User login
One man’s quandary over Fistula First
[Editor's Note: Dr. Sales presents us with both points of view]
POINT: Fistula First for everyone? Sounds good to me
While “do no harm” may be a motto, the truth is, we strive to deliver the best care possible at every moment we interact with patients. There is no argument that an autogenous Arteriovenous Fisutla (AVF) is the best access available for patients—in terms of patency, freedom from intervention and resistance to infection. Thus, providing any other type of access—excluding the bridge catheter—is clearly a “second best” operation.
Other countries boast AVF rates in the upper 80% range and ESRD Network 16 (Alaska, Idaho, Montana, Oregon and Washington) in the U. S. has rates approaching 70%.
If that is the case, why then, does the U. S. still have an overall AVF rate of 63%? It is unlikely that the technical ability of surgeons outside the U. S. or in the upper left corner of our country differs from that of the remainder of the country. Fistula First embarked on a journey to bring this issue to the forefront. Following Drs. Larry Spergel and Tip Jennings advice, many surgeons took to the operating room in an effort to improve the AVF rates in the U. S. In fact, the numbers improved dramatically—but not to the 80+% mark.
Dr. Larry Scher assures us that every arm can have a fistula created and if you are a good interventionalist (or have a good one with whom to work) you can get all of these to mature.
Therefore, all of those 2-2.5mm veins that might not seem “usable,” should be anastomosed carefully to the radial or brachial artery—much as one would perform a distal anastomosis—and coax them into maturing to functional AVFs. If balloon assistance is needed for maturation, make certain the “balloon operator” has a good working knowledge of AV access, lest a nice, big hematoma will greet you, destroying your nicely created AVF. Those of us who cannot achieve the near 100% AVF rate must be technically challenged!
COUNTERPOINT: Fistula First is a bit overdone
Great idea, good execution, too much!
As an early ideologue in the Fistula First movement, I am proud of what we accomplished—education. We got the word out that AVFs were a better option than Arteriovenous Grafts! At the introductory meeting, I did have a rather animated “discussion” with a social worker who was convinced that the reason AVGs outnumbered AVFs was because AVGs reimbursed higher and therefore the surgeons were choosing the AVG for financial reasons (Sometimes it is really hard to maintain your composure!) While it may be true in some quarters, I would venture that was not the real reason affecting the inability of us, as a profession, to deliver the AVF rate that we should.
My alter ego above mentions Drs. Larry Spergel and Tip Jennings—both of whom should be given great credit for opening our eyes to the need for reform. They lead us and we improved. However, the number of AVFs that are being done that are NOT maturing has increased. Just as in any “cult phenomena,” when adherence to a concept supersedes rational thought, the endpoint is blurred. This is what has occurred in the Fistula First.
There are some patients who will never mature an AVF—irrespective of the competence of the vascular surgeon! Many of the veins are simply phlebitic from years of intravenous or venipuncture attacks—now that the PICC teams have learned where the basillic vein is, that vein is often problematic. Additionally, one factor that has never been adequately measured or characterized is skin turgor. Many of our older patients have awful skin turgor and any access (AVF or AVG) will be accompanied by ongoing post-dialysis hematomas with problems.
Our experience has been that octogenarians tend to do better with AVGs than AVFs—heresy to the Fistula First pundits. The number of catheter days is markedly reduced and the long-term outcomes do not vary much in this age group. And guess what, there are some patients who actually do well with a catheter!
Perhaps, two topics are worth mentioning in closing this self-debating article! Fistula First has, appropriately, been renamed Fistula First, Catheter Last! This recognizes the fact that maybe not everyone is a candidate for an AVF—but at the least we should avoid the catheter! The second, much larger issue is whether everyone that is dialyzed should, indeed, be dialyzed! I suspect all readers have been pressured into placing a catheter (or worse) in a patient who has no real chance for a meaningful survival. Additionally, maybe some patients would benefit from nutritional therapy that could delay the onset of dialysis by 6-18 months—imagine how much that would save to an already overburdened system!
Dr. Sales is President, The Cardiovascular Care Group, Westfield , N.J., chief, division of vascular surgery, Overlook Medical Center, Summit, N.J., and clinical assistant professor of Surgery, Mount Sinai School of Medicine, New York, N.Y. Both versions of Dr. Sales are an associate medical editor for Vascular Specialist.
[Editor's Note: Dr. Sales presents us with both points of view]
POINT: Fistula First for everyone? Sounds good to me
While “do no harm” may be a motto, the truth is, we strive to deliver the best care possible at every moment we interact with patients. There is no argument that an autogenous Arteriovenous Fisutla (AVF) is the best access available for patients—in terms of patency, freedom from intervention and resistance to infection. Thus, providing any other type of access—excluding the bridge catheter—is clearly a “second best” operation.
Other countries boast AVF rates in the upper 80% range and ESRD Network 16 (Alaska, Idaho, Montana, Oregon and Washington) in the U. S. has rates approaching 70%.
If that is the case, why then, does the U. S. still have an overall AVF rate of 63%? It is unlikely that the technical ability of surgeons outside the U. S. or in the upper left corner of our country differs from that of the remainder of the country. Fistula First embarked on a journey to bring this issue to the forefront. Following Drs. Larry Spergel and Tip Jennings advice, many surgeons took to the operating room in an effort to improve the AVF rates in the U. S. In fact, the numbers improved dramatically—but not to the 80+% mark.
Dr. Larry Scher assures us that every arm can have a fistula created and if you are a good interventionalist (or have a good one with whom to work) you can get all of these to mature.
Therefore, all of those 2-2.5mm veins that might not seem “usable,” should be anastomosed carefully to the radial or brachial artery—much as one would perform a distal anastomosis—and coax them into maturing to functional AVFs. If balloon assistance is needed for maturation, make certain the “balloon operator” has a good working knowledge of AV access, lest a nice, big hematoma will greet you, destroying your nicely created AVF. Those of us who cannot achieve the near 100% AVF rate must be technically challenged!
COUNTERPOINT: Fistula First is a bit overdone
Great idea, good execution, too much!
As an early ideologue in the Fistula First movement, I am proud of what we accomplished—education. We got the word out that AVFs were a better option than Arteriovenous Grafts! At the introductory meeting, I did have a rather animated “discussion” with a social worker who was convinced that the reason AVGs outnumbered AVFs was because AVGs reimbursed higher and therefore the surgeons were choosing the AVG for financial reasons (Sometimes it is really hard to maintain your composure!) While it may be true in some quarters, I would venture that was not the real reason affecting the inability of us, as a profession, to deliver the AVF rate that we should.
My alter ego above mentions Drs. Larry Spergel and Tip Jennings—both of whom should be given great credit for opening our eyes to the need for reform. They lead us and we improved. However, the number of AVFs that are being done that are NOT maturing has increased. Just as in any “cult phenomena,” when adherence to a concept supersedes rational thought, the endpoint is blurred. This is what has occurred in the Fistula First.
There are some patients who will never mature an AVF—irrespective of the competence of the vascular surgeon! Many of the veins are simply phlebitic from years of intravenous or venipuncture attacks—now that the PICC teams have learned where the basillic vein is, that vein is often problematic. Additionally, one factor that has never been adequately measured or characterized is skin turgor. Many of our older patients have awful skin turgor and any access (AVF or AVG) will be accompanied by ongoing post-dialysis hematomas with problems.
Our experience has been that octogenarians tend to do better with AVGs than AVFs—heresy to the Fistula First pundits. The number of catheter days is markedly reduced and the long-term outcomes do not vary much in this age group. And guess what, there are some patients who actually do well with a catheter!
Perhaps, two topics are worth mentioning in closing this self-debating article! Fistula First has, appropriately, been renamed Fistula First, Catheter Last! This recognizes the fact that maybe not everyone is a candidate for an AVF—but at the least we should avoid the catheter! The second, much larger issue is whether everyone that is dialyzed should, indeed, be dialyzed! I suspect all readers have been pressured into placing a catheter (or worse) in a patient who has no real chance for a meaningful survival. Additionally, maybe some patients would benefit from nutritional therapy that could delay the onset of dialysis by 6-18 months—imagine how much that would save to an already overburdened system!
Dr. Sales is President, The Cardiovascular Care Group, Westfield , N.J., chief, division of vascular surgery, Overlook Medical Center, Summit, N.J., and clinical assistant professor of Surgery, Mount Sinai School of Medicine, New York, N.Y. Both versions of Dr. Sales are an associate medical editor for Vascular Specialist.
[Editor's Note: Dr. Sales presents us with both points of view]
POINT: Fistula First for everyone? Sounds good to me
While “do no harm” may be a motto, the truth is, we strive to deliver the best care possible at every moment we interact with patients. There is no argument that an autogenous Arteriovenous Fisutla (AVF) is the best access available for patients—in terms of patency, freedom from intervention and resistance to infection. Thus, providing any other type of access—excluding the bridge catheter—is clearly a “second best” operation.
Other countries boast AVF rates in the upper 80% range and ESRD Network 16 (Alaska, Idaho, Montana, Oregon and Washington) in the U. S. has rates approaching 70%.
If that is the case, why then, does the U. S. still have an overall AVF rate of 63%? It is unlikely that the technical ability of surgeons outside the U. S. or in the upper left corner of our country differs from that of the remainder of the country. Fistula First embarked on a journey to bring this issue to the forefront. Following Drs. Larry Spergel and Tip Jennings advice, many surgeons took to the operating room in an effort to improve the AVF rates in the U. S. In fact, the numbers improved dramatically—but not to the 80+% mark.
Dr. Larry Scher assures us that every arm can have a fistula created and if you are a good interventionalist (or have a good one with whom to work) you can get all of these to mature.
Therefore, all of those 2-2.5mm veins that might not seem “usable,” should be anastomosed carefully to the radial or brachial artery—much as one would perform a distal anastomosis—and coax them into maturing to functional AVFs. If balloon assistance is needed for maturation, make certain the “balloon operator” has a good working knowledge of AV access, lest a nice, big hematoma will greet you, destroying your nicely created AVF. Those of us who cannot achieve the near 100% AVF rate must be technically challenged!
COUNTERPOINT: Fistula First is a bit overdone
Great idea, good execution, too much!
As an early ideologue in the Fistula First movement, I am proud of what we accomplished—education. We got the word out that AVFs were a better option than Arteriovenous Grafts! At the introductory meeting, I did have a rather animated “discussion” with a social worker who was convinced that the reason AVGs outnumbered AVFs was because AVGs reimbursed higher and therefore the surgeons were choosing the AVG for financial reasons (Sometimes it is really hard to maintain your composure!) While it may be true in some quarters, I would venture that was not the real reason affecting the inability of us, as a profession, to deliver the AVF rate that we should.
My alter ego above mentions Drs. Larry Spergel and Tip Jennings—both of whom should be given great credit for opening our eyes to the need for reform. They lead us and we improved. However, the number of AVFs that are being done that are NOT maturing has increased. Just as in any “cult phenomena,” when adherence to a concept supersedes rational thought, the endpoint is blurred. This is what has occurred in the Fistula First.
There are some patients who will never mature an AVF—irrespective of the competence of the vascular surgeon! Many of the veins are simply phlebitic from years of intravenous or venipuncture attacks—now that the PICC teams have learned where the basillic vein is, that vein is often problematic. Additionally, one factor that has never been adequately measured or characterized is skin turgor. Many of our older patients have awful skin turgor and any access (AVF or AVG) will be accompanied by ongoing post-dialysis hematomas with problems.
Our experience has been that octogenarians tend to do better with AVGs than AVFs—heresy to the Fistula First pundits. The number of catheter days is markedly reduced and the long-term outcomes do not vary much in this age group. And guess what, there are some patients who actually do well with a catheter!
Perhaps, two topics are worth mentioning in closing this self-debating article! Fistula First has, appropriately, been renamed Fistula First, Catheter Last! This recognizes the fact that maybe not everyone is a candidate for an AVF—but at the least we should avoid the catheter! The second, much larger issue is whether everyone that is dialyzed should, indeed, be dialyzed! I suspect all readers have been pressured into placing a catheter (or worse) in a patient who has no real chance for a meaningful survival. Additionally, maybe some patients would benefit from nutritional therapy that could delay the onset of dialysis by 6-18 months—imagine how much that would save to an already overburdened system!
Dr. Sales is President, The Cardiovascular Care Group, Westfield , N.J., chief, division of vascular surgery, Overlook Medical Center, Summit, N.J., and clinical assistant professor of Surgery, Mount Sinai School of Medicine, New York, N.Y. Both versions of Dr. Sales are an associate medical editor for Vascular Specialist.
