User login
Multiple Sclerosis Hub
Neurologists grappling with patients who embrace ‘stem cell tourism’
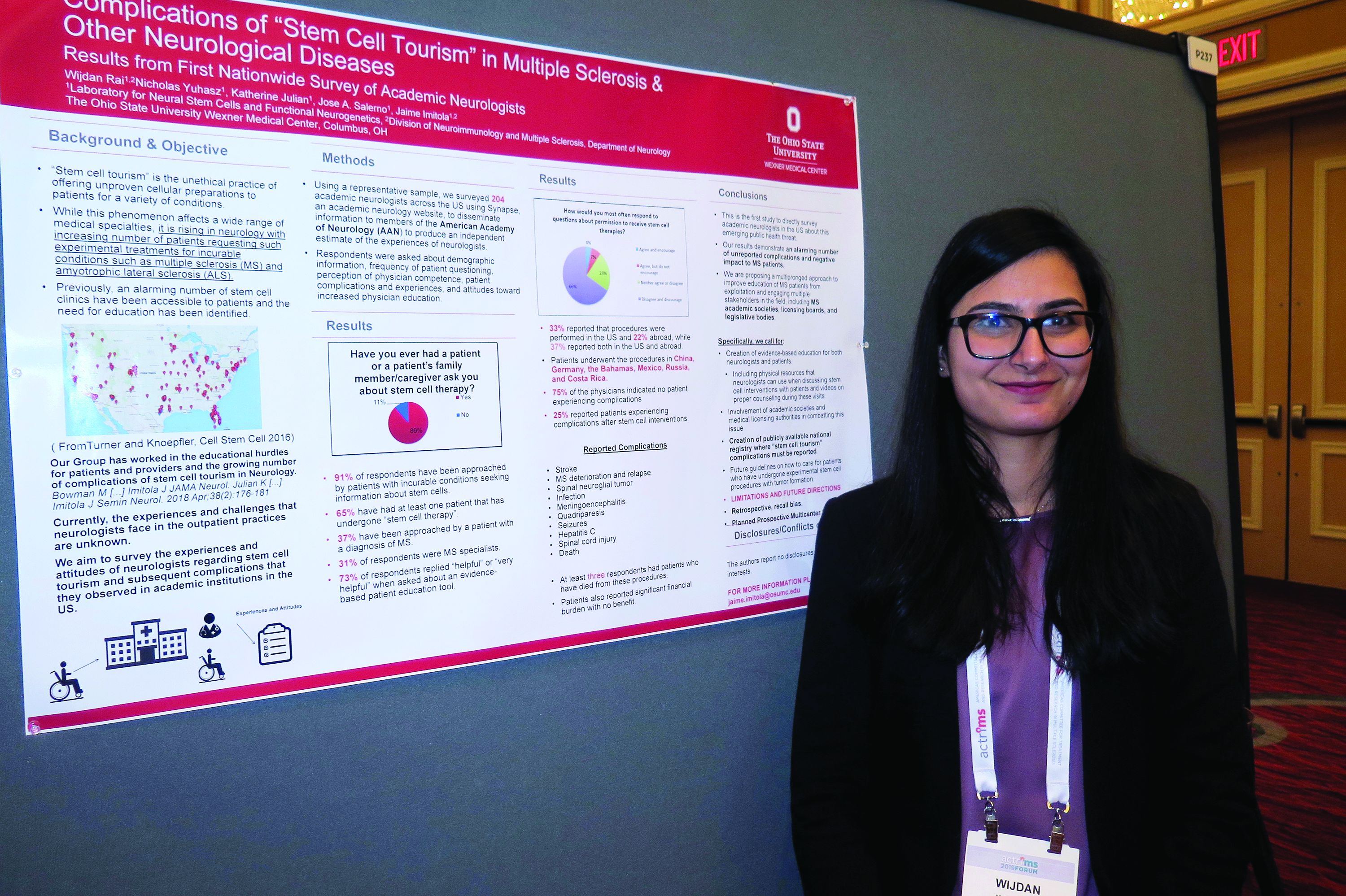
DALLAS – Stem cell tourism – the unethical practice of offering unproven cellular preparations to patients for a variety of conditions – is increasingly sought by patients with incurable conditions such as multiple sclerosis and amyotrophic lateral sclerosis, results from a novel survey suggest.
In fact, most academic neurologists have been approached by patients with incurable conditions who ask them about stem cell therapy, while about two-thirds have had at least one patient who has undergone stem cell therapy.
“It’s really scary,” Wijdan Rai, MBBS, the study’s first author, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “This is a more prevalent issue than we think, and the complication rates are higher than we think.”
According to the study’s senior author, Jaime Imitola, MD, who directs the Progressive Multiple Sclerosis Multidisciplinary Clinic and Translational Research Program at the Ohio State University Wexner Medical Center, Columbus, the results “call for the creation of a nationwide registry where neurologists can document adverse reactions to stem cell procedures and further support dedicated patient and neurologist education as we have proposed before” (See Semin Neurol. 2018; 38[2]:176-81 and JAMA Neurol. 2015;72[11]:1342-5).
In an effort to understand the experiences and attitudes of academic neurologists regarding stem cell tourism and patient-reported complications, the researchers developed a 25-question survey disseminated via Synapse, a web tool from the American Academy of Neurology. Respondents were asked about demographic information, frequency of patient questioning, perception of physician competence, patient complications and experiences, and attitudes toward increased physician education.
Dr. Rai, who is a senior neurology resident at the medical center, presented findings from 204 neurologist respondents, of whom 31% identified themselves as MS specialists. Nearly all respondents (91%) said they have been approached by patients with incurable conditions seeking information about stem cells (37% of whom had diagnosis of MS). In addition, 65% have had at least one patient that has undergone “stem cell therapy,” and 73% said it would be “helpful” or “very helpful” to have an evidence-based patient education tool on the topic. “Patients most often wanted general information,” Dr. Rai said. “However, 50% requested permission to undergo a stem cell procedure, and 31% approached their neurologist after the procedure.”
Survey respondents reported that 33% of the stem cell interventions were performed in the United States and 22% abroad, while 37% reported both in the U.S. and abroad. Patients underwent the procedures in China, Germany, the Bahamas, Mexico, Russia, and Costa Rica. Three-quarters of respondents (75%) indicated no patient experiencing complications from the stem cell interventions. However, 25% reported patients experiencing a variety of complications from the procedures, including strokes, meningoencephalitis, quadriparesis, MS deterioration, sepsis, hepatitis C, seizures, meningitis from intrathecal cell injections, infections, and spinal cord tumors. “At least three respondents had a patient who died as a direct complication from stem cell therapy,” Dr. Rai said.
In their poster, the researchers recommended a “multipronged approach to improve education of MS patients from exploitation and engaging multiple stakeholders in the field, including MS academic societies, licensing boards, and legislative bodies. Specifically, we call for creation of evidence-based education for both neurologists and patients, including physical resources that neurologists can use when discussing stem cell interventions with patients and videos on proper counseling during these visits.”
Colleagues from OSU’s Laboratory for Neural Stem Cells and Functional Neurogenetics contributed to this work. The researchers reported having no financial disclosures.
SOURCE: Rai W et al. ACTRIMS Forum 2019, Poster 237.
DALLAS – Stem cell tourism – the unethical practice of offering unproven cellular preparations to patients for a variety of conditions – is increasingly sought by patients with incurable conditions such as multiple sclerosis and amyotrophic lateral sclerosis, results from a novel survey suggest.
In fact, most academic neurologists have been approached by patients with incurable conditions who ask them about stem cell therapy, while about two-thirds have had at least one patient who has undergone stem cell therapy.
“It’s really scary,” Wijdan Rai, MBBS, the study’s first author, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “This is a more prevalent issue than we think, and the complication rates are higher than we think.”
According to the study’s senior author, Jaime Imitola, MD, who directs the Progressive Multiple Sclerosis Multidisciplinary Clinic and Translational Research Program at the Ohio State University Wexner Medical Center, Columbus, the results “call for the creation of a nationwide registry where neurologists can document adverse reactions to stem cell procedures and further support dedicated patient and neurologist education as we have proposed before” (See Semin Neurol. 2018; 38[2]:176-81 and JAMA Neurol. 2015;72[11]:1342-5).
In an effort to understand the experiences and attitudes of academic neurologists regarding stem cell tourism and patient-reported complications, the researchers developed a 25-question survey disseminated via Synapse, a web tool from the American Academy of Neurology. Respondents were asked about demographic information, frequency of patient questioning, perception of physician competence, patient complications and experiences, and attitudes toward increased physician education.
