User login
THE CASE
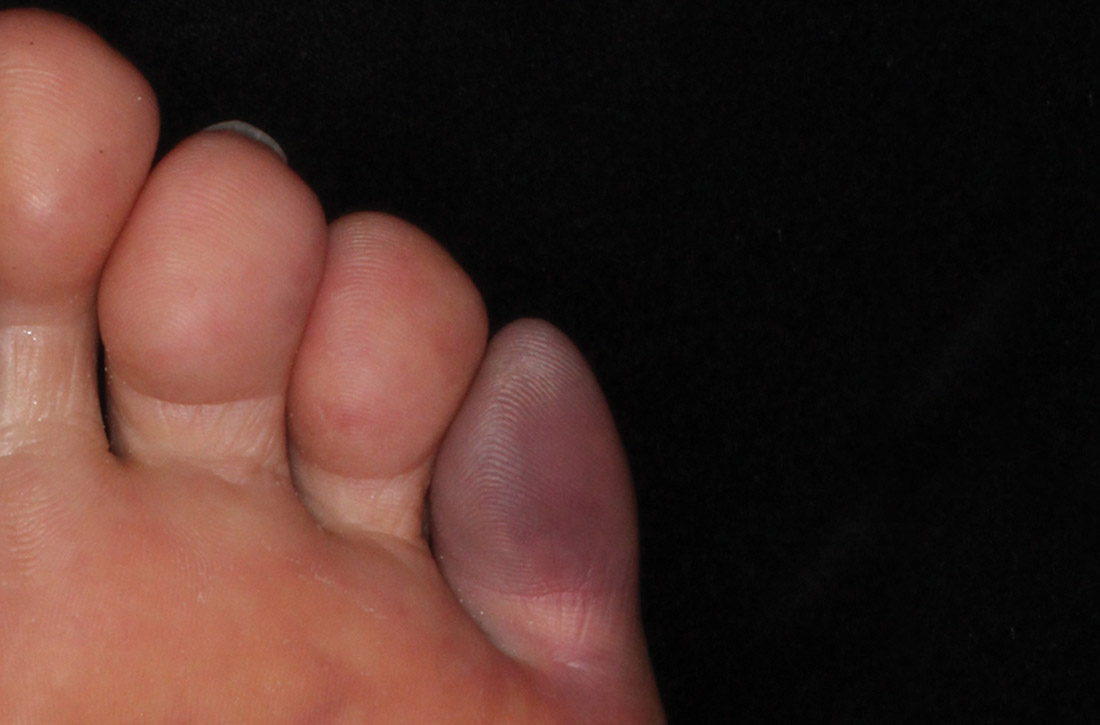
A 50-year-old man presented to the primary care office for evaluation of foot pain. The day before, his left fifth toe had become exquisitely tender. He distinctly remembered that when he awoke, there was no discoloration or pain, but the toe later became “purple.” He denied any trauma. His medical record was notable for an extensive smoking history and a family history of early cardiovascular disease.
The patient appeared well but in obvious distress, secondary to the pain. His vital signs were unremarkable. His head, neck, lung, and cardiac exams revealed no abnormalities. Physical examination revealed a left fifth toe that was dusky purple and warm to the touch. Pain disproportionate to examination was noted on the anterior aspect of the toe, with limited range of motion. The patient walked with a compensated gait. Pulses were palpable on the posterior tibial (PT) and dorsalis pedis (DP) regions.
DIAGNOSIS
Based on our exam findings, we suspected a vascular injury and recommended an emergency consult by Podiatry, for which he was scheduled the following morning. The podiatric evaluation confirmed concern for a vascular injury and prompted a request for an emergent evaluation by Vascular Surgery.
The patient was seen emergently on Day 4 for a vascular surgery evaluation. Examination at that time showed a nearly absent femoral pulse on the left side and diminished and monophasic DP and PT pulses. His left foot demonstrated nonblanchable purpura that was clinically consistent with cholesterol embolization syndrome (CES).
We calculated the patient’s ankle-brachial index, and computed tomography angiography (CTA) was performed. While results were pending, the patient was started on aspirin 81 mg, clopidogrel 75 mg, and atorvastatin 40 mg, for a suspected slowly progressing iliac artery stenosis with a resulting acute atheroembolic event.
The CTA report showed a high-grade stenosis at the bifurcation of the left iliac artery, extending into both external and internal arteries. Of note, mild atherosclerotic disease without significant occlusion and runoff to the foot was observed into the tibial arteries. The stenosis extended into the profonda femoris artery, as well.
