User login
American Heart Association (AHA): Scientific Sessions 2014
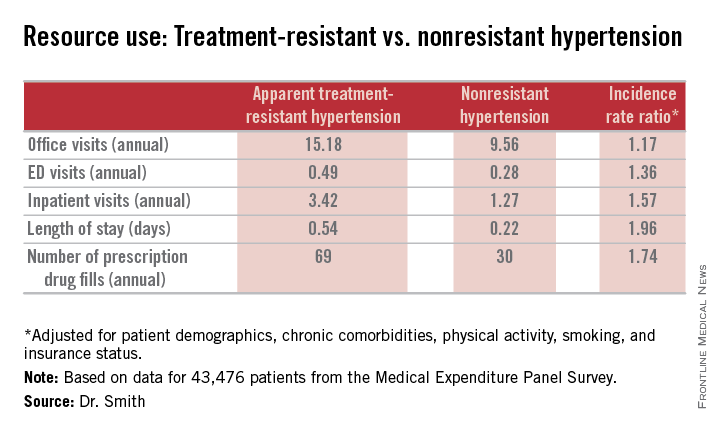
The high cost of treatment-resistant hypertension
CHICAGO– Prepare for sticker shock: Researchers have put a price tag on direct medical expenditures for treatment-resistant hypertension, and it’s a big one.
Indeed, apparent treatment-resistant hypertension (aTRH) is associated with an estimated $11.3-$17.9 billion per year in direct medical expenditures above and beyond expenditures for nonresistant hypertension in the United States, Steven M. Smith, Pharm.D., reported at the American Heart Association scientific sessions.
This is a conservative estimate based on an assumed 5% prevalence of aTRH among U.S. adults with hypertension, which may be a low figure, according to Dr. Smith of the University of Florida, Gainesville.
He presented an analysis of data on direct medical expenditures and health care utilization for 43,476 patients with hypertension in the nationally representative U.S. Medical Expenditure Panel Survey, of whom 1,924 met the criteria for aTRH as defined by a requirement for drugs from at least four antihypertensive medications classes in order to achieve blood pressure control.
While aTRH is known to be associated with higher rates of major adverse cardiovascular events and increased mortality relative to nonresistant hypertension, the financial costs associated with this condition haven’t previously been carefully examined.
Mean annual health care expenditures for individuals with aTRH were $20,018, more than twice the $9,814 figure for patients with nonresistant hypertension. But patients with aTRH were older, heavier, less likely to have a high income, and differed in additional ways from the much larger group with nonresistant hypertension. In a multivariate analysis adjusted for these potential confounders, aTRH was associated with $2,413 in excess annual medical expenditures and $1,253 in excess total prescription expenditures, for a total of $3,647 excess total annual health care expenditures per person, compared with subjects with nonresistant hypertension.
New preventive strategies are clearly in order, Dr. Smith concluded.
He reported having no financial conflicts regarding this study, which was conducted without industry funding.
CHICAGO– Prepare for sticker shock: Researchers have put a price tag on direct medical expenditures for treatment-resistant hypertension, and it’s a big one.
Indeed, apparent treatment-resistant hypertension (aTRH) is associated with an estimated $11.3-$17.9 billion per year in direct medical expenditures above and beyond expenditures for nonresistant hypertension in the United States, Steven M. Smith, Pharm.D., reported at the American Heart Association scientific sessions.
This is a conservative estimate based on an assumed 5% prevalence of aTRH among U.S. adults with hypertension, which may be a low figure, according to Dr. Smith of the University of Florida, Gainesville.
He presented an analysis of data on direct medical expenditures and health care utilization for 43,476 patients with hypertension in the nationally representative U.S. Medical Expenditure Panel Survey, of whom 1,924 met the criteria for aTRH as defined by a requirement for drugs from at least four antihypertensive medications classes in order to achieve blood pressure control.
While aTRH is known to be associated with higher rates of major adverse cardiovascular events and increased mortality relative to nonresistant hypertension, the financial costs associated with this condition haven’t previously been carefully examined.
Mean annual health care expenditures for individuals with aTRH were $20,018, more than twice the $9,814 figure for patients with nonresistant hypertension. But patients with aTRH were older, heavier, less likely to have a high income, and differed in additional ways from the much larger group with nonresistant hypertension. In a multivariate analysis adjusted for these potential confounders, aTRH was associated with $2,413 in excess annual medical expenditures and $1,253 in excess total prescription expenditures, for a total of $3,647 excess total annual health care expenditures per person, compared with subjects with nonresistant hypertension.
New preventive strategies are clearly in order, Dr. Smith concluded.
He reported having no financial conflicts regarding this study, which was conducted without industry funding.
CHICAGO– Prepare for sticker shock: Researchers have put a price tag on direct medical expenditures for treatment-resistant hypertension, and it’s a big one.
Indeed, apparent treatment-resistant hypertension (aTRH) is associated with an estimated $11.3-$17.9 billion per year in direct medical expenditures above and beyond expenditures for nonresistant hypertension in the United States, Steven M. Smith, Pharm.D., reported at the American Heart Association scientific sessions.
This is a conservative estimate based on an assumed 5% prevalence of aTRH among U.S. adults with hypertension, which may be a low figure, according to Dr. Smith of the University of Florida, Gainesville.
He presented an analysis of data on direct medical expenditures and health care utilization for 43,476 patients with hypertension in the nationally representative U.S. Medical Expenditure Panel Survey, of whom 1,924 met the criteria for aTRH as defined by a requirement for drugs from at least four antihypertensive medications classes in order to achieve blood pressure control.
While aTRH is known to be associated with higher rates of major adverse cardiovascular events and increased mortality relative to nonresistant hypertension, the financial costs associated with this condition haven’t previously been carefully examined.
Mean annual health care expenditures for individuals with aTRH were $20,018, more than twice the $9,814 figure for patients with nonresistant hypertension. But patients with aTRH were older, heavier, less likely to have a high income, and differed in additional ways from the much larger group with nonresistant hypertension. In a multivariate analysis adjusted for these potential confounders, aTRH was associated with $2,413 in excess annual medical expenditures and $1,253 in excess total prescription expenditures, for a total of $3,647 excess total annual health care expenditures per person, compared with subjects with nonresistant hypertension.
New preventive strategies are clearly in order, Dr. Smith concluded.
He reported having no financial conflicts regarding this study, which was conducted without industry funding.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Direct medical expenditures for apparent treatment-resistant hypertension amount to up to an additional $17.9 billion annually beyond the cost associated with nonresistant hypertension.
Major finding: Patients with apparent treatment-resistant hypertension averaged twice as many office visits and nearly three times as many inpatient stays annually, compared with individuals with nonresistant hypertension. Moreover, their average hospital stay was twice as long.
Data source: A retrospective analysis of prospectively collected data on 43,476 persons with hypertension included in the U.S. Medical Expenditure Panel Survey.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial funding.
Promising new therapy for critical limb ischemia
CHICAGO – A single set of intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia showed safety as well as evidence of efficacy through 12 months of follow-up in the STOP-CLI trial.
“Patients treated with JVS-100 demonstrated dose-dependent improvement across multiple patient-centered outcomes, including pain, quality of life, and wound healing, with less change in macrovascular objective measures in this small study,” Dr. Melina R. Kibbe reported at the American Heart Association scientific sessions. JVS-100 is a nonviral plasmid encoding human stromal cell–derived factor-1 (SDF-1), a natural chemokine protein that promotes angiogenesis by recruiting endothelial progenitor cells from the bone marrow to ischemic sites, explained Dr. Kibbe, professor and vice chair of surgical research and deputy director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University, Chicago.
STOP-CLI was an exploratory, phase IIa, double-blind, first-in-humans study involving 48 patients with Rutherford classification 4 or 5 critical climb ischemia (CLI). All had an ankle-brachial index of 0.4 or lower, an ankle systolic blood pressure of 70 mm Hg or less or a toe systolic blood pressure of 50 or less, and were poor candidates for surgical revascularization. None had Buerger’s disease.
Participants were randomized to one of four study arms, and within each study arm further randomized 3:1 to stromal cell–derived factor-1 (SDF-1) or placebo injections. The patients received either 8 or 16 injections, each containing either 0.5 or 1.0 mg of SDF-1 or placebo. The injections, given in a single session, were placed at least 0.5 cm apart throughout the ischemic area of the affected limb.
By chance, most patients randomized to the placebo group were Rutherford 4, a category of CLI defined by rest pain, while the majority in the active treatment arms were Rutherford 5, a more severe disease manifestation characterized by ulcers. As a consequence, the SDF-1 recipients also had far larger nonhealing wounds, with an average area of 6.4 cm2, compared with 1.5 cm2, in controls.
The SDF-1 injections proved safe and were well tolerated, with no treatment-related serious adverse events and no safety signals evident in the laboratory results.
Turning to efficacy endpoints, Dr. Kibbe said self-rated visual analog scale pain scores showed clear, dose-dependent improvement over time in the SDF-1 treatment cohorts and no change in controls.
Similarly, the active treatment groups showed improved quality of life scores on all domains of the Short Form-36: physical functioning, bodily pain, general health, social functioning, energy/fatigue, emotional well-being, and overall physical and mental health, the surgeon continued.
Wound area decreased significantly in the SDF-1-treated groups, with the biggest reduction – more than 8 cm2 – being noted in the three patients who received eight 1-mg injections. That was also the group with the largest wounds at baseline, with an average area of 11.4 cm2.
Of note, the major limb amputation rate was “remarkably low” for patients with such severe CLI, according to Dr. Kibbe. The rate was less than 10% over the course of 12 months, with one patient in each of the four active treatment arms having a major amputation at time intervals of 58-112 days post injection. No major limb amputations occurred in the control group.
There was a hint of improvement with SDF-1 therapy over placebo in ankle-brachial index and transcutaneous oxygen pressure, but the between-group differences were too narrow in this study to allow for any conclusions. That must await planned much larger phase III trials, according to Dr. Kibbe.
Audience members, citing the numerous failures of once-promising stem cell therapies for CLI at phase III testing over the last 10-15 years, wondered why Dr. Kibbe thinks SDF-1 will fare any better.
“This is much debated and discussed among all the people involved in these kinds of trials,” she replied. “I’d say, briefly, that a lot of it has to do with patient selection. I think when you have a mixed bag of patients in a trial, including patients with Buerger’s disease, treated in multiple different countries, using different definitions of when to amputate, all those things come into play and could account for why those phase III trials were not successful.”
“Having been involved in lots of the different gene- and cell-based therapy trials, I think one of the unique benefits of this therapy is that it kind of bridges between the two. SDF-1 basically homes your endothelial progenitor cells to the site of ischemic injury for enhanced vasculogenesis. But SDF-1 also has direct effects on endothelial cells, including stimulating proliferation and preventing apoptosis,” she added.
JVS-100 has also successfully completed a phase II clinical trial for the treatment of heart failure. In addition, the agent is being developed as a treatment for acute MI, chronic angina, and for muscle regeneration.
The STOP-CLI study was sponsored by Juventas Therapeutics. Dr. Kibbe reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
CHICAGO – A single set of intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia showed safety as well as evidence of efficacy through 12 months of follow-up in the STOP-CLI trial.
“Patients treated with JVS-100 demonstrated dose-dependent improvement across multiple patient-centered outcomes, including pain, quality of life, and wound healing, with less change in macrovascular objective measures in this small study,” Dr. Melina R. Kibbe reported at the American Heart Association scientific sessions. JVS-100 is a nonviral plasmid encoding human stromal cell–derived factor-1 (SDF-1), a natural chemokine protein that promotes angiogenesis by recruiting endothelial progenitor cells from the bone marrow to ischemic sites, explained Dr. Kibbe, professor and vice chair of surgical research and deputy director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University, Chicago.
STOP-CLI was an exploratory, phase IIa, double-blind, first-in-humans study involving 48 patients with Rutherford classification 4 or 5 critical climb ischemia (CLI). All had an ankle-brachial index of 0.4 or lower, an ankle systolic blood pressure of 70 mm Hg or less or a toe systolic blood pressure of 50 or less, and were poor candidates for surgical revascularization. None had Buerger’s disease.
Participants were randomized to one of four study arms, and within each study arm further randomized 3:1 to stromal cell–derived factor-1 (SDF-1) or placebo injections. The patients received either 8 or 16 injections, each containing either 0.5 or 1.0 mg of SDF-1 or placebo. The injections, given in a single session, were placed at least 0.5 cm apart throughout the ischemic area of the affected limb.
By chance, most patients randomized to the placebo group were Rutherford 4, a category of CLI defined by rest pain, while the majority in the active treatment arms were Rutherford 5, a more severe disease manifestation characterized by ulcers. As a consequence, the SDF-1 recipients also had far larger nonhealing wounds, with an average area of 6.4 cm2, compared with 1.5 cm2, in controls.
The SDF-1 injections proved safe and were well tolerated, with no treatment-related serious adverse events and no safety signals evident in the laboratory results.
Turning to efficacy endpoints, Dr. Kibbe said self-rated visual analog scale pain scores showed clear, dose-dependent improvement over time in the SDF-1 treatment cohorts and no change in controls.
Similarly, the active treatment groups showed improved quality of life scores on all domains of the Short Form-36: physical functioning, bodily pain, general health, social functioning, energy/fatigue, emotional well-being, and overall physical and mental health, the surgeon continued.
Wound area decreased significantly in the SDF-1-treated groups, with the biggest reduction – more than 8 cm2 – being noted in the three patients who received eight 1-mg injections. That was also the group with the largest wounds at baseline, with an average area of 11.4 cm2.
Of note, the major limb amputation rate was “remarkably low” for patients with such severe CLI, according to Dr. Kibbe. The rate was less than 10% over the course of 12 months, with one patient in each of the four active treatment arms having a major amputation at time intervals of 58-112 days post injection. No major limb amputations occurred in the control group.
There was a hint of improvement with SDF-1 therapy over placebo in ankle-brachial index and transcutaneous oxygen pressure, but the between-group differences were too narrow in this study to allow for any conclusions. That must await planned much larger phase III trials, according to Dr. Kibbe.
Audience members, citing the numerous failures of once-promising stem cell therapies for CLI at phase III testing over the last 10-15 years, wondered why Dr. Kibbe thinks SDF-1 will fare any better.
“This is much debated and discussed among all the people involved in these kinds of trials,” she replied. “I’d say, briefly, that a lot of it has to do with patient selection. I think when you have a mixed bag of patients in a trial, including patients with Buerger’s disease, treated in multiple different countries, using different definitions of when to amputate, all those things come into play and could account for why those phase III trials were not successful.”
“Having been involved in lots of the different gene- and cell-based therapy trials, I think one of the unique benefits of this therapy is that it kind of bridges between the two. SDF-1 basically homes your endothelial progenitor cells to the site of ischemic injury for enhanced vasculogenesis. But SDF-1 also has direct effects on endothelial cells, including stimulating proliferation and preventing apoptosis,” she added.
JVS-100 has also successfully completed a phase II clinical trial for the treatment of heart failure. In addition, the agent is being developed as a treatment for acute MI, chronic angina, and for muscle regeneration.
The STOP-CLI study was sponsored by Juventas Therapeutics. Dr. Kibbe reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
CHICAGO – A single set of intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia showed safety as well as evidence of efficacy through 12 months of follow-up in the STOP-CLI trial.
“Patients treated with JVS-100 demonstrated dose-dependent improvement across multiple patient-centered outcomes, including pain, quality of life, and wound healing, with less change in macrovascular objective measures in this small study,” Dr. Melina R. Kibbe reported at the American Heart Association scientific sessions. JVS-100 is a nonviral plasmid encoding human stromal cell–derived factor-1 (SDF-1), a natural chemokine protein that promotes angiogenesis by recruiting endothelial progenitor cells from the bone marrow to ischemic sites, explained Dr. Kibbe, professor and vice chair of surgical research and deputy director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University, Chicago.
