User login
2023 Update on minimally invasive gynecologic surgery
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
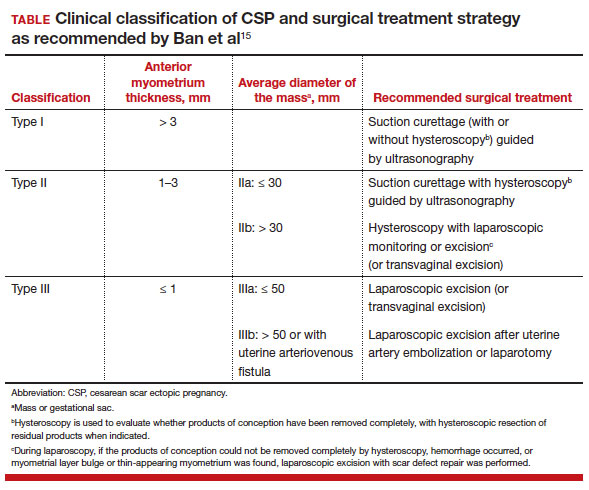
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15
Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
2021 Update on minimally invasive gynecologic surgery

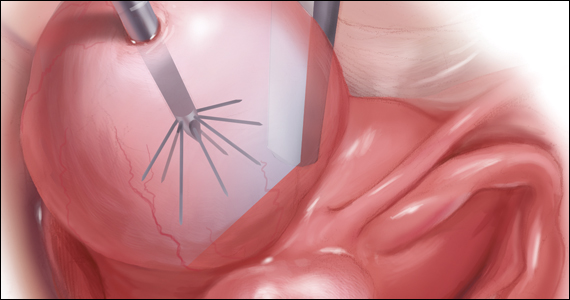
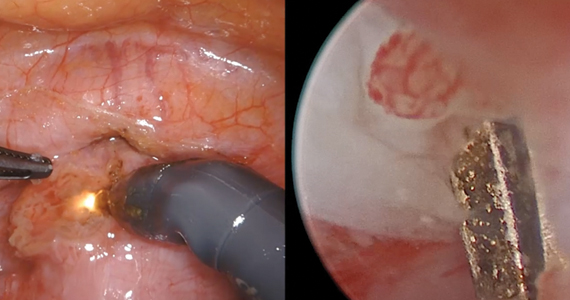
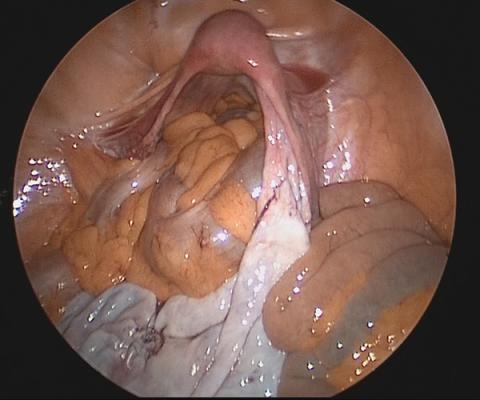
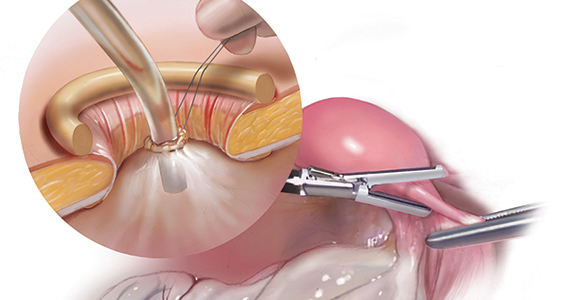
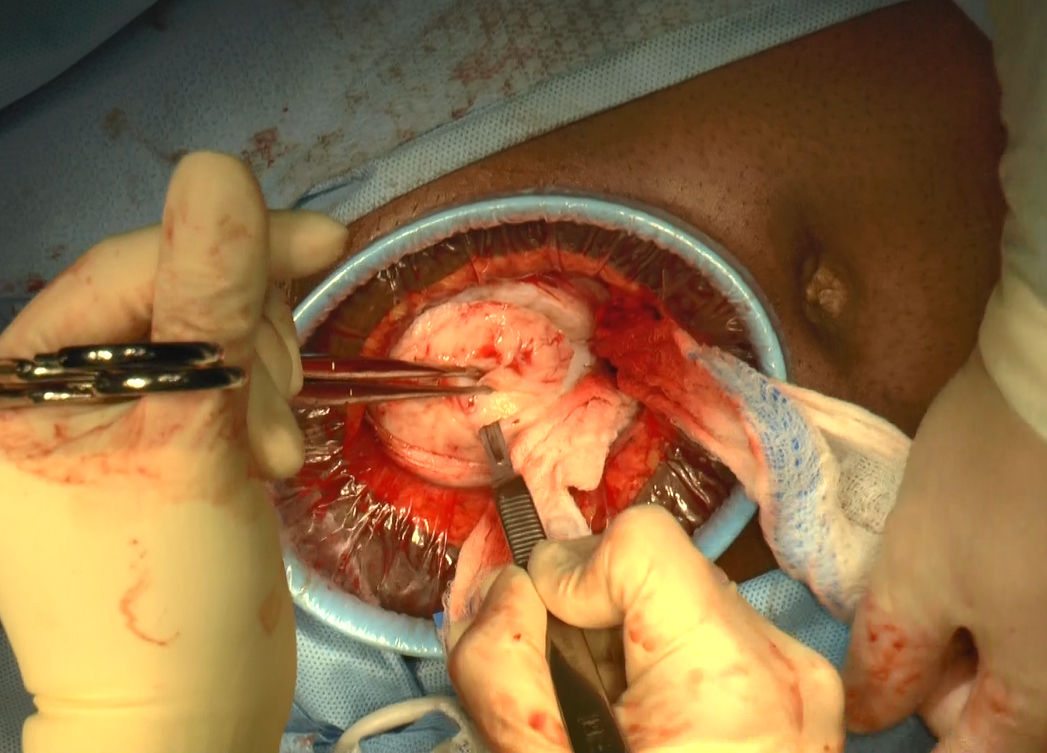
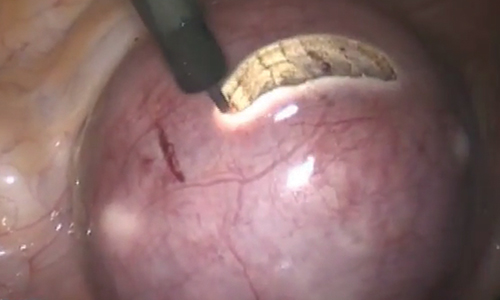
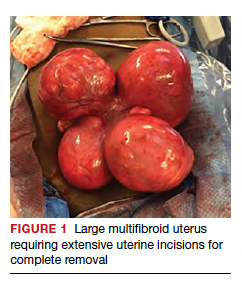
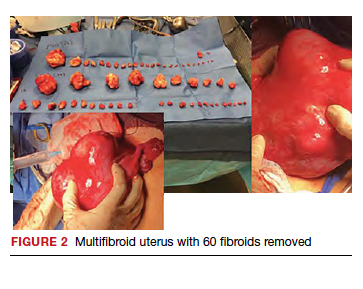
Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5
For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
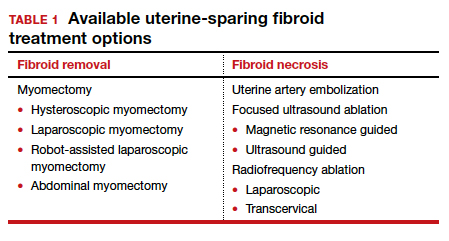
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.
Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
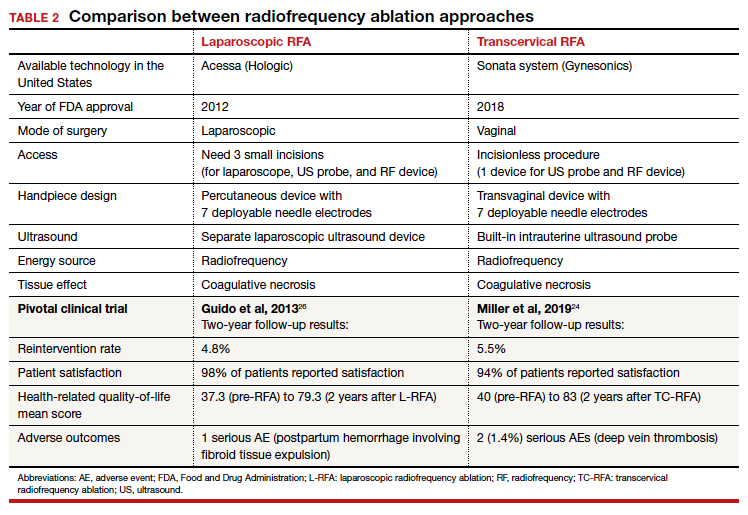
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.
Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
The surgical approach to the obliterated anterior cul-de-sac

Additional videos from SGS are available here, including these recent offerings:
• A minimally invasive modification for fascia lata mid-urethral sling
• Retroperitoneal anatomy and parametrial dissection in robotic uterine artery-sparing radical trachelectomy
• Maintaining and reclaiming hemostasis in laparoscopic surgery

Additional videos from SGS are available here, including these recent offerings:
• A minimally invasive modification for fascia lata mid-urethral sling
• Retroperitoneal anatomy and parametrial dissection in robotic uterine artery-sparing radical trachelectomy
• Maintaining and reclaiming hemostasis in laparoscopic surgery

Additional videos from SGS are available here, including these recent offerings:
• A minimally invasive modification for fascia lata mid-urethral sling
• Retroperitoneal anatomy and parametrial dissection in robotic uterine artery-sparing radical trachelectomy
• Maintaining and reclaiming hemostasis in laparoscopic surgery
Isthmocele repair: Simultaneous hysteroscopy and robotic-assisted laparoscopy

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues
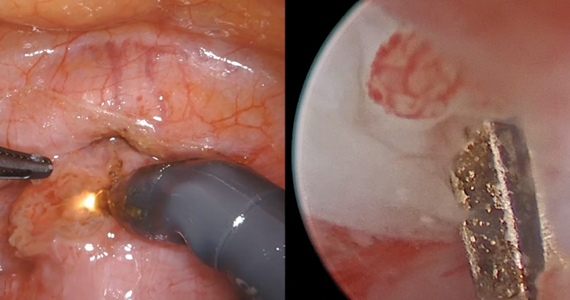
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues


