User login

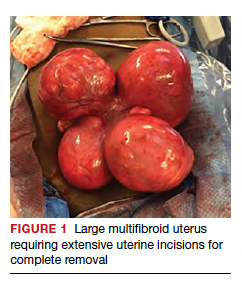
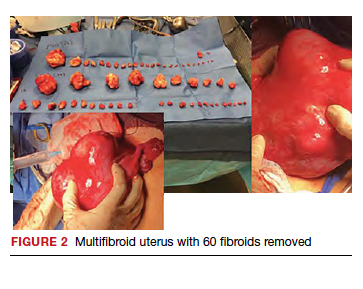
Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5
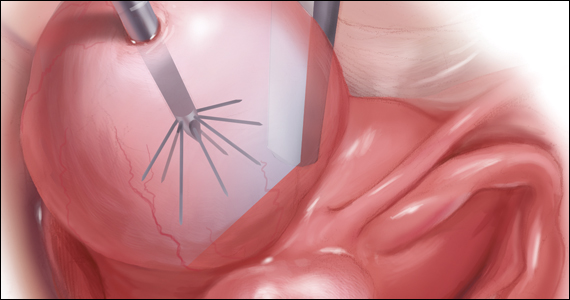
For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
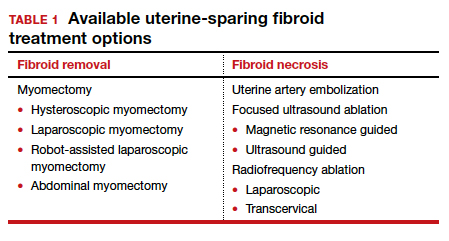
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.
Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
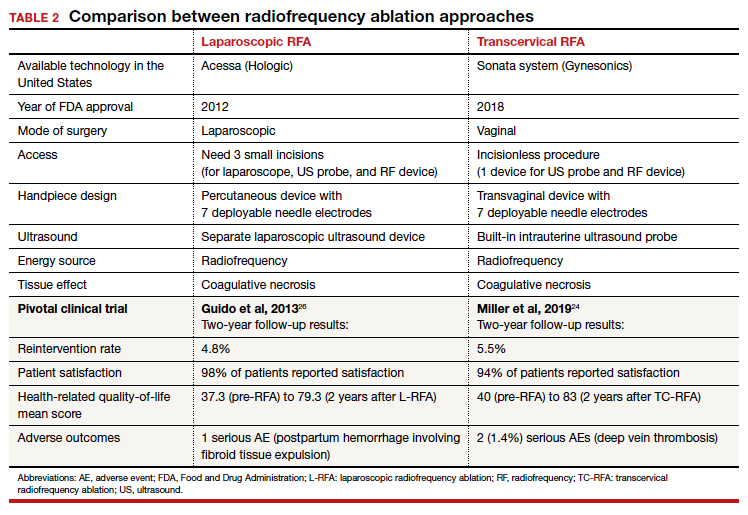
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.
Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.