User login
Stroke Association Reviewing Stroke Definition, New Anticoagulants
NEW ORLEANS – The American Stroke Association is developing a host of new guidelines and statements, including a consensus statement on the definition of stroke and a scientific review of new anticoagulants.
The first new guideline – on the Management of Aneurysmal Subarachnoid Hemorrhage (SAH) – is complete and has been submitted for publication, said Dr. Colin Derdeyn, a member of the ASA’s Stroke Scientific Statement Oversight Committee.
Dr. Derdeyn is the program director for endovascular surgical neuroradiology at Washington University in St. Louis.
The SAH paper was authored by 13 clinicians who represent all disciplines involved in subarachnoid hemorrhage care, said Dr. Alejandro Rabinstein, who is vice chair of the group that wrote the guidelines.
There will be some new sections and some revised sections for 2012, said Dr. Rabinstein, a professor of neurology at the Mayo Clinic, Rochester, Minn. Dr. Rabinstein said that it has evolved into a more balanced document.
Also to be completed soon is a paper on the "Early Management of Patients with Ischemic Stroke." The paper will be very detailed, with more than 1,000 references and 15 authors, Dr. Rabinstein said. The chairman of the writing committee is Dr. Edward Jauch, professor of medicine at the Medical University of South Carolina, Charleston. The vice-chair is Dr. Jeffrey Saver, director of stroke and vascular neurology at the University of California, Los Angeles, Health System.
Two new papers – one reviewing the new anticoagulants on the market, and the other a consensus statement on the definition of stroke – are likely to garner a lot of attention, both as they are developed and when they are completed, Dr. Rabinstein said. "Novel Anticoagulants for the Prevention of Stroke in Atrial Fibrillation" will be completed fairly soon; it will be a science advisory, not a guideline or consensus statement. It will review the evidence on dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban. It will basically attempt to make sense of "the anticoagulant avalanche," Dr. Rabinstein said. The cochairs are Dr. Karen Furie, director of the stroke service and stroke prevention clinic at Massachusetts General Hospital, Boston, and Dr. Larry Goldstein, director of the Duke Stroke Center in Durham, N.C.
The consensus statement on the updated definition of stroke will be "a little bit more contentious," Dr. Rabinstein said. The goal is to update the definition of a transient ischemic attack. Among the questions to be addressed are whether an inclusion of the duration of symptoms is appropriate, and whether imaging should be required. The cochairs are Dr. Ralph Sacco, president of the American Heart Association and chairman of neurology at the University of Miami, and Dr. Scott Kasner, director of the comprehensive stroke center at the University of Pennsylvania, Philadelphia.
Another consensus will be issued on "Risk Adjustment for Stroke Outcome Measures." The goal is to recommend which variables should be included in risk adjustment models to assess the main stroke outcomes – before such benchmarks are imposed from the outside, Dr. Rabinstein said. The measures will be extremely important in both quality of care reporting and reimbursement going forward, he noted at the meeting, which was sponsored by the American Heart Association.
Several additional scientific statements are in development. One, "Stroke as Outcome and Risk Equivalent in Vascular Disease Risk Scores," will review the evidence supporting the inclusion of stroke in the outcome cluster of a risk equivalent and the designation of stroke itself as a risk equivalent to coronary events.
A statement will be issued next year on declining stroke mortality. Stroke was previously the third-leading cause of death; it is now the fourth, Dr. Rabinstein said. The goal is to determine the factors behind the decline: whether it is due to better prevention and treatment, and if so, to further explain how the decline translated into fewer deaths. Another aim is to figure out how to reproduce such knowledge in the future.
Dr. Robert Holloway, a professor of neurology at the University of Rochester (N.Y.) who is also board certified in palliative medicine, is chairing a group that is developing a statement on "Palliative Care and End of Life." The statement will review best end-of-life practices and provide recommendations on how to optimize care for patients with terminal cerebrovascular disease.
Finally, the ASA is trying to develop a statement on "Cervical Dissections and Chiropractic Manipulation." Dr. José Biller, chairman of neurology at Loyola University, Maywood, Ill., will lead the group. Some of the issues to be addressed include determining the risk of cervical arterial dissection in patients undergoing neck manipulations and how to avoid complications. So far, chiropractic organizations have declined to be a part of the statement process, Dr. Rabinstein said.
NEW ORLEANS – The American Stroke Association is developing a host of new guidelines and statements, including a consensus statement on the definition of stroke and a scientific review of new anticoagulants.
The first new guideline – on the Management of Aneurysmal Subarachnoid Hemorrhage (SAH) – is complete and has been submitted for publication, said Dr. Colin Derdeyn, a member of the ASA’s Stroke Scientific Statement Oversight Committee.
Dr. Derdeyn is the program director for endovascular surgical neuroradiology at Washington University in St. Louis.
The SAH paper was authored by 13 clinicians who represent all disciplines involved in subarachnoid hemorrhage care, said Dr. Alejandro Rabinstein, who is vice chair of the group that wrote the guidelines.
There will be some new sections and some revised sections for 2012, said Dr. Rabinstein, a professor of neurology at the Mayo Clinic, Rochester, Minn. Dr. Rabinstein said that it has evolved into a more balanced document.
Also to be completed soon is a paper on the "Early Management of Patients with Ischemic Stroke." The paper will be very detailed, with more than 1,000 references and 15 authors, Dr. Rabinstein said. The chairman of the writing committee is Dr. Edward Jauch, professor of medicine at the Medical University of South Carolina, Charleston. The vice-chair is Dr. Jeffrey Saver, director of stroke and vascular neurology at the University of California, Los Angeles, Health System.
Two new papers – one reviewing the new anticoagulants on the market, and the other a consensus statement on the definition of stroke – are likely to garner a lot of attention, both as they are developed and when they are completed, Dr. Rabinstein said. "Novel Anticoagulants for the Prevention of Stroke in Atrial Fibrillation" will be completed fairly soon; it will be a science advisory, not a guideline or consensus statement. It will review the evidence on dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban. It will basically attempt to make sense of "the anticoagulant avalanche," Dr. Rabinstein said. The cochairs are Dr. Karen Furie, director of the stroke service and stroke prevention clinic at Massachusetts General Hospital, Boston, and Dr. Larry Goldstein, director of the Duke Stroke Center in Durham, N.C.
The consensus statement on the updated definition of stroke will be "a little bit more contentious," Dr. Rabinstein said. The goal is to update the definition of a transient ischemic attack. Among the questions to be addressed are whether an inclusion of the duration of symptoms is appropriate, and whether imaging should be required. The cochairs are Dr. Ralph Sacco, president of the American Heart Association and chairman of neurology at the University of Miami, and Dr. Scott Kasner, director of the comprehensive stroke center at the University of Pennsylvania, Philadelphia.
Another consensus will be issued on "Risk Adjustment for Stroke Outcome Measures." The goal is to recommend which variables should be included in risk adjustment models to assess the main stroke outcomes – before such benchmarks are imposed from the outside, Dr. Rabinstein said. The measures will be extremely important in both quality of care reporting and reimbursement going forward, he noted at the meeting, which was sponsored by the American Heart Association.
Several additional scientific statements are in development. One, "Stroke as Outcome and Risk Equivalent in Vascular Disease Risk Scores," will review the evidence supporting the inclusion of stroke in the outcome cluster of a risk equivalent and the designation of stroke itself as a risk equivalent to coronary events.
A statement will be issued next year on declining stroke mortality. Stroke was previously the third-leading cause of death; it is now the fourth, Dr. Rabinstein said. The goal is to determine the factors behind the decline: whether it is due to better prevention and treatment, and if so, to further explain how the decline translated into fewer deaths. Another aim is to figure out how to reproduce such knowledge in the future.
Dr. Robert Holloway, a professor of neurology at the University of Rochester (N.Y.) who is also board certified in palliative medicine, is chairing a group that is developing a statement on "Palliative Care and End of Life." The statement will review best end-of-life practices and provide recommendations on how to optimize care for patients with terminal cerebrovascular disease.
Finally, the ASA is trying to develop a statement on "Cervical Dissections and Chiropractic Manipulation." Dr. José Biller, chairman of neurology at Loyola University, Maywood, Ill., will lead the group. Some of the issues to be addressed include determining the risk of cervical arterial dissection in patients undergoing neck manipulations and how to avoid complications. So far, chiropractic organizations have declined to be a part of the statement process, Dr. Rabinstein said.
NEW ORLEANS – The American Stroke Association is developing a host of new guidelines and statements, including a consensus statement on the definition of stroke and a scientific review of new anticoagulants.
The first new guideline – on the Management of Aneurysmal Subarachnoid Hemorrhage (SAH) – is complete and has been submitted for publication, said Dr. Colin Derdeyn, a member of the ASA’s Stroke Scientific Statement Oversight Committee.
Dr. Derdeyn is the program director for endovascular surgical neuroradiology at Washington University in St. Louis.
The SAH paper was authored by 13 clinicians who represent all disciplines involved in subarachnoid hemorrhage care, said Dr. Alejandro Rabinstein, who is vice chair of the group that wrote the guidelines.
There will be some new sections and some revised sections for 2012, said Dr. Rabinstein, a professor of neurology at the Mayo Clinic, Rochester, Minn. Dr. Rabinstein said that it has evolved into a more balanced document.
Also to be completed soon is a paper on the "Early Management of Patients with Ischemic Stroke." The paper will be very detailed, with more than 1,000 references and 15 authors, Dr. Rabinstein said. The chairman of the writing committee is Dr. Edward Jauch, professor of medicine at the Medical University of South Carolina, Charleston. The vice-chair is Dr. Jeffrey Saver, director of stroke and vascular neurology at the University of California, Los Angeles, Health System.
Two new papers – one reviewing the new anticoagulants on the market, and the other a consensus statement on the definition of stroke – are likely to garner a lot of attention, both as they are developed and when they are completed, Dr. Rabinstein said. "Novel Anticoagulants for the Prevention of Stroke in Atrial Fibrillation" will be completed fairly soon; it will be a science advisory, not a guideline or consensus statement. It will review the evidence on dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban. It will basically attempt to make sense of "the anticoagulant avalanche," Dr. Rabinstein said. The cochairs are Dr. Karen Furie, director of the stroke service and stroke prevention clinic at Massachusetts General Hospital, Boston, and Dr. Larry Goldstein, director of the Duke Stroke Center in Durham, N.C.
The consensus statement on the updated definition of stroke will be "a little bit more contentious," Dr. Rabinstein said. The goal is to update the definition of a transient ischemic attack. Among the questions to be addressed are whether an inclusion of the duration of symptoms is appropriate, and whether imaging should be required. The cochairs are Dr. Ralph Sacco, president of the American Heart Association and chairman of neurology at the University of Miami, and Dr. Scott Kasner, director of the comprehensive stroke center at the University of Pennsylvania, Philadelphia.
Another consensus will be issued on "Risk Adjustment for Stroke Outcome Measures." The goal is to recommend which variables should be included in risk adjustment models to assess the main stroke outcomes – before such benchmarks are imposed from the outside, Dr. Rabinstein said. The measures will be extremely important in both quality of care reporting and reimbursement going forward, he noted at the meeting, which was sponsored by the American Heart Association.
Several additional scientific statements are in development. One, "Stroke as Outcome and Risk Equivalent in Vascular Disease Risk Scores," will review the evidence supporting the inclusion of stroke in the outcome cluster of a risk equivalent and the designation of stroke itself as a risk equivalent to coronary events.
A statement will be issued next year on declining stroke mortality. Stroke was previously the third-leading cause of death; it is now the fourth, Dr. Rabinstein said. The goal is to determine the factors behind the decline: whether it is due to better prevention and treatment, and if so, to further explain how the decline translated into fewer deaths. Another aim is to figure out how to reproduce such knowledge in the future.
Dr. Robert Holloway, a professor of neurology at the University of Rochester (N.Y.) who is also board certified in palliative medicine, is chairing a group that is developing a statement on "Palliative Care and End of Life." The statement will review best end-of-life practices and provide recommendations on how to optimize care for patients with terminal cerebrovascular disease.
Finally, the ASA is trying to develop a statement on "Cervical Dissections and Chiropractic Manipulation." Dr. José Biller, chairman of neurology at Loyola University, Maywood, Ill., will lead the group. Some of the issues to be addressed include determining the risk of cervical arterial dissection in patients undergoing neck manipulations and how to avoid complications. So far, chiropractic organizations have declined to be a part of the statement process, Dr. Rabinstein said.
FROM THE INTERNATIONAL STROKE CONFERENCE
Filgrastim Fails to Improve Ischemic Stroke Outcomes
NEW ORLEANS –A granulocyte colony-stimulating drug did not protect brain tissue or improve functional outcomes after ischemic stroke.
Researchers had high hopes for filgrastim, which is approved in Europe for chemotherapy-induced neutropenia. In vivo and in vitro studies indicated that filgrastim also had antiapoptotic properties, and stimulated arteriogenesis, neurogenesis and neurite outgrowth, said Dr. E. Bernd Ringelstein, a professor of neurology at the University Hospital of Münster (Germany).
Although the drug was safe and well tolerated, the results confirm the principal problem that has plagued the development of stroke drugs for years: They don’t make the leap from preclinical promise to clinical efficacy, he said. The results seem to echo a research saying from the 1980s: "The outlook for stroke therapy is excellent – if you’re a rat."
The negative findings were noted in the phase II AXIS 2 trial, which randomized 328 patients with acute ischemic stroke to placebo or filgrastim (AX200) at 135 mcg/kg over 72 hours.* Study participants had to have a middle cerebral artery infarct of at least 15 cc, and be younger than 86 years old. The intent-to-treat analysis included 323 patients, 64% of whom had received tissue plasminogen activator (TPA). There was a mean of 7 hours from onset of symptoms to administration of the study drug or placebo.
The study’s primary end point was 90-day function as measured by the modified Rankin Scale. Secondary end points included 30-day lesion volume and 90-day NIHSS (National Institutes of Health Stroke Scale) score and mortality. Imaging was graded by a core lab that was blinded to the treatment.
At 90 days, the modified Rankin Scale scores were 3.26 in the treatment group and 3.06 in the placebo group. The 90-day NIHSS scores were also similar (8.8 and 8.4, respectively). Pretreatment with TPA did not significantly affect outcomes in either group. Survival at 90 days was also not significantly different, at 78% in the treatment group and 82% in the placebo group.
