User login
In this Update, the authors discuss 2 important areas that impact fertility. First, with in vitro fertilization (IVF), successful implantation that leads to live birth requires a normal embryo and a receptive endometrium. While research using advanced molecular array technology has resulted in a clinical test to identify the optimal window of implantation, recent evidence has questioned its clinical effectiveness. Second, recognizing the importance of endometriosis—a common disease with high burden that causes pain, infertility, and other symptoms—the World Health Organization (WHO) last year published an informative fact sheet that highlights the diagnosis, treatment options, and challenges of this significant disease.
Endometrial receptivity array and the quest for optimal endometrial preparation prior to embryo transfer in IVF
Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
A successful pregnancy requires optimal crosstalk between the embryo and the endometrium. Over the past several decades, research efforts to improve IVF outcomes have been focused mainly on the embryo factor and methods to improve embryo selection, such as extended culture to blastocyst, time-lapse imaging (morphokinetic assessment), and more notably, preimplantation genetic testing for aneuploidy (PGT-A). However, the other half of the equation, the endometrium, has not garnered the attention that it deserves. Effort has therefore been renewed to optimize the endometrial factor by better diagnosing and treating various forms of endometrial dysfunction that could lead to infertility in general and lack of success with IVF and euploid embryo transfers in particular.
Historical background on endometrial function
Progesterone has long been recognized as the main effector that transforms the estrogen-primed endometrium into a receptive state that results in successful embryo implantation. Progesterone exposure is required at appropriate levels and duration before the endometrium becomes receptive to the embryo. If implantation does not occur soon after the endometrium has attained receptive status (7–10 days after ovulation), further progesterone exposure results in progression of endometrial changes that no longer permit successful implantation.
As early as the 1950s, “luteal phase deficiency” was defined as due to inadequate progesterone secretion and resulted in a short luteal phase. In the 1970s, histologic “dating” of the endometrium became the gold standard for diagnosing luteal phase defects; this relied on a classic histologic appearance of secretory phase endometrium and its changes throughout the luteal phase. Subsequently, however, results of prospective randomized controlled trials published in 2004 cast significant doubt on the accuracy and reproducibility of these endometrial biopsies and did not show any clinical diagnostic benefit or correlation with pregnancy outcomes.
21st century advances: Endometrial dating 2.0
A decade later, with the advancement of molecular biology tools such as microarray technology, researchers were able to study endometrial gene expression patterns at different stages of the menstrual cycle. They identified different phases of endometrial development with molecular profiles, or “signatures,” for the luteal phase, endometriosis, polycystic ovary syndrome, and uterine fibroids.
In 2013, researchers in Spain introduced a diagnostic test called endometrial receptivity array (ERA) with the stated goal of being able to temporally define the receptive endometrium and identify prereceptive as well as postreceptive states.1 In other words, instead of the histologic dating of the endometrium used in the 1970s, it represented “molecular dating” of the endometrium. Although the initial studies were conducted among women who experienced prior unsuccessful embryo transfers (the so-called recurrent implantation failure, or RIF), the test’s scope was subsequently expanded to include any individual planning on a frozen embryo transfer (FET), regardless of any prior attempts. The term personalized embryo transfer (pET) was coined to suggest the ability to define the best time (up to hours) for embryo transfers on an individual basis. Despite lack of independent validation studies, ERA was then widely adopted by many clinicians (and requested by some patients) with the hope of improving IVF outcomes.
However, not unlike many other novel innovations in assisted reproductive technology, ERA regrettably did not withstand the test of time. Three independent studies in 2021, 1 randomized clinical trial and 2 observational cohort studies, did not show any benefit with regard to implantation rates, pregnancy rates, or live birth rates when ERA was performed in the general infertility population.2-4
Continue to: Study results...
Study results
The cohort study that matched 133 ERA patients with 353 non-ERA patients showed live birth rates of 49.62% for the ERA group and 54.96% for the non-ERA group (odds ratio [OR], 0.8074; 95% confidence interval [CI], 0.5424–1.2018).2 Of note, no difference occurred between subgroups based on the prior number of FETs or the receptivity status (TABLE 1).
