User login
The far-reaching implications of weight gain in pregnancy
It is not surprising that a pregnant woman’s actions heavily influence her developing baby. Ob.gyns. advise patients to stop smoking or to stop using illicit drugs, and limit their alcohol consumption during pregnancy because we know that these substances can cause serious, even fatal, consequences for the fetus. Although we routinely provide nutrition information and guidelines on healthy weight gain in pregnancy, we may not stress the importance of healthy eating to the same degree as we may emphasize the need to eliminate tobacco use. But should we?
In 2011, a study by researchers at Yale University, the University of Texas, and Arizona State University suggested that food can have effects on the brain similar to those of addictive substances (Arch Gen Psychiatry. 2011 Aug;68[8]:808-16). Using MRI, the investigators examined which areas of the brain became active in response to the consumption of a chocolate milkshake, and compared these results to brain scans of people addicted to opioids. The study enrolled 48 women who were lean to obese, based on body mass index. The researchers found that people who were obese had brain activity patterns in response to food that were similar to patterns that people with drug addiction had in response to opioids. Although the sample size was small, the investigators showed, in essence, that food is a “drug.”
Ob.gyns. working with patients who are overweight or obese typically encourage weight loss prior to pregnancy, or suggest limited weight gain during gestation, because obesity increases complications for both the pregnant mother and her unborn baby. If, as the 2011 study suggests, we were to think of food addiction as we do any other drug addiction – tobacco, opioids, alcohol – that should be curbed out of concern for the developing baby, ob.gyns. might tell our patients to reduce or completely eliminate their “trans-fat food habit” before and during pregnancy.
Importantly, a mother’s nutrition, or lack thereof, may exert harmful effects on her child’s long-term health. This idea was intimated decades ago when Dr. David Barker proposed that a person’s future risk for disease began during pregnancy. Exactly how this type of early programming may occur remains to be determined. Therefore, this month we examine the fetal origins of obesity, and have invited Dr. Michael G. Ross, professor of obstetrics and gynecology, and Mina Desai, Ph.D., assistant professor of obstetrics and gynecology, at the University of California, Los Angeles, to discuss this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
It is not surprising that a pregnant woman’s actions heavily influence her developing baby. Ob.gyns. advise patients to stop smoking or to stop using illicit drugs, and limit their alcohol consumption during pregnancy because we know that these substances can cause serious, even fatal, consequences for the fetus. Although we routinely provide nutrition information and guidelines on healthy weight gain in pregnancy, we may not stress the importance of healthy eating to the same degree as we may emphasize the need to eliminate tobacco use. But should we?
In 2011, a study by researchers at Yale University, the University of Texas, and Arizona State University suggested that food can have effects on the brain similar to those of addictive substances (Arch Gen Psychiatry. 2011 Aug;68[8]:808-16). Using MRI, the investigators examined which areas of the brain became active in response to the consumption of a chocolate milkshake, and compared these results to brain scans of people addicted to opioids. The study enrolled 48 women who were lean to obese, based on body mass index. The researchers found that people who were obese had brain activity patterns in response to food that were similar to patterns that people with drug addiction had in response to opioids. Although the sample size was small, the investigators showed, in essence, that food is a “drug.”
Ob.gyns. working with patients who are overweight or obese typically encourage weight loss prior to pregnancy, or suggest limited weight gain during gestation, because obesity increases complications for both the pregnant mother and her unborn baby. If, as the 2011 study suggests, we were to think of food addiction as we do any other drug addiction – tobacco, opioids, alcohol – that should be curbed out of concern for the developing baby, ob.gyns. might tell our patients to reduce or completely eliminate their “trans-fat food habit” before and during pregnancy.
Importantly, a mother’s nutrition, or lack thereof, may exert harmful effects on her child’s long-term health. This idea was intimated decades ago when Dr. David Barker proposed that a person’s future risk for disease began during pregnancy. Exactly how this type of early programming may occur remains to be determined. Therefore, this month we examine the fetal origins of obesity, and have invited Dr. Michael G. Ross, professor of obstetrics and gynecology, and Mina Desai, Ph.D., assistant professor of obstetrics and gynecology, at the University of California, Los Angeles, to discuss this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
It is not surprising that a pregnant woman’s actions heavily influence her developing baby. Ob.gyns. advise patients to stop smoking or to stop using illicit drugs, and limit their alcohol consumption during pregnancy because we know that these substances can cause serious, even fatal, consequences for the fetus. Although we routinely provide nutrition information and guidelines on healthy weight gain in pregnancy, we may not stress the importance of healthy eating to the same degree as we may emphasize the need to eliminate tobacco use. But should we?
In 2011, a study by researchers at Yale University, the University of Texas, and Arizona State University suggested that food can have effects on the brain similar to those of addictive substances (Arch Gen Psychiatry. 2011 Aug;68[8]:808-16). Using MRI, the investigators examined which areas of the brain became active in response to the consumption of a chocolate milkshake, and compared these results to brain scans of people addicted to opioids. The study enrolled 48 women who were lean to obese, based on body mass index. The researchers found that people who were obese had brain activity patterns in response to food that were similar to patterns that people with drug addiction had in response to opioids. Although the sample size was small, the investigators showed, in essence, that food is a “drug.”
Ob.gyns. working with patients who are overweight or obese typically encourage weight loss prior to pregnancy, or suggest limited weight gain during gestation, because obesity increases complications for both the pregnant mother and her unborn baby. If, as the 2011 study suggests, we were to think of food addiction as we do any other drug addiction – tobacco, opioids, alcohol – that should be curbed out of concern for the developing baby, ob.gyns. might tell our patients to reduce or completely eliminate their “trans-fat food habit” before and during pregnancy.
Importantly, a mother’s nutrition, or lack thereof, may exert harmful effects on her child’s long-term health. This idea was intimated decades ago when Dr. David Barker proposed that a person’s future risk for disease began during pregnancy. Exactly how this type of early programming may occur remains to be determined. Therefore, this month we examine the fetal origins of obesity, and have invited Dr. Michael G. Ross, professor of obstetrics and gynecology, and Mina Desai, Ph.D., assistant professor of obstetrics and gynecology, at the University of California, Los Angeles, to discuss this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Managing endometriosis to prevent ovarian cancer
Endometriosis is a common condition, occurring in this country in 1 of 10 women of reproductive age. An association between endometriosis and subsequent ovarian carcinoma has been reported for decades, yet it is only recently that our knowledge has deepened enough to support more rational methods for preventing the malignancy.
Each year, approximately 22,000 new cases of ovarian cancer are diagnosed. The lifetime risk of developing this malignancy is low, but it is the deadliest of the gynecologic malignancies, with diagnosis usually made in advanced stages when prognosis is poor.
Endometriosis shows some characteristics of malignancy, such as the development of local and distant foci, and attachment to and invasion of other tissues with subsequent damage to these tissues. Endometriosis also is characterized by recurrent, unregulated cell proliferation and estrogen-dependent growth.
Our attempts during the past 2 decades to detect ovarian carcinoma at the early stages through a combined screening modality involving transvaginal ultrasound and a test for the serum level of cancer antigen 125 have failed to provide any survival benefit or even any measurable reduction in morbidity. Today, early-stage ovarian carcinoma, which has a 5-year survival rate of more than 90%, is diagnosed in only a minority of women.
There is good news, however. In recent years our insight into the pathophysiology of ovarian cancer has deepened, providing us with a new paradigm for ovarian cancer pathogenesis that divides ovarian epithelial carcinoma into two distinct types with distinct molecular profiles – one which originates largely in the distal portion of the fallopian tube and the other which traces back to endometriosis.
This new paradigm strengthens and helps to explain the reported association between endometriosis and ovarian cancer. It also has important clinical implications for current practice. While we have much more to learn about the etiology of endometriosis and the causes of malignant transformation, our current knowledge provides a strong rationale for identification and close monitoring of some patients with endometriosis deemed at risk for ovarian cancer, risk-reducing medical management, earlier and more meticulous surgical treatment, and close monitoring.
By combining this new approach to endometriosis with consideration of salpingectomy after completion of childbearing, we have an unprecedented opportunity to reduce the incidence of epithelial ovarian cancer.
Dual pathogenesis
The majority of ovarian cancers are of epithelial origin and fall into four histologic categories: serous, endometrioid, clear cell, and mucinous. In recent years, we have gained a deeper understanding of the pathogenesis of ovarian carcinoma, with an array of epidemiologic, histologic, and molecular data showing us that epithelial ovarian cancers are also of two distinct types (Am J Obstet Gynecol. 2015 Sep;213[3]:262-7).
One of these types, a high-grade serous carcinoma, appears to arise in many cases in the epithelium of the fallopian tube. The other type of tumor is a low-grade carcinoma – particularly of the endometrioid and clear cell histologic subtypes – that originates largely from ovarian endometriotic lesions or from borderline serous tumors in the case of serous histology.
The majority of diagnosed stage 1 ovarian cancers are carcinomas of this low-grade type and not high-grade serous carcinomas. In a study of 76 consecutive stage 1 carcinomas, investigators found that ovarian endometriosis was present in 40 of the 76 cases. More than two-thirds of the 76 cases (71%) were nonserous cancers, and almost all of these cases were associated with endometriosis based on histologic examination (Fertil Steril. 2007 Oct;88[4]:906-10).
This study was among the first to show that the majority of stage 1 ovarian carcinomas are not high-grade serous carcinomas, but rather nonserous, primarily endometrioid and clear cell, cancers. The research demonstrated that endometriosis should be viewed as a potential precursor lesion to specific subtypes of ovarian cancer.
The malignant transformation of endometriosis was first suggested by Dr. J. A. Sampson in 1925, and a number of studies – in addition to the 2007 landmark study – have since described ovarian cancer arising from endometriosis, based on the frequent co-occurrence in surgical specimens.
Most recently, a study from the Ovarian Cancer Association Consortium (OCAC) found that women who reported a history of endometriosis had a significantly higher risk of developing ovarian cancer than the general population (odds ratio, 1.46).
Investigators of this critical study pooled data from 13 ovarian cancer case-control studies involving more than 13,226 controls and 7,911 women with invasive epithelial ovarian cancer – 818 (6.2%) and 738 (9.3%) of whom, respectively, reported a history of endometriosis. Specifically, they determined that self-reported endometriosis was associated with a 3.05-fold increased risk for clear cell invasive ovarian cancer and a 2.04-fold increased risk of endometrioid ovarian cancer.
Moreover, a significant association between preexisting endometriosis and low-grade serous invasive ovarian cancer (OR, 2.11) was demonstrated, while no association was found between endometriosis and the risk of high-grade serous invasive ovarian cancer (Lancet Oncol. 2012 Apr;13[4]:385-94).
A second recently published report – a meta-analysis of 20 case-control and 15 cohort studies published between 1990 and 2012 and involving more than 444,000 patients – found that endometriosis increased cancer risk in case-control or two-arm cohort studies by 27% (relative risk, 1.265) and by approximately 80% in single-arm cohort studies (standard incidence ratio, 1.797). Endometrioid and clear cell carcinomas were more common in endometriosis-associated ovarian cancer, while serous carcinoma was less frequent (Br J Cancer. 2014 Apr 2;110[7]:1878-90).
Findings of both of these large studies have served to clarify the association between endometriosis and specific histologic subtypes and suggested that there are important differences in the pathogenesis of low-grade and high-grade serous ovarian carcinomas.
Clinical implications
It is not clear what causes malignant transformation or what predisposes some patients with endometriosis to develop ovarian cancer, but the risk likely involves genetic and epigenetic influences as well as immunologic, inflammatory, and hormonal factors.
The molecular profiles of the main two types of ovarian cancer are different: While the majority of high-grade serous ovarian tumors are characterized by TP53 mutations, the low-grade carcinomas are characterized by a variety of mutations, including KRAS, BRAF, ERBB2, CTNNB1, and BCL2 mutations.
There currently are not enough data to recommend genetic screening tests in patients with endometriosis, but our hope is that we eventually will be able to screen for “high-risk” endometriotic lesions by testing for genes specific to various histologic subtypes of low-grade ovarian cancer, or by finding and utilizing other biomarkers.
In the meantime, we believe it is important to more thoroughly treat endometriosis and to identify and follow women with a history of the condition, especially those with a long-standing history, those with a history of endometriosis associated with infertility, and those with ovarian endometrioma. Each of these factors predisposes patients to a higher risk of malignant transformation.
Complete surgical resection of all visible endometriosis is the most effective treatment and will afford the best cancer prevention, even in women who are asymptomatic. In a recent Swedish national registry case-control study, women who underwent radical surgical excision of all visible endometriosis were significantly less likely (OR, 0.30) to develop ovarian cancer (Acta Obstet Gynecol Scand. 2013 May;92[5]:546-54).
Suppressive hormonal therapy is another treatment option for patients with no interest in conceiving. Most large endometriomas are functional ovarian cysts that have been invaded by cortical ovarian endometriosis or by small primary endometriomas (J Reprod Med. 1992 Sep;37[9]:771-6).
While hormonal therapy will not always result in complete regression of endometriotic lesions, it will decrease the recurrence rate of endometriomas and can be considered for long-term prevention of potentially premalignant lesions. It is most effective when it follows surgical excision of endometriomas and associated endometriosis.
A patient who has completed childbearing at the time of surgical resection may be offered bilateral salpingectomy, regardless of menopausal status. Salpingectomy in both average and high-risk populations (e.g., BRCA 1/2 carriers) not only prevents high-grade serous carcinoma by eliminating the site of origin, but also may decrease the risk of endometrioid and clear cell carcinoma by blocking the passageway that enables the flow of endometrium and factors that induce inflammation. It is estimated that the procedure reduces the risk of ovarian cancer by 40%.
Interestingly, tubal ligation has historically been shown to decrease the risk of ovarian cancer, and recent data have shown that the risk of endometrioid and clear cell carcinoma is cut even more than the risk of high-grade serous carcinoma (Int J Epidemiol. 2013 Apr;42[2]:579-89).
The Society of Gynecologic Oncology recommends that risk-reducing salpingectomy be considered at the time of hysterectomy or other abdominal or pelvic surgery, and in lieu of tubal ligation. The American College of Obstetricians and Gynecologists similarly has stated that prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients. Salpingectomy is an important option for all patients, but is especially important when the fallopian tubes are found to be damaged by endometriosis and/or pelvic inflammatory disease. When imaging studies show that endometriomas are present and resection is not performed, pelvic ultrasound should become part of the patient’s routine examination.
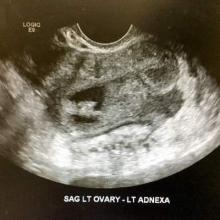
Most endometriomas have a homogeneous appearance; any significant increase in size or a change in the homogeneous cystic characteristics to a more heterogeneous appearance with mural components should raise suspicion about malignant change.
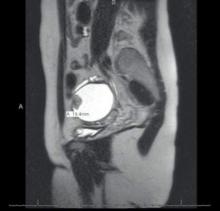
It can be difficult to detect relatively small endocystic components with ultrasound, so if there is any doubt about whether there is some heterogeneous consistency, an MRI should be performed. MRI is showing more promise in detecting malignant change. Hyperdense mural nodules within the ovary and rapid growth of an endometrioma have both been associated with malignant transformation and can be seen on these images.
In a cohort study comparing MRI findings of 10 patients with ovarian adenocarcinoma to 10 patients with benign endometriomas, investigators found mural nodules in all 10 malignancies but in only three of the benign cases (AJR Am J Roentgenol. 2000 Nov;175[5]:1423-30).
Long-term follow-up is necessary to understand the timeline of transformation in patients with mural nodules. This together with increasing knowledge of molecular events underpinning evolution of endometriosis will lead to better screening and preventive strategies.
Dr. Nezhat is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at the State University of New York at Stony Brook. He reported having no financial disclosures.
Endometriosis is a common condition, occurring in this country in 1 of 10 women of reproductive age. An association between endometriosis and subsequent ovarian carcinoma has been reported for decades, yet it is only recently that our knowledge has deepened enough to support more rational methods for preventing the malignancy.
Each year, approximately 22,000 new cases of ovarian cancer are diagnosed. The lifetime risk of developing this malignancy is low, but it is the deadliest of the gynecologic malignancies, with diagnosis usually made in advanced stages when prognosis is poor.
Endometriosis shows some characteristics of malignancy, such as the development of local and distant foci, and attachment to and invasion of other tissues with subsequent damage to these tissues. Endometriosis also is characterized by recurrent, unregulated cell proliferation and estrogen-dependent growth.
Our attempts during the past 2 decades to detect ovarian carcinoma at the early stages through a combined screening modality involving transvaginal ultrasound and a test for the serum level of cancer antigen 125 have failed to provide any survival benefit or even any measurable reduction in morbidity. Today, early-stage ovarian carcinoma, which has a 5-year survival rate of more than 90%, is diagnosed in only a minority of women.
There is good news, however. In recent years our insight into the pathophysiology of ovarian cancer has deepened, providing us with a new paradigm for ovarian cancer pathogenesis that divides ovarian epithelial carcinoma into two distinct types with distinct molecular profiles – one which originates largely in the distal portion of the fallopian tube and the other which traces back to endometriosis.
This new paradigm strengthens and helps to explain the reported association between endometriosis and ovarian cancer. It also has important clinical implications for current practice. While we have much more to learn about the etiology of endometriosis and the causes of malignant transformation, our current knowledge provides a strong rationale for identification and close monitoring of some patients with endometriosis deemed at risk for ovarian cancer, risk-reducing medical management, earlier and more meticulous surgical treatment, and close monitoring.
By combining this new approach to endometriosis with consideration of salpingectomy after completion of childbearing, we have an unprecedented opportunity to reduce the incidence of epithelial ovarian cancer.
Dual pathogenesis
The majority of ovarian cancers are of epithelial origin and fall into four histologic categories: serous, endometrioid, clear cell, and mucinous. In recent years, we have gained a deeper understanding of the pathogenesis of ovarian carcinoma, with an array of epidemiologic, histologic, and molecular data showing us that epithelial ovarian cancers are also of two distinct types (Am J Obstet Gynecol. 2015 Sep;213[3]:262-7).
One of these types, a high-grade serous carcinoma, appears to arise in many cases in the epithelium of the fallopian tube. The other type of tumor is a low-grade carcinoma – particularly of the endometrioid and clear cell histologic subtypes – that originates largely from ovarian endometriotic lesions or from borderline serous tumors in the case of serous histology.
