User login
Although no guidelines to date recommend their widespread use in clinical practice, we believe they will eventually be accepted. For example, markers of bone resorption are excellent indices of disease activity in patients with osteoporosis due to menopause, immobilization, or autoimmune processes, as well as Paget disease of bone or bone metastases. Normalization of the test results can be used to help establish the efficacy of treatment.
Similarly, markers of bone formation are excellent indices of disease activity in Paget disease, osteomalacia and rickets, osteoblastic bone metastases, and to a lesser extent in renal osteodystrophy. Again, successful treatment is associated with normalization of the tests.
This review summarizes some aspects of bone physiology and the pathogenesis of various metabolic bone disorders as a guide for clinicians considering using biochemical markers of osteoblast and osteoclast activity in patient management.
MARKERS OF BONE FORMATION
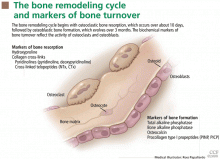
Osteoblasts are mononuclear cells that attach to bone surfaces and form new bone, most commonly at sites that recently underwent resorption. They produce type I collagen and other matrix components of osteoid, and they also mineralize the osteoid with hydroxyapatite.
Growing children have many more osteoblasts than adults.5 In elderly women, osteoblasts may increase in number in response to the increase in bone resorption brought on by estrogen deficiency. In elderly men, osteoblast activity may decrease,6 possibly because of decreasing levels of serum insulin-like growth factor 1 and testosterone.7
Markers of bone formation are measured in serum. Some are enzymes or other proteins secreted by osteoblasts, others are byproducts of type I collagen deposition.
Total alkaline phosphatase
Alkaline phosphatase, introduced into clinical use in 1929, was the first biochemical marker of bone turnover and is still the one most widely used in clinical practice. This enzyme is found in the plasma membrane of osteoblasts and in cells of the liver, kidney, intestine, spleen, and placenta. Its function is still not precisely known, but it is thought to play a role in osteoid formation and mineralization.
Bone alkaline phosphatase
In normal adults, about half the alkaline phosphatase in the serum comes from bone.1 Because alkaline phosphatase from different types of cells differs in its carbohydrate content, workers have been able to develop relatively specific immunoassays for alkaline phosphatase from bone, although there still is cross-reactivity of up to 20% between the bone and liver enzymes.2
Osteocalcin
Osteocalcin is a large peptide that is synthesized by osteoblasts, odontoblasts, and some chondrocytes. It binds to hydroxyapatite, and much of it is deposited in the bone matrix. Because osteocalcin fragments are released from the bone matrix during resorption, assays for circulating osteocalcin and its fragments reflect both bone formation and resorption.8 The exact function of osteocalcin in bone is still unclear, but recent studies raise the surprising possibility that it is a hormone that influences energy metabolism by modulating the production and action of insulin.9
Procollagen type I propeptides
Procollagen type I propeptides are cleaved from the ends of the procollagen molecule and can be detected in the circulation.1 Those from the amino-terminal end are called PINPs; those from the carboxy-terminal end are called PICPs. Although these propeptides are also synthesized in the skin, tendons, ligaments, cornea, blood vessels, fibrocartilage, and many other tissues, their main source is bone. The level of each of the propeptides in blood is thought to reflect the amount of newly synthesized collagen.
MARKERS OF BONE RESORPTION
Osteoclasts are multinucleated cells that resorb bone. They initiate bone remodeling and help shape growing bone and so are more numerous in children. They also liberate skeletal calcium to maintain a normal serum calcium concentration.5 Postmenopausal women who are estrogen-deficient tend to produce more osteoclasts, which accounts for the bone loss that can occur after menopause.
Markers of bone resorption are measured in serum or urine. The most direct indicators are fragments of bone collagen produced by osteoclast activity.1
Hydroxyproline
Hydroxyproline is an amino acid common to and characteristic of all forms of collagen, and urinary hydroxyproline excretion is the oldest test of bone resorption. However, this test lacks specificity for bone resorption because excreted hydroxyproline also comes from other tissues, particularly from skin collagen (which can turn over rapidly in certain disorders), from newly synthesized collagen that is not incorporated into tissue, and from dietary collagen and gelatin. Because it is less specific than newer tests, it is no longer widely used.
Collagen cross-links
Urinary pyridinoline and deoxypyridinoline are more specific markers of bone resorption.1
Pyridinolines are cross-linking amino acids that strengthen collagen fibrils in the extra-cellular matrix. They are found in the main fibril-forming collagens (types I, II, and III) of many tissues. Pyridinoline is the major chemical form, but deoxypyridinoline is also unusually abundant in bone collagen and hence is a relatively selective bone marker.
NTx. Since pyridinolines are not metabolized and are largely excreted as small peptides when produced by osteoclastic bone resorption, immunoassays have been developed that selectively measure cross-link-containing peptide fragments in urine and serum. The first was an assay that recognizes an N-telopeptide of collagen type I (NTx) in urine10 and serum.11 The recognized feature in this sequence is fully generated during the process of osteoclastic proteolysis and so requires no further metabolism by the liver or kidney for its production. Results from second-morning urine collections correlate well with those from 24-hour collections, which simplifies patient evaluation.
CTx. Several other assays target structural variants of a peptide sequence that originates from the carboxy-terminal cross-linking region of collagen type I (CTx).12,13
Other markers of bone resorption
Two enzymes found in osteoclasts have received attention as markers of osteoclast activity.
Serum tartrate-resistant acid phosphatase (TRAP) 5b has not been studied extensively in patients but appears to correlate with other markers of bone resorption.14
Serum cathepsin K is of interest because it is the primary proteolytic enzyme used by osteoclasts to degrade bone type I collagen during resorption. Several studies suggest it may be valuable as a marker of bone resorption,15 but more studies are required to evaluate its performance relative to established bone resorption markers.
Receptor activator of nuclear factor kappa (RANK), RANK ligand, and its decoy receptor osteoprotegerin are the pivotal regulators of osteoclast recruitment and activity.16 They may eventually be used as markers of bone metabolism, though the broad role of RANK ligand signaling in the immune system may limit its specificity.
FACTORS THAT INFLUENCE ASSAY RESULTS
To avoid being misled, clinicians who use biochemical markers of bone turnover should be familiar with factors that influence assay results.3
Diurnal and day-to-day variability
The most important biologic factors probably are diurnal and day-to-day variability in bone-forming and bone-resorbing activities. Levels of bone turnover markers are highest in the early morning and lowest in the afternoon and evening.
Levels of urinary markers can vary 20% to 30% from the highest to lowest value of the day. Serum markers change to a smaller degree except for serum CTx, which can vary by more than 60% during the day.17
In general, the day-to-day variability of urinary markers of bone resorption is similar in range to their diurnal variability. The serum markers of bone formation appear to vary less from day to day.
Eating, calcium intake
Blood for measurement of serum CTx should be taken in the morning after overnight fasting to avoid the large decrease that occurs after eating. An increase in calcium intake also can lower the levels of bone resorption markers, particularly in people whose calcium intake was previously low.18 Presumably, this effect is mediated by inhibition of parathyroid hormone secretion.
Sample handling
Improper collection and handling of specimens can seriously affect assay precision. The optimal time to collect samples is in the morning. Careful sample collection and storage are particularly important in measuring serum osteocalcin and TRAP. It is also important to use the same laboratory for serial measurements, since assay results can vary considerably among laboratories, even if they use identical methods.
BONE TURNOVER THROUGHOUT LIFE
In children, bone turnover can be more than 10 times greater than in adults because of three physiologic processes interacting in the skeleton: bone modeling, remodeling, and growth. Levels of bone formation and resorption markers therefore are much higher in children than in adults.19 Unfortunately, no studies have compared all the available markers in the same pediatric reference population.
In puberty, bone growth accelerates, with an increase in bone turnover markers that reflects the effect of hormones that induce the growth spurt.1
Postmenopausal women who do not use hormone replacement therapy have higher levels of bone resorption and formation markers than premenopausal women.20 Levels in postmenopausal women on hormone replacement are no different than in premenopausal women.20,21 In postmenopausal women not on estrogen, urinary levels of NTx have been reported to discriminate between normal bone mineral density (lowest NTx levels), osteopenia, and osteoporosis (highest levels).22 Normal levels of NTx are found in a small percentage of women. This may be explained by the variable levels of serum estradiol in post-menopausal women.23
Elderly men, in contrast, have variable findings.24–28 However, accelerated bone turnover has been noted in men with full-blown hypogonadism caused by androgen suppression therapy.29
CLINICAL APPLICATIONS OF BONE TURNOVER MARKERS
In postmenopausal osteoporosis
Markers of bone formation are somewhat less likely to be elevated than markers of bone resorption, and if they are elevated, they decrease as expected in response to therapy that inhibits bone resorption, though more gradually and to a lesser extent than the resorption markers.31–35
To monitor bisphosphonate therapy. Antiresorptive drugs such as bisphosphonates reduce the risk of fracture, as they increase bone density and decrease the rate of bone resorption, as shown in many clinical trials.31–35 Because the rate of bone resorption reaches a nadir within 3 to 6 months of starting bisphosphonate therapy and because the increase in bone density after 1 year is quite modest (about 3%–4%), most of the decrease in vertebral fracture incidence, which becomes apparent during the first year of treatment, probably can be attributed more to normalization of bone resorption (and a less perforated structure) than to the increase in bone density.36 This would suggest that it is more appropriate to document that bone resorption has been inhibited than to measure bone density every year when following patients taking antiresorptive agents.
Furthermore, effective antiresorptive therapy reduces the levels of resorption markers by 50% to 70%,32–35 whereas after 1 year bone density has generally not increased more than the error of the bone density measurement. This observation has led to the suggestion that bone density measurements generally should not be done more often than every 2 years when following the effects of antiresorptive therapy. Even with a 20% to 30% day-to-day variation in levels of bone resorption markers, it is easier to document the efficacy of therapy with resorption biomarkers than with bone density.
To document compliance. Another reason to consider measuring a resorption marker (after 3 months of therapy) is to document compliance, a considerable problem in the treatment of an asymptomatic disorder.
To help decide when to restart bisphosphonate therapy. After long-term treatment with a bisphosphonate, the drug may be retained in the skeleton for years. This seems particularly true of alendronate (Fosamax).37 After 5 years of continuous alendronate treatment, bone resorption continues to be suppressed near the maximal level, in some patients for years after they stop taking the drug.38
Once the bone resorption marker begins to approach the pretreatment level, it would signal a possible need to restart the therapy. If a pretreatment level was not measured, an estimate of significant bone resorption would be signaled when the resorption marker is more than 20% above the mean premenopausal level. For urinary NTx this would be more than 42 nmol bone collagen equivalents/mmol creatinine.
In glucocorticoid-induced osteoporosis
Glucocorticoid therapy causes bone loss and an increased incidence of fractures when given in high doses or for prolonged periods by the oral, parenteral, or inhaled routes.41
The pathogenesis of the bone loss has been explored by measurements of bone turnover markers. During glucocorticoid therapy, levels of bone formation markers are generally low and those of bone resorption markers are either normal or low.42–44 Presumably, the reduction in bone resorption is not enough to overcome the reduction in bone formation, and bone loss ensues. In children, the effects on bone formation are particularly profound, as linear growth may be retarded.44
Giving a bisphosphonate during glucocorticoid therapy is quite effective in increasing bone density and preventing fractures.45–47 Patients who receive alendronate have lower levels of bone formation and resorption markers than do untreated subjects.45 Presumably, bone resorption is inhibited more than bone formation, accounting for the skeletal benefits.
In a recent study in patients with glucocorticoid-induced osteoporosis, bone mineral density of the lumbar spine increased more than twice as much with teriparatide than with alendronate over an 18-month period.48 As would be expected from the results of teriparatide therapy in postmenopausal osteoporosis, indices of both bone formation and resorption rose to a peak at 6 months, with formation greater than resorption.
In immobilization-induced osteoporosis
Studies of normal volunteers placed on bed rest indicate that urinary CTx and NTx excretion increase significantly after 24 hours, no doubt reflecting a rapid increase in osteoclast activity.49 In a 16-week study of bed rest in volunteers, markers of bone formation were reduced and markers of bone resorption increased, demonstrating the mechanisms for the profound and rapid loss of bone in immobilized patients.50
In a long-term cross-sectional study of paraplegic men with spinal cord injuries, bone turnover patterns changed over time.51 During the first year after injury, urinary deoxypyridinoline excretion was markedly elevated, whereas blood total alkaline phosphatase and osteocalcin levels were normal to slightly elevated. Over a 30-year period after injury, the bone resorption marker returned to normal levels in most patients and the bone formation markers were normal. Fracture incidence rose but leveled off after 20 years.