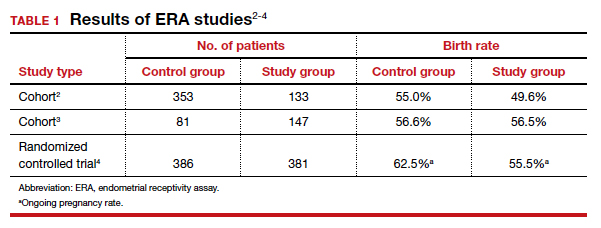
Another cohort study from the University of California, Los Angeles, published in 2021 analyzed 228 single euploid FET cycles.3 This study did not show any benefit for routine ERA testing, with a live birth rate of 56.6% in the non-ERA group and 56.5% in the ERA group.
Still, the most convincing evidence for the lack of benefit from routine ERA was noted from the results of the randomized clinical trial.4 A total of 767 patients were randomly allocated, 381 to the ERA group and 386 to the control group. There was no difference in ongoing pregnancy rates between the 2 groups. Perhaps more important, even after limiting the analysis to individuals with a nonreceptive ERA result, there was no difference in ongoing pregnancy rates between the 2 groups: 62.5% in the control group (default timing of transfer) and 55.5% in the study group (transfer timing adjusted based on ERA) (rate ratio [RR], 0.9; 95% CI, 0.70–1.14).
ERA usefulness is unsupported in general infertility population
The studies discussed collectively suggest with a high degree of certainty that there is no indication for routine ERA testing in the general infertility population prior to frozen embryo transfers.
Although these studies all were conducted in the general infertility population and did not specifically evaluate the performance of ERA in women with recurrent pregnancy loss or recurrent implantation failure, it is important to acknowledge that if ERA were truly able to define the window of receptivity, one would expect a lower implantation rate if the embryos were transferred outside of the window suggested by the ERA. This was not the case in these studies, as they all showed equivalent pregnancy rates in the control (nonadjusted) groups even when ERA suggested a nonreceptive status.
This observation seriously questions the validity of ERA regarding its ability to temporally define the window of receptivity. On the other hand, as stated earlier, there is still a possibility for ERA to be beneficial for a small subgroup of patients whose window of receptivity may not be as wide as expected in the general population. The challenging question would be how best to identify the particular group with a narrow, or displaced, window of receptivity.
The optimal timing for implantation of a normal embryo requires a receptive endometrium. The endometrial biopsy was used widely for many years before research showed it was not clinically useful. More recently, the endometrial receptivity array has been suggested to help time the frozen embryo transfer. Unfortunately, recent studies have shown that this test is not clinically useful for the general infertility population.
Continue to: WHO raises awareness of endometriosis burden and...
WHO raises awareness of endometriosis burden and highlights need to address diagnosis and treatment for women’s reproductive health
World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room /fact-sheets/detail/endometriosis. Accessed January 3, 2022.
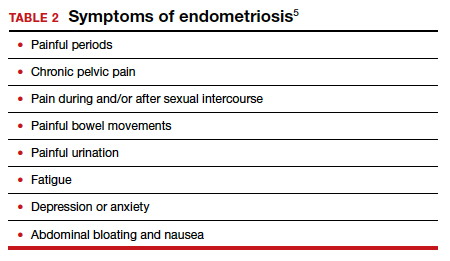
The WHO published its first fact sheet on endometriosis in March 2021, recognizing endometriosis as a severe disease that affects almost 190 million women with life-impacting pain, infertility, other symptoms, and especially with chronic, significant emotional sequelae (TABLE 2).5 The disease’s variable and broad symptoms result in a lack of awareness and diagnosis by both women and health care providers, especially in low- and middle-income countries and in disadvantaged populations in developed countries. Increased awareness to promote earlier diagnosis, improved training for better management, expanded research for greater understanding, and policies that increase access to quality care are needed to ensure the reproductive health and rights of tens of millions of women with endometriosis.
