User login
Alzheimer's Association International Conference 2014 (AAIC)
Alzheimer’s disease antibody crenezumab doesn’t deliver in phase II trial
COPENHAGEN – Once more, an antiamyloid antibody has failed to live up to hopes for the treatment of Alzheimer’s disease.
Roche’s contender, crenezumab, faltered on all of the primary endpoints of its phase II clinical trial, dubbed ABBY. The drug delivered no overall benefit over placebo in measures of both cognition and function, except in a small, unplanned subanalysis, Dr. Jeffrey Cummings said at the Alzheimer’s Association International Conference 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The subanalysis of patients who were the least impaired – with scores of 22-26 on the Mini-Mental State Exam (MMSE) – showed a significant 35% reduction in cognitive decline (P = .036) and a nonsignificant 19.6% reduction in global functional decline (P = .42) after 68 weeks of treatment, said Dr. Cummings, a primary investigator on the study and director of Cleveland Clinic’s Lou Ruvo Center for Brain Health in Las Vegas.
The news is a blow to researchers, who hoped crenezumab might break the string of antiamyloid antibody failures that has hampered immunotherapy clinical trials in Alzheimer’s. The drug’s early promise secured Roche $100 million in federal funds to help launch the first-ever Alzheimer’s primary prevention study.
The Alzheimer’s Prevention Initiative Autosomal Dominant Alzheimer’s Disease Treatment Trial is recruiting Colombian families who carry the presenilin 1 mutation – a virtual guarantee of early-onset Alzheimer’s disease. It was set to randomize cognitively healthy subjects to placebo or to 300 mg subcutaneous crenezumab – the dose that showed absolutely no efficacy signal in ABBY.
The trial’s future seems uncertain now, Dr. Cummings said at the meeting.
"These data require interpretation and then, proper ethical application in that study," he said. "Exactly what the outcome of that decision process will be, I don’t know."
ABBY enrolled 431 patients with mild to moderate Alzheimer’s disease (MMSE 18-26). They were randomized to two placebo-controlled arms: subcutaneous crenezumab 300 mg once every 2 weeks (low dose) or a matching placebo and intravenous crenezumab 15 mg/kg once every 4 weeks (high dose) or a matching placebo. The trial included a 68-week treatment period and a 4-week washout period.
The subjects had a mean age of 70 years and a mean MMSE of 21. Most of the low- and high-dose group included homozygous carriers of the apolipoprotein epsilon-4 allele (65% and 70%, respectively).
For either dosage arm, there were no significant differences in any of the co–primary endpoints, which included a reduction in cognitive decline on the Alzheimer’s Disease Assessment Scale-cognitive domain (ADAS-cog), the Clinical Dementia Rating Scale sum of boxes (CDR-SOB), or in the secondary endpoint of change in the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory scores (ADCS-ADL). In a prespecified subgroup analysis of patients with milder disease (MMSE 20-26), the high-dose intravenous arm experienced a 24% reduction in cognitive decline relative to placebo, but this was not statistically significant (P = .15).
However, the encouraging trend prompted the additional subgroup analysis of some patients with even milder disease (MMSE 22-26). Here there was a significant 35% reduction in cognitive decline (P = .036) and a nonsignificant 19.6% reduction in global functional decline (P = .42).
Crenezumab was safe and generally well tolerated. The 25% discontinuation rate was consistent in each treatment group as well as the placebo groups. There was one case of asymptomatic amyloid-related imaging abnormalities (ARIA) in a patient taking high-dose crenezumab.
There were three deaths: one from disease progression, one from respiratory failure, and one from pneumonia; none were considered related to the study drug.
Roche and its subsidiary Genentech sponsored the study. Dr. Cummings has received financial remuneration from the company.
On Twitter @alz_gal
COPENHAGEN – Once more, an antiamyloid antibody has failed to live up to hopes for the treatment of Alzheimer’s disease.
Roche’s contender, crenezumab, faltered on all of the primary endpoints of its phase II clinical trial, dubbed ABBY. The drug delivered no overall benefit over placebo in measures of both cognition and function, except in a small, unplanned subanalysis, Dr. Jeffrey Cummings said at the Alzheimer’s Association International Conference 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The subanalysis of patients who were the least impaired – with scores of 22-26 on the Mini-Mental State Exam (MMSE) – showed a significant 35% reduction in cognitive decline (P = .036) and a nonsignificant 19.6% reduction in global functional decline (P = .42) after 68 weeks of treatment, said Dr. Cummings, a primary investigator on the study and director of Cleveland Clinic’s Lou Ruvo Center for Brain Health in Las Vegas.
The news is a blow to researchers, who hoped crenezumab might break the string of antiamyloid antibody failures that has hampered immunotherapy clinical trials in Alzheimer’s. The drug’s early promise secured Roche $100 million in federal funds to help launch the first-ever Alzheimer’s primary prevention study.
The Alzheimer’s Prevention Initiative Autosomal Dominant Alzheimer’s Disease Treatment Trial is recruiting Colombian families who carry the presenilin 1 mutation – a virtual guarantee of early-onset Alzheimer’s disease. It was set to randomize cognitively healthy subjects to placebo or to 300 mg subcutaneous crenezumab – the dose that showed absolutely no efficacy signal in ABBY.
The trial’s future seems uncertain now, Dr. Cummings said at the meeting.
"These data require interpretation and then, proper ethical application in that study," he said. "Exactly what the outcome of that decision process will be, I don’t know."
ABBY enrolled 431 patients with mild to moderate Alzheimer’s disease (MMSE 18-26). They were randomized to two placebo-controlled arms: subcutaneous crenezumab 300 mg once every 2 weeks (low dose) or a matching placebo and intravenous crenezumab 15 mg/kg once every 4 weeks (high dose) or a matching placebo. The trial included a 68-week treatment period and a 4-week washout period.
The subjects had a mean age of 70 years and a mean MMSE of 21. Most of the low- and high-dose group included homozygous carriers of the apolipoprotein epsilon-4 allele (65% and 70%, respectively).
For either dosage arm, there were no significant differences in any of the co–primary endpoints, which included a reduction in cognitive decline on the Alzheimer’s Disease Assessment Scale-cognitive domain (ADAS-cog), the Clinical Dementia Rating Scale sum of boxes (CDR-SOB), or in the secondary endpoint of change in the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory scores (ADCS-ADL). In a prespecified subgroup analysis of patients with milder disease (MMSE 20-26), the high-dose intravenous arm experienced a 24% reduction in cognitive decline relative to placebo, but this was not statistically significant (P = .15).
However, the encouraging trend prompted the additional subgroup analysis of some patients with even milder disease (MMSE 22-26). Here there was a significant 35% reduction in cognitive decline (P = .036) and a nonsignificant 19.6% reduction in global functional decline (P = .42).
Crenezumab was safe and generally well tolerated. The 25% discontinuation rate was consistent in each treatment group as well as the placebo groups. There was one case of asymptomatic amyloid-related imaging abnormalities (ARIA) in a patient taking high-dose crenezumab.
There were three deaths: one from disease progression, one from respiratory failure, and one from pneumonia; none were considered related to the study drug.
Roche and its subsidiary Genentech sponsored the study. Dr. Cummings has received financial remuneration from the company.
On Twitter @alz_gal
COPENHAGEN – Once more, an antiamyloid antibody has failed to live up to hopes for the treatment of Alzheimer’s disease.
Roche’s contender, crenezumab, faltered on all of the primary endpoints of its phase II clinical trial, dubbed ABBY. The drug delivered no overall benefit over placebo in measures of both cognition and function, except in a small, unplanned subanalysis, Dr. Jeffrey Cummings said at the Alzheimer’s Association International Conference 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The subanalysis of patients who were the least impaired – with scores of 22-26 on the Mini-Mental State Exam (MMSE) – showed a significant 35% reduction in cognitive decline (P = .036) and a nonsignificant 19.6% reduction in global functional decline (P = .42) after 68 weeks of treatment, said Dr. Cummings, a primary investigator on the study and director of Cleveland Clinic’s Lou Ruvo Center for Brain Health in Las Vegas.
