User login
Despite an abundance of evidenced-based literature supporting monoamine oxidase inhibitors (MAOIs) as an effective treatment for depression, use of these agents has decreased drastically in the past 3 decades. A lack of industry support and the ease of use of other agents are contributing factors, but the biggest impediments to routine use of MAOIs are unfamiliarity with their efficacy advantages and concerns about adverse effects, particularly the risk of hypertensive crises and serotonin syndrome. Many misconceptions regarding these medications are based on outdated data and studies that are no longer reliable.
The goal of this 2-part review is to provide clinicians with updated information regarding MAOIs. Part 1 provides a brief description of:
- the pharmacology of nonselective irreversible MAOIs
- the mechanism by which tyramine induces hypertension
- sources of clinically significant tyramine exposure
- what to tell patients about dietary restrictions and MAOIs.
Part 2 of this guide will cover the risk of serotonin syndrome when MAOIs are combined with inhibitors of serotonin reuptake, how to initiate MAOI therapy, and augmenting MAOIs with other agents.
The pharmacology of MAOIs
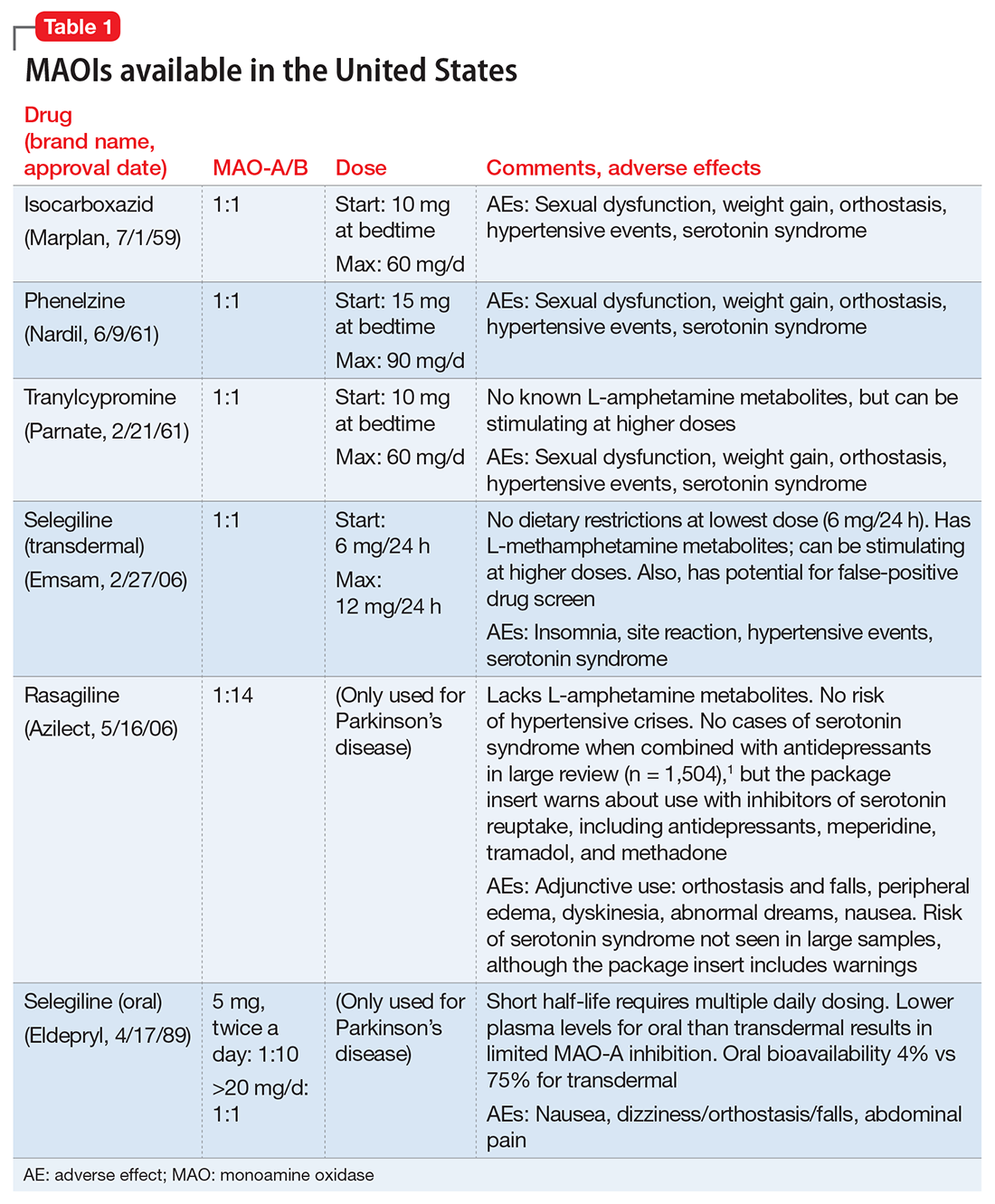
First used clinically in the 1950s to treat tuberculosis, MAOIs have a long and interesting history (see the Box “A brief history of monoamine oxidase inhibitors”). Table 11 lists MAOIs currently available in the United States, including the MAO-B–specific agent rasagiline, which is used for Parkinson’s disease.
Manipulation of the monoamines serotonin, norepinephrine, and dopamine is fundamental to managing major depressive disorder (MDD), yet only nonselective MAOIs directly promote neurotransmission of all 3 by inhibiting MAO-A and MAO-B.2 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that <50% of MDD patients achieve remission in monotherapy trials of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, or bupropion, necessitating consideration of antidepressant combinations, augmentation options, and eventually irreversible, nonselective MAOIs such as phenelzine, tranylcypromine, or isocarboxazid.3,4 Nonselective MAOIs thus offer a therapeutic opportunity for patients who do not respond to single or dual-mechanism strategies; moreover, nonselective MAOIs have compelling effectiveness data for other conditions, including panic disorder and social phobia.5 Although MAOIs are among the most effective pharmacologic agents for MDD,6 they are underutilized because of an inadequate understanding of risk mechanisms and resultant fear of catastrophic outcomes. Because of the difficulties encountered in achieving clinical remission for MDD, the nonselective MAOIs deserve a second look.
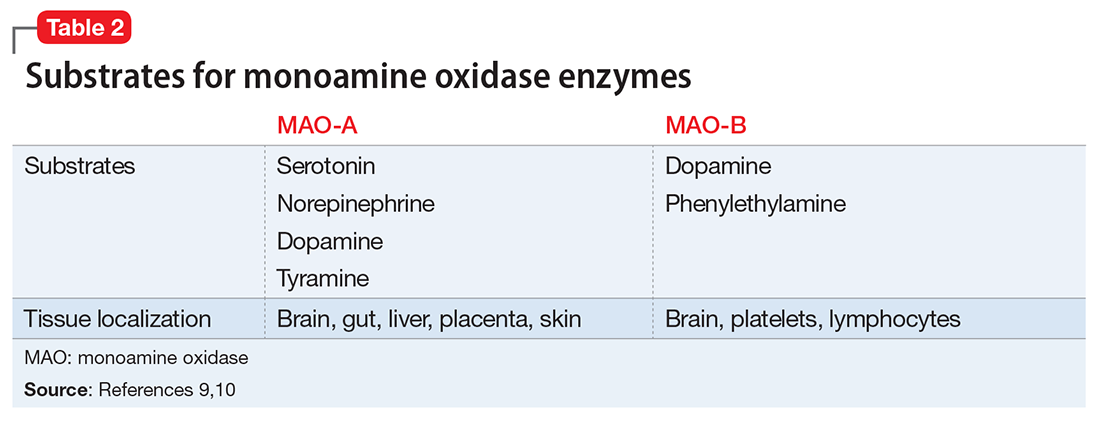
Differentiation of MAO-A from MAO-B. It is essential to understand the mechanism of action of MAOIs, specifically the impact of MAO-A inhibition. Although the enzyme MAO was known in the 1950s, it wasn’t until 1968 that Johnston7 postulated the existence of >1 form. In 1971, Goridis and Neff8 used clorgyline to examine the deamination rate by MAO of tyramine and norepinephrine. They found that tyramine appeared to be a substrate of both MAO isoforms, but only 1 of the MAO types was sensitive to the inhibitory effects of clorgyline. They also discerned that norepinephrine was only a substrate for MAO-A, and that this form of MAO was sensitive to clorgyline inhibition. Thus, the forms of MAO were characterized by their preferred substrates (Table 29,10), and then later by their tissue distribution. Phenylethylamine is a naturally occurring compound found in foods, such as chocolate, and has an in vitro pharmacology similar to amphetamine but with 1 important difference: it has a short half-life of 5 to 10 minutes after oral ingestion, and therefore no appreciable CNS impact.
