User login
Heparin-Coated Stent Graft Produced High SFA Patency
MIAMI BEACH – An investigational, peripheral artery stent graft with heparin bonding showed excellent 1-year performance as long as its size matched the treated vessel, in a multicenter, single-arm study of 119 patients.
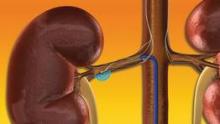
The Viabahn heparin-bonded stent graft placed in superficial femoral arteries (SFA) and oversized at the proximal edge by no more than 20%, produced a 91% primary patency rate at 12 months after the intervention, compared with a 70% primary patency rate among patients who received the graft in vessels where proximal edge oversizing exceeded 20%, Dr. Richard Saxon said at the meeting.
The overall 12-month primary patency rate for the 119 patients in the study was 74%, including a 79% rate in patients who received the 5-mm diameter stent graft, which was "a marked improvement" compared with prior 5-mm devices, said Dr. Saxon, an interventional radiologist and director of research at the San Diego Cardiac and Vascular Institute.
"If we treat the correct subset of patients with this newer stent graft, patency will be excellent and reinterventions low. We need to measure [vessel diameters] and do it correctly, and we’ll get excellent results. Oversizing will lead to occlusions," Dr. Saxon said. Oversizing compared with not oversizing led to "dramatically" different results.
The investigational stent graft used in the Gore Viabahn Endoprosthesis With Heparin Bioactive Surface in the Treatment of SFA Obstructive Disease (VIPER) trial appeared to perform substantially better than historical experience with the similar stent graft without a heparin-coated surface. The 12-month results in the pivotal study of the Viabahn stent graft without heparin showed a primary patency rate of 57%, suggesting that the heparin coating led to a 17% increase in primary patency, he reported.
In addition, the study achieved a 74% 1-year patency rate in patients with long, complex lesions that averaged 19 cm, and 60% of patients had stage III or IV disease based on classification by the Inter-Society Consensus Guidelines for the Management of PAD (TASC II).
The heparin-coated stent graft also featured contoured edges, designed to minimize the stenoses at proximal edges that have posed a problem with prior models. But the results showed that the contoured edge failed to eliminate edge stenosis, Dr. Saxon said.
"These are revolutionary outcomes," said Dr. Gary M. Ansel, an interventional cardiologist and clinical director of peripheral vascular interventions at the Midwest Cardiology Research Foundation in Columbus, Ohio. "The results show it’s not just the engineering, but also the use of the technology" that produces better outcomes. The new findings show the Viabahn heparin-coated stent graft comparable to the investigational Zilver PTX paclitaxel-eluting nitinol stent, said Dr. Ansel, who was a coinvestigator on the pivotal trials for both devices.
"Right now, these two, without a doubt, take it to a different level. They are better than everything else [for treating SFA stenoses], and are the standards against which everyone needs to compare their technology," Dr. Ansel said in an interview.
One-year patency data for the paclitaxel-coated nitinol stent, developed by Cook, were reported last year (Circ. Cardiovasc. Interv. 2011;4:495-504). Last October, a Food and Drug Administration advisory panel voted unanimously to recommend marketing approval of the paclitaxel-eluting nitinol stent, but as of late January, the FDA had not yet issued a decision.
VIPER enrolled patients with SFA lesions greater than 5 cm long at 11 U.S. sites. Their average age was 66 years, 62% were men, one-third had diabetes, 87% had hypertension, and 47% had coronary artery disease. Their average lesion length was 19 cm, and 61% of the SFA lesions had moderate or severe calcification. The study’s primary end point was primary patency in the treated SFA after 12 months, assessed by Doppler ultrasound. The results showed no impact of lesion length on outcomes, with primary patency rates in patients with lesions 20 cm or longer similar to those of patients with shorter lesions.
Within 30 days of treatment, one patient had a major adverse event, a need for bypass due to a target-lesion occlusion. The stent graft placed in this patient was "markedly oversized," Dr. Saxon said. A second patient had target lesion occlusion during follow-up to 1 year, again linked with stent graft oversizing compared with the vessel’s diameter.
Average ankle-brachial index was 0.61 at baseline and 0.9 at 12 months. At 12-month follow-up, 66 (74%) of the 89 patients assessed for their Rutherford-Becker class had class 0 disease, and 74 (83%) had experienced at least a two-class reduction in their Rutherford-Becker status. Twelve patients had a stent graft thrombosis or occlusion, with 10 of these patients having a worsening of their baseline Rutherford-Becker class. Thirteen patients required revisions for stenoses detected by ultrasound; seven of these patients were asymptomatic at the time of stenosis detection.
"We can’t wait for patients to become symptomatic or have their ankle-brachial index drop. We have to follow patients closely with duplex ultrasound," after SFA revascularization, Dr. Saxon said.
The VIPER trial was sponsored by W.L. Gore. Dr. Saxon disclosed ties with W.L. Gore, Cook, Lutonix, Concentric Medical, Vascular Resources, Abbott Vascular, and Reva Medical. Dr. Ansel disclosed ties with Abbott Vascular, Boston Scientific, Access Closure, Angioslide, Arsenal Medical, Atheromed, Atrium Medical, Bard, and Biocardia.
MIAMI BEACH – An investigational, peripheral artery stent graft with heparin bonding showed excellent 1-year performance as long as its size matched the treated vessel, in a multicenter, single-arm study of 119 patients.
The Viabahn heparin-bonded stent graft placed in superficial femoral arteries (SFA) and oversized at the proximal edge by no more than 20%, produced a 91% primary patency rate at 12 months after the intervention, compared with a 70% primary patency rate among patients who received the graft in vessels where proximal edge oversizing exceeded 20%, Dr. Richard Saxon said at the meeting.
The overall 12-month primary patency rate for the 119 patients in the study was 74%, including a 79% rate in patients who received the 5-mm diameter stent graft, which was "a marked improvement" compared with prior 5-mm devices, said Dr. Saxon, an interventional radiologist and director of research at the San Diego Cardiac and Vascular Institute.
"If we treat the correct subset of patients with this newer stent graft, patency will be excellent and reinterventions low. We need to measure [vessel diameters] and do it correctly, and we’ll get excellent results. Oversizing will lead to occlusions," Dr. Saxon said. Oversizing compared with not oversizing led to "dramatically" different results.
The investigational stent graft used in the Gore Viabahn Endoprosthesis With Heparin Bioactive Surface in the Treatment of SFA Obstructive Disease (VIPER) trial appeared to perform substantially better than historical experience with the similar stent graft without a heparin-coated surface. The 12-month results in the pivotal study of the Viabahn stent graft without heparin showed a primary patency rate of 57%, suggesting that the heparin coating led to a 17% increase in primary patency, he reported.
In addition, the study achieved a 74% 1-year patency rate in patients with long, complex lesions that averaged 19 cm, and 60% of patients had stage III or IV disease based on classification by the Inter-Society Consensus Guidelines for the Management of PAD (TASC II).
The heparin-coated stent graft also featured contoured edges, designed to minimize the stenoses at proximal edges that have posed a problem with prior models. But the results showed that the contoured edge failed to eliminate edge stenosis, Dr. Saxon said.
"These are revolutionary outcomes," said Dr. Gary M. Ansel, an interventional cardiologist and clinical director of peripheral vascular interventions at the Midwest Cardiology Research Foundation in Columbus, Ohio. "The results show it’s not just the engineering, but also the use of the technology" that produces better outcomes. The new findings show the Viabahn heparin-coated stent graft comparable to the investigational Zilver PTX paclitaxel-eluting nitinol stent, said Dr. Ansel, who was a coinvestigator on the pivotal trials for both devices.
"Right now, these two, without a doubt, take it to a different level. They are better than everything else [for treating SFA stenoses], and are the standards against which everyone needs to compare their technology," Dr. Ansel said in an interview.
One-year patency data for the paclitaxel-coated nitinol stent, developed by Cook, were reported last year (Circ. Cardiovasc. Interv. 2011;4:495-504). Last October, a Food and Drug Administration advisory panel voted unanimously to recommend marketing approval of the paclitaxel-eluting nitinol stent, but as of late January, the FDA had not yet issued a decision.
VIPER enrolled patients with SFA lesions greater than 5 cm long at 11 U.S. sites. Their average age was 66 years, 62% were men, one-third had diabetes, 87% had hypertension, and 47% had coronary artery disease. Their average lesion length was 19 cm, and 61% of the SFA lesions had moderate or severe calcification. The study’s primary end point was primary patency in the treated SFA after 12 months, assessed by Doppler ultrasound. The results showed no impact of lesion length on outcomes, with primary patency rates in patients with lesions 20 cm or longer similar to those of patients with shorter lesions.
Within 30 days of treatment, one patient had a major adverse event, a need for bypass due to a target-lesion occlusion. The stent graft placed in this patient was "markedly oversized," Dr. Saxon said. A second patient had target lesion occlusion during follow-up to 1 year, again linked with stent graft oversizing compared with the vessel’s diameter.
Average ankle-brachial index was 0.61 at baseline and 0.9 at 12 months. At 12-month follow-up, 66 (74%) of the 89 patients assessed for their Rutherford-Becker class had class 0 disease, and 74 (83%) had experienced at least a two-class reduction in their Rutherford-Becker status. Twelve patients had a stent graft thrombosis or occlusion, with 10 of these patients having a worsening of their baseline Rutherford-Becker class. Thirteen patients required revisions for stenoses detected by ultrasound; seven of these patients were asymptomatic at the time of stenosis detection.
"We can’t wait for patients to become symptomatic or have their ankle-brachial index drop. We have to follow patients closely with duplex ultrasound," after SFA revascularization, Dr. Saxon said.
The VIPER trial was sponsored by W.L. Gore. Dr. Saxon disclosed ties with W.L. Gore, Cook, Lutonix, Concentric Medical, Vascular Resources, Abbott Vascular, and Reva Medical. Dr. Ansel disclosed ties with Abbott Vascular, Boston Scientific, Access Closure, Angioslide, Arsenal Medical, Atheromed, Atrium Medical, Bard, and Biocardia.
MIAMI BEACH – An investigational, peripheral artery stent graft with heparin bonding showed excellent 1-year performance as long as its size matched the treated vessel, in a multicenter, single-arm study of 119 patients.
The Viabahn heparin-bonded stent graft placed in superficial femoral arteries (SFA) and oversized at the proximal edge by no more than 20%, produced a 91% primary patency rate at 12 months after the intervention, compared with a 70% primary patency rate among patients who received the graft in vessels where proximal edge oversizing exceeded 20%, Dr. Richard Saxon said at the meeting.
The overall 12-month primary patency rate for the 119 patients in the study was 74%, including a 79% rate in patients who received the 5-mm diameter stent graft, which was "a marked improvement" compared with prior 5-mm devices, said Dr. Saxon, an interventional radiologist and director of research at the San Diego Cardiac and Vascular Institute.
"If we treat the correct subset of patients with this newer stent graft, patency will be excellent and reinterventions low. We need to measure [vessel diameters] and do it correctly, and we’ll get excellent results. Oversizing will lead to occlusions," Dr. Saxon said. Oversizing compared with not oversizing led to "dramatically" different results.
The investigational stent graft used in the Gore Viabahn Endoprosthesis With Heparin Bioactive Surface in the Treatment of SFA Obstructive Disease (VIPER) trial appeared to perform substantially better than historical experience with the similar stent graft without a heparin-coated surface. The 12-month results in the pivotal study of the Viabahn stent graft without heparin showed a primary patency rate of 57%, suggesting that the heparin coating led to a 17% increase in primary patency, he reported.
In addition, the study achieved a 74% 1-year patency rate in patients with long, complex lesions that averaged 19 cm, and 60% of patients had stage III or IV disease based on classification by the Inter-Society Consensus Guidelines for the Management of PAD (TASC II).
The heparin-coated stent graft also featured contoured edges, designed to minimize the stenoses at proximal edges that have posed a problem with prior models. But the results showed that the contoured edge failed to eliminate edge stenosis, Dr. Saxon said.
"These are revolutionary outcomes," said Dr. Gary M. Ansel, an interventional cardiologist and clinical director of peripheral vascular interventions at the Midwest Cardiology Research Foundation in Columbus, Ohio. "The results show it’s not just the engineering, but also the use of the technology" that produces better outcomes. The new findings show the Viabahn heparin-coated stent graft comparable to the investigational Zilver PTX paclitaxel-eluting nitinol stent, said Dr. Ansel, who was a coinvestigator on the pivotal trials for both devices.
"Right now, these two, without a doubt, take it to a different level. They are better than everything else [for treating SFA stenoses], and are the standards against which everyone needs to compare their technology," Dr. Ansel said in an interview.
One-year patency data for the paclitaxel-coated nitinol stent, developed by Cook, were reported last year (Circ. Cardiovasc. Interv. 2011;4:495-504). Last October, a Food and Drug Administration advisory panel voted unanimously to recommend marketing approval of the paclitaxel-eluting nitinol stent, but as of late January, the FDA had not yet issued a decision.
VIPER enrolled patients with SFA lesions greater than 5 cm long at 11 U.S. sites. Their average age was 66 years, 62% were men, one-third had diabetes, 87% had hypertension, and 47% had coronary artery disease. Their average lesion length was 19 cm, and 61% of the SFA lesions had moderate or severe calcification. The study’s primary end point was primary patency in the treated SFA after 12 months, assessed by Doppler ultrasound. The results showed no impact of lesion length on outcomes, with primary patency rates in patients with lesions 20 cm or longer similar to those of patients with shorter lesions.
Within 30 days of treatment, one patient had a major adverse event, a need for bypass due to a target-lesion occlusion. The stent graft placed in this patient was "markedly oversized," Dr. Saxon said. A second patient had target lesion occlusion during follow-up to 1 year, again linked with stent graft oversizing compared with the vessel’s diameter.
Average ankle-brachial index was 0.61 at baseline and 0.9 at 12 months. At 12-month follow-up, 66 (74%) of the 89 patients assessed for their Rutherford-Becker class had class 0 disease, and 74 (83%) had experienced at least a two-class reduction in their Rutherford-Becker status. Twelve patients had a stent graft thrombosis or occlusion, with 10 of these patients having a worsening of their baseline Rutherford-Becker class. Thirteen patients required revisions for stenoses detected by ultrasound; seven of these patients were asymptomatic at the time of stenosis detection.
"We can’t wait for patients to become symptomatic or have their ankle-brachial index drop. We have to follow patients closely with duplex ultrasound," after SFA revascularization, Dr. Saxon said.
The VIPER trial was sponsored by W.L. Gore. Dr. Saxon disclosed ties with W.L. Gore, Cook, Lutonix, Concentric Medical, Vascular Resources, Abbott Vascular, and Reva Medical. Dr. Ansel disclosed ties with Abbott Vascular, Boston Scientific, Access Closure, Angioslide, Arsenal Medical, Atheromed, Atrium Medical, Bard, and Biocardia.
FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: At 12-month follow-up, superficial femoral arteries treated with a heparin-coated stent graft had a 74% primary patency rate.
Data Source: Results are from VIPER, a single-arm study of 119 patients with long SFA lesions enrolled at 11 U.S. centers.
