User login
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
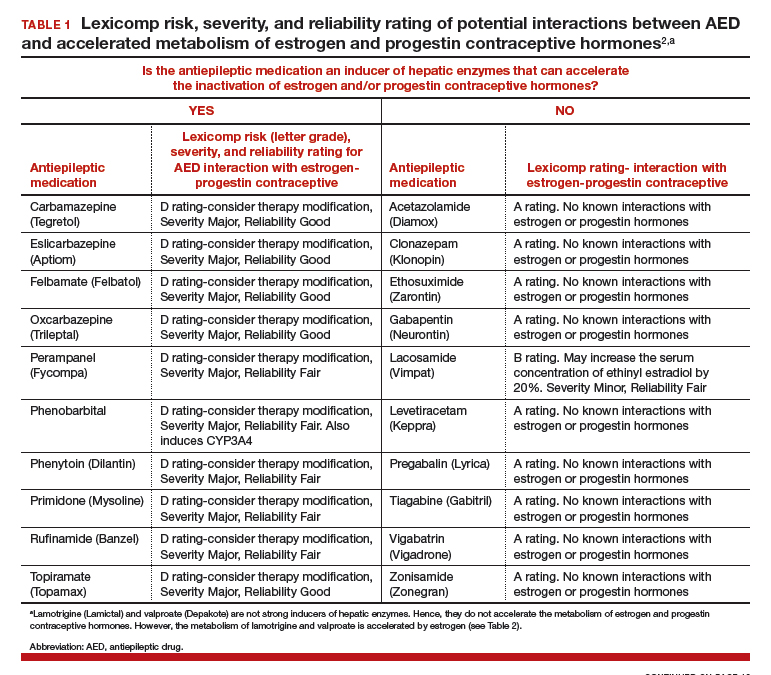
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).

For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.