User login
Heart failure targets African Americans
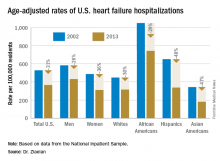
ORLANDO – The disparity in U.S. heart failure incidence continued undiminished during 2002-2013, with African Americans maintaining a steady 2.3-fold increased rate of heart failure, compared with whites, based on national levels of heart failure hospitalizations, a reasonable surrogate for incidence rates, Boback Ziaeian, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
The same period also showed a substantial relative improvement in the heart failure hospitalization rates among U.S. Hispanics, compared with whites, so that, by 2013, the ethnic disparity seen in 2002 between Hispanics and whites largely disappeared, reported Dr. Ziaeian, a cardiologist at the University of California, Los Angeles. The data he analyzed also showed that Asian Americans had the lowest heart failure hospitalization rates of any racial or ethnic group throughout the 11-year period, and that the incidence of heart failure fell more sharply in women than in men during the period, based on the hospitalization numbers.
Age-adjusted heart failure hospitalizations among whites dropped by 30%, and among African Americans by a nearly identical 29%. But this maintained a greater than twofold disparity in rates between the two groups. Among whites, the rate per 100,000 fell from 448 to 315; among African Americans, it dropped from 1,048 to 741. In 2013, the rate of heart failure hospitalizations was 2.4-fold higher in African Americans, compared with whites.
Heart failure hospitalizations fell among Hispanics from 650 per 100,000 to 337 per 100,000 in 2013, a 48% drop that brought the rate among Hispanics to nearly the same as among whites. Asian Americans remained the group with the least heart failure throughout the period, falling from 343 hospitalizations per 100,000 in 2002 to 181 per 100,000 in 2013, a 47% drop.
Among women, the age-adjusted rate per 100,000 fell from 486 to 311, a 36% drop, compared with a decrease from 582 to 431 per 100,000 in men, a 26% reduction. Lower incidence in women may reflect better risk factor control during the study period, compared with men, such as a higher rate of quiting smoking and better treatment compliance, Dr. Ziaeian suggested.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ORLANDO – The disparity in U.S. heart failure incidence continued undiminished during 2002-2013, with African Americans maintaining a steady 2.3-fold increased rate of heart failure, compared with whites, based on national levels of heart failure hospitalizations, a reasonable surrogate for incidence rates, Boback Ziaeian, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
The same period also showed a substantial relative improvement in the heart failure hospitalization rates among U.S. Hispanics, compared with whites, so that, by 2013, the ethnic disparity seen in 2002 between Hispanics and whites largely disappeared, reported Dr. Ziaeian, a cardiologist at the University of California, Los Angeles. The data he analyzed also showed that Asian Americans had the lowest heart failure hospitalization rates of any racial or ethnic group throughout the 11-year period, and that the incidence of heart failure fell more sharply in women than in men during the period, based on the hospitalization numbers.
Age-adjusted heart failure hospitalizations among whites dropped by 30%, and among African Americans by a nearly identical 29%. But this maintained a greater than twofold disparity in rates between the two groups. Among whites, the rate per 100,000 fell from 448 to 315; among African Americans, it dropped from 1,048 to 741. In 2013, the rate of heart failure hospitalizations was 2.4-fold higher in African Americans, compared with whites.
Heart failure hospitalizations fell among Hispanics from 650 per 100,000 to 337 per 100,000 in 2013, a 48% drop that brought the rate among Hispanics to nearly the same as among whites. Asian Americans remained the group with the least heart failure throughout the period, falling from 343 hospitalizations per 100,000 in 2002 to 181 per 100,000 in 2013, a 47% drop.
Among women, the age-adjusted rate per 100,000 fell from 486 to 311, a 36% drop, compared with a decrease from 582 to 431 per 100,000 in men, a 26% reduction. Lower incidence in women may reflect better risk factor control during the study period, compared with men, such as a higher rate of quiting smoking and better treatment compliance, Dr. Ziaeian suggested.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ORLANDO – The disparity in U.S. heart failure incidence continued undiminished during 2002-2013, with African Americans maintaining a steady 2.3-fold increased rate of heart failure, compared with whites, based on national levels of heart failure hospitalizations, a reasonable surrogate for incidence rates, Boback Ziaeian, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
The same period also showed a substantial relative improvement in the heart failure hospitalization rates among U.S. Hispanics, compared with whites, so that, by 2013, the ethnic disparity seen in 2002 between Hispanics and whites largely disappeared, reported Dr. Ziaeian, a cardiologist at the University of California, Los Angeles. The data he analyzed also showed that Asian Americans had the lowest heart failure hospitalization rates of any racial or ethnic group throughout the 11-year period, and that the incidence of heart failure fell more sharply in women than in men during the period, based on the hospitalization numbers.
Age-adjusted heart failure hospitalizations among whites dropped by 30%, and among African Americans by a nearly identical 29%. But this maintained a greater than twofold disparity in rates between the two groups. Among whites, the rate per 100,000 fell from 448 to 315; among African Americans, it dropped from 1,048 to 741. In 2013, the rate of heart failure hospitalizations was 2.4-fold higher in African Americans, compared with whites.
Heart failure hospitalizations fell among Hispanics from 650 per 100,000 to 337 per 100,000 in 2013, a 48% drop that brought the rate among Hispanics to nearly the same as among whites. Asian Americans remained the group with the least heart failure throughout the period, falling from 343 hospitalizations per 100,000 in 2002 to 181 per 100,000 in 2013, a 47% drop.
Among women, the age-adjusted rate per 100,000 fell from 486 to 311, a 36% drop, compared with a decrease from 582 to 431 per 100,000 in men, a 26% reduction. Lower incidence in women may reflect better risk factor control during the study period, compared with men, such as a higher rate of quiting smoking and better treatment compliance, Dr. Ziaeian suggested.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: In 2013, age-adjusted heart failure hospitalization was 741/100,000 in African Americans and 315/100,000 in whites.
Data source: The National Inpatient Sample and U.S. Census data.
Disclosures: Dr. Ziaeian had no disclosures.
Adaptive servo ventilation cuts atrial fib burden
ORLANDO – Adaptive servo ventilation produced a significant and clinically meaningful reduction in atrial fibrillation burden in patients with heart failure and sleep apnea in results from an exploratory, prospective, randomized study with 35 patients.
Adaptive servo ventilation (ASV) “may be an effective antiarrhythmic treatment producing a significant reduction in atrial fibrillation without clear evidence of being proarrhythmogenic,” Jonathan P. Piccini, MD, said at the annual scientific meeting of the Heart Failure Society of America. “Given the potential importance of this finding further studies should validate and quantify the efficacy of ASV for reducing atrial fibrillation in patients with or without heart failure.”
“A mound of data has shown that treating sleep apnea reduced arrhythmias, but until now it’s all been observational and retrospective,” Dr. Piccini, an electrophysiologist at Duke University in Durham, N.C., said in an interview. The study he reported is “the first time” the arrhythmia effects of a sleep apnea intervention, in this case ASV, was studied in a prospective, randomized way while using implanted devices to measure the antiarrhythmic effect of the treatment.
The new finding means that additional, larger studies are now needed, he said. “If patients have sleep apnea, treating the apnea may be an incredibly important way to prevent AF or reduce its burden”
The CAT-HF (Cardiovascular Improvements With Minute Ventilation-Targeted ASV Therapy in Heart Failure) trial was originally designed to randomize 215 heart failure patients with sleep disordered breathing – and who were hospitalized for heart failure – to optimal medical therapy with or without ASV at any of 15 centers in the United States and Germany. But in August 2015, results from the SERVE-HF (Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure) trial, which generally had a similar design to CAT-HF, showed an unexpected danger from ASV in patients with central sleep apnea and heart failure with reduced ejection fraction (N Engl J Med. 2015 Sept 17;373[12]:1095-105). In SERVE-HF, ASV was associated with significant increases in all-cause and cardiovascular mortality. As a result, enrollment into CAT-HF stopped prematurely with just 126 patients entered, and ASV treatment of patients already enrolled came to a halt.
The primary endpoint in the underpowered and shortened CAT-HF study, survival without cardiovascular hospitalization and with improved functional capacity measured on a 6-minute walk test, showed similar outcomes in both the ASV and control arms. But in a prespecified subgroup analysis by baseline ejection fraction, the 24 patients with heart failure with preserved ejection fraction (19% of the CAT-HF enrollment) showed a statistically significant, 62% relative improvement in the primary endpoint linked with ASV treatment compared with similar patients who did not receive ASV, Christopher M. O’Connor, MD, professor of medicine at Duke University, reported in May 2016 at the European Heart Failure meeting in Florence.
Dr. Piccini’s report focused on a prespecified subgroup analysis of CAT-HF designed to examine the impact of ASV on arrhythmias. Assessment of the impact of ASV on atrial fibrillation was possible in 35 of the 126 patients in CAT-HF who had an implanted cardiac device (pacemaker, defibrillator, or cardiac resynchronization device) with an atrial lead, and assessment of ventricular arrhythmias occurred in 46 of the CAT-HF patients with an implanted high-voltage device (a defibrillator or resynchronization device) that allowed monitoring of ventricular arrhythmias.
For the atrial fibrillation analysis, the 35 patients averaged 60 years of age, and about 90% had a reduced ejection fraction. About two-thirds had an apnea-hypopnea index greater than 30.
The results showed that the 19 patients randomized to receive ASV had an average atrial fibrillation burden of 30% at baseline that dropped to 14% after 6 months of treatment. In contrast, the 16 patients in the control arm had a AF burden of 6% at baseline and 8% after 6 months. The between-group difference for change in AF burden was statistically significant, Dr. Piccini reported, with a burden that decreased by a relative 21% with ASV treatment and increased by a relative 31% in the control arm.
Analysis of the ventricular arrhythmia subgroup showed that ASV had no statistically significant impact for either lowering or raising ventricular tachyarrhythmias or fibrillations.
Trying to reconcile this AF benefit and lack of ventricular arrhythmia harm from ASV in CAT-HF with the excess in cardiovascular deaths seen with ASV in SERVE-HF, Dr. Piccini speculated that some of the SERVE-HF deaths may not have been related to arrhythmia.
“Sudden cardiac death adjudication is profoundly difficult, and does not always equal ventricular arrhythmia,” he said. “We need to consider that some of the adverse events in patients with severe central sleep apnea and low left ventricular ejection fraction [enrolled in SERVE-HF] may have been due to causes other than arrhythmias. The CAT-HF results should motivate investigations of alternative mechanisms of death in SERVE-HF.”
