User login
Using social media to change the story on MIGS
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Endometriosis: Expert perspectives on medical and surgical management
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.
Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.
Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.
Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Take-home points
- Endometriosis management involves fluidity of care. Treatment approaches will change throughout a patient's reproductive life, depending on the patient's presenting symptoms and reproductive goals.
- Inform the patient of the disease process and how it may affect her menstrual pain symptoms and family planning.
- Educate patients so they may effectively participate in the management discussion. Hear the voice of the patient to make a tailored plan of care for each individual.
- Endometriosis can be a complex medical problem. Use a comprehensive multidisciplinary approach when appropriate.
Watch: Video roundtable–Endometriosis: Expert perspectives on medical and surgical management
Video roundtable–Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management
Your patient has a large symptomatic fibroid: Tools for decision making
2017 Update on minimally invasive gynecologic surgery
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.
The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
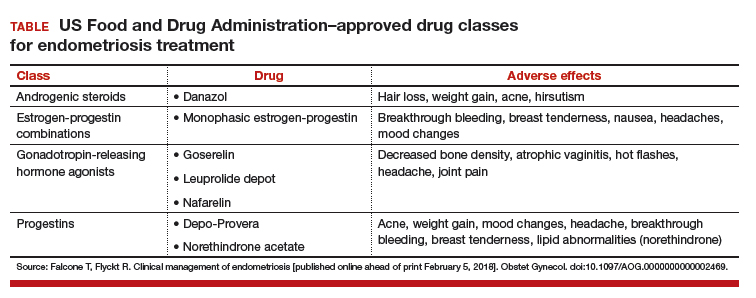
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11
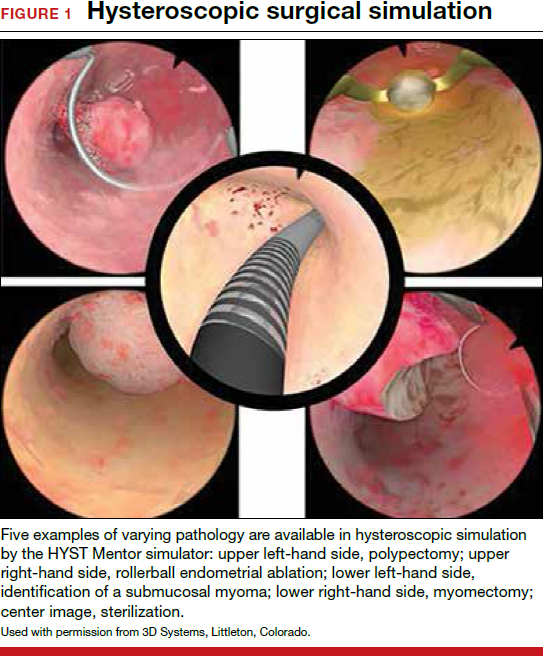
Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
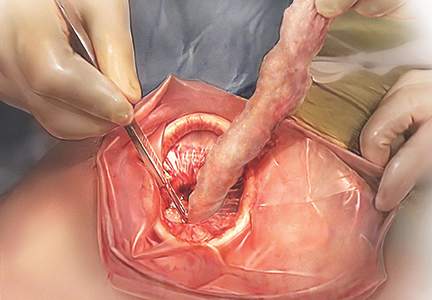
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
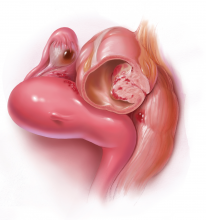
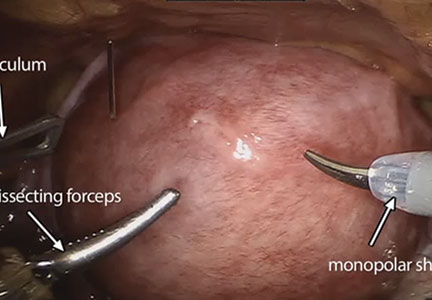
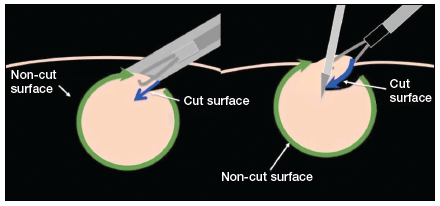
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18
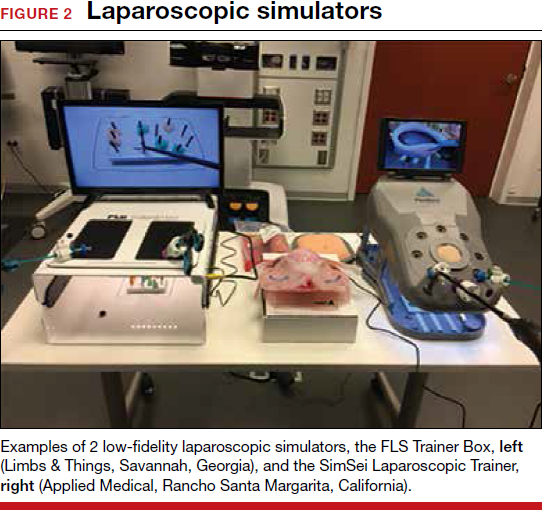
The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
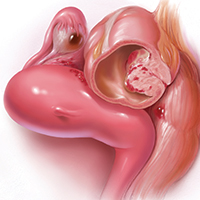
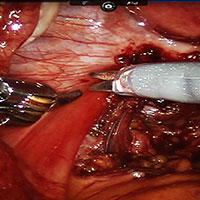
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).
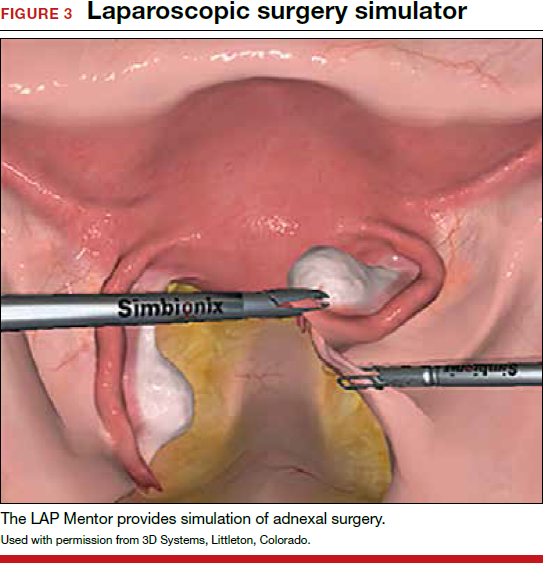
Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
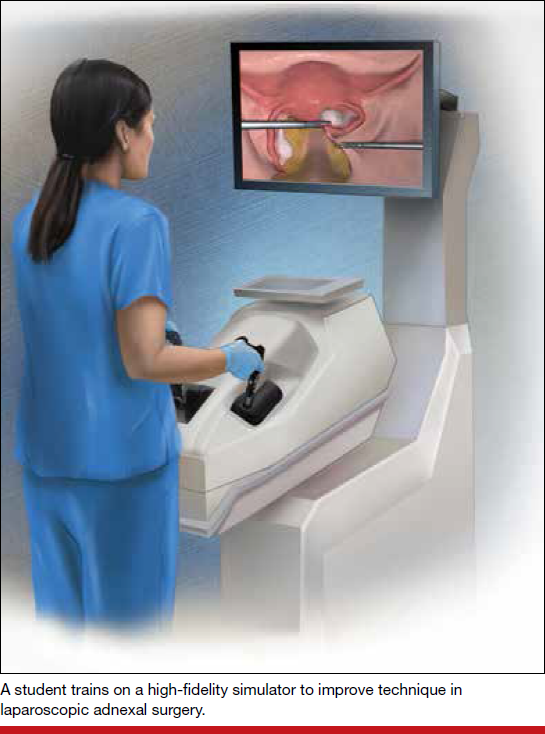
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.

The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11

Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18

The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).

Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.

The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11

Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18

The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).

Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
Robot-assisted laparoscopic excision of a rectovaginal endometriotic nodule
A rectovaginal endometriosis (RVE) is the most severe form of endometriosis. The gold standard for diagnosis is laparoscopy with histologic confirmation. A review of the literature suggests that surgery improves up to 70% of symptoms with generally favorable outcomes.
In this video, we provide a general introduction to endometriosis and a discussion of disease treatment options, ranging from hormonal suppression to radical bowel resections. We also illustrate the steps in robot-assisted laparoscopic excision of an RVE nodule:
- identify the borders of the rectosigmoid
- dissect the pararectal spaces
- release the rectosigmoid from its attachment to the RVE nodule
- identify and isolate the ureter(s)
- determine the margins of the nodule
- ensure complete resection.
Excision of an RVE nodule is a technically challenging surgical procedure. Use of the robot for resection is safe and feasible when performed by a trained and experienced surgeon.
I am pleased to bring you this video, and I hope that it is helpful to your practice.
>> Arnold P. Advincula, MD

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
A rectovaginal endometriosis (RVE) is the most severe form of endometriosis. The gold standard for diagnosis is laparoscopy with histologic confirmation. A review of the literature suggests that surgery improves up to 70% of symptoms with generally favorable outcomes.
In this video, we provide a general introduction to endometriosis and a discussion of disease treatment options, ranging from hormonal suppression to radical bowel resections. We also illustrate the steps in robot-assisted laparoscopic excision of an RVE nodule:
- identify the borders of the rectosigmoid
- dissect the pararectal spaces
- release the rectosigmoid from its attachment to the RVE nodule
- identify and isolate the ureter(s)
- determine the margins of the nodule
- ensure complete resection.
Excision of an RVE nodule is a technically challenging surgical procedure. Use of the robot for resection is safe and feasible when performed by a trained and experienced surgeon.
I am pleased to bring you this video, and I hope that it is helpful to your practice.
>> Arnold P. Advincula, MD