The initial infarct volume was an average of 46 cc. The final lesion volumes were 59 cc in the treatment group and 66 cc in the placebo group, which were not significantly different.
The most common adverse events were cardiac, endocrine, and gastrointestinal disorders, none of which were significantly more common in the treated group. AX200 did significantly increase white blood cell and monocyte counts, and slightly decreased platelets relative to the placebo, as expected, Dr. Ringelstein said.
"In summary, AX200 was a very promising drug with a comprehensive activity package. It was safe and well tolerated, but it did not provide any benefit over placebo. At the moment, we do not know the reasons for this failure, but we are exploring this with additional analyses."
The study was sponsored by SYGNIS Biosience. Dr. Ringelstein disclosed that he was a member of the AXIS study steering board and a consultant and advisory board member for the company.
*Correction, 2/21/2012: An earlier version of this story misstated the phase of this trial.
NEW ORLEANS –A granulocyte colony-stimulating drug did not protect brain tissue or improve functional outcomes after ischemic stroke.
Researchers had high hopes for filgrastim, which is approved in Europe for chemotherapy-induced neutropenia. In vivo and in vitro studies indicated that filgrastim also had antiapoptotic properties, and stimulated arteriogenesis, neurogenesis and neurite outgrowth, said Dr. E. Bernd Ringelstein, a professor of neurology at the University Hospital of Münster (Germany).
Although the drug was safe and well tolerated, the results confirm the principal problem that has plagued the development of stroke drugs for years: They don’t make the leap from preclinical promise to clinical efficacy, he said. The results seem to echo a research saying from the 1980s: "The outlook for stroke therapy is excellent – if you’re a rat."
The negative findings were noted in the phase II AXIS 2 trial, which randomized 328 patients with acute ischemic stroke to placebo or filgrastim (AX200) at 135 mcg/kg over 72 hours.* Study participants had to have a middle cerebral artery infarct of at least 15 cc, and be younger than 86 years old. The intent-to-treat analysis included 323 patients, 64% of whom had received tissue plasminogen activator (TPA). There was a mean of 7 hours from onset of symptoms to administration of the study drug or placebo.
The study’s primary end point was 90-day function as measured by the modified Rankin Scale. Secondary end points included 30-day lesion volume and 90-day NIHSS (National Institutes of Health Stroke Scale) score and mortality. Imaging was graded by a core lab that was blinded to the treatment.
At 90 days, the modified Rankin Scale scores were 3.26 in the treatment group and 3.06 in the placebo group. The 90-day NIHSS scores were also similar (8.8 and 8.4, respectively). Pretreatment with TPA did not significantly affect outcomes in either group. Survival at 90 days was also not significantly different, at 78% in the treatment group and 82% in the placebo group.
The initial infarct volume was an average of 46 cc. The final lesion volumes were 59 cc in the treatment group and 66 cc in the placebo group, which were not significantly different.
The most common adverse events were cardiac, endocrine, and gastrointestinal disorders, none of which were significantly more common in the treated group. AX200 did significantly increase white blood cell and monocyte counts, and slightly decreased platelets relative to the placebo, as expected, Dr. Ringelstein said.
"In summary, AX200 was a very promising drug with a comprehensive activity package. It was safe and well tolerated, but it did not provide any benefit over placebo. At the moment, we do not know the reasons for this failure, but we are exploring this with additional analyses."
The study was sponsored by SYGNIS Biosience. Dr. Ringelstein disclosed that he was a member of the AXIS study steering board and a consultant and advisory board member for the company.
*Correction, 2/21/2012: An earlier version of this story misstated the phase of this trial.
NEW ORLEANS –A granulocyte colony-stimulating drug did not protect brain tissue or improve functional outcomes after ischemic stroke.
Researchers had high hopes for filgrastim, which is approved in Europe for chemotherapy-induced neutropenia. In vivo and in vitro studies indicated that filgrastim also had antiapoptotic properties, and stimulated arteriogenesis, neurogenesis and neurite outgrowth, said Dr. E. Bernd Ringelstein, a professor of neurology at the University Hospital of Münster (Germany).
Although the drug was safe and well tolerated, the results confirm the principal problem that has plagued the development of stroke drugs for years: They don’t make the leap from preclinical promise to clinical efficacy, he said. The results seem to echo a research saying from the 1980s: "The outlook for stroke therapy is excellent – if you’re a rat."
The negative findings were noted in the phase II AXIS 2 trial, which randomized 328 patients with acute ischemic stroke to placebo or filgrastim (AX200) at 135 mcg/kg over 72 hours.* Study participants had to have a middle cerebral artery infarct of at least 15 cc, and be younger than 86 years old. The intent-to-treat analysis included 323 patients, 64% of whom had received tissue plasminogen activator (TPA). There was a mean of 7 hours from onset of symptoms to administration of the study drug or placebo.
The study’s primary end point was 90-day function as measured by the modified Rankin Scale. Secondary end points included 30-day lesion volume and 90-day NIHSS (National Institutes of Health Stroke Scale) score and mortality. Imaging was graded by a core lab that was blinded to the treatment.
At 90 days, the modified Rankin Scale scores were 3.26 in the treatment group and 3.06 in the placebo group. The 90-day NIHSS scores were also similar (8.8 and 8.4, respectively). Pretreatment with TPA did not significantly affect outcomes in either group. Survival at 90 days was also not significantly different, at 78% in the treatment group and 82% in the placebo group.
The initial infarct volume was an average of 46 cc. The final lesion volumes were 59 cc in the treatment group and 66 cc in the placebo group, which were not significantly different.
The most common adverse events were cardiac, endocrine, and gastrointestinal disorders, none of which were significantly more common in the treated group. AX200 did significantly increase white blood cell and monocyte counts, and slightly decreased platelets relative to the placebo, as expected, Dr. Ringelstein said.
"In summary, AX200 was a very promising drug with a comprehensive activity package. It was safe and well tolerated, but it did not provide any benefit over placebo. At the moment, we do not know the reasons for this failure, but we are exploring this with additional analyses."
The study was sponsored by SYGNIS Biosience. Dr. Ringelstein disclosed that he was a member of the AXIS study steering board and a consultant and advisory board member for the company.
*Correction, 2/21/2012: An earlier version of this story misstated the phase of this trial.
FROM AN INTERNATIONAL STROKE CONFERENCE
Major Finding: At 90 days, the modified Rankin Scale scores were 3.26 in the treatment group and 3.06 in the placebo group. The 90-day NIHSS scores were 8.8 and 8.4, respectively. The final lesion volumes were 59 cc in the treatment group and 66 cc in the placebo group.
Data Source: AXIS 2 was a phase II, randomized, placebo-controlled trial with 323 patients in an intent-to-treat analysis.
Disclosures: The study was sponsored by SYGNIS Biosience. Dr. Ringelstein disclosed that he was a member of the AXIS study steering board and a consultant and advisory board member for the company.
Aspirin Plus Clopidogrel Flops for Secondary Stroke Prevention
NEW ORLEANS – The combination of aspirin and clopidogrel proved no more effective than aspirin alone for secondary stroke prevention, and dual therapy significantly boosted the major hemorrhage rate and patient mortality in a randomized, multicenter trial with more than 3,000 patients.
The poor performance of this dual antiplatelet combination in this large study, called the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, leaves the secondary stroke prevention guideline published last year by the American Heart Association and American Stroke Association unchanged in its recommendation of three antiplatelet drug options for patients with a history of stroke or transient ischemic attack: aspirin, aspirin plus dipyridamole, or clopidogrel (Stroke 2011;42:227-76), Dr. Oscar R. Benavente said at the International Stroke Conference.
The new trial results "do not support the use of combination therapy for secondary stroke prevention in patients with lacunar strokes," said Dr. Benavente, the study’s co-principal investigator and research director of the Stroke and Cerebrovascular Disease Program at the Vancouver Coastal Health Research Institute.
The SPS3 study enrolled 3,020 patients within 180 days of a small, subcortical, lacunar stroke at 81 sites in eight countries during 2003-2011. The trial excluded patients with cortical stroke, cardioembolic disease, or carotid stenosis. More than half of the enrolled patients received care at U.S. centers. Their average age was 63 years, about two-thirds were men, and they entered the study an average of 76 days following their index stroke.
The study randomized 1,503 patients to treatment with 325 mg of aspirin daily, and 1,517 patients to the same aspirin dosage plus 75 mg of clopidogrel (Plavix) daily.
The study’s data and safety monitoring board stopped this portion of the trial early last July, after an average follow-up of 3.5 years, because of safety and futility. (The study continues with a concurrent randomization that is comparing the efficacy of usual and "intensive" blood pressure control.) The antiplatelet randomization stopped because the results showed a "surprising," statistically significant increased rate of all-cause mortality and major hemorrhages in patients treated with dual antiplatelet therapy, Dr. Benavente said.
All-cause mortality occurred at a rate of 1.4%/patient-year in the aspirin-only group, and a rate of 2.1%/patient-year in the dual-therapy arm, a 50% relative risk difference. A breakdown of the causes of death showed a statistically significant difference for only one subgroup, "probable vascular" death, which was threefold higher in the aspirin plus clopidogrel patients than in those on aspirin only. The causes of death will require more analysis to better understand the danger posed by the dual-drug regimen, Dr. Benavente said at the meeting, which was sponsored by the American Heart Association.
Major hemorrhages occurred in 1.1% of the aspirin-only patients and in 2.1% of those on the dual regimen. When separated by type of hemorrhage, the only significant difference between the two treatment groups was in the incidence of non–central nervous system hemorrhages, a category that mostly consisted of gastrointestinal bleeds, he said.
The two regimens showed no significant difference for the study’s primary efficacy outcome, the incidence of ischemic and hemorrhagic strokes, which occurred at 2.7%/patient-year among those on aspirin only and 2.5%/patient-year in those on both drugs. The rates of ischemic strokes only, hemorrhagic strokes only, major vascular events, and myocardial infarctions also showed no significant differences between the two treatment arms.
SPS3 did not include a patient group treated only with clopidogrel because previous, large studies had already compared clopidogrel alone with aspirin alone, and with aspirin plus dipyridamole. The results of those studies "have not clearly established that [clopidogrel] is superior to or even equivalent to" aspirin, aspirin plus dipyridamole, and ticlopidine (Ticlid), said last year’s treatment recommendations from the American Heart Association and American Stroke Association.
The SPS3 study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Benavente said that he has received research support from Sanofi-Aventis and Bristol-Myers Squibb.
NEW ORLEANS – The combination of aspirin and clopidogrel proved no more effective than aspirin alone for secondary stroke prevention, and dual therapy significantly boosted the major hemorrhage rate and patient mortality in a randomized, multicenter trial with more than 3,000 patients.
The poor performance of this dual antiplatelet combination in this large study, called the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, leaves the secondary stroke prevention guideline published last year by the American Heart Association and American Stroke Association unchanged in its recommendation of three antiplatelet drug options for patients with a history of stroke or transient ischemic attack: aspirin, aspirin plus dipyridamole, or clopidogrel (Stroke 2011;42:227-76), Dr. Oscar R. Benavente said at the International Stroke Conference.
The new trial results "do not support the use of combination therapy for secondary stroke prevention in patients with lacunar strokes," said Dr. Benavente, the study’s co-principal investigator and research director of the Stroke and Cerebrovascular Disease Program at the Vancouver Coastal Health Research Institute.
The SPS3 study enrolled 3,020 patients within 180 days of a small, subcortical, lacunar stroke at 81 sites in eight countries during 2003-2011. The trial excluded patients with cortical stroke, cardioembolic disease, or carotid stenosis. More than half of the enrolled patients received care at U.S. centers. Their average age was 63 years, about two-thirds were men, and they entered the study an average of 76 days following their index stroke.
The study randomized 1,503 patients to treatment with 325 mg of aspirin daily, and 1,517 patients to the same aspirin dosage plus 75 mg of clopidogrel (Plavix) daily.
The study’s data and safety monitoring board stopped this portion of the trial early last July, after an average follow-up of 3.5 years, because of safety and futility. (The study continues with a concurrent randomization that is comparing the efficacy of usual and "intensive" blood pressure control.) The antiplatelet randomization stopped because the results showed a "surprising," statistically significant increased rate of all-cause mortality and major hemorrhages in patients treated with dual antiplatelet therapy, Dr. Benavente said.
All-cause mortality occurred at a rate of 1.4%/patient-year in the aspirin-only group, and a rate of 2.1%/patient-year in the dual-therapy arm, a 50% relative risk difference. A breakdown of the causes of death showed a statistically significant difference for only one subgroup, "probable vascular" death, which was threefold higher in the aspirin plus clopidogrel patients than in those on aspirin only. The causes of death will require more analysis to better understand the danger posed by the dual-drug regimen, Dr. Benavente said at the meeting, which was sponsored by the American Heart Association.
Major hemorrhages occurred in 1.1% of the aspirin-only patients and in 2.1% of those on the dual regimen. When separated by type of hemorrhage, the only significant difference between the two treatment groups was in the incidence of non–central nervous system hemorrhages, a category that mostly consisted of gastrointestinal bleeds, he said.
The two regimens showed no significant difference for the study’s primary efficacy outcome, the incidence of ischemic and hemorrhagic strokes, which occurred at 2.7%/patient-year among those on aspirin only and 2.5%/patient-year in those on both drugs. The rates of ischemic strokes only, hemorrhagic strokes only, major vascular events, and myocardial infarctions also showed no significant differences between the two treatment arms.
SPS3 did not include a patient group treated only with clopidogrel because previous, large studies had already compared clopidogrel alone with aspirin alone, and with aspirin plus dipyridamole. The results of those studies "have not clearly established that [clopidogrel] is superior to or even equivalent to" aspirin, aspirin plus dipyridamole, and ticlopidine (Ticlid), said last year’s treatment recommendations from the American Heart Association and American Stroke Association.
The SPS3 study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Benavente said that he has received research support from Sanofi-Aventis and Bristol-Myers Squibb.
NEW ORLEANS – The combination of aspirin and clopidogrel proved no more effective than aspirin alone for secondary stroke prevention, and dual therapy significantly boosted the major hemorrhage rate and patient mortality in a randomized, multicenter trial with more than 3,000 patients.