The CAT-HF trial was funded by ResMed, a company that markets adaptive servo ventilation equipment. Dr. Piccini has received research support from ResMed and from Janssen, Gilead, St. Jude, Spectranetics, and he has been a consultant to Janssen, Spectranetics, Medtronic, GSK and BMS-Pfizer. Dr. O’Connor has been a consultant to ResMed and to several other drug and device companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
A small prespecified sub-group of patients in the CAT-HF (Cardiovascuar improvements with minute ventilation-targeted ASV therapy in heart failure) trial randomized to adaptive servo ventilation (ASV) showed a 21% relative reduction in atrial fibrillation burden as compared to the control arm which had only 31% relative reduction. While the CAT-HF study was discontinued following results of SERVE-HF trial, this subgroup analysis included 35 patients (19 ASV arm; 16 control arm), the majority of whom had a reduced ejection fraction. This report poses interesting questions about effects of ASV on atrial fibrillation burden in those with reduced EF given the finding that central sleep apnea and Cheyne-Stokes respiration are shown to be associated with incident atrial fibrillation in older men (May et al. Am J Respir Crit Care Med 2016).
A small prespecified sub-group of patients in the CAT-HF (Cardiovascuar improvements with minute ventilation-targeted ASV therapy in heart failure) trial randomized to adaptive servo ventilation (ASV) showed a 21% relative reduction in atrial fibrillation burden as compared to the control arm which had only 31% relative reduction. While the CAT-HF study was discontinued following results of SERVE-HF trial, this subgroup analysis included 35 patients (19 ASV arm; 16 control arm), the majority of whom had a reduced ejection fraction. This report poses interesting questions about effects of ASV on atrial fibrillation burden in those with reduced EF given the finding that central sleep apnea and Cheyne-Stokes respiration are shown to be associated with incident atrial fibrillation in older men (May et al. Am J Respir Crit Care Med 2016).
A small prespecified sub-group of patients in the CAT-HF (Cardiovascuar improvements with minute ventilation-targeted ASV therapy in heart failure) trial randomized to adaptive servo ventilation (ASV) showed a 21% relative reduction in atrial fibrillation burden as compared to the control arm which had only 31% relative reduction. While the CAT-HF study was discontinued following results of SERVE-HF trial, this subgroup analysis included 35 patients (19 ASV arm; 16 control arm), the majority of whom had a reduced ejection fraction. This report poses interesting questions about effects of ASV on atrial fibrillation burden in those with reduced EF given the finding that central sleep apnea and Cheyne-Stokes respiration are shown to be associated with incident atrial fibrillation in older men (May et al. Am J Respir Crit Care Med 2016).
ORLANDO – Adaptive servo ventilation produced a significant and clinically meaningful reduction in atrial fibrillation burden in patients with heart failure and sleep apnea in results from an exploratory, prospective, randomized study with 35 patients.
Adaptive servo ventilation (ASV) “may be an effective antiarrhythmic treatment producing a significant reduction in atrial fibrillation without clear evidence of being proarrhythmogenic,” Jonathan P. Piccini, MD, said at the annual scientific meeting of the Heart Failure Society of America. “Given the potential importance of this finding further studies should validate and quantify the efficacy of ASV for reducing atrial fibrillation in patients with or without heart failure.”
“A mound of data has shown that treating sleep apnea reduced arrhythmias, but until now it’s all been observational and retrospective,” Dr. Piccini, an electrophysiologist at Duke University in Durham, N.C., said in an interview. The study he reported is “the first time” the arrhythmia effects of a sleep apnea intervention, in this case ASV, was studied in a prospective, randomized way while using implanted devices to measure the antiarrhythmic effect of the treatment.
The new finding means that additional, larger studies are now needed, he said. “If patients have sleep apnea, treating the apnea may be an incredibly important way to prevent AF or reduce its burden”
The CAT-HF (Cardiovascular Improvements With Minute Ventilation-Targeted ASV Therapy in Heart Failure) trial was originally designed to randomize 215 heart failure patients with sleep disordered breathing – and who were hospitalized for heart failure – to optimal medical therapy with or without ASV at any of 15 centers in the United States and Germany. But in August 2015, results from the SERVE-HF (Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure) trial, which generally had a similar design to CAT-HF, showed an unexpected danger from ASV in patients with central sleep apnea and heart failure with reduced ejection fraction (N Engl J Med. 2015 Sept 17;373[12]:1095-105). In SERVE-HF, ASV was associated with significant increases in all-cause and cardiovascular mortality. As a result, enrollment into CAT-HF stopped prematurely with just 126 patients entered, and ASV treatment of patients already enrolled came to a halt.
The primary endpoint in the underpowered and shortened CAT-HF study, survival without cardiovascular hospitalization and with improved functional capacity measured on a 6-minute walk test, showed similar outcomes in both the ASV and control arms. But in a prespecified subgroup analysis by baseline ejection fraction, the 24 patients with heart failure with preserved ejection fraction (19% of the CAT-HF enrollment) showed a statistically significant, 62% relative improvement in the primary endpoint linked with ASV treatment compared with similar patients who did not receive ASV, Christopher M. O’Connor, MD, professor of medicine at Duke University, reported in May 2016 at the European Heart Failure meeting in Florence.
Dr. Piccini’s report focused on a prespecified subgroup analysis of CAT-HF designed to examine the impact of ASV on arrhythmias. Assessment of the impact of ASV on atrial fibrillation was possible in 35 of the 126 patients in CAT-HF who had an implanted cardiac device (pacemaker, defibrillator, or cardiac resynchronization device) with an atrial lead, and assessment of ventricular arrhythmias occurred in 46 of the CAT-HF patients with an implanted high-voltage device (a defibrillator or resynchronization device) that allowed monitoring of ventricular arrhythmias.
For the atrial fibrillation analysis, the 35 patients averaged 60 years of age, and about 90% had a reduced ejection fraction. About two-thirds had an apnea-hypopnea index greater than 30.
The results showed that the 19 patients randomized to receive ASV had an average atrial fibrillation burden of 30% at baseline that dropped to 14% after 6 months of treatment. In contrast, the 16 patients in the control arm had a AF burden of 6% at baseline and 8% after 6 months. The between-group difference for change in AF burden was statistically significant, Dr. Piccini reported, with a burden that decreased by a relative 21% with ASV treatment and increased by a relative 31% in the control arm.
Analysis of the ventricular arrhythmia subgroup showed that ASV had no statistically significant impact for either lowering or raising ventricular tachyarrhythmias or fibrillations.
Trying to reconcile this AF benefit and lack of ventricular arrhythmia harm from ASV in CAT-HF with the excess in cardiovascular deaths seen with ASV in SERVE-HF, Dr. Piccini speculated that some of the SERVE-HF deaths may not have been related to arrhythmia.
“Sudden cardiac death adjudication is profoundly difficult, and does not always equal ventricular arrhythmia,” he said. “We need to consider that some of the adverse events in patients with severe central sleep apnea and low left ventricular ejection fraction [enrolled in SERVE-HF] may have been due to causes other than arrhythmias. The CAT-HF results should motivate investigations of alternative mechanisms of death in SERVE-HF.”
The CAT-HF trial was funded by ResMed, a company that markets adaptive servo ventilation equipment. Dr. Piccini has received research support from ResMed and from Janssen, Gilead, St. Jude, Spectranetics, and he has been a consultant to Janssen, Spectranetics, Medtronic, GSK and BMS-Pfizer. Dr. O’Connor has been a consultant to ResMed and to several other drug and device companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ORLANDO – Adaptive servo ventilation produced a significant and clinically meaningful reduction in atrial fibrillation burden in patients with heart failure and sleep apnea in results from an exploratory, prospective, randomized study with 35 patients.
Adaptive servo ventilation (ASV) “may be an effective antiarrhythmic treatment producing a significant reduction in atrial fibrillation without clear evidence of being proarrhythmogenic,” Jonathan P. Piccini, MD, said at the annual scientific meeting of the Heart Failure Society of America. “Given the potential importance of this finding further studies should validate and quantify the efficacy of ASV for reducing atrial fibrillation in patients with or without heart failure.”
“A mound of data has shown that treating sleep apnea reduced arrhythmias, but until now it’s all been observational and retrospective,” Dr. Piccini, an electrophysiologist at Duke University in Durham, N.C., said in an interview. The study he reported is “the first time” the arrhythmia effects of a sleep apnea intervention, in this case ASV, was studied in a prospective, randomized way while using implanted devices to measure the antiarrhythmic effect of the treatment.
The new finding means that additional, larger studies are now needed, he said. “If patients have sleep apnea, treating the apnea may be an incredibly important way to prevent AF or reduce its burden”
The CAT-HF (Cardiovascular Improvements With Minute Ventilation-Targeted ASV Therapy in Heart Failure) trial was originally designed to randomize 215 heart failure patients with sleep disordered breathing – and who were hospitalized for heart failure – to optimal medical therapy with or without ASV at any of 15 centers in the United States and Germany. But in August 2015, results from the SERVE-HF (Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure) trial, which generally had a similar design to CAT-HF, showed an unexpected danger from ASV in patients with central sleep apnea and heart failure with reduced ejection fraction (N Engl J Med. 2015 Sept 17;373[12]:1095-105). In SERVE-HF, ASV was associated with significant increases in all-cause and cardiovascular mortality. As a result, enrollment into CAT-HF stopped prematurely with just 126 patients entered, and ASV treatment of patients already enrolled came to a halt.
The primary endpoint in the underpowered and shortened CAT-HF study, survival without cardiovascular hospitalization and with improved functional capacity measured on a 6-minute walk test, showed similar outcomes in both the ASV and control arms. But in a prespecified subgroup analysis by baseline ejection fraction, the 24 patients with heart failure with preserved ejection fraction (19% of the CAT-HF enrollment) showed a statistically significant, 62% relative improvement in the primary endpoint linked with ASV treatment compared with similar patients who did not receive ASV, Christopher M. O’Connor, MD, professor of medicine at Duke University, reported in May 2016 at the European Heart Failure meeting in Florence.
Dr. Piccini’s report focused on a prespecified subgroup analysis of CAT-HF designed to examine the impact of ASV on arrhythmias. Assessment of the impact of ASV on atrial fibrillation was possible in 35 of the 126 patients in CAT-HF who had an implanted cardiac device (pacemaker, defibrillator, or cardiac resynchronization device) with an atrial lead, and assessment of ventricular arrhythmias occurred in 46 of the CAT-HF patients with an implanted high-voltage device (a defibrillator or resynchronization device) that allowed monitoring of ventricular arrhythmias.
