User login
A 55-year-old woman presents with an intermittent sensation of food getting stuck in her mid to lower chest. The symptoms have occurred several times per year over the last 2 or 3 years and appear to be slowly worsening. She says she has no trouble swallowing liquids. She has a history of gastroesophageal reflux disease, for which she takes a proton pump inhibitor once a day. She says she has had no odynophagia, cough, regurgitation, or weight loss.
How should her symptoms best be evaluated?
DYSPHAGIA CAN BE OROPHARYNGEAL OR ESOPHAGEAL
Dysphagia is the subjective sensation of difficulty swallowing solids, liquids, or both. Symptoms can range from the inability to initiate a swallow to the sensation of esophageal obstruction. Other symptoms of esophageal disease may also be present, such as chest pain, heartburn, and regurgitation. There may also be nonesophageal symptoms related to the disease process causing the dysphagia.
Dysphagia can be separated into oropharyngeal and esophageal types.
Interestingly, many patients with symptoms of oropharyngeal dysphagia in fact have referred symptoms from primary esophageal dysphagia2; many patients with a distal mucosal ring describe a sense of something sticking in the cervical esophagus.
Esophageal dysphagia arises in the mid to distal esophagus or gastric cardia, and as a result, the symptoms are typically retrosternal.1 It can be caused by structural problems such as strictures, rings, webs, extrinsic compression, or a primary esophageal or gastroesophageal neoplasm, or by a primary motility abnormality such as achalasia (Table 1). Eosinophilic esophagitis is now a frequent cause of esophageal dysphagia, especially in white men.3
ESOPHAGOGRAPHY VS ENDOSCOPY IN EVALUATING DYSPHAGIA
Many gastroenterologists recommend endoscopy rather than barium esophagography as the initial examination in patients with dysphagia.4–8 Each test has certain advantages.
Advantages of endoscopy. Endoscopy is superior to esophagography in detecting milder grades of esophagitis. Further, interventions can be performed endoscopically (eg, dilation, biopsy, attachment of a wireless pH testing probe) that cannot be done during a radiographic procedure, and endoscopy does not expose the patient to radiation.
Advantages of esophagography. Endoscopy cannot detect evidence of gastroesophageal reflux disease unless mucosal injury is present. In dysphagia, the radiologic findings correlate well with endoscopic findings, including the detection of esophageal malignancy and moderate to severe esophagitis. Further, motility disorders can be detected with barium esophagography but not with endoscopy.9,10
Subtle abnormalities, especially rings and strictures, may be missed by narrow-diameter (9.8–10 mm) modern upper-endoscopic equipment. Further, esophagography is noninvasive, costs less, and may be more convenient (it does not require sedation and a chaperone for the patient after sedation). This examination also provides dynamic evaluation of the complex process of swallowing. Causes of dysphagia external to the esophagus can also be determined.
In view of the respective advantages and disadvantages of the two methods, we believe that in most instances barium esophagography should be the initial examination,1,9,11–15 and at our institution most patients presenting with dysphagia undergo barium esophagography before they undergo other examinations.14
OBTAIN A HISTORY BEFORE ORDERING ESOPHAGOGRAPHY
Before a barium examination of the esophagus is done, a focused medical history should be obtained, as it can guide the further workup as well as the esophageal study itself.
An attempt should be made to determine whether the dysphagia is oropharyngeal or if it is esophageal, as the former is generally best initially evaluated by a speech and language pathologist. Generally, the physician who orders the test judges whether the patient has oropharyngeal or esophageal dysphagia. Often, both an oropharyngeal examination, performed by a speech and language pathologist, and an esophageal examination, performed by a radiologist, are ordered.
Rapidly progressive symptoms, especially if accompanied by weight loss, should make one suspect cancer. Chronic symptoms usually point to gastroesophageal reflux disease or a motility disorder such as achalasia. Liquid dysphagia almost always means the patient has a motility disorder such as achalasia.
In view of the possibility of eosinophilic esophagitis, one should ask about food or seasonal allergies, especially in young patients with intermittent difficulty swallowing solids.3
BARIUM ESOPHAGOGRAPHY HAS EIGHT SEPARATE PHASES
Barium esophagography is tailored to the patient with dysphagia on the basis of his or her history. The standard examination is divided into eight separate phases (see below).14 Each phase addresses a specific question or questions concerning the structure and function of the esophagus.