Endometriosis characteristics and symptoms
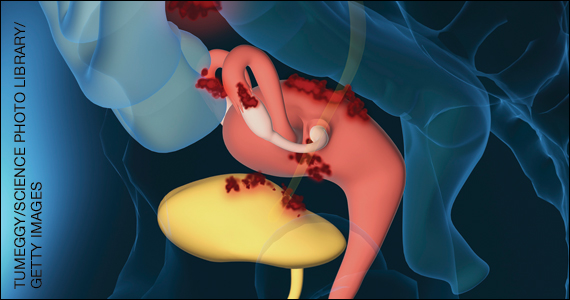
Endometriosis is characterized by the presence of tissue resembling endometrium outside the uterus, where it causes a chronic inflammatory reaction that may result in the formation of scar tissue. Endometriotic lesions may be superficial, cystic ovarian endometriomas, or deep lesions, causing a myriad of pain and related symptoms.6.7
Chronic pain may occur because pain centers in the brain become hyperresponsive over time (central sensitization); this can occur at any point throughout the life course of endometriosis, even when endometriosis lesions are no longer visible. Sometimes, endometriosis is asymptomatic. In addition, endometriosis can cause infertility through anatomic distortion and inflammatory, endocrinologic, and other pathways.
The origins of endometriosis are thought to be multifactorial and include retrograde menstruation, cellular metaplasia, and/or stem cells that spread through blood and lymphatic vessels. Endometriosis is estrogen dependent, but lesion growth also is affected by altered or impaired immunity, localized complex hormonal influences, genetics, and possibly environmental contaminants.
Impact on public health and reproductive rights
Endometriosis has significant social, public health, and economic implications. It can decrease quality of life and prevent girls and women from attending work or school.8 Painful sex can affect sexual health. The WHO states that, “Addressing endometriosis will empower those affected by it, by supporting their human right to the highest standard of sexual and reproductive health, quality of life, and overall well-being.”5
At present, no known way is available to prevent or cure endometriosis. Early diagnosis and treatment, however, may slow or halt its natural progression and associated symptoms.
Diagnostic steps and treatment options
Early suspicion of endometriosis is the most important factor, followed by a careful history of menstrual symptoms and chronic pelvic pain, early referral to specialists for ultrasonography or other imaging, and sometimes surgical or laparoscopic visualization. Empirical treatment can be begun without histologic or laparoscopic confirmation.
Endometriosis can be treated with medications and/or surgery depending on symptoms, lesions, desired outcome, and patient choice.5,6 Common therapies include contraceptive steroids, nonsteroidal anti-inflammatory medications, and analgesics. Medical treatments focus on either lowering estrogen or increasing progesterone levels.
Surgery can remove endometriosis lesions, adhesions, and scar tissue. However, success in reducing pain symptoms and increasing pregnancy rates often depends on the extent of disease.
For infertility due to endometriosis, treatment options include laparoscopic surgical removal of endometriosis, ovarian stimulation with intrauterine insemination (IUI), and IVF. Multidisciplinary treatment addressing different symptoms and overall health often requires referral to pain experts and other specialists.9
The WHO perspective on endometriosis
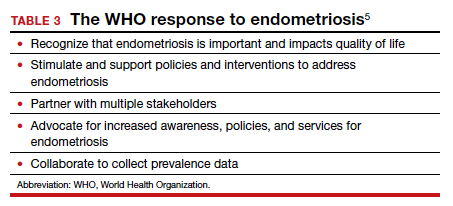
Recognizing the importance of endometriosis and its impact on people’s sexual and reproductive health, quality of life, and overall well-being, the WHO is taking action to improve awareness, diagnosis, and treatment of endometriosis (TABLE 3).5 ●
Endometriosis is now recognized as a disease with significant burden for women everywhere. Widespread lack of awareness of presenting symptoms and management options means that all women’s health care clinicians need to become better informed about endometriosis so they can improve the quality of care they provide.
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818-824.
- Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
- Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
- Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
- World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room/fact-sheets/detail /endometriosis. Accessed January 3, 2022.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244-1256.
- Johnson NP, Hummelshoj L, Adamson GD, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32:315-324.
- Nnoaham K, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366-373.e8.
- Carey ET, Till SR, As-Sanie S. Pharmacological management of chronic pelvic pain in women. Drugs. 2017;77:285-301.