STOP-CLI was an exploratory, phase IIa, double-blind, first-in-humans study involving 48 patients with Rutherford classification 4 or 5 critical climb ischemia (CLI). All had an ankle-brachial index of 0.4 or lower, an ankle systolic blood pressure of 70 mm Hg or less or a toe systolic blood pressure of 50 or less, and were poor candidates for surgical revascularization. None had Buerger’s disease.
Participants were randomized to one of four study arms, and within each study arm further randomized 3:1 to stromal cell–derived factor-1 (SDF-1) or placebo injections. The patients received either 8 or 16 injections, each containing either 0.5 or 1.0 mg of SDF-1 or placebo. The injections, given in a single session, were placed at least 0.5 cm apart throughout the ischemic area of the affected limb.
By chance, most patients randomized to the placebo group were Rutherford 4, a category of CLI defined by rest pain, while the majority in the active treatment arms were Rutherford 5, a more severe disease manifestation characterized by ulcers. As a consequence, the SDF-1 recipients also had far larger nonhealing wounds, with an average area of 6.4 cm2, compared with 1.5 cm2, in controls.
The SDF-1 injections proved safe and were well tolerated, with no treatment-related serious adverse events and no safety signals evident in the laboratory results.
Turning to efficacy endpoints, Dr. Kibbe said self-rated visual analog scale pain scores showed clear, dose-dependent improvement over time in the SDF-1 treatment cohorts and no change in controls.
Similarly, the active treatment groups showed improved quality of life scores on all domains of the Short Form-36: physical functioning, bodily pain, general health, social functioning, energy/fatigue, emotional well-being, and overall physical and mental health, the surgeon continued.
Wound area decreased significantly in the SDF-1-treated groups, with the biggest reduction – more than 8 cm2 – being noted in the three patients who received eight 1-mg injections. That was also the group with the largest wounds at baseline, with an average area of 11.4 cm2.
Of note, the major limb amputation rate was “remarkably low” for patients with such severe CLI, according to Dr. Kibbe. The rate was less than 10% over the course of 12 months, with one patient in each of the four active treatment arms having a major amputation at time intervals of 58-112 days post injection. No major limb amputations occurred in the control group.
There was a hint of improvement with SDF-1 therapy over placebo in ankle-brachial index and transcutaneous oxygen pressure, but the between-group differences were too narrow in this study to allow for any conclusions. That must await planned much larger phase III trials, according to Dr. Kibbe.
Audience members, citing the numerous failures of once-promising stem cell therapies for CLI at phase III testing over the last 10-15 years, wondered why Dr. Kibbe thinks SDF-1 will fare any better.
“This is much debated and discussed among all the people involved in these kinds of trials,” she replied. “I’d say, briefly, that a lot of it has to do with patient selection. I think when you have a mixed bag of patients in a trial, including patients with Buerger’s disease, treated in multiple different countries, using different definitions of when to amputate, all those things come into play and could account for why those phase III trials were not successful.”
“Having been involved in lots of the different gene- and cell-based therapy trials, I think one of the unique benefits of this therapy is that it kind of bridges between the two. SDF-1 basically homes your endothelial progenitor cells to the site of ischemic injury for enhanced vasculogenesis. But SDF-1 also has direct effects on endothelial cells, including stimulating proliferation and preventing apoptosis,” she added.
JVS-100 has also successfully completed a phase II clinical trial for the treatment of heart failure. In addition, the agent is being developed as a treatment for acute MI, chronic angina, and for muscle regeneration.
The STOP-CLI study was sponsored by Juventas Therapeutics. Dr. Kibbe reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia demonstrated safety and efficacy; the therapy is moving forward to phase III testing.
Major finding: The major limb amputation rate was less than 10% during 12 months of follow-up after a single dose of the novel therapy.
Data source: The STOP-CLI trial was a phase IIa, 12-month, randomized, double-blind, placebo-controlled, six-center trial including 48 patients with critical limb ischemia.
Disclosures: The STOP-CLI trial was sponsored by Juventas Therapeutics. The presenter reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
Promising new therapy for critical limb ischemia
CHICAGO– A single set of intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia showed safety as well as evidence of efficacy through 12 months of follow-up in the STOP-CLI trial.
“Patients treated with JVS-100 demonstrated dose-dependent improvement across multiple patient-centered outcomes, including pain, quality of life, and wound healing, with less change in macrovascular objective measures in this small study,” Dr. Melina R. Kibbe reported at the American Heart Association scientific sessions. JVS-100 is a nonviral plasmid encoding human stromal cell–derived factor-1 (SDF-1), a natural chemokine protein that promotes angiogenesis by recruiting endothelial progenitor cells from the bone marrow to ischemic sites, explained Dr. Kibbe, professor and vice chair of surgical research and deputy director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University, Chicago.
STOP-CLI was an exploratory, phase IIa, double-blind, first-in-humans study involving 48 patients with Rutherford classification 4 or 5 critical climb ischemia (CLI). All had an ankle-brachial index of 0.4 or lower, an ankle systolic blood pressure of 70 mm Hg or less or a toe systolic blood pressure of 50 or less, and were poor candidates for surgical revascularization. None had Buerger’s disease.
Participants were randomized to one of four study arms, and within each study arm further randomized 3:1 to stromal cell–derived factor-1 (SDF-1) or placebo injections. The patients received either 8 or 16 injections, each containing either 0.5 or 1.0 mg of SDF-1 or placebo. The injections, given in a single session, were placed at least 0.5 cm apart throughout the ischemic area of the affected limb.
By chance, most patients randomized to the placebo group were Rutherford 4, a category of CLI defined by rest pain, while the majority in the active treatment arms were Rutherford 5, a more severe disease manifestation characterized by ulcers. As a consequence, the SDF-1 recipients also had far larger nonhealing wounds, with an average area of 6.4 cm2, compared with 1.5 cm2, in controls.
The SDF-1 injections proved safe and were well tolerated, with no treatment-related serious adverse events and no safety signals evident in the laboratory results.
Turning to efficacy endpoints, Dr. Kibbe said self-rated visual analog scale pain scores showed clear, dose-dependent improvement over time in the SDF-1 treatment cohorts and no change in controls.
Similarly, the active treatment groups showed improved quality of life scores on all domains of the Short Form-36: physical functioning, bodily pain, general health, social functioning, energy/fatigue, emotional well-being, and overall physical and mental health, the surgeon continued.
Wound area decreased significantly in the SDF-1-treated groups, with the biggest reduction – more than 8 cm2 – being noted in the three patients who received eight 1-mg injections. That was also the group with the largest wounds at baseline, with an average area of 11.4 cm2.
Of note, the major limb amputation rate was “remarkably low” for patients with such severe CLI, according to Dr. Kibbe. The rate was less than 10% over the course of 12 months, with one patient in each of the four active treatment arms having a major amputation at time intervals of 58-112 days post injection. No major limb amputations occurred in the control group.
There was a hint of improvement with SDF-1 therapy over placebo in ankle-brachial index and transcutaneous oxygen pressure, but the between-group differences were too narrow in this study to allow for any conclusions. That must await planned much larger phase III trials, according to Dr. Kibbe.
Audience members, citing the numerous failures of once-promising stem cell therapies for CLI at phase III testing over the last 10-15 years, wondered why Dr. Kibbe thinks SDF-1 will fare any better.
“This is much debated and discussed among all the people involved in these kinds of trials,” she replied. “I’d say, briefly, that a lot of it has to do with patient selection. I think when you have a mixed bag of patients in a trial, including patients with Buerger’s disease, treated in multiple different countries, using different definitions of when to amputate, all those things come into play and could account for why those phase III trials were not successful.”
“Having been involved in lots of the different gene- and cell-based therapy trials, I think one of the unique benefits of this therapy is that it kind of bridges between the two. SDF-1 basically homes your endothelial progenitor cells to the site of ischemic injury for enhanced vasculogenesis. But SDF-1 also has direct effects on endothelial cells, including stimulating proliferation and preventing apoptosis,” she added.
JVS-100 has also successfully completed a phase II clinical trial for the treatment of heart failure. In addition, the agent is being developed as a treatment for acute MI, chronic angina, and for muscle regeneration.
The STOP-CLI study was sponsored by Juventas Therapeutics. Dr. Kibbe reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
CHICAGO– A single set of intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia showed safety as well as evidence of efficacy through 12 months of follow-up in the STOP-CLI trial.
“Patients treated with JVS-100 demonstrated dose-dependent improvement across multiple patient-centered outcomes, including pain, quality of life, and wound healing, with less change in macrovascular objective measures in this small study,” Dr. Melina R. Kibbe reported at the American Heart Association scientific sessions. JVS-100 is a nonviral plasmid encoding human stromal cell–derived factor-1 (SDF-1), a natural chemokine protein that promotes angiogenesis by recruiting endothelial progenitor cells from the bone marrow to ischemic sites, explained Dr. Kibbe, professor and vice chair of surgical research and deputy director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University, Chicago.
STOP-CLI was an exploratory, phase IIa, double-blind, first-in-humans study involving 48 patients with Rutherford classification 4 or 5 critical climb ischemia (CLI). All had an ankle-brachial index of 0.4 or lower, an ankle systolic blood pressure of 70 mm Hg or less or a toe systolic blood pressure of 50 or less, and were poor candidates for surgical revascularization. None had Buerger’s disease.
Participants were randomized to one of four study arms, and within each study arm further randomized 3:1 to stromal cell–derived factor-1 (SDF-1) or placebo injections. The patients received either 8 or 16 injections, each containing either 0.5 or 1.0 mg of SDF-1 or placebo. The injections, given in a single session, were placed at least 0.5 cm apart throughout the ischemic area of the affected limb.
By chance, most patients randomized to the placebo group were Rutherford 4, a category of CLI defined by rest pain, while the majority in the active treatment arms were Rutherford 5, a more severe disease manifestation characterized by ulcers. As a consequence, the SDF-1 recipients also had far larger nonhealing wounds, with an average area of 6.4 cm2, compared with 1.5 cm2, in controls.
The SDF-1 injections proved safe and were well tolerated, with no treatment-related serious adverse events and no safety signals evident in the laboratory results.
Turning to efficacy endpoints, Dr. Kibbe said self-rated visual analog scale pain scores showed clear, dose-dependent improvement over time in the SDF-1 treatment cohorts and no change in controls.
Similarly, the active treatment groups showed improved quality of life scores on all domains of the Short Form-36: physical functioning, bodily pain, general health, social functioning, energy/fatigue, emotional well-being, and overall physical and mental health, the surgeon continued.
Wound area decreased significantly in the SDF-1-treated groups, with the biggest reduction – more than 8 cm2 – being noted in the three patients who received eight 1-mg injections. That was also the group with the largest wounds at baseline, with an average area of 11.4 cm2.
Of note, the major limb amputation rate was “remarkably low” for patients with such severe CLI, according to Dr. Kibbe. The rate was less than 10% over the course of 12 months, with one patient in each of the four active treatment arms having a major amputation at time intervals of 58-112 days post injection. No major limb amputations occurred in the control group.
There was a hint of improvement with SDF-1 therapy over placebo in ankle-brachial index and transcutaneous oxygen pressure, but the between-group differences were too narrow in this study to allow for any conclusions. That must await planned much larger phase III trials, according to Dr. Kibbe.
Audience members, citing the numerous failures of once-promising stem cell therapies for CLI at phase III testing over the last 10-15 years, wondered why Dr. Kibbe thinks SDF-1 will fare any better.
“This is much debated and discussed among all the people involved in these kinds of trials,” she replied. “I’d say, briefly, that a lot of it has to do with patient selection. I think when you have a mixed bag of patients in a trial, including patients with Buerger’s disease, treated in multiple different countries, using different definitions of when to amputate, all those things come into play and could account for why those phase III trials were not successful.”
“Having been involved in lots of the different gene- and cell-based therapy trials, I think one of the unique benefits of this therapy is that it kind of bridges between the two. SDF-1 basically homes your endothelial progenitor cells to the site of ischemic injury for enhanced vasculogenesis. But SDF-1 also has direct effects on endothelial cells, including stimulating proliferation and preventing apoptosis,” she added.
JVS-100 has also successfully completed a phase II clinical trial for the treatment of heart failure. In addition, the agent is being developed as a treatment for acute MI, chronic angina, and for muscle regeneration.
The STOP-CLI study was sponsored by Juventas Therapeutics. Dr. Kibbe reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
CHICAGO– A single set of intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia showed safety as well as evidence of efficacy through 12 months of follow-up in the STOP-CLI trial.
“Patients treated with JVS-100 demonstrated dose-dependent improvement across multiple patient-centered outcomes, including pain, quality of life, and wound healing, with less change in macrovascular objective measures in this small study,” Dr. Melina R. Kibbe reported at the American Heart Association scientific sessions. JVS-100 is a nonviral plasmid encoding human stromal cell–derived factor-1 (SDF-1), a natural chemokine protein that promotes angiogenesis by recruiting endothelial progenitor cells from the bone marrow to ischemic sites, explained Dr. Kibbe, professor and vice chair of surgical research and deputy director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University, Chicago.
STOP-CLI was an exploratory, phase IIa, double-blind, first-in-humans study involving 48 patients with Rutherford classification 4 or 5 critical climb ischemia (CLI). All had an ankle-brachial index of 0.4 or lower, an ankle systolic blood pressure of 70 mm Hg or less or a toe systolic blood pressure of 50 or less, and were poor candidates for surgical revascularization. None had Buerger’s disease.
Participants were randomized to one of four study arms, and within each study arm further randomized 3:1 to stromal cell–derived factor-1 (SDF-1) or placebo injections. The patients received either 8 or 16 injections, each containing either 0.5 or 1.0 mg of SDF-1 or placebo. The injections, given in a single session, were placed at least 0.5 cm apart throughout the ischemic area of the affected limb.
By chance, most patients randomized to the placebo group were Rutherford 4, a category of CLI defined by rest pain, while the majority in the active treatment arms were Rutherford 5, a more severe disease manifestation characterized by ulcers. As a consequence, the SDF-1 recipients also had far larger nonhealing wounds, with an average area of 6.4 cm2, compared with 1.5 cm2, in controls.
The SDF-1 injections proved safe and were well tolerated, with no treatment-related serious adverse events and no safety signals evident in the laboratory results.
Turning to efficacy endpoints, Dr. Kibbe said self-rated visual analog scale pain scores showed clear, dose-dependent improvement over time in the SDF-1 treatment cohorts and no change in controls.
Similarly, the active treatment groups showed improved quality of life scores on all domains of the Short Form-36: physical functioning, bodily pain, general health, social functioning, energy/fatigue, emotional well-being, and overall physical and mental health, the surgeon continued.
Wound area decreased significantly in the SDF-1-treated groups, with the biggest reduction – more than 8 cm2 – being noted in the three patients who received eight 1-mg injections. That was also the group with the largest wounds at baseline, with an average area of 11.4 cm2.
Of note, the major limb amputation rate was “remarkably low” for patients with such severe CLI, according to Dr. Kibbe. The rate was less than 10% over the course of 12 months, with one patient in each of the four active treatment arms having a major amputation at time intervals of 58-112 days post injection. No major limb amputations occurred in the control group.
There was a hint of improvement with SDF-1 therapy over placebo in ankle-brachial index and transcutaneous oxygen pressure, but the between-group differences were too narrow in this study to allow for any conclusions. That must await planned much larger phase III trials, according to Dr. Kibbe.
Audience members, citing the numerous failures of once-promising stem cell therapies for CLI at phase III testing over the last 10-15 years, wondered why Dr. Kibbe thinks SDF-1 will fare any better.
“This is much debated and discussed among all the people involved in these kinds of trials,” she replied. “I’d say, briefly, that a lot of it has to do with patient selection. I think when you have a mixed bag of patients in a trial, including patients with Buerger’s disease, treated in multiple different countries, using different definitions of when to amputate, all those things come into play and could account for why those phase III trials were not successful.”
“Having been involved in lots of the different gene- and cell-based therapy trials, I think one of the unique benefits of this therapy is that it kind of bridges between the two. SDF-1 basically homes your endothelial progenitor cells to the site of ischemic injury for enhanced vasculogenesis. But SDF-1 also has direct effects on endothelial cells, including stimulating proliferation and preventing apoptosis,” she added.