An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
2019 Update on minimally invasive gynecologic surgery
Through the years, the surgical approach to hysterectomy has expanded from its early beginnings of being performed only through an abdominal or transvaginal route with traditional surgical clamps and suture. The late 1980s saw the advent of the laparoscopic-assisted vaginal hysterectomy (LAVH), and from that point forward several additional hysterectomy methods evolved, including today’s robotic approaches.
Although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, it remains one of the least performed among all available routes.1-3 In an analysis of inpatient hysterectomies published by Wright and colleagues in 2013, 16.7% of hysterectomies were performed vaginally, a number that essentially has remained steady throughout the ensuing years.4
Attempts to improve the application of vaginal hysterectomy have been made.5 These include the development of various curriculum and simulation-based medical education programs on vaginal surgical skills training and acquisition in the hopes of improving utilization.6 An interesting recent development is the rethinking of vaginal hysterectomy by several surgeons globally who are applying facets of the various hysterectomy methods to a transvaginal approach known as vaginal natural orifice transluminal endoscopic surgery (vNOTES).7,8 Unique to this thinking is the incorporation of conventional laparoscopic instrumentation.
Although I have not yet incorporated this approach in my surgical armamentarium at Columbia University Medical Center/New York–Presbyterian Hospital, I am intrigued by the possibility that this technique may serve as a rescue for vaginal hysterectomies that are at risk of conversion or of not being performed at all.9
At this time, vNOTES is not a standard of care and should be performed only by highly specialized surgeons. However, in the spirit of this Update on minimally invasive surgery and to keep our readers abreast of burgeoning techniques, I am delighted to bring you this overview by Dr. Xiaoming Guan, one of the pioneers of this surgical approach, and Dr. Tamisa Koythong and Dr. Juan Liu. I hope you find this recent development in hysterectomy of interest.
—Arnold P. Advincula, MD
Continue to: Development and evolution of NOTES...
Development and evolution of NOTES
Over the past few decades, emphasis has shifted from laparotomy to minimally invasive surgery because of its proven significant advantages in patient care, such as improved cosmesis, shorter hospital stay, shorter postoperative recovery, and decreased postoperative pain and blood loss.10 Advances in laparoendoscopic surgery and instrumentation, including robot-assisted laparoscopy (RAL), single-incision laparoscopic surgery (SILS), and most recently natural orifice transluminal endoscopic surgery (NOTES), reflect ongoing innovative developments in the field of minimally invasive surgery.
Here, we provide a brief literature review of the NOTES technique, focus on its application in gynecologic surgery, and describe how we perform NOTES at our institution.
NOTES application in gynecology
With NOTES, peritoneal access is gained through a natural orifice (such as the mouth, vagina, urethra, or anus) to perform endoscopic surgery, occasionally without requiring an abdominal incision. First described in 2004, transgastric peritoneoscopy was performed in a porcine model, and shortly thereafter the first transgastric appendectomy was performed in humans.11,12 The technique has further been adopted in cholecystectomy, appendectomy, gastrectomy, and nephrectomy procedures.13
Given rapid interest in a possible paradigm shift in the field of minimally invasive surgery, the Natural Orifice Surgery Consortiumfor Assessment and Research (NOSCAR) was formed, and the group published an article on potential barriers to accepted practice and adoption of NOTES as a realistic alternative to traditional laparoscopic surgery.14
While transgastric and transanal access to the peritoneum were initially more popular, the risk of anastomotic leaks associated with incomplete closure and subsequent infection were thought to be prohibitively high.15 Transvaginal access was considered a safer and simpler alternative, allowing for complete closure without increased risk of infection, and this is now the route through which the majority of NOTES procedures are completed.16,17
The eventual application of NOTES in the field of gynecology seemed inevitable. The American College of Obstetricians and Gynecologists stated that transvaginal surgery is the most minimally invasive and preferred surgical route in the management of patients with benign gynecologic diseases.18 However, performing it can be challenging at times due to limited visualization and lack of the required skills for single-site surgery. NOTES allows a gynecologic surgeon to improve visualization through the use of laparoendoscopic instruments and to complete surgery through a transvaginal route.
In 2012, Ahn and colleagues demonstrated the feasibility of the NOTES technique in gynecologic surgery after using it to successfully complete benign adnexal surgery in 10 patients.19 Vaginal NOTES (vNOTES) has since been further developed to include successful hysterectomy, myomectomy, sacrocolpopexy, tubal anastomosis, and even lymphadenectomy in the treatment of early- stage endometrial carcinoma.20-26 vNOTES also can be considered a rescue approach for traditional vaginal hysterectomy in instances in which it is necessary to evaluate adnexal pathology.9 Most recently, vNOTES hysterectomy has been reported with da Vinci Si or Xi robotic platforms.27,28
Continue to: Operative time, post-op stay shorter in NAOC-treated patients...
Operative time, post-op stay shorter in NAOC-treated patients
Few studies have compared outcomes with vNOTES to those with traditional laparoscopy. In 2016, Wang and colleagues compared surgical outcomes between NOTES-assisted ovarian cystectomy (NAOC) and laparoscopic ovarian cystectomy (LOC) in a case-matched study that included 277 patients.29 Although mean (SD) blood loss in patients who underwent LOC was significantly less compared with those who underwent NAOC (21.4 [14.7] mL vs 31.6 [24.1] mL; P = .028), absolute blood loss in both groups was deemed minimal. Additionally, mean (SD) operative time and postoperative stay were significantly less in patients undergoing NAOC compared with those having LOC (38.23 [10.19] minutes vs 53.82 [18.61] minutes; P≤.001; and 1.38 [0.55] days vs 1.82 [0.52] days; P≤.001; respectively).29
How vNOTES hysterectomy stacked up against TLH
In 2018, Baekelandt and colleagues compared outcomes between vNOTES hysterectomy and total laparoscopic hysterectomy (TLH) in a noninferiority single-blinded trial of 70 women.8 Compared with TLH, vNOTES hysterectomy was associated with shorter operative time (41 vs 75 minutes; P<.001), shorter hospital stay (0.8 vs 1.3 days; P = .004), and lower postoperative analgesic requirement (8 vs 14 U; P = .006). Additionally, there were no differences between the 2 groups in postoperative infection rate, intraoperative complications, or hospital readmissions within 6 weeks.8
Clearly, vNOTES is the next exciting development in minimally invasive surgery, improving patient outcomes and satisfaction with truly scarless surgery. Compared with traditional transvaginal surgery, vNOTES has the advantage of improved visualization with laparoendoscopic guidance, and it may be beneficial even for patients previously thought to have relative contraindications to successful completion of transvaginal surgery, such as nulliparity or a narrow introitus.
Approach for performing vNOTES procedures
At our institution, Baylor College of Medicine, the majority of gynecologic surgeries are performed via either transumbilical robot-assisted single-incision laparoscopy or vNOTES. Preoperative selection of appropriate candidates for vNOTES includes:
- low suspicion for or prior diagnosis of endometriosis with obliteration of the posterior cul-de-sac
- no surgical history suggestive of severe adhesive disease, and
- adequate vaginal sidewall access and sufficient descent for instrumentation for entry into the peritoneal cavity.
In general, a key concept in vNOTES is "vaginal pull, laparoscopic push," which means that the surgeon must pull the cervix while performing vaginal entry and then push the uterus back in the peritoneal cavity to increase surgical space during laparoscopic surgery.
Continue to: Overview of vNOTES steps...
Overview of vNOTES steps
Below we break down a description of vNOTES in 6 sections. Our patients are always placed in dorsal lithotomy position with TrenGuard (D.A. Surgical) Trendelenburg restraint. We prep the abdomen in case we need to convert to transabdominal surgery via transumbilical single-incision laparoscopic surgery or traditional laparoscopic surgery.
1. Vaginal entry
Accessing the peritoneal cavity through the vagina initially proceeds like a vaginal hysterectomy. We inject dilute vasopressin (20 U in 20 mL of normal saline) circumferentially in the cervix (for hysterectomy) or in the posterior cervix in the cervicovaginal junction (for adnexal surgery without hysterectomy) for vasoconstriction and hydrodissection.
We then incise the vaginal mucosa circumferentially with electrosurgical cautery and follow with posterior colpotomy. We find that reapproximating the posterior peritoneum to the posterior vagina with either figure-of-8 stitches or a running stitch of polyglactin 910 suture (2-0 Vicryl) assists in port placement, bleeding at the peritoneal edge, and closure of the cuff or colpotomy at the end of the case. We tag this suture with a curved hemostat.
Depending on whether a hysterectomy is being performed, anterior colpotomy is made. Again, the anterior peritoneum is then tagged to the anterior vaginal cuff in similar fashion, and this suture is tagged with a different instrument; we typically use a straight hemostat or Sarot clamp (FIGURE 1).
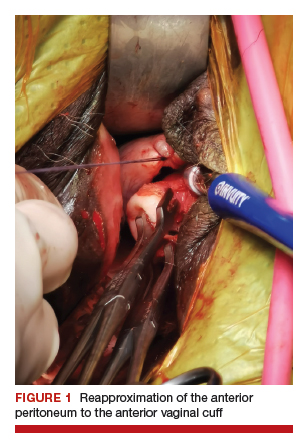
2. Traditional vaginal hysterectomy
After colpotomy, we prefer to perform progressive clamping of the broad ligament from the uterosacral and cardinal ligaments to the level of uterine artery as in traditional vaginal hysterectomy, if feasible.
3. Single-site port placement
The assembled GelPOINT Mini advanced access platform (Applied Medical) (FIGURE 2) is introduced through the vagina after the Alexis wound protector (included with the kit) is first placed through the colpotomy with assistance of Babcock clamps (FIGURE 3).
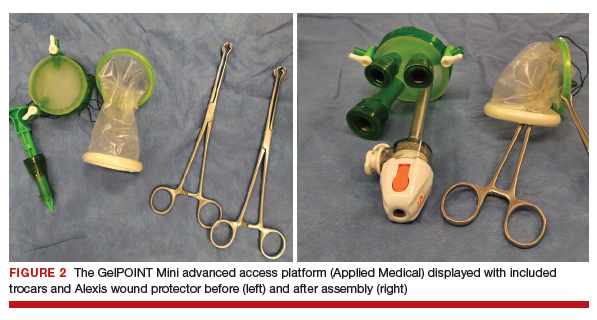