Point/Counterpoint: Are we too quick to treat May-Thurner syndrome?
YES: New tech promotes treatment where none is needed.
BY SAMUEL P. MARTIN, MD
As science and technology continue to advance, we have the ability to treat more and more conditions with less invasive, better-tolerated procedures. In the realm of vascular disease, this has been evidenced by a variable explosion in the endovascular treatment of arterial disease. With new technology, we have witnessed a tremendous relaxation of former standards in the pursuit of “quality of life.” Our new hammer is ever searching for a nail, resulting in the treatment of “anatomical” disease, such as seen in endovascular stenting of renal artery stenosis.
Nowhere is this trend becoming more evident than in the treatment of May-Thurner anatomy.
Despite years of awareness, there is neither an accepted radiologic definition for May-Thurner syndrome, nor established diagnostic criteria. Fortunately, our ability to image has improved from biplanar venography, formerly the gold standard.
Because May-Thurner is a permanent process, the luminal diameter of the iliac vein should not change with patient positioning. Now, with the recent development of blood pool imaging using contrast agents such as gadofosveset trisodium, magnetic resonance venography (MRV) studies can be performed in supine and prone position on a single dose of contrast. This would seem to obviate the former limitations of biplanar venography or contrast CT or traditional MRV, and would appear to provide an objective means of evaluating May-Thurner anatomy. However, upon evaluation of patients with lower-limb venous disorders, a prevalence of left common iliac vein compression was found in 14%-32% of patients, but a prevalence of May-Thurner syndrome in only 2%-5%, leading to the conclusion that left common iliac vein compression is necessary but not sufficient to cause the syndrome.
Thus, the point to be made: May-Thurner anatomy does not equal May-Thurner syndrome (Diagn Interv Radiol. 2013 Jan-Feb;19[1]:44-8).
Sadly, at the present time, there are no clear-cut guidelines.
With the advent of intravascular ultrasound (IVUS), we are seeing a large number of patients with the suspect anatomy undergoing treatment with balloon angioplasty and stents in the iliac system before adequate treatment of chronic venous insufficiency (CVI) in the extremities. What are the consequences? We have no data on primary or secondary patency of these stents (usually Wallstents). How often is anticoagulation necessary, and, is this permanent? I hate to suggest an industry or monetary motivation, but we are even seeing advertising for stent treatment of May-Thurner syndrome for people who have had treatment of their CVI (often with little or no swelling and minimal pain) with angioplasty and stenting. We also have seen patients who have undergone the procedure and had to have secondary procedures and long-term anticoagulation. Worse, they never had the procedure adequately explained, including potential complications or the possibility of future problems, procedures, or permanent anticoagulation.
So, as we face a situation – May-Thurner anatomy – which exists in more than 20% of our population, it raises several questions that need to be answered as we marshal our ever-increasing health care expenditures. Can we clearly define indications for further investigation and possible intervention, realizing that the syndrome of increased pain, swelling, and risk of thrombosis only exists in 2%-3% of those with the anatomy?
As McDermott and associates have shown in gated MRV studies, conditions such as hydration and especially position can significantly affect anatomical findings. My feelings based on 30-plus years of experience is that treatment of the leg should take precedence, and only after this avenue has been exhausted should one progress to suprainguinal investigation unless there is swelling of the entire leg. What are the long-term consequences of a Wallstent in the venous system, and are we “correcting” one risk by supplanting it with another – the long-term risk of stent thrombosis and subsequent interventions with long-term anticoagulation? There have been no reported cases of pulmonary emboli with May-Thurner and it is thought that the “spur” (synechiae) have some protective properties. In contrast, a stent is a definite theoretical risk for thrombosis, and even embolization.
Dr. Samuel P. Martin is a vascular surgeon in Orlando.
NO: Or rather, ‘maybe,’ by unethical practitioners.
BY ENRICO ASCHER, MD
Significant ipsilateral iliac vein stenosis or occlusion may have continued untoward effects in symptomatic patients particularly those with advanced venous stasis changes including venous ulcerations, skin discoloration, edema and/or pain (CEAP class 3-6). Conversely, successful iliac vein stenting (IVS) has been shown to normalize venous outflow, enhance calf vein muscle pump function, improve venous claudication, decrease pain, ameliorate edema, and accelerate wound healing.
Additionally, IVS can be safely performed in an ambulatory/office setting under local anesthesia with minimal or no sedation. The technical success can exceed 95% and long-term patency rates are excellent. Indeed, IVS is much cheaper and more durable than arterial stenting for claudication.
These advantages cannot and should not be used as an alternative to conservative therapy that includes mild exercise, regular use of appropriately measured elastic stockings, and intermittent leg elevation whenever feasible. Moreover, venous ulcers should be treated with compressive bandages placed by well-trained providers. If all else fails then one should consider the minimally invasive procedures available to treat this debilitating, progressive disease. Unfortunately, the conservative approach fails in a substantial number of patients
It is possible that Dr. Martin is correct regarding advertisements for IVS in the presence of minimal symptoms. There is little one can do about this misleading information.
However, the physician who knowingly implants these stents in patients with no potential benefits or in those who did not have the risks, benefits, and alternatives explained should not be allowed to continue this practice. No longer can one remain silent when confronted with such horrendous unprofessional behavior.
Maybe the SVS should create a hotline that can be utilized by anonymous complainers in an attempt to identify potential abusers who fraudulently have the capacity to expose their patients to potential harm. A letter from the SVS will then be sent to the “guilty” party as an alert. Of course such a suggestion needs to be vetted by expert lawyers prior to implementation. It is only a suggestion. Others should come up with more suggestions to stop or minimize these unlawful practices.
I, too, have heard gossip and more gossip about this or that practitioner performing unnecessary procedures. These have included arterial and venous interventions. They were infrainguinal, suprainguinal or both. Some were stents, some were vein ablations. Is an unnecessary IVS worse than an unnecessary great saphenous vein ablation? What if the patient is a candidate for multiple coronary bypasses and has only one good great saphenous vein? What if the patient needs a limb salvage vein bypass operation as the only solution to maintain limb viability? If someone puts a gun to my head and ask me to choose between two unnecessary procedures I may well opt for the IVS. I am a member of the Save the GSV club founded by Dr. Samson. One can argue that the ablated vein is gone forever; the stent may be salvaged if it occludes. All unnecessary procedures are just unnecessary.
I believe that Dr. Martin makes a point to exhaust all infrainguinal options prior to IVS. In fact, he does not advocate IVS at all in any circumstance. I respect his 3 decades of clinical experience coupled to the fact that iliac vein narrowing is a fairly common finding in the general population. Nevertheless, the literature is getting filled up with large and small series of patients highlighting the importance of IVS as an important tool in our armamentarium against this chronic, debilitating disease that affects an important segment of the working population in this country and abroad. Although a small, prospective, randomized study from Brazil published in the Journal of Vascular Surgery conclusively showed the value of IVS in patients with advanced venous stasis (J Vasc Surg Venous Lymphat Disord. 2015;3:117-8), a larger one involving multiple centers will provide many needed answers.
Dr. Ascher is chief of vascular and endovascular surgery, NYU Lutheran Medical Center.
YES: New tech promotes treatment where none is needed.
BY SAMUEL P. MARTIN, MD
As science and technology continue to advance, we have the ability to treat more and more conditions with less invasive, better-tolerated procedures. In the realm of vascular disease, this has been evidenced by a variable explosion in the endovascular treatment of arterial disease. With new technology, we have witnessed a tremendous relaxation of former standards in the pursuit of “quality of life.” Our new hammer is ever searching for a nail, resulting in the treatment of “anatomical” disease, such as seen in endovascular stenting of renal artery stenosis.
Nowhere is this trend becoming more evident than in the treatment of May-Thurner anatomy.
Despite years of awareness, there is neither an accepted radiologic definition for May-Thurner syndrome, nor established diagnostic criteria. Fortunately, our ability to image has improved from biplanar venography, formerly the gold standard.
Because May-Thurner is a permanent process, the luminal diameter of the iliac vein should not change with patient positioning. Now, with the recent development of blood pool imaging using contrast agents such as gadofosveset trisodium, magnetic resonance venography (MRV) studies can be performed in supine and prone position on a single dose of contrast. This would seem to obviate the former limitations of biplanar venography or contrast CT or traditional MRV, and would appear to provide an objective means of evaluating May-Thurner anatomy. However, upon evaluation of patients with lower-limb venous disorders, a prevalence of left common iliac vein compression was found in 14%-32% of patients, but a prevalence of May-Thurner syndrome in only 2%-5%, leading to the conclusion that left common iliac vein compression is necessary but not sufficient to cause the syndrome.
Thus, the point to be made: May-Thurner anatomy does not equal May-Thurner syndrome (Diagn Interv Radiol. 2013 Jan-Feb;19[1]:44-8).
Sadly, at the present time, there are no clear-cut guidelines.
With the advent of intravascular ultrasound (IVUS), we are seeing a large number of patients with the suspect anatomy undergoing treatment with balloon angioplasty and stents in the iliac system before adequate treatment of chronic venous insufficiency (CVI) in the extremities. What are the consequences? We have no data on primary or secondary patency of these stents (usually Wallstents). How often is anticoagulation necessary, and, is this permanent? I hate to suggest an industry or monetary motivation, but we are even seeing advertising for stent treatment of May-Thurner syndrome for people who have had treatment of their CVI (often with little or no swelling and minimal pain) with angioplasty and stenting. We also have seen patients who have undergone the procedure and had to have secondary procedures and long-term anticoagulation. Worse, they never had the procedure adequately explained, including potential complications or the possibility of future problems, procedures, or permanent anticoagulation.
So, as we face a situation – May-Thurner anatomy – which exists in more than 20% of our population, it raises several questions that need to be answered as we marshal our ever-increasing health care expenditures. Can we clearly define indications for further investigation and possible intervention, realizing that the syndrome of increased pain, swelling, and risk of thrombosis only exists in 2%-3% of those with the anatomy?
As McDermott and associates have shown in gated MRV studies, conditions such as hydration and especially position can significantly affect anatomical findings. My feelings based on 30-plus years of experience is that treatment of the leg should take precedence, and only after this avenue has been exhausted should one progress to suprainguinal investigation unless there is swelling of the entire leg. What are the long-term consequences of a Wallstent in the venous system, and are we “correcting” one risk by supplanting it with another – the long-term risk of stent thrombosis and subsequent interventions with long-term anticoagulation? There have been no reported cases of pulmonary emboli with May-Thurner and it is thought that the “spur” (synechiae) have some protective properties. In contrast, a stent is a definite theoretical risk for thrombosis, and even embolization.
Dr. Samuel P. Martin is a vascular surgeon in Orlando.
NO: Or rather, ‘maybe,’ by unethical practitioners.
BY ENRICO ASCHER, MD
Significant ipsilateral iliac vein stenosis or occlusion may have continued untoward effects in symptomatic patients particularly those with advanced venous stasis changes including venous ulcerations, skin discoloration, edema and/or pain (CEAP class 3-6). Conversely, successful iliac vein stenting (IVS) has been shown to normalize venous outflow, enhance calf vein muscle pump function, improve venous claudication, decrease pain, ameliorate edema, and accelerate wound healing.
Additionally, IVS can be safely performed in an ambulatory/office setting under local anesthesia with minimal or no sedation. The technical success can exceed 95% and long-term patency rates are excellent. Indeed, IVS is much cheaper and more durable than arterial stenting for claudication.
These advantages cannot and should not be used as an alternative to conservative therapy that includes mild exercise, regular use of appropriately measured elastic stockings, and intermittent leg elevation whenever feasible. Moreover, venous ulcers should be treated with compressive bandages placed by well-trained providers. If all else fails then one should consider the minimally invasive procedures available to treat this debilitating, progressive disease. Unfortunately, the conservative approach fails in a substantial number of patients
It is possible that Dr. Martin is correct regarding advertisements for IVS in the presence of minimal symptoms. There is little one can do about this misleading information.
However, the physician who knowingly implants these stents in patients with no potential benefits or in those who did not have the risks, benefits, and alternatives explained should not be allowed to continue this practice. No longer can one remain silent when confronted with such horrendous unprofessional behavior.
Maybe the SVS should create a hotline that can be utilized by anonymous complainers in an attempt to identify potential abusers who fraudulently have the capacity to expose their patients to potential harm. A letter from the SVS will then be sent to the “guilty” party as an alert. Of course such a suggestion needs to be vetted by expert lawyers prior to implementation. It is only a suggestion. Others should come up with more suggestions to stop or minimize these unlawful practices.