DISCUSSION
Atherosclerotic plaques are commonly encountered in patients with atherosclerotic disease; however, there are 2 varieties of emboli that arise from these plaques and one is often overlooked.1-4 The more common of these variants, thromboemboli, originates from an atherosclerotic plaque and can become lodged in a medium or large vessel as a single embolus.
Continue to: By contrast...
By contrast, atheroemboli (commonly known as cholesterol emboli or cholesterol crystal embolization) originate from atherosclerotic plaques in the aorta or another large artery,5 which are prone to embolize if the underlying plaque experiences stress. As the plaque erodes, cholesterol crystals break off and embolize distally. These smaller crystals flood into the circulation, allowing a shower of emboli over time to occlude the arterioles. As occlusion spreads through the arterioles, multiple organ systems are affected. (It was previously thought that procedure-associated cases were common, but a literature review has not borne this out.5)
The shower of emboli often triggers a systemic inflammatory response, causing nondescript abnormalities of laboratory inflammatory markers.6,7 Interestingly, hypereosinophilia is noted in about 80% of patients with CES.8
No disease-specific testing. A confounding factor in validating the diagnosis of CES is the lack of disease-specific testing. However, CES should be considered in a patient with acute kidney injury and hypereosinophilia. Making the diagnosis requires a high degree of clinical suspicion. Any organ can be affected, although the brain, kidneys, gastrointestinal tract, skin, and skeletal muscles of the lower extremities are most frequently involved.9 If left undiagnosed, the results can be devastating: slow and chronic injury to a variety of organ systems over time, which may not be recognized as a harbinger of an insidious underlying process causing end-organ damage.
Technically, definitive diagnosis can be made by biopsy of an affected organ. However, biopsy’s utility is limited due to potential for sampling error, accessibility (as noted, the location of the involved organ[s] may make biopsy nearly impossible without additional surgical risk9), and risk of poor healing to the biopsy site.10
Treatment is two-fold: supportive care for the affected end organ and prevention of subsequent embolic events. The latter entails aggressive risk factor reduction strategies, such as smoking cessation, statin therapy, blood pressure control, and blood sugar control. Warfarin is not recommended for treatment of CES due to the risk of further plaque rupture, hemorrhage, acute and chronic renal failure, and cholesterol microembolization to other organs.11,12
Continue to: Our patient
Our patient. After testing confirmed the diagnosis, the patient underwent an angioplasty. A stent was placed in his left iliac artery. He was continued on antiplatelet and statin therapy and was again counseled regarding smoking cessation.
THE TAKEAWAY
When patients present with symptoms suggestive of a vascular origin, consider CES. Although it can affect a multitude of organs, acute kidney injury and hypereosinophilia are the most common signs. Immediate intervention is required to save the affected organ; strategizing to reduce the risk for further embolic events is also key.
Prompt recognition of vascular emergencies, including those that are harbingers of atherosclerotic disease, is essential. As clinicians, it is imperative that we use all resources to address significant population health burdens. If CES is more prevalent than commonly thought, consideration should be given to increasing education about early detection and treatment of this disorder, including the reinforcement of primary prevention and aggressive treatment of risk factors for atherosclerotic cardiovascular disease.
CORRESPONDENCE
Meagan Vermeulen, MD, FAAFP, Department of Family Medicine, Rowan University School of Osteopathic Medicine, 42 East Laurel Road, Suite 2100A, Stratford, NJ 08084; vermeulen@rowan.edu
1. Tunick PA, Kronzon I. Atheromas of the thoracic aorta: clinical and therapeutic update. J Am Coll Cardiol. 2000;35:545-554.
2. Amarenco P, Duyckaerts C, Tzourio C, et al. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221-225.
3. Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474-1479.
4. Amarenco P, Cohen A, et al; French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216-1221.
5. Ong HT, Elmsly WG, Friedlander DH. Cholesterol atheroembolism: an increasingly frequent complication of cardiac catheterisation. Med J Aust. 1991;154:412-414.
6. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
7. Saric M, Kronzon I. Cholesterol embolization syndrome. Curr Opin Cardiol. 2011;26:472-479.
8. Kasinath BS, Lewis EJ. Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Arch Intern Med. 1987;147:1384-1385.
9. Quinones A, Saric M. The cholesterol emboli syndrome in atherosclerosis. Curr Atheroscler Rep. 2013;15:315.
10. Jucgla A, Moreso F, Muniesa C, et al. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol. 2006;55:786-793.
11. Kim H, Zhen DB, Lieske JC, et al. Treatment of cholesterol embolization syndrome in the setting of an acute indication for anticoagulation therapy. J Med Cases. 2014;5:376-379.
