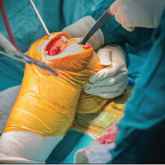
Exsanguination—bleeding to death—is the most common cause of potentially survivable death among wounded military. But a caulk gun–like device loaded with foam could extend valuable time to trauma patients and provide a “bridge to surgery,” says Leigh Anne Alexander, product manager for the U.S. Army Medical Materiel Agency (USAMMA). The foam is intended to stop massive intracavitary abdominal bleeding until the patient can get surgical care.
The device contains expandable foam that is injected into the patient. Two separate chemicals, when mixed, cause the foam to swell rapidly to about 35 times its original volume. It expands around the internal organs and can be left inside the patient for up to 3 hours.
The USAMMA, a subordinate organization of the U.S. Army Medical Research and Materiel Command, is supporting a “pivotal” clinical trial to test the safety and effectiveness of the device, which received an Investigational Device Exemption earlier this year from the FDA. The clinical trial will start in 2018.