For the atrial fibrillation analysis, the 35 patients averaged 60 years of age, and about 90% had a reduced ejection fraction. About two-thirds had an apnea-hypopnea index greater than 30.
The results showed that the 19 patients randomized to receive ASV had an average atrial fibrillation burden of 30% at baseline that dropped to 14% after 6 months of treatment. In contrast, the 16 patients in the control arm had a AF burden of 6% at baseline and 8% after 6 months. The between-group difference for change in AF burden was statistically significant, Dr. Piccini reported, with a burden that decreased by a relative 21% with ASV treatment and increased by a relative 31% in the control arm.
Analysis of the ventricular arrhythmia subgroup showed that ASV had no statistically significant impact for either lowering or raising ventricular tachyarrhythmias or fibrillations.
Trying to reconcile this AF benefit and lack of ventricular arrhythmia harm from ASV in CAT-HF with the excess in cardiovascular deaths seen with ASV in SERVE-HF, Dr. Piccini speculated that some of the SERVE-HF deaths may not have been related to arrhythmia.
“Sudden cardiac death adjudication is profoundly difficult, and does not always equal ventricular arrhythmia,” he said. “We need to consider that some of the adverse events in patients with severe central sleep apnea and low left ventricular ejection fraction [enrolled in SERVE-HF] may have been due to causes other than arrhythmias. The CAT-HF results should motivate investigations of alternative mechanisms of death in SERVE-HF.”
The CAT-HF trial was funded by ResMed, a company that markets adaptive servo ventilation equipment. Dr. Piccini has received research support from ResMed and from Janssen, Gilead, St. Jude, Spectranetics, and he has been a consultant to Janssen, Spectranetics, Medtronic, GSK and BMS-Pfizer. Dr. O’Connor has been a consultant to ResMed and to several other drug and device companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Key clinical point:
Major finding: After 6 months, ASV produced a relative 21% drop in atrial fibrillation burden, compared with increased burden in control patients.
Data source: CAT-HF, a multicenter randomized trial that enrolled 126 heart failure patients with sleep apnea.
Disclosures: The CAT-HF trial was funded by ResMed, a company that markets adaptive servo ventilation equipment. Dr. Piccini has received research support and/or consultant fees from ResMed, Janssen, Gilead, St. Jude, Spectranetics, Medtronic, GSK and BMS-Pfizer.
Advanced heart failure symptoms linked to mortality
ORLANDO – Advanced heart failure patients who are hospitalized for heart failure and have a higher symptom burden at discharge have a significantly increased rate of death or rehospitalization over the next 6 months, based on an analysis of 393 patients enrolled in a heart failure trial.
The strong link between severe symptom burden and poor near-term outcomes persisted despite adjustment for various markers of heart failure severity, suggesting that treatment aimed at reducing symptoms may be able to reduce mortality or heart failure hospitalization in advanced heart failure patients, Ellen K. Hummel, MD, said at the annual scientific meeting of the Heart Failure Society of America.
In her analysis, a severe symptom burden at the time of hospital discharge linked with an adjusted 2.9-fold increased mortality rate and a 2.5-fold increased rate of days dead or hospitalized during the next 6 months, said Dr. Hummel, a geriatric and palliative care specialist at the University of Michigan in Ann Arbor. These elevated rate ratios for patients with severe symptoms at hospital discharge were in comparison to the ratios for advanced heart failure patients in the study with no symptoms at discharge.
Three symptoms contributed to the symptom score she used in her analysis: fatigue, scored on a scale of 0-3; dyspnea, also scored 0-3; and gastrointestinal distress, scored as 0-2, creating a maximum score of 8. Her analysis categorized mild as a total score of 1-4 and severe as 5 or greater. In the study population she used for her analysis, patients enrolled in the multicenter ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial, 111 of the 393 evaluable patients (28%) had none of these symptoms, 239 (61%) had mild symptoms, and 43 (11%) had severe symptoms. Scoring was done by patients based on their subjective self-assessment at the time of hospital discharge.
The absolute, observed 6-month mortality rates were roughly 45% among patients with severe symptoms, about 17% in patients with mild symptoms, and about 12% in those with no symptoms.
The primary purpose of ESCAPE was to assess the impact that routine collection of data from a pulmonary artery catheter during hospitalization has on outcomes; the results showed no significant link between improved outcomes and getting these data (JAMA. 2005 Oct 5;294[13]:1625-33). The study ran during 2000-2003 at 26 centers in the United States and Canada. Of the 433 advanced heart failure patients enrolled in ESCAPE, 393 had complete records to allow the current analysis.
The adjustments that Dr. Hummel made in the proportional hazard analysis took into account New York Heart Association class, and severity of disease at the time of hospital discharge measured by the ESCAPE Discharge Risk Score. This score takes into account age, 6-minute walk distance, blood urea nitrogen, brain natriuretic peptide levels, blood pressure, selected drug treatments, sodium level, and history of cardiopulmonary resuscitation or mechanical ventilation.
Dr. Hummel had no relevant financial disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ORLANDO – Advanced heart failure patients who are hospitalized for heart failure and have a higher symptom burden at discharge have a significantly increased rate of death or rehospitalization over the next 6 months, based on an analysis of 393 patients enrolled in a heart failure trial.
The strong link between severe symptom burden and poor near-term outcomes persisted despite adjustment for various markers of heart failure severity, suggesting that treatment aimed at reducing symptoms may be able to reduce mortality or heart failure hospitalization in advanced heart failure patients, Ellen K. Hummel, MD, said at the annual scientific meeting of the Heart Failure Society of America.
In her analysis, a severe symptom burden at the time of hospital discharge linked with an adjusted 2.9-fold increased mortality rate and a 2.5-fold increased rate of days dead or hospitalized during the next 6 months, said Dr. Hummel, a geriatric and palliative care specialist at the University of Michigan in Ann Arbor. These elevated rate ratios for patients with severe symptoms at hospital discharge were in comparison to the ratios for advanced heart failure patients in the study with no symptoms at discharge.
Three symptoms contributed to the symptom score she used in her analysis: fatigue, scored on a scale of 0-3; dyspnea, also scored 0-3; and gastrointestinal distress, scored as 0-2, creating a maximum score of 8. Her analysis categorized mild as a total score of 1-4 and severe as 5 or greater. In the study population she used for her analysis, patients enrolled in the multicenter ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial, 111 of the 393 evaluable patients (28%) had none of these symptoms, 239 (61%) had mild symptoms, and 43 (11%) had severe symptoms. Scoring was done by patients based on their subjective self-assessment at the time of hospital discharge.
The absolute, observed 6-month mortality rates were roughly 45% among patients with severe symptoms, about 17% in patients with mild symptoms, and about 12% in those with no symptoms.
The primary purpose of ESCAPE was to assess the impact that routine collection of data from a pulmonary artery catheter during hospitalization has on outcomes; the results showed no significant link between improved outcomes and getting these data (JAMA. 2005 Oct 5;294[13]:1625-33). The study ran during 2000-2003 at 26 centers in the United States and Canada. Of the 433 advanced heart failure patients enrolled in ESCAPE, 393 had complete records to allow the current analysis.
The adjustments that Dr. Hummel made in the proportional hazard analysis took into account New York Heart Association class, and severity of disease at the time of hospital discharge measured by the ESCAPE Discharge Risk Score. This score takes into account age, 6-minute walk distance, blood urea nitrogen, brain natriuretic peptide levels, blood pressure, selected drug treatments, sodium level, and history of cardiopulmonary resuscitation or mechanical ventilation.
Dr. Hummel had no relevant financial disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ORLANDO – Advanced heart failure patients who are hospitalized for heart failure and have a higher symptom burden at discharge have a significantly increased rate of death or rehospitalization over the next 6 months, based on an analysis of 393 patients enrolled in a heart failure trial.
The strong link between severe symptom burden and poor near-term outcomes persisted despite adjustment for various markers of heart failure severity, suggesting that treatment aimed at reducing symptoms may be able to reduce mortality or heart failure hospitalization in advanced heart failure patients, Ellen K. Hummel, MD, said at the annual scientific meeting of the Heart Failure Society of America.
In her analysis, a severe symptom burden at the time of hospital discharge linked with an adjusted 2.9-fold increased mortality rate and a 2.5-fold increased rate of days dead or hospitalized during the next 6 months, said Dr. Hummel, a geriatric and palliative care specialist at the University of Michigan in Ann Arbor. These elevated rate ratios for patients with severe symptoms at hospital discharge were in comparison to the ratios for advanced heart failure patients in the study with no symptoms at discharge.
Three symptoms contributed to the symptom score she used in her analysis: fatigue, scored on a scale of 0-3; dyspnea, also scored 0-3; and gastrointestinal distress, scored as 0-2, creating a maximum score of 8. Her analysis categorized mild as a total score of 1-4 and severe as 5 or greater. In the study population she used for her analysis, patients enrolled in the multicenter ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial, 111 of the 393 evaluable patients (28%) had none of these symptoms, 239 (61%) had mild symptoms, and 43 (11%) had severe symptoms. Scoring was done by patients based on their subjective self-assessment at the time of hospital discharge.
The absolute, observed 6-month mortality rates were roughly 45% among patients with severe symptoms, about 17% in patients with mild symptoms, and about 12% in those with no symptoms.
The primary purpose of ESCAPE was to assess the impact that routine collection of data from a pulmonary artery catheter during hospitalization has on outcomes; the results showed no significant link between improved outcomes and getting these data (JAMA. 2005 Oct 5;294[13]:1625-33). The study ran during 2000-2003 at 26 centers in the United States and Canada. Of the 433 advanced heart failure patients enrolled in ESCAPE, 393 had complete records to allow the current analysis.
The adjustments that Dr. Hummel made in the proportional hazard analysis took into account New York Heart Association class, and severity of disease at the time of hospital discharge measured by the ESCAPE Discharge Risk Score. This score takes into account age, 6-minute walk distance, blood urea nitrogen, brain natriuretic peptide levels, blood pressure, selected drug treatments, sodium level, and history of cardiopulmonary resuscitation or mechanical ventilation.
Dr. Hummel had no relevant financial disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: Patients with severe symptoms at discharge had a 2.9-fold increased rate of death, compared with those with no symptoms.
Data source: A post hoc analysis of data collected from 393 patients enrolled in the ESCAPE trial.
