation.
Women who had thoracic radiation before age 30 also should be considered for this screening protocol beginning 8 to 10 years after the radiation exposure or at age 25 years.2
References
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. CRICO Breast Care Management Algorithm. CRICO; Cambridge, Massachusetts; 2014. https://www.rmf.harvard.edu/~/media/Files/_Global/KC/PDFs/Guidelines/cricormfbca2014_locked.pdf. Accessed July 19, 2015.
A high-resource utilization screening approach
There are no randomized trials to help guide recommendations about how to respond to a finding of dense breasts on mammography. In addition to breast density, many factors influence breast cancer risk, including a patient’s:
- age
- family history
- history of previous breast biopsies
- many reproductive factors, including early age of menarche and late childbearing.
Women with both dense breasts and an increased risk of breast cancer may reap the greatest benefit from supplemental imaging, such as ultrasonography. Therefore, a two-step approach can help.
Step 1: Assess breast cancer risk. This can be accomplished using one of many calculators. Three that are commonly used are the:
- National Cancer Institute (NCI) Breast Cancer Surveillance Consortium (BCSC) calculator5
- NCI Breast Cancer Risk Assessment Tool, Gail model (BRCAT)6
- IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model).7
The BCSC calculator uses age, race/ethnicity, first-degree relatives with breast cancer, a history of a breast biopsy, and breast density to calculate a 5-year risk of developing breast cancer.
The BCRAT tool uses current age, race/ethnicity, age at menarche, age at first live-birth of a child, number of first-degree relatives with breast cancer, a history of breast biopsies, and the identification of atypical hyperplasia to calculate a 5-year risk of breast cancer.
The IBIS model uses many more variables, including a detailed family history to calculate a 10-year and lifetime risk of breast cancer. If a patient has ductal carcinoma in situ, lobular carcinoma in situ, chest irradiation before age 30 years, or known BRCA1 or BRCA2 mutations, she is instructed not to use the risk calculators because they are at very high risk for breast cancer, and they need an individualized intensive plan for monitoring and prevention (see MRI section in “Supplemental breast cancer screening modalities” above).
Step 2: Use breast density and breast cancer risk to develop a screening plan. The NIH Breast Cancer Surveillance Consortium has published data estimating the risk that a woman with a mammogram negative for cancer will develop breast cancer within the next 12 months (based on her age, breast density, and breast cancer risk—calculated with the BCSC tool).8
It reported an increased risk of breast cancer diagnosed within 12 months following a mammogram that was negative for cancer in women with extremely dense breasts and a BCSC 5-year risk of breast cancer of 1.67% or greater and in women with heterogeneously dense breasts and a BCSC 5-year risk of breast cancer of 2.5% or greater.8
Using these cutoffs it is estimated that 24% of all women with heterogeneously or extremely dense breasts would be offered supplemental screening with a modality such as ultrasound, and 76% would be guided not to have supplemental screening because their risk of developing breast cancer in the 12 months following their negative mammogram is low.
If this guidance is followed, it would require 694 supplemental ultrasound studies and many biopsies to detect 1 additional breast cancer, significantly increasing overall health care costs.8 In many states insurers do not cover supplemental ultrasound imaging of the breasts. In most states insurers require preauthorization for supplemental MRI of the breasts. You need to know the insurance practices in the state to help guide decision making about supplemental imaging. The approach described above is consistent with the American College of Obstetricians and Gynecologists recommendation that women with dense breasts, who are asymptomaticand have no additional risk factors for breast cancer, do not need to be offered supplemental imaging.9
Case: Next steps
The BCSC calculator reveals that the 51-year-old woman with a family history of breast cancer and a mammogram showing extremely dense breasts has a 5-year risk of breast cancer of 2.68%. Given that this risk is elevated, this patient could be offered supplemental ultrasound screening and annual breast clinical examination. In addition, she could be further counseled about breast cancer chemoprevention options.10
Women with a strong family history of breast and/or ovarian cancer also could be referred for genetic counseling and BRCA testing.11 The risk of having a BRCA mutation can be calculated using the BRCAPRO tool.12
Most women with dense breast tissue on mammography will never develop breast cancer. Yet the presence of dense breast tissue both increases the risk of breast cancer and decreases the sensitivity of mammography to detect cancer. There are no high-quality data from randomized trials to help guide our recommendations concerning the management of dense breasts identified on mammography. Yet many states have laws that suggest patients ask you to provide advice about breast density.