Disclosures: The VIPER trial was sponsored by W.L. Gore. Dr. Saxon disclosed ties with W.L. Gore, Cook, Lutonix, Concentric Medical, Vascular Resources, Abbott Vascular, and Reva Medical. Dr. Ansel disclosed ties with Abbott Vascular, Boston Scientific, Access Closure, Angioslide, Arsenal Medical, Atheromed, Atrium Medical, Bard, and Biocardia.
New EVAR Devices for Abdominal Aortic Aneurysm Evolving
MIAMI BEACH – Devices for endovascular repair of abdominal aortic aneurysms are evolving, with new sizing or other innovations either recently on the market or in studies.
The new products expand the scope of patients who are candidates for endovascular aneurysm repair (EVAR), and in one case bring a new approach to EVAR into the clinic.
During a session at ISET 2012, an international symposium on endovascular therapy, seven experts gave the following quick updates on what’s new in EVAR for 2012:
• Making devices smaller. Downsizing constitutes a major trend for several new EVAR devices. The Endurant device helped blaze the lower-profile path, introducing a device delivered in an 18-Fr catheter for 23- to 25-mm diameter aortic stent grafts. Endurant received Food and Drug Administration marketing approval in 2010, and results from the U.S. pivotal trial for this device appeared last year (J. Vasc. Surg. 2011;54:601-8). In the pivotal trial, the major adverse event rate after 30 days was 4%; after 1 year, the patency rate was 97%, no patients had type I endoleaks, and the rate of secondary procedures was 5%, said Dr. Dittmar Böckler, professor and medical director of vascular surgery at the University of Heidelberg (Germany). An even smaller version of the same device, Endurant II, places a 28-mm bifurcated segment of an aortic stent graft into an 18-Fr delivery catheter – reduced from 20 Fr in the existing Endurant device – and has longer limbs to maximize coverage, changes that will further "expand options" for this device, he said.
Other EVAR devices take the downsizing trend even further. The Ovation Abdominal Stent Graft System, which received FDA marketing approval last November, has a 14-Fr outer diameter. For the time being, Ovation "is the lowest-profile commercially available device, expanding the population that is suitable for EVAR," said Dr. Horst Sievert, director of the cardiovascular center at Sankt Katharinen Hospital in Frankfurt, Germany. The FDA also made Ovation the first EVAR to be approved as a humanitarian use device (defined as an instrument aimed at a U.S. patient population that grows by fewer than 4,000 new cases annually). Ovation is approved for use in patients with iliac or femoral artery access that is less than 7 mm in diameter, and for treating an aorta with an inner neck diameter of 15.5-17.4 mm. The clinical trial results for Ovation showed "excellent short-term results," Dr. Sievert said. In the U.S. pivotal trial with 161 patients, the major adverse event rate after 1 month was 2.5%, he noted.
The Zenith Low Profile, a third low-profile EVAR device delivered in a 16-Fr sheath, is nearing marketing approval. The FDA already has data from a U.S. pivotal trial with 120 patients, and a decision on marketing approval should come sometime this year, said Dr. Roy K. Greenberg, a vascular surgeon at the Cleveland Clinic. Although he voiced enthusiasm for lower-profile devices that allow EVAR in patients who would not be able to accommodate larger devices, he also cautioned that "as we get lower-profile devices, we need to pay more attention to iliac disease. Postimplantation residual stenosis is critical for patients with [calcified] iliac disease."
Another issue with new devices is establishing their long-term performance. "New devices aren’t necessarily better; they’re just different. I think there are some advantages to new devices, but we don’t have a track record with respect to their long-term behavior," Dr. Greenberg said.
Finally, the INCRAFT low-profile EVAR device starts testing in a pivotal U.S. trial in February. INCRAFT matches Ovation with a 14-F outer-diameter delivery sheath, said Dr. Robert M. Bersin, medical director of endovascular services at Swedish Medical Center in Seattle. Its track record so far comes from the first 60 patients who received the device, at six centers in Germany and Italy, he added.
• High aortic neck angles. Aside from size, the aortic-neck angle provides another opportunity to broaden the EVAR device market. The Aorfix EVAR device is highly flexible and designed to deal with aortic neck angles of 60 degrees or greater. The stent has a "unique design, because a 60-degree angle is typically an exclusion for most [EVAR] devices," said Dr. William A. Gray, director of endovascular services at Columbia University in New York.
He reviewed the roll-in phase data from the PYTHAGORAS trial, which enrolled 62 patients with aortic neck angles smaller than 60 degrees; two-thirds of these first-phase patients had necks angled at more than 45 degrees. In the roll-in phase, "the Aorflex device has given excellent results in patients with typical anatomy," and the results serve "as a good prelude to the high-angle cases," he said.
The main phase of the trial, which planned to enroll up to 150 patients with high-angle aortic necks, has also finished, but those data are not yet publicly available. Lombard Medical, the company developing this device, submitted the main-phase data to the FDA, and should make the results public in the next 6 months, he said.
• Prefenestrations. Perhaps one of the more exciting new devices is the Ventana fenestrated system, an "off-the-shelf" device that comes with prefenestrations for the renal arteries that precludes the need for a custom-made device.
A current "unmet clinical need" for EVAR is a device to address patients with a short or nonexistent infrarenal neck, something that occurs in about a quarter of abdominal aortic aneurysm patients, said Dr. Daniel Clair, professor and chairman of vascular surgery at the Cleveland Clinic.
The system can potentially treat even aneurysms that include the renal arteries. The material used in the device is a multilayer polytetrafluoroethylene that forms small branches at the fenestrations that reach out into the renal arteries, and the device is delivered in a 22-Fr sheath.
Pilot studies used this system successfully on 15 patients outside the United States last year, and in February the next phase starts with another 30-50 patients scheduled for treatment both in and outside the United States, he said.
The pivotal U.S. trial (with 122 patients scheduled for treatment) also starts this year, with Dr. Clair as the principal investigator.
• Aneurysm sealing. Also in development is an EVAR-like device that’s not a stent. The Nellix system seals an aneurysm endovascularly, by deploying bags that fill with a liquid polymer that quickly solidifies to seal an aneurysm sac around a pair of stents. The system delivers the polymer at greater than blood pressure to extrude all blood from the aneurysm sac, said Dr. Lindsay Machan, a vascular surgeon at the University of British Columbia in Vancouver. This facilitates treatment of aortas with short or severely angulated necks. "The polymer anchors the devise at the bifurcation," Dr. Machan said, producing "remarkable" stability, better than devices that rely on barbs for anchoring.
So far, operators have used the Nellix system in 47 patients in New Zealand and Latvia, the first 31 with a first-generation device and, more recently, the remaining 16 patients with a second-generation version. More than 1 year of follow-up exists for 34 patients. These cases had 100% technical success with no ruptures, no conversions to open repair, and no endoleaks (either persistent or type II), he said. Two patients needed a secondary procedure, one for renal insufficiency, the other for a type I endoleak after 15 months.
The pivotal U.S. trial is planned to start later this year. "This ‘out-of-the-box’ technology addresses many shortcomings of EVAR, and uses a simple insertion procedure that takes 20-30 minutes," Dr. Machan said.
Dr. Böckler said that he has financial relationships with W.L. Gore, Medtronic, VASCOPS, and Siemens. Dr. Sievert said that he has financial relationships with TriVascular, W.L. Gore, and 55 other device companies. Dr. Greenberg said that he has an ownership interest in Cook Medical. Dr. Bersin said that he has financial relationships with Cordis Endovascular, W.L. Gore, Cook, and Bard. Dr. Gray said that he is a consultant to Cordis. Dr. Clair said that he has financial relationships with Endologix, ev3, Medtronic, Boston Scientific, and Covidien. Dr. Machan said that he has financial relationships with Boston Scientific, Cook, Calgary Scientific, Endologix, and Ikomed.
endovascular aneurysm repair, EVAR, ISET 2012, international symposium on endovascular therapy, Endurant device, aortic stent grafts, Dr. Dittmar Böckler, Endurant II, The Ovation Abdominal Stent Graft System, Dr. Horst Sievert,
The Zenith Low Profile,
MIAMI BEACH – Devices for endovascular repair of abdominal aortic aneurysms are evolving, with new sizing or other innovations either recently on the market or in studies.
The new products expand the scope of patients who are candidates for endovascular aneurysm repair (EVAR), and in one case bring a new approach to EVAR into the clinic.
During a session at ISET 2012, an international symposium on endovascular therapy, seven experts gave the following quick updates on what’s new in EVAR for 2012:
• Making devices smaller. Downsizing constitutes a major trend for several new EVAR devices. The Endurant device helped blaze the lower-profile path, introducing a device delivered in an 18-Fr catheter for 23- to 25-mm diameter aortic stent grafts. Endurant received Food and Drug Administration marketing approval in 2010, and results from the U.S. pivotal trial for this device appeared last year (J. Vasc. Surg. 2011;54:601-8). In the pivotal trial, the major adverse event rate after 30 days was 4%; after 1 year, the patency rate was 97%, no patients had type I endoleaks, and the rate of secondary procedures was 5%, said Dr. Dittmar Böckler, professor and medical director of vascular surgery at the University of Heidelberg (Germany). An even smaller version of the same device, Endurant II, places a 28-mm bifurcated segment of an aortic stent graft into an 18-Fr delivery catheter – reduced from 20 Fr in the existing Endurant device – and has longer limbs to maximize coverage, changes that will further "expand options" for this device, he said.
Other EVAR devices take the downsizing trend even further. The Ovation Abdominal Stent Graft System, which received FDA marketing approval last November, has a 14-Fr outer diameter. For the time being, Ovation "is the lowest-profile commercially available device, expanding the population that is suitable for EVAR," said Dr. Horst Sievert, director of the cardiovascular center at Sankt Katharinen Hospital in Frankfurt, Germany. The FDA also made Ovation the first EVAR to be approved as a humanitarian use device (defined as an instrument aimed at a U.S. patient population that grows by fewer than 4,000 new cases annually). Ovation is approved for use in patients with iliac or femoral artery access that is less than 7 mm in diameter, and for treating an aorta with an inner neck diameter of 15.5-17.4 mm. The clinical trial results for Ovation showed "excellent short-term results," Dr. Sievert said. In the U.S. pivotal trial with 161 patients, the major adverse event rate after 1 month was 2.5%, he noted.
The Zenith Low Profile, a third low-profile EVAR device delivered in a 16-Fr sheath, is nearing marketing approval. The FDA already has data from a U.S. pivotal trial with 120 patients, and a decision on marketing approval should come sometime this year, said Dr. Roy K. Greenberg, a vascular surgeon at the Cleveland Clinic. Although he voiced enthusiasm for lower-profile devices that allow EVAR in patients who would not be able to accommodate larger devices, he also cautioned that "as we get lower-profile devices, we need to pay more attention to iliac disease. Postimplantation residual stenosis is critical for patients with [calcified] iliac disease."
Another issue with new devices is establishing their long-term performance. "New devices aren’t necessarily better; they’re just different. I think there are some advantages to new devices, but we don’t have a track record with respect to their long-term behavior," Dr. Greenberg said.
Finally, the INCRAFT low-profile EVAR device starts testing in a pivotal U.S. trial in February. INCRAFT matches Ovation with a 14-F outer-diameter delivery sheath, said Dr. Robert M. Bersin, medical director of endovascular services at Swedish Medical Center in Seattle. Its track record so far comes from the first 60 patients who received the device, at six centers in Germany and Italy, he added.
• High aortic neck angles. Aside from size, the aortic-neck angle provides another opportunity to broaden the EVAR device market. The Aorfix EVAR device is highly flexible and designed to deal with aortic neck angles of 60 degrees or greater. The stent has a "unique design, because a 60-degree angle is typically an exclusion for most [EVAR] devices," said Dr. William A. Gray, director of endovascular services at Columbia University in New York.
He reviewed the roll-in phase data from the PYTHAGORAS trial, which enrolled 62 patients with aortic neck angles smaller than 60 degrees; two-thirds of these first-phase patients had necks angled at more than 45 degrees. In the roll-in phase, "the Aorflex device has given excellent results in patients with typical anatomy," and the results serve "as a good prelude to the high-angle cases," he said.
The main phase of the trial, which planned to enroll up to 150 patients with high-angle aortic necks, has also finished, but those data are not yet publicly available. Lombard Medical, the company developing this device, submitted the main-phase data to the FDA, and should make the results public in the next 6 months, he said.
• Prefenestrations. Perhaps one of the more exciting new devices is the Ventana fenestrated system, an "off-the-shelf" device that comes with prefenestrations for the renal arteries that precludes the need for a custom-made device.
A current "unmet clinical need" for EVAR is a device to address patients with a short or nonexistent infrarenal neck, something that occurs in about a quarter of abdominal aortic aneurysm patients, said Dr. Daniel Clair, professor and chairman of vascular surgery at the Cleveland Clinic.
The system can potentially treat even aneurysms that include the renal arteries. The material used in the device is a multilayer polytetrafluoroethylene that forms small branches at the fenestrations that reach out into the renal arteries, and the device is delivered in a 22-Fr sheath.
Pilot studies used this system successfully on 15 patients outside the United States last year, and in February the next phase starts with another 30-50 patients scheduled for treatment both in and outside the United States, he said.
The pivotal U.S. trial (with 122 patients scheduled for treatment) also starts this year, with Dr. Clair as the principal investigator.
• Aneurysm sealing. Also in development is an EVAR-like device that’s not a stent. The Nellix system seals an aneurysm endovascularly, by deploying bags that fill with a liquid polymer that quickly solidifies to seal an aneurysm sac around a pair of stents. The system delivers the polymer at greater than blood pressure to extrude all blood from the aneurysm sac, said Dr. Lindsay Machan, a vascular surgeon at the University of British Columbia in Vancouver. This facilitates treatment of aortas with short or severely angulated necks. "The polymer anchors the devise at the bifurcation," Dr. Machan said, producing "remarkable" stability, better than devices that rely on barbs for anchoring.
So far, operators have used the Nellix system in 47 patients in New Zealand and Latvia, the first 31 with a first-generation device and, more recently, the remaining 16 patients with a second-generation version. More than 1 year of follow-up exists for 34 patients. These cases had 100% technical success with no ruptures, no conversions to open repair, and no endoleaks (either persistent or type II), he said. Two patients needed a secondary procedure, one for renal insufficiency, the other for a type I endoleak after 15 months.
The pivotal U.S. trial is planned to start later this year. "This ‘out-of-the-box’ technology addresses many shortcomings of EVAR, and uses a simple insertion procedure that takes 20-30 minutes," Dr. Machan said.
Dr. Böckler said that he has financial relationships with W.L. Gore, Medtronic, VASCOPS, and Siemens. Dr. Sievert said that he has financial relationships with TriVascular, W.L. Gore, and 55 other device companies. Dr. Greenberg said that he has an ownership interest in Cook Medical. Dr. Bersin said that he has financial relationships with Cordis Endovascular, W.L. Gore, Cook, and Bard. Dr. Gray said that he is a consultant to Cordis. Dr. Clair said that he has financial relationships with Endologix, ev3, Medtronic, Boston Scientific, and Covidien. Dr. Machan said that he has financial relationships with Boston Scientific, Cook, Calgary Scientific, Endologix, and Ikomed.