In this Update, the authors discuss 2 important areas that impact fertility. First, with in vitro fertilization (IVF), successful implantation that leads to live birth requires a normal embryo and a receptive endometrium. While research using advanced molecular array technology has resulted in a clinical test to identify the optimal window of implantation, recent evidence has questioned its clinical effectiveness. Second, recognizing the importance of endometriosis—a common disease with high burden that causes pain, infertility, and other symptoms—the World Health Organization (WHO) last year published an informative fact sheet that highlights the diagnosis, treatment options, and challenges of this significant disease.
Endometrial receptivity array and the quest for optimal endometrial preparation prior to embryo transfer in IVF
Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
A successful pregnancy requires optimal crosstalk between the embryo and the endometrium. Over the past several decades, research efforts to improve IVF outcomes have been focused mainly on the embryo factor and methods to improve embryo selection, such as extended culture to blastocyst, time-lapse imaging (morphokinetic assessment), and more notably, preimplantation genetic testing for aneuploidy (PGT-A). However, the other half of the equation, the endometrium, has not garnered the attention that it deserves. Effort has therefore been renewed to optimize the endometrial factor by better diagnosing and treating various forms of endometrial dysfunction that could lead to infertility in general and lack of success with IVF and euploid embryo transfers in particular.
Historical background on endometrial function
Progesterone has long been recognized as the main effector that transforms the estrogen-primed endometrium into a receptive state that results in successful embryo implantation. Progesterone exposure is required at appropriate levels and duration before the endometrium becomes receptive to the embryo. If implantation does not occur soon after the endometrium has attained receptive status (7–10 days after ovulation), further progesterone exposure results in progression of endometrial changes that no longer permit successful implantation.
As early as the 1950s, “luteal phase deficiency” was defined as due to inadequate progesterone secretion and resulted in a short luteal phase. In the 1970s, histologic “dating” of the endometrium became the gold standard for diagnosing luteal phase defects; this relied on a classic histologic appearance of secretory phase endometrium and its changes throughout the luteal phase. Subsequently, however, results of prospective randomized controlled trials published in 2004 cast significant doubt on the accuracy and reproducibility of these endometrial biopsies and did not show any clinical diagnostic benefit or correlation with pregnancy outcomes.
21st century advances: Endometrial dating 2.0
A decade later, with the advancement of molecular biology tools such as microarray technology, researchers were able to study endometrial gene expression patterns at different stages of the menstrual cycle. They identified different phases of endometrial development with molecular profiles, or “signatures,” for the luteal phase, endometriosis, polycystic ovary syndrome, and uterine fibroids.
In 2013, researchers in Spain introduced a diagnostic test called endometrial receptivity array (ERA) with the stated goal of being able to temporally define the receptive endometrium and identify prereceptive as well as postreceptive states.1 In other words, instead of the histologic dating of the endometrium used in the 1970s, it represented “molecular dating” of the endometrium. Although the initial studies were conducted among women who experienced prior unsuccessful embryo transfers (the so-called recurrent implantation failure, or RIF), the test’s scope was subsequently expanded to include any individual planning on a frozen embryo transfer (FET), regardless of any prior attempts. The term personalized embryo transfer (pET) was coined to suggest the ability to define the best time (up to hours) for embryo transfers on an individual basis. Despite lack of independent validation studies, ERA was then widely adopted by many clinicians (and requested by some patients) with the hope of improving IVF outcomes.
However, not unlike many other novel innovations in assisted reproductive technology, ERA regrettably did not withstand the test of time. Three independent studies in 2021, 1 randomized clinical trial and 2 observational cohort studies, did not show any benefit with regard to implantation rates, pregnancy rates, or live birth rates when ERA was performed in the general infertility population.2-4
Continue to: Study results...
Study results
The cohort study that matched 133 ERA patients with 353 non-ERA patients showed live birth rates of 49.62% for the ERA group and 54.96% for the non-ERA group (odds ratio [OR], 0.8074; 95% confidence interval [CI], 0.5424–1.2018).2 Of note, no difference occurred between subgroups based on the prior number of FETs or the receptivity status (TABLE 1).

Another cohort study from the University of California, Los Angeles, published in 2021 analyzed 228 single euploid FET cycles.3 This study did not show any benefit for routine ERA testing, with a live birth rate of 56.6% in the non-ERA group and 56.5% in the ERA group.