12. Igarashi Y, Akimoto T, Kobayashi T, et al. Performing anticoagulation: a puzzling case of cholesterol embolization syndrome. Clin Med Insights Case Rep. 2017;10:1179547616684649. doi:10.1177/1179547616684649.
THE CASE
A 50-year-old man presented to the primary care office for evaluation of foot pain. The day before, his left fifth toe had become exquisitely tender. He distinctly remembered that when he awoke, there was no discoloration or pain, but the toe later became “purple.” He denied any trauma. His medical record was notable for an extensive smoking history and a family history of early cardiovascular disease.
The patient appeared well but in obvious distress, secondary to the pain. His vital signs were unremarkable. His head, neck, lung, and cardiac exams revealed no abnormalities. Physical examination revealed a left fifth toe that was dusky purple and warm to the touch. Pain disproportionate to examination was noted on the anterior aspect of the toe, with limited range of motion. The patient walked with a compensated gait. Pulses were palpable on the posterior tibial (PT) and dorsalis pedis (DP) regions.
DIAGNOSIS
Based on our exam findings, we suspected a vascular injury and recommended an emergency consult by Podiatry, for which he was scheduled the following morning. The podiatric evaluation confirmed concern for a vascular injury and prompted a request for an emergent evaluation by Vascular Surgery.
The patient was seen emergently on Day 4 for a vascular surgery evaluation. Examination at that time showed a nearly absent femoral pulse on the left side and diminished and monophasic DP and PT pulses. His left foot demonstrated nonblanchable purpura that was clinically consistent with cholesterol embolization syndrome (CES).
We calculated the patient’s ankle-brachial index, and computed tomography angiography (CTA) was performed. While results were pending, the patient was started on aspirin 81 mg, clopidogrel 75 mg, and atorvastatin 40 mg, for a suspected slowly progressing iliac artery stenosis with a resulting acute atheroembolic event.
The CTA report showed a high-grade stenosis at the bifurcation of the left iliac artery, extending into both external and internal arteries. Of note, mild atherosclerotic disease without significant occlusion and runoff to the foot was observed into the tibial arteries. The stenosis extended into the profonda femoris artery, as well.
DISCUSSION
Atherosclerotic plaques are commonly encountered in patients with atherosclerotic disease; however, there are 2 varieties of emboli that arise from these plaques and one is often overlooked.1-4 The more common of these variants, thromboemboli, originates from an atherosclerotic plaque and can become lodged in a medium or large vessel as a single embolus.
Continue to: By contrast...
By contrast, atheroemboli (commonly known as cholesterol emboli or cholesterol crystal embolization) originate from atherosclerotic plaques in the aorta or another large artery,5 which are prone to embolize if the underlying plaque experiences stress. As the plaque erodes, cholesterol crystals break off and embolize distally. These smaller crystals flood into the circulation, allowing a shower of emboli over time to occlude the arterioles. As occlusion spreads through the arterioles, multiple organ systems are affected. (It was previously thought that procedure-associated cases were common, but a literature review has not borne this out.5)
The shower of emboli often triggers a systemic inflammatory response, causing nondescript abnormalities of laboratory inflammatory markers.6,7 Interestingly, hypereosinophilia is noted in about 80% of patients with CES.8
No disease-specific testing. A confounding factor in validating the diagnosis of CES is the lack of disease-specific testing. However, CES should be considered in a patient with acute kidney injury and hypereosinophilia. Making the diagnosis requires a high degree of clinical suspicion. Any organ can be affected, although the brain, kidneys, gastrointestinal tract, skin, and skeletal muscles of the lower extremities are most frequently involved.9 If left undiagnosed, the results can be devastating: slow and chronic injury to a variety of organ systems over time, which may not be recognized as a harbinger of an insidious underlying process causing end-organ damage.
Technically, definitive diagnosis can be made by biopsy of an affected organ. However, biopsy’s utility is limited due to potential for sampling error, accessibility (as noted, the location of the involved organ[s] may make biopsy nearly impossible without additional surgical risk9), and risk of poor healing to the biopsy site.10
Treatment is two-fold: supportive care for the affected end organ and prevention of subsequent embolic events. The latter entails aggressive risk factor reduction strategies, such as smoking cessation, statin therapy, blood pressure control, and blood sugar control. Warfarin is not recommended for treatment of CES due to the risk of further plaque rupture, hemorrhage, acute and chronic renal failure, and cholesterol microembolization to other organs.11,12
Continue to: Our patient
Our patient. After testing confirmed the diagnosis, the patient underwent an angioplasty. A stent was placed in his left iliac artery. He was continued on antiplatelet and statin therapy and was again counseled regarding smoking cessation.
