User login
A.G. is a student who misses 2 or 3 days of school each month because of debilitating menstrual migraine (MM) with vomiting. Her referring physician prescribed topiramate, but the drug caused bothersome cognitive effects without relieving her headaches. She is taking a 30-μg oral contraceptive (OC) that contains ethinyl estradiol and drospirenone. Despite starting and stopping the OC several times, she has had no relief. She also takes 100 mg topiramate at bedtime, and 7 to 10 tablets of sumatriptan (100 mg) a month—usually during the menstrual week.
The ObGyn discontinues topiramate and changes dosing of the OC to active pills only for 12 consecutive weeks. During the 13th week, the patient is instructed to take 0.9 mg conjugated equine estrogens twice daily for 7 days before resuming extended-cycle oral contraception. This regimen completely eliminates menstrual migraine. The infrequent migraines the patient does experience—which she attributes to weather fronts—are successfully managed with no more than two doses of a triptan in a month.
A busy gynecology practice sees more migraineurs in a day than a neurology practice sees in a month. But most of the migraineurs who visit gynecology offices have a chief complaint other than headache, and most leave without having mentioned the migraine—returning home to continue treating themselves with the same remedies their mothers used. Many of these women fail to seek a specific diagnosis, or treatment, until they develop chronic daily headaches or become unable to work.
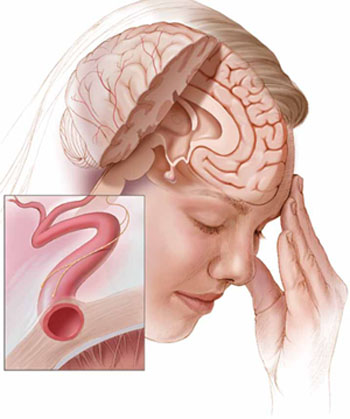
Current theories of migraine implicate spreading cortical depression; trigeminal nerve activation with attendant vasodilation; and neurogenic inflammation.
Menstrual migraine (MM) is, arguably, the most common disabling condition encountered in women’s health. These migraines are more severe, last longer, and are more resistant to treatment than those that occur at other times in the cycle.1,2 And, although headache specialists are adept at diagnosing migraine and prescribing a host of antiepileptic drugs and other migraine preventives, many of these specialists are uncomfortable manipulating the hormonal underpinnings of these “super migraines.”
This article outlines diagnostic criteria, treatment options, and preventive strategies, including hormonal therapy. In the process, it demonstrates how, in many cases, a gynecologist’s expertise in managing hormonal triggers may be the determinant of successful treatment.
Migraine prefers women
Migraine is the most common of all disabling headaches and afflicts 13% of the US population, but with a markedly skewed distribution: It preferentially attacks women in a 3:1 preponderance. The lifetime prevalence of migraine in women is 33%. Migraine strikes women primarily during reproductive years, when many women seek routine health care from a gynecologist rather than an internist or general practitioner.3
Menstrual migraine is defined as migraine without aura that occurs in predictable association with menses. Its onset falls within a 5-day window, spanning 2 days before the onset of menses through the third day of bleeding.5 Although the complete exclusion of migraine with aura from diagnostic criteria is controversial, headache specialists generally agree that aura is uncommonly associated with MM, probably owing to the low-estrogen environment. (Higher concentrations of estrogen are associated with an increased likelihood of aura, similar to the association between estrogen and seizure activity.)
The migraine trigger appears to be estrogen withdrawal in susceptible persons—either during the natural menstrual cycle or as a result of cycling onto inert pills in an oral contraceptive (OC) regimen. A population study found that 39% of menstruating women experience headaches with menses, and almost one third of these headaches meet established criteria for MM or menstrual-related migraine.4
The distinction between these two entities, MM and menstrual-related migraine, is largely semantic. With MM, the menstrual attack is the patient’s only migraine. The broader term implies that she may have other attacks in addition to the menstrual ones. In this article, I’ve gathered both categories under the term MM, which also includes headaches that arise at the time of estrogen withdrawal associated with OC use.
Regardless of what we call them, these headaches have proved to be particularly vexing to headache medicine specialists who hesitate to address hormonal factors.
Diagnostic criteria are clear
Formal diagnosis of migraine requires that at least two of four signature characteristics plus at least one of two associated symptoms be present.5 The four characteristics are:
- moderate or severe pain
- throbbing
- unilateral location
- intensification of headache upon activity.
Any combination of two suffices for diagnosis—much to the astonishment of many patients who mistakenly believe that migraine must be severe or one-sided.
Associated symptoms include either nausea or both photophobia and phonophobia, the latter often signified by the simple preference to be in a dark, quiet room during an attack.
Untreated, migraine usually lasts between 4 and 72 hours.
A practical, clinical approach to diagnosis is to look for the episodic disabling headache. By disabling, I mean the presence of associated nausea or the need to stop one’s activities and lie down. A stable history of attacks with predictable menstrual association offers further confirmation.
Neck tension may be present
Symptoms of neck tension do not rule out migraine. In fact, neck pain is far more common at the time of treatment than is nausea, and migraineurs frequently describe neck pain that radiates forward during an attack.6
Treatment options begin with NSAIDs
Initial treatment of MM is no different than that of other migraines. Mild and moderate attacks can often be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), which concomitantly treat comorbid dysmenorrhea.
A small randomized trial found that mefenamic acid (500 mg every 8 hours, as needed) was superior to placebo for acute MM, and a small crossover trial compared two formulations of diclofenac—50-mg sachets and tablets—with placebo in the treatment of acute migraine (TABLE). Almost one quarter of the patients taking the sachet form were pain-free at 2 hours, compared with 18.5% of patients taking the tablets and 11.7% of those who received placebo.7,8
Injectable ketorolac is more potent and is proving to be as effective as or more effective than triptans or opioids for severe, persistent migraine.
When migraine is more severe, or when NSAIDs no longer suffice, treatment advances to migraine-specific medications—specifically, ergotamines and triptans.
Brand names of drugs mentioned in this article
| Drug | Brand name |
|---|---|
| NSAIDs | |
| Diclofenac sodium | Voltaren (and others) |
| Ketorolac tromethamine | Toradol (and others) |
| Mefenamic acid | Ponstel (and others) |
| Naproxen sodium | Naprelan (and others) |
| Triptans | |
| Eletriptan hydrobromide | Relpax |
| Frovatriptan succinate | Frova |
| Naratriptan hydrochloride | Amerge |
| Sumatriptan succinate | Imitrex |
| Ergotamines | |
| Dihydroergotamine mesylate | D.H.E. 45, Migranal |
| Other | |
| Butalbital, acetaminophen, and caffeine | Fioricet |
| Leuprolide acetate | Lupron, Eligard |
Ergots and ergotamines have varied safety profiles in pregnancy
Dihydroergotamine belongs to the oldest family of migraine-specific drugs, although it is not widely used today. One reason may be that most migraineurs are women of reproductive age, and ergots are oxytocic and potential teratogens. Furthermore, they are not recommended in lactation. Ergotamines, however, are quite diverse, and some have more acceptable FDA use-in-pregnancy ratings. Injectable dihydroergotamine appears to be as effective as or less effective than triptans for migraine pain, but more effective than other drugs, such as NSAIDs or analgesics, for acute attacks.9 The nasal spray is more effective than placebo, but less effective than triptans.9
Triptans are relatively safe for women of reproductive age
Most information on use of triptans in pregnancy concerns sumatriptan. Like all triptans, it falls into Category C; however, its published profile is reassuring.10-12 Data from Sweden on 2,027 first-trimester exposures show a 3.6% risk of birth defects (major and minor), compared with an identical 3.6% risk in the general Swedish population.12 Sumatriptan is considerably less lipophilic than most of the drugs in its class and has been rated as compatible with breastfeeding.13
All triptans except eletriptan have been studied in the treatment of acute MM and found to be superior to placebo.14 These serotonin 1B/1D agonists are tailored therapy for acute migraine because, unlike analgesics, they address the underlying pathology of the attack, inhibiting the release of vasoactive peptides, promoting selective meningeal vasoconstriction, and blocking pain pathways in the brainstem. They also resolve the associated symptoms of nausea and photophobia. Triptans should not be used in the presence of untreated hypertension or vascular disease (cardiovascular, cerebrovascular, or peripheral vascular disease).
