User login
Being “Puzzled” as the First Step to Diagnosis
A 43-year-old Hispanic man has a very itchy rash on his left leg. It manifested within a few weeks of the patient starting a new job, one that involved working more hours than he had previously and on rotating shifts, which interfered with his sleep schedule.
For several years, he tried using different products—mostly OTC creams—on it, with no success. Increasingly annoyed by the rash, he finally consulted his family physician, who prescribed a combination clotrimazole/betamethasone cream. This helped a bit, but over time, the rash steadily worsened, and his primary care provider referred him to dermatology.
The patient claims to be otherwise healthy, with no history of atopy. He takes no prescription medications. He admits finding it difficult to refrain from scratching his legs.
EXAMINATION
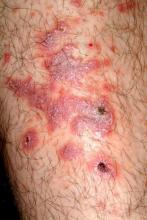
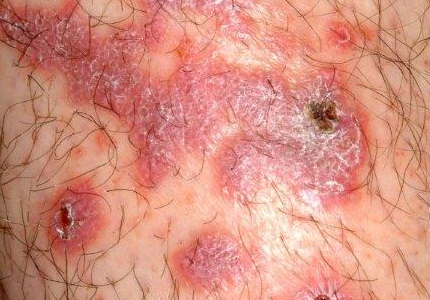
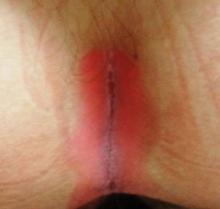
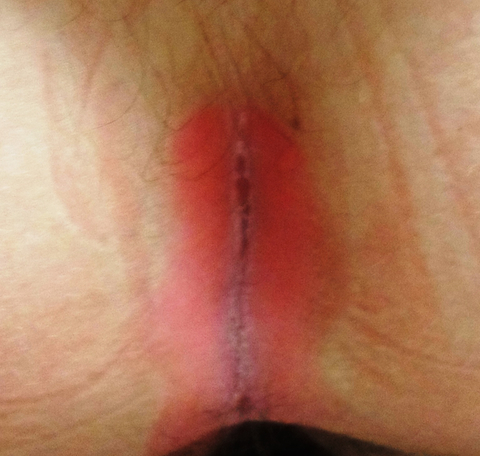
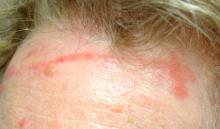
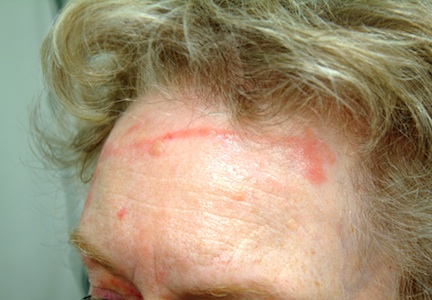
There are large, slightly purplish, hypertrophic papulosquamous plaques of up to 8 cm on both anterior tibial areas. His arms, trunk, and wrists are rash-free, and his oral mucosal surfaces show no obvious signs of disease.
Punch biopsy shows a hypertrophic epidermis. There is marked obliteration of the dermoepidermal junction by a dense bandlike layer of lymphocytic infiltrate, associated with vacuolar change and necrotic keratinocytes in the basal layer of the epidermis.
DISCUSSION
This is a classic clinical and histologic picture of a dermatosis commonly seen in dermatology offices: lichen planus (LP). As is often the case, by the time the patient gets to dermatology, the clinical picture can be confused and atypical. But there is much to learn from such a case.
LP is the prototypical interface lichenoid dermatitis, which can be depended on to present in certain identifiable ways. In this case, what suggested this diagnosis was the word “puzzling.” It’s the perfect example of the intuitive, somewhat subjective side of dermatologic diagnosis: When I see a condition that baffles me momentarily, my puzzled reaction becomes meaningful because the word puzzling starts with a “p.” Lichen planus has long been associated with an extremely useful mnemonic device known as “The Ps.” Besides puzzling, it can stand for purple, plaquish, polygonal (ie, not round), prurtitic, papular, penile (LP has a predilection for this area), and planar (ie, flat-topped).
But it’s the “puzzling” aspect that unlocks the entrance to “The Ps,” and that in turn leads to consideration of this diagnosis. In this case, LP was not totally obvious, but a biopsy confirmed my clinical suspicions by demonstrating the classic findings associated with it.
An LP-like eruption can be provoked by drugs, such as the antimalarials, gold compounds, and penicillamine. It has also been associated with hepatitis C. But in my experience, the most common trigger appears to be stress, since it’s a rare case that doesn’t involve some. (I’ll happily concede that this is my opinion and not established fact.)
This patient’s LP demonstrated two common variants: For one, the darker the patient’s skin, the deeper the purple color of the LP—sometimes to the point of obscuring the diagnosis.
As if that were not enough to confuse the examiner, there was an element of lichen simplex chronicus (LSC; also known as neurodermatitis), which instigates the “itch-scratch-itch” cycle. By its nature, LSC tends to hyperpigmentation (brown). By definition, it involves hypertrophy of the epidermis secondary to scratching. But there is always a primary cause; sometimes mere dry skin will trigger it, or insect bites, eczema, or even psoriasis. This confuses the issue, but the biopsy will establish the true basis for the patient’s condition.
The more common presentation of LP is that of a collection of flat-topped (“planar”), pruritic, purplish papules on the volar wrist, ankles, and sacral area. The surfaces of these lesions often demonstrate a faintly white, shiny surface. LP can also appear on oral mucosal surfaces, where it has a totally different, lacy-white look. In these areas, it tends to burn and not itch. LP appears to have a predilection for the penile glans, where it presents as an annular purplish plaque that sits astride the proximal glans, spilling over onto the coronal area.
LP can present as a widespread generalized eruption mimicking granuloma annulare and secondary syphilis. The differential for simple, classic LP includes psoriasis, granuloma annulare and pityriasis rosea.
Treatment of LP is with topical steroid creams or ointments, tailored in terms of strength to the area of involvement. For example, a class II or III corticosteroid would be used on areas with thicker skin and a class IV or V on thinner skin. Stubborn, solitary plaques can be treated with intralesional steroid injection (2.5 to 10 mg/cc triamcinolone solution).
TAKE-HOME LEARNING POINTS
• Lichen planus (LP) is common and typically presents on the volar wrists, dorsal ankles, and sacrum.
• In a minority of cases, LP can present on the legs as a hypertrophic, plaquish condition with secondary features of lichen simplex chronicus.
• A mnemonic device (“The Ps”) describes LP: papular, pruritic, purple, planar, polygonal, plaquish, and (perhaps most importantly) “puzzling.”
• LP can present as an annular plaque on the penile glans.
• LP has a pathognomic histologic picture on biopsy.
A 43-year-old Hispanic man has a very itchy rash on his left leg. It manifested within a few weeks of the patient starting a new job, one that involved working more hours than he had previously and on rotating shifts, which interfered with his sleep schedule.
For several years, he tried using different products—mostly OTC creams—on it, with no success. Increasingly annoyed by the rash, he finally consulted his family physician, who prescribed a combination clotrimazole/betamethasone cream. This helped a bit, but over time, the rash steadily worsened, and his primary care provider referred him to dermatology.
The patient claims to be otherwise healthy, with no history of atopy. He takes no prescription medications. He admits finding it difficult to refrain from scratching his legs.
EXAMINATION
There are large, slightly purplish, hypertrophic papulosquamous plaques of up to 8 cm on both anterior tibial areas. His arms, trunk, and wrists are rash-free, and his oral mucosal surfaces show no obvious signs of disease.
Punch biopsy shows a hypertrophic epidermis. There is marked obliteration of the dermoepidermal junction by a dense bandlike layer of lymphocytic infiltrate, associated with vacuolar change and necrotic keratinocytes in the basal layer of the epidermis.
DISCUSSION
This is a classic clinical and histologic picture of a dermatosis commonly seen in dermatology offices: lichen planus (LP). As is often the case, by the time the patient gets to dermatology, the clinical picture can be confused and atypical. But there is much to learn from such a case.
LP is the prototypical interface lichenoid dermatitis, which can be depended on to present in certain identifiable ways. In this case, what suggested this diagnosis was the word “puzzling.” It’s the perfect example of the intuitive, somewhat subjective side of dermatologic diagnosis: When I see a condition that baffles me momentarily, my puzzled reaction becomes meaningful because the word puzzling starts with a “p.” Lichen planus has long been associated with an extremely useful mnemonic device known as “The Ps.” Besides puzzling, it can stand for purple, plaquish, polygonal (ie, not round), prurtitic, papular, penile (LP has a predilection for this area), and planar (ie, flat-topped).
But it’s the “puzzling” aspect that unlocks the entrance to “The Ps,” and that in turn leads to consideration of this diagnosis. In this case, LP was not totally obvious, but a biopsy confirmed my clinical suspicions by demonstrating the classic findings associated with it.
An LP-like eruption can be provoked by drugs, such as the antimalarials, gold compounds, and penicillamine. It has also been associated with hepatitis C. But in my experience, the most common trigger appears to be stress, since it’s a rare case that doesn’t involve some. (I’ll happily concede that this is my opinion and not established fact.)
This patient’s LP demonstrated two common variants: For one, the darker the patient’s skin, the deeper the purple color of the LP—sometimes to the point of obscuring the diagnosis.
As if that were not enough to confuse the examiner, there was an element of lichen simplex chronicus (LSC; also known as neurodermatitis), which instigates the “itch-scratch-itch” cycle. By its nature, LSC tends to hyperpigmentation (brown). By definition, it involves hypertrophy of the epidermis secondary to scratching. But there is always a primary cause; sometimes mere dry skin will trigger it, or insect bites, eczema, or even psoriasis. This confuses the issue, but the biopsy will establish the true basis for the patient’s condition.
The more common presentation of LP is that of a collection of flat-topped (“planar”), pruritic, purplish papules on the volar wrist, ankles, and sacral area. The surfaces of these lesions often demonstrate a faintly white, shiny surface. LP can also appear on oral mucosal surfaces, where it has a totally different, lacy-white look. In these areas, it tends to burn and not itch. LP appears to have a predilection for the penile glans, where it presents as an annular purplish plaque that sits astride the proximal glans, spilling over onto the coronal area.
LP can present as a widespread generalized eruption mimicking granuloma annulare and secondary syphilis. The differential for simple, classic LP includes psoriasis, granuloma annulare and pityriasis rosea.
Treatment of LP is with topical steroid creams or ointments, tailored in terms of strength to the area of involvement. For example, a class II or III corticosteroid would be used on areas with thicker skin and a class IV or V on thinner skin. Stubborn, solitary plaques can be treated with intralesional steroid injection (2.5 to 10 mg/cc triamcinolone solution).
TAKE-HOME LEARNING POINTS
• Lichen planus (LP) is common and typically presents on the volar wrists, dorsal ankles, and sacrum.
• In a minority of cases, LP can present on the legs as a hypertrophic, plaquish condition with secondary features of lichen simplex chronicus.
• A mnemonic device (“The Ps”) describes LP: papular, pruritic, purple, planar, polygonal, plaquish, and (perhaps most importantly) “puzzling.”
• LP can present as an annular plaque on the penile glans.
• LP has a pathognomic histologic picture on biopsy.
A 43-year-old Hispanic man has a very itchy rash on his left leg. It manifested within a few weeks of the patient starting a new job, one that involved working more hours than he had previously and on rotating shifts, which interfered with his sleep schedule.
For several years, he tried using different products—mostly OTC creams—on it, with no success. Increasingly annoyed by the rash, he finally consulted his family physician, who prescribed a combination clotrimazole/betamethasone cream. This helped a bit, but over time, the rash steadily worsened, and his primary care provider referred him to dermatology.
The patient claims to be otherwise healthy, with no history of atopy. He takes no prescription medications. He admits finding it difficult to refrain from scratching his legs.
EXAMINATION
There are large, slightly purplish, hypertrophic papulosquamous plaques of up to 8 cm on both anterior tibial areas. His arms, trunk, and wrists are rash-free, and his oral mucosal surfaces show no obvious signs of disease.
Punch biopsy shows a hypertrophic epidermis. There is marked obliteration of the dermoepidermal junction by a dense bandlike layer of lymphocytic infiltrate, associated with vacuolar change and necrotic keratinocytes in the basal layer of the epidermis.
DISCUSSION
This is a classic clinical and histologic picture of a dermatosis commonly seen in dermatology offices: lichen planus (LP). As is often the case, by the time the patient gets to dermatology, the clinical picture can be confused and atypical. But there is much to learn from such a case.
LP is the prototypical interface lichenoid dermatitis, which can be depended on to present in certain identifiable ways. In this case, what suggested this diagnosis was the word “puzzling.” It’s the perfect example of the intuitive, somewhat subjective side of dermatologic diagnosis: When I see a condition that baffles me momentarily, my puzzled reaction becomes meaningful because the word puzzling starts with a “p.” Lichen planus has long been associated with an extremely useful mnemonic device known as “The Ps.” Besides puzzling, it can stand for purple, plaquish, polygonal (ie, not round), prurtitic, papular, penile (LP has a predilection for this area), and planar (ie, flat-topped).
But it’s the “puzzling” aspect that unlocks the entrance to “The Ps,” and that in turn leads to consideration of this diagnosis. In this case, LP was not totally obvious, but a biopsy confirmed my clinical suspicions by demonstrating the classic findings associated with it.
An LP-like eruption can be provoked by drugs, such as the antimalarials, gold compounds, and penicillamine. It has also been associated with hepatitis C. But in my experience, the most common trigger appears to be stress, since it’s a rare case that doesn’t involve some. (I’ll happily concede that this is my opinion and not established fact.)
This patient’s LP demonstrated two common variants: For one, the darker the patient’s skin, the deeper the purple color of the LP—sometimes to the point of obscuring the diagnosis.
As if that were not enough to confuse the examiner, there was an element of lichen simplex chronicus (LSC; also known as neurodermatitis), which instigates the “itch-scratch-itch” cycle. By its nature, LSC tends to hyperpigmentation (brown). By definition, it involves hypertrophy of the epidermis secondary to scratching. But there is always a primary cause; sometimes mere dry skin will trigger it, or insect bites, eczema, or even psoriasis. This confuses the issue, but the biopsy will establish the true basis for the patient’s condition.
The more common presentation of LP is that of a collection of flat-topped (“planar”), pruritic, purplish papules on the volar wrist, ankles, and sacral area. The surfaces of these lesions often demonstrate a faintly white, shiny surface. LP can also appear on oral mucosal surfaces, where it has a totally different, lacy-white look. In these areas, it tends to burn and not itch. LP appears to have a predilection for the penile glans, where it presents as an annular purplish plaque that sits astride the proximal glans, spilling over onto the coronal area.
LP can present as a widespread generalized eruption mimicking granuloma annulare and secondary syphilis. The differential for simple, classic LP includes psoriasis, granuloma annulare and pityriasis rosea.
Treatment of LP is with topical steroid creams or ointments, tailored in terms of strength to the area of involvement. For example, a class II or III corticosteroid would be used on areas with thicker skin and a class IV or V on thinner skin. Stubborn, solitary plaques can be treated with intralesional steroid injection (2.5 to 10 mg/cc triamcinolone solution).
TAKE-HOME LEARNING POINTS
• Lichen planus (LP) is common and typically presents on the volar wrists, dorsal ankles, and sacrum.
• In a minority of cases, LP can present on the legs as a hypertrophic, plaquish condition with secondary features of lichen simplex chronicus.
• A mnemonic device (“The Ps”) describes LP: papular, pruritic, purple, planar, polygonal, plaquish, and (perhaps most importantly) “puzzling.”
• LP can present as an annular plaque on the penile glans.
• LP has a pathognomic histologic picture on biopsy.
When There’s More to the Story …
Three or four weeks ago, this 56-year-old man noticed asymptomatic lesions on his hands and feet. He says he’s never had anything like them before. He denies taking any new medications and claims to “feel fine,” with no fever, joint pain, or malaise.