JVS-100 has also successfully completed a phase II clinical trial for the treatment of heart failure. In addition, the agent is being developed as a treatment for acute MI, chronic angina, and for muscle regeneration.
The STOP-CLI study was sponsored by Juventas Therapeutics. Dr. Kibbe reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia demonstrated safety and efficacy; the therapy is moving forward to phase III testing.
Major finding: The major limb amputation rate was less than 10% during 12 months of follow-up after a single dose of the novel therapy.
Data source: The STOP-CLI trial was a phase IIa, 12-month, randomized, double-blind, placebo-controlled, six-center trial including 48 patients with critical limb ischemia.
Disclosures: The STOP-CLI trial was sponsored by Juventas Therapeutics. The presenter reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
Zero coronary calcium means very low 10-year event risk
CHICAGO – Absence of coronary artery calcium upon imaging results in an impressively low cardiovascular event rate over the next 10 years regardless of an individual’s level of standard risk factors, according to prospective data from the MESA study.
In contrast, a coronary artery calcium (CAC) score of 1-10, often described as minimal CAC, nearly doubles the 10-year risk, compared with a baseline CAC score of 0.
Prior to these new 10-year data, many cardiologists considered a CAC score of 1-10 as tantamount to no CAC. Not so, Dr. Parag H. Joshi said at the American Heart Association scientific sessions.
“A CAC of 0 is presumably identifying someone without any atherosclerosis. Just the presence of minimal calcium suggests that atherosclerosis is building up. Our data suggest that among individuals with a CAC of 1-10, current smoking, elevated non-HDL cholesterol, and particularly hypertension should be treated aggressively,” said Dr. Joshi, a clinical fellow in cardiovascular diseases and prevention at Johns Hopkins University, Baltimore.
Prior studies totaling more than 50,000 subjects with a CAC score of 0 have shown very low cardiovascular event rates over 4-5 years of follow-up. However, current cardiovascular risk estimates focus on 10-year risk. This new analysis from MESA (Multi-Ethnic Study of Atherosclerosis) is the first study to provide prospective, 10-year events data, and those data are highly reassuring, he added.
MESA is a prospective, population-based cohort study. This analysis included 6,814 subjects aged 45-84 who were free of clinical cardiovascular disease at baseline, when their CAC score was determined. At that time, 3,415 participants had a CAC score of 0 and 508 had a score of 1-10.
During a median 10.3 years of follow-up, 123 cardiovascular events occurred, roughly one-third of which were nonfatal acute MIs and half of which were nonfatal strokes; the remainder were cardiovascular deaths.
The event rate was 2.9/1,000 person-years in subjects with a CAC of 0 and significantly greater at 5.5/1,000 person-years with a score of 1-10. However, since the cardiovascular risk factor profile of the zero CAC group was generally more favorable, Dr. Joshi and coinvestigators carried out a Cox proportional hazards analysis factoring in demographics, standard cardiovascular risk factors, body mass index, C-reactive protein level, and carotid intima media thickness. The adjusted 10-year event risk in the group with a CAC score of 1-10 was 1.9-fold greater than with a CAC of 0.
The highest 10-year event rate was noted in subjects with at least three of the following four risk factors at baseline: hypertension, current smoking, diabetes, and hyperlipidemia. The rate was 6.5/1,000 person-years in such individuals if they had a CAC of 0 and doubled at 13.1/1,000 person-years with a score of 1-10.
In a multivariate Cox analysis, age, smoking, and hypertension proved to be significant predictors of cardiovascular events in the group with a CAC of 0 as well as in those with a CAC of 1-10. But there was one important difference between the two groups: While the hazard ratio for cardiovascular events associated with hypertension versus no hypertension was 2.1 in subjects with a CAC of 0, the presence of hypertension in individuals with a CAC of 1-10 increased their event risk by 10.2-fold, or nearly five times greater than the risk increase associated with hypertension in persons with a CAC of 0, Dr. Joshi observed.
Non–HDL cholesterol level was predictive of cardiovascular risk in subjects with a CAC of 1-10 but not in those with a score of 0.
When actual event rates were compared with those predicted by the atherosclerotic cardiovascular disease (ASCVD) risk estimator introduced in the 2013 AHA/American College of Cardiology cholesterol guidelines, the event rate in subjects with an ASCVD 10-year risk estimate of 7.5%-15% but a CAC of 0 was just 4.4%.
Audience members noted that CAC scores didn’t do a very good job of stratifying stroke risk in MESA. That’s not surprising, since the score reflects coronary but not carotid artery calcium. But it is a limitation of CAC as a predictive tool, especially in light of the fact that strokes accounted for half of all cardiovascular events in the study.
Asked where he and his coinvestigators plan to go from here, Dr. Joshi said a randomized, controlled trial would be ideal, but to date funding isn’t available. However, the observational data from MESA and other studies suggest such a trial may not even be needed.
“Certainly the guidelines do allow for CAC scoring to be used in clinical decision making,” he noted.
The MESA study is funded by the National Heart, Lung, and Blood Institute. Dr. Joshi reported having no financial conflicts.
CHICAGO – Absence of coronary artery calcium upon imaging results in an impressively low cardiovascular event rate over the next 10 years regardless of an individual’s level of standard risk factors, according to prospective data from the MESA study.
In contrast, a coronary artery calcium (CAC) score of 1-10, often described as minimal CAC, nearly doubles the 10-year risk, compared with a baseline CAC score of 0.
Prior to these new 10-year data, many cardiologists considered a CAC score of 1-10 as tantamount to no CAC. Not so, Dr. Parag H. Joshi said at the American Heart Association scientific sessions.
“A CAC of 0 is presumably identifying someone without any atherosclerosis. Just the presence of minimal calcium suggests that atherosclerosis is building up. Our data suggest that among individuals with a CAC of 1-10, current smoking, elevated non-HDL cholesterol, and particularly hypertension should be treated aggressively,” said Dr. Joshi, a clinical fellow in cardiovascular diseases and prevention at Johns Hopkins University, Baltimore.
Prior studies totaling more than 50,000 subjects with a CAC score of 0 have shown very low cardiovascular event rates over 4-5 years of follow-up. However, current cardiovascular risk estimates focus on 10-year risk. This new analysis from MESA (Multi-Ethnic Study of Atherosclerosis) is the first study to provide prospective, 10-year events data, and those data are highly reassuring, he added.
MESA is a prospective, population-based cohort study. This analysis included 6,814 subjects aged 45-84 who were free of clinical cardiovascular disease at baseline, when their CAC score was determined. At that time, 3,415 participants had a CAC score of 0 and 508 had a score of 1-10.
During a median 10.3 years of follow-up, 123 cardiovascular events occurred, roughly one-third of which were nonfatal acute MIs and half of which were nonfatal strokes; the remainder were cardiovascular deaths.
The event rate was 2.9/1,000 person-years in subjects with a CAC of 0 and significantly greater at 5.5/1,000 person-years with a score of 1-10. However, since the cardiovascular risk factor profile of the zero CAC group was generally more favorable, Dr. Joshi and coinvestigators carried out a Cox proportional hazards analysis factoring in demographics, standard cardiovascular risk factors, body mass index, C-reactive protein level, and carotid intima media thickness. The adjusted 10-year event risk in the group with a CAC score of 1-10 was 1.9-fold greater than with a CAC of 0.
The highest 10-year event rate was noted in subjects with at least three of the following four risk factors at baseline: hypertension, current smoking, diabetes, and hyperlipidemia. The rate was 6.5/1,000 person-years in such individuals if they had a CAC of 0 and doubled at 13.1/1,000 person-years with a score of 1-10.
In a multivariate Cox analysis, age, smoking, and hypertension proved to be significant predictors of cardiovascular events in the group with a CAC of 0 as well as in those with a CAC of 1-10. But there was one important difference between the two groups: While the hazard ratio for cardiovascular events associated with hypertension versus no hypertension was 2.1 in subjects with a CAC of 0, the presence of hypertension in individuals with a CAC of 1-10 increased their event risk by 10.2-fold, or nearly five times greater than the risk increase associated with hypertension in persons with a CAC of 0, Dr. Joshi observed.
Non–HDL cholesterol level was predictive of cardiovascular risk in subjects with a CAC of 1-10 but not in those with a score of 0.
When actual event rates were compared with those predicted by the atherosclerotic cardiovascular disease (ASCVD) risk estimator introduced in the 2013 AHA/American College of Cardiology cholesterol guidelines, the event rate in subjects with an ASCVD 10-year risk estimate of 7.5%-15% but a CAC of 0 was just 4.4%.
Audience members noted that CAC scores didn’t do a very good job of stratifying stroke risk in MESA. That’s not surprising, since the score reflects coronary but not carotid artery calcium. But it is a limitation of CAC as a predictive tool, especially in light of the fact that strokes accounted for half of all cardiovascular events in the study.
Asked where he and his coinvestigators plan to go from here, Dr. Joshi said a randomized, controlled trial would be ideal, but to date funding isn’t available. However, the observational data from MESA and other studies suggest such a trial may not even be needed.
“Certainly the guidelines do allow for CAC scoring to be used in clinical decision making,” he noted.
The MESA study is funded by the National Heart, Lung, and Blood Institute. Dr. Joshi reported having no financial conflicts.
CHICAGO – Absence of coronary artery calcium upon imaging results in an impressively low cardiovascular event rate over the next 10 years regardless of an individual’s level of standard risk factors, according to prospective data from the MESA study.
In contrast, a coronary artery calcium (CAC) score of 1-10, often described as minimal CAC, nearly doubles the 10-year risk, compared with a baseline CAC score of 0.
Prior to these new 10-year data, many cardiologists considered a CAC score of 1-10 as tantamount to no CAC. Not so, Dr. Parag H. Joshi said at the American Heart Association scientific sessions.
“A CAC of 0 is presumably identifying someone without any atherosclerosis. Just the presence of minimal calcium suggests that atherosclerosis is building up. Our data suggest that among individuals with a CAC of 1-10, current smoking, elevated non-HDL cholesterol, and particularly hypertension should be treated aggressively,” said Dr. Joshi, a clinical fellow in cardiovascular diseases and prevention at Johns Hopkins University, Baltimore.
Prior studies totaling more than 50,000 subjects with a CAC score of 0 have shown very low cardiovascular event rates over 4-5 years of follow-up. However, current cardiovascular risk estimates focus on 10-year risk. This new analysis from MESA (Multi-Ethnic Study of Atherosclerosis) is the first study to provide prospective, 10-year events data, and those data are highly reassuring, he added.
MESA is a prospective, population-based cohort study. This analysis included 6,814 subjects aged 45-84 who were free of clinical cardiovascular disease at baseline, when their CAC score was determined. At that time, 3,415 participants had a CAC score of 0 and 508 had a score of 1-10.
During a median 10.3 years of follow-up, 123 cardiovascular events occurred, roughly one-third of which were nonfatal acute MIs and half of which were nonfatal strokes; the remainder were cardiovascular deaths.
The event rate was 2.9/1,000 person-years in subjects with a CAC of 0 and significantly greater at 5.5/1,000 person-years with a score of 1-10. However, since the cardiovascular risk factor profile of the zero CAC group was generally more favorable, Dr. Joshi and coinvestigators carried out a Cox proportional hazards analysis factoring in demographics, standard cardiovascular risk factors, body mass index, C-reactive protein level, and carotid intima media thickness. The adjusted 10-year event risk in the group with a CAC score of 1-10 was 1.9-fold greater than with a CAC of 0.
The highest 10-year event rate was noted in subjects with at least three of the following four risk factors at baseline: hypertension, current smoking, diabetes, and hyperlipidemia. The rate was 6.5/1,000 person-years in such individuals if they had a CAC of 0 and doubled at 13.1/1,000 person-years with a score of 1-10.
In a multivariate Cox analysis, age, smoking, and hypertension proved to be significant predictors of cardiovascular events in the group with a CAC of 0 as well as in those with a CAC of 1-10. But there was one important difference between the two groups: While the hazard ratio for cardiovascular events associated with hypertension versus no hypertension was 2.1 in subjects with a CAC of 0, the presence of hypertension in individuals with a CAC of 1-10 increased their event risk by 10.2-fold, or nearly five times greater than the risk increase associated with hypertension in persons with a CAC of 0, Dr. Joshi observed.
Non–HDL cholesterol level was predictive of cardiovascular risk in subjects with a CAC of 1-10 but not in those with a score of 0.
When actual event rates were compared with those predicted by the atherosclerotic cardiovascular disease (ASCVD) risk estimator introduced in the 2013 AHA/American College of Cardiology cholesterol guidelines, the event rate in subjects with an ASCVD 10-year risk estimate of 7.5%-15% but a CAC of 0 was just 4.4%.
Audience members noted that CAC scores didn’t do a very good job of stratifying stroke risk in MESA. That’s not surprising, since the score reflects coronary but not carotid artery calcium. But it is a limitation of CAC as a predictive tool, especially in light of the fact that strokes accounted for half of all cardiovascular events in the study.
Asked where he and his coinvestigators plan to go from here, Dr. Joshi said a randomized, controlled trial would be ideal, but to date funding isn’t available. However, the observational data from MESA and other studies suggest such a trial may not even be needed.
“Certainly the guidelines do allow for CAC scoring to be used in clinical decision making,” he noted.
The MESA study is funded by the National Heart, Lung, and Blood Institute. Dr. Joshi reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: A coronary artery calcium score of 0 appears to trump the 10-year atherosclerotic cardiovascular disease risk estimator introduced in the 2013 AHA/ACC cholesterol guidelines.
Major finding: The actual 10-year cardiovascular event rate in subjects with a coronary artery calcium score of 0 was just 4.4% – below the guideline-recommended threshold for statin therapy– even though their predicted risk using the AHA/ACC risk estimator was 7.5%-15%.
Data source: The Multi-Ethnic Study of Atherosclerosis is a prospective, population-based cohort study. This analysis included 6,814 subjects aged 45-84 who were free of clinical cardiovascular disease at baseline.
Disclosures: The MESA study is funded by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts.
Transfusion linked to bad outcomes in percutaneous peripheral vascular interventions
CHICAGO – Periprocedural blood transfusion rates vary greatly among hospitals performing similar percutaneous interventions for peripheral arterial disease, but these rates can be markedly reduced via a focused quality improvement program.
That’s been the lesson learned in Michigan, where blood transfusion rates dropped by 52% statewide at the 44 hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 PCI-VIC), Dr. Peter K. Henke reported at the American Heart Association scientific sessions.
That’s good news because periprocedural blood transfusions in patients undergoing percutaneous interventions for peripheral arterial disease (PAD) are associated with startlingly high major morbidity and mortality rates. Indeed, among 18,127 patients undergoing nonhybrid percutaneous interventions for PAD in the BMC2 PCI-VIC registry, periprocedural blood transfusion was an independent predictor of a 25-fold increased risk of MI, a 12.7-fold increase in in-hospital mortality, a 6-fold increased risk of TIA/stroke, and a 49-fold increase in vascular access complications in a logistic regression analysis adjusted for patient demographics, comorbid disease states, and periprocedural medications, according to Dr. Henke, professor of vascular surgery at the University of Michigan, Ann Arbor.
That being said, he was quick to add that he believes these associations largely reflect correlation, not causality. Transfusion recipients were significantly older and sicker than nontransfused patients undergoing the same percutaneous peripheral vascular interventions. They were far more likely to have critical limb ischemia and undergo an urgent or emergent procedure. Of note, as statewide transfusion rates fell from about 6.6% to 3.2% in response to the quality improvement program, crude in-hospital mortality didn’t change significantly, again suggesting a noncausal relationship.
The quality improvement project was undertaken in response to the observation that periprocedural transfusion rates varied institutionally across the state from 0% to 14% for patients undergoing the same percutaneous interventions for PAD. That was a red flag indicating an opportunity for improved practice.