The poor performance of this dual antiplatelet combination in this large study, called the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, leaves the secondary stroke prevention guideline published last year by the American Heart Association and American Stroke Association unchanged in its recommendation of three antiplatelet drug options for patients with a history of stroke or transient ischemic attack: aspirin, aspirin plus dipyridamole, or clopidogrel (Stroke 2011;42:227-76), Dr. Oscar R. Benavente said at the International Stroke Conference.
The new trial results "do not support the use of combination therapy for secondary stroke prevention in patients with lacunar strokes," said Dr. Benavente, the study’s co-principal investigator and research director of the Stroke and Cerebrovascular Disease Program at the Vancouver Coastal Health Research Institute.
The SPS3 study enrolled 3,020 patients within 180 days of a small, subcortical, lacunar stroke at 81 sites in eight countries during 2003-2011. The trial excluded patients with cortical stroke, cardioembolic disease, or carotid stenosis. More than half of the enrolled patients received care at U.S. centers. Their average age was 63 years, about two-thirds were men, and they entered the study an average of 76 days following their index stroke.
The study randomized 1,503 patients to treatment with 325 mg of aspirin daily, and 1,517 patients to the same aspirin dosage plus 75 mg of clopidogrel (Plavix) daily.
The study’s data and safety monitoring board stopped this portion of the trial early last July, after an average follow-up of 3.5 years, because of safety and futility. (The study continues with a concurrent randomization that is comparing the efficacy of usual and "intensive" blood pressure control.) The antiplatelet randomization stopped because the results showed a "surprising," statistically significant increased rate of all-cause mortality and major hemorrhages in patients treated with dual antiplatelet therapy, Dr. Benavente said.
All-cause mortality occurred at a rate of 1.4%/patient-year in the aspirin-only group, and a rate of 2.1%/patient-year in the dual-therapy arm, a 50% relative risk difference. A breakdown of the causes of death showed a statistically significant difference for only one subgroup, "probable vascular" death, which was threefold higher in the aspirin plus clopidogrel patients than in those on aspirin only. The causes of death will require more analysis to better understand the danger posed by the dual-drug regimen, Dr. Benavente said at the meeting, which was sponsored by the American Heart Association.
Major hemorrhages occurred in 1.1% of the aspirin-only patients and in 2.1% of those on the dual regimen. When separated by type of hemorrhage, the only significant difference between the two treatment groups was in the incidence of non–central nervous system hemorrhages, a category that mostly consisted of gastrointestinal bleeds, he said.
The two regimens showed no significant difference for the study’s primary efficacy outcome, the incidence of ischemic and hemorrhagic strokes, which occurred at 2.7%/patient-year among those on aspirin only and 2.5%/patient-year in those on both drugs. The rates of ischemic strokes only, hemorrhagic strokes only, major vascular events, and myocardial infarctions also showed no significant differences between the two treatment arms.
SPS3 did not include a patient group treated only with clopidogrel because previous, large studies had already compared clopidogrel alone with aspirin alone, and with aspirin plus dipyridamole. The results of those studies "have not clearly established that [clopidogrel] is superior to or even equivalent to" aspirin, aspirin plus dipyridamole, and ticlopidine (Ticlid), said last year’s treatment recommendations from the American Heart Association and American Stroke Association.
The SPS3 study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Benavente said that he has received research support from Sanofi-Aventis and Bristol-Myers Squibb.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: All-cause mortality occurred at a rate of 1.4%/patient-year in the aspirin-only group, and a rate of 2.1%/patient-year in the dual-therapy arm, a 50% relative risk difference.
Data Source: The Secondary Prevention of Subcortical Strokes Study, which randomized 3,020 patients at 81 sites in eight countries.
Disclosures: The SPS3 study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Benavente said that he has received research support from Sanofi-Aventis and Bristol-Myers Squibb.
Scores Help Identify Stroke Patients at Risk of Brain Bleed
NEW ORLEANS – Two new risk assessment scores predicted the likelihood of a symptomatic intracranial hemorrhage after ischemic stroke treatment with more than 70% accuracy.
Predictive rules such as these are important because there is still no large-scale prospective study that clearly identifies which patients with ischemic stroke are more likely to develop a brain bleed after treatment with intravenous tissue plasminogen activator (TPA), Dr. Bijoy Menon said at the International Stroke Conference.
Dr. Menon, a clinical stroke fellow at the University of Calgary (Alta.), and his colleagues developed a 101-point score based on data extracted from the Get With The Guidelines stroke cohort. The cohort consisted of 10,242 patients with ischemic stroke who received TPA within 3 hours of the onset of stroke symptoms. A derivation cohort comprised 70% of the group; the rule was then validated in the remaining 30%.
The cohort’s mean age was 69 years. All patients experienced a moderate to severe ischemic stroke, with a mean National Institutes of Health Stroke Scale (NIHSS) score of 11. They received TPA at a mean of 1.35 hours after symptom onset.
About 5% of the group (496 patients) experienced a symptomatic intracranial hemorrhage (ICH), which the investigators defined as neurologic worsening within 36 hours of TPA administration.
In a multivariate regression model, Dr. Menon and his coinvestigators found six patient characteristics that were significantly associated with a brain bleed. They assigned each of these characteristics a point spread based on the range of measurements:
• Age. From age 60 years or younger (8 points) to older than 80 (17 points).
• NIHSS score. From 1-5 (25 points) to 20 and over (42 points).
• Systolic blood pressure. From less than 120 mm Hg (10 points) to 180 mm Hg or higher (21 points).
• Blood glucose level. From less than 100 mg/dL (2 points) to 150 mg/dL or more (8 points).
• Ethnicity. Asian, 9 points; all others, 0 points.
• Gender. Male, 4 points; female, 0 points.
Diabetes and a history of stroke were significantly associated with ICH in the initial analysis, but the P values were nonsignificant in the multivariate analysis, Dr. Menon noted.
"We also did not find any significantly increased risk associated with warfarin use or with the baseline international normalized ratio," he said at the meeting, sponsored by the American Heart Association.
The score accurately predicted ICH in 71% of the validation cohort, "comparable to most of the other scoring methods out there."
While the score is "well validated and evidence based," neither it nor any other single measure should be the sole factor in determining ischemic stroke treatment, Dr. Menon emphasized. "This is very important. It should not be used to infer which patients would benefit most or least from IV TPA. This is a cohort study, and because treatment was at the discretion of the individual physicians, there may be a selection bias present in it."
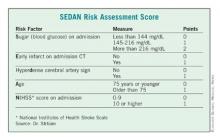
Dr. Daniel Strbian of the University of Helsinki presented a second score, dubbed SEDAN. Dr. Strbian and his colleagues developed their score based on a cohort of 972 ischemic stroke patients who developed an imaging-proven brain bleed after IV TPA treatment.
In a multivariate analysis, he and his associates found five factors that were independently associated with a significantly increased risk of symptomatic ICH: blood glucose level on admission, computed tomography (CT) imaging positive for ischemic stroke, cerebral artery hyperdensity, age, and NIHSS score.
Points were assigned based on the relative risk (RR) associated with each factor – the higher the total score, the greater the risk of symptomatic ICH. Each of the five risk-point categories generated a significant risk ratio:1 point, RR 0.19; 2 points, RR 0.40; 3 points, RR 1.85; 4 points, RR 3.7; 5 points, RR 5.6.
The score was validated in two additional groups totaling about 2,000 patients, resulting in an overall accuracy of about 77%.
"If we have a patient with a very high score, there is a very bad prognosis with a high chance of death or institutionalization at 3 months," Dr. Strbian said. "In this case, we know that the patient may die if we do nothing, but perhaps the SEDAN [score] can help us determine if an endovascular approach might be better than thrombolysis."
The SEDAN score is simple, easy, and quick to calculate, he said. But it’s only part of the treatment decision process.
"We can’t treat based on this score alone. If the patient fulfills the indications for TPA, it’s up to the physician and the patient to decide whether they are willing to accept the risk of an intracranial hemorrhage."
Dr. Menon and Dr. Strbian reported having no financial conflicts.
NEW ORLEANS – Two new risk assessment scores predicted the likelihood of a symptomatic intracranial hemorrhage after ischemic stroke treatment with more than 70% accuracy.
Predictive rules such as these are important because there is still no large-scale prospective study that clearly identifies which patients with ischemic stroke are more likely to develop a brain bleed after treatment with intravenous tissue plasminogen activator (TPA), Dr. Bijoy Menon said at the International Stroke Conference.
Dr. Menon, a clinical stroke fellow at the University of Calgary (Alta.), and his colleagues developed a 101-point score based on data extracted from the Get With The Guidelines stroke cohort. The cohort consisted of 10,242 patients with ischemic stroke who received TPA within 3 hours of the onset of stroke symptoms. A derivation cohort comprised 70% of the group; the rule was then validated in the remaining 30%.
The cohort’s mean age was 69 years. All patients experienced a moderate to severe ischemic stroke, with a mean National Institutes of Health Stroke Scale (NIHSS) score of 11. They received TPA at a mean of 1.35 hours after symptom onset.
About 5% of the group (496 patients) experienced a symptomatic intracranial hemorrhage (ICH), which the investigators defined as neurologic worsening within 36 hours of TPA administration.
In a multivariate regression model, Dr. Menon and his coinvestigators found six patient characteristics that were significantly associated with a brain bleed. They assigned each of these characteristics a point spread based on the range of measurements:
• Age. From age 60 years or younger (8 points) to older than 80 (17 points).
• NIHSS score. From 1-5 (25 points) to 20 and over (42 points).
• Systolic blood pressure. From less than 120 mm Hg (10 points) to 180 mm Hg or higher (21 points).
• Blood glucose level. From less than 100 mg/dL (2 points) to 150 mg/dL or more (8 points).
• Ethnicity. Asian, 9 points; all others, 0 points.
• Gender. Male, 4 points; female, 0 points.
Diabetes and a history of stroke were significantly associated with ICH in the initial analysis, but the P values were nonsignificant in the multivariate analysis, Dr. Menon noted.
"We also did not find any significantly increased risk associated with warfarin use or with the baseline international normalized ratio," he said at the meeting, sponsored by the American Heart Association.
The score accurately predicted ICH in 71% of the validation cohort, "comparable to most of the other scoring methods out there."
While the score is "well validated and evidence based," neither it nor any other single measure should be the sole factor in determining ischemic stroke treatment, Dr. Menon emphasized. "This is very important. It should not be used to infer which patients would benefit most or least from IV TPA. This is a cohort study, and because treatment was at the discretion of the individual physicians, there may be a selection bias present in it."
Dr. Daniel Strbian of the University of Helsinki presented a second score, dubbed SEDAN. Dr. Strbian and his colleagues developed their score based on a cohort of 972 ischemic stroke patients who developed an imaging-proven brain bleed after IV TPA treatment.
In a multivariate analysis, he and his associates found five factors that were independently associated with a significantly increased risk of symptomatic ICH: blood glucose level on admission, computed tomography (CT) imaging positive for ischemic stroke, cerebral artery hyperdensity, age, and NIHSS score.
Points were assigned based on the relative risk (RR) associated with each factor – the higher the total score, the greater the risk of symptomatic ICH. Each of the five risk-point categories generated a significant risk ratio:1 point, RR 0.19; 2 points, RR 0.40; 3 points, RR 1.85; 4 points, RR 3.7; 5 points, RR 5.6.
The score was validated in two additional groups totaling about 2,000 patients, resulting in an overall accuracy of about 77%.
"If we have a patient with a very high score, there is a very bad prognosis with a high chance of death or institutionalization at 3 months," Dr. Strbian said. "In this case, we know that the patient may die if we do nothing, but perhaps the SEDAN [score] can help us determine if an endovascular approach might be better than thrombolysis."
The SEDAN score is simple, easy, and quick to calculate, he said. But it’s only part of the treatment decision process.
"We can’t treat based on this score alone. If the patient fulfills the indications for TPA, it’s up to the physician and the patient to decide whether they are willing to accept the risk of an intracranial hemorrhage."
Dr. Menon and Dr. Strbian reported having no financial conflicts.
NEW ORLEANS – Two new risk assessment scores predicted the likelihood of a symptomatic intracranial hemorrhage after ischemic stroke treatment with more than 70% accuracy.
Predictive rules such as these are important because there is still no large-scale prospective study that clearly identifies which patients with ischemic stroke are more likely to develop a brain bleed after treatment with intravenous tissue plasminogen activator (TPA), Dr. Bijoy Menon said at the International Stroke Conference.
Dr. Menon, a clinical stroke fellow at the University of Calgary (Alta.), and his colleagues developed a 101-point score based on data extracted from the Get With The Guidelines stroke cohort. The cohort consisted of 10,242 patients with ischemic stroke who received TPA within 3 hours of the onset of stroke symptoms. A derivation cohort comprised 70% of the group; the rule was then validated in the remaining 30%.
The cohort’s mean age was 69 years. All patients experienced a moderate to severe ischemic stroke, with a mean National Institutes of Health Stroke Scale (NIHSS) score of 11. They received TPA at a mean of 1.35 hours after symptom onset.
About 5% of the group (496 patients) experienced a symptomatic intracranial hemorrhage (ICH), which the investigators defined as neurologic worsening within 36 hours of TPA administration.
In a multivariate regression model, Dr. Menon and his coinvestigators found six patient characteristics that were significantly associated with a brain bleed. They assigned each of these characteristics a point spread based on the range of measurements:
• Age. From age 60 years or younger (8 points) to older than 80 (17 points).
• NIHSS score. From 1-5 (25 points) to 20 and over (42 points).
• Systolic blood pressure. From less than 120 mm Hg (10 points) to 180 mm Hg or higher (21 points).
• Blood glucose level. From less than 100 mg/dL (2 points) to 150 mg/dL or more (8 points).
• Ethnicity. Asian, 9 points; all others, 0 points.
• Gender. Male, 4 points; female, 0 points.
Diabetes and a history of stroke were significantly associated with ICH in the initial analysis, but the P values were nonsignificant in the multivariate analysis, Dr. Menon noted.
"We also did not find any significantly increased risk associated with warfarin use or with the baseline international normalized ratio," he said at the meeting, sponsored by the American Heart Association.
