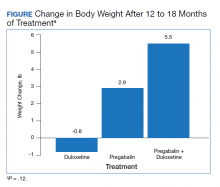
Primary End Point
The change in body weight after 12 to 18 months of treatment was –0.8 lb in the duloxetine group, +2.9 lb in the pregabalin group, and +5.5 lb in the pregabalin plus duloxetine group (P = .12) (Figure).
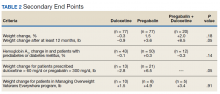
Secondary End Points
The percent change in body weight after 12 to 18 months of treatment was −0.3% in the duloxetine group, +1.5% in the pregabalin group, and +2.0% in the duloxetine plus pregabalin group (P = .18). The change in body weight beyond 12 months of treatment was −0.9 lb in the duloxetine group, +3.6 lb in the pregabalin group, and +8.5 lb in the duloxetine plus pregabalin group (P = .05). The change in HbA1c in patients with DM and pre-DM was −0.1% in the duloxetine group, +0.3% in the pregabalin group, and −0.3% in the duloxetine plus pregabalin group (P = .14). The change in body weight in patients who received increased doses of the study agents was −2.8 lb in the duloxetine group and +6.5 lb in the pregabalin group (P = .05). Among veterans who participated in MOVE!, change in body weight after 12 to 18 months of treatment was +1.5 lb in the duloxetine group, +4.9 lb in the pregabalin group, and +3.4 lb in the pregabalin plus duloxetine group (P = .91)(Table 2).
Discussion
The purpose of this retrospective chart review was to evaluate the association of weight gain in veterans taking duloxetine and/or pregabalin for the treatment of neuropathy. Although the primary end point, weight gain after 12 to 18 months of therapy, was not statistically significant, we found notable trends and associations worthy of discussion.
The secondary end point of the difference in weight gain in veterans taking duloxetine, pregabalin, or both for a treatment duration > 12 months was statistically significant. For this secondary end point, the weight recorded was when the study agent(s) were discontinued or the most recent weight obtained if participants still had an active prescription; the average duration of treatment in the 3 study groups was about 24 months. These weights differed from the primary end point, in which weight closest to 12 months of therapy was recorded.
The other secondary end point that was statistically significant was the difference in weight gain in patients who were on higher doses of duloxetine or pregabalin. This specifically examined participants who were on doses of duloxetine > 60 mg/d and pregabalin > 300 mg/d. Duloxetine was associated with weight loss, whereas pregabalin was associated with weight gain, with a difference of about 10 lb between the groups. The significance of this secondary end point demonstrates that increased doses of duloxetine and pregabalin are more associated with changes in weight compared with standard doses.
The secondary end points of percent change in body weight, change in HBA1c in patients with DM and prediabetes, and weight gain in patients who participated in the MOVE! weight management program were not statistically significant among the 3 study groups. Given the relatively small sample sizes, more significant differences in the evaluation of the primary and secondary end points may have been observed with a larger patient population.
Study investigators made additional observations beyond the primary and secondary end points. Most notably, > 300 patients were excluded from this study because they did not continue treatment beyond 12 months. The investigators found this number staggering, as it may imply that veterans were not satisfied with treatment agent(s) within 1 year of initiation, which could be due to lack of efficacy or intolerable adverse effects.
The mechanism of why combination therapy of duloxetine and pregabalin may be more associated with weight gain compared with either agent alone is unknown. Since this study found duloxetine to be more associated with weight loss, the mechanism does not seem to be an additive effect. The alternative hypothesis proposed prior to the completion of this study stemmed from an observation seen by health care providers at SFVAHCS.