Dr. Rai, who is a senior neurology resident at the medical center, presented findings from 204 neurologist respondents, of whom 31% identified themselves as MS specialists. Nearly all respondents (91%) said they have been approached by patients with incurable conditions seeking information about stem cells (37% of whom had diagnosis of MS). In addition, 65% have had at least one patient that has undergone “stem cell therapy,” and 73% said it would be “helpful” or “very helpful” to have an evidence-based patient education tool on the topic. “Patients most often wanted general information,” Dr. Rai said. “However, 50% requested permission to undergo a stem cell procedure, and 31% approached their neurologist after the procedure.”
Survey respondents reported that 33% of the stem cell interventions were performed in the United States and 22% abroad, while 37% reported both in the U.S. and abroad. Patients underwent the procedures in China, Germany, the Bahamas, Mexico, Russia, and Costa Rica. Three-quarters of respondents (75%) indicated no patient experiencing complications from the stem cell interventions. However, 25% reported patients experiencing a variety of complications from the procedures, including strokes, meningoencephalitis, quadriparesis, MS deterioration, sepsis, hepatitis C, seizures, meningitis from intrathecal cell injections, infections, and spinal cord tumors. “At least three respondents had a patient who died as a direct complication from stem cell therapy,” Dr. Rai said.
In their poster, the researchers recommended a “multipronged approach to improve education of MS patients from exploitation and engaging multiple stakeholders in the field, including MS academic societies, licensing boards, and legislative bodies. Specifically, we call for creation of evidence-based education for both neurologists and patients, including physical resources that neurologists can use when discussing stem cell interventions with patients and videos on proper counseling during these visits.”
Colleagues from OSU’s Laboratory for Neural Stem Cells and Functional Neurogenetics contributed to this work. The researchers reported having no financial disclosures.
SOURCE: Rai W et al. ACTRIMS Forum 2019, Poster 237.
DALLAS – Stem cell tourism – the unethical practice of offering unproven cellular preparations to patients for a variety of conditions – is increasingly sought by patients with incurable conditions such as multiple sclerosis and amyotrophic lateral sclerosis, results from a novel survey suggest.
In fact, most academic neurologists have been approached by patients with incurable conditions who ask them about stem cell therapy, while about two-thirds have had at least one patient who has undergone stem cell therapy.
“It’s really scary,” Wijdan Rai, MBBS, the study’s first author, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “This is a more prevalent issue than we think, and the complication rates are higher than we think.”
According to the study’s senior author, Jaime Imitola, MD, who directs the Progressive Multiple Sclerosis Multidisciplinary Clinic and Translational Research Program at the Ohio State University Wexner Medical Center, Columbus, the results “call for the creation of a nationwide registry where neurologists can document adverse reactions to stem cell procedures and further support dedicated patient and neurologist education as we have proposed before” (See Semin Neurol. 2018; 38[2]:176-81 and JAMA Neurol. 2015;72[11]:1342-5).
In an effort to understand the experiences and attitudes of academic neurologists regarding stem cell tourism and patient-reported complications, the researchers developed a 25-question survey disseminated via Synapse, a web tool from the American Academy of Neurology. Respondents were asked about demographic information, frequency of patient questioning, perception of physician competence, patient complications and experiences, and attitudes toward increased physician education.
Dr. Rai, who is a senior neurology resident at the medical center, presented findings from 204 neurologist respondents, of whom 31% identified themselves as MS specialists. Nearly all respondents (91%) said they have been approached by patients with incurable conditions seeking information about stem cells (37% of whom had diagnosis of MS). In addition, 65% have had at least one patient that has undergone “stem cell therapy,” and 73% said it would be “helpful” or “very helpful” to have an evidence-based patient education tool on the topic. “Patients most often wanted general information,” Dr. Rai said. “However, 50% requested permission to undergo a stem cell procedure, and 31% approached their neurologist after the procedure.”
Survey respondents reported that 33% of the stem cell interventions were performed in the United States and 22% abroad, while 37% reported both in the U.S. and abroad. Patients underwent the procedures in China, Germany, the Bahamas, Mexico, Russia, and Costa Rica. Three-quarters of respondents (75%) indicated no patient experiencing complications from the stem cell interventions. However, 25% reported patients experiencing a variety of complications from the procedures, including strokes, meningoencephalitis, quadriparesis, MS deterioration, sepsis, hepatitis C, seizures, meningitis from intrathecal cell injections, infections, and spinal cord tumors. “At least three respondents had a patient who died as a direct complication from stem cell therapy,” Dr. Rai said.
In their poster, the researchers recommended a “multipronged approach to improve education of MS patients from exploitation and engaging multiple stakeholders in the field, including MS academic societies, licensing boards, and legislative bodies. Specifically, we call for creation of evidence-based education for both neurologists and patients, including physical resources that neurologists can use when discussing stem cell interventions with patients and videos on proper counseling during these visits.”
Colleagues from OSU’s Laboratory for Neural Stem Cells and Functional Neurogenetics contributed to this work. The researchers reported having no financial disclosures.
SOURCE: Rai W et al. ACTRIMS Forum 2019, Poster 237.
REPORTING FROM ACTRIMS FORUM 2019
Don’t forget social determinants of health in minority MS patients
DALLAS – The way Lilyana Amezcua, MD, sees it, clinicians should view race and ethnicity as health disparities when assessing individuals with multiple sclerosis.
Whites are predominately affected with MS, “but we have seen changing demographics,” said Dr. Amezcua, of the University of Southern California MS Comprehensive Care and Research Group. “Why are African Americans now at higher risk ... and why do African Americans appear to have more severe disease? Is it a biological difference ... or is it because of poor access” to health care?
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Amezcua delivered a presentation entitled “Effect of Race and Ethnicity on MS Presentation and Disease Course.” She called on researchers in the field “to not just take race and ethnicity as any small variable. We need to be cognizant and use the correct methodology, depending on what [question] we want to answer. We need to better define how we ascertain race, how we ascertain ethnicity.”
Dr. Amezcua, who is also the MS fellowship program director at the Keck School of Medicine, disclosed that she receives funding from the National MS Society, the National Institutes of Health, the California Community Foundation, and Biogen.
DALLAS – The way Lilyana Amezcua, MD, sees it, clinicians should view race and ethnicity as health disparities when assessing individuals with multiple sclerosis.
Whites are predominately affected with MS, “but we have seen changing demographics,” said Dr. Amezcua, of the University of Southern California MS Comprehensive Care and Research Group. “Why are African Americans now at higher risk ... and why do African Americans appear to have more severe disease? Is it a biological difference ... or is it because of poor access” to health care?
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Amezcua delivered a presentation entitled “Effect of Race and Ethnicity on MS Presentation and Disease Course.” She called on researchers in the field “to not just take race and ethnicity as any small variable. We need to be cognizant and use the correct methodology, depending on what [question] we want to answer. We need to better define how we ascertain race, how we ascertain ethnicity.”
Dr. Amezcua, who is also the MS fellowship program director at the Keck School of Medicine, disclosed that she receives funding from the National MS Society, the National Institutes of Health, the California Community Foundation, and Biogen.
DALLAS – The way Lilyana Amezcua, MD, sees it, clinicians should view race and ethnicity as health disparities when assessing individuals with multiple sclerosis.
Whites are predominately affected with MS, “but we have seen changing demographics,” said Dr. Amezcua, of the University of Southern California MS Comprehensive Care and Research Group. “Why are African Americans now at higher risk ... and why do African Americans appear to have more severe disease? Is it a biological difference ... or is it because of poor access” to health care?
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Amezcua delivered a presentation entitled “Effect of Race and Ethnicity on MS Presentation and Disease Course.” She called on researchers in the field “to not just take race and ethnicity as any small variable. We need to be cognizant and use the correct methodology, depending on what [question] we want to answer. We need to better define how we ascertain race, how we ascertain ethnicity.”
Dr. Amezcua, who is also the MS fellowship program director at the Keck School of Medicine, disclosed that she receives funding from the National MS Society, the National Institutes of Health, the California Community Foundation, and Biogen.
REPORTING FROM ACTRIMS FORUM 2019
CSF biomarker clusters correlate with MS severity
DALLAS – Patients with multiple sclerosis (MS) have elevated levels of specific clusters of cerebrospinal fluid (CSF) biomarkers related to astrocytes and microglia that correlated with disease severity in a blinded analysis of more than 1,000 proteins from the CSF of more than 400 patients with neuroimmunologic disease and healthy volunteers.
Previous studies have indicated that aberrant activation of astrocytes and microglia underlies disability progression in older patients with MS, but researchers have lacked biomarkers of these processes in living subjects. In a presentation at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Ruturaj R. Masvekar, PhD, described developing biomarkers of CNS cell–specific processes and examining how they relate to MS disability progression. Dr. Masvekar, a researcher at the National Institute of Allergy and Infectious Diseases, and his coinvestigators used a modified DNA aptamer assay to measure proteins in the CSF of 431 patients with neuroimmunologic diseases and healthy volunteers, followed by variable cluster analysis and in vitro modeling to define 64 clusters of CSF biomarkers that relate to CNS cell types.