Within the CNS, norepinephrine and dopamine neurons possess both MAO forms, with the MAO-A content greater than MAO-B. Serotonergic neurons only contain MAO-B.11 Outside of the CNS, MAO-A predominates, with only platelets and lymphocytes possessing MAO-B activity.11 The overall relative tissue proportions of MAO-A to MAO-B activity are: brain, 25% MAO-A, 75% MAO-B; liver, 50% MAO-A, 50% MAO-B; intestine, 80% MAO-A, 20% MAO-B; and peripheral adrenergic neurons, 90% MAO-A, 10% MAO-B.
Because of its specificity for serotonin and norepinephrine, CNS MAO-A inhibition is necessary for antidepressant effects. MAO-B inhibition by itself does not appear to raise CNS dopamine levels unless exogenous dopamine is supplied.11 All MAOIs used in the United States to treat depression are irreversible, nonselective inhibitors of MAO-A and MAO-B.
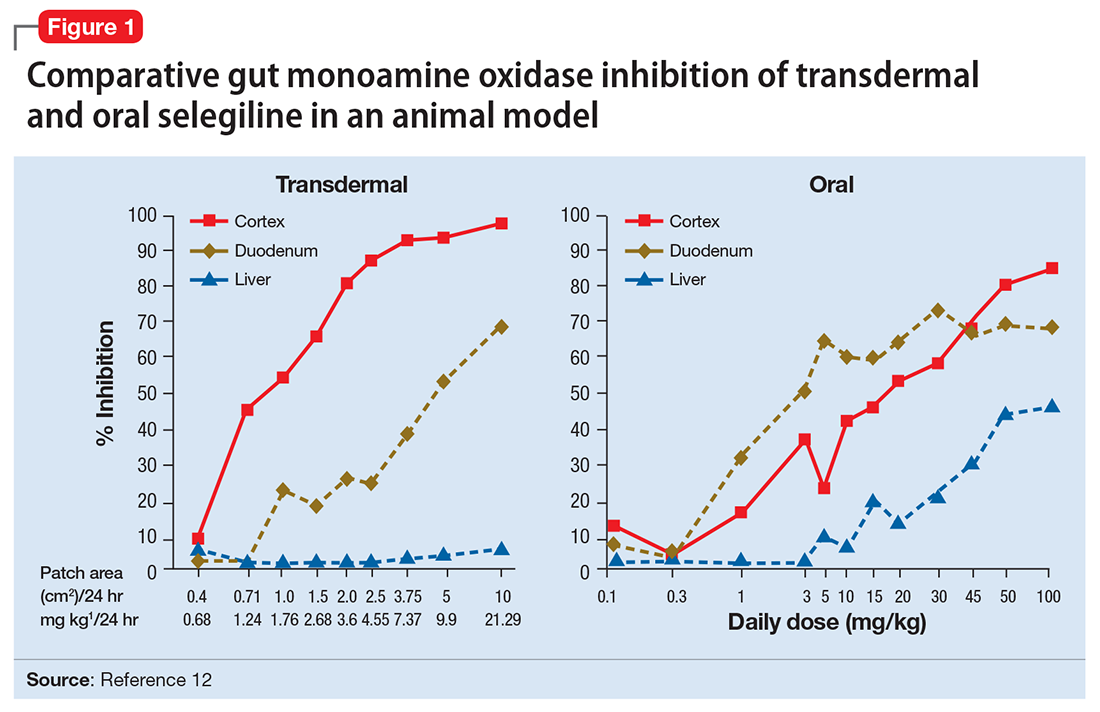
Selegiline in oral form generates low plasma levels and primarily inhibits MAO-B. The transdermal form of selegiline achieves significantly greater systemic exposure, and at these higher plasma levels selegiline is a nonselective, irreversible MAOI effective for MDD (Figure 112). Administering selegiline systemically via a transdermal patch avoids clinically significant MAOI effects in the gut, so no dietary warnings exist for the lowest dose (6 mg/24 hours), although there are warnings for the higher dosages (9 mg/24 hours and 12 mg/24 hours).
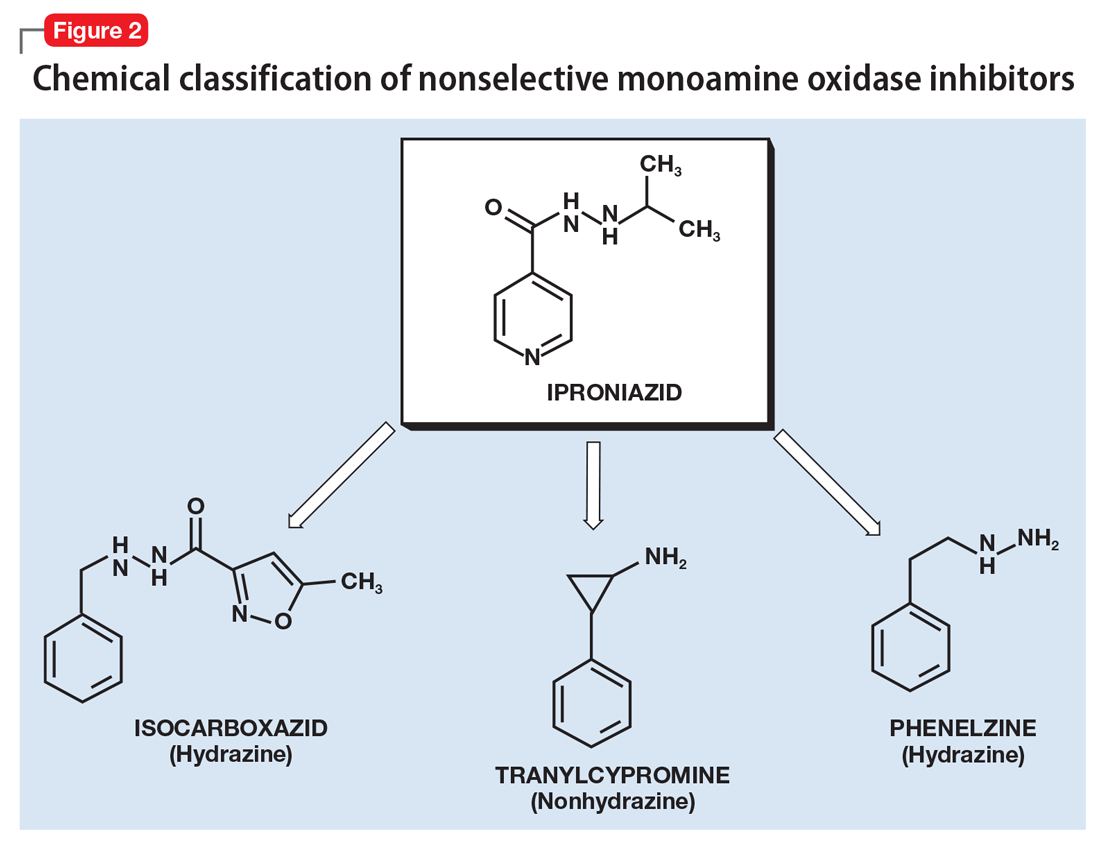
Differentiation of MAOIs by chemical class. The earliest MAOI, iproniazid, was a hydrazine derivative and exhibited hepatotoxicity,13 as did certain other hydrazine MAOIs. This lead to a search for safer hydrazine and nonhydrazine alternatives. Isocarboxazid and phenelzine are the 2 hydrazine MAOIs available in the United States, while tranylcypromine and selegiline transdermal are nonhydrazines (Figure 2).