The news is a blow to researchers, who hoped crenezumab might break the string of antiamyloid antibody failures that has hampered immunotherapy clinical trials in Alzheimer’s. The drug’s early promise secured Roche $100 million in federal funds to help launch the first-ever Alzheimer’s primary prevention study.
The Alzheimer’s Prevention Initiative Autosomal Dominant Alzheimer’s Disease Treatment Trial is recruiting Colombian families who carry the presenilin 1 mutation – a virtual guarantee of early-onset Alzheimer’s disease. It was set to randomize cognitively healthy subjects to placebo or to 300 mg subcutaneous crenezumab – the dose that showed absolutely no efficacy signal in ABBY.
The trial’s future seems uncertain now, Dr. Cummings said at the meeting.
"These data require interpretation and then, proper ethical application in that study," he said. "Exactly what the outcome of that decision process will be, I don’t know."
ABBY enrolled 431 patients with mild to moderate Alzheimer’s disease (MMSE 18-26). They were randomized to two placebo-controlled arms: subcutaneous crenezumab 300 mg once every 2 weeks (low dose) or a matching placebo and intravenous crenezumab 15 mg/kg once every 4 weeks (high dose) or a matching placebo. The trial included a 68-week treatment period and a 4-week washout period.
The subjects had a mean age of 70 years and a mean MMSE of 21. Most of the low- and high-dose group included homozygous carriers of the apolipoprotein epsilon-4 allele (65% and 70%, respectively).
For either dosage arm, there were no significant differences in any of the co–primary endpoints, which included a reduction in cognitive decline on the Alzheimer’s Disease Assessment Scale-cognitive domain (ADAS-cog), the Clinical Dementia Rating Scale sum of boxes (CDR-SOB), or in the secondary endpoint of change in the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory scores (ADCS-ADL). In a prespecified subgroup analysis of patients with milder disease (MMSE 20-26), the high-dose intravenous arm experienced a 24% reduction in cognitive decline relative to placebo, but this was not statistically significant (P = .15).
However, the encouraging trend prompted the additional subgroup analysis of some patients with even milder disease (MMSE 22-26). Here there was a significant 35% reduction in cognitive decline (P = .036) and a nonsignificant 19.6% reduction in global functional decline (P = .42).
Crenezumab was safe and generally well tolerated. The 25% discontinuation rate was consistent in each treatment group as well as the placebo groups. There was one case of asymptomatic amyloid-related imaging abnormalities (ARIA) in a patient taking high-dose crenezumab.
There were three deaths: one from disease progression, one from respiratory failure, and one from pneumonia; none were considered related to the study drug.
Roche and its subsidiary Genentech sponsored the study. Dr. Cummings has received financial remuneration from the company.
On Twitter @alz_gal
AT AAIC 2014
Key clinical point: The antiamyloid antibody crenezumab failed to improve cognitive or functional status in patients with mild to-moderate Alzheimer’s disease.
Major finding: The drug delivered no significant benefits, except in an unplanned subgroup analysis of the milder patients, who experienced a 35% reduction in the rate of cognitive decline relative to placebo.
Data source: The randomized, placebo-controlled ABBY trial that enrolled 431 patients.
Disclosures: Roche and its subsidiary Genentech sponsored the trial. Dr. Cummings has received financial remuneration from the company.
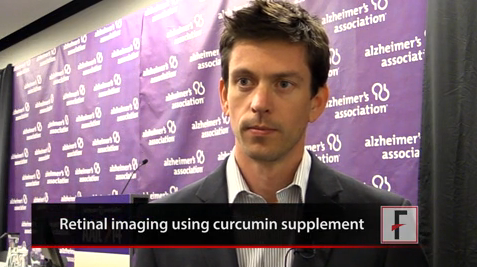
VIDEO: Investigative eye amyloid test shines a light on Alzheimer’s pathology
COPENHAGEN – When it comes to beta-amyloid plaques, the eyes may be more than a poetic window to the soul – they also may be mirrors of pathology developing in the brain.
It turns out that the eye, as a virtual extension of the brain itself, accumulates the same amyloid pathology that damages the brain when Alzheimer’s disease strikes. Visualizing these plaques in the retina and in the lens may eventually become a low-cost, noninvasive screening tool for early disease, according to research presented at the Alzheimer’s Association International Conference 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"Our first 45 subjects showed a strong correlation between retinal amyloid and brain plaques," said Shaun Frost of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) in Australia. "In fact, we had 100% sensitivity and no false positives, something that’s critical for an Alzheimer’s test, because we don’t want to leave anyone behind when it comes to early signs."
Curcumin, an extract of the spice turmeric, is the fluorescent agent in the test. Mr. Frost said investigators were first drawn to it because some studies suggest a lower rate of Alzheimer’s in India, where turmeric is a common seasoning.
Paul Hartung, president of Cognoptix in Acton, Mass., said his company focuses on lens amyloid, which shows a correlation to brain plaques. Cognoptix is accumulating data on a device that identifies plaques using dynamic light scattering and a fluorescent molecule delivered in an ophthalmic ointment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In the phase II study he presented, the test differentiated 20 people with Alzheimer’s from 20 healthy controls with an 85% sensitivity and 95% specificity. It also correlated significantly with brain plaques seen on PET amyloid imaging.
A phase III trial is in the works, after which Cognoptix intends to seek approval from the Food and Drug Administration. Mr. Hartung hopes the device could be marketed by 2016.
The lens amyloid trial is a collaboration between CSIRO; Edith Cowan University in Mt. Lawley, Australia; the McCusker Alzheimer’s Research Foundation; California-based NeuroVision Imaging; and the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing. Mr. Frost had no financial disclosures. Mr. Hartung is a full-time employee of Cognotpix, which is developing the test for commercial use.
On Twitter @alz_gal
COPENHAGEN – When it comes to beta-amyloid plaques, the eyes may be more than a poetic window to the soul – they also may be mirrors of pathology developing in the brain.
It turns out that the eye, as a virtual extension of the brain itself, accumulates the same amyloid pathology that damages the brain when Alzheimer’s disease strikes. Visualizing these plaques in the retina and in the lens may eventually become a low-cost, noninvasive screening tool for early disease, according to research presented at the Alzheimer’s Association International Conference 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"Our first 45 subjects showed a strong correlation between retinal amyloid and brain plaques," said Shaun Frost of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) in Australia. "In fact, we had 100% sensitivity and no false positives, something that’s critical for an Alzheimer’s test, because we don’t want to leave anyone behind when it comes to early signs."
Curcumin, an extract of the spice turmeric, is the fluorescent agent in the test. Mr. Frost said investigators were first drawn to it because some studies suggest a lower rate of Alzheimer’s in India, where turmeric is a common seasoning.
Paul Hartung, president of Cognoptix in Acton, Mass., said his company focuses on lens amyloid, which shows a correlation to brain plaques. Cognoptix is accumulating data on a device that identifies plaques using dynamic light scattering and a fluorescent molecule delivered in an ophthalmic ointment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In the phase II study he presented, the test differentiated 20 people with Alzheimer’s from 20 healthy controls with an 85% sensitivity and 95% specificity. It also correlated significantly with brain plaques seen on PET amyloid imaging.
A phase III trial is in the works, after which Cognoptix intends to seek approval from the Food and Drug Administration. Mr. Hartung hopes the device could be marketed by 2016.
The lens amyloid trial is a collaboration between CSIRO; Edith Cowan University in Mt. Lawley, Australia; the McCusker Alzheimer’s Research Foundation; California-based NeuroVision Imaging; and the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing. Mr. Frost had no financial disclosures. Mr. Hartung is a full-time employee of Cognotpix, which is developing the test for commercial use.
On Twitter @alz_gal
COPENHAGEN – When it comes to beta-amyloid plaques, the eyes may be more than a poetic window to the soul – they also may be mirrors of pathology developing in the brain.
It turns out that the eye, as a virtual extension of the brain itself, accumulates the same amyloid pathology that damages the brain when Alzheimer’s disease strikes. Visualizing these plaques in the retina and in the lens may eventually become a low-cost, noninvasive screening tool for early disease, according to research presented at the Alzheimer’s Association International Conference 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"Our first 45 subjects showed a strong correlation between retinal amyloid and brain plaques," said Shaun Frost of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) in Australia. "In fact, we had 100% sensitivity and no false positives, something that’s critical for an Alzheimer’s test, because we don’t want to leave anyone behind when it comes to early signs."