Disclosures: Dr. Hummel had no relevant financial disclosures.
Palliative care boosts heart failure patient outcomes
ORLANDO – Systematic introduction of palliative care interventions for patients with advanced heart failure improved patients’ quality of life and spurred their development of advanced-care preferences in a pair of independently performed, controlled, pilot studies.
But, despite demonstrating the ability of palliative-care interventions to help heart failure patients during their final months of life, the findings raised questions about the generalizability and reproducibility of palliative-care interventions that may depend on the skills and experience of the individual specialists who deliver the palliative care.
“Palliative care for patients with cardiovascular disease is in desperate need of good-quality evidence,” commented Larry A, Allen, MD, a heart failure cardiologist at the University of Colorado in Aurora and designated discussant for one of the two studies presented at the meeting. “We need large, randomized trials with clinical outcomes to look at patient outcomes from palliative-care interventions.”
The patients average 71 years old, about half were women, and about 40% were African Americans. They had been diagnosed with heart failure for an average of more than 5 years, all had advanced heart failure, about 60% spent at least half of their time awake immobilized in a bed or chair, and they had average NT-proBNP blood levels of greater than 10,000 pg/mL.
After 24 weeks of intervention, the palliative-care program produced both statistically significant and clinically meaningful improvements in two different measures of health-related quality of life, the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Functional Assessment of Chronic Illness Therapy – Palliative Care (FACIT-PAL). The KCCQ showed the palliative care intervention linked with an average rise of more than 9 points compared with patients in the control arm after adjustment for age and sex, a statistically significant increase on a scale where a 5-point rise is considered clinically meaningful. The FACIT-PAL showed an average, adjusted 11-point rise linked with the intervention, a statistically significant increase on a scale where an increase of at least 10 is judged clinically meaningful, reported Dr. Rogers, a heart failure cardiologist and professor of medicine at Duke University.
The palliative-care intervention also led to significant improvements in measures of spirituality, depression, and anxiety, but intervention had no impact on mortality.
“I like these endpoints and the idea that we can make quality-of-life better. These are very sick patients, with a predicted 6-month mortality of 50%. Patients reach a time when they don’t want to live longer but want better life quality for the days they still have,” he said in an interview.
The second report came from a single-center pilot study of 50 patients enrolled when they were hospitalized for acute decompensated heart failure and had at least one addition risk factor for poor prognosis such as age of at least 81 years, renal dysfunction, or a prior heart failure hospitalization within the past year. Patients randomized to the intervention arm underwent a structured evaluation based on the Serious Illness Conversation Guide and performed by a social worker experienced in palliative care and embedded in the heart failure clinical team. The primary endpoint of the SWAP-HF (Social Worker–Aided Palliative Care Intervention in High Risk Patients with Heart Failure) study was clinical-level documentation of advanced-care preferences by 6 months after the program began.
“Although more comprehensive, multidisciplinary palliative care interventions may also be effective, the focused approach [used in this study] may represent a cost-effective and scalable method for shepherding limited specialty resources to enhance the delivery of patient-centered care,” Dr. Desai said. In other words, a program with a social worker costs less than a two-person staff with a palliative-care physician and nurse practitioner.
Despite its relative simplicity, the SWAP-HF intervention had some unique aspects that make it generalizability uncertain, commented Dr. Allen. The embedding of a social worker on the heart failure team placed a professional with a “good understanding of social context” right on the scene with everyone else delivering care to the heart failure patient, a good strategy for minimizing fragmentation, he said. In addition, the place where the study was done, Brigham and Women’s Hospital, “is not your average hospital,” he noted,
In addition, the timing of the intervention studied during hospitalization may be problematic. Clinicians need to “be careful about patients making long-term decisions” about their care while they are hospitalized, a time when patients can be “ill, confused, and scared.” He cited recent findings from a study of hospital-based palliative-care interventions for family members of patients with chronic critical illness that did not reduce anxiety or depression symptoms among the treated family members and may have increased symptoms of posttraumatic stress disorder (JAMA. 2016 July 5;374[1]:51-62).
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
It’s very exciting to have these two studies presented at the Heart Failure Society of America’s annual meeting. Palliative-care research now receives funding from the National Institutes of Health, but consistently and successfully integrating palliative care into heart failure management still has a long way to go. In 2004, my colleagues and I published a set of consensus recommendations on how to apply palliative care methods to patients with advanced heart failure and what research needs existed for the field (J Card Fail. 2004 June;10[3]:200-9). Today, 12 years later, many of those research needs remain inadequately addressed.
Sarah J. Goodlin, MD , is chief of geriatrics at the Portland (Ore.) VA Medical Center. She had no disclosures. She made these comments as the designated discussant for Dr. Rogers’ report.
It’s very exciting to have these two studies presented at the Heart Failure Society of America’s annual meeting. Palliative-care research now receives funding from the National Institutes of Health, but consistently and successfully integrating palliative care into heart failure management still has a long way to go. In 2004, my colleagues and I published a set of consensus recommendations on how to apply palliative care methods to patients with advanced heart failure and what research needs existed for the field (J Card Fail. 2004 June;10[3]:200-9). Today, 12 years later, many of those research needs remain inadequately addressed.
Sarah J. Goodlin, MD , is chief of geriatrics at the Portland (Ore.) VA Medical Center. She had no disclosures. She made these comments as the designated discussant for Dr. Rogers’ report.
It’s very exciting to have these two studies presented at the Heart Failure Society of America’s annual meeting. Palliative-care research now receives funding from the National Institutes of Health, but consistently and successfully integrating palliative care into heart failure management still has a long way to go. In 2004, my colleagues and I published a set of consensus recommendations on how to apply palliative care methods to patients with advanced heart failure and what research needs existed for the field (J Card Fail. 2004 June;10[3]:200-9). Today, 12 years later, many of those research needs remain inadequately addressed.
Sarah J. Goodlin, MD , is chief of geriatrics at the Portland (Ore.) VA Medical Center. She had no disclosures. She made these comments as the designated discussant for Dr. Rogers’ report.
ORLANDO – Systematic introduction of palliative care interventions for patients with advanced heart failure improved patients’ quality of life and spurred their development of advanced-care preferences in a pair of independently performed, controlled, pilot studies.
But, despite demonstrating the ability of palliative-care interventions to help heart failure patients during their final months of life, the findings raised questions about the generalizability and reproducibility of palliative-care interventions that may depend on the skills and experience of the individual specialists who deliver the palliative care.
“Palliative care for patients with cardiovascular disease is in desperate need of good-quality evidence,” commented Larry A, Allen, MD, a heart failure cardiologist at the University of Colorado in Aurora and designated discussant for one of the two studies presented at the meeting. “We need large, randomized trials with clinical outcomes to look at patient outcomes from palliative-care interventions.”
The patients average 71 years old, about half were women, and about 40% were African Americans. They had been diagnosed with heart failure for an average of more than 5 years, all had advanced heart failure, about 60% spent at least half of their time awake immobilized in a bed or chair, and they had average NT-proBNP blood levels of greater than 10,000 pg/mL.
After 24 weeks of intervention, the palliative-care program produced both statistically significant and clinically meaningful improvements in two different measures of health-related quality of life, the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Functional Assessment of Chronic Illness Therapy – Palliative Care (FACIT-PAL). The KCCQ showed the palliative care intervention linked with an average rise of more than 9 points compared with patients in the control arm after adjustment for age and sex, a statistically significant increase on a scale where a 5-point rise is considered clinically meaningful. The FACIT-PAL showed an average, adjusted 11-point rise linked with the intervention, a statistically significant increase on a scale where an increase of at least 10 is judged clinically meaningful, reported Dr. Rogers, a heart failure cardiologist and professor of medicine at Duke University.
The palliative-care intervention also led to significant improvements in measures of spirituality, depression, and anxiety, but intervention had no impact on mortality.
“I like these endpoints and the idea that we can make quality-of-life better. These are very sick patients, with a predicted 6-month mortality of 50%. Patients reach a time when they don’t want to live longer but want better life quality for the days they still have,” he said in an interview.
The second report came from a single-center pilot study of 50 patients enrolled when they were hospitalized for acute decompensated heart failure and had at least one addition risk factor for poor prognosis such as age of at least 81 years, renal dysfunction, or a prior heart failure hospitalization within the past year. Patients randomized to the intervention arm underwent a structured evaluation based on the Serious Illness Conversation Guide and performed by a social worker experienced in palliative care and embedded in the heart failure clinical team. The primary endpoint of the SWAP-HF (Social Worker–Aided Palliative Care Intervention in High Risk Patients with Heart Failure) study was clinical-level documentation of advanced-care preferences by 6 months after the program began.
“Although more comprehensive, multidisciplinary palliative care interventions may also be effective, the focused approach [used in this study] may represent a cost-effective and scalable method for shepherding limited specialty resources to enhance the delivery of patient-centered care,” Dr. Desai said. In other words, a program with a social worker costs less than a two-person staff with a palliative-care physician and nurse practitioner.
Despite its relative simplicity, the SWAP-HF intervention had some unique aspects that make it generalizability uncertain, commented Dr. Allen. The embedding of a social worker on the heart failure team placed a professional with a “good understanding of social context” right on the scene with everyone else delivering care to the heart failure patient, a good strategy for minimizing fragmentation, he said. In addition, the place where the study was done, Brigham and Women’s Hospital, “is not your average hospital,” he noted,
In addition, the timing of the intervention studied during hospitalization may be problematic. Clinicians need to “be careful about patients making long-term decisions” about their care while they are hospitalized, a time when patients can be “ill, confused, and scared.” He cited recent findings from a study of hospital-based palliative-care interventions for family members of patients with chronic critical illness that did not reduce anxiety or depression symptoms among the treated family members and may have increased symptoms of posttraumatic stress disorder (JAMA. 2016 July 5;374[1]:51-62).
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ORLANDO – Systematic introduction of palliative care interventions for patients with advanced heart failure improved patients’ quality of life and spurred their development of advanced-care preferences in a pair of independently performed, controlled, pilot studies.
But, despite demonstrating the ability of palliative-care interventions to help heart failure patients during their final months of life, the findings raised questions about the generalizability and reproducibility of palliative-care interventions that may depend on the skills and experience of the individual specialists who deliver the palliative care.
“Palliative care for patients with cardiovascular disease is in desperate need of good-quality evidence,” commented Larry A, Allen, MD, a heart failure cardiologist at the University of Colorado in Aurora and designated discussant for one of the two studies presented at the meeting. “We need large, randomized trials with clinical outcomes to look at patient outcomes from palliative-care interventions.”