MIAMI BEACH – Devices for endovascular repair of abdominal aortic aneurysms are evolving, with new sizing or other innovations either recently on the market or in studies.
The new products expand the scope of patients who are candidates for endovascular aneurysm repair (EVAR), and in one case bring a new approach to EVAR into the clinic.
During a session at ISET 2012, an international symposium on endovascular therapy, seven experts gave the following quick updates on what’s new in EVAR for 2012:
• Making devices smaller. Downsizing constitutes a major trend for several new EVAR devices. The Endurant device helped blaze the lower-profile path, introducing a device delivered in an 18-Fr catheter for 23- to 25-mm diameter aortic stent grafts. Endurant received Food and Drug Administration marketing approval in 2010, and results from the U.S. pivotal trial for this device appeared last year (J. Vasc. Surg. 2011;54:601-8). In the pivotal trial, the major adverse event rate after 30 days was 4%; after 1 year, the patency rate was 97%, no patients had type I endoleaks, and the rate of secondary procedures was 5%, said Dr. Dittmar Böckler, professor and medical director of vascular surgery at the University of Heidelberg (Germany). An even smaller version of the same device, Endurant II, places a 28-mm bifurcated segment of an aortic stent graft into an 18-Fr delivery catheter – reduced from 20 Fr in the existing Endurant device – and has longer limbs to maximize coverage, changes that will further "expand options" for this device, he said.
Other EVAR devices take the downsizing trend even further. The Ovation Abdominal Stent Graft System, which received FDA marketing approval last November, has a 14-Fr outer diameter. For the time being, Ovation "is the lowest-profile commercially available device, expanding the population that is suitable for EVAR," said Dr. Horst Sievert, director of the cardiovascular center at Sankt Katharinen Hospital in Frankfurt, Germany. The FDA also made Ovation the first EVAR to be approved as a humanitarian use device (defined as an instrument aimed at a U.S. patient population that grows by fewer than 4,000 new cases annually). Ovation is approved for use in patients with iliac or femoral artery access that is less than 7 mm in diameter, and for treating an aorta with an inner neck diameter of 15.5-17.4 mm. The clinical trial results for Ovation showed "excellent short-term results," Dr. Sievert said. In the U.S. pivotal trial with 161 patients, the major adverse event rate after 1 month was 2.5%, he noted.
The Zenith Low Profile, a third low-profile EVAR device delivered in a 16-Fr sheath, is nearing marketing approval. The FDA already has data from a U.S. pivotal trial with 120 patients, and a decision on marketing approval should come sometime this year, said Dr. Roy K. Greenberg, a vascular surgeon at the Cleveland Clinic. Although he voiced enthusiasm for lower-profile devices that allow EVAR in patients who would not be able to accommodate larger devices, he also cautioned that "as we get lower-profile devices, we need to pay more attention to iliac disease. Postimplantation residual stenosis is critical for patients with [calcified] iliac disease."
Another issue with new devices is establishing their long-term performance. "New devices aren’t necessarily better; they’re just different. I think there are some advantages to new devices, but we don’t have a track record with respect to their long-term behavior," Dr. Greenberg said.
Finally, the INCRAFT low-profile EVAR device starts testing in a pivotal U.S. trial in February. INCRAFT matches Ovation with a 14-F outer-diameter delivery sheath, said Dr. Robert M. Bersin, medical director of endovascular services at Swedish Medical Center in Seattle. Its track record so far comes from the first 60 patients who received the device, at six centers in Germany and Italy, he added.
• High aortic neck angles. Aside from size, the aortic-neck angle provides another opportunity to broaden the EVAR device market. The Aorfix EVAR device is highly flexible and designed to deal with aortic neck angles of 60 degrees or greater. The stent has a "unique design, because a 60-degree angle is typically an exclusion for most [EVAR] devices," said Dr. William A. Gray, director of endovascular services at Columbia University in New York.
He reviewed the roll-in phase data from the PYTHAGORAS trial, which enrolled 62 patients with aortic neck angles smaller than 60 degrees; two-thirds of these first-phase patients had necks angled at more than 45 degrees. In the roll-in phase, "the Aorflex device has given excellent results in patients with typical anatomy," and the results serve "as a good prelude to the high-angle cases," he said.
The main phase of the trial, which planned to enroll up to 150 patients with high-angle aortic necks, has also finished, but those data are not yet publicly available. Lombard Medical, the company developing this device, submitted the main-phase data to the FDA, and should make the results public in the next 6 months, he said.
• Prefenestrations. Perhaps one of the more exciting new devices is the Ventana fenestrated system, an "off-the-shelf" device that comes with prefenestrations for the renal arteries that precludes the need for a custom-made device.
A current "unmet clinical need" for EVAR is a device to address patients with a short or nonexistent infrarenal neck, something that occurs in about a quarter of abdominal aortic aneurysm patients, said Dr. Daniel Clair, professor and chairman of vascular surgery at the Cleveland Clinic.
The system can potentially treat even aneurysms that include the renal arteries. The material used in the device is a multilayer polytetrafluoroethylene that forms small branches at the fenestrations that reach out into the renal arteries, and the device is delivered in a 22-Fr sheath.
Pilot studies used this system successfully on 15 patients outside the United States last year, and in February the next phase starts with another 30-50 patients scheduled for treatment both in and outside the United States, he said.
The pivotal U.S. trial (with 122 patients scheduled for treatment) also starts this year, with Dr. Clair as the principal investigator.
• Aneurysm sealing. Also in development is an EVAR-like device that’s not a stent. The Nellix system seals an aneurysm endovascularly, by deploying bags that fill with a liquid polymer that quickly solidifies to seal an aneurysm sac around a pair of stents. The system delivers the polymer at greater than blood pressure to extrude all blood from the aneurysm sac, said Dr. Lindsay Machan, a vascular surgeon at the University of British Columbia in Vancouver. This facilitates treatment of aortas with short or severely angulated necks. "The polymer anchors the devise at the bifurcation," Dr. Machan said, producing "remarkable" stability, better than devices that rely on barbs for anchoring.
So far, operators have used the Nellix system in 47 patients in New Zealand and Latvia, the first 31 with a first-generation device and, more recently, the remaining 16 patients with a second-generation version. More than 1 year of follow-up exists for 34 patients. These cases had 100% technical success with no ruptures, no conversions to open repair, and no endoleaks (either persistent or type II), he said. Two patients needed a secondary procedure, one for renal insufficiency, the other for a type I endoleak after 15 months.
The pivotal U.S. trial is planned to start later this year. "This ‘out-of-the-box’ technology addresses many shortcomings of EVAR, and uses a simple insertion procedure that takes 20-30 minutes," Dr. Machan said.
Dr. Böckler said that he has financial relationships with W.L. Gore, Medtronic, VASCOPS, and Siemens. Dr. Sievert said that he has financial relationships with TriVascular, W.L. Gore, and 55 other device companies. Dr. Greenberg said that he has an ownership interest in Cook Medical. Dr. Bersin said that he has financial relationships with Cordis Endovascular, W.L. Gore, Cook, and Bard. Dr. Gray said that he is a consultant to Cordis. Dr. Clair said that he has financial relationships with Endologix, ev3, Medtronic, Boston Scientific, and Covidien. Dr. Machan said that he has financial relationships with Boston Scientific, Cook, Calgary Scientific, Endologix, and Ikomed.
endovascular aneurysm repair, EVAR, ISET 2012, international symposium on endovascular therapy, Endurant device, aortic stent grafts, Dr. Dittmar Böckler, Endurant II, The Ovation Abdominal Stent Graft System, Dr. Horst Sievert,
The Zenith Low Profile,
endovascular aneurysm repair, EVAR, ISET 2012, international symposium on endovascular therapy, Endurant device, aortic stent grafts, Dr. Dittmar Böckler, Endurant II, The Ovation Abdominal Stent Graft System, Dr. Horst Sievert,
The Zenith Low Profile,
EXPERT ANALYSIS FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Pregnancy Does Not Preclude Safe DVT Treatment
MIAMI BEACH – Women who develop a deep vein thrombosis while pregnant can safely undergo removal of the clot without jeopardizing their pregnancy, based on a recent experience with 11 cases at one U.S. center.
"Not many vascular surgeons are willing to perform thrombectomy in pregnant women, and most physicians fear thrombolytic therapy in pregnant patients," Dr. Anthony J. Comerota said at ISET 2012, an international symposium on endovascular therapy.
But in reality, there is no evidence that thrombolysis poses any special risk during pregnancy, in part because the drugs that are usually used – urokinase, streptokinase, or tissue plasminogen activator (TPA) – do not cross the placenta, and therefore do not exert any fibrinolytic effect on a fetus, he said. In addition, the relatively recent introduction of pharmacomechanical methods for thrombolysis have "accelerated our enthusiasm and strengthened our confidence that this is an effective and safe strategy for treating extensive DVT [deep vein thrombosis] during pregnancy," said Dr. Comerota, a vascular surgeon and director of the Jobst Vascular Institute in Toledo, Ohio.
"Pregnancy need not be a major contraindication for successful management of extensive DVT. We need to minimize radiation, monitor the fetus, and control uterine contractions, but we should treat healthy, pregnant women [who have] a DVT the same way we treat healthy nonpregnant women" who have a DVT, he said in an interview. "The extraordinary concern for risk to the pregnancy [from clot removal] seems substantially overstated."
Because pregnancy produces a prothrombotic state, the risk that a healthy, pregnant woman has for DVT is about sixfold higher than her risk when not pregnant. Until now, most physicians deferred endovascular or surgical management of women who develop a DVT during pregnancy, and have relied exclusively on medical treatment with heparin or low-molecular-weight heparin, a strategy that is often ineffective.
"What pushed me to treat women earlier during their pregnancy was that I saw patients who did not receive therapy until after delivery, and by then the clot became more organized – a mix of collagen and scar inside the vein – -and caused severe postthrombotic disability," Dr. Comerota said.
Recently, he treated 11 pregnant women (10 with an iliofemoral thrombosis and 1 with the clot in her superior vena cava). One woman was in the first trimester, two were in the second, and eight were in the third trimester. Three patients underwent clot removal by open surgery, with the other eight treated by endovascular methods, including six who were treated with catheter-delivered TPA and one who received urokinase via catheter. Other endovascular tools that were used as needed included ultrasound clot treatment and rheolytic thrombectomy. In all cases, these treatments successfully removed the clot. One women developed hematuria and required a transfusion following her clot removal, and another patient developed a popliteal artery pseudoaneurysm. None of the patients had a recurrent DVT; two developed mild postthrombotic symptoms.
In one case, the fetus spontaneously aborted 5 days after the procedure secondary to antiphospholipid antibody syndrome, which had also triggered the thrombosis. The other 10 cases resulted in successful pregnancies and live deliveries, one surgically and the other nine vaginally. Three of the women had successful subsequent pregnancies, Dr. Comerota said.
Dr. Comerota said that he has been a speaker for and a consultant to Covidien, a company that makes some of the devices used in these procedures.
MIAMI BEACH – Women who develop a deep vein thrombosis while pregnant can safely undergo removal of the clot without jeopardizing their pregnancy, based on a recent experience with 11 cases at one U.S. center.
"Not many vascular surgeons are willing to perform thrombectomy in pregnant women, and most physicians fear thrombolytic therapy in pregnant patients," Dr. Anthony J. Comerota said at ISET 2012, an international symposium on endovascular therapy.
But in reality, there is no evidence that thrombolysis poses any special risk during pregnancy, in part because the drugs that are usually used – urokinase, streptokinase, or tissue plasminogen activator (TPA) – do not cross the placenta, and therefore do not exert any fibrinolytic effect on a fetus, he said. In addition, the relatively recent introduction of pharmacomechanical methods for thrombolysis have "accelerated our enthusiasm and strengthened our confidence that this is an effective and safe strategy for treating extensive DVT [deep vein thrombosis] during pregnancy," said Dr. Comerota, a vascular surgeon and director of the Jobst Vascular Institute in Toledo, Ohio.
"Pregnancy need not be a major contraindication for successful management of extensive DVT. We need to minimize radiation, monitor the fetus, and control uterine contractions, but we should treat healthy, pregnant women [who have] a DVT the same way we treat healthy nonpregnant women" who have a DVT, he said in an interview. "The extraordinary concern for risk to the pregnancy [from clot removal] seems substantially overstated."
Because pregnancy produces a prothrombotic state, the risk that a healthy, pregnant woman has for DVT is about sixfold higher than her risk when not pregnant. Until now, most physicians deferred endovascular or surgical management of women who develop a DVT during pregnancy, and have relied exclusively on medical treatment with heparin or low-molecular-weight heparin, a strategy that is often ineffective.
"What pushed me to treat women earlier during their pregnancy was that I saw patients who did not receive therapy until after delivery, and by then the clot became more organized – a mix of collagen and scar inside the vein – -and caused severe postthrombotic disability," Dr. Comerota said.
Recently, he treated 11 pregnant women (10 with an iliofemoral thrombosis and 1 with the clot in her superior vena cava). One woman was in the first trimester, two were in the second, and eight were in the third trimester. Three patients underwent clot removal by open surgery, with the other eight treated by endovascular methods, including six who were treated with catheter-delivered TPA and one who received urokinase via catheter. Other endovascular tools that were used as needed included ultrasound clot treatment and rheolytic thrombectomy. In all cases, these treatments successfully removed the clot. One women developed hematuria and required a transfusion following her clot removal, and another patient developed a popliteal artery pseudoaneurysm. None of the patients had a recurrent DVT; two developed mild postthrombotic symptoms.
In one case, the fetus spontaneously aborted 5 days after the procedure secondary to antiphospholipid antibody syndrome, which had also triggered the thrombosis. The other 10 cases resulted in successful pregnancies and live deliveries, one surgically and the other nine vaginally. Three of the women had successful subsequent pregnancies, Dr. Comerota said.
Dr. Comerota said that he has been a speaker for and a consultant to Covidien, a company that makes some of the devices used in these procedures.
MIAMI BEACH – Women who develop a deep vein thrombosis while pregnant can safely undergo removal of the clot without jeopardizing their pregnancy, based on a recent experience with 11 cases at one U.S. center.
"Not many vascular surgeons are willing to perform thrombectomy in pregnant women, and most physicians fear thrombolytic therapy in pregnant patients," Dr. Anthony J. Comerota said at ISET 2012, an international symposium on endovascular therapy.
But in reality, there is no evidence that thrombolysis poses any special risk during pregnancy, in part because the drugs that are usually used – urokinase, streptokinase, or tissue plasminogen activator (TPA) – do not cross the placenta, and therefore do not exert any fibrinolytic effect on a fetus, he said. In addition, the relatively recent introduction of pharmacomechanical methods for thrombolysis have "accelerated our enthusiasm and strengthened our confidence that this is an effective and safe strategy for treating extensive DVT [deep vein thrombosis] during pregnancy," said Dr. Comerota, a vascular surgeon and director of the Jobst Vascular Institute in Toledo, Ohio.