At our institution, the first phase of the examination is determined by the presenting symptoms. If the patient has liquid dysphagia, we start with a timed barium swallow to assess esophageal emptying. If the patient does not have liquid dysphagia, we start with an air-contrast mucosal examination.
The patient must be cooperative and mobile to complete all phases of the examination.
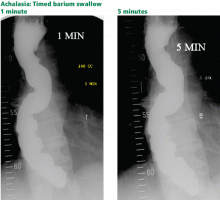
Timed barium swallow to measure esophageal emptying
The timed barium swallow is an objective measure of esophageal emptying.16–18 This technique is essential in the initial evaluation of a patient with liquid dysphagia, a symptom common in patients with severe dysmotility, usually achalasia.
We use this examination in our patients with suspected or confirmed achalasia and to follow up patients who have been treated with pneumatic dilatation, botulinum toxin injection, and Heller myotomy.17,18 In addition, this timed test is an objective measure of emptying in patients who have undergone intervention but whose symptoms have not subjectively improved, and can suggest that further intervention may be required.
Air-contrast or mucosal phase
Although this phase is not as sensitive as endoscopy, it can detect masses, mucosal erosions, ulcers, and—most importantly in our experience—fixed hernias. Patients with a fixed hernia have a foreshortened esophagus, which is important to know about before repairing the hernia. Many esophageal surgeons believe that a foreshortened esophagus precludes a standard Nissen fundoplication and necessitates an esophageal lengthening procedure (ie, Collis gastroplasty with a Nissen fundoplication).14
Motility phase
The third phase examines esophageal motility. With the patient in a semiprone position, low-density barium is given in single swallows, separated by 25 to 30 seconds. The images are recorded on digital media to allow one to review them frame by frame.
The findings on this phase correlate well with those of manometry.19 This portion of the examination also uses impedance monitoring to assess bolus transfer, an aspect not evaluated by manometry.20,21 Impedance monitoring detects changes in resistance to current flow and correlates well with esophagraphic findings regarding bolus transfer.
While many patients with dysphagia also undergo esophageal manometry, the findings from this phase of the esophagographic examination may be the first indication of an esophageal motility disorder. In fact, this portion of the examination shows the distinct advantage of esophagography over endoscopy as the initial test in patients with dysphagia, as endoscopy may not identify patients with achalasia, especially early on.4
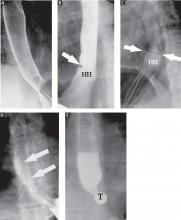
Single-contrast (full-column) phase to detect strictures, rings
The fourth phase of the esophagographic evaluation is the distended, single-contrast examination (Figure 2B). This is performed in the semiprone position with the patient rapidly drinking thin barium. It is done to detect esophageal strictures, rings, and contour abnormalities caused by extrinsic processes. Subtle abnormalities shown by this technique, including benign strictures and rings, are often not visualized with endoscopy.
Mucosal relief phase
The fifth phase is performed with a collapsed esophagus immediately after the distended, single-contrast phase, where spot films are taken of the barium-coated, collapsed esophagus (Figure 2C). This phase is used to evaluate thickened mucosal folds, a common finding in moderate to severe reflux esophagitis.
Reflux evaluation
Provocative maneuvers are used in the sixth phase to elicit gastroesophageal reflux (Figure 2D). With the patient supine, he or she is asked to roll side to side, do a Valsalva maneuver, and do a straight-leg raise. The patient then sips water in the supine position to assess for reflux (the water siphon test). If reflux is seen, the cause, the height of the reflux, and the duration of reflux retention are recorded.
Solid-bolus phase to assess strictures
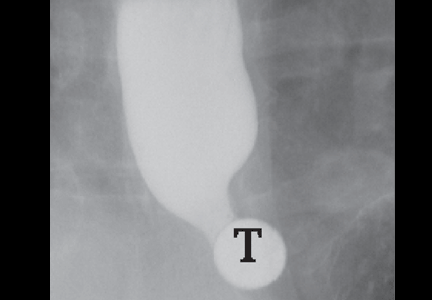
In the seventh phase, the patient swallows a 13-mm barium tablet (Figure 2E). This allows one to assess the significance of a ring or stricture and to assess if dysphagia symptoms recur as a result of tablet obstruction. Subtle strictures that were not detected during the prior phases can also be detected using a tablet. If obstruction or impaired passage occurs, the site of obstruction and the presence or absence of symptoms are recorded.