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
A rectovaginal endometriosis (RVE) is the most severe form of endometriosis. The gold standard for diagnosis is laparoscopy with histologic confirmation. A review of the literature suggests that surgery improves up to 70% of symptoms with generally favorable outcomes.
In this video, we provide a general introduction to endometriosis and a discussion of disease treatment options, ranging from hormonal suppression to radical bowel resections. We also illustrate the steps in robot-assisted laparoscopic excision of an RVE nodule:
- identify the borders of the rectosigmoid
- dissect the pararectal spaces
- release the rectosigmoid from its attachment to the RVE nodule
- identify and isolate the ureter(s)
- determine the margins of the nodule
- ensure complete resection.
Excision of an RVE nodule is a technically challenging surgical procedure. Use of the robot for resection is safe and feasible when performed by a trained and experienced surgeon.
I am pleased to bring you this video, and I hope that it is helpful to your practice.
>> Arnold P. Advincula, MD

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Robot-assisted laparoscopic resection of a noncommunicating cavitary rudimentary horn
A unicornuate uterus with a noncommunicating rudimentary horn is a rare mullerian duct anomaly (MDA). It often goes undiagnosed due to the absence of functional endometrium in the anomalous horn. However, when the rudimentary horn is lined with endometrium, obstructed menstrual flow can lead to severe cyclic pelvic pain, development of a pelvic mass, and endometriosis from retrograde menstruation. For these reasons, surgical resection is recommended for patients with this anomaly.
In this video the surgical patient is a 15-year-old adolescent with a 1-year history of progressive dysmenorrhea. Imaging studies revealed a noncommunicating cavitary right uterine horn and confirmed a normal urinary tract system.
We present a stepwise demonstration of our technique for surgical resection of a noncommunicating cavitary uterine horn and conclude that robotic surgery is a safe and feasible route for surgical management of this pathology.
I am pleased to bring you this video to kick off the New Year. We are delighted that our work won "Best Video on Robotic Technology" at the annual AAGL meeting in November 2016, and I hope that it is helpful to your practice.

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
A unicornuate uterus with a noncommunicating rudimentary horn is a rare mullerian duct anomaly (MDA). It often goes undiagnosed due to the absence of functional endometrium in the anomalous horn. However, when the rudimentary horn is lined with endometrium, obstructed menstrual flow can lead to severe cyclic pelvic pain, development of a pelvic mass, and endometriosis from retrograde menstruation. For these reasons, surgical resection is recommended for patients with this anomaly.
In this video the surgical patient is a 15-year-old adolescent with a 1-year history of progressive dysmenorrhea. Imaging studies revealed a noncommunicating cavitary right uterine horn and confirmed a normal urinary tract system.
We present a stepwise demonstration of our technique for surgical resection of a noncommunicating cavitary uterine horn and conclude that robotic surgery is a safe and feasible route for surgical management of this pathology.
I am pleased to bring you this video to kick off the New Year. We are delighted that our work won "Best Video on Robotic Technology" at the annual AAGL meeting in November 2016, and I hope that it is helpful to your practice.

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
A unicornuate uterus with a noncommunicating rudimentary horn is a rare mullerian duct anomaly (MDA). It often goes undiagnosed due to the absence of functional endometrium in the anomalous horn. However, when the rudimentary horn is lined with endometrium, obstructed menstrual flow can lead to severe cyclic pelvic pain, development of a pelvic mass, and endometriosis from retrograde menstruation. For these reasons, surgical resection is recommended for patients with this anomaly.
In this video the surgical patient is a 15-year-old adolescent with a 1-year history of progressive dysmenorrhea. Imaging studies revealed a noncommunicating cavitary right uterine horn and confirmed a normal urinary tract system.
We present a stepwise demonstration of our technique for surgical resection of a noncommunicating cavitary uterine horn and conclude that robotic surgery is a safe and feasible route for surgical management of this pathology.
I am pleased to bring you this video to kick off the New Year. We are delighted that our work won "Best Video on Robotic Technology" at the annual AAGL meeting in November 2016, and I hope that it is helpful to your practice.