Still, the most convincing evidence for the lack of benefit from routine ERA was noted from the results of the randomized clinical trial.4 A total of 767 patients were randomly allocated, 381 to the ERA group and 386 to the control group. There was no difference in ongoing pregnancy rates between the 2 groups. Perhaps more important, even after limiting the analysis to individuals with a nonreceptive ERA result, there was no difference in ongoing pregnancy rates between the 2 groups: 62.5% in the control group (default timing of transfer) and 55.5% in the study group (transfer timing adjusted based on ERA) (rate ratio [RR], 0.9; 95% CI, 0.70–1.14).
ERA usefulness is unsupported in general infertility population
The studies discussed collectively suggest with a high degree of certainty that there is no indication for routine ERA testing in the general infertility population prior to frozen embryo transfers.
Although these studies all were conducted in the general infertility population and did not specifically evaluate the performance of ERA in women with recurrent pregnancy loss or recurrent implantation failure, it is important to acknowledge that if ERA were truly able to define the window of receptivity, one would expect a lower implantation rate if the embryos were transferred outside of the window suggested by the ERA. This was not the case in these studies, as they all showed equivalent pregnancy rates in the control (nonadjusted) groups even when ERA suggested a nonreceptive status.
This observation seriously questions the validity of ERA regarding its ability to temporally define the window of receptivity. On the other hand, as stated earlier, there is still a possibility for ERA to be beneficial for a small subgroup of patients whose window of receptivity may not be as wide as expected in the general population. The challenging question would be how best to identify the particular group with a narrow, or displaced, window of receptivity.
The optimal timing for implantation of a normal embryo requires a receptive endometrium. The endometrial biopsy was used widely for many years before research showed it was not clinically useful. More recently, the endometrial receptivity array has been suggested to help time the frozen embryo transfer. Unfortunately, recent studies have shown that this test is not clinically useful for the general infertility population.
Continue to: WHO raises awareness of endometriosis burden and...
WHO raises awareness of endometriosis burden and highlights need to address diagnosis and treatment for women’s reproductive health
World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room /fact-sheets/detail/endometriosis. Accessed January 3, 2022.
The WHO published its first fact sheet on endometriosis in March 2021, recognizing endometriosis as a severe disease that affects almost 190 million women with life-impacting pain, infertility, other symptoms, and especially with chronic, significant emotional sequelae (TABLE 2).5 The disease’s variable and broad symptoms result in a lack of awareness and diagnosis by both women and health care providers, especially in low- and middle-income countries and in disadvantaged populations in developed countries. Increased awareness to promote earlier diagnosis, improved training for better management, expanded research for greater understanding, and policies that increase access to quality care are needed to ensure the reproductive health and rights of tens of millions of women with endometriosis.

Endometriosis characteristics and symptoms
Endometriosis is characterized by the presence of tissue resembling endometrium outside the uterus, where it causes a chronic inflammatory reaction that may result in the formation of scar tissue. Endometriotic lesions may be superficial, cystic ovarian endometriomas, or deep lesions, causing a myriad of pain and related symptoms.6.7
Chronic pain may occur because pain centers in the brain become hyperresponsive over time (central sensitization); this can occur at any point throughout the life course of endometriosis, even when endometriosis lesions are no longer visible. Sometimes, endometriosis is asymptomatic. In addition, endometriosis can cause infertility through anatomic distortion and inflammatory, endocrinologic, and other pathways.
The origins of endometriosis are thought to be multifactorial and include retrograde menstruation, cellular metaplasia, and/or stem cells that spread through blood and lymphatic vessels. Endometriosis is estrogen dependent, but lesion growth also is affected by altered or impaired immunity, localized complex hormonal influences, genetics, and possibly environmental contaminants.
Impact on public health and reproductive rights
Endometriosis has significant social, public health, and economic implications. It can decrease quality of life and prevent girls and women from attending work or school.8 Painful sex can affect sexual health. The WHO states that, “Addressing endometriosis will empower those affected by it, by supporting their human right to the highest standard of sexual and reproductive health, quality of life, and overall well-being.”5
At present, no known way is available to prevent or cure endometriosis. Early diagnosis and treatment, however, may slow or halt its natural progression and associated symptoms.