Modified esophagography to assess the oropharynx
The final or eighth phase of barium esophagography is called “modified barium esophagography” or the modified barium swallow. However, it may be the first phase of the examination performed or the only portion of the examination performed, or it may not be performed at all.
Modified barium esophagography is used to define the anatomy of the oropharynx and to assess its function in swallowing.12 It may also guide rehabilitation strategies aimed at eliminating a patient’s swallowing symptoms.
Most patients referred for this test have sustained damage to the central nervous system or structures of the oropharynx, such as stroke or radiation therapy for laryngeal cancer. Many have difficulty in starting to swallow, aspirate when they try to swallow, or both.
The final esophagographic report should document the findings of each phase of the examination (Table 2).
WHAT HAPPENED TO OUR PATIENT?
Our patient underwent barium esophagography (Figure 2). A distal mucosal ring that transiently obstructed a 13-mm tablet was found. The patient underwent endoscopy and the ring was dilated. No biopsies were necessary.
- Levine MS, Rubesin SE. Radiologic investigation of dysphagia. AJR Am J Roentgenol 1990; 154:1157–1163.
- Smith DF, Ott DJ, Gelfand DW, Chen MY. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol 1998; 171:1361–1365.
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Spechler SJ. American Gastroenterological Association medical position statement on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology 1999; 117:229–233.
- American Society for Gastrointestinal Endoscopy. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc 2000; 52:831–837.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Standards of Practice Committee. Role of endoscopy in the management of GERD. Gastrointest Endosc 2007; 66:219–224.
- Halpert RD, Feczko PJ, Spickler EM, Ackerman LV. Radiological assessment of dysphagia with endoscopic correlation. Radiology 1985; 157:599–602.
- Ott DJ. Gastroesophageal reflux disease. Radiol Clin North Am 1994; 32:1147–1166.
- Ekberg O, Pokieser P. Radiologic evaluation of the dysphagic patient. Eur Radiol 1997; 7:1285–1295.
- Logemann JA. Role of the modified barium swallow in management of patients with dysphagia. Otolaryngol Head Neck Surg 1997; 116:335–338.
- Baker ME, Rice TW. Radiologic evaluation of the esophagus: methods and value in motility disorders and GERD. Semin Thorac Cardiovasc Surg 2001; 13:201–225.
- Baker ME, Einstein DM, Herts BR, et al. Gastroesophageal reflux disease: integrating the barium esophagram before and after antire-flux surgery. Radiology 2007; 243:329–339.
- Levine MS, Rubesin SE, Laufer I. Barium esophagography: a study for all seasons. Clin Gastroenterol Hepatol 2008; 6:11–25.
- deOliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol 1997; 169:473–479.
- Kostic SV, Rice TW, Baker ME, et al. Time barium esophagram: a simple physiologic assessment for achalasia. J Thorac Cardiovasc Surg 2000; 120:935–943.
- Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002; 50:765–770.
- Hewson EG, Ott DJ, Dalton CB, Chen YM, Wu WC, Richter JE. Manometry and radiology. Complementary studies in the assessment of esophageal motility disorders. Gastroenterology 1990; 98:626–632.
- Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, video-esophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol 2005;G1000–G1006.
- Imam H, Baker M, Shay S. Simultaneous barium esophagram, impedance monitoring and manometry in patients with dysphagia due to a tight fundoplication [abstract]. Gastroenterology 2004; 126:A-639.
A 55-year-old woman presents with an intermittent sensation of food getting stuck in her mid to lower chest. The symptoms have occurred several times per year over the last 2 or 3 years and appear to be slowly worsening. She says she has no trouble swallowing liquids. She has a history of gastroesophageal reflux disease, for which she takes a proton pump inhibitor once a day. She says she has had no odynophagia, cough, regurgitation, or weight loss.
How should her symptoms best be evaluated?
DYSPHAGIA CAN BE OROPHARYNGEAL OR ESOPHAGEAL
Dysphagia is the subjective sensation of difficulty swallowing solids, liquids, or both. Symptoms can range from the inability to initiate a swallow to the sensation of esophageal obstruction. Other symptoms of esophageal disease may also be present, such as chest pain, heartburn, and regurgitation. There may also be nonesophageal symptoms related to the disease process causing the dysphagia.
Dysphagia can be separated into oropharyngeal and esophageal types.
Interestingly, many patients with symptoms of oropharyngeal dysphagia in fact have referred symptoms from primary esophageal dysphagia2; many patients with a distal mucosal ring describe a sense of something sticking in the cervical esophagus.