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Tissue extraction: Can the pendulum change direction?
On April 17, 2014, the US Food and Drug Administration (FDA) released a safety communication that discouraged the use of laparoscopic power morcellation during hysterectomy or myomectomy for the treatment of women with uterine fibroids. This recommendation was based mainly on the premise that fibroids may contain an underlying malignancy (in 1 in 350 women) that laparoscopic power morcellation could spread, thereby potentially worsening a cancer prognosis.1 Whether we liked it or not, minimally invasive gynecologic surgery as we knew it had changed forever on that fateful day.
During the last 2 years, the implications of that initial safety communication, along with its update in November of 2014,2 have been far reaching. From a health care industry perspective, the largest manufacturer of power morcellators, Johnson & Johnson, completely pulled its device out of the market within months of the initial FDA safety communication.3 Although several other companies remained in the market, health insurers such as Highmark and Aetna took the stance of not paying for the routine use of a power morcellator.4,5 Soon government officials became involved in the campaign against power morcellators and trial lawyers made this surgical device the latest “hot target.”6,7
The power morcellator’s absence impactFor clinicians, the narrative painted had moved the pendulum so far against power morcellation that it did not come as a surprise that practice patterns were significantly impacted. Harris and colleagues recently measured that impact by evaluating practice patterns and postoperative complications before and after the FDA safety communication on power morcellation. In their retrospective cohort study of patients within the Michigan Surgical Quality Collaborative, utilization of minimally invasive hysterectomy decreased and major surgical, nontransfusion complications and 30-day hospital readmission rates increased after release of the safety communication.8
Further support of this change in practice patterns was demonstrated in a recent survey conducted by the AAGL and American College of Obstetricians and Gynecologists Collaborative Ambulatory Research Network (ACOG CARN). In this survey study, Lum and colleagues were able to show that power morcellation use decreased among AAGL and ACOG CARN members after the FDA warnings, and rates of laparotomy increased.9
Critical to the discussion, yet apparently overlooked during the formulation of the initial FDA warning, was the potential clinical significance of the downstream impact of converting minimally invasive surgical cases to more invasive laparotomies. A decision-tree analysis published by Siedhoff and colleagues highlighted this point by predicting fewer overall deaths for laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy.10 An ability to weigh the benefits and risks of procedure-related complications that are associated with laparotomy, including death, should have been part of the conversation from the beginning.
There is a silver lining emergingBased on this domino effect, it would seem that the damage done to minimally invasive gynecologic surgery over the past 2 years is irreparable. So is there any silver lining to the current state of affairs in tissue extraction? I would argue yes.
We have updated estimates for incidence of unsuspected leiomyosarcoma. First of all, discussions surrounding the FDA’s estimated 1 in 350 risk of encountering an unsuspected uterine sarcoma during the treatment of fibroids prompted a more critical evaluation of the scientific literature. In fact, Parker and colleagues, in a commentary published in Obstetrics & Gynecology, summarized very nicely the flawed methodology in the FDA’s determination of risk and in turn presented several updated calculations and studies that placed the prevalence somewhere in the range of 2 in 8,720 (0.023%) to 1 in 1,550 (0.064%).11
We are speaking with the FDA. Channels of communication with the FDA have since been developed and societies such as the AAGL were invited by the Center for Devices and Radiological Health (CDRH) at the FDA to serve in their Network of Experts. This Network of Experts is an FDA-vetted group of non−FDA affiliated scientists, clinicians, and engineers who provide the CDRH at the FDA with rapid access to scientific, engineering, and medical expertise when it is needed to supplement existing knowledge and expertise within the CDRH. By developing these lines of communication, the CDRH is able to broaden its exposure to scientific viewpoints without the influence of external, or non−FDA, policy advice or opinions.
We have been innovating our techniques. Clinicians also began to develop innovative techniques for tissue extraction that also included contained, in-bag power morcellation. Vargas and colleagues showed similar perioperative outcomes when comparing open power morcellation with contained power morcellation within an insufflated isolation bag. The mean operative time was prolonged by only 26 minutes with in-bag morcellation.12
Although the initial experience of surgeons involved various containment systems that were off label in their usage and not designed to be paired with a power morcellator, it allowed for the identification of potential limitations, such as the risk of leakage. Cohen and colleagues, in their published experience, demonstrated a 9.2% spillage in 76 patients who underwent contained power morcellation.13
Could a new FDA approval turn the tide?In an interesting turn of events, the FDA, on April 7, 2016, nearly 2 years after their initial warning about power morcellation, gave de novo classification clearance to Advanced Surgical Concepts for its PneumoLiner device.14 This first-of-a-kind medical device was developed for the purpose of completely containing fluid, cells, and tissue fragments during laparoscopic power morcellation, isolating uterine tissue that is not suspected to contain cancer (FIGURES 1 and 2). It is important to note, however, that it has not been shown to reduce the risk of spreading cancer during this procedure. Although a surprise to some, the approval of such a product is in keeping with the FDA’s safety communication update in November 2014 that encouraged the development of containment systems designed specifically for gynecologic surgery.
| FIGURE 1 PneumoLiner tissue containment device | FIGURE 2 PneumoLiner device placement | |
 |  |
Currently, the PneumoLiner is not yet available for purchase until Olympus, who will be the exclusive distributor, finalizes its formal training program rollout for ensuring safe and effective use of the device by gynecologic surgeons. As part of the FDA approval process, a training protocol was validated in a laboratory setting by surgeons with varying levels of experience in order to demonstrate that adherence to this process showed no damage to any of the containment systems used during the study.
As I look forward to the opportunity to learn more about this new containment system and potentially incorporate it into my surgical armamentarium, several questions immediately come to mind.
- Will hospitals, many of which pulled morcellators off their shelves in the wake of the FDA safety communication, be willing to embrace such innovative new containment technology and make it available to their surgeons?
- Will the litigious landscape painted by trial lawyers prevent surgeons from offering this option to their patients?
- Can surgeons undo much of the misinformation being propagated by the media as it relates to tissue extraction and the risk of encountering a malignancy?
I hope the answer to all of these questions is yes.
I do believe the pendulum can change directionAs surgeons it is our duty to evaluate potentially innovative solutions to the surgical challenges we face in clinical practice while maintaining an evidence-based approach. Most importantly, all of this must be balanced by sound clinical judgment that no technology can replace. With these guiding principles in mind, I believe that the pendulum regarding tissue extraction can change direction.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed May 24, 2016.
- Updated laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Updated April 7, 2014. Accessed May 24, 2016.
- Kamp J, Levitz J. Johnson & Johnson pulls hysterectomy device from hospitals. The Wall Street Journal. July 30, 2014. http://www.wsj.com/articles/johnson-johnson-to-call-for-voluntary-return-of-morcellators-1406754350. Accessed May 25, 2016.
- Levitz J. Health insurer to stop covering uterine procedure. The Wall Street Journal. August 2, 2014. http://www.wsj.com/articles/health-insurer-to-stop-covering-uterine-procedure-1406999176. Accessed May 25, 2016.
- Kamp J. Aetna to stop covering routine use of power morcellator. The Wall Street Journal. May 5, 2015. http://www.wsj.com/articles/aetna-to-stop-covering-routine-use-of-power-morcellator-1430838666. Accessed May 25, 2016.
- Kamp J. Senators want more companies to pull surgical device from market. The Wall Street Journal. August 19, 2014. http://www.wsj.com/articles/senators-want-more-companies-to-pull-surgical-device-from-market-1408405349. Accessed May 25, 2016.
- Silverman E. Power morcellators are the latest hot target among trial lawyers. The Wall Street Journal. December 2, 2014. http://blogs.wsj.com/pharmalot/2014/12/02/power-morcellators-are-the-latest-hot-target-among-trial-lawyers/. Accessed May 25, 2016.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after the US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1−e13.
- Lum DA, Sokol ER, Berek JS, et al. Impact of the 2014 Food and Drug Administration warnings against power morcellation. J Minim Invasiv Gynecol. 2016;23(4):548−556.
- Siedhoff M, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212(5):591.e1−e8.
- Parker WH, Kaunitz AM, Pritts EA, et al; Leiomyoma Morcellation Review Group. US Food and Drug Administration’s guidance regarding morcellation of leiomyomas: well-intentioned, but is it harmful for women? Obstet Gynecol. 2016;127(1):18−22.
- Vargas MV, Cohen SL, Fuchs-Weizman N, et al. Open power morcellation versus contained power morcellation within an insufflated isolation bag: comparison of perioperative outcomes. J Minim Invasiv Gynecol. 2015;22(3):433−438.
- Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257.e1−e6.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. April 7, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed May 24, 2016.
On April 17, 2014, the US Food and Drug Administration (FDA) released a safety communication that discouraged the use of laparoscopic power morcellation during hysterectomy or myomectomy for the treatment of women with uterine fibroids. This recommendation was based mainly on the premise that fibroids may contain an underlying malignancy (in 1 in 350 women) that laparoscopic power morcellation could spread, thereby potentially worsening a cancer prognosis.1 Whether we liked it or not, minimally invasive gynecologic surgery as we knew it had changed forever on that fateful day.
During the last 2 years, the implications of that initial safety communication, along with its update in November of 2014,2 have been far reaching. From a health care industry perspective, the largest manufacturer of power morcellators, Johnson & Johnson, completely pulled its device out of the market within months of the initial FDA safety communication.3 Although several other companies remained in the market, health insurers such as Highmark and Aetna took the stance of not paying for the routine use of a power morcellator.4,5 Soon government officials became involved in the campaign against power morcellators and trial lawyers made this surgical device the latest “hot target.”6,7
The power morcellator’s absence impactFor clinicians, the narrative painted had moved the pendulum so far against power morcellation that it did not come as a surprise that practice patterns were significantly impacted. Harris and colleagues recently measured that impact by evaluating practice patterns and postoperative complications before and after the FDA safety communication on power morcellation. In their retrospective cohort study of patients within the Michigan Surgical Quality Collaborative, utilization of minimally invasive hysterectomy decreased and major surgical, nontransfusion complications and 30-day hospital readmission rates increased after release of the safety communication.8
Further support of this change in practice patterns was demonstrated in a recent survey conducted by the AAGL and American College of Obstetricians and Gynecologists Collaborative Ambulatory Research Network (ACOG CARN). In this survey study, Lum and colleagues were able to show that power morcellation use decreased among AAGL and ACOG CARN members after the FDA warnings, and rates of laparotomy increased.9
Critical to the discussion, yet apparently overlooked during the formulation of the initial FDA warning, was the potential clinical significance of the downstream impact of converting minimally invasive surgical cases to more invasive laparotomies. A decision-tree analysis published by Siedhoff and colleagues highlighted this point by predicting fewer overall deaths for laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy.10 An ability to weigh the benefits and risks of procedure-related complications that are associated with laparotomy, including death, should have been part of the conversation from the beginning.
There is a silver lining emergingBased on this domino effect, it would seem that the damage done to minimally invasive gynecologic surgery over the past 2 years is irreparable. So is there any silver lining to the current state of affairs in tissue extraction? I would argue yes.
We have updated estimates for incidence of unsuspected leiomyosarcoma. First of all, discussions surrounding the FDA’s estimated 1 in 350 risk of encountering an unsuspected uterine sarcoma during the treatment of fibroids prompted a more critical evaluation of the scientific literature. In fact, Parker and colleagues, in a commentary published in Obstetrics & Gynecology, summarized very nicely the flawed methodology in the FDA’s determination of risk and in turn presented several updated calculations and studies that placed the prevalence somewhere in the range of 2 in 8,720 (0.023%) to 1 in 1,550 (0.064%).11
We are speaking with the FDA. Channels of communication with the FDA have since been developed and societies such as the AAGL were invited by the Center for Devices and Radiological Health (CDRH) at the FDA to serve in their Network of Experts. This Network of Experts is an FDA-vetted group of non−FDA affiliated scientists, clinicians, and engineers who provide the CDRH at the FDA with rapid access to scientific, engineering, and medical expertise when it is needed to supplement existing knowledge and expertise within the CDRH. By developing these lines of communication, the CDRH is able to broaden its exposure to scientific viewpoints without the influence of external, or non−FDA, policy advice or opinions.
We have been innovating our techniques. Clinicians also began to develop innovative techniques for tissue extraction that also included contained, in-bag power morcellation. Vargas and colleagues showed similar perioperative outcomes when comparing open power morcellation with contained power morcellation within an insufflated isolation bag. The mean operative time was prolonged by only 26 minutes with in-bag morcellation.12
Although the initial experience of surgeons involved various containment systems that were off label in their usage and not designed to be paired with a power morcellator, it allowed for the identification of potential limitations, such as the risk of leakage. Cohen and colleagues, in their published experience, demonstrated a 9.2% spillage in 76 patients who underwent contained power morcellation.13
Could a new FDA approval turn the tide?In an interesting turn of events, the FDA, on April 7, 2016, nearly 2 years after their initial warning about power morcellation, gave de novo classification clearance to Advanced Surgical Concepts for its PneumoLiner device.14 This first-of-a-kind medical device was developed for the purpose of completely containing fluid, cells, and tissue fragments during laparoscopic power morcellation, isolating uterine tissue that is not suspected to contain cancer (FIGURES 1 and 2). It is important to note, however, that it has not been shown to reduce the risk of spreading cancer during this procedure. Although a surprise to some, the approval of such a product is in keeping with the FDA’s safety communication update in November 2014 that encouraged the development of containment systems designed specifically for gynecologic surgery.
| FIGURE 1 PneumoLiner tissue containment device | FIGURE 2 PneumoLiner device placement | |
 |  |
Currently, the PneumoLiner is not yet available for purchase until Olympus, who will be the exclusive distributor, finalizes its formal training program rollout for ensuring safe and effective use of the device by gynecologic surgeons. As part of the FDA approval process, a training protocol was validated in a laboratory setting by surgeons with varying levels of experience in order to demonstrate that adherence to this process showed no damage to any of the containment systems used during the study.
As I look forward to the opportunity to learn more about this new containment system and potentially incorporate it into my surgical armamentarium, several questions immediately come to mind.
- Will hospitals, many of which pulled morcellators off their shelves in the wake of the FDA safety communication, be willing to embrace such innovative new containment technology and make it available to their surgeons?
- Will the litigious landscape painted by trial lawyers prevent surgeons from offering this option to their patients?
- Can surgeons undo much of the misinformation being propagated by the media as it relates to tissue extraction and the risk of encountering a malignancy?
I hope the answer to all of these questions is yes.
I do believe the pendulum can change directionAs surgeons it is our duty to evaluate potentially innovative solutions to the surgical challenges we face in clinical practice while maintaining an evidence-based approach. Most importantly, all of this must be balanced by sound clinical judgment that no technology can replace. With these guiding principles in mind, I believe that the pendulum regarding tissue extraction can change direction.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