“The median nadir hemoglobin varied within a rather narrow range of 6.8-8.5 g/dL, yet the transfusion rates were quite wide ranging,” the surgeon observed.
Over a 2-year period, the BMC2 PCI-VIC quality improvement team made repeated site visits to the hospitals with the lowest transfusion rates. They performed detailed analysis of peripheral vascular procedure processes, protocols, and order sets in order to identify best practices. Those best practices were then shared at meetings with representatives of all the participating hospitals. Feedback was provided. And transfusion rates began dropping.
Analysis of the 18,000-plus patients enrolled in the registry led to identification of a specific set of risk factors for blood transfusions, most of which occurred after patients had left the catheterization lab. These risk factors included low creatinine clearance, preprocedural anemia, chronic obstructive pulmonary disease, use of warfarin, cerebrovascular disease, critical limb ischemia, and urgent or emergent procedures.
This was the largest-ever study focused on transfusion in patients undergoing endovascular procedures for PAD, according to Dr. Henke. He noted that the results are consistent with a recent report by other investigators regarding the implications of periprocedural blood transfusion in patients undergoing percutaneous coronary intervention. In more than 2.2 million patients who underwent PCI in 2009-2013, transfusion rates varied institutionally from 0% to 13%. Transfusion was associated with 4.6-fold in-hospital mortality, a 3.6-fold increase in acute MI, and a 7.7-fold increased risk of stroke (JAMA 2014;311:836-43).
Dr. Henke reported no financial conflicts of interest regarding the PAD transfusion study, which was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network.
CHICAGO – Periprocedural blood transfusion rates vary greatly among hospitals performing similar percutaneous interventions for peripheral arterial disease, but these rates can be markedly reduced via a focused quality improvement program.
That’s been the lesson learned in Michigan, where blood transfusion rates dropped by 52% statewide at the 44 hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 PCI-VIC), Dr. Peter K. Henke reported at the American Heart Association scientific sessions.
That’s good news because periprocedural blood transfusions in patients undergoing percutaneous interventions for peripheral arterial disease (PAD) are associated with startlingly high major morbidity and mortality rates. Indeed, among 18,127 patients undergoing nonhybrid percutaneous interventions for PAD in the BMC2 PCI-VIC registry, periprocedural blood transfusion was an independent predictor of a 25-fold increased risk of MI, a 12.7-fold increase in in-hospital mortality, a 6-fold increased risk of TIA/stroke, and a 49-fold increase in vascular access complications in a logistic regression analysis adjusted for patient demographics, comorbid disease states, and periprocedural medications, according to Dr. Henke, professor of vascular surgery at the University of Michigan, Ann Arbor.
That being said, he was quick to add that he believes these associations largely reflect correlation, not causality. Transfusion recipients were significantly older and sicker than nontransfused patients undergoing the same percutaneous peripheral vascular interventions. They were far more likely to have critical limb ischemia and undergo an urgent or emergent procedure. Of note, as statewide transfusion rates fell from about 6.6% to 3.2% in response to the quality improvement program, crude in-hospital mortality didn’t change significantly, again suggesting a noncausal relationship.
The quality improvement project was undertaken in response to the observation that periprocedural transfusion rates varied institutionally across the state from 0% to 14% for patients undergoing the same percutaneous interventions for PAD. That was a red flag indicating an opportunity for improved practice.
“The median nadir hemoglobin varied within a rather narrow range of 6.8-8.5 g/dL, yet the transfusion rates were quite wide ranging,” the surgeon observed.
Over a 2-year period, the BMC2 PCI-VIC quality improvement team made repeated site visits to the hospitals with the lowest transfusion rates. They performed detailed analysis of peripheral vascular procedure processes, protocols, and order sets in order to identify best practices. Those best practices were then shared at meetings with representatives of all the participating hospitals. Feedback was provided. And transfusion rates began dropping.
Analysis of the 18,000-plus patients enrolled in the registry led to identification of a specific set of risk factors for blood transfusions, most of which occurred after patients had left the catheterization lab. These risk factors included low creatinine clearance, preprocedural anemia, chronic obstructive pulmonary disease, use of warfarin, cerebrovascular disease, critical limb ischemia, and urgent or emergent procedures.
This was the largest-ever study focused on transfusion in patients undergoing endovascular procedures for PAD, according to Dr. Henke. He noted that the results are consistent with a recent report by other investigators regarding the implications of periprocedural blood transfusion in patients undergoing percutaneous coronary intervention. In more than 2.2 million patients who underwent PCI in 2009-2013, transfusion rates varied institutionally from 0% to 13%. Transfusion was associated with 4.6-fold in-hospital mortality, a 3.6-fold increase in acute MI, and a 7.7-fold increased risk of stroke (JAMA 2014;311:836-43).
Dr. Henke reported no financial conflicts of interest regarding the PAD transfusion study, which was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network.
CHICAGO – Periprocedural blood transfusion rates vary greatly among hospitals performing similar percutaneous interventions for peripheral arterial disease, but these rates can be markedly reduced via a focused quality improvement program.
That’s been the lesson learned in Michigan, where blood transfusion rates dropped by 52% statewide at the 44 hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 PCI-VIC), Dr. Peter K. Henke reported at the American Heart Association scientific sessions.
That’s good news because periprocedural blood transfusions in patients undergoing percutaneous interventions for peripheral arterial disease (PAD) are associated with startlingly high major morbidity and mortality rates. Indeed, among 18,127 patients undergoing nonhybrid percutaneous interventions for PAD in the BMC2 PCI-VIC registry, periprocedural blood transfusion was an independent predictor of a 25-fold increased risk of MI, a 12.7-fold increase in in-hospital mortality, a 6-fold increased risk of TIA/stroke, and a 49-fold increase in vascular access complications in a logistic regression analysis adjusted for patient demographics, comorbid disease states, and periprocedural medications, according to Dr. Henke, professor of vascular surgery at the University of Michigan, Ann Arbor.
That being said, he was quick to add that he believes these associations largely reflect correlation, not causality. Transfusion recipients were significantly older and sicker than nontransfused patients undergoing the same percutaneous peripheral vascular interventions. They were far more likely to have critical limb ischemia and undergo an urgent or emergent procedure. Of note, as statewide transfusion rates fell from about 6.6% to 3.2% in response to the quality improvement program, crude in-hospital mortality didn’t change significantly, again suggesting a noncausal relationship.
The quality improvement project was undertaken in response to the observation that periprocedural transfusion rates varied institutionally across the state from 0% to 14% for patients undergoing the same percutaneous interventions for PAD. That was a red flag indicating an opportunity for improved practice.
“The median nadir hemoglobin varied within a rather narrow range of 6.8-8.5 g/dL, yet the transfusion rates were quite wide ranging,” the surgeon observed.
Over a 2-year period, the BMC2 PCI-VIC quality improvement team made repeated site visits to the hospitals with the lowest transfusion rates. They performed detailed analysis of peripheral vascular procedure processes, protocols, and order sets in order to identify best practices. Those best practices were then shared at meetings with representatives of all the participating hospitals. Feedback was provided. And transfusion rates began dropping.
Analysis of the 18,000-plus patients enrolled in the registry led to identification of a specific set of risk factors for blood transfusions, most of which occurred after patients had left the catheterization lab. These risk factors included low creatinine clearance, preprocedural anemia, chronic obstructive pulmonary disease, use of warfarin, cerebrovascular disease, critical limb ischemia, and urgent or emergent procedures.
This was the largest-ever study focused on transfusion in patients undergoing endovascular procedures for PAD, according to Dr. Henke. He noted that the results are consistent with a recent report by other investigators regarding the implications of periprocedural blood transfusion in patients undergoing percutaneous coronary intervention. In more than 2.2 million patients who underwent PCI in 2009-2013, transfusion rates varied institutionally from 0% to 13%. Transfusion was associated with 4.6-fold in-hospital mortality, a 3.6-fold increase in acute MI, and a 7.7-fold increased risk of stroke (JAMA 2014;311:836-43).
Dr. Henke reported no financial conflicts of interest regarding the PAD transfusion study, which was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: High institutional blood transfusion rates in conjunction with percutaneous interventions for peripheral arterial disease can be sharply and safely lowered through a focused quality improvement program.
Major finding: The average periprocedural transfusion rate at 44 Michigan hospitals fell from 6.6% to 3.2% in response to the performance improvement program.
Data source: A retrospective analysis of prospectively gathered data on 18,127 Michigan patients who underwent nonhybrid percutaneous interventions for peripheral arterial disease.
Disclosures: The study was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network. The presenter reported having no financial conflicts.
How physicians are using ‘the power of zero’ in primary prevention
CHICAGO – Coronary artery calcium testing has established itself as a true “game changer” in primary cardiovascular prevention, proponents of the risk-stratification tool said at the American Heart Association scientific sessions.
Knowing a patient’s coronary artery calcium score facilitates a more informed physician-patient discussion and shared decision making regarding whether to go on decades-long statin therapy, according to Dr. Khurram Nasir of the center for prevention and wellness research at Baptist Health Medical Center in Miami Beach.
“In our view, a much underappreciated value of coronary artery calcium testing lies in the power of zero. Roughly half of adults have a coronary artery calcium score of 0, and this results in a very low cardiovascular event rate,” the cardiologist said.
He presented an analysis of 4,758 nondiabetic participants in the prospective, population-based MESA (Multi-Ethnic Study of Atherosclerosis) in which he examined how they fared in terms of cardiovascular events over a median 10.3 years of follow-up. All were free of known cardiovascular disease at baseline. With the risk estimator included in the 2013 AHA/ACC cholesterol management guidelines, 2,377 subjects would be recommended for high-intensity statin therapy at baseline on the basis of a 10-year atherosclerotic cardiovascular disease risk estimate of at least 7.5%. Another 589 participants were recommended for consideration of a moderate-intensity statin based on an estimated 10-year risk of 5%-7.4%.
Forty-one percent of MESA subjects recommended for a high-intensity statin according to the AHA/ACC risk estimator had a coronary artery calcium (CAC) score of 0, and their 10-year composite rate of MI, stroke, or cardiovascular death was just 4.9% – well below the 7.5% threshold recommended for statin therapy. In contrast, if any CAC was present, the event rate was 10.5%.
With a relative risk reduction with statin therapy of 30%, the number needed to treat for 5 years to prevent one cardiovascular event in the group with a CAC of 0 would be 128. In the presence of any CAC, the number needed to treat fell to a far more reasonable 56, Dr. Nasir said.
Similarly, among the group recommended for consideration of statin therapy on the basis of a 10-year risk of 5%-7.4%, the actual event rate in the 57% of subjects with a CAC of 0 was just 1.5%. If any CAC was present, the event rate shot up to 7.2%. The number needed to treat in this cohort was 445 among those with a CAC of 0 and 90 with any CAC present.
“I think coronary artery calcium is a game changer in primary prevention,” Dr. Michael J. Blaha commented. “It sufficiently moves the needle to make you think differently about a patient. I’m not sure some of the other tests have sufficient evidence to say, ‘I’m going to think about not treating you if it’s negative and treating you if it’s positive,’ but coronary artery calcium has that evidence.”
In his own cardiology practice at Johns Hopkins University, Baltimore, Dr. Blaha finds himself using CAC testing often, especially in his many statin-reluctant patients.
“I have a lot of patients who would fit under a recommendation for statin therapy under the 2013 AHA/ACC cholesterol management guidelines, but who really don’t want to take medications. I know you see these patients in your practices, too. This is lifelong therapy, and they want a really good reason to take it or not to take it. If a patient is reluctant to take a statin and has a CAC score of 0, I will sometimes emphasize lifestyle therapy. It certainly redoubles my interest in lifestyle therapy. But if the CAC score is elevated, then I can make a specific case that the number needed to treat is very favorable, compared to the number needed to harm,” explained Dr. Blaha, a coinvestigator with Dr. Nasir in the MESA study.
Other situations where he finds CAC testing useful in daily practice include uncertainty as to a patient’s true risk level because the individual’s situation isn’t adequately captured by the AHA/ACC risk estimator. A patient with rheumatologic disease would be one example; another would be an individual who is neither white nor African American. He said he also utilizes CAC testing in statin-intolerant patients, where the results are useful in deciding how many different statins to try before saying “enough.”
Audience members asked what it’s going to take to get insurers to cover CAC testing for risk stratification. Dr. Blaha replied that more long-term outcomes and cost-effectiveness data are coming. In the meantime, at an out-of-pocket cost of $75-$100, a lot of his statin-reluctant patients consider CAC testing a good buy.
“They say, ‘I’ll take this test to help me decide whether to take a pill for the rest of my life,’” according to Dr. Blaha.
Dr. Nasir said the evidence in support of CAC testing is now so strong that he believes physicians have an obligation to mention it as an option during the statin treatment decision discussion.
“At this moment, most patients are making their decision based on the guesstimate of their risk we are giving them using the risk calculator. If they have the ability through a $75-$100 test that costs about the same as 18 months of statin therapy to know that their true risk is not, say, 10%, but actually 5%, they’re less likely to choose therapy. Is it even ethical to withhold from our patients that there is a test out there that can reduce their estimated risk to a point that they can avoid statin therapy?” the cardiologist asked.
Dr. Nasir reported serving on an advisory board for Quest Diagnostics. Dr. Blaha reported having no financial conflicts.
CHICAGO – Coronary artery calcium testing has established itself as a true “game changer” in primary cardiovascular prevention, proponents of the risk-stratification tool said at the American Heart Association scientific sessions.
Knowing a patient’s coronary artery calcium score facilitates a more informed physician-patient discussion and shared decision making regarding whether to go on decades-long statin therapy, according to Dr. Khurram Nasir of the center for prevention and wellness research at Baptist Health Medical Center in Miami Beach.
“In our view, a much underappreciated value of coronary artery calcium testing lies in the power of zero. Roughly half of adults have a coronary artery calcium score of 0, and this results in a very low cardiovascular event rate,” the cardiologist said.
He presented an analysis of 4,758 nondiabetic participants in the prospective, population-based MESA (Multi-Ethnic Study of Atherosclerosis) in which he examined how they fared in terms of cardiovascular events over a median 10.3 years of follow-up. All were free of known cardiovascular disease at baseline. With the risk estimator included in the 2013 AHA/ACC cholesterol management guidelines, 2,377 subjects would be recommended for high-intensity statin therapy at baseline on the basis of a 10-year atherosclerotic cardiovascular disease risk estimate of at least 7.5%. Another 589 participants were recommended for consideration of a moderate-intensity statin based on an estimated 10-year risk of 5%-7.4%.
Forty-one percent of MESA subjects recommended for a high-intensity statin according to the AHA/ACC risk estimator had a coronary artery calcium (CAC) score of 0, and their 10-year composite rate of MI, stroke, or cardiovascular death was just 4.9% – well below the 7.5% threshold recommended for statin therapy. In contrast, if any CAC was present, the event rate was 10.5%.
With a relative risk reduction with statin therapy of 30%, the number needed to treat for 5 years to prevent one cardiovascular event in the group with a CAC of 0 would be 128. In the presence of any CAC, the number needed to treat fell to a far more reasonable 56, Dr. Nasir said.
Similarly, among the group recommended for consideration of statin therapy on the basis of a 10-year risk of 5%-7.4%, the actual event rate in the 57% of subjects with a CAC of 0 was just 1.5%. If any CAC was present, the event rate shot up to 7.2%. The number needed to treat in this cohort was 445 among those with a CAC of 0 and 90 with any CAC present.
“I think coronary artery calcium is a game changer in primary prevention,” Dr. Michael J. Blaha commented. “It sufficiently moves the needle to make you think differently about a patient. I’m not sure some of the other tests have sufficient evidence to say, ‘I’m going to think about not treating you if it’s negative and treating you if it’s positive,’ but coronary artery calcium has that evidence.”
In his own cardiology practice at Johns Hopkins University, Baltimore, Dr. Blaha finds himself using CAC testing often, especially in his many statin-reluctant patients.
