User login
The Role of Fidaxomicin in Clostridium difficile Infection
The incidence of Clostridium difficile (C difficile) infection (CDI) in the U.S. has been steadily increasing. In U.S. hospitals between 1996 and 2003, the rate of CDI diagnosis doubled, and in 2011, almost half a million CDIs contributed to 29,000 deaths.1,2 Recurrence rates after successful metronidazole or vancomycin treatment are as high as 35%.3-5 After a second recurrence, rates are as high as 40% to 60%.6
Historically, CDI was almost exclusively associated with the elderly, with exposure to health care facilities, or in individuals with a history of previous antibiotic use.1,7 Risk factors for CDI recurrence are similar, including the elderly, antibiotic use during or after initial CDI treatment, and an impaired immune response against C difficile toxins.8 However, more recently CDI has been linked to individuals who were previously considered low risk, including the young and previously healthy individuals without exposure to a health care environment or recent antibiotic use.9
Community-acquired CDIs occurring in populations previously at low risk may be due to increased virulence of the disease. A hypervirulent C difficile strain, the North American Pulsed field type 1 (NAP1)/B1/027 strain, has emerged. This strain is more resistant to fluoroquinolone antibiotics and has caused multiple CDI outbreaks in the U.S.7 Along with the increased rate of CDI occurrence, mortality rates due to CDI have been rising.10 Recent studies have shown increased rates of CDI recurrence and treatment failure in response to standard therapy (metronidazole or vancomycin).11-13
To combat emerging treatment challenges of CDIs, the FDA approved a new antibiotic, fidaxomicin, for the treatment of C difficile-associated diarrhea in 2011.14 Fidaxomicin is a first-in-class macrocyclic antibiotic that has low systemic absorption, low activity against intestinal flora, and high fecal concentrations.15 Fidaxomicin also has been shown to be less likely to promote vancomycin-resistant enterococci (VRE) than does vancomycin in CDI treatment.16 Fidaxomicin is an emerging treatment strategy for CDIs, and this article reviews its role in the treatment of CDI.
CDI Standard of Therapy
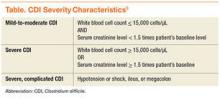
Before the approval of fidaxomicin, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America released the 2010 update to the clinical practice guidelines for the treatment of CDI. Due to initial concern that use of oral vancomycin would select for VRE, guidelines recommend oral metronidazole for mild-to-moderate disease, oral vancomycin for severe CDI, and combination therapy of oral vancomycin with or without IV metronidazole for severe, complicated CDI (disease severity is defined in the Table).8,17
Fidaxomicin Approval
Prior to the approval of fidaxomicin for CDI in 2011, the FDA evaluated 2 noninferiority (NI) clinical trials comparing oral fidaxomicin to oral vancomycin for the treatment of CDI. Given that a clinical trial had previously demonstrated superior clinical cure rates of vancomycin over metronidazole, vancomycin was used as the comparator in the fidaxomicin NI trials.15 Louie and colleagues conducted a double-blind, randomized phase 3 trial comparing 10 days of fidaxomicin (200 mg twice daily) to vancomycin (125 mg 4 times daily) for the treatment of CDI (n = 629).3 Fidaxomicin was found to be noninferior to vancomycin for rate of clinical cure in the modified intention-to-treat (ITT) analysis (88% vs 86%, respectively) and the per-protocol analysis (92% vs 90%, respectively) with a NI margin of 10%. There were lower recurrence rates of CDI with fidaxomicin compared with that of vancomycin in the modified ITT analysis (15% vs 25%, respectively; P = .005). When infection with the NAP1/BI/027 strain was evaluated, fidaxomicin was shown to be noninferior to vancomycin for both clinical cure and recurrence rates.
The Louie and colleagues results were further validated when a second NI trial (n = 535) was published by Cornely and colleagues, which demonstrated similar clinical cure and recurrence rates with fidaxomicin compared with that of vancomycin.4 It is important to note that both trials used a modified ITT analysis, which included postrandomization exclusions that may have biased the results. Additionally, both trials were industry sponsored and had industry representation throughout the data collection, analysis, and manuscript preparation processes.