EXAMINATION
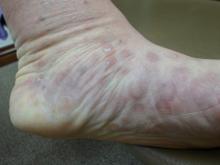
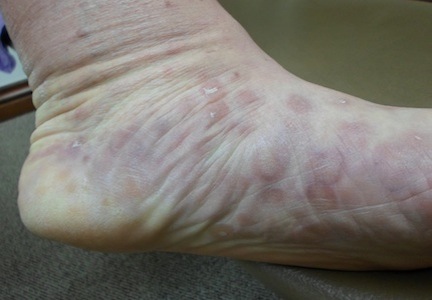
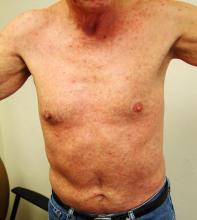
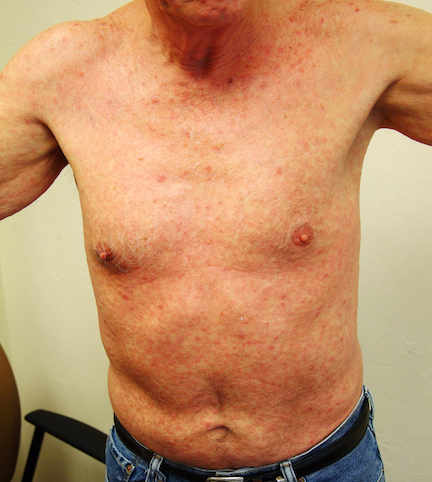
Numerous round macules and papules are seen on the patient’s palms and soles, many of which have scaly peripheral margins. Most are brownish-red, and they are especially dense on the periphery of the feet.
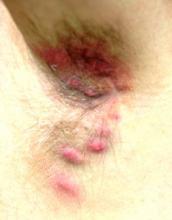
Looking elsewhere, similar lesions are noted on the penile corona and glans. There is a faint but definite morbiliform, blanchable pink rash covering most of the patient’s trunk, taking on a “shawl” distribution across the shoulders.
These findings prompt a more directed and thorough history, which reveals that the patient is exclusively homosexual and recently engaged in high-risk sexual activity. He denies being HIV-positive, though he admits he hasn’t been tested for several years.
At this point, the patient admits to consulting a urologist two weeks ago. However, he came away from that visit with an assurance that serious disease was “unlikely.”
Accordingly, laboratory tests, including a rapid plasma reagin (RPR), are obtained. The RPR results are positive (1:64). The case is reported to the county health department.
DISCUSSION
It’s been said (by me and others) that half the job of any clinician is staying awake, in advance of the inevitable appearance of serious disease. These diseases are out there but don’t always appear the way they do in the textbooks. “Monroe’s rule #4” says the more serious the skin disease, the less likely it is to be diagnosed in a timely fashion.
It would be hard to imagine a more classic example of secondary syphilis than was seen in this case, occurring in a patient so obviously at risk. But it’s only “obvious” if you’re ready and aware of how syphilis manifests. It also helps if you understand how common it is and who’s likely to get it.
This patient’s primary care provider didn’t recognize the condition and referred the patient to a provider whose specialty is surgical diseases of the urogenital system. I mean no disrespect to urologists (or to urology PAs/NPs), but there’s no particular reason they would know what this was. It’s analogous to the referral of patients to podiatry for a rash or other lesion on the foot. Being a surgical expert on the structural maladies of the foot and ankle in no way imparts expertise in skin diseases of the same areas.
The point is: Skin diseases belong with the experts in skin disease (ie, dermatology providers). They are uniquely qualified, not only in terms of recognizing what is being seen, but in being able to see those findings in the context of other physical and historical data. This case is the perfect example.
Here’s the thought process that occurred in this case: Rashes of the feet and palms are unusual, though far from unknown. But when the rash is composed of round, slightly scaly lesions concentrated on the peripheral feet and hands, the differential narrows significantly. Pointed questions regarding sexual history become necessary. Homosexuality, by itself, is not a risk factor, but engaging in high-risk behaviors performed with exclusively homosexual partners is. These facts, combined with the discovery of the widespread truncal rash, mandated specific blood tests; once those tests confirmed the suspected diagnosis, the law mandated reporting of the case to the health department.
Representatives of said entity will likely confirm the diagnosis with more specific testing, treat the patient (probably with penicillin injection), then take a detailed history of sexual exposure in order to stop the spread of the disease in the community. Physically, this patient will be fine. But, as one might imagine, more fallout can be expected in terms of accusations and denials.
Other items in the differential for such rashes include: lichen planus, psoriasis, and erythema multiforme. A biopsy would have been necessary had the tests for syphilis been negative.
TAKE-HOME LEARNING POINTS
• Palmar and plantar rashes are unusual and should prompt the examiner to expand the history and physical.
• Secondary syphilis, though uncommon, is far from rare, especially among gay men engaging in high-risk sexual behavior.
• It’s common for the patient to deny the appearance of the chancre of primary syphilis, and such a lesion would be long gone by the time those of secondary syphilis manifest.
• Conditions involving the skin should be seen by a dermatology provider, regardless of location. This includes diseases of the skin, hair, nails, oral mucosa, genitals, feet, or palms. One potential exception is the eye itself, though most diseases “of the eye” are, in reality, diseases of the periocular skin—and belong with a dermatology provider.
Three or four weeks ago, this 56-year-old man noticed asymptomatic lesions on his hands and feet. He says he’s never had anything like them before. He denies taking any new medications and claims to “feel fine,” with no fever, joint pain, or malaise.
EXAMINATION
Numerous round macules and papules are seen on the patient’s palms and soles, many of which have scaly peripheral margins. Most are brownish-red, and they are especially dense on the periphery of the feet.
Looking elsewhere, similar lesions are noted on the penile corona and glans. There is a faint but definite morbiliform, blanchable pink rash covering most of the patient’s trunk, taking on a “shawl” distribution across the shoulders.
These findings prompt a more directed and thorough history, which reveals that the patient is exclusively homosexual and recently engaged in high-risk sexual activity. He denies being HIV-positive, though he admits he hasn’t been tested for several years.
At this point, the patient admits to consulting a urologist two weeks ago. However, he came away from that visit with an assurance that serious disease was “unlikely.”
Accordingly, laboratory tests, including a rapid plasma reagin (RPR), are obtained. The RPR results are positive (1:64). The case is reported to the county health department.
DISCUSSION
It’s been said (by me and others) that half the job of any clinician is staying awake, in advance of the inevitable appearance of serious disease. These diseases are out there but don’t always appear the way they do in the textbooks. “Monroe’s rule #4” says the more serious the skin disease, the less likely it is to be diagnosed in a timely fashion.
It would be hard to imagine a more classic example of secondary syphilis than was seen in this case, occurring in a patient so obviously at risk. But it’s only “obvious” if you’re ready and aware of how syphilis manifests. It also helps if you understand how common it is and who’s likely to get it.
This patient’s primary care provider didn’t recognize the condition and referred the patient to a provider whose specialty is surgical diseases of the urogenital system. I mean no disrespect to urologists (or to urology PAs/NPs), but there’s no particular reason they would know what this was. It’s analogous to the referral of patients to podiatry for a rash or other lesion on the foot. Being a surgical expert on the structural maladies of the foot and ankle in no way imparts expertise in skin diseases of the same areas.
The point is: Skin diseases belong with the experts in skin disease (ie, dermatology providers). They are uniquely qualified, not only in terms of recognizing what is being seen, but in being able to see those findings in the context of other physical and historical data. This case is the perfect example.
Here’s the thought process that occurred in this case: Rashes of the feet and palms are unusual, though far from unknown. But when the rash is composed of round, slightly scaly lesions concentrated on the peripheral feet and hands, the differential narrows significantly. Pointed questions regarding sexual history become necessary. Homosexuality, by itself, is not a risk factor, but engaging in high-risk behaviors performed with exclusively homosexual partners is. These facts, combined with the discovery of the widespread truncal rash, mandated specific blood tests; once those tests confirmed the suspected diagnosis, the law mandated reporting of the case to the health department.
Representatives of said entity will likely confirm the diagnosis with more specific testing, treat the patient (probably with penicillin injection), then take a detailed history of sexual exposure in order to stop the spread of the disease in the community. Physically, this patient will be fine. But, as one might imagine, more fallout can be expected in terms of accusations and denials.
Other items in the differential for such rashes include: lichen planus, psoriasis, and erythema multiforme. A biopsy would have been necessary had the tests for syphilis been negative.
TAKE-HOME LEARNING POINTS
• Palmar and plantar rashes are unusual and should prompt the examiner to expand the history and physical.
• Secondary syphilis, though uncommon, is far from rare, especially among gay men engaging in high-risk sexual behavior.
• It’s common for the patient to deny the appearance of the chancre of primary syphilis, and such a lesion would be long gone by the time those of secondary syphilis manifest.
• Conditions involving the skin should be seen by a dermatology provider, regardless of location. This includes diseases of the skin, hair, nails, oral mucosa, genitals, feet, or palms. One potential exception is the eye itself, though most diseases “of the eye” are, in reality, diseases of the periocular skin—and belong with a dermatology provider.
Three or four weeks ago, this 56-year-old man noticed asymptomatic lesions on his hands and feet. He says he’s never had anything like them before. He denies taking any new medications and claims to “feel fine,” with no fever, joint pain, or malaise.
EXAMINATION
Numerous round macules and papules are seen on the patient’s palms and soles, many of which have scaly peripheral margins. Most are brownish-red, and they are especially dense on the periphery of the feet.
Looking elsewhere, similar lesions are noted on the penile corona and glans. There is a faint but definite morbiliform, blanchable pink rash covering most of the patient’s trunk, taking on a “shawl” distribution across the shoulders.
These findings prompt a more directed and thorough history, which reveals that the patient is exclusively homosexual and recently engaged in high-risk sexual activity. He denies being HIV-positive, though he admits he hasn’t been tested for several years.
At this point, the patient admits to consulting a urologist two weeks ago. However, he came away from that visit with an assurance that serious disease was “unlikely.”
Accordingly, laboratory tests, including a rapid plasma reagin (RPR), are obtained. The RPR results are positive (1:64). The case is reported to the county health department.
DISCUSSION
It’s been said (by me and others) that half the job of any clinician is staying awake, in advance of the inevitable appearance of serious disease. These diseases are out there but don’t always appear the way they do in the textbooks. “Monroe’s rule #4” says the more serious the skin disease, the less likely it is to be diagnosed in a timely fashion.
It would be hard to imagine a more classic example of secondary syphilis than was seen in this case, occurring in a patient so obviously at risk. But it’s only “obvious” if you’re ready and aware of how syphilis manifests. It also helps if you understand how common it is and who’s likely to get it.
This patient’s primary care provider didn’t recognize the condition and referred the patient to a provider whose specialty is surgical diseases of the urogenital system. I mean no disrespect to urologists (or to urology PAs/NPs), but there’s no particular reason they would know what this was. It’s analogous to the referral of patients to podiatry for a rash or other lesion on the foot. Being a surgical expert on the structural maladies of the foot and ankle in no way imparts expertise in skin diseases of the same areas.
The point is: Skin diseases belong with the experts in skin disease (ie, dermatology providers). They are uniquely qualified, not only in terms of recognizing what is being seen, but in being able to see those findings in the context of other physical and historical data. This case is the perfect example.
Here’s the thought process that occurred in this case: Rashes of the feet and palms are unusual, though far from unknown. But when the rash is composed of round, slightly scaly lesions concentrated on the peripheral feet and hands, the differential narrows significantly. Pointed questions regarding sexual history become necessary. Homosexuality, by itself, is not a risk factor, but engaging in high-risk behaviors performed with exclusively homosexual partners is. These facts, combined with the discovery of the widespread truncal rash, mandated specific blood tests; once those tests confirmed the suspected diagnosis, the law mandated reporting of the case to the health department.
Representatives of said entity will likely confirm the diagnosis with more specific testing, treat the patient (probably with penicillin injection), then take a detailed history of sexual exposure in order to stop the spread of the disease in the community. Physically, this patient will be fine. But, as one might imagine, more fallout can be expected in terms of accusations and denials.
Other items in the differential for such rashes include: lichen planus, psoriasis, and erythema multiforme. A biopsy would have been necessary had the tests for syphilis been negative.
TAKE-HOME LEARNING POINTS
• Palmar and plantar rashes are unusual and should prompt the examiner to expand the history and physical.
• Secondary syphilis, though uncommon, is far from rare, especially among gay men engaging in high-risk sexual behavior.
• It’s common for the patient to deny the appearance of the chancre of primary syphilis, and such a lesion would be long gone by the time those of secondary syphilis manifest.
• Conditions involving the skin should be seen by a dermatology provider, regardless of location. This includes diseases of the skin, hair, nails, oral mucosa, genitals, feet, or palms. One potential exception is the eye itself, though most diseases “of the eye” are, in reality, diseases of the periocular skin—and belong with a dermatology provider.
Painless Lesion Interferes With Man’s Vision
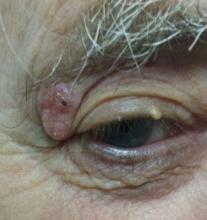
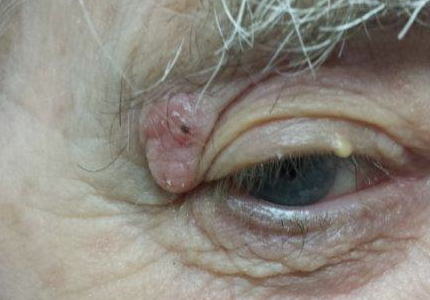
For years, this 80-year-old man has had a lesion on his right upper eyelid. It has slowly grown, though it causes no pain or other symptoms, and is now interfering with his lateral vision. This is what prompts him to seek evaluation.
His primary care providers over the years have seen the lesion. All have assured him of its benignancy.
In the distant past, the patient had a great deal of overexposure to the sun. Several skin cancers have been removed from his face.
At this time, he is residing in a rehab center, where he is recovering from a stroke. With his daughter’s assistance, the patient, who is not ambulatory and is a bit confused, is able to understand what is happening.
EXAMINATION
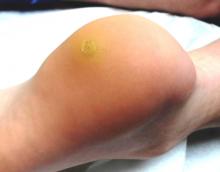
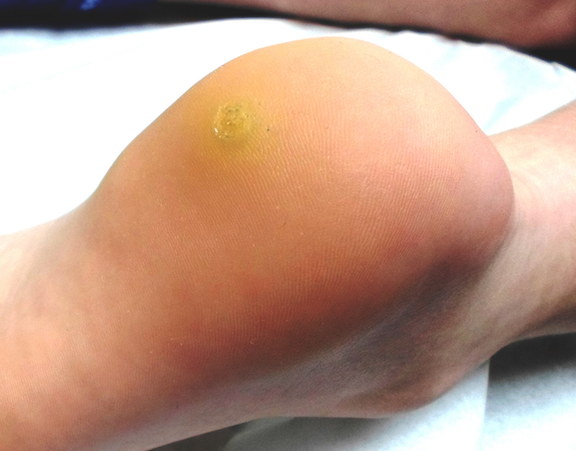
The lesion is a well-defined, 5 mm x 1.1–cm pearly plaque located on the lateral portion of his left upper eyelid, within 2 to 3 mm of the lateral palpebral margin. It is seen in the context of heavily sun-damaged type II facial skin.
A shave biopsy indicates the lesion is a basal cell carcinoma (BCC).
DISCUSSION
BCCs, though rarely fatal, can be associated with a great deal of morbidity. This case highlights several issues regarding the diagnosis and treatment of nonmelanoma skin cancers (eg, basal or squamous cell carcinoma)—not the least of which is the delayed diagnosis. In this instance, the delay is unlikely to harm the patient; however, that is not always the case. If this BCC had been a bit more aggressive, it could have invaded the periocular structures, which might have necessitated extensive surgery and possibly postoperative radiation.
Fortunately, this particular BCC was exceptionally slow to grow, to the extent that doing nothing was a serious consideration. If the patient had been older and/or less capable of cooperating with the surgical process, taking no action might have been the best choice.
But his BCC had grown, and he was able to state his preference (as did the family) to have it surgically removed. As of this writing, he has been scheduled for an appointment with a Mohs surgeon, who is likely to remove the lesion with margins. If the sample tests negative for residual cancer, the patient may not require further treatment. (Given the lesion’s location, the surgical wound does not even require closure, since they usually heal nicely by secondary intention.)
If microscopic examination reveals that the cancer extends into the eyelid itself, the patient will probably be referred to an oculoplastic surgeon for definitive excision and repair, which can be complex and difficult.
While the initial biopsy identified the lesion as a BCC, other items in the differential include seborrheic keratosis and even sebaceous carcinoma (an unusual diagnosis, but one common in patients this age). Squamous cell carcinoma and wart were also possibilities.
TAKE-HOME LEARNING POINTS
• Changing lesions require investigation, usually in the form of a simple shave biopsy.
• Patients with a history of skin cancer tend to develop additional skin cancers.
• Not all basal cell carcinomas are aggressive. Some are remarkably slow-growing.
• For nonaggressive basal cell carcinomas in patients who are unable to cooperate with treatment decisions, consider doing nothing.
• The eyelid is a favorite location for an unusual type of skin cancer: sebaceous carcinoma.