“I have a lot of patients who would fit under a recommendation for statin therapy under the 2013 AHA/ACC cholesterol management guidelines, but who really don’t want to take medications. I know you see these patients in your practices, too. This is lifelong therapy, and they want a really good reason to take it or not to take it. If a patient is reluctant to take a statin and has a CAC score of 0, I will sometimes emphasize lifestyle therapy. It certainly redoubles my interest in lifestyle therapy. But if the CAC score is elevated, then I can make a specific case that the number needed to treat is very favorable, compared to the number needed to harm,” explained Dr. Blaha, a coinvestigator with Dr. Nasir in the MESA study.
Other situations where he finds CAC testing useful in daily practice include uncertainty as to a patient’s true risk level because the individual’s situation isn’t adequately captured by the AHA/ACC risk estimator. A patient with rheumatologic disease would be one example; another would be an individual who is neither white nor African American. He said he also utilizes CAC testing in statin-intolerant patients, where the results are useful in deciding how many different statins to try before saying “enough.”
Audience members asked what it’s going to take to get insurers to cover CAC testing for risk stratification. Dr. Blaha replied that more long-term outcomes and cost-effectiveness data are coming. In the meantime, at an out-of-pocket cost of $75-$100, a lot of his statin-reluctant patients consider CAC testing a good buy.
“They say, ‘I’ll take this test to help me decide whether to take a pill for the rest of my life,’” according to Dr. Blaha.
Dr. Nasir said the evidence in support of CAC testing is now so strong that he believes physicians have an obligation to mention it as an option during the statin treatment decision discussion.
“At this moment, most patients are making their decision based on the guesstimate of their risk we are giving them using the risk calculator. If they have the ability through a $75-$100 test that costs about the same as 18 months of statin therapy to know that their true risk is not, say, 10%, but actually 5%, they’re less likely to choose therapy. Is it even ethical to withhold from our patients that there is a test out there that can reduce their estimated risk to a point that they can avoid statin therapy?” the cardiologist asked.
Dr. Nasir reported serving on an advisory board for Quest Diagnostics. Dr. Blaha reported having no financial conflicts.
CHICAGO – Coronary artery calcium testing has established itself as a true “game changer” in primary cardiovascular prevention, proponents of the risk-stratification tool said at the American Heart Association scientific sessions.
Knowing a patient’s coronary artery calcium score facilitates a more informed physician-patient discussion and shared decision making regarding whether to go on decades-long statin therapy, according to Dr. Khurram Nasir of the center for prevention and wellness research at Baptist Health Medical Center in Miami Beach.
“In our view, a much underappreciated value of coronary artery calcium testing lies in the power of zero. Roughly half of adults have a coronary artery calcium score of 0, and this results in a very low cardiovascular event rate,” the cardiologist said.
He presented an analysis of 4,758 nondiabetic participants in the prospective, population-based MESA (Multi-Ethnic Study of Atherosclerosis) in which he examined how they fared in terms of cardiovascular events over a median 10.3 years of follow-up. All were free of known cardiovascular disease at baseline. With the risk estimator included in the 2013 AHA/ACC cholesterol management guidelines, 2,377 subjects would be recommended for high-intensity statin therapy at baseline on the basis of a 10-year atherosclerotic cardiovascular disease risk estimate of at least 7.5%. Another 589 participants were recommended for consideration of a moderate-intensity statin based on an estimated 10-year risk of 5%-7.4%.
Forty-one percent of MESA subjects recommended for a high-intensity statin according to the AHA/ACC risk estimator had a coronary artery calcium (CAC) score of 0, and their 10-year composite rate of MI, stroke, or cardiovascular death was just 4.9% – well below the 7.5% threshold recommended for statin therapy. In contrast, if any CAC was present, the event rate was 10.5%.
With a relative risk reduction with statin therapy of 30%, the number needed to treat for 5 years to prevent one cardiovascular event in the group with a CAC of 0 would be 128. In the presence of any CAC, the number needed to treat fell to a far more reasonable 56, Dr. Nasir said.
Similarly, among the group recommended for consideration of statin therapy on the basis of a 10-year risk of 5%-7.4%, the actual event rate in the 57% of subjects with a CAC of 0 was just 1.5%. If any CAC was present, the event rate shot up to 7.2%. The number needed to treat in this cohort was 445 among those with a CAC of 0 and 90 with any CAC present.
“I think coronary artery calcium is a game changer in primary prevention,” Dr. Michael J. Blaha commented. “It sufficiently moves the needle to make you think differently about a patient. I’m not sure some of the other tests have sufficient evidence to say, ‘I’m going to think about not treating you if it’s negative and treating you if it’s positive,’ but coronary artery calcium has that evidence.”
In his own cardiology practice at Johns Hopkins University, Baltimore, Dr. Blaha finds himself using CAC testing often, especially in his many statin-reluctant patients.
“I have a lot of patients who would fit under a recommendation for statin therapy under the 2013 AHA/ACC cholesterol management guidelines, but who really don’t want to take medications. I know you see these patients in your practices, too. This is lifelong therapy, and they want a really good reason to take it or not to take it. If a patient is reluctant to take a statin and has a CAC score of 0, I will sometimes emphasize lifestyle therapy. It certainly redoubles my interest in lifestyle therapy. But if the CAC score is elevated, then I can make a specific case that the number needed to treat is very favorable, compared to the number needed to harm,” explained Dr. Blaha, a coinvestigator with Dr. Nasir in the MESA study.
Other situations where he finds CAC testing useful in daily practice include uncertainty as to a patient’s true risk level because the individual’s situation isn’t adequately captured by the AHA/ACC risk estimator. A patient with rheumatologic disease would be one example; another would be an individual who is neither white nor African American. He said he also utilizes CAC testing in statin-intolerant patients, where the results are useful in deciding how many different statins to try before saying “enough.”
Audience members asked what it’s going to take to get insurers to cover CAC testing for risk stratification. Dr. Blaha replied that more long-term outcomes and cost-effectiveness data are coming. In the meantime, at an out-of-pocket cost of $75-$100, a lot of his statin-reluctant patients consider CAC testing a good buy.
“They say, ‘I’ll take this test to help me decide whether to take a pill for the rest of my life,’” according to Dr. Blaha.
Dr. Nasir said the evidence in support of CAC testing is now so strong that he believes physicians have an obligation to mention it as an option during the statin treatment decision discussion.
“At this moment, most patients are making their decision based on the guesstimate of their risk we are giving them using the risk calculator. If they have the ability through a $75-$100 test that costs about the same as 18 months of statin therapy to know that their true risk is not, say, 10%, but actually 5%, they’re less likely to choose therapy. Is it even ethical to withhold from our patients that there is a test out there that can reduce their estimated risk to a point that they can avoid statin therapy?” the cardiologist asked.
Dr. Nasir reported serving on an advisory board for Quest Diagnostics. Dr. Blaha reported having no financial conflicts.
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
WOSCOPS 20-year follow-up shows impressive statin ‘legacy effect’
CHICAGO – Five years of statin therapy for primary prevention provided an impressive lifetime benefit expressed as reduced risks of a range of cardiovascular disease outcomes in a 20-year follow-up of the landmark WOSCOPS trial.
“There is a rather remarkable persistence of benefit in terms of risk reduction over a long period. You’ve changed the natural history of the disease in some way by lowering LDL,” Dr. Chris J. Packard said in presenting the 20-year WOSCOPS (West of Scotland Coronary Prevention Study) follow-up at the American Heart Association scientific sessions.
The primary prevention study randomized 6,595 middle-aged Scotsmen with an average baseline LDL cholesterol of 190 mg/dL to 4 years of pravastatin at 40 mg/day or placebo. This was one of the early large statin trials, and publication of the 5-year outcomes showing a 31% reduction in the relative risk of cardiovascular death or MI (N. Engl. J. Med. 1995;333:1301-8) caused a great stir.
At that point, WOSCOPS leaders advised study participants’ primary care physicians to seriously consider putting their patients on long-term statin therapy. However, only an identically low 31% of subjects in each of the two study arms did so.
Because the Scottish national health care system effectively captures all utilization of medical services, Dr. Packard and coinvestigators were able to analyze 20-year outcomes in the former study participants. No other major statin trial has come close in terms of length of reported follow-up.

At 20 years, with the only treatment difference between the two original study arms being that the pravastatin group had been on statin therapy for an additional 5 years, the 20-year coronary heart disease mortality rate in the original statin group was reduced by 27%, compared with the original controls. All-cause mortality was reduced by 13%. And numerous other benefits were noted at 20 years with 5 years of statin therapy.
Indeed, patients treated with pravastatin for 5 years during the trial gained an average of 5 extra years free of nonfatal MI or cardiovascular death at the 20-year mark, he said.
“The average age of the men was 55 years during the trial and 20 years on they’re now 75 years old. This covers the entire period of premature cardiovascular morbidity and mortality. We would argue that this is a good picture of the lifetime benefit, which is different from lifetime risk. This is real events happening to real people, not predictions,” observed Dr. Packard, professor of cardiovascular and medical sciences at the University of Glasgow, Scotland.
The group given pravastatin for 5 years collectively spent 24,038 days in the hospital for any cardiovascular event, compared with 30,342 days for controls.
Particularly noteworthy was the divergence in the risk of heart failure, with 96 cases being diagnosed during 20 years in patients who received 5 years of pravastatin, compared with 128 in controls.
“Heart failure was our biggest surprise,” he said. “We got no result for heart failure at all at 5 years; these were middle-aged men with just high cholesterol, so we didn’t see much in the way of incident heart failure. But extrapolating 20 years, there is a 31% risk reduction in the incidence of hospitalization for heart failure in the statin-treated group, compared to the placebo-treated group. This is a remarkable finding.”
For WOSCOPS participants who got a 20% drop in LDL during 5 years of pravastatin from a baseline of 190 mg/dL, as was typical, the number of patients who needed to be treated (NNT) for 5 years to prevent one cardiovascular hospital admission over a 20-year period was six. Moreover, for every 10 patients on statin therapy for 5 years, an average of 19 hospital days were avoided over the course of 20 years. For patients with a baseline LDL of 120 mg/dL, the NNT was 10, and 12 hospital days were saved over a 20-year period for every 10 patients treated for 5 years.
“This is a real savings to the health service and the health care providers to offset the cost of drugs,” Dr. Packard continued.
He urged physicians to look beyond the initial reports of primary outcomes of the statin clinical trials and take a long-term view.
“If you take a lifetime approach to benefit so you’re looking not only at the first event, but the second event, the third, at heart failure, and at death, you can see tremendous benefits, whereas usually we only focus on the first event. And that’s not the full cost evaluation that you need to do,” according to Dr. Packard.
The overall incidence of cancer during 20 years of follow-up was 24.8% in the initial placebo arm and 24.5% in the pravastatin group, with no differences between the two groups in any type of cancer. Nor did the original pravastatin group show any increase in noncardiovascular mortality.
“This is a very important study – the first study to show a legacy effect, with reduced mortality and a gain of 5 event-free years over 20 years attributable to a 5-year treatment allocation,” said discussant Harvey White of Auckland (New Zealand) City Hospital.
This legacy effect, he added, can be viewed as an ongoing carryover effect related to statin-induced slowing and/or stabilization of existing coronary artery plaque. The mechanism is unknown, Dr. White said, but the key to why the legacy effect was seen in WOSCOPS despite the use of pravastatin – a less potent statin – but not to date in other statin trials may lie in the fact that WOSCOPS was a primary prevention study and its participants had the youngest mean age of all the major statin trials.
“Their plaques may not have been calcified yet and therefore were more able to be modified and stabilized. If you treat very early you might get a bigger effect,” said to Dr. White.
Undercutting that argument, however, was the WOSCOPS finding that the long-term benefits of 5 years of pravastatin were independent of age at treatment, Dr. Packard said.
He believes based upon other studies that statins’ coronary disease prevention benefits are expressed within the first 12 months after starting therapy.
“It suggests that whatever is happening to the pathobiology of atherosclerosis happens within a year, and somehow a statis is introduced into plaque. That’s my guess, that an unstable plaque is reduced to a stable one. People then form a new trajectory going forward and they never catch up. We should think of atherosclerosis as a rate effect rather than something that either happens or doesn’t happen. It’s a rate of happening,” Dr. Packard asserted.
Asked why in the aftermath of the strongly positive 5-year results of WOSCOPS only 31% of patients in each treatment arm were on long-term statin therapy, he replied that 20 years ago in Scotland there really was no push for primary prevention.
“The 4S trial had come out the year before [Lancet 1994;344:1383-9] and placed the focus on secondary prevention. Statins were relatively expensive and everybody was putting their money into secondary prevention. Our health care system, which is socialized, had not put any emphasis at all on primary prevention. We were actually amazed that even 31% got treated,” the physician explained.
Dr. Packard reported serving as a consultant to Merck, Roche, and AstraZeneca.
CHICAGO – Five years of statin therapy for primary prevention provided an impressive lifetime benefit expressed as reduced risks of a range of cardiovascular disease outcomes in a 20-year follow-up of the landmark WOSCOPS trial.
“There is a rather remarkable persistence of benefit in terms of risk reduction over a long period. You’ve changed the natural history of the disease in some way by lowering LDL,” Dr. Chris J. Packard said in presenting the 20-year WOSCOPS (West of Scotland Coronary Prevention Study) follow-up at the American Heart Association scientific sessions.
The primary prevention study randomized 6,595 middle-aged Scotsmen with an average baseline LDL cholesterol of 190 mg/dL to 4 years of pravastatin at 40 mg/day or placebo. This was one of the early large statin trials, and publication of the 5-year outcomes showing a 31% reduction in the relative risk of cardiovascular death or MI (N. Engl. J. Med. 1995;333:1301-8) caused a great stir.
At that point, WOSCOPS leaders advised study participants’ primary care physicians to seriously consider putting their patients on long-term statin therapy. However, only an identically low 31% of subjects in each of the two study arms did so.
Because the Scottish national health care system effectively captures all utilization of medical services, Dr. Packard and coinvestigators were able to analyze 20-year outcomes in the former study participants. No other major statin trial has come close in terms of length of reported follow-up.

At 20 years, with the only treatment difference between the two original study arms being that the pravastatin group had been on statin therapy for an additional 5 years, the 20-year coronary heart disease mortality rate in the original statin group was reduced by 27%, compared with the original controls. All-cause mortality was reduced by 13%. And numerous other benefits were noted at 20 years with 5 years of statin therapy.
Indeed, patients treated with pravastatin for 5 years during the trial gained an average of 5 extra years free of nonfatal MI or cardiovascular death at the 20-year mark, he said.
“The average age of the men was 55 years during the trial and 20 years on they’re now 75 years old. This covers the entire period of premature cardiovascular morbidity and mortality. We would argue that this is a good picture of the lifetime benefit, which is different from lifetime risk. This is real events happening to real people, not predictions,” observed Dr. Packard, professor of cardiovascular and medical sciences at the University of Glasgow, Scotland.
The group given pravastatin for 5 years collectively spent 24,038 days in the hospital for any cardiovascular event, compared with 30,342 days for controls.
Particularly noteworthy was the divergence in the risk of heart failure, with 96 cases being diagnosed during 20 years in patients who received 5 years of pravastatin, compared with 128 in controls.
“Heart failure was our biggest surprise,” he said. “We got no result for heart failure at all at 5 years; these were middle-aged men with just high cholesterol, so we didn’t see much in the way of incident heart failure. But extrapolating 20 years, there is a 31% risk reduction in the incidence of hospitalization for heart failure in the statin-treated group, compared to the placebo-treated group. This is a remarkable finding.”
For WOSCOPS participants who got a 20% drop in LDL during 5 years of pravastatin from a baseline of 190 mg/dL, as was typical, the number of patients who needed to be treated (NNT) for 5 years to prevent one cardiovascular hospital admission over a 20-year period was six. Moreover, for every 10 patients on statin therapy for 5 years, an average of 19 hospital days were avoided over the course of 20 years. For patients with a baseline LDL of 120 mg/dL, the NNT was 10, and 12 hospital days were saved over a 20-year period for every 10 patients treated for 5 years.