Esophageal dysphagia arises in the mid to distal esophagus or gastric cardia, and as a result, the symptoms are typically retrosternal.1 It can be caused by structural problems such as strictures, rings, webs, extrinsic compression, or a primary esophageal or gastroesophageal neoplasm, or by a primary motility abnormality such as achalasia (Table 1). Eosinophilic esophagitis is now a frequent cause of esophageal dysphagia, especially in white men.3
ESOPHAGOGRAPHY VS ENDOSCOPY IN EVALUATING DYSPHAGIA
Many gastroenterologists recommend endoscopy rather than barium esophagography as the initial examination in patients with dysphagia.4–8 Each test has certain advantages.
Advantages of endoscopy. Endoscopy is superior to esophagography in detecting milder grades of esophagitis. Further, interventions can be performed endoscopically (eg, dilation, biopsy, attachment of a wireless pH testing probe) that cannot be done during a radiographic procedure, and endoscopy does not expose the patient to radiation.
Advantages of esophagography. Endoscopy cannot detect evidence of gastroesophageal reflux disease unless mucosal injury is present. In dysphagia, the radiologic findings correlate well with endoscopic findings, including the detection of esophageal malignancy and moderate to severe esophagitis. Further, motility disorders can be detected with barium esophagography but not with endoscopy.9,10
Subtle abnormalities, especially rings and strictures, may be missed by narrow-diameter (9.8–10 mm) modern upper-endoscopic equipment. Further, esophagography is noninvasive, costs less, and may be more convenient (it does not require sedation and a chaperone for the patient after sedation). This examination also provides dynamic evaluation of the complex process of swallowing. Causes of dysphagia external to the esophagus can also be determined.
In view of the respective advantages and disadvantages of the two methods, we believe that in most instances barium esophagography should be the initial examination,1,9,11–15 and at our institution most patients presenting with dysphagia undergo barium esophagography before they undergo other examinations.14
OBTAIN A HISTORY BEFORE ORDERING ESOPHAGOGRAPHY
Before a barium examination of the esophagus is done, a focused medical history should be obtained, as it can guide the further workup as well as the esophageal study itself.
An attempt should be made to determine whether the dysphagia is oropharyngeal or if it is esophageal, as the former is generally best initially evaluated by a speech and language pathologist. Generally, the physician who orders the test judges whether the patient has oropharyngeal or esophageal dysphagia. Often, both an oropharyngeal examination, performed by a speech and language pathologist, and an esophageal examination, performed by a radiologist, are ordered.
Rapidly progressive symptoms, especially if accompanied by weight loss, should make one suspect cancer. Chronic symptoms usually point to gastroesophageal reflux disease or a motility disorder such as achalasia. Liquid dysphagia almost always means the patient has a motility disorder such as achalasia.
In view of the possibility of eosinophilic esophagitis, one should ask about food or seasonal allergies, especially in young patients with intermittent difficulty swallowing solids.3
BARIUM ESOPHAGOGRAPHY HAS EIGHT SEPARATE PHASES
Barium esophagography is tailored to the patient with dysphagia on the basis of his or her history. The standard examination is divided into eight separate phases (see below).14 Each phase addresses a specific question or questions concerning the structure and function of the esophagus.
At our institution, the first phase of the examination is determined by the presenting symptoms. If the patient has liquid dysphagia, we start with a timed barium swallow to assess esophageal emptying. If the patient does not have liquid dysphagia, we start with an air-contrast mucosal examination.
The patient must be cooperative and mobile to complete all phases of the examination.
Timed barium swallow to measure esophageal emptying
The timed barium swallow is an objective measure of esophageal emptying.16–18 This technique is essential in the initial evaluation of a patient with liquid dysphagia, a symptom common in patients with severe dysmotility, usually achalasia.
We use this examination in our patients with suspected or confirmed achalasia and to follow up patients who have been treated with pneumatic dilatation, botulinum toxin injection, and Heller myotomy.17,18 In addition, this timed test is an objective measure of emptying in patients who have undergone intervention but whose symptoms have not subjectively improved, and can suggest that further intervention may be required.
Air-contrast or mucosal phase
Although this phase is not as sensitive as endoscopy, it can detect masses, mucosal erosions, ulcers, and—most importantly in our experience—fixed hernias. Patients with a fixed hernia have a foreshortened esophagus, which is important to know about before repairing the hernia. Many esophageal surgeons believe that a foreshortened esophagus precludes a standard Nissen fundoplication and necessitates an esophageal lengthening procedure (ie, Collis gastroplasty with a Nissen fundoplication).14
Motility phase
The third phase examines esophageal motility. With the patient in a semiprone position, low-density barium is given in single swallows, separated by 25 to 30 seconds. The images are recorded on digital media to allow one to review them frame by frame.