For years, this 80-year-old man has had a lesion on his right upper eyelid. It has slowly grown, though it causes no pain or other symptoms, and is now interfering with his lateral vision. This is what prompts him to seek evaluation.
His primary care providers over the years have seen the lesion. All have assured him of its benignancy.
In the distant past, the patient had a great deal of overexposure to the sun. Several skin cancers have been removed from his face.
At this time, he is residing in a rehab center, where he is recovering from a stroke. With his daughter’s assistance, the patient, who is not ambulatory and is a bit confused, is able to understand what is happening.
EXAMINATION
The lesion is a well-defined, 5 mm x 1.1–cm pearly plaque located on the lateral portion of his left upper eyelid, within 2 to 3 mm of the lateral palpebral margin. It is seen in the context of heavily sun-damaged type II facial skin.
A shave biopsy indicates the lesion is a basal cell carcinoma (BCC).
DISCUSSION
BCCs, though rarely fatal, can be associated with a great deal of morbidity. This case highlights several issues regarding the diagnosis and treatment of nonmelanoma skin cancers (eg, basal or squamous cell carcinoma)—not the least of which is the delayed diagnosis. In this instance, the delay is unlikely to harm the patient; however, that is not always the case. If this BCC had been a bit more aggressive, it could have invaded the periocular structures, which might have necessitated extensive surgery and possibly postoperative radiation.
Fortunately, this particular BCC was exceptionally slow to grow, to the extent that doing nothing was a serious consideration. If the patient had been older and/or less capable of cooperating with the surgical process, taking no action might have been the best choice.
But his BCC had grown, and he was able to state his preference (as did the family) to have it surgically removed. As of this writing, he has been scheduled for an appointment with a Mohs surgeon, who is likely to remove the lesion with margins. If the sample tests negative for residual cancer, the patient may not require further treatment. (Given the lesion’s location, the surgical wound does not even require closure, since they usually heal nicely by secondary intention.)
If microscopic examination reveals that the cancer extends into the eyelid itself, the patient will probably be referred to an oculoplastic surgeon for definitive excision and repair, which can be complex and difficult.
While the initial biopsy identified the lesion as a BCC, other items in the differential include seborrheic keratosis and even sebaceous carcinoma (an unusual diagnosis, but one common in patients this age). Squamous cell carcinoma and wart were also possibilities.
TAKE-HOME LEARNING POINTS
• Changing lesions require investigation, usually in the form of a simple shave biopsy.
• Patients with a history of skin cancer tend to develop additional skin cancers.
• Not all basal cell carcinomas are aggressive. Some are remarkably slow-growing.
• For nonaggressive basal cell carcinomas in patients who are unable to cooperate with treatment decisions, consider doing nothing.
• The eyelid is a favorite location for an unusual type of skin cancer: sebaceous carcinoma.
For years, this 80-year-old man has had a lesion on his right upper eyelid. It has slowly grown, though it causes no pain or other symptoms, and is now interfering with his lateral vision. This is what prompts him to seek evaluation.
His primary care providers over the years have seen the lesion. All have assured him of its benignancy.
In the distant past, the patient had a great deal of overexposure to the sun. Several skin cancers have been removed from his face.
At this time, he is residing in a rehab center, where he is recovering from a stroke. With his daughter’s assistance, the patient, who is not ambulatory and is a bit confused, is able to understand what is happening.
EXAMINATION
The lesion is a well-defined, 5 mm x 1.1–cm pearly plaque located on the lateral portion of his left upper eyelid, within 2 to 3 mm of the lateral palpebral margin. It is seen in the context of heavily sun-damaged type II facial skin.
A shave biopsy indicates the lesion is a basal cell carcinoma (BCC).
DISCUSSION
BCCs, though rarely fatal, can be associated with a great deal of morbidity. This case highlights several issues regarding the diagnosis and treatment of nonmelanoma skin cancers (eg, basal or squamous cell carcinoma)—not the least of which is the delayed diagnosis. In this instance, the delay is unlikely to harm the patient; however, that is not always the case. If this BCC had been a bit more aggressive, it could have invaded the periocular structures, which might have necessitated extensive surgery and possibly postoperative radiation.
Fortunately, this particular BCC was exceptionally slow to grow, to the extent that doing nothing was a serious consideration. If the patient had been older and/or less capable of cooperating with the surgical process, taking no action might have been the best choice.
But his BCC had grown, and he was able to state his preference (as did the family) to have it surgically removed. As of this writing, he has been scheduled for an appointment with a Mohs surgeon, who is likely to remove the lesion with margins. If the sample tests negative for residual cancer, the patient may not require further treatment. (Given the lesion’s location, the surgical wound does not even require closure, since they usually heal nicely by secondary intention.)
If microscopic examination reveals that the cancer extends into the eyelid itself, the patient will probably be referred to an oculoplastic surgeon for definitive excision and repair, which can be complex and difficult.
While the initial biopsy identified the lesion as a BCC, other items in the differential include seborrheic keratosis and even sebaceous carcinoma (an unusual diagnosis, but one common in patients this age). Squamous cell carcinoma and wart were also possibilities.
TAKE-HOME LEARNING POINTS
• Changing lesions require investigation, usually in the form of a simple shave biopsy.
• Patients with a history of skin cancer tend to develop additional skin cancers.
• Not all basal cell carcinomas are aggressive. Some are remarkably slow-growing.
• For nonaggressive basal cell carcinomas in patients who are unable to cooperate with treatment decisions, consider doing nothing.
• The eyelid is a favorite location for an unusual type of skin cancer: sebaceous carcinoma.
Cryotherapy: A Beginner’s Guide
Without a doubt, the most useful treatment modality I’ve acquired in my career is the proper use of liquid nitrogen. Cryotherapy is the standard treatment for small epidermal lesions, because it allows for removal without the need for anesthesia, with no broken skin or bleeding, and with a tolerable amount of pain.
Over the years, I’ve learned—often the hard way!—how to work with liquid nitrogen. For example, for the past 25 years, I’ve used a cryogun (or “unit,” as it’s sometimes called), having discovered that applying LN2 with anything else (eg, a cotton-tipped applicator) is a huge exercise in futility.
As with any tool in medicine, there are uses and misuses of cryotherapy. It is not intuitive—but it does yield to common sense. So allow me to give you the benefit of my experience.
APPROPRIATE AND INAPPROPRIATE TARGETS
In general, only epidermal lesions of obvious origin are treated with liquid nitrogen. This includes ordinary skin tags, small warts, and seborrheic and actinic keratosis. Utterly common and easy to treat, these lesions have no potential for malignant transformation and have relatively poor vasculature, which makes them ideal candidates for controlled, localized frostbite. This process (which can take as long as two weeks) kills the cells, disrupts the blood supply, and causes the lesions to die and fall away.
Other uses for cryotherapy include softening keloids or hypertrophic scars sufficiently to facilitate intralesional injection; treating condyloma, molluscum contagiosum, and small chondromdermatitis nodules; and even to destroy skin cancers (basal cell and squamous cell carcinomas)—the latter, however, only by clinicians with specialized training.
Inappropriate targets for cryotherapy include “moles” (nevi), vascular lesions such as hemangiomas, “birthmarks,” or any lesion of unknown nature. There are two reasons not to use liquid nitrogen in these cases: First, relatively well–vascularized lesions will be superficially blistered but will survive the treatment. Second, these lesions have at least a theoretical chance of having undergone malignant transformation. (As such, these lesions may need to be sampled to determine whether they are malignant; but that is a discussion for another column.)
THE NEED FOR CAUTION
For all its positive features, there are drawbacks to using cryotherapy. Here are six to consider:
• Blistering, which can be severe in sensitive patients
• Dyschromia (color changes in treated skin), especially in darker-skinned patients
• Pain, particularly in children
• Loss of function (eg, nerve or cartilage damage)
• Scarring, usually from overtreatment
• Disability—walking (let alone running or working) may be painful for a day or two after treatment, especially following brisk cryotherapy of a larger plantar wart
TREATING WARTS WITH CRYOTHERAPY
First, you must confirm the diagnosis: “Seeds” (black dots on the surface, which really represent vertically aligned thrombosed capillaries) are pathognomic. Warts lack surface skin lines (dermatoglyphics) and do not umbilicate on paring (unlike clavi or corns).
Always discuss indications, procedure, alternatives, and risks prior to performing cryotherapy. Patients (and parents) need to understand that the “perfect” treatment for warts has yet to be devised. All currently available methods have shortcomings.
Consider using an ear speculum (3 to 5 mm) to concentrate the spray of liquid nitrogen, reducing pain and shortening the length of treatment. Particularly with thicker plantar warts, you might want to pare away surface keratotic material first, then use the “freeze-thaw-freeze” technique. The average length of a single treatment seldom exceeds five seconds, particularly if a speculum or other dam is used.
Arrange for follow-up, typically one month later, since it’s a rare wart that clears with a single treatment.
Without a doubt, the most useful treatment modality I’ve acquired in my career is the proper use of liquid nitrogen. Cryotherapy is the standard treatment for small epidermal lesions, because it allows for removal without the need for anesthesia, with no broken skin or bleeding, and with a tolerable amount of pain.
Over the years, I’ve learned—often the hard way!—how to work with liquid nitrogen. For example, for the past 25 years, I’ve used a cryogun (or “unit,” as it’s sometimes called), having discovered that applying LN2 with anything else (eg, a cotton-tipped applicator) is a huge exercise in futility.
As with any tool in medicine, there are uses and misuses of cryotherapy. It is not intuitive—but it does yield to common sense. So allow me to give you the benefit of my experience.
APPROPRIATE AND INAPPROPRIATE TARGETS
In general, only epidermal lesions of obvious origin are treated with liquid nitrogen. This includes ordinary skin tags, small warts, and seborrheic and actinic keratosis. Utterly common and easy to treat, these lesions have no potential for malignant transformation and have relatively poor vasculature, which makes them ideal candidates for controlled, localized frostbite. This process (which can take as long as two weeks) kills the cells, disrupts the blood supply, and causes the lesions to die and fall away.
Other uses for cryotherapy include softening keloids or hypertrophic scars sufficiently to facilitate intralesional injection; treating condyloma, molluscum contagiosum, and small chondromdermatitis nodules; and even to destroy skin cancers (basal cell and squamous cell carcinomas)—the latter, however, only by clinicians with specialized training.
Inappropriate targets for cryotherapy include “moles” (nevi), vascular lesions such as hemangiomas, “birthmarks,” or any lesion of unknown nature. There are two reasons not to use liquid nitrogen in these cases: First, relatively well–vascularized lesions will be superficially blistered but will survive the treatment. Second, these lesions have at least a theoretical chance of having undergone malignant transformation. (As such, these lesions may need to be sampled to determine whether they are malignant; but that is a discussion for another column.)
THE NEED FOR CAUTION
For all its positive features, there are drawbacks to using cryotherapy. Here are six to consider:
• Blistering, which can be severe in sensitive patients
• Dyschromia (color changes in treated skin), especially in darker-skinned patients
• Pain, particularly in children
• Loss of function (eg, nerve or cartilage damage)
• Scarring, usually from overtreatment
• Disability—walking (let alone running or working) may be painful for a day or two after treatment, especially following brisk cryotherapy of a larger plantar wart
TREATING WARTS WITH CRYOTHERAPY
First, you must confirm the diagnosis: “Seeds” (black dots on the surface, which really represent vertically aligned thrombosed capillaries) are pathognomic. Warts lack surface skin lines (dermatoglyphics) and do not umbilicate on paring (unlike clavi or corns).
Always discuss indications, procedure, alternatives, and risks prior to performing cryotherapy. Patients (and parents) need to understand that the “perfect” treatment for warts has yet to be devised. All currently available methods have shortcomings.
Consider using an ear speculum (3 to 5 mm) to concentrate the spray of liquid nitrogen, reducing pain and shortening the length of treatment. Particularly with thicker plantar warts, you might want to pare away surface keratotic material first, then use the “freeze-thaw-freeze” technique. The average length of a single treatment seldom exceeds five seconds, particularly if a speculum or other dam is used.
Arrange for follow-up, typically one month later, since it’s a rare wart that clears with a single treatment.
Without a doubt, the most useful treatment modality I’ve acquired in my career is the proper use of liquid nitrogen. Cryotherapy is the standard treatment for small epidermal lesions, because it allows for removal without the need for anesthesia, with no broken skin or bleeding, and with a tolerable amount of pain.
Over the years, I’ve learned—often the hard way!—how to work with liquid nitrogen. For example, for the past 25 years, I’ve used a cryogun (or “unit,” as it’s sometimes called), having discovered that applying LN2 with anything else (eg, a cotton-tipped applicator) is a huge exercise in futility.
As with any tool in medicine, there are uses and misuses of cryotherapy. It is not intuitive—but it does yield to common sense. So allow me to give you the benefit of my experience.
APPROPRIATE AND INAPPROPRIATE TARGETS
In general, only epidermal lesions of obvious origin are treated with liquid nitrogen. This includes ordinary skin tags, small warts, and seborrheic and actinic keratosis. Utterly common and easy to treat, these lesions have no potential for malignant transformation and have relatively poor vasculature, which makes them ideal candidates for controlled, localized frostbite. This process (which can take as long as two weeks) kills the cells, disrupts the blood supply, and causes the lesions to die and fall away.
Other uses for cryotherapy include softening keloids or hypertrophic scars sufficiently to facilitate intralesional injection; treating condyloma, molluscum contagiosum, and small chondromdermatitis nodules; and even to destroy skin cancers (basal cell and squamous cell carcinomas)—the latter, however, only by clinicians with specialized training.
Inappropriate targets for cryotherapy include “moles” (nevi), vascular lesions such as hemangiomas, “birthmarks,” or any lesion of unknown nature. There are two reasons not to use liquid nitrogen in these cases: First, relatively well–vascularized lesions will be superficially blistered but will survive the treatment. Second, these lesions have at least a theoretical chance of having undergone malignant transformation. (As such, these lesions may need to be sampled to determine whether they are malignant; but that is a discussion for another column.)
THE NEED FOR CAUTION
For all its positive features, there are drawbacks to using cryotherapy. Here are six to consider:
• Blistering, which can be severe in sensitive patients
• Dyschromia (color changes in treated skin), especially in darker-skinned patients
• Pain, particularly in children
• Loss of function (eg, nerve or cartilage damage)
• Scarring, usually from overtreatment
• Disability—walking (let alone running or working) may be painful for a day or two after treatment, especially following brisk cryotherapy of a larger plantar wart
TREATING WARTS WITH CRYOTHERAPY
First, you must confirm the diagnosis: “Seeds” (black dots on the surface, which really represent vertically aligned thrombosed capillaries) are pathognomic. Warts lack surface skin lines (dermatoglyphics) and do not umbilicate on paring (unlike clavi or corns).
Always discuss indications, procedure, alternatives, and risks prior to performing cryotherapy. Patients (and parents) need to understand that the “perfect” treatment for warts has yet to be devised. All currently available methods have shortcomings.
Consider using an ear speculum (3 to 5 mm) to concentrate the spray of liquid nitrogen, reducing pain and shortening the length of treatment. Particularly with thicker plantar warts, you might want to pare away surface keratotic material first, then use the “freeze-thaw-freeze” technique. The average length of a single treatment seldom exceeds five seconds, particularly if a speculum or other dam is used.
Arrange for follow-up, typically one month later, since it’s a rare wart that clears with a single treatment.
A Little Pink in the Cheeks Signals Serious Problem
A 37-year-old man presents with a complaint of a scalp rash that first appeared several months ago. On his friends’ advice, he tried changing shampoos and applying tea tree oil, with no discernible improvement. He has never had problems with his scalp before, but there is a family history of “unknown skin disease” (mentioned by his father in past conversations). The patient denies any other skin problems.
Recently, he’s experienced a lot of stress: job loss, marital strife, and a subsequent increase in alcohol intake. He has also gained weight, adding that he isn’t getting any exercise. He denies joint pain or swelling.
EXAMINATION
Faint scaling is seen in the patient’s scalp, most of it over and behind the ears. These areas are modestly excoriated as well. There is similar faint scale in both external auditory meati. Examination of the fingernails reveals modest, scattered pits in four nail plates, which the patient says have been there “on and off for years.”
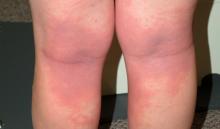
Looking elsewhere, focal heavy white scaling is noted on one knee and both elbows. These findings prompt examination of the patient’s upper intergluteal area, where definite pinkish erythema is observed. It covers an area of 7 x 4 cm, with no significant scaling.