The patients average 71 years old, about half were women, and about 40% were African Americans. They had been diagnosed with heart failure for an average of more than 5 years, all had advanced heart failure, about 60% spent at least half of their time awake immobilized in a bed or chair, and they had average NT-proBNP blood levels of greater than 10,000 pg/mL.
After 24 weeks of intervention, the palliative-care program produced both statistically significant and clinically meaningful improvements in two different measures of health-related quality of life, the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Functional Assessment of Chronic Illness Therapy – Palliative Care (FACIT-PAL). The KCCQ showed the palliative care intervention linked with an average rise of more than 9 points compared with patients in the control arm after adjustment for age and sex, a statistically significant increase on a scale where a 5-point rise is considered clinically meaningful. The FACIT-PAL showed an average, adjusted 11-point rise linked with the intervention, a statistically significant increase on a scale where an increase of at least 10 is judged clinically meaningful, reported Dr. Rogers, a heart failure cardiologist and professor of medicine at Duke University.
The palliative-care intervention also led to significant improvements in measures of spirituality, depression, and anxiety, but intervention had no impact on mortality.
“I like these endpoints and the idea that we can make quality-of-life better. These are very sick patients, with a predicted 6-month mortality of 50%. Patients reach a time when they don’t want to live longer but want better life quality for the days they still have,” he said in an interview.
The second report came from a single-center pilot study of 50 patients enrolled when they were hospitalized for acute decompensated heart failure and had at least one addition risk factor for poor prognosis such as age of at least 81 years, renal dysfunction, or a prior heart failure hospitalization within the past year. Patients randomized to the intervention arm underwent a structured evaluation based on the Serious Illness Conversation Guide and performed by a social worker experienced in palliative care and embedded in the heart failure clinical team. The primary endpoint of the SWAP-HF (Social Worker–Aided Palliative Care Intervention in High Risk Patients with Heart Failure) study was clinical-level documentation of advanced-care preferences by 6 months after the program began.
“Although more comprehensive, multidisciplinary palliative care interventions may also be effective, the focused approach [used in this study] may represent a cost-effective and scalable method for shepherding limited specialty resources to enhance the delivery of patient-centered care,” Dr. Desai said. In other words, a program with a social worker costs less than a two-person staff with a palliative-care physician and nurse practitioner.
Despite its relative simplicity, the SWAP-HF intervention had some unique aspects that make it generalizability uncertain, commented Dr. Allen. The embedding of a social worker on the heart failure team placed a professional with a “good understanding of social context” right on the scene with everyone else delivering care to the heart failure patient, a good strategy for minimizing fragmentation, he said. In addition, the place where the study was done, Brigham and Women’s Hospital, “is not your average hospital,” he noted,
In addition, the timing of the intervention studied during hospitalization may be problematic. Clinicians need to “be careful about patients making long-term decisions” about their care while they are hospitalized, a time when patients can be “ill, confused, and scared.” He cited recent findings from a study of hospital-based palliative-care interventions for family members of patients with chronic critical illness that did not reduce anxiety or depression symptoms among the treated family members and may have increased symptoms of posttraumatic stress disorder (JAMA. 2016 July 5;374[1]:51-62).
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: Palliative care measures boosted patients’ Kansas City Cardiomyopathy Questionnaire score by an average of 9 points over that of controls.
Data source: PAL-HF, a single-center study with 150 randomized patients with heart failure and SWAP-HF, a single-center study with 50 randomized patients.
Disclosures: Dr. Rogers, Dr. Allen, and Dr. Desai had no relevant disclosures.
Reimbursement hurdles hinder Entresto use in HFrEF
ORLANDO – Use of sacubitril/valsartan to treat patients with heart failure with reduced ejection fraction (HFrEF) became a class I recommendation in both the U.S. and European heart failure guidelines in May 2016, but virtually all U.S. health insurers continue to regard the potent and effective sacubitril/valsartan formulation as a second-line treatment that needs special preauthorization before patients receive reimbursement for the prescription.
“What is really morally sad is that U.S. payers are requiring physicians to fill out extensive, patient-by-patient paperwork” to allow patients with HFrEF to receive health insurance coverage for sacubitril/valsartan (Entresto), Milton Packer, MD, said at the annual scientific meeting of the Heart Failure Society of America.
“It is very difficult to understand why third-party payers would intentionally try to slow adoption of this life-saving drug simply because it is considered expensive. It is much cheaper than many drugs they cover for patients with cancer that don’t work half as well,” said Dr. Packer, a cardiologist and heart failure specialist at Baylor University Medical Center in Dallas.
The “excessive paperwork” for insurers when starting patients on sacubitril/valsartan is a “new and unique phenomenon among the cardiovascular drugs I prescribe,” agreed Nancy K. Sweitzer, MD, PhD, professor and chief of cardiology at the University of Arizona in Tuscon. “This approach by insurers seems based on cost; they do not want to pay” for sacubitril/valsartan, and to successfully arrange for coverage patients need to exactly match the enrollment criteria used in the PARADIGM-HF (Prospective Comparison of ARNI [Angiotensin Receptor – Neprilysin Inhibitor] with ACEI [Angiotensin-Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial ), the pivotal study that supplied the evidence base for making sacubitril/valsartan a class I agent for treating HFrEF.
“I don’t put some HFrEF patients on sacubitil/valsartan just because they don’t meet the trial’s entry criteria,” Dr. Sweitzer said in an interview. “Insurers seem to scrutinize every single parameter to make sure patients match the PARADIGM-HF patients. Coverage is denied if their BNP [brain natriuretic peptide] level is too low.” Dr. Sweitzer added that in one instance she had to submit a second preauthorization to simply uptitrate the dosage of sacubitril/valsartan she wanted a patient to receive.
“Based on the data it seems like you could easily identify HFrEF patients who are good candidates for sacubitril/valsartan, but your hands are tied by payers because you can’t prescribe it until you’ve first tried something else, and even then you still need to deal with a lot of paperwork,” agreed Robert O. Bonow, MD, professor of medicine at Northwestern University in Chicago. “The paperwork burden is really cumbersome for physicians with busy practices; it impedes taking care of patients,” Dr. Bonow said in an interview.
Sales figures for sacubitril/valsartan that the drug’s manufacturer, Novartis, has reported since the agent received U.S. marketing approval a little over a year ago reflect these challenges in prescribing the compound to patients. During the first quarter of 2016, Novartis reported $17 million in worldwide sales of the agent, followed by $32 million in worldwide sales during the second quarter of 2016, through June 30. With a total of $49 million in sacubitril/valsartan sales during the first 6 months of 2016, it seems like Novartis may be challenged to meet its stated target of $200 million in total sales of the compound during 2016. In April, one commentator called the $17 million sales figure for first quarter 2016 “an astonishingly small amount for a drug that was widely expected to be a blockbuster.”
Dr. Packer has in the past been a consultant to Novartis and was one of the lead investigators for the PARADIGM-HF trial. He said that currently he has no financial relationship with Novartis but he does serve as a consultant to several other drug companies. Dr. Sweitzer has received research support from Novartis and was an investigator for PARADIGM-HF. Dr. Bonow has been a consultant to Gilead.
On Twitter @mitchelzoler
ORLANDO – Use of sacubitril/valsartan to treat patients with heart failure with reduced ejection fraction (HFrEF) became a class I recommendation in both the U.S. and European heart failure guidelines in May 2016, but virtually all U.S. health insurers continue to regard the potent and effective sacubitril/valsartan formulation as a second-line treatment that needs special preauthorization before patients receive reimbursement for the prescription.
“What is really morally sad is that U.S. payers are requiring physicians to fill out extensive, patient-by-patient paperwork” to allow patients with HFrEF to receive health insurance coverage for sacubitril/valsartan (Entresto), Milton Packer, MD, said at the annual scientific meeting of the Heart Failure Society of America.
“It is very difficult to understand why third-party payers would intentionally try to slow adoption of this life-saving drug simply because it is considered expensive. It is much cheaper than many drugs they cover for patients with cancer that don’t work half as well,” said Dr. Packer, a cardiologist and heart failure specialist at Baylor University Medical Center in Dallas.
The “excessive paperwork” for insurers when starting patients on sacubitril/valsartan is a “new and unique phenomenon among the cardiovascular drugs I prescribe,” agreed Nancy K. Sweitzer, MD, PhD, professor and chief of cardiology at the University of Arizona in Tuscon. “This approach by insurers seems based on cost; they do not want to pay” for sacubitril/valsartan, and to successfully arrange for coverage patients need to exactly match the enrollment criteria used in the PARADIGM-HF (Prospective Comparison of ARNI [Angiotensin Receptor – Neprilysin Inhibitor] with ACEI [Angiotensin-Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial ), the pivotal study that supplied the evidence base for making sacubitril/valsartan a class I agent for treating HFrEF.
“I don’t put some HFrEF patients on sacubitil/valsartan just because they don’t meet the trial’s entry criteria,” Dr. Sweitzer said in an interview. “Insurers seem to scrutinize every single parameter to make sure patients match the PARADIGM-HF patients. Coverage is denied if their BNP [brain natriuretic peptide] level is too low.” Dr. Sweitzer added that in one instance she had to submit a second preauthorization to simply uptitrate the dosage of sacubitril/valsartan she wanted a patient to receive.
“Based on the data it seems like you could easily identify HFrEF patients who are good candidates for sacubitril/valsartan, but your hands are tied by payers because you can’t prescribe it until you’ve first tried something else, and even then you still need to deal with a lot of paperwork,” agreed Robert O. Bonow, MD, professor of medicine at Northwestern University in Chicago. “The paperwork burden is really cumbersome for physicians with busy practices; it impedes taking care of patients,” Dr. Bonow said in an interview.
Sales figures for sacubitril/valsartan that the drug’s manufacturer, Novartis, has reported since the agent received U.S. marketing approval a little over a year ago reflect these challenges in prescribing the compound to patients. During the first quarter of 2016, Novartis reported $17 million in worldwide sales of the agent, followed by $32 million in worldwide sales during the second quarter of 2016, through June 30. With a total of $49 million in sacubitril/valsartan sales during the first 6 months of 2016, it seems like Novartis may be challenged to meet its stated target of $200 million in total sales of the compound during 2016. In April, one commentator called the $17 million sales figure for first quarter 2016 “an astonishingly small amount for a drug that was widely expected to be a blockbuster.”