Bisphosphonate therapy in spinal cord injury patients reduces urinary NTx and prevents bone loss.52,53 These agents have also proven effective in reversing hypercalcemia in immobilized patients.54
In inflammatory bowel disease
Patients with inflammatory bowel disease, especially Crohn disease, have low bone mass and are at risk of fractures.55 These complications could be due to glucocorticoid therapy, hypogonadism, vitamin D defeciency, weight loss, and high circulating levels of bone-active cytokines released by inflammatory cells residing in the diseased intestine.
Bone formation markers have not been found to be outside the normal range, although both interleukin 1 and tumor necrosis factor alpha are known to inhibit bone formation.
Bisphosphonate treatment produces an increase in bone density concomitant with decreases in markers of bone resorption and formation.57,58 Of considerable interest is the observation that infliximab (anti-tumor necrosis factor alpha; Remicade) generally produces a rise in bone formation markers, with a smaller and inconsistent effect on bone resorption.59,60
In rheumatoid arthritis
The incidence of osteoporosis and fractures is also increased In patients with rheumatoid arthritis.61 As in patients with inflammatory bowel disease, a variety of factors can contribute to bone loss, including glucocorticoid therapy, hypogonadism, vitamin D deficiency, immobility, and elevated levels of bone-active cytokines.
Generally, studies have reported increased bone resorption based on type I collagen markers,62,63 whereas patients with osteoarthritis have levels of these bone resorption markers no different from those of control subjects.62 Although serum total TRAP protein is elevated in rheumatoid arthritis patients, this is probably due to the 5a isoform, the origin of which may be macrophages and dendritric cells.64
The influence of abnormalities in bone formation on bone loss is less clear. Levels of bone formation markers have been reported to be normal,65 elevated,66 or reduced.67
Treatment of rheumatoid arthritis with high-dose glucocorticoid pulse therapy is effective in controlling the symptoms and some manifestations of the immune system in patients with the disorder. The latter effect would be expected to have a beneficial effect on bone metabolism. This appears to be the case, as there are only transient decreases in bone formation markers and no significant reduction in bone density.68 Similarly, there is only a transient decrease in serum osteocalcin after an intra-articular injection of a glucocorticoid, and no effect on urinary pyridino-line.69
As would be expected, bisphosphonate therapy prevents bone loss in rheumatoid arthritis patients treated with glucocorticoids.70,71 Both oral and intravenous therapy decrease the levels of bone turnover markers.70–72 Infliximab therapy was shown to reduce the levels of bone resorption markers but not of PINP (a bone formation marker).73
In primary hyperparathyroidism
Hypersecretion of parathyroid hormone increases osteoclastic activity, with a secondary increase in osteoblastic activity. Bone loss may ensue and an increase in fracture incidence may be a consequence, particularly in post-menopausal women, who have the highest incidence of the disorder.74
Before screening chemistry panels became widely used during routine medical evaluations, it was not unusual to find elevated serum total alkaline phosphatase levels in patients discovered to have primary hyperparathyroidism. Today, this finding is not so common, as the disorder is diagnosed at a much earlier stage. Nevertheless, more specific and sensitive markers of bone turnover have made it possible to demonstrate the metabolic abnormalities that reflect the skeletal pathology in patients with primary hyperparathyroidism and its response to various therapies.75,76
On average, patients with untreated primary hyperparathyroidism have high levels of markers of bone resorption and formation, except in the mildest cases.73,74 Bone turnover returns to normal within 6 months to a year after successful parathyroidectomy.77,78 This response correlates with improvement in bone density, primarily in the lumbar spine.77,78
In patients who do not undergo surgery, alternative means of preventing bone loss include estrogen replacement in estrogendeficient postmenopausal women,76 bisphosphonates,79,80 and cinacalcet (Sensipar).81 Estrogen,76 raloxifene (Evista),82 and alendronate79,80 all reduce levels of bone resorption and formation markers, and estrogen76 and alendronate79,80 increase bone density. Although cinacalcet usually restores the serum calcium to the normal range and prevents bone loss, it only reduces serum parathyroid hormone levels by about 20%, and both bone resorption and formation markers increase above baseline.81 This could be related to fluctuations in serum parathyroid hormone that occur during each day of therapy.
In osteomalacia and rickets
Osteomalacia and rickets of any cause are characterized by increased osteoblastic activity. If the underlying cause is vitamin D deficiency, genetic or acquired defects in calcitriol synthesis, or vitamin D resistance, then hyper-parathyroidism with increased bone resorption is a secondary feature.
Serum total alkaline phosphatase activity has been a useful marker of disease activity for many years, although the newer markers, except for serum osteocalcin,83 are potentially more sensitive. The insensitivity of osteocalcin as an index of osteoblastic activity is unexplained but could be related to the state of differentiation of the osteoblasts. Bone resorption markers are elevated in vitamin D deficiency84 but are not widely used in clinical practice, as serum parathyroid hormone is an excellent indirect means of assessing the presence of increased bone resorption and the response to therapy.
In renal osteodystrophy
Bone disease associated with renal failure is termed renal osteodystrophy and is quite heterogeneous.85 Microscopic examination of a bone biopsy specimen is still considered the gold standard for diagnosis, and measurement in serum of intact parathyroid hormone is an important guide to diagnosis and response to therapy.
Nevertheless, recent studies suggest that serum markers of bone formation and resorption may be of additional help in assessing bone turnover.86 At present it is not certain whether any of the newer markers are superior to serum total alkaline phosphatase activity. Future studies that correlate bone histology with bone turnover markers should clarify the value of the various markers.
In cancer
Bone metastases are a common complication in cancer patients. They are classified as osteolytic, osteoblastic, or mixed on the basis of radiographic features. Biochemical markers of bone turnover have proven useful in assessing the magnitude of the metastases, the response to therapy, and even the prognosis for survival.87
Osteolytic metastases, which are common in breast cancer, are associated with increases in bone resorption markers, and after treatment with intravenous bisphosphonates the levels can decrease nearly 70%.88,89
Patients with higher levels of urinary NTx had a higher risk of skeletal complications and disease progression than patients with low levels across multiple tumor groups, including multiple myeloma.87
In osteoblastic metastases. Prostate cancer patients, who typically have predominantly osteoblastic lesions, have elevations of serum total alkaline phosphatase activity and other markers of bone formation.90 In addition, they have elevated bone resorption markers. Urinary NTx decreased markedly but serum bone-specific alkaline phosphatase decreased only slightly after treatment with intravenous zoledronic acid (Zometa),91 whereas androgen ablation therapy has inconsistent effects on bone turnover.92,93 High levels of these markers again predict poor prognosis.93,94
In hormone-suppression therapy. Two of the most successful cancer therapies, aromatase inhibitors for breast cancer95 and androgen ablation for prostate cancer,96 accelerate bone loss through marked suppression of gonadal steroids. Bone resorption and formation markers increase and bone loss ensues, with resorption exceeding formation. Estrogen suppression appears mainly responsible in both sexes, since raloxifene prevents bone loss in prostate cancer patients.97
Bisphosphonates are highly effective in preventing bone loss in either sex.98–100 A single infusion of zoledronic acid in androgen-ablated prostate cancer patients can prevent bone loss for at least 1 year.100
In Paget disease of bone
Paget disease of bone evolves over many years, from an early osteolytic phase to dominance of secondary osteoblastic activity. In patients with extensive polyostotic disease, bone resorption and formation marker levels may be higher than in almost any other skeletal disorder. An exception is serum osteocalcin,101 which once again usually does not accurately reflect the rate of bone formation.
Bisphosphonates, given orally or intravenously, produce an early decrease in bone resorption followed by a fall in bone formation.102 In clinical practice it appears adequate to use the least expensive test, serum total alkaline phosphatase activity, to assess disease activity and the response to therapy.103
Acknowledgments: Grant support to FRS from the Edythe and Eli Broad Foundation and Lois Rosen. Grant support to DRE from the National Institutes of Health (NIAMS: AR37318, AR36794).
- Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996; 17:333–368.
- Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005; 26:97–122.
- Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005; 2:504–517.
- Seibel MJ. Biochemical markers of bone turnover: part II: clinical applications in the management of osteoporosis. Clin Biochem Rev. 2006; 27:123–138.
- Glorieux FH, Travers R, Taylor A, et al. Normative data for iliac bone histomorphometry in growing children. Bone. 2000; 26:103–109.
- Clarke BL, Ebeling PR, Jones JD, et al. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996; 81:2264–2270.
- Rucker D, Ezzat S, Diamandi A, Khosravi J, Hanley DA. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf). 2004; 60:491–499.
- Cloos PA, Christgau S. Characterization of aged osteocalcin fragments derived from bone resorption. Clin Lab. 2004; 50:585–598.
- Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130:456–469.
- Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992; 7:1251–1258.
- Clemens JD, Herrick MV, Singer FR, Eyre DR. Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem. 1997; 43:2058–2063.
- Garnero P, Gineyts E, Riou JP, Delmas PD. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab. 1994; 79:780–785.
- Christgau S, Rosenquist C, Alexandersen P, et al. Clinical evaluation of the Serum CrossLaps One Step, ELISA a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem. 1998; 44:2290–2300.
- Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell R, Blumsohn A. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004; 34:187–194.
- Meier C, Meinhardt U, Greenfield JR, et al. Serum cathepsin K concentrations reflect osteoclastic activity in women with postmenopausal osteoporosis and patients with Paget’s disease. Clin Lab. 2006; 52:1–10.
- Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 2007; 9(suppl 1):S1.
- Wichers M, Schmidt E, Bidlingmaier F, Klingmuller D. Diurnal rhythm of CrossLaps in human serum. Clin Chem. 1999; 45:1858–1860.
- Kenny AM, Prestwood KM, Biskup B, et al. Comparison of the effects of calcium loading with calcium citrate or calcium carbonate on bone turnover in postmenopausal women. Osteoporos Int. 2004; 15:290–294.
- Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex-and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007; 92:443–449.
- Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996; 81:3366–3371.
- Prestwood KM, Pilbeam CC, Burleson JA, et al. The short-term effects of conjugated estrogen on bone turnover in older women. J Clin Endocrinol Metab. 1994; 79:366–371.
- Schneider DL, Barrett-Connor EL. Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med. 1997; 157:1241–1245.
- Huang AJ, Ettinger B, Vittinghoff E, Ensrud KE, Johnson KC, Cummings SR. Endogenous estrogen levels and the effects of ultra low-dose transdermal estradiol therapy on bone turnover and bone density in postmenopausal women. J Bone Miner Res. 2007; 22:1791–1797.
- Khosla S, Melton LJ, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998; 83:2266–2274.
- Goemaere S, Van Pottelbergh I, Zmierczak H, et al. Inverse association between bone turnover rate and bone mineral density in community-dwelling men >70 years of age: no major role of sex steroid status. Bone. 2001; 29:286–291.
- Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD. Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res. 2001; 16:1642–1650.
- Scopacasa F, Wishart JM, Need AG, Horowitz M, Morris HA, Nordin BE. Bone density and bone-related biochemical variables in normal men: a longitudinal study. J Gerontol A Biol Sci Med Sci 2002; 57:M385–M391.
- Nguyen TV, Meier C, Center JR, Eisman JA, Seibel MJ. Bone turnover in elderly men: relationships to change in bone mineral density. BMC Musculoskelet Disord 2007; 8:13.
- Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001; 345:948–955.
- Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–1538.
- Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995; 333:1437–1443.
- Bauer DC, Black DM, Garnero P, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004; 19:1250–1258.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999; 282:1344–1352.
- Delmas PD, Recker RR, Chesnut CH, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004; 15:792–798.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356:1809–1822.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003; 18:1051–1056.
- Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004; 20:1291–1300.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006; 296:2927–2938.
- Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2000; 11:434–442.
- Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005; 20:962–970.
- Shaker JL, Lukert BP. Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am. 2005; 34:341–356.
- Ebeling PR, Erbas B, Hopper JL, Wark JD, Rubinfeld AR. Bone mineral density and bone turnover in asthmatics treated with long-term inhaled or oral glucocorticoids. J Bone Miner Res. 1998; 13:1283–1289.
- Siomou E, Challa A, Tzoufi M, Papadopoulou ZL, Lapatsanis PD, Siamopoulou A. Biochemical markers of bone metabolism in infants and children under intravenous corticosteroid therapy. Calcif Tissue Int. 2003; 73:319–325.
- Ahmed SF, Tucker P, Mushtaq T, Wallace AM, Williams DM, Hughes IA. Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin Endocrinol (Oxf). 2002; 57:185–191.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001; 44:202–211.
- Reid DM, Adami S, Devogelaer JP, Chines AA. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int. 2001; 69:242–247.
- Ringe JD, Dorst A, Faber H, Ibach K, Sorenson F. Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative study. Osteoporos Int. 2003; 14:801–807.
- Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007; 357:2028–2039.
- Heer M, Baecker N, Mika C, Boese A, Gerzer R. Immobilization induces a very rapid increase in osteoclast activity. Acta Astronaut. 2005; 57:31–36.
- Scheld K, Zittermann A, Heer M, et al. Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin Chem. 2001; 47:1688–1695.
- Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004; 15:180–189.
- Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999; 80:243–251.
- Gilchrist NL, Frampton CM, Acland RH, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007; 92:1385–1390.
- Massagli TL, Cardenas DD. Immobilization hypercalcemia treatment with pamidronate disodium after spinal cord injury. Arch Phys Med Rehabil. 1999; 80:998–1000.
- van Staa TP, Cooper C, Brusse LS, Leufkens H, Javaid MK, Arden NK. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003; 125:1591–1597.
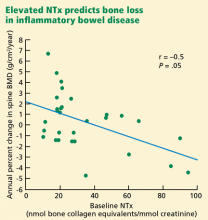
- Dresner-Pollak R, Karmeli F, Eliakim R, Ackerman Z, Rachmilewitz D. Increased urinary N-telopeptide cross-linked type 1 collagen predicts bone loss in patients with inflammatory bowel disease. Am J Gastroenterol. 2000; 95:699–704.
- Haderslev KV, Tjellesen L, Sorensen HA, Staun M. Alendronate increases lumbar spine bone mineral density in patients with Crohn’s disease. Gastroenterology. 2000; 119:639–646.
- Palomba S, Orio F, Manguso F, et al. Efficacy of risedronate administration in osteoporotic postmenopausal women affected by inflammatory bowel disease. Osteoporos Int. 2005; 16:1141–1149.
- Franchimont N, Putzeys V, Collette J, et al. Rapid improvement of bone metabolism after infliximab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2004; 20:607–614.
- Ryan BM, Russel MG, Schurgers L, et al. Effect of antitumour necrosis factor-alpha therapy on bone turnover in patients with active Crohn’s disease: a prospective study. Aliment Pharmacol Ther. 2004; 20:851–857.
- van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006; 54:3104–3112.
- Wong PK, Young L, Vaile JH, et al. Telopeptides as markers of bone turnover in rheumatoid arthritis and osteoarthritis. Intern Med J. 2004; 34:539–544.
- Momohara S, Okamoto H, Yago T, et al. The study of bone mineral density and bone turnover markers in postmenopausal women with active rheumatoid arthritis. Mod Rheumatol. 2005; 15:410–414.
- Janckila AJ, Neustadt DH, Nakasato YR, Halleen JM, Hentunen T, Yam LT. Serum tartrate-resistant acid phosphatase isoforms in rheumatoid arthritis. Clin Chim Acta. 2002; 320:49–58.
- Lems WF, Gerrits MI, Jacobs JW, van Vugt RM, van Rijn HJ, Bijlsma JW. Changes in (markers of) bone metabolism during high dose corticosteroid pulse treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 1996; 55:288–293.
- Manrique F, Gamardo J, de Elguezabal K, et al. Abnormalities of bone mineral density and bone metabolism in Venezuelan patients with rheumatoid arthritis. J Clin Rheumatol. 2003; 9:219–227.
- Garnero P, Jouvenne P, Buchs N, Delmas PD, Miossec P. Uncoupling of bone metabolism in rheumatoid arthritis patients with or without joint destruction: assessment with serum type I collagen breakdown products. Bone. 1999; 24:381–385.
- Frediani B, Falsetti P, Bisogno S, et al. Effects of high dose methyl-prednisolone pulse therapy on bone mass and biochemical markers of bone metabolism in patients with active rheumatoid arthritis: a 12-month randomized prospective controlled study. J Rheumatol. 2004; 31:1083–1087.
- Emkey RD, Lindsay R, Lyssy J, Weisberg JS, Dempster DW, Shen V. The systemic effect of intraarticular administration of corticosteroid on markers of bone formation and bone resorption in patients with rheumatoid arthritis. Arthritis Rheum. 1996; 39:277–282.
- Lange U, Illgner U, Teichmann J, Schleenbecker H. Skeletal benefit after one year of risedronate therapy in patients with rheumatoid arthritis and glucocorticoid-induced osteoporosis: a prospective study. Int J Clin Pharmacol Res. 2004; 24:33–38.
- Tascioglu F, Colak O, Armagan O, Alatas O, Oner C. The treatment of osteoporosis in patients with rheumatoid arthritis receiving glucocorticoids: a comparison of alendronate and intranasal salmon calcitonin. Rheumatol Int. 2005; 26:21–29.
- Cremers SC, Lodder MC, Den Hartigh J, et al. Short term whole body retention in relation to rate of bone resorption and cartilage degradation after intravenous bisphosphonate (pamidronate) in rheumatoid arthritis. J Rheumatol. 2004; 31:1732–1737.
- Chopin F, Garnero P, Le Henanff A, et al. Long term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis. 2007; 67:353–357.
- Khosla S, Melton LJ, Wermers RA, Crowson CS, O’Fallon W, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999; 14:1700–1707.
- Guo CY, Thomas WE, al-Dehaimi AW, Assiri AM, Eastell R. Longitudinal changes in bone mineral density and bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 1996; 81:3487–3491.
- Orr-Walker BJ, Evans MC, Clearwater JM, Horne A, Grey AB, Reid IR. Effects of hormone replacement therapy on bone mineral density in postmenopausal women with primary hyperparathyroidism: four-year follow-up and comparison with healthy postmenopausal women. Arch Intern Med. 2000; 160:2161–2166.
- Christiansen P, Steiniche T, Brixen K, et al. Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone. 1999; 25:237–244.
- Tamura Y, Araki A, Chiba Y, Mori S, Hosoi T, Horiuchi T. Remarkable increase in lumbar spine bone mineral density and amelioration in biochemical markers of bone turnover after parathyroidectomy in elderly patients with primary hyperparathyroidism: a 5-year follow-up study. J Bone Miner Metab. 2007; 25:226–231.
- Chow CC, Chan WB, Li JK, et al. Oral alendronate increases bone mineral density in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:581–587.
- Khan AA, Bilezikian JP, Kung AW, et al. Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004; 89:3319–3325.
- Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005; 90:135–141.
- Rubin MR, Lee KH, McMahon DJ, Silverberg SJ. Raloxifene lowers serum calcium and markers of bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:1174–1178.
- Daniels ED, Pettifor JM, Moodley GP. Serum osteocalcin has limited usefulness as a diagnostic marker for rickets. Eur J Pediatr. 2000; 159:730–733.
- Need AG. Bone resorption markers in vitamin D insufficiency. Clin Chim Acta. 2006; 368:48–52.
- Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004; 43:558–565.
- Malyszko J, Wolczynski S, Malyszko JS, Konstantynowicz J, Kaczmarski M, Mysliwiec M. Correlations of new markers of bone formation and resorption in kidney transplant recipients. Transplant Proc. 2003; 35:1351–1354.
- Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005; 23:4925–4935.
- Body JJ, Dumon JC, Gineyts E, Delmas PD. Comparative evaluation of markers of bone resorption in patients with breast cancer-induced osteolysis before and after bisphosphonate therapy. Br J Cancer. 1997; 75:408–412.
- Coleman RE Efficacy of zoledronic acid and pamidronate in breast cancer patients: a comparative analysis of randomized phase III trials. Am J Clin Oncol 2002; 25(suppl 1):S25–S31.
- Smith MR. Markers of bone metabolism in prostate cancer. Cancer Treat Rev 2006; 32(suppl 1):23–26.
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94:1458–1468.
- Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998; 83:1561–1566.
- Johansen JS, Brasso K, Iversen P, et al. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res. 2007; 13:3244–3249.
- Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006; 12:3361–3367.
- Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res. 2006; 21:1215–1223.
- Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res 2006; 12:6315s–6319s.
- Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004; 89:3841–3846.
- Confavreux CB, Fontana A, Guastalla JP, Munoz F, Brun J, Delmas PD. Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prevention with bisphosphonates. Bone. 2007; 41:346–352.
- Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007; 146:416–424.
- Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007; 25:1038–1042.
- Kaddam IM, Iqbal SJ, Holland S, Wong M, Manning D. Comparison of serum osteocalcin with total and bone specific alkaline phosphatase and urinary hydroxyproline:creatinine ratio in patients with Paget’s disease of bone. Ann Clin Biochem. 1994; 31:327–330.
- Reid IR, Miller P, Lyles K, et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med. 2005; 353:898–908.
- Reid IR, Davidson JS, Wattie D, et al. Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone. 2004; 35:224–230.
Although no guidelines to date recommend their widespread use in clinical practice, we believe they will eventually be accepted. For example, markers of bone resorption are excellent indices of disease activity in patients with osteoporosis due to menopause, immobilization, or autoimmune processes, as well as Paget disease of bone or bone metastases. Normalization of the test results can be used to help establish the efficacy of treatment.
Similarly, markers of bone formation are excellent indices of disease activity in Paget disease, osteomalacia and rickets, osteoblastic bone metastases, and to a lesser extent in renal osteodystrophy. Again, successful treatment is associated with normalization of the tests.
This review summarizes some aspects of bone physiology and the pathogenesis of various metabolic bone disorders as a guide for clinicians considering using biochemical markers of osteoblast and osteoclast activity in patient management.
MARKERS OF BONE FORMATION
Osteoblasts are mononuclear cells that attach to bone surfaces and form new bone, most commonly at sites that recently underwent resorption. They produce type I collagen and other matrix components of osteoid, and they also mineralize the osteoid with hydroxyapatite.
Growing children have many more osteoblasts than adults.5 In elderly women, osteoblasts may increase in number in response to the increase in bone resorption brought on by estrogen deficiency. In elderly men, osteoblast activity may decrease,6 possibly because of decreasing levels of serum insulin-like growth factor 1 and testosterone.7
Markers of bone formation are measured in serum. Some are enzymes or other proteins secreted by osteoblasts, others are byproducts of type I collagen deposition.
Total alkaline phosphatase
Alkaline phosphatase, introduced into clinical use in 1929, was the first biochemical marker of bone turnover and is still the one most widely used in clinical practice. This enzyme is found in the plasma membrane of osteoblasts and in cells of the liver, kidney, intestine, spleen, and placenta. Its function is still not precisely known, but it is thought to play a role in osteoid formation and mineralization.
Bone alkaline phosphatase
In normal adults, about half the alkaline phosphatase in the serum comes from bone.1 Because alkaline phosphatase from different types of cells differs in its carbohydrate content, workers have been able to develop relatively specific immunoassays for alkaline phosphatase from bone, although there still is cross-reactivity of up to 20% between the bone and liver enzymes.2
Osteocalcin
Osteocalcin is a large peptide that is synthesized by osteoblasts, odontoblasts, and some chondrocytes. It binds to hydroxyapatite, and much of it is deposited in the bone matrix. Because osteocalcin fragments are released from the bone matrix during resorption, assays for circulating osteocalcin and its fragments reflect both bone formation and resorption.8 The exact function of osteocalcin in bone is still unclear, but recent studies raise the surprising possibility that it is a hormone that influences energy metabolism by modulating the production and action of insulin.9
Procollagen type I propeptides
Procollagen type I propeptides are cleaved from the ends of the procollagen molecule and can be detected in the circulation.1 Those from the amino-terminal end are called PINPs; those from the carboxy-terminal end are called PICPs. Although these propeptides are also synthesized in the skin, tendons, ligaments, cornea, blood vessels, fibrocartilage, and many other tissues, their main source is bone. The level of each of the propeptides in blood is thought to reflect the amount of newly synthesized collagen.
MARKERS OF BONE RESORPTION
Osteoclasts are multinucleated cells that resorb bone. They initiate bone remodeling and help shape growing bone and so are more numerous in children. They also liberate skeletal calcium to maintain a normal serum calcium concentration.5 Postmenopausal women who are estrogen-deficient tend to produce more osteoclasts, which accounts for the bone loss that can occur after menopause.
Markers of bone resorption are measured in serum or urine. The most direct indicators are fragments of bone collagen produced by osteoclast activity.1
Hydroxyproline
Hydroxyproline is an amino acid common to and characteristic of all forms of collagen, and urinary hydroxyproline excretion is the oldest test of bone resorption. However, this test lacks specificity for bone resorption because excreted hydroxyproline also comes from other tissues, particularly from skin collagen (which can turn over rapidly in certain disorders), from newly synthesized collagen that is not incorporated into tissue, and from dietary collagen and gelatin. Because it is less specific than newer tests, it is no longer widely used.
Collagen cross-links
Urinary pyridinoline and deoxypyridinoline are more specific markers of bone resorption.1
Pyridinolines are cross-linking amino acids that strengthen collagen fibrils in the extra-cellular matrix. They are found in the main fibril-forming collagens (types I, II, and III) of many tissues. Pyridinoline is the major chemical form, but deoxypyridinoline is also unusually abundant in bone collagen and hence is a relatively selective bone marker.