The findings on this phase correlate well with those of manometry.19 This portion of the examination also uses impedance monitoring to assess bolus transfer, an aspect not evaluated by manometry.20,21 Impedance monitoring detects changes in resistance to current flow and correlates well with esophagraphic findings regarding bolus transfer.
While many patients with dysphagia also undergo esophageal manometry, the findings from this phase of the esophagographic examination may be the first indication of an esophageal motility disorder. In fact, this portion of the examination shows the distinct advantage of esophagography over endoscopy as the initial test in patients with dysphagia, as endoscopy may not identify patients with achalasia, especially early on.4
Single-contrast (full-column) phase to detect strictures, rings
The fourth phase of the esophagographic evaluation is the distended, single-contrast examination (Figure 2B). This is performed in the semiprone position with the patient rapidly drinking thin barium. It is done to detect esophageal strictures, rings, and contour abnormalities caused by extrinsic processes. Subtle abnormalities shown by this technique, including benign strictures and rings, are often not visualized with endoscopy.
Mucosal relief phase
The fifth phase is performed with a collapsed esophagus immediately after the distended, single-contrast phase, where spot films are taken of the barium-coated, collapsed esophagus (Figure 2C). This phase is used to evaluate thickened mucosal folds, a common finding in moderate to severe reflux esophagitis.
Reflux evaluation
Provocative maneuvers are used in the sixth phase to elicit gastroesophageal reflux (Figure 2D). With the patient supine, he or she is asked to roll side to side, do a Valsalva maneuver, and do a straight-leg raise. The patient then sips water in the supine position to assess for reflux (the water siphon test). If reflux is seen, the cause, the height of the reflux, and the duration of reflux retention are recorded.
Solid-bolus phase to assess strictures
In the seventh phase, the patient swallows a 13-mm barium tablet (Figure 2E). This allows one to assess the significance of a ring or stricture and to assess if dysphagia symptoms recur as a result of tablet obstruction. Subtle strictures that were not detected during the prior phases can also be detected using a tablet. If obstruction or impaired passage occurs, the site of obstruction and the presence or absence of symptoms are recorded.
Modified esophagography to assess the oropharynx
The final or eighth phase of barium esophagography is called “modified barium esophagography” or the modified barium swallow. However, it may be the first phase of the examination performed or the only portion of the examination performed, or it may not be performed at all.
Modified barium esophagography is used to define the anatomy of the oropharynx and to assess its function in swallowing.12 It may also guide rehabilitation strategies aimed at eliminating a patient’s swallowing symptoms.
Most patients referred for this test have sustained damage to the central nervous system or structures of the oropharynx, such as stroke or radiation therapy for laryngeal cancer. Many have difficulty in starting to swallow, aspirate when they try to swallow, or both.
The final esophagographic report should document the findings of each phase of the examination (Table 2).
WHAT HAPPENED TO OUR PATIENT?
Our patient underwent barium esophagography (Figure 2). A distal mucosal ring that transiently obstructed a 13-mm tablet was found. The patient underwent endoscopy and the ring was dilated. No biopsies were necessary.
A 55-year-old woman presents with an intermittent sensation of food getting stuck in her mid to lower chest. The symptoms have occurred several times per year over the last 2 or 3 years and appear to be slowly worsening. She says she has no trouble swallowing liquids. She has a history of gastroesophageal reflux disease, for which she takes a proton pump inhibitor once a day. She says she has had no odynophagia, cough, regurgitation, or weight loss.
How should her symptoms best be evaluated?
DYSPHAGIA CAN BE OROPHARYNGEAL OR ESOPHAGEAL
Dysphagia is the subjective sensation of difficulty swallowing solids, liquids, or both. Symptoms can range from the inability to initiate a swallow to the sensation of esophageal obstruction. Other symptoms of esophageal disease may also be present, such as chest pain, heartburn, and regurgitation. There may also be nonesophageal symptoms related to the disease process causing the dysphagia.
Dysphagia can be separated into oropharyngeal and esophageal types.
Interestingly, many patients with symptoms of oropharyngeal dysphagia in fact have referred symptoms from primary esophageal dysphagia2; many patients with a distal mucosal ring describe a sense of something sticking in the cervical esophagus.