On April 17, 2014, the US Food and Drug Administration (FDA) released a safety communication that discouraged the use of laparoscopic power morcellation during hysterectomy or myomectomy for the treatment of women with uterine fibroids. This recommendation was based mainly on the premise that fibroids may contain an underlying malignancy (in 1 in 350 women) that laparoscopic power morcellation could spread, thereby potentially worsening a cancer prognosis.1 Whether we liked it or not, minimally invasive gynecologic surgery as we knew it had changed forever on that fateful day.
During the last 2 years, the implications of that initial safety communication, along with its update in November of 2014,2 have been far reaching. From a health care industry perspective, the largest manufacturer of power morcellators, Johnson & Johnson, completely pulled its device out of the market within months of the initial FDA safety communication.3 Although several other companies remained in the market, health insurers such as Highmark and Aetna took the stance of not paying for the routine use of a power morcellator.4,5 Soon government officials became involved in the campaign against power morcellators and trial lawyers made this surgical device the latest “hot target.”6,7
The power morcellator’s absence impactFor clinicians, the narrative painted had moved the pendulum so far against power morcellation that it did not come as a surprise that practice patterns were significantly impacted. Harris and colleagues recently measured that impact by evaluating practice patterns and postoperative complications before and after the FDA safety communication on power morcellation. In their retrospective cohort study of patients within the Michigan Surgical Quality Collaborative, utilization of minimally invasive hysterectomy decreased and major surgical, nontransfusion complications and 30-day hospital readmission rates increased after release of the safety communication.8
Further support of this change in practice patterns was demonstrated in a recent survey conducted by the AAGL and American College of Obstetricians and Gynecologists Collaborative Ambulatory Research Network (ACOG CARN). In this survey study, Lum and colleagues were able to show that power morcellation use decreased among AAGL and ACOG CARN members after the FDA warnings, and rates of laparotomy increased.9
Critical to the discussion, yet apparently overlooked during the formulation of the initial FDA warning, was the potential clinical significance of the downstream impact of converting minimally invasive surgical cases to more invasive laparotomies. A decision-tree analysis published by Siedhoff and colleagues highlighted this point by predicting fewer overall deaths for laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy.10 An ability to weigh the benefits and risks of procedure-related complications that are associated with laparotomy, including death, should have been part of the conversation from the beginning.
There is a silver lining emergingBased on this domino effect, it would seem that the damage done to minimally invasive gynecologic surgery over the past 2 years is irreparable. So is there any silver lining to the current state of affairs in tissue extraction? I would argue yes.
We have updated estimates for incidence of unsuspected leiomyosarcoma. First of all, discussions surrounding the FDA’s estimated 1 in 350 risk of encountering an unsuspected uterine sarcoma during the treatment of fibroids prompted a more critical evaluation of the scientific literature. In fact, Parker and colleagues, in a commentary published in Obstetrics & Gynecology, summarized very nicely the flawed methodology in the FDA’s determination of risk and in turn presented several updated calculations and studies that placed the prevalence somewhere in the range of 2 in 8,720 (0.023%) to 1 in 1,550 (0.064%).11
We are speaking with the FDA. Channels of communication with the FDA have since been developed and societies such as the AAGL were invited by the Center for Devices and Radiological Health (CDRH) at the FDA to serve in their Network of Experts. This Network of Experts is an FDA-vetted group of non−FDA affiliated scientists, clinicians, and engineers who provide the CDRH at the FDA with rapid access to scientific, engineering, and medical expertise when it is needed to supplement existing knowledge and expertise within the CDRH. By developing these lines of communication, the CDRH is able to broaden its exposure to scientific viewpoints without the influence of external, or non−FDA, policy advice or opinions.
We have been innovating our techniques. Clinicians also began to develop innovative techniques for tissue extraction that also included contained, in-bag power morcellation. Vargas and colleagues showed similar perioperative outcomes when comparing open power morcellation with contained power morcellation within an insufflated isolation bag. The mean operative time was prolonged by only 26 minutes with in-bag morcellation.12
Although the initial experience of surgeons involved various containment systems that were off label in their usage and not designed to be paired with a power morcellator, it allowed for the identification of potential limitations, such as the risk of leakage. Cohen and colleagues, in their published experience, demonstrated a 9.2% spillage in 76 patients who underwent contained power morcellation.13
Could a new FDA approval turn the tide?In an interesting turn of events, the FDA, on April 7, 2016, nearly 2 years after their initial warning about power morcellation, gave de novo classification clearance to Advanced Surgical Concepts for its PneumoLiner device.14 This first-of-a-kind medical device was developed for the purpose of completely containing fluid, cells, and tissue fragments during laparoscopic power morcellation, isolating uterine tissue that is not suspected to contain cancer (FIGURES 1 and 2). It is important to note, however, that it has not been shown to reduce the risk of spreading cancer during this procedure. Although a surprise to some, the approval of such a product is in keeping with the FDA’s safety communication update in November 2014 that encouraged the development of containment systems designed specifically for gynecologic surgery.
| FIGURE 1 PneumoLiner tissue containment device | FIGURE 2 PneumoLiner device placement | |
 |  |
Currently, the PneumoLiner is not yet available for purchase until Olympus, who will be the exclusive distributor, finalizes its formal training program rollout for ensuring safe and effective use of the device by gynecologic surgeons. As part of the FDA approval process, a training protocol was validated in a laboratory setting by surgeons with varying levels of experience in order to demonstrate that adherence to this process showed no damage to any of the containment systems used during the study.
As I look forward to the opportunity to learn more about this new containment system and potentially incorporate it into my surgical armamentarium, several questions immediately come to mind.
- Will hospitals, many of which pulled morcellators off their shelves in the wake of the FDA safety communication, be willing to embrace such innovative new containment technology and make it available to their surgeons?
- Will the litigious landscape painted by trial lawyers prevent surgeons from offering this option to their patients?
- Can surgeons undo much of the misinformation being propagated by the media as it relates to tissue extraction and the risk of encountering a malignancy?
I hope the answer to all of these questions is yes.
I do believe the pendulum can change directionAs surgeons it is our duty to evaluate potentially innovative solutions to the surgical challenges we face in clinical practice while maintaining an evidence-based approach. Most importantly, all of this must be balanced by sound clinical judgment that no technology can replace. With these guiding principles in mind, I believe that the pendulum regarding tissue extraction can change direction.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed May 24, 2016.
- Updated laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Updated April 7, 2014. Accessed May 24, 2016.
- Kamp J, Levitz J. Johnson & Johnson pulls hysterectomy device from hospitals. The Wall Street Journal. July 30, 2014. http://www.wsj.com/articles/johnson-johnson-to-call-for-voluntary-return-of-morcellators-1406754350. Accessed May 25, 2016.
- Levitz J. Health insurer to stop covering uterine procedure. The Wall Street Journal. August 2, 2014. http://www.wsj.com/articles/health-insurer-to-stop-covering-uterine-procedure-1406999176. Accessed May 25, 2016.
- Kamp J. Aetna to stop covering routine use of power morcellator. The Wall Street Journal. May 5, 2015. http://www.wsj.com/articles/aetna-to-stop-covering-routine-use-of-power-morcellator-1430838666. Accessed May 25, 2016.
- Kamp J. Senators want more companies to pull surgical device from market. The Wall Street Journal. August 19, 2014. http://www.wsj.com/articles/senators-want-more-companies-to-pull-surgical-device-from-market-1408405349. Accessed May 25, 2016.
- Silverman E. Power morcellators are the latest hot target among trial lawyers. The Wall Street Journal. December 2, 2014. http://blogs.wsj.com/pharmalot/2014/12/02/power-morcellators-are-the-latest-hot-target-among-trial-lawyers/. Accessed May 25, 2016.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after the US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1−e13.
- Lum DA, Sokol ER, Berek JS, et al. Impact of the 2014 Food and Drug Administration warnings against power morcellation. J Minim Invasiv Gynecol. 2016;23(4):548−556.
- Siedhoff M, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212(5):591.e1−e8.
- Parker WH, Kaunitz AM, Pritts EA, et al; Leiomyoma Morcellation Review Group. US Food and Drug Administration’s guidance regarding morcellation of leiomyomas: well-intentioned, but is it harmful for women? Obstet Gynecol. 2016;127(1):18−22.
- Vargas MV, Cohen SL, Fuchs-Weizman N, et al. Open power morcellation versus contained power morcellation within an insufflated isolation bag: comparison of perioperative outcomes. J Minim Invasiv Gynecol. 2015;22(3):433−438.
- Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257.e1−e6.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. April 7, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed May 24, 2016.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed May 24, 2016.
- Updated laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Updated April 7, 2014. Accessed May 24, 2016.
- Kamp J, Levitz J. Johnson & Johnson pulls hysterectomy device from hospitals. The Wall Street Journal. July 30, 2014. http://www.wsj.com/articles/johnson-johnson-to-call-for-voluntary-return-of-morcellators-1406754350. Accessed May 25, 2016.
- Levitz J. Health insurer to stop covering uterine procedure. The Wall Street Journal. August 2, 2014. http://www.wsj.com/articles/health-insurer-to-stop-covering-uterine-procedure-1406999176. Accessed May 25, 2016.
- Kamp J. Aetna to stop covering routine use of power morcellator. The Wall Street Journal. May 5, 2015. http://www.wsj.com/articles/aetna-to-stop-covering-routine-use-of-power-morcellator-1430838666. Accessed May 25, 2016.
- Kamp J. Senators want more companies to pull surgical device from market. The Wall Street Journal. August 19, 2014. http://www.wsj.com/articles/senators-want-more-companies-to-pull-surgical-device-from-market-1408405349. Accessed May 25, 2016.
- Silverman E. Power morcellators are the latest hot target among trial lawyers. The Wall Street Journal. December 2, 2014. http://blogs.wsj.com/pharmalot/2014/12/02/power-morcellators-are-the-latest-hot-target-among-trial-lawyers/. Accessed May 25, 2016.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after the US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1−e13.
- Lum DA, Sokol ER, Berek JS, et al. Impact of the 2014 Food and Drug Administration warnings against power morcellation. J Minim Invasiv Gynecol. 2016;23(4):548−556.
- Siedhoff M, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212(5):591.e1−e8.
- Parker WH, Kaunitz AM, Pritts EA, et al; Leiomyoma Morcellation Review Group. US Food and Drug Administration’s guidance regarding morcellation of leiomyomas: well-intentioned, but is it harmful for women? Obstet Gynecol. 2016;127(1):18−22.
- Vargas MV, Cohen SL, Fuchs-Weizman N, et al. Open power morcellation versus contained power morcellation within an insufflated isolation bag: comparison of perioperative outcomes. J Minim Invasiv Gynecol. 2015;22(3):433−438.
- Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257.e1−e6.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. April 7, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed May 24, 2016.
Robot-assisted laparoscopic myomectomy
The management of symptomatic uterine fibroids in the patient desiring conservative surgical therapy can be challenging at times. The advent of robot-assisted laparoscopy has provided surgeons with an enabling tool and patients with the option for a minimally invasive approach to myomectomy.
This month’s video was produced in order to demonstrate a systematic approach to the robot-assisted laparoscopic myomectomy in patients who are candidates. The example case is removal of a 5-cm, intrauterine posterior myoma in a 39-year-old woman (G3P1021) with heavy menstrual bleeding who desires future fertility.
Key objectives of the video include:
- understanding the role of radiologic imaging as part of preoperative surgical planning
- recognizing the key robotic instruments and suture selected to perform the procedure
- discussing robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair.
Also integrated into this video is the application of the ExCITE technique—a manual cold knife tissue extraction technique utilizing an extracorporeal semi-circle “C-incision” approach—for tissue extraction. This technique was featured in an earlier installment of the video channel.1
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Truong M, Advincula A. Minimally invasive tissue extraction made simple: the Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
The management of symptomatic uterine fibroids in the patient desiring conservative surgical therapy can be challenging at times. The advent of robot-assisted laparoscopy has provided surgeons with an enabling tool and patients with the option for a minimally invasive approach to myomectomy.
This month’s video was produced in order to demonstrate a systematic approach to the robot-assisted laparoscopic myomectomy in patients who are candidates. The example case is removal of a 5-cm, intrauterine posterior myoma in a 39-year-old woman (G3P1021) with heavy menstrual bleeding who desires future fertility.
Key objectives of the video include:
- understanding the role of radiologic imaging as part of preoperative surgical planning
- recognizing the key robotic instruments and suture selected to perform the procedure
- discussing robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair.
Also integrated into this video is the application of the ExCITE technique—a manual cold knife tissue extraction technique utilizing an extracorporeal semi-circle “C-incision” approach—for tissue extraction. This technique was featured in an earlier installment of the video channel.1
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The management of symptomatic uterine fibroids in the patient desiring conservative surgical therapy can be challenging at times. The advent of robot-assisted laparoscopy has provided surgeons with an enabling tool and patients with the option for a minimally invasive approach to myomectomy.
This month’s video was produced in order to demonstrate a systematic approach to the robot-assisted laparoscopic myomectomy in patients who are candidates. The example case is removal of a 5-cm, intrauterine posterior myoma in a 39-year-old woman (G3P1021) with heavy menstrual bleeding who desires future fertility.
Key objectives of the video include:
- understanding the role of radiologic imaging as part of preoperative surgical planning
- recognizing the key robotic instruments and suture selected to perform the procedure
- discussing robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair.
Also integrated into this video is the application of the ExCITE technique—a manual cold knife tissue extraction technique utilizing an extracorporeal semi-circle “C-incision” approach—for tissue extraction. This technique was featured in an earlier installment of the video channel.1
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Truong M, Advincula A. Minimally invasive tissue extraction made simple: the Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
- Truong M, Advincula A. Minimally invasive tissue extraction made simple: the Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
ExCITE: Minimally invasive tissue extraction made simple with simulation
In November 2014, following concerns regarding the use of electromechanical, or power, morcellation, we published a surgical technique called the extracorporeal C-incision tissue extraction, or ExCITE, technique, as an alternative to contained tissue extraction during minimally invasive gynecologic procedures such as myomectomy and hysterectomy.1 This technique was developed to create a simple, reproducible, and minimally invasive approach to tissue extraction without the need for power morcellation. ExCITE is trainee-friendly and teachable.
In this article, we will review the steps for successful execution of the ExCITE technique. In addition, we will describe how to create your own cost-effective simulation model for teaching, learning, and practicing this technique with a few simple materials found in any craft or grocery store. Simulation is essential. It helps to troubleshoot issues that may arise in an actual case and allows for learning and practicing of surgical techniques to improve the learning curve and efficiency in the operating room (OR).
The model described here is viewable in the video, “The ExCITE technique, Part 2: Simulation made simple.” It is archived in Arnold Advincula’s Surgical Techniques Video Channel, which also is accessible through the “multimedia” section of this Web site.
ExCITE operative technique
“Traditional” intracorporeal tissue extraction techniques use power morcellation without specimen containment. The specimen is grasped with a tenaculum and pulled through the device. The specimen is essentially peeled like an apple and results in long strips of tissue with both a “cut” and “noncut” or intact surface due to the way the blade incises the tissue (FIGURE 1). When performing extracorporeal tissue extraction, we are replicating essentially the same mechanism of tissue removal. With ExCITE, however, the specimen is contained, there is no power morcellator, and tissue extraction is performed manually (FIGURE 2).