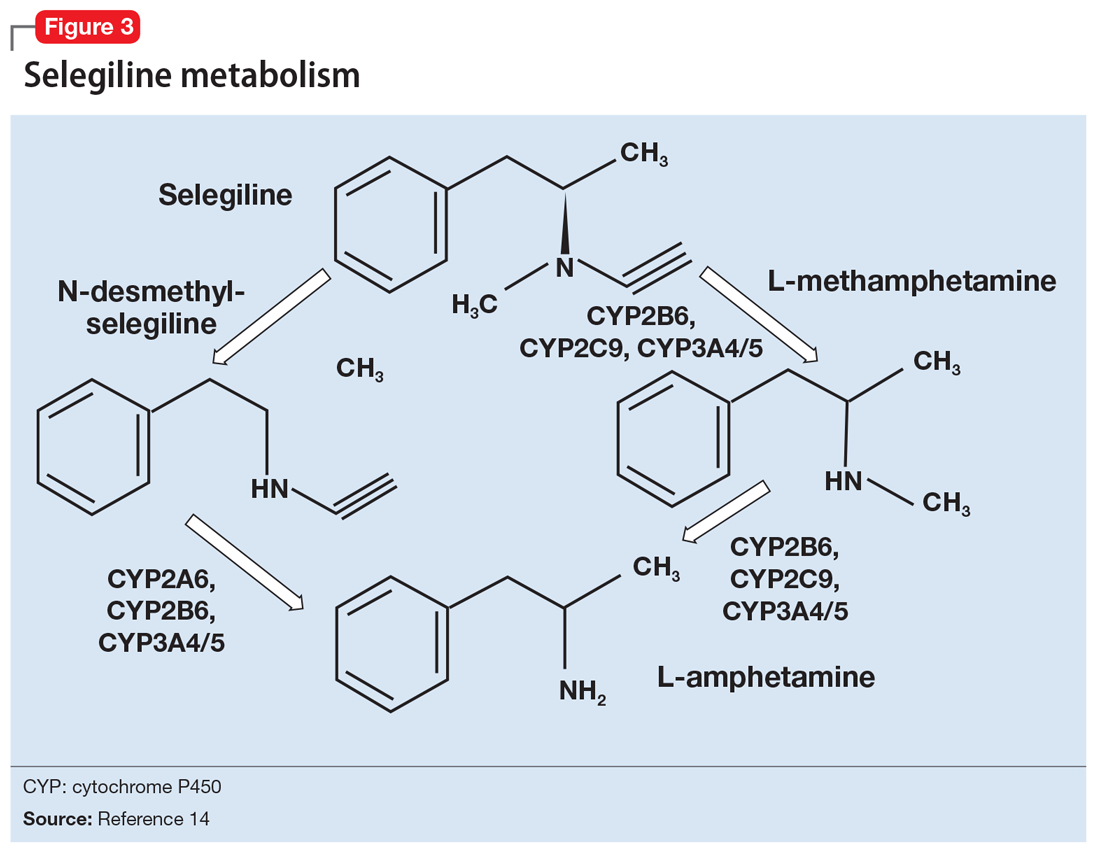
What distinguishes the nonhydrazine medication selegiline is that its metabolism generates L-amphetamine metabolites (Figure 314). This property was thought to be shared by other nonhydrazines, but recent studies indicate than neither tranylcypromine15 nor the MAO-B–selective rasagiline possess amphetamine metabolites.16 Unlike the dextro isomers, L-amphetamine structures do not inhibit dopamine reuptake or cause euphoria, but can cause stimulation (eg, sleep disturbance) by inhibiting norepinephrine reuptake, and also by interacting with the trace amine-associated receptor 1 (TAAR1), an intracellular receptor expressed within the presynaptic terminal of monoamine neurons. Activation of TAAR1 by tyramine is an important part of the hypertensive effects related to excessive tyramine exposure.17 (The importance of TAAR1 and the interaction with tyramine is discussed in the next section.) Importantly, patients taking selegiline must be warned that certain drug screens may not discriminate between levo and dextro isomers of amphetamines, and that the use of selegiline should be disclosed prior to drug testing procedures.
MAOIs and tyramine: Dietary requirements
Clinicians who are familiar with MAOIs recognize that there are dietary restrictions to minimize patients’ exposure to tyramine. As most clinicians know, significant tyramine ingestion may cause an increase in blood pressure (BP) in patients taking an MAOI, but many overestimate the prevalence of foods high in tyramine content since the original reports emerged in the early 1960s.18 In a recent monograph, one of the leading experts on MAOIs, Professor Ken Gillman, stated:
Very few foods now contain problematically high tyramine levels, that is a result of great changes in international food production methods and hygiene regulations. Cheese is the only food that, in the past, has been associated with documented fatalities resulting from hypertension. Nowadays most cheeses are quite safe, and even ‘matured’ cheeses are usually safe in healthy-sized portions. The variability of sensitivity to tyramine between individuals, and the sometimes unpredictable amount of tyramine content in foods, means a little knowledge and care are still required.19
What is tyramine? Tyramine is a biogenic amine that is virtually absent in fresh animal protein sources but is enriched after decay or fermentation.20 Modern food processing and handling methods have significantly limited the tyramine content in processed foods, with the exception of certain cheeses and sauces, as discussed below. Moreover, modern assaying techniques using high-performance liquid chromatography have generated extremely accurate assessments of the tyramine content of specific foods.21 Data published prior to 2000 are not reliable, because many of these publications employed outdated methods.17
When ingested, tyramine is metabolized by gut MAO-A, with doses up to 400 mg causing no known effects, although most people rarely ingest >25 mg during a meal.22 In addition to being a substrate for MAO-A, tyramine is also a substrate for the dopamine transporter, norepinephrine transporter (NET), the vesicular monoamine transporter 2, and TAAR1.23 Tyramine enters the cell via NET, where it interacts with TAAR1, a G protein-coupled receptor that is responsive to trace amines, such as tyramine, as well as amphetamines.20 The agonist properties at TAAR1 are the presumed site of action for the BP effects of tyramine, because binding results in potent release of norepinephrine.20,24 When tyramine is supplied to an animal in which MAO-A is inhibited, the decreased peripheral catabolism of tyramine results in markedly increased norepinephrine release by peripheral adrenergic neurons. Moreover, the absence of MAO-A activity in those neurons prevents any norepinephrine breakdown, resulting in robust synaptic norepinephrine delivery and peripheral effects.