“This is a real savings to the health service and the health care providers to offset the cost of drugs,” Dr. Packard continued.
He urged physicians to look beyond the initial reports of primary outcomes of the statin clinical trials and take a long-term view.
“If you take a lifetime approach to benefit so you’re looking not only at the first event, but the second event, the third, at heart failure, and at death, you can see tremendous benefits, whereas usually we only focus on the first event. And that’s not the full cost evaluation that you need to do,” according to Dr. Packard.
The overall incidence of cancer during 20 years of follow-up was 24.8% in the initial placebo arm and 24.5% in the pravastatin group, with no differences between the two groups in any type of cancer. Nor did the original pravastatin group show any increase in noncardiovascular mortality.
“This is a very important study – the first study to show a legacy effect, with reduced mortality and a gain of 5 event-free years over 20 years attributable to a 5-year treatment allocation,” said discussant Harvey White of Auckland (New Zealand) City Hospital.
This legacy effect, he added, can be viewed as an ongoing carryover effect related to statin-induced slowing and/or stabilization of existing coronary artery plaque. The mechanism is unknown, Dr. White said, but the key to why the legacy effect was seen in WOSCOPS despite the use of pravastatin – a less potent statin – but not to date in other statin trials may lie in the fact that WOSCOPS was a primary prevention study and its participants had the youngest mean age of all the major statin trials.
“Their plaques may not have been calcified yet and therefore were more able to be modified and stabilized. If you treat very early you might get a bigger effect,” said to Dr. White.
Undercutting that argument, however, was the WOSCOPS finding that the long-term benefits of 5 years of pravastatin were independent of age at treatment, Dr. Packard said.
He believes based upon other studies that statins’ coronary disease prevention benefits are expressed within the first 12 months after starting therapy.
“It suggests that whatever is happening to the pathobiology of atherosclerosis happens within a year, and somehow a statis is introduced into plaque. That’s my guess, that an unstable plaque is reduced to a stable one. People then form a new trajectory going forward and they never catch up. We should think of atherosclerosis as a rate effect rather than something that either happens or doesn’t happen. It’s a rate of happening,” Dr. Packard asserted.
Asked why in the aftermath of the strongly positive 5-year results of WOSCOPS only 31% of patients in each treatment arm were on long-term statin therapy, he replied that 20 years ago in Scotland there really was no push for primary prevention.
“The 4S trial had come out the year before [Lancet 1994;344:1383-9] and placed the focus on secondary prevention. Statins were relatively expensive and everybody was putting their money into secondary prevention. Our health care system, which is socialized, had not put any emphasis at all on primary prevention. We were actually amazed that even 31% got treated,” the physician explained.
Dr. Packard reported serving as a consultant to Merck, Roche, and AstraZeneca.
CHICAGO – Five years of statin therapy for primary prevention provided an impressive lifetime benefit expressed as reduced risks of a range of cardiovascular disease outcomes in a 20-year follow-up of the landmark WOSCOPS trial.
“There is a rather remarkable persistence of benefit in terms of risk reduction over a long period. You’ve changed the natural history of the disease in some way by lowering LDL,” Dr. Chris J. Packard said in presenting the 20-year WOSCOPS (West of Scotland Coronary Prevention Study) follow-up at the American Heart Association scientific sessions.
The primary prevention study randomized 6,595 middle-aged Scotsmen with an average baseline LDL cholesterol of 190 mg/dL to 4 years of pravastatin at 40 mg/day or placebo. This was one of the early large statin trials, and publication of the 5-year outcomes showing a 31% reduction in the relative risk of cardiovascular death or MI (N. Engl. J. Med. 1995;333:1301-8) caused a great stir.
At that point, WOSCOPS leaders advised study participants’ primary care physicians to seriously consider putting their patients on long-term statin therapy. However, only an identically low 31% of subjects in each of the two study arms did so.
Because the Scottish national health care system effectively captures all utilization of medical services, Dr. Packard and coinvestigators were able to analyze 20-year outcomes in the former study participants. No other major statin trial has come close in terms of length of reported follow-up.

At 20 years, with the only treatment difference between the two original study arms being that the pravastatin group had been on statin therapy for an additional 5 years, the 20-year coronary heart disease mortality rate in the original statin group was reduced by 27%, compared with the original controls. All-cause mortality was reduced by 13%. And numerous other benefits were noted at 20 years with 5 years of statin therapy.
Indeed, patients treated with pravastatin for 5 years during the trial gained an average of 5 extra years free of nonfatal MI or cardiovascular death at the 20-year mark, he said.
“The average age of the men was 55 years during the trial and 20 years on they’re now 75 years old. This covers the entire period of premature cardiovascular morbidity and mortality. We would argue that this is a good picture of the lifetime benefit, which is different from lifetime risk. This is real events happening to real people, not predictions,” observed Dr. Packard, professor of cardiovascular and medical sciences at the University of Glasgow, Scotland.
The group given pravastatin for 5 years collectively spent 24,038 days in the hospital for any cardiovascular event, compared with 30,342 days for controls.
Particularly noteworthy was the divergence in the risk of heart failure, with 96 cases being diagnosed during 20 years in patients who received 5 years of pravastatin, compared with 128 in controls.
“Heart failure was our biggest surprise,” he said. “We got no result for heart failure at all at 5 years; these were middle-aged men with just high cholesterol, so we didn’t see much in the way of incident heart failure. But extrapolating 20 years, there is a 31% risk reduction in the incidence of hospitalization for heart failure in the statin-treated group, compared to the placebo-treated group. This is a remarkable finding.”
For WOSCOPS participants who got a 20% drop in LDL during 5 years of pravastatin from a baseline of 190 mg/dL, as was typical, the number of patients who needed to be treated (NNT) for 5 years to prevent one cardiovascular hospital admission over a 20-year period was six. Moreover, for every 10 patients on statin therapy for 5 years, an average of 19 hospital days were avoided over the course of 20 years. For patients with a baseline LDL of 120 mg/dL, the NNT was 10, and 12 hospital days were saved over a 20-year period for every 10 patients treated for 5 years.
“This is a real savings to the health service and the health care providers to offset the cost of drugs,” Dr. Packard continued.
He urged physicians to look beyond the initial reports of primary outcomes of the statin clinical trials and take a long-term view.
“If you take a lifetime approach to benefit so you’re looking not only at the first event, but the second event, the third, at heart failure, and at death, you can see tremendous benefits, whereas usually we only focus on the first event. And that’s not the full cost evaluation that you need to do,” according to Dr. Packard.
The overall incidence of cancer during 20 years of follow-up was 24.8% in the initial placebo arm and 24.5% in the pravastatin group, with no differences between the two groups in any type of cancer. Nor did the original pravastatin group show any increase in noncardiovascular mortality.
“This is a very important study – the first study to show a legacy effect, with reduced mortality and a gain of 5 event-free years over 20 years attributable to a 5-year treatment allocation,” said discussant Harvey White of Auckland (New Zealand) City Hospital.
This legacy effect, he added, can be viewed as an ongoing carryover effect related to statin-induced slowing and/or stabilization of existing coronary artery plaque. The mechanism is unknown, Dr. White said, but the key to why the legacy effect was seen in WOSCOPS despite the use of pravastatin – a less potent statin – but not to date in other statin trials may lie in the fact that WOSCOPS was a primary prevention study and its participants had the youngest mean age of all the major statin trials.
“Their plaques may not have been calcified yet and therefore were more able to be modified and stabilized. If you treat very early you might get a bigger effect,” said to Dr. White.
Undercutting that argument, however, was the WOSCOPS finding that the long-term benefits of 5 years of pravastatin were independent of age at treatment, Dr. Packard said.
He believes based upon other studies that statins’ coronary disease prevention benefits are expressed within the first 12 months after starting therapy.
“It suggests that whatever is happening to the pathobiology of atherosclerosis happens within a year, and somehow a statis is introduced into plaque. That’s my guess, that an unstable plaque is reduced to a stable one. People then form a new trajectory going forward and they never catch up. We should think of atherosclerosis as a rate effect rather than something that either happens or doesn’t happen. It’s a rate of happening,” Dr. Packard asserted.
Asked why in the aftermath of the strongly positive 5-year results of WOSCOPS only 31% of patients in each treatment arm were on long-term statin therapy, he replied that 20 years ago in Scotland there really was no push for primary prevention.
“The 4S trial had come out the year before [Lancet 1994;344:1383-9] and placed the focus on secondary prevention. Statins were relatively expensive and everybody was putting their money into secondary prevention. Our health care system, which is socialized, had not put any emphasis at all on primary prevention. We were actually amazed that even 31% got treated,” the physician explained.
Dr. Packard reported serving as a consultant to Merck, Roche, and AstraZeneca.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Five years of statin therapy during midlife for primary prevention may reset the clock for atherosclerotic progression, providing persistently lower cardiovascular event rates 20 years later.
Major finding: The number of patients who needed to be treated with pravastatin for 5 years to prevent one cardiovascular hospitalization over a 20-year period was six.
Data source: A 20-year follow-up of the West of Scotland Coronary Prevention Study, in which 6,595 middle-aged men with high cholesterol were randomized to 5 years of pravastatin or placebo.
Disclosures: WOSCOPS was sponsored by Bristol-Myers Squibb. The presenter reported serving as a consultant to Merck, Roche, and AstraZeneca.
Unrecognized MI common with impaired fasting glucose
CHICAGO– Impaired fasting glucose was associated with a doubled risk of asymptomatic or unrecognized myocardial infarction during prospective follow-up of subjects without overt cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis.
The clinical relevance of this finding lies in previous work establishing that asymptomatic, unrecognized MIs are associated with a prognosis as serious as for symptomatic MIs.
“Because of the high prevalence of impaired fasting glucose, the implications of this finding may have ramifications for a large proportion of the adult population,” Dr. Richard B. Stacey observed at the American Heart Association Scientific Sessions.
The magnitude of the risk of unrecognized MI was associated with duration of exposure to abnormal blood glucose levels in the landmark population-based MESA study. Subjects with impaired fasting glucose (IFG) noted on at least three of five biannual examinations conducted over a 10-year period had an adjusted 1.97-fold greater risk of experiencing an unrecognized MI during that time, compared with MESA participants who had normal blood glucose, added Dr. Stacey of Wake Forest University in Winston-Salem, N.C.
He presented two new analyses from the MESA study. One was cross-sectional: Among 4,955 study participants with normal fasting glucose and 930 with IFG upon study enrollment, a baseline 12-lead ECG revealed a prior unrecognized MI in 1.4% of those with normal fasting glucose and 3.2% of those with IFG, a 2.3-fold increase. MESA participants were on average in their mid-60s, and none had clinical cardiovascular disease.
In a multivariate logistic regression analysis fully adjusted for demographics and the standard cardiovascular risk factors as well as body mass index and the use of antihypertensive and lipid-lowering medications, IFG was associated with a 1.63-fold increased risk of prior asymptomatic or unrecognized MI (P < .001).
The other, prospective, longitudinal analysis, included 872 subjects with baseline normal fasting glucose and 545 with IFG, none of whom had evidence of a prior unrecognized MI at entry. This analysis excluded individuals who developed diabetes mellitus, had a clinically recognized MI, or underwent coronary revascularization during follow-up. During 10 years of follow-up, 14.9% of the group who continued to have normal fasting glucose experienced an unrecognized MI, as did 23.8% of those with IFG at entry. The unrecognized MIs were detected either by the presence of pathologic Q waves or minor Q waves with ST-T abnormalities on ECG or by an MRI showing late gadolinium enhancement indicative of myocardial scar.
The MESA study is funded by the National Institutes of Health. Dr. Stacey reported having no financial conflicts.
CHICAGO– Impaired fasting glucose was associated with a doubled risk of asymptomatic or unrecognized myocardial infarction during prospective follow-up of subjects without overt cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis.
The clinical relevance of this finding lies in previous work establishing that asymptomatic, unrecognized MIs are associated with a prognosis as serious as for symptomatic MIs.
“Because of the high prevalence of impaired fasting glucose, the implications of this finding may have ramifications for a large proportion of the adult population,” Dr. Richard B. Stacey observed at the American Heart Association Scientific Sessions.
The magnitude of the risk of unrecognized MI was associated with duration of exposure to abnormal blood glucose levels in the landmark population-based MESA study. Subjects with impaired fasting glucose (IFG) noted on at least three of five biannual examinations conducted over a 10-year period had an adjusted 1.97-fold greater risk of experiencing an unrecognized MI during that time, compared with MESA participants who had normal blood glucose, added Dr. Stacey of Wake Forest University in Winston-Salem, N.C.
He presented two new analyses from the MESA study. One was cross-sectional: Among 4,955 study participants with normal fasting glucose and 930 with IFG upon study enrollment, a baseline 12-lead ECG revealed a prior unrecognized MI in 1.4% of those with normal fasting glucose and 3.2% of those with IFG, a 2.3-fold increase. MESA participants were on average in their mid-60s, and none had clinical cardiovascular disease.
In a multivariate logistic regression analysis fully adjusted for demographics and the standard cardiovascular risk factors as well as body mass index and the use of antihypertensive and lipid-lowering medications, IFG was associated with a 1.63-fold increased risk of prior asymptomatic or unrecognized MI (P < .001).
The other, prospective, longitudinal analysis, included 872 subjects with baseline normal fasting glucose and 545 with IFG, none of whom had evidence of a prior unrecognized MI at entry. This analysis excluded individuals who developed diabetes mellitus, had a clinically recognized MI, or underwent coronary revascularization during follow-up. During 10 years of follow-up, 14.9% of the group who continued to have normal fasting glucose experienced an unrecognized MI, as did 23.8% of those with IFG at entry. The unrecognized MIs were detected either by the presence of pathologic Q waves or minor Q waves with ST-T abnormalities on ECG or by an MRI showing late gadolinium enhancement indicative of myocardial scar.
The MESA study is funded by the National Institutes of Health. Dr. Stacey reported having no financial conflicts.
CHICAGO– Impaired fasting glucose was associated with a doubled risk of asymptomatic or unrecognized myocardial infarction during prospective follow-up of subjects without overt cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis.
The clinical relevance of this finding lies in previous work establishing that asymptomatic, unrecognized MIs are associated with a prognosis as serious as for symptomatic MIs.
“Because of the high prevalence of impaired fasting glucose, the implications of this finding may have ramifications for a large proportion of the adult population,” Dr. Richard B. Stacey observed at the American Heart Association Scientific Sessions.
The magnitude of the risk of unrecognized MI was associated with duration of exposure to abnormal blood glucose levels in the landmark population-based MESA study. Subjects with impaired fasting glucose (IFG) noted on at least three of five biannual examinations conducted over a 10-year period had an adjusted 1.97-fold greater risk of experiencing an unrecognized MI during that time, compared with MESA participants who had normal blood glucose, added Dr. Stacey of Wake Forest University in Winston-Salem, N.C.
He presented two new analyses from the MESA study. One was cross-sectional: Among 4,955 study participants with normal fasting glucose and 930 with IFG upon study enrollment, a baseline 12-lead ECG revealed a prior unrecognized MI in 1.4% of those with normal fasting glucose and 3.2% of those with IFG, a 2.3-fold increase. MESA participants were on average in their mid-60s, and none had clinical cardiovascular disease.
In a multivariate logistic regression analysis fully adjusted for demographics and the standard cardiovascular risk factors as well as body mass index and the use of antihypertensive and lipid-lowering medications, IFG was associated with a 1.63-fold increased risk of prior asymptomatic or unrecognized MI (P < .001).
The other, prospective, longitudinal analysis, included 872 subjects with baseline normal fasting glucose and 545 with IFG, none of whom had evidence of a prior unrecognized MI at entry. This analysis excluded individuals who developed diabetes mellitus, had a clinically recognized MI, or underwent coronary revascularization during follow-up. During 10 years of follow-up, 14.9% of the group who continued to have normal fasting glucose experienced an unrecognized MI, as did 23.8% of those with IFG at entry. The unrecognized MIs were detected either by the presence of pathologic Q waves or minor Q waves with ST-T abnormalities on ECG or by an MRI showing late gadolinium enhancement indicative of myocardial scar.
