The incidence of Clostridium difficile (C difficile) infection (CDI) in the U.S. has been steadily increasing. In U.S. hospitals between 1996 and 2003, the rate of CDI diagnosis doubled, and in 2011, almost half a million CDIs contributed to 29,000 deaths.1,2 Recurrence rates after successful metronidazole or vancomycin treatment are as high as 35%.3-5 After a second recurrence, rates are as high as 40% to 60%.6
Historically, CDI was almost exclusively associated with the elderly, with exposure to health care facilities, or in individuals with a history of previous antibiotic use.1,7 Risk factors for CDI recurrence are similar, including the elderly, antibiotic use during or after initial CDI treatment, and an impaired immune response against C difficile toxins.8 However, more recently CDI has been linked to individuals who were previously considered low risk, including the young and previously healthy individuals without exposure to a health care environment or recent antibiotic use.9
Community-acquired CDIs occurring in populations previously at low risk may be due to increased virulence of the disease. A hypervirulent C difficile strain, the North American Pulsed field type 1 (NAP1)/B1/027 strain, has emerged. This strain is more resistant to fluoroquinolone antibiotics and has caused multiple CDI outbreaks in the U.S.7 Along with the increased rate of CDI occurrence, mortality rates due to CDI have been rising.10 Recent studies have shown increased rates of CDI recurrence and treatment failure in response to standard therapy (metronidazole or vancomycin).11-13
To combat emerging treatment challenges of CDIs, the FDA approved a new antibiotic, fidaxomicin, for the treatment of C difficile-associated diarrhea in 2011.14 Fidaxomicin is a first-in-class macrocyclic antibiotic that has low systemic absorption, low activity against intestinal flora, and high fecal concentrations.15 Fidaxomicin also has been shown to be less likely to promote vancomycin-resistant enterococci (VRE) than does vancomycin in CDI treatment.16 Fidaxomicin is an emerging treatment strategy for CDIs, and this article reviews its role in the treatment of CDI.
CDI Standard of Therapy
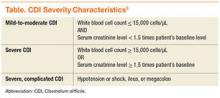
Before the approval of fidaxomicin, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America released the 2010 update to the clinical practice guidelines for the treatment of CDI. Due to initial concern that use of oral vancomycin would select for VRE, guidelines recommend oral metronidazole for mild-to-moderate disease, oral vancomycin for severe CDI, and combination therapy of oral vancomycin with or without IV metronidazole for severe, complicated CDI (disease severity is defined in the Table).8,17
However, treatment failure and CDI recurrence rates after treatment with standard therapy (metronidazole or vancomycin) have been increasing. Treatment failure with metronidazole has increased since 2000 from 0% to 6% to 16% to 38%,17 and recurrence occurs with both metronidazole and vancomycin in rates up to 35%.3-5Fidaxomicin Approval
Prior to the approval of fidaxomicin for CDI in 2011, the FDA evaluated 2 noninferiority (NI) clinical trials comparing oral fidaxomicin to oral vancomycin for the treatment of CDI. Given that a clinical trial had previously demonstrated superior clinical cure rates of vancomycin over metronidazole, vancomycin was used as the comparator in the fidaxomicin NI trials.15 Louie and colleagues conducted a double-blind, randomized phase 3 trial comparing 10 days of fidaxomicin (200 mg twice daily) to vancomycin (125 mg 4 times daily) for the treatment of CDI (n = 629).3 Fidaxomicin was found to be noninferior to vancomycin for rate of clinical cure in the modified intention-to-treat (ITT) analysis (88% vs 86%, respectively) and the per-protocol analysis (92% vs 90%, respectively) with a NI margin of 10%. There were lower recurrence rates of CDI with fidaxomicin compared with that of vancomycin in the modified ITT analysis (15% vs 25%, respectively; P = .005). When infection with the NAP1/BI/027 strain was evaluated, fidaxomicin was shown to be noninferior to vancomycin for both clinical cure and recurrence rates.
The Louie and colleagues results were further validated when a second NI trial (n = 535) was published by Cornely and colleagues, which demonstrated similar clinical cure and recurrence rates with fidaxomicin compared with that of vancomycin.4 It is important to note that both trials used a modified ITT analysis, which included postrandomization exclusions that may have biased the results. Additionally, both trials were industry sponsored and had industry representation throughout the data collection, analysis, and manuscript preparation processes.
Use of Fidaxomicin
Fidaxomicin has been considered for use in the treatment of recurrent CDI. According to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of CDI, fidaxomicin is an option for both first and multiple recurrences (ESCMID does not recommend fidaxomicin for an initial episode of CDI). These guidelines state that either oral fidaxomicin or vancomycin for 10 days is an appropriate first recurrence treatment option. For multiple recurrences, the recommendations are oral fidaxomicin for 10 days or oral vancomycin for 10 days followed by a tapered or pulsed regimen.18 The IDSA C difficile treatment guidelines have not been updated since the approval of fidaxomicin and, therefore, do not contain recommendations for its use.