The study included 42 healthy donors, 20 patients with clinically isolated syndrome, 57 patients with noninflammatory neurologic disorders, 127 patients with relapsing-remitting MS, 72 patients with secondary progressive MS, and 113 patients with primary progressive MS. In a training cohort of 217 participants, the researchers assessed how biomarkers differed between the diagnostic categories. The researchers then validated the results in an independent cohort of 214 participants.
One astrocyte-related cluster (MMP7, SERPINA3, GZMA, and CLIC1) and one microglia-related cluster (DSG2 and TNFRSF25) was significantly elevated in all MS subgroups, compared with healthy controls and patients with noninflammatory neurologic disorders.
In addition, these clusters “significantly correlated with clinical measures of disability, CNS tissue destruction, and MS severity,” Dr. Masvekar said.
The microglial cluster was significantly elevated in all MS subgroups, whereas neuronal endothelial, astrocytic, and oligodendroglial biomarker clusters were elevated only in patients with progressive MS.
“Microglial activation is present in all stages of MS, while toxic astrogliosis increases with MS duration, concomitantly with neuronal and oligodendroglial degeneration,” Dr. Masvekar said. “Microglial activation and toxic astrogliosis likely partake in CNS tissue destruction and enhance MS severity.”
This study, which was recently published in Multiple Sclerosis and Related Disorders (2019 Feb;28:34-43), was supported by the intramural research program at NIAID.
jremaly@mdedge.com
SOURCE: Masvekar RR et al. ACTRIMS Forum 2019, Abstract 281.
DALLAS – Patients with multiple sclerosis (MS) have elevated levels of specific clusters of cerebrospinal fluid (CSF) biomarkers related to astrocytes and microglia that correlated with disease severity in a blinded analysis of more than 1,000 proteins from the CSF of more than 400 patients with neuroimmunologic disease and healthy volunteers.
Previous studies have indicated that aberrant activation of astrocytes and microglia underlies disability progression in older patients with MS, but researchers have lacked biomarkers of these processes in living subjects. In a presentation at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Ruturaj R. Masvekar, PhD, described developing biomarkers of CNS cell–specific processes and examining how they relate to MS disability progression. Dr. Masvekar, a researcher at the National Institute of Allergy and Infectious Diseases, and his coinvestigators used a modified DNA aptamer assay to measure proteins in the CSF of 431 patients with neuroimmunologic diseases and healthy volunteers, followed by variable cluster analysis and in vitro modeling to define 64 clusters of CSF biomarkers that relate to CNS cell types.
The study included 42 healthy donors, 20 patients with clinically isolated syndrome, 57 patients with noninflammatory neurologic disorders, 127 patients with relapsing-remitting MS, 72 patients with secondary progressive MS, and 113 patients with primary progressive MS. In a training cohort of 217 participants, the researchers assessed how biomarkers differed between the diagnostic categories. The researchers then validated the results in an independent cohort of 214 participants.
One astrocyte-related cluster (MMP7, SERPINA3, GZMA, and CLIC1) and one microglia-related cluster (DSG2 and TNFRSF25) was significantly elevated in all MS subgroups, compared with healthy controls and patients with noninflammatory neurologic disorders.
In addition, these clusters “significantly correlated with clinical measures of disability, CNS tissue destruction, and MS severity,” Dr. Masvekar said.
The microglial cluster was significantly elevated in all MS subgroups, whereas neuronal endothelial, astrocytic, and oligodendroglial biomarker clusters were elevated only in patients with progressive MS.
“Microglial activation is present in all stages of MS, while toxic astrogliosis increases with MS duration, concomitantly with neuronal and oligodendroglial degeneration,” Dr. Masvekar said. “Microglial activation and toxic astrogliosis likely partake in CNS tissue destruction and enhance MS severity.”
This study, which was recently published in Multiple Sclerosis and Related Disorders (2019 Feb;28:34-43), was supported by the intramural research program at NIAID.
jremaly@mdedge.com
SOURCE: Masvekar RR et al. ACTRIMS Forum 2019, Abstract 281.
DALLAS – Patients with multiple sclerosis (MS) have elevated levels of specific clusters of cerebrospinal fluid (CSF) biomarkers related to astrocytes and microglia that correlated with disease severity in a blinded analysis of more than 1,000 proteins from the CSF of more than 400 patients with neuroimmunologic disease and healthy volunteers.
Previous studies have indicated that aberrant activation of astrocytes and microglia underlies disability progression in older patients with MS, but researchers have lacked biomarkers of these processes in living subjects. In a presentation at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Ruturaj R. Masvekar, PhD, described developing biomarkers of CNS cell–specific processes and examining how they relate to MS disability progression. Dr. Masvekar, a researcher at the National Institute of Allergy and Infectious Diseases, and his coinvestigators used a modified DNA aptamer assay to measure proteins in the CSF of 431 patients with neuroimmunologic diseases and healthy volunteers, followed by variable cluster analysis and in vitro modeling to define 64 clusters of CSF biomarkers that relate to CNS cell types.
The study included 42 healthy donors, 20 patients with clinically isolated syndrome, 57 patients with noninflammatory neurologic disorders, 127 patients with relapsing-remitting MS, 72 patients with secondary progressive MS, and 113 patients with primary progressive MS. In a training cohort of 217 participants, the researchers assessed how biomarkers differed between the diagnostic categories. The researchers then validated the results in an independent cohort of 214 participants.
One astrocyte-related cluster (MMP7, SERPINA3, GZMA, and CLIC1) and one microglia-related cluster (DSG2 and TNFRSF25) was significantly elevated in all MS subgroups, compared with healthy controls and patients with noninflammatory neurologic disorders.
In addition, these clusters “significantly correlated with clinical measures of disability, CNS tissue destruction, and MS severity,” Dr. Masvekar said.
The microglial cluster was significantly elevated in all MS subgroups, whereas neuronal endothelial, astrocytic, and oligodendroglial biomarker clusters were elevated only in patients with progressive MS.
“Microglial activation is present in all stages of MS, while toxic astrogliosis increases with MS duration, concomitantly with neuronal and oligodendroglial degeneration,” Dr. Masvekar said. “Microglial activation and toxic astrogliosis likely partake in CNS tissue destruction and enhance MS severity.”
This study, which was recently published in Multiple Sclerosis and Related Disorders (2019 Feb;28:34-43), was supported by the intramural research program at NIAID.
jremaly@mdedge.com
SOURCE: Masvekar RR et al. ACTRIMS Forum 2019, Abstract 281.
REPORTING FROM ACTRIMS FORUM 2019
Tanuja Chitnis: “It’s the right time” for precision medicine in MS
DALLAS – In an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, the conference’s cochair, Tanuja Chitnis, MD, explained why this is the right time to take a deep dive into what precision medicine means in MS, for patients and physicians alike.
“We chose the topic of precision medicine for this forum because it’s a really timely issue,” said Dr. Chitnis, noting that there are now over 16 approved treatments for MS, and an increasing recognition that “not every patient has the same disease course.”
“It’s the right time to think about individualized treatment, and not a one-size-fits-all approach,” she said, noting that clinicians and patients stand to benefit from guidance about treatment choices.
“In addition, we are aided by the number of biomarkers that are becoming available,” including quantitative MRI and serum biomarkers. “I think we – as a field – need to understand how to use these in clinical settings in order to guide treatment decisions,” said Dr. Chitnis, professor of neurology at Harvard Medical School, Boston.
Advances in data science are allowing the connection of disparate kinds of data for discovery and hypothesis testing and validation, said Dr. Chitnis, who serves as medical director for the large longitudinal CLIMB study. The study follows about 2,000 patients who have yearly neurologic examinations and brain MRI; serum biomarkers and self-report data are also acquired annually.
“Network science can help in the precision medicine approach to multiple sclerosis, because we have a very clear understanding that MS is a complex disease. It is not one gene; it is not one modality,” she said.
Dr. Chitnis reported that she has received research funding from multiple pharmaceutical companies.
koakes@mdedge.com
DALLAS – In an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, the conference’s cochair, Tanuja Chitnis, MD, explained why this is the right time to take a deep dive into what precision medicine means in MS, for patients and physicians alike.
“We chose the topic of precision medicine for this forum because it’s a really timely issue,” said Dr. Chitnis, noting that there are now over 16 approved treatments for MS, and an increasing recognition that “not every patient has the same disease course.”
“It’s the right time to think about individualized treatment, and not a one-size-fits-all approach,” she said, noting that clinicians and patients stand to benefit from guidance about treatment choices.