I, too, have heard gossip and more gossip about this or that practitioner performing unnecessary procedures. These have included arterial and venous interventions. They were infrainguinal, suprainguinal or both. Some were stents, some were vein ablations. Is an unnecessary IVS worse than an unnecessary great saphenous vein ablation? What if the patient is a candidate for multiple coronary bypasses and has only one good great saphenous vein? What if the patient needs a limb salvage vein bypass operation as the only solution to maintain limb viability? If someone puts a gun to my head and ask me to choose between two unnecessary procedures I may well opt for the IVS. I am a member of the Save the GSV club founded by Dr. Samson. One can argue that the ablated vein is gone forever; the stent may be salvaged if it occludes. All unnecessary procedures are just unnecessary.
I believe that Dr. Martin makes a point to exhaust all infrainguinal options prior to IVS. In fact, he does not advocate IVS at all in any circumstance. I respect his 3 decades of clinical experience coupled to the fact that iliac vein narrowing is a fairly common finding in the general population. Nevertheless, the literature is getting filled up with large and small series of patients highlighting the importance of IVS as an important tool in our armamentarium against this chronic, debilitating disease that affects an important segment of the working population in this country and abroad. Although a small, prospective, randomized study from Brazil published in the Journal of Vascular Surgery conclusively showed the value of IVS in patients with advanced venous stasis (J Vasc Surg Venous Lymphat Disord. 2015;3:117-8), a larger one involving multiple centers will provide many needed answers.
Dr. Ascher is chief of vascular and endovascular surgery, NYU Lutheran Medical Center.
YES: New tech promotes treatment where none is needed.
BY SAMUEL P. MARTIN, MD
As science and technology continue to advance, we have the ability to treat more and more conditions with less invasive, better-tolerated procedures. In the realm of vascular disease, this has been evidenced by a variable explosion in the endovascular treatment of arterial disease. With new technology, we have witnessed a tremendous relaxation of former standards in the pursuit of “quality of life.” Our new hammer is ever searching for a nail, resulting in the treatment of “anatomical” disease, such as seen in endovascular stenting of renal artery stenosis.
Nowhere is this trend becoming more evident than in the treatment of May-Thurner anatomy.
Despite years of awareness, there is neither an accepted radiologic definition for May-Thurner syndrome, nor established diagnostic criteria. Fortunately, our ability to image has improved from biplanar venography, formerly the gold standard.
Because May-Thurner is a permanent process, the luminal diameter of the iliac vein should not change with patient positioning. Now, with the recent development of blood pool imaging using contrast agents such as gadofosveset trisodium, magnetic resonance venography (MRV) studies can be performed in supine and prone position on a single dose of contrast. This would seem to obviate the former limitations of biplanar venography or contrast CT or traditional MRV, and would appear to provide an objective means of evaluating May-Thurner anatomy. However, upon evaluation of patients with lower-limb venous disorders, a prevalence of left common iliac vein compression was found in 14%-32% of patients, but a prevalence of May-Thurner syndrome in only 2%-5%, leading to the conclusion that left common iliac vein compression is necessary but not sufficient to cause the syndrome.
Thus, the point to be made: May-Thurner anatomy does not equal May-Thurner syndrome (Diagn Interv Radiol. 2013 Jan-Feb;19[1]:44-8).
Sadly, at the present time, there are no clear-cut guidelines.
With the advent of intravascular ultrasound (IVUS), we are seeing a large number of patients with the suspect anatomy undergoing treatment with balloon angioplasty and stents in the iliac system before adequate treatment of chronic venous insufficiency (CVI) in the extremities. What are the consequences? We have no data on primary or secondary patency of these stents (usually Wallstents). How often is anticoagulation necessary, and, is this permanent? I hate to suggest an industry or monetary motivation, but we are even seeing advertising for stent treatment of May-Thurner syndrome for people who have had treatment of their CVI (often with little or no swelling and minimal pain) with angioplasty and stenting. We also have seen patients who have undergone the procedure and had to have secondary procedures and long-term anticoagulation. Worse, they never had the procedure adequately explained, including potential complications or the possibility of future problems, procedures, or permanent anticoagulation.
So, as we face a situation – May-Thurner anatomy – which exists in more than 20% of our population, it raises several questions that need to be answered as we marshal our ever-increasing health care expenditures. Can we clearly define indications for further investigation and possible intervention, realizing that the syndrome of increased pain, swelling, and risk of thrombosis only exists in 2%-3% of those with the anatomy?
As McDermott and associates have shown in gated MRV studies, conditions such as hydration and especially position can significantly affect anatomical findings. My feelings based on 30-plus years of experience is that treatment of the leg should take precedence, and only after this avenue has been exhausted should one progress to suprainguinal investigation unless there is swelling of the entire leg. What are the long-term consequences of a Wallstent in the venous system, and are we “correcting” one risk by supplanting it with another – the long-term risk of stent thrombosis and subsequent interventions with long-term anticoagulation? There have been no reported cases of pulmonary emboli with May-Thurner and it is thought that the “spur” (synechiae) have some protective properties. In contrast, a stent is a definite theoretical risk for thrombosis, and even embolization.
Dr. Samuel P. Martin is a vascular surgeon in Orlando.
NO: Or rather, ‘maybe,’ by unethical practitioners.
BY ENRICO ASCHER, MD
Significant ipsilateral iliac vein stenosis or occlusion may have continued untoward effects in symptomatic patients particularly those with advanced venous stasis changes including venous ulcerations, skin discoloration, edema and/or pain (CEAP class 3-6). Conversely, successful iliac vein stenting (IVS) has been shown to normalize venous outflow, enhance calf vein muscle pump function, improve venous claudication, decrease pain, ameliorate edema, and accelerate wound healing.
Additionally, IVS can be safely performed in an ambulatory/office setting under local anesthesia with minimal or no sedation. The technical success can exceed 95% and long-term patency rates are excellent. Indeed, IVS is much cheaper and more durable than arterial stenting for claudication.
These advantages cannot and should not be used as an alternative to conservative therapy that includes mild exercise, regular use of appropriately measured elastic stockings, and intermittent leg elevation whenever feasible. Moreover, venous ulcers should be treated with compressive bandages placed by well-trained providers. If all else fails then one should consider the minimally invasive procedures available to treat this debilitating, progressive disease. Unfortunately, the conservative approach fails in a substantial number of patients
It is possible that Dr. Martin is correct regarding advertisements for IVS in the presence of minimal symptoms. There is little one can do about this misleading information.
However, the physician who knowingly implants these stents in patients with no potential benefits or in those who did not have the risks, benefits, and alternatives explained should not be allowed to continue this practice. No longer can one remain silent when confronted with such horrendous unprofessional behavior.
Maybe the SVS should create a hotline that can be utilized by anonymous complainers in an attempt to identify potential abusers who fraudulently have the capacity to expose their patients to potential harm. A letter from the SVS will then be sent to the “guilty” party as an alert. Of course such a suggestion needs to be vetted by expert lawyers prior to implementation. It is only a suggestion. Others should come up with more suggestions to stop or minimize these unlawful practices.
I, too, have heard gossip and more gossip about this or that practitioner performing unnecessary procedures. These have included arterial and venous interventions. They were infrainguinal, suprainguinal or both. Some were stents, some were vein ablations. Is an unnecessary IVS worse than an unnecessary great saphenous vein ablation? What if the patient is a candidate for multiple coronary bypasses and has only one good great saphenous vein? What if the patient needs a limb salvage vein bypass operation as the only solution to maintain limb viability? If someone puts a gun to my head and ask me to choose between two unnecessary procedures I may well opt for the IVS. I am a member of the Save the GSV club founded by Dr. Samson. One can argue that the ablated vein is gone forever; the stent may be salvaged if it occludes. All unnecessary procedures are just unnecessary.
I believe that Dr. Martin makes a point to exhaust all infrainguinal options prior to IVS. In fact, he does not advocate IVS at all in any circumstance. I respect his 3 decades of clinical experience coupled to the fact that iliac vein narrowing is a fairly common finding in the general population. Nevertheless, the literature is getting filled up with large and small series of patients highlighting the importance of IVS as an important tool in our armamentarium against this chronic, debilitating disease that affects an important segment of the working population in this country and abroad. Although a small, prospective, randomized study from Brazil published in the Journal of Vascular Surgery conclusively showed the value of IVS in patients with advanced venous stasis (J Vasc Surg Venous Lymphat Disord. 2015;3:117-8), a larger one involving multiple centers will provide many needed answers.
Dr. Ascher is chief of vascular and endovascular surgery, NYU Lutheran Medical Center.
Point/Counterpoint: So you think you can make a vascular surgeon in 5 years?
YES
BY MALACHI G. SHEAHAN III, M.D.
Believe it or not, one thing just about all vascular surgeons will agree upon is the proper way to train. For most of us, the best way to become a surgeon is the way we became a surgeon. Therefore, unless there is some aberration in the readership circulation of Vascular Specialist, I begin this debate facing an uphill battle with most of you.
The question of how to become a vascular surgeon should not be some esoteric matter left to be debated in the late Friday session of some educational symposium. Indeed, I commend the editors for bringing this issue to a more public forum. As much as I enjoy listening to the twenty-seventh abstract redefining the risks of type 2 endoleaks at our national meeting, the matter of how to create a vascular surgeon will define our profession for years to come.
Data from the Association of American Medical Colleges shows that there is now one vascular surgeon for every 100,000 people in the U.S. That is one vascular surgeon for every 350 dialysis patients or one for every 2,600 individuals with peripheral artery disease. We are already in short supply and 40% of us are over 55 years old. Applicant numbers to traditional 5 + 2 programs have plateaued over the past 10 years, suggesting that expanding fellowship positions is not the answer. Who then will fill this gap? As Dr. Ian Malcolm warned us in “Jurassic Park,” life will find a way.
If vascular surgeons don’t act to address this need, I know two candidates who are interested. Both interventional cardiology (10% over 55) and interventional radiology (12% over 55) have younger workforces that are growing at a superior rate. Between 2008 and 2013, the largest increases in training positions offered among all medical specialties were seen in interventional cardiology and interventional radiology.
Luckily our profession has not been caught completely off guard. Integrated vascular residency positions were first offered in 2007. Based on the quality and quantity of applicants, the number of institutions offering the integrated 0 + 5 vascular residency has grown from 17 in 2009 to 51 in 2015.
As practiced today, vascular surgery bears little similarity to even a decade ago. Limb salvage, aortic interventions, vein care, and access management all require highly specific training not typically offered in a general surgery residency. Our new board certification emphasizes the ability to supervise and interpret radiologic tests. Vascular surgery training is no longer a honing of general surgical skills. We must teach and develop completely new areas of expertise in our trainees. I propose the longer we have to focus on these specific abilities, the better our product will be.
A classic argument against traditional 5 + 2 training is why have a postgraduate-year 4 or 5 performing a pancreaticoduodenectomy (Whipple procedure) when they will never perform one in practice? This, however, is a flawed point, as open abdominal cases contain many aspects that translate well to vascular surgery. I believe the enemy is not Allen O. Whipple, but rather Harvey J. Laparoscope.1 Much like the declining numbers of open aortic cases, laparoscopic surgery has replaced much of the open surgical volume in general surgery training programs. How well these skills translate to vascular is unknown, but at face value, the cross-applicability doesn’t seem to pass muster. So while no case is wasted, perhaps our trainees’ time could be spent more efficiently.
Integrated 0 + 5 programs give total control of the rotations and curriculum to the vascular program director. This allows a truly cohesive approach to developing vascular skills and knowledge over a five year period interspersed with core general surgery skills and principals. Surgery rotations such as trauma, ICU, and cardiothoracic surgery that provide the best educational content to our trainees can now be handpicked, while avoiding lower-yield content like advanced laparoscopy and breast. Quality control is now in the hands of a vascular surgeon.
After all, Erica, if the sanctity of the five year general surgery residency must be preserved, why do you run one of the world’s only 4 + 2 programs? Clearly you believe we can condense our trainees’ education without losing quality.
Using the available metrics and data points it would be difficult to prove superiority of the 0 + 5 pathway to the 5 + 2. Therefore, I will borrow a technique from my clinical trials’ friends and claim noninferiority. Follow my logic here and I promise not to include a convoluted endpoint like strokes, deaths, and non-Q wave MIs induced in training directors.
Our best test for measuring cognitive development during vascular training remains the Vascular Surgery In-Training Examination (VSITE). Looking at the 2015 results, the L5 integrated residents received a better average standard score (565 vs. 542) than their L2 fellowship counterparts. In fact, the L5 integrated residents had a superior score on seven of the nine vascular sub-tests.