"Pregnancy need not be a major contraindication for successful management of extensive DVT. We need to minimize radiation, monitor the fetus, and control uterine contractions, but we should treat healthy, pregnant women [who have] a DVT the same way we treat healthy nonpregnant women" who have a DVT, he said in an interview. "The extraordinary concern for risk to the pregnancy [from clot removal] seems substantially overstated."
Because pregnancy produces a prothrombotic state, the risk that a healthy, pregnant woman has for DVT is about sixfold higher than her risk when not pregnant. Until now, most physicians deferred endovascular or surgical management of women who develop a DVT during pregnancy, and have relied exclusively on medical treatment with heparin or low-molecular-weight heparin, a strategy that is often ineffective.
"What pushed me to treat women earlier during their pregnancy was that I saw patients who did not receive therapy until after delivery, and by then the clot became more organized – a mix of collagen and scar inside the vein – -and caused severe postthrombotic disability," Dr. Comerota said.
Recently, he treated 11 pregnant women (10 with an iliofemoral thrombosis and 1 with the clot in her superior vena cava). One woman was in the first trimester, two were in the second, and eight were in the third trimester. Three patients underwent clot removal by open surgery, with the other eight treated by endovascular methods, including six who were treated with catheter-delivered TPA and one who received urokinase via catheter. Other endovascular tools that were used as needed included ultrasound clot treatment and rheolytic thrombectomy. In all cases, these treatments successfully removed the clot. One women developed hematuria and required a transfusion following her clot removal, and another patient developed a popliteal artery pseudoaneurysm. None of the patients had a recurrent DVT; two developed mild postthrombotic symptoms.
In one case, the fetus spontaneously aborted 5 days after the procedure secondary to antiphospholipid antibody syndrome, which had also triggered the thrombosis. The other 10 cases resulted in successful pregnancies and live deliveries, one surgically and the other nine vaginally. Three of the women had successful subsequent pregnancies, Dr. Comerota said.
Dr. Comerota said that he has been a speaker for and a consultant to Covidien, a company that makes some of the devices used in these procedures.
FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: Treatment of 11 pregnant women with DVT resulted in 10 successful live births.
Data Source: A retrospective review of 11 cases managed at one U.S. center.
Disclosures: Dr. Comerota said that he has been a speaker for and a consultant to Covidien, a company that makes some of the devices used in these procedures.
Two Stents Show Peripheral Artery Efficacy
MIAMI BEACH – Two different self-expandable nitinol stents showed efficacy for treating peripheral arterial stenoses, according to results from two separate, pivotal trials. The Food and Drug Administration is reviewing data from both studies, with the agency’s decisions expected later this year, researchers said at ISET 2012, an international symposium on endovascular therapy.
The EverFlex stent underwent testing in femoropopliteal arteries in 287 patients with peripheral artery disease at 44 centers in the United States and Europe. The trial enrolled patients with Rutherford-Becker clinical categories of 2, 3, or 4, and at least a 50% stenosis. The average age of the patients enrolled was 68 years, two-thirds were men, 43% had diabetes, 88% had hypertension, 40% had Rutherford category 2 disease, and 56% had category 3 disease. The average lesion length was 9 cm, with 29% of patients having a lesion that was 20 cm long.
One of the study’s major goals was testing a single-stent strategy, and 95% of enrolled patients received a single stent, said Dr. Jon S. Matsumura, the trial’s principal investigator. Using single stents for long lesions may reduce the risk of stent fracture.
The primary efficacy end point of DURABILITY II (Safety and Effectiveness Study of EverFlex Stent to Treat Symptomatic Femoral-Popliteal Atherosclerosis) was primary patency in the treated vessel 1 year after stent placement. Study results from 226 patients with follow-up data showed primary patency in 77% after 1 year in an actuarial analysis. This level of success exceeded the 33% performance goal, set on the basis of literature controls using angioplasty interventions, said Dr. Matsumura, professor and chairman of the division of vascular surgery at the University of Wisconsin in Madison.
The study’s primary safety end point was the combined rate of major adverse effects after 30 days. The results showed no such events within that period, with no deaths, no amputations of treated limbs, and no instances of clinically driven target vessel revascularizations.
At 1 year after treatment, a single episode of stent fracture – a class V fracture in the stent’s transaxial spiral configuration – had occurred.
Also at 1 year, 84% of patients had at least a single-level improvement, compared with their baseline Rutherford clinical category, with 65% of patients improving by two or more categories. The researchers collected data on the baseline and 1-year follow-up treadmill walking tests in 29 patients, and 69% of these patients showed improvements in walking distance, with an average increase of about 420 feet per patient.
ORION (A Boston Scientific Trial of the Epic Nitinol Stent in the Treatment of Atherosclerotic Lesions in Iliac Arteries) enrolled 125 patients with a new or restenotic lesion in the common or external iliac artery, or in both, and with a Rutherford-Becker clinical category of 1, 2, 3, or 4. Lesions had to be no longer than 13 cm, with a reference vessel diameter of 5-11 mm. The study enrolled patients at 28 U.S. centers during May 2009–December 2010.
The study population had an average age of 61 years, two-thirds were men, a third of the patients had diabetes, and 76% had hypertension. The average lesion length was just over 3 cm, and the average vessel diameter was just under 8 mm. The operators in the study placed a total of 187 stents into 166 lesions in the enrolled patients.
This study’s primary end point was the combined rate of major adverse events after 9 months of follow-up. These events occurred in four of the 117 patients who were followed for 9 months, a 3.4% rate that consisted entirely of target vessel revascularizations; in three cases, these revascularizations occurred because of stent thrombosis episodes, said Dr. Daniel G. Clair, principal investigator for the study and chairman of vascular surgery at the Cleveland Clinic. The study patients had no other events that made up the major adverse event composite: no deaths within 30 days, no MIs during hospitalization, and no amputations of the index limb during 9 months of follow-up.
The 3.4% observed major adverse event rate ran significantly below the study’s prespecified performance goal of 17%. The 17% goal derived from an expected 8% rate based on the historical literature, plus an added margin of 9%. The actual rate "was much lower than predicted by the literature," based on how iliac artery stents performed in prior studies, Dr. Clair said.
The percentage of patients with a Rutherford-Becker clinical category of 2-4 dropped from 93% at baseline to 18% at 9 months. Average ankle brachial index increased from an average of 0.79 at baseline to 0.97 at 9 months. The primary patency rate at 9 months was 96%.
The results "support the safety and efficacy of the stent in iliac arteries," Dr. Clair concluded.
The DURABILITY II trial was sponsored by Covidien, the company developing the EverFlex stent. The ORION trial was sponsored by Boston Scientific, the company developing the Epic stent. Dr. Matsumura said that he has received research grants from Covidien and from Abbott, Cook, Endologix, and W.L. Gore. Dr. Clair said that he has been a consultant to Boston Scientific, and also to Covidien, Endologix, ev3, and Medtronic.
MIAMI BEACH – Two different self-expandable nitinol stents showed efficacy for treating peripheral arterial stenoses, according to results from two separate, pivotal trials. The Food and Drug Administration is reviewing data from both studies, with the agency’s decisions expected later this year, researchers said at ISET 2012, an international symposium on endovascular therapy.
The EverFlex stent underwent testing in femoropopliteal arteries in 287 patients with peripheral artery disease at 44 centers in the United States and Europe. The trial enrolled patients with Rutherford-Becker clinical categories of 2, 3, or 4, and at least a 50% stenosis. The average age of the patients enrolled was 68 years, two-thirds were men, 43% had diabetes, 88% had hypertension, 40% had Rutherford category 2 disease, and 56% had category 3 disease. The average lesion length was 9 cm, with 29% of patients having a lesion that was 20 cm long.
One of the study’s major goals was testing a single-stent strategy, and 95% of enrolled patients received a single stent, said Dr. Jon S. Matsumura, the trial’s principal investigator. Using single stents for long lesions may reduce the risk of stent fracture.
The primary efficacy end point of DURABILITY II (Safety and Effectiveness Study of EverFlex Stent to Treat Symptomatic Femoral-Popliteal Atherosclerosis) was primary patency in the treated vessel 1 year after stent placement. Study results from 226 patients with follow-up data showed primary patency in 77% after 1 year in an actuarial analysis. This level of success exceeded the 33% performance goal, set on the basis of literature controls using angioplasty interventions, said Dr. Matsumura, professor and chairman of the division of vascular surgery at the University of Wisconsin in Madison.
The study’s primary safety end point was the combined rate of major adverse effects after 30 days. The results showed no such events within that period, with no deaths, no amputations of treated limbs, and no instances of clinically driven target vessel revascularizations.
At 1 year after treatment, a single episode of stent fracture – a class V fracture in the stent’s transaxial spiral configuration – had occurred.
Also at 1 year, 84% of patients had at least a single-level improvement, compared with their baseline Rutherford clinical category, with 65% of patients improving by two or more categories. The researchers collected data on the baseline and 1-year follow-up treadmill walking tests in 29 patients, and 69% of these patients showed improvements in walking distance, with an average increase of about 420 feet per patient.
ORION (A Boston Scientific Trial of the Epic Nitinol Stent in the Treatment of Atherosclerotic Lesions in Iliac Arteries) enrolled 125 patients with a new or restenotic lesion in the common or external iliac artery, or in both, and with a Rutherford-Becker clinical category of 1, 2, 3, or 4. Lesions had to be no longer than 13 cm, with a reference vessel diameter of 5-11 mm. The study enrolled patients at 28 U.S. centers during May 2009–December 2010.
The study population had an average age of 61 years, two-thirds were men, a third of the patients had diabetes, and 76% had hypertension. The average lesion length was just over 3 cm, and the average vessel diameter was just under 8 mm. The operators in the study placed a total of 187 stents into 166 lesions in the enrolled patients.
This study’s primary end point was the combined rate of major adverse events after 9 months of follow-up. These events occurred in four of the 117 patients who were followed for 9 months, a 3.4% rate that consisted entirely of target vessel revascularizations; in three cases, these revascularizations occurred because of stent thrombosis episodes, said Dr. Daniel G. Clair, principal investigator for the study and chairman of vascular surgery at the Cleveland Clinic. The study patients had no other events that made up the major adverse event composite: no deaths within 30 days, no MIs during hospitalization, and no amputations of the index limb during 9 months of follow-up.
The 3.4% observed major adverse event rate ran significantly below the study’s prespecified performance goal of 17%. The 17% goal derived from an expected 8% rate based on the historical literature, plus an added margin of 9%. The actual rate "was much lower than predicted by the literature," based on how iliac artery stents performed in prior studies, Dr. Clair said.
The percentage of patients with a Rutherford-Becker clinical category of 2-4 dropped from 93% at baseline to 18% at 9 months. Average ankle brachial index increased from an average of 0.79 at baseline to 0.97 at 9 months. The primary patency rate at 9 months was 96%.
The results "support the safety and efficacy of the stent in iliac arteries," Dr. Clair concluded.
The DURABILITY II trial was sponsored by Covidien, the company developing the EverFlex stent. The ORION trial was sponsored by Boston Scientific, the company developing the Epic stent. Dr. Matsumura said that he has received research grants from Covidien and from Abbott, Cook, Endologix, and W.L. Gore. Dr. Clair said that he has been a consultant to Boston Scientific, and also to Covidien, Endologix, ev3, and Medtronic.
MIAMI BEACH – Two different self-expandable nitinol stents showed efficacy for treating peripheral arterial stenoses, according to results from two separate, pivotal trials. The Food and Drug Administration is reviewing data from both studies, with the agency’s decisions expected later this year, researchers said at ISET 2012, an international symposium on endovascular therapy.
The EverFlex stent underwent testing in femoropopliteal arteries in 287 patients with peripheral artery disease at 44 centers in the United States and Europe. The trial enrolled patients with Rutherford-Becker clinical categories of 2, 3, or 4, and at least a 50% stenosis. The average age of the patients enrolled was 68 years, two-thirds were men, 43% had diabetes, 88% had hypertension, 40% had Rutherford category 2 disease, and 56% had category 3 disease. The average lesion length was 9 cm, with 29% of patients having a lesion that was 20 cm long.
One of the study’s major goals was testing a single-stent strategy, and 95% of enrolled patients received a single stent, said Dr. Jon S. Matsumura, the trial’s principal investigator. Using single stents for long lesions may reduce the risk of stent fracture.
The primary efficacy end point of DURABILITY II (Safety and Effectiveness Study of EverFlex Stent to Treat Symptomatic Femoral-Popliteal Atherosclerosis) was primary patency in the treated vessel 1 year after stent placement. Study results from 226 patients with follow-up data showed primary patency in 77% after 1 year in an actuarial analysis. This level of success exceeded the 33% performance goal, set on the basis of literature controls using angioplasty interventions, said Dr. Matsumura, professor and chairman of the division of vascular surgery at the University of Wisconsin in Madison.
The study’s primary safety end point was the combined rate of major adverse effects after 30 days. The results showed no such events within that period, with no deaths, no amputations of treated limbs, and no instances of clinically driven target vessel revascularizations.
At 1 year after treatment, a single episode of stent fracture – a class V fracture in the stent’s transaxial spiral configuration – had occurred.
Also at 1 year, 84% of patients had at least a single-level improvement, compared with their baseline Rutherford clinical category, with 65% of patients improving by two or more categories. The researchers collected data on the baseline and 1-year follow-up treadmill walking tests in 29 patients, and 69% of these patients showed improvements in walking distance, with an average increase of about 420 feet per patient.
ORION (A Boston Scientific Trial of the Epic Nitinol Stent in the Treatment of Atherosclerotic Lesions in Iliac Arteries) enrolled 125 patients with a new or restenotic lesion in the common or external iliac artery, or in both, and with a Rutherford-Becker clinical category of 1, 2, 3, or 4. Lesions had to be no longer than 13 cm, with a reference vessel diameter of 5-11 mm. The study enrolled patients at 28 U.S. centers during May 2009–December 2010.
The study population had an average age of 61 years, two-thirds were men, a third of the patients had diabetes, and 76% had hypertension. The average lesion length was just over 3 cm, and the average vessel diameter was just under 8 mm. The operators in the study placed a total of 187 stents into 166 lesions in the enrolled patients.
This study’s primary end point was the combined rate of major adverse events after 9 months of follow-up. These events occurred in four of the 117 patients who were followed for 9 months, a 3.4% rate that consisted entirely of target vessel revascularizations; in three cases, these revascularizations occurred because of stent thrombosis episodes, said Dr. Daniel G. Clair, principal investigator for the study and chairman of vascular surgery at the Cleveland Clinic. The study patients had no other events that made up the major adverse event composite: no deaths within 30 days, no MIs during hospitalization, and no amputations of the index limb during 9 months of follow-up.
The 3.4% observed major adverse event rate ran significantly below the study’s prespecified performance goal of 17%. The 17% goal derived from an expected 8% rate based on the historical literature, plus an added margin of 9%. The actual rate "was much lower than predicted by the literature," based on how iliac artery stents performed in prior studies, Dr. Clair said.