“In addition, we are aided by the number of biomarkers that are becoming available,” including quantitative MRI and serum biomarkers. “I think we – as a field – need to understand how to use these in clinical settings in order to guide treatment decisions,” said Dr. Chitnis, professor of neurology at Harvard Medical School, Boston.
Advances in data science are allowing the connection of disparate kinds of data for discovery and hypothesis testing and validation, said Dr. Chitnis, who serves as medical director for the large longitudinal CLIMB study. The study follows about 2,000 patients who have yearly neurologic examinations and brain MRI; serum biomarkers and self-report data are also acquired annually.
“Network science can help in the precision medicine approach to multiple sclerosis, because we have a very clear understanding that MS is a complex disease. It is not one gene; it is not one modality,” she said.
Dr. Chitnis reported that she has received research funding from multiple pharmaceutical companies.
koakes@mdedge.com
DALLAS – In an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, the conference’s cochair, Tanuja Chitnis, MD, explained why this is the right time to take a deep dive into what precision medicine means in MS, for patients and physicians alike.
“We chose the topic of precision medicine for this forum because it’s a really timely issue,” said Dr. Chitnis, noting that there are now over 16 approved treatments for MS, and an increasing recognition that “not every patient has the same disease course.”
“It’s the right time to think about individualized treatment, and not a one-size-fits-all approach,” she said, noting that clinicians and patients stand to benefit from guidance about treatment choices.
“In addition, we are aided by the number of biomarkers that are becoming available,” including quantitative MRI and serum biomarkers. “I think we – as a field – need to understand how to use these in clinical settings in order to guide treatment decisions,” said Dr. Chitnis, professor of neurology at Harvard Medical School, Boston.
Advances in data science are allowing the connection of disparate kinds of data for discovery and hypothesis testing and validation, said Dr. Chitnis, who serves as medical director for the large longitudinal CLIMB study. The study follows about 2,000 patients who have yearly neurologic examinations and brain MRI; serum biomarkers and self-report data are also acquired annually.
“Network science can help in the precision medicine approach to multiple sclerosis, because we have a very clear understanding that MS is a complex disease. It is not one gene; it is not one modality,” she said.
Dr. Chitnis reported that she has received research funding from multiple pharmaceutical companies.
koakes@mdedge.com
REPORTING FROM ACTRIMS FORUM 2019
Large survey reveals that few MS patients have long-term care insurance
DALLAS – A number of sociodemographic factors may influence health and disability insurance access by individuals with multiple sclerosis, including employment, age, gender, disease duration, marital status, and ethnicity, results from a large survey suggest.
“The last similar work was conducted over 10 years ago and so much has happened in the meantime, including the Great Recession and the introduction of the Affordable Care Act, that offers protection for health care but not for other important types of insurance (short- and long-term disability, long-term care, and life),” lead study author Sarah Planchon, PhD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “MS is one of the most costly chronic diseases today. That is not only because of the cost of disease-modifying therapies but also because of lost employment and income. We wanted to better understand the insurance landscape so that we could in turn educate patients and professionals about the protection these insurances offer and advise them on how to obtain these policies.”
In an effort to evaluate factors that affect insurance access in MS, Dr. Planchon, a project scientist at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic in Ohio, and her colleagues used the North American Research Committee on MS (NARCOMS), iConquerMS, and the National Multiple Sclerosis Society to survey 2,507 individuals with the disease regarding insurance, demographic, health, disability, and employment status. They used covariate-adjusted nominal logistic regression to estimate odds ratios for the likelihood of having or not having a type of insurance. The majority of respondents (83%) were female, their mean age was 54 years, 91% were white, 65% were currently married, and their mean disease duration at the time of the survey was 16 years. In addition, 43% were employed full/part-time, and 29% were not employed or retired because of disability. Nearly all respondents (96%) reported having health insurance, while 59% had life insurance, 29% had private long-term disability insurance, 18% had short-term disability insurance, and 10% had long-term care insurance.
The researchers found that employment status had the greatest impact on insurance coverage. Of those with health insurance, 33% were employed full-time, compared with 89% of those with short-term disability insurance, 42% of those with private long-term disability insurance, 44% of those with long-term care insurance, and 41% of those with life insurance. Logistic regression analyses indicated that respondents employed part time were significantly more likely to have short-term disability insurance if they were currently married (odds ratio, 4.4). Short-term disability insurance was significantly more likely among fully employed patients with disease duration of 5-10 years vs. more than 20 years (OR, 2.0). Private long-term disability insurance was significantly associated with female gender (OR, 1.6), age 50-59 years vs. younger than 40 (OR, 1.6), full-time vs. part-time employment (OR, 2.3), and shorter disease duration (ORs, 1.4-1.6 for 6-10, 11-15, and 16-20 years’ duration). Long-term care insurance was associated with older age (ORs, 2.5 and 4.3 for those aged 50-59 and 60-65 vs. younger than 40), and having excellent or good general health status vs. fair or poor (OR, 1.8). Life insurance was associated with non-Hispanic ethnicity (OR, 1.6), full-time vs. part-time employment (OR, 2.4), older age (ORs, 1.6-1.7 for ages 40-49 and 50-59 vs. younger than 40), and marital status (currently/previously married, ORs, 1.6-2.6). Considering the high rate of survey respondents with health insurance, covariate-adjusted modeling was not applicable.
“The number of people with MS who do not have long-term care insurance was surprisingly high,” Dr. Planchon said. “Although the improved treatment climate recently may decrease the long-term disability levels, we do not yet know this with certainty. A large number of people with MS are likely to need long-term care in the future, which often is a significant financial burden to families.” The findings suggest that clinical care teams “need to initiate early discussions of possible long-term needs with their patients,” she continued. “Incorporation of social work teams, who are familiar with the needs of people with MS and insurance options available to them, within MS specialty practices will bolster the comprehensive care of patients and their families.”
She acknowledged certain limitations of the study, including the low proportion of respondents who were Hispanic/Latino and African American (about 4% each). “The insurance landscape may differ in these groups compared to the majority Caucasian population who responded to this survey,” Dr. Planchon said.
The National Multiple Sclerosis Society funded the study. Dr. Planchon reported having no relevant financial disclosures.
SOURCE: Planchon S et al. ACTRIMS Forum 2019, Abstract P295.
DALLAS – A number of sociodemographic factors may influence health and disability insurance access by individuals with multiple sclerosis, including employment, age, gender, disease duration, marital status, and ethnicity, results from a large survey suggest.
“The last similar work was conducted over 10 years ago and so much has happened in the meantime, including the Great Recession and the introduction of the Affordable Care Act, that offers protection for health care but not for other important types of insurance (short- and long-term disability, long-term care, and life),” lead study author Sarah Planchon, PhD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “MS is one of the most costly chronic diseases today. That is not only because of the cost of disease-modifying therapies but also because of lost employment and income. We wanted to better understand the insurance landscape so that we could in turn educate patients and professionals about the protection these insurances offer and advise them on how to obtain these policies.”
In an effort to evaluate factors that affect insurance access in MS, Dr. Planchon, a project scientist at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic in Ohio, and her colleagues used the North American Research Committee on MS (NARCOMS), iConquerMS, and the National Multiple Sclerosis Society to survey 2,507 individuals with the disease regarding insurance, demographic, health, disability, and employment status. They used covariate-adjusted nominal logistic regression to estimate odds ratios for the likelihood of having or not having a type of insurance. The majority of respondents (83%) were female, their mean age was 54 years, 91% were white, 65% were currently married, and their mean disease duration at the time of the survey was 16 years. In addition, 43% were employed full/part-time, and 29% were not employed or retired because of disability. Nearly all respondents (96%) reported having health insurance, while 59% had life insurance, 29% had private long-term disability insurance, 18% had short-term disability insurance, and 10% had long-term care insurance.
The researchers found that employment status had the greatest impact on insurance coverage. Of those with health insurance, 33% were employed full-time, compared with 89% of those with short-term disability insurance, 42% of those with private long-term disability insurance, 44% of those with long-term care insurance, and 41% of those with life insurance. Logistic regression analyses indicated that respondents employed part time were significantly more likely to have short-term disability insurance if they were currently married (odds ratio, 4.4). Short-term disability insurance was significantly more likely among fully employed patients with disease duration of 5-10 years vs. more than 20 years (OR, 2.0). Private long-term disability insurance was significantly associated with female gender (OR, 1.6), age 50-59 years vs. younger than 40 (OR, 1.6), full-time vs. part-time employment (OR, 2.3), and shorter disease duration (ORs, 1.4-1.6 for 6-10, 11-15, and 16-20 years’ duration). Long-term care insurance was associated with older age (ORs, 2.5 and 4.3 for those aged 50-59 and 60-65 vs. younger than 40), and having excellent or good general health status vs. fair or poor (OR, 1.8). Life insurance was associated with non-Hispanic ethnicity (OR, 1.6), full-time vs. part-time employment (OR, 2.4), older age (ORs, 1.6-1.7 for ages 40-49 and 50-59 vs. younger than 40), and marital status (currently/previously married, ORs, 1.6-2.6). Considering the high rate of survey respondents with health insurance, covariate-adjusted modeling was not applicable.