For technical skill acquisition, we can look at both Accreditation Council for Graduate Medical Education (ACGME) case logs and the Fundamentals of Vascular Surgery (FVS) exam. The largest study of vascular surgical experience was published by P. Batista and colleagues from Thomas Jefferson University, Philadelphia, in 2015. They found integrated residents had performed 12% more vascular procedures than traditional 5 + 2 residents (851 vs. 758) despite 2 years less training time. Our own FVS exam was conducted on more than 280 vascular trainees representing all levels from both paradigms. On this validated exam of technical skill, 94% of PGY 5 integrated residents received a passing score, compared with 92% of PGY 7 fellows. Interestingly, means scores were significantly higher for PGY 5 integrated residents vs. first year fellows (P less than .005) despite the former group receiving one year less training.
Perhaps the final barrier to the success of the integrated pathway is our own preconceived notions. Doubters often cite some unmeasurable like “maturity” as a deterrent. Do we question the maturity of the general surgeon with five years of residency? How about the pediatrician or general practitioner with fewer? Isn’t maturity a key aspect of any physician?
I believe it is time to put our doubts to rest and embrace this new paradigm. We now have ample evidence that under the supervision of a vascular program director, a competent surgeon can be produced in five years.
These young people may not have followed our exact path, but they are our future.
Dr. Sheahan is an associate professor and the program director of the Vascular Surgery Fellowship at the Health Sciences Center, School of Medicine, Louisiana State University, New Orleans.
References
1. Possibly not the actual name of the inventor of the laparoscope, but I’m working on a deadline here. [Editor’s Note; A summary of the complex history of the development of laparascopy can be found here: J Laparoendosc Adv Surg Tech A. 1997 Dec;7:369-73.]
NO
BY ERICA L. MITCHELL, M.D.
Dr. Sheahan has already convinced himself that he has won this debate because he honestly believes that he has persuaded the Vascular Specialist readers of the merits and benefits of the integrated vascular surgery training paradigm. While I respect Mal for supporting a 5 year training paradigm, I am prepared to argue for a potentially even shorter surgical training model than that set by the integrated 0 + 5 (and in some cases 0 + 6 or 0 + 7) time-based archetype.
I propose, and implore, that vascular surgery educators adopt a competency-based educational (CBE) framework in which trainees complete their training when competence has been met and demonstrated through objective performance benchmarks, whether that is after 7 years, 5 years, or even 4 or fewer years of vascular surgical training.
The goal of all graduate medical education is to ensure that the graduating physician is competent to practice independently in his or her chosen field of medicine. For nearly a century, surgical training has been based on the apprenticeship model as articulated by Halsted. Residents work with faculty members on clinical rotations, gaining experience while providing service to patients. The rotations have formal educational goals and objectives, but resident experience relies heavily on the patients who present to the clinical service. The time in training is set and for vascular surgery, the required time in training is either 5 years via the integrated 0 + 5 track or 6-7 years via the early-specialization or traditional training tracks. Board eligibility requires completion of this training time, documentation of operative case logs, and a “ready to practice independently” attestation from the vascular surgery program director. It is unusual for surgical residents not to complete their program or to remain in their program for additional training, despite recent evidence suggesting that current surgical training may be resulting in suboptimal experiences.1
As a consequence of time-based residency training, residents completing vascular surgical training vary in competence, and currently there is no mechanism to solve this situation. While, I am sure you will agree, none of us think we are graduating incompetent vascular surgeons, we do, however, come across residents or fellows whom we believe are not yet ready for autonomous practice at completion of their training, regardless of their training paradigm. With time determining completion of training these residents, unfortunately, at the end of their designated training period the training is done, regardless of demonstrated skills or knowledge. While this is concerning, we also see the counter to this unprepared resident.
We have all witnessed exceptional trainees in our programs. These trainees, regardless of their training program, sail through their surgical residencies. They meet all of the defined educational milestones, finish all of the program requirements, and demonstrate ability to care for patients unsupervised way before their set graduation date. For both these types of residents, educational landmarks, as defined by the ACGME, are of secondary importance and since only time determines completion of training, the curriculum becomes irrelevant. The question then becomes: why work to define a body of vascular surgical knowledge or a required set of technical and non-technical skills if competence is defined as time in training? Mal, surely you don’t support graduating a trainee simply because they have spent five years in training? Hopefully you would want to know that this graduating trainee is ready and competent to safely and autonomously practice the full scope of vascular surgical practice.
Competency-based education is gaining momentum around the world as medical educators, physicians, and policy makers try to ensure that our graduating specialists are acquiring and demonstrating the competencies needed to practice in today’s rapidly evolving heath care systems. It is becoming the standard in training of physicians because of the perception that it provides more transparent standards and increased public accountability. Competency-based training is learner centric, outcomes based, and differentiated. A key distinguishing feature of CBE is that residents can progress through the educational process at different rates: the most capable and talented individuals should be able to make career transitions earlier, thus allowing them to enter the workforce at an accelerated rate. Others, requiring more time, would still attain the appropriate level of knowledge, skills, and attitudes needed to enter independent practice, and leave the program only when competent.
With the emerging reality of numerous nonsurgical specialties encroaching upon various traditional domains of vascular surgery, it is essential that our specialty lead the field in vascular education so as to maintain our stronghold on these areas of expertise. Competency-based training is a logical evolutionary step from our traditional years-in-place based system. Such training should improve, or at least verify, the quality of educational outcomes for our vascular trainees and our varying training programs. This model of education will allow comparisons among training programs, differing training tracks and even differing specialty practices. I urge the vascular surgery community to discuss this concept and ultimately to implement it.
Dr. Mitchell is a professor of surgery, program director for vascular surgery, and vice-chair of Quality, Department of Surgery, Division of Vascular Surgery, Oregon Health and Science University, Portland.
References
YES
BY MALACHI G. SHEAHAN III, M.D.
Believe it or not, one thing just about all vascular surgeons will agree upon is the proper way to train. For most of us, the best way to become a surgeon is the way we became a surgeon. Therefore, unless there is some aberration in the readership circulation of Vascular Specialist, I begin this debate facing an uphill battle with most of you.
The question of how to become a vascular surgeon should not be some esoteric matter left to be debated in the late Friday session of some educational symposium. Indeed, I commend the editors for bringing this issue to a more public forum. As much as I enjoy listening to the twenty-seventh abstract redefining the risks of type 2 endoleaks at our national meeting, the matter of how to create a vascular surgeon will define our profession for years to come.
Data from the Association of American Medical Colleges shows that there is now one vascular surgeon for every 100,000 people in the U.S. That is one vascular surgeon for every 350 dialysis patients or one for every 2,600 individuals with peripheral artery disease. We are already in short supply and 40% of us are over 55 years old. Applicant numbers to traditional 5 + 2 programs have plateaued over the past 10 years, suggesting that expanding fellowship positions is not the answer. Who then will fill this gap? As Dr. Ian Malcolm warned us in “Jurassic Park,” life will find a way.
If vascular surgeons don’t act to address this need, I know two candidates who are interested. Both interventional cardiology (10% over 55) and interventional radiology (12% over 55) have younger workforces that are growing at a superior rate. Between 2008 and 2013, the largest increases in training positions offered among all medical specialties were seen in interventional cardiology and interventional radiology.
Luckily our profession has not been caught completely off guard. Integrated vascular residency positions were first offered in 2007. Based on the quality and quantity of applicants, the number of institutions offering the integrated 0 + 5 vascular residency has grown from 17 in 2009 to 51 in 2015.
As practiced today, vascular surgery bears little similarity to even a decade ago. Limb salvage, aortic interventions, vein care, and access management all require highly specific training not typically offered in a general surgery residency. Our new board certification emphasizes the ability to supervise and interpret radiologic tests. Vascular surgery training is no longer a honing of general surgical skills. We must teach and develop completely new areas of expertise in our trainees. I propose the longer we have to focus on these specific abilities, the better our product will be.
A classic argument against traditional 5 + 2 training is why have a postgraduate-year 4 or 5 performing a pancreaticoduodenectomy (Whipple procedure) when they will never perform one in practice? This, however, is a flawed point, as open abdominal cases contain many aspects that translate well to vascular surgery. I believe the enemy is not Allen O. Whipple, but rather Harvey J. Laparoscope.1 Much like the declining numbers of open aortic cases, laparoscopic surgery has replaced much of the open surgical volume in general surgery training programs. How well these skills translate to vascular is unknown, but at face value, the cross-applicability doesn’t seem to pass muster. So while no case is wasted, perhaps our trainees’ time could be spent more efficiently.
Integrated 0 + 5 programs give total control of the rotations and curriculum to the vascular program director. This allows a truly cohesive approach to developing vascular skills and knowledge over a five year period interspersed with core general surgery skills and principals. Surgery rotations such as trauma, ICU, and cardiothoracic surgery that provide the best educational content to our trainees can now be handpicked, while avoiding lower-yield content like advanced laparoscopy and breast. Quality control is now in the hands of a vascular surgeon.
After all, Erica, if the sanctity of the five year general surgery residency must be preserved, why do you run one of the world’s only 4 + 2 programs? Clearly you believe we can condense our trainees’ education without losing quality.
Using the available metrics and data points it would be difficult to prove superiority of the 0 + 5 pathway to the 5 + 2. Therefore, I will borrow a technique from my clinical trials’ friends and claim noninferiority. Follow my logic here and I promise not to include a convoluted endpoint like strokes, deaths, and non-Q wave MIs induced in training directors.
Our best test for measuring cognitive development during vascular training remains the Vascular Surgery In-Training Examination (VSITE). Looking at the 2015 results, the L5 integrated residents received a better average standard score (565 vs. 542) than their L2 fellowship counterparts. In fact, the L5 integrated residents had a superior score on seven of the nine vascular sub-tests.
For technical skill acquisition, we can look at both Accreditation Council for Graduate Medical Education (ACGME) case logs and the Fundamentals of Vascular Surgery (FVS) exam. The largest study of vascular surgical experience was published by P. Batista and colleagues from Thomas Jefferson University, Philadelphia, in 2015. They found integrated residents had performed 12% more vascular procedures than traditional 5 + 2 residents (851 vs. 758) despite 2 years less training time. Our own FVS exam was conducted on more than 280 vascular trainees representing all levels from both paradigms. On this validated exam of technical skill, 94% of PGY 5 integrated residents received a passing score, compared with 92% of PGY 7 fellows. Interestingly, means scores were significantly higher for PGY 5 integrated residents vs. first year fellows (P less than .005) despite the former group receiving one year less training.
Perhaps the final barrier to the success of the integrated pathway is our own preconceived notions. Doubters often cite some unmeasurable like “maturity” as a deterrent. Do we question the maturity of the general surgeon with five years of residency? How about the pediatrician or general practitioner with fewer? Isn’t maturity a key aspect of any physician?
I believe it is time to put our doubts to rest and embrace this new paradigm. We now have ample evidence that under the supervision of a vascular program director, a competent surgeon can be produced in five years.
These young people may not have followed our exact path, but they are our future.
Dr. Sheahan is an associate professor and the program director of the Vascular Surgery Fellowship at the Health Sciences Center, School of Medicine, Louisiana State University, New Orleans.
References
1. Possibly not the actual name of the inventor of the laparoscope, but I’m working on a deadline here. [Editor’s Note; A summary of the complex history of the development of laparascopy can be found here: J Laparoendosc Adv Surg Tech A. 1997 Dec;7:369-73.]
NO
BY ERICA L. MITCHELL, M.D.
Dr. Sheahan has already convinced himself that he has won this debate because he honestly believes that he has persuaded the Vascular Specialist readers of the merits and benefits of the integrated vascular surgery training paradigm. While I respect Mal for supporting a 5 year training paradigm, I am prepared to argue for a potentially even shorter surgical training model than that set by the integrated 0 + 5 (and in some cases 0 + 6 or 0 + 7) time-based archetype.
I propose, and implore, that vascular surgery educators adopt a competency-based educational (CBE) framework in which trainees complete their training when competence has been met and demonstrated through objective performance benchmarks, whether that is after 7 years, 5 years, or even 4 or fewer years of vascular surgical training.