NTx. Since pyridinolines are not metabolized and are largely excreted as small peptides when produced by osteoclastic bone resorption, immunoassays have been developed that selectively measure cross-link-containing peptide fragments in urine and serum. The first was an assay that recognizes an N-telopeptide of collagen type I (NTx) in urine10 and serum.11 The recognized feature in this sequence is fully generated during the process of osteoclastic proteolysis and so requires no further metabolism by the liver or kidney for its production. Results from second-morning urine collections correlate well with those from 24-hour collections, which simplifies patient evaluation.
CTx. Several other assays target structural variants of a peptide sequence that originates from the carboxy-terminal cross-linking region of collagen type I (CTx).12,13
Other markers of bone resorption
Two enzymes found in osteoclasts have received attention as markers of osteoclast activity.
Serum tartrate-resistant acid phosphatase (TRAP) 5b has not been studied extensively in patients but appears to correlate with other markers of bone resorption.14
Serum cathepsin K is of interest because it is the primary proteolytic enzyme used by osteoclasts to degrade bone type I collagen during resorption. Several studies suggest it may be valuable as a marker of bone resorption,15 but more studies are required to evaluate its performance relative to established bone resorption markers.
Receptor activator of nuclear factor kappa (RANK), RANK ligand, and its decoy receptor osteoprotegerin are the pivotal regulators of osteoclast recruitment and activity.16 They may eventually be used as markers of bone metabolism, though the broad role of RANK ligand signaling in the immune system may limit its specificity.
FACTORS THAT INFLUENCE ASSAY RESULTS
To avoid being misled, clinicians who use biochemical markers of bone turnover should be familiar with factors that influence assay results.3
Diurnal and day-to-day variability
The most important biologic factors probably are diurnal and day-to-day variability in bone-forming and bone-resorbing activities. Levels of bone turnover markers are highest in the early morning and lowest in the afternoon and evening.
Levels of urinary markers can vary 20% to 30% from the highest to lowest value of the day. Serum markers change to a smaller degree except for serum CTx, which can vary by more than 60% during the day.17
In general, the day-to-day variability of urinary markers of bone resorption is similar in range to their diurnal variability. The serum markers of bone formation appear to vary less from day to day.
Eating, calcium intake
Blood for measurement of serum CTx should be taken in the morning after overnight fasting to avoid the large decrease that occurs after eating. An increase in calcium intake also can lower the levels of bone resorption markers, particularly in people whose calcium intake was previously low.18 Presumably, this effect is mediated by inhibition of parathyroid hormone secretion.
Sample handling
Improper collection and handling of specimens can seriously affect assay precision. The optimal time to collect samples is in the morning. Careful sample collection and storage are particularly important in measuring serum osteocalcin and TRAP. It is also important to use the same laboratory for serial measurements, since assay results can vary considerably among laboratories, even if they use identical methods.
BONE TURNOVER THROUGHOUT LIFE
In children, bone turnover can be more than 10 times greater than in adults because of three physiologic processes interacting in the skeleton: bone modeling, remodeling, and growth. Levels of bone formation and resorption markers therefore are much higher in children than in adults.19 Unfortunately, no studies have compared all the available markers in the same pediatric reference population.
In puberty, bone growth accelerates, with an increase in bone turnover markers that reflects the effect of hormones that induce the growth spurt.1
Postmenopausal women who do not use hormone replacement therapy have higher levels of bone resorption and formation markers than premenopausal women.20 Levels in postmenopausal women on hormone replacement are no different than in premenopausal women.20,21 In postmenopausal women not on estrogen, urinary levels of NTx have been reported to discriminate between normal bone mineral density (lowest NTx levels), osteopenia, and osteoporosis (highest levels).22 Normal levels of NTx are found in a small percentage of women. This may be explained by the variable levels of serum estradiol in post-menopausal women.23
Elderly men, in contrast, have variable findings.24–28 However, accelerated bone turnover has been noted in men with full-blown hypogonadism caused by androgen suppression therapy.29
CLINICAL APPLICATIONS OF BONE TURNOVER MARKERS
In postmenopausal osteoporosis
Markers of bone formation are somewhat less likely to be elevated than markers of bone resorption, and if they are elevated, they decrease as expected in response to therapy that inhibits bone resorption, though more gradually and to a lesser extent than the resorption markers.31–35
To monitor bisphosphonate therapy. Antiresorptive drugs such as bisphosphonates reduce the risk of fracture, as they increase bone density and decrease the rate of bone resorption, as shown in many clinical trials.31–35 Because the rate of bone resorption reaches a nadir within 3 to 6 months of starting bisphosphonate therapy and because the increase in bone density after 1 year is quite modest (about 3%–4%), most of the decrease in vertebral fracture incidence, which becomes apparent during the first year of treatment, probably can be attributed more to normalization of bone resorption (and a less perforated structure) than to the increase in bone density.36 This would suggest that it is more appropriate to document that bone resorption has been inhibited than to measure bone density every year when following patients taking antiresorptive agents.
Furthermore, effective antiresorptive therapy reduces the levels of resorption markers by 50% to 70%,32–35 whereas after 1 year bone density has generally not increased more than the error of the bone density measurement. This observation has led to the suggestion that bone density measurements generally should not be done more often than every 2 years when following the effects of antiresorptive therapy. Even with a 20% to 30% day-to-day variation in levels of bone resorption markers, it is easier to document the efficacy of therapy with resorption biomarkers than with bone density.
To document compliance. Another reason to consider measuring a resorption marker (after 3 months of therapy) is to document compliance, a considerable problem in the treatment of an asymptomatic disorder.
To help decide when to restart bisphosphonate therapy. After long-term treatment with a bisphosphonate, the drug may be retained in the skeleton for years. This seems particularly true of alendronate (Fosamax).37 After 5 years of continuous alendronate treatment, bone resorption continues to be suppressed near the maximal level, in some patients for years after they stop taking the drug.38
Once the bone resorption marker begins to approach the pretreatment level, it would signal a possible need to restart the therapy. If a pretreatment level was not measured, an estimate of significant bone resorption would be signaled when the resorption marker is more than 20% above the mean premenopausal level. For urinary NTx this would be more than 42 nmol bone collagen equivalents/mmol creatinine.
In glucocorticoid-induced osteoporosis
Glucocorticoid therapy causes bone loss and an increased incidence of fractures when given in high doses or for prolonged periods by the oral, parenteral, or inhaled routes.41
The pathogenesis of the bone loss has been explored by measurements of bone turnover markers. During glucocorticoid therapy, levels of bone formation markers are generally low and those of bone resorption markers are either normal or low.42–44 Presumably, the reduction in bone resorption is not enough to overcome the reduction in bone formation, and bone loss ensues. In children, the effects on bone formation are particularly profound, as linear growth may be retarded.44
Giving a bisphosphonate during glucocorticoid therapy is quite effective in increasing bone density and preventing fractures.45–47 Patients who receive alendronate have lower levels of bone formation and resorption markers than do untreated subjects.45 Presumably, bone resorption is inhibited more than bone formation, accounting for the skeletal benefits.
In a recent study in patients with glucocorticoid-induced osteoporosis, bone mineral density of the lumbar spine increased more than twice as much with teriparatide than with alendronate over an 18-month period.48 As would be expected from the results of teriparatide therapy in postmenopausal osteoporosis, indices of both bone formation and resorption rose to a peak at 6 months, with formation greater than resorption.
In immobilization-induced osteoporosis
Studies of normal volunteers placed on bed rest indicate that urinary CTx and NTx excretion increase significantly after 24 hours, no doubt reflecting a rapid increase in osteoclast activity.49 In a 16-week study of bed rest in volunteers, markers of bone formation were reduced and markers of bone resorption increased, demonstrating the mechanisms for the profound and rapid loss of bone in immobilized patients.50
In a long-term cross-sectional study of paraplegic men with spinal cord injuries, bone turnover patterns changed over time.51 During the first year after injury, urinary deoxypyridinoline excretion was markedly elevated, whereas blood total alkaline phosphatase and osteocalcin levels were normal to slightly elevated. Over a 30-year period after injury, the bone resorption marker returned to normal levels in most patients and the bone formation markers were normal. Fracture incidence rose but leveled off after 20 years.
Bisphosphonate therapy in spinal cord injury patients reduces urinary NTx and prevents bone loss.52,53 These agents have also proven effective in reversing hypercalcemia in immobilized patients.54
In inflammatory bowel disease
Patients with inflammatory bowel disease, especially Crohn disease, have low bone mass and are at risk of fractures.55 These complications could be due to glucocorticoid therapy, hypogonadism, vitamin D defeciency, weight loss, and high circulating levels of bone-active cytokines released by inflammatory cells residing in the diseased intestine.
Bone formation markers have not been found to be outside the normal range, although both interleukin 1 and tumor necrosis factor alpha are known to inhibit bone formation.
Bisphosphonate treatment produces an increase in bone density concomitant with decreases in markers of bone resorption and formation.57,58 Of considerable interest is the observation that infliximab (anti-tumor necrosis factor alpha; Remicade) generally produces a rise in bone formation markers, with a smaller and inconsistent effect on bone resorption.59,60
In rheumatoid arthritis
The incidence of osteoporosis and fractures is also increased In patients with rheumatoid arthritis.61 As in patients with inflammatory bowel disease, a variety of factors can contribute to bone loss, including glucocorticoid therapy, hypogonadism, vitamin D deficiency, immobility, and elevated levels of bone-active cytokines.
Generally, studies have reported increased bone resorption based on type I collagen markers,62,63 whereas patients with osteoarthritis have levels of these bone resorption markers no different from those of control subjects.62 Although serum total TRAP protein is elevated in rheumatoid arthritis patients, this is probably due to the 5a isoform, the origin of which may be macrophages and dendritric cells.64
The influence of abnormalities in bone formation on bone loss is less clear. Levels of bone formation markers have been reported to be normal,65 elevated,66 or reduced.67
Treatment of rheumatoid arthritis with high-dose glucocorticoid pulse therapy is effective in controlling the symptoms and some manifestations of the immune system in patients with the disorder. The latter effect would be expected to have a beneficial effect on bone metabolism. This appears to be the case, as there are only transient decreases in bone formation markers and no significant reduction in bone density.68 Similarly, there is only a transient decrease in serum osteocalcin after an intra-articular injection of a glucocorticoid, and no effect on urinary pyridino-line.69
As would be expected, bisphosphonate therapy prevents bone loss in rheumatoid arthritis patients treated with glucocorticoids.70,71 Both oral and intravenous therapy decrease the levels of bone turnover markers.70–72 Infliximab therapy was shown to reduce the levels of bone resorption markers but not of PINP (a bone formation marker).73
In primary hyperparathyroidism
Hypersecretion of parathyroid hormone increases osteoclastic activity, with a secondary increase in osteoblastic activity. Bone loss may ensue and an increase in fracture incidence may be a consequence, particularly in post-menopausal women, who have the highest incidence of the disorder.74
Before screening chemistry panels became widely used during routine medical evaluations, it was not unusual to find elevated serum total alkaline phosphatase levels in patients discovered to have primary hyperparathyroidism. Today, this finding is not so common, as the disorder is diagnosed at a much earlier stage. Nevertheless, more specific and sensitive markers of bone turnover have made it possible to demonstrate the metabolic abnormalities that reflect the skeletal pathology in patients with primary hyperparathyroidism and its response to various therapies.75,76
On average, patients with untreated primary hyperparathyroidism have high levels of markers of bone resorption and formation, except in the mildest cases.73,74 Bone turnover returns to normal within 6 months to a year after successful parathyroidectomy.77,78 This response correlates with improvement in bone density, primarily in the lumbar spine.77,78
In patients who do not undergo surgery, alternative means of preventing bone loss include estrogen replacement in estrogendeficient postmenopausal women,76 bisphosphonates,79,80 and cinacalcet (Sensipar).81 Estrogen,76 raloxifene (Evista),82 and alendronate79,80 all reduce levels of bone resorption and formation markers, and estrogen76 and alendronate79,80 increase bone density. Although cinacalcet usually restores the serum calcium to the normal range and prevents bone loss, it only reduces serum parathyroid hormone levels by about 20%, and both bone resorption and formation markers increase above baseline.81 This could be related to fluctuations in serum parathyroid hormone that occur during each day of therapy.
In osteomalacia and rickets
Osteomalacia and rickets of any cause are characterized by increased osteoblastic activity. If the underlying cause is vitamin D deficiency, genetic or acquired defects in calcitriol synthesis, or vitamin D resistance, then hyper-parathyroidism with increased bone resorption is a secondary feature.