The majority of diagnosed stage 1 ovarian cancers are carcinomas of this low-grade type and not high-grade serous carcinomas. In a study of 76 consecutive stage 1 carcinomas, investigators found that ovarian endometriosis was present in 40 of the 76 cases. More than two-thirds of the 76 cases (71%) were nonserous cancers, and almost all of these cases were associated with endometriosis based on histologic examination (Fertil Steril. 2007 Oct;88[4]:906-10).
This study was among the first to show that the majority of stage 1 ovarian carcinomas are not high-grade serous carcinomas, but rather nonserous, primarily endometrioid and clear cell, cancers. The research demonstrated that endometriosis should be viewed as a potential precursor lesion to specific subtypes of ovarian cancer.
The malignant transformation of endometriosis was first suggested by Dr. J. A. Sampson in 1925, and a number of studies – in addition to the 2007 landmark study – have since described ovarian cancer arising from endometriosis, based on the frequent co-occurrence in surgical specimens.
Most recently, a study from the Ovarian Cancer Association Consortium (OCAC) found that women who reported a history of endometriosis had a significantly higher risk of developing ovarian cancer than the general population (odds ratio, 1.46).
Investigators of this critical study pooled data from 13 ovarian cancer case-control studies involving more than 13,226 controls and 7,911 women with invasive epithelial ovarian cancer – 818 (6.2%) and 738 (9.3%) of whom, respectively, reported a history of endometriosis. Specifically, they determined that self-reported endometriosis was associated with a 3.05-fold increased risk for clear cell invasive ovarian cancer and a 2.04-fold increased risk of endometrioid ovarian cancer.
Moreover, a significant association between preexisting endometriosis and low-grade serous invasive ovarian cancer (OR, 2.11) was demonstrated, while no association was found between endometriosis and the risk of high-grade serous invasive ovarian cancer (Lancet Oncol. 2012 Apr;13[4]:385-94).
A second recently published report – a meta-analysis of 20 case-control and 15 cohort studies published between 1990 and 2012 and involving more than 444,000 patients – found that endometriosis increased cancer risk in case-control or two-arm cohort studies by 27% (relative risk, 1.265) and by approximately 80% in single-arm cohort studies (standard incidence ratio, 1.797). Endometrioid and clear cell carcinomas were more common in endometriosis-associated ovarian cancer, while serous carcinoma was less frequent (Br J Cancer. 2014 Apr 2;110[7]:1878-90).
Findings of both of these large studies have served to clarify the association between endometriosis and specific histologic subtypes and suggested that there are important differences in the pathogenesis of low-grade and high-grade serous ovarian carcinomas.
Clinical implications
It is not clear what causes malignant transformation or what predisposes some patients with endometriosis to develop ovarian cancer, but the risk likely involves genetic and epigenetic influences as well as immunologic, inflammatory, and hormonal factors.
The molecular profiles of the main two types of ovarian cancer are different: While the majority of high-grade serous ovarian tumors are characterized by TP53 mutations, the low-grade carcinomas are characterized by a variety of mutations, including KRAS, BRAF, ERBB2, CTNNB1, and BCL2 mutations.
There currently are not enough data to recommend genetic screening tests in patients with endometriosis, but our hope is that we eventually will be able to screen for “high-risk” endometriotic lesions by testing for genes specific to various histologic subtypes of low-grade ovarian cancer, or by finding and utilizing other biomarkers.
In the meantime, we believe it is important to more thoroughly treat endometriosis and to identify and follow women with a history of the condition, especially those with a long-standing history, those with a history of endometriosis associated with infertility, and those with ovarian endometrioma. Each of these factors predisposes patients to a higher risk of malignant transformation.
Complete surgical resection of all visible endometriosis is the most effective treatment and will afford the best cancer prevention, even in women who are asymptomatic. In a recent Swedish national registry case-control study, women who underwent radical surgical excision of all visible endometriosis were significantly less likely (OR, 0.30) to develop ovarian cancer (Acta Obstet Gynecol Scand. 2013 May;92[5]:546-54).
Suppressive hormonal therapy is another treatment option for patients with no interest in conceiving. Most large endometriomas are functional ovarian cysts that have been invaded by cortical ovarian endometriosis or by small primary endometriomas (J Reprod Med. 1992 Sep;37[9]:771-6).
While hormonal therapy will not always result in complete regression of endometriotic lesions, it will decrease the recurrence rate of endometriomas and can be considered for long-term prevention of potentially premalignant lesions. It is most effective when it follows surgical excision of endometriomas and associated endometriosis.
A patient who has completed childbearing at the time of surgical resection may be offered bilateral salpingectomy, regardless of menopausal status. Salpingectomy in both average and high-risk populations (e.g., BRCA 1/2 carriers) not only prevents high-grade serous carcinoma by eliminating the site of origin, but also may decrease the risk of endometrioid and clear cell carcinoma by blocking the passageway that enables the flow of endometrium and factors that induce inflammation. It is estimated that the procedure reduces the risk of ovarian cancer by 40%.
Interestingly, tubal ligation has historically been shown to decrease the risk of ovarian cancer, and recent data have shown that the risk of endometrioid and clear cell carcinoma is cut even more than the risk of high-grade serous carcinoma (Int J Epidemiol. 2013 Apr;42[2]:579-89).
The Society of Gynecologic Oncology recommends that risk-reducing salpingectomy be considered at the time of hysterectomy or other abdominal or pelvic surgery, and in lieu of tubal ligation. The American College of Obstetricians and Gynecologists similarly has stated that prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients. Salpingectomy is an important option for all patients, but is especially important when the fallopian tubes are found to be damaged by endometriosis and/or pelvic inflammatory disease. When imaging studies show that endometriomas are present and resection is not performed, pelvic ultrasound should become part of the patient’s routine examination.
Most endometriomas have a homogeneous appearance; any significant increase in size or a change in the homogeneous cystic characteristics to a more heterogeneous appearance with mural components should raise suspicion about malignant change.
It can be difficult to detect relatively small endocystic components with ultrasound, so if there is any doubt about whether there is some heterogeneous consistency, an MRI should be performed. MRI is showing more promise in detecting malignant change. Hyperdense mural nodules within the ovary and rapid growth of an endometrioma have both been associated with malignant transformation and can be seen on these images.
In a cohort study comparing MRI findings of 10 patients with ovarian adenocarcinoma to 10 patients with benign endometriomas, investigators found mural nodules in all 10 malignancies but in only three of the benign cases (AJR Am J Roentgenol. 2000 Nov;175[5]:1423-30).
Long-term follow-up is necessary to understand the timeline of transformation in patients with mural nodules. This together with increasing knowledge of molecular events underpinning evolution of endometriosis will lead to better screening and preventive strategies.
Dr. Nezhat is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at the State University of New York at Stony Brook. He reported having no financial disclosures.
Endometriosis is a common condition, occurring in this country in 1 of 10 women of reproductive age. An association between endometriosis and subsequent ovarian carcinoma has been reported for decades, yet it is only recently that our knowledge has deepened enough to support more rational methods for preventing the malignancy.
Each year, approximately 22,000 new cases of ovarian cancer are diagnosed. The lifetime risk of developing this malignancy is low, but it is the deadliest of the gynecologic malignancies, with diagnosis usually made in advanced stages when prognosis is poor.
Endometriosis shows some characteristics of malignancy, such as the development of local and distant foci, and attachment to and invasion of other tissues with subsequent damage to these tissues. Endometriosis also is characterized by recurrent, unregulated cell proliferation and estrogen-dependent growth.
Our attempts during the past 2 decades to detect ovarian carcinoma at the early stages through a combined screening modality involving transvaginal ultrasound and a test for the serum level of cancer antigen 125 have failed to provide any survival benefit or even any measurable reduction in morbidity. Today, early-stage ovarian carcinoma, which has a 5-year survival rate of more than 90%, is diagnosed in only a minority of women.
There is good news, however. In recent years our insight into the pathophysiology of ovarian cancer has deepened, providing us with a new paradigm for ovarian cancer pathogenesis that divides ovarian epithelial carcinoma into two distinct types with distinct molecular profiles – one which originates largely in the distal portion of the fallopian tube and the other which traces back to endometriosis.
This new paradigm strengthens and helps to explain the reported association between endometriosis and ovarian cancer. It also has important clinical implications for current practice. While we have much more to learn about the etiology of endometriosis and the causes of malignant transformation, our current knowledge provides a strong rationale for identification and close monitoring of some patients with endometriosis deemed at risk for ovarian cancer, risk-reducing medical management, earlier and more meticulous surgical treatment, and close monitoring.
By combining this new approach to endometriosis with consideration of salpingectomy after completion of childbearing, we have an unprecedented opportunity to reduce the incidence of epithelial ovarian cancer.
Dual pathogenesis
The majority of ovarian cancers are of epithelial origin and fall into four histologic categories: serous, endometrioid, clear cell, and mucinous. In recent years, we have gained a deeper understanding of the pathogenesis of ovarian carcinoma, with an array of epidemiologic, histologic, and molecular data showing us that epithelial ovarian cancers are also of two distinct types (Am J Obstet Gynecol. 2015 Sep;213[3]:262-7).
One of these types, a high-grade serous carcinoma, appears to arise in many cases in the epithelium of the fallopian tube. The other type of tumor is a low-grade carcinoma – particularly of the endometrioid and clear cell histologic subtypes – that originates largely from ovarian endometriotic lesions or from borderline serous tumors in the case of serous histology.
The majority of diagnosed stage 1 ovarian cancers are carcinomas of this low-grade type and not high-grade serous carcinomas. In a study of 76 consecutive stage 1 carcinomas, investigators found that ovarian endometriosis was present in 40 of the 76 cases. More than two-thirds of the 76 cases (71%) were nonserous cancers, and almost all of these cases were associated with endometriosis based on histologic examination (Fertil Steril. 2007 Oct;88[4]:906-10).
This study was among the first to show that the majority of stage 1 ovarian carcinomas are not high-grade serous carcinomas, but rather nonserous, primarily endometrioid and clear cell, cancers. The research demonstrated that endometriosis should be viewed as a potential precursor lesion to specific subtypes of ovarian cancer.
The malignant transformation of endometriosis was first suggested by Dr. J. A. Sampson in 1925, and a number of studies – in addition to the 2007 landmark study – have since described ovarian cancer arising from endometriosis, based on the frequent co-occurrence in surgical specimens.
Most recently, a study from the Ovarian Cancer Association Consortium (OCAC) found that women who reported a history of endometriosis had a significantly higher risk of developing ovarian cancer than the general population (odds ratio, 1.46).
Investigators of this critical study pooled data from 13 ovarian cancer case-control studies involving more than 13,226 controls and 7,911 women with invasive epithelial ovarian cancer – 818 (6.2%) and 738 (9.3%) of whom, respectively, reported a history of endometriosis. Specifically, they determined that self-reported endometriosis was associated with a 3.05-fold increased risk for clear cell invasive ovarian cancer and a 2.04-fold increased risk of endometrioid ovarian cancer.
Moreover, a significant association between preexisting endometriosis and low-grade serous invasive ovarian cancer (OR, 2.11) was demonstrated, while no association was found between endometriosis and the risk of high-grade serous invasive ovarian cancer (Lancet Oncol. 2012 Apr;13[4]:385-94).
A second recently published report – a meta-analysis of 20 case-control and 15 cohort studies published between 1990 and 2012 and involving more than 444,000 patients – found that endometriosis increased cancer risk in case-control or two-arm cohort studies by 27% (relative risk, 1.265) and by approximately 80% in single-arm cohort studies (standard incidence ratio, 1.797). Endometrioid and clear cell carcinomas were more common in endometriosis-associated ovarian cancer, while serous carcinoma was less frequent (Br J Cancer. 2014 Apr 2;110[7]:1878-90).
Findings of both of these large studies have served to clarify the association between endometriosis and specific histologic subtypes and suggested that there are important differences in the pathogenesis of low-grade and high-grade serous ovarian carcinomas.
Clinical implications
It is not clear what causes malignant transformation or what predisposes some patients with endometriosis to develop ovarian cancer, but the risk likely involves genetic and epigenetic influences as well as immunologic, inflammatory, and hormonal factors.
The molecular profiles of the main two types of ovarian cancer are different: While the majority of high-grade serous ovarian tumors are characterized by TP53 mutations, the low-grade carcinomas are characterized by a variety of mutations, including KRAS, BRAF, ERBB2, CTNNB1, and BCL2 mutations.
There currently are not enough data to recommend genetic screening tests in patients with endometriosis, but our hope is that we eventually will be able to screen for “high-risk” endometriotic lesions by testing for genes specific to various histologic subtypes of low-grade ovarian cancer, or by finding and utilizing other biomarkers.
In the meantime, we believe it is important to more thoroughly treat endometriosis and to identify and follow women with a history of the condition, especially those with a long-standing history, those with a history of endometriosis associated with infertility, and those with ovarian endometrioma. Each of these factors predisposes patients to a higher risk of malignant transformation.
Complete surgical resection of all visible endometriosis is the most effective treatment and will afford the best cancer prevention, even in women who are asymptomatic. In a recent Swedish national registry case-control study, women who underwent radical surgical excision of all visible endometriosis were significantly less likely (OR, 0.30) to develop ovarian cancer (Acta Obstet Gynecol Scand. 2013 May;92[5]:546-54).
Suppressive hormonal therapy is another treatment option for patients with no interest in conceiving. Most large endometriomas are functional ovarian cysts that have been invaded by cortical ovarian endometriosis or by small primary endometriomas (J Reprod Med. 1992 Sep;37[9]:771-6).
While hormonal therapy will not always result in complete regression of endometriotic lesions, it will decrease the recurrence rate of endometriomas and can be considered for long-term prevention of potentially premalignant lesions. It is most effective when it follows surgical excision of endometriomas and associated endometriosis.
A patient who has completed childbearing at the time of surgical resection may be offered bilateral salpingectomy, regardless of menopausal status. Salpingectomy in both average and high-risk populations (e.g., BRCA 1/2 carriers) not only prevents high-grade serous carcinoma by eliminating the site of origin, but also may decrease the risk of endometrioid and clear cell carcinoma by blocking the passageway that enables the flow of endometrium and factors that induce inflammation. It is estimated that the procedure reduces the risk of ovarian cancer by 40%.
Interestingly, tubal ligation has historically been shown to decrease the risk of ovarian cancer, and recent data have shown that the risk of endometrioid and clear cell carcinoma is cut even more than the risk of high-grade serous carcinoma (Int J Epidemiol. 2013 Apr;42[2]:579-89).
The Society of Gynecologic Oncology recommends that risk-reducing salpingectomy be considered at the time of hysterectomy or other abdominal or pelvic surgery, and in lieu of tubal ligation. The American College of Obstetricians and Gynecologists similarly has stated that prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients. Salpingectomy is an important option for all patients, but is especially important when the fallopian tubes are found to be damaged by endometriosis and/or pelvic inflammatory disease. When imaging studies show that endometriomas are present and resection is not performed, pelvic ultrasound should become part of the patient’s routine examination.
Most endometriomas have a homogeneous appearance; any significant increase in size or a change in the homogeneous cystic characteristics to a more heterogeneous appearance with mural components should raise suspicion about malignant change.
It can be difficult to detect relatively small endocystic components with ultrasound, so if there is any doubt about whether there is some heterogeneous consistency, an MRI should be performed. MRI is showing more promise in detecting malignant change. Hyperdense mural nodules within the ovary and rapid growth of an endometrioma have both been associated with malignant transformation and can be seen on these images.
In a cohort study comparing MRI findings of 10 patients with ovarian adenocarcinoma to 10 patients with benign endometriomas, investigators found mural nodules in all 10 malignancies but in only three of the benign cases (AJR Am J Roentgenol. 2000 Nov;175[5]:1423-30).
Long-term follow-up is necessary to understand the timeline of transformation in patients with mural nodules. This together with increasing knowledge of molecular events underpinning evolution of endometriosis will lead to better screening and preventive strategies.
Dr. Nezhat is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at the State University of New York at Stony Brook. He reported having no financial disclosures.
The impact of endometriosis on ovarian cancer
During an ob.gyn. rotation, a medical student quickly learns the risks related to endometriosis; that is, pelvic pain, abnormal uterine bleeding, and infertility. With more experience, the young practitioner realizes the concern of unopposed estrogen therapy in patients with a history of endometriosis (i.e., cancer).
Now, in this excellent discussion by Dr. Farr Nezhat, for the current edition of the Master Class in Gynecologic Surgery, he describes the risk of endometriosis and ovarian cancer. Not only does Dr. Nezhat present data revealing the increased association between ovarian cancer and endometriosis, but he goes on to describe the usual type of epithelial ovarian cancer that is noted in the patient with endometriosis.
Dr. Nezhat describes women who appear to be predisposed to malignant transformation and provides current recommendations to lower the risk of malignancy in patients with endometriosis. This includes complete surgical resection of endometriosis, routine ultrasound/MRI if endometriosis is not resected, suppressive hormonal therapy, and bilateral salpingectomy. Moreover, Dr. Nezhat looks to the future and the possibility of genetic screening tests.
Dr. Nezhat is board certified in gynecologic oncology and is world renowned for his work with advanced laparoscopic and robotic surgery for the treatment of gynecologic cancers and complex benign conditions. He is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at State University of New York at Stony Brook.
His main areas of interest and research include early detection and treatment of early and advanced ovarian cancer, as well as cancer arising in endometriosis. Dr. Nezhat has authored and coauthored more than 200 medical and scientific manuscripts and book chapters.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this column.
During an ob.gyn. rotation, a medical student quickly learns the risks related to endometriosis; that is, pelvic pain, abnormal uterine bleeding, and infertility. With more experience, the young practitioner realizes the concern of unopposed estrogen therapy in patients with a history of endometriosis (i.e., cancer).
Now, in this excellent discussion by Dr. Farr Nezhat, for the current edition of the Master Class in Gynecologic Surgery, he describes the risk of endometriosis and ovarian cancer. Not only does Dr. Nezhat present data revealing the increased association between ovarian cancer and endometriosis, but he goes on to describe the usual type of epithelial ovarian cancer that is noted in the patient with endometriosis.
Dr. Nezhat describes women who appear to be predisposed to malignant transformation and provides current recommendations to lower the risk of malignancy in patients with endometriosis. This includes complete surgical resection of endometriosis, routine ultrasound/MRI if endometriosis is not resected, suppressive hormonal therapy, and bilateral salpingectomy. Moreover, Dr. Nezhat looks to the future and the possibility of genetic screening tests.