Curcumin, an extract of the spice turmeric, is the fluorescent agent in the test. Mr. Frost said investigators were first drawn to it because some studies suggest a lower rate of Alzheimer’s in India, where turmeric is a common seasoning.
Paul Hartung, president of Cognoptix in Acton, Mass., said his company focuses on lens amyloid, which shows a correlation to brain plaques. Cognoptix is accumulating data on a device that identifies plaques using dynamic light scattering and a fluorescent molecule delivered in an ophthalmic ointment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In the phase II study he presented, the test differentiated 20 people with Alzheimer’s from 20 healthy controls with an 85% sensitivity and 95% specificity. It also correlated significantly with brain plaques seen on PET amyloid imaging.
A phase III trial is in the works, after which Cognoptix intends to seek approval from the Food and Drug Administration. Mr. Hartung hopes the device could be marketed by 2016.
The lens amyloid trial is a collaboration between CSIRO; Edith Cowan University in Mt. Lawley, Australia; the McCusker Alzheimer’s Research Foundation; California-based NeuroVision Imaging; and the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing. Mr. Frost had no financial disclosures. Mr. Hartung is a full-time employee of Cognotpix, which is developing the test for commercial use.
On Twitter @alz_gal
AT AAIC 2014
VIDEO: Don’t brush off subjective memory decline
COPENHAGEN – Researchers are paying more attention to subjective cognitive decline – a patient’s perceived notion of memory loss – even if the patients complete various cognitive tests with flying colors.
In a video interview at the Alzheimer’s Association International Conference 2014, Wiesje M. van der Flier, Ph.D., head of clinical research for the Alzheimer’s Center at the VU University Medical Center in Amsterdam, explained the issue and how physicians should handle a patient’s perceptions of cognitive decline.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
COPENHAGEN – Researchers are paying more attention to subjective cognitive decline – a patient’s perceived notion of memory loss – even if the patients complete various cognitive tests with flying colors.
In a video interview at the Alzheimer’s Association International Conference 2014, Wiesje M. van der Flier, Ph.D., head of clinical research for the Alzheimer’s Center at the VU University Medical Center in Amsterdam, explained the issue and how physicians should handle a patient’s perceptions of cognitive decline.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
COPENHAGEN – Researchers are paying more attention to subjective cognitive decline – a patient’s perceived notion of memory loss – even if the patients complete various cognitive tests with flying colors.
In a video interview at the Alzheimer’s Association International Conference 2014, Wiesje M. van der Flier, Ph.D., head of clinical research for the Alzheimer’s Center at the VU University Medical Center in Amsterdam, explained the issue and how physicians should handle a patient’s perceptions of cognitive decline.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
At AAIC 2014
VIDEO: New genetic research could identify Alzheimer’s therapy targets
COPENHAGEN – The National Institutes of Health recently awarded $24 million to eight academic centers to analyze the human genome and identify genes that either increase the risk of Alzheimer’s disease or have a protective effect.
It’s a very exciting time in Alzheimer’s disease genetics, said Marilyn Miller, Ph.D., program director of the National Institute of Aging’s Genetics of Alzheimer’s Disease, Tau, and Hormone Research portfolios in the division of neuroscience.
In a video interview at the Alzheimer’s Association International Conference 2014, Dr. Miller explains the ongoing research and the implications for potential therapies.
Alzheimer’s genetics research resources:
• The Alzheimer’s Disease Sequencing Project.
• The NIA Genetics of Alzheimer’s Disease Data Storage Site.
• The National Plan to Address Alzheimer’s Disease.
• The National Human Genome Research Institute.
On Twitter @naseemmiller
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
COPENHAGEN – The National Institutes of Health recently awarded $24 million to eight academic centers to analyze the human genome and identify genes that either increase the risk of Alzheimer’s disease or have a protective effect.
It’s a very exciting time in Alzheimer’s disease genetics, said Marilyn Miller, Ph.D., program director of the National Institute of Aging’s Genetics of Alzheimer’s Disease, Tau, and Hormone Research portfolios in the division of neuroscience.
In a video interview at the Alzheimer’s Association International Conference 2014, Dr. Miller explains the ongoing research and the implications for potential therapies.
Alzheimer’s genetics research resources:
• The Alzheimer’s Disease Sequencing Project.
• The NIA Genetics of Alzheimer’s Disease Data Storage Site.
• The National Plan to Address Alzheimer’s Disease.
• The National Human Genome Research Institute.
On Twitter @naseemmiller
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
COPENHAGEN – The National Institutes of Health recently awarded $24 million to eight academic centers to analyze the human genome and identify genes that either increase the risk of Alzheimer’s disease or have a protective effect.
It’s a very exciting time in Alzheimer’s disease genetics, said Marilyn Miller, Ph.D., program director of the National Institute of Aging’s Genetics of Alzheimer’s Disease, Tau, and Hormone Research portfolios in the division of neuroscience.
In a video interview at the Alzheimer’s Association International Conference 2014, Dr. Miller explains the ongoing research and the implications for potential therapies.
Alzheimer’s genetics research resources:
• The Alzheimer’s Disease Sequencing Project.
• The NIA Genetics of Alzheimer’s Disease Data Storage Site.
• The National Plan to Address Alzheimer’s Disease.
• The National Human Genome Research Institute.
On Twitter @naseemmiller
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At AAIC 2014
Alzheimer’s may be declining in U.S. and Germany
COPENHAGEN – The incidence of Alzheimer’s disease appears to be declining in the United States and may be following a similar positive trend in Germany as well.
An analysis of the ongoing Framingham Heart Study suggests that the disease has declined by 44% since 1978 in the United States, Claudia Satizabal, Ph.D., said at the Alzheimer’s Association International Conference 2014. Better management of cardiovascular risk factors and a generally higher education level seem to be driving the change.
"There has been a progressive decrease over that period," Dr. Satizabal of Boston University said during a press briefing. "Our results are in line with other studies from developed countries, and offer cautious hope that some cases might be preventable by managing risk – especially hypertension – and continuing to improve educational status."
She divided the Framingham study period into four epochs, beginning in 1978, 1989, and 1996, and finally, running from 2006 to 2013. She then calculated the 5-year incidence of Alzheimer’s disease in each of those periods, and according to gender and age of onset.
Using epoch 1 as the baseline, Dr. Satizabal found that overall incidence had decreased 22% by epoch 2. From epoch 2 to 3, she found a total decrease of 38% from baseline. From epoch 3 to 4, the total overall decrease was 44% from baseline.
The incidence reduction was somewhat greater in women, dropping 30% from baseline to epoch 1 to 2 and 48% from baseline to epoch 2 to 3. The decline then stabilized at a 47% decrease from baseline to epoch 3 to 4. The corresponding decreases among men were 4%, 11%, and 36%.
People who developed the disease also did so at a later age as the years progressed. The mean age of onset was 80 years in epoch 1, 82 in epoch 2, 84 in epoch 3, and 85 in epoch 4.
Education also influenced the reduction in incidence. Those with at least high school education had a consistent reduction in dementia incidence across all time periods, while those less educated did not.
"We also observed significant improvements in the use of antihypertensive and statin medication, blood pressure and cholesterol levels, and prevalence of smoking, heart disease, and stroke," Dr. Satizabal added.
However, she said, "our study looks at a small population of white Americans, so we can’t make the same assumptions about other populations."
Gabriele Doblhammer, Ph.D., of the German Center for Neurodegenerative Diseases in Rostock, had some similarly encouraging findings. Her study of short-term dementia trends in Germany – the first ever conducted – concluded that incidence is declining, age at onset is being pushed back, and disease duration has shortened.
"People seem to be enjoying more healthy years of life, and who would not want that?" she said.
Her study examined claims from the country’s largest public health insurer, which covers about 13% of the population overall, and 50% of older citizens.
The analysis comprised 6.5 million people and 600,000 dementia cases in those aged 65 and older, for the years 2007, 2008, and 2009. Over that period, new cases of dementia decreased for both men and women.