Patients, clinicians, and health systems vary in their confidence in the clinical value of breast cancer screening programs. Consequently, there is no “right answer” to this vexing problem. The standard of care is to support a range of options tailored to the specific clinical characteristics and needs of each patient.
Many states mandate that patients receive letters from their mammography center that report on breast density. In many states the law requires that the letter contain a statement that dense breasts increase the risk of breast cancer and reduce the ability of mammography to detect breast cancer. Do you believe these letters:
b) are beneficial because they provide the patient important information
c) both a and b
To weigh in and send your Letter to the Editor, visit obgmanagement.com and look for the “Quick Poll” on the right side of the home page.
Case: Patient seeks clarification and next steps on her breast density classification
Your patient, a 51-year-old postmenopausal woman (G0P0) in good health, had an annual screening mammogram that showed no evidence of malignancy. She is white and has a mother with a history of breast cancer. She has never had a breast biopsy. Following the mammogram, she received a letter from the imaging center, stating:
She calls your office and asks, “What should I do next?”
Breasts are composed of fibrous, glandular, and adipose tissue. If the breasts contain a lot of fibrous and glandular tissue, and little adipose tissue, they are considered to be “dense.” Using mammography, the current standard is to report the density of breast tissue using 4 categories:
- almost entirely fatty
- scattered fibroglandular densities
- heterogeneously dense
- extremely dense.
Dense breast tissue is defined to include the 2 categories heterogeneously dense and extremely dense.
Observational studies have reported that dense breast tissue is associated with an increased risk of breast cancer, and dense breast tissue makes it more difficult to detect breast cancer on mammography. According to data from the Breast Cancer Surveillance Consortium, among women aged 50 or older, the relative risk of breast cancer stratified by the 4 categories of breast density is 0.59, 1.00, 1.46, and 1.77, for almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, and extremely dense, respectively.1 In one study, the sensitivity of mammography to detect breast cancer was 82% to 88% for women with nondense breasts and 62% to 69% in women with dense breasts.2 These data have catalyzed investigators to explore the use of supplemental imaging to enhance cancer detection in women with dense breasts.
The link between breast density and breast cancer risk and reduced sensitivity of mammography also has catalyzed activists and legislators to champion breast density notification laws, which have passed in more than 20 states. These laws require facilities that perform mammography to notify women with dense breasts that this finding is associated with an increased risk of breast cancer and that dense breasts reduce the ability of mammography to detect cancer. In some states, the law mandates that women with dense breasts be offered supplemental ultrasound imaging and that insurers must cover the cost of the ultrasound studies. Many of the laws recommend that the patient discuss the situation with the clinician who ordered the mammogram.
When I first saw the recommendation for patients to contact me about how to manage dense breasts, my initial response was, “Who? Me?” I felt ill equipped to provide any useful advice and suspected that many of my patients knew more than I about this issue.
Based on a review of the evidence, my current clinical recommendation is outlined in the 2 options below, including a low-resource utilization option and a high-resource utilization option. For patients, physicians, and health systems that are concerned that excessive breast cancer screening tests might cause more harm than benefit, the identification of dense breasts on mammogram is unlikely to be a trigger to perform any additional testing. In this situation, the pragmatic low-resource option is most relevant.
Alternatively, for patients and physicians who strongly believe in the value of screening mammography (see “Utilize tomosynthesis digital mammography technology for your patients” below), a reasonable strategy is to recommend that women with dense breasts and an increased risk for breast cancer be offered supplemental imaging.
In this editorial I elaborate these 2 approaches to breast cancer screening in women with dense breasts.