Use of Fidaxomicin
Fidaxomicin has been considered for use in the treatment of recurrent CDI. According to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of CDI, fidaxomicin is an option for both first and multiple recurrences (ESCMID does not recommend fidaxomicin for an initial episode of CDI). These guidelines state that either oral fidaxomicin or vancomycin for 10 days is an appropriate first recurrence treatment option. For multiple recurrences, the recommendations are oral fidaxomicin for 10 days or oral vancomycin for 10 days followed by a tapered or pulsed regimen.18 The IDSA C difficile treatment guidelines have not been updated since the approval of fidaxomicin and, therefore, do not contain recommendations for its use.
Given the low propensity for fidaxomicin to disrupt colonic flora, it may be hypothesized that its greatest benefit would be for use as first-line therapy in patients with a high risk of CDI recurrence prior to disruption of colonic flora with the treatment of vancomycin or metronidazole. A clinical prediction tool is needed to identify patients at high risk of CDI recurrence who would most benefit from initial fidaxomicin therapy. However, clinically relevant prediction tools are not currently used.19
Evidence exists that demonstrates the role of fidaxomicin for the treatment of recurrent CDI episodes. Cornely and colleagues pooled data from the 2 NI trials that led to the approval of fidaxomicin (n = 1,164).3,4,20 Of these, 128 participants had a recent CDI episode prior to study enrollment. For the treatment of first recurrence, 20% of patients treated with fidaxomicin experienced a second recurrence within 28 days compared with 36% of vancomycin patients (P = .045). Similarly, fewer fidaxomicin patients experienced an early recurrence within 14 days of treatment than with vancomycin for early recurrence within 14 days of treatment (8% vs 27%, respectively; P = .003).
Limitations of Fidaxomicin
One limitation of fidaxomicin is the paucity of data existing for its use in severe, life-threatening CDI, and it is currently not recommended in this indication.18 The main limitation behind the use of fidaxomicin is cost. The average wholesale price of a 10-day course of therapy of fidaxomicin is $3,360 compared with $1,273 for vancomycin capsules, $32 for compounded vancomycin oral solution, and $21 for oral metronidazole.21,22 Despite the price, cost-analysis studies have found that fidaxomicin compared with oral vancomycin is cost-effective for clinical cure rates and recurrences.23,24 Fidaxomicin also was found to be cost-effective in patients with mild-to-moderate CDI and in those using concomitant antibiotics.23 Given that 2 studies demonstrated that fidaxomicin has lower recurrence rates than that of oral vancomycin, the economical use of fidaxomicin would be of most benefit to patients at highest risk for CDI recurrence.3,4
Conclusion
In light of increased CDI treatment failure, recurrence rates, and virulence of CDI, fidaxomicin is an emerging treatment strategy. Through 2 pivotal trials, fidaxomicin has been shown to be a safe and effective first-line agent for CDI.3,4 New U.S. clinical guidelines for CDI are expected to be published in spring 2016, which will likely include the recommendation for fidaxomicin use in recurrent CDI. Current evidence suggests the most cost-effective use of fidaxomicin is in patients at highest risk of CDI recurrence.
1. McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis. 2006;12(3):409-415.
2. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
3. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
4. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281-289.
5. Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5(9):549-557.
6. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:403-410.
7. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene—variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433-2441.
8. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
9. Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk—4 states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(47):1201-1205.
10. Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999-2004. Emerg Infect Dis. 2007;13(9):1417-1419.
11. Maroo S, Lamont JT. Recurrent Clostridium difficile. Gastroenterology. 2006;130(4):1311-1316.
12. Pépin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol. 2007;102(12):2781-2788.
13. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40(11):1586-1590.
14. U.S. Food and Drug Administration. FDA approves treatment for Clostridium difficile infection; May 27, 2011 [news release]. U.S. Food and Drug Administration website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm257024.htm. Updated April 10, 2014. Accessed March 15, 2016.