Serum total alkaline phosphatase activity has been a useful marker of disease activity for many years, although the newer markers, except for serum osteocalcin,83 are potentially more sensitive. The insensitivity of osteocalcin as an index of osteoblastic activity is unexplained but could be related to the state of differentiation of the osteoblasts. Bone resorption markers are elevated in vitamin D deficiency84 but are not widely used in clinical practice, as serum parathyroid hormone is an excellent indirect means of assessing the presence of increased bone resorption and the response to therapy.
In renal osteodystrophy
Bone disease associated with renal failure is termed renal osteodystrophy and is quite heterogeneous.85 Microscopic examination of a bone biopsy specimen is still considered the gold standard for diagnosis, and measurement in serum of intact parathyroid hormone is an important guide to diagnosis and response to therapy.
Nevertheless, recent studies suggest that serum markers of bone formation and resorption may be of additional help in assessing bone turnover.86 At present it is not certain whether any of the newer markers are superior to serum total alkaline phosphatase activity. Future studies that correlate bone histology with bone turnover markers should clarify the value of the various markers.
In cancer
Bone metastases are a common complication in cancer patients. They are classified as osteolytic, osteoblastic, or mixed on the basis of radiographic features. Biochemical markers of bone turnover have proven useful in assessing the magnitude of the metastases, the response to therapy, and even the prognosis for survival.87
Osteolytic metastases, which are common in breast cancer, are associated with increases in bone resorption markers, and after treatment with intravenous bisphosphonates the levels can decrease nearly 70%.88,89
Patients with higher levels of urinary NTx had a higher risk of skeletal complications and disease progression than patients with low levels across multiple tumor groups, including multiple myeloma.87
In osteoblastic metastases. Prostate cancer patients, who typically have predominantly osteoblastic lesions, have elevations of serum total alkaline phosphatase activity and other markers of bone formation.90 In addition, they have elevated bone resorption markers. Urinary NTx decreased markedly but serum bone-specific alkaline phosphatase decreased only slightly after treatment with intravenous zoledronic acid (Zometa),91 whereas androgen ablation therapy has inconsistent effects on bone turnover.92,93 High levels of these markers again predict poor prognosis.93,94
In hormone-suppression therapy. Two of the most successful cancer therapies, aromatase inhibitors for breast cancer95 and androgen ablation for prostate cancer,96 accelerate bone loss through marked suppression of gonadal steroids. Bone resorption and formation markers increase and bone loss ensues, with resorption exceeding formation. Estrogen suppression appears mainly responsible in both sexes, since raloxifene prevents bone loss in prostate cancer patients.97
Bisphosphonates are highly effective in preventing bone loss in either sex.98–100 A single infusion of zoledronic acid in androgen-ablated prostate cancer patients can prevent bone loss for at least 1 year.100
In Paget disease of bone
Paget disease of bone evolves over many years, from an early osteolytic phase to dominance of secondary osteoblastic activity. In patients with extensive polyostotic disease, bone resorption and formation marker levels may be higher than in almost any other skeletal disorder. An exception is serum osteocalcin,101 which once again usually does not accurately reflect the rate of bone formation.
Bisphosphonates, given orally or intravenously, produce an early decrease in bone resorption followed by a fall in bone formation.102 In clinical practice it appears adequate to use the least expensive test, serum total alkaline phosphatase activity, to assess disease activity and the response to therapy.103
Acknowledgments: Grant support to FRS from the Edythe and Eli Broad Foundation and Lois Rosen. Grant support to DRE from the National Institutes of Health (NIAMS: AR37318, AR36794).
Although no guidelines to date recommend their widespread use in clinical practice, we believe they will eventually be accepted. For example, markers of bone resorption are excellent indices of disease activity in patients with osteoporosis due to menopause, immobilization, or autoimmune processes, as well as Paget disease of bone or bone metastases. Normalization of the test results can be used to help establish the efficacy of treatment.
Similarly, markers of bone formation are excellent indices of disease activity in Paget disease, osteomalacia and rickets, osteoblastic bone metastases, and to a lesser extent in renal osteodystrophy. Again, successful treatment is associated with normalization of the tests.
This review summarizes some aspects of bone physiology and the pathogenesis of various metabolic bone disorders as a guide for clinicians considering using biochemical markers of osteoblast and osteoclast activity in patient management.
MARKERS OF BONE FORMATION
Osteoblasts are mononuclear cells that attach to bone surfaces and form new bone, most commonly at sites that recently underwent resorption. They produce type I collagen and other matrix components of osteoid, and they also mineralize the osteoid with hydroxyapatite.
Growing children have many more osteoblasts than adults.5 In elderly women, osteoblasts may increase in number in response to the increase in bone resorption brought on by estrogen deficiency. In elderly men, osteoblast activity may decrease,6 possibly because of decreasing levels of serum insulin-like growth factor 1 and testosterone.7
Markers of bone formation are measured in serum. Some are enzymes or other proteins secreted by osteoblasts, others are byproducts of type I collagen deposition.
Total alkaline phosphatase
Alkaline phosphatase, introduced into clinical use in 1929, was the first biochemical marker of bone turnover and is still the one most widely used in clinical practice. This enzyme is found in the plasma membrane of osteoblasts and in cells of the liver, kidney, intestine, spleen, and placenta. Its function is still not precisely known, but it is thought to play a role in osteoid formation and mineralization.
Bone alkaline phosphatase
In normal adults, about half the alkaline phosphatase in the serum comes from bone.1 Because alkaline phosphatase from different types of cells differs in its carbohydrate content, workers have been able to develop relatively specific immunoassays for alkaline phosphatase from bone, although there still is cross-reactivity of up to 20% between the bone and liver enzymes.2
Osteocalcin
Osteocalcin is a large peptide that is synthesized by osteoblasts, odontoblasts, and some chondrocytes. It binds to hydroxyapatite, and much of it is deposited in the bone matrix. Because osteocalcin fragments are released from the bone matrix during resorption, assays for circulating osteocalcin and its fragments reflect both bone formation and resorption.8 The exact function of osteocalcin in bone is still unclear, but recent studies raise the surprising possibility that it is a hormone that influences energy metabolism by modulating the production and action of insulin.9
Procollagen type I propeptides
Procollagen type I propeptides are cleaved from the ends of the procollagen molecule and can be detected in the circulation.1 Those from the amino-terminal end are called PINPs; those from the carboxy-terminal end are called PICPs. Although these propeptides are also synthesized in the skin, tendons, ligaments, cornea, blood vessels, fibrocartilage, and many other tissues, their main source is bone. The level of each of the propeptides in blood is thought to reflect the amount of newly synthesized collagen.
MARKERS OF BONE RESORPTION
Osteoclasts are multinucleated cells that resorb bone. They initiate bone remodeling and help shape growing bone and so are more numerous in children. They also liberate skeletal calcium to maintain a normal serum calcium concentration.5 Postmenopausal women who are estrogen-deficient tend to produce more osteoclasts, which accounts for the bone loss that can occur after menopause.
Markers of bone resorption are measured in serum or urine. The most direct indicators are fragments of bone collagen produced by osteoclast activity.1
Hydroxyproline
Hydroxyproline is an amino acid common to and characteristic of all forms of collagen, and urinary hydroxyproline excretion is the oldest test of bone resorption. However, this test lacks specificity for bone resorption because excreted hydroxyproline also comes from other tissues, particularly from skin collagen (which can turn over rapidly in certain disorders), from newly synthesized collagen that is not incorporated into tissue, and from dietary collagen and gelatin. Because it is less specific than newer tests, it is no longer widely used.
Collagen cross-links
Urinary pyridinoline and deoxypyridinoline are more specific markers of bone resorption.1
Pyridinolines are cross-linking amino acids that strengthen collagen fibrils in the extra-cellular matrix. They are found in the main fibril-forming collagens (types I, II, and III) of many tissues. Pyridinoline is the major chemical form, but deoxypyridinoline is also unusually abundant in bone collagen and hence is a relatively selective bone marker.
NTx. Since pyridinolines are not metabolized and are largely excreted as small peptides when produced by osteoclastic bone resorption, immunoassays have been developed that selectively measure cross-link-containing peptide fragments in urine and serum. The first was an assay that recognizes an N-telopeptide of collagen type I (NTx) in urine10 and serum.11 The recognized feature in this sequence is fully generated during the process of osteoclastic proteolysis and so requires no further metabolism by the liver or kidney for its production. Results from second-morning urine collections correlate well with those from 24-hour collections, which simplifies patient evaluation.
CTx. Several other assays target structural variants of a peptide sequence that originates from the carboxy-terminal cross-linking region of collagen type I (CTx).12,13
Other markers of bone resorption
Two enzymes found in osteoclasts have received attention as markers of osteoclast activity.
Serum tartrate-resistant acid phosphatase (TRAP) 5b has not been studied extensively in patients but appears to correlate with other markers of bone resorption.14
Serum cathepsin K is of interest because it is the primary proteolytic enzyme used by osteoclasts to degrade bone type I collagen during resorption. Several studies suggest it may be valuable as a marker of bone resorption,15 but more studies are required to evaluate its performance relative to established bone resorption markers.
Receptor activator of nuclear factor kappa (RANK), RANK ligand, and its decoy receptor osteoprotegerin are the pivotal regulators of osteoclast recruitment and activity.16 They may eventually be used as markers of bone metabolism, though the broad role of RANK ligand signaling in the immune system may limit its specificity.
FACTORS THAT INFLUENCE ASSAY RESULTS
To avoid being misled, clinicians who use biochemical markers of bone turnover should be familiar with factors that influence assay results.3
Diurnal and day-to-day variability
The most important biologic factors probably are diurnal and day-to-day variability in bone-forming and bone-resorbing activities. Levels of bone turnover markers are highest in the early morning and lowest in the afternoon and evening.
Levels of urinary markers can vary 20% to 30% from the highest to lowest value of the day. Serum markers change to a smaller degree except for serum CTx, which can vary by more than 60% during the day.17
In general, the day-to-day variability of urinary markers of bone resorption is similar in range to their diurnal variability. The serum markers of bone formation appear to vary less from day to day.
Eating, calcium intake
Blood for measurement of serum CTx should be taken in the morning after overnight fasting to avoid the large decrease that occurs after eating. An increase in calcium intake also can lower the levels of bone resorption markers, particularly in people whose calcium intake was previously low.18 Presumably, this effect is mediated by inhibition of parathyroid hormone secretion.
Sample handling
Improper collection and handling of specimens can seriously affect assay precision. The optimal time to collect samples is in the morning. Careful sample collection and storage are particularly important in measuring serum osteocalcin and TRAP. It is also important to use the same laboratory for serial measurements, since assay results can vary considerably among laboratories, even if they use identical methods.
BONE TURNOVER THROUGHOUT LIFE
In children, bone turnover can be more than 10 times greater than in adults because of three physiologic processes interacting in the skeleton: bone modeling, remodeling, and growth. Levels of bone formation and resorption markers therefore are much higher in children than in adults.19 Unfortunately, no studies have compared all the available markers in the same pediatric reference population.
In puberty, bone growth accelerates, with an increase in bone turnover markers that reflects the effect of hormones that induce the growth spurt.1
Postmenopausal women who do not use hormone replacement therapy have higher levels of bone resorption and formation markers than premenopausal women.20 Levels in postmenopausal women on hormone replacement are no different than in premenopausal women.20,21 In postmenopausal women not on estrogen, urinary levels of NTx have been reported to discriminate between normal bone mineral density (lowest NTx levels), osteopenia, and osteoporosis (highest levels).22 Normal levels of NTx are found in a small percentage of women. This may be explained by the variable levels of serum estradiol in post-menopausal women.23
Elderly men, in contrast, have variable findings.24–28 However, accelerated bone turnover has been noted in men with full-blown hypogonadism caused by androgen suppression therapy.29
CLINICAL APPLICATIONS OF BONE TURNOVER MARKERS
In postmenopausal osteoporosis
Markers of bone formation are somewhat less likely to be elevated than markers of bone resorption, and if they are elevated, they decrease as expected in response to therapy that inhibits bone resorption, though more gradually and to a lesser extent than the resorption markers.31–35
To monitor bisphosphonate therapy. Antiresorptive drugs such as bisphosphonates reduce the risk of fracture, as they increase bone density and decrease the rate of bone resorption, as shown in many clinical trials.31–35 Because the rate of bone resorption reaches a nadir within 3 to 6 months of starting bisphosphonate therapy and because the increase in bone density after 1 year is quite modest (about 3%–4%), most of the decrease in vertebral fracture incidence, which becomes apparent during the first year of treatment, probably can be attributed more to normalization of bone resorption (and a less perforated structure) than to the increase in bone density.36 This would suggest that it is more appropriate to document that bone resorption has been inhibited than to measure bone density every year when following patients taking antiresorptive agents.