The score accurately predicted ICH in 71% of the validation cohort, "comparable to most of the other scoring methods out there."
While the score is "well validated and evidence based," neither it nor any other single measure should be the sole factor in determining ischemic stroke treatment, Dr. Menon emphasized. "This is very important. It should not be used to infer which patients would benefit most or least from IV TPA. This is a cohort study, and because treatment was at the discretion of the individual physicians, there may be a selection bias present in it."
Dr. Daniel Strbian of the University of Helsinki presented a second score, dubbed SEDAN. Dr. Strbian and his colleagues developed their score based on a cohort of 972 ischemic stroke patients who developed an imaging-proven brain bleed after IV TPA treatment.
In a multivariate analysis, he and his associates found five factors that were independently associated with a significantly increased risk of symptomatic ICH: blood glucose level on admission, computed tomography (CT) imaging positive for ischemic stroke, cerebral artery hyperdensity, age, and NIHSS score.
Points were assigned based on the relative risk (RR) associated with each factor – the higher the total score, the greater the risk of symptomatic ICH. Each of the five risk-point categories generated a significant risk ratio:1 point, RR 0.19; 2 points, RR 0.40; 3 points, RR 1.85; 4 points, RR 3.7; 5 points, RR 5.6.
The score was validated in two additional groups totaling about 2,000 patients, resulting in an overall accuracy of about 77%.
"If we have a patient with a very high score, there is a very bad prognosis with a high chance of death or institutionalization at 3 months," Dr. Strbian said. "In this case, we know that the patient may die if we do nothing, but perhaps the SEDAN [score] can help us determine if an endovascular approach might be better than thrombolysis."
The SEDAN score is simple, easy, and quick to calculate, he said. But it’s only part of the treatment decision process.
"We can’t treat based on this score alone. If the patient fulfills the indications for TPA, it’s up to the physician and the patient to decide whether they are willing to accept the risk of an intracranial hemorrhage."
Dr. Menon and Dr. Strbian reported having no financial conflicts.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: Two new scores helped predict intracranial hemorrhage after TPA treatment with more than 70% accuracy.
Data Source: Each score was derived from and validated in large, heterogeneous groups of patients who received intravenous TPA after an ischemic stroke.
Disclosures: Dr. Menon and Dr. Strbian reported having no financial conflicts.
Nearly Half of U.S. Stroke Patients Remain Hypertensive
NEW ORLEANS – Nearly half of adult Americans who self-reported a history of stroke also had poorly-controlled hypertension, based on a review of data collected from a U.S. nationwide sample during 1999-2004.
In addition, another 8% of stroke survivors had undiagnosed hypertension, Dr. Amytis Towfighi and her associates reported in a poster at the International Stroke Conference.
"Several medical and lifestyle modification factors could be potential targets of intervention to bridge this evidence practice gap," said Dr. Towfighi, a neurologist at the University of Southern California, Los Angeles and director of the acute neurology/acute stroke unit at Rancho Los Amigos National Rehabilitation Center in Downey, Calif.
Dr. Towfighi and her associates used data collected in the National Health and Nutrition Examination Survey (NHANES) during 1999-2004. Among the 9,145 Americans aged 40 years or older included in the NHANES surveys during those years, 490 (5%) self-reported a history of stroke.
Within this subgroup of stroke survivors, 72% said that they had been diagnosed with hypertension, and 47% had hypertension that was poorly controlled, with a blood pressure at the time of NHANES data collected that exceeded 140/90 mm Hg. An additional 8% of the stroke survivors had hypertension based on their NHANES data but did not self-report a previous diagnosis of hypertension.
Mortality follow-up of the 490 stroke patients through the end of 2006 showed that the mortality rates of these people did not differ significantly regardless of their initially measured blood pressure in the NHANES survey, nor by the number of antihypertensive medications these people reported receiving at baseline.
A multivariate analysis that adjusted for several demographic and clinical features identified Hispanic ethnicity, female sex, and diabetes as correlates of poor hypertension control, while hyperlipidemia and male gender were correlates of stroke patients not receiving any antihypertensive medications.
Dr. Towfighi said that she had no disclosures.
NEW ORLEANS – Nearly half of adult Americans who self-reported a history of stroke also had poorly-controlled hypertension, based on a review of data collected from a U.S. nationwide sample during 1999-2004.
In addition, another 8% of stroke survivors had undiagnosed hypertension, Dr. Amytis Towfighi and her associates reported in a poster at the International Stroke Conference.
"Several medical and lifestyle modification factors could be potential targets of intervention to bridge this evidence practice gap," said Dr. Towfighi, a neurologist at the University of Southern California, Los Angeles and director of the acute neurology/acute stroke unit at Rancho Los Amigos National Rehabilitation Center in Downey, Calif.
Dr. Towfighi and her associates used data collected in the National Health and Nutrition Examination Survey (NHANES) during 1999-2004. Among the 9,145 Americans aged 40 years or older included in the NHANES surveys during those years, 490 (5%) self-reported a history of stroke.
Within this subgroup of stroke survivors, 72% said that they had been diagnosed with hypertension, and 47% had hypertension that was poorly controlled, with a blood pressure at the time of NHANES data collected that exceeded 140/90 mm Hg. An additional 8% of the stroke survivors had hypertension based on their NHANES data but did not self-report a previous diagnosis of hypertension.
Mortality follow-up of the 490 stroke patients through the end of 2006 showed that the mortality rates of these people did not differ significantly regardless of their initially measured blood pressure in the NHANES survey, nor by the number of antihypertensive medications these people reported receiving at baseline.
A multivariate analysis that adjusted for several demographic and clinical features identified Hispanic ethnicity, female sex, and diabetes as correlates of poor hypertension control, while hyperlipidemia and male gender were correlates of stroke patients not receiving any antihypertensive medications.
Dr. Towfighi said that she had no disclosures.
NEW ORLEANS – Nearly half of adult Americans who self-reported a history of stroke also had poorly-controlled hypertension, based on a review of data collected from a U.S. nationwide sample during 1999-2004.
In addition, another 8% of stroke survivors had undiagnosed hypertension, Dr. Amytis Towfighi and her associates reported in a poster at the International Stroke Conference.
"Several medical and lifestyle modification factors could be potential targets of intervention to bridge this evidence practice gap," said Dr. Towfighi, a neurologist at the University of Southern California, Los Angeles and director of the acute neurology/acute stroke unit at Rancho Los Amigos National Rehabilitation Center in Downey, Calif.
Dr. Towfighi and her associates used data collected in the National Health and Nutrition Examination Survey (NHANES) during 1999-2004. Among the 9,145 Americans aged 40 years or older included in the NHANES surveys during those years, 490 (5%) self-reported a history of stroke.
Within this subgroup of stroke survivors, 72% said that they had been diagnosed with hypertension, and 47% had hypertension that was poorly controlled, with a blood pressure at the time of NHANES data collected that exceeded 140/90 mm Hg. An additional 8% of the stroke survivors had hypertension based on their NHANES data but did not self-report a previous diagnosis of hypertension.
Mortality follow-up of the 490 stroke patients through the end of 2006 showed that the mortality rates of these people did not differ significantly regardless of their initially measured blood pressure in the NHANES survey, nor by the number of antihypertensive medications these people reported receiving at baseline.
A multivariate analysis that adjusted for several demographic and clinical features identified Hispanic ethnicity, female sex, and diabetes as correlates of poor hypertension control, while hyperlipidemia and male gender were correlates of stroke patients not receiving any antihypertensive medications.
Dr. Towfighi said that she had no disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: Among a representative sample of 490 Americans aged 40 years or older with a history of stroke, 47% had uncontrolled hypertension.
Data Source: An analysis of data from the 1999-2004 National Health and Nutrition Examination Survey.
Disclosures: Dr. Towfighi said that she had no disclosures.
Clot-Busters Safely Treated Wake-Up Stroke Patients
NEW ORLEANS – A review of a stroke registry shows that it may be safe to give clot-busting therapy to "wake-up" stroke patients who have the same clinical and imaging features as those of patients who are traditionally considered eligible for the therapies.
Clinicians have traditionally shied away from giving clot-busters to patients who have stroke symptoms upon awakening because the time of onset is generally unknown. But many of these patients likely have experienced the stroke within a few hours of arriving for treatment and could benefit from a clot-busting intervention, Dr. Dulka Manawadu said during a press briefing at the International Stroke Conference.
Determining how to identify which wake-up patients could benefit "is an area of growing importance because it may allow us to extend the indication for this effective treatment," said Dr. Manawadu, a stroke and general medicine consultant at King’s College Hospital in London.
Dr. Manawadu and her colleagues at King’s College analyzed patients in the hospital’s stroke registry who received alteplase (Activase) between January 2009 and December 2010. The study analyzed data for 326 unselected and consecutive patients with a stroke onset of 0 to 4.5 hours with a National Institute of Health Stroke Scale (NIHSS) score of 5 or greater, and 68 unselected, consecutive patients who awoke with symptoms, had an NIHSS of 5 or greater, and had an unknown time of onset.
At King’s College Hospital, clinicians can give thrombolysis to wake-up patients on a compassionate basis. Decisions are made on a case-by-case basis, and treatment is given with consent, said Dr. Manawadu. The patient’s history, clinical signs of stroke, and scan findings are all taken into consideration. Outside of the time constraint, wake-up patients have to meet all other eligibility criteria for clot-busting treatment. A nonenhanced CT scan must show an Alberta Stroke Program Early CT Score (ASPECTS) of 7 or greater. CT Perfusion (CTP) mismatch was also used to assess eligibility in some wake-up patients, but was not mandatory.
The mean age was 73 years for both arms. There was no significant difference between the groups in most baseline characteristics. However, there was significantly greater use of CTP in the wake-up group: 44 patients, or 65%, compared with 84 patients, or 26% of the comparator group. The wake-up patients also had a higher incidence of large-vessel stroke.
The outcomes were statistically similar for both groups, except for mean NIHSS scores at 24 hours. The mean score was significantly lower for the wake-up group than for the reference group (7.2 vs. 11.5). The rates were similar between the groups for having any intracranial hemorrhage ([ICH], 22% vs. 20%, respectively) or symptomatic ICH (2.9% vs. 3.4%). At 3 months after stroke, wake-up patients had a lower death rate (15% vs. 25%).
When the data for wake-up stroke patients were stratified by age, patients older than 80 years fared significantly worse than did those under 80.
The study was limited in that it was retrospective, but it did include all comers, Dr. Manawadu said. Despite the findings from the registry analysis, there have been no changes in protocol at King’s College; wake-up patients are still given thrombolysis on a case-by-case basis, she said.
Dr. Lee H. Schwamm, director of the telestroke and acute stroke services at Massachusetts General Hospital, Boston, said that parts of the study are reassuring and consistent with what would be expected. A surprising finding was that "the use of CT scanning alone was able to identify this cohort safely, and they could be treated with similar outcomes."
It is surprising in part because CT generally is not very sensitive in detecting ischemic stroke. If the findings hold up, it would be important because CT is more widely available than is MRI, Dr. Schwamm said in an interview.
Currently, few American clinicians are using clot-busting therapy for wake-up patients, and it would be premature to use the King’s College study as a basis for thrombolysis in those patients, Dr. Schwamm said.
The conference was sponsored by the American Heart Association. The study was funded by the Institutional Research and Development Board at King’s College Hospital, London. Dr. Manawadu and her colleagues reported having no relevant disclosures.
NEW ORLEANS – A review of a stroke registry shows that it may be safe to give clot-busting therapy to "wake-up" stroke patients who have the same clinical and imaging features as those of patients who are traditionally considered eligible for the therapies.
Clinicians have traditionally shied away from giving clot-busters to patients who have stroke symptoms upon awakening because the time of onset is generally unknown. But many of these patients likely have experienced the stroke within a few hours of arriving for treatment and could benefit from a clot-busting intervention, Dr. Dulka Manawadu said during a press briefing at the International Stroke Conference.
Determining how to identify which wake-up patients could benefit "is an area of growing importance because it may allow us to extend the indication for this effective treatment," said Dr. Manawadu, a stroke and general medicine consultant at King’s College Hospital in London.
Dr. Manawadu and her colleagues at King’s College analyzed patients in the hospital’s stroke registry who received alteplase (Activase) between January 2009 and December 2010. The study analyzed data for 326 unselected and consecutive patients with a stroke onset of 0 to 4.5 hours with a National Institute of Health Stroke Scale (NIHSS) score of 5 or greater, and 68 unselected, consecutive patients who awoke with symptoms, had an NIHSS of 5 or greater, and had an unknown time of onset.
At King’s College Hospital, clinicians can give thrombolysis to wake-up patients on a compassionate basis. Decisions are made on a case-by-case basis, and treatment is given with consent, said Dr. Manawadu. The patient’s history, clinical signs of stroke, and scan findings are all taken into consideration. Outside of the time constraint, wake-up patients have to meet all other eligibility criteria for clot-busting treatment. A nonenhanced CT scan must show an Alberta Stroke Program Early CT Score (ASPECTS) of 7 or greater. CT Perfusion (CTP) mismatch was also used to assess eligibility in some wake-up patients, but was not mandatory.
The mean age was 73 years for both arms. There was no significant difference between the groups in most baseline characteristics. However, there was significantly greater use of CTP in the wake-up group: 44 patients, or 65%, compared with 84 patients, or 26% of the comparator group. The wake-up patients also had a higher incidence of large-vessel stroke.
The outcomes were statistically similar for both groups, except for mean NIHSS scores at 24 hours. The mean score was significantly lower for the wake-up group than for the reference group (7.2 vs. 11.5). The rates were similar between the groups for having any intracranial hemorrhage ([ICH], 22% vs. 20%, respectively) or symptomatic ICH (2.9% vs. 3.4%). At 3 months after stroke, wake-up patients had a lower death rate (15% vs. 25%).
When the data for wake-up stroke patients were stratified by age, patients older than 80 years fared significantly worse than did those under 80.
The study was limited in that it was retrospective, but it did include all comers, Dr. Manawadu said. Despite the findings from the registry analysis, there have been no changes in protocol at King’s College; wake-up patients are still given thrombolysis on a case-by-case basis, she said.