The results were better for women, however. For them, prevalence declined significantly: –3.6% from 2007 to 2008, and –1.8% from 2008 to 2009. Prevalence among men also decreased, but the change was not statistically significant. When women developed the disease, they died sooner – 10% more quickly in 2007 than in a sample obtained from 2004, suggesting that the disease was developing later in life, and much sooner to the time of normal expected death.
Neither Dr. Satizabal nor Dr. Doblhammer had any relevant financial disclosures.
On Twitter @alz_gal
COPENHAGEN – The incidence of Alzheimer’s disease appears to be declining in the United States and may be following a similar positive trend in Germany as well.
An analysis of the ongoing Framingham Heart Study suggests that the disease has declined by 44% since 1978 in the United States, Claudia Satizabal, Ph.D., said at the Alzheimer’s Association International Conference 2014. Better management of cardiovascular risk factors and a generally higher education level seem to be driving the change.
"There has been a progressive decrease over that period," Dr. Satizabal of Boston University said during a press briefing. "Our results are in line with other studies from developed countries, and offer cautious hope that some cases might be preventable by managing risk – especially hypertension – and continuing to improve educational status."
She divided the Framingham study period into four epochs, beginning in 1978, 1989, and 1996, and finally, running from 2006 to 2013. She then calculated the 5-year incidence of Alzheimer’s disease in each of those periods, and according to gender and age of onset.
Using epoch 1 as the baseline, Dr. Satizabal found that overall incidence had decreased 22% by epoch 2. From epoch 2 to 3, she found a total decrease of 38% from baseline. From epoch 3 to 4, the total overall decrease was 44% from baseline.
The incidence reduction was somewhat greater in women, dropping 30% from baseline to epoch 1 to 2 and 48% from baseline to epoch 2 to 3. The decline then stabilized at a 47% decrease from baseline to epoch 3 to 4. The corresponding decreases among men were 4%, 11%, and 36%.
People who developed the disease also did so at a later age as the years progressed. The mean age of onset was 80 years in epoch 1, 82 in epoch 2, 84 in epoch 3, and 85 in epoch 4.
Education also influenced the reduction in incidence. Those with at least high school education had a consistent reduction in dementia incidence across all time periods, while those less educated did not.
"We also observed significant improvements in the use of antihypertensive and statin medication, blood pressure and cholesterol levels, and prevalence of smoking, heart disease, and stroke," Dr. Satizabal added.
However, she said, "our study looks at a small population of white Americans, so we can’t make the same assumptions about other populations."
Gabriele Doblhammer, Ph.D., of the German Center for Neurodegenerative Diseases in Rostock, had some similarly encouraging findings. Her study of short-term dementia trends in Germany – the first ever conducted – concluded that incidence is declining, age at onset is being pushed back, and disease duration has shortened.
"People seem to be enjoying more healthy years of life, and who would not want that?" she said.
Her study examined claims from the country’s largest public health insurer, which covers about 13% of the population overall, and 50% of older citizens.
The analysis comprised 6.5 million people and 600,000 dementia cases in those aged 65 and older, for the years 2007, 2008, and 2009. Over that period, new cases of dementia decreased for both men and women.
The results were better for women, however. For them, prevalence declined significantly: –3.6% from 2007 to 2008, and –1.8% from 2008 to 2009. Prevalence among men also decreased, but the change was not statistically significant. When women developed the disease, they died sooner – 10% more quickly in 2007 than in a sample obtained from 2004, suggesting that the disease was developing later in life, and much sooner to the time of normal expected death.
Neither Dr. Satizabal nor Dr. Doblhammer had any relevant financial disclosures.
On Twitter @alz_gal
COPENHAGEN – The incidence of Alzheimer’s disease appears to be declining in the United States and may be following a similar positive trend in Germany as well.
An analysis of the ongoing Framingham Heart Study suggests that the disease has declined by 44% since 1978 in the United States, Claudia Satizabal, Ph.D., said at the Alzheimer’s Association International Conference 2014. Better management of cardiovascular risk factors and a generally higher education level seem to be driving the change.
"There has been a progressive decrease over that period," Dr. Satizabal of Boston University said during a press briefing. "Our results are in line with other studies from developed countries, and offer cautious hope that some cases might be preventable by managing risk – especially hypertension – and continuing to improve educational status."
She divided the Framingham study period into four epochs, beginning in 1978, 1989, and 1996, and finally, running from 2006 to 2013. She then calculated the 5-year incidence of Alzheimer’s disease in each of those periods, and according to gender and age of onset.
Using epoch 1 as the baseline, Dr. Satizabal found that overall incidence had decreased 22% by epoch 2. From epoch 2 to 3, she found a total decrease of 38% from baseline. From epoch 3 to 4, the total overall decrease was 44% from baseline.
The incidence reduction was somewhat greater in women, dropping 30% from baseline to epoch 1 to 2 and 48% from baseline to epoch 2 to 3. The decline then stabilized at a 47% decrease from baseline to epoch 3 to 4. The corresponding decreases among men were 4%, 11%, and 36%.
People who developed the disease also did so at a later age as the years progressed. The mean age of onset was 80 years in epoch 1, 82 in epoch 2, 84 in epoch 3, and 85 in epoch 4.
Education also influenced the reduction in incidence. Those with at least high school education had a consistent reduction in dementia incidence across all time periods, while those less educated did not.
"We also observed significant improvements in the use of antihypertensive and statin medication, blood pressure and cholesterol levels, and prevalence of smoking, heart disease, and stroke," Dr. Satizabal added.
However, she said, "our study looks at a small population of white Americans, so we can’t make the same assumptions about other populations."
Gabriele Doblhammer, Ph.D., of the German Center for Neurodegenerative Diseases in Rostock, had some similarly encouraging findings. Her study of short-term dementia trends in Germany – the first ever conducted – concluded that incidence is declining, age at onset is being pushed back, and disease duration has shortened.
"People seem to be enjoying more healthy years of life, and who would not want that?" she said.
Her study examined claims from the country’s largest public health insurer, which covers about 13% of the population overall, and 50% of older citizens.
The analysis comprised 6.5 million people and 600,000 dementia cases in those aged 65 and older, for the years 2007, 2008, and 2009. Over that period, new cases of dementia decreased for both men and women.
The results were better for women, however. For them, prevalence declined significantly: –3.6% from 2007 to 2008, and –1.8% from 2008 to 2009. Prevalence among men also decreased, but the change was not statistically significant. When women developed the disease, they died sooner – 10% more quickly in 2007 than in a sample obtained from 2004, suggesting that the disease was developing later in life, and much sooner to the time of normal expected death.
Neither Dr. Satizabal nor Dr. Doblhammer had any relevant financial disclosures.
On Twitter @alz_gal
AT AAIC 2014
Rising projection of Alzheimer’s cases stems mainly from better reporting
COPENHAGEN – Researchers appear to be tracking contradictory trends in the global incidence and prevalence of Alzheimer’s disease.
There’s no doubt that both are declining in the United States and other high-income countries. But the disease appears to be claiming more people in less-developed countries, particularly in Asia and sub-Saharan Africa, and that disparity will become much worse by 2050.
Half of the 7.7 million new cases diagnosed per year are occurring in China alone, Dr. Martin Prince said at the Alzheimer’s Association International Conference 2014. And while African and Latin American countries contribute a lower portion of the total (7% and 5%, respectively), they are projected to proportionally outstrip even China’s expected tripling of cases, with each poised for a fourfold increase by 2050.
Taken together with the doubling of cases expected in the United States and European countries, the global incidence could exceed previous projections by more than 30 million cases worldwide by 2050, said Dr. Prince, a primary author of the G8 report "The Global Impact of Dementia, 2013-2050." The report contains updated numbers based on data supplied by the United Nations.
"Because of this new U.N. data, and new data coming out of China, we realized that our 2009 global estimates were about 10% too low," said Dr. Prince, who is also a professor of epidemiological psychiatry in the Institute of Psychiatry at King’s College London. "We are talking about not 36 million right now, but 44 million. Not 66 million by 2030, but 76 million. And by 2050, not 115 million as we previously thought, but 135 million."
The upward trend doesn’t reflect so much an increase in disease incidence as an increase in disease reporting, he noted. While China has published papers on incidence and prevalence, they were almost exclusively in Chinese journals and unavailable outside the country. The biggest change came from a groundbreaking paper in 2013 (Lancet 2013;381:2016-23) – the first widely accessible English language paper on the topic. It concluded that the burden of dementia in China is increasing faster than previously assumed.