Dr. Nezhat is board certified in gynecologic oncology and is world renowned for his work with advanced laparoscopic and robotic surgery for the treatment of gynecologic cancers and complex benign conditions. He is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at State University of New York at Stony Brook.
His main areas of interest and research include early detection and treatment of early and advanced ovarian cancer, as well as cancer arising in endometriosis. Dr. Nezhat has authored and coauthored more than 200 medical and scientific manuscripts and book chapters.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this column.
During an ob.gyn. rotation, a medical student quickly learns the risks related to endometriosis; that is, pelvic pain, abnormal uterine bleeding, and infertility. With more experience, the young practitioner realizes the concern of unopposed estrogen therapy in patients with a history of endometriosis (i.e., cancer).
Now, in this excellent discussion by Dr. Farr Nezhat, for the current edition of the Master Class in Gynecologic Surgery, he describes the risk of endometriosis and ovarian cancer. Not only does Dr. Nezhat present data revealing the increased association between ovarian cancer and endometriosis, but he goes on to describe the usual type of epithelial ovarian cancer that is noted in the patient with endometriosis.
Dr. Nezhat describes women who appear to be predisposed to malignant transformation and provides current recommendations to lower the risk of malignancy in patients with endometriosis. This includes complete surgical resection of endometriosis, routine ultrasound/MRI if endometriosis is not resected, suppressive hormonal therapy, and bilateral salpingectomy. Moreover, Dr. Nezhat looks to the future and the possibility of genetic screening tests.
Dr. Nezhat is board certified in gynecologic oncology and is world renowned for his work with advanced laparoscopic and robotic surgery for the treatment of gynecologic cancers and complex benign conditions. He is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at State University of New York at Stony Brook.
His main areas of interest and research include early detection and treatment of early and advanced ovarian cancer, as well as cancer arising in endometriosis. Dr. Nezhat has authored and coauthored more than 200 medical and scientific manuscripts and book chapters.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported having no financial disclosures relevant to this column.
New data points to slower course of labor
Only recently has evidence emerged that challenges our long-held understanding of “normal” and “abnormal” labor. We now know there is a much wider range of normal labor progress in women who go on to have good labor outcomes. We have a new labor curve to guide us – one that shows us, for example, that active labor occurs most commonly after 6 cm dilation rather than 4 cm as we’d previously thought.
By appreciating this new labor paradigm, we can potentially have a significant impact on the cesarean rate in the United States. While our use of the older labor curve is not the only reason for the rise in cesarean deliveries over the last 30 years, it very likely has played a role. A study published in 2011 of more than 32,000 live births at a major academic hospital demonstrated that one of the most common reasons for primary cesarean is abnormal labor or arrest (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Another study by the Consortium on Safe Labor – an analysis of labor and delivery information from more than 228,000 women across the United States – showed that half of the cesarean deliveries performed for dystocia in women undergoing labor induction were performed before 6 cm of cervical dilation and relatively soon after the previous cervical examination (Am J Obstet Gynecol. 2010 Oct; 203[4]: 326.e1–326.e10).
Our new labor paradigm brings to the forefront a host of new issues and questions about how we can best manage labor to optimize outcomes. In a way, recent discoveries about labor progress have highlighted a dearth of evidence and made “old” issues in labor management seem new and urgent.
As we strive to learn more, however, we are challenged to change our practices and behavior at the bedside with the evidence we currently have. By appreciating both the new labor curve and our current understanding of how labor induction, obesity, and other patient characteristics and clinical conditions can affect labor progress, we can expect that many women will simply progress much more slowly than was historically expected.
As long as we have indications of the well-being of the baby and the well-being of the mother, a slower but progressive labor in the first stage should not prompt us to intervene. We should no longer apply the standards of active-phase progress – standards that have traditionally driven our diagnoses of labor dystocia – until the patient has achieved 6 cm of dilation.
The labor curve that had shaped our thinking about normal and abnormal labor progress until recently was developed by Dr. Emanuel Friedman. Based on findings from a prospective cohort study of 500 nulliparous women, Dr. Friedman plotted labor progress with centimeters of cervical dilation on the Y-axis and time on the X-axis, and divided labor into several stages and phases. In this curve, the rate of change of cervical dilation over time started increasing significantly at 4 cm; this period of increasing slope defined the active phase of labor.
Abnormal labor progress in the active phase was then defined, based on the 95th percentile, as cervical dilation of less than 1.2 cm per hour for nulliparous women and less than 1.5 cm per hour for multiparous women. Based on Dr. Friedman’s work, a woman was deemed to be in active-phase arrest when she had no cervical changes for 2 hours or more while having adequate uterine contractions and cervical dilation of at least 4 cm. These concepts came to govern labor management.
The paradigm shifted when the Consortium on Safe Labor reported in 2010 on a retrospective cohort study of more than 62,000 women at 19 U.S. hospitals. The women had a singleton term gestation, spontaneous labor, vertex presentation, vaginal delivery, and a normal perinatal outcome. In their analysis of labor and delivery information, Dr. Jun Zhang of the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and his colleagues accounted for the fact that the exact times of cervical change are unknown.
They used modern statistical methods and analytical tools that took into account the specific nature of cervical dilation data – that cervical measurements are interval-censored (we never know the exact time when a woman’s cervix changes) and that multiple exams of the cervix in the same patient are not independent (Obstet Gynecol. 2010 Dec;116[6]:1281-7).
The methodology used in the Consortium study accounted for both the interval-censored and repeated-measures nature of cervical dilation data. It thus addressed analytical flaws in the previous approach to labor data, which was purely descriptive of the exam findings and did not consider the nature of the data itself.
Under the new analysis and in the larger, contemporary population of patients, the period of increasing slope was found to occur most commonly after 6 cm, not 4 cm. The slowest 5% of nulliparous women had cervical dilation of 0.4 cm per hour (with the median at 1.9 cm per hour), compared with 1.2 cm per hour (with a median of 3.0 cm per hour) as in the Friedman data.
Dr. Zhang’s study showed us that labor may take more than 6 hours to progress from 4 to 5 cm dilation, and more than 3 hours to progress from 5 to 6 cm dilation – a rate of progress that is significantly slower than what Dr. Friedman had described. The new data showed us, moreover, that from 4 cm-6 cm dilation, nulliparous and multiparous women progressed similarly slowly. Beyond 6 cm, multiparous women dilated more rapidly, with a steeper acceleration phase than previously described.
A consensus statement published in 2014 by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) on “Safe Prevention of the Primary Cesarean Delivery” encourages use of the Consortium data to revisit the definition of labor dystocia. While the data “do not directly address an optimal duration for the diagnosis of active-phase protraction or labor arrest, [they] do suggest that neither should be diagnosed before 6 cm dilation” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
The ACOG-SMFM statement makes a series of recommendations for managing the first and second stages of labor, based not only on the Consortium data but on a broader literature review. It recommends that if mother and fetus appear well, cesarean delivery for active-phase arrest in the first stage of labor be reserved for women of at least 6 cm of dilation with ruptured membranes who fail to progress despite 4 hours of adequate uterine activity, or at least 6 hours of oxytocin administration with inadequate uterine activity and no cervical change.
Regarding the latent phase of labor, the statement says that most women with a prolonged latent phase ultimately will enter the active phase with expectant management. It advises that a prolonged latent phase (for example, greater than 20 hours in nulliparous women and greater than 14 hours in multiparous women) should not be an isolated indication for cesarean delivery.
The consensus statement also recognizes recent data showing that women who undergo labor induction have an even slower “normal” course of labor, particularly a longer latent phase, than women who labor spontaneously. A retrospective cohort study of more than 5,000 women, for instance, found that before 6 cm, women whose labor is induced can spend up to 10 hours to achieve each 1 cm of dilation (Obstet Gynecol. 2012 Jun;119[6]:1113-8).
As long as maternal and fetal status are reassuring, the statement says, cesarean deliveries for failed induction of labor in the latent phase can be avoided by allowing longer durations of the latent phase (up to 24 hours) and by requiring that oxytocin be administered for 12-18 hours after membrane rupture before deeming induction a failure.
Each of these described recommendations were graded in the ACOG-SMFM consensus document as “strong” recommendations with “moderate quality evidence.”
Examining our standards
Moving forward, we must further develop and define our thresholds for identifying who will most benefit from a cesarean delivery. We have many specific aspects of labor management to address as well, such as the optimal timing of artificial membrane rupture and the safety and efficacy of different oxytocin protocols. We may also want to revisit recommendations for serial cervical assessment, possibly adjusting the intervals given our understanding of the new labor curve.
Under the new labor paradigm, moreover, we must think not only about the clinical decisions we make at the bedside, but about the decisions we make early in the labor management process.
The timing of admission is one such decision. A statement published in 2012 on “Preventing the First Cesarean Delivery” by ACOG, SMFM, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development advises us to avoid admittance of women during the early latent phase of labor (Obstet Gynecol. 2012 Nov;120[5]:1181-93).
It may even be advisable that we consider admittance at higher cervical dilation. A study published this year shows that women admitted at less than 6 cm of dilation had an increased risk of cesarean delivery, compared with women admitted at higher cervical dilation (Am J Perinatol. 2016 Jan;33[2]:188-94). We have more to learn, but certainly, given what we know now about labor progress and the start of active labor, the timing of admission is an important factor to consider.
The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, presents many questions as well. There is a paucity of quality published data concerning what is normal, how long the stage should last, and how we should manage it. Historically, we have been taught to allow 2 hours of pushing for nulliparous women and 1 hour for multiparous women, when epidural anesthesia has not been administered, and to add an additional hour when epidural is used.
The 2014 ACOG-SMFM consensus statement recommends extending each of these limits by an hour, if maternal and fetal conditions permit, so that we allow at least 3 hours of pushing for nulliparous women and at least 2 hours for multiparous women before diagnosing arrest of labor in the second stage. Longer durations may be appropriate with the use of epidural anesthesia and on an individualized basis.
At this time, it is unclear whether there is any absolute maximum length of time beyond which all women in the second stage of labor should undergo cesarean delivery. We also still do not know the optimal technique for managing maternal pushing during the second stage. Should women with an epidural push right away or should they allow for a period of spontaneous descent? Many of the high-quality studies reported thus far that compare delayed and immediate pushing have limited applicability to current practice because they involved now-obsolete midpelvic forceps deliveries. A large multicenter randomized trial currently underway should provide us with some answers.
Dr. Cahill is an associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University School of Medicine in St. Louis. She reported having no relevant financial disclosures.
Only recently has evidence emerged that challenges our long-held understanding of “normal” and “abnormal” labor. We now know there is a much wider range of normal labor progress in women who go on to have good labor outcomes. We have a new labor curve to guide us – one that shows us, for example, that active labor occurs most commonly after 6 cm dilation rather than 4 cm as we’d previously thought.
By appreciating this new labor paradigm, we can potentially have a significant impact on the cesarean rate in the United States. While our use of the older labor curve is not the only reason for the rise in cesarean deliveries over the last 30 years, it very likely has played a role. A study published in 2011 of more than 32,000 live births at a major academic hospital demonstrated that one of the most common reasons for primary cesarean is abnormal labor or arrest (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Another study by the Consortium on Safe Labor – an analysis of labor and delivery information from more than 228,000 women across the United States – showed that half of the cesarean deliveries performed for dystocia in women undergoing labor induction were performed before 6 cm of cervical dilation and relatively soon after the previous cervical examination (Am J Obstet Gynecol. 2010 Oct; 203[4]: 326.e1–326.e10).
Our new labor paradigm brings to the forefront a host of new issues and questions about how we can best manage labor to optimize outcomes. In a way, recent discoveries about labor progress have highlighted a dearth of evidence and made “old” issues in labor management seem new and urgent.
As we strive to learn more, however, we are challenged to change our practices and behavior at the bedside with the evidence we currently have. By appreciating both the new labor curve and our current understanding of how labor induction, obesity, and other patient characteristics and clinical conditions can affect labor progress, we can expect that many women will simply progress much more slowly than was historically expected.
As long as we have indications of the well-being of the baby and the well-being of the mother, a slower but progressive labor in the first stage should not prompt us to intervene. We should no longer apply the standards of active-phase progress – standards that have traditionally driven our diagnoses of labor dystocia – until the patient has achieved 6 cm of dilation.
The labor curve that had shaped our thinking about normal and abnormal labor progress until recently was developed by Dr. Emanuel Friedman. Based on findings from a prospective cohort study of 500 nulliparous women, Dr. Friedman plotted labor progress with centimeters of cervical dilation on the Y-axis and time on the X-axis, and divided labor into several stages and phases. In this curve, the rate of change of cervical dilation over time started increasing significantly at 4 cm; this period of increasing slope defined the active phase of labor.
Abnormal labor progress in the active phase was then defined, based on the 95th percentile, as cervical dilation of less than 1.2 cm per hour for nulliparous women and less than 1.5 cm per hour for multiparous women. Based on Dr. Friedman’s work, a woman was deemed to be in active-phase arrest when she had no cervical changes for 2 hours or more while having adequate uterine contractions and cervical dilation of at least 4 cm. These concepts came to govern labor management.
The paradigm shifted when the Consortium on Safe Labor reported in 2010 on a retrospective cohort study of more than 62,000 women at 19 U.S. hospitals. The women had a singleton term gestation, spontaneous labor, vertex presentation, vaginal delivery, and a normal perinatal outcome. In their analysis of labor and delivery information, Dr. Jun Zhang of the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and his colleagues accounted for the fact that the exact times of cervical change are unknown.
They used modern statistical methods and analytical tools that took into account the specific nature of cervical dilation data – that cervical measurements are interval-censored (we never know the exact time when a woman’s cervix changes) and that multiple exams of the cervix in the same patient are not independent (Obstet Gynecol. 2010 Dec;116[6]:1281-7).
The methodology used in the Consortium study accounted for both the interval-censored and repeated-measures nature of cervical dilation data. It thus addressed analytical flaws in the previous approach to labor data, which was purely descriptive of the exam findings and did not consider the nature of the data itself.
Under the new analysis and in the larger, contemporary population of patients, the period of increasing slope was found to occur most commonly after 6 cm, not 4 cm. The slowest 5% of nulliparous women had cervical dilation of 0.4 cm per hour (with the median at 1.9 cm per hour), compared with 1.2 cm per hour (with a median of 3.0 cm per hour) as in the Friedman data.
Dr. Zhang’s study showed us that labor may take more than 6 hours to progress from 4 to 5 cm dilation, and more than 3 hours to progress from 5 to 6 cm dilation – a rate of progress that is significantly slower than what Dr. Friedman had described. The new data showed us, moreover, that from 4 cm-6 cm dilation, nulliparous and multiparous women progressed similarly slowly. Beyond 6 cm, multiparous women dilated more rapidly, with a steeper acceleration phase than previously described.
A consensus statement published in 2014 by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) on “Safe Prevention of the Primary Cesarean Delivery” encourages use of the Consortium data to revisit the definition of labor dystocia. While the data “do not directly address an optimal duration for the diagnosis of active-phase protraction or labor arrest, [they] do suggest that neither should be diagnosed before 6 cm dilation” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
The ACOG-SMFM statement makes a series of recommendations for managing the first and second stages of labor, based not only on the Consortium data but on a broader literature review. It recommends that if mother and fetus appear well, cesarean delivery for active-phase arrest in the first stage of labor be reserved for women of at least 6 cm of dilation with ruptured membranes who fail to progress despite 4 hours of adequate uterine activity, or at least 6 hours of oxytocin administration with inadequate uterine activity and no cervical change.
Regarding the latent phase of labor, the statement says that most women with a prolonged latent phase ultimately will enter the active phase with expectant management. It advises that a prolonged latent phase (for example, greater than 20 hours in nulliparous women and greater than 14 hours in multiparous women) should not be an isolated indication for cesarean delivery.
The consensus statement also recognizes recent data showing that women who undergo labor induction have an even slower “normal” course of labor, particularly a longer latent phase, than women who labor spontaneously. A retrospective cohort study of more than 5,000 women, for instance, found that before 6 cm, women whose labor is induced can spend up to 10 hours to achieve each 1 cm of dilation (Obstet Gynecol. 2012 Jun;119[6]:1113-8).
As long as maternal and fetal status are reassuring, the statement says, cesarean deliveries for failed induction of labor in the latent phase can be avoided by allowing longer durations of the latent phase (up to 24 hours) and by requiring that oxytocin be administered for 12-18 hours after membrane rupture before deeming induction a failure.
Each of these described recommendations were graded in the ACOG-SMFM consensus document as “strong” recommendations with “moderate quality evidence.”
Examining our standards
Moving forward, we must further develop and define our thresholds for identifying who will most benefit from a cesarean delivery. We have many specific aspects of labor management to address as well, such as the optimal timing of artificial membrane rupture and the safety and efficacy of different oxytocin protocols. We may also want to revisit recommendations for serial cervical assessment, possibly adjusting the intervals given our understanding of the new labor curve.
Under the new labor paradigm, moreover, we must think not only about the clinical decisions we make at the bedside, but about the decisions we make early in the labor management process.
The timing of admission is one such decision. A statement published in 2012 on “Preventing the First Cesarean Delivery” by ACOG, SMFM, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development advises us to avoid admittance of women during the early latent phase of labor (Obstet Gynecol. 2012 Nov;120[5]:1181-93).
It may even be advisable that we consider admittance at higher cervical dilation. A study published this year shows that women admitted at less than 6 cm of dilation had an increased risk of cesarean delivery, compared with women admitted at higher cervical dilation (Am J Perinatol. 2016 Jan;33[2]:188-94). We have more to learn, but certainly, given what we know now about labor progress and the start of active labor, the timing of admission is an important factor to consider.
The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, presents many questions as well. There is a paucity of quality published data concerning what is normal, how long the stage should last, and how we should manage it. Historically, we have been taught to allow 2 hours of pushing for nulliparous women and 1 hour for multiparous women, when epidural anesthesia has not been administered, and to add an additional hour when epidural is used.
The 2014 ACOG-SMFM consensus statement recommends extending each of these limits by an hour, if maternal and fetal conditions permit, so that we allow at least 3 hours of pushing for nulliparous women and at least 2 hours for multiparous women before diagnosing arrest of labor in the second stage. Longer durations may be appropriate with the use of epidural anesthesia and on an individualized basis.
At this time, it is unclear whether there is any absolute maximum length of time beyond which all women in the second stage of labor should undergo cesarean delivery. We also still do not know the optimal technique for managing maternal pushing during the second stage. Should women with an epidural push right away or should they allow for a period of spontaneous descent? Many of the high-quality studies reported thus far that compare delayed and immediate pushing have limited applicability to current practice because they involved now-obsolete midpelvic forceps deliveries. A large multicenter randomized trial currently underway should provide us with some answers.