“The number of people with MS who do not have long-term care insurance was surprisingly high,” Dr. Planchon said. “Although the improved treatment climate recently may decrease the long-term disability levels, we do not yet know this with certainty. A large number of people with MS are likely to need long-term care in the future, which often is a significant financial burden to families.” The findings suggest that clinical care teams “need to initiate early discussions of possible long-term needs with their patients,” she continued. “Incorporation of social work teams, who are familiar with the needs of people with MS and insurance options available to them, within MS specialty practices will bolster the comprehensive care of patients and their families.”
She acknowledged certain limitations of the study, including the low proportion of respondents who were Hispanic/Latino and African American (about 4% each). “The insurance landscape may differ in these groups compared to the majority Caucasian population who responded to this survey,” Dr. Planchon said.
The National Multiple Sclerosis Society funded the study. Dr. Planchon reported having no relevant financial disclosures.
SOURCE: Planchon S et al. ACTRIMS Forum 2019, Abstract P295.
DALLAS – A number of sociodemographic factors may influence health and disability insurance access by individuals with multiple sclerosis, including employment, age, gender, disease duration, marital status, and ethnicity, results from a large survey suggest.
“The last similar work was conducted over 10 years ago and so much has happened in the meantime, including the Great Recession and the introduction of the Affordable Care Act, that offers protection for health care but not for other important types of insurance (short- and long-term disability, long-term care, and life),” lead study author Sarah Planchon, PhD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “MS is one of the most costly chronic diseases today. That is not only because of the cost of disease-modifying therapies but also because of lost employment and income. We wanted to better understand the insurance landscape so that we could in turn educate patients and professionals about the protection these insurances offer and advise them on how to obtain these policies.”
In an effort to evaluate factors that affect insurance access in MS, Dr. Planchon, a project scientist at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic in Ohio, and her colleagues used the North American Research Committee on MS (NARCOMS), iConquerMS, and the National Multiple Sclerosis Society to survey 2,507 individuals with the disease regarding insurance, demographic, health, disability, and employment status. They used covariate-adjusted nominal logistic regression to estimate odds ratios for the likelihood of having or not having a type of insurance. The majority of respondents (83%) were female, their mean age was 54 years, 91% were white, 65% were currently married, and their mean disease duration at the time of the survey was 16 years. In addition, 43% were employed full/part-time, and 29% were not employed or retired because of disability. Nearly all respondents (96%) reported having health insurance, while 59% had life insurance, 29% had private long-term disability insurance, 18% had short-term disability insurance, and 10% had long-term care insurance.
The researchers found that employment status had the greatest impact on insurance coverage. Of those with health insurance, 33% were employed full-time, compared with 89% of those with short-term disability insurance, 42% of those with private long-term disability insurance, 44% of those with long-term care insurance, and 41% of those with life insurance. Logistic regression analyses indicated that respondents employed part time were significantly more likely to have short-term disability insurance if they were currently married (odds ratio, 4.4). Short-term disability insurance was significantly more likely among fully employed patients with disease duration of 5-10 years vs. more than 20 years (OR, 2.0). Private long-term disability insurance was significantly associated with female gender (OR, 1.6), age 50-59 years vs. younger than 40 (OR, 1.6), full-time vs. part-time employment (OR, 2.3), and shorter disease duration (ORs, 1.4-1.6 for 6-10, 11-15, and 16-20 years’ duration). Long-term care insurance was associated with older age (ORs, 2.5 and 4.3 for those aged 50-59 and 60-65 vs. younger than 40), and having excellent or good general health status vs. fair or poor (OR, 1.8). Life insurance was associated with non-Hispanic ethnicity (OR, 1.6), full-time vs. part-time employment (OR, 2.4), older age (ORs, 1.6-1.7 for ages 40-49 and 50-59 vs. younger than 40), and marital status (currently/previously married, ORs, 1.6-2.6). Considering the high rate of survey respondents with health insurance, covariate-adjusted modeling was not applicable.
“The number of people with MS who do not have long-term care insurance was surprisingly high,” Dr. Planchon said. “Although the improved treatment climate recently may decrease the long-term disability levels, we do not yet know this with certainty. A large number of people with MS are likely to need long-term care in the future, which often is a significant financial burden to families.” The findings suggest that clinical care teams “need to initiate early discussions of possible long-term needs with their patients,” she continued. “Incorporation of social work teams, who are familiar with the needs of people with MS and insurance options available to them, within MS specialty practices will bolster the comprehensive care of patients and their families.”
She acknowledged certain limitations of the study, including the low proportion of respondents who were Hispanic/Latino and African American (about 4% each). “The insurance landscape may differ in these groups compared to the majority Caucasian population who responded to this survey,” Dr. Planchon said.
The National Multiple Sclerosis Society funded the study. Dr. Planchon reported having no relevant financial disclosures.
SOURCE: Planchon S et al. ACTRIMS Forum 2019, Abstract P295.
REPORTING FROM ACTRIMS FORUM 2019
Smartphone-based visual tests for MS patients show promise
DALLAS – A battery of smartphone-based tests has been developed to help detect visual pathway disturbances in MS patients and to follow them over time.
“One of the ideas is, can you design something that’s so easy to use and quick that it’s not a burden on the patient?” Randy H. Kardon, MD, PhD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “The other was to test a couple of different modalities. By that I mean we test visual acuity, contrast sensitivity, and critical flicker fusion, which is a way of measuring the speed of conduction of nerves in the visual system.”
Dr. Kardon, professor of neuro-ophthalmology at the University of Iowa, Iowa City, worked with colleagues from Aalborg University, Denmark, to study these tests and a novel measure known as the vanishing optotype on 117 patients with MS and 103 age-matched controls. They found that the tests “very nicely discriminated between normal eyes from patients that had MS,” said Dr. Kardon, director of the Iowa City VA Center for Prevention and Treatment of Visual Loss. “Furthermore, we could determine which eyes from the MS patients had previous optic neuritis and which eyes hadn’t. We’re now looking for partners to go forward with larger studies to validate it further and refine these tests even more.”
Dr. Kardon disclosed that he has received funding from the National Eye Institute, the Department of Defense, and from VA Rehabilitation Research and Development. He was also a member of the Novartis steering committee for the OCTiMS study and is a cofounder of MedFace and FaceX.
DALLAS – A battery of smartphone-based tests has been developed to help detect visual pathway disturbances in MS patients and to follow them over time.
“One of the ideas is, can you design something that’s so easy to use and quick that it’s not a burden on the patient?” Randy H. Kardon, MD, PhD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “The other was to test a couple of different modalities. By that I mean we test visual acuity, contrast sensitivity, and critical flicker fusion, which is a way of measuring the speed of conduction of nerves in the visual system.”
Dr. Kardon, professor of neuro-ophthalmology at the University of Iowa, Iowa City, worked with colleagues from Aalborg University, Denmark, to study these tests and a novel measure known as the vanishing optotype on 117 patients with MS and 103 age-matched controls. They found that the tests “very nicely discriminated between normal eyes from patients that had MS,” said Dr. Kardon, director of the Iowa City VA Center for Prevention and Treatment of Visual Loss. “Furthermore, we could determine which eyes from the MS patients had previous optic neuritis and which eyes hadn’t. We’re now looking for partners to go forward with larger studies to validate it further and refine these tests even more.”
Dr. Kardon disclosed that he has received funding from the National Eye Institute, the Department of Defense, and from VA Rehabilitation Research and Development. He was also a member of the Novartis steering committee for the OCTiMS study and is a cofounder of MedFace and FaceX.
DALLAS – A battery of smartphone-based tests has been developed to help detect visual pathway disturbances in MS patients and to follow them over time.
“One of the ideas is, can you design something that’s so easy to use and quick that it’s not a burden on the patient?” Randy H. Kardon, MD, PhD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “The other was to test a couple of different modalities. By that I mean we test visual acuity, contrast sensitivity, and critical flicker fusion, which is a way of measuring the speed of conduction of nerves in the visual system.”
Dr. Kardon, professor of neuro-ophthalmology at the University of Iowa, Iowa City, worked with colleagues from Aalborg University, Denmark, to study these tests and a novel measure known as the vanishing optotype on 117 patients with MS and 103 age-matched controls. They found that the tests “very nicely discriminated between normal eyes from patients that had MS,” said Dr. Kardon, director of the Iowa City VA Center for Prevention and Treatment of Visual Loss. “Furthermore, we could determine which eyes from the MS patients had previous optic neuritis and which eyes hadn’t. We’re now looking for partners to go forward with larger studies to validate it further and refine these tests even more.”