All orally administered irreversible MAOIs potently inhibit gut and systemic MAO-A, and are susceptible to the impact of significant tyramine ingestion. The exception is selegiline transdermal (Figure 112), as appreciable gut MAO-A inhibition does not occur until doses >6 mg/24 hours are reached.22 No significant pressor response was seen in participants taking selegiline transdermal, 6 mg/24 hours for 13 days, who consumed a meal that provided 400 mg of tyramine.22 Conversely, for oral agents that produce gut MAO-A inhibition, tyramine doses as low as 8 to 10 mg (when administered as tyramine capsules) may increase systolic pressure by 30 mm Hg.25 The dietary warnings do not apply to rasagiline, which is a selective MAO-B inhibitor, although rasagiline may have an impact on resting BP; the prescribing information for rasagiline includes warnings about hypotension and hypertension.26
What to tell patients about tyramine. Although administering pure tyramine capsules can induce a measurable change in systolic BP, when ingested as food, tyramine doses <50 mg are unlikely to cause an increase in BP sufficient to warrant clinical intervention, although some individuals can be sensitive to 10 to 25 mg.19 When discussing with patients safety issues related to diet, there are a few important concepts to remember19:
- In an era when the tyramine content of foods was much higher (1960 to 1964) and MAOI users received no dietary guidance, only 14 deaths were reported among an estimated 1.5 million patients who took MAOIs.
- MAOIs do not raise BP, and their use is associated with orthostasis in some patients.
- Routine exercise or other vigorous activities (eg, weightlifting) can raise systolic pressure well above 200 mm Hg, and routine baseline systolic pressures, ranging from 180 to 220 mm Hg, do not increase the risk of subarachnoid hemorrhage.
- Hospital evaluation is needed only if a substantial amount of tyramine is ingested (eg, estimated ≥100 mg), and self-monitoring shows a systolic BP ≥220 mm Hg over a prolonged period (eg, 2 hours). Ingestion of 100 mg of tyramine would almost certainly have to be intentional, as it would require one to consume 3.5 oz of the most highly tyramine-laden cheeses.
Emphasize to patients that only a small number of highly aged cheeses, foods, and sauces contain high quantities of tyramine, and that even these foods can be enjoyed in small amounts. All patients who are prescribed an MAOI also should purchase a portable BP cuff for those rare instances when a dietary indiscretion may have occurred and the person experiences a headache within 1 to 2 hours after tyramine ingestion. Most reactions are self-limited and resolve over 2 to 4 hours.
Patients who ingest ≥100 mg of tyramine should be evaluated by a physician. Under no circumstances should a patient be given a prescription for nifedipine or other medications that can abruptly lower BP, because this may result in complications, including myocardial infarction.27,28 Counsel patients to remain calm. Some clinicians endorse the use of low doses of benzodiazepines (the equivalent of alprazolam 0.5 mg) to facilitate this, because anxiety elevates BP. A recent emergency room study of patients with an initial systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg without end organ damage demonstrated that alprazolam, 0.5 mg, was as effective as captopril, 25 mg, in lowering BP.29
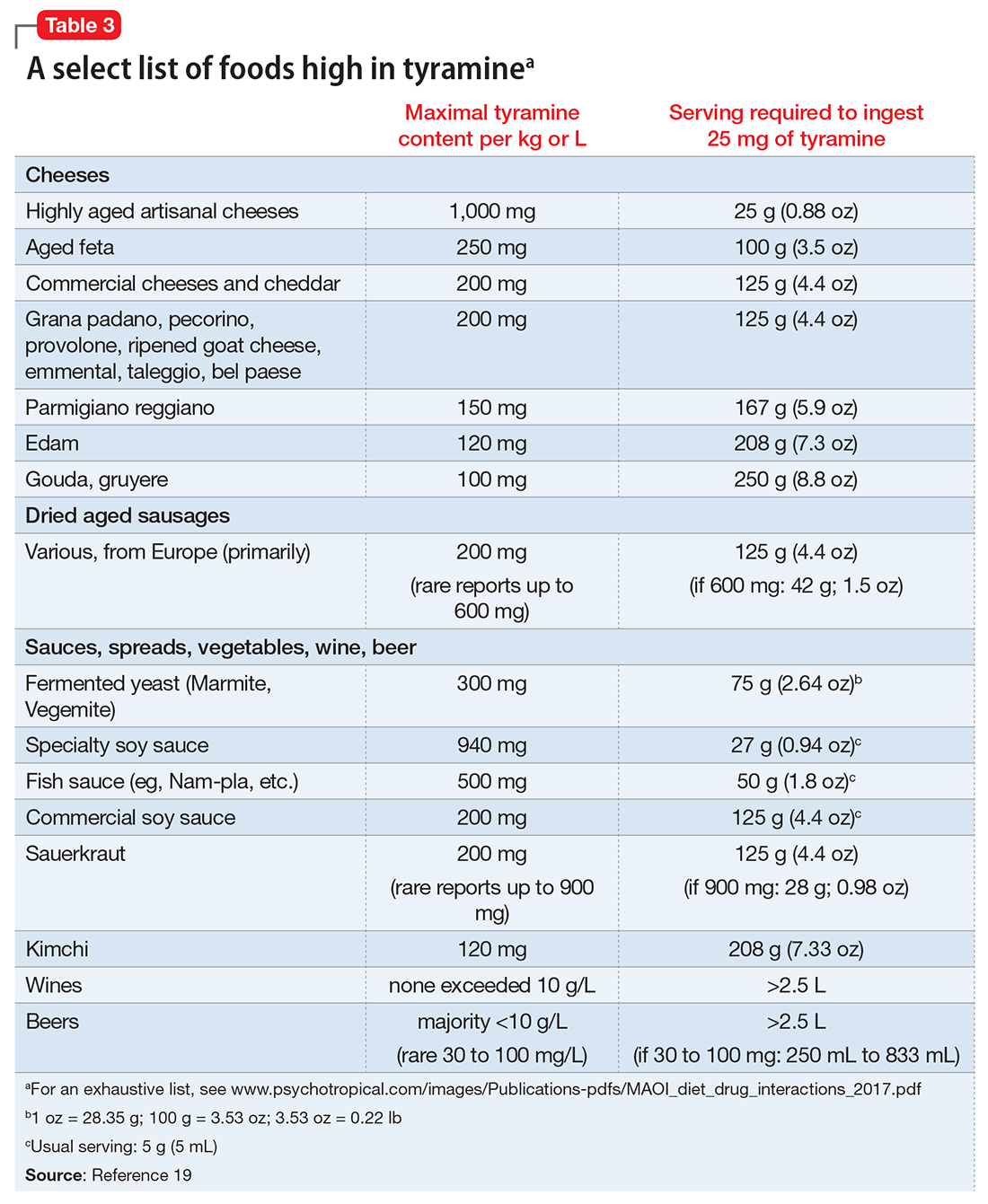
Also, tell patients that if a food is unfamiliar and highly aged or fermented, they should avoid it until they can further inquire about it. In a review, Gillman19 provides the tyramine content of an exhaustive list of cheeses, aged meats, and sauces (see Related Resources). For other products, patients often can obtain information directly from the manufacturer. In many parts of the world, assays for tyramine content are required as a demonstration of adequate product safety procedures. Even the most highly aged cheeses with a tyramine content of 1,000 g/kg can be enjoyed in small amounts (<1 oz), and most products would require heroic intake to achieve clinically significant tyramine ingestion (Table 319).
Improved education can clarify the risks
Medications such as lithium, clozapine, and MAOIs have a proven record of efficacy, yet often are underused due to fears engendered by lack of systematic training. A recent initiative in New York thus aimed to increase rates of
1. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
2. López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563-1586.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
4. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354(12):1243-1252.
5. Bandelow B, Zohar J, Hollander E, et al; World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Posttraumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychiatry. 2002;3(4):171-199.
6. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797.
7. Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285-1297.
8. Goridis C, Neff NH. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type. Br J Pharmacol. 1971;43(4):814-818.
9. Geha RM, Rebrin I, Chen K, et al. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877-9882.
10. O’Carroll AM, Fowler CJ, Phillips JP, et al. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(3):198-202.
11. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
12. Mawhinney M, Cole D, Azzaro AJ. Daily transdermal administration of selegiline to guinea-pigs preferentially inhibits monoamine oxidase activity in brain when compared with intestinal and hepatic tissues. J Pharm Pharmacol. 2003;55(1):27-34.