Dr. Packer has in the past been a consultant to Novartis and was one of the lead investigators for the PARADIGM-HF trial. He said that currently he has no financial relationship with Novartis but he does serve as a consultant to several other drug companies. Dr. Sweitzer has received research support from Novartis and was an investigator for PARADIGM-HF. Dr. Bonow has been a consultant to Gilead.
On Twitter @mitchelzoler
ORLANDO – Use of sacubitril/valsartan to treat patients with heart failure with reduced ejection fraction (HFrEF) became a class I recommendation in both the U.S. and European heart failure guidelines in May 2016, but virtually all U.S. health insurers continue to regard the potent and effective sacubitril/valsartan formulation as a second-line treatment that needs special preauthorization before patients receive reimbursement for the prescription.
“What is really morally sad is that U.S. payers are requiring physicians to fill out extensive, patient-by-patient paperwork” to allow patients with HFrEF to receive health insurance coverage for sacubitril/valsartan (Entresto), Milton Packer, MD, said at the annual scientific meeting of the Heart Failure Society of America.
“It is very difficult to understand why third-party payers would intentionally try to slow adoption of this life-saving drug simply because it is considered expensive. It is much cheaper than many drugs they cover for patients with cancer that don’t work half as well,” said Dr. Packer, a cardiologist and heart failure specialist at Baylor University Medical Center in Dallas.
The “excessive paperwork” for insurers when starting patients on sacubitril/valsartan is a “new and unique phenomenon among the cardiovascular drugs I prescribe,” agreed Nancy K. Sweitzer, MD, PhD, professor and chief of cardiology at the University of Arizona in Tuscon. “This approach by insurers seems based on cost; they do not want to pay” for sacubitril/valsartan, and to successfully arrange for coverage patients need to exactly match the enrollment criteria used in the PARADIGM-HF (Prospective Comparison of ARNI [Angiotensin Receptor – Neprilysin Inhibitor] with ACEI [Angiotensin-Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial ), the pivotal study that supplied the evidence base for making sacubitril/valsartan a class I agent for treating HFrEF.
“I don’t put some HFrEF patients on sacubitil/valsartan just because they don’t meet the trial’s entry criteria,” Dr. Sweitzer said in an interview. “Insurers seem to scrutinize every single parameter to make sure patients match the PARADIGM-HF patients. Coverage is denied if their BNP [brain natriuretic peptide] level is too low.” Dr. Sweitzer added that in one instance she had to submit a second preauthorization to simply uptitrate the dosage of sacubitril/valsartan she wanted a patient to receive.
“Based on the data it seems like you could easily identify HFrEF patients who are good candidates for sacubitril/valsartan, but your hands are tied by payers because you can’t prescribe it until you’ve first tried something else, and even then you still need to deal with a lot of paperwork,” agreed Robert O. Bonow, MD, professor of medicine at Northwestern University in Chicago. “The paperwork burden is really cumbersome for physicians with busy practices; it impedes taking care of patients,” Dr. Bonow said in an interview.
Sales figures for sacubitril/valsartan that the drug’s manufacturer, Novartis, has reported since the agent received U.S. marketing approval a little over a year ago reflect these challenges in prescribing the compound to patients. During the first quarter of 2016, Novartis reported $17 million in worldwide sales of the agent, followed by $32 million in worldwide sales during the second quarter of 2016, through June 30. With a total of $49 million in sacubitril/valsartan sales during the first 6 months of 2016, it seems like Novartis may be challenged to meet its stated target of $200 million in total sales of the compound during 2016. In April, one commentator called the $17 million sales figure for first quarter 2016 “an astonishingly small amount for a drug that was widely expected to be a blockbuster.”
Dr. Packer has in the past been a consultant to Novartis and was one of the lead investigators for the PARADIGM-HF trial. He said that currently he has no financial relationship with Novartis but he does serve as a consultant to several other drug companies. Dr. Sweitzer has received research support from Novartis and was an investigator for PARADIGM-HF. Dr. Bonow has been a consultant to Gilead.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE HFSA ANNUAL SCIENTIFIC MEETING
CardioMEMS shows real-world heart failure benefit
ORLANDO – Pulmonary artery pressure monitoring using an implanted device was even more effective for controlling pulmonary artery pressures in 2,000 real-world U.S. heart failure patients than it was in the pivotal trial that led to the device’s regulatory approval.
Data from the first 2,000 U.S. heart failure patients to receive the CardioMEMS pulmonary artery (PA) pressure monitoring device and have at least 6 months of follow-up data since the device received Food and Drug Administration approval in 2014 showed that cumulative PA pressure reductions in these patients during the first 6 months of use averaged 434 mm Hg per patient when compared with their baseline PA pressure when they first received the device. This was nearly threefold better than the average 150–mm Hg cumulative reduction in PA pressure per patient during 6 months of use seen in the CHAMPION trial, Dr. William T. Abraham reported at the annual scientific meeting of the Heart Failure Society of America.
Although this analysis of data from the registry maintained for U.S. patients who receive the CardioMEMS device does not yet include information on how these patients fared clinically, and specifically how often they required rehospitalization for heart failure, the strikingly high level of PA pressure control seen in the first 2,000 U.S. patients bodes well for what the clinical findings will show once they are available.
“In our experience with PA pressure monitoring, there is almost a linear relationship between reduced PA pressures and reduced numbers of events” in the form of rehospitalizations for heart failure, said Dr. Abraham, professor of medicine and director of cardiovascular medicine at Ohio State University in Columbus. Once data on outcomes are analyzed for the registry patients, “I think they will be even better than they were in the trial,” he said in an interview.
The PA pressure data in these initial patients “are very important because they tell us that in general use, clinicians – many of whom are at community hospitals – are very capable of using the CardioMEMS data to control patient pressures, and in CHAMPION we showed that there is a relationship between controlled pressures and improved outcomes,” he said. The findings also help allay a key concern about the potential benefit from implanting a device to monitor PA pressure, which is that clinicians must respond to the information and tweak a patient’s diuretic and vasodilator treatments in order for pressure monitoring to have an effect on heart failure outcomes.
“These data clearly refute that concern,” Dr. Abraham said.
He expressed some surprise that PA pressure control with monitoring was so much more effective in real-world use than in the CHAMPION pivotal trial. “In the trial, it was a paradigm shift to manage heart failure patients based on their PA pressures and not according to their symptoms,” he said. With CardioMEMS pressure monitoring, clinicians are supposed to treat high PA pressure with dose adjustments even if the patient feels okay. The new data suggest that clinicians now using the device “have gotten the message that if you don’t do something with the data the patients won’t improve.”
The registry patients came from 47 states and 427 unique physicians who worked in a range of settings including large and small centers, and academic and nonacademic community centers. The patients averaged 70 years, 40% were women, a third had a left ventricular ejection fraction at or above 40%, and their average PA pressure at the time they had their device implanted was 34.9 mm Hg. This pressure was notably higher than the average 31.6 mm Hg pressure among patients enrolled in CHAMPION, a fact that also helps explain why the registry patients received a larger pressure-reduction benefit: They started from a higher level than the trial patients, and during follow-up, their achieved pressures were always compared back to their high baseline pressures.
The registry patients were also substantially older than the trial patients, who had averaged 62 years, and the registry included substantially more women and more patients with higher ejection fractions. Dr. Abraham did not report data on their New York Heart Association class at entry, but labeling for CardioMEMS specifies that patients should have class III heart failure as well as a recent heart failure hospitalization.
Dr. Abraham’s analysis also showed that the greatest degree of PA pressure control occurred in the patients who began device-based treatment with the highest PA pressures. Nearly half the 2,000 registry patients had an entry PA pressure at or above 35 mm Hg, and over a period of 6 months, they averaged a cumulative 876–mm Hg reduction in their PA pressure relative to their baseline level. The third of patients who began with a PA pressure of 25-34 mm Hg had an average 169–mm Hg cumulative pressure reduction over the 6 month period, and the 18% of patients who began with a PA pressure of less than 25 mm Hg actually had an average cumulative increase in the PA pressure of 163 mm Hg. Target PA pressures are usually in the normal range of 18-25 mm Hg.
The analyses also showed that the impact of PA pressure monitoring on pressure was roughly similar regardless of the left ventricular ejection fraction patients had at baseline, and regardless of their sex.
The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
On Twitter @mitchelzoler
ORLANDO – Pulmonary artery pressure monitoring using an implanted device was even more effective for controlling pulmonary artery pressures in 2,000 real-world U.S. heart failure patients than it was in the pivotal trial that led to the device’s regulatory approval.
Data from the first 2,000 U.S. heart failure patients to receive the CardioMEMS pulmonary artery (PA) pressure monitoring device and have at least 6 months of follow-up data since the device received Food and Drug Administration approval in 2014 showed that cumulative PA pressure reductions in these patients during the first 6 months of use averaged 434 mm Hg per patient when compared with their baseline PA pressure when they first received the device. This was nearly threefold better than the average 150–mm Hg cumulative reduction in PA pressure per patient during 6 months of use seen in the CHAMPION trial, Dr. William T. Abraham reported at the annual scientific meeting of the Heart Failure Society of America.
Although this analysis of data from the registry maintained for U.S. patients who receive the CardioMEMS device does not yet include information on how these patients fared clinically, and specifically how often they required rehospitalization for heart failure, the strikingly high level of PA pressure control seen in the first 2,000 U.S. patients bodes well for what the clinical findings will show once they are available.
“In our experience with PA pressure monitoring, there is almost a linear relationship between reduced PA pressures and reduced numbers of events” in the form of rehospitalizations for heart failure, said Dr. Abraham, professor of medicine and director of cardiovascular medicine at Ohio State University in Columbus. Once data on outcomes are analyzed for the registry patients, “I think they will be even better than they were in the trial,” he said in an interview.
The PA pressure data in these initial patients “are very important because they tell us that in general use, clinicians – many of whom are at community hospitals – are very capable of using the CardioMEMS data to control patient pressures, and in CHAMPION we showed that there is a relationship between controlled pressures and improved outcomes,” he said. The findings also help allay a key concern about the potential benefit from implanting a device to monitor PA pressure, which is that clinicians must respond to the information and tweak a patient’s diuretic and vasodilator treatments in order for pressure monitoring to have an effect on heart failure outcomes.
“These data clearly refute that concern,” Dr. Abraham said.
He expressed some surprise that PA pressure control with monitoring was so much more effective in real-world use than in the CHAMPION pivotal trial. “In the trial, it was a paradigm shift to manage heart failure patients based on their PA pressures and not according to their symptoms,” he said. With CardioMEMS pressure monitoring, clinicians are supposed to treat high PA pressure with dose adjustments even if the patient feels okay. The new data suggest that clinicians now using the device “have gotten the message that if you don’t do something with the data the patients won’t improve.”