Dr. Kardon disclosed that he has received funding from the National Eye Institute, the Department of Defense, and from VA Rehabilitation Research and Development. He was also a member of the Novartis steering committee for the OCTiMS study and is a cofounder of MedFace and FaceX.
REPORTING FROM ACTRIMS FORUM 2019
Spinal cord atrophy found to be accelerated in subset of RRMS patients
DALLAS – The rate of spinal cord atrophy at the C1 level is promising as a prognostic biomarker for the future conversion to secondary progressive disease in patients with relapsing remitting multiple sclerosis (RRMS), results from a novel, single-center study suggest.
“Among all magnetic resonance imaging measures, spinal cord area shows the strongest correlations with MS disability and has been shown to discriminate progressive from relapsing remitting disease subtypes,” lead study author Antje Bischof, MD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “In our work, we used a novel method to accurately measure upper cervical cord area at C1 vertebral level from brain MRI. This enabled us to show for the first time that compared to a matched group of patients who remained relapsing remitting MS over 2 decades.”
Dr. Bischof, a postdoctoral research fellow in the department of neurology at the University of California, San Francisco, and her colleagues matched 54 RRMS patients who converted to secondary progressive MS (SPMS) during the 12-year observation period with 54 RRMS patients who remained RRMS during the observation period, based on demographic and clinical criteria. Additionally, they evaluated 54 age- and sex-matched healthy controls at baseline. From routine T1-weighted brain MRI, they analyzed brain measures and spinal cord area at C1 level over 12 years to evaluate their potential to discriminate between the two matched groups during the preconversion period.
Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001). Their data suggest that this difference exists at least 4 years before conversion to SPMS. “None of the commonly used measures of the brain including global brain volumes like white matter and gray matter, regional brain volumes like thalamus, and MS lesion volumes, discriminated between the patients with relapsing remitting MS who later converted to secondary progressive disease and the patients who remained RRMS,” Dr. Bischof said.
She acknowledged certain limitations of the study, including the small sample size and the fact that the results require confirmation in a second MS cohort in order to be generalizable. “These results suggest cervical cord atrophy rate at C1 level as a prognostic biomarker for the conversion to secondary progressive MS and could be useful for treatment decisions early in the disease course, and for the study of genetic, epidemiologic, and immune variables in MS,” Dr. Bischof concluded.
She reported having no financial disclosures.
SOURCE: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
DALLAS – The rate of spinal cord atrophy at the C1 level is promising as a prognostic biomarker for the future conversion to secondary progressive disease in patients with relapsing remitting multiple sclerosis (RRMS), results from a novel, single-center study suggest.
“Among all magnetic resonance imaging measures, spinal cord area shows the strongest correlations with MS disability and has been shown to discriminate progressive from relapsing remitting disease subtypes,” lead study author Antje Bischof, MD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “In our work, we used a novel method to accurately measure upper cervical cord area at C1 vertebral level from brain MRI. This enabled us to show for the first time that compared to a matched group of patients who remained relapsing remitting MS over 2 decades.”
Dr. Bischof, a postdoctoral research fellow in the department of neurology at the University of California, San Francisco, and her colleagues matched 54 RRMS patients who converted to secondary progressive MS (SPMS) during the 12-year observation period with 54 RRMS patients who remained RRMS during the observation period, based on demographic and clinical criteria. Additionally, they evaluated 54 age- and sex-matched healthy controls at baseline. From routine T1-weighted brain MRI, they analyzed brain measures and spinal cord area at C1 level over 12 years to evaluate their potential to discriminate between the two matched groups during the preconversion period.
Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001). Their data suggest that this difference exists at least 4 years before conversion to SPMS. “None of the commonly used measures of the brain including global brain volumes like white matter and gray matter, regional brain volumes like thalamus, and MS lesion volumes, discriminated between the patients with relapsing remitting MS who later converted to secondary progressive disease and the patients who remained RRMS,” Dr. Bischof said.
She acknowledged certain limitations of the study, including the small sample size and the fact that the results require confirmation in a second MS cohort in order to be generalizable. “These results suggest cervical cord atrophy rate at C1 level as a prognostic biomarker for the conversion to secondary progressive MS and could be useful for treatment decisions early in the disease course, and for the study of genetic, epidemiologic, and immune variables in MS,” Dr. Bischof concluded.
She reported having no financial disclosures.
SOURCE: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
DALLAS – The rate of spinal cord atrophy at the C1 level is promising as a prognostic biomarker for the future conversion to secondary progressive disease in patients with relapsing remitting multiple sclerosis (RRMS), results from a novel, single-center study suggest.
“Among all magnetic resonance imaging measures, spinal cord area shows the strongest correlations with MS disability and has been shown to discriminate progressive from relapsing remitting disease subtypes,” lead study author Antje Bischof, MD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “In our work, we used a novel method to accurately measure upper cervical cord area at C1 vertebral level from brain MRI. This enabled us to show for the first time that compared to a matched group of patients who remained relapsing remitting MS over 2 decades.”
Dr. Bischof, a postdoctoral research fellow in the department of neurology at the University of California, San Francisco, and her colleagues matched 54 RRMS patients who converted to secondary progressive MS (SPMS) during the 12-year observation period with 54 RRMS patients who remained RRMS during the observation period, based on demographic and clinical criteria. Additionally, they evaluated 54 age- and sex-matched healthy controls at baseline. From routine T1-weighted brain MRI, they analyzed brain measures and spinal cord area at C1 level over 12 years to evaluate their potential to discriminate between the two matched groups during the preconversion period.
Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001). Their data suggest that this difference exists at least 4 years before conversion to SPMS. “None of the commonly used measures of the brain including global brain volumes like white matter and gray matter, regional brain volumes like thalamus, and MS lesion volumes, discriminated between the patients with relapsing remitting MS who later converted to secondary progressive disease and the patients who remained RRMS,” Dr. Bischof said.
She acknowledged certain limitations of the study, including the small sample size and the fact that the results require confirmation in a second MS cohort in order to be generalizable. “These results suggest cervical cord atrophy rate at C1 level as a prognostic biomarker for the conversion to secondary progressive MS and could be useful for treatment decisions early in the disease course, and for the study of genetic, epidemiologic, and immune variables in MS,” Dr. Bischof concluded.
She reported having no financial disclosures.
SOURCE: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
REPORTING FROM ACTRIMS FORUM 2019
Key clinical point: In patients with relapsing remitting MS (RRMS), upper cervical cord atrophy, as obtained from routine T1-weighted brain MRI, is a strong indicator of impending conversion to secondary progressive MS (SPMS).
Major finding: Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001).
Study details: A single-center, observational study of 54 RRMS patients who converted to SPMS during the 12-year observation and 54 RRMS patients who remained RRMS during the observation period.
Disclosures: Dr. Bischof reported having no financial disclosures.
Source: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
Early intensive treatment of MS may benefit patients
First-line treatment of multiple sclerosis with a high-efficacy therapy may produce better long-term outcomes than does an escalation treatment approach, data from a real-world cohort study suggest.
In a population-based cohort of patients with multiple sclerosis (MS) in southeast Wales, those who initiated treatment with a high-efficacy therapy had a smaller average increase in Expanded Disability Status Scale (EDSS) score after 5 years, compared with patients who started on moderate-efficacy therapy, researchers reported Feb. 18 in JAMA Neurology. These outcomes occurred “despite clinical surveillance and targeted escalation” in the group of patients who started on moderate-efficacy drugs, said first author Katharine Harding, PhD, of Cardiff University and the University Hospital of Wales in Cardiff and the Royal Gwent Hospital, Newport, Wales, and her colleagues. “These findings suggest that real-world escalation approaches may be inadequate to prevent unfavorable long-term outcomes and support the need for a prospective clinical trial to compare disease-modifying therapy algorithms.”
The investigators analyzed data collected between January 1998 and December 2016 from 592 patients with MS. Of the 592 patients, 104 initiated treatment with alemtuzumab (Lemtrada) or natalizumab (Tysabri), which the researchers classified as high-efficacy therapies (i.e., early intensive treatment), and 488 initiated treatment with interferons, glatiramer acetate (Copaxone), dimethyl fumarate (Tecfidera), fingolimod (Gilenya), or teriflunomide (Aubagio), which were considered moderate-efficacy therapies (i.e., escalation approach).
At baseline, patients who received early intensive treatment had higher average EDSS scores, compared with patients treated with an escalation approach (4.2 vs. 3.5). After 5 years, the average increase in EDSS score was lower among patients who received early intensive treatment, compared with patients treated with an escalation approach (0.3 vs. 1.2). The researchers adjusted for patients’ sex, age at treatment, year of starting treatment, and escalation to high-efficacy treatment in the escalation treatment approach group.