Watch for “rebound” headache. Like all acute treatment—be it a simple analgesic, NSAID, caffeine-containing compound, butalbital, ergotamine, or opioid—too-frequent use of triptans can produce medication-overuse headache, also referred to as rebound headache. In general, try to limit the use of agents for acute migraine, whether prescription or over-the-counter, to no more than 2 days a week to avoid this consequence. Also, be aware that some agents—notably, butalbital-containing compounds—may cause rebound headache, even when given as infrequently as 5 days of the month.15
Some preventive strategies are “nonspecific”
When treatment of acute MM is inadequate, management shifts toward prevention. Options include nonspecific (those that do not address the hormonal trigger) and specific (hormonal) strategies. Nonspecific strategies rely on the predictable onset of MM for proper timing of the therapeutic intervention; therefore, women who have irregular cycles are not good candidates for this approach. The presence of comorbid conditions such as dysmenorrhea, menometrorrhagia, and endometriosis may argue for early adoption of specific strategies that improve both conditions at once.
The fact that menstrual migraine (MM) is associated with estrogen withdrawal prompts a question: What happens immediately postpartum, when estrogen levels decline with loss of the placenta?
Although hormonal fluctuations generally stabilize during pregnancy, and most menstrual migraineurs experience fewer headaches during gestation, that protective effect erodes at delivery, when the incidence of migraine can be as high as 40% in the first week.41
Investigators who prospectively studied 49 migraineurs—two of whom were affected by migraine with aura and 47 by migraine without aura—found improvement in 46.8% of these women during the first trimester of pregnancy, 83% during the second trimester, and 87.2% during the third trimester, with complete remission rates of 10.6%, 53.2%, and 78.7%, respectively.42 During the first postpartum week, migraine recurred in 34% of women.42 Interestingly, women who had a history of MM were less likely to improve during the first and third trimesters of pregnancy.42
In a separate prospective investigation of 985 women who delivered over a 3-month period in one tertiary-care facility, 381 experienced postpartum headache.43 The median time to onset of the headache was 2 days, and the median duration was 4 hours. More than 75% of these headaches were primary headaches.43 Only a small percentage (4%) were incapacitating.43
When migraine may signal a more serious condition
Another question arises when a woman experiences a severe headache shortly after delivery: Could the headache be related to preeclampsia or another serious complication of pregnancy and delivery? According to Contag and colleagues, immediate assessment may be warranted.44
“Characteristics to consider are the association of the headache with elevated blood pressure (which could signal postpartum preeclampsia), the sudden onset of an atypical headache, and variations to the usual nature of the migraine, such as the onset of new neurological symptoms. Postpartum women with any of these characteristics should be evaluated in the emergency department, and neuroimaging should be strongly considered,” they write.44
Treatment reverts to prepregnancy options
Assuming preeclampsia or another serious condition is not the culprit, the treatment options for postpartum migraine revert to prepregnancy choices, as effect on the developing fetus is no longer a concern. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the first line of therapy. If they are ineffective, proceed to an ergotamine or triptan.—Janelle Yates, Senior Editor
Nonhormonal (nonspecific) therapy NSAIDs may provide some relief. In one small study, naproxen sodium (550 mg twice daily) was administered for 2 weeks, beginning 1 week before the anticipated onset of menses. It modestly reduced the overall duration and severity of menstrual migraine16—a benefit that must be weighed against the risk of adverse events and the more recent FDA black box warning about its potentially serious heart and gastrointestinal risks.
Triptan regimens may prevent MM. Several triptans have been investigated for prevention of MM. A small, open-label study evaluated the use of oral sumatriptan (25 mg three times daily), beginning 2 to 3 days before the anticipated onset of MM and continuing for 5 days.17 Menstrual attacks were prevented in just over 50% of cases.
Naratriptan (1 mg twice daily), beginning 2 days before anticipated menses and continuing for 5 days, reduced the number of MM attacks by 50%, with side effects comparable to placebo.18 A higher dosage (2.5 mg twice daily) did not prove to be superior to placebo, for unexplained reasons.
In a similar trial, frovatriptan (2.5 mg daily or 2.5 mg twice daily), beginning 2 days before the anticipated onset of MM and continuing for 6 days, prevented at least 50% of MM.19 Twice-daily dosing was superior to daily administration.
Magnesium may shorten MM. In one small study, oral magnesium (360 mg daily), beginning on the 15th day of the cycle and continuing through the menses, shortened the duration of MM and improved menstrual complaints better than placebo.20
The goal of hormonal therapy is to eliminate or sufficiently minimize the premenstrual decline in estrogen that is believed to precipitate MM.21 An observational study of 229 women found that hormonal strategies prevented MM in 73% of cases (81% when taken as directed).22
It is fortunate that MM, by definition, is migraine without aura, because the use of combined OCs has been controversial in the setting of migraine with aura. International studies have reported a small but increased risk of stroke associated with their use, although a subset of women with migraine had no increased risk.23-25 In contrast, no U.S. study since 1975 has found an increased risk of stroke associated with the use of OCs.26 One large domestic study reviewed 3.6 million woman-years of use and found no increased risk of ischemic stroke with the low-dose OCs currently available, nor did a pooled analysis of U.S. studies.27,28
The discrepancy is likely explained by the strong relative contraindication, in the United States, to the use of OCs in smokers older than 35 years (the smoking prevalence in most of the European case series was more than 50%), as well as the more prevalent use of high-dose OCs in the international studies. High-dose pills were implicated in the majority of stroke cases in the World Health Organization (WHO) study, but were used by only 0.7% of cases and controls in the pooled U.S. studies.23 Nevertheless, both ACOG and WHO concluded that the risk of OC use usually outweighs the benefit in women older than 35 years whose migraines are complicated by focal neurologic deficits.
Extended-cycle OCs may offer a lengthy reprieve. Regimens that forego monthly withdrawal bleeds and provide extended administration of active pills can afford migraineurs a lengthy reprieve from MM.29 Breakthrough bleeding is the most common side effect but tends to decrease over time. It is preferable for the patient to take the pill at bedtime to avoid a drug nadir during susceptible stages of sleep, which may be associated with migraine generation. It also is prudent to avoid concomitant administration of drugs that might increase the rate of hepatic metabolism of estrogen (e.g., a high dosage of topiramate) and lead to a more rapid decline in concentration.
When using an extended-cycle regimen that allows for periodic withdrawal bleeds, give the patient supplemental estrogen during the withdrawal week if the decline in ethinyl estradiol (EE) exceeds 10 μg, a threshold that appears to elicit MM in susceptible women.21,22 For example, with an extended-cycle regimen that contains 30 μg of EE, it may be necessary to add 20 μg of estrogen during the placebo week to prevent MM, whereas an extended-cycle regimen that contains 20 μg EE, declining to 10 μg during the 13th week, would be adequate as packaged.
21/7 hormonal OCs may require supplemental estrogen to prevent MM. The late luteal-phase decline in estradiol concentration during the natural menstrual cycle is equivalent to the decline experienced with the transition to inert pills in 20- to 25-μg EE formulations. Therefore, these regimens confer the same risk of MM as the woman’s natural cycle. Products that contain incrementally higher dosages of EE (30, 35, and 50 μg) confer an increasingly greater risk of MM. Supplementation of estrogen during the placebo week may prevent MM by limiting the decline in estrogen.
In a small, open-label study, women took an OC containing 20 μg EE at bedtime on days 1 to 21 of the cycle, followed by 0.9 mg of conjugated equine estrogens (CEE) on days 22 to 28. All patients reported a reduction in migraine frequency of at least 50%, with a mean reduction of 78%.30
Parenteral options can be created utilizing a transdermal 20-μg EE/norelgestromin patch or a 15-μg EE/etonogestrel vaginal ring. With the first approach, I recommend adding a 0.1-mg EE patch during the withdrawal week to prevent MM. With the latter, a 0.075-mg EE patch may be used during the week following ring removal.31
Consider a menstrually targeted estrogen supplement. Some women who have contraindications to use of an OC may still be candidates for targeted strategies using lower dosages of supplemental estrogen rather than a combination OC. Among the options is perimenstrual administration of an estradiol patch or gel.32 One study found a 0.1-mg estrogen patch (worn 7 days and applied just before the expected onset of menses) to be effective, but lower dosages (0.025 to 0.05 mg EE) were not.33 Daily application of 1.5 mg estradiol gel for 7 days, beginning before the onset of MM, was also effective in preventing MM.34-36
With targeted strategies, timing is critical; if estrogen is begun too early, the incidence of migraine may rise after cessation.36
GnRH agonists may benefit women who have isolated MM. Administration of gonadotropin-releasing hormone (GnRH) agonists has been shown to ease MM, but the baseline frequency of headaches appears to be an important variable in the success of these agents.37-39 Leuprolide acetate markedly diminished MM in women who seldom experienced headaches other than their menstrual attacks.37 It did not benefit women who experienced migraine in the setting of chronic headache (≥15 days/month), probably because chronic migraine is influenced by other variables, such as nonrestorative sleep and overuse of medication.15,38,40
No evidence that progestin-only contraceptives are effective. Although these agents have been proposed for treatment of migraine, evidence of their benefit is lacking. It is my opinion that, more often than not, they exacerbate migraine.
1. MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63(2):351-353.
2. Granella F, Sances G, Allais G, et al. Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia. 2004;24(9):707-716.
3. Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53(3):537-542.
4. Couturier EG, Bomhof MA, Neven AK, van Duijn NP. Menstrual migraine in a representative Dutch population sample: prevalence, disability and treatment. Cephalalgia. 2003;23(4):302-308.
5. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2nd ed. Cephalalgia. 2004;24(suppl 1):9-160.
6. Calhoun AH, Ford S, Millen C, Finkel AG, Truong Y, Nie Y. The prevalence of neck pain in migraine [abstract]. Headache. 2010 Jan 20.-[Epub ahead of print].
7. Al-Waili NS. Treatment of menstrual migraine with prostaglandin synthesis inhibitor mefenamic acid: double-blind study with placebo. Eur J Med Res. 2000;5(4):176-182.
8. Diener HC, Montagna P, Gács G, et al. Efficacy and tolerability of diclofenac potassium sachets in migraine: a randomized, double-blind, cross-over study in comparison with diclofenac potassium tablets and placebo. Cephalalgia. 2006;26(5):537-547.
9. Schürks M. Dihydroergotamine: role in the treatment of migraine. Expert Opin Drug Metab Toxicol. 2009;5(9):1141-1148.
10. Loder E. Safety of sumatriptan in pregnancy: a review of the data so far. CNS Drugs. 2003;17(1):1-7.
11. Shuhaiber S, Pastuszak A, Schick B, et al. Pregnancy outcome following first trimester exposure to sumatriptan. Neurology. 1998;51(2):581-583.
12. Cunnington M, Ephross S, Churchill P. The safety of sumatriptan and naratriptan in pregnancy: what have we learned? Headache. 2009;49(10):1414-1422.
13. Ressel G. AAP updates statement for transfer of drugs and other chemicals into breast milk. American Academy of Pediatrics. Am Fam Physician. 2002;65(5):979-980.
14. Ashkenazi A, Silberstein S. Menstrual migraine: a review of hormonal causes, prophylaxis and treatment. Expert Opin Pharmacother. 2007;8(11):1605-1613.
15. Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157-1168.
16. Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705-709.
17. Newman LC, Lipton RB, Lay CL, Solomon S. A pilot study of oral sumatriptan as intermittent prophylaxis of menstruation-related migraine. Neurology. 1998;51(1):307-309.
18. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled study. Headache. 2001;41(3):248-256.
19. Silberstein SD, Elkind AH, Schreiber C, Keywood C. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63(2):261-269.
20. Facchinetti F, Sances G, Borella P, Genazzani AR, Nappi G. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31(5):298-301.
21. Calhoun A. Women’s Issues in Headache. In: Loder E, ed. Headache. Philadelphia: American College of Physicians; 2004:157-177.
22. Calhoun A, Ford S. Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache. 2008;48(8):1186-1193.
23. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348:498-505.
24. Chan WS, Ray J, Wai EK, et al. Risk of stroke in women exposed to low-dose oral contraceptives: a critical evaluation of the evidence. Arch Intern Med. 2004;164(7):741-747.
25. Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63.-
26. Oral contraceptives and stroke in young women. Associated risk factors. JAMA. 1975;231:718-722.
27. Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8-15.
28. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29(11):2277-2284.
29. Sulak PJ, Scow RD, Preece C, Riggs MW, Kuehl TJ. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol. 2000;95(2):261-266.
30. Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J. 2004;97(9):819-822.
31. Calhoun AH, Hutchinson S. Hormonal therapies for menstrual migraine. Curr Pain Headache Rep. 2009;13(5):381-385.
32. Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70(17):1555-1563.
33. Pradalier A VD, Beaulieu PH, et al. Correlation between oestradiol plasma level and therapeutic effect on menstrual migraine. In: FC Rose, ed. New advances in headache research. London: Smith-Gordon; 1994:129-132.
34. de Lignières B, Vincens M, Mauvais-Jarvis P, Mas JL, Touboul PJ, Bousser MG. Prevention of menstrual migraine by percutaneous oestradiol. Br Med J (Clin Res Ed). 1986;293(6561):1540.-
35. Dennerstein L, Morse C, Burrows G, Oats J, Brown J, Smith M. Menstrual migraine: a double-blind trial of percutaneous estradiol. Gynecol Endocrinol. 1988;2(2):113-120.
36. MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Prevention of menstrual attacks of migraine: a double-blind placebo-controlled crossover study. Neurology. 2006;67(12):2159-2163.
37. Murray SC, Muse KN. Effective treatment of severe menstrual migraine headaches with gonadotropin-releasing hormone agonist and “add-back” therapy. Fertil Steril. 1997;67(2):390-393.
38. Martin V, Wernke S, Mandell K, et al. Medical oophorectomy with and without estrogen add-back therapy in the prevention of migraine headache. Headache. 2003;43(4):309-321.
39. Lichten EM, Lichten JB, Whitty A, Pieper D. The confirmation of a biochemical marker for women’s hormonal migraine: the depo-estradiol challenge test. Headache. 1996;36(6):367-371.
40. Calhoun AH, Ford S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache. 2007;47(8):1178-1183.
41. Stein GS. Headaches in the first postpartum week and their relationship to migraine. Headache. 1981;21(5):201-205.
42. Sances G, Granella F, Nappi RE, et al. Course of migraine during pregnancy and postpartum: a prospective study. Cephalalgia. 2003;23(3):197-205.
43. Goldszmidt E, Kern R, Chaput A, Macarthur A. The incidence and etiology of postpartum headaches: a prospective cohort study. Can J Anaesth. 2005;52(9):971-977.
44. Contag SA, Mertz HL, Bushnell CD. Migraine during pregnancy: Is it more than a headache? Nat Rev Neurol. 2009;5(8):449-456.
A.G. is a student who misses 2 or 3 days of school each month because of debilitating menstrual migraine (MM) with vomiting. Her referring physician prescribed topiramate, but the drug caused bothersome cognitive effects without relieving her headaches. She is taking a 30-μg oral contraceptive (OC) that contains ethinyl estradiol and drospirenone. Despite starting and stopping the OC several times, she has had no relief. She also takes 100 mg topiramate at bedtime, and 7 to 10 tablets of sumatriptan (100 mg) a month—usually during the menstrual week.
The ObGyn discontinues topiramate and changes dosing of the OC to active pills only for 12 consecutive weeks. During the 13th week, the patient is instructed to take 0.9 mg conjugated equine estrogens twice daily for 7 days before resuming extended-cycle oral contraception. This regimen completely eliminates menstrual migraine. The infrequent migraines the patient does experience—which she attributes to weather fronts—are successfully managed with no more than two doses of a triptan in a month.
A busy gynecology practice sees more migraineurs in a day than a neurology practice sees in a month. But most of the migraineurs who visit gynecology offices have a chief complaint other than headache, and most leave without having mentioned the migraine—returning home to continue treating themselves with the same remedies their mothers used. Many of these women fail to seek a specific diagnosis, or treatment, until they develop chronic daily headaches or become unable to work.

Current theories of migraine implicate spreading cortical depression; trigeminal nerve activation with attendant vasodilation; and neurogenic inflammation.
Menstrual migraine (MM) is, arguably, the most common disabling condition encountered in women’s health. These migraines are more severe, last longer, and are more resistant to treatment than those that occur at other times in the cycle.1,2 And, although headache specialists are adept at diagnosing migraine and prescribing a host of antiepileptic drugs and other migraine preventives, many of these specialists are uncomfortable manipulating the hormonal underpinnings of these “super migraines.”
This article outlines diagnostic criteria, treatment options, and preventive strategies, including hormonal therapy. In the process, it demonstrates how, in many cases, a gynecologist’s expertise in managing hormonal triggers may be the determinant of successful treatment.
Migraine prefers women
Migraine is the most common of all disabling headaches and afflicts 13% of the US population, but with a markedly skewed distribution: It preferentially attacks women in a 3:1 preponderance. The lifetime prevalence of migraine in women is 33%. Migraine strikes women primarily during reproductive years, when many women seek routine health care from a gynecologist rather than an internist or general practitioner.3
Menstrual migraine is defined as migraine without aura that occurs in predictable association with menses. Its onset falls within a 5-day window, spanning 2 days before the onset of menses through the third day of bleeding.5 Although the complete exclusion of migraine with aura from diagnostic criteria is controversial, headache specialists generally agree that aura is uncommonly associated with MM, probably owing to the low-estrogen environment. (Higher concentrations of estrogen are associated with an increased likelihood of aura, similar to the association between estrogen and seizure activity.)