Diagnostic steps and treatment options
Early suspicion of endometriosis is the most important factor, followed by a careful history of menstrual symptoms and chronic pelvic pain, early referral to specialists for ultrasonography or other imaging, and sometimes surgical or laparoscopic visualization. Empirical treatment can be begun without histologic or laparoscopic confirmation.
Endometriosis can be treated with medications and/or surgery depending on symptoms, lesions, desired outcome, and patient choice.5,6 Common therapies include contraceptive steroids, nonsteroidal anti-inflammatory medications, and analgesics. Medical treatments focus on either lowering estrogen or increasing progesterone levels.
Surgery can remove endometriosis lesions, adhesions, and scar tissue. However, success in reducing pain symptoms and increasing pregnancy rates often depends on the extent of disease.
For infertility due to endometriosis, treatment options include laparoscopic surgical removal of endometriosis, ovarian stimulation with intrauterine insemination (IUI), and IVF. Multidisciplinary treatment addressing different symptoms and overall health often requires referral to pain experts and other specialists.9
The WHO perspective on endometriosis
Recognizing the importance of endometriosis and its impact on people’s sexual and reproductive health, quality of life, and overall well-being, the WHO is taking action to improve awareness, diagnosis, and treatment of endometriosis (TABLE 3).5 ●

Endometriosis is now recognized as a disease with significant burden for women everywhere. Widespread lack of awareness of presenting symptoms and management options means that all women’s health care clinicians need to become better informed about endometriosis so they can improve the quality of care they provide.
In this Update, the authors discuss 2 important areas that impact fertility. First, with in vitro fertilization (IVF), successful implantation that leads to live birth requires a normal embryo and a receptive endometrium. While research using advanced molecular array technology has resulted in a clinical test to identify the optimal window of implantation, recent evidence has questioned its clinical effectiveness. Second, recognizing the importance of endometriosis—a common disease with high burden that causes pain, infertility, and other symptoms—the World Health Organization (WHO) last year published an informative fact sheet that highlights the diagnosis, treatment options, and challenges of this significant disease.
Endometrial receptivity array and the quest for optimal endometrial preparation prior to embryo transfer in IVF
Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
A successful pregnancy requires optimal crosstalk between the embryo and the endometrium. Over the past several decades, research efforts to improve IVF outcomes have been focused mainly on the embryo factor and methods to improve embryo selection, such as extended culture to blastocyst, time-lapse imaging (morphokinetic assessment), and more notably, preimplantation genetic testing for aneuploidy (PGT-A). However, the other half of the equation, the endometrium, has not garnered the attention that it deserves. Effort has therefore been renewed to optimize the endometrial factor by better diagnosing and treating various forms of endometrial dysfunction that could lead to infertility in general and lack of success with IVF and euploid embryo transfers in particular.
Historical background on endometrial function
Progesterone has long been recognized as the main effector that transforms the estrogen-primed endometrium into a receptive state that results in successful embryo implantation. Progesterone exposure is required at appropriate levels and duration before the endometrium becomes receptive to the embryo. If implantation does not occur soon after the endometrium has attained receptive status (7–10 days after ovulation), further progesterone exposure results in progression of endometrial changes that no longer permit successful implantation.
As early as the 1950s, “luteal phase deficiency” was defined as due to inadequate progesterone secretion and resulted in a short luteal phase. In the 1970s, histologic “dating” of the endometrium became the gold standard for diagnosing luteal phase defects; this relied on a classic histologic appearance of secretory phase endometrium and its changes throughout the luteal phase. Subsequently, however, results of prospective randomized controlled trials published in 2004 cast significant doubt on the accuracy and reproducibility of these endometrial biopsies and did not show any clinical diagnostic benefit or correlation with pregnancy outcomes.
21st century advances: Endometrial dating 2.0
A decade later, with the advancement of molecular biology tools such as microarray technology, researchers were able to study endometrial gene expression patterns at different stages of the menstrual cycle. They identified different phases of endometrial development with molecular profiles, or “signatures,” for the luteal phase, endometriosis, polycystic ovary syndrome, and uterine fibroids.