Esophageal dysphagia arises in the mid to distal esophagus or gastric cardia, and as a result, the symptoms are typically retrosternal.1 It can be caused by structural problems such as strictures, rings, webs, extrinsic compression, or a primary esophageal or gastroesophageal neoplasm, or by a primary motility abnormality such as achalasia (Table 1). Eosinophilic esophagitis is now a frequent cause of esophageal dysphagia, especially in white men.3
ESOPHAGOGRAPHY VS ENDOSCOPY IN EVALUATING DYSPHAGIA
Many gastroenterologists recommend endoscopy rather than barium esophagography as the initial examination in patients with dysphagia.4–8 Each test has certain advantages.
Advantages of endoscopy. Endoscopy is superior to esophagography in detecting milder grades of esophagitis. Further, interventions can be performed endoscopically (eg, dilation, biopsy, attachment of a wireless pH testing probe) that cannot be done during a radiographic procedure, and endoscopy does not expose the patient to radiation.
Advantages of esophagography. Endoscopy cannot detect evidence of gastroesophageal reflux disease unless mucosal injury is present. In dysphagia, the radiologic findings correlate well with endoscopic findings, including the detection of esophageal malignancy and moderate to severe esophagitis. Further, motility disorders can be detected with barium esophagography but not with endoscopy.9,10
Subtle abnormalities, especially rings and strictures, may be missed by narrow-diameter (9.8–10 mm) modern upper-endoscopic equipment. Further, esophagography is noninvasive, costs less, and may be more convenient (it does not require sedation and a chaperone for the patient after sedation). This examination also provides dynamic evaluation of the complex process of swallowing. Causes of dysphagia external to the esophagus can also be determined.
In view of the respective advantages and disadvantages of the two methods, we believe that in most instances barium esophagography should be the initial examination,1,9,11–15 and at our institution most patients presenting with dysphagia undergo barium esophagography before they undergo other examinations.14
OBTAIN A HISTORY BEFORE ORDERING ESOPHAGOGRAPHY
Before a barium examination of the esophagus is done, a focused medical history should be obtained, as it can guide the further workup as well as the esophageal study itself.
An attempt should be made to determine whether the dysphagia is oropharyngeal or if it is esophageal, as the former is generally best initially evaluated by a speech and language pathologist. Generally, the physician who orders the test judges whether the patient has oropharyngeal or esophageal dysphagia. Often, both an oropharyngeal examination, performed by a speech and language pathologist, and an esophageal examination, performed by a radiologist, are ordered.
Rapidly progressive symptoms, especially if accompanied by weight loss, should make one suspect cancer. Chronic symptoms usually point to gastroesophageal reflux disease or a motility disorder such as achalasia. Liquid dysphagia almost always means the patient has a motility disorder such as achalasia.
In view of the possibility of eosinophilic esophagitis, one should ask about food or seasonal allergies, especially in young patients with intermittent difficulty swallowing solids.3
BARIUM ESOPHAGOGRAPHY HAS EIGHT SEPARATE PHASES
Barium esophagography is tailored to the patient with dysphagia on the basis of his or her history. The standard examination is divided into eight separate phases (see below).14 Each phase addresses a specific question or questions concerning the structure and function of the esophagus.
At our institution, the first phase of the examination is determined by the presenting symptoms. If the patient has liquid dysphagia, we start with a timed barium swallow to assess esophageal emptying. If the patient does not have liquid dysphagia, we start with an air-contrast mucosal examination.
The patient must be cooperative and mobile to complete all phases of the examination.
Timed barium swallow to measure esophageal emptying
The timed barium swallow is an objective measure of esophageal emptying.16–18 This technique is essential in the initial evaluation of a patient with liquid dysphagia, a symptom common in patients with severe dysmotility, usually achalasia.
We use this examination in our patients with suspected or confirmed achalasia and to follow up patients who have been treated with pneumatic dilatation, botulinum toxin injection, and Heller myotomy.17,18 In addition, this timed test is an objective measure of emptying in patients who have undergone intervention but whose symptoms have not subjectively improved, and can suggest that further intervention may be required.