The goal of all graduate medical education is to ensure that the graduating physician is competent to practice independently in his or her chosen field of medicine. For nearly a century, surgical training has been based on the apprenticeship model as articulated by Halsted. Residents work with faculty members on clinical rotations, gaining experience while providing service to patients. The rotations have formal educational goals and objectives, but resident experience relies heavily on the patients who present to the clinical service. The time in training is set and for vascular surgery, the required time in training is either 5 years via the integrated 0 + 5 track or 6-7 years via the early-specialization or traditional training tracks. Board eligibility requires completion of this training time, documentation of operative case logs, and a “ready to practice independently” attestation from the vascular surgery program director. It is unusual for surgical residents not to complete their program or to remain in their program for additional training, despite recent evidence suggesting that current surgical training may be resulting in suboptimal experiences.1
As a consequence of time-based residency training, residents completing vascular surgical training vary in competence, and currently there is no mechanism to solve this situation. While, I am sure you will agree, none of us think we are graduating incompetent vascular surgeons, we do, however, come across residents or fellows whom we believe are not yet ready for autonomous practice at completion of their training, regardless of their training paradigm. With time determining completion of training these residents, unfortunately, at the end of their designated training period the training is done, regardless of demonstrated skills or knowledge. While this is concerning, we also see the counter to this unprepared resident.
We have all witnessed exceptional trainees in our programs. These trainees, regardless of their training program, sail through their surgical residencies. They meet all of the defined educational milestones, finish all of the program requirements, and demonstrate ability to care for patients unsupervised way before their set graduation date. For both these types of residents, educational landmarks, as defined by the ACGME, are of secondary importance and since only time determines completion of training, the curriculum becomes irrelevant. The question then becomes: why work to define a body of vascular surgical knowledge or a required set of technical and non-technical skills if competence is defined as time in training? Mal, surely you don’t support graduating a trainee simply because they have spent five years in training? Hopefully you would want to know that this graduating trainee is ready and competent to safely and autonomously practice the full scope of vascular surgical practice.
Competency-based education is gaining momentum around the world as medical educators, physicians, and policy makers try to ensure that our graduating specialists are acquiring and demonstrating the competencies needed to practice in today’s rapidly evolving heath care systems. It is becoming the standard in training of physicians because of the perception that it provides more transparent standards and increased public accountability. Competency-based training is learner centric, outcomes based, and differentiated. A key distinguishing feature of CBE is that residents can progress through the educational process at different rates: the most capable and talented individuals should be able to make career transitions earlier, thus allowing them to enter the workforce at an accelerated rate. Others, requiring more time, would still attain the appropriate level of knowledge, skills, and attitudes needed to enter independent practice, and leave the program only when competent.
With the emerging reality of numerous nonsurgical specialties encroaching upon various traditional domains of vascular surgery, it is essential that our specialty lead the field in vascular education so as to maintain our stronghold on these areas of expertise. Competency-based training is a logical evolutionary step from our traditional years-in-place based system. Such training should improve, or at least verify, the quality of educational outcomes for our vascular trainees and our varying training programs. This model of education will allow comparisons among training programs, differing training tracks and even differing specialty practices. I urge the vascular surgery community to discuss this concept and ultimately to implement it.
Dr. Mitchell is a professor of surgery, program director for vascular surgery, and vice-chair of Quality, Department of Surgery, Division of Vascular Surgery, Oregon Health and Science University, Portland.
References
YES
BY MALACHI G. SHEAHAN III, M.D.
Believe it or not, one thing just about all vascular surgeons will agree upon is the proper way to train. For most of us, the best way to become a surgeon is the way we became a surgeon. Therefore, unless there is some aberration in the readership circulation of Vascular Specialist, I begin this debate facing an uphill battle with most of you.
The question of how to become a vascular surgeon should not be some esoteric matter left to be debated in the late Friday session of some educational symposium. Indeed, I commend the editors for bringing this issue to a more public forum. As much as I enjoy listening to the twenty-seventh abstract redefining the risks of type 2 endoleaks at our national meeting, the matter of how to create a vascular surgeon will define our profession for years to come.
Data from the Association of American Medical Colleges shows that there is now one vascular surgeon for every 100,000 people in the U.S. That is one vascular surgeon for every 350 dialysis patients or one for every 2,600 individuals with peripheral artery disease. We are already in short supply and 40% of us are over 55 years old. Applicant numbers to traditional 5 + 2 programs have plateaued over the past 10 years, suggesting that expanding fellowship positions is not the answer. Who then will fill this gap? As Dr. Ian Malcolm warned us in “Jurassic Park,” life will find a way.
If vascular surgeons don’t act to address this need, I know two candidates who are interested. Both interventional cardiology (10% over 55) and interventional radiology (12% over 55) have younger workforces that are growing at a superior rate. Between 2008 and 2013, the largest increases in training positions offered among all medical specialties were seen in interventional cardiology and interventional radiology.
Luckily our profession has not been caught completely off guard. Integrated vascular residency positions were first offered in 2007. Based on the quality and quantity of applicants, the number of institutions offering the integrated 0 + 5 vascular residency has grown from 17 in 2009 to 51 in 2015.
As practiced today, vascular surgery bears little similarity to even a decade ago. Limb salvage, aortic interventions, vein care, and access management all require highly specific training not typically offered in a general surgery residency. Our new board certification emphasizes the ability to supervise and interpret radiologic tests. Vascular surgery training is no longer a honing of general surgical skills. We must teach and develop completely new areas of expertise in our trainees. I propose the longer we have to focus on these specific abilities, the better our product will be.
A classic argument against traditional 5 + 2 training is why have a postgraduate-year 4 or 5 performing a pancreaticoduodenectomy (Whipple procedure) when they will never perform one in practice? This, however, is a flawed point, as open abdominal cases contain many aspects that translate well to vascular surgery. I believe the enemy is not Allen O. Whipple, but rather Harvey J. Laparoscope.1 Much like the declining numbers of open aortic cases, laparoscopic surgery has replaced much of the open surgical volume in general surgery training programs. How well these skills translate to vascular is unknown, but at face value, the cross-applicability doesn’t seem to pass muster. So while no case is wasted, perhaps our trainees’ time could be spent more efficiently.
Integrated 0 + 5 programs give total control of the rotations and curriculum to the vascular program director. This allows a truly cohesive approach to developing vascular skills and knowledge over a five year period interspersed with core general surgery skills and principals. Surgery rotations such as trauma, ICU, and cardiothoracic surgery that provide the best educational content to our trainees can now be handpicked, while avoiding lower-yield content like advanced laparoscopy and breast. Quality control is now in the hands of a vascular surgeon.
After all, Erica, if the sanctity of the five year general surgery residency must be preserved, why do you run one of the world’s only 4 + 2 programs? Clearly you believe we can condense our trainees’ education without losing quality.
Using the available metrics and data points it would be difficult to prove superiority of the 0 + 5 pathway to the 5 + 2. Therefore, I will borrow a technique from my clinical trials’ friends and claim noninferiority. Follow my logic here and I promise not to include a convoluted endpoint like strokes, deaths, and non-Q wave MIs induced in training directors.
Our best test for measuring cognitive development during vascular training remains the Vascular Surgery In-Training Examination (VSITE). Looking at the 2015 results, the L5 integrated residents received a better average standard score (565 vs. 542) than their L2 fellowship counterparts. In fact, the L5 integrated residents had a superior score on seven of the nine vascular sub-tests.
For technical skill acquisition, we can look at both Accreditation Council for Graduate Medical Education (ACGME) case logs and the Fundamentals of Vascular Surgery (FVS) exam. The largest study of vascular surgical experience was published by P. Batista and colleagues from Thomas Jefferson University, Philadelphia, in 2015. They found integrated residents had performed 12% more vascular procedures than traditional 5 + 2 residents (851 vs. 758) despite 2 years less training time. Our own FVS exam was conducted on more than 280 vascular trainees representing all levels from both paradigms. On this validated exam of technical skill, 94% of PGY 5 integrated residents received a passing score, compared with 92% of PGY 7 fellows. Interestingly, means scores were significantly higher for PGY 5 integrated residents vs. first year fellows (P less than .005) despite the former group receiving one year less training.
Perhaps the final barrier to the success of the integrated pathway is our own preconceived notions. Doubters often cite some unmeasurable like “maturity” as a deterrent. Do we question the maturity of the general surgeon with five years of residency? How about the pediatrician or general practitioner with fewer? Isn’t maturity a key aspect of any physician?
I believe it is time to put our doubts to rest and embrace this new paradigm. We now have ample evidence that under the supervision of a vascular program director, a competent surgeon can be produced in five years.
These young people may not have followed our exact path, but they are our future.
Dr. Sheahan is an associate professor and the program director of the Vascular Surgery Fellowship at the Health Sciences Center, School of Medicine, Louisiana State University, New Orleans.
References
1. Possibly not the actual name of the inventor of the laparoscope, but I’m working on a deadline here. [Editor’s Note; A summary of the complex history of the development of laparascopy can be found here: J Laparoendosc Adv Surg Tech A. 1997 Dec;7:369-73.]
NO
BY ERICA L. MITCHELL, M.D.
Dr. Sheahan has already convinced himself that he has won this debate because he honestly believes that he has persuaded the Vascular Specialist readers of the merits and benefits of the integrated vascular surgery training paradigm. While I respect Mal for supporting a 5 year training paradigm, I am prepared to argue for a potentially even shorter surgical training model than that set by the integrated 0 + 5 (and in some cases 0 + 6 or 0 + 7) time-based archetype.
I propose, and implore, that vascular surgery educators adopt a competency-based educational (CBE) framework in which trainees complete their training when competence has been met and demonstrated through objective performance benchmarks, whether that is after 7 years, 5 years, or even 4 or fewer years of vascular surgical training.
The goal of all graduate medical education is to ensure that the graduating physician is competent to practice independently in his or her chosen field of medicine. For nearly a century, surgical training has been based on the apprenticeship model as articulated by Halsted. Residents work with faculty members on clinical rotations, gaining experience while providing service to patients. The rotations have formal educational goals and objectives, but resident experience relies heavily on the patients who present to the clinical service. The time in training is set and for vascular surgery, the required time in training is either 5 years via the integrated 0 + 5 track or 6-7 years via the early-specialization or traditional training tracks. Board eligibility requires completion of this training time, documentation of operative case logs, and a “ready to practice independently” attestation from the vascular surgery program director. It is unusual for surgical residents not to complete their program or to remain in their program for additional training, despite recent evidence suggesting that current surgical training may be resulting in suboptimal experiences.1
As a consequence of time-based residency training, residents completing vascular surgical training vary in competence, and currently there is no mechanism to solve this situation. While, I am sure you will agree, none of us think we are graduating incompetent vascular surgeons, we do, however, come across residents or fellows whom we believe are not yet ready for autonomous practice at completion of their training, regardless of their training paradigm. With time determining completion of training these residents, unfortunately, at the end of their designated training period the training is done, regardless of demonstrated skills or knowledge. While this is concerning, we also see the counter to this unprepared resident.
We have all witnessed exceptional trainees in our programs. These trainees, regardless of their training program, sail through their surgical residencies. They meet all of the defined educational milestones, finish all of the program requirements, and demonstrate ability to care for patients unsupervised way before their set graduation date. For both these types of residents, educational landmarks, as defined by the ACGME, are of secondary importance and since only time determines completion of training, the curriculum becomes irrelevant. The question then becomes: why work to define a body of vascular surgical knowledge or a required set of technical and non-technical skills if competence is defined as time in training? Mal, surely you don’t support graduating a trainee simply because they have spent five years in training? Hopefully you would want to know that this graduating trainee is ready and competent to safely and autonomously practice the full scope of vascular surgical practice.
Competency-based education is gaining momentum around the world as medical educators, physicians, and policy makers try to ensure that our graduating specialists are acquiring and demonstrating the competencies needed to practice in today’s rapidly evolving heath care systems. It is becoming the standard in training of physicians because of the perception that it provides more transparent standards and increased public accountability. Competency-based training is learner centric, outcomes based, and differentiated. A key distinguishing feature of CBE is that residents can progress through the educational process at different rates: the most capable and talented individuals should be able to make career transitions earlier, thus allowing them to enter the workforce at an accelerated rate. Others, requiring more time, would still attain the appropriate level of knowledge, skills, and attitudes needed to enter independent practice, and leave the program only when competent.
With the emerging reality of numerous nonsurgical specialties encroaching upon various traditional domains of vascular surgery, it is essential that our specialty lead the field in vascular education so as to maintain our stronghold on these areas of expertise. Competency-based training is a logical evolutionary step from our traditional years-in-place based system. Such training should improve, or at least verify, the quality of educational outcomes for our vascular trainees and our varying training programs. This model of education will allow comparisons among training programs, differing training tracks and even differing specialty practices. I urge the vascular surgery community to discuss this concept and ultimately to implement it.
Dr. Mitchell is a professor of surgery, program director for vascular surgery, and vice-chair of Quality, Department of Surgery, Division of Vascular Surgery, Oregon Health and Science University, Portland.