In 2013, researchers in Spain introduced a diagnostic test called endometrial receptivity array (ERA) with the stated goal of being able to temporally define the receptive endometrium and identify prereceptive as well as postreceptive states.1 In other words, instead of the histologic dating of the endometrium used in the 1970s, it represented “molecular dating” of the endometrium. Although the initial studies were conducted among women who experienced prior unsuccessful embryo transfers (the so-called recurrent implantation failure, or RIF), the test’s scope was subsequently expanded to include any individual planning on a frozen embryo transfer (FET), regardless of any prior attempts. The term personalized embryo transfer (pET) was coined to suggest the ability to define the best time (up to hours) for embryo transfers on an individual basis. Despite lack of independent validation studies, ERA was then widely adopted by many clinicians (and requested by some patients) with the hope of improving IVF outcomes.
However, not unlike many other novel innovations in assisted reproductive technology, ERA regrettably did not withstand the test of time. Three independent studies in 2021, 1 randomized clinical trial and 2 observational cohort studies, did not show any benefit with regard to implantation rates, pregnancy rates, or live birth rates when ERA was performed in the general infertility population.2-4
Continue to: Study results...
Study results
The cohort study that matched 133 ERA patients with 353 non-ERA patients showed live birth rates of 49.62% for the ERA group and 54.96% for the non-ERA group (odds ratio [OR], 0.8074; 95% confidence interval [CI], 0.5424–1.2018).2 Of note, no difference occurred between subgroups based on the prior number of FETs or the receptivity status (TABLE 1).

Another cohort study from the University of California, Los Angeles, published in 2021 analyzed 228 single euploid FET cycles.3 This study did not show any benefit for routine ERA testing, with a live birth rate of 56.6% in the non-ERA group and 56.5% in the ERA group.
Still, the most convincing evidence for the lack of benefit from routine ERA was noted from the results of the randomized clinical trial.4 A total of 767 patients were randomly allocated, 381 to the ERA group and 386 to the control group. There was no difference in ongoing pregnancy rates between the 2 groups. Perhaps more important, even after limiting the analysis to individuals with a nonreceptive ERA result, there was no difference in ongoing pregnancy rates between the 2 groups: 62.5% in the control group (default timing of transfer) and 55.5% in the study group (transfer timing adjusted based on ERA) (rate ratio [RR], 0.9; 95% CI, 0.70–1.14).
ERA usefulness is unsupported in general infertility population
The studies discussed collectively suggest with a high degree of certainty that there is no indication for routine ERA testing in the general infertility population prior to frozen embryo transfers.
Although these studies all were conducted in the general infertility population and did not specifically evaluate the performance of ERA in women with recurrent pregnancy loss or recurrent implantation failure, it is important to acknowledge that if ERA were truly able to define the window of receptivity, one would expect a lower implantation rate if the embryos were transferred outside of the window suggested by the ERA. This was not the case in these studies, as they all showed equivalent pregnancy rates in the control (nonadjusted) groups even when ERA suggested a nonreceptive status.
This observation seriously questions the validity of ERA regarding its ability to temporally define the window of receptivity. On the other hand, as stated earlier, there is still a possibility for ERA to be beneficial for a small subgroup of patients whose window of receptivity may not be as wide as expected in the general population. The challenging question would be how best to identify the particular group with a narrow, or displaced, window of receptivity.
The optimal timing for implantation of a normal embryo requires a receptive endometrium. The endometrial biopsy was used widely for many years before research showed it was not clinically useful. More recently, the endometrial receptivity array has been suggested to help time the frozen embryo transfer. Unfortunately, recent studies have shown that this test is not clinically useful for the general infertility population.
Continue to: WHO raises awareness of endometriosis burden and...
WHO raises awareness of endometriosis burden and highlights need to address diagnosis and treatment for women’s reproductive health
World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room /fact-sheets/detail/endometriosis. Accessed January 3, 2022.