The migraine trigger appears to be estrogen withdrawal in susceptible persons—either during the natural menstrual cycle or as a result of cycling onto inert pills in an oral contraceptive (OC) regimen. A population study found that 39% of menstruating women experience headaches with menses, and almost one third of these headaches meet established criteria for MM or menstrual-related migraine.4
The distinction between these two entities, MM and menstrual-related migraine, is largely semantic. With MM, the menstrual attack is the patient’s only migraine. The broader term implies that she may have other attacks in addition to the menstrual ones. In this article, I’ve gathered both categories under the term MM, which also includes headaches that arise at the time of estrogen withdrawal associated with OC use.
Regardless of what we call them, these headaches have proved to be particularly vexing to headache medicine specialists who hesitate to address hormonal factors.
Diagnostic criteria are clear
Formal diagnosis of migraine requires that at least two of four signature characteristics plus at least one of two associated symptoms be present.5 The four characteristics are:
- moderate or severe pain
- throbbing
- unilateral location
- intensification of headache upon activity.
Any combination of two suffices for diagnosis—much to the astonishment of many patients who mistakenly believe that migraine must be severe or one-sided.
Associated symptoms include either nausea or both photophobia and phonophobia, the latter often signified by the simple preference to be in a dark, quiet room during an attack.
Untreated, migraine usually lasts between 4 and 72 hours.
A practical, clinical approach to diagnosis is to look for the episodic disabling headache. By disabling, I mean the presence of associated nausea or the need to stop one’s activities and lie down. A stable history of attacks with predictable menstrual association offers further confirmation.
Neck tension may be present
Symptoms of neck tension do not rule out migraine. In fact, neck pain is far more common at the time of treatment than is nausea, and migraineurs frequently describe neck pain that radiates forward during an attack.6
Treatment options begin with NSAIDs
Initial treatment of MM is no different than that of other migraines. Mild and moderate attacks can often be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), which concomitantly treat comorbid dysmenorrhea.
A small randomized trial found that mefenamic acid (500 mg every 8 hours, as needed) was superior to placebo for acute MM, and a small crossover trial compared two formulations of diclofenac—50-mg sachets and tablets—with placebo in the treatment of acute migraine (TABLE). Almost one quarter of the patients taking the sachet form were pain-free at 2 hours, compared with 18.5% of patients taking the tablets and 11.7% of those who received placebo.7,8
Injectable ketorolac is more potent and is proving to be as effective as or more effective than triptans or opioids for severe, persistent migraine.
When migraine is more severe, or when NSAIDs no longer suffice, treatment advances to migraine-specific medications—specifically, ergotamines and triptans.
Brand names of drugs mentioned in this article
| Drug | Brand name |
|---|---|
| NSAIDs | |
| Diclofenac sodium | Voltaren (and others) |
| Ketorolac tromethamine | Toradol (and others) |
| Mefenamic acid | Ponstel (and others) |
| Naproxen sodium | Naprelan (and others) |
| Triptans | |
| Eletriptan hydrobromide | Relpax |
| Frovatriptan succinate | Frova |
| Naratriptan hydrochloride | Amerge |
| Sumatriptan succinate | Imitrex |
| Ergotamines | |
| Dihydroergotamine mesylate | D.H.E. 45, Migranal |
| Other | |
| Butalbital, acetaminophen, and caffeine | Fioricet |
| Leuprolide acetate | Lupron, Eligard |
Ergots and ergotamines have varied safety profiles in pregnancy
Dihydroergotamine belongs to the oldest family of migraine-specific drugs, although it is not widely used today. One reason may be that most migraineurs are women of reproductive age, and ergots are oxytocic and potential teratogens. Furthermore, they are not recommended in lactation. Ergotamines, however, are quite diverse, and some have more acceptable FDA use-in-pregnancy ratings. Injectable dihydroergotamine appears to be as effective as or less effective than triptans for migraine pain, but more effective than other drugs, such as NSAIDs or analgesics, for acute attacks.9 The nasal spray is more effective than placebo, but less effective than triptans.9
Triptans are relatively safe for women of reproductive age
Most information on use of triptans in pregnancy concerns sumatriptan. Like all triptans, it falls into Category C; however, its published profile is reassuring.10-12 Data from Sweden on 2,027 first-trimester exposures show a 3.6% risk of birth defects (major and minor), compared with an identical 3.6% risk in the general Swedish population.12 Sumatriptan is considerably less lipophilic than most of the drugs in its class and has been rated as compatible with breastfeeding.13
All triptans except eletriptan have been studied in the treatment of acute MM and found to be superior to placebo.14 These serotonin 1B/1D agonists are tailored therapy for acute migraine because, unlike analgesics, they address the underlying pathology of the attack, inhibiting the release of vasoactive peptides, promoting selective meningeal vasoconstriction, and blocking pain pathways in the brainstem. They also resolve the associated symptoms of nausea and photophobia. Triptans should not be used in the presence of untreated hypertension or vascular disease (cardiovascular, cerebrovascular, or peripheral vascular disease).
Watch for “rebound” headache. Like all acute treatment—be it a simple analgesic, NSAID, caffeine-containing compound, butalbital, ergotamine, or opioid—too-frequent use of triptans can produce medication-overuse headache, also referred to as rebound headache. In general, try to limit the use of agents for acute migraine, whether prescription or over-the-counter, to no more than 2 days a week to avoid this consequence. Also, be aware that some agents—notably, butalbital-containing compounds—may cause rebound headache, even when given as infrequently as 5 days of the month.15
Some preventive strategies are “nonspecific”
When treatment of acute MM is inadequate, management shifts toward prevention. Options include nonspecific (those that do not address the hormonal trigger) and specific (hormonal) strategies. Nonspecific strategies rely on the predictable onset of MM for proper timing of the therapeutic intervention; therefore, women who have irregular cycles are not good candidates for this approach. The presence of comorbid conditions such as dysmenorrhea, menometrorrhagia, and endometriosis may argue for early adoption of specific strategies that improve both conditions at once.
The fact that menstrual migraine (MM) is associated with estrogen withdrawal prompts a question: What happens immediately postpartum, when estrogen levels decline with loss of the placenta?
Although hormonal fluctuations generally stabilize during pregnancy, and most menstrual migraineurs experience fewer headaches during gestation, that protective effect erodes at delivery, when the incidence of migraine can be as high as 40% in the first week.41
Investigators who prospectively studied 49 migraineurs—two of whom were affected by migraine with aura and 47 by migraine without aura—found improvement in 46.8% of these women during the first trimester of pregnancy, 83% during the second trimester, and 87.2% during the third trimester, with complete remission rates of 10.6%, 53.2%, and 78.7%, respectively.42 During the first postpartum week, migraine recurred in 34% of women.42 Interestingly, women who had a history of MM were less likely to improve during the first and third trimesters of pregnancy.42
In a separate prospective investigation of 985 women who delivered over a 3-month period in one tertiary-care facility, 381 experienced postpartum headache.43 The median time to onset of the headache was 2 days, and the median duration was 4 hours. More than 75% of these headaches were primary headaches.43 Only a small percentage (4%) were incapacitating.43
When migraine may signal a more serious condition
Another question arises when a woman experiences a severe headache shortly after delivery: Could the headache be related to preeclampsia or another serious complication of pregnancy and delivery? According to Contag and colleagues, immediate assessment may be warranted.44
“Characteristics to consider are the association of the headache with elevated blood pressure (which could signal postpartum preeclampsia), the sudden onset of an atypical headache, and variations to the usual nature of the migraine, such as the onset of new neurological symptoms. Postpartum women with any of these characteristics should be evaluated in the emergency department, and neuroimaging should be strongly considered,” they write.44
Treatment reverts to prepregnancy options
Assuming preeclampsia or another serious condition is not the culprit, the treatment options for postpartum migraine revert to prepregnancy choices, as effect on the developing fetus is no longer a concern. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the first line of therapy. If they are ineffective, proceed to an ergotamine or triptan.—Janelle Yates, Senior Editor
Nonhormonal (nonspecific) therapy NSAIDs may provide some relief. In one small study, naproxen sodium (550 mg twice daily) was administered for 2 weeks, beginning 1 week before the anticipated onset of menses. It modestly reduced the overall duration and severity of menstrual migraine16—a benefit that must be weighed against the risk of adverse events and the more recent FDA black box warning about its potentially serious heart and gastrointestinal risks.