Dr. Lee H. Schwamm, director of the telestroke and acute stroke services at Massachusetts General Hospital, Boston, said that parts of the study are reassuring and consistent with what would be expected. A surprising finding was that "the use of CT scanning alone was able to identify this cohort safely, and they could be treated with similar outcomes."
It is surprising in part because CT generally is not very sensitive in detecting ischemic stroke. If the findings hold up, it would be important because CT is more widely available than is MRI, Dr. Schwamm said in an interview.
Currently, few American clinicians are using clot-busting therapy for wake-up patients, and it would be premature to use the King’s College study as a basis for thrombolysis in those patients, Dr. Schwamm said.
The conference was sponsored by the American Heart Association. The study was funded by the Institutional Research and Development Board at King’s College Hospital, London. Dr. Manawadu and her colleagues reported having no relevant disclosures.
NEW ORLEANS – A review of a stroke registry shows that it may be safe to give clot-busting therapy to "wake-up" stroke patients who have the same clinical and imaging features as those of patients who are traditionally considered eligible for the therapies.
Clinicians have traditionally shied away from giving clot-busters to patients who have stroke symptoms upon awakening because the time of onset is generally unknown. But many of these patients likely have experienced the stroke within a few hours of arriving for treatment and could benefit from a clot-busting intervention, Dr. Dulka Manawadu said during a press briefing at the International Stroke Conference.
Determining how to identify which wake-up patients could benefit "is an area of growing importance because it may allow us to extend the indication for this effective treatment," said Dr. Manawadu, a stroke and general medicine consultant at King’s College Hospital in London.
Dr. Manawadu and her colleagues at King’s College analyzed patients in the hospital’s stroke registry who received alteplase (Activase) between January 2009 and December 2010. The study analyzed data for 326 unselected and consecutive patients with a stroke onset of 0 to 4.5 hours with a National Institute of Health Stroke Scale (NIHSS) score of 5 or greater, and 68 unselected, consecutive patients who awoke with symptoms, had an NIHSS of 5 or greater, and had an unknown time of onset.
At King’s College Hospital, clinicians can give thrombolysis to wake-up patients on a compassionate basis. Decisions are made on a case-by-case basis, and treatment is given with consent, said Dr. Manawadu. The patient’s history, clinical signs of stroke, and scan findings are all taken into consideration. Outside of the time constraint, wake-up patients have to meet all other eligibility criteria for clot-busting treatment. A nonenhanced CT scan must show an Alberta Stroke Program Early CT Score (ASPECTS) of 7 or greater. CT Perfusion (CTP) mismatch was also used to assess eligibility in some wake-up patients, but was not mandatory.
The mean age was 73 years for both arms. There was no significant difference between the groups in most baseline characteristics. However, there was significantly greater use of CTP in the wake-up group: 44 patients, or 65%, compared with 84 patients, or 26% of the comparator group. The wake-up patients also had a higher incidence of large-vessel stroke.
The outcomes were statistically similar for both groups, except for mean NIHSS scores at 24 hours. The mean score was significantly lower for the wake-up group than for the reference group (7.2 vs. 11.5). The rates were similar between the groups for having any intracranial hemorrhage ([ICH], 22% vs. 20%, respectively) or symptomatic ICH (2.9% vs. 3.4%). At 3 months after stroke, wake-up patients had a lower death rate (15% vs. 25%).
When the data for wake-up stroke patients were stratified by age, patients older than 80 years fared significantly worse than did those under 80.
The study was limited in that it was retrospective, but it did include all comers, Dr. Manawadu said. Despite the findings from the registry analysis, there have been no changes in protocol at King’s College; wake-up patients are still given thrombolysis on a case-by-case basis, she said.
Dr. Lee H. Schwamm, director of the telestroke and acute stroke services at Massachusetts General Hospital, Boston, said that parts of the study are reassuring and consistent with what would be expected. A surprising finding was that "the use of CT scanning alone was able to identify this cohort safely, and they could be treated with similar outcomes."
It is surprising in part because CT generally is not very sensitive in detecting ischemic stroke. If the findings hold up, it would be important because CT is more widely available than is MRI, Dr. Schwamm said in an interview.
Currently, few American clinicians are using clot-busting therapy for wake-up patients, and it would be premature to use the King’s College study as a basis for thrombolysis in those patients, Dr. Schwamm said.
The conference was sponsored by the American Heart Association. The study was funded by the Institutional Research and Development Board at King’s College Hospital, London. Dr. Manawadu and her colleagues reported having no relevant disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: The mean score was significantly lower for the wake-up group than for the reference group (7.2 vs. 11.5, respectively), but a similar percentage of patients in each group died at 3 months (15% vs. 25%).
Data Source: A retrospective analysis of a single-center registry of 326 unselected, consecutive patients who presented within 4.5 hours of stroke onset and 68 unselected, consecutive patients who awoke with stroke symptoms.
Disclosures: The study was funded by the Institutional Research and Development Board at King’s College Hospital, London. Dr. Manawadu and her colleagues reported having no relevant disclosures.
Framingham Score Flags MI Risk After Stroke
NEW ORLEANS – An elevated Framingham Risk Score identified recent ischemic stroke patients at high risk for a myocardial infarction or vascular death over the next 2 years, in a retrospective analysis of data from more than 2,500 stroke survivors without documented coronary heart disease.
If prospective study results confirm this finding, it could establish the Framingham Risk Score (FRS) as an important prognostic assessment for patients with a recent ischemic stroke, Dr. Amytis Towfighi said at the International Stroke Conference. If used this way, the FRS could identify stroke survivors who would benefit from more intensive risk-factor modification, she said.
"Considering every stroke to be a coronary risk equivalent could expose patients with a low risk for subsequent coronary events to unnecessary treatment," said Dr. Towfighi, a neurologist at the University of Southern California in Los Angeles and director of the acute neurology/acute stroke unit at Rancho Los Amigos National Rehabilitation Center in Downey, Calif.
"Unlike coronary atherosclerosis, there are lots of different causes of stroke, including nonatherosclerotic mechanisms.
"Many stroke survivors harbor asymptomatic coronary heart disease, and beyond the acute period they are often at higher risk for cardiac death than for recurrent cerebrovascular events," she said. Identifying patients with high FRSs of 20% or greater could flag those who would benefit from treatment with a beta- blocker; coronary diagnostic imaging; coronary revascularization; or more intensive vascular risk reduction by reducing body mass index, lowering triglycerides, exercising tighter diabetes management, and smoking cessation.
"Today, the FRS is usually not calculated for stroke patients," Dr. Towfighi said in an interview. "Part of the issue is that the FRS was initially developed for people who had not yet had a [cardiovascular] event, so it has not been studied in patients who had a stroke. We didn’t know going into our study whether or not a high FRS would predict a myocardial infarction or vascular death in patients who had a stroke."
To test whether the FRS could help stratify coronary risk in stroke patients, Dr. Towfighi and her associates analyzed data collected from 3,509 patients who had a recent ischemic stroke and who participated in the Vitamin Intervention for Stroke Prevention trial during 1996-2003 (JAMA 2004;291:565-75). The analysis primarily focused on the 2,547 patients from this group who did not have documented coronary heart disease at the time of their enrollment.
Calculation of the FRS for these patients identified 933 (37%) with a score of 20% or greater, and 1,614 (63%) with a score of less than 20%.
The researchers then tallied the rates of myocardial infarction, vascular death, or stroke in these two subgroups during 2 years of follow-up, and compared the rates between the two FRS groups in a multivariate analysis that controlled for baseline differences in age, race, prior stroke, body mass index, heart failure, carotid endarterectomy, stroke severity, alcohol use, low-density lipoprotein cholesterol, triglyceride levels, mean systolic blood pressure while in the study, antidyslipidemia drug treatment, antithrombotic drug treatment.
In this analysis, patients with an FRS of 20% or greater at baseline had a 2.8-fold increased risk for a myocardial infarction during 2 years of follow-up compared with patients with a lower FRS. The risk of vascular death during follow-up was 80% higher in the high-FRS subgroup than in patients with a lower score. However, a higher FRS had no link with an increased risk for a subsequent stroke.
In addition, the FRS components most strongly linked to subsequent myocardial infarction risk were smoking, which raised the risk by 70%, and diabetes, which doubled the risk.
These findings are not conclusive because they came from a retrospective analysis and may have been influenced by unmeasured confounding. Ideally, the findings need confirmation in a prospective study, Dr. Towfighi said.
Despite this limitation, Dr. Towfighi noted that she and her associates at Rancho Los Amigos now calculate an FRS for their stroke patients "because we feel it’s useful," she said at the meeting, which was sponsored by the American Heart Association.
Dr. Towfighi said that she had no disclosures.
NEW ORLEANS – An elevated Framingham Risk Score identified recent ischemic stroke patients at high risk for a myocardial infarction or vascular death over the next 2 years, in a retrospective analysis of data from more than 2,500 stroke survivors without documented coronary heart disease.
If prospective study results confirm this finding, it could establish the Framingham Risk Score (FRS) as an important prognostic assessment for patients with a recent ischemic stroke, Dr. Amytis Towfighi said at the International Stroke Conference. If used this way, the FRS could identify stroke survivors who would benefit from more intensive risk-factor modification, she said.
"Considering every stroke to be a coronary risk equivalent could expose patients with a low risk for subsequent coronary events to unnecessary treatment," said Dr. Towfighi, a neurologist at the University of Southern California in Los Angeles and director of the acute neurology/acute stroke unit at Rancho Los Amigos National Rehabilitation Center in Downey, Calif.
"Unlike coronary atherosclerosis, there are lots of different causes of stroke, including nonatherosclerotic mechanisms.
"Many stroke survivors harbor asymptomatic coronary heart disease, and beyond the acute period they are often at higher risk for cardiac death than for recurrent cerebrovascular events," she said. Identifying patients with high FRSs of 20% or greater could flag those who would benefit from treatment with a beta- blocker; coronary diagnostic imaging; coronary revascularization; or more intensive vascular risk reduction by reducing body mass index, lowering triglycerides, exercising tighter diabetes management, and smoking cessation.
"Today, the FRS is usually not calculated for stroke patients," Dr. Towfighi said in an interview. "Part of the issue is that the FRS was initially developed for people who had not yet had a [cardiovascular] event, so it has not been studied in patients who had a stroke. We didn’t know going into our study whether or not a high FRS would predict a myocardial infarction or vascular death in patients who had a stroke."
To test whether the FRS could help stratify coronary risk in stroke patients, Dr. Towfighi and her associates analyzed data collected from 3,509 patients who had a recent ischemic stroke and who participated in the Vitamin Intervention for Stroke Prevention trial during 1996-2003 (JAMA 2004;291:565-75). The analysis primarily focused on the 2,547 patients from this group who did not have documented coronary heart disease at the time of their enrollment.
Calculation of the FRS for these patients identified 933 (37%) with a score of 20% or greater, and 1,614 (63%) with a score of less than 20%.
The researchers then tallied the rates of myocardial infarction, vascular death, or stroke in these two subgroups during 2 years of follow-up, and compared the rates between the two FRS groups in a multivariate analysis that controlled for baseline differences in age, race, prior stroke, body mass index, heart failure, carotid endarterectomy, stroke severity, alcohol use, low-density lipoprotein cholesterol, triglyceride levels, mean systolic blood pressure while in the study, antidyslipidemia drug treatment, antithrombotic drug treatment.
In this analysis, patients with an FRS of 20% or greater at baseline had a 2.8-fold increased risk for a myocardial infarction during 2 years of follow-up compared with patients with a lower FRS. The risk of vascular death during follow-up was 80% higher in the high-FRS subgroup than in patients with a lower score. However, a higher FRS had no link with an increased risk for a subsequent stroke.
In addition, the FRS components most strongly linked to subsequent myocardial infarction risk were smoking, which raised the risk by 70%, and diabetes, which doubled the risk.
These findings are not conclusive because they came from a retrospective analysis and may have been influenced by unmeasured confounding. Ideally, the findings need confirmation in a prospective study, Dr. Towfighi said.
Despite this limitation, Dr. Towfighi noted that she and her associates at Rancho Los Amigos now calculate an FRS for their stroke patients "because we feel it’s useful," she said at the meeting, which was sponsored by the American Heart Association.
Dr. Towfighi said that she had no disclosures.
NEW ORLEANS – An elevated Framingham Risk Score identified recent ischemic stroke patients at high risk for a myocardial infarction or vascular death over the next 2 years, in a retrospective analysis of data from more than 2,500 stroke survivors without documented coronary heart disease.
If prospective study results confirm this finding, it could establish the Framingham Risk Score (FRS) as an important prognostic assessment for patients with a recent ischemic stroke, Dr. Amytis Towfighi said at the International Stroke Conference. If used this way, the FRS could identify stroke survivors who would benefit from more intensive risk-factor modification, she said.
"Considering every stroke to be a coronary risk equivalent could expose patients with a low risk for subsequent coronary events to unnecessary treatment," said Dr. Towfighi, a neurologist at the University of Southern California in Los Angeles and director of the acute neurology/acute stroke unit at Rancho Los Amigos National Rehabilitation Center in Downey, Calif.
"Unlike coronary atherosclerosis, there are lots of different causes of stroke, including nonatherosclerotic mechanisms.
"Many stroke survivors harbor asymptomatic coronary heart disease, and beyond the acute period they are often at higher risk for cardiac death than for recurrent cerebrovascular events," she said. Identifying patients with high FRSs of 20% or greater could flag those who would benefit from treatment with a beta- blocker; coronary diagnostic imaging; coronary revascularization; or more intensive vascular risk reduction by reducing body mass index, lowering triglycerides, exercising tighter diabetes management, and smoking cessation.
"Today, the FRS is usually not calculated for stroke patients," Dr. Towfighi said in an interview. "Part of the issue is that the FRS was initially developed for people who had not yet had a [cardiovascular] event, so it has not been studied in patients who had a stroke. We didn’t know going into our study whether or not a high FRS would predict a myocardial infarction or vascular death in patients who had a stroke."
To test whether the FRS could help stratify coronary risk in stroke patients, Dr. Towfighi and her associates analyzed data collected from 3,509 patients who had a recent ischemic stroke and who participated in the Vitamin Intervention for Stroke Prevention trial during 1996-2003 (JAMA 2004;291:565-75). The analysis primarily focused on the 2,547 patients from this group who did not have documented coronary heart disease at the time of their enrollment.