THE TAKEAWAY
When patients present with symptoms suggestive of a vascular origin, consider CES. Although it can affect a multitude of organs, acute kidney injury and hypereosinophilia are the most common signs. Immediate intervention is required to save the affected organ; strategizing to reduce the risk for further embolic events is also key.
Prompt recognition of vascular emergencies, including those that are harbingers of atherosclerotic disease, is essential. As clinicians, it is imperative that we use all resources to address significant population health burdens. If CES is more prevalent than commonly thought, consideration should be given to increasing education about early detection and treatment of this disorder, including the reinforcement of primary prevention and aggressive treatment of risk factors for atherosclerotic cardiovascular disease.
CORRESPONDENCE
Meagan Vermeulen, MD, FAAFP, Department of Family Medicine, Rowan University School of Osteopathic Medicine, 42 East Laurel Road, Suite 2100A, Stratford, NJ 08084; vermeulen@rowan.edu
THE CASE
A 50-year-old man presented to the primary care office for evaluation of foot pain. The day before, his left fifth toe had become exquisitely tender. He distinctly remembered that when he awoke, there was no discoloration or pain, but the toe later became “purple.” He denied any trauma. His medical record was notable for an extensive smoking history and a family history of early cardiovascular disease.
The patient appeared well but in obvious distress, secondary to the pain. His vital signs were unremarkable. His head, neck, lung, and cardiac exams revealed no abnormalities. Physical examination revealed a left fifth toe that was dusky purple and warm to the touch. Pain disproportionate to examination was noted on the anterior aspect of the toe, with limited range of motion. The patient walked with a compensated gait. Pulses were palpable on the posterior tibial (PT) and dorsalis pedis (DP) regions.
DIAGNOSIS
Based on our exam findings, we suspected a vascular injury and recommended an emergency consult by Podiatry, for which he was scheduled the following morning. The podiatric evaluation confirmed concern for a vascular injury and prompted a request for an emergent evaluation by Vascular Surgery.
The patient was seen emergently on Day 4 for a vascular surgery evaluation. Examination at that time showed a nearly absent femoral pulse on the left side and diminished and monophasic DP and PT pulses. His left foot demonstrated nonblanchable purpura that was clinically consistent with cholesterol embolization syndrome (CES).
We calculated the patient’s ankle-brachial index, and computed tomography angiography (CTA) was performed. While results were pending, the patient was started on aspirin 81 mg, clopidogrel 75 mg, and atorvastatin 40 mg, for a suspected slowly progressing iliac artery stenosis with a resulting acute atheroembolic event.
The CTA report showed a high-grade stenosis at the bifurcation of the left iliac artery, extending into both external and internal arteries. Of note, mild atherosclerotic disease without significant occlusion and runoff to the foot was observed into the tibial arteries. The stenosis extended into the profonda femoris artery, as well.
DISCUSSION
Atherosclerotic plaques are commonly encountered in patients with atherosclerotic disease; however, there are 2 varieties of emboli that arise from these plaques and one is often overlooked.1-4 The more common of these variants, thromboemboli, originates from an atherosclerotic plaque and can become lodged in a medium or large vessel as a single embolus.
Continue to: By contrast...
By contrast, atheroemboli (commonly known as cholesterol emboli or cholesterol crystal embolization) originate from atherosclerotic plaques in the aorta or another large artery,5 which are prone to embolize if the underlying plaque experiences stress. As the plaque erodes, cholesterol crystals break off and embolize distally. These smaller crystals flood into the circulation, allowing a shower of emboli over time to occlude the arterioles. As occlusion spreads through the arterioles, multiple organ systems are affected. (It was previously thought that procedure-associated cases were common, but a literature review has not borne this out.5)
The shower of emboli often triggers a systemic inflammatory response, causing nondescript abnormalities of laboratory inflammatory markers.6,7 Interestingly, hypereosinophilia is noted in about 80% of patients with CES.8
No disease-specific testing. A confounding factor in validating the diagnosis of CES is the lack of disease-specific testing. However, CES should be considered in a patient with acute kidney injury and hypereosinophilia. Making the diagnosis requires a high degree of clinical suspicion. Any organ can be affected, although the brain, kidneys, gastrointestinal tract, skin, and skeletal muscles of the lower extremities are most frequently involved.9 If left undiagnosed, the results can be devastating: slow and chronic injury to a variety of organ systems over time, which may not be recognized as a harbinger of an insidious underlying process causing end-organ damage.