The percentage of patients with a Rutherford-Becker clinical category of 2-4 dropped from 93% at baseline to 18% at 9 months. Average ankle brachial index increased from an average of 0.79 at baseline to 0.97 at 9 months. The primary patency rate at 9 months was 96%.
The results "support the safety and efficacy of the stent in iliac arteries," Dr. Clair concluded.
The DURABILITY II trial was sponsored by Covidien, the company developing the EverFlex stent. The ORION trial was sponsored by Boston Scientific, the company developing the Epic stent. Dr. Matsumura said that he has received research grants from Covidien and from Abbott, Cook, Endologix, and W.L. Gore. Dr. Clair said that he has been a consultant to Boston Scientific, and also to Covidien, Endologix, ev3, and Medtronic.
FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: In femoropopliteal arteries, the EverFlex stent produced a 77% actuarial primary patency rate after 1 year. In iliac arteries, the Epic stent produced a 3.4% major adverse event rate after 9 months.
Data Source: The DURABILITY II trial enrolled 287 patients in a single-arm study at 44 U.S. and European centers. The ORION study enrolled 125 patients in a single-arm study at 28 U.S. centers.
Disclosures: The DURABILITY II trial was sponsored by Covidien, the company developing the EverFlex stent. The ORION trial was sponsored by Boston Scientific, the company developing the Epic stent. Dr. Matsumura said that he has received research grants from Covidien and from Abbott, Cook, Endologix, and W.L. Gore. Dr. Clair said that he has been a consultant to Boston Scientific, and also to Covidien, Endologix, ev3, and Medtronic.
Aortic Regurgitation After TAVR Poses Threat
MIAMI BEACH – Now that transcatheter aortic valve replacement is available for routine U.S. use, interventionalists have focused on making the procedure better and safer, such as cutting the incidence of significant regurgitation through the replacement aortic valve.
Toward that end, researchers have developed a way to quickly and objectively assess the risk for aortic valve regurgitation based on the measurement of aortic blood pressures immediately after transcatheter valve replacement (TAVR). This new measure, the "aortic regurgitation index," showed significant correlation with periprocedural aortic regurgitation as well as with patients’ 1-year survival following their procedure, Dr. Eberhard Grube said at ISET 2012, an international symposium on endovascular therapy.
Until now, "there has been no way to quantify aortic regurgitation; it’s subjective," said Dr. Grube, professor and chief of cardiology and angiology at the Helios Heart Centre in Siegburg, Germany. The aortic regurgitation index (ARI) allows physicians "to quantify aortic regurgitation periprocedurally by objectively looking at the patient’s hemodynamics, regardless of the subjective evaluation of aortic insufficiency by angiography or by echo," he said. "We can see the ARI and know what we need to do for postdeployment treatment."
Dr. Grube and his associates set the ARI as equal to the patient’s diastolic aortic pressure minus the left ventricular end diastolic pressure, then divided by the aortic systolic pressure, and multiplied by 100.
For example, a patient with mild periprocedural aortic regurgitation might have an aortic diastolic pressure of 60 mm Hg, a left ventricular end diastolic pressure of 15 mm Hg, and an aortic systolic pressure of 150 mm Hg, which would produce an ARI of 30, showing that the patient had a low risk for dying during the subsequent year. In contrast, a patient with moderate or severe periprocedural aortic regurgitation could have an aortic diastolic pressure of 40 mm Hg, a left ventricular end diastolic pressure of 20 mm Hg, and an aortic systolic pressure of 120 mm Hg, which would produce an ARI of 16.7, flagging a higher mortality risk during the following year.
His group assessed the prognostic ability of the ARI in a series of 146 patients who underwent TAVR. The group included 124 patients with no or mild aortic regurgitation following TAVR and 22 who showed moderate or severe regurgitation. During 1-year follow-up, the 96 patients with a periprocedural ARI of 25 or greater had an 83% survival rate; the 50 patients with a periprocedural ARI of less than 25 had a survival rate of 54%, a statistically significant difference. The analysis also showed a significant correlation between the ARI and the severity of aortic regurgitation. Among patients with no discernible regurgitation, the average ARI was about 30, in those with mild regurgitation the average ARI was about 25, in patients with moderate regurgitation the average was about 18, and in patients with severe regurgitation the ARI averaged about 10.
One recently identified key to limiting aortic regurgitation following TAVR is proper valve sizing relative to the patient’s aortic annulus. "Appropriate valve sizing is likely the most important factor that will influence both the degree of perivalvular regurgitation and pacemaker need after TAVR," said Dr. Jeffrey J. Popma in a separate talk at the meeting.
Until recently, many operators used transthoracic echocardiography for annulus sizing, but recently published evidence showed that imaging by CT or by MRI provide superior information, said Dr. Popma, director of interventional cardiology at Beth Israel Deaconess Medical Center in Boston. He cited a British report published last November that compared transthoracic echo, CT, and MRI in 202 patients who underwent TAVR, which found both CT and MRI superior to echo for annulus measurement prior to TAVR (J. Am. Coll. Cardiol. 2011;58:2165-73).
Dr. Grube said that he has financial relationships with Direct Flow, Claret, Biosensors, Medtronic, Mitralign, Boston Scientific, Cordis, Abbott Vascular, and InSeal. Dr. Popma said that he has financial relationships with Abbott, Boston Scientific, ev3 (Covidien), Medtronic, and Cordis.
MIAMI BEACH – Now that transcatheter aortic valve replacement is available for routine U.S. use, interventionalists have focused on making the procedure better and safer, such as cutting the incidence of significant regurgitation through the replacement aortic valve.
Toward that end, researchers have developed a way to quickly and objectively assess the risk for aortic valve regurgitation based on the measurement of aortic blood pressures immediately after transcatheter valve replacement (TAVR). This new measure, the "aortic regurgitation index," showed significant correlation with periprocedural aortic regurgitation as well as with patients’ 1-year survival following their procedure, Dr. Eberhard Grube said at ISET 2012, an international symposium on endovascular therapy.
Until now, "there has been no way to quantify aortic regurgitation; it’s subjective," said Dr. Grube, professor and chief of cardiology and angiology at the Helios Heart Centre in Siegburg, Germany. The aortic regurgitation index (ARI) allows physicians "to quantify aortic regurgitation periprocedurally by objectively looking at the patient’s hemodynamics, regardless of the subjective evaluation of aortic insufficiency by angiography or by echo," he said. "We can see the ARI and know what we need to do for postdeployment treatment."
Dr. Grube and his associates set the ARI as equal to the patient’s diastolic aortic pressure minus the left ventricular end diastolic pressure, then divided by the aortic systolic pressure, and multiplied by 100.
For example, a patient with mild periprocedural aortic regurgitation might have an aortic diastolic pressure of 60 mm Hg, a left ventricular end diastolic pressure of 15 mm Hg, and an aortic systolic pressure of 150 mm Hg, which would produce an ARI of 30, showing that the patient had a low risk for dying during the subsequent year. In contrast, a patient with moderate or severe periprocedural aortic regurgitation could have an aortic diastolic pressure of 40 mm Hg, a left ventricular end diastolic pressure of 20 mm Hg, and an aortic systolic pressure of 120 mm Hg, which would produce an ARI of 16.7, flagging a higher mortality risk during the following year.
His group assessed the prognostic ability of the ARI in a series of 146 patients who underwent TAVR. The group included 124 patients with no or mild aortic regurgitation following TAVR and 22 who showed moderate or severe regurgitation. During 1-year follow-up, the 96 patients with a periprocedural ARI of 25 or greater had an 83% survival rate; the 50 patients with a periprocedural ARI of less than 25 had a survival rate of 54%, a statistically significant difference. The analysis also showed a significant correlation between the ARI and the severity of aortic regurgitation. Among patients with no discernible regurgitation, the average ARI was about 30, in those with mild regurgitation the average ARI was about 25, in patients with moderate regurgitation the average was about 18, and in patients with severe regurgitation the ARI averaged about 10.
One recently identified key to limiting aortic regurgitation following TAVR is proper valve sizing relative to the patient’s aortic annulus. "Appropriate valve sizing is likely the most important factor that will influence both the degree of perivalvular regurgitation and pacemaker need after TAVR," said Dr. Jeffrey J. Popma in a separate talk at the meeting.
Until recently, many operators used transthoracic echocardiography for annulus sizing, but recently published evidence showed that imaging by CT or by MRI provide superior information, said Dr. Popma, director of interventional cardiology at Beth Israel Deaconess Medical Center in Boston. He cited a British report published last November that compared transthoracic echo, CT, and MRI in 202 patients who underwent TAVR, which found both CT and MRI superior to echo for annulus measurement prior to TAVR (J. Am. Coll. Cardiol. 2011;58:2165-73).
Dr. Grube said that he has financial relationships with Direct Flow, Claret, Biosensors, Medtronic, Mitralign, Boston Scientific, Cordis, Abbott Vascular, and InSeal. Dr. Popma said that he has financial relationships with Abbott, Boston Scientific, ev3 (Covidien), Medtronic, and Cordis.
MIAMI BEACH – Now that transcatheter aortic valve replacement is available for routine U.S. use, interventionalists have focused on making the procedure better and safer, such as cutting the incidence of significant regurgitation through the replacement aortic valve.
Toward that end, researchers have developed a way to quickly and objectively assess the risk for aortic valve regurgitation based on the measurement of aortic blood pressures immediately after transcatheter valve replacement (TAVR). This new measure, the "aortic regurgitation index," showed significant correlation with periprocedural aortic regurgitation as well as with patients’ 1-year survival following their procedure, Dr. Eberhard Grube said at ISET 2012, an international symposium on endovascular therapy.
Until now, "there has been no way to quantify aortic regurgitation; it’s subjective," said Dr. Grube, professor and chief of cardiology and angiology at the Helios Heart Centre in Siegburg, Germany. The aortic regurgitation index (ARI) allows physicians "to quantify aortic regurgitation periprocedurally by objectively looking at the patient’s hemodynamics, regardless of the subjective evaluation of aortic insufficiency by angiography or by echo," he said. "We can see the ARI and know what we need to do for postdeployment treatment."
Dr. Grube and his associates set the ARI as equal to the patient’s diastolic aortic pressure minus the left ventricular end diastolic pressure, then divided by the aortic systolic pressure, and multiplied by 100.
For example, a patient with mild periprocedural aortic regurgitation might have an aortic diastolic pressure of 60 mm Hg, a left ventricular end diastolic pressure of 15 mm Hg, and an aortic systolic pressure of 150 mm Hg, which would produce an ARI of 30, showing that the patient had a low risk for dying during the subsequent year. In contrast, a patient with moderate or severe periprocedural aortic regurgitation could have an aortic diastolic pressure of 40 mm Hg, a left ventricular end diastolic pressure of 20 mm Hg, and an aortic systolic pressure of 120 mm Hg, which would produce an ARI of 16.7, flagging a higher mortality risk during the following year.
His group assessed the prognostic ability of the ARI in a series of 146 patients who underwent TAVR. The group included 124 patients with no or mild aortic regurgitation following TAVR and 22 who showed moderate or severe regurgitation. During 1-year follow-up, the 96 patients with a periprocedural ARI of 25 or greater had an 83% survival rate; the 50 patients with a periprocedural ARI of less than 25 had a survival rate of 54%, a statistically significant difference. The analysis also showed a significant correlation between the ARI and the severity of aortic regurgitation. Among patients with no discernible regurgitation, the average ARI was about 30, in those with mild regurgitation the average ARI was about 25, in patients with moderate regurgitation the average was about 18, and in patients with severe regurgitation the ARI averaged about 10.
One recently identified key to limiting aortic regurgitation following TAVR is proper valve sizing relative to the patient’s aortic annulus. "Appropriate valve sizing is likely the most important factor that will influence both the degree of perivalvular regurgitation and pacemaker need after TAVR," said Dr. Jeffrey J. Popma in a separate talk at the meeting.
Until recently, many operators used transthoracic echocardiography for annulus sizing, but recently published evidence showed that imaging by CT or by MRI provide superior information, said Dr. Popma, director of interventional cardiology at Beth Israel Deaconess Medical Center in Boston. He cited a British report published last November that compared transthoracic echo, CT, and MRI in 202 patients who underwent TAVR, which found both CT and MRI superior to echo for annulus measurement prior to TAVR (J. Am. Coll. Cardiol. 2011;58:2165-73).
Dr. Grube said that he has financial relationships with Direct Flow, Claret, Biosensors, Medtronic, Mitralign, Boston Scientific, Cordis, Abbott Vascular, and InSeal. Dr. Popma said that he has financial relationships with Abbott, Boston Scientific, ev3 (Covidien), Medtronic, and Cordis.
FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: An aortic regurgitation index of 25 or higher was linked with a 1-year survival post TAVR of 83%, compared with 54% among patients with an index of less than 25.
Data Source: One-year follow-up of 146 patients who underwent transcatheter aortic valve replacement.
Disclosures: Dr. Grube said that he has financial relationships with Direct Flow, Claret, Biosensors, Medtronic, Mitralign, Boston Scientific, Cordis, Abbott Vascular, and InSeal. Dr. Popma said that he has financial relationships with Abbott, Boston Scientific, ev3 (Covidien), Medtronic, and Cordis.
Excitement builds for renal denervation in hypertension
MIAMI BEACH – The U.S. pivotal trial assessing renal denervation for blood pressure reduction began in October and a report on its final results won’t appear until next year, but excitement based on earlier study findings and the European clinical experience is already running high for this new approach to treating drug-resistant hypertension.
"Resistant hypertension is common, and is becoming more common despite a huge armamentarium of pharmacologic agents. The early literature [on renal denervation] is very impressive" for safety and efficacy, and the approach is plausibly "based on physiology," Dr. Michael R. Jaff said at ISET 2012, an international symposium on endovascular therapy. Moreover, on top of the expected antihypertensive effect of renal denervation, recent results from a pilot, randomized controlled study with 50 hypertensive patients suggested that the intervention could also help normalize glucose metabolism and reverse type 2 diabetes in some patients.
Renal denervation "could arguably be the most exciting advance in interventional vascular medicine," said Dr. Jaff, medical director of the vascular center at Massachusetts General Hospital in Boston. "People’s imaginations are running wild about the potential implications of this type of therapy for a whole host of patients. In the near term I’m incredibly bullish [about this treatment] and in the far term I’m even more enthusiastic."
In addition to the clinical efficacy that renal denervation so far has shown, it has the pluses of being relatively simple and easy to do, easy to learn, and fairly quick; the procedure involves modestly priced equipment and appears very safe as well, noted Dr. Horst Sievert, an interventional cardiologist who collaborated on one of the efficacy trials and now routinely performs renal denervations at the cardiovascular center of Saint Katharinen Hospital in Frankfurt, Germany.
The Symplicity Renal Denervation System, developed by Ardian, which is now owned by Medtronic, has been available for routine use in Europe and Australia since 2010. The U.S. pivotal trial, Symplicity HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension), plans to enroll 530 patients at 60 U.S. centers and should be completed by March of next year.