DISCUSSION
You might expect a skin disease that “runs in the family” to be well known to all of them, but in truth, it’s quite common for family members to suffer for years without seeking evaluation by dermatology. Even worse, they might be misdiagnosed by someone else and spend a lifetime thinking they have “eczema” or “ringworm,” and passing this misinformation on to their kin.
Psoriasis is like that; it can present in so many ways, and it can also vary extensively in severity and morphology from one generation to another. As such, while most cases of psoriasis are obvious and therefore easy to diagnose, some are more obscure.
It helps to know how common psoriasis is: It affects 3% of the population, which equates to about 9 million people in the United States. Many, like this patient, have relatively mild cases that flare with stress. Known stressors include infection (especially strep) or introduction of certain medications (eg, β-blockers and lithium). A history of genetic predisposition can be obtained in about 30% of such cases.
This case illustrates a major point about the diagnosis of psoriasis: Often, it must be cobbled together from a collection of findings that appear disconnected on first glance. The scalp findings, by themselves, could have simply represented dry skin or seborrhea. However, taken in context with the nail changes, the family history, the extensor involvement, and intergluteal “pinking,” an almost certain diagnosis emerges.
The intergluteal pinking, by the way, can also be seen with seborrhea—so it’s not pathognomic for psoriasis but is highly suggestive of that diagnosis. The salmon-pink color and lack of scaling (because of the friction and moisture in the affected area) are especially typical.
Those tempted to dismiss all of this as mere sophistry have likely never experienced the effects of this disease, which is notorious for its association with serious negative psychologic repercussions, such as depression, isolation, and suicide. Imagine, for example, having to vacuum out your bed and surrounding carpet every morning just to remove the skin that was shed overnight, then having to be seen and judged by the public.
And that’s not even the worst of it. Up to 30% of psoriatic patients go on to develop psoriatic arthropathy, a destructive form of inflammatory arthritis that is relentless in its course without correct diagnosis and treatment. We know a great deal more about this autoimmune disease now than when I started out in dermatology, and we have marvelous treatment for it, more of which are in the research pipeline.
But these are of little use without the one essential ingredient the clinician must supply: a correct diagnosis.
TAKE-HOME LEARNING POINTS
• Weight gain, smoking, and stress are all documented triggers for psoriasis.
• A positive family history of psoriasis can be obtained in about 30% of suspected cases, but a negative history does not rule out the diagnosis.
• Certain medications, such as β-blockers and lithium, can trigger exacerbations of psoriasis.
• Psoriasis often presents with what appear to be unrelated findings, such as scalp rash, nail pits, genital involvement, and intergluteal pinking.
• Psoriasis is associated with significant psychologic pathology, including poor body image, depression, isolation, increased incidence of drug and alcohol abuse, and suicide.
• Almost 30% of psoriasis patients will develop a related form of arthritis called psoriatic arthropathy.
• Effective treatment is available for psoriasis and psoriatic arthropathy, but these conditions must first be suspected and diagnosed.
A 37-year-old man presents with a complaint of a scalp rash that first appeared several months ago. On his friends’ advice, he tried changing shampoos and applying tea tree oil, with no discernible improvement. He has never had problems with his scalp before, but there is a family history of “unknown skin disease” (mentioned by his father in past conversations). The patient denies any other skin problems.
Recently, he’s experienced a lot of stress: job loss, marital strife, and a subsequent increase in alcohol intake. He has also gained weight, adding that he isn’t getting any exercise. He denies joint pain or swelling.
EXAMINATION
Faint scaling is seen in the patient’s scalp, most of it over and behind the ears. These areas are modestly excoriated as well. There is similar faint scale in both external auditory meati. Examination of the fingernails reveals modest, scattered pits in four nail plates, which the patient says have been there “on and off for years.”
Looking elsewhere, focal heavy white scaling is noted on one knee and both elbows. These findings prompt examination of the patient’s upper intergluteal area, where definite pinkish erythema is observed. It covers an area of 7 x 4 cm, with no significant scaling.
DISCUSSION
You might expect a skin disease that “runs in the family” to be well known to all of them, but in truth, it’s quite common for family members to suffer for years without seeking evaluation by dermatology. Even worse, they might be misdiagnosed by someone else and spend a lifetime thinking they have “eczema” or “ringworm,” and passing this misinformation on to their kin.
Psoriasis is like that; it can present in so many ways, and it can also vary extensively in severity and morphology from one generation to another. As such, while most cases of psoriasis are obvious and therefore easy to diagnose, some are more obscure.
It helps to know how common psoriasis is: It affects 3% of the population, which equates to about 9 million people in the United States. Many, like this patient, have relatively mild cases that flare with stress. Known stressors include infection (especially strep) or introduction of certain medications (eg, β-blockers and lithium). A history of genetic predisposition can be obtained in about 30% of such cases.
This case illustrates a major point about the diagnosis of psoriasis: Often, it must be cobbled together from a collection of findings that appear disconnected on first glance. The scalp findings, by themselves, could have simply represented dry skin or seborrhea. However, taken in context with the nail changes, the family history, the extensor involvement, and intergluteal “pinking,” an almost certain diagnosis emerges.
The intergluteal pinking, by the way, can also be seen with seborrhea—so it’s not pathognomic for psoriasis but is highly suggestive of that diagnosis. The salmon-pink color and lack of scaling (because of the friction and moisture in the affected area) are especially typical.
Those tempted to dismiss all of this as mere sophistry have likely never experienced the effects of this disease, which is notorious for its association with serious negative psychologic repercussions, such as depression, isolation, and suicide. Imagine, for example, having to vacuum out your bed and surrounding carpet every morning just to remove the skin that was shed overnight, then having to be seen and judged by the public.
And that’s not even the worst of it. Up to 30% of psoriatic patients go on to develop psoriatic arthropathy, a destructive form of inflammatory arthritis that is relentless in its course without correct diagnosis and treatment. We know a great deal more about this autoimmune disease now than when I started out in dermatology, and we have marvelous treatment for it, more of which are in the research pipeline.
But these are of little use without the one essential ingredient the clinician must supply: a correct diagnosis.
TAKE-HOME LEARNING POINTS
• Weight gain, smoking, and stress are all documented triggers for psoriasis.
• A positive family history of psoriasis can be obtained in about 30% of suspected cases, but a negative history does not rule out the diagnosis.
• Certain medications, such as β-blockers and lithium, can trigger exacerbations of psoriasis.
• Psoriasis often presents with what appear to be unrelated findings, such as scalp rash, nail pits, genital involvement, and intergluteal pinking.
• Psoriasis is associated with significant psychologic pathology, including poor body image, depression, isolation, increased incidence of drug and alcohol abuse, and suicide.
• Almost 30% of psoriasis patients will develop a related form of arthritis called psoriatic arthropathy.
• Effective treatment is available for psoriasis and psoriatic arthropathy, but these conditions must first be suspected and diagnosed.
A 37-year-old man presents with a complaint of a scalp rash that first appeared several months ago. On his friends’ advice, he tried changing shampoos and applying tea tree oil, with no discernible improvement. He has never had problems with his scalp before, but there is a family history of “unknown skin disease” (mentioned by his father in past conversations). The patient denies any other skin problems.
Recently, he’s experienced a lot of stress: job loss, marital strife, and a subsequent increase in alcohol intake. He has also gained weight, adding that he isn’t getting any exercise. He denies joint pain or swelling.
EXAMINATION
Faint scaling is seen in the patient’s scalp, most of it over and behind the ears. These areas are modestly excoriated as well. There is similar faint scale in both external auditory meati. Examination of the fingernails reveals modest, scattered pits in four nail plates, which the patient says have been there “on and off for years.”
Looking elsewhere, focal heavy white scaling is noted on one knee and both elbows. These findings prompt examination of the patient’s upper intergluteal area, where definite pinkish erythema is observed. It covers an area of 7 x 4 cm, with no significant scaling.
DISCUSSION
You might expect a skin disease that “runs in the family” to be well known to all of them, but in truth, it’s quite common for family members to suffer for years without seeking evaluation by dermatology. Even worse, they might be misdiagnosed by someone else and spend a lifetime thinking they have “eczema” or “ringworm,” and passing this misinformation on to their kin.
Psoriasis is like that; it can present in so many ways, and it can also vary extensively in severity and morphology from one generation to another. As such, while most cases of psoriasis are obvious and therefore easy to diagnose, some are more obscure.
It helps to know how common psoriasis is: It affects 3% of the population, which equates to about 9 million people in the United States. Many, like this patient, have relatively mild cases that flare with stress. Known stressors include infection (especially strep) or introduction of certain medications (eg, β-blockers and lithium). A history of genetic predisposition can be obtained in about 30% of such cases.
This case illustrates a major point about the diagnosis of psoriasis: Often, it must be cobbled together from a collection of findings that appear disconnected on first glance. The scalp findings, by themselves, could have simply represented dry skin or seborrhea. However, taken in context with the nail changes, the family history, the extensor involvement, and intergluteal “pinking,” an almost certain diagnosis emerges.
The intergluteal pinking, by the way, can also be seen with seborrhea—so it’s not pathognomic for psoriasis but is highly suggestive of that diagnosis. The salmon-pink color and lack of scaling (because of the friction and moisture in the affected area) are especially typical.
Those tempted to dismiss all of this as mere sophistry have likely never experienced the effects of this disease, which is notorious for its association with serious negative psychologic repercussions, such as depression, isolation, and suicide. Imagine, for example, having to vacuum out your bed and surrounding carpet every morning just to remove the skin that was shed overnight, then having to be seen and judged by the public.
And that’s not even the worst of it. Up to 30% of psoriatic patients go on to develop psoriatic arthropathy, a destructive form of inflammatory arthritis that is relentless in its course without correct diagnosis and treatment. We know a great deal more about this autoimmune disease now than when I started out in dermatology, and we have marvelous treatment for it, more of which are in the research pipeline.
But these are of little use without the one essential ingredient the clinician must supply: a correct diagnosis.
TAKE-HOME LEARNING POINTS
• Weight gain, smoking, and stress are all documented triggers for psoriasis.
• A positive family history of psoriasis can be obtained in about 30% of suspected cases, but a negative history does not rule out the diagnosis.
• Certain medications, such as β-blockers and lithium, can trigger exacerbations of psoriasis.
• Psoriasis often presents with what appear to be unrelated findings, such as scalp rash, nail pits, genital involvement, and intergluteal pinking.
• Psoriasis is associated with significant psychologic pathology, including poor body image, depression, isolation, increased incidence of drug and alcohol abuse, and suicide.
• Almost 30% of psoriasis patients will develop a related form of arthritis called psoriatic arthropathy.
• Effective treatment is available for psoriasis and psoriatic arthropathy, but these conditions must first be suspected and diagnosed.
The Most Problematic Warts Have No Sure Treatment
A 23-year-old woman presents with an 18-month history of a lesion on her heel that has persisted despite treatment with an OTC salicylic acid preparation, several attempts with an OTC “freezing unit,” and cryotherapy performed by her primary care provider. The lesion is continually aggravated by weight-bearing.
As a child, she reports, she had several warts on her hands. However, they resolved without treatment.
EXAMINATION
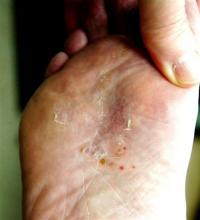
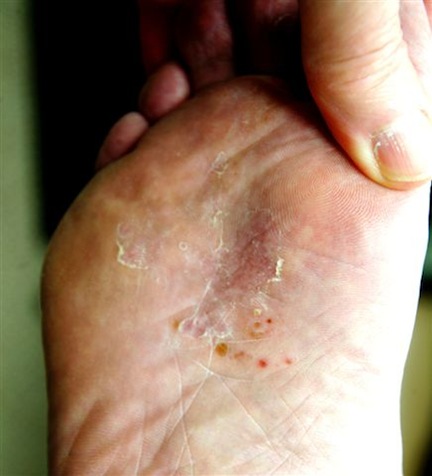
The lesion in question is almost 1 cm in diameter and clearly intradermal in nature. It has a rough, dry feel. On its surface, there are tiny black dots. Normal skin lines flow over the surface of the heel until they reach the lesion; at that point, they curve around its periphery and rejoin on the other side.
DISCUSSION
These features—the black dots, which represent vertically aligned thrombosed capillaries, and the curving skin lines—are diagnostic for plantar warts. This case represents a classic plantar (not “planter”) wart, so named because of its location, not because it has anything to do with planting.
There are good reasons for distinguishing plantar from ordinary warts. For one thing, the outer layer of skin on the sole (the stratum lucidum) is avascular and quite thick, allowing the wart not only to become relatively deep but also to escape detection by the immune system. Since they are almost always on the weight-bearing surface of the sole, even small warts can cause considerable discomfort as they grow.
These same features—the depth and location of the wart—also get in the way of successful treatment, since it may or may not reach the deep margin of the lesion without undue pain and scarring. As in other areas of medicine, we in dermatology strive to avoid treatments that are worse than the disease.
While this is especially true with children, many adults are intolerant of the usual liquid nitrogen treatment (cryotherapy), not only because of the pain associated with the initial application but also because the blistering and pain can persist for days afterward. Perhaps worst of all, no treatment modality is a “sure thing,” so the patient may go through the process and get very little in return.
This combination of issues is why, in dermatology, the first thing we do is to discuss the situation thoroughly with the patient (and parents). My typical conversation starts like this: “You know, you’ve brought us a very difficult problem to treat. This is an infection caused by the human papillomavirus (HPV), which can grow deep and does not elicit much of an immune response. We don’t have drugs or shots to kill the virus. And, as if this were not enough of a problem, none of our treatment choices is remotely perfect. They might not work, and most are sure to cause pain.” Then, of course, I review the treatment options one by one.
TREATMENT
If the patient is lucky and the wart is small and shallow, we typically treat with a liquid nitrogen gun, almost always through a 3 to 5 mm speculum to concentrate the spray. We might shave down the surface of the wart with a #10 blade first, to reduce the thickness and increase the likelihood of successful treatment.
In my opinion, based on many years of treating these warts, using a cotton-tipped applicator to apply the liquid nitrogen is a waste of time and pain. Warts on thin-skinned areas such as arms and legs might be the exception.
Often, with small children, I discuss another option with the parents: that of doing nothing. Warts are warts, not dangerous in any way. Virtually all of them will eventually resolve on their own. The trouble is, of course, that we can’t promise when this will happen, or how big the wart might become in the interim.
There are nonpainful treatment choices, albeit ones with little chance of ultimate success. These include the OTC wart treatment products, virtually all of which contain salicylic acid as their main ingredient. Applied two or three times a week, these products can at least hold smaller warts in check, and could result in a cure.
Much the same could be said for products like cantharidin, a chemical derived from blister beetles, which causes nonpainful and slight blistering at the site. Unfortunately, we can’t prescribe it, which dooms the patient to returning to the office every two or three weeks. As with many such treatment options, it is unlikely to result in a cure.
Another treatment option is dinitrochlorobenzene (DNCB) as the main ingredient in a compounded product. DNCB is a potent T-cell stimulator—another way of saying that we can make the patient allergic to the substance, bringing the body’s immune system to bear on the treated area, producing slight blistering and hopefully destroying the wart in the process. It has to be used with caution, lest the substance wind up on unaffected skin. Parents need to understand that DNCB is slow to work (often taking a month or more) and again, by no means a sure thing. Squaric acid is an alternative to DNCB.
Surgical curettage and electrodessication, under local anesthesia, is potentially the most effective treatment we have for plantar warts—but as you might imagine, piercing the sole of a young child’s foot with a 30-gauge needle is rarely our first choice. It’s the method we used before liquid nitrogen was widely available, and it was a nightmare for all involved. Three outcomes were possible, two quite negative: Serious post-procedure pain and scarring were almost certain. But worst of all, the wart could easily return despite all that. The only time I use this is when the plantar wart is totally resistant to treatment and so large as to interfere with walking.
TAKE-AWAY LEARNING POINTS
• The reason there are 20 or more treatments for warts is that none are remotely perfect.
• Plantar warts are a special problem because they develop on weight-bearing portions of the sole, growing inward (endophytic) in a thick skin layer that allows the virus to avoid detection by the immune system. Plantar warts often cause pain, which treatment can worsen.
• Parents/patients need to understand all of this prior to selecting an appropriate treatment choice.
• They also need to understand that warts do not have to be treated. Most will resolve on their own, eventually.
• Terrorizing children is to be avoided if at all possible. Parents may want their child’s warts to be “taken care of,” but they need to understand this may not be possible.
• Consider referral of problematic plantar warts to dermatology.
A 23-year-old woman presents with an 18-month history of a lesion on her heel that has persisted despite treatment with an OTC salicylic acid preparation, several attempts with an OTC “freezing unit,” and cryotherapy performed by her primary care provider. The lesion is continually aggravated by weight-bearing.