Utilize tomosynthesis digital mammography technology for your patients
Mammograms are the primary modality used for breast cancer screening because screening mammography has been shown to reduce breast cancer deaths by 15% to 30%.1,2 Annual or biennial mammograms are recommended for women aged 40 years or older by many professional organizations, including the American College of Obstetricians and Gynecologists and the American College of Radiology. However, mammography screening programs have been criticized because of false-positive tests resulting in unnecessary biopsies, limited sensitivity, and the theoretical risk of over-diagnosing clinically insignificant cancers.3,4
Mammography technology continues to evolve. Film-based mammography has been replaced by digital mammography. Tomosynthesis digital mammography, also known as 3-D mammography, is now replacing standard digital mammography.5
With tomosynthesis, digital mammography image acquisition is performed using an x-ray source that moves through an arc across the breast with the capture of a series of images from different angles and reconstruction of the data into thin slices approximately 1 mm in width. The presentation of breast images in thin slices permits superior detection of lesions. In addition, the collected images can be reconstructed to present a virtual 2-D image for analysis.
Tomosynthesis has been demonstrated to increase the sensitivity of mammography to detect cancer and reduce false-positive examinations. In a study of 454,850 mammography examinations, investigators found that the invasive cancer detection rate per 1,000 studies increased from 2.9 with standard digital mammography to 4.1 with tomosynthesis.6
Tomosynthesis also reduces the patient recall rate to perform additional views or subsequent ultrasound. In one large study, the recall rate was 12% for standard digital mammography and 8.4% for tomosynthesis.7
The limitations of tomosynthesis include higher costs and higher radiation doses.
If the technology is available, I recommend that women have their mammograms using the best technology, tomosynthesis digital mammography.8
References
1. Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806.
2. Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786.
3. US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendations statement. Ann Intern Med. 2009;151(10):716–726, W-236.
4. Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammograms. JAMA Intern Med. 2014;174(3):448–454.
5. Destounis SV, Morgan R, Areino A. Screening for dense breasts: digital tomosynthesis. AJR Am J Roentgenol. 2015;204(2):261–264.
6. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
7. Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269(3):694–700.
8. Pisano ED, Yaffe MJ. Breast cancer screening: should tomosynthesis replace digital mammography? JAMA. 2014;311(24):2488–2489.
A pragmatic, low-resource utilization screening approach for women with dense breasts
There are no published randomized clinical trials that provide high-quality evidence on what to do if dense breasts are identified on mammography.3 Authors of observational studies have evaluated the potential role of supplemental imaging, including ultrasound and magnetic resonance imaging (MRI), in the management of dense breast tissue (see “Supplemental breast cancer screening modalities” below). Supplemental imaging involves complex trade-offs, balancing the potential benefit of identifying occult early breast cancer lesions not identified by mammography with the risk of subjecting many women without cancer to additional testing and unnecessary biopsies.
A pragmatic, low-resource utilization plan for women with dense breasts involves emphasizing that mammography is the best available screening tool and that annual or biennial mammography is the foundation of all current approaches to breast cancer screening. Supplemental imaging is unnecessary with this approach because there is no evidence that it reduces breast cancer mortality. There is, however, substantial evidence that using supplemental imaging for all women with dense breasts will result in little benefit and great costs, including many unnecessary biopsies.1,4 Women with dense breasts also could consider annual clinical breast examination.
Supplemental breast cancer screening modalities
Ultrasound and magnetic resonance imaging (MRI) are available as supplemental imaging, although ultrasound is the only supplemental imaging test that is specifically approved for women with dense breasts. Among the clinically available imaging modalities, MRI can detect the greatest number of cancers.
Ultrasound
In women with dense breasts, ultrasound can detect another 3 to 4 cancers that were not detected by mammography. However, ultrasound imaging generates many false positive results that lead to additional biopsies. According to one analysis, compared with mammography alone, mammography plus ultrasound would prevent 0.36 breast cancer deaths and cause 354 additional biopsies per 1,000 women with dense breasts screened biennially for 25 years.1
Ultrasound commonly is used to follow up an abnormal mammogram to further evaluate masses and differentiate cysts from solid tumors. Ultrasound is also a useful breast-imaging tool for women who are pregnant. In 2012, the US Food and Drug Administration approved an automated breast ultrasound device to be used for supplemental imaging of asymptomatic women with dense breasts and a mammogram negative for cancer. This device may facilitate the use of ultrasound for supplemental imaging of women with dense breasts on mammography.
Magnetic resonance imaging
MRI can detect the greatest number of cancers of any clinically available modality.