After ensuring that the green rigid ring of the Alexis wound protector is contained and completely expanded within the peritoneal cavity, we cross our previously tagged sutures as we find this helps with preventing the GelPOINT Mini access platform from inadvertently shifting out of the peritoneal cavity during surgery. The GelSeal cap is then secured and pneumoperitoneum is established (FIGURE 4).
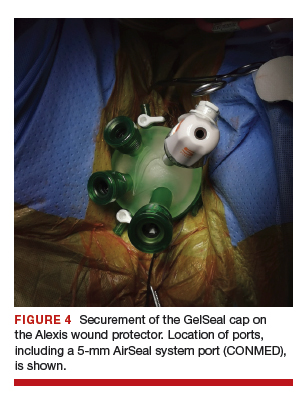
Continue to: 4. Laparoendoscopic surgery...
4. Laparoendoscopic surgery
Instruments used in our surgeries include a 10-mm rigid 30° 43-cm working length laparoscope; a 44-cm LigaSure device (Medtronic); a 5-mm, 37-cm laparoscopic cobra grasping forceps and fenestrated grasper (Karl Storz); and a 5-mm, 45-cm laparoscopic suction with hydrodissection tip (Stryker) (FIGURE 5).
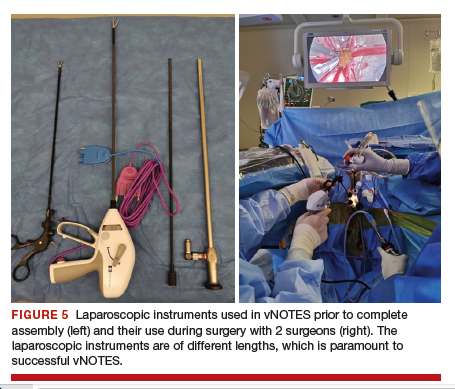
vNOTES allows a gynecologic surgeon the unique ability to survey the upper abdomen. The remainder of the surgery proceeds using basic laparoscopic single-site skills.
During vNOTES, as with all single-site surgical procedures, understanding the optimal placement of crossed instruments is important for successful completion. For example, when securing the right uterine artery, the surgeon needs to push the cervix toward the patient's left and slightly into the peritoneal cavity using a laparoscopic cobra grasper with his or her left hand while then securing the uterine pedicle using the LigaSure device with his or her right hand. This is then reversed when securing the left uterine artery, where the assistant surgeon pushes the cervix toward the patient's right while the surgeon secures the pedicle ("vaginal pull, laparoscopic push") (FIGURE 6).
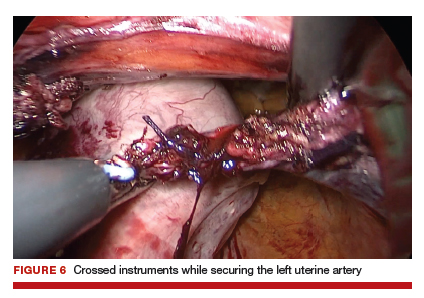
This again is reiterated in securing the ovarian pedicles, which are pushed into the peritoneal cavity while being secured with the LigaSure device.
5. Specimen removal
For large uteri or specimens that need morcellation, a 15-mm Endo Catch specimen retrieval bag (Medtronic) is introduced through the GelPOINT Mini system. The specimen is then placed in the bag and delivered to the vagina, where contained bag morcellation is performed in standard fashion (FIGURES 7 AND 8). We utilized the "big C" technique by first grasping the specimen with a penetrating clamp. The clamp is then held in our nondominant hand and a No. 10 blade scalpel is used to create a reverse c-incision, keeping one surface of the specimen intact. This is continued until the specimen can be completely delivered through the vagina.
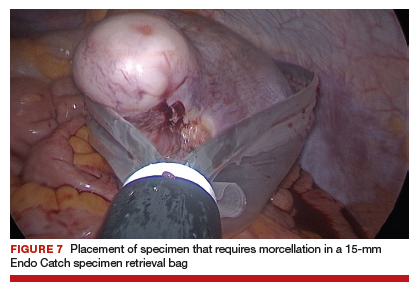
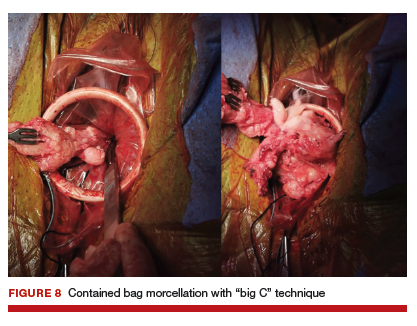
Specimens that do not require morcellation can be grasped laparoscopically, brought to the GelPOINT Mini port, which is quickly disassembled, and delivered. The GelSeal cap is then reassembled.
6. Vaginal cuff closure
The colpotomy or vaginal cuff is closed with barbed suture continuously, as in traditional vaginal hysterectomy cuff closure. Uterosacral ligament suspension should be performed for vaginal cuff support.
vNOTES is the most recent innovative development in the field of minimally invasive surgery, and it has demonstrated feasibility and safety in the fields of general surgery, urology, and gynecology. Adopting vNOTES in clinical practice can improve patient satisfaction and cosmesis as well as surgical outcomes. Gynecologic surgeons can think of vNOTES hysterectomy as "placing an eye" in the vagina while performing transvaginal hysterectomy. The surgical principle of "vaginal pull, laparoscopic push" facilitates the learning process.
1. ACOG Committee on Gynecologic Practice. Committee opinion no. 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
2. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18:1-3.
3. Whiteside JL, Kaeser CT, Ridgeway B. Achieving high value in the surgical approach to hysterectomy. Am J Obstet Gynecol. 2019;220:242-245.
4. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
5. Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585-588.
6. Balgobin S, Owens DM, Florian-Rodriguez ME, et al. Vaginal hysterectomy suturing skills training model and curriculum. Obstet Gynecol. 2019;134:553-558.
7. Baekelandt J. Total vaginal NOTES hysterectomy: a new approach to hysterectomy. J Minim Invasive Gynecol. 2015;22:1088-1094.
8. Baekelandt JF, De Mulder PA, Le Roy I, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG. 2019;126:105-113.
9. Guan X, Bardawil E, Liu J, et al. Transvaginal natural orifice transluminal endoscopic surgery as a rescue for total vaginal hysterectomy. J Minim Invasive Gynecol. 2018;25:1135-1136.
10. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677.
11. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
12. Reddy N, Rao P. Per oral transgastric endoscopic appendectomy in human. Paper Presented at: 45th Annual Conference of the Society of Gastrointestinal Endoscopy of India; February 28-29, 2004; Jaipur, India.
13. Clark MP, Qayed ES, Kooby DA, et al. Natural orifice translumenal endoscopic surgery in humans: a review. Minim Invasive Surg. 2012;189296.
14. Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/ SAGES Working Group on natural orifice translumenal endoscopic surgery, October 2005. Surg Endosc. 2006;20:329-333.
15. Autorino R, Yakoubi R, White WM, et al. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16.
16. Santos BF, Hungness ES. Natural orifice transluminal endoscopic surgery: progress in humans since the white paper. World J Gastroenterol. 2011;17:1655-1665.
17. Tolcher MC, Kalogera E, Hopkins MR, et al. Safety of culdotomy as a surgical approach: implications for natural orifice transluminal endoscopic surgery. JSLS. 2012;16:413-420.
18. ACOG Committee on Gynecologic Practice. Committee opinion no. 701. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017:129:e155-e159.
19. Ahn KH, Song JY, Kim SH, et al. Transvaginal single-port natural orifice transluminal endoscopic surgery for benign uterine adnexal pathologies. J Minim Invasive Gynecol. 2012;19:631-635.
20. Liu J, Kohn J, Sun B, et al. Transvaginal natural orifice transluminal endoscopic surgery sacrocolpopexy: tips and tricks. Minim Invasive Gynecol. 2019;26:38-39.
21. Liu J, Kohn J, Fu H, et al. Transvaginal natural orifice transluminal endoscopic surgery for sacrocolpopexy: a pilot study of 26 cases. J Minim Invasive Gynecol. 2019;26:748-753.
22. Su H, Yen CF, Wu KY, et al. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery (NOTES): feasibility of an innovative approach. Taiwan J Obstet Gynecol. 2012;51:217-221.
23. Lee CL, Huang CY, Wu KY, et al. Natural orifice transvaginal endoscopic surgery myomectomy: an innovative approach to myomectomy. Gynecol Minim Invasive Ther. 2014;3:127-130.
24. Chen Y, Li J, Zhang Y, et al. Transvaginal single-port laparoscopy sacrocolpopexy. J Minim Invasive Gynecol. 2018;25:585- 588.
25. Lee CL, Wu KY, Tsao FY, et al. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol Minim Invasive Ther. 2014;3:89-92.
26. Leblanc E, Narducci F, Bresson L, et al. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol Oncol. 2016;143:686-687.
27. Lee CL, Wu KY, Su H, et al. Robot-assisted natural orifice transluminal endoscopic surgery for hysterectomy. Taiwan J Obstet Gynecol. 2015;54:761-765.
28. Rezai S, Giovane RA, Johnson SN, et al. Robotic natural orifice transluminal endoscopic surgery (R-NOTES) in gynecologic surgeries, a case report and review of literature. Obstet Gynecol Int J. 2019;10:287-289.
29. Wang CJ, Wu PY, Kuo HH, et al. Natural orifice transluminal endoscopic surgery-assisted versus laparoscopic ovarian cystectomy (NAOC vs. LOC): a case-matched study. Surg Endosc. 2016;30:1227-1234.
Through the years, the surgical approach to hysterectomy has expanded from its early beginnings of being performed only through an abdominal or transvaginal route with traditional surgical clamps and suture. The late 1980s saw the advent of the laparoscopic-assisted vaginal hysterectomy (LAVH), and from that point forward several additional hysterectomy methods evolved, including today’s robotic approaches.
Although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, it remains one of the least performed among all available routes.1-3 In an analysis of inpatient hysterectomies published by Wright and colleagues in 2013, 16.7% of hysterectomies were performed vaginally, a number that essentially has remained steady throughout the ensuing years.4
Attempts to improve the application of vaginal hysterectomy have been made.5 These include the development of various curriculum and simulation-based medical education programs on vaginal surgical skills training and acquisition in the hopes of improving utilization.6 An interesting recent development is the rethinking of vaginal hysterectomy by several surgeons globally who are applying facets of the various hysterectomy methods to a transvaginal approach known as vaginal natural orifice transluminal endoscopic surgery (vNOTES).7,8 Unique to this thinking is the incorporation of conventional laparoscopic instrumentation.
Although I have not yet incorporated this approach in my surgical armamentarium at Columbia University Medical Center/New York–Presbyterian Hospital, I am intrigued by the possibility that this technique may serve as a rescue for vaginal hysterectomies that are at risk of conversion or of not being performed at all.9
At this time, vNOTES is not a standard of care and should be performed only by highly specialized surgeons. However, in the spirit of this Update on minimally invasive surgery and to keep our readers abreast of burgeoning techniques, I am delighted to bring you this overview by Dr. Xiaoming Guan, one of the pioneers of this surgical approach, and Dr. Tamisa Koythong and Dr. Juan Liu. I hope you find this recent development in hysterectomy of interest.
—Arnold P. Advincula, MD
Continue to: Development and evolution of NOTES...
Development and evolution of NOTES
Over the past few decades, emphasis has shifted from laparotomy to minimally invasive surgery because of its proven significant advantages in patient care, such as improved cosmesis, shorter hospital stay, shorter postoperative recovery, and decreased postoperative pain and blood loss.10 Advances in laparoendoscopic surgery and instrumentation, including robot-assisted laparoscopy (RAL), single-incision laparoscopic surgery (SILS), and most recently natural orifice transluminal endoscopic surgery (NOTES), reflect ongoing innovative developments in the field of minimally invasive surgery.
Here, we provide a brief literature review of the NOTES technique, focus on its application in gynecologic surgery, and describe how we perform NOTES at our institution.
NOTES application in gynecology
With NOTES, peritoneal access is gained through a natural orifice (such as the mouth, vagina, urethra, or anus) to perform endoscopic surgery, occasionally without requiring an abdominal incision. First described in 2004, transgastric peritoneoscopy was performed in a porcine model, and shortly thereafter the first transgastric appendectomy was performed in humans.11,12 The technique has further been adopted in cholecystectomy, appendectomy, gastrectomy, and nephrectomy procedures.13
Given rapid interest in a possible paradigm shift in the field of minimally invasive surgery, the Natural Orifice Surgery Consortiumfor Assessment and Research (NOSCAR) was formed, and the group published an article on potential barriers to accepted practice and adoption of NOTES as a realistic alternative to traditional laparoscopic surgery.14
While transgastric and transanal access to the peritoneum were initially more popular, the risk of anastomotic leaks associated with incomplete closure and subsequent infection were thought to be prohibitively high.15 Transvaginal access was considered a safer and simpler alternative, allowing for complete closure without increased risk of infection, and this is now the route through which the majority of NOTES procedures are completed.16,17
The eventual application of NOTES in the field of gynecology seemed inevitable. The American College of Obstetricians and Gynecologists stated that transvaginal surgery is the most minimally invasive and preferred surgical route in the management of patients with benign gynecologic diseases.18 However, performing it can be challenging at times due to limited visualization and lack of the required skills for single-site surgery. NOTES allows a gynecologic surgeon to improve visualization through the use of laparoendoscopic instruments and to complete surgery through a transvaginal route.
In 2012, Ahn and colleagues demonstrated the feasibility of the NOTES technique in gynecologic surgery after using it to successfully complete benign adnexal surgery in 10 patients.19 Vaginal NOTES (vNOTES) has since been further developed to include successful hysterectomy, myomectomy, sacrocolpopexy, tubal anastomosis, and even lymphadenectomy in the treatment of early- stage endometrial carcinoma.20-26 vNOTES also can be considered a rescue approach for traditional vaginal hysterectomy in instances in which it is necessary to evaluate adnexal pathology.9 Most recently, vNOTES hysterectomy has been reported with da Vinci Si or Xi robotic platforms.27,28
Continue to: Operative time, post-op stay shorter in NAOC-treated patients...
Operative time, post-op stay shorter in NAOC-treated patients
Few studies have compared outcomes with vNOTES to those with traditional laparoscopy. In 2016, Wang and colleagues compared surgical outcomes between NOTES-assisted ovarian cystectomy (NAOC) and laparoscopic ovarian cystectomy (LOC) in a case-matched study that included 277 patients.29 Although mean (SD) blood loss in patients who underwent LOC was significantly less compared with those who underwent NAOC (21.4 [14.7] mL vs 31.6 [24.1] mL; P = .028), absolute blood loss in both groups was deemed minimal. Additionally, mean (SD) operative time and postoperative stay were significantly less in patients undergoing NAOC compared with those having LOC (38.23 [10.19] minutes vs 53.82 [18.61] minutes; P≤.001; and 1.38 [0.55] days vs 1.82 [0.52] days; P≤.001; respectively).29
How vNOTES hysterectomy stacked up against TLH
In 2018, Baekelandt and colleagues compared outcomes between vNOTES hysterectomy and total laparoscopic hysterectomy (TLH) in a noninferiority single-blinded trial of 70 women.8 Compared with TLH, vNOTES hysterectomy was associated with shorter operative time (41 vs 75 minutes; P<.001), shorter hospital stay (0.8 vs 1.3 days; P = .004), and lower postoperative analgesic requirement (8 vs 14 U; P = .006). Additionally, there were no differences between the 2 groups in postoperative infection rate, intraoperative complications, or hospital readmissions within 6 weeks.8
Clearly, vNOTES is the next exciting development in minimally invasive surgery, improving patient outcomes and satisfaction with truly scarless surgery. Compared with traditional transvaginal surgery, vNOTES has the advantage of improved visualization with laparoendoscopic guidance, and it may be beneficial even for patients previously thought to have relative contraindications to successful completion of transvaginal surgery, such as nulliparity or a narrow introitus.
Approach for performing vNOTES procedures
At our institution, Baylor College of Medicine, the majority of gynecologic surgeries are performed via either transumbilical robot-assisted single-incision laparoscopy or vNOTES. Preoperative selection of appropriate candidates for vNOTES includes:
- low suspicion for or prior diagnosis of endometriosis with obliteration of the posterior cul-de-sac
- no surgical history suggestive of severe adhesive disease, and
- adequate vaginal sidewall access and sufficient descent for instrumentation for entry into the peritoneal cavity.
In general, a key concept in vNOTES is "vaginal pull, laparoscopic push," which means that the surgeon must pull the cervix while performing vaginal entry and then push the uterus back in the peritoneal cavity to increase surgical space during laparoscopic surgery.
Continue to: Overview of vNOTES steps...
Overview of vNOTES steps
Below we break down a description of vNOTES in 6 sections. Our patients are always placed in dorsal lithotomy position with TrenGuard (D.A. Surgical) Trendelenburg restraint. We prep the abdomen in case we need to convert to transabdominal surgery via transumbilical single-incision laparoscopic surgery or traditional laparoscopic surgery.
1. Vaginal entry
Accessing the peritoneal cavity through the vagina initially proceeds like a vaginal hysterectomy. We inject dilute vasopressin (20 U in 20 mL of normal saline) circumferentially in the cervix (for hysterectomy) or in the posterior cervix in the cervicovaginal junction (for adnexal surgery without hysterectomy) for vasoconstriction and hydrodissection.
We then incise the vaginal mucosa circumferentially with electrosurgical cautery and follow with posterior colpotomy. We find that reapproximating the posterior peritoneum to the posterior vagina with either figure-of-8 stitches or a running stitch of polyglactin 910 suture (2-0 Vicryl) assists in port placement, bleeding at the peritoneal edge, and closure of the cuff or colpotomy at the end of the case. We tag this suture with a curved hemostat.
Depending on whether a hysterectomy is being performed, anterior colpotomy is made. Again, the anterior peritoneum is then tagged to the anterior vaginal cuff in similar fashion, and this suture is tagged with a different instrument; we typically use a straight hemostat or Sarot clamp (FIGURE 1).