Triptan regimens may prevent MM. Several triptans have been investigated for prevention of MM. A small, open-label study evaluated the use of oral sumatriptan (25 mg three times daily), beginning 2 to 3 days before the anticipated onset of MM and continuing for 5 days.17 Menstrual attacks were prevented in just over 50% of cases.
Naratriptan (1 mg twice daily), beginning 2 days before anticipated menses and continuing for 5 days, reduced the number of MM attacks by 50%, with side effects comparable to placebo.18 A higher dosage (2.5 mg twice daily) did not prove to be superior to placebo, for unexplained reasons.
In a similar trial, frovatriptan (2.5 mg daily or 2.5 mg twice daily), beginning 2 days before the anticipated onset of MM and continuing for 6 days, prevented at least 50% of MM.19 Twice-daily dosing was superior to daily administration.
Magnesium may shorten MM. In one small study, oral magnesium (360 mg daily), beginning on the 15th day of the cycle and continuing through the menses, shortened the duration of MM and improved menstrual complaints better than placebo.20
The goal of hormonal therapy is to eliminate or sufficiently minimize the premenstrual decline in estrogen that is believed to precipitate MM.21 An observational study of 229 women found that hormonal strategies prevented MM in 73% of cases (81% when taken as directed).22
It is fortunate that MM, by definition, is migraine without aura, because the use of combined OCs has been controversial in the setting of migraine with aura. International studies have reported a small but increased risk of stroke associated with their use, although a subset of women with migraine had no increased risk.23-25 In contrast, no U.S. study since 1975 has found an increased risk of stroke associated with the use of OCs.26 One large domestic study reviewed 3.6 million woman-years of use and found no increased risk of ischemic stroke with the low-dose OCs currently available, nor did a pooled analysis of U.S. studies.27,28
The discrepancy is likely explained by the strong relative contraindication, in the United States, to the use of OCs in smokers older than 35 years (the smoking prevalence in most of the European case series was more than 50%), as well as the more prevalent use of high-dose OCs in the international studies. High-dose pills were implicated in the majority of stroke cases in the World Health Organization (WHO) study, but were used by only 0.7% of cases and controls in the pooled U.S. studies.23 Nevertheless, both ACOG and WHO concluded that the risk of OC use usually outweighs the benefit in women older than 35 years whose migraines are complicated by focal neurologic deficits.
Extended-cycle OCs may offer a lengthy reprieve. Regimens that forego monthly withdrawal bleeds and provide extended administration of active pills can afford migraineurs a lengthy reprieve from MM.29 Breakthrough bleeding is the most common side effect but tends to decrease over time. It is preferable for the patient to take the pill at bedtime to avoid a drug nadir during susceptible stages of sleep, which may be associated with migraine generation. It also is prudent to avoid concomitant administration of drugs that might increase the rate of hepatic metabolism of estrogen (e.g., a high dosage of topiramate) and lead to a more rapid decline in concentration.
When using an extended-cycle regimen that allows for periodic withdrawal bleeds, give the patient supplemental estrogen during the withdrawal week if the decline in ethinyl estradiol (EE) exceeds 10 μg, a threshold that appears to elicit MM in susceptible women.21,22 For example, with an extended-cycle regimen that contains 30 μg of EE, it may be necessary to add 20 μg of estrogen during the placebo week to prevent MM, whereas an extended-cycle regimen that contains 20 μg EE, declining to 10 μg during the 13th week, would be adequate as packaged.
21/7 hormonal OCs may require supplemental estrogen to prevent MM. The late luteal-phase decline in estradiol concentration during the natural menstrual cycle is equivalent to the decline experienced with the transition to inert pills in 20- to 25-μg EE formulations. Therefore, these regimens confer the same risk of MM as the woman’s natural cycle. Products that contain incrementally higher dosages of EE (30, 35, and 50 μg) confer an increasingly greater risk of MM. Supplementation of estrogen during the placebo week may prevent MM by limiting the decline in estrogen.
In a small, open-label study, women took an OC containing 20 μg EE at bedtime on days 1 to 21 of the cycle, followed by 0.9 mg of conjugated equine estrogens (CEE) on days 22 to 28. All patients reported a reduction in migraine frequency of at least 50%, with a mean reduction of 78%.30
Parenteral options can be created utilizing a transdermal 20-μg EE/norelgestromin patch or a 15-μg EE/etonogestrel vaginal ring. With the first approach, I recommend adding a 0.1-mg EE patch during the withdrawal week to prevent MM. With the latter, a 0.075-mg EE patch may be used during the week following ring removal.31
Consider a menstrually targeted estrogen supplement. Some women who have contraindications to use of an OC may still be candidates for targeted strategies using lower dosages of supplemental estrogen rather than a combination OC. Among the options is perimenstrual administration of an estradiol patch or gel.32 One study found a 0.1-mg estrogen patch (worn 7 days and applied just before the expected onset of menses) to be effective, but lower dosages (0.025 to 0.05 mg EE) were not.33 Daily application of 1.5 mg estradiol gel for 7 days, beginning before the onset of MM, was also effective in preventing MM.34-36
With targeted strategies, timing is critical; if estrogen is begun too early, the incidence of migraine may rise after cessation.36
GnRH agonists may benefit women who have isolated MM. Administration of gonadotropin-releasing hormone (GnRH) agonists has been shown to ease MM, but the baseline frequency of headaches appears to be an important variable in the success of these agents.37-39 Leuprolide acetate markedly diminished MM in women who seldom experienced headaches other than their menstrual attacks.37 It did not benefit women who experienced migraine in the setting of chronic headache (≥15 days/month), probably because chronic migraine is influenced by other variables, such as nonrestorative sleep and overuse of medication.15,38,40
No evidence that progestin-only contraceptives are effective. Although these agents have been proposed for treatment of migraine, evidence of their benefit is lacking. It is my opinion that, more often than not, they exacerbate migraine.
A.G. is a student who misses 2 or 3 days of school each month because of debilitating menstrual migraine (MM) with vomiting. Her referring physician prescribed topiramate, but the drug caused bothersome cognitive effects without relieving her headaches. She is taking a 30-μg oral contraceptive (OC) that contains ethinyl estradiol and drospirenone. Despite starting and stopping the OC several times, she has had no relief. She also takes 100 mg topiramate at bedtime, and 7 to 10 tablets of sumatriptan (100 mg) a month—usually during the menstrual week.
The ObGyn discontinues topiramate and changes dosing of the OC to active pills only for 12 consecutive weeks. During the 13th week, the patient is instructed to take 0.9 mg conjugated equine estrogens twice daily for 7 days before resuming extended-cycle oral contraception. This regimen completely eliminates menstrual migraine. The infrequent migraines the patient does experience—which she attributes to weather fronts—are successfully managed with no more than two doses of a triptan in a month.
A busy gynecology practice sees more migraineurs in a day than a neurology practice sees in a month. But most of the migraineurs who visit gynecology offices have a chief complaint other than headache, and most leave without having mentioned the migraine—returning home to continue treating themselves with the same remedies their mothers used. Many of these women fail to seek a specific diagnosis, or treatment, until they develop chronic daily headaches or become unable to work.

Current theories of migraine implicate spreading cortical depression; trigeminal nerve activation with attendant vasodilation; and neurogenic inflammation.
Menstrual migraine (MM) is, arguably, the most common disabling condition encountered in women’s health. These migraines are more severe, last longer, and are more resistant to treatment than those that occur at other times in the cycle.1,2 And, although headache specialists are adept at diagnosing migraine and prescribing a host of antiepileptic drugs and other migraine preventives, many of these specialists are uncomfortable manipulating the hormonal underpinnings of these “super migraines.”
This article outlines diagnostic criteria, treatment options, and preventive strategies, including hormonal therapy. In the process, it demonstrates how, in many cases, a gynecologist’s expertise in managing hormonal triggers may be the determinant of successful treatment.
Migraine prefers women
Migraine is the most common of all disabling headaches and afflicts 13% of the US population, but with a markedly skewed distribution: It preferentially attacks women in a 3:1 preponderance. The lifetime prevalence of migraine in women is 33%. Migraine strikes women primarily during reproductive years, when many women seek routine health care from a gynecologist rather than an internist or general practitioner.3
Menstrual migraine is defined as migraine without aura that occurs in predictable association with menses. Its onset falls within a 5-day window, spanning 2 days before the onset of menses through the third day of bleeding.5 Although the complete exclusion of migraine with aura from diagnostic criteria is controversial, headache specialists generally agree that aura is uncommonly associated with MM, probably owing to the low-estrogen environment. (Higher concentrations of estrogen are associated with an increased likelihood of aura, similar to the association between estrogen and seizure activity.)