Calculation of the FRS for these patients identified 933 (37%) with a score of 20% or greater, and 1,614 (63%) with a score of less than 20%.
The researchers then tallied the rates of myocardial infarction, vascular death, or stroke in these two subgroups during 2 years of follow-up, and compared the rates between the two FRS groups in a multivariate analysis that controlled for baseline differences in age, race, prior stroke, body mass index, heart failure, carotid endarterectomy, stroke severity, alcohol use, low-density lipoprotein cholesterol, triglyceride levels, mean systolic blood pressure while in the study, antidyslipidemia drug treatment, antithrombotic drug treatment.
In this analysis, patients with an FRS of 20% or greater at baseline had a 2.8-fold increased risk for a myocardial infarction during 2 years of follow-up compared with patients with a lower FRS. The risk of vascular death during follow-up was 80% higher in the high-FRS subgroup than in patients with a lower score. However, a higher FRS had no link with an increased risk for a subsequent stroke.
In addition, the FRS components most strongly linked to subsequent myocardial infarction risk were smoking, which raised the risk by 70%, and diabetes, which doubled the risk.
These findings are not conclusive because they came from a retrospective analysis and may have been influenced by unmeasured confounding. Ideally, the findings need confirmation in a prospective study, Dr. Towfighi said.
Despite this limitation, Dr. Towfighi noted that she and her associates at Rancho Los Amigos now calculate an FRS for their stroke patients "because we feel it’s useful," she said at the meeting, which was sponsored by the American Heart Association.
Dr. Towfighi said that she had no disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: In ischemic stroke patients with no coronary disease, a Framingham Risk Score of 20% or greater was associated with a threefold increased risk for myocardial infarction compared with lower-score patients.
Data Source: Retrospective analysis of data from 2,547 patients with a recent ischemic stroke, and no coronary heart disease.
Disclosures: Dr. Towfighi said that she had no disclosures.
Solitaire Trumped Merci in Stroke Clot Retrieval Trial
NEW ORLEANS – An investigational clot-retrieval device completely recanalized blocked cerebral arteries, improved neurologic outcomes, and significantly reduced mortality from acute ischemic stroke more often than did the widely used Merci device in a randomized, open-label trial.
The SWIFT (SOLITAIRE-FR With the Intention for Thrombectomy) study intended to randomize 200 patients with ischemic stroke to treatment with either of the devices, but after 18 months, the trial’s data safety monitoring board stopped the study with 144 patients treated, citing "an overwhelming benefit" of the Solitaire-Flow Restoration device over the Merci device, Dr. Jeffrey Saver said at the International Stroke Conference.
"With these results, we see for the first time a highly effective cerebral recanalization procedure for ischemic stroke," said Dr. Saver, director of the stroke unit at the University of California, Los Angeles, in an interview. "[Tissue plasminogen activator] opens the arteries partially 40% of the time and completely just 5% of the time. This device opens them completely 60% of the time. It’s a dramatic step forward and a device that will probably be a game-changer for our systems, once it becomes available."
Solitaire is a columnar metal cage which, when expanded, engages the clot at multiple points, facilitating extraction and decreasing the chance of symptomatic intracranial bleeding. The Merci device, a corkscrew-like coil, does release pressure on the atrial wall, "but has a tendency to uncoil and fail to engage the clot," he said.
The patients’ average age was 67 years; most (68%) were male. The median pre-stroke modified Rankin Scale score was 0.5. Time to first angiogram was 4-5 hours.
The primary end point – successful recanalization without symptomatic intracranial hemorrhage – occurred in 61% of the Solitaire group and 24% of the Merci group. This was a highly significant difference with a P value of .0001 in both noninferiority and superiority analyses.
The trial’s secondary end points also indicated the superiority and noninferiority of Solitaire in comparison with Merci:
• Use of rescue therapy (21% vs. 44%).
• Symptomatic intracranial hemorrhage (2% vs. 11%).
• All intracranial hemorrhage (17% vs. 38%).
• Good 90-day neurologic outcome (58% vs. 33%).
• 90-day mortality (17% vs. 38%).
The investigators defined a good neurologic outcome as a modified Rankin Scale score of 0-2 if a patient came in at that level, if the patient returned to their baseline score after coming in with a worse score, or for patients who achieved at least a 10-point increase in their National Institutes of Health Stroke Scale score also were assessed as having a good outcome.
The results demonstrate an excellent number-needed-to-treat analysis, Dr. Saver said.
"For every 2.7 patients treated, 1 additional patient had a successful recanalization with no brain bleed. For every five patients treated, one patient was saved from death. And for every four patients treated, there was one more with a good neurologic outcome."
Device fracture occurred in two instances with Solitaire but in no cases with Merci. There were two instances with each device in which the device could not be delivered appropriately. Air embolism occurred in one patient with each device. Vasospasms occurred in eight cases with Solitaire and six cases with Merci. Two patients undergoing interventions with the Merci device had vessel perforations, compared with none who were treated with the Solitaire device. Distal emboli occurred with Solitaire in two patients and with Merci in three patients.
The study design included a "roll-in" period in which the investigators learned how to perform the interventions; 31 patients were treated during this time, while the remaining 113 were randomized to treatment with the Merci device (55) or the Solitaire (58). All of the patients either were ineligible for tissue plasminogen activator or had failed a treatment.
Each patient received up to three passes of the assigned device. A separate lab, blinded to treatment arms, reviewed scans of the treated arteries. If the clot remained, physicians could try other appropriate treatments.
While SWIFT decisively proved Solitaire’s capability as a clot retriever, it didn’t have the chance to explore the device’s other possible indication as a stent, Dr. Saver added.
‘This device is designed to pull the clot out or, if that fails, you can detach the stent portion and leave it in place. But what we experienced in this study was that it was getting the clot out so often, we weren’t able to test it as a stenting device."
In fact, for some patients, Solitaire may be more suited as a stent than as a retrieval device. "I think that for a patient who has plaques in the intracranial arteries and a little bit of clot on top of the plaque, retrieval is not the best strategy. The devices tend to snag on the hard edges of the plaque. Those patients are best treated with a stenting approach, which may possible with his device."
Solitaire has already been approved in Europe. These new data should help propel it to a U.S. approval, he said.
"The data from this trial have been submitted to the Food and Drug Administration, and we are hopeful that within a few weeks or months these results will allow the FDA to approve it in the U.S."
Ev3, the company that makes Solitaire, funded the SWIFT trial. Dr. Saver disclosed that he is on the company’s speakers advisory board.
This trial brings us into the third generation of new and potentially better devices to open blocked arteries in acute ischemic stroke. Solitaire showed pretty impressive recanalization rates, compared with Merci, and improved clinical and functional outcomes.
This device also looks at little easier to deploy than Merci, and it seems to be somewhat more practical and better-designed.
Dr. Ralph L. Sacco |
I think the SWIFT trial will bring very important information to the FDA review, and hopefully we will have another device entering the market to help us treat acute stroke.
But even after interventionists get the Solitaire device, they must be well trained before they start using it. Additionally, devices like this are primarily used in comprehensive stroke centers. To increase the number of patients who might benefit from it, we need to increase the numbers of our comprehensive stroke centers.
Dr. Ralph L. Sacco is the immediate past president of the American Heart Association and chairman of neurology at the University of Miami. He had no relevant disclosures.
This trial brings us into the third generation of new and potentially better devices to open blocked arteries in acute ischemic stroke. Solitaire showed pretty impressive recanalization rates, compared with Merci, and improved clinical and functional outcomes.
This device also looks at little easier to deploy than Merci, and it seems to be somewhat more practical and better-designed.
Dr. Ralph L. Sacco |
I think the SWIFT trial will bring very important information to the FDA review, and hopefully we will have another device entering the market to help us treat acute stroke.
But even after interventionists get the Solitaire device, they must be well trained before they start using it. Additionally, devices like this are primarily used in comprehensive stroke centers. To increase the number of patients who might benefit from it, we need to increase the numbers of our comprehensive stroke centers.
Dr. Ralph L. Sacco is the immediate past president of the American Heart Association and chairman of neurology at the University of Miami. He had no relevant disclosures.
This trial brings us into the third generation of new and potentially better devices to open blocked arteries in acute ischemic stroke. Solitaire showed pretty impressive recanalization rates, compared with Merci, and improved clinical and functional outcomes.
This device also looks at little easier to deploy than Merci, and it seems to be somewhat more practical and better-designed.
Dr. Ralph L. Sacco |
I think the SWIFT trial will bring very important information to the FDA review, and hopefully we will have another device entering the market to help us treat acute stroke.
But even after interventionists get the Solitaire device, they must be well trained before they start using it. Additionally, devices like this are primarily used in comprehensive stroke centers. To increase the number of patients who might benefit from it, we need to increase the numbers of our comprehensive stroke centers.
Dr. Ralph L. Sacco is the immediate past president of the American Heart Association and chairman of neurology at the University of Miami. He had no relevant disclosures.
NEW ORLEANS – An investigational clot-retrieval device completely recanalized blocked cerebral arteries, improved neurologic outcomes, and significantly reduced mortality from acute ischemic stroke more often than did the widely used Merci device in a randomized, open-label trial.
The SWIFT (SOLITAIRE-FR With the Intention for Thrombectomy) study intended to randomize 200 patients with ischemic stroke to treatment with either of the devices, but after 18 months, the trial’s data safety monitoring board stopped the study with 144 patients treated, citing "an overwhelming benefit" of the Solitaire-Flow Restoration device over the Merci device, Dr. Jeffrey Saver said at the International Stroke Conference.
"With these results, we see for the first time a highly effective cerebral recanalization procedure for ischemic stroke," said Dr. Saver, director of the stroke unit at the University of California, Los Angeles, in an interview. "[Tissue plasminogen activator] opens the arteries partially 40% of the time and completely just 5% of the time. This device opens them completely 60% of the time. It’s a dramatic step forward and a device that will probably be a game-changer for our systems, once it becomes available."
Solitaire is a columnar metal cage which, when expanded, engages the clot at multiple points, facilitating extraction and decreasing the chance of symptomatic intracranial bleeding. The Merci device, a corkscrew-like coil, does release pressure on the atrial wall, "but has a tendency to uncoil and fail to engage the clot," he said.
The patients’ average age was 67 years; most (68%) were male. The median pre-stroke modified Rankin Scale score was 0.5. Time to first angiogram was 4-5 hours.
The primary end point – successful recanalization without symptomatic intracranial hemorrhage – occurred in 61% of the Solitaire group and 24% of the Merci group. This was a highly significant difference with a P value of .0001 in both noninferiority and superiority analyses.
The trial’s secondary end points also indicated the superiority and noninferiority of Solitaire in comparison with Merci:
• Use of rescue therapy (21% vs. 44%).
• Symptomatic intracranial hemorrhage (2% vs. 11%).
• All intracranial hemorrhage (17% vs. 38%).
• Good 90-day neurologic outcome (58% vs. 33%).
• 90-day mortality (17% vs. 38%).
The investigators defined a good neurologic outcome as a modified Rankin Scale score of 0-2 if a patient came in at that level, if the patient returned to their baseline score after coming in with a worse score, or for patients who achieved at least a 10-point increase in their National Institutes of Health Stroke Scale score also were assessed as having a good outcome.
The results demonstrate an excellent number-needed-to-treat analysis, Dr. Saver said.
"For every 2.7 patients treated, 1 additional patient had a successful recanalization with no brain bleed. For every five patients treated, one patient was saved from death. And for every four patients treated, there was one more with a good neurologic outcome."
Device fracture occurred in two instances with Solitaire but in no cases with Merci. There were two instances with each device in which the device could not be delivered appropriately. Air embolism occurred in one patient with each device. Vasospasms occurred in eight cases with Solitaire and six cases with Merci. Two patients undergoing interventions with the Merci device had vessel perforations, compared with none who were treated with the Solitaire device. Distal emboli occurred with Solitaire in two patients and with Merci in three patients.
The study design included a "roll-in" period in which the investigators learned how to perform the interventions; 31 patients were treated during this time, while the remaining 113 were randomized to treatment with the Merci device (55) or the Solitaire (58). All of the patients either were ineligible for tissue plasminogen activator or had failed a treatment.
Each patient received up to three passes of the assigned device. A separate lab, blinded to treatment arms, reviewed scans of the treated arteries. If the clot remained, physicians could try other appropriate treatments.
While SWIFT decisively proved Solitaire’s capability as a clot retriever, it didn’t have the chance to explore the device’s other possible indication as a stent, Dr. Saver added.
‘This device is designed to pull the clot out or, if that fails, you can detach the stent portion and leave it in place. But what we experienced in this study was that it was getting the clot out so often, we weren’t able to test it as a stenting device."
In fact, for some patients, Solitaire may be more suited as a stent than as a retrieval device. "I think that for a patient who has plaques in the intracranial arteries and a little bit of clot on top of the plaque, retrieval is not the best strategy. The devices tend to snag on the hard edges of the plaque. Those patients are best treated with a stenting approach, which may possible with his device."
Solitaire has already been approved in Europe. These new data should help propel it to a U.S. approval, he said.
"The data from this trial have been submitted to the Food and Drug Administration, and we are hopeful that within a few weeks or months these results will allow the FDA to approve it in the U.S."
Ev3, the company that makes Solitaire, funded the SWIFT trial. Dr. Saver disclosed that he is on the company’s speakers advisory board.
NEW ORLEANS – An investigational clot-retrieval device completely recanalized blocked cerebral arteries, improved neurologic outcomes, and significantly reduced mortality from acute ischemic stroke more often than did the widely used Merci device in a randomized, open-label trial.
The SWIFT (SOLITAIRE-FR With the Intention for Thrombectomy) study intended to randomize 200 patients with ischemic stroke to treatment with either of the devices, but after 18 months, the trial’s data safety monitoring board stopped the study with 144 patients treated, citing "an overwhelming benefit" of the Solitaire-Flow Restoration device over the Merci device, Dr. Jeffrey Saver said at the International Stroke Conference.
"With these results, we see for the first time a highly effective cerebral recanalization procedure for ischemic stroke," said Dr. Saver, director of the stroke unit at the University of California, Los Angeles, in an interview. "[Tissue plasminogen activator] opens the arteries partially 40% of the time and completely just 5% of the time. This device opens them completely 60% of the time. It’s a dramatic step forward and a device that will probably be a game-changer for our systems, once it becomes available."