Dr. Cahill is an associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University School of Medicine in St. Louis. She reported having no relevant financial disclosures.
Only recently has evidence emerged that challenges our long-held understanding of “normal” and “abnormal” labor. We now know there is a much wider range of normal labor progress in women who go on to have good labor outcomes. We have a new labor curve to guide us – one that shows us, for example, that active labor occurs most commonly after 6 cm dilation rather than 4 cm as we’d previously thought.
By appreciating this new labor paradigm, we can potentially have a significant impact on the cesarean rate in the United States. While our use of the older labor curve is not the only reason for the rise in cesarean deliveries over the last 30 years, it very likely has played a role. A study published in 2011 of more than 32,000 live births at a major academic hospital demonstrated that one of the most common reasons for primary cesarean is abnormal labor or arrest (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Another study by the Consortium on Safe Labor – an analysis of labor and delivery information from more than 228,000 women across the United States – showed that half of the cesarean deliveries performed for dystocia in women undergoing labor induction were performed before 6 cm of cervical dilation and relatively soon after the previous cervical examination (Am J Obstet Gynecol. 2010 Oct; 203[4]: 326.e1–326.e10).
Our new labor paradigm brings to the forefront a host of new issues and questions about how we can best manage labor to optimize outcomes. In a way, recent discoveries about labor progress have highlighted a dearth of evidence and made “old” issues in labor management seem new and urgent.
As we strive to learn more, however, we are challenged to change our practices and behavior at the bedside with the evidence we currently have. By appreciating both the new labor curve and our current understanding of how labor induction, obesity, and other patient characteristics and clinical conditions can affect labor progress, we can expect that many women will simply progress much more slowly than was historically expected.
As long as we have indications of the well-being of the baby and the well-being of the mother, a slower but progressive labor in the first stage should not prompt us to intervene. We should no longer apply the standards of active-phase progress – standards that have traditionally driven our diagnoses of labor dystocia – until the patient has achieved 6 cm of dilation.
The labor curve that had shaped our thinking about normal and abnormal labor progress until recently was developed by Dr. Emanuel Friedman. Based on findings from a prospective cohort study of 500 nulliparous women, Dr. Friedman plotted labor progress with centimeters of cervical dilation on the Y-axis and time on the X-axis, and divided labor into several stages and phases. In this curve, the rate of change of cervical dilation over time started increasing significantly at 4 cm; this period of increasing slope defined the active phase of labor.
Abnormal labor progress in the active phase was then defined, based on the 95th percentile, as cervical dilation of less than 1.2 cm per hour for nulliparous women and less than 1.5 cm per hour for multiparous women. Based on Dr. Friedman’s work, a woman was deemed to be in active-phase arrest when she had no cervical changes for 2 hours or more while having adequate uterine contractions and cervical dilation of at least 4 cm. These concepts came to govern labor management.
The paradigm shifted when the Consortium on Safe Labor reported in 2010 on a retrospective cohort study of more than 62,000 women at 19 U.S. hospitals. The women had a singleton term gestation, spontaneous labor, vertex presentation, vaginal delivery, and a normal perinatal outcome. In their analysis of labor and delivery information, Dr. Jun Zhang of the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and his colleagues accounted for the fact that the exact times of cervical change are unknown.
They used modern statistical methods and analytical tools that took into account the specific nature of cervical dilation data – that cervical measurements are interval-censored (we never know the exact time when a woman’s cervix changes) and that multiple exams of the cervix in the same patient are not independent (Obstet Gynecol. 2010 Dec;116[6]:1281-7).
The methodology used in the Consortium study accounted for both the interval-censored and repeated-measures nature of cervical dilation data. It thus addressed analytical flaws in the previous approach to labor data, which was purely descriptive of the exam findings and did not consider the nature of the data itself.
Under the new analysis and in the larger, contemporary population of patients, the period of increasing slope was found to occur most commonly after 6 cm, not 4 cm. The slowest 5% of nulliparous women had cervical dilation of 0.4 cm per hour (with the median at 1.9 cm per hour), compared with 1.2 cm per hour (with a median of 3.0 cm per hour) as in the Friedman data.
Dr. Zhang’s study showed us that labor may take more than 6 hours to progress from 4 to 5 cm dilation, and more than 3 hours to progress from 5 to 6 cm dilation – a rate of progress that is significantly slower than what Dr. Friedman had described. The new data showed us, moreover, that from 4 cm-6 cm dilation, nulliparous and multiparous women progressed similarly slowly. Beyond 6 cm, multiparous women dilated more rapidly, with a steeper acceleration phase than previously described.
A consensus statement published in 2014 by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) on “Safe Prevention of the Primary Cesarean Delivery” encourages use of the Consortium data to revisit the definition of labor dystocia. While the data “do not directly address an optimal duration for the diagnosis of active-phase protraction or labor arrest, [they] do suggest that neither should be diagnosed before 6 cm dilation” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
The ACOG-SMFM statement makes a series of recommendations for managing the first and second stages of labor, based not only on the Consortium data but on a broader literature review. It recommends that if mother and fetus appear well, cesarean delivery for active-phase arrest in the first stage of labor be reserved for women of at least 6 cm of dilation with ruptured membranes who fail to progress despite 4 hours of adequate uterine activity, or at least 6 hours of oxytocin administration with inadequate uterine activity and no cervical change.
Regarding the latent phase of labor, the statement says that most women with a prolonged latent phase ultimately will enter the active phase with expectant management. It advises that a prolonged latent phase (for example, greater than 20 hours in nulliparous women and greater than 14 hours in multiparous women) should not be an isolated indication for cesarean delivery.
The consensus statement also recognizes recent data showing that women who undergo labor induction have an even slower “normal” course of labor, particularly a longer latent phase, than women who labor spontaneously. A retrospective cohort study of more than 5,000 women, for instance, found that before 6 cm, women whose labor is induced can spend up to 10 hours to achieve each 1 cm of dilation (Obstet Gynecol. 2012 Jun;119[6]:1113-8).
As long as maternal and fetal status are reassuring, the statement says, cesarean deliveries for failed induction of labor in the latent phase can be avoided by allowing longer durations of the latent phase (up to 24 hours) and by requiring that oxytocin be administered for 12-18 hours after membrane rupture before deeming induction a failure.
Each of these described recommendations were graded in the ACOG-SMFM consensus document as “strong” recommendations with “moderate quality evidence.”
Examining our standards
Moving forward, we must further develop and define our thresholds for identifying who will most benefit from a cesarean delivery. We have many specific aspects of labor management to address as well, such as the optimal timing of artificial membrane rupture and the safety and efficacy of different oxytocin protocols. We may also want to revisit recommendations for serial cervical assessment, possibly adjusting the intervals given our understanding of the new labor curve.
Under the new labor paradigm, moreover, we must think not only about the clinical decisions we make at the bedside, but about the decisions we make early in the labor management process.
The timing of admission is one such decision. A statement published in 2012 on “Preventing the First Cesarean Delivery” by ACOG, SMFM, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development advises us to avoid admittance of women during the early latent phase of labor (Obstet Gynecol. 2012 Nov;120[5]:1181-93).
It may even be advisable that we consider admittance at higher cervical dilation. A study published this year shows that women admitted at less than 6 cm of dilation had an increased risk of cesarean delivery, compared with women admitted at higher cervical dilation (Am J Perinatol. 2016 Jan;33[2]:188-94). We have more to learn, but certainly, given what we know now about labor progress and the start of active labor, the timing of admission is an important factor to consider.
The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, presents many questions as well. There is a paucity of quality published data concerning what is normal, how long the stage should last, and how we should manage it. Historically, we have been taught to allow 2 hours of pushing for nulliparous women and 1 hour for multiparous women, when epidural anesthesia has not been administered, and to add an additional hour when epidural is used.
The 2014 ACOG-SMFM consensus statement recommends extending each of these limits by an hour, if maternal and fetal conditions permit, so that we allow at least 3 hours of pushing for nulliparous women and at least 2 hours for multiparous women before diagnosing arrest of labor in the second stage. Longer durations may be appropriate with the use of epidural anesthesia and on an individualized basis.
At this time, it is unclear whether there is any absolute maximum length of time beyond which all women in the second stage of labor should undergo cesarean delivery. We also still do not know the optimal technique for managing maternal pushing during the second stage. Should women with an epidural push right away or should they allow for a period of spontaneous descent? Many of the high-quality studies reported thus far that compare delayed and immediate pushing have limited applicability to current practice because they involved now-obsolete midpelvic forceps deliveries. A large multicenter randomized trial currently underway should provide us with some answers.
Dr. Cahill is an associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University School of Medicine in St. Louis. She reported having no relevant financial disclosures.
Rethinking the management of labor
Over the last 50 years, we have witnessed some incredible advancements that have vastly improved maternal and fetal outcomes, even in the face of the most complex obstetrical dilemmas. As our practice and the research continues to evolve, it is increasingly important that we carefully review our practice standards to ensure that every woman and her baby receives the most up-to-date medical care.
This month’s Master Class highlights a critical area of obstetrics where the convergence of technology, clinical observation, and research stimulated a change in practice guidelines: the use of the labor curve to monitor normal versus abnormal labor. Until quite recently, ob.gyns. had based labor criteria on the “Friedman Curve,” first established in the mid-1950s, and supported by other smaller and less comprehensive studies. This work was adopted by the American College of Obstetricians and Gynecologists.
For more than half a century, we used these parameters to determine if a woman had entered active-phase arrest, and to make the very important decision of whether to perform a cesarean section. However, work in the early 2000s strongly suggested that the old criteria no longer applied to the full course of labor in contemporary patients (Am J Obstet Gynecol. 2002 Oct;187[4]:824-8). A 2010 comprehensive study showed that we needed to consider a new approach to labor management (Am J Obstet Gynecol. 2010 Oct;203[4]:326.e1-326.e10).
It may seem incredible that it took such a long time to update our thinking about what constitutes normal versus abnormal labor progression. However, we must keep in mind that many studies supported the original labor curve, and advanced tools to assess fetal health during labor were just being developed. The first commercially available fetal heart rate monitor would not be produced until 1968, and debates about the utility of these devices would continue into the early 1990s.
Additionally, our patient population has changed. As we have discussed in previous columns, the incidence and severity of other chronic conditions, such as diabetes and obesity, has increased significantly and deeply impacted labor progression.
Just as technology has advanced and our patients’ needs have changed, so, too, must our practice standards. We have invited Dr. Alison G. Cahill, associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University, St. Louis, to discuss the importance and implications of the new labor curve.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Over the last 50 years, we have witnessed some incredible advancements that have vastly improved maternal and fetal outcomes, even in the face of the most complex obstetrical dilemmas. As our practice and the research continues to evolve, it is increasingly important that we carefully review our practice standards to ensure that every woman and her baby receives the most up-to-date medical care.
This month’s Master Class highlights a critical area of obstetrics where the convergence of technology, clinical observation, and research stimulated a change in practice guidelines: the use of the labor curve to monitor normal versus abnormal labor. Until quite recently, ob.gyns. had based labor criteria on the “Friedman Curve,” first established in the mid-1950s, and supported by other smaller and less comprehensive studies. This work was adopted by the American College of Obstetricians and Gynecologists.
For more than half a century, we used these parameters to determine if a woman had entered active-phase arrest, and to make the very important decision of whether to perform a cesarean section. However, work in the early 2000s strongly suggested that the old criteria no longer applied to the full course of labor in contemporary patients (Am J Obstet Gynecol. 2002 Oct;187[4]:824-8). A 2010 comprehensive study showed that we needed to consider a new approach to labor management (Am J Obstet Gynecol. 2010 Oct;203[4]:326.e1-326.e10).
It may seem incredible that it took such a long time to update our thinking about what constitutes normal versus abnormal labor progression. However, we must keep in mind that many studies supported the original labor curve, and advanced tools to assess fetal health during labor were just being developed. The first commercially available fetal heart rate monitor would not be produced until 1968, and debates about the utility of these devices would continue into the early 1990s.
Additionally, our patient population has changed. As we have discussed in previous columns, the incidence and severity of other chronic conditions, such as diabetes and obesity, has increased significantly and deeply impacted labor progression.
Just as technology has advanced and our patients’ needs have changed, so, too, must our practice standards. We have invited Dr. Alison G. Cahill, associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University, St. Louis, to discuss the importance and implications of the new labor curve.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Over the last 50 years, we have witnessed some incredible advancements that have vastly improved maternal and fetal outcomes, even in the face of the most complex obstetrical dilemmas. As our practice and the research continues to evolve, it is increasingly important that we carefully review our practice standards to ensure that every woman and her baby receives the most up-to-date medical care.
This month’s Master Class highlights a critical area of obstetrics where the convergence of technology, clinical observation, and research stimulated a change in practice guidelines: the use of the labor curve to monitor normal versus abnormal labor. Until quite recently, ob.gyns. had based labor criteria on the “Friedman Curve,” first established in the mid-1950s, and supported by other smaller and less comprehensive studies. This work was adopted by the American College of Obstetricians and Gynecologists.
For more than half a century, we used these parameters to determine if a woman had entered active-phase arrest, and to make the very important decision of whether to perform a cesarean section. However, work in the early 2000s strongly suggested that the old criteria no longer applied to the full course of labor in contemporary patients (Am J Obstet Gynecol. 2002 Oct;187[4]:824-8). A 2010 comprehensive study showed that we needed to consider a new approach to labor management (Am J Obstet Gynecol. 2010 Oct;203[4]:326.e1-326.e10).
It may seem incredible that it took such a long time to update our thinking about what constitutes normal versus abnormal labor progression. However, we must keep in mind that many studies supported the original labor curve, and advanced tools to assess fetal health during labor were just being developed. The first commercially available fetal heart rate monitor would not be produced until 1968, and debates about the utility of these devices would continue into the early 1990s.
Additionally, our patient population has changed. As we have discussed in previous columns, the incidence and severity of other chronic conditions, such as diabetes and obesity, has increased significantly and deeply impacted labor progression.
Just as technology has advanced and our patients’ needs have changed, so, too, must our practice standards. We have invited Dr. Alison G. Cahill, associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University, St. Louis, to discuss the importance and implications of the new labor curve.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Understanding stillbirth
When a couple learns that “they are pregnant,” it is often one of the most joyous moments in their lives. However, despite the modern prenatal care available to women in the United States, pregnancy loss remains a real concern. Miscarriage is estimated to occur in 15%-20% of pregnancies; recurrent pregnancy loss in about 1%-2% of pregnancies; and stillbirth in as many as 1% of pregnancies. The causes of pregnancy loss can range from those we can diagnose, such as genetic factors, anatomic complications, and thrombophilia, to those that elude us completely.
In December 2015, investigators from Karolinska Institutet in Stockholm published a study indicating that women who gained weight between their first and second pregnancies, but who were a healthy weight prior to their first pregnancy, had an increased risk of experiencing a stillbirth (30%-50%), or having an infant who died within the first year (27%-60%) (Lancet 2015. doi: 10.1016/S0140-6736(15)00990-3). We have devoted a number of Master Class columns to the link between obesity and pregnancy complications, and this study further reinforces the influence of a healthy weight on pregnancy outcomes.
In addition to lifestyle modifications, evidence has suggested that low-molecular-weight heparin, aspirin, or vitamin supplements, in combination with appropriate surveillance and management, may reduce risk of pregnancy loss. However, more work is needed to fully understand why fetal death occurs if we are to better equip ourselves, and our patients, with all the information necessary to prevent loss from happening.
For this reason, we have invited Dr. Uma M. Reddy of the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health, to address one of the most devastating types of pregnancy losses: stillbirth. As a program scientist for large research studies, such as the Stillbirth Collaborative Research Network, Dr. Reddy’s unique perspective will add greatly to our understanding of pregnancy loss.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column.
When a couple learns that “they are pregnant,” it is often one of the most joyous moments in their lives. However, despite the modern prenatal care available to women in the United States, pregnancy loss remains a real concern. Miscarriage is estimated to occur in 15%-20% of pregnancies; recurrent pregnancy loss in about 1%-2% of pregnancies; and stillbirth in as many as 1% of pregnancies. The causes of pregnancy loss can range from those we can diagnose, such as genetic factors, anatomic complications, and thrombophilia, to those that elude us completely.
In December 2015, investigators from Karolinska Institutet in Stockholm published a study indicating that women who gained weight between their first and second pregnancies, but who were a healthy weight prior to their first pregnancy, had an increased risk of experiencing a stillbirth (30%-50%), or having an infant who died within the first year (27%-60%) (Lancet 2015. doi: 10.1016/S0140-6736(15)00990-3). We have devoted a number of Master Class columns to the link between obesity and pregnancy complications, and this study further reinforces the influence of a healthy weight on pregnancy outcomes.
In addition to lifestyle modifications, evidence has suggested that low-molecular-weight heparin, aspirin, or vitamin supplements, in combination with appropriate surveillance and management, may reduce risk of pregnancy loss. However, more work is needed to fully understand why fetal death occurs if we are to better equip ourselves, and our patients, with all the information necessary to prevent loss from happening.
For this reason, we have invited Dr. Uma M. Reddy of the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health, to address one of the most devastating types of pregnancy losses: stillbirth. As a program scientist for large research studies, such as the Stillbirth Collaborative Research Network, Dr. Reddy’s unique perspective will add greatly to our understanding of pregnancy loss.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column.
When a couple learns that “they are pregnant,” it is often one of the most joyous moments in their lives. However, despite the modern prenatal care available to women in the United States, pregnancy loss remains a real concern. Miscarriage is estimated to occur in 15%-20% of pregnancies; recurrent pregnancy loss in about 1%-2% of pregnancies; and stillbirth in as many as 1% of pregnancies. The causes of pregnancy loss can range from those we can diagnose, such as genetic factors, anatomic complications, and thrombophilia, to those that elude us completely.
In December 2015, investigators from Karolinska Institutet in Stockholm published a study indicating that women who gained weight between their first and second pregnancies, but who were a healthy weight prior to their first pregnancy, had an increased risk of experiencing a stillbirth (30%-50%), or having an infant who died within the first year (27%-60%) (Lancet 2015. doi: 10.1016/S0140-6736(15)00990-3). We have devoted a number of Master Class columns to the link between obesity and pregnancy complications, and this study further reinforces the influence of a healthy weight on pregnancy outcomes.