15. Shue YK, Sears PS, Shangle S, et al. Safety, tolerance, and pharmacokinetic studies of OPT-80 in healthy volunteers following single and multiple oral doses. Antimicrob Agents Chemother. 2008;52(4):1391-1395.
16. Nerandzic MM, Mullane K, Miller MA, Babakhani F, Donskey CJ. Reduced acquisition and overgrowth of vancomycin-resistant enterococci and Candida species in patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Clin Infect Dis. 2012;55(2):S121-S126.
17. Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5(9):549-557.
18. Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(suppl 2):1-26.
19. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
20. Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
21. Murray L, ed. Red Book: Pharmacy’s Fundamental Reference. 2010 ed. Montvale, NJ: Thomson Reuters (Healthcare) Inc; 2010.
22. Cruz MP. Fidaxomicin (Dificid), a novel oral macrocyclic antibacterial agent for the treatment of Clostridium difficile-associated diarrhea in adults. Pharm Ther. 2012;37(5):278-281.
23. Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health. 2013;16(2):297-304.
24. Sclar DA, Robison LM, Oganov AM, Schmidt JM, Bowen KA, Castillo LV. Fidaxomicin for Clostridium difficile-associated diarrhoea: epidemiological method for estimation of warranted price. Clin Drug Investig. 2012;32(8):e17-e24.
The incidence of Clostridium difficile (C difficile) infection (CDI) in the U.S. has been steadily increasing. In U.S. hospitals between 1996 and 2003, the rate of CDI diagnosis doubled, and in 2011, almost half a million CDIs contributed to 29,000 deaths.1,2 Recurrence rates after successful metronidazole or vancomycin treatment are as high as 35%.3-5 After a second recurrence, rates are as high as 40% to 60%.6
Historically, CDI was almost exclusively associated with the elderly, with exposure to health care facilities, or in individuals with a history of previous antibiotic use.1,7 Risk factors for CDI recurrence are similar, including the elderly, antibiotic use during or after initial CDI treatment, and an impaired immune response against C difficile toxins.8 However, more recently CDI has been linked to individuals who were previously considered low risk, including the young and previously healthy individuals without exposure to a health care environment or recent antibiotic use.9
Community-acquired CDIs occurring in populations previously at low risk may be due to increased virulence of the disease. A hypervirulent C difficile strain, the North American Pulsed field type 1 (NAP1)/B1/027 strain, has emerged. This strain is more resistant to fluoroquinolone antibiotics and has caused multiple CDI outbreaks in the U.S.7 Along with the increased rate of CDI occurrence, mortality rates due to CDI have been rising.10 Recent studies have shown increased rates of CDI recurrence and treatment failure in response to standard therapy (metronidazole or vancomycin).11-13
To combat emerging treatment challenges of CDIs, the FDA approved a new antibiotic, fidaxomicin, for the treatment of C difficile-associated diarrhea in 2011.14 Fidaxomicin is a first-in-class macrocyclic antibiotic that has low systemic absorption, low activity against intestinal flora, and high fecal concentrations.15 Fidaxomicin also has been shown to be less likely to promote vancomycin-resistant enterococci (VRE) than does vancomycin in CDI treatment.16 Fidaxomicin is an emerging treatment strategy for CDIs, and this article reviews its role in the treatment of CDI.