The MESA study is funded by the National Institutes of Health. Dr. Stacey reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Consistent impaired fasting glucose may be associated with asymptomatic myocardial infarction.
Major finding: Individuals with impaired fasting glucose on at least three out of five structured clinic visits during 10 years of prospective follow-up were at twice the risk of experiencing an unrecognized MI, compared with those who had normal fasting glucose.
Data source: The Multi-Ethnic Study of Atherosclerosis, a prospective, longitudinal, population-based study.
Disclosures: MESA is funded by the National Institutes of Health. The presenter reported having no financial conflicts.
Bare-metal stent superior safety debunked in DAPT analysis
CHICAGO – Drug-eluting stents were associated with significantly lower risks of both stent thrombosis and major adverse cardio- and cerebrovascular events, compared with bare-metal stents in a prespecified secondary analysis of the landmark Dual Antiplatelet Therapy (DAPT) trial.
“What I’ve learned from this is the myth of the safety of the bare-metal stent: It is not safer. So the concept that we still see in practice every day, where colleagues put in bare-metal stents because they perceive them to be safer and they perceive the optimal duration of dual antiplatelet therapy to be shorter, I’m not sure that’s the right thought process,” Dr. Dean J. Kereiakes said in presenting the study findings at the American Heart Association scientific sessions.
Another eye-opening finding in the secondary analysis was that when it comes to the duration of DAPT after stent placement, be it a drug-eluting stent (DES) or bare-metal stent (BMS), longer appears to be better. A surprising proportion of cases of stent thrombosis in both groups occurred after they stopped DAPT at the 12-month mark.
“Patients who don’t bleed on DAPT should be left on it a long time. I have evolved my thought process such that I’m thinking of this like a statin,” said Dr. Kereiakes, medical director of the Christ Hospital Heart and Vascular Center in Cincinnati.
The DAPT trial included 9,961 patients who underwent percutaneous coronary intervention with one of four types of drug-eluting stent (DES) and 1,687 who got a bare-metal stent (BMS), all of whom received 12 months of DAPT and then were randomized to blinded placebo or an additional 18 months of DAPT. The primary outcome was presented earlier at the AHA meeting and simultaneously published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1409312]).
The secondary analysis addressed two key questions: Do the risks of stent thrombosis and major adverse cardio- and cerebrovascular events (MACCE) differ between DES and BMS? And does the optimal duration of DAPT differ between the two stent platforms?
Dr. Kereiakes presented a propensity-matched analysis that included 8,308 patients with DES and 1,178 with BMS. The key finding here was that the rate of stent thrombosis was significantly lower in the DES group. This was the case both through the first 12 months of DAPT, where the stent thrombosis rates were 0.7% and 1.7% in the DES and BMS groups, respectively, and at 33 months, where the cumulative stent thrombosis rates were 1.7% and 2.6%.
The MACCE rate – defined as death, MI, or stroke – at 12 months was also significantly lower in the DES group: 5.1%, compared with 6.8% with BMS. The same was true at 33 months, when the rate was 11.4% in the DES group, compared with 13.4% in patients with a BMS. However, because of the smaller sample size of the BMS propensity-matched comparison group, this absolute 2% difference in MACCE at 33 months was sufficient to show DES were noninferior to BMS on this endpoint, but not superior to BMS, the cardiologist continued.
The advantage for DES over BMS in terms of MACCE appeared to be similar across all four DES types utilized in the trial: everolimus-, sirolimus-, paclitaxel-, and zotarolimus-eluting stents. While paclitaxel-eluting stents weren’t significantly better than BMS in terms of stent thrombosis rates, the other three DES types were, and to a comparable degree.
The hazard ratio for stent thrombosis with 30 months of DAPT as compared to 12 months was 0.29 in the DES group and 0.49 in BMS recipients.
“The magnitude of reduction in stent thrombosis risk with the longer, 30-month duration thienopyridine therapy appears consistent for both bare-metal stents and drug-eluting stents,” Dr. Kereiakes said.
There was no significant difference between DES and BMS in the rate of moderate to severe bleeding through 33 months: 4.04% with DES and 3.67% in the BMS group. Stroke rates were similar as well.
Dr. Kereiakes also presented another prespecified secondary analysis from the DAPT trial, this one a comparison of event rates in 1,687 BMS patients randomized to 12 versus 30 months of DAPT. At 33 months’ follow-up, the stent thrombosis rate was 0.5% in BMS patients who got 30 months of DAPT, compared with 1.1% in those who got 12 months of DAPT followed by aspirin plus placebo. The MACCE rate was 4.0% in BMS recipients who received 30 months of DAPT and 4.7% with 12 months.
Discussant Dr. Daniel B. Mark said these secondary analyses of the DAPT trial have brought home for him several key points: First, BMS are not safer than DES from the patient’s viewpoint; second, the risk of stent thrombosis continues after 12 months for both stent platforms; and third, current DAPT regimens can decrease but certainly don’t eliminate either stent thrombosis or non–stent related cardiovascular events.
The late occurrence of stent thromboses between 12 and 30 months in both study arms “really makes us think of this issue of DAPT in a different way than we have in the past,” added Dr. Mark, professor of medicine at Duke University and director of outcomes research at the Duke Clinical Research Institute in Durham, N.C.
He observed that “a perfect storm” of observational evidence arose beginning in 2006 which convinced physicians that BMS were safer – wrongly, as it now turns out.
The conventional wisdom was that BMS had a restenosis problem seen as “a benign nuisance requiring repeat revascularization procedures but having no discernible effect on death or MI,” Dr. Marks said, “while the DES were extremely effective at reducing restenosis but had this stent thrombosis problem” that was viewed in hyperbolic terms in the literature. The DAPT trial has gone a long way towards correcting those misperceptions, he said.
He wondered, however, just how confident Dr. Kereiakes is in the validity of his propensity matching.
“The matching was based on 55 different variables,” Dr. Kereiakes replied. “With all the limitations inherent in propensity-matched analysis, I think this is about as good as it gets.”
Dr. Mark noted that while cardiologists ponder the fine points between DES and BMS, it’s important not to lose sight of the big picture as highlighted in a recent study by Dr. Mark A. Hlatky of Stanford (Calif.) University and coinvestigators. Their analysis of Medicare beneficiaries who underwent either multivessel coronary artery bypass surgery or multivessel percutaneous coronary intervention concluded that the introduction of DES didn’t alter the comparative effectiveness of CABG and PCI. The 5-year survival rate following CABG has been about 10% better than with PCI both in the pre-DES era of BMS and in the DES era. Moreover, the 5-year rate of freedom from MI has been about 18% better with multivessel CABG than multivessel PCI in both stent platform eras (Am. Heart J. 2014;169:149-54).
The DAPT study was supported by more than half a dozen pharmaceutical and medical device companies as well as the U.S. Department of Health and Human Services. Dr. Kereiakes reported receiving payments as an advisor to Boston Scientific and Abbott Vascular. Dr. Marks reported receiving research grants from the National Institutes of Health and serving as a consultant to Somahlution, Milestone, Medtronic, and CardioDx.
CHICAGO – Drug-eluting stents were associated with significantly lower risks of both stent thrombosis and major adverse cardio- and cerebrovascular events, compared with bare-metal stents in a prespecified secondary analysis of the landmark Dual Antiplatelet Therapy (DAPT) trial.
“What I’ve learned from this is the myth of the safety of the bare-metal stent: It is not safer. So the concept that we still see in practice every day, where colleagues put in bare-metal stents because they perceive them to be safer and they perceive the optimal duration of dual antiplatelet therapy to be shorter, I’m not sure that’s the right thought process,” Dr. Dean J. Kereiakes said in presenting the study findings at the American Heart Association scientific sessions.
Another eye-opening finding in the secondary analysis was that when it comes to the duration of DAPT after stent placement, be it a drug-eluting stent (DES) or bare-metal stent (BMS), longer appears to be better. A surprising proportion of cases of stent thrombosis in both groups occurred after they stopped DAPT at the 12-month mark.
“Patients who don’t bleed on DAPT should be left on it a long time. I have evolved my thought process such that I’m thinking of this like a statin,” said Dr. Kereiakes, medical director of the Christ Hospital Heart and Vascular Center in Cincinnati.
The DAPT trial included 9,961 patients who underwent percutaneous coronary intervention with one of four types of drug-eluting stent (DES) and 1,687 who got a bare-metal stent (BMS), all of whom received 12 months of DAPT and then were randomized to blinded placebo or an additional 18 months of DAPT. The primary outcome was presented earlier at the AHA meeting and simultaneously published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1409312]).
The secondary analysis addressed two key questions: Do the risks of stent thrombosis and major adverse cardio- and cerebrovascular events (MACCE) differ between DES and BMS? And does the optimal duration of DAPT differ between the two stent platforms?
Dr. Kereiakes presented a propensity-matched analysis that included 8,308 patients with DES and 1,178 with BMS. The key finding here was that the rate of stent thrombosis was significantly lower in the DES group. This was the case both through the first 12 months of DAPT, where the stent thrombosis rates were 0.7% and 1.7% in the DES and BMS groups, respectively, and at 33 months, where the cumulative stent thrombosis rates were 1.7% and 2.6%.
The MACCE rate – defined as death, MI, or stroke – at 12 months was also significantly lower in the DES group: 5.1%, compared with 6.8% with BMS. The same was true at 33 months, when the rate was 11.4% in the DES group, compared with 13.4% in patients with a BMS. However, because of the smaller sample size of the BMS propensity-matched comparison group, this absolute 2% difference in MACCE at 33 months was sufficient to show DES were noninferior to BMS on this endpoint, but not superior to BMS, the cardiologist continued.
The advantage for DES over BMS in terms of MACCE appeared to be similar across all four DES types utilized in the trial: everolimus-, sirolimus-, paclitaxel-, and zotarolimus-eluting stents. While paclitaxel-eluting stents weren’t significantly better than BMS in terms of stent thrombosis rates, the other three DES types were, and to a comparable degree.
The hazard ratio for stent thrombosis with 30 months of DAPT as compared to 12 months was 0.29 in the DES group and 0.49 in BMS recipients.
“The magnitude of reduction in stent thrombosis risk with the longer, 30-month duration thienopyridine therapy appears consistent for both bare-metal stents and drug-eluting stents,” Dr. Kereiakes said.
There was no significant difference between DES and BMS in the rate of moderate to severe bleeding through 33 months: 4.04% with DES and 3.67% in the BMS group. Stroke rates were similar as well.
Dr. Kereiakes also presented another prespecified secondary analysis from the DAPT trial, this one a comparison of event rates in 1,687 BMS patients randomized to 12 versus 30 months of DAPT. At 33 months’ follow-up, the stent thrombosis rate was 0.5% in BMS patients who got 30 months of DAPT, compared with 1.1% in those who got 12 months of DAPT followed by aspirin plus placebo. The MACCE rate was 4.0% in BMS recipients who received 30 months of DAPT and 4.7% with 12 months.
Discussant Dr. Daniel B. Mark said these secondary analyses of the DAPT trial have brought home for him several key points: First, BMS are not safer than DES from the patient’s viewpoint; second, the risk of stent thrombosis continues after 12 months for both stent platforms; and third, current DAPT regimens can decrease but certainly don’t eliminate either stent thrombosis or non–stent related cardiovascular events.
The late occurrence of stent thromboses between 12 and 30 months in both study arms “really makes us think of this issue of DAPT in a different way than we have in the past,” added Dr. Mark, professor of medicine at Duke University and director of outcomes research at the Duke Clinical Research Institute in Durham, N.C.
He observed that “a perfect storm” of observational evidence arose beginning in 2006 which convinced physicians that BMS were safer – wrongly, as it now turns out.
The conventional wisdom was that BMS had a restenosis problem seen as “a benign nuisance requiring repeat revascularization procedures but having no discernible effect on death or MI,” Dr. Marks said, “while the DES were extremely effective at reducing restenosis but had this stent thrombosis problem” that was viewed in hyperbolic terms in the literature. The DAPT trial has gone a long way towards correcting those misperceptions, he said.
He wondered, however, just how confident Dr. Kereiakes is in the validity of his propensity matching.
“The matching was based on 55 different variables,” Dr. Kereiakes replied. “With all the limitations inherent in propensity-matched analysis, I think this is about as good as it gets.”
Dr. Mark noted that while cardiologists ponder the fine points between DES and BMS, it’s important not to lose sight of the big picture as highlighted in a recent study by Dr. Mark A. Hlatky of Stanford (Calif.) University and coinvestigators. Their analysis of Medicare beneficiaries who underwent either multivessel coronary artery bypass surgery or multivessel percutaneous coronary intervention concluded that the introduction of DES didn’t alter the comparative effectiveness of CABG and PCI. The 5-year survival rate following CABG has been about 10% better than with PCI both in the pre-DES era of BMS and in the DES era. Moreover, the 5-year rate of freedom from MI has been about 18% better with multivessel CABG than multivessel PCI in both stent platform eras (Am. Heart J. 2014;169:149-54).
The DAPT study was supported by more than half a dozen pharmaceutical and medical device companies as well as the U.S. Department of Health and Human Services. Dr. Kereiakes reported receiving payments as an advisor to Boston Scientific and Abbott Vascular. Dr. Marks reported receiving research grants from the National Institutes of Health and serving as a consultant to Somahlution, Milestone, Medtronic, and CardioDx.
CHICAGO – Drug-eluting stents were associated with significantly lower risks of both stent thrombosis and major adverse cardio- and cerebrovascular events, compared with bare-metal stents in a prespecified secondary analysis of the landmark Dual Antiplatelet Therapy (DAPT) trial.
“What I’ve learned from this is the myth of the safety of the bare-metal stent: It is not safer. So the concept that we still see in practice every day, where colleagues put in bare-metal stents because they perceive them to be safer and they perceive the optimal duration of dual antiplatelet therapy to be shorter, I’m not sure that’s the right thought process,” Dr. Dean J. Kereiakes said in presenting the study findings at the American Heart Association scientific sessions.
Another eye-opening finding in the secondary analysis was that when it comes to the duration of DAPT after stent placement, be it a drug-eluting stent (DES) or bare-metal stent (BMS), longer appears to be better. A surprising proportion of cases of stent thrombosis in both groups occurred after they stopped DAPT at the 12-month mark.
“Patients who don’t bleed on DAPT should be left on it a long time. I have evolved my thought process such that I’m thinking of this like a statin,” said Dr. Kereiakes, medical director of the Christ Hospital Heart and Vascular Center in Cincinnati.
The DAPT trial included 9,961 patients who underwent percutaneous coronary intervention with one of four types of drug-eluting stent (DES) and 1,687 who got a bare-metal stent (BMS), all of whom received 12 months of DAPT and then were randomized to blinded placebo or an additional 18 months of DAPT. The primary outcome was presented earlier at the AHA meeting and simultaneously published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1409312]).
The secondary analysis addressed two key questions: Do the risks of stent thrombosis and major adverse cardio- and cerebrovascular events (MACCE) differ between DES and BMS? And does the optimal duration of DAPT differ between the two stent platforms?
Dr. Kereiakes presented a propensity-matched analysis that included 8,308 patients with DES and 1,178 with BMS. The key finding here was that the rate of stent thrombosis was significantly lower in the DES group. This was the case both through the first 12 months of DAPT, where the stent thrombosis rates were 0.7% and 1.7% in the DES and BMS groups, respectively, and at 33 months, where the cumulative stent thrombosis rates were 1.7% and 2.6%.
The MACCE rate – defined as death, MI, or stroke – at 12 months was also significantly lower in the DES group: 5.1%, compared with 6.8% with BMS. The same was true at 33 months, when the rate was 11.4% in the DES group, compared with 13.4% in patients with a BMS. However, because of the smaller sample size of the BMS propensity-matched comparison group, this absolute 2% difference in MACCE at 33 months was sufficient to show DES were noninferior to BMS on this endpoint, but not superior to BMS, the cardiologist continued.