In addition to lifestyle modifications, evidence has suggested that low-molecular-weight heparin, aspirin, or vitamin supplements, in combination with appropriate surveillance and management, may reduce risk of pregnancy loss. However, more work is needed to fully understand why fetal death occurs if we are to better equip ourselves, and our patients, with all the information necessary to prevent loss from happening.
For this reason, we have invited Dr. Uma M. Reddy of the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health, to address one of the most devastating types of pregnancy losses: stillbirth. As a program scientist for large research studies, such as the Stillbirth Collaborative Research Network, Dr. Reddy’s unique perspective will add greatly to our understanding of pregnancy loss.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column.
Research adds insight on stillbirth risk factors
Stillbirth is a major public health problem, occurring in approximately 1 of every 160 pregnancies in the United States. The rate has remained stagnant since 2006. Prior to that time, from 1990 to 2006, the rate declined somewhat, but only half as much as the decline in infant mortality during this time period. Racial disparities also have persisted, with non-Hispanic black women having more than a twofold increase in risk (Natl Vital Stat Rep. 2012;60:1-22).
Research conducted by the Stillbirth Collaborative Research Network (SCRN) and others has provided us with insight on risk factors and on probable and possible causes of death among stillbirths, which are defined as fetal deaths at 20 or more weeks’ gestation. We know from SCRN data, for instance, that black women are more likely to have stillbirths associated with obstetric complications and infections than white and Hispanic women. However, we still cannot explain a substantial proportion of stillbirths, despite a complete evaluation, or predict who will have a stillbirth.
What we can do as obstetricians is be aware that stillbirth is one of the most common adverse pregnancy outcomes in the United States and counsel women regarding risk factors that are modifiable. Moreover, when stillbirth happens, a complete postmortem evaluation that includes autopsy, placental pathology, karyotype or microarray analysis, and fetal-maternal hemorrhage testing is recommended (Obstet Gynecol. 2009;113[3]:748-61). Recent data show that each of these four components is valuable and should be considered the basic work-up for stillbirth.
Risks and causes
Pregnancy history was the strongest baseline risk factor for stillbirth in an analysis of 614 stillbirths and 1,816 live births in the SCRN’s population-based, case-control study conducted between 2006 and 2008. The SCRN was initiated by the Eunice Kennedy Shriver National Institute of Child Health and Human Development in 2003. This critical population-based study was conducted at 59 U.S. tertiary care and community hospitals in five catchment areas and has been analyzed in more than 15 published reports.
Women with a previous stillbirth have been known to be at 5- to 10-fold increased risk of a recurrence of stillbirth, and the SCRN findings confirmed this. The study added to our knowledge, however, with the finding that even a prior pregnancy loss at less than 20 weeks’ gestation increased the risk for stillbirth.
Other risk factors identified in the study, in addition to race, included having a multifetal pregnancy (adjusted odds ratio of 4.59), diabetes (AOR of 2.50), maternal age of 40 years or older (AOR of 2.41), maternal AB blood type (AOR of 1.96, compared with type O), a history of drug addiction (AOR of 2.08), smoking during the 3 months prior to pregnancy (AOR of 1.55-1.57, depending on amount), and being unmarried and not cohabitating (AOR of 1.69). Regarding racial disparity, the study showed that elevated risk of stillbirth for non-Hispanic blacks occurred predominantly prior to 24 weeks of gestation.
As in prior research, overweight and obesity also conferred elevated risks in the SCRN study (AORs of 1.43 and 1.72, respectively), and these risks were not explained by either diabetes or hypertension (JAMA. 2011;306:2469-79).
The use of assisted reproductive technology was not included in the study’s multivariate model, but previous research has shown a fourfold increased risk of stillbirth for singleton IVF/ICSI pregnancies. The reason is unclear, but the risk appears to be more related to IVF/ICSI rather than the underlying infertility (Hum Reprod. 2010 May;25[5]:1312-6).
A previous preterm or small-for-gestational-age birth has also been shown in prior research to be a significant risk factor for stillbirth. Less clear is the role of previous cesarean delivery in stillbirth risk. An association has been demonstrated in several studies, however, including one involving about 180,000 singleton pregnancies of 23 or more weeks’ gestation. Women in this cohort who had a previous cesarean delivery had a 1.3-fold increased risk of antepartum stillbirth, after controlling for important factors such as race, body mass index (BMI), and maternal disease (Obstet Gynecol. 2010 Nov;116[5]:1119-26).
In another analysis of the SCRN study looking specifically at causes of stillbirth, a “probable” cause of death was found in 61% of cases and a “possible or probable” cause of death in more than 76% of cases. The most common causes were obstetric complications (29.3%), placental abnormalities (23.6%), fetal genetic/structural abnormalities (13.7%), infection (12.9%), umbilical cord abnormalities (10.4%), hypertensive disorders (9.2%), and other maternal medical conditions (7.8%).
A higher proportion of stillbirths in non-Hispanic black women, compared with non-Hispanic white women and Hispanic women was associated with obstetric complications (43.5%) and infections (25.2%). This finding combined with the finding that stillbirth in black women often occurs at less than 24 weeks’ gestation suggests that measures aimed at reducing the rate of spontaneous preterm birth in black women could potentially reduce the rate of stillbirth as well (JAMA. 2011 Dec 14;306[22]:2459-68).
Work-up and prevention
Prevention of stillbirth requires that we identify the women at highest risk, and thus far this ability still eludes us. Apart from occurrence of previous stillbirth or pregnancy loss, other risk factors have had limited predictive value in the SCRN analyses and other research.
Biomarkers such as a low PAPP-A during the first trimester and a high AFP in the second trimester – as well as Doppler imaging of the uterine artery – have also been associated with stillbirth, but again, the positive predictive value has been shown to be low (Clin Obstet Gynecol. 2010 Sep;53[3]:597-606). More research is needed to determine if some combination of biochemical markers, imaging, and other risk factors can predict which women are at highest risk.
In the meantime, attention can be paid – in the preconception period if possible – to modifiable risk factors such as maternal obesity, diabetes, and smoking. About 10% of stillbirths are associated with maternal conditions such as hypertension and diabetes, and late stillbirths in particular (28 weeks or later) are associated with maternal medical conditions that are potentially preventable.
Normalization of prepregnancy weight should be a goal, since the overall risk of stillbirth appears to increase independently with increasing BMI. Glycemic control should also be achieved: A recent meta-analysis of preconception and prenatal care of diabetic women estimated “conservatively” that 10% of diabetes-associated stillbirths could be prevented with early detection and glycemic control (BMC Public Health. 2011;11 Suppl 3:S2). Research has also shown that women who quit smoking between their first and second pregnancy reduce their stillbirth risk to that of nonsmokers in the second pregnancy (BJOG. 2007 Jun;114[6]:699-704).
When stillbirth happens, a thorough work-up is recommended in order to counsel for future pregnancies and decrease the risk of recurrence. Evaluations for causes of stillbirth are too often incomplete in the United States for various reasons, including emotional, cultural, and resource factors. Even if a cause is not found, many families appreciate knowing that every effort has been made to determine a cause of death.
Four components of evaluation – autopsy, placental examination, karyotype or microarray analysis, and fetal-maternal hemorrhage testing – have proven to be high-yield tests when performed in all cases of stillbirth.
In the SCRN study, of 512 stillbirths undergoing a complete evaluation, 66.4% had a positive result – defined as abnormalities contributing to a probable or possible cause – for at least one of the first three tests (JAMA. 2011 Dec 14;306[22]:2459-68).
A Dutch study of 1,025 stillbirths similarly demonstrated that all four tests are justified. A test was defined as valuable in this study if it established or excluded a cause of stillbirth. Placental examination was determined to be the most valuable test, helping to determine a cause of death in 95.7% of cases. Autopsy was valuable 72.6% of the time, and cytogenetic analysis was valuable in 29% of cases.
Kleihauer-Betke testing for fetal-maternal hemorrhage was positive in 11.9% of women. However, fetal maternal hemorrhage was considered the cause of death in only 1.3%.of cases because, beyond a positive Kleihauer-Betke test, evidence of fetal anemia confirmed by placental examination and/or autopsy was required for hemorrhage to be considered the cause of death (Am. J. Obstet. Gynecol. 2012;206:53.e1-12). Because Kleihauer-Betke testing is ideally performed before induction, authors of both the SCRN study and the Dutch study believe it is a valuable test to be offered in all cases.
In both studies, the yield of other stillbirth diagnostic tests (for example, maternal serology, hormone assessment, and toxicology screen) was low, indicating that these tests are considered sequential and can be performed only when the clinical history or findings of the four core tests raise suspicion of particular potential causes. Antinuclear antibody testing and TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex) titers have an extremely low yield and are generally not useful.
For detecting genetic abnormalities after stillbirth, it appears that microarray analysis is superior to karyotype analysis. In a SCRN analysis of samples from 532 stillbirths, microarray yielded results more often and identified more genetic abnormalities. Unlike karyotype, it does not require live cells, which makes it preferable for stillbirth evaluation (N Engl J Med. 2012 Dec 6;367[23]:2185-93).
Current research
One of the more significant studies underway on prevention is looking at labor induction as an intervention for reducing stillbirths and improving other perinatal outcomes. The ARRIVE trial (“A Randomized Trial of Induction Versus Expectant Management”), currently in the recruitment stage, will examine outcomes after induction at 39 weeks’ gestation, compared with expectant management in 6,000 patients (clinicaltrials.gov/ct2/show/NCT01990612).
Common wisdom informed by retrospective cohort studies has long told us that inducing labor prior to 41 weeks’ gestation is associated with a higher risk of cesarean delivery in nulliparous women. However, recent observational data have suggested that women whose labor is induced actually have fewer cesarean deliveries and better perinatal outcomes, including a lower risk of stillbirth (AJOG 2012;207:502.e1-8).
In addition, a meta-analysis published in 2014, as the ARRIVE trial was taking shape, reported a 12% reduction in cesarean delivery, and a reduced risk of stillbirth, among women whose labor was induced. The initial cervical score did not impact the main findings (CMAJ. 2014 Jun 10;186[9]:665-73). If these findings are confirmed in the ARRIVE trial, we could see a new opportunity for stillbirth prevention.
Another ongoing study of 10,000 singleton pregnancies – the Nulliparous Pregnancy Outcomes: Monitoring Mothers-to-Be (nuMoM2b) study – may also lead to prevention strategies in women for whom the current pregnancy will lead to their first delivery. Among the questions being examined in this eight-site study are whether sleep-disordered breathing, or apnea, and a supine sleep position are risk factors for adverse pregnancy outcomes including stillbirth.
Supine sleeping in the last month of pregnancy was strongly associated with stillbirth in a recent analysis from the Sydney Stillbirth Study (Obstet Gynecol. 2015 Feb;125[2]:347-55), and an early analysis of a nuMoM2b subset has shown associations between sleep-disordered breathing in midpregnancy and the development of hypertensive disorders of pregnancy, and between sleep-disordered breathing in early- and mid-pregnancy and gestational diabetes (Am J Obstet Gynecol. 2015;212:S424-425).
The possible role of low-dose aspirin in preventing stillbirth also needs more exploration. A recent randomized trial of women attempting to become pregnant after having had one or two prior pregnancy losses found no difference overall in live birth rates between those who took low-dose aspirin and those assigned to placebo. However, there was one subgroup – women with a single loss at less than 20 weeks’ gestation during the previous year – in which live birth rates were higher in the aspirin group (Lancet. 2014 Jul 5;384[9937]:29-36). More research is necessary to determine if low-dose aspirin administration in women with a previous stillbirth improves pregnancy outcome.
Dr. Reddy is a member at the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. She is a board-certified ob.gyn. and maternal-fetal medicine specialist. She is the program scientist for the Maternal-Fetal Medicine Units Network and for the Stillbirth Collaborative Research Network. The comments and views of the author do not necessarily represent the views of the NICHD.
Stillbirth is a major public health problem, occurring in approximately 1 of every 160 pregnancies in the United States. The rate has remained stagnant since 2006. Prior to that time, from 1990 to 2006, the rate declined somewhat, but only half as much as the decline in infant mortality during this time period. Racial disparities also have persisted, with non-Hispanic black women having more than a twofold increase in risk (Natl Vital Stat Rep. 2012;60:1-22).
Research conducted by the Stillbirth Collaborative Research Network (SCRN) and others has provided us with insight on risk factors and on probable and possible causes of death among stillbirths, which are defined as fetal deaths at 20 or more weeks’ gestation. We know from SCRN data, for instance, that black women are more likely to have stillbirths associated with obstetric complications and infections than white and Hispanic women. However, we still cannot explain a substantial proportion of stillbirths, despite a complete evaluation, or predict who will have a stillbirth.
What we can do as obstetricians is be aware that stillbirth is one of the most common adverse pregnancy outcomes in the United States and counsel women regarding risk factors that are modifiable. Moreover, when stillbirth happens, a complete postmortem evaluation that includes autopsy, placental pathology, karyotype or microarray analysis, and fetal-maternal hemorrhage testing is recommended (Obstet Gynecol. 2009;113[3]:748-61). Recent data show that each of these four components is valuable and should be considered the basic work-up for stillbirth.
Risks and causes
Pregnancy history was the strongest baseline risk factor for stillbirth in an analysis of 614 stillbirths and 1,816 live births in the SCRN’s population-based, case-control study conducted between 2006 and 2008. The SCRN was initiated by the Eunice Kennedy Shriver National Institute of Child Health and Human Development in 2003. This critical population-based study was conducted at 59 U.S. tertiary care and community hospitals in five catchment areas and has been analyzed in more than 15 published reports.
Women with a previous stillbirth have been known to be at 5- to 10-fold increased risk of a recurrence of stillbirth, and the SCRN findings confirmed this. The study added to our knowledge, however, with the finding that even a prior pregnancy loss at less than 20 weeks’ gestation increased the risk for stillbirth.
Other risk factors identified in the study, in addition to race, included having a multifetal pregnancy (adjusted odds ratio of 4.59), diabetes (AOR of 2.50), maternal age of 40 years or older (AOR of 2.41), maternal AB blood type (AOR of 1.96, compared with type O), a history of drug addiction (AOR of 2.08), smoking during the 3 months prior to pregnancy (AOR of 1.55-1.57, depending on amount), and being unmarried and not cohabitating (AOR of 1.69). Regarding racial disparity, the study showed that elevated risk of stillbirth for non-Hispanic blacks occurred predominantly prior to 24 weeks of gestation.
As in prior research, overweight and obesity also conferred elevated risks in the SCRN study (AORs of 1.43 and 1.72, respectively), and these risks were not explained by either diabetes or hypertension (JAMA. 2011;306:2469-79).
The use of assisted reproductive technology was not included in the study’s multivariate model, but previous research has shown a fourfold increased risk of stillbirth for singleton IVF/ICSI pregnancies. The reason is unclear, but the risk appears to be more related to IVF/ICSI rather than the underlying infertility (Hum Reprod. 2010 May;25[5]:1312-6).
A previous preterm or small-for-gestational-age birth has also been shown in prior research to be a significant risk factor for stillbirth. Less clear is the role of previous cesarean delivery in stillbirth risk. An association has been demonstrated in several studies, however, including one involving about 180,000 singleton pregnancies of 23 or more weeks’ gestation. Women in this cohort who had a previous cesarean delivery had a 1.3-fold increased risk of antepartum stillbirth, after controlling for important factors such as race, body mass index (BMI), and maternal disease (Obstet Gynecol. 2010 Nov;116[5]:1119-26).
In another analysis of the SCRN study looking specifically at causes of stillbirth, a “probable” cause of death was found in 61% of cases and a “possible or probable” cause of death in more than 76% of cases. The most common causes were obstetric complications (29.3%), placental abnormalities (23.6%), fetal genetic/structural abnormalities (13.7%), infection (12.9%), umbilical cord abnormalities (10.4%), hypertensive disorders (9.2%), and other maternal medical conditions (7.8%).
A higher proportion of stillbirths in non-Hispanic black women, compared with non-Hispanic white women and Hispanic women was associated with obstetric complications (43.5%) and infections (25.2%). This finding combined with the finding that stillbirth in black women often occurs at less than 24 weeks’ gestation suggests that measures aimed at reducing the rate of spontaneous preterm birth in black women could potentially reduce the rate of stillbirth as well (JAMA. 2011 Dec 14;306[22]:2459-68).
Work-up and prevention
Prevention of stillbirth requires that we identify the women at highest risk, and thus far this ability still eludes us. Apart from occurrence of previous stillbirth or pregnancy loss, other risk factors have had limited predictive value in the SCRN analyses and other research.
Biomarkers such as a low PAPP-A during the first trimester and a high AFP in the second trimester – as well as Doppler imaging of the uterine artery – have also been associated with stillbirth, but again, the positive predictive value has been shown to be low (Clin Obstet Gynecol. 2010 Sep;53[3]:597-606). More research is needed to determine if some combination of biochemical markers, imaging, and other risk factors can predict which women are at highest risk.
In the meantime, attention can be paid – in the preconception period if possible – to modifiable risk factors such as maternal obesity, diabetes, and smoking. About 10% of stillbirths are associated with maternal conditions such as hypertension and diabetes, and late stillbirths in particular (28 weeks or later) are associated with maternal medical conditions that are potentially preventable.
Normalization of prepregnancy weight should be a goal, since the overall risk of stillbirth appears to increase independently with increasing BMI. Glycemic control should also be achieved: A recent meta-analysis of preconception and prenatal care of diabetic women estimated “conservatively” that 10% of diabetes-associated stillbirths could be prevented with early detection and glycemic control (BMC Public Health. 2011;11 Suppl 3:S2). Research has also shown that women who quit smoking between their first and second pregnancy reduce their stillbirth risk to that of nonsmokers in the second pregnancy (BJOG. 2007 Jun;114[6]:699-704).
When stillbirth happens, a thorough work-up is recommended in order to counsel for future pregnancies and decrease the risk of recurrence. Evaluations for causes of stillbirth are too often incomplete in the United States for various reasons, including emotional, cultural, and resource factors. Even if a cause is not found, many families appreciate knowing that every effort has been made to determine a cause of death.
Four components of evaluation – autopsy, placental examination, karyotype or microarray analysis, and fetal-maternal hemorrhage testing – have proven to be high-yield tests when performed in all cases of stillbirth.
In the SCRN study, of 512 stillbirths undergoing a complete evaluation, 66.4% had a positive result – defined as abnormalities contributing to a probable or possible cause – for at least one of the first three tests (JAMA. 2011 Dec 14;306[22]:2459-68).