References
Point/Counterpoint: Self-employed community practice is still a viable proposition
YES
The recent 2-year bipartisan budget deal signed by President Obama and sent up by Congress brought the hammer down on hospitals so quickly that they did not see it coming. It is highly unusual for Congress to keep anything secreted from the American Hospital Association (AHA) lobby. The AHA spent $4.6 million in the first quarter of 2015 for an annual estimated expenditure of about $18 million. This does not include dollars spent by local and state hospital associations. The SVS is clearly dwarfed by these powerful interests. Our society spent less than $100,000 in that same quarter on advocating for over 4,000 members, the majority of whom are United States residents and most of whom depend solely on the SVS to look out for them.
As a result of the budget deal, Medicare will not pay most hospital-owned physician practices higher rates than those of independently owned practices. The reimbursement changes will apply to those hospital-owned physician practices acquired or opened since the date the law was signed and also located farther than 250 yards from a hospital’s main campus. It does grandfather in facilities prior to the signing that were being reimbursed with hospital outpatient department (HOPD) rates. The savings will prevent an increase in premiums for about 15 million Medicare beneficiaries. The AHA expressed its outrage while the AARP celebrated. So did independent physicians who have been protesting all along that costs were rising because of excessive payments to hospitals for essentially the same services.
Margot Sanger-Katz, in a column for “The Upshot” in the New York Times, wrote that it had been estimated that correcting this payment differential would save Medicare $30 billion over 10 years, more than Medicare could save if it raised the Medicare eligibility age to 67!1 She also pointed out that the Medicare Payment Advisory Committee (MedPAC), an independent group that advises Congress, thinks “that the pay differences should be narrowed, but only for a select set of medical services in which it’s really clear that there’s no difference between the care offered by a hospital and a physician office.”
The rush to buy physician practices is being done for many reasons but the disparate payment schedule favoring hospital-owned practices for many of the same services is one reason. The hospital brings in a lot more revenue through its hired physicians providing the same service in their offices that are now under the banner of the health system. The hospitals cite several justifications for the “surcharge” on care provided by employed physicians in hospital facilities, some of which may be valid. Regulatory requirements, sicker inpatients, increased cost due to training programs, and being required to support money-losing services such as burn care are some reasons. But, independent physicians say they provide the same or better quality care at a lower cost without resources such as legal, accounting, self-insurance against professional liability, and robust lobbying firms.
Hospitals have also contended that vertical integration by buying physician practices should lead to lower health care costs by squeezing efficiencies within the system. There have been conflicting reports on whether physician hospital integration leads to lower health care expenditures.2 The public debate has caught the attention of government regulators. In the recent case of Saint Lukes-Saltzer, the question before the Federal Trade Commission (the agency responsible for federal antitrust action) was: Did total medical expenditures increase or decrease for patients cared for by physician practices acquired by St. Luke’s? Indeed, the conclusions were that not only did overall costs not go down but evidence showed that the merger may have resulted in increased costs.
On appeal, the Ninth Circuit Court ruled that any future efficiency must be “substantial, verifiable and specific” to the merger. Ciliberto and Dranove looked at hospital prices after physician hospital affiliations in California and found no evidence of increase in prices.3 Baker and coauthors analyzed privately insured patients between 2001 and 2007 and the effect of physician hospital integration on hospital prices, admission volumes, and spending.4 They reported higher hospital prices and spending in hospitals with the tightest vertically integrated relationship with physicians. In one of the few studies of the issue, Capps and colleagues reviewed 7 years of administrative data from multiple insurers across the United States to estimate postintegration costs. From 2007 to 2013, they found that there was a 57% increase in the share of spending by physicians whose practices are owned by hospitals. In addition, this led to an increase in physician prices of 14% post integration.5 The larger the market share of inpatients by a hospital the larger the price increase. The authors estimate that about 25% of the price increases are precisely due to “exploitation of reimbursement rules” by charging the facility fees for their employed physicians. If these “surcharges” led to decreased utilization as one measure of increased efficiency and therefore reduced overall health care costs, it would be acceptable. But, Capps et al. found no such evidence and speculate that this scenario could lead to higher expenditures.
In a recent study, total expenditures for over 4 million patients by private physician groups or integrated groups covered by health maintenance organizations (HMOs) in California between 2009 and 2012 were analyzed.6 Mean annual expenditures were highest for large multihospital systems followed by hospital-owned physician groups and, lastly, physician-owned groups. The expenditures for multihospital systems were 19.8% higher and for local hospital-employed physician groups 10% higher compared to physician-owned organizations.
Why should prices increase after tighter physician hospital integration on a large scale? Market power. Once health systems have a large enough number of physicians in their panel, hospitals could charge insurers higher prices to access their specialists. Similarly, by employing a large number of physicians in a particular specialty, which then attracts a large pool of patients with a particular illness, they could dominate the other health systems in the region. One action specifically forbidden by anti-kickback laws is compensating physicians based on the number of referrals they make to the hospital. But, there are enough loopholes that allow hospitals to indirectly tie compensation to “productivity.” This may change with bundled payments or compensation tied to “value,” although there will always be incentives for work volume to some degree.
A further roadblock for basing merger decisions entirely on possible efficiencies is how the courts will see these activities in terms of antitrust actions. Most arguments using efficiency as the basis for merging physician groups with hospitals are vague and in general courts have not superseded antitrust actions with economic efficiency arguments.
What should be genuine reasons for hospitals employing and aligning with physicians? Addressing uneven quality of care, access and, of course, ever spiraling costs. If the object was to share responsibility for attacking these problems, health care systems and physicians would be cut a lot of slack. But, some health care systems want to not only survive the existing chaos but also dominate their local market.
I guess health care is really no different from Wall Street corporations in its focus on short-term gains versus long-term benefits. Until broader incentives change, health systems will continue to look to survive and gain market share and power. Competition, in isolation, drives tactics where the only objective may be to increase market share. However, it appears that the FTC will be busy wielding the Sherman Act of the antitrust law to keep a check on health systems to ensure consumers, payers, physicians, and the country at large are all on a fair playing field.7
Dr. Satiani is professor of clinical surgery, division of vascular diseases & surgery, department of surgery, associate director, FAME; director, Faculty Leadership Institute, and medical director, Vascular Labs, at Ohio State University College of Medicine, Columbus. He is also an associate medical editor for Vascular Specialist.
References
2. Journal of Health Economics 2006; 25: 1-28.
3. Journal of Health Economics 2006; 25: 29-38.
4. Health Affairs 2014; 33(5): 756-63.
5. www.ipr.northwestern.edu/publications/docs/workingpapers/2015/IPR-WP-15-02.pdf
6. JAMA. 2014; 312(16):1663-9.
7. Plastic & Reconstructive Surgery. 2006; 117(3): 1012-22.
NO
The days of hanging one’s shingle on a door and starting a self-employed practice are rapidly fading. While some fondly remember the practice of medicine as it was in Norman Rockwell’s classic “Before the Shot,” the realities of a current practice couldn’t be more different. Reusable syringes, analog weighing stations, an unaccompanied minor, and lack of regard for universal precautions are just a few examples from that painting that have long since disappeared. However, the humor in this painting comes from the young boy scrutinizing the doctor’s credentials, implying a sense of distrust and fear as he stands there with his buttocks partially exposed waiting for the vaccination.
This scrutiny of physician performance and results is more relevant today than ever before. Perhaps if we were to update the painting today, it would depict the boy furiously tapping away at his tablet searching through ProPublica to see what the doctor’s complication rate with the intended procedure truly is.
This is just one of the many pressures physicians are facing today. Navigating the publicly reported complication data is but one tiny portion of the regulatory red tape physicians face in taking care of their patients. If you add in the need to negotiate and contact with insurers, manage an office staff, acquire and maintain an electronic medical record (EMR) while ensuring that your EMR is properly secured against potential cyber threats and compliant with meaningful use regulations, audit your billing and coding, keep up to date with upcoming changes to bundled payments, mail out and track Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), as well as an endless list of other requirements, it is no wonder physicians are less willing to take these challenges on as solo practitioners. In fact, based on Medscape’s 2014 Employed Doctors Report, which compiled responses from over 4,600 physicians, the top three reasons for being an employed physician were not having to deal with the business of running an office (58%), not having to deal with insurers and billing (45%), and guaranteed income/even cash flow (42%).1
Multiple sources continue to confirm the trend that more physicians are moving to an employed practice.2-4 In the last decade, the rate of hospital employment has increased from 11% to 64%.1 There are many factors that have pushed physicians away from self-employment. Some of these are related to physicians’ personal choices, and many are from external pressures. As various parts of the Affordable Care Act come into play, there will continue to be increasing regulatory demands. These have the potential of increasing overhead costs, and, coupled with decreasing reimbursement, will inevitably make staying profitable more challenging in a self-employed model.
There are two other very telling trends that foretell the inevitable decline of self-employed physicians. Fewer and fewer new graduates are reporting that they are self employed. In the most recent surveys, twice as many physicians under the age of 40 are employed than self-employed.1 Furthermore, 92% of residents surveyed in their final year would prefer employment with a salary, and only 2% would consider solo practice.5 Of these graduating residents, 36% specifically were considering hospital employment, which is nearly a 10-fold increase from a decade ago. The second factor affecting new hires is their confidence that they have the necessary skills to manage a self-employed model. During the same decade, there was only a small increase in graduating residents who felt very prepared to deal with the business side of medicine (10% vs. 2%).5 This lack of knowledge will undoubtedly make it difficult for those who would consider self-employment to feel comfortable in that practice model. Some have speculated that there is soon to be a “push back” from the physicians and specifically from specialists who don’t have as much to gain in large group practices. With so few graduates considering solo and small group practice, and the overwhelming majority not feeling very prepared to manage the business of medicine, who can help lead this trend reversal?
Not only are fewer new graduates choosing self-employment, but fewer opportunities for self-employment are available as more physician groups are being bought by hospitals or other large group practices. Specifically with vascular surgery, there is a significant overhead cost requirement. Advantages to joining a large group practice include better ability to negotiate cost savings with the frequent capital requirements for new equipment, updates and maintenance of the electronic records, and professional liability. In fact, one study in California shows that as the proportion of physicians employed by the health system increased, supply chain expenses and inventory costs improved.6 Furthermore, hospitals have administrators who are hired to negotiate with insurers regarding reimbursement and respond to audits and other regulatory changes. As mentioned above, the top two reasons for avoiding self-employment are precisely these. This will no doubt draw even more physicians and specifically vascular surgeons into employed models.
Dr. Haurani is assistant professor of surgery in the division of vascular diseases and surgery, Ohio State University Medical Center, Columbus.
References
1. www.medscape.com/features/slideshow/public/employed-doctors#1
3.Perspect Vasc Surg Endovasc Ther. 2013;25:46-52.
4 Tenn Med. 2012;105:38-39.
5.www.merritthawkins.com/uploadedFiles/MerrittHawkings/Surveys/2014_MerrittHawkins_FYMR_Survey.pdf.
6. Health Care Manage Rev. 2015 Jul 23. [Epub ahead of print] www.ncbi.nlm.nih.gov/pubmed/26207654
YES
The recent 2-year bipartisan budget deal signed by President Obama and sent up by Congress brought the hammer down on hospitals so quickly that they did not see it coming. It is highly unusual for Congress to keep anything secreted from the American Hospital Association (AHA) lobby. The AHA spent $4.6 million in the first quarter of 2015 for an annual estimated expenditure of about $18 million. This does not include dollars spent by local and state hospital associations. The SVS is clearly dwarfed by these powerful interests. Our society spent less than $100,000 in that same quarter on advocating for over 4,000 members, the majority of whom are United States residents and most of whom depend solely on the SVS to look out for them.
As a result of the budget deal, Medicare will not pay most hospital-owned physician practices higher rates than those of independently owned practices. The reimbursement changes will apply to those hospital-owned physician practices acquired or opened since the date the law was signed and also located farther than 250 yards from a hospital’s main campus. It does grandfather in facilities prior to the signing that were being reimbursed with hospital outpatient department (HOPD) rates. The savings will prevent an increase in premiums for about 15 million Medicare beneficiaries. The AHA expressed its outrage while the AARP celebrated. So did independent physicians who have been protesting all along that costs were rising because of excessive payments to hospitals for essentially the same services.
Margot Sanger-Katz, in a column for “The Upshot” in the New York Times, wrote that it had been estimated that correcting this payment differential would save Medicare $30 billion over 10 years, more than Medicare could save if it raised the Medicare eligibility age to 67!1 She also pointed out that the Medicare Payment Advisory Committee (MedPAC), an independent group that advises Congress, thinks “that the pay differences should be narrowed, but only for a select set of medical services in which it’s really clear that there’s no difference between the care offered by a hospital and a physician office.”