According to the G8 report, about 67 million are living with Alzheimer’s in China now, and that number could increase to nearly 200 million by 2050.
But that paper probably doesn’t tell the whole story, Dr. Prince added. China is suffering from the same epidemics of obesity and diabetes that plague other countries, and in contrast to many, cigarette smoking is on a huge rise in China. All of these factors contribute to the development of Alzheimer’s and other dementias, so the numbers will most likely continue to change.
Perhaps not surprisingly, national prosperity is directly tied to Alzheimer’s incidence. By 2050, the incidence is projected to increase by 106% in the G8 countries and by 185% in the G20 countries. This is mainly due to higher educational levels and better risk management, Dr. Prince said. Reducing the prevalence of diabetes, physical inactivity, smoking, depression, low education, and midlife hypertension and obesity could reduce the Alzheimer’s population-attributable risk fraction by more than 50%.
"Even a 10% reduction in risk exposure would result in 250,000 fewer new cases [3.3%]. A 25% reduction in risk exposure would result in 680,000 fewer new cases," an incidence reduction of almost 9% worldwide, he said.
No one has yet modeled the global economic impact of the rising Alzheimer’s tide, Dr. Prince said. Current worldwide spending on Alzheimer’s disease exceeds $600 billion per year. In developed countries, most of this is associated with care in long-term facilities and the cost of professional caregivers. "In less-developed countries, the largest proportion is the informal costs of family care, especially lost employment opportunities. This needs to be calculated, and I’m not aware that’s being done. But it will be a lot of money."
Dr. Prince had no financial disclosures.
On Twitter @alz_gal
COPENHAGEN – Researchers appear to be tracking contradictory trends in the global incidence and prevalence of Alzheimer’s disease.
There’s no doubt that both are declining in the United States and other high-income countries. But the disease appears to be claiming more people in less-developed countries, particularly in Asia and sub-Saharan Africa, and that disparity will become much worse by 2050.
Half of the 7.7 million new cases diagnosed per year are occurring in China alone, Dr. Martin Prince said at the Alzheimer’s Association International Conference 2014. And while African and Latin American countries contribute a lower portion of the total (7% and 5%, respectively), they are projected to proportionally outstrip even China’s expected tripling of cases, with each poised for a fourfold increase by 2050.
Taken together with the doubling of cases expected in the United States and European countries, the global incidence could exceed previous projections by more than 30 million cases worldwide by 2050, said Dr. Prince, a primary author of the G8 report "The Global Impact of Dementia, 2013-2050." The report contains updated numbers based on data supplied by the United Nations.
"Because of this new U.N. data, and new data coming out of China, we realized that our 2009 global estimates were about 10% too low," said Dr. Prince, who is also a professor of epidemiological psychiatry in the Institute of Psychiatry at King’s College London. "We are talking about not 36 million right now, but 44 million. Not 66 million by 2030, but 76 million. And by 2050, not 115 million as we previously thought, but 135 million."
The upward trend doesn’t reflect so much an increase in disease incidence as an increase in disease reporting, he noted. While China has published papers on incidence and prevalence, they were almost exclusively in Chinese journals and unavailable outside the country. The biggest change came from a groundbreaking paper in 2013 (Lancet 2013;381:2016-23) – the first widely accessible English language paper on the topic. It concluded that the burden of dementia in China is increasing faster than previously assumed.
According to the G8 report, about 67 million are living with Alzheimer’s in China now, and that number could increase to nearly 200 million by 2050.
But that paper probably doesn’t tell the whole story, Dr. Prince added. China is suffering from the same epidemics of obesity and diabetes that plague other countries, and in contrast to many, cigarette smoking is on a huge rise in China. All of these factors contribute to the development of Alzheimer’s and other dementias, so the numbers will most likely continue to change.
Perhaps not surprisingly, national prosperity is directly tied to Alzheimer’s incidence. By 2050, the incidence is projected to increase by 106% in the G8 countries and by 185% in the G20 countries. This is mainly due to higher educational levels and better risk management, Dr. Prince said. Reducing the prevalence of diabetes, physical inactivity, smoking, depression, low education, and midlife hypertension and obesity could reduce the Alzheimer’s population-attributable risk fraction by more than 50%.
"Even a 10% reduction in risk exposure would result in 250,000 fewer new cases [3.3%]. A 25% reduction in risk exposure would result in 680,000 fewer new cases," an incidence reduction of almost 9% worldwide, he said.
No one has yet modeled the global economic impact of the rising Alzheimer’s tide, Dr. Prince said. Current worldwide spending on Alzheimer’s disease exceeds $600 billion per year. In developed countries, most of this is associated with care in long-term facilities and the cost of professional caregivers. "In less-developed countries, the largest proportion is the informal costs of family care, especially lost employment opportunities. This needs to be calculated, and I’m not aware that’s being done. But it will be a lot of money."
Dr. Prince had no financial disclosures.
On Twitter @alz_gal
COPENHAGEN – Researchers appear to be tracking contradictory trends in the global incidence and prevalence of Alzheimer’s disease.
There’s no doubt that both are declining in the United States and other high-income countries. But the disease appears to be claiming more people in less-developed countries, particularly in Asia and sub-Saharan Africa, and that disparity will become much worse by 2050.
Half of the 7.7 million new cases diagnosed per year are occurring in China alone, Dr. Martin Prince said at the Alzheimer’s Association International Conference 2014. And while African and Latin American countries contribute a lower portion of the total (7% and 5%, respectively), they are projected to proportionally outstrip even China’s expected tripling of cases, with each poised for a fourfold increase by 2050.
Taken together with the doubling of cases expected in the United States and European countries, the global incidence could exceed previous projections by more than 30 million cases worldwide by 2050, said Dr. Prince, a primary author of the G8 report "The Global Impact of Dementia, 2013-2050." The report contains updated numbers based on data supplied by the United Nations.
"Because of this new U.N. data, and new data coming out of China, we realized that our 2009 global estimates were about 10% too low," said Dr. Prince, who is also a professor of epidemiological psychiatry in the Institute of Psychiatry at King’s College London. "We are talking about not 36 million right now, but 44 million. Not 66 million by 2030, but 76 million. And by 2050, not 115 million as we previously thought, but 135 million."
The upward trend doesn’t reflect so much an increase in disease incidence as an increase in disease reporting, he noted. While China has published papers on incidence and prevalence, they were almost exclusively in Chinese journals and unavailable outside the country. The biggest change came from a groundbreaking paper in 2013 (Lancet 2013;381:2016-23) – the first widely accessible English language paper on the topic. It concluded that the burden of dementia in China is increasing faster than previously assumed.
According to the G8 report, about 67 million are living with Alzheimer’s in China now, and that number could increase to nearly 200 million by 2050.
But that paper probably doesn’t tell the whole story, Dr. Prince added. China is suffering from the same epidemics of obesity and diabetes that plague other countries, and in contrast to many, cigarette smoking is on a huge rise in China. All of these factors contribute to the development of Alzheimer’s and other dementias, so the numbers will most likely continue to change.
Perhaps not surprisingly, national prosperity is directly tied to Alzheimer’s incidence. By 2050, the incidence is projected to increase by 106% in the G8 countries and by 185% in the G20 countries. This is mainly due to higher educational levels and better risk management, Dr. Prince said. Reducing the prevalence of diabetes, physical inactivity, smoking, depression, low education, and midlife hypertension and obesity could reduce the Alzheimer’s population-attributable risk fraction by more than 50%.
"Even a 10% reduction in risk exposure would result in 250,000 fewer new cases [3.3%]. A 25% reduction in risk exposure would result in 680,000 fewer new cases," an incidence reduction of almost 9% worldwide, he said.
No one has yet modeled the global economic impact of the rising Alzheimer’s tide, Dr. Prince said. Current worldwide spending on Alzheimer’s disease exceeds $600 billion per year. In developed countries, most of this is associated with care in long-term facilities and the cost of professional caregivers. "In less-developed countries, the largest proportion is the informal costs of family care, especially lost employment opportunities. This needs to be calculated, and I’m not aware that’s being done. But it will be a lot of money."