2. Traditional vaginal hysterectomy
After colpotomy, we prefer to perform progressive clamping of the broad ligament from the uterosacral and cardinal ligaments to the level of uterine artery as in traditional vaginal hysterectomy, if feasible.
3. Single-site port placement
The assembled GelPOINT Mini advanced access platform (Applied Medical) (FIGURE 2) is introduced through the vagina after the Alexis wound protector (included with the kit) is first placed through the colpotomy with assistance of Babcock clamps (FIGURE 3).


After ensuring that the green rigid ring of the Alexis wound protector is contained and completely expanded within the peritoneal cavity, we cross our previously tagged sutures as we find this helps with preventing the GelPOINT Mini access platform from inadvertently shifting out of the peritoneal cavity during surgery. The GelSeal cap is then secured and pneumoperitoneum is established (FIGURE 4).

Continue to: 4. Laparoendoscopic surgery...
4. Laparoendoscopic surgery
Instruments used in our surgeries include a 10-mm rigid 30° 43-cm working length laparoscope; a 44-cm LigaSure device (Medtronic); a 5-mm, 37-cm laparoscopic cobra grasping forceps and fenestrated grasper (Karl Storz); and a 5-mm, 45-cm laparoscopic suction with hydrodissection tip (Stryker) (FIGURE 5).

vNOTES allows a gynecologic surgeon the unique ability to survey the upper abdomen. The remainder of the surgery proceeds using basic laparoscopic single-site skills.
During vNOTES, as with all single-site surgical procedures, understanding the optimal placement of crossed instruments is important for successful completion. For example, when securing the right uterine artery, the surgeon needs to push the cervix toward the patient's left and slightly into the peritoneal cavity using a laparoscopic cobra grasper with his or her left hand while then securing the uterine pedicle using the LigaSure device with his or her right hand. This is then reversed when securing the left uterine artery, where the assistant surgeon pushes the cervix toward the patient's right while the surgeon secures the pedicle ("vaginal pull, laparoscopic push") (FIGURE 6).