CDI Standard of Therapy
Before the approval of fidaxomicin, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America released the 2010 update to the clinical practice guidelines for the treatment of CDI. Due to initial concern that use of oral vancomycin would select for VRE, guidelines recommend oral metronidazole for mild-to-moderate disease, oral vancomycin for severe CDI, and combination therapy of oral vancomycin with or without IV metronidazole for severe, complicated CDI (disease severity is defined in the Table).8,17
Fidaxomicin Approval
Prior to the approval of fidaxomicin for CDI in 2011, the FDA evaluated 2 noninferiority (NI) clinical trials comparing oral fidaxomicin to oral vancomycin for the treatment of CDI. Given that a clinical trial had previously demonstrated superior clinical cure rates of vancomycin over metronidazole, vancomycin was used as the comparator in the fidaxomicin NI trials.15 Louie and colleagues conducted a double-blind, randomized phase 3 trial comparing 10 days of fidaxomicin (200 mg twice daily) to vancomycin (125 mg 4 times daily) for the treatment of CDI (n = 629).3 Fidaxomicin was found to be noninferior to vancomycin for rate of clinical cure in the modified intention-to-treat (ITT) analysis (88% vs 86%, respectively) and the per-protocol analysis (92% vs 90%, respectively) with a NI margin of 10%. There were lower recurrence rates of CDI with fidaxomicin compared with that of vancomycin in the modified ITT analysis (15% vs 25%, respectively; P = .005). When infection with the NAP1/BI/027 strain was evaluated, fidaxomicin was shown to be noninferior to vancomycin for both clinical cure and recurrence rates.
The Louie and colleagues results were further validated when a second NI trial (n = 535) was published by Cornely and colleagues, which demonstrated similar clinical cure and recurrence rates with fidaxomicin compared with that of vancomycin.4 It is important to note that both trials used a modified ITT analysis, which included postrandomization exclusions that may have biased the results. Additionally, both trials were industry sponsored and had industry representation throughout the data collection, analysis, and manuscript preparation processes.
Use of Fidaxomicin
Fidaxomicin has been considered for use in the treatment of recurrent CDI. According to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of CDI, fidaxomicin is an option for both first and multiple recurrences (ESCMID does not recommend fidaxomicin for an initial episode of CDI). These guidelines state that either oral fidaxomicin or vancomycin for 10 days is an appropriate first recurrence treatment option. For multiple recurrences, the recommendations are oral fidaxomicin for 10 days or oral vancomycin for 10 days followed by a tapered or pulsed regimen.18 The IDSA C difficile treatment guidelines have not been updated since the approval of fidaxomicin and, therefore, do not contain recommendations for its use.
Given the low propensity for fidaxomicin to disrupt colonic flora, it may be hypothesized that its greatest benefit would be for use as first-line therapy in patients with a high risk of CDI recurrence prior to disruption of colonic flora with the treatment of vancomycin or metronidazole. A clinical prediction tool is needed to identify patients at high risk of CDI recurrence who would most benefit from initial fidaxomicin therapy. However, clinically relevant prediction tools are not currently used.19
Evidence exists that demonstrates the role of fidaxomicin for the treatment of recurrent CDI episodes. Cornely and colleagues pooled data from the 2 NI trials that led to the approval of fidaxomicin (n = 1,164).3,4,20 Of these, 128 participants had a recent CDI episode prior to study enrollment. For the treatment of first recurrence, 20% of patients treated with fidaxomicin experienced a second recurrence within 28 days compared with 36% of vancomycin patients (P = .045). Similarly, fewer fidaxomicin patients experienced an early recurrence within 14 days of treatment than with vancomycin for early recurrence within 14 days of treatment (8% vs 27%, respectively; P = .003).
Limitations of Fidaxomicin
One limitation of fidaxomicin is the paucity of data existing for its use in severe, life-threatening CDI, and it is currently not recommended in this indication.18 The main limitation behind the use of fidaxomicin is cost. The average wholesale price of a 10-day course of therapy of fidaxomicin is $3,360 compared with $1,273 for vancomycin capsules, $32 for compounded vancomycin oral solution, and $21 for oral metronidazole.21,22 Despite the price, cost-analysis studies have found that fidaxomicin compared with oral vancomycin is cost-effective for clinical cure rates and recurrences.23,24 Fidaxomicin also was found to be cost-effective in patients with mild-to-moderate CDI and in those using concomitant antibiotics.23 Given that 2 studies demonstrated that fidaxomicin has lower recurrence rates than that of oral vancomycin, the economical use of fidaxomicin would be of most benefit to patients at highest risk for CDI recurrence.3,4
Conclusion
In light of increased CDI treatment failure, recurrence rates, and virulence of CDI, fidaxomicin is an emerging treatment strategy. Through 2 pivotal trials, fidaxomicin has been shown to be a safe and effective first-line agent for CDI.3,4 New U.S. clinical guidelines for CDI are expected to be published in spring 2016, which will likely include the recommendation for fidaxomicin use in recurrent CDI. Current evidence suggests the most cost-effective use of fidaxomicin is in patients at highest risk of CDI recurrence.