Dr. Prince had no financial disclosures.
On Twitter @alz_gal
AT AAIC 2014
Cerebral microbleeds’ depth may reveal dementia type
COPENHAGEN – The location of cerebral microbleeds appears to be strongly associated with dementia subtypes, perhaps reflecting the disorders’ underlying pathologies.
"This regional association with dementia subtypes is consistent with the hypothesis that lobar cerebral microbleeds reflect cerebral amyloid angiopathy, which is consistent with Alzheimer’s disease, while those in deep areas reflect hypertensive arteriopathy, which is important in vascular dementia," Lenore Launer, Ph.D., said at the Alzheimer’s Association International Conference 2014.
Dr. Launer, chief of the neuroepidemiology section in the laboratory of epidemiology and population science at the National Institute on Aging, and her colleagues examined the relationship between incident microbleeds and dementia in the AGES (Age, Gene/Environment Susceptibility) Reykjavik Study.
The population-based study is a project of the Icelandic Heart Association. It was established in 1967 and has followed more than 9,000 people, all of whom were born between 1907 and 1935. In 2000, the group partnered with the National Institute on Aging to further study diseases of old age, with an emphasis on imaging.
Since then, participants have undergone extensive phenotyping and repeat brain imaging to look specifically at cerebral microbleeds, infarcts, white-matter hyperintensities, and whole-brain volume.
Dr. Launer’s study comprised 2,482 people who were without stroke or dementia at baseline and who had complete brain MRI data during two 5-year periods: 2002-2006 and 2007-2011.
At baseline, patients’ mean age was 75 years. About 25% were carriers of the apolipoprotein E epsilon 4 allele. Hypertension was common (77% of patients). Almost a third of patients (29%) had brain infarct–like lesions, 11% had white-matter hyperintensities, and 16% had cerebral microbleeds.
Over the study period, 458 (18%) of the cohort’s patients developed new microbleeds, with 30% of those developing multiple bleeds. Of those new microbleeds, 64% were strictly lobar, with 1-15 bleeds per person. The remainder were deep lesions, numbering 1-19 per person.
There were 111 new dementia cases; of those, 83 were diagnosed as Alzheimer’s and 17 as vascular dementia. The rest were designated as "other dementia."
Two multivariate regression analyses examined the relationship between microbleed location and dementia subtype. Both controlled for a number of clinical and demographic factors. The first analysis included age, gender, and baseline cerebral microbleeds. The second analysis included all of those factors, plus education, depression, baseline vascular risk factors (hypertension, smoking, diabetes, body mass index, and total cholesterol), and baseline MRI markers (infarcts, total brain volume, and hyperintense lesions).
In the fully adjusted model, microbleed location showed a significant relationship with dementia subtypes. Lobar microbleeds were associated with a doubling in the risk of Alzheimer’s disease, while deep bleeds increased the risk of vascular dementia sixfold.
"It’s difficult to disentangle the temporal relationship here," Dr. Launer said. "But incident cerebral microbleeds may indicate more severe small-vessel disease, and be the thing that pushes a person off the cliff into the clinical presentation of dementia."
The Icelandic Heart Association and the National Institute on Aging sponsored the study. As a government employee, Dr. Launer has no financial disclosures.
On Twitter @alz_gal
COPENHAGEN – The location of cerebral microbleeds appears to be strongly associated with dementia subtypes, perhaps reflecting the disorders’ underlying pathologies.
"This regional association with dementia subtypes is consistent with the hypothesis that lobar cerebral microbleeds reflect cerebral amyloid angiopathy, which is consistent with Alzheimer’s disease, while those in deep areas reflect hypertensive arteriopathy, which is important in vascular dementia," Lenore Launer, Ph.D., said at the Alzheimer’s Association International Conference 2014.
Dr. Launer, chief of the neuroepidemiology section in the laboratory of epidemiology and population science at the National Institute on Aging, and her colleagues examined the relationship between incident microbleeds and dementia in the AGES (Age, Gene/Environment Susceptibility) Reykjavik Study.
The population-based study is a project of the Icelandic Heart Association. It was established in 1967 and has followed more than 9,000 people, all of whom were born between 1907 and 1935. In 2000, the group partnered with the National Institute on Aging to further study diseases of old age, with an emphasis on imaging.
Since then, participants have undergone extensive phenotyping and repeat brain imaging to look specifically at cerebral microbleeds, infarcts, white-matter hyperintensities, and whole-brain volume.
Dr. Launer’s study comprised 2,482 people who were without stroke or dementia at baseline and who had complete brain MRI data during two 5-year periods: 2002-2006 and 2007-2011.
At baseline, patients’ mean age was 75 years. About 25% were carriers of the apolipoprotein E epsilon 4 allele. Hypertension was common (77% of patients). Almost a third of patients (29%) had brain infarct–like lesions, 11% had white-matter hyperintensities, and 16% had cerebral microbleeds.
Over the study period, 458 (18%) of the cohort’s patients developed new microbleeds, with 30% of those developing multiple bleeds. Of those new microbleeds, 64% were strictly lobar, with 1-15 bleeds per person. The remainder were deep lesions, numbering 1-19 per person.
There were 111 new dementia cases; of those, 83 were diagnosed as Alzheimer’s and 17 as vascular dementia. The rest were designated as "other dementia."
Two multivariate regression analyses examined the relationship between microbleed location and dementia subtype. Both controlled for a number of clinical and demographic factors. The first analysis included age, gender, and baseline cerebral microbleeds. The second analysis included all of those factors, plus education, depression, baseline vascular risk factors (hypertension, smoking, diabetes, body mass index, and total cholesterol), and baseline MRI markers (infarcts, total brain volume, and hyperintense lesions).
In the fully adjusted model, microbleed location showed a significant relationship with dementia subtypes. Lobar microbleeds were associated with a doubling in the risk of Alzheimer’s disease, while deep bleeds increased the risk of vascular dementia sixfold.
"It’s difficult to disentangle the temporal relationship here," Dr. Launer said. "But incident cerebral microbleeds may indicate more severe small-vessel disease, and be the thing that pushes a person off the cliff into the clinical presentation of dementia."
The Icelandic Heart Association and the National Institute on Aging sponsored the study. As a government employee, Dr. Launer has no financial disclosures.
On Twitter @alz_gal
COPENHAGEN – The location of cerebral microbleeds appears to be strongly associated with dementia subtypes, perhaps reflecting the disorders’ underlying pathologies.
"This regional association with dementia subtypes is consistent with the hypothesis that lobar cerebral microbleeds reflect cerebral amyloid angiopathy, which is consistent with Alzheimer’s disease, while those in deep areas reflect hypertensive arteriopathy, which is important in vascular dementia," Lenore Launer, Ph.D., said at the Alzheimer’s Association International Conference 2014.
Dr. Launer, chief of the neuroepidemiology section in the laboratory of epidemiology and population science at the National Institute on Aging, and her colleagues examined the relationship between incident microbleeds and dementia in the AGES (Age, Gene/Environment Susceptibility) Reykjavik Study.
The population-based study is a project of the Icelandic Heart Association. It was established in 1967 and has followed more than 9,000 people, all of whom were born between 1907 and 1935. In 2000, the group partnered with the National Institute on Aging to further study diseases of old age, with an emphasis on imaging.
Since then, participants have undergone extensive phenotyping and repeat brain imaging to look specifically at cerebral microbleeds, infarcts, white-matter hyperintensities, and whole-brain volume.
Dr. Launer’s study comprised 2,482 people who were without stroke or dementia at baseline and who had complete brain MRI data during two 5-year periods: 2002-2006 and 2007-2011.
At baseline, patients’ mean age was 75 years. About 25% were carriers of the apolipoprotein E epsilon 4 allele. Hypertension was common (77% of patients). Almost a third of patients (29%) had brain infarct–like lesions, 11% had white-matter hyperintensities, and 16% had cerebral microbleeds.
Over the study period, 458 (18%) of the cohort’s patients developed new microbleeds, with 30% of those developing multiple bleeds. Of those new microbleeds, 64% were strictly lobar, with 1-15 bleeds per person. The remainder were deep lesions, numbering 1-19 per person.