13. Maille F, Duvoux C, Cherqui D, et al. Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France? [in French]. Gastroenterol Clin Biol. 1999;23(10):1083-1085.
14. Salonen JS, Nyman L, Boobis AR, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003;31(9):1093-1102.
15. Iwersen S, Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20(5):301-304.
16. Müller T, Hoffmann JA, Dimpfel W, et al. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm (Vienna). 2013;120(5):761-765.
17. Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044-7048.
18. Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2(7313):849-850.
19. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-97.
20. Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
21. Fiechter G, Sivec G, Mayer HK. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:191-200.
22. Blob LF, Sharoky M, Campbell BJ, et al. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007;12(1):25-34.
23. Partilla JS, Dempsey AG, Nagpal AS, et al. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319(1):237-246.
24. Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966-8971.
25. Azzaro AJ, Vandenberg CM, Blob LF, et al. Tyramine pressor sensitivity during treatment with the selegiline transdermal system 6 mg/24 h in healthy subjects. J Clin Pharmacol. 2006;46(8):933-944.
26. Azilect [package insert]. Overland Park, KS: Teva Neuroscience, Inc.; 2014.
27. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
28. Burton TJ, Wilkinson IB. The dangers of immediate-release nifedipine in the emergency treatment of hypertension. J Hum Hypertens. 2008;22(4):301-302.
29. Yilmaz S, Pekdemir M, Tural U, et al. Comparison of alprazolam versus captopril in high blood pressure: a randomized controlled trial. Blood Press. 2011;20(4):239-243.
30. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv. 2016;67(4):369-371.
Despite an abundance of evidenced-based literature supporting monoamine oxidase inhibitors (MAOIs) as an effective treatment for depression, use of these agents has decreased drastically in the past 3 decades. A lack of industry support and the ease of use of other agents are contributing factors, but the biggest impediments to routine use of MAOIs are unfamiliarity with their efficacy advantages and concerns about adverse effects, particularly the risk of hypertensive crises and serotonin syndrome. Many misconceptions regarding these medications are based on outdated data and studies that are no longer reliable.
The goal of this 2-part review is to provide clinicians with updated information regarding MAOIs. Part 1 provides a brief description of:
- the pharmacology of nonselective irreversible MAOIs
- the mechanism by which tyramine induces hypertension
- sources of clinically significant tyramine exposure
- what to tell patients about dietary restrictions and MAOIs.
Part 2 of this guide will cover the risk of serotonin syndrome when MAOIs are combined with inhibitors of serotonin reuptake, how to initiate MAOI therapy, and augmenting MAOIs with other agents.
The pharmacology of MAOIs
First used clinically in the 1950s to treat tuberculosis, MAOIs have a long and interesting history (see the Box “A brief history of monoamine oxidase inhibitors”). Table 11 lists MAOIs currently available in the United States, including the MAO-B–specific agent rasagiline, which is used for Parkinson’s disease.
Manipulation of the monoamines serotonin, norepinephrine, and dopamine is fundamental to managing major depressive disorder (MDD), yet only nonselective MAOIs directly promote neurotransmission of all 3 by inhibiting MAO-A and MAO-B.2 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that <50% of MDD patients achieve remission in monotherapy trials of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, or bupropion, necessitating consideration of antidepressant combinations, augmentation options, and eventually irreversible, nonselective MAOIs such as phenelzine, tranylcypromine, or isocarboxazid.3,4 Nonselective MAOIs thus offer a therapeutic opportunity for patients who do not respond to single or dual-mechanism strategies; moreover, nonselective MAOIs have compelling effectiveness data for other conditions, including panic disorder and social phobia.5 Although MAOIs are among the most effective pharmacologic agents for MDD,6 they are underutilized because of an inadequate understanding of risk mechanisms and resultant fear of catastrophic outcomes. Because of the difficulties encountered in achieving clinical remission for MDD, the nonselective MAOIs deserve a second look.
Differentiation of MAO-A from MAO-B. It is essential to understand the mechanism of action of MAOIs, specifically the impact of MAO-A inhibition. Although the enzyme MAO was known in the 1950s, it wasn’t until 1968 that Johnston7 postulated the existence of >1 form. In 1971, Goridis and Neff8 used clorgyline to examine the deamination rate by MAO of tyramine and norepinephrine. They found that tyramine appeared to be a substrate of both MAO isoforms, but only 1 of the MAO types was sensitive to the inhibitory effects of clorgyline. They also discerned that norepinephrine was only a substrate for MAO-A, and that this form of MAO was sensitive to clorgyline inhibition. Thus, the forms of MAO were characterized by their preferred substrates (Table 29,10), and then later by their tissue distribution. Phenylethylamine is a naturally occurring compound found in foods, such as chocolate, and has an in vitro pharmacology similar to amphetamine but with 1 important difference: it has a short half-life of 5 to 10 minutes after oral ingestion, and therefore no appreciable CNS impact.
Within the CNS, norepinephrine and dopamine neurons possess both MAO forms, with the MAO-A content greater than MAO-B. Serotonergic neurons only contain MAO-B.11 Outside of the CNS, MAO-A predominates, with only platelets and lymphocytes possessing MAO-B activity.11 The overall relative tissue proportions of MAO-A to MAO-B activity are: brain, 25% MAO-A, 75% MAO-B; liver, 50% MAO-A, 50% MAO-B; intestine, 80% MAO-A, 20% MAO-B; and peripheral adrenergic neurons, 90% MAO-A, 10% MAO-B.
Because of its specificity for serotonin and norepinephrine, CNS MAO-A inhibition is necessary for antidepressant effects. MAO-B inhibition by itself does not appear to raise CNS dopamine levels unless exogenous dopamine is supplied.11 All MAOIs used in the United States to treat depression are irreversible, nonselective inhibitors of MAO-A and MAO-B.
Selegiline in oral form generates low plasma levels and primarily inhibits MAO-B. The transdermal form of selegiline achieves significantly greater systemic exposure, and at these higher plasma levels selegiline is a nonselective, irreversible MAOI effective for MDD (Figure 112). Administering selegiline systemically via a transdermal patch avoids clinically significant MAOI effects in the gut, so no dietary warnings exist for the lowest dose (6 mg/24 hours), although there are warnings for the higher dosages (9 mg/24 hours and 12 mg/24 hours).
Differentiation of MAOIs by chemical class. The earliest MAOI, iproniazid, was a hydrazine derivative and exhibited hepatotoxicity,13 as did certain other hydrazine MAOIs. This lead to a search for safer hydrazine and nonhydrazine alternatives. Isocarboxazid and phenelzine are the 2 hydrazine MAOIs available in the United States, while tranylcypromine and selegiline transdermal are nonhydrazines (Figure 2).
What distinguishes the nonhydrazine medication selegiline is that its metabolism generates L-amphetamine metabolites (Figure 314). This property was thought to be shared by other nonhydrazines, but recent studies indicate than neither tranylcypromine15 nor the MAO-B–selective rasagiline possess amphetamine metabolites.16 Unlike the dextro isomers, L-amphetamine structures do not inhibit dopamine reuptake or cause euphoria, but can cause stimulation (eg, sleep disturbance) by inhibiting norepinephrine reuptake, and also by interacting with the trace amine-associated receptor 1 (TAAR1), an intracellular receptor expressed within the presynaptic terminal of monoamine neurons. Activation of TAAR1 by tyramine is an important part of the hypertensive effects related to excessive tyramine exposure.17 (The importance of TAAR1 and the interaction with tyramine is discussed in the next section.) Importantly, patients taking selegiline must be warned that certain drug screens may not discriminate between levo and dextro isomers of amphetamines, and that the use of selegiline should be disclosed prior to drug testing procedures.