Furthermore, effective antiresorptive therapy reduces the levels of resorption markers by 50% to 70%,32–35 whereas after 1 year bone density has generally not increased more than the error of the bone density measurement. This observation has led to the suggestion that bone density measurements generally should not be done more often than every 2 years when following the effects of antiresorptive therapy. Even with a 20% to 30% day-to-day variation in levels of bone resorption markers, it is easier to document the efficacy of therapy with resorption biomarkers than with bone density.
To document compliance. Another reason to consider measuring a resorption marker (after 3 months of therapy) is to document compliance, a considerable problem in the treatment of an asymptomatic disorder.
To help decide when to restart bisphosphonate therapy. After long-term treatment with a bisphosphonate, the drug may be retained in the skeleton for years. This seems particularly true of alendronate (Fosamax).37 After 5 years of continuous alendronate treatment, bone resorption continues to be suppressed near the maximal level, in some patients for years after they stop taking the drug.38
Once the bone resorption marker begins to approach the pretreatment level, it would signal a possible need to restart the therapy. If a pretreatment level was not measured, an estimate of significant bone resorption would be signaled when the resorption marker is more than 20% above the mean premenopausal level. For urinary NTx this would be more than 42 nmol bone collagen equivalents/mmol creatinine.
In glucocorticoid-induced osteoporosis
Glucocorticoid therapy causes bone loss and an increased incidence of fractures when given in high doses or for prolonged periods by the oral, parenteral, or inhaled routes.41
The pathogenesis of the bone loss has been explored by measurements of bone turnover markers. During glucocorticoid therapy, levels of bone formation markers are generally low and those of bone resorption markers are either normal or low.42–44 Presumably, the reduction in bone resorption is not enough to overcome the reduction in bone formation, and bone loss ensues. In children, the effects on bone formation are particularly profound, as linear growth may be retarded.44
Giving a bisphosphonate during glucocorticoid therapy is quite effective in increasing bone density and preventing fractures.45–47 Patients who receive alendronate have lower levels of bone formation and resorption markers than do untreated subjects.45 Presumably, bone resorption is inhibited more than bone formation, accounting for the skeletal benefits.
In a recent study in patients with glucocorticoid-induced osteoporosis, bone mineral density of the lumbar spine increased more than twice as much with teriparatide than with alendronate over an 18-month period.48 As would be expected from the results of teriparatide therapy in postmenopausal osteoporosis, indices of both bone formation and resorption rose to a peak at 6 months, with formation greater than resorption.
In immobilization-induced osteoporosis
Studies of normal volunteers placed on bed rest indicate that urinary CTx and NTx excretion increase significantly after 24 hours, no doubt reflecting a rapid increase in osteoclast activity.49 In a 16-week study of bed rest in volunteers, markers of bone formation were reduced and markers of bone resorption increased, demonstrating the mechanisms for the profound and rapid loss of bone in immobilized patients.50
In a long-term cross-sectional study of paraplegic men with spinal cord injuries, bone turnover patterns changed over time.51 During the first year after injury, urinary deoxypyridinoline excretion was markedly elevated, whereas blood total alkaline phosphatase and osteocalcin levels were normal to slightly elevated. Over a 30-year period after injury, the bone resorption marker returned to normal levels in most patients and the bone formation markers were normal. Fracture incidence rose but leveled off after 20 years.
Bisphosphonate therapy in spinal cord injury patients reduces urinary NTx and prevents bone loss.52,53 These agents have also proven effective in reversing hypercalcemia in immobilized patients.54
In inflammatory bowel disease
Patients with inflammatory bowel disease, especially Crohn disease, have low bone mass and are at risk of fractures.55 These complications could be due to glucocorticoid therapy, hypogonadism, vitamin D defeciency, weight loss, and high circulating levels of bone-active cytokines released by inflammatory cells residing in the diseased intestine.
Bone formation markers have not been found to be outside the normal range, although both interleukin 1 and tumor necrosis factor alpha are known to inhibit bone formation.
Bisphosphonate treatment produces an increase in bone density concomitant with decreases in markers of bone resorption and formation.57,58 Of considerable interest is the observation that infliximab (anti-tumor necrosis factor alpha; Remicade) generally produces a rise in bone formation markers, with a smaller and inconsistent effect on bone resorption.59,60
In rheumatoid arthritis
The incidence of osteoporosis and fractures is also increased In patients with rheumatoid arthritis.61 As in patients with inflammatory bowel disease, a variety of factors can contribute to bone loss, including glucocorticoid therapy, hypogonadism, vitamin D deficiency, immobility, and elevated levels of bone-active cytokines.
Generally, studies have reported increased bone resorption based on type I collagen markers,62,63 whereas patients with osteoarthritis have levels of these bone resorption markers no different from those of control subjects.62 Although serum total TRAP protein is elevated in rheumatoid arthritis patients, this is probably due to the 5a isoform, the origin of which may be macrophages and dendritric cells.64
The influence of abnormalities in bone formation on bone loss is less clear. Levels of bone formation markers have been reported to be normal,65 elevated,66 or reduced.67
Treatment of rheumatoid arthritis with high-dose glucocorticoid pulse therapy is effective in controlling the symptoms and some manifestations of the immune system in patients with the disorder. The latter effect would be expected to have a beneficial effect on bone metabolism. This appears to be the case, as there are only transient decreases in bone formation markers and no significant reduction in bone density.68 Similarly, there is only a transient decrease in serum osteocalcin after an intra-articular injection of a glucocorticoid, and no effect on urinary pyridino-line.69
As would be expected, bisphosphonate therapy prevents bone loss in rheumatoid arthritis patients treated with glucocorticoids.70,71 Both oral and intravenous therapy decrease the levels of bone turnover markers.70–72 Infliximab therapy was shown to reduce the levels of bone resorption markers but not of PINP (a bone formation marker).73
In primary hyperparathyroidism
Hypersecretion of parathyroid hormone increases osteoclastic activity, with a secondary increase in osteoblastic activity. Bone loss may ensue and an increase in fracture incidence may be a consequence, particularly in post-menopausal women, who have the highest incidence of the disorder.74
Before screening chemistry panels became widely used during routine medical evaluations, it was not unusual to find elevated serum total alkaline phosphatase levels in patients discovered to have primary hyperparathyroidism. Today, this finding is not so common, as the disorder is diagnosed at a much earlier stage. Nevertheless, more specific and sensitive markers of bone turnover have made it possible to demonstrate the metabolic abnormalities that reflect the skeletal pathology in patients with primary hyperparathyroidism and its response to various therapies.75,76
On average, patients with untreated primary hyperparathyroidism have high levels of markers of bone resorption and formation, except in the mildest cases.73,74 Bone turnover returns to normal within 6 months to a year after successful parathyroidectomy.77,78 This response correlates with improvement in bone density, primarily in the lumbar spine.77,78
In patients who do not undergo surgery, alternative means of preventing bone loss include estrogen replacement in estrogendeficient postmenopausal women,76 bisphosphonates,79,80 and cinacalcet (Sensipar).81 Estrogen,76 raloxifene (Evista),82 and alendronate79,80 all reduce levels of bone resorption and formation markers, and estrogen76 and alendronate79,80 increase bone density. Although cinacalcet usually restores the serum calcium to the normal range and prevents bone loss, it only reduces serum parathyroid hormone levels by about 20%, and both bone resorption and formation markers increase above baseline.81 This could be related to fluctuations in serum parathyroid hormone that occur during each day of therapy.
In osteomalacia and rickets
Osteomalacia and rickets of any cause are characterized by increased osteoblastic activity. If the underlying cause is vitamin D deficiency, genetic or acquired defects in calcitriol synthesis, or vitamin D resistance, then hyper-parathyroidism with increased bone resorption is a secondary feature.
Serum total alkaline phosphatase activity has been a useful marker of disease activity for many years, although the newer markers, except for serum osteocalcin,83 are potentially more sensitive. The insensitivity of osteocalcin as an index of osteoblastic activity is unexplained but could be related to the state of differentiation of the osteoblasts. Bone resorption markers are elevated in vitamin D deficiency84 but are not widely used in clinical practice, as serum parathyroid hormone is an excellent indirect means of assessing the presence of increased bone resorption and the response to therapy.
In renal osteodystrophy
Bone disease associated with renal failure is termed renal osteodystrophy and is quite heterogeneous.85 Microscopic examination of a bone biopsy specimen is still considered the gold standard for diagnosis, and measurement in serum of intact parathyroid hormone is an important guide to diagnosis and response to therapy.
Nevertheless, recent studies suggest that serum markers of bone formation and resorption may be of additional help in assessing bone turnover.86 At present it is not certain whether any of the newer markers are superior to serum total alkaline phosphatase activity. Future studies that correlate bone histology with bone turnover markers should clarify the value of the various markers.
In cancer
Bone metastases are a common complication in cancer patients. They are classified as osteolytic, osteoblastic, or mixed on the basis of radiographic features. Biochemical markers of bone turnover have proven useful in assessing the magnitude of the metastases, the response to therapy, and even the prognosis for survival.87
Osteolytic metastases, which are common in breast cancer, are associated with increases in bone resorption markers, and after treatment with intravenous bisphosphonates the levels can decrease nearly 70%.88,89
Patients with higher levels of urinary NTx had a higher risk of skeletal complications and disease progression than patients with low levels across multiple tumor groups, including multiple myeloma.87
In osteoblastic metastases. Prostate cancer patients, who typically have predominantly osteoblastic lesions, have elevations of serum total alkaline phosphatase activity and other markers of bone formation.90 In addition, they have elevated bone resorption markers. Urinary NTx decreased markedly but serum bone-specific alkaline phosphatase decreased only slightly after treatment with intravenous zoledronic acid (Zometa),91 whereas androgen ablation therapy has inconsistent effects on bone turnover.92,93 High levels of these markers again predict poor prognosis.93,94
In hormone-suppression therapy. Two of the most successful cancer therapies, aromatase inhibitors for breast cancer95 and androgen ablation for prostate cancer,96 accelerate bone loss through marked suppression of gonadal steroids. Bone resorption and formation markers increase and bone loss ensues, with resorption exceeding formation. Estrogen suppression appears mainly responsible in both sexes, since raloxifene prevents bone loss in prostate cancer patients.97
Bisphosphonates are highly effective in preventing bone loss in either sex.98–100 A single infusion of zoledronic acid in androgen-ablated prostate cancer patients can prevent bone loss for at least 1 year.100
In Paget disease of bone
Paget disease of bone evolves over many years, from an early osteolytic phase to dominance of secondary osteoblastic activity. In patients with extensive polyostotic disease, bone resorption and formation marker levels may be higher than in almost any other skeletal disorder. An exception is serum osteocalcin,101 which once again usually does not accurately reflect the rate of bone formation.
Bisphosphonates, given orally or intravenously, produce an early decrease in bone resorption followed by a fall in bone formation.102 In clinical practice it appears adequate to use the least expensive test, serum total alkaline phosphatase activity, to assess disease activity and the response to therapy.103
Acknowledgments: Grant support to FRS from the Edythe and Eli Broad Foundation and Lois Rosen. Grant support to DRE from the National Institutes of Health (NIAMS: AR37318, AR36794).
- Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996; 17:333–368.
- Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005; 26:97–122.
- Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005; 2:504–517.
- Seibel MJ. Biochemical markers of bone turnover: part II: clinical applications in the management of osteoporosis. Clin Biochem Rev. 2006; 27:123–138.
- Glorieux FH, Travers R, Taylor A, et al. Normative data for iliac bone histomorphometry in growing children. Bone. 2000; 26:103–109.
- Clarke BL, Ebeling PR, Jones JD, et al. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996; 81:2264–2270.
- Rucker D, Ezzat S, Diamandi A, Khosravi J, Hanley DA. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf). 2004; 60:491–499.
- Cloos PA, Christgau S. Characterization of aged osteocalcin fragments derived from bone resorption. Clin Lab. 2004; 50:585–598.
- Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130:456–469.
- Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992; 7:1251–1258.
- Clemens JD, Herrick MV, Singer FR, Eyre DR. Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem. 1997; 43:2058–2063.
- Garnero P, Gineyts E, Riou JP, Delmas PD. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab. 1994; 79:780–785.
- Christgau S, Rosenquist C, Alexandersen P, et al. Clinical evaluation of the Serum CrossLaps One Step, ELISA a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem. 1998; 44:2290–2300.
- Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell R, Blumsohn A. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004; 34:187–194.
- Meier C, Meinhardt U, Greenfield JR, et al. Serum cathepsin K concentrations reflect osteoclastic activity in women with postmenopausal osteoporosis and patients with Paget’s disease. Clin Lab. 2006; 52:1–10.
- Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 2007; 9(suppl 1):S1.
- Wichers M, Schmidt E, Bidlingmaier F, Klingmuller D. Diurnal rhythm of CrossLaps in human serum. Clin Chem. 1999; 45:1858–1860.
- Kenny AM, Prestwood KM, Biskup B, et al. Comparison of the effects of calcium loading with calcium citrate or calcium carbonate on bone turnover in postmenopausal women. Osteoporos Int. 2004; 15:290–294.
- Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex-and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007; 92:443–449.
- Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996; 81:3366–3371.
- Prestwood KM, Pilbeam CC, Burleson JA, et al. The short-term effects of conjugated estrogen on bone turnover in older women. J Clin Endocrinol Metab. 1994; 79:366–371.
- Schneider DL, Barrett-Connor EL. Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med. 1997; 157:1241–1245.
- Huang AJ, Ettinger B, Vittinghoff E, Ensrud KE, Johnson KC, Cummings SR. Endogenous estrogen levels and the effects of ultra low-dose transdermal estradiol therapy on bone turnover and bone density in postmenopausal women. J Bone Miner Res. 2007; 22:1791–1797.
- Khosla S, Melton LJ, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998; 83:2266–2274.
- Goemaere S, Van Pottelbergh I, Zmierczak H, et al. Inverse association between bone turnover rate and bone mineral density in community-dwelling men >70 years of age: no major role of sex steroid status. Bone. 2001; 29:286–291.
- Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD. Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res. 2001; 16:1642–1650.
- Scopacasa F, Wishart JM, Need AG, Horowitz M, Morris HA, Nordin BE. Bone density and bone-related biochemical variables in normal men: a longitudinal study. J Gerontol A Biol Sci Med Sci 2002; 57:M385–M391.
- Nguyen TV, Meier C, Center JR, Eisman JA, Seibel MJ. Bone turnover in elderly men: relationships to change in bone mineral density. BMC Musculoskelet Disord 2007; 8:13.
- Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001; 345:948–955.
- Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–1538.
- Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995; 333:1437–1443.
- Bauer DC, Black DM, Garnero P, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004; 19:1250–1258.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999; 282:1344–1352.
- Delmas PD, Recker RR, Chesnut CH, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004; 15:792–798.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356:1809–1822.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003; 18:1051–1056.
- Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004; 20:1291–1300.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006; 296:2927–2938.
- Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2000; 11:434–442.
- Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005; 20:962–970.
- Shaker JL, Lukert BP. Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am. 2005; 34:341–356.
- Ebeling PR, Erbas B, Hopper JL, Wark JD, Rubinfeld AR. Bone mineral density and bone turnover in asthmatics treated with long-term inhaled or oral glucocorticoids. J Bone Miner Res. 1998; 13:1283–1289.
- Siomou E, Challa A, Tzoufi M, Papadopoulou ZL, Lapatsanis PD, Siamopoulou A. Biochemical markers of bone metabolism in infants and children under intravenous corticosteroid therapy. Calcif Tissue Int. 2003; 73:319–325.
- Ahmed SF, Tucker P, Mushtaq T, Wallace AM, Williams DM, Hughes IA. Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin Endocrinol (Oxf). 2002; 57:185–191.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001; 44:202–211.
- Reid DM, Adami S, Devogelaer JP, Chines AA. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int. 2001; 69:242–247.
- Ringe JD, Dorst A, Faber H, Ibach K, Sorenson F. Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative study. Osteoporos Int. 2003; 14:801–807.
- Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007; 357:2028–2039.
- Heer M, Baecker N, Mika C, Boese A, Gerzer R. Immobilization induces a very rapid increase in osteoclast activity. Acta Astronaut. 2005; 57:31–36.
- Scheld K, Zittermann A, Heer M, et al. Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin Chem. 2001; 47:1688–1695.
- Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004; 15:180–189.
- Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999; 80:243–251.
- Gilchrist NL, Frampton CM, Acland RH, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007; 92:1385–1390.
- Massagli TL, Cardenas DD. Immobilization hypercalcemia treatment with pamidronate disodium after spinal cord injury. Arch Phys Med Rehabil. 1999; 80:998–1000.
- van Staa TP, Cooper C, Brusse LS, Leufkens H, Javaid MK, Arden NK. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003; 125:1591–1597.
- Dresner-Pollak R, Karmeli F, Eliakim R, Ackerman Z, Rachmilewitz D. Increased urinary N-telopeptide cross-linked type 1 collagen predicts bone loss in patients with inflammatory bowel disease. Am J Gastroenterol. 2000; 95:699–704.
- Haderslev KV, Tjellesen L, Sorensen HA, Staun M. Alendronate increases lumbar spine bone mineral density in patients with Crohn’s disease. Gastroenterology. 2000; 119:639–646.
- Palomba S, Orio F, Manguso F, et al. Efficacy of risedronate administration in osteoporotic postmenopausal women affected by inflammatory bowel disease. Osteoporos Int. 2005; 16:1141–1149.
- Franchimont N, Putzeys V, Collette J, et al. Rapid improvement of bone metabolism after infliximab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2004; 20:607–614.
- Ryan BM, Russel MG, Schurgers L, et al. Effect of antitumour necrosis factor-alpha therapy on bone turnover in patients with active Crohn’s disease: a prospective study. Aliment Pharmacol Ther. 2004; 20:851–857.
- van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006; 54:3104–3112.
- Wong PK, Young L, Vaile JH, et al. Telopeptides as markers of bone turnover in rheumatoid arthritis and osteoarthritis. Intern Med J. 2004; 34:539–544.
- Momohara S, Okamoto H, Yago T, et al. The study of bone mineral density and bone turnover markers in postmenopausal women with active rheumatoid arthritis. Mod Rheumatol. 2005; 15:410–414.
- Janckila AJ, Neustadt DH, Nakasato YR, Halleen JM, Hentunen T, Yam LT. Serum tartrate-resistant acid phosphatase isoforms in rheumatoid arthritis. Clin Chim Acta. 2002; 320:49–58.
- Lems WF, Gerrits MI, Jacobs JW, van Vugt RM, van Rijn HJ, Bijlsma JW. Changes in (markers of) bone metabolism during high dose corticosteroid pulse treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 1996; 55:288–293.
- Manrique F, Gamardo J, de Elguezabal K, et al. Abnormalities of bone mineral density and bone metabolism in Venezuelan patients with rheumatoid arthritis. J Clin Rheumatol. 2003; 9:219–227.
- Garnero P, Jouvenne P, Buchs N, Delmas PD, Miossec P. Uncoupling of bone metabolism in rheumatoid arthritis patients with or without joint destruction: assessment with serum type I collagen breakdown products. Bone. 1999; 24:381–385.
- Frediani B, Falsetti P, Bisogno S, et al. Effects of high dose methyl-prednisolone pulse therapy on bone mass and biochemical markers of bone metabolism in patients with active rheumatoid arthritis: a 12-month randomized prospective controlled study. J Rheumatol. 2004; 31:1083–1087.
- Emkey RD, Lindsay R, Lyssy J, Weisberg JS, Dempster DW, Shen V. The systemic effect of intraarticular administration of corticosteroid on markers of bone formation and bone resorption in patients with rheumatoid arthritis. Arthritis Rheum. 1996; 39:277–282.
- Lange U, Illgner U, Teichmann J, Schleenbecker H. Skeletal benefit after one year of risedronate therapy in patients with rheumatoid arthritis and glucocorticoid-induced osteoporosis: a prospective study. Int J Clin Pharmacol Res. 2004; 24:33–38.
- Tascioglu F, Colak O, Armagan O, Alatas O, Oner C. The treatment of osteoporosis in patients with rheumatoid arthritis receiving glucocorticoids: a comparison of alendronate and intranasal salmon calcitonin. Rheumatol Int. 2005; 26:21–29.
- Cremers SC, Lodder MC, Den Hartigh J, et al. Short term whole body retention in relation to rate of bone resorption and cartilage degradation after intravenous bisphosphonate (pamidronate) in rheumatoid arthritis. J Rheumatol. 2004; 31:1732–1737.
- Chopin F, Garnero P, Le Henanff A, et al. Long term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis. 2007; 67:353–357.
- Khosla S, Melton LJ, Wermers RA, Crowson CS, O’Fallon W, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999; 14:1700–1707.
- Guo CY, Thomas WE, al-Dehaimi AW, Assiri AM, Eastell R. Longitudinal changes in bone mineral density and bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 1996; 81:3487–3491.
- Orr-Walker BJ, Evans MC, Clearwater JM, Horne A, Grey AB, Reid IR. Effects of hormone replacement therapy on bone mineral density in postmenopausal women with primary hyperparathyroidism: four-year follow-up and comparison with healthy postmenopausal women. Arch Intern Med. 2000; 160:2161–2166.
- Christiansen P, Steiniche T, Brixen K, et al. Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone. 1999; 25:237–244.
- Tamura Y, Araki A, Chiba Y, Mori S, Hosoi T, Horiuchi T. Remarkable increase in lumbar spine bone mineral density and amelioration in biochemical markers of bone turnover after parathyroidectomy in elderly patients with primary hyperparathyroidism: a 5-year follow-up study. J Bone Miner Metab. 2007; 25:226–231.
- Chow CC, Chan WB, Li JK, et al. Oral alendronate increases bone mineral density in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:581–587.
- Khan AA, Bilezikian JP, Kung AW, et al. Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004; 89:3319–3325.
- Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005; 90:135–141.
- Rubin MR, Lee KH, McMahon DJ, Silverberg SJ. Raloxifene lowers serum calcium and markers of bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:1174–1178.
- Daniels ED, Pettifor JM, Moodley GP. Serum osteocalcin has limited usefulness as a diagnostic marker for rickets. Eur J Pediatr. 2000; 159:730–733.
- Need AG. Bone resorption markers in vitamin D insufficiency. Clin Chim Acta. 2006; 368:48–52.
- Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004; 43:558–565.
- Malyszko J, Wolczynski S, Malyszko JS, Konstantynowicz J, Kaczmarski M, Mysliwiec M. Correlations of new markers of bone formation and resorption in kidney transplant recipients. Transplant Proc. 2003; 35:1351–1354.
- Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005; 23:4925–4935.
- Body JJ, Dumon JC, Gineyts E, Delmas PD. Comparative evaluation of markers of bone resorption in patients with breast cancer-induced osteolysis before and after bisphosphonate therapy. Br J Cancer. 1997; 75:408–412.
- Coleman RE Efficacy of zoledronic acid and pamidronate in breast cancer patients: a comparative analysis of randomized phase III trials. Am J Clin Oncol 2002; 25(suppl 1):S25–S31.
- Smith MR. Markers of bone metabolism in prostate cancer. Cancer Treat Rev 2006; 32(suppl 1):23–26.
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94:1458–1468.
- Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998; 83:1561–1566.
- Johansen JS, Brasso K, Iversen P, et al. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res. 2007; 13:3244–3249.
- Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006; 12:3361–3367.
- Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res. 2006; 21:1215–1223.
- Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res 2006; 12:6315s–6319s.
- Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004; 89:3841–3846.
- Confavreux CB, Fontana A, Guastalla JP, Munoz F, Brun J, Delmas PD. Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prevention with bisphosphonates. Bone. 2007; 41:346–352.
- Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007; 146:416–424.
- Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007; 25:1038–1042.
- Kaddam IM, Iqbal SJ, Holland S, Wong M, Manning D. Comparison of serum osteocalcin with total and bone specific alkaline phosphatase and urinary hydroxyproline:creatinine ratio in patients with Paget’s disease of bone. Ann Clin Biochem. 1994; 31:327–330.
- Reid IR, Miller P, Lyles K, et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med. 2005; 353:898–908.
- Reid IR, Davidson JS, Wattie D, et al. Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone. 2004; 35:224–230.
- Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996; 17:333–368.
- Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005; 26:97–122.
- Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005; 2:504–517.
- Seibel MJ. Biochemical markers of bone turnover: part II: clinical applications in the management of osteoporosis. Clin Biochem Rev. 2006; 27:123–138.
- Glorieux FH, Travers R, Taylor A, et al. Normative data for iliac bone histomorphometry in growing children. Bone. 2000; 26:103–109.
- Clarke BL, Ebeling PR, Jones JD, et al. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996; 81:2264–2270.
- Rucker D, Ezzat S, Diamandi A, Khosravi J, Hanley DA. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf). 2004; 60:491–499.
- Cloos PA, Christgau S. Characterization of aged osteocalcin fragments derived from bone resorption. Clin Lab. 2004; 50:585–598.
- Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130:456–469.
- Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992; 7:1251–1258.
- Clemens JD, Herrick MV, Singer FR, Eyre DR. Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem. 1997; 43:2058–2063.
- Garnero P, Gineyts E, Riou JP, Delmas PD. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab. 1994; 79:780–785.