This again is reiterated in securing the ovarian pedicles, which are pushed into the peritoneal cavity while being secured with the LigaSure device.
5. Specimen removal
For large uteri or specimens that need morcellation, a 15-mm Endo Catch specimen retrieval bag (Medtronic) is introduced through the GelPOINT Mini system. The specimen is then placed in the bag and delivered to the vagina, where contained bag morcellation is performed in standard fashion (FIGURES 7 AND 8). We utilized the "big C" technique by first grasping the specimen with a penetrating clamp. The clamp is then held in our nondominant hand and a No. 10 blade scalpel is used to create a reverse c-incision, keeping one surface of the specimen intact. This is continued until the specimen can be completely delivered through the vagina.


Specimens that do not require morcellation can be grasped laparoscopically, brought to the GelPOINT Mini port, which is quickly disassembled, and delivered. The GelSeal cap is then reassembled.
6. Vaginal cuff closure
The colpotomy or vaginal cuff is closed with barbed suture continuously, as in traditional vaginal hysterectomy cuff closure. Uterosacral ligament suspension should be performed for vaginal cuff support.
vNOTES is the most recent innovative development in the field of minimally invasive surgery, and it has demonstrated feasibility and safety in the fields of general surgery, urology, and gynecology. Adopting vNOTES in clinical practice can improve patient satisfaction and cosmesis as well as surgical outcomes. Gynecologic surgeons can think of vNOTES hysterectomy as "placing an eye" in the vagina while performing transvaginal hysterectomy. The surgical principle of "vaginal pull, laparoscopic push" facilitates the learning process.
Through the years, the surgical approach to hysterectomy has expanded from its early beginnings of being performed only through an abdominal or transvaginal route with traditional surgical clamps and suture. The late 1980s saw the advent of the laparoscopic-assisted vaginal hysterectomy (LAVH), and from that point forward several additional hysterectomy methods evolved, including today’s robotic approaches.
Although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, it remains one of the least performed among all available routes.1-3 In an analysis of inpatient hysterectomies published by Wright and colleagues in 2013, 16.7% of hysterectomies were performed vaginally, a number that essentially has remained steady throughout the ensuing years.4
Attempts to improve the application of vaginal hysterectomy have been made.5 These include the development of various curriculum and simulation-based medical education programs on vaginal surgical skills training and acquisition in the hopes of improving utilization.6 An interesting recent development is the rethinking of vaginal hysterectomy by several surgeons globally who are applying facets of the various hysterectomy methods to a transvaginal approach known as vaginal natural orifice transluminal endoscopic surgery (vNOTES).7,8 Unique to this thinking is the incorporation of conventional laparoscopic instrumentation.
Although I have not yet incorporated this approach in my surgical armamentarium at Columbia University Medical Center/New York–Presbyterian Hospital, I am intrigued by the possibility that this technique may serve as a rescue for vaginal hysterectomies that are at risk of conversion or of not being performed at all.9
At this time, vNOTES is not a standard of care and should be performed only by highly specialized surgeons. However, in the spirit of this Update on minimally invasive surgery and to keep our readers abreast of burgeoning techniques, I am delighted to bring you this overview by Dr. Xiaoming Guan, one of the pioneers of this surgical approach, and Dr. Tamisa Koythong and Dr. Juan Liu. I hope you find this recent development in hysterectomy of interest.
—Arnold P. Advincula, MD
Continue to: Development and evolution of NOTES...
Development and evolution of NOTES
Over the past few decades, emphasis has shifted from laparotomy to minimally invasive surgery because of its proven significant advantages in patient care, such as improved cosmesis, shorter hospital stay, shorter postoperative recovery, and decreased postoperative pain and blood loss.10 Advances in laparoendoscopic surgery and instrumentation, including robot-assisted laparoscopy (RAL), single-incision laparoscopic surgery (SILS), and most recently natural orifice transluminal endoscopic surgery (NOTES), reflect ongoing innovative developments in the field of minimally invasive surgery.
Here, we provide a brief literature review of the NOTES technique, focus on its application in gynecologic surgery, and describe how we perform NOTES at our institution.
NOTES application in gynecology
With NOTES, peritoneal access is gained through a natural orifice (such as the mouth, vagina, urethra, or anus) to perform endoscopic surgery, occasionally without requiring an abdominal incision. First described in 2004, transgastric peritoneoscopy was performed in a porcine model, and shortly thereafter the first transgastric appendectomy was performed in humans.11,12 The technique has further been adopted in cholecystectomy, appendectomy, gastrectomy, and nephrectomy procedures.13
Given rapid interest in a possible paradigm shift in the field of minimally invasive surgery, the Natural Orifice Surgery Consortiumfor Assessment and Research (NOSCAR) was formed, and the group published an article on potential barriers to accepted practice and adoption of NOTES as a realistic alternative to traditional laparoscopic surgery.14
While transgastric and transanal access to the peritoneum were initially more popular, the risk of anastomotic leaks associated with incomplete closure and subsequent infection were thought to be prohibitively high.15 Transvaginal access was considered a safer and simpler alternative, allowing for complete closure without increased risk of infection, and this is now the route through which the majority of NOTES procedures are completed.16,17
The eventual application of NOTES in the field of gynecology seemed inevitable. The American College of Obstetricians and Gynecologists stated that transvaginal surgery is the most minimally invasive and preferred surgical route in the management of patients with benign gynecologic diseases.18 However, performing it can be challenging at times due to limited visualization and lack of the required skills for single-site surgery. NOTES allows a gynecologic surgeon to improve visualization through the use of laparoendoscopic instruments and to complete surgery through a transvaginal route.
In 2012, Ahn and colleagues demonstrated the feasibility of the NOTES technique in gynecologic surgery after using it to successfully complete benign adnexal surgery in 10 patients.19 Vaginal NOTES (vNOTES) has since been further developed to include successful hysterectomy, myomectomy, sacrocolpopexy, tubal anastomosis, and even lymphadenectomy in the treatment of early- stage endometrial carcinoma.20-26 vNOTES also can be considered a rescue approach for traditional vaginal hysterectomy in instances in which it is necessary to evaluate adnexal pathology.9 Most recently, vNOTES hysterectomy has been reported with da Vinci Si or Xi robotic platforms.27,28
Continue to: Operative time, post-op stay shorter in NAOC-treated patients...
Operative time, post-op stay shorter in NAOC-treated patients
Few studies have compared outcomes with vNOTES to those with traditional laparoscopy. In 2016, Wang and colleagues compared surgical outcomes between NOTES-assisted ovarian cystectomy (NAOC) and laparoscopic ovarian cystectomy (LOC) in a case-matched study that included 277 patients.29 Although mean (SD) blood loss in patients who underwent LOC was significantly less compared with those who underwent NAOC (21.4 [14.7] mL vs 31.6 [24.1] mL; P = .028), absolute blood loss in both groups was deemed minimal. Additionally, mean (SD) operative time and postoperative stay were significantly less in patients undergoing NAOC compared with those having LOC (38.23 [10.19] minutes vs 53.82 [18.61] minutes; P≤.001; and 1.38 [0.55] days vs 1.82 [0.52] days; P≤.001; respectively).29
How vNOTES hysterectomy stacked up against TLH
In 2018, Baekelandt and colleagues compared outcomes between vNOTES hysterectomy and total laparoscopic hysterectomy (TLH) in a noninferiority single-blinded trial of 70 women.8 Compared with TLH, vNOTES hysterectomy was associated with shorter operative time (41 vs 75 minutes; P<.001), shorter hospital stay (0.8 vs 1.3 days; P = .004), and lower postoperative analgesic requirement (8 vs 14 U; P = .006). Additionally, there were no differences between the 2 groups in postoperative infection rate, intraoperative complications, or hospital readmissions within 6 weeks.8
Clearly, vNOTES is the next exciting development in minimally invasive surgery, improving patient outcomes and satisfaction with truly scarless surgery. Compared with traditional transvaginal surgery, vNOTES has the advantage of improved visualization with laparoendoscopic guidance, and it may be beneficial even for patients previously thought to have relative contraindications to successful completion of transvaginal surgery, such as nulliparity or a narrow introitus.
Approach for performing vNOTES procedures
At our institution, Baylor College of Medicine, the majority of gynecologic surgeries are performed via either transumbilical robot-assisted single-incision laparoscopy or vNOTES. Preoperative selection of appropriate candidates for vNOTES includes:
- low suspicion for or prior diagnosis of endometriosis with obliteration of the posterior cul-de-sac
- no surgical history suggestive of severe adhesive disease, and
- adequate vaginal sidewall access and sufficient descent for instrumentation for entry into the peritoneal cavity.
In general, a key concept in vNOTES is "vaginal pull, laparoscopic push," which means that the surgeon must pull the cervix while performing vaginal entry and then push the uterus back in the peritoneal cavity to increase surgical space during laparoscopic surgery.
Continue to: Overview of vNOTES steps...
Overview of vNOTES steps
Below we break down a description of vNOTES in 6 sections. Our patients are always placed in dorsal lithotomy position with TrenGuard (D.A. Surgical) Trendelenburg restraint. We prep the abdomen in case we need to convert to transabdominal surgery via transumbilical single-incision laparoscopic surgery or traditional laparoscopic surgery.
1. Vaginal entry
Accessing the peritoneal cavity through the vagina initially proceeds like a vaginal hysterectomy. We inject dilute vasopressin (20 U in 20 mL of normal saline) circumferentially in the cervix (for hysterectomy) or in the posterior cervix in the cervicovaginal junction (for adnexal surgery without hysterectomy) for vasoconstriction and hydrodissection.
We then incise the vaginal mucosa circumferentially with electrosurgical cautery and follow with posterior colpotomy. We find that reapproximating the posterior peritoneum to the posterior vagina with either figure-of-8 stitches or a running stitch of polyglactin 910 suture (2-0 Vicryl) assists in port placement, bleeding at the peritoneal edge, and closure of the cuff or colpotomy at the end of the case. We tag this suture with a curved hemostat.
Depending on whether a hysterectomy is being performed, anterior colpotomy is made. Again, the anterior peritoneum is then tagged to the anterior vaginal cuff in similar fashion, and this suture is tagged with a different instrument; we typically use a straight hemostat or Sarot clamp (FIGURE 1).