Air-contrast or mucosal phase
Although this phase is not as sensitive as endoscopy, it can detect masses, mucosal erosions, ulcers, and—most importantly in our experience—fixed hernias. Patients with a fixed hernia have a foreshortened esophagus, which is important to know about before repairing the hernia. Many esophageal surgeons believe that a foreshortened esophagus precludes a standard Nissen fundoplication and necessitates an esophageal lengthening procedure (ie, Collis gastroplasty with a Nissen fundoplication).14
Motility phase
The third phase examines esophageal motility. With the patient in a semiprone position, low-density barium is given in single swallows, separated by 25 to 30 seconds. The images are recorded on digital media to allow one to review them frame by frame.
The findings on this phase correlate well with those of manometry.19 This portion of the examination also uses impedance monitoring to assess bolus transfer, an aspect not evaluated by manometry.20,21 Impedance monitoring detects changes in resistance to current flow and correlates well with esophagraphic findings regarding bolus transfer.
While many patients with dysphagia also undergo esophageal manometry, the findings from this phase of the esophagographic examination may be the first indication of an esophageal motility disorder. In fact, this portion of the examination shows the distinct advantage of esophagography over endoscopy as the initial test in patients with dysphagia, as endoscopy may not identify patients with achalasia, especially early on.4
Single-contrast (full-column) phase to detect strictures, rings
The fourth phase of the esophagographic evaluation is the distended, single-contrast examination (Figure 2B). This is performed in the semiprone position with the patient rapidly drinking thin barium. It is done to detect esophageal strictures, rings, and contour abnormalities caused by extrinsic processes. Subtle abnormalities shown by this technique, including benign strictures and rings, are often not visualized with endoscopy.
Mucosal relief phase
The fifth phase is performed with a collapsed esophagus immediately after the distended, single-contrast phase, where spot films are taken of the barium-coated, collapsed esophagus (Figure 2C). This phase is used to evaluate thickened mucosal folds, a common finding in moderate to severe reflux esophagitis.
Reflux evaluation
Provocative maneuvers are used in the sixth phase to elicit gastroesophageal reflux (Figure 2D). With the patient supine, he or she is asked to roll side to side, do a Valsalva maneuver, and do a straight-leg raise. The patient then sips water in the supine position to assess for reflux (the water siphon test). If reflux is seen, the cause, the height of the reflux, and the duration of reflux retention are recorded.
Solid-bolus phase to assess strictures
In the seventh phase, the patient swallows a 13-mm barium tablet (Figure 2E). This allows one to assess the significance of a ring or stricture and to assess if dysphagia symptoms recur as a result of tablet obstruction. Subtle strictures that were not detected during the prior phases can also be detected using a tablet. If obstruction or impaired passage occurs, the site of obstruction and the presence or absence of symptoms are recorded.
Modified esophagography to assess the oropharynx
The final or eighth phase of barium esophagography is called “modified barium esophagography” or the modified barium swallow. However, it may be the first phase of the examination performed or the only portion of the examination performed, or it may not be performed at all.
Modified barium esophagography is used to define the anatomy of the oropharynx and to assess its function in swallowing.12 It may also guide rehabilitation strategies aimed at eliminating a patient’s swallowing symptoms.
Most patients referred for this test have sustained damage to the central nervous system or structures of the oropharynx, such as stroke or radiation therapy for laryngeal cancer. Many have difficulty in starting to swallow, aspirate when they try to swallow, or both.
The final esophagographic report should document the findings of each phase of the examination (Table 2).
WHAT HAPPENED TO OUR PATIENT?
Our patient underwent barium esophagography (Figure 2). A distal mucosal ring that transiently obstructed a 13-mm tablet was found. The patient underwent endoscopy and the ring was dilated. No biopsies were necessary.
- Levine MS, Rubesin SE. Radiologic investigation of dysphagia. AJR Am J Roentgenol 1990; 154:1157–1163.
- Smith DF, Ott DJ, Gelfand DW, Chen MY. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol 1998; 171:1361–1365.
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Spechler SJ. American Gastroenterological Association medical position statement on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology 1999; 117:229–233.
- American Society for Gastrointestinal Endoscopy. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc 2000; 52:831–837.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Standards of Practice Committee. Role of endoscopy in the management of GERD. Gastrointest Endosc 2007; 66:219–224.
- Halpert RD, Feczko PJ, Spickler EM, Ackerman LV. Radiological assessment of dysphagia with endoscopic correlation. Radiology 1985; 157:599–602.
- Ott DJ. Gastroesophageal reflux disease. Radiol Clin North Am 1994; 32:1147–1166.
- Ekberg O, Pokieser P. Radiologic evaluation of the dysphagic patient. Eur Radiol 1997; 7:1285–1295.