MAOIs and tyramine: Dietary requirements
Clinicians who are familiar with MAOIs recognize that there are dietary restrictions to minimize patients’ exposure to tyramine. As most clinicians know, significant tyramine ingestion may cause an increase in blood pressure (BP) in patients taking an MAOI, but many overestimate the prevalence of foods high in tyramine content since the original reports emerged in the early 1960s.18 In a recent monograph, one of the leading experts on MAOIs, Professor Ken Gillman, stated:
Very few foods now contain problematically high tyramine levels, that is a result of great changes in international food production methods and hygiene regulations. Cheese is the only food that, in the past, has been associated with documented fatalities resulting from hypertension. Nowadays most cheeses are quite safe, and even ‘matured’ cheeses are usually safe in healthy-sized portions. The variability of sensitivity to tyramine between individuals, and the sometimes unpredictable amount of tyramine content in foods, means a little knowledge and care are still required.19
What is tyramine? Tyramine is a biogenic amine that is virtually absent in fresh animal protein sources but is enriched after decay or fermentation.20 Modern food processing and handling methods have significantly limited the tyramine content in processed foods, with the exception of certain cheeses and sauces, as discussed below. Moreover, modern assaying techniques using high-performance liquid chromatography have generated extremely accurate assessments of the tyramine content of specific foods.21 Data published prior to 2000 are not reliable, because many of these publications employed outdated methods.17
When ingested, tyramine is metabolized by gut MAO-A, with doses up to 400 mg causing no known effects, although most people rarely ingest >25 mg during a meal.22 In addition to being a substrate for MAO-A, tyramine is also a substrate for the dopamine transporter, norepinephrine transporter (NET), the vesicular monoamine transporter 2, and TAAR1.23 Tyramine enters the cell via NET, where it interacts with TAAR1, a G protein-coupled receptor that is responsive to trace amines, such as tyramine, as well as amphetamines.20 The agonist properties at TAAR1 are the presumed site of action for the BP effects of tyramine, because binding results in potent release of norepinephrine.20,24 When tyramine is supplied to an animal in which MAO-A is inhibited, the decreased peripheral catabolism of tyramine results in markedly increased norepinephrine release by peripheral adrenergic neurons. Moreover, the absence of MAO-A activity in those neurons prevents any norepinephrine breakdown, resulting in robust synaptic norepinephrine delivery and peripheral effects.
All orally administered irreversible MAOIs potently inhibit gut and systemic MAO-A, and are susceptible to the impact of significant tyramine ingestion. The exception is selegiline transdermal (Figure 112), as appreciable gut MAO-A inhibition does not occur until doses >6 mg/24 hours are reached.22 No significant pressor response was seen in participants taking selegiline transdermal, 6 mg/24 hours for 13 days, who consumed a meal that provided 400 mg of tyramine.22 Conversely, for oral agents that produce gut MAO-A inhibition, tyramine doses as low as 8 to 10 mg (when administered as tyramine capsules) may increase systolic pressure by 30 mm Hg.25 The dietary warnings do not apply to rasagiline, which is a selective MAO-B inhibitor, although rasagiline may have an impact on resting BP; the prescribing information for rasagiline includes warnings about hypotension and hypertension.26
What to tell patients about tyramine. Although administering pure tyramine capsules can induce a measurable change in systolic BP, when ingested as food, tyramine doses <50 mg are unlikely to cause an increase in BP sufficient to warrant clinical intervention, although some individuals can be sensitive to 10 to 25 mg.19 When discussing with patients safety issues related to diet, there are a few important concepts to remember19:
- In an era when the tyramine content of foods was much higher (1960 to 1964) and MAOI users received no dietary guidance, only 14 deaths were reported among an estimated 1.5 million patients who took MAOIs.
- MAOIs do not raise BP, and their use is associated with orthostasis in some patients.
- Routine exercise or other vigorous activities (eg, weightlifting) can raise systolic pressure well above 200 mm Hg, and routine baseline systolic pressures, ranging from 180 to 220 mm Hg, do not increase the risk of subarachnoid hemorrhage.
- Hospital evaluation is needed only if a substantial amount of tyramine is ingested (eg, estimated ≥100 mg), and self-monitoring shows a systolic BP ≥220 mm Hg over a prolonged period (eg, 2 hours). Ingestion of 100 mg of tyramine would almost certainly have to be intentional, as it would require one to consume 3.5 oz of the most highly tyramine-laden cheeses.
Emphasize to patients that only a small number of highly aged cheeses, foods, and sauces contain high quantities of tyramine, and that even these foods can be enjoyed in small amounts. All patients who are prescribed an MAOI also should purchase a portable BP cuff for those rare instances when a dietary indiscretion may have occurred and the person experiences a headache within 1 to 2 hours after tyramine ingestion. Most reactions are self-limited and resolve over 2 to 4 hours.
Patients who ingest ≥100 mg of tyramine should be evaluated by a physician. Under no circumstances should a patient be given a prescription for nifedipine or other medications that can abruptly lower BP, because this may result in complications, including myocardial infarction.27,28 Counsel patients to remain calm. Some clinicians endorse the use of low doses of benzodiazepines (the equivalent of alprazolam 0.5 mg) to facilitate this, because anxiety elevates BP. A recent emergency room study of patients with an initial systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg without end organ damage demonstrated that alprazolam, 0.5 mg, was as effective as captopril, 25 mg, in lowering BP.29
Also, tell patients that if a food is unfamiliar and highly aged or fermented, they should avoid it until they can further inquire about it. In a review, Gillman19 provides the tyramine content of an exhaustive list of cheeses, aged meats, and sauces (see Related Resources). For other products, patients often can obtain information directly from the manufacturer. In many parts of the world, assays for tyramine content are required as a demonstration of adequate product safety procedures. Even the most highly aged cheeses with a tyramine content of 1,000 g/kg can be enjoyed in small amounts (<1 oz), and most products would require heroic intake to achieve clinically significant tyramine ingestion (Table 319).
Improved education can clarify the risks
Medications such as lithium, clozapine, and MAOIs have a proven record of efficacy, yet often are underused due to fears engendered by lack of systematic training. A recent initiative in New York thus aimed to increase rates of
Despite an abundance of evidenced-based literature supporting monoamine oxidase inhibitors (MAOIs) as an effective treatment for depression, use of these agents has decreased drastically in the past 3 decades. A lack of industry support and the ease of use of other agents are contributing factors, but the biggest impediments to routine use of MAOIs are unfamiliarity with their efficacy advantages and concerns about adverse effects, particularly the risk of hypertensive crises and serotonin syndrome. Many misconceptions regarding these medications are based on outdated data and studies that are no longer reliable.
The goal of this 2-part review is to provide clinicians with updated information regarding MAOIs. Part 1 provides a brief description of:
- the pharmacology of nonselective irreversible MAOIs
- the mechanism by which tyramine induces hypertension
- sources of clinically significant tyramine exposure
- what to tell patients about dietary restrictions and MAOIs.
Part 2 of this guide will cover the risk of serotonin syndrome when MAOIs are combined with inhibitors of serotonin reuptake, how to initiate MAOI therapy, and augmenting MAOIs with other agents.
The pharmacology of MAOIs
First used clinically in the 1950s to treat tuberculosis, MAOIs have a long and interesting history (see the Box “A brief history of monoamine oxidase inhibitors”). Table 11 lists MAOIs currently available in the United States, including the MAO-B–specific agent rasagiline, which is used for Parkinson’s disease.