As a child, she reports, she had several warts on her hands. However, they resolved without treatment.
EXAMINATION
The lesion in question is almost 1 cm in diameter and clearly intradermal in nature. It has a rough, dry feel. On its surface, there are tiny black dots. Normal skin lines flow over the surface of the heel until they reach the lesion; at that point, they curve around its periphery and rejoin on the other side.
DISCUSSION
These features—the black dots, which represent vertically aligned thrombosed capillaries, and the curving skin lines—are diagnostic for plantar warts. This case represents a classic plantar (not “planter”) wart, so named because of its location, not because it has anything to do with planting.
There are good reasons for distinguishing plantar from ordinary warts. For one thing, the outer layer of skin on the sole (the stratum lucidum) is avascular and quite thick, allowing the wart not only to become relatively deep but also to escape detection by the immune system. Since they are almost always on the weight-bearing surface of the sole, even small warts can cause considerable discomfort as they grow.
These same features—the depth and location of the wart—also get in the way of successful treatment, since it may or may not reach the deep margin of the lesion without undue pain and scarring. As in other areas of medicine, we in dermatology strive to avoid treatments that are worse than the disease.
While this is especially true with children, many adults are intolerant of the usual liquid nitrogen treatment (cryotherapy), not only because of the pain associated with the initial application but also because the blistering and pain can persist for days afterward. Perhaps worst of all, no treatment modality is a “sure thing,” so the patient may go through the process and get very little in return.
This combination of issues is why, in dermatology, the first thing we do is to discuss the situation thoroughly with the patient (and parents). My typical conversation starts like this: “You know, you’ve brought us a very difficult problem to treat. This is an infection caused by the human papillomavirus (HPV), which can grow deep and does not elicit much of an immune response. We don’t have drugs or shots to kill the virus. And, as if this were not enough of a problem, none of our treatment choices is remotely perfect. They might not work, and most are sure to cause pain.” Then, of course, I review the treatment options one by one.
TREATMENT
If the patient is lucky and the wart is small and shallow, we typically treat with a liquid nitrogen gun, almost always through a 3 to 5 mm speculum to concentrate the spray. We might shave down the surface of the wart with a #10 blade first, to reduce the thickness and increase the likelihood of successful treatment.
In my opinion, based on many years of treating these warts, using a cotton-tipped applicator to apply the liquid nitrogen is a waste of time and pain. Warts on thin-skinned areas such as arms and legs might be the exception.
Often, with small children, I discuss another option with the parents: that of doing nothing. Warts are warts, not dangerous in any way. Virtually all of them will eventually resolve on their own. The trouble is, of course, that we can’t promise when this will happen, or how big the wart might become in the interim.
There are nonpainful treatment choices, albeit ones with little chance of ultimate success. These include the OTC wart treatment products, virtually all of which contain salicylic acid as their main ingredient. Applied two or three times a week, these products can at least hold smaller warts in check, and could result in a cure.
Much the same could be said for products like cantharidin, a chemical derived from blister beetles, which causes nonpainful and slight blistering at the site. Unfortunately, we can’t prescribe it, which dooms the patient to returning to the office every two or three weeks. As with many such treatment options, it is unlikely to result in a cure.
Another treatment option is dinitrochlorobenzene (DNCB) as the main ingredient in a compounded product. DNCB is a potent T-cell stimulator—another way of saying that we can make the patient allergic to the substance, bringing the body’s immune system to bear on the treated area, producing slight blistering and hopefully destroying the wart in the process. It has to be used with caution, lest the substance wind up on unaffected skin. Parents need to understand that DNCB is slow to work (often taking a month or more) and again, by no means a sure thing. Squaric acid is an alternative to DNCB.
Surgical curettage and electrodessication, under local anesthesia, is potentially the most effective treatment we have for plantar warts—but as you might imagine, piercing the sole of a young child’s foot with a 30-gauge needle is rarely our first choice. It’s the method we used before liquid nitrogen was widely available, and it was a nightmare for all involved. Three outcomes were possible, two quite negative: Serious post-procedure pain and scarring were almost certain. But worst of all, the wart could easily return despite all that. The only time I use this is when the plantar wart is totally resistant to treatment and so large as to interfere with walking.
TAKE-AWAY LEARNING POINTS
• The reason there are 20 or more treatments for warts is that none are remotely perfect.
• Plantar warts are a special problem because they develop on weight-bearing portions of the sole, growing inward (endophytic) in a thick skin layer that allows the virus to avoid detection by the immune system. Plantar warts often cause pain, which treatment can worsen.
• Parents/patients need to understand all of this prior to selecting an appropriate treatment choice.
• They also need to understand that warts do not have to be treated. Most will resolve on their own, eventually.
• Terrorizing children is to be avoided if at all possible. Parents may want their child’s warts to be “taken care of,” but they need to understand this may not be possible.
• Consider referral of problematic plantar warts to dermatology.
A 23-year-old woman presents with an 18-month history of a lesion on her heel that has persisted despite treatment with an OTC salicylic acid preparation, several attempts with an OTC “freezing unit,” and cryotherapy performed by her primary care provider. The lesion is continually aggravated by weight-bearing.
As a child, she reports, she had several warts on her hands. However, they resolved without treatment.
EXAMINATION
The lesion in question is almost 1 cm in diameter and clearly intradermal in nature. It has a rough, dry feel. On its surface, there are tiny black dots. Normal skin lines flow over the surface of the heel until they reach the lesion; at that point, they curve around its periphery and rejoin on the other side.
DISCUSSION
These features—the black dots, which represent vertically aligned thrombosed capillaries, and the curving skin lines—are diagnostic for plantar warts. This case represents a classic plantar (not “planter”) wart, so named because of its location, not because it has anything to do with planting.
There are good reasons for distinguishing plantar from ordinary warts. For one thing, the outer layer of skin on the sole (the stratum lucidum) is avascular and quite thick, allowing the wart not only to become relatively deep but also to escape detection by the immune system. Since they are almost always on the weight-bearing surface of the sole, even small warts can cause considerable discomfort as they grow.
These same features—the depth and location of the wart—also get in the way of successful treatment, since it may or may not reach the deep margin of the lesion without undue pain and scarring. As in other areas of medicine, we in dermatology strive to avoid treatments that are worse than the disease.
While this is especially true with children, many adults are intolerant of the usual liquid nitrogen treatment (cryotherapy), not only because of the pain associated with the initial application but also because the blistering and pain can persist for days afterward. Perhaps worst of all, no treatment modality is a “sure thing,” so the patient may go through the process and get very little in return.
This combination of issues is why, in dermatology, the first thing we do is to discuss the situation thoroughly with the patient (and parents). My typical conversation starts like this: “You know, you’ve brought us a very difficult problem to treat. This is an infection caused by the human papillomavirus (HPV), which can grow deep and does not elicit much of an immune response. We don’t have drugs or shots to kill the virus. And, as if this were not enough of a problem, none of our treatment choices is remotely perfect. They might not work, and most are sure to cause pain.” Then, of course, I review the treatment options one by one.
TREATMENT
If the patient is lucky and the wart is small and shallow, we typically treat with a liquid nitrogen gun, almost always through a 3 to 5 mm speculum to concentrate the spray. We might shave down the surface of the wart with a #10 blade first, to reduce the thickness and increase the likelihood of successful treatment.
In my opinion, based on many years of treating these warts, using a cotton-tipped applicator to apply the liquid nitrogen is a waste of time and pain. Warts on thin-skinned areas such as arms and legs might be the exception.
Often, with small children, I discuss another option with the parents: that of doing nothing. Warts are warts, not dangerous in any way. Virtually all of them will eventually resolve on their own. The trouble is, of course, that we can’t promise when this will happen, or how big the wart might become in the interim.
There are nonpainful treatment choices, albeit ones with little chance of ultimate success. These include the OTC wart treatment products, virtually all of which contain salicylic acid as their main ingredient. Applied two or three times a week, these products can at least hold smaller warts in check, and could result in a cure.
Much the same could be said for products like cantharidin, a chemical derived from blister beetles, which causes nonpainful and slight blistering at the site. Unfortunately, we can’t prescribe it, which dooms the patient to returning to the office every two or three weeks. As with many such treatment options, it is unlikely to result in a cure.
Another treatment option is dinitrochlorobenzene (DNCB) as the main ingredient in a compounded product. DNCB is a potent T-cell stimulator—another way of saying that we can make the patient allergic to the substance, bringing the body’s immune system to bear on the treated area, producing slight blistering and hopefully destroying the wart in the process. It has to be used with caution, lest the substance wind up on unaffected skin. Parents need to understand that DNCB is slow to work (often taking a month or more) and again, by no means a sure thing. Squaric acid is an alternative to DNCB.
Surgical curettage and electrodessication, under local anesthesia, is potentially the most effective treatment we have for plantar warts—but as you might imagine, piercing the sole of a young child’s foot with a 30-gauge needle is rarely our first choice. It’s the method we used before liquid nitrogen was widely available, and it was a nightmare for all involved. Three outcomes were possible, two quite negative: Serious post-procedure pain and scarring were almost certain. But worst of all, the wart could easily return despite all that. The only time I use this is when the plantar wart is totally resistant to treatment and so large as to interfere with walking.
TAKE-AWAY LEARNING POINTS
• The reason there are 20 or more treatments for warts is that none are remotely perfect.
• Plantar warts are a special problem because they develop on weight-bearing portions of the sole, growing inward (endophytic) in a thick skin layer that allows the virus to avoid detection by the immune system. Plantar warts often cause pain, which treatment can worsen.
• Parents/patients need to understand all of this prior to selecting an appropriate treatment choice.
• They also need to understand that warts do not have to be treated. Most will resolve on their own, eventually.
• Terrorizing children is to be avoided if at all possible. Parents may want their child’s warts to be “taken care of,” but they need to understand this may not be possible.
• Consider referral of problematic plantar warts to dermatology.
The Truth About Poison Ivy
Picture it: It’s 1948. You’re 12, and you’re itching. Then a rash develops. Your mother says you probably have poison ivy—and sure enough, you’ve been thrashing about in the woods where they told you not to go (but you didn’t listen). It never hurt you before, but now the blisters are coming, in long streaks, on your legs and arms. There’s no sleeping and no holding still, not even in church. The itching is so bad, and vinegar and the pink stuff they put on you don’t help at all; they only call more attention to your rash. Everyone, even your own family, is giving you a wide berth, because they all believe poison ivy is contagious.
Six weeks later, the rash and itching are finally gone—but not the memory of this common condition, for which no effective treatment will exist until topical corticosteroids are introduced in 1951.
All these years later, many myths about poison ivy persist. Some are such powerful misconceptions that many people cannot be swayed from them, even by facts. For example, poison ivy is not in the least contagious. Nor is it caused by any kind of “poison.”
The culprit is an oleoresin known as urushiol. This oily resin is contained in the stems, leaves, and flowers of plants from the Toxicodendron genus, which includes poison ivy (by far the most common source), poison oak (found almost exclusively west of the Rockies) and poison sumac (found in limited areas of the southeastern United States). Similar reactions can be caused by exposure to other botanicals, including ficus, fern, fig, mango, and Japanese lacquer trees. (Another member of the Toxicodendron family is the Rhus tree, found mostly in Australia, where it is notorious for causing rashes.)
A history of recent exposure to the great outdoors can therefore usually be obtained, although exposure can also occur through contact with pets or with the clothing of family members who have been exposed. A live plant is not necessary. Many a victim has acquired poison ivy from clearing brush or handling firewood in the dead of winter. (The antigen can also be acquired through an airborne route, such as from the smoke generated by burning brush—even at a considerable distance.) Repeated exposure to poison ivy over several years’ time is required, which explains why children and city-dwelling adults may appear to be immune.
Typically, the blisters begin to appear within 12 to 48 hours of exposure (with some notable exceptions). Much to the consternation of the patient and family, new lesions can continue to manifest for up to two weeks after initial exposure, which is probably why so many people think poison ivy is contagious. The truth is, there is no urushiol in the fluid from the blisters, nor is the antigen “poison” in any way. In short, poison ivy cannot be transmitted from one person to another.
Poison ivy rashes manifest in several different ways. The most common is a type IV delayed hypersensitivity reaction, with pathognomic linear streaks of erythematous patches of edematous, blistery skin. Figure 1 shows a classic linear lesion on the face of a 69-year-old woman who had cleaned out her fence line a few days previously. This particular patient had a similar rash on her chest.
Figure 2 shows a more atypical form of poison ivy, entirely lacking linear lesions or even blisters. The patient’s rash was widespread, but concentrated on popliteal and antecubital areas and medial thighs. It comprised large sheets of highly erythematous skin, in the centers of which were targetoid reddish blue patches. This is a variant of erythema multiforme, which is by definition a secondary condition usually triggered by bugs (strep, herpes simplex virus) or drugs (sulfa, tetracycline, aspirin, penicillin) but occasionally by an exaggerated reaction to antigens such as poison ivy. In this case, this 185-pound 47-year-old woman and her family acquired poison ivy while picking wildflowers in wet weeds—bringing a rapid end to the family vacation.
The most effective treatment for severe, symptomatic poison ivy is the use of systemic glucocorticoids (eg, prednisone) and/or intramuscular injection of a corticosteroid (eg, triamcinolone). Patient 2’s condition was so severe that she couldn’t sleep, eat, or even drive a car. (Interestingly enough, she was the only one who sought medical evaluation. The rest of her family was content to clean out the local pharmacy’s supply of calamine lotion and diphenhydramine capsules.) Severity of this nature demands serious medication—in this case, a two-week taper of prednisone (from 60 mg down to 20 mg) plus an IM injection of triamcinolone (60 mg).
We made this treatment decision with the knowledge that such doses might produce adverse effects, including an increase in appetite, fluid accumulation, irritability, and sleeplessness. Before prescribing these medications, the potential effects were thoroughly discussed with the patient and a history was taken to rule out antecedent diabetes, severe hypertension, intercurrent infection, peptic ulcer disease, or bipolar affective disorder, all of which could be worsened by the use of corticosteroids.
So-called “dosepaks” of corticosteroids are far too weak for such a serious condition, and topical medications—even the most powerful—will be of little benefit. I gave Patient 2 one more thing: a prescription for hydroxyzine hydrochloride (25 mg tablets, to be taken at bedtime), both for sedative and antipruritic effects. For milder cases of poison ivy, I often advise no treatment except topical because the one thing that can be depended on is that poison ivy will resolve on its own, eventually.
I also educate patients about the appearance of the poison ivy plant (see Figure 3). In my part of the country (Oklahoma), where poison ivy is found in virtually every fence line, every creek bank, and every backyard, most people don’t really know what it looks like. Part of that confusion results from the fact that it can take a number of forms, including a vine, often wrapped around trees, a small tree, or even a root, coursing along the ground. Its distinguishing features include the “leaves of three” in typical shapes (darts, with notches, or “thumbs” along the leaf margins), a shiny surface, and white flowers turning into berries.
TAKE-AWAY LEARNING POINTS
• Poison ivy is predominantly found east of the Rocky Mountains and poison oak almost exclusively to the west of them. Poison sumac is limited to the southeastern United States.
• Poison ivy is also known as a form of phytodermatitis, with other botanical sources such as ficus, fig, fern, mango, rhus, and Japanese lacquer trees.
• Poison ivy can grow in the form of a bush, a vine, or even a small tree. However, it also demonstrates the “leaves of three.”
• Linear blisters on an erythematous base is the classic description of a poison ivy reaction, but it can also present in a more diffuse form that concentrates on the popliteal areas and medial thighs.
• Poison ivy is not “poison.” It is the quintessential contact dermatitis, a type IV delayed hypersensitivity reaction to the oil contained in the plant.
• Poison ivy is not contagious and cannot be spread on the patient’s body or to anyone else.
Picture it: It’s 1948. You’re 12, and you’re itching. Then a rash develops. Your mother says you probably have poison ivy—and sure enough, you’ve been thrashing about in the woods where they told you not to go (but you didn’t listen). It never hurt you before, but now the blisters are coming, in long streaks, on your legs and arms. There’s no sleeping and no holding still, not even in church. The itching is so bad, and vinegar and the pink stuff they put on you don’t help at all; they only call more attention to your rash. Everyone, even your own family, is giving you a wide berth, because they all believe poison ivy is contagious.
Six weeks later, the rash and itching are finally gone—but not the memory of this common condition, for which no effective treatment will exist until topical corticosteroids are introduced in 1951.
All these years later, many myths about poison ivy persist. Some are such powerful misconceptions that many people cannot be swayed from them, even by facts. For example, poison ivy is not in the least contagious. Nor is it caused by any kind of “poison.”