The ExCITE technique can be broken down into 5 major steps:
1. specimen retrieval and containment
2. self-retaining retractor placement
3. creation of the C-incision
4. tissue extraction
5. fascial closure.
1. Specimen retrieval and containment
First, place the specimen in an endoscopic specimen retrieval bag. Extend the incision at the umbilicus, to approximately 2.5 to 3.5 cm (roughly 2 good fingerbreadths), and exteriorize the bag at the level of the umbilicus.
2. Self-retaining retractor placement
Next, place a small disposable self-retaining retractor, (we prefer the extra-small Alexis-O) inside the bag, which helps keep the bag in position at the umbilicus (FIGURE 3).

Tip. When inserting the retractor, push it in all the way until the entire bottom ring is inside of the bag. This allows for the retractor ring to deploy. Allow some space between the specimen and the opening of the bag when placing the retractor. Do not pull the bag too tightly against the anterior abdominal wall as this may prevent the retractor ring from deploying fully and make the specimen extraction step more difficult.
3. Creation of C-incision
Grasp the specimen with a penetrating clamp (such as a tenaculum, Lahey, or towel clamp) and pull the specimen flush against the incision and retractor. Use a #11 or #10 blade scalpel to create a reverse “C-incision,” with the clamp in your nondominant hand and the scalpel starting the C-incision from your nondominant side moving toward your dominant side. (The curve of the “C” faces your dominant side.)
Tip. It is important to make your C-incision wide enough to get an adequate sized specimen strip through the umbilicus but not too wide (ie, too flush with the retractor), as this will decrease your workspace and increase the risk of cutting the retractor or the bag. It is helpful to hold the scalpel like a pencil and use a sawing-like motion rather than trying to advance the scalpel through the tissue in one motion.
4. Tissue extraction
Re-grasp the tissue flap, or “nub,” created by the C-incision with the penetrating clamp. While maintaining tension on the specimen, continue cutting with a sawing-like motion, using a reverse C coring technique, keeping one surface completely intact. (Generally this is the surface facing your nondominant side.) When cutting, the tissue becomes a strip, similar in appearance to when using a power morcellator. In fact, the technique is very similar to peeling an apple all the way around while trying to keep the skin of the fruit intact.
Tip. Try to angle the scalpel slightly when cutting the tissue, especially at the curve of the C. In other words, keep the tip of the scalpel toward the center of the strip and the handle away from the center, angled closer to the abdominal wall. When achieving an adequate strip of tissue, often the specimen will start rolling (similar to power morcellation). If this occurs, “go with the roll” by modifying the C-coring incision to a half C and incising along the top part of the C repeatedly until the specimen stops rolling. At that point, complete the C. Be sure to re-grasp near the specimen base as you continue the procedure and as the strip gets longer to prevent premature breakage of the strip and for ease of maintaining tension.
5. Fascial closure
After the specimen is completely extracted, remove the self-retaining retractor and specimen bag. Close the fascia at the umbilical incision. We prefer to close the fascia with an 0-polysorb (absorbable) suture in a running fashion, but you may consider an interrupted closure or use delayed absorbable sutures such as polyglyconate/polydioxanone (maxon/PDS).
Tip. To facilitate removal of the self-retaining retractor, pull on the specimen retrieval bag at one apex in order to collapse the retractor ring inside the bag. This allows removal of the bag and retractor simultaneously.
Keys to success
- Perfect the cutting technique; it is imperative to achieve tissue removal in long strip-like pieces for efficiency. Achieving the “saw cut” is like connecting the dots on a piece of paper with a pencil, where you try not to lift up the pencil (or the scalpel in this case). Rock the tissue back and forth with your nondominant hand and pull the specimen flush to the incision. This helps expose maximal surface area so you can continue to cut tissue pieces that are as large as possible. When rocking, move your dominant (cutting) and nondominant (holding the specimen with the tenaculum) hands in opposite directions.
- Ensure that the appropriate amount of tissue is cut when performing the C-incision. If the tissue strip is too thick, it becomes hard to see and incise the tissue, especially as you come around the back curve of the C. Limited visualization will increase your risk of cutting the retractor or the bag. If the cut is too thick, angle the scalpel in to make the tissue strip thinner (ie, make a “V-like” incision into the noncut surface). If the tissue strip becomes small, do the opposite; instead of cutting at a diagonal toward the noncut surface, aim out from your last incision (“V-out”). You should re-grasp below the narrowed area of the strip in this case before continuing to cut to prevent premature breakage of the strip.
- Maintain traction on the specimen. Keep it flush against the abdominal wall and the opening of the self-retaining rectractor. Use your finger to help “roll” the specimen when continuing the C-incision, if necessary. Maintaining traction will help avoid the need to use your finger.
- If you cannot remove the tissue fully intact, reorient or resect, and move forward. When the tissue is not easily extractable, try to roll the specimen by pushing near or behind the junction of the cut surface and the specimen. This helps reorient the specimen and exposes more smooth, noncut surfaces so coring can continue. The strip of tissue may need to be completely incised at times. If this occurs, drop the specimen back into the bag, find a smoother surface, re-grasp, and begin the C-incision again.
To view ExCITE performed in real-time during removal of an 8-cm, 130-g fibroid after a robot-assisted laparoscopic myomectomy, access the video “The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique” at obgmanagement.com, found in Arnold Advincula’s Surgical Techniques Video Channel.
Building the ExCITE simulation model
Creation of the ExCITE simulation model can be broken down into 4 simple steps: creating the self-retaining retractor, building the torso, preparing the specimen, and simulating the ExCITE technique.
Supplies
To complete all 4 steps, you will need several materials, all of which are easily accessible and easy to prepare for simulation (FIGURE 4).