The incidence of Clostridium difficile (C difficile) infection (CDI) in the U.S. has been steadily increasing. In U.S. hospitals between 1996 and 2003, the rate of CDI diagnosis doubled, and in 2011, almost half a million CDIs contributed to 29,000 deaths.1,2 Recurrence rates after successful metronidazole or vancomycin treatment are as high as 35%.3-5 After a second recurrence, rates are as high as 40% to 60%.6
Historically, CDI was almost exclusively associated with the elderly, with exposure to health care facilities, or in individuals with a history of previous antibiotic use.1,7 Risk factors for CDI recurrence are similar, including the elderly, antibiotic use during or after initial CDI treatment, and an impaired immune response against C difficile toxins.8 However, more recently CDI has been linked to individuals who were previously considered low risk, including the young and previously healthy individuals without exposure to a health care environment or recent antibiotic use.9
Community-acquired CDIs occurring in populations previously at low risk may be due to increased virulence of the disease. A hypervirulent C difficile strain, the North American Pulsed field type 1 (NAP1)/B1/027 strain, has emerged. This strain is more resistant to fluoroquinolone antibiotics and has caused multiple CDI outbreaks in the U.S.7 Along with the increased rate of CDI occurrence, mortality rates due to CDI have been rising.10 Recent studies have shown increased rates of CDI recurrence and treatment failure in response to standard therapy (metronidazole or vancomycin).11-13
To combat emerging treatment challenges of CDIs, the FDA approved a new antibiotic, fidaxomicin, for the treatment of C difficile-associated diarrhea in 2011.14 Fidaxomicin is a first-in-class macrocyclic antibiotic that has low systemic absorption, low activity against intestinal flora, and high fecal concentrations.15 Fidaxomicin also has been shown to be less likely to promote vancomycin-resistant enterococci (VRE) than does vancomycin in CDI treatment.16 Fidaxomicin is an emerging treatment strategy for CDIs, and this article reviews its role in the treatment of CDI.
CDI Standard of Therapy
Before the approval of fidaxomicin, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America released the 2010 update to the clinical practice guidelines for the treatment of CDI. Due to initial concern that use of oral vancomycin would select for VRE, guidelines recommend oral metronidazole for mild-to-moderate disease, oral vancomycin for severe CDI, and combination therapy of oral vancomycin with or without IV metronidazole for severe, complicated CDI (disease severity is defined in the Table).8,17
Fidaxomicin Approval
Prior to the approval of fidaxomicin for CDI in 2011, the FDA evaluated 2 noninferiority (NI) clinical trials comparing oral fidaxomicin to oral vancomycin for the treatment of CDI. Given that a clinical trial had previously demonstrated superior clinical cure rates of vancomycin over metronidazole, vancomycin was used as the comparator in the fidaxomicin NI trials.15 Louie and colleagues conducted a double-blind, randomized phase 3 trial comparing 10 days of fidaxomicin (200 mg twice daily) to vancomycin (125 mg 4 times daily) for the treatment of CDI (n = 629).3 Fidaxomicin was found to be noninferior to vancomycin for rate of clinical cure in the modified intention-to-treat (ITT) analysis (88% vs 86%, respectively) and the per-protocol analysis (92% vs 90%, respectively) with a NI margin of 10%. There were lower recurrence rates of CDI with fidaxomicin compared with that of vancomycin in the modified ITT analysis (15% vs 25%, respectively; P = .005). When infection with the NAP1/BI/027 strain was evaluated, fidaxomicin was shown to be noninferior to vancomycin for both clinical cure and recurrence rates.