Manipulation of the monoamines serotonin, norepinephrine, and dopamine is fundamental to managing major depressive disorder (MDD), yet only nonselective MAOIs directly promote neurotransmission of all 3 by inhibiting MAO-A and MAO-B.2 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that <50% of MDD patients achieve remission in monotherapy trials of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, or bupropion, necessitating consideration of antidepressant combinations, augmentation options, and eventually irreversible, nonselective MAOIs such as phenelzine, tranylcypromine, or isocarboxazid.3,4 Nonselective MAOIs thus offer a therapeutic opportunity for patients who do not respond to single or dual-mechanism strategies; moreover, nonselective MAOIs have compelling effectiveness data for other conditions, including panic disorder and social phobia.5 Although MAOIs are among the most effective pharmacologic agents for MDD,6 they are underutilized because of an inadequate understanding of risk mechanisms and resultant fear of catastrophic outcomes. Because of the difficulties encountered in achieving clinical remission for MDD, the nonselective MAOIs deserve a second look.
Differentiation of MAO-A from MAO-B. It is essential to understand the mechanism of action of MAOIs, specifically the impact of MAO-A inhibition. Although the enzyme MAO was known in the 1950s, it wasn’t until 1968 that Johnston7 postulated the existence of >1 form. In 1971, Goridis and Neff8 used clorgyline to examine the deamination rate by MAO of tyramine and norepinephrine. They found that tyramine appeared to be a substrate of both MAO isoforms, but only 1 of the MAO types was sensitive to the inhibitory effects of clorgyline. They also discerned that norepinephrine was only a substrate for MAO-A, and that this form of MAO was sensitive to clorgyline inhibition. Thus, the forms of MAO were characterized by their preferred substrates (Table 29,10), and then later by their tissue distribution. Phenylethylamine is a naturally occurring compound found in foods, such as chocolate, and has an in vitro pharmacology similar to amphetamine but with 1 important difference: it has a short half-life of 5 to 10 minutes after oral ingestion, and therefore no appreciable CNS impact.
Within the CNS, norepinephrine and dopamine neurons possess both MAO forms, with the MAO-A content greater than MAO-B. Serotonergic neurons only contain MAO-B.11 Outside of the CNS, MAO-A predominates, with only platelets and lymphocytes possessing MAO-B activity.11 The overall relative tissue proportions of MAO-A to MAO-B activity are: brain, 25% MAO-A, 75% MAO-B; liver, 50% MAO-A, 50% MAO-B; intestine, 80% MAO-A, 20% MAO-B; and peripheral adrenergic neurons, 90% MAO-A, 10% MAO-B.
Because of its specificity for serotonin and norepinephrine, CNS MAO-A inhibition is necessary for antidepressant effects. MAO-B inhibition by itself does not appear to raise CNS dopamine levels unless exogenous dopamine is supplied.11 All MAOIs used in the United States to treat depression are irreversible, nonselective inhibitors of MAO-A and MAO-B.
Selegiline in oral form generates low plasma levels and primarily inhibits MAO-B. The transdermal form of selegiline achieves significantly greater systemic exposure, and at these higher plasma levels selegiline is a nonselective, irreversible MAOI effective for MDD (Figure 112). Administering selegiline systemically via a transdermal patch avoids clinically significant MAOI effects in the gut, so no dietary warnings exist for the lowest dose (6 mg/24 hours), although there are warnings for the higher dosages (9 mg/24 hours and 12 mg/24 hours).
Differentiation of MAOIs by chemical class. The earliest MAOI, iproniazid, was a hydrazine derivative and exhibited hepatotoxicity,13 as did certain other hydrazine MAOIs. This lead to a search for safer hydrazine and nonhydrazine alternatives. Isocarboxazid and phenelzine are the 2 hydrazine MAOIs available in the United States, while tranylcypromine and selegiline transdermal are nonhydrazines (Figure 2).
What distinguishes the nonhydrazine medication selegiline is that its metabolism generates L-amphetamine metabolites (Figure 314). This property was thought to be shared by other nonhydrazines, but recent studies indicate than neither tranylcypromine15 nor the MAO-B–selective rasagiline possess amphetamine metabolites.16 Unlike the dextro isomers, L-amphetamine structures do not inhibit dopamine reuptake or cause euphoria, but can cause stimulation (eg, sleep disturbance) by inhibiting norepinephrine reuptake, and also by interacting with the trace amine-associated receptor 1 (TAAR1), an intracellular receptor expressed within the presynaptic terminal of monoamine neurons. Activation of TAAR1 by tyramine is an important part of the hypertensive effects related to excessive tyramine exposure.17 (The importance of TAAR1 and the interaction with tyramine is discussed in the next section.) Importantly, patients taking selegiline must be warned that certain drug screens may not discriminate between levo and dextro isomers of amphetamines, and that the use of selegiline should be disclosed prior to drug testing procedures.
MAOIs and tyramine: Dietary requirements
Clinicians who are familiar with MAOIs recognize that there are dietary restrictions to minimize patients’ exposure to tyramine. As most clinicians know, significant tyramine ingestion may cause an increase in blood pressure (BP) in patients taking an MAOI, but many overestimate the prevalence of foods high in tyramine content since the original reports emerged in the early 1960s.18 In a recent monograph, one of the leading experts on MAOIs, Professor Ken Gillman, stated:
Very few foods now contain problematically high tyramine levels, that is a result of great changes in international food production methods and hygiene regulations. Cheese is the only food that, in the past, has been associated with documented fatalities resulting from hypertension. Nowadays most cheeses are quite safe, and even ‘matured’ cheeses are usually safe in healthy-sized portions. The variability of sensitivity to tyramine between individuals, and the sometimes unpredictable amount of tyramine content in foods, means a little knowledge and care are still required.19
What is tyramine? Tyramine is a biogenic amine that is virtually absent in fresh animal protein sources but is enriched after decay or fermentation.20 Modern food processing and handling methods have significantly limited the tyramine content in processed foods, with the exception of certain cheeses and sauces, as discussed below. Moreover, modern assaying techniques using high-performance liquid chromatography have generated extremely accurate assessments of the tyramine content of specific foods.21 Data published prior to 2000 are not reliable, because many of these publications employed outdated methods.17
When ingested, tyramine is metabolized by gut MAO-A, with doses up to 400 mg causing no known effects, although most people rarely ingest >25 mg during a meal.22 In addition to being a substrate for MAO-A, tyramine is also a substrate for the dopamine transporter, norepinephrine transporter (NET), the vesicular monoamine transporter 2, and TAAR1.23 Tyramine enters the cell via NET, where it interacts with TAAR1, a G protein-coupled receptor that is responsive to trace amines, such as tyramine, as well as amphetamines.20 The agonist properties at TAAR1 are the presumed site of action for the BP effects of tyramine, because binding results in potent release of norepinephrine.20,24 When tyramine is supplied to an animal in which MAO-A is inhibited, the decreased peripheral catabolism of tyramine results in markedly increased norepinephrine release by peripheral adrenergic neurons. Moreover, the absence of MAO-A activity in those neurons prevents any norepinephrine breakdown, resulting in robust synaptic norepinephrine delivery and peripheral effects.
All orally administered irreversible MAOIs potently inhibit gut and systemic MAO-A, and are susceptible to the impact of significant tyramine ingestion. The exception is selegiline transdermal (Figure 112), as appreciable gut MAO-A inhibition does not occur until doses >6 mg/24 hours are reached.22 No significant pressor response was seen in participants taking selegiline transdermal, 6 mg/24 hours for 13 days, who consumed a meal that provided 400 mg of tyramine.22 Conversely, for oral agents that produce gut MAO-A inhibition, tyramine doses as low as 8 to 10 mg (when administered as tyramine capsules) may increase systolic pressure by 30 mm Hg.25 The dietary warnings do not apply to rasagiline, which is a selective MAO-B inhibitor, although rasagiline may have an impact on resting BP; the prescribing information for rasagiline includes warnings about hypotension and hypertension.26
What to tell patients about tyramine. Although administering pure tyramine capsules can induce a measurable change in systolic BP, when ingested as food, tyramine doses <50 mg are unlikely to cause an increase in BP sufficient to warrant clinical intervention, although some individuals can be sensitive to 10 to 25 mg.19 When discussing with patients safety issues related to diet, there are a few important concepts to remember19:
- In an era when the tyramine content of foods was much higher (1960 to 1964) and MAOI users received no dietary guidance, only 14 deaths were reported among an estimated 1.5 million patients who took MAOIs.