A Dutch study of 1,025 stillbirths similarly demonstrated that all four tests are justified. A test was defined as valuable in this study if it established or excluded a cause of stillbirth. Placental examination was determined to be the most valuable test, helping to determine a cause of death in 95.7% of cases. Autopsy was valuable 72.6% of the time, and cytogenetic analysis was valuable in 29% of cases.
Kleihauer-Betke testing for fetal-maternal hemorrhage was positive in 11.9% of women. However, fetal maternal hemorrhage was considered the cause of death in only 1.3%.of cases because, beyond a positive Kleihauer-Betke test, evidence of fetal anemia confirmed by placental examination and/or autopsy was required for hemorrhage to be considered the cause of death (Am. J. Obstet. Gynecol. 2012;206:53.e1-12). Because Kleihauer-Betke testing is ideally performed before induction, authors of both the SCRN study and the Dutch study believe it is a valuable test to be offered in all cases.
In both studies, the yield of other stillbirth diagnostic tests (for example, maternal serology, hormone assessment, and toxicology screen) was low, indicating that these tests are considered sequential and can be performed only when the clinical history or findings of the four core tests raise suspicion of particular potential causes. Antinuclear antibody testing and TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex) titers have an extremely low yield and are generally not useful.
For detecting genetic abnormalities after stillbirth, it appears that microarray analysis is superior to karyotype analysis. In a SCRN analysis of samples from 532 stillbirths, microarray yielded results more often and identified more genetic abnormalities. Unlike karyotype, it does not require live cells, which makes it preferable for stillbirth evaluation (N Engl J Med. 2012 Dec 6;367[23]:2185-93).
Current research
One of the more significant studies underway on prevention is looking at labor induction as an intervention for reducing stillbirths and improving other perinatal outcomes. The ARRIVE trial (“A Randomized Trial of Induction Versus Expectant Management”), currently in the recruitment stage, will examine outcomes after induction at 39 weeks’ gestation, compared with expectant management in 6,000 patients (clinicaltrials.gov/ct2/show/NCT01990612).
Common wisdom informed by retrospective cohort studies has long told us that inducing labor prior to 41 weeks’ gestation is associated with a higher risk of cesarean delivery in nulliparous women. However, recent observational data have suggested that women whose labor is induced actually have fewer cesarean deliveries and better perinatal outcomes, including a lower risk of stillbirth (AJOG 2012;207:502.e1-8).
In addition, a meta-analysis published in 2014, as the ARRIVE trial was taking shape, reported a 12% reduction in cesarean delivery, and a reduced risk of stillbirth, among women whose labor was induced. The initial cervical score did not impact the main findings (CMAJ. 2014 Jun 10;186[9]:665-73). If these findings are confirmed in the ARRIVE trial, we could see a new opportunity for stillbirth prevention.
Another ongoing study of 10,000 singleton pregnancies – the Nulliparous Pregnancy Outcomes: Monitoring Mothers-to-Be (nuMoM2b) study – may also lead to prevention strategies in women for whom the current pregnancy will lead to their first delivery. Among the questions being examined in this eight-site study are whether sleep-disordered breathing, or apnea, and a supine sleep position are risk factors for adverse pregnancy outcomes including stillbirth.
Supine sleeping in the last month of pregnancy was strongly associated with stillbirth in a recent analysis from the Sydney Stillbirth Study (Obstet Gynecol. 2015 Feb;125[2]:347-55), and an early analysis of a nuMoM2b subset has shown associations between sleep-disordered breathing in midpregnancy and the development of hypertensive disorders of pregnancy, and between sleep-disordered breathing in early- and mid-pregnancy and gestational diabetes (Am J Obstet Gynecol. 2015;212:S424-425).
The possible role of low-dose aspirin in preventing stillbirth also needs more exploration. A recent randomized trial of women attempting to become pregnant after having had one or two prior pregnancy losses found no difference overall in live birth rates between those who took low-dose aspirin and those assigned to placebo. However, there was one subgroup – women with a single loss at less than 20 weeks’ gestation during the previous year – in which live birth rates were higher in the aspirin group (Lancet. 2014 Jul 5;384[9937]:29-36). More research is necessary to determine if low-dose aspirin administration in women with a previous stillbirth improves pregnancy outcome.
Dr. Reddy is a member at the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. She is a board-certified ob.gyn. and maternal-fetal medicine specialist. She is the program scientist for the Maternal-Fetal Medicine Units Network and for the Stillbirth Collaborative Research Network. The comments and views of the author do not necessarily represent the views of the NICHD.
Stillbirth is a major public health problem, occurring in approximately 1 of every 160 pregnancies in the United States. The rate has remained stagnant since 2006. Prior to that time, from 1990 to 2006, the rate declined somewhat, but only half as much as the decline in infant mortality during this time period. Racial disparities also have persisted, with non-Hispanic black women having more than a twofold increase in risk (Natl Vital Stat Rep. 2012;60:1-22).
Research conducted by the Stillbirth Collaborative Research Network (SCRN) and others has provided us with insight on risk factors and on probable and possible causes of death among stillbirths, which are defined as fetal deaths at 20 or more weeks’ gestation. We know from SCRN data, for instance, that black women are more likely to have stillbirths associated with obstetric complications and infections than white and Hispanic women. However, we still cannot explain a substantial proportion of stillbirths, despite a complete evaluation, or predict who will have a stillbirth.
What we can do as obstetricians is be aware that stillbirth is one of the most common adverse pregnancy outcomes in the United States and counsel women regarding risk factors that are modifiable. Moreover, when stillbirth happens, a complete postmortem evaluation that includes autopsy, placental pathology, karyotype or microarray analysis, and fetal-maternal hemorrhage testing is recommended (Obstet Gynecol. 2009;113[3]:748-61). Recent data show that each of these four components is valuable and should be considered the basic work-up for stillbirth.
Risks and causes
Pregnancy history was the strongest baseline risk factor for stillbirth in an analysis of 614 stillbirths and 1,816 live births in the SCRN’s population-based, case-control study conducted between 2006 and 2008. The SCRN was initiated by the Eunice Kennedy Shriver National Institute of Child Health and Human Development in 2003. This critical population-based study was conducted at 59 U.S. tertiary care and community hospitals in five catchment areas and has been analyzed in more than 15 published reports.
Women with a previous stillbirth have been known to be at 5- to 10-fold increased risk of a recurrence of stillbirth, and the SCRN findings confirmed this. The study added to our knowledge, however, with the finding that even a prior pregnancy loss at less than 20 weeks’ gestation increased the risk for stillbirth.
Other risk factors identified in the study, in addition to race, included having a multifetal pregnancy (adjusted odds ratio of 4.59), diabetes (AOR of 2.50), maternal age of 40 years or older (AOR of 2.41), maternal AB blood type (AOR of 1.96, compared with type O), a history of drug addiction (AOR of 2.08), smoking during the 3 months prior to pregnancy (AOR of 1.55-1.57, depending on amount), and being unmarried and not cohabitating (AOR of 1.69). Regarding racial disparity, the study showed that elevated risk of stillbirth for non-Hispanic blacks occurred predominantly prior to 24 weeks of gestation.
As in prior research, overweight and obesity also conferred elevated risks in the SCRN study (AORs of 1.43 and 1.72, respectively), and these risks were not explained by either diabetes or hypertension (JAMA. 2011;306:2469-79).
The use of assisted reproductive technology was not included in the study’s multivariate model, but previous research has shown a fourfold increased risk of stillbirth for singleton IVF/ICSI pregnancies. The reason is unclear, but the risk appears to be more related to IVF/ICSI rather than the underlying infertility (Hum Reprod. 2010 May;25[5]:1312-6).
A previous preterm or small-for-gestational-age birth has also been shown in prior research to be a significant risk factor for stillbirth. Less clear is the role of previous cesarean delivery in stillbirth risk. An association has been demonstrated in several studies, however, including one involving about 180,000 singleton pregnancies of 23 or more weeks’ gestation. Women in this cohort who had a previous cesarean delivery had a 1.3-fold increased risk of antepartum stillbirth, after controlling for important factors such as race, body mass index (BMI), and maternal disease (Obstet Gynecol. 2010 Nov;116[5]:1119-26).
In another analysis of the SCRN study looking specifically at causes of stillbirth, a “probable” cause of death was found in 61% of cases and a “possible or probable” cause of death in more than 76% of cases. The most common causes were obstetric complications (29.3%), placental abnormalities (23.6%), fetal genetic/structural abnormalities (13.7%), infection (12.9%), umbilical cord abnormalities (10.4%), hypertensive disorders (9.2%), and other maternal medical conditions (7.8%).
A higher proportion of stillbirths in non-Hispanic black women, compared with non-Hispanic white women and Hispanic women was associated with obstetric complications (43.5%) and infections (25.2%). This finding combined with the finding that stillbirth in black women often occurs at less than 24 weeks’ gestation suggests that measures aimed at reducing the rate of spontaneous preterm birth in black women could potentially reduce the rate of stillbirth as well (JAMA. 2011 Dec 14;306[22]:2459-68).
Work-up and prevention
Prevention of stillbirth requires that we identify the women at highest risk, and thus far this ability still eludes us. Apart from occurrence of previous stillbirth or pregnancy loss, other risk factors have had limited predictive value in the SCRN analyses and other research.
Biomarkers such as a low PAPP-A during the first trimester and a high AFP in the second trimester – as well as Doppler imaging of the uterine artery – have also been associated with stillbirth, but again, the positive predictive value has been shown to be low (Clin Obstet Gynecol. 2010 Sep;53[3]:597-606). More research is needed to determine if some combination of biochemical markers, imaging, and other risk factors can predict which women are at highest risk.
In the meantime, attention can be paid – in the preconception period if possible – to modifiable risk factors such as maternal obesity, diabetes, and smoking. About 10% of stillbirths are associated with maternal conditions such as hypertension and diabetes, and late stillbirths in particular (28 weeks or later) are associated with maternal medical conditions that are potentially preventable.
Normalization of prepregnancy weight should be a goal, since the overall risk of stillbirth appears to increase independently with increasing BMI. Glycemic control should also be achieved: A recent meta-analysis of preconception and prenatal care of diabetic women estimated “conservatively” that 10% of diabetes-associated stillbirths could be prevented with early detection and glycemic control (BMC Public Health. 2011;11 Suppl 3:S2). Research has also shown that women who quit smoking between their first and second pregnancy reduce their stillbirth risk to that of nonsmokers in the second pregnancy (BJOG. 2007 Jun;114[6]:699-704).
When stillbirth happens, a thorough work-up is recommended in order to counsel for future pregnancies and decrease the risk of recurrence. Evaluations for causes of stillbirth are too often incomplete in the United States for various reasons, including emotional, cultural, and resource factors. Even if a cause is not found, many families appreciate knowing that every effort has been made to determine a cause of death.
Four components of evaluation – autopsy, placental examination, karyotype or microarray analysis, and fetal-maternal hemorrhage testing – have proven to be high-yield tests when performed in all cases of stillbirth.
In the SCRN study, of 512 stillbirths undergoing a complete evaluation, 66.4% had a positive result – defined as abnormalities contributing to a probable or possible cause – for at least one of the first three tests (JAMA. 2011 Dec 14;306[22]:2459-68).
A Dutch study of 1,025 stillbirths similarly demonstrated that all four tests are justified. A test was defined as valuable in this study if it established or excluded a cause of stillbirth. Placental examination was determined to be the most valuable test, helping to determine a cause of death in 95.7% of cases. Autopsy was valuable 72.6% of the time, and cytogenetic analysis was valuable in 29% of cases.
Kleihauer-Betke testing for fetal-maternal hemorrhage was positive in 11.9% of women. However, fetal maternal hemorrhage was considered the cause of death in only 1.3%.of cases because, beyond a positive Kleihauer-Betke test, evidence of fetal anemia confirmed by placental examination and/or autopsy was required for hemorrhage to be considered the cause of death (Am. J. Obstet. Gynecol. 2012;206:53.e1-12). Because Kleihauer-Betke testing is ideally performed before induction, authors of both the SCRN study and the Dutch study believe it is a valuable test to be offered in all cases.
In both studies, the yield of other stillbirth diagnostic tests (for example, maternal serology, hormone assessment, and toxicology screen) was low, indicating that these tests are considered sequential and can be performed only when the clinical history or findings of the four core tests raise suspicion of particular potential causes. Antinuclear antibody testing and TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex) titers have an extremely low yield and are generally not useful.
For detecting genetic abnormalities after stillbirth, it appears that microarray analysis is superior to karyotype analysis. In a SCRN analysis of samples from 532 stillbirths, microarray yielded results more often and identified more genetic abnormalities. Unlike karyotype, it does not require live cells, which makes it preferable for stillbirth evaluation (N Engl J Med. 2012 Dec 6;367[23]:2185-93).
Current research
One of the more significant studies underway on prevention is looking at labor induction as an intervention for reducing stillbirths and improving other perinatal outcomes. The ARRIVE trial (“A Randomized Trial of Induction Versus Expectant Management”), currently in the recruitment stage, will examine outcomes after induction at 39 weeks’ gestation, compared with expectant management in 6,000 patients (clinicaltrials.gov/ct2/show/NCT01990612).
Common wisdom informed by retrospective cohort studies has long told us that inducing labor prior to 41 weeks’ gestation is associated with a higher risk of cesarean delivery in nulliparous women. However, recent observational data have suggested that women whose labor is induced actually have fewer cesarean deliveries and better perinatal outcomes, including a lower risk of stillbirth (AJOG 2012;207:502.e1-8).
In addition, a meta-analysis published in 2014, as the ARRIVE trial was taking shape, reported a 12% reduction in cesarean delivery, and a reduced risk of stillbirth, among women whose labor was induced. The initial cervical score did not impact the main findings (CMAJ. 2014 Jun 10;186[9]:665-73). If these findings are confirmed in the ARRIVE trial, we could see a new opportunity for stillbirth prevention.
Another ongoing study of 10,000 singleton pregnancies – the Nulliparous Pregnancy Outcomes: Monitoring Mothers-to-Be (nuMoM2b) study – may also lead to prevention strategies in women for whom the current pregnancy will lead to their first delivery. Among the questions being examined in this eight-site study are whether sleep-disordered breathing, or apnea, and a supine sleep position are risk factors for adverse pregnancy outcomes including stillbirth.
Supine sleeping in the last month of pregnancy was strongly associated with stillbirth in a recent analysis from the Sydney Stillbirth Study (Obstet Gynecol. 2015 Feb;125[2]:347-55), and an early analysis of a nuMoM2b subset has shown associations between sleep-disordered breathing in midpregnancy and the development of hypertensive disorders of pregnancy, and between sleep-disordered breathing in early- and mid-pregnancy and gestational diabetes (Am J Obstet Gynecol. 2015;212:S424-425).
The possible role of low-dose aspirin in preventing stillbirth also needs more exploration. A recent randomized trial of women attempting to become pregnant after having had one or two prior pregnancy losses found no difference overall in live birth rates between those who took low-dose aspirin and those assigned to placebo. However, there was one subgroup – women with a single loss at less than 20 weeks’ gestation during the previous year – in which live birth rates were higher in the aspirin group (Lancet. 2014 Jul 5;384[9937]:29-36). More research is necessary to determine if low-dose aspirin administration in women with a previous stillbirth improves pregnancy outcome.
Dr. Reddy is a member at the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. She is a board-certified ob.gyn. and maternal-fetal medicine specialist. She is the program scientist for the Maternal-Fetal Medicine Units Network and for the Stillbirth Collaborative Research Network. The comments and views of the author do not necessarily represent the views of the NICHD.
Radiofrequency volumetric thermal ablation for symptomatic uterine fibroids
In 2002, Dr. Bruce B. Lee first described a laparoscopic technique to ablate symptomatic uterine fibroids utilizing radiofrequency under ultrasound guidance. Since this time, several papers have documented the procedure’s feasibility and efficacy, including reduction in menstrual blood loss, fibroid volume decrease, and improvement in quality of life.
In a randomized, prospective, single-center, longitudinal study that compared laparoscopic radiofrequency volumetric thermal ablation (RFVTA) of fibroids with laparoscopic myomectomy, Dr. Sara Y. Brucker and her colleagues concluded that RFVTA resulted in the treatment of more fibroids, a significantly shorter hospital stay, and less intraoperative blood loss than did laparoscopic myomectomy (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
More recently, in the literature and at the 2015 American Association of Gynecologic Laparoscopists (AAGL) Global Congress in November, viable, full-term pregnancies have been reported in patients previously treated for symptomatic fibroids via RFVTA (J Reprod Med. 2015 May-Jun;60[5-6]:194-8).
The system for performing RFVTA of symptomatic fibroids – the Acessa System (Halt Medical) – has continued to improve. Earlier this year, Dr. Donald I. Galen described the use of electromagnetic image guidance, which has been cleared by the Food and Drug Administration and incorporated into the Acessa Guidance System. Dr. Galen’s feasibility study showed that the guidance system enhances the ultrasonic image of Acessa’s handpiece to facilitate accurate tip placement during the targeting and ablation of uterine fibroids (Biomed Eng Online. 2015 Oct 15;14:90).
In this edition of the Master Class in Gynecologic Surgery, Dr. Jay M. Berman discusses the use of RFVTA for the treatment of symptomatic uterine fibroids. Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist in chief for obstetrics and gynecology at the Detroit Medical Center. He served as a principal investigator of the pivotal trial of Acessa and has reported on reproductive outcomes. Dr. Berman has long been interested in alternatives to hysterectomy for fibroid management and has incorporated RFVTA into his armamentarium of therapies.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported that he is a consultant for Halt Medical Inc., which developed the Acessa System.
In 2002, Dr. Bruce B. Lee first described a laparoscopic technique to ablate symptomatic uterine fibroids utilizing radiofrequency under ultrasound guidance. Since this time, several papers have documented the procedure’s feasibility and efficacy, including reduction in menstrual blood loss, fibroid volume decrease, and improvement in quality of life.
In a randomized, prospective, single-center, longitudinal study that compared laparoscopic radiofrequency volumetric thermal ablation (RFVTA) of fibroids with laparoscopic myomectomy, Dr. Sara Y. Brucker and her colleagues concluded that RFVTA resulted in the treatment of more fibroids, a significantly shorter hospital stay, and less intraoperative blood loss than did laparoscopic myomectomy (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
More recently, in the literature and at the 2015 American Association of Gynecologic Laparoscopists (AAGL) Global Congress in November, viable, full-term pregnancies have been reported in patients previously treated for symptomatic fibroids via RFVTA (J Reprod Med. 2015 May-Jun;60[5-6]:194-8).