The culprit is an oleoresin known as urushiol. This oily resin is contained in the stems, leaves, and flowers of plants from the Toxicodendron genus, which includes poison ivy (by far the most common source), poison oak (found almost exclusively west of the Rockies) and poison sumac (found in limited areas of the southeastern United States). Similar reactions can be caused by exposure to other botanicals, including ficus, fern, fig, mango, and Japanese lacquer trees. (Another member of the Toxicodendron family is the Rhus tree, found mostly in Australia, where it is notorious for causing rashes.)
A history of recent exposure to the great outdoors can therefore usually be obtained, although exposure can also occur through contact with pets or with the clothing of family members who have been exposed. A live plant is not necessary. Many a victim has acquired poison ivy from clearing brush or handling firewood in the dead of winter. (The antigen can also be acquired through an airborne route, such as from the smoke generated by burning brush—even at a considerable distance.) Repeated exposure to poison ivy over several years’ time is required, which explains why children and city-dwelling adults may appear to be immune.
Typically, the blisters begin to appear within 12 to 48 hours of exposure (with some notable exceptions). Much to the consternation of the patient and family, new lesions can continue to manifest for up to two weeks after initial exposure, which is probably why so many people think poison ivy is contagious. The truth is, there is no urushiol in the fluid from the blisters, nor is the antigen “poison” in any way. In short, poison ivy cannot be transmitted from one person to another.
Poison ivy rashes manifest in several different ways. The most common is a type IV delayed hypersensitivity reaction, with pathognomic linear streaks of erythematous patches of edematous, blistery skin. Figure 1 shows a classic linear lesion on the face of a 69-year-old woman who had cleaned out her fence line a few days previously. This particular patient had a similar rash on her chest.
Figure 2 shows a more atypical form of poison ivy, entirely lacking linear lesions or even blisters. The patient’s rash was widespread, but concentrated on popliteal and antecubital areas and medial thighs. It comprised large sheets of highly erythematous skin, in the centers of which were targetoid reddish blue patches. This is a variant of erythema multiforme, which is by definition a secondary condition usually triggered by bugs (strep, herpes simplex virus) or drugs (sulfa, tetracycline, aspirin, penicillin) but occasionally by an exaggerated reaction to antigens such as poison ivy. In this case, this 185-pound 47-year-old woman and her family acquired poison ivy while picking wildflowers in wet weeds—bringing a rapid end to the family vacation.
The most effective treatment for severe, symptomatic poison ivy is the use of systemic glucocorticoids (eg, prednisone) and/or intramuscular injection of a corticosteroid (eg, triamcinolone). Patient 2’s condition was so severe that she couldn’t sleep, eat, or even drive a car. (Interestingly enough, she was the only one who sought medical evaluation. The rest of her family was content to clean out the local pharmacy’s supply of calamine lotion and diphenhydramine capsules.) Severity of this nature demands serious medication—in this case, a two-week taper of prednisone (from 60 mg down to 20 mg) plus an IM injection of triamcinolone (60 mg).
We made this treatment decision with the knowledge that such doses might produce adverse effects, including an increase in appetite, fluid accumulation, irritability, and sleeplessness. Before prescribing these medications, the potential effects were thoroughly discussed with the patient and a history was taken to rule out antecedent diabetes, severe hypertension, intercurrent infection, peptic ulcer disease, or bipolar affective disorder, all of which could be worsened by the use of corticosteroids.
So-called “dosepaks” of corticosteroids are far too weak for such a serious condition, and topical medications—even the most powerful—will be of little benefit. I gave Patient 2 one more thing: a prescription for hydroxyzine hydrochloride (25 mg tablets, to be taken at bedtime), both for sedative and antipruritic effects. For milder cases of poison ivy, I often advise no treatment except topical because the one thing that can be depended on is that poison ivy will resolve on its own, eventually.
I also educate patients about the appearance of the poison ivy plant (see Figure 3). In my part of the country (Oklahoma), where poison ivy is found in virtually every fence line, every creek bank, and every backyard, most people don’t really know what it looks like. Part of that confusion results from the fact that it can take a number of forms, including a vine, often wrapped around trees, a small tree, or even a root, coursing along the ground. Its distinguishing features include the “leaves of three” in typical shapes (darts, with notches, or “thumbs” along the leaf margins), a shiny surface, and white flowers turning into berries.
TAKE-AWAY LEARNING POINTS
• Poison ivy is predominantly found east of the Rocky Mountains and poison oak almost exclusively to the west of them. Poison sumac is limited to the southeastern United States.
• Poison ivy is also known as a form of phytodermatitis, with other botanical sources such as ficus, fig, fern, mango, rhus, and Japanese lacquer trees.
• Poison ivy can grow in the form of a bush, a vine, or even a small tree. However, it also demonstrates the “leaves of three.”
• Linear blisters on an erythematous base is the classic description of a poison ivy reaction, but it can also present in a more diffuse form that concentrates on the popliteal areas and medial thighs.
• Poison ivy is not “poison.” It is the quintessential contact dermatitis, a type IV delayed hypersensitivity reaction to the oil contained in the plant.
• Poison ivy is not contagious and cannot be spread on the patient’s body or to anyone else.
Picture it: It’s 1948. You’re 12, and you’re itching. Then a rash develops. Your mother says you probably have poison ivy—and sure enough, you’ve been thrashing about in the woods where they told you not to go (but you didn’t listen). It never hurt you before, but now the blisters are coming, in long streaks, on your legs and arms. There’s no sleeping and no holding still, not even in church. The itching is so bad, and vinegar and the pink stuff they put on you don’t help at all; they only call more attention to your rash. Everyone, even your own family, is giving you a wide berth, because they all believe poison ivy is contagious.
Six weeks later, the rash and itching are finally gone—but not the memory of this common condition, for which no effective treatment will exist until topical corticosteroids are introduced in 1951.
All these years later, many myths about poison ivy persist. Some are such powerful misconceptions that many people cannot be swayed from them, even by facts. For example, poison ivy is not in the least contagious. Nor is it caused by any kind of “poison.”
The culprit is an oleoresin known as urushiol. This oily resin is contained in the stems, leaves, and flowers of plants from the Toxicodendron genus, which includes poison ivy (by far the most common source), poison oak (found almost exclusively west of the Rockies) and poison sumac (found in limited areas of the southeastern United States). Similar reactions can be caused by exposure to other botanicals, including ficus, fern, fig, mango, and Japanese lacquer trees. (Another member of the Toxicodendron family is the Rhus tree, found mostly in Australia, where it is notorious for causing rashes.)
A history of recent exposure to the great outdoors can therefore usually be obtained, although exposure can also occur through contact with pets or with the clothing of family members who have been exposed. A live plant is not necessary. Many a victim has acquired poison ivy from clearing brush or handling firewood in the dead of winter. (The antigen can also be acquired through an airborne route, such as from the smoke generated by burning brush—even at a considerable distance.) Repeated exposure to poison ivy over several years’ time is required, which explains why children and city-dwelling adults may appear to be immune.
Typically, the blisters begin to appear within 12 to 48 hours of exposure (with some notable exceptions). Much to the consternation of the patient and family, new lesions can continue to manifest for up to two weeks after initial exposure, which is probably why so many people think poison ivy is contagious. The truth is, there is no urushiol in the fluid from the blisters, nor is the antigen “poison” in any way. In short, poison ivy cannot be transmitted from one person to another.
Poison ivy rashes manifest in several different ways. The most common is a type IV delayed hypersensitivity reaction, with pathognomic linear streaks of erythematous patches of edematous, blistery skin. Figure 1 shows a classic linear lesion on the face of a 69-year-old woman who had cleaned out her fence line a few days previously. This particular patient had a similar rash on her chest.
Figure 2 shows a more atypical form of poison ivy, entirely lacking linear lesions or even blisters. The patient’s rash was widespread, but concentrated on popliteal and antecubital areas and medial thighs. It comprised large sheets of highly erythematous skin, in the centers of which were targetoid reddish blue patches. This is a variant of erythema multiforme, which is by definition a secondary condition usually triggered by bugs (strep, herpes simplex virus) or drugs (sulfa, tetracycline, aspirin, penicillin) but occasionally by an exaggerated reaction to antigens such as poison ivy. In this case, this 185-pound 47-year-old woman and her family acquired poison ivy while picking wildflowers in wet weeds—bringing a rapid end to the family vacation.
The most effective treatment for severe, symptomatic poison ivy is the use of systemic glucocorticoids (eg, prednisone) and/or intramuscular injection of a corticosteroid (eg, triamcinolone). Patient 2’s condition was so severe that she couldn’t sleep, eat, or even drive a car. (Interestingly enough, she was the only one who sought medical evaluation. The rest of her family was content to clean out the local pharmacy’s supply of calamine lotion and diphenhydramine capsules.) Severity of this nature demands serious medication—in this case, a two-week taper of prednisone (from 60 mg down to 20 mg) plus an IM injection of triamcinolone (60 mg).
We made this treatment decision with the knowledge that such doses might produce adverse effects, including an increase in appetite, fluid accumulation, irritability, and sleeplessness. Before prescribing these medications, the potential effects were thoroughly discussed with the patient and a history was taken to rule out antecedent diabetes, severe hypertension, intercurrent infection, peptic ulcer disease, or bipolar affective disorder, all of which could be worsened by the use of corticosteroids.
So-called “dosepaks” of corticosteroids are far too weak for such a serious condition, and topical medications—even the most powerful—will be of little benefit. I gave Patient 2 one more thing: a prescription for hydroxyzine hydrochloride (25 mg tablets, to be taken at bedtime), both for sedative and antipruritic effects. For milder cases of poison ivy, I often advise no treatment except topical because the one thing that can be depended on is that poison ivy will resolve on its own, eventually.
I also educate patients about the appearance of the poison ivy plant (see Figure 3). In my part of the country (Oklahoma), where poison ivy is found in virtually every fence line, every creek bank, and every backyard, most people don’t really know what it looks like. Part of that confusion results from the fact that it can take a number of forms, including a vine, often wrapped around trees, a small tree, or even a root, coursing along the ground. Its distinguishing features include the “leaves of three” in typical shapes (darts, with notches, or “thumbs” along the leaf margins), a shiny surface, and white flowers turning into berries.
TAKE-AWAY LEARNING POINTS
• Poison ivy is predominantly found east of the Rocky Mountains and poison oak almost exclusively to the west of them. Poison sumac is limited to the southeastern United States.
• Poison ivy is also known as a form of phytodermatitis, with other botanical sources such as ficus, fig, fern, mango, rhus, and Japanese lacquer trees.
• Poison ivy can grow in the form of a bush, a vine, or even a small tree. However, it also demonstrates the “leaves of three.”
• Linear blisters on an erythematous base is the classic description of a poison ivy reaction, but it can also present in a more diffuse form that concentrates on the popliteal areas and medial thighs.
• Poison ivy is not “poison.” It is the quintessential contact dermatitis, a type IV delayed hypersensitivity reaction to the oil contained in the plant.
• Poison ivy is not contagious and cannot be spread on the patient’s body or to anyone else.
An Itch for Which OTC Creams Fail
A 50-year-old man self-refers to dermatology for an acute-onset, very itchy rash on the bottom of his foot. The rash appeared a week ago and is so symptomatic that he is unable to sleep. The patient tried applying OTC hydrocortisone 0.5% cream, but it only seemed to worsen his condition.
The patient denies any other significant health concerns. There is no personal or family history of skin problems; no one else in his household has been affected by this outbreak. He has not traveled internationally or domestically in the recent past. The only thing new in his life is a kitten that was rescued from a local animal shelter and given to his teenage daughter by a friend.
EXAMINATION
The rash, confined to the right instep, is composed of tiny, low vesicles surrounded by peripheral scaling. No overt inflammation is noted, although several of the vesicles (which appear to be drying up) have a slightly inflamed appearance. No rash is observed between the toes or elsewhere on the feet.
Scale is carefully collected (with a #10 blade) from the periphery of the site, as well as the dried roofs of some of the old vesicles. KOH examination shows abundant hyphae.
With his condition thus diagnosed as fungal in origin, the patient is given a written prescription for oxiconazole cream. But when he takes the script to his pharmacy, he discovers his insurance will not cover the cost. With advice from the pharmacist, he buys tolnaftate cream and applies it twice a day.
Despite the use of this product, his symptoms continue unabated throughout the intervening weekend. Very distressed, the patient calls in bright and early on Monday morning to complain. He is able to come in and get samples of the originally prescribed oxiconazole cream, which clears the problem within a week.
DISCUSSION
While “athlete’s foot” is a common problem, neither diagnosis nor treatment is always as simple as one might think. First of all, there are three types of tinea pedis—although interdigital is, by far, the most common. Almost always caused by Trichophyton rubrum, it manifests with a white, macerated look between the fourth and fifth toes (occasionally the third and fourth).
Although heredity may play a significant role in terms of susceptibility, sweat and heat are the major factors in the acquisition of tinea pedis. Persistent sweating (and the wearing of shoes, such as boots, that encourage it) often results in a chronic condition that is unlikely to be “cured.”
Almost any imidazole cream (eg econazole, oxiconazole, or clotrimazole) will at least control it, as will allylamine topical preparations (eg, terbinafine and naftin). Patients should understand that, no matter how earnestly the TV pitchman promotes an antifungal cream as “fast actin’,” nothing will change the fact that tolnaftate is ineffective compared to the medications mentioned above.
The second-most common type is moccasin-variety tinea pedis, which is usually asymptomatic and chronic, presenting with a powdery white fine scale that covers the sides and bottoms of both feet. It rarely needs treatment, in part because it’s asymptomatic but also because most affected patients don’t complain about it. This is a good thing, because a cure is virtually impossible.
This brings us to our case patient, who has the most unusual type of tinea pedis: the inflammatory type, which can be anthropophilic or zoophilic (contracted from man or animal). As this case illustrates, it is usually of acute origin, affects the instep, and is highly symptomatic. Unlike the other types, it manifests with inflammatory vesicles, from which peripheral scaling spreads as the lesions resolve. Abundant hyphae are reliably found in the roofs of resolved vesicles, confirming the diagnosis—although this relatively thick skin may take time to be digested by the alkaline KOH.
Trichophyton mentagrophytes, a common pathogen shed in cat hair, can be difficult to treat. Had this case been more severe, oral terbinafine (250 mg/d for two weeks) might have been added to the oxiconazole to clear the problem. The good news is that this type of tinea pedis tends to be episodic and not chronic. The bad news? If the cat is the culprit (by no means is this certain), the condition may recur.
The differential for this condition includes dyshidrotic eczema, contact dermatitis, and scabies.
TAKE-AWAY LEARNING POINTS
• The least common form of tinea pedis is the inflammatory type, which tends to be of acute onset and highly symptomatic (itchy), typically affects the instep, and presents with vesicles.
• KOH prep must be performed to diagnose this condition; it will reveal large numbers of obvious hyphae. The richest source of sample material is the roof of dried vesicles.
• The hydrocortisone the patient used probably made the fungal infection worse.
• Tolnaftate cream, available OTC, is a relatively ineffective treatment for tinea pedis, compared to the imidazoles and allylamines (no matter what John Madden says).
• Inflammatory tinea pedis is often of zoophilic origin, with cats being a common source.
A 50-year-old man self-refers to dermatology for an acute-onset, very itchy rash on the bottom of his foot. The rash appeared a week ago and is so symptomatic that he is unable to sleep. The patient tried applying OTC hydrocortisone 0.5% cream, but it only seemed to worsen his condition.
The patient denies any other significant health concerns. There is no personal or family history of skin problems; no one else in his household has been affected by this outbreak. He has not traveled internationally or domestically in the recent past. The only thing new in his life is a kitten that was rescued from a local animal shelter and given to his teenage daughter by a friend.
EXAMINATION
The rash, confined to the right instep, is composed of tiny, low vesicles surrounded by peripheral scaling. No overt inflammation is noted, although several of the vesicles (which appear to be drying up) have a slightly inflamed appearance. No rash is observed between the toes or elsewhere on the feet.
Scale is carefully collected (with a #10 blade) from the periphery of the site, as well as the dried roofs of some of the old vesicles. KOH examination shows abundant hyphae.
With his condition thus diagnosed as fungal in origin, the patient is given a written prescription for oxiconazole cream. But when he takes the script to his pharmacy, he discovers his insurance will not cover the cost. With advice from the pharmacist, he buys tolnaftate cream and applies it twice a day.
Despite the use of this product, his symptoms continue unabated throughout the intervening weekend. Very distressed, the patient calls in bright and early on Monday morning to complain. He is able to come in and get samples of the originally prescribed oxiconazole cream, which clears the problem within a week.