The WHO published its first fact sheet on endometriosis in March 2021, recognizing endometriosis as a severe disease that affects almost 190 million women with life-impacting pain, infertility, other symptoms, and especially with chronic, significant emotional sequelae (TABLE 2).5 The disease’s variable and broad symptoms result in a lack of awareness and diagnosis by both women and health care providers, especially in low- and middle-income countries and in disadvantaged populations in developed countries. Increased awareness to promote earlier diagnosis, improved training for better management, expanded research for greater understanding, and policies that increase access to quality care are needed to ensure the reproductive health and rights of tens of millions of women with endometriosis.

Endometriosis characteristics and symptoms
Endometriosis is characterized by the presence of tissue resembling endometrium outside the uterus, where it causes a chronic inflammatory reaction that may result in the formation of scar tissue. Endometriotic lesions may be superficial, cystic ovarian endometriomas, or deep lesions, causing a myriad of pain and related symptoms.6.7
Chronic pain may occur because pain centers in the brain become hyperresponsive over time (central sensitization); this can occur at any point throughout the life course of endometriosis, even when endometriosis lesions are no longer visible. Sometimes, endometriosis is asymptomatic. In addition, endometriosis can cause infertility through anatomic distortion and inflammatory, endocrinologic, and other pathways.
The origins of endometriosis are thought to be multifactorial and include retrograde menstruation, cellular metaplasia, and/or stem cells that spread through blood and lymphatic vessels. Endometriosis is estrogen dependent, but lesion growth also is affected by altered or impaired immunity, localized complex hormonal influences, genetics, and possibly environmental contaminants.
Impact on public health and reproductive rights
Endometriosis has significant social, public health, and economic implications. It can decrease quality of life and prevent girls and women from attending work or school.8 Painful sex can affect sexual health. The WHO states that, “Addressing endometriosis will empower those affected by it, by supporting their human right to the highest standard of sexual and reproductive health, quality of life, and overall well-being.”5
At present, no known way is available to prevent or cure endometriosis. Early diagnosis and treatment, however, may slow or halt its natural progression and associated symptoms.
Diagnostic steps and treatment options
Early suspicion of endometriosis is the most important factor, followed by a careful history of menstrual symptoms and chronic pelvic pain, early referral to specialists for ultrasonography or other imaging, and sometimes surgical or laparoscopic visualization. Empirical treatment can be begun without histologic or laparoscopic confirmation.
Endometriosis can be treated with medications and/or surgery depending on symptoms, lesions, desired outcome, and patient choice.5,6 Common therapies include contraceptive steroids, nonsteroidal anti-inflammatory medications, and analgesics. Medical treatments focus on either lowering estrogen or increasing progesterone levels.
Surgery can remove endometriosis lesions, adhesions, and scar tissue. However, success in reducing pain symptoms and increasing pregnancy rates often depends on the extent of disease.
For infertility due to endometriosis, treatment options include laparoscopic surgical removal of endometriosis, ovarian stimulation with intrauterine insemination (IUI), and IVF. Multidisciplinary treatment addressing different symptoms and overall health often requires referral to pain experts and other specialists.9
The WHO perspective on endometriosis
Recognizing the importance of endometriosis and its impact on people’s sexual and reproductive health, quality of life, and overall well-being, the WHO is taking action to improve awareness, diagnosis, and treatment of endometriosis (TABLE 3).5 ●

Endometriosis is now recognized as a disease with significant burden for women everywhere. Widespread lack of awareness of presenting symptoms and management options means that all women’s health care clinicians need to become better informed about endometriosis so they can improve the quality of care they provide.
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818-824.
- Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
- Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
- Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
- World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room/fact-sheets/detail /endometriosis. Accessed January 3, 2022.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244-1256.
- Johnson NP, Hummelshoj L, Adamson GD, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32:315-324.
- Nnoaham K, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366-373.e8.
- Carey ET, Till SR, As-Sanie S. Pharmacological management of chronic pelvic pain in women. Drugs. 2017;77:285-301.
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818-824.
- Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
- Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
- Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
- World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room/fact-sheets/detail /endometriosis. Accessed January 3, 2022.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244-1256.
- Johnson NP, Hummelshoj L, Adamson GD, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32:315-324.
- Nnoaham K, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366-373.e8.
- Carey ET, Till SR, As-Sanie S. Pharmacological management of chronic pelvic pain in women. Drugs. 2017;77:285-301.