The system for performing RFVTA of symptomatic fibroids – the Acessa System (Halt Medical) – has continued to improve. Earlier this year, Dr. Donald I. Galen described the use of electromagnetic image guidance, which has been cleared by the Food and Drug Administration and incorporated into the Acessa Guidance System. Dr. Galen’s feasibility study showed that the guidance system enhances the ultrasonic image of Acessa’s handpiece to facilitate accurate tip placement during the targeting and ablation of uterine fibroids (Biomed Eng Online. 2015 Oct 15;14:90).
In this edition of the Master Class in Gynecologic Surgery, Dr. Jay M. Berman discusses the use of RFVTA for the treatment of symptomatic uterine fibroids. Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist in chief for obstetrics and gynecology at the Detroit Medical Center. He served as a principal investigator of the pivotal trial of Acessa and has reported on reproductive outcomes. Dr. Berman has long been interested in alternatives to hysterectomy for fibroid management and has incorporated RFVTA into his armamentarium of therapies.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported that he is a consultant for Halt Medical Inc., which developed the Acessa System.
In 2002, Dr. Bruce B. Lee first described a laparoscopic technique to ablate symptomatic uterine fibroids utilizing radiofrequency under ultrasound guidance. Since this time, several papers have documented the procedure’s feasibility and efficacy, including reduction in menstrual blood loss, fibroid volume decrease, and improvement in quality of life.
In a randomized, prospective, single-center, longitudinal study that compared laparoscopic radiofrequency volumetric thermal ablation (RFVTA) of fibroids with laparoscopic myomectomy, Dr. Sara Y. Brucker and her colleagues concluded that RFVTA resulted in the treatment of more fibroids, a significantly shorter hospital stay, and less intraoperative blood loss than did laparoscopic myomectomy (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
More recently, in the literature and at the 2015 American Association of Gynecologic Laparoscopists (AAGL) Global Congress in November, viable, full-term pregnancies have been reported in patients previously treated for symptomatic fibroids via RFVTA (J Reprod Med. 2015 May-Jun;60[5-6]:194-8).
The system for performing RFVTA of symptomatic fibroids – the Acessa System (Halt Medical) – has continued to improve. Earlier this year, Dr. Donald I. Galen described the use of electromagnetic image guidance, which has been cleared by the Food and Drug Administration and incorporated into the Acessa Guidance System. Dr. Galen’s feasibility study showed that the guidance system enhances the ultrasonic image of Acessa’s handpiece to facilitate accurate tip placement during the targeting and ablation of uterine fibroids (Biomed Eng Online. 2015 Oct 15;14:90).
In this edition of the Master Class in Gynecologic Surgery, Dr. Jay M. Berman discusses the use of RFVTA for the treatment of symptomatic uterine fibroids. Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist in chief for obstetrics and gynecology at the Detroit Medical Center. He served as a principal investigator of the pivotal trial of Acessa and has reported on reproductive outcomes. Dr. Berman has long been interested in alternatives to hysterectomy for fibroid management and has incorporated RFVTA into his armamentarium of therapies.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, and a past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller reported that he is a consultant for Halt Medical Inc., which developed the Acessa System.
RFVTA system offers alternative to myomectomy
Uterine myomas cause heavy menstrual bleeding and other clinically significant symptoms in 35%-50% of affected women and have been shown to be the leading indication for hysterectomy in the United States among women aged 35-54 years.
Research has shown that a significant number of women who undergo hysterectomy for treatment of fibroids later regret the loss of their uterus and have other concerns and complications. Other options for therapy include various pharmacologic treatments, a progestin-releasing intrauterine device, uterine artery embolization, endometrial ablation, MRI-guided focused ultrasound surgery, and myomectomy performed laparoscopically, robotically, or hysteroscopically.
Myomectomy seems largely to preserve fertility, but rates of recurrence and additional procedures for bleeding and myoma symptoms are still high – upward of 30% in some studies. Overall, we need other more efficacious and minimally invasive options.
Radiofrequency volumetric thermal ablation (RFVTA) achieved through the Acessa System (Halt Medical) has been the newest addition to our armamentarium for treatment of symptomatic fibroids. It is suitable for every type of fibroid except for type 0 pedunculated intracavitary fibroids and type 7 pedunculated subserosal fibroids, which is significant because deep intramural fibroids have been difficult to target and treat by other methods.
Three-year outcome data show sustained improvements in fibroid symptoms and quality of life, with an incidence of recurrences and additional procedures – approximately 11% – that appears to be substantially lower than for other uterine-sparing fibroid treatments. In addition, while the technology is not indicated for women seeking future childbearing, successful pregnancies are being reported, suggesting that full-term pregnancies – and vaginal delivery in some cases – may be possible after RFVTA.
The principles
Radiofrequency ablation has been used for years in the treatment of liver and kidney tumors. The basic concept is that volumetric thermal ablation results in coagulative necrosis.
The Acessa System, approved by the Food and Drug Administration in late 2012, was designed to treat fibroids, which have much firmer tissue than the tissues being targeted in other radiofrequency ablation procedures. It uses a specially designed intrauterine ultrasound probe and radiofrequency probe, and it combines three fundamental gynecologic skills: Laparoscopy using two trocars and requiring no special suturing skills; ultrasound using a laparoscopic ultrasound probe to scan and manipulate; and probe placement under laparoscopic ultrasound guidance.
Specifically, the system allows for percutaneous, laparoscopic ultrasound–guided radiofrequency ablation of fibroids with a disposable 3.4-mm handpiece coupled to a dual-function radiofrequency generator. The handpiece contains a retractable array of electrodes, so that the fibroid may be ablated with one electrode or with the deployed electrode array.
The generator controls and monitors the ablation with real-time feedback from thermocouples. It monitors and displays the temperature at each needle tip, the average temperature of the array, and the return temperatures on two dispersive electrode pads that are placed on the anterior thighs. The electrode pads are designed to reduce the incidence of pad burns, which are a complication with other radiofrequency ablation devices. The system will automatically stop treatment if either of the pad thermocouples registers a skin temperature greater than 40° C (JSLS. 2014 Apr-Jun;18[2]:182-90).
The outcomes
Laparoscopic ultrasound–guided RFVTA has been studied in five prospective trials, including one multicenter international trial of 135 premenopausal women – the pivotal trial for FDA clearance – in which 104 women were followed for 3 years and found to have prolonged symptom relief and improved quality of life.
At baseline, the women had symptomatic uterine myomas and moderate to severe heavy menstrual bleeding measured by alkaline hematin analysis of returned sanitary products. Their mean symptom severity scores on the Uterine Fibroid Symptom and Quality-of-Life Questionnaire (UFS-QOL) decreased significantly from baseline to 3 months and changed little after that, for a total change of –32.6 over the study period.
The cumulative repeat intervention rate at 3 years was 11%, with 14 of the 135 participants having repeat interventions to treat bleeding and myoma symptoms. Seven of these women were found to have adenomyosis (J Minim Invasive Gynecol. 2014 Sep-Oct;21[5]:767-74).
The surprisingly low reintervention rates may stem from the benefits of direct contact imaging of the uterus. A comparison of images from the pivotal trial has shown that intraoperative ultrasound detected more than twice as many fibroids as did preoperative transvaginal ultrasound, and about one-third more than preoperative MRIs (J Minim Invasive Gynecol. 2013 Nov-Dec;20[6]:770-4).
Interestingly, four women became pregnant over the study’s 3-year follow-up, despite the inclusion requirement that women desire uterine conservation but not future childbearing.
We have followed reproductive outcomes in women after RFVTA of symptomatic fibroids in other studies as well. In our most recent analysis, presented in November at the 2015 American Association of Gynecologic Laparoscopists Global Congress, we identified 10 pregnancies among participants of the five prospective trials.
Of 232 women enrolled in premarket RFVTA studies – trials in which completing childbearing and continuing contraception were requirements – six conceived at 3.5-15 months post ablation. The number of myomas treated ranged from one to seven and included multiple types and dimensions. Five of these six women delivered full-term healthy babies – one by vaginal delivery and four by cesarean section. The sixth patient had a spontaneous abortion in the first trimester.
Of 43 women who participated in two randomized clinical trials undertaken after FDA clearance, four conceived at 4-23.5 months post ablation. Three of these women had uneventful, full-term pregnancies with vaginal births. The fourth had a cesarean section at 38 weeks.
Considering the theoretical advantages of the Acessa procedure – that it is less damaging to healthy myometrium – and the outcomes reported thus far, it appears likely that Acessa will be preferable to myomectomy. Early results from an ongoing 5-year German study that randomized 50 women to RFVTA or laparoscopic myomectomy show that RFVTA resulted in the treatment of more fibroids and involved a significantly shorter hospital stay and post-operative recovery (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
The technique
The patient is pretreated with a nonsteroidal anti-inflammatory agent and prophylactic antibiotic. She is placed in a supine position with arms tucked, and a single-toothed tenaculum is placed on the cervix from 12 to 6 o’clock, without any instrument for manipulation of the uterus. The system’s dispersive electrode pads are placed symmetrically just above the patella on the anterior thighs; asymmetrical placement could potentially increase the risk of a pad burn.
Two standard laparoscopic ports are placed. A 5-mm trocar for the camera and video laparoscope is placed through the umbilicus or at a supraumbilical or left upper–quadrant level, depending on the patient’s anatomy, her surgical history, and the size of the uterus. A thorough visual inspection of the abdomen should be performed to look for unsuspected findings.
A 10-mm trocar is then placed at the level of the top of the fundus for the intra-abdominal ultrasound probe. Laparoscopic ultrasound is used to survey the entire uterus, map the fibroids, and plan an approach. Once the fibroid to be treated first is identified, the ability to stabilize the uterus accordingly is assessed, and the dimensions of the fibroid are taken. The dimensions will be used by the surgeon with a volume algorithm to calculate the length of ablation time based on the size of the fibroid and electrode deployment.
Under ultrasound guidance, the Acessa radiofrequency ablation handpiece is inserted percutaneously at 12, 3, 6, or 9 o’clock relative to the ultrasound trocar, based on the location of the target fibroid. The uterus must be stabilized, with the handpiece and ultrasound probe parallel and in plane. The handpiece is then inserted 1 cm into the target fibroid through the uterine serosal surface, utilizing a combination of laparoscopic and ultrasound views. Care must be taken to use gentle rotation and minimal downward pressure as the tip of the handpiece is quite sharp.
The location of the tip is confirmed by laparoscopic ultrasound, and the 7-needle electrode array can then be deployed to the ablation site. All three dimensions of the fibroid should be viewed for placement and deployment of the electrodes. Care is taken to avoid large blood vessels and ensure that the electrodes are confined within the fibroid and within the uterus.
Radiofrequency ablation is carried out with a low-voltage, high-frequency alternating current. The radiofrequency waves heat the tissue to an average temperature of 95° C for a length of time determined by a treatment algorithm. The wattage automatically adjusts to maintain the treatment temperature for the calculated duration of ablation.
Small fibroids can be treated in a manual mode without deployment of the electrode array at a current output of 15 W.
At the conclusion of the ablation, the electrodes are withdrawn into the handpiece, the generator is changed to coagulation mode, and the handpiece is slowly withdrawn under ultrasound visualization. The tract is simultaneously coagulated. A bit of additional coagulation is facilitated by pausing at the serosal surface.
Additional fibroids can be ablated through another insertion of the handpiece, either through the same tract or through a new tract.
Larger fibroids may require multiple ablations. The maximum size of ablation is about 5 cm, so it is important to plan the treatment of larger fibroids. This can be accomplished by carefully scanning large fibroids and visualizing the number of overlapping ablations needed to treat the entire volume. I ask my assistant to record the size and location of each ablation; I find this helpful both for organizing the treatment of large fibroids and for dictating the operative report.
It is important to appreciate that treatment of one area can make it difficult to visualize nearby fibroids with ultrasound. The effect dissipates in about 30-45 minutes. It is one reason why having a fibroid map prior to treatment is so important.
Once all fibroids are treated, a final inspection is performed. We usually use a suction irrigator to clean out whatever small amounts of blood are present, and the laparoscopic and port sites are closed in standard fashion.
Patients are seen 1 week postoperatively and are instructed to call in cases of pain, fever, bleeding, or chills. Most patients require only NSAIDs for pain relief and return to work in 2-7 days.
Many patients experience a slightly heavier than normal first menses after treatment. Pelvic rest is recommended for 3 weeks as a precaution, and avoidance of intrauterine procedures is advised because the uterus will be soft and thus may be easily perforated. Patients who have had type 1, type 2, or type 2-5 fibroids ablated may experience drainage for several weeks as the fibroid tissue is reabsorbed.
Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist-in-chief for obstetrics and gynecology at the Detroit Medical Center. He was a principal investigator of the 3-year outcome study of Acessa sponsored by Halt Medical. He is a consultant for Halt Medical and directs physician training in the use of Acessa.
Uterine myomas cause heavy menstrual bleeding and other clinically significant symptoms in 35%-50% of affected women and have been shown to be the leading indication for hysterectomy in the United States among women aged 35-54 years.
Research has shown that a significant number of women who undergo hysterectomy for treatment of fibroids later regret the loss of their uterus and have other concerns and complications. Other options for therapy include various pharmacologic treatments, a progestin-releasing intrauterine device, uterine artery embolization, endometrial ablation, MRI-guided focused ultrasound surgery, and myomectomy performed laparoscopically, robotically, or hysteroscopically.
Myomectomy seems largely to preserve fertility, but rates of recurrence and additional procedures for bleeding and myoma symptoms are still high – upward of 30% in some studies. Overall, we need other more efficacious and minimally invasive options.
Radiofrequency volumetric thermal ablation (RFVTA) achieved through the Acessa System (Halt Medical) has been the newest addition to our armamentarium for treatment of symptomatic fibroids. It is suitable for every type of fibroid except for type 0 pedunculated intracavitary fibroids and type 7 pedunculated subserosal fibroids, which is significant because deep intramural fibroids have been difficult to target and treat by other methods.
Three-year outcome data show sustained improvements in fibroid symptoms and quality of life, with an incidence of recurrences and additional procedures – approximately 11% – that appears to be substantially lower than for other uterine-sparing fibroid treatments. In addition, while the technology is not indicated for women seeking future childbearing, successful pregnancies are being reported, suggesting that full-term pregnancies – and vaginal delivery in some cases – may be possible after RFVTA.
The principles
Radiofrequency ablation has been used for years in the treatment of liver and kidney tumors. The basic concept is that volumetric thermal ablation results in coagulative necrosis.
The Acessa System, approved by the Food and Drug Administration in late 2012, was designed to treat fibroids, which have much firmer tissue than the tissues being targeted in other radiofrequency ablation procedures. It uses a specially designed intrauterine ultrasound probe and radiofrequency probe, and it combines three fundamental gynecologic skills: Laparoscopy using two trocars and requiring no special suturing skills; ultrasound using a laparoscopic ultrasound probe to scan and manipulate; and probe placement under laparoscopic ultrasound guidance.
Specifically, the system allows for percutaneous, laparoscopic ultrasound–guided radiofrequency ablation of fibroids with a disposable 3.4-mm handpiece coupled to a dual-function radiofrequency generator. The handpiece contains a retractable array of electrodes, so that the fibroid may be ablated with one electrode or with the deployed electrode array.
The generator controls and monitors the ablation with real-time feedback from thermocouples. It monitors and displays the temperature at each needle tip, the average temperature of the array, and the return temperatures on two dispersive electrode pads that are placed on the anterior thighs. The electrode pads are designed to reduce the incidence of pad burns, which are a complication with other radiofrequency ablation devices. The system will automatically stop treatment if either of the pad thermocouples registers a skin temperature greater than 40° C (JSLS. 2014 Apr-Jun;18[2]:182-90).
The outcomes
Laparoscopic ultrasound–guided RFVTA has been studied in five prospective trials, including one multicenter international trial of 135 premenopausal women – the pivotal trial for FDA clearance – in which 104 women were followed for 3 years and found to have prolonged symptom relief and improved quality of life.
At baseline, the women had symptomatic uterine myomas and moderate to severe heavy menstrual bleeding measured by alkaline hematin analysis of returned sanitary products. Their mean symptom severity scores on the Uterine Fibroid Symptom and Quality-of-Life Questionnaire (UFS-QOL) decreased significantly from baseline to 3 months and changed little after that, for a total change of –32.6 over the study period.
The cumulative repeat intervention rate at 3 years was 11%, with 14 of the 135 participants having repeat interventions to treat bleeding and myoma symptoms. Seven of these women were found to have adenomyosis (J Minim Invasive Gynecol. 2014 Sep-Oct;21[5]:767-74).
The surprisingly low reintervention rates may stem from the benefits of direct contact imaging of the uterus. A comparison of images from the pivotal trial has shown that intraoperative ultrasound detected more than twice as many fibroids as did preoperative transvaginal ultrasound, and about one-third more than preoperative MRIs (J Minim Invasive Gynecol. 2013 Nov-Dec;20[6]:770-4).
Interestingly, four women became pregnant over the study’s 3-year follow-up, despite the inclusion requirement that women desire uterine conservation but not future childbearing.
We have followed reproductive outcomes in women after RFVTA of symptomatic fibroids in other studies as well. In our most recent analysis, presented in November at the 2015 American Association of Gynecologic Laparoscopists Global Congress, we identified 10 pregnancies among participants of the five prospective trials.
Of 232 women enrolled in premarket RFVTA studies – trials in which completing childbearing and continuing contraception were requirements – six conceived at 3.5-15 months post ablation. The number of myomas treated ranged from one to seven and included multiple types and dimensions. Five of these six women delivered full-term healthy babies – one by vaginal delivery and four by cesarean section. The sixth patient had a spontaneous abortion in the first trimester.
Of 43 women who participated in two randomized clinical trials undertaken after FDA clearance, four conceived at 4-23.5 months post ablation. Three of these women had uneventful, full-term pregnancies with vaginal births. The fourth had a cesarean section at 38 weeks.