Median time to sustained accumulation of disability was 6.0 years for the early intensive therapy group and 3.1 years for the escalation therapy group, but the risk of sustained accumulation of disability did not differ between the groups after adjustment for covariates.
“Although patients were selected to receive early intensive treatment on the basis of poor prognostic factors, including more active disease, it was this patient group that had better long-term outcomes,” Dr. Harding and her colleagues wrote.
There were no treatment-related deaths in the study. Among patients who received alemtuzumab, 87% developed infusion-related adverse events, and 47% developed autoimmunity. Among patients receiving natalizumab, there were no serious adverse events and no cases of progressive multifocal leukoencephalopathy. In patients receiving moderate-efficacy disease-modifying therapies, there were seven serious adverse events (1.4%).
Dr. Harding disclosed grants from Novartis UK outside the present study. Coauthors reported honoraria, support to attend educational meetings, and travel expenses, as well as grants and salary outside the present study, from various pharmaceutical companies, including Biogen, Teva, Roche, MedDay Pharma, Merck, Genzyme, and Novartis.
SOURCE: Harding K et al. JAMA Neurol. 2019 Feb 18. doi: 10.1001/jamaneurol.2018.4905
First-line treatment of multiple sclerosis with a high-efficacy therapy may produce better long-term outcomes than does an escalation treatment approach, data from a real-world cohort study suggest.
In a population-based cohort of patients with multiple sclerosis (MS) in southeast Wales, those who initiated treatment with a high-efficacy therapy had a smaller average increase in Expanded Disability Status Scale (EDSS) score after 5 years, compared with patients who started on moderate-efficacy therapy, researchers reported Feb. 18 in JAMA Neurology. These outcomes occurred “despite clinical surveillance and targeted escalation” in the group of patients who started on moderate-efficacy drugs, said first author Katharine Harding, PhD, of Cardiff University and the University Hospital of Wales in Cardiff and the Royal Gwent Hospital, Newport, Wales, and her colleagues. “These findings suggest that real-world escalation approaches may be inadequate to prevent unfavorable long-term outcomes and support the need for a prospective clinical trial to compare disease-modifying therapy algorithms.”
The investigators analyzed data collected between January 1998 and December 2016 from 592 patients with MS. Of the 592 patients, 104 initiated treatment with alemtuzumab (Lemtrada) or natalizumab (Tysabri), which the researchers classified as high-efficacy therapies (i.e., early intensive treatment), and 488 initiated treatment with interferons, glatiramer acetate (Copaxone), dimethyl fumarate (Tecfidera), fingolimod (Gilenya), or teriflunomide (Aubagio), which were considered moderate-efficacy therapies (i.e., escalation approach).
At baseline, patients who received early intensive treatment had higher average EDSS scores, compared with patients treated with an escalation approach (4.2 vs. 3.5). After 5 years, the average increase in EDSS score was lower among patients who received early intensive treatment, compared with patients treated with an escalation approach (0.3 vs. 1.2). The researchers adjusted for patients’ sex, age at treatment, year of starting treatment, and escalation to high-efficacy treatment in the escalation treatment approach group.
Median time to sustained accumulation of disability was 6.0 years for the early intensive therapy group and 3.1 years for the escalation therapy group, but the risk of sustained accumulation of disability did not differ between the groups after adjustment for covariates.
“Although patients were selected to receive early intensive treatment on the basis of poor prognostic factors, including more active disease, it was this patient group that had better long-term outcomes,” Dr. Harding and her colleagues wrote.
There were no treatment-related deaths in the study. Among patients who received alemtuzumab, 87% developed infusion-related adverse events, and 47% developed autoimmunity. Among patients receiving natalizumab, there were no serious adverse events and no cases of progressive multifocal leukoencephalopathy. In patients receiving moderate-efficacy disease-modifying therapies, there were seven serious adverse events (1.4%).
Dr. Harding disclosed grants from Novartis UK outside the present study. Coauthors reported honoraria, support to attend educational meetings, and travel expenses, as well as grants and salary outside the present study, from various pharmaceutical companies, including Biogen, Teva, Roche, MedDay Pharma, Merck, Genzyme, and Novartis.
SOURCE: Harding K et al. JAMA Neurol. 2019 Feb 18. doi: 10.1001/jamaneurol.2018.4905
First-line treatment of multiple sclerosis with a high-efficacy therapy may produce better long-term outcomes than does an escalation treatment approach, data from a real-world cohort study suggest.
In a population-based cohort of patients with multiple sclerosis (MS) in southeast Wales, those who initiated treatment with a high-efficacy therapy had a smaller average increase in Expanded Disability Status Scale (EDSS) score after 5 years, compared with patients who started on moderate-efficacy therapy, researchers reported Feb. 18 in JAMA Neurology. These outcomes occurred “despite clinical surveillance and targeted escalation” in the group of patients who started on moderate-efficacy drugs, said first author Katharine Harding, PhD, of Cardiff University and the University Hospital of Wales in Cardiff and the Royal Gwent Hospital, Newport, Wales, and her colleagues. “These findings suggest that real-world escalation approaches may be inadequate to prevent unfavorable long-term outcomes and support the need for a prospective clinical trial to compare disease-modifying therapy algorithms.”
The investigators analyzed data collected between January 1998 and December 2016 from 592 patients with MS. Of the 592 patients, 104 initiated treatment with alemtuzumab (Lemtrada) or natalizumab (Tysabri), which the researchers classified as high-efficacy therapies (i.e., early intensive treatment), and 488 initiated treatment with interferons, glatiramer acetate (Copaxone), dimethyl fumarate (Tecfidera), fingolimod (Gilenya), or teriflunomide (Aubagio), which were considered moderate-efficacy therapies (i.e., escalation approach).
At baseline, patients who received early intensive treatment had higher average EDSS scores, compared with patients treated with an escalation approach (4.2 vs. 3.5). After 5 years, the average increase in EDSS score was lower among patients who received early intensive treatment, compared with patients treated with an escalation approach (0.3 vs. 1.2). The researchers adjusted for patients’ sex, age at treatment, year of starting treatment, and escalation to high-efficacy treatment in the escalation treatment approach group.
Median time to sustained accumulation of disability was 6.0 years for the early intensive therapy group and 3.1 years for the escalation therapy group, but the risk of sustained accumulation of disability did not differ between the groups after adjustment for covariates.
“Although patients were selected to receive early intensive treatment on the basis of poor prognostic factors, including more active disease, it was this patient group that had better long-term outcomes,” Dr. Harding and her colleagues wrote.
There were no treatment-related deaths in the study. Among patients who received alemtuzumab, 87% developed infusion-related adverse events, and 47% developed autoimmunity. Among patients receiving natalizumab, there were no serious adverse events and no cases of progressive multifocal leukoencephalopathy. In patients receiving moderate-efficacy disease-modifying therapies, there were seven serious adverse events (1.4%).
Dr. Harding disclosed grants from Novartis UK outside the present study. Coauthors reported honoraria, support to attend educational meetings, and travel expenses, as well as grants and salary outside the present study, from various pharmaceutical companies, including Biogen, Teva, Roche, MedDay Pharma, Merck, Genzyme, and Novartis.
SOURCE: Harding K et al. JAMA Neurol. 2019 Feb 18. doi: 10.1001/jamaneurol.2018.4905
FROM JAMA NEUROLOGY
Key clinical point:
Major finding: After 5 years, the average increase in Expanded Disability Status Scale score was lower among patients who received early intensive treatment, compared with patients treated with an escalation approach (0.3 vs. 1.2).
Study details: A population-based cohort study of 592 patients with MS in southeast Wales.
Disclosures: Dr. Harding disclosed grants from Novartis UK outside the present study. Coauthors reported honoraria, support to attend educational meetings, and travel expenses, as well as grants and salary outside the present study, from various pharmaceutical companies, including Biogen, Teva, Roche, MedDay Pharma, Merck, Genzyme, and Novartis.
Source: Harding K et al. JAMA Neurol. 2019 Feb 18. doi: 10.1001/jamaneurol.2018.4905.
DMTs, stem cell transplants both reduce disease progression in MS
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
FROM JAMA
Autologous Hematopoietic Stem Cells May Treat Aggressive MS Effectively
The therapy may halt disease activity and promote sustained functional improvement.
BERLIN—Autologous hematopoietic stem cell transplantation (HSCT) could prevent disease activity and promote functional recovery in patients with aggressive multiple sclerosis (MS), according to a retrospective case series presented at ECTRIMS 2018.