The migraine trigger appears to be estrogen withdrawal in susceptible persons—either during the natural menstrual cycle or as a result of cycling onto inert pills in an oral contraceptive (OC) regimen. A population study found that 39% of menstruating women experience headaches with menses, and almost one third of these headaches meet established criteria for MM or menstrual-related migraine.4
The distinction between these two entities, MM and menstrual-related migraine, is largely semantic. With MM, the menstrual attack is the patient’s only migraine. The broader term implies that she may have other attacks in addition to the menstrual ones. In this article, I’ve gathered both categories under the term MM, which also includes headaches that arise at the time of estrogen withdrawal associated with OC use.
Regardless of what we call them, these headaches have proved to be particularly vexing to headache medicine specialists who hesitate to address hormonal factors.
Diagnostic criteria are clear
Formal diagnosis of migraine requires that at least two of four signature characteristics plus at least one of two associated symptoms be present.5 The four characteristics are:
- moderate or severe pain
- throbbing
- unilateral location
- intensification of headache upon activity.
Any combination of two suffices for diagnosis—much to the astonishment of many patients who mistakenly believe that migraine must be severe or one-sided.
Associated symptoms include either nausea or both photophobia and phonophobia, the latter often signified by the simple preference to be in a dark, quiet room during an attack.
Untreated, migraine usually lasts between 4 and 72 hours.
A practical, clinical approach to diagnosis is to look for the episodic disabling headache. By disabling, I mean the presence of associated nausea or the need to stop one’s activities and lie down. A stable history of attacks with predictable menstrual association offers further confirmation.
Neck tension may be present
Symptoms of neck tension do not rule out migraine. In fact, neck pain is far more common at the time of treatment than is nausea, and migraineurs frequently describe neck pain that radiates forward during an attack.6
Treatment options begin with NSAIDs
Initial treatment of MM is no different than that of other migraines. Mild and moderate attacks can often be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), which concomitantly treat comorbid dysmenorrhea.
A small randomized trial found that mefenamic acid (500 mg every 8 hours, as needed) was superior to placebo for acute MM, and a small crossover trial compared two formulations of diclofenac—50-mg sachets and tablets—with placebo in the treatment of acute migraine (TABLE). Almost one quarter of the patients taking the sachet form were pain-free at 2 hours, compared with 18.5% of patients taking the tablets and 11.7% of those who received placebo.7,8
Injectable ketorolac is more potent and is proving to be as effective as or more effective than triptans or opioids for severe, persistent migraine.
When migraine is more severe, or when NSAIDs no longer suffice, treatment advances to migraine-specific medications—specifically, ergotamines and triptans.
Brand names of drugs mentioned in this article
| Drug | Brand name |
|---|---|
| NSAIDs | |
| Diclofenac sodium | Voltaren (and others) |
| Ketorolac tromethamine | Toradol (and others) |
| Mefenamic acid | Ponstel (and others) |
| Naproxen sodium | Naprelan (and others) |
| Triptans | |
| Eletriptan hydrobromide | Relpax |
| Frovatriptan succinate | Frova |
| Naratriptan hydrochloride | Amerge |
| Sumatriptan succinate | Imitrex |
| Ergotamines | |
| Dihydroergotamine mesylate | D.H.E. 45, Migranal |
| Other | |
| Butalbital, acetaminophen, and caffeine | Fioricet |
| Leuprolide acetate | Lupron, Eligard |
Ergots and ergotamines have varied safety profiles in pregnancy
Dihydroergotamine belongs to the oldest family of migraine-specific drugs, although it is not widely used today. One reason may be that most migraineurs are women of reproductive age, and ergots are oxytocic and potential teratogens. Furthermore, they are not recommended in lactation. Ergotamines, however, are quite diverse, and some have more acceptable FDA use-in-pregnancy ratings. Injectable dihydroergotamine appears to be as effective as or less effective than triptans for migraine pain, but more effective than other drugs, such as NSAIDs or analgesics, for acute attacks.9 The nasal spray is more effective than placebo, but less effective than triptans.9
Triptans are relatively safe for women of reproductive age
Most information on use of triptans in pregnancy concerns sumatriptan. Like all triptans, it falls into Category C; however, its published profile is reassuring.10-12 Data from Sweden on 2,027 first-trimester exposures show a 3.6% risk of birth defects (major and minor), compared with an identical 3.6% risk in the general Swedish population.12 Sumatriptan is considerably less lipophilic than most of the drugs in its class and has been rated as compatible with breastfeeding.13
All triptans except eletriptan have been studied in the treatment of acute MM and found to be superior to placebo.14 These serotonin 1B/1D agonists are tailored therapy for acute migraine because, unlike analgesics, they address the underlying pathology of the attack, inhibiting the release of vasoactive peptides, promoting selective meningeal vasoconstriction, and blocking pain pathways in the brainstem. They also resolve the associated symptoms of nausea and photophobia. Triptans should not be used in the presence of untreated hypertension or vascular disease (cardiovascular, cerebrovascular, or peripheral vascular disease).
Watch for “rebound” headache. Like all acute treatment—be it a simple analgesic, NSAID, caffeine-containing compound, butalbital, ergotamine, or opioid—too-frequent use of triptans can produce medication-overuse headache, also referred to as rebound headache. In general, try to limit the use of agents for acute migraine, whether prescription or over-the-counter, to no more than 2 days a week to avoid this consequence. Also, be aware that some agents—notably, butalbital-containing compounds—may cause rebound headache, even when given as infrequently as 5 days of the month.15
Some preventive strategies are “nonspecific”
When treatment of acute MM is inadequate, management shifts toward prevention. Options include nonspecific (those that do not address the hormonal trigger) and specific (hormonal) strategies. Nonspecific strategies rely on the predictable onset of MM for proper timing of the therapeutic intervention; therefore, women who have irregular cycles are not good candidates for this approach. The presence of comorbid conditions such as dysmenorrhea, menometrorrhagia, and endometriosis may argue for early adoption of specific strategies that improve both conditions at once.
The fact that menstrual migraine (MM) is associated with estrogen withdrawal prompts a question: What happens immediately postpartum, when estrogen levels decline with loss of the placenta?
Although hormonal fluctuations generally stabilize during pregnancy, and most menstrual migraineurs experience fewer headaches during gestation, that protective effect erodes at delivery, when the incidence of migraine can be as high as 40% in the first week.41
Investigators who prospectively studied 49 migraineurs—two of whom were affected by migraine with aura and 47 by migraine without aura—found improvement in 46.8% of these women during the first trimester of pregnancy, 83% during the second trimester, and 87.2% during the third trimester, with complete remission rates of 10.6%, 53.2%, and 78.7%, respectively.42 During the first postpartum week, migraine recurred in 34% of women.42 Interestingly, women who had a history of MM were less likely to improve during the first and third trimesters of pregnancy.42
In a separate prospective investigation of 985 women who delivered over a 3-month period in one tertiary-care facility, 381 experienced postpartum headache.43 The median time to onset of the headache was 2 days, and the median duration was 4 hours. More than 75% of these headaches were primary headaches.43 Only a small percentage (4%) were incapacitating.43
When migraine may signal a more serious condition
Another question arises when a woman experiences a severe headache shortly after delivery: Could the headache be related to preeclampsia or another serious complication of pregnancy and delivery? According to Contag and colleagues, immediate assessment may be warranted.44
“Characteristics to consider are the association of the headache with elevated blood pressure (which could signal postpartum preeclampsia), the sudden onset of an atypical headache, and variations to the usual nature of the migraine, such as the onset of new neurological symptoms. Postpartum women with any of these characteristics should be evaluated in the emergency department, and neuroimaging should be strongly considered,” they write.44
Treatment reverts to prepregnancy options
Assuming preeclampsia or another serious condition is not the culprit, the treatment options for postpartum migraine revert to prepregnancy choices, as effect on the developing fetus is no longer a concern. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the first line of therapy. If they are ineffective, proceed to an ergotamine or triptan.—Janelle Yates, Senior Editor
Nonhormonal (nonspecific) therapy NSAIDs may provide some relief. In one small study, naproxen sodium (550 mg twice daily) was administered for 2 weeks, beginning 1 week before the anticipated onset of menses. It modestly reduced the overall duration and severity of menstrual migraine16—a benefit that must be weighed against the risk of adverse events and the more recent FDA black box warning about its potentially serious heart and gastrointestinal risks.
