User login
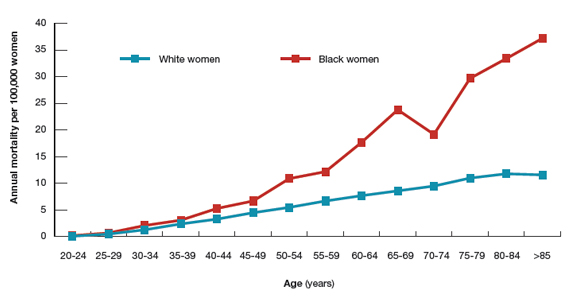
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1
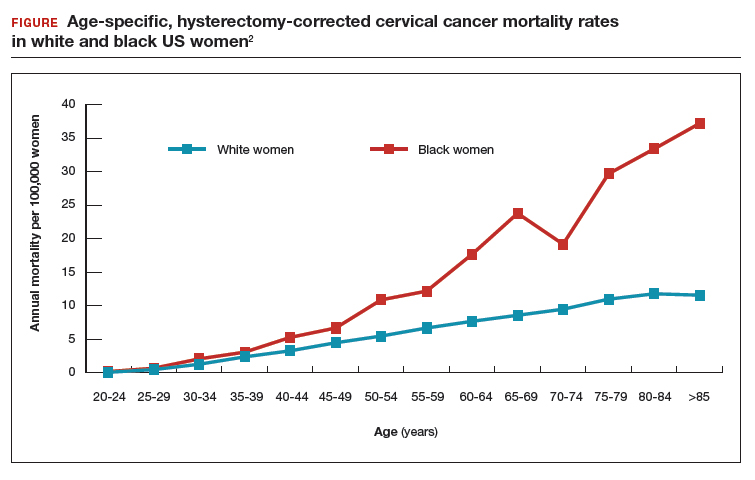
A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.