The registry patients came from 47 states and 427 unique physicians who worked in a range of settings including large and small centers, and academic and nonacademic community centers. The patients averaged 70 years, 40% were women, a third had a left ventricular ejection fraction at or above 40%, and their average PA pressure at the time they had their device implanted was 34.9 mm Hg. This pressure was notably higher than the average 31.6 mm Hg pressure among patients enrolled in CHAMPION, a fact that also helps explain why the registry patients received a larger pressure-reduction benefit: They started from a higher level than the trial patients, and during follow-up, their achieved pressures were always compared back to their high baseline pressures.
The registry patients were also substantially older than the trial patients, who had averaged 62 years, and the registry included substantially more women and more patients with higher ejection fractions. Dr. Abraham did not report data on their New York Heart Association class at entry, but labeling for CardioMEMS specifies that patients should have class III heart failure as well as a recent heart failure hospitalization.
Dr. Abraham’s analysis also showed that the greatest degree of PA pressure control occurred in the patients who began device-based treatment with the highest PA pressures. Nearly half the 2,000 registry patients had an entry PA pressure at or above 35 mm Hg, and over a period of 6 months, they averaged a cumulative 876–mm Hg reduction in their PA pressure relative to their baseline level. The third of patients who began with a PA pressure of 25-34 mm Hg had an average 169–mm Hg cumulative pressure reduction over the 6 month period, and the 18% of patients who began with a PA pressure of less than 25 mm Hg actually had an average cumulative increase in the PA pressure of 163 mm Hg. Target PA pressures are usually in the normal range of 18-25 mm Hg.
The analyses also showed that the impact of PA pressure monitoring on pressure was roughly similar regardless of the left ventricular ejection fraction patients had at baseline, and regardless of their sex.
The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
On Twitter @mitchelzoler
ORLANDO – Pulmonary artery pressure monitoring using an implanted device was even more effective for controlling pulmonary artery pressures in 2,000 real-world U.S. heart failure patients than it was in the pivotal trial that led to the device’s regulatory approval.
Data from the first 2,000 U.S. heart failure patients to receive the CardioMEMS pulmonary artery (PA) pressure monitoring device and have at least 6 months of follow-up data since the device received Food and Drug Administration approval in 2014 showed that cumulative PA pressure reductions in these patients during the first 6 months of use averaged 434 mm Hg per patient when compared with their baseline PA pressure when they first received the device. This was nearly threefold better than the average 150–mm Hg cumulative reduction in PA pressure per patient during 6 months of use seen in the CHAMPION trial, Dr. William T. Abraham reported at the annual scientific meeting of the Heart Failure Society of America.
Although this analysis of data from the registry maintained for U.S. patients who receive the CardioMEMS device does not yet include information on how these patients fared clinically, and specifically how often they required rehospitalization for heart failure, the strikingly high level of PA pressure control seen in the first 2,000 U.S. patients bodes well for what the clinical findings will show once they are available.
“In our experience with PA pressure monitoring, there is almost a linear relationship between reduced PA pressures and reduced numbers of events” in the form of rehospitalizations for heart failure, said Dr. Abraham, professor of medicine and director of cardiovascular medicine at Ohio State University in Columbus. Once data on outcomes are analyzed for the registry patients, “I think they will be even better than they were in the trial,” he said in an interview.
The PA pressure data in these initial patients “are very important because they tell us that in general use, clinicians – many of whom are at community hospitals – are very capable of using the CardioMEMS data to control patient pressures, and in CHAMPION we showed that there is a relationship between controlled pressures and improved outcomes,” he said. The findings also help allay a key concern about the potential benefit from implanting a device to monitor PA pressure, which is that clinicians must respond to the information and tweak a patient’s diuretic and vasodilator treatments in order for pressure monitoring to have an effect on heart failure outcomes.
“These data clearly refute that concern,” Dr. Abraham said.
He expressed some surprise that PA pressure control with monitoring was so much more effective in real-world use than in the CHAMPION pivotal trial. “In the trial, it was a paradigm shift to manage heart failure patients based on their PA pressures and not according to their symptoms,” he said. With CardioMEMS pressure monitoring, clinicians are supposed to treat high PA pressure with dose adjustments even if the patient feels okay. The new data suggest that clinicians now using the device “have gotten the message that if you don’t do something with the data the patients won’t improve.”
The registry patients came from 47 states and 427 unique physicians who worked in a range of settings including large and small centers, and academic and nonacademic community centers. The patients averaged 70 years, 40% were women, a third had a left ventricular ejection fraction at or above 40%, and their average PA pressure at the time they had their device implanted was 34.9 mm Hg. This pressure was notably higher than the average 31.6 mm Hg pressure among patients enrolled in CHAMPION, a fact that also helps explain why the registry patients received a larger pressure-reduction benefit: They started from a higher level than the trial patients, and during follow-up, their achieved pressures were always compared back to their high baseline pressures.
The registry patients were also substantially older than the trial patients, who had averaged 62 years, and the registry included substantially more women and more patients with higher ejection fractions. Dr. Abraham did not report data on their New York Heart Association class at entry, but labeling for CardioMEMS specifies that patients should have class III heart failure as well as a recent heart failure hospitalization.
Dr. Abraham’s analysis also showed that the greatest degree of PA pressure control occurred in the patients who began device-based treatment with the highest PA pressures. Nearly half the 2,000 registry patients had an entry PA pressure at or above 35 mm Hg, and over a period of 6 months, they averaged a cumulative 876–mm Hg reduction in their PA pressure relative to their baseline level. The third of patients who began with a PA pressure of 25-34 mm Hg had an average 169–mm Hg cumulative pressure reduction over the 6 month period, and the 18% of patients who began with a PA pressure of less than 25 mm Hg actually had an average cumulative increase in the PA pressure of 163 mm Hg. Target PA pressures are usually in the normal range of 18-25 mm Hg.
The analyses also showed that the impact of PA pressure monitoring on pressure was roughly similar regardless of the left ventricular ejection fraction patients had at baseline, and regardless of their sex.
The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Registry data from the first 2,000 U.S. heart failure patients who received an implanted pulmonary artery pressure monitor showed a level of pressure control over a period of 6 months nearly triple that seen in the pivotal trial.
Major finding: Cumulative pulmonary artery pressure reductions averaged 434 mm Hg over a period of 6 months, compared with an average reduction of 150 mm Hg in the pivotal trial.
Data source: The first 2,000 U.S. patients who received a CardioMEMS device and were followed for at least 6 months.
Disclosures: The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
More TOPCAT flaws back spironolactone’s HFpEF efficacy
ORLANDO – Spironolactone inched a little closer toward becoming the first and only agent with proven efficacy for treating patients with heart failure with preserved ejection fraction based on further evidence for the drug’s efficacy in a subgroup of patients enrolled in the TOPCAT trial.
In 2014, the initial TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) report showed that spironolactone treatment of patients with heart failure with preserved ejection fraction (HFpEF) for 3 years produced a small, 11% relative reduction in the primary risk endpoint, compared with placebo that was not statistically significant (N Engl J Med. 2014 Apr 10;370[15]:1383-92).
But a follow-up post hoc analysis a year later showed evidence that the roughly half of patients in TOPCAT enrolled at centers in Russia and the Republic of Georgia may not have had HFpEF and also may not have received the planned dosage of spironolactone (Circulation. 2015 Jan 6;131[1]:34-42). An analysis that focused only on the 1,767 HFpEF patients (51% of the total TOPCAT cohort) enrolled in the Americas (United States, Canada, Argentina, and Brazil) showed that, compared with placebo, treatment with spironolactone cut the combined rate of cardiovascular death, nonfatal cardiac arrest, and heart failure hospitalization by 4.5 percentage points, an 18% relative risk reduction that was statistically significant. In the Americas, spironolactone also cut cardiovascular death alone by a relative 26%, and reduced heart failure hospitalization by a relative 18%, both statistically significant.
Additional analysis reported at the annual scientific meeting of the Heart Failure Society of America further supported the idea that many TOPCAT patients enrolled in Russia did not receive a physiologically meaningful dosage of spironolactone. Among 66 Russian patients randomized to the spironolactone arm who reported taking their drug as prescribed and who participated in a random draw of blood specimens 1 year into the study, 20 (30%) failed to show detectable blood levels of canrenone, a characteristic spironolactone metabolite, reported Eileen O’Meara, MD, at the meeting. In contrast, 2 (3%) of 76 enrolled U.S. patients failed to show detectable blood levels of the canrenone metabolite, a 10-fold difference said Dr. O’Meara, a cardiologist at the Montreal Heart Institute.The tested U.S. patients also showed a clear dose-response relationship between their reported spironolactone dosage and their canrenone levels, something not seen in the Russian patients. These new findings, plus the evidence cited in the 2015 analysis, create a compelling case that “actual use of spironolactone in Russia was lower than reported” by the trial participants in Russia and probably in Georgia as well, Dr. O’Meara said. The implication is that spironolactone’s real impact on HFpEF patients is best represented in the 51% of TOPCAT patients from the Americas, she added.
“We believe these findings emphasize the reliability of the Americas data,” said Marc A. Pfeffer, MD, a coinvestigator for TOPCAT and lead author of the 2015 post hoc analysis. “Until someone comes up with a better treatment for patients with HFpEF, we should pay attention to this. People need to get this message. And spironolactone costs 7 cents a day,” said Dr. Pfeffer, professor of medicine at Harvard Medical School in Boston.Currently, no agent is considered proven effective for improving outcomes in HFpEF patients. The 2016 guidelinesfor heart failure treatment from the European Society of Cardiology said “no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.”
After the TOPCAT results and post hoc analysis came out in 2014 and 2015 and word spread of spironolactone’s apparent efficacy in the American half of the trial, use of spironolactone to treat HFpEF patient has increased, commented Margaret M. Redfield, MD, a heart failure physician and professor at the Mayo Clinic in Rochester, Minn. She said she often prescribes spironolactone patients to HFpEF patients who require potassium supplementation, generally because of their diuretic treatment. These are the “safest” HFpEF patients for spironolactone treatment, she said, because they face the lowest risk for hyperkalemia, the major adverse effect from spironolactone.