Triptan regimens may prevent MM. Several triptans have been investigated for prevention of MM. A small, open-label study evaluated the use of oral sumatriptan (25 mg three times daily), beginning 2 to 3 days before the anticipated onset of MM and continuing for 5 days.17 Menstrual attacks were prevented in just over 50% of cases.
Naratriptan (1 mg twice daily), beginning 2 days before anticipated menses and continuing for 5 days, reduced the number of MM attacks by 50%, with side effects comparable to placebo.18 A higher dosage (2.5 mg twice daily) did not prove to be superior to placebo, for unexplained reasons.
In a similar trial, frovatriptan (2.5 mg daily or 2.5 mg twice daily), beginning 2 days before the anticipated onset of MM and continuing for 6 days, prevented at least 50% of MM.19 Twice-daily dosing was superior to daily administration.
Magnesium may shorten MM. In one small study, oral magnesium (360 mg daily), beginning on the 15th day of the cycle and continuing through the menses, shortened the duration of MM and improved menstrual complaints better than placebo.20
The goal of hormonal therapy is to eliminate or sufficiently minimize the premenstrual decline in estrogen that is believed to precipitate MM.21 An observational study of 229 women found that hormonal strategies prevented MM in 73% of cases (81% when taken as directed).22
It is fortunate that MM, by definition, is migraine without aura, because the use of combined OCs has been controversial in the setting of migraine with aura. International studies have reported a small but increased risk of stroke associated with their use, although a subset of women with migraine had no increased risk.23-25 In contrast, no U.S. study since 1975 has found an increased risk of stroke associated with the use of OCs.26 One large domestic study reviewed 3.6 million woman-years of use and found no increased risk of ischemic stroke with the low-dose OCs currently available, nor did a pooled analysis of U.S. studies.27,28
The discrepancy is likely explained by the strong relative contraindication, in the United States, to the use of OCs in smokers older than 35 years (the smoking prevalence in most of the European case series was more than 50%), as well as the more prevalent use of high-dose OCs in the international studies. High-dose pills were implicated in the majority of stroke cases in the World Health Organization (WHO) study, but were used by only 0.7% of cases and controls in the pooled U.S. studies.23 Nevertheless, both ACOG and WHO concluded that the risk of OC use usually outweighs the benefit in women older than 35 years whose migraines are complicated by focal neurologic deficits.
Extended-cycle OCs may offer a lengthy reprieve. Regimens that forego monthly withdrawal bleeds and provide extended administration of active pills can afford migraineurs a lengthy reprieve from MM.29 Breakthrough bleeding is the most common side effect but tends to decrease over time. It is preferable for the patient to take the pill at bedtime to avoid a drug nadir during susceptible stages of sleep, which may be associated with migraine generation. It also is prudent to avoid concomitant administration of drugs that might increase the rate of hepatic metabolism of estrogen (e.g., a high dosage of topiramate) and lead to a more rapid decline in concentration.
When using an extended-cycle regimen that allows for periodic withdrawal bleeds, give the patient supplemental estrogen during the withdrawal week if the decline in ethinyl estradiol (EE) exceeds 10 μg, a threshold that appears to elicit MM in susceptible women.21,22 For example, with an extended-cycle regimen that contains 30 μg of EE, it may be necessary to add 20 μg of estrogen during the placebo week to prevent MM, whereas an extended-cycle regimen that contains 20 μg EE, declining to 10 μg during the 13th week, would be adequate as packaged.
21/7 hormonal OCs may require supplemental estrogen to prevent MM. The late luteal-phase decline in estradiol concentration during the natural menstrual cycle is equivalent to the decline experienced with the transition to inert pills in 20- to 25-μg EE formulations. Therefore, these regimens confer the same risk of MM as the woman’s natural cycle. Products that contain incrementally higher dosages of EE (30, 35, and 50 μg) confer an increasingly greater risk of MM. Supplementation of estrogen during the placebo week may prevent MM by limiting the decline in estrogen.
In a small, open-label study, women took an OC containing 20 μg EE at bedtime on days 1 to 21 of the cycle, followed by 0.9 mg of conjugated equine estrogens (CEE) on days 22 to 28. All patients reported a reduction in migraine frequency of at least 50%, with a mean reduction of 78%.30
Parenteral options can be created utilizing a transdermal 20-μg EE/norelgestromin patch or a 15-μg EE/etonogestrel vaginal ring. With the first approach, I recommend adding a 0.1-mg EE patch during the withdrawal week to prevent MM. With the latter, a 0.075-mg EE patch may be used during the week following ring removal.31
Consider a menstrually targeted estrogen supplement. Some women who have contraindications to use of an OC may still be candidates for targeted strategies using lower dosages of supplemental estrogen rather than a combination OC. Among the options is perimenstrual administration of an estradiol patch or gel.32 One study found a 0.1-mg estrogen patch (worn 7 days and applied just before the expected onset of menses) to be effective, but lower dosages (0.025 to 0.05 mg EE) were not.33 Daily application of 1.5 mg estradiol gel for 7 days, beginning before the onset of MM, was also effective in preventing MM.34-36
With targeted strategies, timing is critical; if estrogen is begun too early, the incidence of migraine may rise after cessation.36
GnRH agonists may benefit women who have isolated MM. Administration of gonadotropin-releasing hormone (GnRH) agonists has been shown to ease MM, but the baseline frequency of headaches appears to be an important variable in the success of these agents.37-39 Leuprolide acetate markedly diminished MM in women who seldom experienced headaches other than their menstrual attacks.37 It did not benefit women who experienced migraine in the setting of chronic headache (≥15 days/month), probably because chronic migraine is influenced by other variables, such as nonrestorative sleep and overuse of medication.15,38,40
No evidence that progestin-only contraceptives are effective. Although these agents have been proposed for treatment of migraine, evidence of their benefit is lacking. It is my opinion that, more often than not, they exacerbate migraine.
1. MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63(2):351-353.
2. Granella F, Sances G, Allais G, et al. Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia. 2004;24(9):707-716.
3. Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53(3):537-542.
4. Couturier EG, Bomhof MA, Neven AK, van Duijn NP. Menstrual migraine in a representative Dutch population sample: prevalence, disability and treatment. Cephalalgia. 2003;23(4):302-308.
5. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2nd ed. Cephalalgia. 2004;24(suppl 1):9-160.
6. Calhoun AH, Ford S, Millen C, Finkel AG, Truong Y, Nie Y. The prevalence of neck pain in migraine [abstract]. Headache. 2010 Jan 20.-[Epub ahead of print].
7. Al-Waili NS. Treatment of menstrual migraine with prostaglandin synthesis inhibitor mefenamic acid: double-blind study with placebo. Eur J Med Res. 2000;5(4):176-182.
8. Diener HC, Montagna P, Gács G, et al. Efficacy and tolerability of diclofenac potassium sachets in migraine: a randomized, double-blind, cross-over study in comparison with diclofenac potassium tablets and placebo. Cephalalgia. 2006;26(5):537-547.
9. Schürks M. Dihydroergotamine: role in the treatment of migraine. Expert Opin Drug Metab Toxicol. 2009;5(9):1141-1148.
10. Loder E. Safety of sumatriptan in pregnancy: a review of the data so far. CNS Drugs. 2003;17(1):1-7.
11. Shuhaiber S, Pastuszak A, Schick B, et al. Pregnancy outcome following first trimester exposure to sumatriptan. Neurology. 1998;51(2):581-583.
12. Cunnington M, Ephross S, Churchill P. The safety of sumatriptan and naratriptan in pregnancy: what have we learned? Headache. 2009;49(10):1414-1422.
13. Ressel G. AAP updates statement for transfer of drugs and other chemicals into breast milk. American Academy of Pediatrics. Am Fam Physician. 2002;65(5):979-980.
14. Ashkenazi A, Silberstein S. Menstrual migraine: a review of hormonal causes, prophylaxis and treatment. Expert Opin Pharmacother. 2007;8(11):1605-1613.
15. Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157-1168.
16. Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705-709.
17. Newman LC, Lipton RB, Lay CL, Solomon S. A pilot study of oral sumatriptan as intermittent prophylaxis of menstruation-related migraine. Neurology. 1998;51(1):307-309.
18. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled study. Headache. 2001;41(3):248-256.
19. Silberstein SD, Elkind AH, Schreiber C, Keywood C. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63(2):261-269.
20. Facchinetti F, Sances G, Borella P, Genazzani AR, Nappi G. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31(5):298-301.
21. Calhoun A. Women’s Issues in Headache. In: Loder E, ed. Headache. Philadelphia: American College of Physicians; 2004:157-177.
22. Calhoun A, Ford S. Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache. 2008;48(8):1186-1193.
23. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348:498-505.