The procedure, which requires just a flexible catheter and radiofrequency generator, delivers four to eight low-power treatments along the length of both main renal arteries using a 6F sheath, targeting the renal nerves in the adventitia of the renal arteries and producing reduced sympathetic activity and renin release. According to Dr. Sievert, the typical procedure takes 45-60 minutes, and is successful in significantly reducing systolic and diastolic pressure in 75%-80% of drug-refractory patients. The worldwide case experience so far has led to few complications, no reported thrombus formations, no detrimental effects on renal blood flow or function, and no reported cases of severe hypotension following treatment, he said.
"I believe that renal denervation will become as important as PCI [percutaneous coronary intervention] or PTA [percutaneous transluminal angioplasty]," said Dr. Sievert, director of the cardiovascular center and also on the faculty of the University of Frankfurt.
"The risks are so few and the complication rate so low that it’s not worth figuring out which patients will respond and who won’t. I would just do the treatment and see if the patient responds," Dr. Sievert said at the meeting. No explanation exists as to why some patients don’t respond, and no marker exists to distinguish patients who will respond and those who won’t.
The largest systematic assessment of the antihypertensive impact of renal denervation came in a randomized trial, Symplicity HTN-2, with 106 patients, which was sponsored by Ardian and published in 2010. All patients had an entry systolic pressure of at least 160 mm Hg (or at least 150 mm Hg if they also had type 2 diabetes) despite current treatment with at least three antihypertensive medications. At 6 months after treatment, the 52 patients treated with denervation had an average blood pressure reduction of 32/12 mm Hg, compared with an average systolic pressure rise of 1 mm Hg and no average diastolic change in the 54 control patients (Lancet 2010;376:1903-9).
In the denervation group, 84% of patients had at least a 10-mm Hg drop in their systolic pressure 6 months after treatment compared with 35% of the control patients having this response, and 39% of the denervation patients had their systolic pressure fall below 140 mm Hg at 6 months compared with 6% of control patients. A subsequent report on a total of 153 patients treated with renal denervation documented that the blood pressure reductions seen at 6 months persisted out to 2 years (Hypertension 2011;57:911-7).
Striking as this effect was, perhaps even more notable was a report last year from a separate pilot study that randomized 50 patients who met the same definition of refractory hypertension. In addition to showing similar blood pressure results in the denervated patients, the researchers on this study also looked at several measures of glucose metabolism. At 3 months after intervention, the 37 denervated patients had an average 9% cut in their fasting blood glucose level and an average 12% drop in their average fasting blood insulin levels, both statistically significant changes from baseline, compared with no significant change in the 13 control patients. The percent of patients with normal glucose tolerance rose by 11% in the denervated group, and dropped by 7% in the control group. The authors of the report concluded that renal sympathetic activity plays a role in insulin resistance (Circulation 2011;123:1940-6).
Future studies will likely examine the possible impact of renal denervation on heart failure and on obstructive sleep apnea, noted Dr. Jaff.
Dr. Jaff said that he has financial relationships with several cardiovascular device companies including Medtronic Hansen Medical and Medtronic Vascular. Dr. Sievert said that he has financial relationships with several cardiovascular device and pharmaceutical companies including Ardian and Medtronic.
MIAMI BEACH – The U.S. pivotal trial assessing renal denervation for blood pressure reduction began in October and a report on its final results won’t appear until next year, but excitement based on earlier study findings and the European clinical experience is already running high for this new approach to treating drug-resistant hypertension.
"Resistant hypertension is common, and is becoming more common despite a huge armamentarium of pharmacologic agents. The early literature [on renal denervation] is very impressive" for safety and efficacy, and the approach is plausibly "based on physiology," Dr. Michael R. Jaff said at ISET 2012, an international symposium on endovascular therapy. Moreover, on top of the expected antihypertensive effect of renal denervation, recent results from a pilot, randomized controlled study with 50 hypertensive patients suggested that the intervention could also help normalize glucose metabolism and reverse type 2 diabetes in some patients.
Renal denervation "could arguably be the most exciting advance in interventional vascular medicine," said Dr. Jaff, medical director of the vascular center at Massachusetts General Hospital in Boston. "People’s imaginations are running wild about the potential implications of this type of therapy for a whole host of patients. In the near term I’m incredibly bullish [about this treatment] and in the far term I’m even more enthusiastic."
In addition to the clinical efficacy that renal denervation so far has shown, it has the pluses of being relatively simple and easy to do, easy to learn, and fairly quick; the procedure involves modestly priced equipment and appears very safe as well, noted Dr. Horst Sievert, an interventional cardiologist who collaborated on one of the efficacy trials and now routinely performs renal denervations at the cardiovascular center of Saint Katharinen Hospital in Frankfurt, Germany.
The Symplicity Renal Denervation System, developed by Ardian, which is now owned by Medtronic, has been available for routine use in Europe and Australia since 2010. The U.S. pivotal trial, Symplicity HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension), plans to enroll 530 patients at 60 U.S. centers and should be completed by March of next year.
The procedure, which requires just a flexible catheter and radiofrequency generator, delivers four to eight low-power treatments along the length of both main renal arteries using a 6F sheath, targeting the renal nerves in the adventitia of the renal arteries and producing reduced sympathetic activity and renin release. According to Dr. Sievert, the typical procedure takes 45-60 minutes, and is successful in significantly reducing systolic and diastolic pressure in 75%-80% of drug-refractory patients. The worldwide case experience so far has led to few complications, no reported thrombus formations, no detrimental effects on renal blood flow or function, and no reported cases of severe hypotension following treatment, he said.
"I believe that renal denervation will become as important as PCI [percutaneous coronary intervention] or PTA [percutaneous transluminal angioplasty]," said Dr. Sievert, director of the cardiovascular center and also on the faculty of the University of Frankfurt.
"The risks are so few and the complication rate so low that it’s not worth figuring out which patients will respond and who won’t. I would just do the treatment and see if the patient responds," Dr. Sievert said at the meeting. No explanation exists as to why some patients don’t respond, and no marker exists to distinguish patients who will respond and those who won’t.
The largest systematic assessment of the antihypertensive impact of renal denervation came in a randomized trial, Symplicity HTN-2, with 106 patients, which was sponsored by Ardian and published in 2010. All patients had an entry systolic pressure of at least 160 mm Hg (or at least 150 mm Hg if they also had type 2 diabetes) despite current treatment with at least three antihypertensive medications. At 6 months after treatment, the 52 patients treated with denervation had an average blood pressure reduction of 32/12 mm Hg, compared with an average systolic pressure rise of 1 mm Hg and no average diastolic change in the 54 control patients (Lancet 2010;376:1903-9).
In the denervation group, 84% of patients had at least a 10-mm Hg drop in their systolic pressure 6 months after treatment compared with 35% of the control patients having this response, and 39% of the denervation patients had their systolic pressure fall below 140 mm Hg at 6 months compared with 6% of control patients. A subsequent report on a total of 153 patients treated with renal denervation documented that the blood pressure reductions seen at 6 months persisted out to 2 years (Hypertension 2011;57:911-7).
Striking as this effect was, perhaps even more notable was a report last year from a separate pilot study that randomized 50 patients who met the same definition of refractory hypertension. In addition to showing similar blood pressure results in the denervated patients, the researchers on this study also looked at several measures of glucose metabolism. At 3 months after intervention, the 37 denervated patients had an average 9% cut in their fasting blood glucose level and an average 12% drop in their average fasting blood insulin levels, both statistically significant changes from baseline, compared with no significant change in the 13 control patients. The percent of patients with normal glucose tolerance rose by 11% in the denervated group, and dropped by 7% in the control group. The authors of the report concluded that renal sympathetic activity plays a role in insulin resistance (Circulation 2011;123:1940-6).
Future studies will likely examine the possible impact of renal denervation on heart failure and on obstructive sleep apnea, noted Dr. Jaff.
Dr. Jaff said that he has financial relationships with several cardiovascular device companies including Medtronic Hansen Medical and Medtronic Vascular. Dr. Sievert said that he has financial relationships with several cardiovascular device and pharmaceutical companies including Ardian and Medtronic.
MIAMI BEACH – The U.S. pivotal trial assessing renal denervation for blood pressure reduction began in October and a report on its final results won’t appear until next year, but excitement based on earlier study findings and the European clinical experience is already running high for this new approach to treating drug-resistant hypertension.
"Resistant hypertension is common, and is becoming more common despite a huge armamentarium of pharmacologic agents. The early literature [on renal denervation] is very impressive" for safety and efficacy, and the approach is plausibly "based on physiology," Dr. Michael R. Jaff said at ISET 2012, an international symposium on endovascular therapy. Moreover, on top of the expected antihypertensive effect of renal denervation, recent results from a pilot, randomized controlled study with 50 hypertensive patients suggested that the intervention could also help normalize glucose metabolism and reverse type 2 diabetes in some patients.
Renal denervation "could arguably be the most exciting advance in interventional vascular medicine," said Dr. Jaff, medical director of the vascular center at Massachusetts General Hospital in Boston. "People’s imaginations are running wild about the potential implications of this type of therapy for a whole host of patients. In the near term I’m incredibly bullish [about this treatment] and in the far term I’m even more enthusiastic."
In addition to the clinical efficacy that renal denervation so far has shown, it has the pluses of being relatively simple and easy to do, easy to learn, and fairly quick; the procedure involves modestly priced equipment and appears very safe as well, noted Dr. Horst Sievert, an interventional cardiologist who collaborated on one of the efficacy trials and now routinely performs renal denervations at the cardiovascular center of Saint Katharinen Hospital in Frankfurt, Germany.
The Symplicity Renal Denervation System, developed by Ardian, which is now owned by Medtronic, has been available for routine use in Europe and Australia since 2010. The U.S. pivotal trial, Symplicity HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension), plans to enroll 530 patients at 60 U.S. centers and should be completed by March of next year.
The procedure, which requires just a flexible catheter and radiofrequency generator, delivers four to eight low-power treatments along the length of both main renal arteries using a 6F sheath, targeting the renal nerves in the adventitia of the renal arteries and producing reduced sympathetic activity and renin release. According to Dr. Sievert, the typical procedure takes 45-60 minutes, and is successful in significantly reducing systolic and diastolic pressure in 75%-80% of drug-refractory patients. The worldwide case experience so far has led to few complications, no reported thrombus formations, no detrimental effects on renal blood flow or function, and no reported cases of severe hypotension following treatment, he said.
"I believe that renal denervation will become as important as PCI [percutaneous coronary intervention] or PTA [percutaneous transluminal angioplasty]," said Dr. Sievert, director of the cardiovascular center and also on the faculty of the University of Frankfurt.
"The risks are so few and the complication rate so low that it’s not worth figuring out which patients will respond and who won’t. I would just do the treatment and see if the patient responds," Dr. Sievert said at the meeting. No explanation exists as to why some patients don’t respond, and no marker exists to distinguish patients who will respond and those who won’t.
The largest systematic assessment of the antihypertensive impact of renal denervation came in a randomized trial, Symplicity HTN-2, with 106 patients, which was sponsored by Ardian and published in 2010. All patients had an entry systolic pressure of at least 160 mm Hg (or at least 150 mm Hg if they also had type 2 diabetes) despite current treatment with at least three antihypertensive medications. At 6 months after treatment, the 52 patients treated with denervation had an average blood pressure reduction of 32/12 mm Hg, compared with an average systolic pressure rise of 1 mm Hg and no average diastolic change in the 54 control patients (Lancet 2010;376:1903-9).
In the denervation group, 84% of patients had at least a 10-mm Hg drop in their systolic pressure 6 months after treatment compared with 35% of the control patients having this response, and 39% of the denervation patients had their systolic pressure fall below 140 mm Hg at 6 months compared with 6% of control patients. A subsequent report on a total of 153 patients treated with renal denervation documented that the blood pressure reductions seen at 6 months persisted out to 2 years (Hypertension 2011;57:911-7).
Striking as this effect was, perhaps even more notable was a report last year from a separate pilot study that randomized 50 patients who met the same definition of refractory hypertension. In addition to showing similar blood pressure results in the denervated patients, the researchers on this study also looked at several measures of glucose metabolism. At 3 months after intervention, the 37 denervated patients had an average 9% cut in their fasting blood glucose level and an average 12% drop in their average fasting blood insulin levels, both statistically significant changes from baseline, compared with no significant change in the 13 control patients. The percent of patients with normal glucose tolerance rose by 11% in the denervated group, and dropped by 7% in the control group. The authors of the report concluded that renal sympathetic activity plays a role in insulin resistance (Circulation 2011;123:1940-6).
Future studies will likely examine the possible impact of renal denervation on heart failure and on obstructive sleep apnea, noted Dr. Jaff.
Dr. Jaff said that he has financial relationships with several cardiovascular device companies including Medtronic Hansen Medical and Medtronic Vascular. Dr. Sievert said that he has financial relationships with several cardiovascular device and pharmaceutical companies including Ardian and Medtronic.
EXPERT ANALYSIS FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
First U.S. Fibromuscular Dysplasia Registry Yields New Clues
MIAMI BEACH – The mystery shrouding fibromuscular dysplasia, a clinically important and surprisingly prevalent vascular disease of unknown etiology, began to lift with initial findings from 339 patients enrolled in the first U.S. registry for the disease.
Baseline observations from the U.S. Registry for Fibromuscular Dysplasia (FMD), which enrolled its first patient in 2008 and currently has women as 91% of enrolled patients, show that the disease first occurs across a broad swath of age groups, with an age at first diagnosis ranging from 5 to 83 years old. In addition to the distinctive "string of beads" arterial fibrosis appearance that defines the disease and is apparent on imaging, and which usually occurs in the renal or carotid artery, or both, 19% of patients also had a dissection in an artery somewhere in their body (14% with a dissection in a carotid artery) and 17% had an arterial aneurysm somewhere (5% with a renal artery aneurysm and 4% with a carotid aneurysm).
"A patient can have normal blood pressure and normal-appearing carotid and renal arteries but have terrible headaches and FMD. Why? We don’t know."
These dissection and aneurysm prevalence rates had not previously been appreciated to run so high in FMD patients. "It suggests a diffuse arteriopathy that can present in several different ways," Dr. Jeffrey W. Olin said at ISET 2012, an international symposium on endovascular therapy.
Another notable finding was that, besides hypertension, which was the most common presenting manifestation of FMD (seen in 66% of the registry patients at initial diagnosis), other common presenting symptoms included significant, often migraine-like headache in 53%, pulsatile tinnitus (a whooshing sound patients hear) in 30%, and dizziness in 28%. The high prevalence of headache in FMD had been "first reported 30 years ago, but sort of got lost," Dr. Olin said in an interview.
"A patient can have normal blood pressure and normal-appearing carotid and renal arteries but have terrible headaches and FMD. Why? We don’t know. We have no idea what causes the headaches," said Dr. Olin, a cardiologist who is professor of medicine and director of vascular medicine at Mount Sinai Medical Center in New York. Four percent of the registry patients had no symptoms on presentation; physicians found their FMD incidentally during imaging examinations for other reasons.