- 1 beef tongue (2−3 lb)
- 1 pantyhose
- 2 silicone rings (4−5 cm in diameter, such as those used as wrist bracelets for cancer awareness)
- 1-gallon resealable (Ziploc) plastic bags
- 8x12 cardboard/corrugated box (or plastic storage box)
- duct or masking tape
- instruments:
– #11-blade (or your preference) scalpel
– penetrating clamps (tenaculum, Lahey, or towel clamps)
Note that beef tongue, given its muscular texture, closely mimics uterine tissue and therefore is used to represent the fibroid or uterus during simulation. Sometimes, a piece of beef tongue can be marbleized, or fatty, in which case it can simulate a degenerated fibroid. Beef tongue usually comes in one large piece, which could be suitable for up to 4 surgical exercises. The cost of a single tongue is approximately $20 to $30, so it averages about $5 to $7 per exercise/surgical trainee.
1. Create the self-retaining retractor
Supplies: pantyhose, 2 silicone rings
A self-retaining retractor is tubular and made up of a thin plastic material that has a pliable ring on either end. The pantyhose is used to simulate the tubular plastic material, and the silicone bracelets serve as the ring ends of the retractor. The retractor should be large enough so that it does not slip through the incision.
First, cut off the toe end of the pantyhose. Measure and cut a pantyhose strip to approximately 38 cm (15 in). Place one end of the pantyhose through the center of one of the silicone bracelets and wrap it around the edges of the bracelet. Make it as even as possible all the way around the ring. Roll the pantyhose over the bracelet twice more to secure it. Repeat these steps for the other end of the pantyhose to create the simulated self-retaining retractor (FIGURE 5).

2. Build the torso
Supplies: cardboard (ie, office paper box) or plastic box, scissors, duct tape
Place the cardboard box upside down and cut a hole (approximately 2−3 cm wide) at the center of the box top (technically the bottom of the box) to simulate the umbilical incision. Cut another opening on either side of the box (large enough to fit a hand so that the specimen can be inserted inside the box). When performing the ExCITE technique, a constant upward traction is required. In order to keep the box from lifting off the table, tape the box to the table with masking or duct tape. Alternatively, place weights in the bottom of the inside of the box.
3. Prepare the specimen
Supplies: beef tongue, resealable plastic bag
To simulate the contained fibroid or uterus, slice the beef tongue into 3 to 4 pieces (approximately 1-lb pieces) and place one piece of beef tongue inside the resealable plastic bag. Using the side opening in the box, place the bag with the specimen inside the box, and pull the bag through the “umbilical incision” hole, just as you would in a real case. When exteriorizing the bag, ensure some slack so the simulated self-retaining retractor can be placed inside the bag with the ring rolled over it (FIGURE 6).

 |
4. ExCITE technique simulation: Grasp, cut, extract
Supplies: #11-blade scalpel, penetrating clamps (tenaculum, Lahey, or towel clamps).
After exteriorizing the bag, place the self-retaining retractor inside the bag and roll the silicone ring until the retractor is flush with the anterior abdominal wall. Grab the specimen (beef tongue) inside the bag. Perform the ExCITE technique using the beef tongue and the simulated model to fully remove the specimen (FIGURE 7).

Ready, set, simulate
There are many advantages to being able to teach and practice the ExCITE technique outside of the OR. Simulation helps the surgeon to better understand the nuances of tissue extraction in a risk-free environment, and it can improve efficiency in the OR. Building the simulation model as we have described is simple, quick, and inexpensive. We hope that this technique will add to your surgical armamentarium so that you can continue to provide your patients minimally invasive gynecologic surgical options. We recommend that you view both of our videos related to the ExCITE technique and its simulation model at obgmanagement.com, and soon you will be ready to teach or practice the ExCITE technique.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
1. Truong MD, Advincula AP. The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
In November 2014, following concerns regarding the use of electromechanical, or power, morcellation, we published a surgical technique called the extracorporeal C-incision tissue extraction, or ExCITE, technique, as an alternative to contained tissue extraction during minimally invasive gynecologic procedures such as myomectomy and hysterectomy.1 This technique was developed to create a simple, reproducible, and minimally invasive approach to tissue extraction without the need for power morcellation. ExCITE is trainee-friendly and teachable.
In this article, we will review the steps for successful execution of the ExCITE technique. In addition, we will describe how to create your own cost-effective simulation model for teaching, learning, and practicing this technique with a few simple materials found in any craft or grocery store. Simulation is essential. It helps to troubleshoot issues that may arise in an actual case and allows for learning and practicing of surgical techniques to improve the learning curve and efficiency in the operating room (OR).
The model described here is viewable in the video, “The ExCITE technique, Part 2: Simulation made simple.” It is archived in Arnold Advincula’s Surgical Techniques Video Channel, which also is accessible through the “multimedia” section of this Web site.
ExCITE operative technique
“Traditional” intracorporeal tissue extraction techniques use power morcellation without specimen containment. The specimen is grasped with a tenaculum and pulled through the device. The specimen is essentially peeled like an apple and results in long strips of tissue with both a “cut” and “noncut” or intact surface due to the way the blade incises the tissue (FIGURE 1). When performing extracorporeal tissue extraction, we are replicating essentially the same mechanism of tissue removal. With ExCITE, however, the specimen is contained, there is no power morcellator, and tissue extraction is performed manually (FIGURE 2).

The ExCITE technique can be broken down into 5 major steps:
1. specimen retrieval and containment
2. self-retaining retractor placement
3. creation of the C-incision
4. tissue extraction
5. fascial closure.
1. Specimen retrieval and containment
First, place the specimen in an endoscopic specimen retrieval bag. Extend the incision at the umbilicus, to approximately 2.5 to 3.5 cm (roughly 2 good fingerbreadths), and exteriorize the bag at the level of the umbilicus.
2. Self-retaining retractor placement
Next, place a small disposable self-retaining retractor, (we prefer the extra-small Alexis-O) inside the bag, which helps keep the bag in position at the umbilicus (FIGURE 3).