Considering the theoretical advantages of the Acessa procedure – that it is less damaging to healthy myometrium – and the outcomes reported thus far, it appears likely that Acessa will be preferable to myomectomy. Early results from an ongoing 5-year German study that randomized 50 women to RFVTA or laparoscopic myomectomy show that RFVTA resulted in the treatment of more fibroids and involved a significantly shorter hospital stay and post-operative recovery (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
The technique
The patient is pretreated with a nonsteroidal anti-inflammatory agent and prophylactic antibiotic. She is placed in a supine position with arms tucked, and a single-toothed tenaculum is placed on the cervix from 12 to 6 o’clock, without any instrument for manipulation of the uterus. The system’s dispersive electrode pads are placed symmetrically just above the patella on the anterior thighs; asymmetrical placement could potentially increase the risk of a pad burn.
Two standard laparoscopic ports are placed. A 5-mm trocar for the camera and video laparoscope is placed through the umbilicus or at a supraumbilical or left upper–quadrant level, depending on the patient’s anatomy, her surgical history, and the size of the uterus. A thorough visual inspection of the abdomen should be performed to look for unsuspected findings.
A 10-mm trocar is then placed at the level of the top of the fundus for the intra-abdominal ultrasound probe. Laparoscopic ultrasound is used to survey the entire uterus, map the fibroids, and plan an approach. Once the fibroid to be treated first is identified, the ability to stabilize the uterus accordingly is assessed, and the dimensions of the fibroid are taken. The dimensions will be used by the surgeon with a volume algorithm to calculate the length of ablation time based on the size of the fibroid and electrode deployment.
Under ultrasound guidance, the Acessa radiofrequency ablation handpiece is inserted percutaneously at 12, 3, 6, or 9 o’clock relative to the ultrasound trocar, based on the location of the target fibroid. The uterus must be stabilized, with the handpiece and ultrasound probe parallel and in plane. The handpiece is then inserted 1 cm into the target fibroid through the uterine serosal surface, utilizing a combination of laparoscopic and ultrasound views. Care must be taken to use gentle rotation and minimal downward pressure as the tip of the handpiece is quite sharp.
The location of the tip is confirmed by laparoscopic ultrasound, and the 7-needle electrode array can then be deployed to the ablation site. All three dimensions of the fibroid should be viewed for placement and deployment of the electrodes. Care is taken to avoid large blood vessels and ensure that the electrodes are confined within the fibroid and within the uterus.
Radiofrequency ablation is carried out with a low-voltage, high-frequency alternating current. The radiofrequency waves heat the tissue to an average temperature of 95° C for a length of time determined by a treatment algorithm. The wattage automatically adjusts to maintain the treatment temperature for the calculated duration of ablation.
Small fibroids can be treated in a manual mode without deployment of the electrode array at a current output of 15 W.
At the conclusion of the ablation, the electrodes are withdrawn into the handpiece, the generator is changed to coagulation mode, and the handpiece is slowly withdrawn under ultrasound visualization. The tract is simultaneously coagulated. A bit of additional coagulation is facilitated by pausing at the serosal surface.
Additional fibroids can be ablated through another insertion of the handpiece, either through the same tract or through a new tract.
Larger fibroids may require multiple ablations. The maximum size of ablation is about 5 cm, so it is important to plan the treatment of larger fibroids. This can be accomplished by carefully scanning large fibroids and visualizing the number of overlapping ablations needed to treat the entire volume. I ask my assistant to record the size and location of each ablation; I find this helpful both for organizing the treatment of large fibroids and for dictating the operative report.
It is important to appreciate that treatment of one area can make it difficult to visualize nearby fibroids with ultrasound. The effect dissipates in about 30-45 minutes. It is one reason why having a fibroid map prior to treatment is so important.
Once all fibroids are treated, a final inspection is performed. We usually use a suction irrigator to clean out whatever small amounts of blood are present, and the laparoscopic and port sites are closed in standard fashion.
Patients are seen 1 week postoperatively and are instructed to call in cases of pain, fever, bleeding, or chills. Most patients require only NSAIDs for pain relief and return to work in 2-7 days.
Many patients experience a slightly heavier than normal first menses after treatment. Pelvic rest is recommended for 3 weeks as a precaution, and avoidance of intrauterine procedures is advised because the uterus will be soft and thus may be easily perforated. Patients who have had type 1, type 2, or type 2-5 fibroids ablated may experience drainage for several weeks as the fibroid tissue is reabsorbed.
Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist-in-chief for obstetrics and gynecology at the Detroit Medical Center. He was a principal investigator of the 3-year outcome study of Acessa sponsored by Halt Medical. He is a consultant for Halt Medical and directs physician training in the use of Acessa.
Uterine myomas cause heavy menstrual bleeding and other clinically significant symptoms in 35%-50% of affected women and have been shown to be the leading indication for hysterectomy in the United States among women aged 35-54 years.
Research has shown that a significant number of women who undergo hysterectomy for treatment of fibroids later regret the loss of their uterus and have other concerns and complications. Other options for therapy include various pharmacologic treatments, a progestin-releasing intrauterine device, uterine artery embolization, endometrial ablation, MRI-guided focused ultrasound surgery, and myomectomy performed laparoscopically, robotically, or hysteroscopically.
Myomectomy seems largely to preserve fertility, but rates of recurrence and additional procedures for bleeding and myoma symptoms are still high – upward of 30% in some studies. Overall, we need other more efficacious and minimally invasive options.
Radiofrequency volumetric thermal ablation (RFVTA) achieved through the Acessa System (Halt Medical) has been the newest addition to our armamentarium for treatment of symptomatic fibroids. It is suitable for every type of fibroid except for type 0 pedunculated intracavitary fibroids and type 7 pedunculated subserosal fibroids, which is significant because deep intramural fibroids have been difficult to target and treat by other methods.
Three-year outcome data show sustained improvements in fibroid symptoms and quality of life, with an incidence of recurrences and additional procedures – approximately 11% – that appears to be substantially lower than for other uterine-sparing fibroid treatments. In addition, while the technology is not indicated for women seeking future childbearing, successful pregnancies are being reported, suggesting that full-term pregnancies – and vaginal delivery in some cases – may be possible after RFVTA.
The principles
Radiofrequency ablation has been used for years in the treatment of liver and kidney tumors. The basic concept is that volumetric thermal ablation results in coagulative necrosis.
The Acessa System, approved by the Food and Drug Administration in late 2012, was designed to treat fibroids, which have much firmer tissue than the tissues being targeted in other radiofrequency ablation procedures. It uses a specially designed intrauterine ultrasound probe and radiofrequency probe, and it combines three fundamental gynecologic skills: Laparoscopy using two trocars and requiring no special suturing skills; ultrasound using a laparoscopic ultrasound probe to scan and manipulate; and probe placement under laparoscopic ultrasound guidance.
Specifically, the system allows for percutaneous, laparoscopic ultrasound–guided radiofrequency ablation of fibroids with a disposable 3.4-mm handpiece coupled to a dual-function radiofrequency generator. The handpiece contains a retractable array of electrodes, so that the fibroid may be ablated with one electrode or with the deployed electrode array.
The generator controls and monitors the ablation with real-time feedback from thermocouples. It monitors and displays the temperature at each needle tip, the average temperature of the array, and the return temperatures on two dispersive electrode pads that are placed on the anterior thighs. The electrode pads are designed to reduce the incidence of pad burns, which are a complication with other radiofrequency ablation devices. The system will automatically stop treatment if either of the pad thermocouples registers a skin temperature greater than 40° C (JSLS. 2014 Apr-Jun;18[2]:182-90).
The outcomes
Laparoscopic ultrasound–guided RFVTA has been studied in five prospective trials, including one multicenter international trial of 135 premenopausal women – the pivotal trial for FDA clearance – in which 104 women were followed for 3 years and found to have prolonged symptom relief and improved quality of life.
At baseline, the women had symptomatic uterine myomas and moderate to severe heavy menstrual bleeding measured by alkaline hematin analysis of returned sanitary products. Their mean symptom severity scores on the Uterine Fibroid Symptom and Quality-of-Life Questionnaire (UFS-QOL) decreased significantly from baseline to 3 months and changed little after that, for a total change of –32.6 over the study period.
The cumulative repeat intervention rate at 3 years was 11%, with 14 of the 135 participants having repeat interventions to treat bleeding and myoma symptoms. Seven of these women were found to have adenomyosis (J Minim Invasive Gynecol. 2014 Sep-Oct;21[5]:767-74).
The surprisingly low reintervention rates may stem from the benefits of direct contact imaging of the uterus. A comparison of images from the pivotal trial has shown that intraoperative ultrasound detected more than twice as many fibroids as did preoperative transvaginal ultrasound, and about one-third more than preoperative MRIs (J Minim Invasive Gynecol. 2013 Nov-Dec;20[6]:770-4).
Interestingly, four women became pregnant over the study’s 3-year follow-up, despite the inclusion requirement that women desire uterine conservation but not future childbearing.
We have followed reproductive outcomes in women after RFVTA of symptomatic fibroids in other studies as well. In our most recent analysis, presented in November at the 2015 American Association of Gynecologic Laparoscopists Global Congress, we identified 10 pregnancies among participants of the five prospective trials.
Of 232 women enrolled in premarket RFVTA studies – trials in which completing childbearing and continuing contraception were requirements – six conceived at 3.5-15 months post ablation. The number of myomas treated ranged from one to seven and included multiple types and dimensions. Five of these six women delivered full-term healthy babies – one by vaginal delivery and four by cesarean section. The sixth patient had a spontaneous abortion in the first trimester.
Of 43 women who participated in two randomized clinical trials undertaken after FDA clearance, four conceived at 4-23.5 months post ablation. Three of these women had uneventful, full-term pregnancies with vaginal births. The fourth had a cesarean section at 38 weeks.
Considering the theoretical advantages of the Acessa procedure – that it is less damaging to healthy myometrium – and the outcomes reported thus far, it appears likely that Acessa will be preferable to myomectomy. Early results from an ongoing 5-year German study that randomized 50 women to RFVTA or laparoscopic myomectomy show that RFVTA resulted in the treatment of more fibroids and involved a significantly shorter hospital stay and post-operative recovery (Int J Gynaecol Obstet. 2014 Jun;125[3]:261-5).
The technique
The patient is pretreated with a nonsteroidal anti-inflammatory agent and prophylactic antibiotic. She is placed in a supine position with arms tucked, and a single-toothed tenaculum is placed on the cervix from 12 to 6 o’clock, without any instrument for manipulation of the uterus. The system’s dispersive electrode pads are placed symmetrically just above the patella on the anterior thighs; asymmetrical placement could potentially increase the risk of a pad burn.
Two standard laparoscopic ports are placed. A 5-mm trocar for the camera and video laparoscope is placed through the umbilicus or at a supraumbilical or left upper–quadrant level, depending on the patient’s anatomy, her surgical history, and the size of the uterus. A thorough visual inspection of the abdomen should be performed to look for unsuspected findings.
A 10-mm trocar is then placed at the level of the top of the fundus for the intra-abdominal ultrasound probe. Laparoscopic ultrasound is used to survey the entire uterus, map the fibroids, and plan an approach. Once the fibroid to be treated first is identified, the ability to stabilize the uterus accordingly is assessed, and the dimensions of the fibroid are taken. The dimensions will be used by the surgeon with a volume algorithm to calculate the length of ablation time based on the size of the fibroid and electrode deployment.
Under ultrasound guidance, the Acessa radiofrequency ablation handpiece is inserted percutaneously at 12, 3, 6, or 9 o’clock relative to the ultrasound trocar, based on the location of the target fibroid. The uterus must be stabilized, with the handpiece and ultrasound probe parallel and in plane. The handpiece is then inserted 1 cm into the target fibroid through the uterine serosal surface, utilizing a combination of laparoscopic and ultrasound views. Care must be taken to use gentle rotation and minimal downward pressure as the tip of the handpiece is quite sharp.
The location of the tip is confirmed by laparoscopic ultrasound, and the 7-needle electrode array can then be deployed to the ablation site. All three dimensions of the fibroid should be viewed for placement and deployment of the electrodes. Care is taken to avoid large blood vessels and ensure that the electrodes are confined within the fibroid and within the uterus.
Radiofrequency ablation is carried out with a low-voltage, high-frequency alternating current. The radiofrequency waves heat the tissue to an average temperature of 95° C for a length of time determined by a treatment algorithm. The wattage automatically adjusts to maintain the treatment temperature for the calculated duration of ablation.
Small fibroids can be treated in a manual mode without deployment of the electrode array at a current output of 15 W.
At the conclusion of the ablation, the electrodes are withdrawn into the handpiece, the generator is changed to coagulation mode, and the handpiece is slowly withdrawn under ultrasound visualization. The tract is simultaneously coagulated. A bit of additional coagulation is facilitated by pausing at the serosal surface.
Additional fibroids can be ablated through another insertion of the handpiece, either through the same tract or through a new tract.
Larger fibroids may require multiple ablations. The maximum size of ablation is about 5 cm, so it is important to plan the treatment of larger fibroids. This can be accomplished by carefully scanning large fibroids and visualizing the number of overlapping ablations needed to treat the entire volume. I ask my assistant to record the size and location of each ablation; I find this helpful both for organizing the treatment of large fibroids and for dictating the operative report.
It is important to appreciate that treatment of one area can make it difficult to visualize nearby fibroids with ultrasound. The effect dissipates in about 30-45 minutes. It is one reason why having a fibroid map prior to treatment is so important.
Once all fibroids are treated, a final inspection is performed. We usually use a suction irrigator to clean out whatever small amounts of blood are present, and the laparoscopic and port sites are closed in standard fashion.
Patients are seen 1 week postoperatively and are instructed to call in cases of pain, fever, bleeding, or chills. Most patients require only NSAIDs for pain relief and return to work in 2-7 days.
Many patients experience a slightly heavier than normal first menses after treatment. Pelvic rest is recommended for 3 weeks as a precaution, and avoidance of intrauterine procedures is advised because the uterus will be soft and thus may be easily perforated. Patients who have had type 1, type 2, or type 2-5 fibroids ablated may experience drainage for several weeks as the fibroid tissue is reabsorbed.
Dr. Berman is interim chairman of Wayne State University’s department of obstetrics and gynecology and interim specialist-in-chief for obstetrics and gynecology at the Detroit Medical Center. He was a principal investigator of the 3-year outcome study of Acessa sponsored by Halt Medical. He is a consultant for Halt Medical and directs physician training in the use of Acessa.
Lessons learned from the history of VBAC
In December 2014, The Wall Street Journal ran an article about a young mother who wanted a vaginal birth after C-section (VBAC) for her second child. After her hospital stopped offering VBACs, the woman had to find another place to deliver. She did have a successful VBAC, but her story is not unique – many women may not receive adequate consultations about or provider support for VBAC as a delivery option.
According to the article, a lack of clinical support was the reason the hospital discontinued VBACs. Although the hospital’s decision may have frustrated the mother, this ensured that she would not be promised a birthing option that the hospital could not deliver – in all senses of this word. Successful VBAC requires proper patient selection, appropriate consent and adequate provisions in case of emergencies.
Not every hospital has made such a choice. Based on studies of a trial of labor after cesarean, conducted after the 1960s, the rate of VBACs increased. As VBACs became more common, the approach to the procedure became more relaxed. VBACs went from only being performed in tertiary care hospitals with appropriate support for emergencies, to community hospitals with no backup. Patient selection became less rigorous, and the rate of complications went up, which, in turn, caused the number of associated legal claims to rise. Hospitals started discouraging VBACs, and ob.gyns. no longer counseled their patients about this option. The VBAC rate decreased, and the C-section rate increased.
Today, many women want to pursue a trial of labor after cesarean. Data from large clinical studies have demonstrated the safety and success of VBAC with proper care. Because of the storied history and a revival of interest in VBACs, we have invited Dr. Mark Landon, the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, and the lead on one of the recent seminal VBAC studies, to address this topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
In December 2014, The Wall Street Journal ran an article about a young mother who wanted a vaginal birth after C-section (VBAC) for her second child. After her hospital stopped offering VBACs, the woman had to find another place to deliver. She did have a successful VBAC, but her story is not unique – many women may not receive adequate consultations about or provider support for VBAC as a delivery option.
According to the article, a lack of clinical support was the reason the hospital discontinued VBACs. Although the hospital’s decision may have frustrated the mother, this ensured that she would not be promised a birthing option that the hospital could not deliver – in all senses of this word. Successful VBAC requires proper patient selection, appropriate consent and adequate provisions in case of emergencies.
Not every hospital has made such a choice. Based on studies of a trial of labor after cesarean, conducted after the 1960s, the rate of VBACs increased. As VBACs became more common, the approach to the procedure became more relaxed. VBACs went from only being performed in tertiary care hospitals with appropriate support for emergencies, to community hospitals with no backup. Patient selection became less rigorous, and the rate of complications went up, which, in turn, caused the number of associated legal claims to rise. Hospitals started discouraging VBACs, and ob.gyns. no longer counseled their patients about this option. The VBAC rate decreased, and the C-section rate increased.
Today, many women want to pursue a trial of labor after cesarean. Data from large clinical studies have demonstrated the safety and success of VBAC with proper care. Because of the storied history and a revival of interest in VBACs, we have invited Dr. Mark Landon, the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, and the lead on one of the recent seminal VBAC studies, to address this topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
In December 2014, The Wall Street Journal ran an article about a young mother who wanted a vaginal birth after C-section (VBAC) for her second child. After her hospital stopped offering VBACs, the woman had to find another place to deliver. She did have a successful VBAC, but her story is not unique – many women may not receive adequate consultations about or provider support for VBAC as a delivery option.
According to the article, a lack of clinical support was the reason the hospital discontinued VBACs. Although the hospital’s decision may have frustrated the mother, this ensured that she would not be promised a birthing option that the hospital could not deliver – in all senses of this word. Successful VBAC requires proper patient selection, appropriate consent and adequate provisions in case of emergencies.
Not every hospital has made such a choice. Based on studies of a trial of labor after cesarean, conducted after the 1960s, the rate of VBACs increased. As VBACs became more common, the approach to the procedure became more relaxed. VBACs went from only being performed in tertiary care hospitals with appropriate support for emergencies, to community hospitals with no backup. Patient selection became less rigorous, and the rate of complications went up, which, in turn, caused the number of associated legal claims to rise. Hospitals started discouraging VBACs, and ob.gyns. no longer counseled their patients about this option. The VBAC rate decreased, and the C-section rate increased.
Today, many women want to pursue a trial of labor after cesarean. Data from large clinical studies have demonstrated the safety and success of VBAC with proper care. Because of the storied history and a revival of interest in VBACs, we have invited Dr. Mark Landon, the Richard L. Meiling Professor and chairman of the department of obstetrics and gynecology at the Ohio State University, and the lead on one of the recent seminal VBAC studies, to address this topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.