Article
In reply: Barium esophagography
- Author:
- Brian C. Allen, MD
- Mark E. Baker, MD
- Gary W. Falk, MD, MS
Article
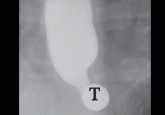
Role of barium esophagography in evaluating dysphagia
- Author:
- Brian C. Allen, MD
- Mark E. Baker, MD
- Gary W. Falk, MD, MS
A 55-year-old woman presents with an intermittent sensation of food getting stuck in her mid to lower chest. How should her symptoms best be...
Article
Eosinophilic esophagitis: An increasingly recognized cause of dysphagia, food impaction, and refractory heartburn
- Author:
- Ilche T. Nonevski, MD, MBA
- Erinn Downs-Kelly, DO
- Gary W. Falk, MD, MS
This disease, which was not described as a distinct clinical entity until 1993, may be due to allergic and immune-mediated mechanisms similar to...
Article
Current challenges in Barrett's esophagus
- Author:
- Gary W. Falk, MD, MS
The diagnosis and treatment of Barrett's esophagus are fraught with unresolved questions.
Article
Treatment of Helicobacter pylori in nonulcer dyspepsia: Should we or shouldn’t we?
- Author:
- Gary W. Falk, MD, MS
Although it may be tempting to treat for Helicobacter pylori in every patient with dyspepsia, two recent trials indicate we should temper...
Article
H pylori 1997: Testing and treatment options
- Author:
- Gary W. Falk, MD, MS
Which tests for H pylori are most useful? Which treatment regimens are most effective?