The rush to buy physician practices is being done for many reasons but the disparate payment schedule favoring hospital-owned practices for many of the same services is one reason. The hospital brings in a lot more revenue through its hired physicians providing the same service in their offices that are now under the banner of the health system. The hospitals cite several justifications for the “surcharge” on care provided by employed physicians in hospital facilities, some of which may be valid. Regulatory requirements, sicker inpatients, increased cost due to training programs, and being required to support money-losing services such as burn care are some reasons. But, independent physicians say they provide the same or better quality care at a lower cost without resources such as legal, accounting, self-insurance against professional liability, and robust lobbying firms.
Hospitals have also contended that vertical integration by buying physician practices should lead to lower health care costs by squeezing efficiencies within the system. There have been conflicting reports on whether physician hospital integration leads to lower health care expenditures.2 The public debate has caught the attention of government regulators. In the recent case of Saint Lukes-Saltzer, the question before the Federal Trade Commission (the agency responsible for federal antitrust action) was: Did total medical expenditures increase or decrease for patients cared for by physician practices acquired by St. Luke’s? Indeed, the conclusions were that not only did overall costs not go down but evidence showed that the merger may have resulted in increased costs.
On appeal, the Ninth Circuit Court ruled that any future efficiency must be “substantial, verifiable and specific” to the merger. Ciliberto and Dranove looked at hospital prices after physician hospital affiliations in California and found no evidence of increase in prices.3 Baker and coauthors analyzed privately insured patients between 2001 and 2007 and the effect of physician hospital integration on hospital prices, admission volumes, and spending.4 They reported higher hospital prices and spending in hospitals with the tightest vertically integrated relationship with physicians. In one of the few studies of the issue, Capps and colleagues reviewed 7 years of administrative data from multiple insurers across the United States to estimate postintegration costs. From 2007 to 2013, they found that there was a 57% increase in the share of spending by physicians whose practices are owned by hospitals. In addition, this led to an increase in physician prices of 14% post integration.5 The larger the market share of inpatients by a hospital the larger the price increase. The authors estimate that about 25% of the price increases are precisely due to “exploitation of reimbursement rules” by charging the facility fees for their employed physicians. If these “surcharges” led to decreased utilization as one measure of increased efficiency and therefore reduced overall health care costs, it would be acceptable. But, Capps et al. found no such evidence and speculate that this scenario could lead to higher expenditures.
In a recent study, total expenditures for over 4 million patients by private physician groups or integrated groups covered by health maintenance organizations (HMOs) in California between 2009 and 2012 were analyzed.6 Mean annual expenditures were highest for large multihospital systems followed by hospital-owned physician groups and, lastly, physician-owned groups. The expenditures for multihospital systems were 19.8% higher and for local hospital-employed physician groups 10% higher compared to physician-owned organizations.
Why should prices increase after tighter physician hospital integration on a large scale? Market power. Once health systems have a large enough number of physicians in their panel, hospitals could charge insurers higher prices to access their specialists. Similarly, by employing a large number of physicians in a particular specialty, which then attracts a large pool of patients with a particular illness, they could dominate the other health systems in the region. One action specifically forbidden by anti-kickback laws is compensating physicians based on the number of referrals they make to the hospital. But, there are enough loopholes that allow hospitals to indirectly tie compensation to “productivity.” This may change with bundled payments or compensation tied to “value,” although there will always be incentives for work volume to some degree.
A further roadblock for basing merger decisions entirely on possible efficiencies is how the courts will see these activities in terms of antitrust actions. Most arguments using efficiency as the basis for merging physician groups with hospitals are vague and in general courts have not superseded antitrust actions with economic efficiency arguments.
What should be genuine reasons for hospitals employing and aligning with physicians? Addressing uneven quality of care, access and, of course, ever spiraling costs. If the object was to share responsibility for attacking these problems, health care systems and physicians would be cut a lot of slack. But, some health care systems want to not only survive the existing chaos but also dominate their local market.
I guess health care is really no different from Wall Street corporations in its focus on short-term gains versus long-term benefits. Until broader incentives change, health systems will continue to look to survive and gain market share and power. Competition, in isolation, drives tactics where the only objective may be to increase market share. However, it appears that the FTC will be busy wielding the Sherman Act of the antitrust law to keep a check on health systems to ensure consumers, payers, physicians, and the country at large are all on a fair playing field.7
Dr. Satiani is professor of clinical surgery, division of vascular diseases & surgery, department of surgery, associate director, FAME; director, Faculty Leadership Institute, and medical director, Vascular Labs, at Ohio State University College of Medicine, Columbus. He is also an associate medical editor for Vascular Specialist.
References
2. Journal of Health Economics 2006; 25: 1-28.
3. Journal of Health Economics 2006; 25: 29-38.
4. Health Affairs 2014; 33(5): 756-63.
5. www.ipr.northwestern.edu/publications/docs/workingpapers/2015/IPR-WP-15-02.pdf
6. JAMA. 2014; 312(16):1663-9.
7. Plastic & Reconstructive Surgery. 2006; 117(3): 1012-22.
NO
The days of hanging one’s shingle on a door and starting a self-employed practice are rapidly fading. While some fondly remember the practice of medicine as it was in Norman Rockwell’s classic “Before the Shot,” the realities of a current practice couldn’t be more different. Reusable syringes, analog weighing stations, an unaccompanied minor, and lack of regard for universal precautions are just a few examples from that painting that have long since disappeared. However, the humor in this painting comes from the young boy scrutinizing the doctor’s credentials, implying a sense of distrust and fear as he stands there with his buttocks partially exposed waiting for the vaccination.
This scrutiny of physician performance and results is more relevant today than ever before. Perhaps if we were to update the painting today, it would depict the boy furiously tapping away at his tablet searching through ProPublica to see what the doctor’s complication rate with the intended procedure truly is.
This is just one of the many pressures physicians are facing today. Navigating the publicly reported complication data is but one tiny portion of the regulatory red tape physicians face in taking care of their patients. If you add in the need to negotiate and contact with insurers, manage an office staff, acquire and maintain an electronic medical record (EMR) while ensuring that your EMR is properly secured against potential cyber threats and compliant with meaningful use regulations, audit your billing and coding, keep up to date with upcoming changes to bundled payments, mail out and track Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), as well as an endless list of other requirements, it is no wonder physicians are less willing to take these challenges on as solo practitioners. In fact, based on Medscape’s 2014 Employed Doctors Report, which compiled responses from over 4,600 physicians, the top three reasons for being an employed physician were not having to deal with the business of running an office (58%), not having to deal with insurers and billing (45%), and guaranteed income/even cash flow (42%).1
Multiple sources continue to confirm the trend that more physicians are moving to an employed practice.2-4 In the last decade, the rate of hospital employment has increased from 11% to 64%.1 There are many factors that have pushed physicians away from self-employment. Some of these are related to physicians’ personal choices, and many are from external pressures. As various parts of the Affordable Care Act come into play, there will continue to be increasing regulatory demands. These have the potential of increasing overhead costs, and, coupled with decreasing reimbursement, will inevitably make staying profitable more challenging in a self-employed model.
There are two other very telling trends that foretell the inevitable decline of self-employed physicians. Fewer and fewer new graduates are reporting that they are self employed. In the most recent surveys, twice as many physicians under the age of 40 are employed than self-employed.1 Furthermore, 92% of residents surveyed in their final year would prefer employment with a salary, and only 2% would consider solo practice.5 Of these graduating residents, 36% specifically were considering hospital employment, which is nearly a 10-fold increase from a decade ago. The second factor affecting new hires is their confidence that they have the necessary skills to manage a self-employed model. During the same decade, there was only a small increase in graduating residents who felt very prepared to deal with the business side of medicine (10% vs. 2%).5 This lack of knowledge will undoubtedly make it difficult for those who would consider self-employment to feel comfortable in that practice model. Some have speculated that there is soon to be a “push back” from the physicians and specifically from specialists who don’t have as much to gain in large group practices. With so few graduates considering solo and small group practice, and the overwhelming majority not feeling very prepared to manage the business of medicine, who can help lead this trend reversal?
Not only are fewer new graduates choosing self-employment, but fewer opportunities for self-employment are available as more physician groups are being bought by hospitals or other large group practices. Specifically with vascular surgery, there is a significant overhead cost requirement. Advantages to joining a large group practice include better ability to negotiate cost savings with the frequent capital requirements for new equipment, updates and maintenance of the electronic records, and professional liability. In fact, one study in California shows that as the proportion of physicians employed by the health system increased, supply chain expenses and inventory costs improved.6 Furthermore, hospitals have administrators who are hired to negotiate with insurers regarding reimbursement and respond to audits and other regulatory changes. As mentioned above, the top two reasons for avoiding self-employment are precisely these. This will no doubt draw even more physicians and specifically vascular surgeons into employed models.
Dr. Haurani is assistant professor of surgery in the division of vascular diseases and surgery, Ohio State University Medical Center, Columbus.
References
1. www.medscape.com/features/slideshow/public/employed-doctors#1
3.Perspect Vasc Surg Endovasc Ther. 2013;25:46-52.
4 Tenn Med. 2012;105:38-39.
5.www.merritthawkins.com/uploadedFiles/MerrittHawkings/Surveys/2014_MerrittHawkins_FYMR_Survey.pdf.
6. Health Care Manage Rev. 2015 Jul 23. [Epub ahead of print] www.ncbi.nlm.nih.gov/pubmed/26207654
YES
The recent 2-year bipartisan budget deal signed by President Obama and sent up by Congress brought the hammer down on hospitals so quickly that they did not see it coming. It is highly unusual for Congress to keep anything secreted from the American Hospital Association (AHA) lobby. The AHA spent $4.6 million in the first quarter of 2015 for an annual estimated expenditure of about $18 million. This does not include dollars spent by local and state hospital associations. The SVS is clearly dwarfed by these powerful interests. Our society spent less than $100,000 in that same quarter on advocating for over 4,000 members, the majority of whom are United States residents and most of whom depend solely on the SVS to look out for them.
As a result of the budget deal, Medicare will not pay most hospital-owned physician practices higher rates than those of independently owned practices. The reimbursement changes will apply to those hospital-owned physician practices acquired or opened since the date the law was signed and also located farther than 250 yards from a hospital’s main campus. It does grandfather in facilities prior to the signing that were being reimbursed with hospital outpatient department (HOPD) rates. The savings will prevent an increase in premiums for about 15 million Medicare beneficiaries. The AHA expressed its outrage while the AARP celebrated. So did independent physicians who have been protesting all along that costs were rising because of excessive payments to hospitals for essentially the same services.
Margot Sanger-Katz, in a column for “The Upshot” in the New York Times, wrote that it had been estimated that correcting this payment differential would save Medicare $30 billion over 10 years, more than Medicare could save if it raised the Medicare eligibility age to 67!1 She also pointed out that the Medicare Payment Advisory Committee (MedPAC), an independent group that advises Congress, thinks “that the pay differences should be narrowed, but only for a select set of medical services in which it’s really clear that there’s no difference between the care offered by a hospital and a physician office.”
The rush to buy physician practices is being done for many reasons but the disparate payment schedule favoring hospital-owned practices for many of the same services is one reason. The hospital brings in a lot more revenue through its hired physicians providing the same service in their offices that are now under the banner of the health system. The hospitals cite several justifications for the “surcharge” on care provided by employed physicians in hospital facilities, some of which may be valid. Regulatory requirements, sicker inpatients, increased cost due to training programs, and being required to support money-losing services such as burn care are some reasons. But, independent physicians say they provide the same or better quality care at a lower cost without resources such as legal, accounting, self-insurance against professional liability, and robust lobbying firms.
Hospitals have also contended that vertical integration by buying physician practices should lead to lower health care costs by squeezing efficiencies within the system. There have been conflicting reports on whether physician hospital integration leads to lower health care expenditures.2 The public debate has caught the attention of government regulators. In the recent case of Saint Lukes-Saltzer, the question before the Federal Trade Commission (the agency responsible for federal antitrust action) was: Did total medical expenditures increase or decrease for patients cared for by physician practices acquired by St. Luke’s? Indeed, the conclusions were that not only did overall costs not go down but evidence showed that the merger may have resulted in increased costs.