- Logemann JA. Role of the modified barium swallow in management of patients with dysphagia. Otolaryngol Head Neck Surg 1997; 116:335–338.
- Baker ME, Rice TW. Radiologic evaluation of the esophagus: methods and value in motility disorders and GERD. Semin Thorac Cardiovasc Surg 2001; 13:201–225.
- Baker ME, Einstein DM, Herts BR, et al. Gastroesophageal reflux disease: integrating the barium esophagram before and after antire-flux surgery. Radiology 2007; 243:329–339.
- Levine MS, Rubesin SE, Laufer I. Barium esophagography: a study for all seasons. Clin Gastroenterol Hepatol 2008; 6:11–25.
- deOliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol 1997; 169:473–479.
- Kostic SV, Rice TW, Baker ME, et al. Time barium esophagram: a simple physiologic assessment for achalasia. J Thorac Cardiovasc Surg 2000; 120:935–943.
- Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002; 50:765–770.
- Hewson EG, Ott DJ, Dalton CB, Chen YM, Wu WC, Richter JE. Manometry and radiology. Complementary studies in the assessment of esophageal motility disorders. Gastroenterology 1990; 98:626–632.
- Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, video-esophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol 2005;G1000–G1006.
- Imam H, Baker M, Shay S. Simultaneous barium esophagram, impedance monitoring and manometry in patients with dysphagia due to a tight fundoplication [abstract]. Gastroenterology 2004; 126:A-639.
- Levine MS, Rubesin SE. Radiologic investigation of dysphagia. AJR Am J Roentgenol 1990; 154:1157–1163.
- Smith DF, Ott DJ, Gelfand DW, Chen MY. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol 1998; 171:1361–1365.
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Spechler SJ. American Gastroenterological Association medical position statement on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology 1999; 117:229–233.
- American Society for Gastrointestinal Endoscopy. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc 2000; 52:831–837.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Standards of Practice Committee. Role of endoscopy in the management of GERD. Gastrointest Endosc 2007; 66:219–224.
- Halpert RD, Feczko PJ, Spickler EM, Ackerman LV. Radiological assessment of dysphagia with endoscopic correlation. Radiology 1985; 157:599–602.
- Ott DJ. Gastroesophageal reflux disease. Radiol Clin North Am 1994; 32:1147–1166.
- Ekberg O, Pokieser P. Radiologic evaluation of the dysphagic patient. Eur Radiol 1997; 7:1285–1295.
- Logemann JA. Role of the modified barium swallow in management of patients with dysphagia. Otolaryngol Head Neck Surg 1997; 116:335–338.
- Baker ME, Rice TW. Radiologic evaluation of the esophagus: methods and value in motility disorders and GERD. Semin Thorac Cardiovasc Surg 2001; 13:201–225.
- Baker ME, Einstein DM, Herts BR, et al. Gastroesophageal reflux disease: integrating the barium esophagram before and after antire-flux surgery. Radiology 2007; 243:329–339.
- Levine MS, Rubesin SE, Laufer I. Barium esophagography: a study for all seasons. Clin Gastroenterol Hepatol 2008; 6:11–25.
- deOliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol 1997; 169:473–479.
- Kostic SV, Rice TW, Baker ME, et al. Time barium esophagram: a simple physiologic assessment for achalasia. J Thorac Cardiovasc Surg 2000; 120:935–943.
- Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002; 50:765–770.
- Hewson EG, Ott DJ, Dalton CB, Chen YM, Wu WC, Richter JE. Manometry and radiology. Complementary studies in the assessment of esophageal motility disorders. Gastroenterology 1990; 98:626–632.
- Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, video-esophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol 2005;G1000–G1006.
- Imam H, Baker M, Shay S. Simultaneous barium esophagram, impedance monitoring and manometry in patients with dysphagia due to a tight fundoplication [abstract]. Gastroenterology 2004; 126:A-639.
KEY POINTS
- Dysphagia can be due to problems in the oropharynx and cervical esophagus or in the distal esophagus.
- Radiologic evaluation of dysphagia has distinct advantages over endoscopy, including its ability to diagnose both structural changes and motility disorders.
- A barium evaluation can include a modified barium-swallowing study to evaluate the oropharynx, barium esophagography to evaluate the esophagus, and a timed study to evaluate esophageal emptying.
- Often, the true cause of dysphagia is best approached with a combination of radiographic and endoscopic studies.