On Twitter @mitchelzoler
This is extraordinarily important information from an extraordinarily important study. I’m strongly persuaded that the data from the Americas in TOPCAT show that spironolactone worked. The new data presented on canrenone levels make me even more ready to exclude from consideration the TOPCAT data from Russia and Georgia.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Barry H. Greenberg |
The heart failure community is left to decide what conclusions to draw from TOPCAT. I think guideline committees will struggle over what to make of the TOPCAT evidence. Any recommendation in favor of spironolactone needs to be somewhat guarded, but if a group made recommendations in support of spironolactone it would add an impetus for using it.
There has been long-standing interest in treating patients with heart failure with preserved ejection fraction with spironolactone. Currently, about a quarter of these patients take spironolactone. I’m not sure this level of use will increase dramatically because of what we now know about TOPCAT.
Dr. Barry H. Greenberg is professor of medicine and director of the advanced heart failure treatment program at the University of California, San Diego. He had no relevant disclosures. He made these comments in an interview.
This is extraordinarily important information from an extraordinarily important study. I’m strongly persuaded that the data from the Americas in TOPCAT show that spironolactone worked. The new data presented on canrenone levels make me even more ready to exclude from consideration the TOPCAT data from Russia and Georgia.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Barry H. Greenberg |
The heart failure community is left to decide what conclusions to draw from TOPCAT. I think guideline committees will struggle over what to make of the TOPCAT evidence. Any recommendation in favor of spironolactone needs to be somewhat guarded, but if a group made recommendations in support of spironolactone it would add an impetus for using it.
There has been long-standing interest in treating patients with heart failure with preserved ejection fraction with spironolactone. Currently, about a quarter of these patients take spironolactone. I’m not sure this level of use will increase dramatically because of what we now know about TOPCAT.
Dr. Barry H. Greenberg is professor of medicine and director of the advanced heart failure treatment program at the University of California, San Diego. He had no relevant disclosures. He made these comments in an interview.
This is extraordinarily important information from an extraordinarily important study. I’m strongly persuaded that the data from the Americas in TOPCAT show that spironolactone worked. The new data presented on canrenone levels make me even more ready to exclude from consideration the TOPCAT data from Russia and Georgia.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Barry H. Greenberg |
The heart failure community is left to decide what conclusions to draw from TOPCAT. I think guideline committees will struggle over what to make of the TOPCAT evidence. Any recommendation in favor of spironolactone needs to be somewhat guarded, but if a group made recommendations in support of spironolactone it would add an impetus for using it.
There has been long-standing interest in treating patients with heart failure with preserved ejection fraction with spironolactone. Currently, about a quarter of these patients take spironolactone. I’m not sure this level of use will increase dramatically because of what we now know about TOPCAT.
Dr. Barry H. Greenberg is professor of medicine and director of the advanced heart failure treatment program at the University of California, San Diego. He had no relevant disclosures. He made these comments in an interview.
ORLANDO – Spironolactone inched a little closer toward becoming the first and only agent with proven efficacy for treating patients with heart failure with preserved ejection fraction based on further evidence for the drug’s efficacy in a subgroup of patients enrolled in the TOPCAT trial.
In 2014, the initial TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) report showed that spironolactone treatment of patients with heart failure with preserved ejection fraction (HFpEF) for 3 years produced a small, 11% relative reduction in the primary risk endpoint, compared with placebo that was not statistically significant (N Engl J Med. 2014 Apr 10;370[15]:1383-92).
But a follow-up post hoc analysis a year later showed evidence that the roughly half of patients in TOPCAT enrolled at centers in Russia and the Republic of Georgia may not have had HFpEF and also may not have received the planned dosage of spironolactone (Circulation. 2015 Jan 6;131[1]:34-42). An analysis that focused only on the 1,767 HFpEF patients (51% of the total TOPCAT cohort) enrolled in the Americas (United States, Canada, Argentina, and Brazil) showed that, compared with placebo, treatment with spironolactone cut the combined rate of cardiovascular death, nonfatal cardiac arrest, and heart failure hospitalization by 4.5 percentage points, an 18% relative risk reduction that was statistically significant. In the Americas, spironolactone also cut cardiovascular death alone by a relative 26%, and reduced heart failure hospitalization by a relative 18%, both statistically significant.
Additional analysis reported at the annual scientific meeting of the Heart Failure Society of America further supported the idea that many TOPCAT patients enrolled in Russia did not receive a physiologically meaningful dosage of spironolactone. Among 66 Russian patients randomized to the spironolactone arm who reported taking their drug as prescribed and who participated in a random draw of blood specimens 1 year into the study, 20 (30%) failed to show detectable blood levels of canrenone, a characteristic spironolactone metabolite, reported Eileen O’Meara, MD, at the meeting. In contrast, 2 (3%) of 76 enrolled U.S. patients failed to show detectable blood levels of the canrenone metabolite, a 10-fold difference said Dr. O’Meara, a cardiologist at the Montreal Heart Institute.The tested U.S. patients also showed a clear dose-response relationship between their reported spironolactone dosage and their canrenone levels, something not seen in the Russian patients. These new findings, plus the evidence cited in the 2015 analysis, create a compelling case that “actual use of spironolactone in Russia was lower than reported” by the trial participants in Russia and probably in Georgia as well, Dr. O’Meara said. The implication is that spironolactone’s real impact on HFpEF patients is best represented in the 51% of TOPCAT patients from the Americas, she added.
“We believe these findings emphasize the reliability of the Americas data,” said Marc A. Pfeffer, MD, a coinvestigator for TOPCAT and lead author of the 2015 post hoc analysis. “Until someone comes up with a better treatment for patients with HFpEF, we should pay attention to this. People need to get this message. And spironolactone costs 7 cents a day,” said Dr. Pfeffer, professor of medicine at Harvard Medical School in Boston.Currently, no agent is considered proven effective for improving outcomes in HFpEF patients. The 2016 guidelinesfor heart failure treatment from the European Society of Cardiology said “no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.”
After the TOPCAT results and post hoc analysis came out in 2014 and 2015 and word spread of spironolactone’s apparent efficacy in the American half of the trial, use of spironolactone to treat HFpEF patient has increased, commented Margaret M. Redfield, MD, a heart failure physician and professor at the Mayo Clinic in Rochester, Minn. She said she often prescribes spironolactone patients to HFpEF patients who require potassium supplementation, generally because of their diuretic treatment. These are the “safest” HFpEF patients for spironolactone treatment, she said, because they face the lowest risk for hyperkalemia, the major adverse effect from spironolactone.
On Twitter @mitchelzoler
ORLANDO – Spironolactone inched a little closer toward becoming the first and only agent with proven efficacy for treating patients with heart failure with preserved ejection fraction based on further evidence for the drug’s efficacy in a subgroup of patients enrolled in the TOPCAT trial.
In 2014, the initial TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) report showed that spironolactone treatment of patients with heart failure with preserved ejection fraction (HFpEF) for 3 years produced a small, 11% relative reduction in the primary risk endpoint, compared with placebo that was not statistically significant (N Engl J Med. 2014 Apr 10;370[15]:1383-92).
But a follow-up post hoc analysis a year later showed evidence that the roughly half of patients in TOPCAT enrolled at centers in Russia and the Republic of Georgia may not have had HFpEF and also may not have received the planned dosage of spironolactone (Circulation. 2015 Jan 6;131[1]:34-42). An analysis that focused only on the 1,767 HFpEF patients (51% of the total TOPCAT cohort) enrolled in the Americas (United States, Canada, Argentina, and Brazil) showed that, compared with placebo, treatment with spironolactone cut the combined rate of cardiovascular death, nonfatal cardiac arrest, and heart failure hospitalization by 4.5 percentage points, an 18% relative risk reduction that was statistically significant. In the Americas, spironolactone also cut cardiovascular death alone by a relative 26%, and reduced heart failure hospitalization by a relative 18%, both statistically significant.
Additional analysis reported at the annual scientific meeting of the Heart Failure Society of America further supported the idea that many TOPCAT patients enrolled in Russia did not receive a physiologically meaningful dosage of spironolactone. Among 66 Russian patients randomized to the spironolactone arm who reported taking their drug as prescribed and who participated in a random draw of blood specimens 1 year into the study, 20 (30%) failed to show detectable blood levels of canrenone, a characteristic spironolactone metabolite, reported Eileen O’Meara, MD, at the meeting. In contrast, 2 (3%) of 76 enrolled U.S. patients failed to show detectable blood levels of the canrenone metabolite, a 10-fold difference said Dr. O’Meara, a cardiologist at the Montreal Heart Institute.The tested U.S. patients also showed a clear dose-response relationship between their reported spironolactone dosage and their canrenone levels, something not seen in the Russian patients. These new findings, plus the evidence cited in the 2015 analysis, create a compelling case that “actual use of spironolactone in Russia was lower than reported” by the trial participants in Russia and probably in Georgia as well, Dr. O’Meara said. The implication is that spironolactone’s real impact on HFpEF patients is best represented in the 51% of TOPCAT patients from the Americas, she added.
“We believe these findings emphasize the reliability of the Americas data,” said Marc A. Pfeffer, MD, a coinvestigator for TOPCAT and lead author of the 2015 post hoc analysis. “Until someone comes up with a better treatment for patients with HFpEF, we should pay attention to this. People need to get this message. And spironolactone costs 7 cents a day,” said Dr. Pfeffer, professor of medicine at Harvard Medical School in Boston.Currently, no agent is considered proven effective for improving outcomes in HFpEF patients. The 2016 guidelinesfor heart failure treatment from the European Society of Cardiology said “no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.”
After the TOPCAT results and post hoc analysis came out in 2014 and 2015 and word spread of spironolactone’s apparent efficacy in the American half of the trial, use of spironolactone to treat HFpEF patient has increased, commented Margaret M. Redfield, MD, a heart failure physician and professor at the Mayo Clinic in Rochester, Minn. She said she often prescribes spironolactone patients to HFpEF patients who require potassium supplementation, generally because of their diuretic treatment. These are the “safest” HFpEF patients for spironolactone treatment, she said, because they face the lowest risk for hyperkalemia, the major adverse effect from spironolactone.
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: A post hoc analysis of spironolactone use among TOPCAT participants further fueled the idea that spironolactone provides real benefit to patients with HFpEF.
Major finding: Among patients reportedly taking spironolactone, 30% of tested Russians and 3% of tested Americans did not have detectable canrenone levels.
Data source: TOPCAT, a multicenter, randomized trial with 3,445 HFpEF patients.
Disclosures: TOPCAT received no commercial funding. Dr. O’Meara, Dr. Pfeffer, and Dr. Redfield had no relevant disclosures.