Other flags to trigger suspicion of FMD include hypertension that begins before age 35 (although it can also start later in life), treatment-resistant hypertension, epigastric bruit and hypertension, renal infarction, cervical bruit in a patient less than 60 years old, transient ischemic attack or stroke in a patient less than 60 years old, or an aortic aneurysm in a patient less than 60 years old.
Perhaps the most surprising new finding so far from the registry is evidence for a strong family history of cardiovascular disease, with 81% of first- or second-degree relatives of FMD patients having hypertension, 59% with hyperlipidemia, 53% with a history of a stroke, 22% with an aneurysm, and 21% with a history of sudden death. The prevalence of a first- or second-degree relative also having a diagnosis of FMD was 7%, not much higher than the currently estimated 4% prevalence of FMD in the general population.
Dr. Olin and his associates from the registry hypothesize that patients who develop FMD have an as-yet unidentified genetic predisposition that interacts with an environmental trigger. His hope is that, by continuing to expand the registry and by receiving substantially more research support than FMD now gets, a more concerted research effort can address the genetic questions raised by the family-history findings.
Current treatment of FMD is symptom driven and usually focuses on trying to resolve patients’ hypertension, which is often treatment resistant.
"FMD is potentially treatable for hypertension," with endovascular treatment of affected renal arteries the standard intervention for patients with resistant hypertension, Dr. Robert A. Lookstein said in a separate talk at the meeting. Hypertension cure rates from balloon angioplasty, however, are "surprisingly low" – 45% in one meta-analysis – probably because of suboptimal interventional treatment, said Dr. Lookstein, chief of the division of interventional radiology at Mount Sinai.
Assessing a patient’s renal arteries before and during treatment using intravascular ultrasound (IVUS) and a pressure wire provides the key to successful resolution of hypertension by renal-artery angioplasty in FMD patients, he said.
These two techniques, especially pressure-wire measurements, allow the operator to assess the patient’s renal arteries before and during treatment to determine whether the intervention is having a meaningful effect. "You can’t tell [whether the angioplasty produced benefit] by the angiography appearance alone," he cautioned. "You need to be compulsive with IVUS and measuring pressure gradients to determine where to start treatment and when to stop."
The U. S. Registry for FMD began with seven U.S. centers enrolling patients and now involves 10 centers and has enrolled more than 500 patients. The first 339 enrollees included 44% who were patients at the Cleveland Clinic and 20% who were patients at Mount Sinai.
The average age of the enrolled patients at first symptom appearance was 47 years old; first diagnosis did not occur until an average of more than 4 years later, at an average age of 52 years old.
Vascular bed involvement among the first registry patients included one or both renal arteries in 69%, at least one extracranial carotid in 62%, vertebral arteries in 19%, and mesenteric arteries in 12%. At the time of enrollment, 7% of patients had a history of coronary artery disease and 10% had a history of stroke. The arterial fibrosis characteristic of FMD notably appears in portions of the renal and carotid arteries where atherosclerotic disease usually does not occur – in the distal renal artery and in the distal carotid, "higher than physicians usually look when they do carotid ultrasound" examinations, Dr. Olin said.
When he finds carotid fibrosis, Dr. Olin starts those patients on a daily aspirin, although the benefit of this strategy hasn’t been proven. If carotid lesions are substantial, treatment by angioplasty is possible; carotid stenting is not used on FMD patients, nor is endarterectomy, as there is no atherosclerotic plaque to remove, he said.
Dr. Olin said that he has been a consultant to Merck, and that he chairs the advisory board of the Fibromuscular Dysplasia Society of America. Dr. Lookstein said that he has been a consultant to Medrad and Cordis.
MIAMI BEACH – The mystery shrouding fibromuscular dysplasia, a clinically important and surprisingly prevalent vascular disease of unknown etiology, began to lift with initial findings from 339 patients enrolled in the first U.S. registry for the disease.
Baseline observations from the U.S. Registry for Fibromuscular Dysplasia (FMD), which enrolled its first patient in 2008 and currently has women as 91% of enrolled patients, show that the disease first occurs across a broad swath of age groups, with an age at first diagnosis ranging from 5 to 83 years old. In addition to the distinctive "string of beads" arterial fibrosis appearance that defines the disease and is apparent on imaging, and which usually occurs in the renal or carotid artery, or both, 19% of patients also had a dissection in an artery somewhere in their body (14% with a dissection in a carotid artery) and 17% had an arterial aneurysm somewhere (5% with a renal artery aneurysm and 4% with a carotid aneurysm).
"A patient can have normal blood pressure and normal-appearing carotid and renal arteries but have terrible headaches and FMD. Why? We don’t know."
These dissection and aneurysm prevalence rates had not previously been appreciated to run so high in FMD patients. "It suggests a diffuse arteriopathy that can present in several different ways," Dr. Jeffrey W. Olin said at ISET 2012, an international symposium on endovascular therapy.
Another notable finding was that, besides hypertension, which was the most common presenting manifestation of FMD (seen in 66% of the registry patients at initial diagnosis), other common presenting symptoms included significant, often migraine-like headache in 53%, pulsatile tinnitus (a whooshing sound patients hear) in 30%, and dizziness in 28%. The high prevalence of headache in FMD had been "first reported 30 years ago, but sort of got lost," Dr. Olin said in an interview.
"A patient can have normal blood pressure and normal-appearing carotid and renal arteries but have terrible headaches and FMD. Why? We don’t know. We have no idea what causes the headaches," said Dr. Olin, a cardiologist who is professor of medicine and director of vascular medicine at Mount Sinai Medical Center in New York. Four percent of the registry patients had no symptoms on presentation; physicians found their FMD incidentally during imaging examinations for other reasons.
Other flags to trigger suspicion of FMD include hypertension that begins before age 35 (although it can also start later in life), treatment-resistant hypertension, epigastric bruit and hypertension, renal infarction, cervical bruit in a patient less than 60 years old, transient ischemic attack or stroke in a patient less than 60 years old, or an aortic aneurysm in a patient less than 60 years old.
Perhaps the most surprising new finding so far from the registry is evidence for a strong family history of cardiovascular disease, with 81% of first- or second-degree relatives of FMD patients having hypertension, 59% with hyperlipidemia, 53% with a history of a stroke, 22% with an aneurysm, and 21% with a history of sudden death. The prevalence of a first- or second-degree relative also having a diagnosis of FMD was 7%, not much higher than the currently estimated 4% prevalence of FMD in the general population.
Dr. Olin and his associates from the registry hypothesize that patients who develop FMD have an as-yet unidentified genetic predisposition that interacts with an environmental trigger. His hope is that, by continuing to expand the registry and by receiving substantially more research support than FMD now gets, a more concerted research effort can address the genetic questions raised by the family-history findings.
Current treatment of FMD is symptom driven and usually focuses on trying to resolve patients’ hypertension, which is often treatment resistant.
"FMD is potentially treatable for hypertension," with endovascular treatment of affected renal arteries the standard intervention for patients with resistant hypertension, Dr. Robert A. Lookstein said in a separate talk at the meeting. Hypertension cure rates from balloon angioplasty, however, are "surprisingly low" – 45% in one meta-analysis – probably because of suboptimal interventional treatment, said Dr. Lookstein, chief of the division of interventional radiology at Mount Sinai.
Assessing a patient’s renal arteries before and during treatment using intravascular ultrasound (IVUS) and a pressure wire provides the key to successful resolution of hypertension by renal-artery angioplasty in FMD patients, he said.
These two techniques, especially pressure-wire measurements, allow the operator to assess the patient’s renal arteries before and during treatment to determine whether the intervention is having a meaningful effect. "You can’t tell [whether the angioplasty produced benefit] by the angiography appearance alone," he cautioned. "You need to be compulsive with IVUS and measuring pressure gradients to determine where to start treatment and when to stop."
The U. S. Registry for FMD began with seven U.S. centers enrolling patients and now involves 10 centers and has enrolled more than 500 patients. The first 339 enrollees included 44% who were patients at the Cleveland Clinic and 20% who were patients at Mount Sinai.
The average age of the enrolled patients at first symptom appearance was 47 years old; first diagnosis did not occur until an average of more than 4 years later, at an average age of 52 years old.
Vascular bed involvement among the first registry patients included one or both renal arteries in 69%, at least one extracranial carotid in 62%, vertebral arteries in 19%, and mesenteric arteries in 12%. At the time of enrollment, 7% of patients had a history of coronary artery disease and 10% had a history of stroke. The arterial fibrosis characteristic of FMD notably appears in portions of the renal and carotid arteries where atherosclerotic disease usually does not occur – in the distal renal artery and in the distal carotid, "higher than physicians usually look when they do carotid ultrasound" examinations, Dr. Olin said.
When he finds carotid fibrosis, Dr. Olin starts those patients on a daily aspirin, although the benefit of this strategy hasn’t been proven. If carotid lesions are substantial, treatment by angioplasty is possible; carotid stenting is not used on FMD patients, nor is endarterectomy, as there is no atherosclerotic plaque to remove, he said.
Dr. Olin said that he has been a consultant to Merck, and that he chairs the advisory board of the Fibromuscular Dysplasia Society of America. Dr. Lookstein said that he has been a consultant to Medrad and Cordis.
MIAMI BEACH – The mystery shrouding fibromuscular dysplasia, a clinically important and surprisingly prevalent vascular disease of unknown etiology, began to lift with initial findings from 339 patients enrolled in the first U.S. registry for the disease.
Baseline observations from the U.S. Registry for Fibromuscular Dysplasia (FMD), which enrolled its first patient in 2008 and currently has women as 91% of enrolled patients, show that the disease first occurs across a broad swath of age groups, with an age at first diagnosis ranging from 5 to 83 years old. In addition to the distinctive "string of beads" arterial fibrosis appearance that defines the disease and is apparent on imaging, and which usually occurs in the renal or carotid artery, or both, 19% of patients also had a dissection in an artery somewhere in their body (14% with a dissection in a carotid artery) and 17% had an arterial aneurysm somewhere (5% with a renal artery aneurysm and 4% with a carotid aneurysm).
"A patient can have normal blood pressure and normal-appearing carotid and renal arteries but have terrible headaches and FMD. Why? We don’t know."
These dissection and aneurysm prevalence rates had not previously been appreciated to run so high in FMD patients. "It suggests a diffuse arteriopathy that can present in several different ways," Dr. Jeffrey W. Olin said at ISET 2012, an international symposium on endovascular therapy.
Another notable finding was that, besides hypertension, which was the most common presenting manifestation of FMD (seen in 66% of the registry patients at initial diagnosis), other common presenting symptoms included significant, often migraine-like headache in 53%, pulsatile tinnitus (a whooshing sound patients hear) in 30%, and dizziness in 28%. The high prevalence of headache in FMD had been "first reported 30 years ago, but sort of got lost," Dr. Olin said in an interview.
"A patient can have normal blood pressure and normal-appearing carotid and renal arteries but have terrible headaches and FMD. Why? We don’t know. We have no idea what causes the headaches," said Dr. Olin, a cardiologist who is professor of medicine and director of vascular medicine at Mount Sinai Medical Center in New York. Four percent of the registry patients had no symptoms on presentation; physicians found their FMD incidentally during imaging examinations for other reasons.
Other flags to trigger suspicion of FMD include hypertension that begins before age 35 (although it can also start later in life), treatment-resistant hypertension, epigastric bruit and hypertension, renal infarction, cervical bruit in a patient less than 60 years old, transient ischemic attack or stroke in a patient less than 60 years old, or an aortic aneurysm in a patient less than 60 years old.
Perhaps the most surprising new finding so far from the registry is evidence for a strong family history of cardiovascular disease, with 81% of first- or second-degree relatives of FMD patients having hypertension, 59% with hyperlipidemia, 53% with a history of a stroke, 22% with an aneurysm, and 21% with a history of sudden death. The prevalence of a first- or second-degree relative also having a diagnosis of FMD was 7%, not much higher than the currently estimated 4% prevalence of FMD in the general population.
Dr. Olin and his associates from the registry hypothesize that patients who develop FMD have an as-yet unidentified genetic predisposition that interacts with an environmental trigger. His hope is that, by continuing to expand the registry and by receiving substantially more research support than FMD now gets, a more concerted research effort can address the genetic questions raised by the family-history findings.
Current treatment of FMD is symptom driven and usually focuses on trying to resolve patients’ hypertension, which is often treatment resistant.
"FMD is potentially treatable for hypertension," with endovascular treatment of affected renal arteries the standard intervention for patients with resistant hypertension, Dr. Robert A. Lookstein said in a separate talk at the meeting. Hypertension cure rates from balloon angioplasty, however, are "surprisingly low" – 45% in one meta-analysis – probably because of suboptimal interventional treatment, said Dr. Lookstein, chief of the division of interventional radiology at Mount Sinai.
Assessing a patient’s renal arteries before and during treatment using intravascular ultrasound (IVUS) and a pressure wire provides the key to successful resolution of hypertension by renal-artery angioplasty in FMD patients, he said.
These two techniques, especially pressure-wire measurements, allow the operator to assess the patient’s renal arteries before and during treatment to determine whether the intervention is having a meaningful effect. "You can’t tell [whether the angioplasty produced benefit] by the angiography appearance alone," he cautioned. "You need to be compulsive with IVUS and measuring pressure gradients to determine where to start treatment and when to stop."
The U. S. Registry for FMD began with seven U.S. centers enrolling patients and now involves 10 centers and has enrolled more than 500 patients. The first 339 enrollees included 44% who were patients at the Cleveland Clinic and 20% who were patients at Mount Sinai.
The average age of the enrolled patients at first symptom appearance was 47 years old; first diagnosis did not occur until an average of more than 4 years later, at an average age of 52 years old.
Vascular bed involvement among the first registry patients included one or both renal arteries in 69%, at least one extracranial carotid in 62%, vertebral arteries in 19%, and mesenteric arteries in 12%. At the time of enrollment, 7% of patients had a history of coronary artery disease and 10% had a history of stroke. The arterial fibrosis characteristic of FMD notably appears in portions of the renal and carotid arteries where atherosclerotic disease usually does not occur – in the distal renal artery and in the distal carotid, "higher than physicians usually look when they do carotid ultrasound" examinations, Dr. Olin said.
When he finds carotid fibrosis, Dr. Olin starts those patients on a daily aspirin, although the benefit of this strategy hasn’t been proven. If carotid lesions are substantial, treatment by angioplasty is possible; carotid stenting is not used on FMD patients, nor is endarterectomy, as there is no atherosclerotic plaque to remove, he said.
Dr. Olin said that he has been a consultant to Merck, and that he chairs the advisory board of the Fibromuscular Dysplasia Society of America. Dr. Lookstein said that he has been a consultant to Medrad and Cordis.
FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: The most common presenting symptoms in patients with fibromuscular dysplasia were hypertension in 66%, headache in 53%, pulsatile tinnitus in 30%, and dizziness in 28%.
Data Source: Baseline findings from the first 339 patients enrolled in the U.S. Registry for Fibromuscular Dysplasia.
Disclosures: Dr. Olin said that he has been a consultant to Merck, and that he chairs the advisory board of the Fibromuscular Dysplasia Society of America. Dr. Lookstein said that he has been a consultant to Medrad and Cordis.
