Endpoints
The primary endpoint assessed was the change in rate of falls between the time preceding and during supplementation. The number of falls during the 60 days preceding ergocalciferol supplementation was standardized to falls per person per 30 days and compared with the same parameter during the 60-day period following initiation of ergocalciferol.
The secondary outcome was the rate of falls according to the level of 25(OH)D achieved as a result of supplementation in those patients who achieved a minimum 25(OH)D level of 30 ng/mL according to AGS recommendations. Those patients who achieved a minimum 25(OH)D concentration of 30 ng/mL were separated into 2 equal groups according to their respective concentration relative to the median.
Statistical Analysis
Numerical variables were compared using a Student t test. For the primary outcome, 64 participants were required in order to achieve 80% power at a significance of .05 for a 2-tailed assessment, each serving as his or her own control in the case-crossover study design. For the secondary outcome of falls according to 25(OH)D level following supplementation in order to achieve 80% power at a significance of 0.05 for a 2-tailed assessment, a total of 128 participants who reach a minimum 25(OH)D level of 30 ng/mL were required.
Results
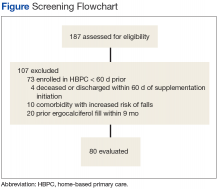
After screening 187 subjects who met the inclusion criteria, 107 subjects were excluded (Figure ).
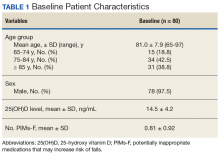
Of the 80 study enrollees, 78 were male. The mean age was 81 years with 81.3% (n = 65) aged ≥ 75 years. The mean 25(OH)D level prior to supplementation was 14.5 ng/mL (SD 4.2). The mean number of potentially inappropriate medications that may increase risk of falls (PIMs-F) was 0.81 PIMs-F per person (SD 0.92). Baseline patient characteristic data are summarized in Table 1.Primary Endpoint
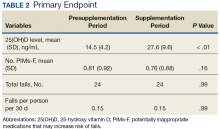
Following once weekly supplementation with ergocalciferol 50,000 IU, 25(OH)D levels increased from 14.5 ng/mL (SD 4.2) to 27.6 (SD 9.6) (P < .01). Of note, the timing of the 25(OH)D level obtained following initiation of supplementation ranged between 8 weeks and 24 weeks. The number of PIMs-F decreased marginally, although to a not statistically significant degree, from 0.81 PIMs-F per person (SD 0.92) to 0.76 PIMs-F per person (SD 0.88).
The number of falls among the group was identical both preceding and during supplementation, totaling 24 falls in each 60-day period and equating to a rate of 0.15 falls per person per 30 days to which this was standardized (P = .99) (Table 2).Secondary Endpoint
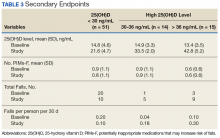
Although 51 of the subjects (63.8%) failed to achieve the target 25(OH)D level of ≥ 30 ng/mL, 29 were successful (Table 3).
Of those, 14 subjects achieved a 25(OH)D level of 30 to 36 ng/mL (mean 33.5 ng/mL, SD 2.0), and the remaining 15 subjects achieved a 25(OH)D level of > 36 ng/mL (mean 42.8 ng/mL, SD 5.2). In subjects whose achieved 25(OH)D level was < 30 ng/mL, the rate of falls per person per 30 days decreased from 0.2 during the 60 days preceding ergocalciferol supplementation to 0.1 on initiation of supplementation.In subjects whose achieved 25(OH)D level was 30 to 36 ng/mL, the rate of falls per person per 30 days increased from 0.036 to 0.18. Similarly, an increase in rate of falls per person per 30 days from 0.1 to 0.3 was noted in subjects whose attained 25(OH)D level was > 36.0 ng/mL. However, study enrollment was underpowered to claim statistical significance in these findings related to the secondary endpoint.
Discussion
In this retrospective chart review, individuals aged ≥ 65 years who were prescribed once weekly ergocalciferol 50,000 IU for increase of 25(OH)D levels < 20 ng/mL experienced no change in rate of falls across the entire study population. In those individuals whose achieved 25(OH)D level met the AGS recommendation of ≥ 30 ng/mL, there was a trend toward an increased rate of falls while the rate of falls decreased for subjects whose achieved 25(OH)D level was < 30 ng/mL.
High-dose vitamin D supplementation, albeit with vitamin D3, and its effect on falls have been evaluated in the geriatric population previously, most notably and recently, by Bischoff-Ferrari and colleagues.16 In a study comparing 24,000 IU vitamin D3 per month vs 60,000 IU vitamin D3 per month vs 24,000 IU vitamin D3 plus calcifediol 300 µg per month, lower extremity function did not differ in the 3 groups. However, an increased number of falls was noted in the second and third arm, respectively. Furthermore, after 12 months of treatment, those individuals who achieved the highest quartile of 25(OH)D level (44.7-98.9 ng/mL) had starkly increased odds of falling and number of falls compared with those achieving the lowest quartile (21.3-30.3 ng/mL).
The results of this study suggest that once-weekly high-dose vitamin D2 may carry a similar risk of increasing falls as found with high-dose vitamin D3, particularly at higher achieved levels of 25(OH)D. A possible explanation for a lower rate of falls in those individuals who did not achieve a 25(OH)D level of at least 30 ng/mL could be that these individuals may not have initiated the medication appropriately or administered it adherently, thereby avoiding a possible deleterious effect that the high-dose preparation may pose in this population.
Given the retrospective nature of the study and the evaluation of the change in the 25(OH)D level following approximately a 90-day supply of ergocalciferol, adherence was not addressed. In this case, although increased 25(OH)D level was the desired outcome of vitamin D supplementation, the increase in rate of falls may be attributable to the high-dose preparation itself. Alternatively, the 25(OH)D target of ≥ 30 ng/mL may be worth reconsidering in favor of a lower target with an upper limit.
The rate of falls in this study was collected over the 60 days following initiation of ergocalciferol. However, the achieved 25(OH)D level was not evaluated until between 8 and 24 weeks following initiation. In this context, it may be more likely that the increased rate of falls could be attributable to the high-dose nature of vitamin D2 supplementation or the rate of 25(OH)D repletion rather than the 25(OH)D level ultimately achieved.