Methods
A retrospective, single-center, chart review was conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS). Data were collected through manual chart review of US Department of Veterans Affairs (VA) electronic health records (EHRs). Patients included were veterans aged 18 to 89 years who were initiated on duloxetine and/or pregabalin between October 2015 and September 2018. The monotherapy groups were made equal by randomization based on whichever group had the lesser number of participants. The indication of neuropathy was determined by the presence of neuropathic pain, neuropathy, nerve pain, or otherwise similar terminology in the prescription directions for use in the EHR. Patients were excluded if they did not have an active prescription of the study agent(s) for ≥ 12 months or if there was a lack of documented body weight(s) at baseline and/or at follow-up outpatient visit(s) occurring 12 to 18 months after initiation of therapy. Additional exclusion criteria were health conditions, including active cancer; pregnancy; history of bariatric surgery; if the patient received hospice care; was morbidly obese (body mass index [BMI] > 40; or if estimated glomerular filtration rate (eGFR) was < 30 mL/min/1.83m2. Patients also were excluded if they were concurrently taking any of the following medications: antipsychotics, tricyclic antidepressants, venlafaxine, divalproex/valproic acid, lithium, mirtazapine, weight loss medications (orlistat, lorcaserin, phentermine-topiramate, naltrexone-bupropion, liraglutide), chronic corticosteroids, or chronic opioids; chronic being defined as receiving more than a 30-day supply.
The primary end point of this study was the change in body weight, expressed in pounds, after 12 to 18 months of treatment. If multiple weights were obtained during the 12- to 18-month period, the weight recorded closest to 12 months was used. The secondary end points included the percent change in body weight and dose effect, which evaluated change in weight at doses of duloxetine > 60 mg/d, and pregabalin at doses > 300 mg/d. Duration of effect was evaluated as a secondary end point; contrary to the primary end point, the weight furthest from 12 months was recorded. The change in hemoglobin A1c (HbA1c) in patients with prediabetes and DM also was investigated as a secondary end point. Last, involvement in the Managing Overweight Veterans Everywhere (MOVE!) weight management program at SFVAHCS and its effect on weight gain was reviewed.
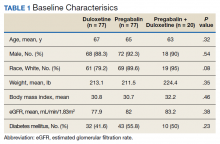
Baseline characteristics were collected to determine the variability between each study group. Data collected during the study included age, sex, race, weight, BMI, HbA1c, eGFR, DM diagnosis, insulin therapy prescription, duration of use, and MOVE! program participation.
Statistical Analysis
The primary and secondary end points were analyzed using an analysis of variance statistical test. Results were considered statistically significant at P < .05.
Results
A total of 174 participants were included in this study, with 77 in each monotherapy group, and 22 in the combination therapy group. More than 300 patients were excluded from the study due to prespecified inclusion and exclusion criteria. Baseline characteristics were similar among the 3 groups, with no statistically significant differences identified (Table 1).