- Christgau S, Rosenquist C, Alexandersen P, et al. Clinical evaluation of the Serum CrossLaps One Step, ELISA a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem. 1998; 44:2290–2300.
- Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell R, Blumsohn A. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004; 34:187–194.
- Meier C, Meinhardt U, Greenfield JR, et al. Serum cathepsin K concentrations reflect osteoclastic activity in women with postmenopausal osteoporosis and patients with Paget’s disease. Clin Lab. 2006; 52:1–10.
- Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 2007; 9(suppl 1):S1.
- Wichers M, Schmidt E, Bidlingmaier F, Klingmuller D. Diurnal rhythm of CrossLaps in human serum. Clin Chem. 1999; 45:1858–1860.
- Kenny AM, Prestwood KM, Biskup B, et al. Comparison of the effects of calcium loading with calcium citrate or calcium carbonate on bone turnover in postmenopausal women. Osteoporos Int. 2004; 15:290–294.
- Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex-and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007; 92:443–449.
- Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996; 81:3366–3371.
- Prestwood KM, Pilbeam CC, Burleson JA, et al. The short-term effects of conjugated estrogen on bone turnover in older women. J Clin Endocrinol Metab. 1994; 79:366–371.
- Schneider DL, Barrett-Connor EL. Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med. 1997; 157:1241–1245.
- Huang AJ, Ettinger B, Vittinghoff E, Ensrud KE, Johnson KC, Cummings SR. Endogenous estrogen levels and the effects of ultra low-dose transdermal estradiol therapy on bone turnover and bone density in postmenopausal women. J Bone Miner Res. 2007; 22:1791–1797.
- Khosla S, Melton LJ, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998; 83:2266–2274.
- Goemaere S, Van Pottelbergh I, Zmierczak H, et al. Inverse association between bone turnover rate and bone mineral density in community-dwelling men >70 years of age: no major role of sex steroid status. Bone. 2001; 29:286–291.
- Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD. Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res. 2001; 16:1642–1650.
- Scopacasa F, Wishart JM, Need AG, Horowitz M, Morris HA, Nordin BE. Bone density and bone-related biochemical variables in normal men: a longitudinal study. J Gerontol A Biol Sci Med Sci 2002; 57:M385–M391.
- Nguyen TV, Meier C, Center JR, Eisman JA, Seibel MJ. Bone turnover in elderly men: relationships to change in bone mineral density. BMC Musculoskelet Disord 2007; 8:13.
- Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001; 345:948–955.
- Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–1538.
- Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995; 333:1437–1443.
- Bauer DC, Black DM, Garnero P, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004; 19:1250–1258.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999; 282:1344–1352.
- Delmas PD, Recker RR, Chesnut CH, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004; 15:792–798.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356:1809–1822.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003; 18:1051–1056.
- Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004; 20:1291–1300.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006; 296:2927–2938.
- Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2000; 11:434–442.
- Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005; 20:962–970.
- Shaker JL, Lukert BP. Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am. 2005; 34:341–356.
- Ebeling PR, Erbas B, Hopper JL, Wark JD, Rubinfeld AR. Bone mineral density and bone turnover in asthmatics treated with long-term inhaled or oral glucocorticoids. J Bone Miner Res. 1998; 13:1283–1289.
- Siomou E, Challa A, Tzoufi M, Papadopoulou ZL, Lapatsanis PD, Siamopoulou A. Biochemical markers of bone metabolism in infants and children under intravenous corticosteroid therapy. Calcif Tissue Int. 2003; 73:319–325.
- Ahmed SF, Tucker P, Mushtaq T, Wallace AM, Williams DM, Hughes IA. Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin Endocrinol (Oxf). 2002; 57:185–191.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001; 44:202–211.
- Reid DM, Adami S, Devogelaer JP, Chines AA. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int. 2001; 69:242–247.
- Ringe JD, Dorst A, Faber H, Ibach K, Sorenson F. Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative study. Osteoporos Int. 2003; 14:801–807.
- Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007; 357:2028–2039.
- Heer M, Baecker N, Mika C, Boese A, Gerzer R. Immobilization induces a very rapid increase in osteoclast activity. Acta Astronaut. 2005; 57:31–36.
- Scheld K, Zittermann A, Heer M, et al. Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin Chem. 2001; 47:1688–1695.
- Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004; 15:180–189.
- Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999; 80:243–251.
- Gilchrist NL, Frampton CM, Acland RH, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007; 92:1385–1390.
- Massagli TL, Cardenas DD. Immobilization hypercalcemia treatment with pamidronate disodium after spinal cord injury. Arch Phys Med Rehabil. 1999; 80:998–1000.
- van Staa TP, Cooper C, Brusse LS, Leufkens H, Javaid MK, Arden NK. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003; 125:1591–1597.
- Dresner-Pollak R, Karmeli F, Eliakim R, Ackerman Z, Rachmilewitz D. Increased urinary N-telopeptide cross-linked type 1 collagen predicts bone loss in patients with inflammatory bowel disease. Am J Gastroenterol. 2000; 95:699–704.
- Haderslev KV, Tjellesen L, Sorensen HA, Staun M. Alendronate increases lumbar spine bone mineral density in patients with Crohn’s disease. Gastroenterology. 2000; 119:639–646.
- Palomba S, Orio F, Manguso F, et al. Efficacy of risedronate administration in osteoporotic postmenopausal women affected by inflammatory bowel disease. Osteoporos Int. 2005; 16:1141–1149.
- Franchimont N, Putzeys V, Collette J, et al. Rapid improvement of bone metabolism after infliximab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2004; 20:607–614.
- Ryan BM, Russel MG, Schurgers L, et al. Effect of antitumour necrosis factor-alpha therapy on bone turnover in patients with active Crohn’s disease: a prospective study. Aliment Pharmacol Ther. 2004; 20:851–857.
- van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006; 54:3104–3112.
- Wong PK, Young L, Vaile JH, et al. Telopeptides as markers of bone turnover in rheumatoid arthritis and osteoarthritis. Intern Med J. 2004; 34:539–544.
- Momohara S, Okamoto H, Yago T, et al. The study of bone mineral density and bone turnover markers in postmenopausal women with active rheumatoid arthritis. Mod Rheumatol. 2005; 15:410–414.
- Janckila AJ, Neustadt DH, Nakasato YR, Halleen JM, Hentunen T, Yam LT. Serum tartrate-resistant acid phosphatase isoforms in rheumatoid arthritis. Clin Chim Acta. 2002; 320:49–58.
- Lems WF, Gerrits MI, Jacobs JW, van Vugt RM, van Rijn HJ, Bijlsma JW. Changes in (markers of) bone metabolism during high dose corticosteroid pulse treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 1996; 55:288–293.
- Manrique F, Gamardo J, de Elguezabal K, et al. Abnormalities of bone mineral density and bone metabolism in Venezuelan patients with rheumatoid arthritis. J Clin Rheumatol. 2003; 9:219–227.
- Garnero P, Jouvenne P, Buchs N, Delmas PD, Miossec P. Uncoupling of bone metabolism in rheumatoid arthritis patients with or without joint destruction: assessment with serum type I collagen breakdown products. Bone. 1999; 24:381–385.
- Frediani B, Falsetti P, Bisogno S, et al. Effects of high dose methyl-prednisolone pulse therapy on bone mass and biochemical markers of bone metabolism in patients with active rheumatoid arthritis: a 12-month randomized prospective controlled study. J Rheumatol. 2004; 31:1083–1087.
- Emkey RD, Lindsay R, Lyssy J, Weisberg JS, Dempster DW, Shen V. The systemic effect of intraarticular administration of corticosteroid on markers of bone formation and bone resorption in patients with rheumatoid arthritis. Arthritis Rheum. 1996; 39:277–282.
- Lange U, Illgner U, Teichmann J, Schleenbecker H. Skeletal benefit after one year of risedronate therapy in patients with rheumatoid arthritis and glucocorticoid-induced osteoporosis: a prospective study. Int J Clin Pharmacol Res. 2004; 24:33–38.
- Tascioglu F, Colak O, Armagan O, Alatas O, Oner C. The treatment of osteoporosis in patients with rheumatoid arthritis receiving glucocorticoids: a comparison of alendronate and intranasal salmon calcitonin. Rheumatol Int. 2005; 26:21–29.
- Cremers SC, Lodder MC, Den Hartigh J, et al. Short term whole body retention in relation to rate of bone resorption and cartilage degradation after intravenous bisphosphonate (pamidronate) in rheumatoid arthritis. J Rheumatol. 2004; 31:1732–1737.
- Chopin F, Garnero P, Le Henanff A, et al. Long term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis. 2007; 67:353–357.
- Khosla S, Melton LJ, Wermers RA, Crowson CS, O’Fallon W, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999; 14:1700–1707.
- Guo CY, Thomas WE, al-Dehaimi AW, Assiri AM, Eastell R. Longitudinal changes in bone mineral density and bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 1996; 81:3487–3491.
- Orr-Walker BJ, Evans MC, Clearwater JM, Horne A, Grey AB, Reid IR. Effects of hormone replacement therapy on bone mineral density in postmenopausal women with primary hyperparathyroidism: four-year follow-up and comparison with healthy postmenopausal women. Arch Intern Med. 2000; 160:2161–2166.
- Christiansen P, Steiniche T, Brixen K, et al. Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone. 1999; 25:237–244.
- Tamura Y, Araki A, Chiba Y, Mori S, Hosoi T, Horiuchi T. Remarkable increase in lumbar spine bone mineral density and amelioration in biochemical markers of bone turnover after parathyroidectomy in elderly patients with primary hyperparathyroidism: a 5-year follow-up study. J Bone Miner Metab. 2007; 25:226–231.
- Chow CC, Chan WB, Li JK, et al. Oral alendronate increases bone mineral density in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:581–587.
- Khan AA, Bilezikian JP, Kung AW, et al. Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004; 89:3319–3325.
- Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005; 90:135–141.
- Rubin MR, Lee KH, McMahon DJ, Silverberg SJ. Raloxifene lowers serum calcium and markers of bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:1174–1178.
- Daniels ED, Pettifor JM, Moodley GP. Serum osteocalcin has limited usefulness as a diagnostic marker for rickets. Eur J Pediatr. 2000; 159:730–733.
- Need AG. Bone resorption markers in vitamin D insufficiency. Clin Chim Acta. 2006; 368:48–52.
- Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004; 43:558–565.
- Malyszko J, Wolczynski S, Malyszko JS, Konstantynowicz J, Kaczmarski M, Mysliwiec M. Correlations of new markers of bone formation and resorption in kidney transplant recipients. Transplant Proc. 2003; 35:1351–1354.
- Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005; 23:4925–4935.
- Body JJ, Dumon JC, Gineyts E, Delmas PD. Comparative evaluation of markers of bone resorption in patients with breast cancer-induced osteolysis before and after bisphosphonate therapy. Br J Cancer. 1997; 75:408–412.
- Coleman RE Efficacy of zoledronic acid and pamidronate in breast cancer patients: a comparative analysis of randomized phase III trials. Am J Clin Oncol 2002; 25(suppl 1):S25–S31.
- Smith MR. Markers of bone metabolism in prostate cancer. Cancer Treat Rev 2006; 32(suppl 1):23–26.
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94:1458–1468.
- Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998; 83:1561–1566.
- Johansen JS, Brasso K, Iversen P, et al. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res. 2007; 13:3244–3249.
- Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006; 12:3361–3367.
- Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res. 2006; 21:1215–1223.
- Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res 2006; 12:6315s–6319s.
- Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004; 89:3841–3846.
- Confavreux CB, Fontana A, Guastalla JP, Munoz F, Brun J, Delmas PD. Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prevention with bisphosphonates. Bone. 2007; 41:346–352.
- Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007; 146:416–424.
- Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007; 25:1038–1042.
- Kaddam IM, Iqbal SJ, Holland S, Wong M, Manning D. Comparison of serum osteocalcin with total and bone specific alkaline phosphatase and urinary hydroxyproline:creatinine ratio in patients with Paget’s disease of bone. Ann Clin Biochem. 1994; 31:327–330.
- Reid IR, Miller P, Lyles K, et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med. 2005; 353:898–908.
- Reid IR, Davidson JS, Wattie D, et al. Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone. 2004; 35:224–230.
KEY POINTS
- Biomarkers of bone formation and resorption reflect the overall osteoblastic and osteoclastic activity in the skeleton and in some situations may serve as surrogates for histologic examination of bone.
- Biomarkers of bone turnover can be used to document the effects of therapeutic agents in some patients with osteoporosis and possibly reduce the need for frequent bone density testing.
- In cancer patients with bone metastases, biomarkers of bone resorption provide evidence of the efficacy of antiresorptive therapy. The baseline levels also have prognostic value: patients with the highest levels have the worst prognosis.