Local, Regional Anesthesia Surpass General for AAA EVAR
MIAMI BEACH – Local or regional anesthesia is a better option than is general anesthesia for patients undergoing endovascular repair of an abdominal aortic aneurysm, based on findings from a registry with nearly 1,200 patients.
Both local anesthesia and regional anesthesia each surpassed general anesthesia in two periprocedural measures: significantly reducing procedure time, and significantly reducing postoperative hospitalization, Dr. Rutger A. Stokmans said at ISET 2012, an international symposium on endovascular therapy.
In addition, both local and regional anesthesia led to trends in reduced rates of major adverse events during the 30 days following surgery, although these differences did not reach statistical significance. All three anesthesia types linked with similar rates of both technical and clinical success of the aneurysm repairs. Regional anesthesia also led to a significantly lower rate of ICU admission, compared with both general and local anesthesia; local anesthesia showed no significant difference for this measure, compared with general anesthesia.
Based on these findings, local or regional anesthesia should be preferred when performing endovascular aneurysm repair (EVAR), whereas general anesthesia should usually be avoided, said Dr. Stokmans, a vascular surgeon at Catharina Hospital in Eindhoven, the Netherlands.
These findings support the most recent anesthesia recommendations of the European Society for Vascular Surgery, which in 2011 guidelines for managing abdominal aortic aneurysms (AAA) cited local anesthesia as preferred for EVAR, with regional or general anesthesia reserved for patients with contraindications for local anesthesia, he said (Euro. J. Vasc. Endovasc. Surg .2011;41[suppl. 1]:S1-S58). The most recent guidelines for AAA management from the Society for Vascular Surgery suggested using local or regional anesthesia over general anesthesia, he added (J. Vasc. Surg. 2009[suppl.]:50:S2-S49).
But despite these recommendations, the most commonly used anesthesia type worldwide for EVAR repair of AAA has been general anesthesia, followed by regional anesthesia, with local treatment used least often, according to the registry data reported by Dr. Stokmans. Among the 1,199 patients enrolled in ENGAGE (Endurant Stent Graft Natural Selection Global Postmarketing Registry) during March 2009 to December 2010 in 30 countries on five continents, 749 (62%) underwent their EVAR with general anesthesia, 325 (27%) with regional, and 125 (10%) with local anesthesia. (Percentages do not add up to 100% because of rounding.)
The registry data also showed striking regional variations in anesthesia use, with general anesthesia used on about 90% of patients in Canada, Australia, and New Zealand, and on about 70% of patients in Scandinavian countries and the United Kingdom. But in Central Europe, regional anesthesia – used on nearly 70% of EVAR patients – dominated. The only region favoring local anesthesia was South America (Argentina, Columbia, and Uruguay), where about 50% of patients received local, but more than 40% received general anesthesia, he said. The registry contained no U.S. patients, although the Endurant AAA stent graft system is marketed in the United States.
The average age of the EVAR patients in the registry was about 73 years. Those patients who underwent general anesthesia were significantly older, by an average of about 18 months, compared with those who received local or regional anesthesia.
The proportions of patients undergoing general, regional, or local anesthesia were similar in the subgroups of patients with American Society of Anesthesiologists (ASA) physical status scores of 1, 2, or 3. However, among the highest-risk patients included in the study – those with an ASA score of 4 – a significantly greater proportion of patients received general anesthesia. The multivariate models used in the analysis, therefore, were adjusted for age and for ASA score. About 42% of patients were in ASA class 2, and another 42% in class 3.
All patients were hemodynamically stable at the time of their enrollment. The maximum AAA diameter of registry patients was 6 cm, and about 88% of patients had an AAA diameter greater than 5 cm.
Average procedure times were 106 minutes in the general anesthesia patients, 95 minutes in the regional patients, and 81 minutes in the local anesthesia patients – statistically significant differences among the three groups.
The average postoperative hospitalization was 5.2 days in the general anesthesia patients, 4.3 days in the regional patients, and 3.6 days in the local anesthesia patients, differences that were statistically significant among each of the three groups.
The rate of technical surgical success was 98% in all three subgroups, and the rate of clinical success reached 97%-98% in all three groups.
The rates of major adverse events during the 30 days following surgery were 5.1% in the general anesthesia patients, 3.2% in the local patients, and 2.2% in the regional anesthesia patients. None of these differences reached statistical significance. Major adverse events included death, MI, stroke, renal failure, blood loss greater than 1 L, and bowel ischemia. No patients developed paraplegia or respiratory failure.
Postoperative ICU admission occurred for 27% of the regional anesthesia patients, 35% of the general patients, and 42% of the local anesthesia patients. The rate among the regional patients was significantly less than in the other two groups, but the difference in rates between the general and local anesthesia patients did not reach statistical significance.
The ENGAGE registry was organized and sponsored by Medtronic, which markets the Endurant stent. Dr. Stokmans and his associates received an unrestricted research grant from Medtronic. Dr. Stokmans said that he had no other disclosures.
MIAMI BEACH – Local or regional anesthesia is a better option than is general anesthesia for patients undergoing endovascular repair of an abdominal aortic aneurysm, based on findings from a registry with nearly 1,200 patients.
Both local anesthesia and regional anesthesia each surpassed general anesthesia in two periprocedural measures: significantly reducing procedure time, and significantly reducing postoperative hospitalization, Dr. Rutger A. Stokmans said at ISET 2012, an international symposium on endovascular therapy.
In addition, both local and regional anesthesia led to trends in reduced rates of major adverse events during the 30 days following surgery, although these differences did not reach statistical significance. All three anesthesia types linked with similar rates of both technical and clinical success of the aneurysm repairs. Regional anesthesia also led to a significantly lower rate of ICU admission, compared with both general and local anesthesia; local anesthesia showed no significant difference for this measure, compared with general anesthesia.
Based on these findings, local or regional anesthesia should be preferred when performing endovascular aneurysm repair (EVAR), whereas general anesthesia should usually be avoided, said Dr. Stokmans, a vascular surgeon at Catharina Hospital in Eindhoven, the Netherlands.
These findings support the most recent anesthesia recommendations of the European Society for Vascular Surgery, which in 2011 guidelines for managing abdominal aortic aneurysms (AAA) cited local anesthesia as preferred for EVAR, with regional or general anesthesia reserved for patients with contraindications for local anesthesia, he said (Euro. J. Vasc. Endovasc. Surg .2011;41[suppl. 1]:S1-S58). The most recent guidelines for AAA management from the Society for Vascular Surgery suggested using local or regional anesthesia over general anesthesia, he added (J. Vasc. Surg. 2009[suppl.]:50:S2-S49).
But despite these recommendations, the most commonly used anesthesia type worldwide for EVAR repair of AAA has been general anesthesia, followed by regional anesthesia, with local treatment used least often, according to the registry data reported by Dr. Stokmans. Among the 1,199 patients enrolled in ENGAGE (Endurant Stent Graft Natural Selection Global Postmarketing Registry) during March 2009 to December 2010 in 30 countries on five continents, 749 (62%) underwent their EVAR with general anesthesia, 325 (27%) with regional, and 125 (10%) with local anesthesia. (Percentages do not add up to 100% because of rounding.)
The registry data also showed striking regional variations in anesthesia use, with general anesthesia used on about 90% of patients in Canada, Australia, and New Zealand, and on about 70% of patients in Scandinavian countries and the United Kingdom. But in Central Europe, regional anesthesia – used on nearly 70% of EVAR patients – dominated. The only region favoring local anesthesia was South America (Argentina, Columbia, and Uruguay), where about 50% of patients received local, but more than 40% received general anesthesia, he said. The registry contained no U.S. patients, although the Endurant AAA stent graft system is marketed in the United States.
The average age of the EVAR patients in the registry was about 73 years. Those patients who underwent general anesthesia were significantly older, by an average of about 18 months, compared with those who received local or regional anesthesia.
The proportions of patients undergoing general, regional, or local anesthesia were similar in the subgroups of patients with American Society of Anesthesiologists (ASA) physical status scores of 1, 2, or 3. However, among the highest-risk patients included in the study – those with an ASA score of 4 – a significantly greater proportion of patients received general anesthesia. The multivariate models used in the analysis, therefore, were adjusted for age and for ASA score. About 42% of patients were in ASA class 2, and another 42% in class 3.
All patients were hemodynamically stable at the time of their enrollment. The maximum AAA diameter of registry patients was 6 cm, and about 88% of patients had an AAA diameter greater than 5 cm.
Average procedure times were 106 minutes in the general anesthesia patients, 95 minutes in the regional patients, and 81 minutes in the local anesthesia patients – statistically significant differences among the three groups.
The average postoperative hospitalization was 5.2 days in the general anesthesia patients, 4.3 days in the regional patients, and 3.6 days in the local anesthesia patients, differences that were statistically significant among each of the three groups.
The rate of technical surgical success was 98% in all three subgroups, and the rate of clinical success reached 97%-98% in all three groups.
The rates of major adverse events during the 30 days following surgery were 5.1% in the general anesthesia patients, 3.2% in the local patients, and 2.2% in the regional anesthesia patients. None of these differences reached statistical significance. Major adverse events included death, MI, stroke, renal failure, blood loss greater than 1 L, and bowel ischemia. No patients developed paraplegia or respiratory failure.
Postoperative ICU admission occurred for 27% of the regional anesthesia patients, 35% of the general patients, and 42% of the local anesthesia patients. The rate among the regional patients was significantly less than in the other two groups, but the difference in rates between the general and local anesthesia patients did not reach statistical significance.
The ENGAGE registry was organized and sponsored by Medtronic, which markets the Endurant stent. Dr. Stokmans and his associates received an unrestricted research grant from Medtronic. Dr. Stokmans said that he had no other disclosures.
MIAMI BEACH – Local or regional anesthesia is a better option than is general anesthesia for patients undergoing endovascular repair of an abdominal aortic aneurysm, based on findings from a registry with nearly 1,200 patients.
Both local anesthesia and regional anesthesia each surpassed general anesthesia in two periprocedural measures: significantly reducing procedure time, and significantly reducing postoperative hospitalization, Dr. Rutger A. Stokmans said at ISET 2012, an international symposium on endovascular therapy.
In addition, both local and regional anesthesia led to trends in reduced rates of major adverse events during the 30 days following surgery, although these differences did not reach statistical significance. All three anesthesia types linked with similar rates of both technical and clinical success of the aneurysm repairs. Regional anesthesia also led to a significantly lower rate of ICU admission, compared with both general and local anesthesia; local anesthesia showed no significant difference for this measure, compared with general anesthesia.
Based on these findings, local or regional anesthesia should be preferred when performing endovascular aneurysm repair (EVAR), whereas general anesthesia should usually be avoided, said Dr. Stokmans, a vascular surgeon at Catharina Hospital in Eindhoven, the Netherlands.
These findings support the most recent anesthesia recommendations of the European Society for Vascular Surgery, which in 2011 guidelines for managing abdominal aortic aneurysms (AAA) cited local anesthesia as preferred for EVAR, with regional or general anesthesia reserved for patients with contraindications for local anesthesia, he said (Euro. J. Vasc. Endovasc. Surg .2011;41[suppl. 1]:S1-S58). The most recent guidelines for AAA management from the Society for Vascular Surgery suggested using local or regional anesthesia over general anesthesia, he added (J. Vasc. Surg. 2009[suppl.]:50:S2-S49).
But despite these recommendations, the most commonly used anesthesia type worldwide for EVAR repair of AAA has been general anesthesia, followed by regional anesthesia, with local treatment used least often, according to the registry data reported by Dr. Stokmans. Among the 1,199 patients enrolled in ENGAGE (Endurant Stent Graft Natural Selection Global Postmarketing Registry) during March 2009 to December 2010 in 30 countries on five continents, 749 (62%) underwent their EVAR with general anesthesia, 325 (27%) with regional, and 125 (10%) with local anesthesia. (Percentages do not add up to 100% because of rounding.)
The registry data also showed striking regional variations in anesthesia use, with general anesthesia used on about 90% of patients in Canada, Australia, and New Zealand, and on about 70% of patients in Scandinavian countries and the United Kingdom. But in Central Europe, regional anesthesia – used on nearly 70% of EVAR patients – dominated. The only region favoring local anesthesia was South America (Argentina, Columbia, and Uruguay), where about 50% of patients received local, but more than 40% received general anesthesia, he said. The registry contained no U.S. patients, although the Endurant AAA stent graft system is marketed in the United States.
The average age of the EVAR patients in the registry was about 73 years. Those patients who underwent general anesthesia were significantly older, by an average of about 18 months, compared with those who received local or regional anesthesia.
The proportions of patients undergoing general, regional, or local anesthesia were similar in the subgroups of patients with American Society of Anesthesiologists (ASA) physical status scores of 1, 2, or 3. However, among the highest-risk patients included in the study – those with an ASA score of 4 – a significantly greater proportion of patients received general anesthesia. The multivariate models used in the analysis, therefore, were adjusted for age and for ASA score. About 42% of patients were in ASA class 2, and another 42% in class 3.
All patients were hemodynamically stable at the time of their enrollment. The maximum AAA diameter of registry patients was 6 cm, and about 88% of patients had an AAA diameter greater than 5 cm.
Average procedure times were 106 minutes in the general anesthesia patients, 95 minutes in the regional patients, and 81 minutes in the local anesthesia patients – statistically significant differences among the three groups.
The average postoperative hospitalization was 5.2 days in the general anesthesia patients, 4.3 days in the regional patients, and 3.6 days in the local anesthesia patients, differences that were statistically significant among each of the three groups.
The rate of technical surgical success was 98% in all three subgroups, and the rate of clinical success reached 97%-98% in all three groups.
The rates of major adverse events during the 30 days following surgery were 5.1% in the general anesthesia patients, 3.2% in the local patients, and 2.2% in the regional anesthesia patients. None of these differences reached statistical significance. Major adverse events included death, MI, stroke, renal failure, blood loss greater than 1 L, and bowel ischemia. No patients developed paraplegia or respiratory failure.
Postoperative ICU admission occurred for 27% of the regional anesthesia patients, 35% of the general patients, and 42% of the local anesthesia patients. The rate among the regional patients was significantly less than in the other two groups, but the difference in rates between the general and local anesthesia patients did not reach statistical significance.
The ENGAGE registry was organized and sponsored by Medtronic, which markets the Endurant stent. Dr. Stokmans and his associates received an unrestricted research grant from Medtronic. Dr. Stokmans said that he had no other disclosures.
FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: Postoperative hospitalization averaged 5.2 days in EVAR patients treated with general anesthesia, 4.3 days with regional anesthesia, and 3.6 days with local anesthesia.
Data Source: ENGAGE, an international registry of 1,199 patients who underwent EVAR for an abdominal aortic aneurysm during 2009 or 2010.
Disclosures: The ENGAGE registry was organized and sponsored by Medtronic, which markets the Endurant EVAR stent. Dr. Stokmans and his associates received an unrestricted research grant from Medtronic. Dr. Stokmans said that he had no other disclosures.