It is almost never covered by insurance for women whose only breast cancer risk factor is the identification of dense breasts on mammography. The cost of MRI testing is, however, typically covered for women at very high risk for breast cancer.
Women who are known to be at very high risk for breast cancer should begin annual clinical breast examinations at age 25 years and alternate between screening mammography and screening MRI every 6 months or annually. These women include:
- carriers of clinically significant BRCA1 or BRCA2 mutations
- carriers of other high-risk genetic mutations such as Cowden syndrome (PTEN mutation), Lai-Fraumeni syndrome (TP53 mutation), and Peutz-Jeghers syndrome
- genetically untested women with a first-degree relative with a BRCA mutation.
Women who had thoracic radiation before age 30 also should be considered for this screening protocol beginning 8 to 10 years after the radiation exposure or at age 25 years.2
References
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. CRICO Breast Care Management Algorithm. CRICO; Cambridge, Massachusetts; 2014. https://www.rmf.harvard.edu/~/media/Files/_Global/KC/PDFs/Guidelines/cricormfbca2014_locked.pdf. Accessed July 19, 2015.
A high-resource utilization screening approach
There are no randomized trials to help guide recommendations about how to respond to a finding of dense breasts on mammography. In addition to breast density, many factors influence breast cancer risk, including a patient’s:
- age
- family history
- history of previous breast biopsies
- many reproductive factors, including early age of menarche and late childbearing.
Women with both dense breasts and an increased risk of breast cancer may reap the greatest benefit from supplemental imaging, such as ultrasonography. Therefore, a two-step approach can help.
Step 1: Assess breast cancer risk. This can be accomplished using one of many calculators. Three that are commonly used are the:
- National Cancer Institute (NCI) Breast Cancer Surveillance Consortium (BCSC) calculator5
- NCI Breast Cancer Risk Assessment Tool, Gail model (BRCAT)6
- IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model).7
The BCSC calculator uses age, race/ethnicity, first-degree relatives with breast cancer, a history of a breast biopsy, and breast density to calculate a 5-year risk of developing breast cancer.
The BCRAT tool uses current age, race/ethnicity, age at menarche, age at first live-birth of a child, number of first-degree relatives with breast cancer, a history of breast biopsies, and the identification of atypical hyperplasia to calculate a 5-year risk of breast cancer.
The IBIS model uses many more variables, including a detailed family history to calculate a 10-year and lifetime risk of breast cancer. If a patient has ductal carcinoma in situ, lobular carcinoma in situ, chest irradiation before age 30 years, or known BRCA1 or BRCA2 mutations, she is instructed not to use the risk calculators because they are at very high risk for breast cancer, and they need an individualized intensive plan for monitoring and prevention (see MRI section in “Supplemental breast cancer screening modalities” above).
Step 2: Use breast density and breast cancer risk to develop a screening plan. The NIH Breast Cancer Surveillance Consortium has published data estimating the risk that a woman with a mammogram negative for cancer will develop breast cancer within the next 12 months (based on her age, breast density, and breast cancer risk—calculated with the BCSC tool).8
It reported an increased risk of breast cancer diagnosed within 12 months following a mammogram that was negative for cancer in women with extremely dense breasts and a BCSC 5-year risk of breast cancer of 1.67% or greater and in women with heterogeneously dense breasts and a BCSC 5-year risk of breast cancer of 2.5% or greater.8
Using these cutoffs it is estimated that 24% of all women with heterogeneously or extremely dense breasts would be offered supplemental screening with a modality such as ultrasound, and 76% would be guided not to have supplemental screening because their risk of developing breast cancer in the 12 months following their negative mammogram is low.