There were 111 new dementia cases; of those, 83 were diagnosed as Alzheimer’s and 17 as vascular dementia. The rest were designated as "other dementia."
Two multivariate regression analyses examined the relationship between microbleed location and dementia subtype. Both controlled for a number of clinical and demographic factors. The first analysis included age, gender, and baseline cerebral microbleeds. The second analysis included all of those factors, plus education, depression, baseline vascular risk factors (hypertension, smoking, diabetes, body mass index, and total cholesterol), and baseline MRI markers (infarcts, total brain volume, and hyperintense lesions).
In the fully adjusted model, microbleed location showed a significant relationship with dementia subtypes. Lobar microbleeds were associated with a doubling in the risk of Alzheimer’s disease, while deep bleeds increased the risk of vascular dementia sixfold.
"It’s difficult to disentangle the temporal relationship here," Dr. Launer said. "But incident cerebral microbleeds may indicate more severe small-vessel disease, and be the thing that pushes a person off the cliff into the clinical presentation of dementia."
The Icelandic Heart Association and the National Institute on Aging sponsored the study. As a government employee, Dr. Launer has no financial disclosures.
On Twitter @alz_gal
AT AAIC 2014
Key clinical point: The location of cerebral microbleeds is strongly associated with the type of dementia that may develop.
Major finding: Lobar microbleeds doubled the risk of Alzheimer’s disease, while deep bleeds increased the risk of vascular dementia sixfold.
Data source: The population-based AGES Reykjavik Study has followed more than 9,000 patients since 1967.
Disclosures: The Icelandic Heart Association and the National Institute on Aging sponsored the study. As a government employee, Dr. Launer has no financial disclosures.
Smelling test makes progress in identifying preclinical Alzheimer’s
COPENHAGEN – A test that measures how well patients recognize scents was highly correlated with progression from mild cognitive impairment to Alzheimer’s disease as well as neurodegeneration and beta-amyloid deposition in the brain in two separate studies.
Although the test is not ready for use in the clinic, it has the potential to be part of a panel of screening tests to predict the development of Alzheimer’s disease, researchers said at a press briefing during the Alzheimer’s Association International Conference 2014.
Timely detection of asymptomatic patients is currently lacking, said Dr. David S. Knopman, who moderated the session.
"Our principal clinical tools, including PET and MRI imaging, are excellent, but they are of little value for detecting patients in the preclinical stage of the disease," said Dr. Knopman, professor of neurology at the Mayo Clinic, Rochester, Minn. "These tests, on the other hand, are much simpler, less expensive and invasive, and would be feasible to use in the field, as opposed to only in a large research center."
The studies used the 40-item University of Pennsylvania Smell Identification Test (UPSIT), a scratch-and-sniff test that measures anosmia of mild, moderate, or severe levels, and costs about $25. For patients with Alzheimer’s disease, the test is a measure of memory, revealing an inability to recall once-familiar scent associations.
In one study, Matthew Growdon, a fourth-year medical student at Harvard University, Boston, and his colleagues found a significant relationship between olfactory impairment and pathologic markers of Alzheimer’s disease. The study involved 212 participants of the ongoing Harvard Aging Brain Study, which seeks to identify structural brain changes associated with memory impairment in aging. Participants were cognitively and physically healthy at baseline.
The investigators administered the UPSIT to all subjects, who also underwent a full evaluation to detect signs and symptoms of Alzheimer’s disease. The evaluation included cognitive testing, structural brain scanning, and amyloid imaging, as well as genetic testing and obtaining blood and spinal fluid biomarkers.
A linear regression model evaluated associations between the UPSIT and amyloid burden, hippocampal size, and thinning of the entorhinal cortex. A separate model looked at associations between UPSIT scores and memory test results.
A total of 100 participants were high performers, scoring in the upper 75th percentile of both the UPSIT and the memory tests. The remaining 112 were considered low performers. Of these, 59 scored in the lower 25th percentile of the UPSIT, but in the upper 25th percentile of memory. Nineteen scored in the upper 75th percentile of the UPSIT but the lower 25th percentile of memory, and 34 in the lower 25th percentile of both the UPSIT and memory.
The investigators found a linear correlation between poor UPSIT performance and both lower hippocampal volume on MRI and higher amyloid burden based on Pittsburgh compound B PET imaging. There was a marginal association between poorer UPSIT scores and entorhinal cortex thinning, Mr. Growdon noted.
The associations may indicate that Alzheimer’s pathology affects neurons in the olfactory bulb as well as other brain regions, said Mr. Growdon. "We seem to be looking at neuronal death in all these regions. And what’s really interesting is that this could be the basis of a very simple, low-cost screening tool for a clinically normal, asymptomatic older population. I envision it as something that could flag those at risk of developing symptoms or as a gateway to more expensive or invasive testing.
"It’s not ready for prime-time, though. This is a snapshot of a single moment in time. We would need longitudinal studies before it could be incorporated into care – maybe as part of a panel of screening tests," he said.
Dr. Davangere Devanand presented a second, unrelated study in which he and his colleagues measured the association between olfactory identification impairment and progression from mild cognitive impairment to Alzheimer’s dementia.
The investigation followed 757 participants of the Northern Manhattan Study, a multiethnic community sample in New York that has been used in large part to examine stroke risk factors. The participants initially took the UPSIT during 2004-2006, followed by additional testing during 2006-2008 and 2008-2010.
A total of 109 transitioned from normal cognition to mild cognitive impairment, and then 101 progressed to Alzheimer’s disease.
A low score on the UPSIT test significantly predicted the development of Alzheimer’s with a specificity of 65%. For each one-point decline in UPSIT score, the risk of developing Alzheimer’s increased by 5%.
The specificity of a low score on the UPSIT improved significantly to 77% when combined with the Selective Reminding Test immediate recall subscore. Even alone, the olfactory test was a better predictor of Alzheimer’s than was the memory test, said Dr. Devanand, director of the division of geriatric psychiatry and professor of psychiatry and neurology at Columbia University, New York.
Dr. Devanand said this test would also require further study before it could be incorporated into clinical practice. "We think of this not as a gold standard test, but as one we would add to other tests in making a prediction."
The Harvard Aging Brain Study is funded by the National Institute on Aging. The Northern Manhattan Study is funded by the National Institute of Neurological Disorders and Stroke. Neither Mr. Growdon nor Dr. Devanand had any relevant financial disclosures.
On Twitter @alz_gal
COPENHAGEN – A test that measures how well patients recognize scents was highly correlated with progression from mild cognitive impairment to Alzheimer’s disease as well as neurodegeneration and beta-amyloid deposition in the brain in two separate studies.
Although the test is not ready for use in the clinic, it has the potential to be part of a panel of screening tests to predict the development of Alzheimer’s disease, researchers said at a press briefing during the Alzheimer’s Association International Conference 2014.
Timely detection of asymptomatic patients is currently lacking, said Dr. David S. Knopman, who moderated the session.
"Our principal clinical tools, including PET and MRI imaging, are excellent, but they are of little value for detecting patients in the preclinical stage of the disease," said Dr. Knopman, professor of neurology at the Mayo Clinic, Rochester, Minn. "These tests, on the other hand, are much simpler, less expensive and invasive, and would be feasible to use in the field, as opposed to only in a large research center."
The studies used the 40-item University of Pennsylvania Smell Identification Test (UPSIT), a scratch-and-sniff test that measures anosmia of mild, moderate, or severe levels, and costs about $25. For patients with Alzheimer’s disease, the test is a measure of memory, revealing an inability to recall once-familiar scent associations.
In one study, Matthew Growdon, a fourth-year medical student at Harvard University, Boston, and his colleagues found a significant relationship between olfactory impairment and pathologic markers of Alzheimer’s disease. The study involved 212 participants of the ongoing Harvard Aging Brain Study, which seeks to identify structural brain changes associated with memory impairment in aging. Participants were cognitively and physically healthy at baseline.
The investigators administered the UPSIT to all subjects, who also underwent a full evaluation to detect signs and symptoms of Alzheimer’s disease. The evaluation included cognitive testing, structural brain scanning, and amyloid imaging, as well as genetic testing and obtaining blood and spinal fluid biomarkers.
A linear regression model evaluated associations between the UPSIT and amyloid burden, hippocampal size, and thinning of the entorhinal cortex. A separate model looked at associations between UPSIT scores and memory test results.