Solitaire is a columnar metal cage which, when expanded, engages the clot at multiple points, facilitating extraction and decreasing the chance of symptomatic intracranial bleeding. The Merci device, a corkscrew-like coil, does release pressure on the atrial wall, "but has a tendency to uncoil and fail to engage the clot," he said.
The patients’ average age was 67 years; most (68%) were male. The median pre-stroke modified Rankin Scale score was 0.5. Time to first angiogram was 4-5 hours.
The primary end point – successful recanalization without symptomatic intracranial hemorrhage – occurred in 61% of the Solitaire group and 24% of the Merci group. This was a highly significant difference with a P value of .0001 in both noninferiority and superiority analyses.
The trial’s secondary end points also indicated the superiority and noninferiority of Solitaire in comparison with Merci:
• Use of rescue therapy (21% vs. 44%).
• Symptomatic intracranial hemorrhage (2% vs. 11%).
• All intracranial hemorrhage (17% vs. 38%).
• Good 90-day neurologic outcome (58% vs. 33%).
• 90-day mortality (17% vs. 38%).
The investigators defined a good neurologic outcome as a modified Rankin Scale score of 0-2 if a patient came in at that level, if the patient returned to their baseline score after coming in with a worse score, or for patients who achieved at least a 10-point increase in their National Institutes of Health Stroke Scale score also were assessed as having a good outcome.
The results demonstrate an excellent number-needed-to-treat analysis, Dr. Saver said.
"For every 2.7 patients treated, 1 additional patient had a successful recanalization with no brain bleed. For every five patients treated, one patient was saved from death. And for every four patients treated, there was one more with a good neurologic outcome."
Device fracture occurred in two instances with Solitaire but in no cases with Merci. There were two instances with each device in which the device could not be delivered appropriately. Air embolism occurred in one patient with each device. Vasospasms occurred in eight cases with Solitaire and six cases with Merci. Two patients undergoing interventions with the Merci device had vessel perforations, compared with none who were treated with the Solitaire device. Distal emboli occurred with Solitaire in two patients and with Merci in three patients.
The study design included a "roll-in" period in which the investigators learned how to perform the interventions; 31 patients were treated during this time, while the remaining 113 were randomized to treatment with the Merci device (55) or the Solitaire (58). All of the patients either were ineligible for tissue plasminogen activator or had failed a treatment.
Each patient received up to three passes of the assigned device. A separate lab, blinded to treatment arms, reviewed scans of the treated arteries. If the clot remained, physicians could try other appropriate treatments.
While SWIFT decisively proved Solitaire’s capability as a clot retriever, it didn’t have the chance to explore the device’s other possible indication as a stent, Dr. Saver added.
‘This device is designed to pull the clot out or, if that fails, you can detach the stent portion and leave it in place. But what we experienced in this study was that it was getting the clot out so often, we weren’t able to test it as a stenting device."
In fact, for some patients, Solitaire may be more suited as a stent than as a retrieval device. "I think that for a patient who has plaques in the intracranial arteries and a little bit of clot on top of the plaque, retrieval is not the best strategy. The devices tend to snag on the hard edges of the plaque. Those patients are best treated with a stenting approach, which may possible with his device."
Solitaire has already been approved in Europe. These new data should help propel it to a U.S. approval, he said.
"The data from this trial have been submitted to the Food and Drug Administration, and we are hopeful that within a few weeks or months these results will allow the FDA to approve it in the U.S."
Ev3, the company that makes Solitaire, funded the SWIFT trial. Dr. Saver disclosed that he is on the company’s speakers advisory board.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: Successful recanalization without symptomatic intracranial hemorrhage occurred in a significantly larger proportion of patients who received treatment with Solitaire than did with Merci (61% vs. 24%).
Data Source: SWIFT randomized 113 patients with acute ischemic stroke to treatment with the investigational Solitaire device or the approved Merci device.
Disclosures: Ev3, the company that makes Solitaire, funded the SWIFT trial. Dr. Saver disclosed that he is on the company’s speakers advisory board.
Aspirin, Warfarin Show Equal Heart Failure Efficacy
NEW ORLEANS – The largest, best controlled comparison of aspirin and warfarin ever done in patients with heart failure showed no overall difference between the two drugs for preventing thromboembolic events that cause death or stroke.
As a consequence, for the time being physicians should feel comfortable prescribing either drug to patients with a left ventricular ejection fraction of 35% or less and in sinus rhythm, Dr. Shunichi Homma reported at the International Stroke Conference.
"Most heart failure patients are put on aspirin or warfarin" to deal with their elevated risk for thromboembolic events," said Dr. Homma, a cardiologist and professor of medicine at Columbia University in New York, and until now it’s remained an open question whether one drug surpassed the other. The study compared a daily 325-mg aspirin dosage with a warfarin regimen targeted to maintain patients at a target international normalized ratio (INR) of 2.75.
"Overall, there was no benefit of one over the other" for the study’s primary end point of the combined rate of death, ischemic stroke, or intracerebral hemorrhage, he said in an interview. Aspirin is more convenient and cheaper than a warfarin regimen that requires regular INR monitoring, and the results also showed that aspirin led to significantly fewer hemorrhagic events. In addition, total major hemorrhagic events, including intracranial, intracerebral, and gastrointestinal bleeding, ran 0.9% in the aspirin patients and 1.8% in the warfarin patients, a statistically significant difference.
But findings from the Warfarin Versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) Trial also showed advantages for warfarin. While the primary, combined end point showed a virtual dead heat of a 6.6% event rate with warfarin and a 6.5% rate with aspirin, warfarin treatment showed a statistically significant edge over aspirin for preventing ischemic strokes. This end point occurred in 0.7% of the warfarin patients and in 1.4% of those on aspirin, a significant difference.
In addition, warfarin treatment produced a hard-to-explain advantage for the study’s primary end point that appeared once patients were followed for more than 3 years. Although average follow-up for all 2,305 patients in the study was 3.5 years, follow-up for more than 1,000 of the enrollees extended to 4 years, and nearly 700 patients had a follow-up of 5 years. After 4 years, warfarin produced a statistically significant, roughly 25% reduction in the study’s primary end point compared with aspirin, an advantage maintained through 5 years and then out to 6 years.
Because this separate analysis of the study results after prolonged follow-up was a prespecified end point, it represented a major finding of the trial, although its full interpretation will need additional analysis and its clinical implications are not yet clear, Dr. Homma said.
"While there was no overall difference in the outcomes, there is a group of patients, those followed for 4 or more years, who benefited from warfarin. We don’t know who they are or why this happens. It’s important to find out, before we decide to [routinely use] warfarin," he said. "Also, in years 1-3 there was no significant difference between the two drugs, and given the bleeding risk with warfarin we need to clarify the characteristics of the patients who might benefit from one medication or the other."
For the time, being, until a better understanding of the long-term warfarin effect is available, "I think the results of our study will tip physicians toward using aspirin over warfarin," he said.
"We suspected, based on prior results, that there might be a change in the effect [of warfarin] over time," said J.L.P. Thompson, Ph.D., professor of clinical biostatistics and clinical neurology at Columbia and co-principal investigator on the study. "The benefit from warfarin is modest, but it is there. It does not lead to a clinical recommendation at the moment, but it is an unexpected finding and the clinical investigators want to look at it further," he said in an interview.
An additional, important observation was that daily aspirin treatment led to a strong trend toward a reduced rate of heart failure hospitalizations compared with the warfarin arm. Results from a prior study that compared aspirin and warfarin in heart failure patients, the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial, had indicated that aspirin boosted the rate of heart failure hospitalizations for acute exacerbations, and at the least the new findings "show that aspirin certainly did not make hospitalization any worse," and may possibly have even reduced the heart-failure hospitalization rate, Dr. Homma said.
"It is a very important finding. Aspirin use in heart failure was a concern, but we didn’t see that," he said at the meeting, which was sponsored by the American Heart Association.
WARCEF enrolled patients at 176 centers in 11 countries between 2002 and January 2010, with more than a third of the patients enrolled in the United States. The study was funded by the National Institute of Neurological Disorders and Stroke.
The study also featured an unusual design, in that it was completely double-blinded even though half the patients received warfarin and half did not. To maintain the blind, all patients regularly went to warfarin clinics where their blood was drawn and INR reports generated that their physicians then used to adjust their warfarin dosages, even for the patients who received dummy warfarin pills.
"Neither patients nor their physicians knew who was on warfarin and who was on aspirin, so this gives us great confidence about the study results. It removes the possibility that one group received more intensive care," Dr. Thompson said.
WARCEF was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Homma and Dr. Thompson said that they had no disclosures.
NEW ORLEANS – The largest, best controlled comparison of aspirin and warfarin ever done in patients with heart failure showed no overall difference between the two drugs for preventing thromboembolic events that cause death or stroke.
As a consequence, for the time being physicians should feel comfortable prescribing either drug to patients with a left ventricular ejection fraction of 35% or less and in sinus rhythm, Dr. Shunichi Homma reported at the International Stroke Conference.
"Most heart failure patients are put on aspirin or warfarin" to deal with their elevated risk for thromboembolic events," said Dr. Homma, a cardiologist and professor of medicine at Columbia University in New York, and until now it’s remained an open question whether one drug surpassed the other. The study compared a daily 325-mg aspirin dosage with a warfarin regimen targeted to maintain patients at a target international normalized ratio (INR) of 2.75.
"Overall, there was no benefit of one over the other" for the study’s primary end point of the combined rate of death, ischemic stroke, or intracerebral hemorrhage, he said in an interview. Aspirin is more convenient and cheaper than a warfarin regimen that requires regular INR monitoring, and the results also showed that aspirin led to significantly fewer hemorrhagic events. In addition, total major hemorrhagic events, including intracranial, intracerebral, and gastrointestinal bleeding, ran 0.9% in the aspirin patients and 1.8% in the warfarin patients, a statistically significant difference.
But findings from the Warfarin Versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) Trial also showed advantages for warfarin. While the primary, combined end point showed a virtual dead heat of a 6.6% event rate with warfarin and a 6.5% rate with aspirin, warfarin treatment showed a statistically significant edge over aspirin for preventing ischemic strokes. This end point occurred in 0.7% of the warfarin patients and in 1.4% of those on aspirin, a significant difference.
In addition, warfarin treatment produced a hard-to-explain advantage for the study’s primary end point that appeared once patients were followed for more than 3 years. Although average follow-up for all 2,305 patients in the study was 3.5 years, follow-up for more than 1,000 of the enrollees extended to 4 years, and nearly 700 patients had a follow-up of 5 years. After 4 years, warfarin produced a statistically significant, roughly 25% reduction in the study’s primary end point compared with aspirin, an advantage maintained through 5 years and then out to 6 years.
Because this separate analysis of the study results after prolonged follow-up was a prespecified end point, it represented a major finding of the trial, although its full interpretation will need additional analysis and its clinical implications are not yet clear, Dr. Homma said.
"While there was no overall difference in the outcomes, there is a group of patients, those followed for 4 or more years, who benefited from warfarin. We don’t know who they are or why this happens. It’s important to find out, before we decide to [routinely use] warfarin," he said. "Also, in years 1-3 there was no significant difference between the two drugs, and given the bleeding risk with warfarin we need to clarify the characteristics of the patients who might benefit from one medication or the other."
For the time, being, until a better understanding of the long-term warfarin effect is available, "I think the results of our study will tip physicians toward using aspirin over warfarin," he said.
"We suspected, based on prior results, that there might be a change in the effect [of warfarin] over time," said J.L.P. Thompson, Ph.D., professor of clinical biostatistics and clinical neurology at Columbia and co-principal investigator on the study. "The benefit from warfarin is modest, but it is there. It does not lead to a clinical recommendation at the moment, but it is an unexpected finding and the clinical investigators want to look at it further," he said in an interview.
An additional, important observation was that daily aspirin treatment led to a strong trend toward a reduced rate of heart failure hospitalizations compared with the warfarin arm. Results from a prior study that compared aspirin and warfarin in heart failure patients, the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial, had indicated that aspirin boosted the rate of heart failure hospitalizations for acute exacerbations, and at the least the new findings "show that aspirin certainly did not make hospitalization any worse," and may possibly have even reduced the heart-failure hospitalization rate, Dr. Homma said.
"It is a very important finding. Aspirin use in heart failure was a concern, but we didn’t see that," he said at the meeting, which was sponsored by the American Heart Association.
WARCEF enrolled patients at 176 centers in 11 countries between 2002 and January 2010, with more than a third of the patients enrolled in the United States. The study was funded by the National Institute of Neurological Disorders and Stroke.
The study also featured an unusual design, in that it was completely double-blinded even though half the patients received warfarin and half did not. To maintain the blind, all patients regularly went to warfarin clinics where their blood was drawn and INR reports generated that their physicians then used to adjust their warfarin dosages, even for the patients who received dummy warfarin pills.
"Neither patients nor their physicians knew who was on warfarin and who was on aspirin, so this gives us great confidence about the study results. It removes the possibility that one group received more intensive care," Dr. Thompson said.
WARCEF was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Homma and Dr. Thompson said that they had no disclosures.
NEW ORLEANS – The largest, best controlled comparison of aspirin and warfarin ever done in patients with heart failure showed no overall difference between the two drugs for preventing thromboembolic events that cause death or stroke.
As a consequence, for the time being physicians should feel comfortable prescribing either drug to patients with a left ventricular ejection fraction of 35% or less and in sinus rhythm, Dr. Shunichi Homma reported at the International Stroke Conference.
"Most heart failure patients are put on aspirin or warfarin" to deal with their elevated risk for thromboembolic events," said Dr. Homma, a cardiologist and professor of medicine at Columbia University in New York, and until now it’s remained an open question whether one drug surpassed the other. The study compared a daily 325-mg aspirin dosage with a warfarin regimen targeted to maintain patients at a target international normalized ratio (INR) of 2.75.