A review article suggested that the likelihood of achieving no evidence of disease activity (NEDA) after two years of treatment ranges between 10% and 60%
Like other highly effective therapies, HSCT has been considered to entail significant safety risks. When the European Society for Blood and Marrow Transplantation (EBMT) reviewed their data, however, they identified one death related to HSCT between 2012 and 2016. The estimated risk of death from HSCT is thus approximately 0.2%. “Mortality associated with transplantation has decreased so much that it is almost into the range of other standard disease-modifying therapies,” said Joyutpal Das, MBBS, a neuroscientist at Royal Hallamshire Hospital in Sheffield, United Kingdom.
EBMT recommended that neurologists consider HSCT for patients with highly active radiologic and clinical disease who have failed to respond to standard disease-modifying therapy. The treatment can be considered as first-line therapy for patients with exceptionally active disease who have become disabled, they added.
A Retrospective Case Series
To examine the efficacy of HSCT in this patient population, Dr. Das and colleagues conducted a retrospective case series of 20 patients with MS from five centers in various countries. The patients’ treating physicians decided that HSCT should be their first-line therapy. Dr. Das and colleagues used NEDA-3 (which includes relapses, disability progression, and MRI activity) as their primary outcome. Each patient underwent brain MRI during the first six months of treatment and at six- to 12-month intervals thereafter.
The case series included equal numbers of men and women. All patients had frequent relapses, incomplete recovery, and multiple gadolinium enhancing lesions on serial MRI scans. The lesions often affected the brainstem, cerebellum, and spine. Patients’ median age of diagnosis and median age of treatment were 28. The time between the first onset of symptoms and treatment was nine months, and that between diagnosis and treatment was five months. Patients’ median pretreatment Expanded Disability Status Scale (EDSS) score was 6.5. Median follow-up duration was 2.5 years.
EDSS Score Improved
Three patients had new lesions during the first six months of treatment, but no patients had new lesions on subsequent MRI scans. “It has been suggested … that if you want to use NEDA to measure efficacy, the patient should have rebaseline imaging after the initiation of treatment,” said Dr. Das. “If we use our six-month scan as rebaseline imaging, then we have no further disease activity on MRI scan.”
After treatment initiation, the median EDSS score decreased from 6.5 to 2. Patients’ median improvement on EDSS score was 2.5 points, which was statistically significant. Seven patients had an EDSS score improvement of 3 points or greater. EDSS score improved for all but one patient. The results suggest that HSCT induced rapid and sustained remission, said Dr. Das.
The investigators observed typical transplant-related toxicity in the population, and no patient died. One woman conceived and gave birth to a healthy baby, and one man fathered a healthy baby.
—Erik Greb
Suggested Reading
Sormani MP, Muraro PA, Saccardi R, Mancardi G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler. 2017;23(2):201-204.
The therapy may halt disease activity and promote sustained functional improvement.
The therapy may halt disease activity and promote sustained functional improvement.
BERLIN—Autologous hematopoietic stem cell transplantation (HSCT) could prevent disease activity and promote functional recovery in patients with aggressive multiple sclerosis (MS), according to a retrospective case series presented at ECTRIMS 2018.
A review article suggested that the likelihood of achieving no evidence of disease activity (NEDA) after two years of treatment ranges between 10% and 60%
Like other highly effective therapies, HSCT has been considered to entail significant safety risks. When the European Society for Blood and Marrow Transplantation (EBMT) reviewed their data, however, they identified one death related to HSCT between 2012 and 2016. The estimated risk of death from HSCT is thus approximately 0.2%. “Mortality associated with transplantation has decreased so much that it is almost into the range of other standard disease-modifying therapies,” said Joyutpal Das, MBBS, a neuroscientist at Royal Hallamshire Hospital in Sheffield, United Kingdom.
EBMT recommended that neurologists consider HSCT for patients with highly active radiologic and clinical disease who have failed to respond to standard disease-modifying therapy. The treatment can be considered as first-line therapy for patients with exceptionally active disease who have become disabled, they added.
A Retrospective Case Series
To examine the efficacy of HSCT in this patient population, Dr. Das and colleagues conducted a retrospective case series of 20 patients with MS from five centers in various countries. The patients’ treating physicians decided that HSCT should be their first-line therapy. Dr. Das and colleagues used NEDA-3 (which includes relapses, disability progression, and MRI activity) as their primary outcome. Each patient underwent brain MRI during the first six months of treatment and at six- to 12-month intervals thereafter.
The case series included equal numbers of men and women. All patients had frequent relapses, incomplete recovery, and multiple gadolinium enhancing lesions on serial MRI scans. The lesions often affected the brainstem, cerebellum, and spine. Patients’ median age of diagnosis and median age of treatment were 28. The time between the first onset of symptoms and treatment was nine months, and that between diagnosis and treatment was five months. Patients’ median pretreatment Expanded Disability Status Scale (EDSS) score was 6.5. Median follow-up duration was 2.5 years.
EDSS Score Improved
Three patients had new lesions during the first six months of treatment, but no patients had new lesions on subsequent MRI scans. “It has been suggested … that if you want to use NEDA to measure efficacy, the patient should have rebaseline imaging after the initiation of treatment,” said Dr. Das. “If we use our six-month scan as rebaseline imaging, then we have no further disease activity on MRI scan.”
After treatment initiation, the median EDSS score decreased from 6.5 to 2. Patients’ median improvement on EDSS score was 2.5 points, which was statistically significant. Seven patients had an EDSS score improvement of 3 points or greater. EDSS score improved for all but one patient. The results suggest that HSCT induced rapid and sustained remission, said Dr. Das.
The investigators observed typical transplant-related toxicity in the population, and no patient died. One woman conceived and gave birth to a healthy baby, and one man fathered a healthy baby.
—Erik Greb
Suggested Reading
Sormani MP, Muraro PA, Saccardi R, Mancardi G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler. 2017;23(2):201-204.
BERLIN—Autologous hematopoietic stem cell transplantation (HSCT) could prevent disease activity and promote functional recovery in patients with aggressive multiple sclerosis (MS), according to a retrospective case series presented at ECTRIMS 2018.
A review article suggested that the likelihood of achieving no evidence of disease activity (NEDA) after two years of treatment ranges between 10% and 60%
Like other highly effective therapies, HSCT has been considered to entail significant safety risks. When the European Society for Blood and Marrow Transplantation (EBMT) reviewed their data, however, they identified one death related to HSCT between 2012 and 2016. The estimated risk of death from HSCT is thus approximately 0.2%. “Mortality associated with transplantation has decreased so much that it is almost into the range of other standard disease-modifying therapies,” said Joyutpal Das, MBBS, a neuroscientist at Royal Hallamshire Hospital in Sheffield, United Kingdom.
EBMT recommended that neurologists consider HSCT for patients with highly active radiologic and clinical disease who have failed to respond to standard disease-modifying therapy. The treatment can be considered as first-line therapy for patients with exceptionally active disease who have become disabled, they added.
A Retrospective Case Series
To examine the efficacy of HSCT in this patient population, Dr. Das and colleagues conducted a retrospective case series of 20 patients with MS from five centers in various countries. The patients’ treating physicians decided that HSCT should be their first-line therapy. Dr. Das and colleagues used NEDA-3 (which includes relapses, disability progression, and MRI activity) as their primary outcome. Each patient underwent brain MRI during the first six months of treatment and at six- to 12-month intervals thereafter.
The case series included equal numbers of men and women. All patients had frequent relapses, incomplete recovery, and multiple gadolinium enhancing lesions on serial MRI scans. The lesions often affected the brainstem, cerebellum, and spine. Patients’ median age of diagnosis and median age of treatment were 28. The time between the first onset of symptoms and treatment was nine months, and that between diagnosis and treatment was five months. Patients’ median pretreatment Expanded Disability Status Scale (EDSS) score was 6.5. Median follow-up duration was 2.5 years.
EDSS Score Improved
Three patients had new lesions during the first six months of treatment, but no patients had new lesions on subsequent MRI scans. “It has been suggested … that if you want to use NEDA to measure efficacy, the patient should have rebaseline imaging after the initiation of treatment,” said Dr. Das. “If we use our six-month scan as rebaseline imaging, then we have no further disease activity on MRI scan.”
After treatment initiation, the median EDSS score decreased from 6.5 to 2. Patients’ median improvement on EDSS score was 2.5 points, which was statistically significant. Seven patients had an EDSS score improvement of 3 points or greater. EDSS score improved for all but one patient. The results suggest that HSCT induced rapid and sustained remission, said Dr. Das.
The investigators observed typical transplant-related toxicity in the population, and no patient died. One woman conceived and gave birth to a healthy baby, and one man fathered a healthy baby.
—Erik Greb
Suggested Reading
Sormani MP, Muraro PA, Saccardi R, Mancardi G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler. 2017;23(2):201-204.