A total of 100 participants were high performers, scoring in the upper 75th percentile of both the UPSIT and the memory tests. The remaining 112 were considered low performers. Of these, 59 scored in the lower 25th percentile of the UPSIT, but in the upper 25th percentile of memory. Nineteen scored in the upper 75th percentile of the UPSIT but the lower 25th percentile of memory, and 34 in the lower 25th percentile of both the UPSIT and memory.
The investigators found a linear correlation between poor UPSIT performance and both lower hippocampal volume on MRI and higher amyloid burden based on Pittsburgh compound B PET imaging. There was a marginal association between poorer UPSIT scores and entorhinal cortex thinning, Mr. Growdon noted.
The associations may indicate that Alzheimer’s pathology affects neurons in the olfactory bulb as well as other brain regions, said Mr. Growdon. "We seem to be looking at neuronal death in all these regions. And what’s really interesting is that this could be the basis of a very simple, low-cost screening tool for a clinically normal, asymptomatic older population. I envision it as something that could flag those at risk of developing symptoms or as a gateway to more expensive or invasive testing.
"It’s not ready for prime-time, though. This is a snapshot of a single moment in time. We would need longitudinal studies before it could be incorporated into care – maybe as part of a panel of screening tests," he said.
Dr. Davangere Devanand presented a second, unrelated study in which he and his colleagues measured the association between olfactory identification impairment and progression from mild cognitive impairment to Alzheimer’s dementia.
The investigation followed 757 participants of the Northern Manhattan Study, a multiethnic community sample in New York that has been used in large part to examine stroke risk factors. The participants initially took the UPSIT during 2004-2006, followed by additional testing during 2006-2008 and 2008-2010.
A total of 109 transitioned from normal cognition to mild cognitive impairment, and then 101 progressed to Alzheimer’s disease.
A low score on the UPSIT test significantly predicted the development of Alzheimer’s with a specificity of 65%. For each one-point decline in UPSIT score, the risk of developing Alzheimer’s increased by 5%.
The specificity of a low score on the UPSIT improved significantly to 77% when combined with the Selective Reminding Test immediate recall subscore. Even alone, the olfactory test was a better predictor of Alzheimer’s than was the memory test, said Dr. Devanand, director of the division of geriatric psychiatry and professor of psychiatry and neurology at Columbia University, New York.
Dr. Devanand said this test would also require further study before it could be incorporated into clinical practice. "We think of this not as a gold standard test, but as one we would add to other tests in making a prediction."
The Harvard Aging Brain Study is funded by the National Institute on Aging. The Northern Manhattan Study is funded by the National Institute of Neurological Disorders and Stroke. Neither Mr. Growdon nor Dr. Devanand had any relevant financial disclosures.
On Twitter @alz_gal
COPENHAGEN – A test that measures how well patients recognize scents was highly correlated with progression from mild cognitive impairment to Alzheimer’s disease as well as neurodegeneration and beta-amyloid deposition in the brain in two separate studies.
Although the test is not ready for use in the clinic, it has the potential to be part of a panel of screening tests to predict the development of Alzheimer’s disease, researchers said at a press briefing during the Alzheimer’s Association International Conference 2014.
Timely detection of asymptomatic patients is currently lacking, said Dr. David S. Knopman, who moderated the session.
"Our principal clinical tools, including PET and MRI imaging, are excellent, but they are of little value for detecting patients in the preclinical stage of the disease," said Dr. Knopman, professor of neurology at the Mayo Clinic, Rochester, Minn. "These tests, on the other hand, are much simpler, less expensive and invasive, and would be feasible to use in the field, as opposed to only in a large research center."
The studies used the 40-item University of Pennsylvania Smell Identification Test (UPSIT), a scratch-and-sniff test that measures anosmia of mild, moderate, or severe levels, and costs about $25. For patients with Alzheimer’s disease, the test is a measure of memory, revealing an inability to recall once-familiar scent associations.
In one study, Matthew Growdon, a fourth-year medical student at Harvard University, Boston, and his colleagues found a significant relationship between olfactory impairment and pathologic markers of Alzheimer’s disease. The study involved 212 participants of the ongoing Harvard Aging Brain Study, which seeks to identify structural brain changes associated with memory impairment in aging. Participants were cognitively and physically healthy at baseline.
The investigators administered the UPSIT to all subjects, who also underwent a full evaluation to detect signs and symptoms of Alzheimer’s disease. The evaluation included cognitive testing, structural brain scanning, and amyloid imaging, as well as genetic testing and obtaining blood and spinal fluid biomarkers.
A linear regression model evaluated associations between the UPSIT and amyloid burden, hippocampal size, and thinning of the entorhinal cortex. A separate model looked at associations between UPSIT scores and memory test results.
A total of 100 participants were high performers, scoring in the upper 75th percentile of both the UPSIT and the memory tests. The remaining 112 were considered low performers. Of these, 59 scored in the lower 25th percentile of the UPSIT, but in the upper 25th percentile of memory. Nineteen scored in the upper 75th percentile of the UPSIT but the lower 25th percentile of memory, and 34 in the lower 25th percentile of both the UPSIT and memory.
The investigators found a linear correlation between poor UPSIT performance and both lower hippocampal volume on MRI and higher amyloid burden based on Pittsburgh compound B PET imaging. There was a marginal association between poorer UPSIT scores and entorhinal cortex thinning, Mr. Growdon noted.
The associations may indicate that Alzheimer’s pathology affects neurons in the olfactory bulb as well as other brain regions, said Mr. Growdon. "We seem to be looking at neuronal death in all these regions. And what’s really interesting is that this could be the basis of a very simple, low-cost screening tool for a clinically normal, asymptomatic older population. I envision it as something that could flag those at risk of developing symptoms or as a gateway to more expensive or invasive testing.
"It’s not ready for prime-time, though. This is a snapshot of a single moment in time. We would need longitudinal studies before it could be incorporated into care – maybe as part of a panel of screening tests," he said.
Dr. Davangere Devanand presented a second, unrelated study in which he and his colleagues measured the association between olfactory identification impairment and progression from mild cognitive impairment to Alzheimer’s dementia.
The investigation followed 757 participants of the Northern Manhattan Study, a multiethnic community sample in New York that has been used in large part to examine stroke risk factors. The participants initially took the UPSIT during 2004-2006, followed by additional testing during 2006-2008 and 2008-2010.
A total of 109 transitioned from normal cognition to mild cognitive impairment, and then 101 progressed to Alzheimer’s disease.
A low score on the UPSIT test significantly predicted the development of Alzheimer’s with a specificity of 65%. For each one-point decline in UPSIT score, the risk of developing Alzheimer’s increased by 5%.
The specificity of a low score on the UPSIT improved significantly to 77% when combined with the Selective Reminding Test immediate recall subscore. Even alone, the olfactory test was a better predictor of Alzheimer’s than was the memory test, said Dr. Devanand, director of the division of geriatric psychiatry and professor of psychiatry and neurology at Columbia University, New York.
Dr. Devanand said this test would also require further study before it could be incorporated into clinical practice. "We think of this not as a gold standard test, but as one we would add to other tests in making a prediction."
The Harvard Aging Brain Study is funded by the National Institute on Aging. The Northern Manhattan Study is funded by the National Institute of Neurological Disorders and Stroke. Neither Mr. Growdon nor Dr. Devanand had any relevant financial disclosures.
On Twitter @alz_gal
AT AAIC 2014
Key clinical point: Two studies found that a simple, inexpensive test measuring olfactory memory could help identify patients with presymptomatic Alzheimer’s disease.
Major finding: Poor performance on the UPSIT significantly correlated with a smaller hippocampal volume and higher amyloid burden, and had 77% specificity for Alzheimer’s when combined with a measure of memory.
Data source: A cross-sectional study of 212 participants in the Harvard Aging Brain Study and a longitudinal study of 757 patients in the Northern Manhattan Study.
Disclosures: The Harvard Aging Brain Study is funded by the National Institute on Aging. The Northern Manhattan Study is funded by the National Institute of Neurological Disorders and Stroke. Neither Mr. Matthew Growdon nor Dr. Devangere Devanand had any relevant financial disclosures.