2. Traditional vaginal hysterectomy
After colpotomy, we prefer to perform progressive clamping of the broad ligament from the uterosacral and cardinal ligaments to the level of uterine artery as in traditional vaginal hysterectomy, if feasible.
3. Single-site port placement
The assembled GelPOINT Mini advanced access platform (Applied Medical) (FIGURE 2) is introduced through the vagina after the Alexis wound protector (included with the kit) is first placed through the colpotomy with assistance of Babcock clamps (FIGURE 3).


After ensuring that the green rigid ring of the Alexis wound protector is contained and completely expanded within the peritoneal cavity, we cross our previously tagged sutures as we find this helps with preventing the GelPOINT Mini access platform from inadvertently shifting out of the peritoneal cavity during surgery. The GelSeal cap is then secured and pneumoperitoneum is established (FIGURE 4).

Continue to: 4. Laparoendoscopic surgery...
4. Laparoendoscopic surgery
Instruments used in our surgeries include a 10-mm rigid 30° 43-cm working length laparoscope; a 44-cm LigaSure device (Medtronic); a 5-mm, 37-cm laparoscopic cobra grasping forceps and fenestrated grasper (Karl Storz); and a 5-mm, 45-cm laparoscopic suction with hydrodissection tip (Stryker) (FIGURE 5).

vNOTES allows a gynecologic surgeon the unique ability to survey the upper abdomen. The remainder of the surgery proceeds using basic laparoscopic single-site skills.
During vNOTES, as with all single-site surgical procedures, understanding the optimal placement of crossed instruments is important for successful completion. For example, when securing the right uterine artery, the surgeon needs to push the cervix toward the patient's left and slightly into the peritoneal cavity using a laparoscopic cobra grasper with his or her left hand while then securing the uterine pedicle using the LigaSure device with his or her right hand. This is then reversed when securing the left uterine artery, where the assistant surgeon pushes the cervix toward the patient's right while the surgeon secures the pedicle ("vaginal pull, laparoscopic push") (FIGURE 6).

This again is reiterated in securing the ovarian pedicles, which are pushed into the peritoneal cavity while being secured with the LigaSure device.
5. Specimen removal
For large uteri or specimens that need morcellation, a 15-mm Endo Catch specimen retrieval bag (Medtronic) is introduced through the GelPOINT Mini system. The specimen is then placed in the bag and delivered to the vagina, where contained bag morcellation is performed in standard fashion (FIGURES 7 AND 8). We utilized the "big C" technique by first grasping the specimen with a penetrating clamp. The clamp is then held in our nondominant hand and a No. 10 blade scalpel is used to create a reverse c-incision, keeping one surface of the specimen intact. This is continued until the specimen can be completely delivered through the vagina.


Specimens that do not require morcellation can be grasped laparoscopically, brought to the GelPOINT Mini port, which is quickly disassembled, and delivered. The GelSeal cap is then reassembled.
6. Vaginal cuff closure
The colpotomy or vaginal cuff is closed with barbed suture continuously, as in traditional vaginal hysterectomy cuff closure. Uterosacral ligament suspension should be performed for vaginal cuff support.
vNOTES is the most recent innovative development in the field of minimally invasive surgery, and it has demonstrated feasibility and safety in the fields of general surgery, urology, and gynecology. Adopting vNOTES in clinical practice can improve patient satisfaction and cosmesis as well as surgical outcomes. Gynecologic surgeons can think of vNOTES hysterectomy as "placing an eye" in the vagina while performing transvaginal hysterectomy. The surgical principle of "vaginal pull, laparoscopic push" facilitates the learning process.
1. ACOG Committee on Gynecologic Practice. Committee opinion no. 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
2. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18:1-3.
3. Whiteside JL, Kaeser CT, Ridgeway B. Achieving high value in the surgical approach to hysterectomy. Am J Obstet Gynecol. 2019;220:242-245.
4. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
5. Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585-588.
6. Balgobin S, Owens DM, Florian-Rodriguez ME, et al. Vaginal hysterectomy suturing skills training model and curriculum. Obstet Gynecol. 2019;134:553-558.
7. Baekelandt J. Total vaginal NOTES hysterectomy: a new approach to hysterectomy. J Minim Invasive Gynecol. 2015;22:1088-1094.
8. Baekelandt JF, De Mulder PA, Le Roy I, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG. 2019;126:105-113.
9. Guan X, Bardawil E, Liu J, et al. Transvaginal natural orifice transluminal endoscopic surgery as a rescue for total vaginal hysterectomy. J Minim Invasive Gynecol. 2018;25:1135-1136.
10. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677.
11. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
12. Reddy N, Rao P. Per oral transgastric endoscopic appendectomy in human. Paper Presented at: 45th Annual Conference of the Society of Gastrointestinal Endoscopy of India; February 28-29, 2004; Jaipur, India.
13. Clark MP, Qayed ES, Kooby DA, et al. Natural orifice translumenal endoscopic surgery in humans: a review. Minim Invasive Surg. 2012;189296.
14. Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/ SAGES Working Group on natural orifice translumenal endoscopic surgery, October 2005. Surg Endosc. 2006;20:329-333.
15. Autorino R, Yakoubi R, White WM, et al. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16.
16. Santos BF, Hungness ES. Natural orifice transluminal endoscopic surgery: progress in humans since the white paper. World J Gastroenterol. 2011;17:1655-1665.
17. Tolcher MC, Kalogera E, Hopkins MR, et al. Safety of culdotomy as a surgical approach: implications for natural orifice transluminal endoscopic surgery. JSLS. 2012;16:413-420.
18. ACOG Committee on Gynecologic Practice. Committee opinion no. 701. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017:129:e155-e159.
19. Ahn KH, Song JY, Kim SH, et al. Transvaginal single-port natural orifice transluminal endoscopic surgery for benign uterine adnexal pathologies. J Minim Invasive Gynecol. 2012;19:631-635.
20. Liu J, Kohn J, Sun B, et al. Transvaginal natural orifice transluminal endoscopic surgery sacrocolpopexy: tips and tricks. Minim Invasive Gynecol. 2019;26:38-39.
21. Liu J, Kohn J, Fu H, et al. Transvaginal natural orifice transluminal endoscopic surgery for sacrocolpopexy: a pilot study of 26 cases. J Minim Invasive Gynecol. 2019;26:748-753.
22. Su H, Yen CF, Wu KY, et al. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery (NOTES): feasibility of an innovative approach. Taiwan J Obstet Gynecol. 2012;51:217-221.
23. Lee CL, Huang CY, Wu KY, et al. Natural orifice transvaginal endoscopic surgery myomectomy: an innovative approach to myomectomy. Gynecol Minim Invasive Ther. 2014;3:127-130.
24. Chen Y, Li J, Zhang Y, et al. Transvaginal single-port laparoscopy sacrocolpopexy. J Minim Invasive Gynecol. 2018;25:585- 588.
25. Lee CL, Wu KY, Tsao FY, et al. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol Minim Invasive Ther. 2014;3:89-92.
26. Leblanc E, Narducci F, Bresson L, et al. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol Oncol. 2016;143:686-687.
27. Lee CL, Wu KY, Su H, et al. Robot-assisted natural orifice transluminal endoscopic surgery for hysterectomy. Taiwan J Obstet Gynecol. 2015;54:761-765.
28. Rezai S, Giovane RA, Johnson SN, et al. Robotic natural orifice transluminal endoscopic surgery (R-NOTES) in gynecologic surgeries, a case report and review of literature. Obstet Gynecol Int J. 2019;10:287-289.
29. Wang CJ, Wu PY, Kuo HH, et al. Natural orifice transluminal endoscopic surgery-assisted versus laparoscopic ovarian cystectomy (NAOC vs. LOC): a case-matched study. Surg Endosc. 2016;30:1227-1234.
1. ACOG Committee on Gynecologic Practice. Committee opinion no. 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
2. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18:1-3.
3. Whiteside JL, Kaeser CT, Ridgeway B. Achieving high value in the surgical approach to hysterectomy. Am J Obstet Gynecol. 2019;220:242-245.
4. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
5. Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585-588.
6. Balgobin S, Owens DM, Florian-Rodriguez ME, et al. Vaginal hysterectomy suturing skills training model and curriculum. Obstet Gynecol. 2019;134:553-558.
7. Baekelandt J. Total vaginal NOTES hysterectomy: a new approach to hysterectomy. J Minim Invasive Gynecol. 2015;22:1088-1094.
8. Baekelandt JF, De Mulder PA, Le Roy I, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG. 2019;126:105-113.
9. Guan X, Bardawil E, Liu J, et al. Transvaginal natural orifice transluminal endoscopic surgery as a rescue for total vaginal hysterectomy. J Minim Invasive Gynecol. 2018;25:1135-1136.
10. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677.
11. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
12. Reddy N, Rao P. Per oral transgastric endoscopic appendectomy in human. Paper Presented at: 45th Annual Conference of the Society of Gastrointestinal Endoscopy of India; February 28-29, 2004; Jaipur, India.
13. Clark MP, Qayed ES, Kooby DA, et al. Natural orifice translumenal endoscopic surgery in humans: a review. Minim Invasive Surg. 2012;189296.
14. Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/ SAGES Working Group on natural orifice translumenal endoscopic surgery, October 2005. Surg Endosc. 2006;20:329-333.
15. Autorino R, Yakoubi R, White WM, et al. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16.
16. Santos BF, Hungness ES. Natural orifice transluminal endoscopic surgery: progress in humans since the white paper. World J Gastroenterol. 2011;17:1655-1665.
17. Tolcher MC, Kalogera E, Hopkins MR, et al. Safety of culdotomy as a surgical approach: implications for natural orifice transluminal endoscopic surgery. JSLS. 2012;16:413-420.
18. ACOG Committee on Gynecologic Practice. Committee opinion no. 701. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017:129:e155-e159.
19. Ahn KH, Song JY, Kim SH, et al. Transvaginal single-port natural orifice transluminal endoscopic surgery for benign uterine adnexal pathologies. J Minim Invasive Gynecol. 2012;19:631-635.
20. Liu J, Kohn J, Sun B, et al. Transvaginal natural orifice transluminal endoscopic surgery sacrocolpopexy: tips and tricks. Minim Invasive Gynecol. 2019;26:38-39.
21. Liu J, Kohn J, Fu H, et al. Transvaginal natural orifice transluminal endoscopic surgery for sacrocolpopexy: a pilot study of 26 cases. J Minim Invasive Gynecol. 2019;26:748-753.
22. Su H, Yen CF, Wu KY, et al. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery (NOTES): feasibility of an innovative approach. Taiwan J Obstet Gynecol. 2012;51:217-221.
23. Lee CL, Huang CY, Wu KY, et al. Natural orifice transvaginal endoscopic surgery myomectomy: an innovative approach to myomectomy. Gynecol Minim Invasive Ther. 2014;3:127-130.
24. Chen Y, Li J, Zhang Y, et al. Transvaginal single-port laparoscopy sacrocolpopexy. J Minim Invasive Gynecol. 2018;25:585- 588.
25. Lee CL, Wu KY, Tsao FY, et al. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol Minim Invasive Ther. 2014;3:89-92.
26. Leblanc E, Narducci F, Bresson L, et al. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol Oncol. 2016;143:686-687.
27. Lee CL, Wu KY, Su H, et al. Robot-assisted natural orifice transluminal endoscopic surgery for hysterectomy. Taiwan J Obstet Gynecol. 2015;54:761-765.
28. Rezai S, Giovane RA, Johnson SN, et al. Robotic natural orifice transluminal endoscopic surgery (R-NOTES) in gynecologic surgeries, a case report and review of literature. Obstet Gynecol Int J. 2019;10:287-289.
29. Wang CJ, Wu PY, Kuo HH, et al. Natural orifice transluminal endoscopic surgery-assisted versus laparoscopic ovarian cystectomy (NAOC vs. LOC): a case-matched study. Surg Endosc. 2016;30:1227-1234.
Excision of abdominal wall endometriosis
Endometriosis, defined by the ectopic growth of functioning endometrial glands and stroma,1,2 usually affects the peritoneal cavity. However, endometriosis has been identified in the pneumothorax, brain, and within the extraperitoneum, such as the abdominal wall.1-3 Incidence of abdominal wall endometriosis can be up to 12%.3-5 If patients report symptoms, they can include abdominal pain, a palpable mass, pelvic pain consistent with endometriosis, and bleeding from involvement of the overlying skin. Abdominal wall endometriosis can be surgically resected, with complete resolution and a low rate of recurrence.
In the following video, we review the diagnosis of abdominal wall endometriosis, including our imaging of choice, and treatment options. In addition, we illustrate a surgical technique for the excision of abdominal wall endometriosis in a 38-year-old patient with symptomatic disease. We conclude with a review of key surgical steps.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211:363.e1-e5.
- Horton JD, Dezee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207-212.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41-44.
- Chang Y, Tsai EM, Long CY, et al. Abdominal wall endometriosis. J Reproductive Med. 2009;54:155-159.
Endometriosis, defined by the ectopic growth of functioning endometrial glands and stroma,1,2 usually affects the peritoneal cavity. However, endometriosis has been identified in the pneumothorax, brain, and within the extraperitoneum, such as the abdominal wall.1-3 Incidence of abdominal wall endometriosis can be up to 12%.3-5 If patients report symptoms, they can include abdominal pain, a palpable mass, pelvic pain consistent with endometriosis, and bleeding from involvement of the overlying skin. Abdominal wall endometriosis can be surgically resected, with complete resolution and a low rate of recurrence.
In the following video, we review the diagnosis of abdominal wall endometriosis, including our imaging of choice, and treatment options. In addition, we illustrate a surgical technique for the excision of abdominal wall endometriosis in a 38-year-old patient with symptomatic disease. We conclude with a review of key surgical steps.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