If this guidance is followed, it would require 694 supplemental ultrasound studies and many biopsies to detect 1 additional breast cancer, significantly increasing overall health care costs.8 In many states insurers do not cover supplemental ultrasound imaging of the breasts. In most states insurers require preauthorization for supplemental MRI of the breasts. You need to know the insurance practices in the state to help guide decision making about supplemental imaging. The approach described above is consistent with the American College of Obstetricians and Gynecologists recommendation that women with dense breasts, who are asymptomaticand have no additional risk factors for breast cancer, do not need to be offered supplemental imaging.9
Case: Next steps
The BCSC calculator reveals that the 51-year-old woman with a family history of breast cancer and a mammogram showing extremely dense breasts has a 5-year risk of breast cancer of 2.68%. Given that this risk is elevated, this patient could be offered supplemental ultrasound screening and annual breast clinical examination. In addition, she could be further counseled about breast cancer chemoprevention options.10
Women with a strong family history of breast and/or ovarian cancer also could be referred for genetic counseling and BRCA testing.11 The risk of having a BRCA mutation can be calculated using the BRCAPRO tool.12
Most women with dense breast tissue on mammography will never develop breast cancer. Yet the presence of dense breast tissue both increases the risk of breast cancer and decreases the sensitivity of mammography to detect cancer. There are no high-quality data from randomized trials to help guide our recommendations concerning the management of dense breasts identified on mammography. Yet many states have laws that suggest patients ask you to provide advice about breast density.
Patients, clinicians, and health systems vary in their confidence in the clinical value of breast cancer screening programs. Consequently, there is no “right answer” to this vexing problem. The standard of care is to support a range of options tailored to the specific clinical characteristics and needs of each patient.
Many states mandate that patients receive letters from their mammography center that report on breast density. In many states the law requires that the letter contain a statement that dense breasts increase the risk of breast cancer and reduce the ability of mammography to detect breast cancer. Do you believe these letters:
b) are beneficial because they provide the patient important information
c) both a and b
To weigh in and send your Letter to the Editor, visit obgmanagement.com and look for the “Quick Poll” on the right side of the home page.
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175.
3. Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013;4:CD009632.
4. Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs. mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–2163.
5. Breast Cancer Surveillance Consortium risk calculator. BCSC Web site. https://tools.bcsc-scc.org/BC5yearRisk/intro.htm. Updated February 13, 2015. Accessed July 17, 2015.
6. NCI Breast Cancer Risk Assessment Tool (Gail model). National Cancer Institute Web site. http://www.cancer.gov/BCRISKTOOL/. Accessed July 17, 2015.
7. IBIS Breast Cancer Risk Evaluation Tool. http://www.ems-trials.org/riskevaluator/. Updated January 9, 2015. Accessed July 17, 2015.
8. Kerlikowske K, Zhu W, Tosteson AN, et al; Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer. Ann Intern Med. 2015;162(10):673–681.
9. Committee on Gynecologic Practice. Committee Opinion No. 625: Management of women with dense breasts diagnosed by mammography. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;125(3): 750–751.
10. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(34):2942–2962.
11. Profato JL, Arun BK. Genetic risk assessment for breast and gynecological malignancies. Curr Opin Obstet Gynecol. 2015;27(1):1–5.
12. BRCAPRO. BayesMendel Lab. Harvard University Web site. http://bcb.dfci.harvard.edu/bayesmendel/brcapro.php. Accessed July 19, 2015.
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175.
3. Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013;4:CD009632.
4. Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs. mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–2163.
5. Breast Cancer Surveillance Consortium risk calculator. BCSC Web site. https://tools.bcsc-scc.org/BC5yearRisk/intro.htm. Updated February 13, 2015. Accessed July 17, 2015.
6. NCI Breast Cancer Risk Assessment Tool (Gail model). National Cancer Institute Web site. http://www.cancer.gov/BCRISKTOOL/. Accessed July 17, 2015.
7. IBIS Breast Cancer Risk Evaluation Tool. http://www.ems-trials.org/riskevaluator/. Updated January 9, 2015. Accessed July 17, 2015.
8. Kerlikowske K, Zhu W, Tosteson AN, et al; Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer. Ann Intern Med. 2015;162(10):673–681.
9. Committee on Gynecologic Practice. Committee Opinion No. 625: Management of women with dense breasts diagnosed by mammography. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;125(3): 750–751.
10. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(34):2942–2962.
11. Profato JL, Arun BK. Genetic risk assessment for breast and gynecological malignancies. Curr Opin Obstet Gynecol. 2015;27(1):1–5.
12. BRCAPRO. BayesMendel Lab. Harvard University Web site. http://bcb.dfci.harvard.edu/bayesmendel/brcapro.php. Accessed July 19, 2015.