"Overall, there was no benefit of one over the other" for the study’s primary end point of the combined rate of death, ischemic stroke, or intracerebral hemorrhage, he said in an interview. Aspirin is more convenient and cheaper than a warfarin regimen that requires regular INR monitoring, and the results also showed that aspirin led to significantly fewer hemorrhagic events. In addition, total major hemorrhagic events, including intracranial, intracerebral, and gastrointestinal bleeding, ran 0.9% in the aspirin patients and 1.8% in the warfarin patients, a statistically significant difference.
But findings from the Warfarin Versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) Trial also showed advantages for warfarin. While the primary, combined end point showed a virtual dead heat of a 6.6% event rate with warfarin and a 6.5% rate with aspirin, warfarin treatment showed a statistically significant edge over aspirin for preventing ischemic strokes. This end point occurred in 0.7% of the warfarin patients and in 1.4% of those on aspirin, a significant difference.
In addition, warfarin treatment produced a hard-to-explain advantage for the study’s primary end point that appeared once patients were followed for more than 3 years. Although average follow-up for all 2,305 patients in the study was 3.5 years, follow-up for more than 1,000 of the enrollees extended to 4 years, and nearly 700 patients had a follow-up of 5 years. After 4 years, warfarin produced a statistically significant, roughly 25% reduction in the study’s primary end point compared with aspirin, an advantage maintained through 5 years and then out to 6 years.
Because this separate analysis of the study results after prolonged follow-up was a prespecified end point, it represented a major finding of the trial, although its full interpretation will need additional analysis and its clinical implications are not yet clear, Dr. Homma said.
"While there was no overall difference in the outcomes, there is a group of patients, those followed for 4 or more years, who benefited from warfarin. We don’t know who they are or why this happens. It’s important to find out, before we decide to [routinely use] warfarin," he said. "Also, in years 1-3 there was no significant difference between the two drugs, and given the bleeding risk with warfarin we need to clarify the characteristics of the patients who might benefit from one medication or the other."
For the time, being, until a better understanding of the long-term warfarin effect is available, "I think the results of our study will tip physicians toward using aspirin over warfarin," he said.
"We suspected, based on prior results, that there might be a change in the effect [of warfarin] over time," said J.L.P. Thompson, Ph.D., professor of clinical biostatistics and clinical neurology at Columbia and co-principal investigator on the study. "The benefit from warfarin is modest, but it is there. It does not lead to a clinical recommendation at the moment, but it is an unexpected finding and the clinical investigators want to look at it further," he said in an interview.
An additional, important observation was that daily aspirin treatment led to a strong trend toward a reduced rate of heart failure hospitalizations compared with the warfarin arm. Results from a prior study that compared aspirin and warfarin in heart failure patients, the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial, had indicated that aspirin boosted the rate of heart failure hospitalizations for acute exacerbations, and at the least the new findings "show that aspirin certainly did not make hospitalization any worse," and may possibly have even reduced the heart-failure hospitalization rate, Dr. Homma said.
"It is a very important finding. Aspirin use in heart failure was a concern, but we didn’t see that," he said at the meeting, which was sponsored by the American Heart Association.
WARCEF enrolled patients at 176 centers in 11 countries between 2002 and January 2010, with more than a third of the patients enrolled in the United States. The study was funded by the National Institute of Neurological Disorders and Stroke.
The study also featured an unusual design, in that it was completely double-blinded even though half the patients received warfarin and half did not. To maintain the blind, all patients regularly went to warfarin clinics where their blood was drawn and INR reports generated that their physicians then used to adjust their warfarin dosages, even for the patients who received dummy warfarin pills.
"Neither patients nor their physicians knew who was on warfarin and who was on aspirin, so this gives us great confidence about the study results. It removes the possibility that one group received more intensive care," Dr. Thompson said.
WARCEF was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Homma and Dr. Thompson said that they had no disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: Heart failure patients treated with aspirin or warfarin had an identical 7% rate of death, ischemic stroke, or intracerebral hemorrhage.
Data Source: The WARCEF trial, which randomized 2,305 patients with a left ventricular ejection fraction of 35% or less and normal sinus rhythm at 176 centers in 11 countries.
Disclosures: WARCEF was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Homma and Dr. Thompson said that they had no disclosures.
Anemia Triples Post-Stroke Mortality Risk
NEW ORLEANS – Patients with a very low or a very high hematocrit are at higher risk for death after a stroke, and anemic patients are at greatest risk, according to a study presented at the International Stroke Conference sponsored by the American Heart Association on Feb. 2.
Previous studies have shown that extremes of hematocrit increase mortality after myocardial infarction, congestive heart failure, and kidney disease. Dr. Jason J. Sico of the VA Connecticut Healthcare System and researchers from the Department of Veterans Affairs medical system explored whether there was a similar association in stroke. Previous stroke studies had not adjusted for stroke severity or a large number of comorbidities.
They found that "among stroke patients, severe anemia is a potent predictor of dying throughout the first year after a stroke," said Dr. Sico, who is also an assistant professor of neurology at Yale University, New Haven, Conn.
The researchers abstracted medical records for a sample from 131 Veterans Health Administration (VHA) hospitals of 3,965 patients admitted for a confirmed diagnosis of ischemic stroke in fiscal 2007. Patients with unavailable hematocrits, those who received thrombolytics, or those whose charts had inconsistent death dates were also excluded.
"Among stroke patients, severe anemia is a potent predictor of dying throughout the first year after a stroke."
The hematocrit, taken from 24 hours of admission, was divided into six tiers: less than or equal to 27% (defined as severe anemia); 28%-32% (moderate anemia); 33%-37% (mild anemia); 38%-42% (normal); 43%-47% (normal); and greater than or equal to 48% (polycythemia).
Researchers adjusted for age, National Institutes of Health Stroke Scale (NIHSS) score, comorbidities (including pneumonia, hypertension, hypercholesterolemia, diabetes, and history of cancer and heart disease), and Acute Physiology and Chronic Health Evaluation (APACHE)-III scores.
A total of 2% of the 3,750 patients analyzed had severe anemia, 6.2% had moderate anemia, and 17.9% had mild anemia. About 64% were in the normal categories. A total of 9% had a high hematocrit of greater than or equal to 48%.
People with lower hematocrits tended to be older and have higher APACHE scores, a higher Charlson index, a history of heart disease, and were more likely to have diabetes.
The risk of death was 2.5 to 3.5 times higher for patients with severe anemia (P = .013 for in-hospital and 30-day mortality; P = .002 at 6 months and P = .001 at 1 year). A high hematocrit was independently associated only with in-hospital mortality (OR 2.9, P = .004).
The study showed that having a history of severe anemia put stroke patients at a higher risk of death than having a history of other conditions, including cancer and heart disease, said Dr. Sico.
There are several potential mechanisms to explain why anemia might increase the risk of death, he said in an interview. With lower hematocrits, less blood and less oxygen circulate to various parts of the body. Long-term anemia also impairs the ability of the brain’s blood vessels to respond appropriately to a stroke.
A higher hematocrit also decreases blood flow to brain, said Dr. Sico. It causes a more turbulent blood flow to the brain, which could predispose the stroke patient to having a bad outcome.
Dr. Sico said that the study’s findings were limited to men because no women had been analyzed.
But he concluded that stroke patients with low or high hematocrits should be evaluated for potentially reversible causes, and that they should also be closely monitored during the post-stroke period.
Dr. Sico reported having no financial disclosures. The study was funded by the VA Health Services Research and Development Quality Enhancement Research Initiative.
NEW ORLEANS – Patients with a very low or a very high hematocrit are at higher risk for death after a stroke, and anemic patients are at greatest risk, according to a study presented at the International Stroke Conference sponsored by the American Heart Association on Feb. 2.
Previous studies have shown that extremes of hematocrit increase mortality after myocardial infarction, congestive heart failure, and kidney disease. Dr. Jason J. Sico of the VA Connecticut Healthcare System and researchers from the Department of Veterans Affairs medical system explored whether there was a similar association in stroke. Previous stroke studies had not adjusted for stroke severity or a large number of comorbidities.
They found that "among stroke patients, severe anemia is a potent predictor of dying throughout the first year after a stroke," said Dr. Sico, who is also an assistant professor of neurology at Yale University, New Haven, Conn.
The researchers abstracted medical records for a sample from 131 Veterans Health Administration (VHA) hospitals of 3,965 patients admitted for a confirmed diagnosis of ischemic stroke in fiscal 2007. Patients with unavailable hematocrits, those who received thrombolytics, or those whose charts had inconsistent death dates were also excluded.
"Among stroke patients, severe anemia is a potent predictor of dying throughout the first year after a stroke."
The hematocrit, taken from 24 hours of admission, was divided into six tiers: less than or equal to 27% (defined as severe anemia); 28%-32% (moderate anemia); 33%-37% (mild anemia); 38%-42% (normal); 43%-47% (normal); and greater than or equal to 48% (polycythemia).
Researchers adjusted for age, National Institutes of Health Stroke Scale (NIHSS) score, comorbidities (including pneumonia, hypertension, hypercholesterolemia, diabetes, and history of cancer and heart disease), and Acute Physiology and Chronic Health Evaluation (APACHE)-III scores.
A total of 2% of the 3,750 patients analyzed had severe anemia, 6.2% had moderate anemia, and 17.9% had mild anemia. About 64% were in the normal categories. A total of 9% had a high hematocrit of greater than or equal to 48%.
People with lower hematocrits tended to be older and have higher APACHE scores, a higher Charlson index, a history of heart disease, and were more likely to have diabetes.
The risk of death was 2.5 to 3.5 times higher for patients with severe anemia (P = .013 for in-hospital and 30-day mortality; P = .002 at 6 months and P = .001 at 1 year). A high hematocrit was independently associated only with in-hospital mortality (OR 2.9, P = .004).
The study showed that having a history of severe anemia put stroke patients at a higher risk of death than having a history of other conditions, including cancer and heart disease, said Dr. Sico.
There are several potential mechanisms to explain why anemia might increase the risk of death, he said in an interview. With lower hematocrits, less blood and less oxygen circulate to various parts of the body. Long-term anemia also impairs the ability of the brain’s blood vessels to respond appropriately to a stroke.
A higher hematocrit also decreases blood flow to brain, said Dr. Sico. It causes a more turbulent blood flow to the brain, which could predispose the stroke patient to having a bad outcome.
Dr. Sico said that the study’s findings were limited to men because no women had been analyzed.
But he concluded that stroke patients with low or high hematocrits should be evaluated for potentially reversible causes, and that they should also be closely monitored during the post-stroke period.
Dr. Sico reported having no financial disclosures. The study was funded by the VA Health Services Research and Development Quality Enhancement Research Initiative.
NEW ORLEANS – Patients with a very low or a very high hematocrit are at higher risk for death after a stroke, and anemic patients are at greatest risk, according to a study presented at the International Stroke Conference sponsored by the American Heart Association on Feb. 2.
Previous studies have shown that extremes of hematocrit increase mortality after myocardial infarction, congestive heart failure, and kidney disease. Dr. Jason J. Sico of the VA Connecticut Healthcare System and researchers from the Department of Veterans Affairs medical system explored whether there was a similar association in stroke. Previous stroke studies had not adjusted for stroke severity or a large number of comorbidities.
They found that "among stroke patients, severe anemia is a potent predictor of dying throughout the first year after a stroke," said Dr. Sico, who is also an assistant professor of neurology at Yale University, New Haven, Conn.
The researchers abstracted medical records for a sample from 131 Veterans Health Administration (VHA) hospitals of 3,965 patients admitted for a confirmed diagnosis of ischemic stroke in fiscal 2007. Patients with unavailable hematocrits, those who received thrombolytics, or those whose charts had inconsistent death dates were also excluded.
"Among stroke patients, severe anemia is a potent predictor of dying throughout the first year after a stroke."
The hematocrit, taken from 24 hours of admission, was divided into six tiers: less than or equal to 27% (defined as severe anemia); 28%-32% (moderate anemia); 33%-37% (mild anemia); 38%-42% (normal); 43%-47% (normal); and greater than or equal to 48% (polycythemia).
Researchers adjusted for age, National Institutes of Health Stroke Scale (NIHSS) score, comorbidities (including pneumonia, hypertension, hypercholesterolemia, diabetes, and history of cancer and heart disease), and Acute Physiology and Chronic Health Evaluation (APACHE)-III scores.
A total of 2% of the 3,750 patients analyzed had severe anemia, 6.2% had moderate anemia, and 17.9% had mild anemia. About 64% were in the normal categories. A total of 9% had a high hematocrit of greater than or equal to 48%.
People with lower hematocrits tended to be older and have higher APACHE scores, a higher Charlson index, a history of heart disease, and were more likely to have diabetes.
The risk of death was 2.5 to 3.5 times higher for patients with severe anemia (P = .013 for in-hospital and 30-day mortality; P = .002 at 6 months and P = .001 at 1 year). A high hematocrit was independently associated only with in-hospital mortality (OR 2.9, P = .004).
The study showed that having a history of severe anemia put stroke patients at a higher risk of death than having a history of other conditions, including cancer and heart disease, said Dr. Sico.
There are several potential mechanisms to explain why anemia might increase the risk of death, he said in an interview. With lower hematocrits, less blood and less oxygen circulate to various parts of the body. Long-term anemia also impairs the ability of the brain’s blood vessels to respond appropriately to a stroke.
A higher hematocrit also decreases blood flow to brain, said Dr. Sico. It causes a more turbulent blood flow to the brain, which could predispose the stroke patient to having a bad outcome.
Dr. Sico said that the study’s findings were limited to men because no women had been analyzed.
But he concluded that stroke patients with low or high hematocrits should be evaluated for potentially reversible causes, and that they should also be closely monitored during the post-stroke period.
Dr. Sico reported having no financial disclosures. The study was funded by the VA Health Services Research and Development Quality Enhancement Research Initiative.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: A history of severe anemia put stroke patients at a higher risk of death than having a history of other conditions, including cancer and heart disease. The risk of death was 2.5 to 3.5 times higher for patients with severe anemia. A high hematocrit was independently associated only with in-hospital mortality.
Data Source: The researchers abstracted medical records for a sample from 131 Veterans Health Administration (VHA) hospitals of 3,965 patients admitted for a confirmed diagnosis of ischemic stroke in fiscal 2007
Disclosures: Dr. Sico reported having no financial disclosures. The study was funded by the VA Health Services Research and Development Quality Enhancement Research Initiative.