24. Chan WS, Ray J, Wai EK, et al. Risk of stroke in women exposed to low-dose oral contraceptives: a critical evaluation of the evidence. Arch Intern Med. 2004;164(7):741-747.
25. Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63.-
26. Oral contraceptives and stroke in young women. Associated risk factors. JAMA. 1975;231:718-722.
27. Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8-15.
28. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29(11):2277-2284.
29. Sulak PJ, Scow RD, Preece C, Riggs MW, Kuehl TJ. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol. 2000;95(2):261-266.
30. Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J. 2004;97(9):819-822.
31. Calhoun AH, Hutchinson S. Hormonal therapies for menstrual migraine. Curr Pain Headache Rep. 2009;13(5):381-385.
32. Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70(17):1555-1563.
33. Pradalier A VD, Beaulieu PH, et al. Correlation between oestradiol plasma level and therapeutic effect on menstrual migraine. In: FC Rose, ed. New advances in headache research. London: Smith-Gordon; 1994:129-132.
34. de Lignières B, Vincens M, Mauvais-Jarvis P, Mas JL, Touboul PJ, Bousser MG. Prevention of menstrual migraine by percutaneous oestradiol. Br Med J (Clin Res Ed). 1986;293(6561):1540.-
35. Dennerstein L, Morse C, Burrows G, Oats J, Brown J, Smith M. Menstrual migraine: a double-blind trial of percutaneous estradiol. Gynecol Endocrinol. 1988;2(2):113-120.
36. MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Prevention of menstrual attacks of migraine: a double-blind placebo-controlled crossover study. Neurology. 2006;67(12):2159-2163.
37. Murray SC, Muse KN. Effective treatment of severe menstrual migraine headaches with gonadotropin-releasing hormone agonist and “add-back” therapy. Fertil Steril. 1997;67(2):390-393.
38. Martin V, Wernke S, Mandell K, et al. Medical oophorectomy with and without estrogen add-back therapy in the prevention of migraine headache. Headache. 2003;43(4):309-321.
39. Lichten EM, Lichten JB, Whitty A, Pieper D. The confirmation of a biochemical marker for women’s hormonal migraine: the depo-estradiol challenge test. Headache. 1996;36(6):367-371.
40. Calhoun AH, Ford S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache. 2007;47(8):1178-1183.
41. Stein GS. Headaches in the first postpartum week and their relationship to migraine. Headache. 1981;21(5):201-205.
42. Sances G, Granella F, Nappi RE, et al. Course of migraine during pregnancy and postpartum: a prospective study. Cephalalgia. 2003;23(3):197-205.
43. Goldszmidt E, Kern R, Chaput A, Macarthur A. The incidence and etiology of postpartum headaches: a prospective cohort study. Can J Anaesth. 2005;52(9):971-977.
44. Contag SA, Mertz HL, Bushnell CD. Migraine during pregnancy: Is it more than a headache? Nat Rev Neurol. 2009;5(8):449-456.
1. MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63(2):351-353.
2. Granella F, Sances G, Allais G, et al. Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia. 2004;24(9):707-716.
3. Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53(3):537-542.
4. Couturier EG, Bomhof MA, Neven AK, van Duijn NP. Menstrual migraine in a representative Dutch population sample: prevalence, disability and treatment. Cephalalgia. 2003;23(4):302-308.
5. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2nd ed. Cephalalgia. 2004;24(suppl 1):9-160.
6. Calhoun AH, Ford S, Millen C, Finkel AG, Truong Y, Nie Y. The prevalence of neck pain in migraine [abstract]. Headache. 2010 Jan 20.-[Epub ahead of print].
7. Al-Waili NS. Treatment of menstrual migraine with prostaglandin synthesis inhibitor mefenamic acid: double-blind study with placebo. Eur J Med Res. 2000;5(4):176-182.
8. Diener HC, Montagna P, Gács G, et al. Efficacy and tolerability of diclofenac potassium sachets in migraine: a randomized, double-blind, cross-over study in comparison with diclofenac potassium tablets and placebo. Cephalalgia. 2006;26(5):537-547.
9. Schürks M. Dihydroergotamine: role in the treatment of migraine. Expert Opin Drug Metab Toxicol. 2009;5(9):1141-1148.
10. Loder E. Safety of sumatriptan in pregnancy: a review of the data so far. CNS Drugs. 2003;17(1):1-7.
11. Shuhaiber S, Pastuszak A, Schick B, et al. Pregnancy outcome following first trimester exposure to sumatriptan. Neurology. 1998;51(2):581-583.
12. Cunnington M, Ephross S, Churchill P. The safety of sumatriptan and naratriptan in pregnancy: what have we learned? Headache. 2009;49(10):1414-1422.
13. Ressel G. AAP updates statement for transfer of drugs and other chemicals into breast milk. American Academy of Pediatrics. Am Fam Physician. 2002;65(5):979-980.
14. Ashkenazi A, Silberstein S. Menstrual migraine: a review of hormonal causes, prophylaxis and treatment. Expert Opin Pharmacother. 2007;8(11):1605-1613.
15. Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157-1168.
16. Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705-709.
17. Newman LC, Lipton RB, Lay CL, Solomon S. A pilot study of oral sumatriptan as intermittent prophylaxis of menstruation-related migraine. Neurology. 1998;51(1):307-309.
18. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled study. Headache. 2001;41(3):248-256.
19. Silberstein SD, Elkind AH, Schreiber C, Keywood C. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63(2):261-269.
20. Facchinetti F, Sances G, Borella P, Genazzani AR, Nappi G. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31(5):298-301.
21. Calhoun A. Women’s Issues in Headache. In: Loder E, ed. Headache. Philadelphia: American College of Physicians; 2004:157-177.
22. Calhoun A, Ford S. Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache. 2008;48(8):1186-1193.
23. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348:498-505.
24. Chan WS, Ray J, Wai EK, et al. Risk of stroke in women exposed to low-dose oral contraceptives: a critical evaluation of the evidence. Arch Intern Med. 2004;164(7):741-747.
25. Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63.-
26. Oral contraceptives and stroke in young women. Associated risk factors. JAMA. 1975;231:718-722.
27. Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8-15.
28. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29(11):2277-2284.
29. Sulak PJ, Scow RD, Preece C, Riggs MW, Kuehl TJ. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol. 2000;95(2):261-266.
30. Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J. 2004;97(9):819-822.
31. Calhoun AH, Hutchinson S. Hormonal therapies for menstrual migraine. Curr Pain Headache Rep. 2009;13(5):381-385.
32. Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70(17):1555-1563.
33. Pradalier A VD, Beaulieu PH, et al. Correlation between oestradiol plasma level and therapeutic effect on menstrual migraine. In: FC Rose, ed. New advances in headache research. London: Smith-Gordon; 1994:129-132.
34. de Lignières B, Vincens M, Mauvais-Jarvis P, Mas JL, Touboul PJ, Bousser MG. Prevention of menstrual migraine by percutaneous oestradiol. Br Med J (Clin Res Ed). 1986;293(6561):1540.-
35. Dennerstein L, Morse C, Burrows G, Oats J, Brown J, Smith M. Menstrual migraine: a double-blind trial of percutaneous estradiol. Gynecol Endocrinol. 1988;2(2):113-120.
36. MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Prevention of menstrual attacks of migraine: a double-blind placebo-controlled crossover study. Neurology. 2006;67(12):2159-2163.
37. Murray SC, Muse KN. Effective treatment of severe menstrual migraine headaches with gonadotropin-releasing hormone agonist and “add-back” therapy. Fertil Steril. 1997;67(2):390-393.
38. Martin V, Wernke S, Mandell K, et al. Medical oophorectomy with and without estrogen add-back therapy in the prevention of migraine headache. Headache. 2003;43(4):309-321.
39. Lichten EM, Lichten JB, Whitty A, Pieper D. The confirmation of a biochemical marker for women’s hormonal migraine: the depo-estradiol challenge test. Headache. 1996;36(6):367-371.
40. Calhoun AH, Ford S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache. 2007;47(8):1178-1183.
41. Stein GS. Headaches in the first postpartum week and their relationship to migraine. Headache. 1981;21(5):201-205.
42. Sances G, Granella F, Nappi RE, et al. Course of migraine during pregnancy and postpartum: a prospective study. Cephalalgia. 2003;23(3):197-205.
43. Goldszmidt E, Kern R, Chaput A, Macarthur A. The incidence and etiology of postpartum headaches: a prospective cohort study. Can J Anaesth. 2005;52(9):971-977.
44. Contag SA, Mertz HL, Bushnell CD. Migraine during pregnancy: Is it more than a headache? Nat Rev Neurol. 2009;5(8):449-456.