Tip. When inserting the retractor, push it in all the way until the entire bottom ring is inside of the bag. This allows for the retractor ring to deploy. Allow some space between the specimen and the opening of the bag when placing the retractor. Do not pull the bag too tightly against the anterior abdominal wall as this may prevent the retractor ring from deploying fully and make the specimen extraction step more difficult.
3. Creation of C-incision
Grasp the specimen with a penetrating clamp (such as a tenaculum, Lahey, or towel clamp) and pull the specimen flush against the incision and retractor. Use a #11 or #10 blade scalpel to create a reverse “C-incision,” with the clamp in your nondominant hand and the scalpel starting the C-incision from your nondominant side moving toward your dominant side. (The curve of the “C” faces your dominant side.)
Tip. It is important to make your C-incision wide enough to get an adequate sized specimen strip through the umbilicus but not too wide (ie, too flush with the retractor), as this will decrease your workspace and increase the risk of cutting the retractor or the bag. It is helpful to hold the scalpel like a pencil and use a sawing-like motion rather than trying to advance the scalpel through the tissue in one motion.
4. Tissue extraction
Re-grasp the tissue flap, or “nub,” created by the C-incision with the penetrating clamp. While maintaining tension on the specimen, continue cutting with a sawing-like motion, using a reverse C coring technique, keeping one surface completely intact. (Generally this is the surface facing your nondominant side.) When cutting, the tissue becomes a strip, similar in appearance to when using a power morcellator. In fact, the technique is very similar to peeling an apple all the way around while trying to keep the skin of the fruit intact.
Tip. Try to angle the scalpel slightly when cutting the tissue, especially at the curve of the C. In other words, keep the tip of the scalpel toward the center of the strip and the handle away from the center, angled closer to the abdominal wall. When achieving an adequate strip of tissue, often the specimen will start rolling (similar to power morcellation). If this occurs, “go with the roll” by modifying the C-coring incision to a half C and incising along the top part of the C repeatedly until the specimen stops rolling. At that point, complete the C. Be sure to re-grasp near the specimen base as you continue the procedure and as the strip gets longer to prevent premature breakage of the strip and for ease of maintaining tension.
5. Fascial closure
After the specimen is completely extracted, remove the self-retaining retractor and specimen bag. Close the fascia at the umbilical incision. We prefer to close the fascia with an 0-polysorb (absorbable) suture in a running fashion, but you may consider an interrupted closure or use delayed absorbable sutures such as polyglyconate/polydioxanone (maxon/PDS).
Tip. To facilitate removal of the self-retaining retractor, pull on the specimen retrieval bag at one apex in order to collapse the retractor ring inside the bag. This allows removal of the bag and retractor simultaneously.
Keys to success
- Perfect the cutting technique; it is imperative to achieve tissue removal in long strip-like pieces for efficiency. Achieving the “saw cut” is like connecting the dots on a piece of paper with a pencil, where you try not to lift up the pencil (or the scalpel in this case). Rock the tissue back and forth with your nondominant hand and pull the specimen flush to the incision. This helps expose maximal surface area so you can continue to cut tissue pieces that are as large as possible. When rocking, move your dominant (cutting) and nondominant (holding the specimen with the tenaculum) hands in opposite directions.
- Ensure that the appropriate amount of tissue is cut when performing the C-incision. If the tissue strip is too thick, it becomes hard to see and incise the tissue, especially as you come around the back curve of the C. Limited visualization will increase your risk of cutting the retractor or the bag. If the cut is too thick, angle the scalpel in to make the tissue strip thinner (ie, make a “V-like” incision into the noncut surface). If the tissue strip becomes small, do the opposite; instead of cutting at a diagonal toward the noncut surface, aim out from your last incision (“V-out”). You should re-grasp below the narrowed area of the strip in this case before continuing to cut to prevent premature breakage of the strip.
- Maintain traction on the specimen. Keep it flush against the abdominal wall and the opening of the self-retaining rectractor. Use your finger to help “roll” the specimen when continuing the C-incision, if necessary. Maintaining traction will help avoid the need to use your finger.
- If you cannot remove the tissue fully intact, reorient or resect, and move forward. When the tissue is not easily extractable, try to roll the specimen by pushing near or behind the junction of the cut surface and the specimen. This helps reorient the specimen and exposes more smooth, noncut surfaces so coring can continue. The strip of tissue may need to be completely incised at times. If this occurs, drop the specimen back into the bag, find a smoother surface, re-grasp, and begin the C-incision again.
To view ExCITE performed in real-time during removal of an 8-cm, 130-g fibroid after a robot-assisted laparoscopic myomectomy, access the video “The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique” at obgmanagement.com, found in Arnold Advincula’s Surgical Techniques Video Channel.
Building the ExCITE simulation model
Creation of the ExCITE simulation model can be broken down into 4 simple steps: creating the self-retaining retractor, building the torso, preparing the specimen, and simulating the ExCITE technique.
Supplies
To complete all 4 steps, you will need several materials, all of which are easily accessible and easy to prepare for simulation (FIGURE 4).

- 1 beef tongue (2−3 lb)
- 1 pantyhose
- 2 silicone rings (4−5 cm in diameter, such as those used as wrist bracelets for cancer awareness)
- 1-gallon resealable (Ziploc) plastic bags
- 8x12 cardboard/corrugated box (or plastic storage box)
- duct or masking tape
- instruments:
– #11-blade (or your preference) scalpel
– penetrating clamps (tenaculum, Lahey, or towel clamps)
Note that beef tongue, given its muscular texture, closely mimics uterine tissue and therefore is used to represent the fibroid or uterus during simulation. Sometimes, a piece of beef tongue can be marbleized, or fatty, in which case it can simulate a degenerated fibroid. Beef tongue usually comes in one large piece, which could be suitable for up to 4 surgical exercises. The cost of a single tongue is approximately $20 to $30, so it averages about $5 to $7 per exercise/surgical trainee.
1. Create the self-retaining retractor
Supplies: pantyhose, 2 silicone rings
A self-retaining retractor is tubular and made up of a thin plastic material that has a pliable ring on either end. The pantyhose is used to simulate the tubular plastic material, and the silicone bracelets serve as the ring ends of the retractor. The retractor should be large enough so that it does not slip through the incision.
First, cut off the toe end of the pantyhose. Measure and cut a pantyhose strip to approximately 38 cm (15 in). Place one end of the pantyhose through the center of one of the silicone bracelets and wrap it around the edges of the bracelet. Make it as even as possible all the way around the ring. Roll the pantyhose over the bracelet twice more to secure it. Repeat these steps for the other end of the pantyhose to create the simulated self-retaining retractor (FIGURE 5).

2. Build the torso
Supplies: cardboard (ie, office paper box) or plastic box, scissors, duct tape
Place the cardboard box upside down and cut a hole (approximately 2−3 cm wide) at the center of the box top (technically the bottom of the box) to simulate the umbilical incision. Cut another opening on either side of the box (large enough to fit a hand so that the specimen can be inserted inside the box). When performing the ExCITE technique, a constant upward traction is required. In order to keep the box from lifting off the table, tape the box to the table with masking or duct tape. Alternatively, place weights in the bottom of the inside of the box.
3. Prepare the specimen
Supplies: beef tongue, resealable plastic bag
To simulate the contained fibroid or uterus, slice the beef tongue into 3 to 4 pieces (approximately 1-lb pieces) and place one piece of beef tongue inside the resealable plastic bag. Using the side opening in the box, place the bag with the specimen inside the box, and pull the bag through the “umbilical incision” hole, just as you would in a real case. When exteriorizing the bag, ensure some slack so the simulated self-retaining retractor can be placed inside the bag with the ring rolled over it (FIGURE 6).

 |
4. ExCITE technique simulation: Grasp, cut, extract
Supplies: #11-blade scalpel, penetrating clamps (tenaculum, Lahey, or towel clamps).
After exteriorizing the bag, place the self-retaining retractor inside the bag and roll the silicone ring until the retractor is flush with the anterior abdominal wall. Grab the specimen (beef tongue) inside the bag. Perform the ExCITE technique using the beef tongue and the simulated model to fully remove the specimen (FIGURE 7).

Ready, set, simulate
There are many advantages to being able to teach and practice the ExCITE technique outside of the OR. Simulation helps the surgeon to better understand the nuances of tissue extraction in a risk-free environment, and it can improve efficiency in the OR. Building the simulation model as we have described is simple, quick, and inexpensive. We hope that this technique will add to your surgical armamentarium so that you can continue to provide your patients minimally invasive gynecologic surgical options. We recommend that you view both of our videos related to the ExCITE technique and its simulation model at obgmanagement.com, and soon you will be ready to teach or practice the ExCITE technique.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In November 2014, following concerns regarding the use of electromechanical, or power, morcellation, we published a surgical technique called the extracorporeal C-incision tissue extraction, or ExCITE, technique, as an alternative to contained tissue extraction during minimally invasive gynecologic procedures such as myomectomy and hysterectomy.1 This technique was developed to create a simple, reproducible, and minimally invasive approach to tissue extraction without the need for power morcellation. ExCITE is trainee-friendly and teachable.
In this article, we will review the steps for successful execution of the ExCITE technique. In addition, we will describe how to create your own cost-effective simulation model for teaching, learning, and practicing this technique with a few simple materials found in any craft or grocery store. Simulation is essential. It helps to troubleshoot issues that may arise in an actual case and allows for learning and practicing of surgical techniques to improve the learning curve and efficiency in the operating room (OR).
The model described here is viewable in the video, “The ExCITE technique, Part 2: Simulation made simple.” It is archived in Arnold Advincula’s Surgical Techniques Video Channel, which also is accessible through the “multimedia” section of this Web site.
ExCITE operative technique
“Traditional” intracorporeal tissue extraction techniques use power morcellation without specimen containment. The specimen is grasped with a tenaculum and pulled through the device. The specimen is essentially peeled like an apple and results in long strips of tissue with both a “cut” and “noncut” or intact surface due to the way the blade incises the tissue (FIGURE 1). When performing extracorporeal tissue extraction, we are replicating essentially the same mechanism of tissue removal. With ExCITE, however, the specimen is contained, there is no power morcellator, and tissue extraction is performed manually (FIGURE 2).