Endometriosis, defined by the ectopic growth of functioning endometrial glands and stroma,1,2 usually affects the peritoneal cavity. However, endometriosis has been identified in the pneumothorax, brain, and within the extraperitoneum, such as the abdominal wall.1-3 Incidence of abdominal wall endometriosis can be up to 12%.3-5 If patients report symptoms, they can include abdominal pain, a palpable mass, pelvic pain consistent with endometriosis, and bleeding from involvement of the overlying skin. Abdominal wall endometriosis can be surgically resected, with complete resolution and a low rate of recurrence.
In the following video, we review the diagnosis of abdominal wall endometriosis, including our imaging of choice, and treatment options. In addition, we illustrate a surgical technique for the excision of abdominal wall endometriosis in a 38-year-old patient with symptomatic disease. We conclude with a review of key surgical steps.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211:363.e1-e5.
- Horton JD, Dezee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207-212.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41-44.
- Chang Y, Tsai EM, Long CY, et al. Abdominal wall endometriosis. J Reproductive Med. 2009;54:155-159.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211:363.e1-e5.
- Horton JD, Dezee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207-212.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41-44.
- Chang Y, Tsai EM, Long CY, et al. Abdominal wall endometriosis. J Reproductive Med. 2009;54:155-159.
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass

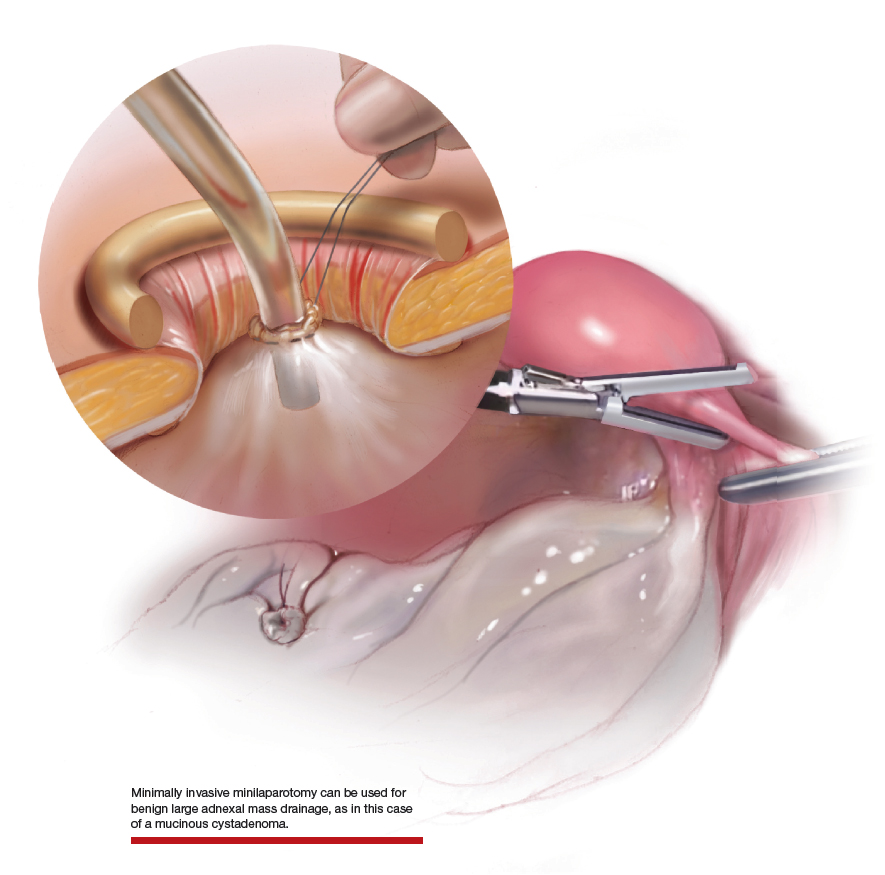
Large adnexal masses traditionally are removed surgically via laparotomy through a midline vertical incision to achieve adequate exposure and to avoid spillage of cyst contents. However, large laparotomies carry significant morbidity compared with minimally invasive techniques. Minilaparotomy is a minimally invasive approach that is associated with shorter operating times and lower estimated blood loss compared with laparoscopy in gynecologic surgery.1 The procedure also provides adequate exposure and can be used for carefully selected patients with a large adnexal mass.2,3 Preoperative assessment for the risk of malignancy typically includes an evaluation of risk factors, physical examination, imaging, and tumor markers.4
In this video, we illustrate a minimally invasive technique for the removal of a massively enlarged adnexal mass through laparoscopic bilateral salpingo-oophorectomy with minilaparotomy assistance. We conclude that this procedure is a safe and feasible option for women with a large benign adnexal mass, such as the highlighted patient whose final pathology resulted in a mucinous cystadenoma. Careful patient selection and preoperative assessment of malignancy risk is critical.5,6
We hope that you find this innovative approach useful in your clinical practice.
>> Dr. Arnold P. Advincula and colleagues
- Kumar A, Pearl M. Mini-laparotomy versus laparoscopy for gynecologic conditions. J Minim Invasive Gynecol. 2014;21:109-114.
- Pelosi MA. A novel minilaparotomy approach for large ovarian cysts. OBG Manag. 2004;16(2):17-30.
- Rhode JM, Advincula AP, Reynolds RK, et al. A minimally invasive technique for management of the large adnexal mass. J Minim Invasive Gynecol. 2006;13:476-479.
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol. 2016;128:e210-e226.
- Roman LD, Muderspach LI, Stein SM, et al. Pelvic examination, tumor marker level, and gray-scale and Doppler sonography in the prediction of pelvic cancer. Obstet Gynecol. 1997;89:493-500.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol. 2012:126:157-166.