DISCUSSION
While “athlete’s foot” is a common problem, neither diagnosis nor treatment is always as simple as one might think. First of all, there are three types of tinea pedis—although interdigital is, by far, the most common. Almost always caused by Trichophyton rubrum, it manifests with a white, macerated look between the fourth and fifth toes (occasionally the third and fourth).
Although heredity may play a significant role in terms of susceptibility, sweat and heat are the major factors in the acquisition of tinea pedis. Persistent sweating (and the wearing of shoes, such as boots, that encourage it) often results in a chronic condition that is unlikely to be “cured.”
Almost any imidazole cream (eg econazole, oxiconazole, or clotrimazole) will at least control it, as will allylamine topical preparations (eg, terbinafine and naftin). Patients should understand that, no matter how earnestly the TV pitchman promotes an antifungal cream as “fast actin’,” nothing will change the fact that tolnaftate is ineffective compared to the medications mentioned above.
The second-most common type is moccasin-variety tinea pedis, which is usually asymptomatic and chronic, presenting with a powdery white fine scale that covers the sides and bottoms of both feet. It rarely needs treatment, in part because it’s asymptomatic but also because most affected patients don’t complain about it. This is a good thing, because a cure is virtually impossible.
This brings us to our case patient, who has the most unusual type of tinea pedis: the inflammatory type, which can be anthropophilic or zoophilic (contracted from man or animal). As this case illustrates, it is usually of acute origin, affects the instep, and is highly symptomatic. Unlike the other types, it manifests with inflammatory vesicles, from which peripheral scaling spreads as the lesions resolve. Abundant hyphae are reliably found in the roofs of resolved vesicles, confirming the diagnosis—although this relatively thick skin may take time to be digested by the alkaline KOH.
Trichophyton mentagrophytes, a common pathogen shed in cat hair, can be difficult to treat. Had this case been more severe, oral terbinafine (250 mg/d for two weeks) might have been added to the oxiconazole to clear the problem. The good news is that this type of tinea pedis tends to be episodic and not chronic. The bad news? If the cat is the culprit (by no means is this certain), the condition may recur.
The differential for this condition includes dyshidrotic eczema, contact dermatitis, and scabies.
TAKE-AWAY LEARNING POINTS
• The least common form of tinea pedis is the inflammatory type, which tends to be of acute onset and highly symptomatic (itchy), typically affects the instep, and presents with vesicles.
• KOH prep must be performed to diagnose this condition; it will reveal large numbers of obvious hyphae. The richest source of sample material is the roof of dried vesicles.
• The hydrocortisone the patient used probably made the fungal infection worse.
• Tolnaftate cream, available OTC, is a relatively ineffective treatment for tinea pedis, compared to the imidazoles and allylamines (no matter what John Madden says).
• Inflammatory tinea pedis is often of zoophilic origin, with cats being a common source.
A 50-year-old man self-refers to dermatology for an acute-onset, very itchy rash on the bottom of his foot. The rash appeared a week ago and is so symptomatic that he is unable to sleep. The patient tried applying OTC hydrocortisone 0.5% cream, but it only seemed to worsen his condition.
The patient denies any other significant health concerns. There is no personal or family history of skin problems; no one else in his household has been affected by this outbreak. He has not traveled internationally or domestically in the recent past. The only thing new in his life is a kitten that was rescued from a local animal shelter and given to his teenage daughter by a friend.
EXAMINATION
The rash, confined to the right instep, is composed of tiny, low vesicles surrounded by peripheral scaling. No overt inflammation is noted, although several of the vesicles (which appear to be drying up) have a slightly inflamed appearance. No rash is observed between the toes or elsewhere on the feet.
Scale is carefully collected (with a #10 blade) from the periphery of the site, as well as the dried roofs of some of the old vesicles. KOH examination shows abundant hyphae.
With his condition thus diagnosed as fungal in origin, the patient is given a written prescription for oxiconazole cream. But when he takes the script to his pharmacy, he discovers his insurance will not cover the cost. With advice from the pharmacist, he buys tolnaftate cream and applies it twice a day.
Despite the use of this product, his symptoms continue unabated throughout the intervening weekend. Very distressed, the patient calls in bright and early on Monday morning to complain. He is able to come in and get samples of the originally prescribed oxiconazole cream, which clears the problem within a week.
DISCUSSION
While “athlete’s foot” is a common problem, neither diagnosis nor treatment is always as simple as one might think. First of all, there are three types of tinea pedis—although interdigital is, by far, the most common. Almost always caused by Trichophyton rubrum, it manifests with a white, macerated look between the fourth and fifth toes (occasionally the third and fourth).
Although heredity may play a significant role in terms of susceptibility, sweat and heat are the major factors in the acquisition of tinea pedis. Persistent sweating (and the wearing of shoes, such as boots, that encourage it) often results in a chronic condition that is unlikely to be “cured.”
Almost any imidazole cream (eg econazole, oxiconazole, or clotrimazole) will at least control it, as will allylamine topical preparations (eg, terbinafine and naftin). Patients should understand that, no matter how earnestly the TV pitchman promotes an antifungal cream as “fast actin’,” nothing will change the fact that tolnaftate is ineffective compared to the medications mentioned above.
The second-most common type is moccasin-variety tinea pedis, which is usually asymptomatic and chronic, presenting with a powdery white fine scale that covers the sides and bottoms of both feet. It rarely needs treatment, in part because it’s asymptomatic but also because most affected patients don’t complain about it. This is a good thing, because a cure is virtually impossible.
This brings us to our case patient, who has the most unusual type of tinea pedis: the inflammatory type, which can be anthropophilic or zoophilic (contracted from man or animal). As this case illustrates, it is usually of acute origin, affects the instep, and is highly symptomatic. Unlike the other types, it manifests with inflammatory vesicles, from which peripheral scaling spreads as the lesions resolve. Abundant hyphae are reliably found in the roofs of resolved vesicles, confirming the diagnosis—although this relatively thick skin may take time to be digested by the alkaline KOH.
Trichophyton mentagrophytes, a common pathogen shed in cat hair, can be difficult to treat. Had this case been more severe, oral terbinafine (250 mg/d for two weeks) might have been added to the oxiconazole to clear the problem. The good news is that this type of tinea pedis tends to be episodic and not chronic. The bad news? If the cat is the culprit (by no means is this certain), the condition may recur.
The differential for this condition includes dyshidrotic eczema, contact dermatitis, and scabies.
TAKE-AWAY LEARNING POINTS
• The least common form of tinea pedis is the inflammatory type, which tends to be of acute onset and highly symptomatic (itchy), typically affects the instep, and presents with vesicles.
• KOH prep must be performed to diagnose this condition; it will reveal large numbers of obvious hyphae. The richest source of sample material is the roof of dried vesicles.
• The hydrocortisone the patient used probably made the fungal infection worse.
• Tolnaftate cream, available OTC, is a relatively ineffective treatment for tinea pedis, compared to the imidazoles and allylamines (no matter what John Madden says).
• Inflammatory tinea pedis is often of zoophilic origin, with cats being a common source.
Unusual Cause for Asymptomatic Rash
A 52-year-old man is referred to dermatology by his primary care provider for evaluation of an extensive rash. When it first appeared a month ago, the rash was confined to his abdomen. It has subsequently spread—slowly but steadily—to most of his body.
Although it is asymptomatic, the rash is nonetheless of considerable concern to the patient. He denies any history of atopy and has not experienced fever or malaise during the rash’s manifestation. A number of OTC products, including calamine lotion and hydrocortisone 1% cream, have been tried without success.
During history taking, the patient indicates that he is HIV-positive. He was diagnosed more than 10 years ago, and he reports that his disease is well controlled with medication. Homosexually active, he denies having any new contacts.
EXAMINATION
The patient is in no acute distress, although his complexion appears somewhat sallow. The rash is composed of blanchable, erythematous papules and nodules averaging a centimeter in diameter. It is fairly dense, uniformly covering most of his skin but sparing his face and soles. Two 7-mm scaly brown nodules are seen on his right palm. There are no palpable nodes in the usual locations.
Records from his primary care provider are examined. These confirm the patient’s report of his HIV-positive status.
A 4-mm punch biopsy is performed on one of his truncal lesions, with the sample submitted for routine handling.
HISTOPATHOLOGY
Superficial bandlike perivascular infiltrates composed of lymphocytes, macrophages, and plasma cells are noted, along with psoriasiform epidermal hyperplasia and hyperkeratosis. Stains for spirochetes are negative.
DISCUSSION
The differential for generalized red rashes is prodigious and includes granuloma annulare, lichen planus, lupus, and pityriasis rosea, just to name a few. However, this case presents a fairly typical clinical picture of secondary syphilis—a diagnosis that requires confirmation with syphilis serology: rapid plasma reagin (RPR) or Venereal Disease Research Laboratory (VDRL) testing. The latter measures antibodies to the lipids formed by the host against lipids formed on the treponemal cell surface.
In this case, the diagnosis had to be confirmed by more specific treponemal tests, usually conducted by the local health department, to which positive results must be reported. If further testing confirms the diagnosis (as expected), the patient will be treated by the health department. Investigators will question him, attempting to determine the source of the infection and thereby quell an outbreak.
Primary syphilis usually involves development of the characteristic asymptomatic ulcer with rolled edges, called a chancre, on the genitals, lips, or other area. This lesion is often overlooked or (as is often the case) no history of it can be obtained. Four to 10 weeks later, if left untreated, secondary syphilis develops, manifesting with the variable appearance of lesions on the palms and soles, generalized lymphadenopathy, patchy alopecia (termed a “moth-eaten” look), and generalized rash.
Left undiagnosed and treated, secondary syphilis can progress, over several years, to tertiary syphilis, which can manifest in a number of ways (eg, involvement of the heart or nervous system). It may play a role in the formation of “gummatous” lesions, foci of necrotic tissue that can develop in internal organs or in the mouth, where “punched out” defects can manifest.
The causative organism of syphilis is Treponema pallidum, a spirochete usually transmitted directly from the infected individual through breaks in genital or oral tissues. Though it is usually sexually transmitted, syphilis can be acquired congenitally, through direct contact with open lesions, or through exposure to blood products. Since T pallidum only lives for a very short time away from the body, acquiring it from exposure to fomites (eg, toilet seats or door knobs) is virtually impossible.
Huge spikes in rates of this infection occur periodically in the United States. For example, approximately one-tenth of all military draftees in WW1 tested positive for syphilis, a figure that so alarmed public health officials that it lead to the origins of the monitoring of syphilis by governmental agencies. Another spike was noted during WW2, reaching a rate of 66 cases per 100,000 population in 1947. But with the end of warfare and the introduction of penicillin, the rate fell rapidly and today stands at about 4 cases per 100,000 population, 80% of which occur in the South.
There is a high correlation between a history of men having sex with men (MSM) and infection with syphilis, with 64% of new cases currently coming from those ranks. As might be expected, the correlation between syphilis and HIV-positive status is high. The male to female ratio is approximately 6:1.
TREATMENT
Although this man’s treatment is pending as of this writing, he is likely to receive a single dose of benzathine penicillin G (2.4 million units given IM). Penicillin is so much more effective than alternative treatments that it is strongly recommended even when the patient is allergic to it. (In those cases, desensitization is advised if the allergy is confirmed.) Other treatment options include ceftriaxone and doxycycline.
TAKE-HOME LEARNING POINTS
• Secondary syphilis can present with a generalized rash, the appearance of which can vary greatly from one case to another.
• Persistent, unexplained generalized rashes require biopsy to attempt to explain their origin.
• The presence of plasma cells predominating in the inflammatory infiltrate of a rash is highly suggestive of secondary syphilis.
• Indiscriminate and unprotected sex, particularly among men who have sex with men, correlates with risk for a number of conditions, including syphilis and HIV; this information must be sought in the history-taking process.
• Secondary syphilis must be reported to the local health department for investigation and definitive treatment.
A 52-year-old man is referred to dermatology by his primary care provider for evaluation of an extensive rash. When it first appeared a month ago, the rash was confined to his abdomen. It has subsequently spread—slowly but steadily—to most of his body.
Although it is asymptomatic, the rash is nonetheless of considerable concern to the patient. He denies any history of atopy and has not experienced fever or malaise during the rash’s manifestation. A number of OTC products, including calamine lotion and hydrocortisone 1% cream, have been tried without success.
During history taking, the patient indicates that he is HIV-positive. He was diagnosed more than 10 years ago, and he reports that his disease is well controlled with medication. Homosexually active, he denies having any new contacts.
EXAMINATION
The patient is in no acute distress, although his complexion appears somewhat sallow. The rash is composed of blanchable, erythematous papules and nodules averaging a centimeter in diameter. It is fairly dense, uniformly covering most of his skin but sparing his face and soles. Two 7-mm scaly brown nodules are seen on his right palm. There are no palpable nodes in the usual locations.
Records from his primary care provider are examined. These confirm the patient’s report of his HIV-positive status.
A 4-mm punch biopsy is performed on one of his truncal lesions, with the sample submitted for routine handling.
HISTOPATHOLOGY
Superficial bandlike perivascular infiltrates composed of lymphocytes, macrophages, and plasma cells are noted, along with psoriasiform epidermal hyperplasia and hyperkeratosis. Stains for spirochetes are negative.
DISCUSSION
The differential for generalized red rashes is prodigious and includes granuloma annulare, lichen planus, lupus, and pityriasis rosea, just to name a few. However, this case presents a fairly typical clinical picture of secondary syphilis—a diagnosis that requires confirmation with syphilis serology: rapid plasma reagin (RPR) or Venereal Disease Research Laboratory (VDRL) testing. The latter measures antibodies to the lipids formed by the host against lipids formed on the treponemal cell surface.
In this case, the diagnosis had to be confirmed by more specific treponemal tests, usually conducted by the local health department, to which positive results must be reported. If further testing confirms the diagnosis (as expected), the patient will be treated by the health department. Investigators will question him, attempting to determine the source of the infection and thereby quell an outbreak.
Primary syphilis usually involves development of the characteristic asymptomatic ulcer with rolled edges, called a chancre, on the genitals, lips, or other area. This lesion is often overlooked or (as is often the case) no history of it can be obtained. Four to 10 weeks later, if left untreated, secondary syphilis develops, manifesting with the variable appearance of lesions on the palms and soles, generalized lymphadenopathy, patchy alopecia (termed a “moth-eaten” look), and generalized rash.
Left undiagnosed and treated, secondary syphilis can progress, over several years, to tertiary syphilis, which can manifest in a number of ways (eg, involvement of the heart or nervous system). It may play a role in the formation of “gummatous” lesions, foci of necrotic tissue that can develop in internal organs or in the mouth, where “punched out” defects can manifest.
The causative organism of syphilis is Treponema pallidum, a spirochete usually transmitted directly from the infected individual through breaks in genital or oral tissues. Though it is usually sexually transmitted, syphilis can be acquired congenitally, through direct contact with open lesions, or through exposure to blood products. Since T pallidum only lives for a very short time away from the body, acquiring it from exposure to fomites (eg, toilet seats or door knobs) is virtually impossible.
Huge spikes in rates of this infection occur periodically in the United States. For example, approximately one-tenth of all military draftees in WW1 tested positive for syphilis, a figure that so alarmed public health officials that it lead to the origins of the monitoring of syphilis by governmental agencies. Another spike was noted during WW2, reaching a rate of 66 cases per 100,000 population in 1947. But with the end of warfare and the introduction of penicillin, the rate fell rapidly and today stands at about 4 cases per 100,000 population, 80% of which occur in the South.
There is a high correlation between a history of men having sex with men (MSM) and infection with syphilis, with 64% of new cases currently coming from those ranks. As might be expected, the correlation between syphilis and HIV-positive status is high. The male to female ratio is approximately 6:1.
TREATMENT
Although this man’s treatment is pending as of this writing, he is likely to receive a single dose of benzathine penicillin G (2.4 million units given IM). Penicillin is so much more effective than alternative treatments that it is strongly recommended even when the patient is allergic to it. (In those cases, desensitization is advised if the allergy is confirmed.) Other treatment options include ceftriaxone and doxycycline.
TAKE-HOME LEARNING POINTS
• Secondary syphilis can present with a generalized rash, the appearance of which can vary greatly from one case to another.
• Persistent, unexplained generalized rashes require biopsy to attempt to explain their origin.
• The presence of plasma cells predominating in the inflammatory infiltrate of a rash is highly suggestive of secondary syphilis.
• Indiscriminate and unprotected sex, particularly among men who have sex with men, correlates with risk for a number of conditions, including syphilis and HIV; this information must be sought in the history-taking process.