Technically, definitive diagnosis can be made by biopsy of an affected organ. However, biopsy’s utility is limited due to potential for sampling error, accessibility (as noted, the location of the involved organ[s] may make biopsy nearly impossible without additional surgical risk9), and risk of poor healing to the biopsy site.10
Treatment is two-fold: supportive care for the affected end organ and prevention of subsequent embolic events. The latter entails aggressive risk factor reduction strategies, such as smoking cessation, statin therapy, blood pressure control, and blood sugar control. Warfarin is not recommended for treatment of CES due to the risk of further plaque rupture, hemorrhage, acute and chronic renal failure, and cholesterol microembolization to other organs.11,12
Continue to: Our patient
Our patient. After testing confirmed the diagnosis, the patient underwent an angioplasty. A stent was placed in his left iliac artery. He was continued on antiplatelet and statin therapy and was again counseled regarding smoking cessation.
THE TAKEAWAY
When patients present with symptoms suggestive of a vascular origin, consider CES. Although it can affect a multitude of organs, acute kidney injury and hypereosinophilia are the most common signs. Immediate intervention is required to save the affected organ; strategizing to reduce the risk for further embolic events is also key.
Prompt recognition of vascular emergencies, including those that are harbingers of atherosclerotic disease, is essential. As clinicians, it is imperative that we use all resources to address significant population health burdens. If CES is more prevalent than commonly thought, consideration should be given to increasing education about early detection and treatment of this disorder, including the reinforcement of primary prevention and aggressive treatment of risk factors for atherosclerotic cardiovascular disease.
CORRESPONDENCE
Meagan Vermeulen, MD, FAAFP, Department of Family Medicine, Rowan University School of Osteopathic Medicine, 42 East Laurel Road, Suite 2100A, Stratford, NJ 08084; vermeulen@rowan.edu
1. Tunick PA, Kronzon I. Atheromas of the thoracic aorta: clinical and therapeutic update. J Am Coll Cardiol. 2000;35:545-554.
2. Amarenco P, Duyckaerts C, Tzourio C, et al. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221-225.
3. Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474-1479.
4. Amarenco P, Cohen A, et al; French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216-1221.
5. Ong HT, Elmsly WG, Friedlander DH. Cholesterol atheroembolism: an increasingly frequent complication of cardiac catheterisation. Med J Aust. 1991;154:412-414.
6. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
7. Saric M, Kronzon I. Cholesterol embolization syndrome. Curr Opin Cardiol. 2011;26:472-479.
8. Kasinath BS, Lewis EJ. Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Arch Intern Med. 1987;147:1384-1385.
9. Quinones A, Saric M. The cholesterol emboli syndrome in atherosclerosis. Curr Atheroscler Rep. 2013;15:315.
10. Jucgla A, Moreso F, Muniesa C, et al. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol. 2006;55:786-793.
11. Kim H, Zhen DB, Lieske JC, et al. Treatment of cholesterol embolization syndrome in the setting of an acute indication for anticoagulation therapy. J Med Cases. 2014;5:376-379.
12. Igarashi Y, Akimoto T, Kobayashi T, et al. Performing anticoagulation: a puzzling case of cholesterol embolization syndrome. Clin Med Insights Case Rep. 2017;10:1179547616684649. doi:10.1177/1179547616684649.
1. Tunick PA, Kronzon I. Atheromas of the thoracic aorta: clinical and therapeutic update. J Am Coll Cardiol. 2000;35:545-554.
2. Amarenco P, Duyckaerts C, Tzourio C, et al. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221-225.
3. Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474-1479.
4. Amarenco P, Cohen A, et al; French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216-1221.
5. Ong HT, Elmsly WG, Friedlander DH. Cholesterol atheroembolism: an increasingly frequent complication of cardiac catheterisation. Med J Aust. 1991;154:412-414.
6. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
7. Saric M, Kronzon I. Cholesterol embolization syndrome. Curr Opin Cardiol. 2011;26:472-479.
8. Kasinath BS, Lewis EJ. Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Arch Intern Med. 1987;147:1384-1385.
9. Quinones A, Saric M. The cholesterol emboli syndrome in atherosclerosis. Curr Atheroscler Rep. 2013;15:315.
10. Jucgla A, Moreso F, Muniesa C, et al. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol. 2006;55:786-793.
11. Kim H, Zhen DB, Lieske JC, et al. Treatment of cholesterol embolization syndrome in the setting of an acute indication for anticoagulation therapy. J Med Cases. 2014;5:376-379.
12. Igarashi Y, Akimoto T, Kobayashi T, et al. Performing anticoagulation: a puzzling case of cholesterol embolization syndrome. Clin Med Insights Case Rep. 2017;10:1179547616684649. doi:10.1177/1179547616684649.