Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
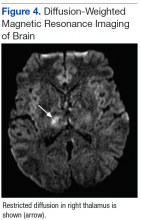
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus. 13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT. 14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT. 15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months. 14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE. 16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism. 17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT. 20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection. 21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.