- MAOIs do not raise BP, and their use is associated with orthostasis in some patients.
- Routine exercise or other vigorous activities (eg, weightlifting) can raise systolic pressure well above 200 mm Hg, and routine baseline systolic pressures, ranging from 180 to 220 mm Hg, do not increase the risk of subarachnoid hemorrhage.
- Hospital evaluation is needed only if a substantial amount of tyramine is ingested (eg, estimated ≥100 mg), and self-monitoring shows a systolic BP ≥220 mm Hg over a prolonged period (eg, 2 hours). Ingestion of 100 mg of tyramine would almost certainly have to be intentional, as it would require one to consume 3.5 oz of the most highly tyramine-laden cheeses.
Emphasize to patients that only a small number of highly aged cheeses, foods, and sauces contain high quantities of tyramine, and that even these foods can be enjoyed in small amounts. All patients who are prescribed an MAOI also should purchase a portable BP cuff for those rare instances when a dietary indiscretion may have occurred and the person experiences a headache within 1 to 2 hours after tyramine ingestion. Most reactions are self-limited and resolve over 2 to 4 hours.
Patients who ingest ≥100 mg of tyramine should be evaluated by a physician. Under no circumstances should a patient be given a prescription for nifedipine or other medications that can abruptly lower BP, because this may result in complications, including myocardial infarction.27,28 Counsel patients to remain calm. Some clinicians endorse the use of low doses of benzodiazepines (the equivalent of alprazolam 0.5 mg) to facilitate this, because anxiety elevates BP. A recent emergency room study of patients with an initial systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg without end organ damage demonstrated that alprazolam, 0.5 mg, was as effective as captopril, 25 mg, in lowering BP.29
Also, tell patients that if a food is unfamiliar and highly aged or fermented, they should avoid it until they can further inquire about it. In a review, Gillman19 provides the tyramine content of an exhaustive list of cheeses, aged meats, and sauces (see Related Resources). For other products, patients often can obtain information directly from the manufacturer. In many parts of the world, assays for tyramine content are required as a demonstration of adequate product safety procedures. Even the most highly aged cheeses with a tyramine content of 1,000 g/kg can be enjoyed in small amounts (<1 oz), and most products would require heroic intake to achieve clinically significant tyramine ingestion (Table 319).
Improved education can clarify the risks
Medications such as lithium, clozapine, and MAOIs have a proven record of efficacy, yet often are underused due to fears engendered by lack of systematic training. A recent initiative in New York thus aimed to increase rates of
1. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
2. López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563-1586.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
4. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354(12):1243-1252.
5. Bandelow B, Zohar J, Hollander E, et al; World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Posttraumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychiatry. 2002;3(4):171-199.
6. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797.
7. Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285-1297.
8. Goridis C, Neff NH. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type. Br J Pharmacol. 1971;43(4):814-818.
9. Geha RM, Rebrin I, Chen K, et al. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877-9882.
10. O’Carroll AM, Fowler CJ, Phillips JP, et al. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(3):198-202.
11. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
12. Mawhinney M, Cole D, Azzaro AJ. Daily transdermal administration of selegiline to guinea-pigs preferentially inhibits monoamine oxidase activity in brain when compared with intestinal and hepatic tissues. J Pharm Pharmacol. 2003;55(1):27-34.
13. Maille F, Duvoux C, Cherqui D, et al. Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France? [in French]. Gastroenterol Clin Biol. 1999;23(10):1083-1085.
14. Salonen JS, Nyman L, Boobis AR, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003;31(9):1093-1102.
15. Iwersen S, Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20(5):301-304.
16. Müller T, Hoffmann JA, Dimpfel W, et al. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm (Vienna). 2013;120(5):761-765.
17. Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044-7048.
18. Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2(7313):849-850.
19. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-97.
20. Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
21. Fiechter G, Sivec G, Mayer HK. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:191-200.
22. Blob LF, Sharoky M, Campbell BJ, et al. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007;12(1):25-34.
23. Partilla JS, Dempsey AG, Nagpal AS, et al. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319(1):237-246.
24. Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966-8971.
25. Azzaro AJ, Vandenberg CM, Blob LF, et al. Tyramine pressor sensitivity during treatment with the selegiline transdermal system 6 mg/24 h in healthy subjects. J Clin Pharmacol. 2006;46(8):933-944.
26. Azilect [package insert]. Overland Park, KS: Teva Neuroscience, Inc.; 2014.
27. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
28. Burton TJ, Wilkinson IB. The dangers of immediate-release nifedipine in the emergency treatment of hypertension. J Hum Hypertens. 2008;22(4):301-302.
29. Yilmaz S, Pekdemir M, Tural U, et al. Comparison of alprazolam versus captopril in high blood pressure: a randomized controlled trial. Blood Press. 2011;20(4):239-243.
30. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv. 2016;67(4):369-371.
1. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
2. López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563-1586.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
4. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354(12):1243-1252.
5. Bandelow B, Zohar J, Hollander E, et al; World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Posttraumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychiatry. 2002;3(4):171-199.
6. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797.
7. Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285-1297.
8. Goridis C, Neff NH. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type. Br J Pharmacol. 1971;43(4):814-818.
9. Geha RM, Rebrin I, Chen K, et al. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877-9882.
10. O’Carroll AM, Fowler CJ, Phillips JP, et al. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(3):198-202.
11. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
12. Mawhinney M, Cole D, Azzaro AJ. Daily transdermal administration of selegiline to guinea-pigs preferentially inhibits monoamine oxidase activity in brain when compared with intestinal and hepatic tissues. J Pharm Pharmacol. 2003;55(1):27-34.
13. Maille F, Duvoux C, Cherqui D, et al. Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France? [in French]. Gastroenterol Clin Biol. 1999;23(10):1083-1085.
14. Salonen JS, Nyman L, Boobis AR, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003;31(9):1093-1102.
15. Iwersen S, Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20(5):301-304.
16. Müller T, Hoffmann JA, Dimpfel W, et al. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm (Vienna). 2013;120(5):761-765.
17. Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044-7048.
18. Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2(7313):849-850.
19. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-97.
20. Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
21. Fiechter G, Sivec G, Mayer HK. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:191-200.
22. Blob LF, Sharoky M, Campbell BJ, et al. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007;12(1):25-34.
23. Partilla JS, Dempsey AG, Nagpal AS, et al. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319(1):237-246.
24. Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966-8971.
25. Azzaro AJ, Vandenberg CM, Blob LF, et al. Tyramine pressor sensitivity during treatment with the selegiline transdermal system 6 mg/24 h in healthy subjects. J Clin Pharmacol. 2006;46(8):933-944.
26. Azilect [package insert]. Overland Park, KS: Teva Neuroscience, Inc.; 2014.
27. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
28. Burton TJ, Wilkinson IB. The dangers of immediate-release nifedipine in the emergency treatment of hypertension. J Hum Hypertens. 2008;22(4):301-302.
29. Yilmaz S, Pekdemir M, Tural U, et al. Comparison of alprazolam versus captopril in high blood pressure: a randomized controlled trial. Blood Press. 2011;20(4):239-243.
30. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv. 2016;67(4):369-371.