The Louie and colleagues results were further validated when a second NI trial (n = 535) was published by Cornely and colleagues, which demonstrated similar clinical cure and recurrence rates with fidaxomicin compared with that of vancomycin.4 It is important to note that both trials used a modified ITT analysis, which included postrandomization exclusions that may have biased the results. Additionally, both trials were industry sponsored and had industry representation throughout the data collection, analysis, and manuscript preparation processes.
Use of Fidaxomicin
Fidaxomicin has been considered for use in the treatment of recurrent CDI. According to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of CDI, fidaxomicin is an option for both first and multiple recurrences (ESCMID does not recommend fidaxomicin for an initial episode of CDI). These guidelines state that either oral fidaxomicin or vancomycin for 10 days is an appropriate first recurrence treatment option. For multiple recurrences, the recommendations are oral fidaxomicin for 10 days or oral vancomycin for 10 days followed by a tapered or pulsed regimen.18 The IDSA C difficile treatment guidelines have not been updated since the approval of fidaxomicin and, therefore, do not contain recommendations for its use.
Given the low propensity for fidaxomicin to disrupt colonic flora, it may be hypothesized that its greatest benefit would be for use as first-line therapy in patients with a high risk of CDI recurrence prior to disruption of colonic flora with the treatment of vancomycin or metronidazole. A clinical prediction tool is needed to identify patients at high risk of CDI recurrence who would most benefit from initial fidaxomicin therapy. However, clinically relevant prediction tools are not currently used.19
Evidence exists that demonstrates the role of fidaxomicin for the treatment of recurrent CDI episodes. Cornely and colleagues pooled data from the 2 NI trials that led to the approval of fidaxomicin (n = 1,164).3,4,20 Of these, 128 participants had a recent CDI episode prior to study enrollment. For the treatment of first recurrence, 20% of patients treated with fidaxomicin experienced a second recurrence within 28 days compared with 36% of vancomycin patients (P = .045). Similarly, fewer fidaxomicin patients experienced an early recurrence within 14 days of treatment than with vancomycin for early recurrence within 14 days of treatment (8% vs 27%, respectively; P = .003).
Limitations of Fidaxomicin
One limitation of fidaxomicin is the paucity of data existing for its use in severe, life-threatening CDI, and it is currently not recommended in this indication.18 The main limitation behind the use of fidaxomicin is cost. The average wholesale price of a 10-day course of therapy of fidaxomicin is $3,360 compared with $1,273 for vancomycin capsules, $32 for compounded vancomycin oral solution, and $21 for oral metronidazole.21,22 Despite the price, cost-analysis studies have found that fidaxomicin compared with oral vancomycin is cost-effective for clinical cure rates and recurrences.23,24 Fidaxomicin also was found to be cost-effective in patients with mild-to-moderate CDI and in those using concomitant antibiotics.23 Given that 2 studies demonstrated that fidaxomicin has lower recurrence rates than that of oral vancomycin, the economical use of fidaxomicin would be of most benefit to patients at highest risk for CDI recurrence.3,4
Conclusion
In light of increased CDI treatment failure, recurrence rates, and virulence of CDI, fidaxomicin is an emerging treatment strategy. Through 2 pivotal trials, fidaxomicin has been shown to be a safe and effective first-line agent for CDI.3,4 New U.S. clinical guidelines for CDI are expected to be published in spring 2016, which will likely include the recommendation for fidaxomicin use in recurrent CDI. Current evidence suggests the most cost-effective use of fidaxomicin is in patients at highest risk of CDI recurrence.
1. McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis. 2006;12(3):409-415.
2. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
3. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
4. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281-289.
5. Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5(9):549-557.
6. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:403-410.
7. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene—variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433-2441.
8. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
9. Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk—4 states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(47):1201-1205.
10. Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999-2004. Emerg Infect Dis. 2007;13(9):1417-1419.
11. Maroo S, Lamont JT. Recurrent Clostridium difficile. Gastroenterology. 2006;130(4):1311-1316.
12. Pépin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol. 2007;102(12):2781-2788.
13. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40(11):1586-1590.
14. U.S. Food and Drug Administration. FDA approves treatment for Clostridium difficile infection; May 27, 2011 [news release]. U.S. Food and Drug Administration website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm257024.htm. Updated April 10, 2014. Accessed March 15, 2016.
15. Shue YK, Sears PS, Shangle S, et al. Safety, tolerance, and pharmacokinetic studies of OPT-80 in healthy volunteers following single and multiple oral doses. Antimicrob Agents Chemother. 2008;52(4):1391-1395.
16. Nerandzic MM, Mullane K, Miller MA, Babakhani F, Donskey CJ. Reduced acquisition and overgrowth of vancomycin-resistant enterococci and Candida species in patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Clin Infect Dis. 2012;55(2):S121-S126.
17. Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5(9):549-557.
18. Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(suppl 2):1-26.
19. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
20. Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
21. Murray L, ed. Red Book: Pharmacy’s Fundamental Reference. 2010 ed. Montvale, NJ: Thomson Reuters (Healthcare) Inc; 2010.
22. Cruz MP. Fidaxomicin (Dificid), a novel oral macrocyclic antibacterial agent for the treatment of Clostridium difficile-associated diarrhea in adults. Pharm Ther. 2012;37(5):278-281.
23. Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health. 2013;16(2):297-304.
24. Sclar DA, Robison LM, Oganov AM, Schmidt JM, Bowen KA, Castillo LV. Fidaxomicin for Clostridium difficile-associated diarrhoea: epidemiological method for estimation of warranted price. Clin Drug Investig. 2012;32(8):e17-e24.
1. McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis. 2006;12(3):409-415.
2. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
3. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
4. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281-289.
5. Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5(9):549-557.
6. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:403-410.
7. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene—variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433-2441.
8. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
9. Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk—4 states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(47):1201-1205.
10. Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999-2004. Emerg Infect Dis. 2007;13(9):1417-1419.
11. Maroo S, Lamont JT. Recurrent Clostridium difficile. Gastroenterology. 2006;130(4):1311-1316.
12. Pépin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol. 2007;102(12):2781-2788.
13. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40(11):1586-1590.
14. U.S. Food and Drug Administration. FDA approves treatment for Clostridium difficile infection; May 27, 2011 [news release]. U.S. Food and Drug Administration website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm257024.htm. Updated April 10, 2014. Accessed March 15, 2016.
15. Shue YK, Sears PS, Shangle S, et al. Safety, tolerance, and pharmacokinetic studies of OPT-80 in healthy volunteers following single and multiple oral doses. Antimicrob Agents Chemother. 2008;52(4):1391-1395.
16. Nerandzic MM, Mullane K, Miller MA, Babakhani F, Donskey CJ. Reduced acquisition and overgrowth of vancomycin-resistant enterococci and Candida species in patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Clin Infect Dis. 2012;55(2):S121-S126.
17. Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5(9):549-557.
18. Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(suppl 2):1-26.
19. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
20. Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
21. Murray L, ed. Red Book: Pharmacy’s Fundamental Reference. 2010 ed. Montvale, NJ: Thomson Reuters (Healthcare) Inc; 2010.
22. Cruz MP. Fidaxomicin (Dificid), a novel oral macrocyclic antibacterial agent for the treatment of Clostridium difficile-associated diarrhea in adults. Pharm Ther. 2012;37(5):278-281.
23. Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health. 2013;16(2):297-304.
24. Sclar DA, Robison LM, Oganov AM, Schmidt JM, Bowen KA, Castillo LV. Fidaxomicin for Clostridium difficile-associated diarrhoea: epidemiological method for estimation of warranted price. Clin Drug Investig. 2012;32(8):e17-e24.