Large adnexal masses traditionally are removed surgically via laparotomy through a midline vertical incision to achieve adequate exposure and to avoid spillage of cyst contents. However, large laparotomies carry significant morbidity compared with minimally invasive techniques. Minilaparotomy is a minimally invasive approach that is associated with shorter operating times and lower estimated blood loss compared with laparoscopy in gynecologic surgery.1 The procedure also provides adequate exposure and can be used for carefully selected patients with a large adnexal mass.2,3 Preoperative assessment for the risk of malignancy typically includes an evaluation of risk factors, physical examination, imaging, and tumor markers.4
In this video, we illustrate a minimally invasive technique for the removal of a massively enlarged adnexal mass through laparoscopic bilateral salpingo-oophorectomy with minilaparotomy assistance. We conclude that this procedure is a safe and feasible option for women with a large benign adnexal mass, such as the highlighted patient whose final pathology resulted in a mucinous cystadenoma. Careful patient selection and preoperative assessment of malignancy risk is critical.5,6
We hope that you find this innovative approach useful in your clinical practice.
>> Dr. Arnold P. Advincula and colleagues


Large adnexal masses traditionally are removed surgically via laparotomy through a midline vertical incision to achieve adequate exposure and to avoid spillage of cyst contents. However, large laparotomies carry significant morbidity compared with minimally invasive techniques. Minilaparotomy is a minimally invasive approach that is associated with shorter operating times and lower estimated blood loss compared with laparoscopy in gynecologic surgery.1 The procedure also provides adequate exposure and can be used for carefully selected patients with a large adnexal mass.2,3 Preoperative assessment for the risk of malignancy typically includes an evaluation of risk factors, physical examination, imaging, and tumor markers.4
In this video, we illustrate a minimally invasive technique for the removal of a massively enlarged adnexal mass through laparoscopic bilateral salpingo-oophorectomy with minilaparotomy assistance. We conclude that this procedure is a safe and feasible option for women with a large benign adnexal mass, such as the highlighted patient whose final pathology resulted in a mucinous cystadenoma. Careful patient selection and preoperative assessment of malignancy risk is critical.5,6
We hope that you find this innovative approach useful in your clinical practice.
>> Dr. Arnold P. Advincula and colleagues
- Kumar A, Pearl M. Mini-laparotomy versus laparoscopy for gynecologic conditions. J Minim Invasive Gynecol. 2014;21:109-114.
- Pelosi MA. A novel minilaparotomy approach for large ovarian cysts. OBG Manag. 2004;16(2):17-30.
- Rhode JM, Advincula AP, Reynolds RK, et al. A minimally invasive technique for management of the large adnexal mass. J Minim Invasive Gynecol. 2006;13:476-479.
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol. 2016;128:e210-e226.
- Roman LD, Muderspach LI, Stein SM, et al. Pelvic examination, tumor marker level, and gray-scale and Doppler sonography in the prediction of pelvic cancer. Obstet Gynecol. 1997;89:493-500.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol. 2012:126:157-166.
- Kumar A, Pearl M. Mini-laparotomy versus laparoscopy for gynecologic conditions. J Minim Invasive Gynecol. 2014;21:109-114.
- Pelosi MA. A novel minilaparotomy approach for large ovarian cysts. OBG Manag. 2004;16(2):17-30.
- Rhode JM, Advincula AP, Reynolds RK, et al. A minimally invasive technique for management of the large adnexal mass. J Minim Invasive Gynecol. 2006;13:476-479.
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol. 2016;128:e210-e226.
- Roman LD, Muderspach LI, Stein SM, et al. Pelvic examination, tumor marker level, and gray-scale and Doppler sonography in the prediction of pelvic cancer. Obstet Gynecol. 1997;89:493-500.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol. 2012:126:157-166.
Minilaparotomy: Minimally invasive approach to abdominal myomectomy
A minilaparotomy is loosely defined as a laparotomy measuring between 4 cm and 6 cm. For the appropriate surgical candidate, a minilaparotomy is a useful alternative to laparotomy or laparoscopy, especially for large pathology.1 Benefits of minilaparotomy include improved pain management and postoperative recovery, as well as improved cosmetic outcome, with comparable blood loss and operative time.2,3

In this video, we illustrate the key surgical steps of a minilaparotomy for the removal of large fibroids. These steps include:
- strategic vertical skin incision
- use of a self-retaining retractor
- infiltrate myometrium with dilute vasopressin
- strategic hysterotomy
- use of tenaculum for upward traction
- 10# blade scalpels for the “lemon wedge” coring technique
- layered closure.
Minilaparotomy myomectomy can be an excellent minimally invasive alternative to a traditional “full laparotomy” for women with large fibroids.
We hope that you find this video beneficial to your clinical practice.
>> Arnold P. Advincula, MD
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass
- Pelosi MA 2nd, Pelosi MA 3rd. Pelosi minilaparotomy hysterectomy: a non-endoscopic minimally invasive alternative to laparoscopy and laparotomy. Surg Technol Int. 2004;13:157-167.
- Fanafani F, Fagotti A, Longo R. Minilaparotomy in the management of benign gynecologic disease. Eur J Obstet Gynecol Reprod Biol. 2005;119:232-236.
- Glasser MH. Minilaparotomy: a minimally invasive alternative for major gynecologic abdominal surgery. Perm J. 2005;9:41-45.
A minilaparotomy is loosely defined as a laparotomy measuring between 4 cm and 6 cm. For the appropriate surgical candidate, a minilaparotomy is a useful alternative to laparotomy or laparoscopy, especially for large pathology.1 Benefits of minilaparotomy include improved pain management and postoperative recovery, as well as improved cosmetic outcome, with comparable blood loss and operative time.2,3

In this video, we illustrate the key surgical steps of a minilaparotomy for the removal of large fibroids. These steps include:
- strategic vertical skin incision
- use of a self-retaining retractor
- infiltrate myometrium with dilute vasopressin
- strategic hysterotomy
- use of tenaculum for upward traction
- 10# blade scalpels for the “lemon wedge” coring technique
- layered closure.
Minilaparotomy myomectomy can be an excellent minimally invasive alternative to a traditional “full laparotomy” for women with large fibroids.
We hope that you find this video beneficial to your clinical practice.
>> Arnold P. Advincula, MD
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass
A minilaparotomy is loosely defined as a laparotomy measuring between 4 cm and 6 cm. For the appropriate surgical candidate, a minilaparotomy is a useful alternative to laparotomy or laparoscopy, especially for large pathology.1 Benefits of minilaparotomy include improved pain management and postoperative recovery, as well as improved cosmetic outcome, with comparable blood loss and operative time.2,3

In this video, we illustrate the key surgical steps of a minilaparotomy for the removal of large fibroids. These steps include:
- strategic vertical skin incision
- use of a self-retaining retractor
- infiltrate myometrium with dilute vasopressin
- strategic hysterotomy
- use of tenaculum for upward traction
- 10# blade scalpels for the “lemon wedge” coring technique
- layered closure.
Minilaparotomy myomectomy can be an excellent minimally invasive alternative to a traditional “full laparotomy” for women with large fibroids.
We hope that you find this video beneficial to your clinical practice.
>> Arnold P. Advincula, MD
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass
- Pelosi MA 2nd, Pelosi MA 3rd. Pelosi minilaparotomy hysterectomy: a non-endoscopic minimally invasive alternative to laparoscopy and laparotomy. Surg Technol Int. 2004;13:157-167.
- Fanafani F, Fagotti A, Longo R. Minilaparotomy in the management of benign gynecologic disease. Eur J Obstet Gynecol Reprod Biol. 2005;119:232-236.
- Glasser MH. Minilaparotomy: a minimally invasive alternative for major gynecologic abdominal surgery. Perm J. 2005;9:41-45.
- Pelosi MA 2nd, Pelosi MA 3rd. Pelosi minilaparotomy hysterectomy: a non-endoscopic minimally invasive alternative to laparoscopy and laparotomy. Surg Technol Int. 2004;13:157-167.
- Fanafani F, Fagotti A, Longo R. Minilaparotomy in the management of benign gynecologic disease. Eur J Obstet Gynecol Reprod Biol. 2005;119:232-236.
- Glasser MH. Minilaparotomy: a minimally invasive alternative for major gynecologic abdominal surgery. Perm J. 2005;9:41-45.
2018 Update on minimally invasive gynecologic surgery
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel
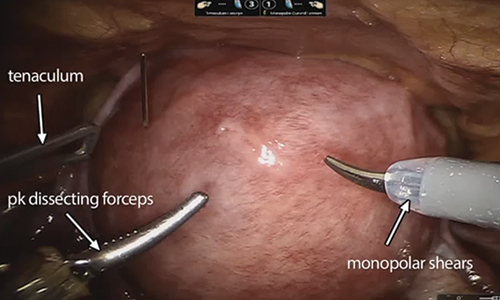
Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.
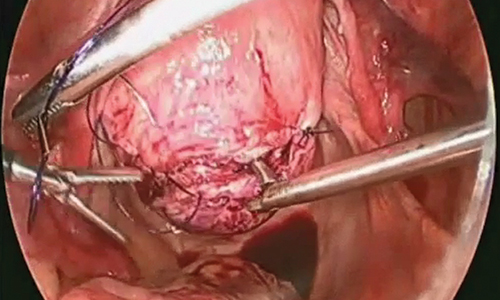
Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28
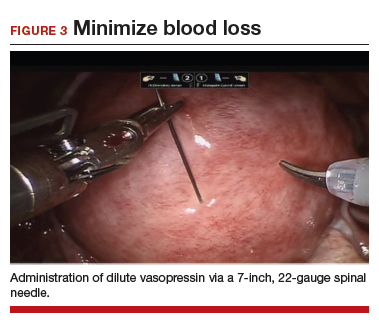
Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.
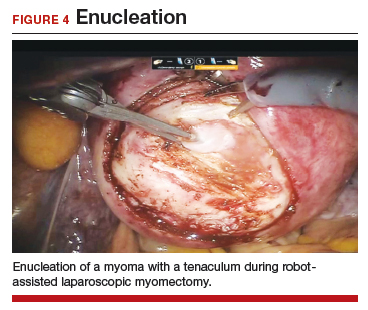
As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.
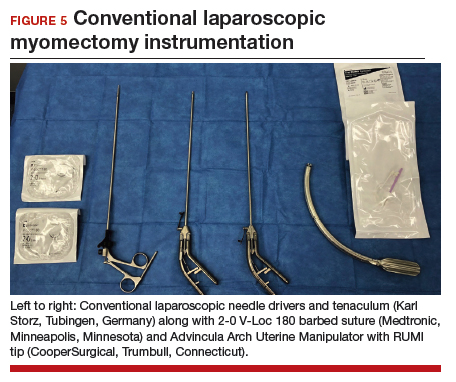
Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
Robot-assisted laparoscopic tubal anastomosis following sterilization

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.