• Secondary syphilis must be reported to the local health department for investigation and definitive treatment.
A 52-year-old man is referred to dermatology by his primary care provider for evaluation of an extensive rash. When it first appeared a month ago, the rash was confined to his abdomen. It has subsequently spread—slowly but steadily—to most of his body.
Although it is asymptomatic, the rash is nonetheless of considerable concern to the patient. He denies any history of atopy and has not experienced fever or malaise during the rash’s manifestation. A number of OTC products, including calamine lotion and hydrocortisone 1% cream, have been tried without success.
During history taking, the patient indicates that he is HIV-positive. He was diagnosed more than 10 years ago, and he reports that his disease is well controlled with medication. Homosexually active, he denies having any new contacts.
EXAMINATION
The patient is in no acute distress, although his complexion appears somewhat sallow. The rash is composed of blanchable, erythematous papules and nodules averaging a centimeter in diameter. It is fairly dense, uniformly covering most of his skin but sparing his face and soles. Two 7-mm scaly brown nodules are seen on his right palm. There are no palpable nodes in the usual locations.
Records from his primary care provider are examined. These confirm the patient’s report of his HIV-positive status.
A 4-mm punch biopsy is performed on one of his truncal lesions, with the sample submitted for routine handling.
HISTOPATHOLOGY
Superficial bandlike perivascular infiltrates composed of lymphocytes, macrophages, and plasma cells are noted, along with psoriasiform epidermal hyperplasia and hyperkeratosis. Stains for spirochetes are negative.
DISCUSSION
The differential for generalized red rashes is prodigious and includes granuloma annulare, lichen planus, lupus, and pityriasis rosea, just to name a few. However, this case presents a fairly typical clinical picture of secondary syphilis—a diagnosis that requires confirmation with syphilis serology: rapid plasma reagin (RPR) or Venereal Disease Research Laboratory (VDRL) testing. The latter measures antibodies to the lipids formed by the host against lipids formed on the treponemal cell surface.
In this case, the diagnosis had to be confirmed by more specific treponemal tests, usually conducted by the local health department, to which positive results must be reported. If further testing confirms the diagnosis (as expected), the patient will be treated by the health department. Investigators will question him, attempting to determine the source of the infection and thereby quell an outbreak.
Primary syphilis usually involves development of the characteristic asymptomatic ulcer with rolled edges, called a chancre, on the genitals, lips, or other area. This lesion is often overlooked or (as is often the case) no history of it can be obtained. Four to 10 weeks later, if left untreated, secondary syphilis develops, manifesting with the variable appearance of lesions on the palms and soles, generalized lymphadenopathy, patchy alopecia (termed a “moth-eaten” look), and generalized rash.
Left undiagnosed and treated, secondary syphilis can progress, over several years, to tertiary syphilis, which can manifest in a number of ways (eg, involvement of the heart or nervous system). It may play a role in the formation of “gummatous” lesions, foci of necrotic tissue that can develop in internal organs or in the mouth, where “punched out” defects can manifest.
The causative organism of syphilis is Treponema pallidum, a spirochete usually transmitted directly from the infected individual through breaks in genital or oral tissues. Though it is usually sexually transmitted, syphilis can be acquired congenitally, through direct contact with open lesions, or through exposure to blood products. Since T pallidum only lives for a very short time away from the body, acquiring it from exposure to fomites (eg, toilet seats or door knobs) is virtually impossible.
Huge spikes in rates of this infection occur periodically in the United States. For example, approximately one-tenth of all military draftees in WW1 tested positive for syphilis, a figure that so alarmed public health officials that it lead to the origins of the monitoring of syphilis by governmental agencies. Another spike was noted during WW2, reaching a rate of 66 cases per 100,000 population in 1947. But with the end of warfare and the introduction of penicillin, the rate fell rapidly and today stands at about 4 cases per 100,000 population, 80% of which occur in the South.
There is a high correlation between a history of men having sex with men (MSM) and infection with syphilis, with 64% of new cases currently coming from those ranks. As might be expected, the correlation between syphilis and HIV-positive status is high. The male to female ratio is approximately 6:1.
TREATMENT
Although this man’s treatment is pending as of this writing, he is likely to receive a single dose of benzathine penicillin G (2.4 million units given IM). Penicillin is so much more effective than alternative treatments that it is strongly recommended even when the patient is allergic to it. (In those cases, desensitization is advised if the allergy is confirmed.) Other treatment options include ceftriaxone and doxycycline.
TAKE-HOME LEARNING POINTS
• Secondary syphilis can present with a generalized rash, the appearance of which can vary greatly from one case to another.
• Persistent, unexplained generalized rashes require biopsy to attempt to explain their origin.
• The presence of plasma cells predominating in the inflammatory infiltrate of a rash is highly suggestive of secondary syphilis.
• Indiscriminate and unprotected sex, particularly among men who have sex with men, correlates with risk for a number of conditions, including syphilis and HIV; this information must be sought in the history-taking process.
• Secondary syphilis must be reported to the local health department for investigation and definitive treatment.
What Actually Qualifies as a “Boil”?
A 49-year-old man self-refers to dermatology for evaluation of new, painful nodules confined to his left axilla. He has never experienced anything like this, although he’s had the occasional “boil” on his legs and trunk in the past two years.
His medical history includes a coronary artery bypass three years ago and a recent follow-up arteriogram, performed in the hospital. It was shortly after this procedure that his axillary lesions appeared.
EXAMINATION
The patient is afebrile but in no distress. His fairly impressive lesions—red, fluctuant, and pus-filled—are confined to the left axilla. There are about 10, each averaging more than a centimeter in diameter. Each lesion is discrete, but none display a central comedone. The right axilla is unaffected.
One of the lesions is incised, releasing a modest amount of pus that is collected and submitted for bacterial culture. With a presumptive diagnosis of methicillin-resistant Staphylococcus aureus (MRSA), the patient is started on trimethoprim/sulfamethoxazole (double strength, bid for a month).
DISCUSSION
This case was clinically consistent with mild furunculosis caused by MRSA—an impression borne out by the bacterial culture and sensitivity. To be more precise, this patient had community-acquired MRSA (CA-MRSA), a relatively minor problem compared to more serious variants such as hospital-acquired MRSA; the latter usually requires hospitalization for delivery of IV antibiotics. (To further confuse the situation, I had to explain to the patient that, although he probably had CA-MRSA, he easily could have acquired it at the hospital where he had his arteriogram.)
The word boil is commonly used by the public to refer to almost any new, red, fluctuant mass. Inflamed epidermal cysts—which don’t usually involve infection—are classic examples of how the term is misused. But what the medical world terms a boil entails skin infection, and two common types of such lesions are furuncles and carbuncles.
Furuncles are red follicular pustules that develop around a hair. Usually of acute onset, furuncles are almost always bright red and often uncomfortable, if not painful. Staph, as in this case, is the most common cause, but not all furunculosis is associated with MRSA. More often, it is caused by relatively trivial normal flora, such as staph epidermidis, by pseudomonas (as in hot tub folliculitis), or even by a yeast-like fungus called Malassezia furfur. Bacterial culture is indicated when furunculosis is significant.
When furuncles coalesce, forming a single large, pus-filled mass in the skin, they are called carbuncles. Common on the neck, groin, and axillae, carbuncles are treated by liberating the contents (incision and drainage) and sometimes are packed to encourage continued drainage. Culture and sensitivity of the contents can guide rational treatment.
As far as the differential, cysts—even inflamed ones—can be of apocrine derivation, especially in the axillae. They can manifest singly or in multiples; one common condition, hidradenitis supprativa, manifests during puberty/menarche with multiple inflamed cysts in intertriginous (skin on skin) locations. There are typically multiple comedones on the surfaces of these lesions. Other types of cysts include pilar cysts (scalp), keratinous cysts (trunk and extremities), digital mucous cysts (fingers and occasionally toes), and ganglion cysts (overlying tendons).
Most cysts have organized, well-defined walls—a useful feature in distinguishing them from furuncles, carbuncles, and abscesses. The last are collections of pus usually associated with inflammation but not with hair follicles. As a result, they are able to form anywhere on the body (most notably, in the mouth). Some abscesses are sterile, such as those caused by heroin or medication injected accidentally into extravascular tissue. Incision and drainage, culture and sensitivity of the contents, and packing of the space are still indicated.
TAKE-HOME LEARNING POINTS
1) Furuncles are collections of pus associated with a hair follicle and are often caused by staph infection.
2) Carbuncles are composed of multiple furuncles that coalesce into a single larger pus-filled lesion, which is often called a “boil.”
3) MRSA is a form of furunculosis caused by Staphylococcus aureus.
4) Cysts can be inflamed but are almost never infected. Cysts have organized walls, a feature that helps distinguish them from furuncles and carbuncles.
5) Abscesses are collections of pus, often associated with inflammation but not with hair follicles.
6) Abscesses can be sterile, or they can be caused by anaerobic bacteria (eg, dental abscesses).
A 49-year-old man self-refers to dermatology for evaluation of new, painful nodules confined to his left axilla. He has never experienced anything like this, although he’s had the occasional “boil” on his legs and trunk in the past two years.
His medical history includes a coronary artery bypass three years ago and a recent follow-up arteriogram, performed in the hospital. It was shortly after this procedure that his axillary lesions appeared.
EXAMINATION
The patient is afebrile but in no distress. His fairly impressive lesions—red, fluctuant, and pus-filled—are confined to the left axilla. There are about 10, each averaging more than a centimeter in diameter. Each lesion is discrete, but none display a central comedone. The right axilla is unaffected.
One of the lesions is incised, releasing a modest amount of pus that is collected and submitted for bacterial culture. With a presumptive diagnosis of methicillin-resistant Staphylococcus aureus (MRSA), the patient is started on trimethoprim/sulfamethoxazole (double strength, bid for a month).
DISCUSSION
This case was clinically consistent with mild furunculosis caused by MRSA—an impression borne out by the bacterial culture and sensitivity. To be more precise, this patient had community-acquired MRSA (CA-MRSA), a relatively minor problem compared to more serious variants such as hospital-acquired MRSA; the latter usually requires hospitalization for delivery of IV antibiotics. (To further confuse the situation, I had to explain to the patient that, although he probably had CA-MRSA, he easily could have acquired it at the hospital where he had his arteriogram.)
The word boil is commonly used by the public to refer to almost any new, red, fluctuant mass. Inflamed epidermal cysts—which don’t usually involve infection—are classic examples of how the term is misused. But what the medical world terms a boil entails skin infection, and two common types of such lesions are furuncles and carbuncles.
Furuncles are red follicular pustules that develop around a hair. Usually of acute onset, furuncles are almost always bright red and often uncomfortable, if not painful. Staph, as in this case, is the most common cause, but not all furunculosis is associated with MRSA. More often, it is caused by relatively trivial normal flora, such as staph epidermidis, by pseudomonas (as in hot tub folliculitis), or even by a yeast-like fungus called Malassezia furfur. Bacterial culture is indicated when furunculosis is significant.
When furuncles coalesce, forming a single large, pus-filled mass in the skin, they are called carbuncles. Common on the neck, groin, and axillae, carbuncles are treated by liberating the contents (incision and drainage) and sometimes are packed to encourage continued drainage. Culture and sensitivity of the contents can guide rational treatment.
As far as the differential, cysts—even inflamed ones—can be of apocrine derivation, especially in the axillae. They can manifest singly or in multiples; one common condition, hidradenitis supprativa, manifests during puberty/menarche with multiple inflamed cysts in intertriginous (skin on skin) locations. There are typically multiple comedones on the surfaces of these lesions. Other types of cysts include pilar cysts (scalp), keratinous cysts (trunk and extremities), digital mucous cysts (fingers and occasionally toes), and ganglion cysts (overlying tendons).
Most cysts have organized, well-defined walls—a useful feature in distinguishing them from furuncles, carbuncles, and abscesses. The last are collections of pus usually associated with inflammation but not with hair follicles. As a result, they are able to form anywhere on the body (most notably, in the mouth). Some abscesses are sterile, such as those caused by heroin or medication injected accidentally into extravascular tissue. Incision and drainage, culture and sensitivity of the contents, and packing of the space are still indicated.
TAKE-HOME LEARNING POINTS
1) Furuncles are collections of pus associated with a hair follicle and are often caused by staph infection.
2) Carbuncles are composed of multiple furuncles that coalesce into a single larger pus-filled lesion, which is often called a “boil.”
3) MRSA is a form of furunculosis caused by Staphylococcus aureus.
4) Cysts can be inflamed but are almost never infected. Cysts have organized walls, a feature that helps distinguish them from furuncles and carbuncles.
5) Abscesses are collections of pus, often associated with inflammation but not with hair follicles.
6) Abscesses can be sterile, or they can be caused by anaerobic bacteria (eg, dental abscesses).
A 49-year-old man self-refers to dermatology for evaluation of new, painful nodules confined to his left axilla. He has never experienced anything like this, although he’s had the occasional “boil” on his legs and trunk in the past two years.
His medical history includes a coronary artery bypass three years ago and a recent follow-up arteriogram, performed in the hospital. It was shortly after this procedure that his axillary lesions appeared.
EXAMINATION
The patient is afebrile but in no distress. His fairly impressive lesions—red, fluctuant, and pus-filled—are confined to the left axilla. There are about 10, each averaging more than a centimeter in diameter. Each lesion is discrete, but none display a central comedone. The right axilla is unaffected.
One of the lesions is incised, releasing a modest amount of pus that is collected and submitted for bacterial culture. With a presumptive diagnosis of methicillin-resistant Staphylococcus aureus (MRSA), the patient is started on trimethoprim/sulfamethoxazole (double strength, bid for a month).
DISCUSSION
This case was clinically consistent with mild furunculosis caused by MRSA—an impression borne out by the bacterial culture and sensitivity. To be more precise, this patient had community-acquired MRSA (CA-MRSA), a relatively minor problem compared to more serious variants such as hospital-acquired MRSA; the latter usually requires hospitalization for delivery of IV antibiotics. (To further confuse the situation, I had to explain to the patient that, although he probably had CA-MRSA, he easily could have acquired it at the hospital where he had his arteriogram.)
The word boil is commonly used by the public to refer to almost any new, red, fluctuant mass. Inflamed epidermal cysts—which don’t usually involve infection—are classic examples of how the term is misused. But what the medical world terms a boil entails skin infection, and two common types of such lesions are furuncles and carbuncles.
Furuncles are red follicular pustules that develop around a hair. Usually of acute onset, furuncles are almost always bright red and often uncomfortable, if not painful. Staph, as in this case, is the most common cause, but not all furunculosis is associated with MRSA. More often, it is caused by relatively trivial normal flora, such as staph epidermidis, by pseudomonas (as in hot tub folliculitis), or even by a yeast-like fungus called Malassezia furfur. Bacterial culture is indicated when furunculosis is significant.
When furuncles coalesce, forming a single large, pus-filled mass in the skin, they are called carbuncles. Common on the neck, groin, and axillae, carbuncles are treated by liberating the contents (incision and drainage) and sometimes are packed to encourage continued drainage. Culture and sensitivity of the contents can guide rational treatment.
As far as the differential, cysts—even inflamed ones—can be of apocrine derivation, especially in the axillae. They can manifest singly or in multiples; one common condition, hidradenitis supprativa, manifests during puberty/menarche with multiple inflamed cysts in intertriginous (skin on skin) locations. There are typically multiple comedones on the surfaces of these lesions. Other types of cysts include pilar cysts (scalp), keratinous cysts (trunk and extremities), digital mucous cysts (fingers and occasionally toes), and ganglion cysts (overlying tendons).
Most cysts have organized, well-defined walls—a useful feature in distinguishing them from furuncles, carbuncles, and abscesses. The last are collections of pus usually associated with inflammation but not with hair follicles. As a result, they are able to form anywhere on the body (most notably, in the mouth). Some abscesses are sterile, such as those caused by heroin or medication injected accidentally into extravascular tissue. Incision and drainage, culture and sensitivity of the contents, and packing of the space are still indicated.
TAKE-HOME LEARNING POINTS
1) Furuncles are collections of pus associated with a hair follicle and are often caused by staph infection.
2) Carbuncles are composed of multiple furuncles that coalesce into a single larger pus-filled lesion, which is often called a “boil.”
3) MRSA is a form of furunculosis caused by Staphylococcus aureus.
4) Cysts can be inflamed but are almost never infected. Cysts have organized walls, a feature that helps distinguish them from furuncles and carbuncles.
5) Abscesses are collections of pus, often associated with inflammation but not with hair follicles.
6) Abscesses can be sterile, or they can be caused by anaerobic bacteria (eg, dental abscesses).