The advantage for DES over BMS in terms of MACCE appeared to be similar across all four DES types utilized in the trial: everolimus-, sirolimus-, paclitaxel-, and zotarolimus-eluting stents. While paclitaxel-eluting stents weren’t significantly better than BMS in terms of stent thrombosis rates, the other three DES types were, and to a comparable degree.
The hazard ratio for stent thrombosis with 30 months of DAPT as compared to 12 months was 0.29 in the DES group and 0.49 in BMS recipients.
“The magnitude of reduction in stent thrombosis risk with the longer, 30-month duration thienopyridine therapy appears consistent for both bare-metal stents and drug-eluting stents,” Dr. Kereiakes said.
There was no significant difference between DES and BMS in the rate of moderate to severe bleeding through 33 months: 4.04% with DES and 3.67% in the BMS group. Stroke rates were similar as well.
Dr. Kereiakes also presented another prespecified secondary analysis from the DAPT trial, this one a comparison of event rates in 1,687 BMS patients randomized to 12 versus 30 months of DAPT. At 33 months’ follow-up, the stent thrombosis rate was 0.5% in BMS patients who got 30 months of DAPT, compared with 1.1% in those who got 12 months of DAPT followed by aspirin plus placebo. The MACCE rate was 4.0% in BMS recipients who received 30 months of DAPT and 4.7% with 12 months.
Discussant Dr. Daniel B. Mark said these secondary analyses of the DAPT trial have brought home for him several key points: First, BMS are not safer than DES from the patient’s viewpoint; second, the risk of stent thrombosis continues after 12 months for both stent platforms; and third, current DAPT regimens can decrease but certainly don’t eliminate either stent thrombosis or non–stent related cardiovascular events.
The late occurrence of stent thromboses between 12 and 30 months in both study arms “really makes us think of this issue of DAPT in a different way than we have in the past,” added Dr. Mark, professor of medicine at Duke University and director of outcomes research at the Duke Clinical Research Institute in Durham, N.C.
He observed that “a perfect storm” of observational evidence arose beginning in 2006 which convinced physicians that BMS were safer – wrongly, as it now turns out.
The conventional wisdom was that BMS had a restenosis problem seen as “a benign nuisance requiring repeat revascularization procedures but having no discernible effect on death or MI,” Dr. Marks said, “while the DES were extremely effective at reducing restenosis but had this stent thrombosis problem” that was viewed in hyperbolic terms in the literature. The DAPT trial has gone a long way towards correcting those misperceptions, he said.
He wondered, however, just how confident Dr. Kereiakes is in the validity of his propensity matching.
“The matching was based on 55 different variables,” Dr. Kereiakes replied. “With all the limitations inherent in propensity-matched analysis, I think this is about as good as it gets.”
Dr. Mark noted that while cardiologists ponder the fine points between DES and BMS, it’s important not to lose sight of the big picture as highlighted in a recent study by Dr. Mark A. Hlatky of Stanford (Calif.) University and coinvestigators. Their analysis of Medicare beneficiaries who underwent either multivessel coronary artery bypass surgery or multivessel percutaneous coronary intervention concluded that the introduction of DES didn’t alter the comparative effectiveness of CABG and PCI. The 5-year survival rate following CABG has been about 10% better than with PCI both in the pre-DES era of BMS and in the DES era. Moreover, the 5-year rate of freedom from MI has been about 18% better with multivessel CABG than multivessel PCI in both stent platform eras (Am. Heart J. 2014;169:149-54).
The DAPT study was supported by more than half a dozen pharmaceutical and medical device companies as well as the U.S. Department of Health and Human Services. Dr. Kereiakes reported receiving payments as an advisor to Boston Scientific and Abbott Vascular. Dr. Marks reported receiving research grants from the National Institutes of Health and serving as a consultant to Somahlution, Milestone, Medtronic, and CardioDx.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Bare-metal stents aren’t safer than drug-eluting stents.
Major finding: The stent thrombosis rate in drug-eluting stent recipients through 33 months of follow-up was 1.7%, compared with 2.6% in patients with bare-metal stents.
Data source: A prespecified secondary propensity-matched comparison of stent thrombosis and major cardiovascular and cerebrovascular rates in 8,308 drug-eluting stent recipients and 1,178 bare-metal stent recipients in the Dual Antiplatelet Therapy trial.
Disclosures: DAPT was supported by more than half a dozen pharmaceutical and medical device companies as well as the U.S. Department of Health and Human Services. The presenter reported receiving payments as an advisor to Boston Scientific and Abbott Vascular.
Overanticoagulation in AF boosts dementia risk
CHICAGO – Patients with atrial fibrillation who frequently have a supratherapeutic international normalized ratio are at sharply increased risk for developing dementia, according to a large observational study.
“We postulate that the mechanism is an accumulation of microbleeds in the brain,” Dr. T. Jared Bunch said at the American Heart Association Scientific Sessions.
“In patients with hypertension, a condition that’s extremely common with atrial fibrillation, these repetitive small bleeds are preferentially in the hippocampus, where memory is stored,” added Dr. Bunch, who is medical director for heart rhythm services at the Intermountain Medical Center Heart Institute in Salt Lake City.
He presented a study of 1,031 patients with atrial fibrillation (AF) in Intermountain’s centralized anticoagulation service. All were on dual therapy with warfarin plus aspirin or, much less commonly, another antiplatelet agent. At baseline, their average age was in the early- to mid-70s, and none of the subjects had a history of stroke or any notes in their medical record suggestive of early cognitive decline. At this dedicated anticoagulation center, their INR was measured on a weekly or biweekly basis, as a result of which their average time spent in the therapeutic range of 2.0-3.0 was relatively high at nearly 70%.
The increased risk of dementia in patients with AF has previously been recognized. The association is stronger in patients under age 75 than in those who are older. But the mechanism has been unknown. Dr. Bunch and coinvestigators decided to test their hypothesis that the mechanism involves microbleeds secondary to long-term overanticoagulation by dividing the patients into three groups based upon their percentage of INR measurements above 3.0 during a mean follow-up of more than 4 and up to a maximum of 10 years: 240 patients had a supratherapeutic INR 25% of the time or more; 374 did so less than 10% of the time; and 417 had an elevated INR 10%-24% of the time.
The incidence of dementia diagnosed by a consultant neurologist during follow-up was 5.8% in the group with an INR above 3.0 at least 25% of the time, more than twice the 2.7% rate in patients with a high INR less than 10% of the time. In the middle group, the incidence of dementia was 4.1%. In a multivariate Cox regression analysis, having an INR above 3.0 on at least 25% of occasions was independently associated with a 2.59-fold increased risk of developing dementia, making it by far the most potent risk factor in their analysis.
The next step in their research will be to perform serial brain imaging and volumetric scans, Dr. Bunch said. Also, he and his coworkers are 3 years into an ongoing study looking at the incidence of dementia in AF patients on the various novel oral anticoagulants, where INR is a nonissue. Their hypothesis is the dementia risk will be lower than in patients on warfarin. Dr. Bunch has particularly high hopes for AF patients on apixaban (Eliquis) because it’s known to have a reduced risk of large bleeds, stroke, and GI bleeding; the hope is it will cause fewer cerebral microbleeds as well.
In an interview, the cardiologist said he believes his study showing an increased risk of dementia in AF patients with supratherapeutic INRs on warfarin plus antiplatelet therapy holds several important lessons for AF patients and physicians alike.
For patients, the message is don’t just start taking aspirin on your own because you’ve read it’s good for your heart or may reduce cancer risk; consult your physician.
And for physicians, it’s important to ask all patients on warfarin if they’re using aspirin; many don’t ask. Also, periodically reconsider the need for dual therapy with warfarin and aspirin.
“We find the risks of stroke and bleeding change dynamically over time, so the initial therapy for stroke prevention may not be the ideal therapy after 5-10 years,” Dr. Bunch said.
Lastly, for patients who are overanticoagulated on a substantial percentage of their INR measurements, it’s essential to consider a change in strategy. Either follow their INRs more closely and adjust warfarin dosing accordingly, or switch to one of the novel, more predictable oral anticoagulants, he concluded.
This study was funded internally by Intermountain Healthcare. Dr. Bunch reported having no financial conflicts.
CHICAGO – Patients with atrial fibrillation who frequently have a supratherapeutic international normalized ratio are at sharply increased risk for developing dementia, according to a large observational study.
“We postulate that the mechanism is an accumulation of microbleeds in the brain,” Dr. T. Jared Bunch said at the American Heart Association Scientific Sessions.
“In patients with hypertension, a condition that’s extremely common with atrial fibrillation, these repetitive small bleeds are preferentially in the hippocampus, where memory is stored,” added Dr. Bunch, who is medical director for heart rhythm services at the Intermountain Medical Center Heart Institute in Salt Lake City.
He presented a study of 1,031 patients with atrial fibrillation (AF) in Intermountain’s centralized anticoagulation service. All were on dual therapy with warfarin plus aspirin or, much less commonly, another antiplatelet agent. At baseline, their average age was in the early- to mid-70s, and none of the subjects had a history of stroke or any notes in their medical record suggestive of early cognitive decline. At this dedicated anticoagulation center, their INR was measured on a weekly or biweekly basis, as a result of which their average time spent in the therapeutic range of 2.0-3.0 was relatively high at nearly 70%.
The increased risk of dementia in patients with AF has previously been recognized. The association is stronger in patients under age 75 than in those who are older. But the mechanism has been unknown. Dr. Bunch and coinvestigators decided to test their hypothesis that the mechanism involves microbleeds secondary to long-term overanticoagulation by dividing the patients into three groups based upon their percentage of INR measurements above 3.0 during a mean follow-up of more than 4 and up to a maximum of 10 years: 240 patients had a supratherapeutic INR 25% of the time or more; 374 did so less than 10% of the time; and 417 had an elevated INR 10%-24% of the time.
The incidence of dementia diagnosed by a consultant neurologist during follow-up was 5.8% in the group with an INR above 3.0 at least 25% of the time, more than twice the 2.7% rate in patients with a high INR less than 10% of the time. In the middle group, the incidence of dementia was 4.1%. In a multivariate Cox regression analysis, having an INR above 3.0 on at least 25% of occasions was independently associated with a 2.59-fold increased risk of developing dementia, making it by far the most potent risk factor in their analysis.
The next step in their research will be to perform serial brain imaging and volumetric scans, Dr. Bunch said. Also, he and his coworkers are 3 years into an ongoing study looking at the incidence of dementia in AF patients on the various novel oral anticoagulants, where INR is a nonissue. Their hypothesis is the dementia risk will be lower than in patients on warfarin. Dr. Bunch has particularly high hopes for AF patients on apixaban (Eliquis) because it’s known to have a reduced risk of large bleeds, stroke, and GI bleeding; the hope is it will cause fewer cerebral microbleeds as well.
In an interview, the cardiologist said he believes his study showing an increased risk of dementia in AF patients with supratherapeutic INRs on warfarin plus antiplatelet therapy holds several important lessons for AF patients and physicians alike.
For patients, the message is don’t just start taking aspirin on your own because you’ve read it’s good for your heart or may reduce cancer risk; consult your physician.
And for physicians, it’s important to ask all patients on warfarin if they’re using aspirin; many don’t ask. Also, periodically reconsider the need for dual therapy with warfarin and aspirin.
“We find the risks of stroke and bleeding change dynamically over time, so the initial therapy for stroke prevention may not be the ideal therapy after 5-10 years,” Dr. Bunch said.
Lastly, for patients who are overanticoagulated on a substantial percentage of their INR measurements, it’s essential to consider a change in strategy. Either follow their INRs more closely and adjust warfarin dosing accordingly, or switch to one of the novel, more predictable oral anticoagulants, he concluded.
This study was funded internally by Intermountain Healthcare. Dr. Bunch reported having no financial conflicts.
CHICAGO – Patients with atrial fibrillation who frequently have a supratherapeutic international normalized ratio are at sharply increased risk for developing dementia, according to a large observational study.
“We postulate that the mechanism is an accumulation of microbleeds in the brain,” Dr. T. Jared Bunch said at the American Heart Association Scientific Sessions.
“In patients with hypertension, a condition that’s extremely common with atrial fibrillation, these repetitive small bleeds are preferentially in the hippocampus, where memory is stored,” added Dr. Bunch, who is medical director for heart rhythm services at the Intermountain Medical Center Heart Institute in Salt Lake City.
He presented a study of 1,031 patients with atrial fibrillation (AF) in Intermountain’s centralized anticoagulation service. All were on dual therapy with warfarin plus aspirin or, much less commonly, another antiplatelet agent. At baseline, their average age was in the early- to mid-70s, and none of the subjects had a history of stroke or any notes in their medical record suggestive of early cognitive decline. At this dedicated anticoagulation center, their INR was measured on a weekly or biweekly basis, as a result of which their average time spent in the therapeutic range of 2.0-3.0 was relatively high at nearly 70%.
The increased risk of dementia in patients with AF has previously been recognized. The association is stronger in patients under age 75 than in those who are older. But the mechanism has been unknown. Dr. Bunch and coinvestigators decided to test their hypothesis that the mechanism involves microbleeds secondary to long-term overanticoagulation by dividing the patients into three groups based upon their percentage of INR measurements above 3.0 during a mean follow-up of more than 4 and up to a maximum of 10 years: 240 patients had a supratherapeutic INR 25% of the time or more; 374 did so less than 10% of the time; and 417 had an elevated INR 10%-24% of the time.
The incidence of dementia diagnosed by a consultant neurologist during follow-up was 5.8% in the group with an INR above 3.0 at least 25% of the time, more than twice the 2.7% rate in patients with a high INR less than 10% of the time. In the middle group, the incidence of dementia was 4.1%. In a multivariate Cox regression analysis, having an INR above 3.0 on at least 25% of occasions was independently associated with a 2.59-fold increased risk of developing dementia, making it by far the most potent risk factor in their analysis.
The next step in their research will be to perform serial brain imaging and volumetric scans, Dr. Bunch said. Also, he and his coworkers are 3 years into an ongoing study looking at the incidence of dementia in AF patients on the various novel oral anticoagulants, where INR is a nonissue. Their hypothesis is the dementia risk will be lower than in patients on warfarin. Dr. Bunch has particularly high hopes for AF patients on apixaban (Eliquis) because it’s known to have a reduced risk of large bleeds, stroke, and GI bleeding; the hope is it will cause fewer cerebral microbleeds as well.
In an interview, the cardiologist said he believes his study showing an increased risk of dementia in AF patients with supratherapeutic INRs on warfarin plus antiplatelet therapy holds several important lessons for AF patients and physicians alike.
For patients, the message is don’t just start taking aspirin on your own because you’ve read it’s good for your heart or may reduce cancer risk; consult your physician.
And for physicians, it’s important to ask all patients on warfarin if they’re using aspirin; many don’t ask. Also, periodically reconsider the need for dual therapy with warfarin and aspirin.
“We find the risks of stroke and bleeding change dynamically over time, so the initial therapy for stroke prevention may not be the ideal therapy after 5-10 years,” Dr. Bunch said.
Lastly, for patients who are overanticoagulated on a substantial percentage of their INR measurements, it’s essential to consider a change in strategy. Either follow their INRs more closely and adjust warfarin dosing accordingly, or switch to one of the novel, more predictable oral anticoagulants, he concluded.
This study was funded internally by Intermountain Healthcare. Dr. Bunch reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: The more time patients with atrial fibrillation who are on warfarin plus aspirin spend with a supratherapeutic INR, the greater their risk of developing dementia.
Major finding: Atrial fibrillation patients on warfarin plus an antiplatelet agent who had an INR above 3.0 on at least 25% of occasions had a 5.8% incidence of dementia during follow-up, compared with a 2.7% incidence in those with a high INR less than 10% of the time.
Data source: This was a retrospective, case-control study involving 1,031 patients with atrial fibrillation on warfarin plus aspirin or another antiplatelet agent followed for a mean of more than 4 years.
Disclosures: This study was funded internally by Intermountain Healthcare. Dr. Bunch reported having no financial conflicts.