The ExCITE technique can be broken down into 5 major steps:
1. specimen retrieval and containment
2. self-retaining retractor placement
3. creation of the C-incision
4. tissue extraction
5. fascial closure.
1. Specimen retrieval and containment
First, place the specimen in an endoscopic specimen retrieval bag. Extend the incision at the umbilicus, to approximately 2.5 to 3.5 cm (roughly 2 good fingerbreadths), and exteriorize the bag at the level of the umbilicus.
2. Self-retaining retractor placement
Next, place a small disposable self-retaining retractor, (we prefer the extra-small Alexis-O) inside the bag, which helps keep the bag in position at the umbilicus (FIGURE 3).

Tip. When inserting the retractor, push it in all the way until the entire bottom ring is inside of the bag. This allows for the retractor ring to deploy. Allow some space between the specimen and the opening of the bag when placing the retractor. Do not pull the bag too tightly against the anterior abdominal wall as this may prevent the retractor ring from deploying fully and make the specimen extraction step more difficult.
3. Creation of C-incision
Grasp the specimen with a penetrating clamp (such as a tenaculum, Lahey, or towel clamp) and pull the specimen flush against the incision and retractor. Use a #11 or #10 blade scalpel to create a reverse “C-incision,” with the clamp in your nondominant hand and the scalpel starting the C-incision from your nondominant side moving toward your dominant side. (The curve of the “C” faces your dominant side.)
Tip. It is important to make your C-incision wide enough to get an adequate sized specimen strip through the umbilicus but not too wide (ie, too flush with the retractor), as this will decrease your workspace and increase the risk of cutting the retractor or the bag. It is helpful to hold the scalpel like a pencil and use a sawing-like motion rather than trying to advance the scalpel through the tissue in one motion.
4. Tissue extraction
Re-grasp the tissue flap, or “nub,” created by the C-incision with the penetrating clamp. While maintaining tension on the specimen, continue cutting with a sawing-like motion, using a reverse C coring technique, keeping one surface completely intact. (Generally this is the surface facing your nondominant side.) When cutting, the tissue becomes a strip, similar in appearance to when using a power morcellator. In fact, the technique is very similar to peeling an apple all the way around while trying to keep the skin of the fruit intact.
Tip. Try to angle the scalpel slightly when cutting the tissue, especially at the curve of the C. In other words, keep the tip of the scalpel toward the center of the strip and the handle away from the center, angled closer to the abdominal wall. When achieving an adequate strip of tissue, often the specimen will start rolling (similar to power morcellation). If this occurs, “go with the roll” by modifying the C-coring incision to a half C and incising along the top part of the C repeatedly until the specimen stops rolling. At that point, complete the C. Be sure to re-grasp near the specimen base as you continue the procedure and as the strip gets longer to prevent premature breakage of the strip and for ease of maintaining tension.
5. Fascial closure
After the specimen is completely extracted, remove the self-retaining retractor and specimen bag. Close the fascia at the umbilical incision. We prefer to close the fascia with an 0-polysorb (absorbable) suture in a running fashion, but you may consider an interrupted closure or use delayed absorbable sutures such as polyglyconate/polydioxanone (maxon/PDS).
Tip. To facilitate removal of the self-retaining retractor, pull on the specimen retrieval bag at one apex in order to collapse the retractor ring inside the bag. This allows removal of the bag and retractor simultaneously.
Keys to success
- Perfect the cutting technique; it is imperative to achieve tissue removal in long strip-like pieces for efficiency. Achieving the “saw cut” is like connecting the dots on a piece of paper with a pencil, where you try not to lift up the pencil (or the scalpel in this case). Rock the tissue back and forth with your nondominant hand and pull the specimen flush to the incision. This helps expose maximal surface area so you can continue to cut tissue pieces that are as large as possible. When rocking, move your dominant (cutting) and nondominant (holding the specimen with the tenaculum) hands in opposite directions.
- Ensure that the appropriate amount of tissue is cut when performing the C-incision. If the tissue strip is too thick, it becomes hard to see and incise the tissue, especially as you come around the back curve of the C. Limited visualization will increase your risk of cutting the retractor or the bag. If the cut is too thick, angle the scalpel in to make the tissue strip thinner (ie, make a “V-like” incision into the noncut surface). If the tissue strip becomes small, do the opposite; instead of cutting at a diagonal toward the noncut surface, aim out from your last incision (“V-out”). You should re-grasp below the narrowed area of the strip in this case before continuing to cut to prevent premature breakage of the strip.
- Maintain traction on the specimen. Keep it flush against the abdominal wall and the opening of the self-retaining rectractor. Use your finger to help “roll” the specimen when continuing the C-incision, if necessary. Maintaining traction will help avoid the need to use your finger.
- If you cannot remove the tissue fully intact, reorient or resect, and move forward. When the tissue is not easily extractable, try to roll the specimen by pushing near or behind the junction of the cut surface and the specimen. This helps reorient the specimen and exposes more smooth, noncut surfaces so coring can continue. The strip of tissue may need to be completely incised at times. If this occurs, drop the specimen back into the bag, find a smoother surface, re-grasp, and begin the C-incision again.
To view ExCITE performed in real-time during removal of an 8-cm, 130-g fibroid after a robot-assisted laparoscopic myomectomy, access the video “The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique” at obgmanagement.com, found in Arnold Advincula’s Surgical Techniques Video Channel.
Building the ExCITE simulation model
Creation of the ExCITE simulation model can be broken down into 4 simple steps: creating the self-retaining retractor, building the torso, preparing the specimen, and simulating the ExCITE technique.
Supplies
To complete all 4 steps, you will need several materials, all of which are easily accessible and easy to prepare for simulation (FIGURE 4).

- 1 beef tongue (2−3 lb)
- 1 pantyhose
- 2 silicone rings (4−5 cm in diameter, such as those used as wrist bracelets for cancer awareness)
- 1-gallon resealable (Ziploc) plastic bags
- 8x12 cardboard/corrugated box (or plastic storage box)
- duct or masking tape
- instruments:
– #11-blade (or your preference) scalpel
– penetrating clamps (tenaculum, Lahey, or towel clamps)
Note that beef tongue, given its muscular texture, closely mimics uterine tissue and therefore is used to represent the fibroid or uterus during simulation. Sometimes, a piece of beef tongue can be marbleized, or fatty, in which case it can simulate a degenerated fibroid. Beef tongue usually comes in one large piece, which could be suitable for up to 4 surgical exercises. The cost of a single tongue is approximately $20 to $30, so it averages about $5 to $7 per exercise/surgical trainee.
1. Create the self-retaining retractor
Supplies: pantyhose, 2 silicone rings
A self-retaining retractor is tubular and made up of a thin plastic material that has a pliable ring on either end. The pantyhose is used to simulate the tubular plastic material, and the silicone bracelets serve as the ring ends of the retractor. The retractor should be large enough so that it does not slip through the incision.
First, cut off the toe end of the pantyhose. Measure and cut a pantyhose strip to approximately 38 cm (15 in). Place one end of the pantyhose through the center of one of the silicone bracelets and wrap it around the edges of the bracelet. Make it as even as possible all the way around the ring. Roll the pantyhose over the bracelet twice more to secure it. Repeat these steps for the other end of the pantyhose to create the simulated self-retaining retractor (FIGURE 5).

2. Build the torso
Supplies: cardboard (ie, office paper box) or plastic box, scissors, duct tape
Place the cardboard box upside down and cut a hole (approximately 2−3 cm wide) at the center of the box top (technically the bottom of the box) to simulate the umbilical incision. Cut another opening on either side of the box (large enough to fit a hand so that the specimen can be inserted inside the box). When performing the ExCITE technique, a constant upward traction is required. In order to keep the box from lifting off the table, tape the box to the table with masking or duct tape. Alternatively, place weights in the bottom of the inside of the box.
3. Prepare the specimen
Supplies: beef tongue, resealable plastic bag
To simulate the contained fibroid or uterus, slice the beef tongue into 3 to 4 pieces (approximately 1-lb pieces) and place one piece of beef tongue inside the resealable plastic bag. Using the side opening in the box, place the bag with the specimen inside the box, and pull the bag through the “umbilical incision” hole, just as you would in a real case. When exteriorizing the bag, ensure some slack so the simulated self-retaining retractor can be placed inside the bag with the ring rolled over it (FIGURE 6).

 |
4. ExCITE technique simulation: Grasp, cut, extract
Supplies: #11-blade scalpel, penetrating clamps (tenaculum, Lahey, or towel clamps).
After exteriorizing the bag, place the self-retaining retractor inside the bag and roll the silicone ring until the retractor is flush with the anterior abdominal wall. Grab the specimen (beef tongue) inside the bag. Perform the ExCITE technique using the beef tongue and the simulated model to fully remove the specimen (FIGURE 7).

Ready, set, simulate
There are many advantages to being able to teach and practice the ExCITE technique outside of the OR. Simulation helps the surgeon to better understand the nuances of tissue extraction in a risk-free environment, and it can improve efficiency in the OR. Building the simulation model as we have described is simple, quick, and inexpensive. We hope that this technique will add to your surgical armamentarium so that you can continue to provide your patients minimally invasive gynecologic surgical options. We recommend that you view both of our videos related to the ExCITE technique and its simulation model at obgmanagement.com, and soon you will be ready to teach or practice the ExCITE technique.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
1. Truong MD, Advincula AP. The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
Reference
1. Truong MD, Advincula AP. The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
In this Article
- 5 steps to execute ExCITE
- Keys to technique success
- Building the simulation model