On appeal, the Ninth Circuit Court ruled that any future efficiency must be “substantial, verifiable and specific” to the merger. Ciliberto and Dranove looked at hospital prices after physician hospital affiliations in California and found no evidence of increase in prices.3 Baker and coauthors analyzed privately insured patients between 2001 and 2007 and the effect of physician hospital integration on hospital prices, admission volumes, and spending.4 They reported higher hospital prices and spending in hospitals with the tightest vertically integrated relationship with physicians. In one of the few studies of the issue, Capps and colleagues reviewed 7 years of administrative data from multiple insurers across the United States to estimate postintegration costs. From 2007 to 2013, they found that there was a 57% increase in the share of spending by physicians whose practices are owned by hospitals. In addition, this led to an increase in physician prices of 14% post integration.5 The larger the market share of inpatients by a hospital the larger the price increase. The authors estimate that about 25% of the price increases are precisely due to “exploitation of reimbursement rules” by charging the facility fees for their employed physicians. If these “surcharges” led to decreased utilization as one measure of increased efficiency and therefore reduced overall health care costs, it would be acceptable. But, Capps et al. found no such evidence and speculate that this scenario could lead to higher expenditures.
In a recent study, total expenditures for over 4 million patients by private physician groups or integrated groups covered by health maintenance organizations (HMOs) in California between 2009 and 2012 were analyzed.6 Mean annual expenditures were highest for large multihospital systems followed by hospital-owned physician groups and, lastly, physician-owned groups. The expenditures for multihospital systems were 19.8% higher and for local hospital-employed physician groups 10% higher compared to physician-owned organizations.
Why should prices increase after tighter physician hospital integration on a large scale? Market power. Once health systems have a large enough number of physicians in their panel, hospitals could charge insurers higher prices to access their specialists. Similarly, by employing a large number of physicians in a particular specialty, which then attracts a large pool of patients with a particular illness, they could dominate the other health systems in the region. One action specifically forbidden by anti-kickback laws is compensating physicians based on the number of referrals they make to the hospital. But, there are enough loopholes that allow hospitals to indirectly tie compensation to “productivity.” This may change with bundled payments or compensation tied to “value,” although there will always be incentives for work volume to some degree.
A further roadblock for basing merger decisions entirely on possible efficiencies is how the courts will see these activities in terms of antitrust actions. Most arguments using efficiency as the basis for merging physician groups with hospitals are vague and in general courts have not superseded antitrust actions with economic efficiency arguments.
What should be genuine reasons for hospitals employing and aligning with physicians? Addressing uneven quality of care, access and, of course, ever spiraling costs. If the object was to share responsibility for attacking these problems, health care systems and physicians would be cut a lot of slack. But, some health care systems want to not only survive the existing chaos but also dominate their local market.
I guess health care is really no different from Wall Street corporations in its focus on short-term gains versus long-term benefits. Until broader incentives change, health systems will continue to look to survive and gain market share and power. Competition, in isolation, drives tactics where the only objective may be to increase market share. However, it appears that the FTC will be busy wielding the Sherman Act of the antitrust law to keep a check on health systems to ensure consumers, payers, physicians, and the country at large are all on a fair playing field.7
Dr. Satiani is professor of clinical surgery, division of vascular diseases & surgery, department of surgery, associate director, FAME; director, Faculty Leadership Institute, and medical director, Vascular Labs, at Ohio State University College of Medicine, Columbus. He is also an associate medical editor for Vascular Specialist.
References
2. Journal of Health Economics 2006; 25: 1-28.
3. Journal of Health Economics 2006; 25: 29-38.
4. Health Affairs 2014; 33(5): 756-63.
5. www.ipr.northwestern.edu/publications/docs/workingpapers/2015/IPR-WP-15-02.pdf
6. JAMA. 2014; 312(16):1663-9.
7. Plastic & Reconstructive Surgery. 2006; 117(3): 1012-22.
NO
The days of hanging one’s shingle on a door and starting a self-employed practice are rapidly fading. While some fondly remember the practice of medicine as it was in Norman Rockwell’s classic “Before the Shot,” the realities of a current practice couldn’t be more different. Reusable syringes, analog weighing stations, an unaccompanied minor, and lack of regard for universal precautions are just a few examples from that painting that have long since disappeared. However, the humor in this painting comes from the young boy scrutinizing the doctor’s credentials, implying a sense of distrust and fear as he stands there with his buttocks partially exposed waiting for the vaccination.
This scrutiny of physician performance and results is more relevant today than ever before. Perhaps if we were to update the painting today, it would depict the boy furiously tapping away at his tablet searching through ProPublica to see what the doctor’s complication rate with the intended procedure truly is.
This is just one of the many pressures physicians are facing today. Navigating the publicly reported complication data is but one tiny portion of the regulatory red tape physicians face in taking care of their patients. If you add in the need to negotiate and contact with insurers, manage an office staff, acquire and maintain an electronic medical record (EMR) while ensuring that your EMR is properly secured against potential cyber threats and compliant with meaningful use regulations, audit your billing and coding, keep up to date with upcoming changes to bundled payments, mail out and track Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), as well as an endless list of other requirements, it is no wonder physicians are less willing to take these challenges on as solo practitioners. In fact, based on Medscape’s 2014 Employed Doctors Report, which compiled responses from over 4,600 physicians, the top three reasons for being an employed physician were not having to deal with the business of running an office (58%), not having to deal with insurers and billing (45%), and guaranteed income/even cash flow (42%).1
Multiple sources continue to confirm the trend that more physicians are moving to an employed practice.2-4 In the last decade, the rate of hospital employment has increased from 11% to 64%.1 There are many factors that have pushed physicians away from self-employment. Some of these are related to physicians’ personal choices, and many are from external pressures. As various parts of the Affordable Care Act come into play, there will continue to be increasing regulatory demands. These have the potential of increasing overhead costs, and, coupled with decreasing reimbursement, will inevitably make staying profitable more challenging in a self-employed model.
There are two other very telling trends that foretell the inevitable decline of self-employed physicians. Fewer and fewer new graduates are reporting that they are self employed. In the most recent surveys, twice as many physicians under the age of 40 are employed than self-employed.1 Furthermore, 92% of residents surveyed in their final year would prefer employment with a salary, and only 2% would consider solo practice.5 Of these graduating residents, 36% specifically were considering hospital employment, which is nearly a 10-fold increase from a decade ago. The second factor affecting new hires is their confidence that they have the necessary skills to manage a self-employed model. During the same decade, there was only a small increase in graduating residents who felt very prepared to deal with the business side of medicine (10% vs. 2%).5 This lack of knowledge will undoubtedly make it difficult for those who would consider self-employment to feel comfortable in that practice model. Some have speculated that there is soon to be a “push back” from the physicians and specifically from specialists who don’t have as much to gain in large group practices. With so few graduates considering solo and small group practice, and the overwhelming majority not feeling very prepared to manage the business of medicine, who can help lead this trend reversal?
Not only are fewer new graduates choosing self-employment, but fewer opportunities for self-employment are available as more physician groups are being bought by hospitals or other large group practices. Specifically with vascular surgery, there is a significant overhead cost requirement. Advantages to joining a large group practice include better ability to negotiate cost savings with the frequent capital requirements for new equipment, updates and maintenance of the electronic records, and professional liability. In fact, one study in California shows that as the proportion of physicians employed by the health system increased, supply chain expenses and inventory costs improved.6 Furthermore, hospitals have administrators who are hired to negotiate with insurers regarding reimbursement and respond to audits and other regulatory changes. As mentioned above, the top two reasons for avoiding self-employment are precisely these. This will no doubt draw even more physicians and specifically vascular surgeons into employed models.
Dr. Haurani is assistant professor of surgery in the division of vascular diseases and surgery, Ohio State University Medical Center, Columbus.
References
1. www.medscape.com/features/slideshow/public/employed-doctors#1
3.Perspect Vasc Surg Endovasc Ther. 2013;25:46-52.
4 Tenn Med. 2012;105:38-39.
5.www.merritthawkins.com/uploadedFiles/MerrittHawkings/Surveys/2014_MerrittHawkins_FYMR_Survey.pdf.
6. Health Care Manage Rev. 2015 Jul 23. [Epub ahead of print] www.ncbi.nlm.nih.gov/pubmed/26207654
Do we need lower extremity physiologic studies in the age of duplex scanners?
Lower-Extremity Physiologic Studies Are No Longer Required – Wrong!
Okay Gene, consider an example from the cardiology world.
You walk up a flight of stairs and develop chest pain. Is it angina? If subsequent coronary catheterization reveals a 60% stenosis in your proximal right coronary artery, then you have an anatomic coronary lesion. But is this lesion causing angina? Maybe. If the catheter is pushed across the stenosis and a 12mm Hg resting pressure gradient is identified, the lesion also has hemodynamic significance. But is it causing angina? Possibly.
To clarify things, a functional study is necessary; specifically, you need to stress the heart (using treadmill exercise, biking, infusion of a catecholamine, etc.) and determine the subsequent cardiac response (pain, ECG changes, wall motion abnormalities, sestamibi/thallium uptake, etc.). If stress produces an abnormal cardiac response, does that mean you have angina? Probably.
Yes, it’s so simple a cardiologist can get it. Yet many vascular specialists seem to struggle with the concept that anatomic, hemodynamic, and functional tests provide different types of information about the arterial system. These tests are complimentary; when used properly they combine to yield information that no single test can provide. But the siren song of an image is hard to resist – after all, everyone loves pictures, and Duplex provides nice ones. With imaging, you can scan for aneurysms. You can see what plaque looks like. In some instances you can even perform operations or procedures solely on the basis of Duplex scanning. What more do you need? (Insert my sarcastic sneer here, Gene.)
Duplex even provides basic hemodynamic information about specific lesions (based on blood flow velocity changes across the lesion). However, it’s a mistake to think that hemodynamic changes across a particular lesion tell us much about the overall “hemodynamics” in a limb. Major arteries may be narrowed (or even completely occluded), but if the collateral vessels are good, the resulting hemodynamic compromise may be minimal. Unfortunately, Duplex scanning is relatively poor at identifying and assessing collateral flow. Tests like segmental pressures (and the ankle-brachial index, or ABI) often provide a better assessment of overall arterial status because the adequacy of collateral flow influences (and is reflected in) distal blood pressure measurements. It’s also naive to think that Duplex is the only means for noninvasively obtaining anatomic information about arterial disease; nonimaging tests using segmental pressures, multilevel pulse-volume recordings (PVRs), continuous-wave (CW) Doppler, etc. may not produce pretty pictures, but they can still localize arterial lesions to a particular anatomic level (aortoiliac vs. femoral-popliteal vs. infrapopliteal, etc.).
The combination of hemodynamic and functional testing is typically referred to as “physiologic” testing, but there’s much more to this than just CW Dopplerand pneumatic cuffs. Specific tests using Laser-Doppler to measure skin blood flow or microvascular perfusion pressure make it possible to (physiologically) assess the microcirculation. Transcutaneous Oxygen (TcPO2) measurement is available in many labs; this test provides valuable physiological information about skin viability and healing potential.
Where Duplex scanning meets its biggest challenge is in the area of functional testing. Yes, it’s possible to exercise the legs and identify subsequent flow (velocity) changes across certain lesions or vessels, but any seasoned sonographer knows that these studies are extremely difficult and time consuming. And because they typically can’t assess/account for collateral flow, they are often of questionable value for determining the adverse impact of arterial disease on the patient. In contrast, functional (exercise) studies performed with CW Doppler and air cuffs are simple, inexpensive, reliable, reproducible, and widely applicable.
Bottom line – physiologic studies are an indispensable part of noninvasive arterial testing and will certainly remain so. For any given patient, they are essential for identifying and quantifying the scope and impact of arterial disease. For populations, they are the test of choice for arterial screening. And, from a practical standpoint, they are required for IAC [intersocietal accreditation commission] laboratory accreditation in peripheral arterial testing (as you know, Duplex scanning is optional for arterial accreditation – but physiologic studies are mandatory!)
Finally, the same characteristics that make physiologic tests important for assessing arterial disease are equally – or perhaps even more – relevant for the assessment of venous disease, but the application of this approach remains in its infancy. It’s a good topic for another discussion.
Dr. Rooke is the Krehbiel Professor of Vascular Medicine at the Mayo Clinic, Rochester, Minn. He has no relevant conflicts.
Lower-Extremity Physiologic Studies Are No Longer Required – Yes ... Maybe!
My friend, Thom, makes a strong case for the value of physiologic studies in patients with lower extremity arterial disease – so strong, in fact, that one might wonder if there is any truly cogent “counterpoint” at all. To provide some perspective on this issue, let’s take a look back to see how this field started and consider why someone would even dare make the statement that is being debated here.
When clinical vascular laboratories appeared in the late 1960s, the concept of “nondestructive” or “noninvasive” testing was a novelty that was not widely accepted. The first vascular laboratory tests for the assessment of carotid, peripheral arterial, and venous disease were described as “indirect,” because they relied on detection of the physiologic alterations produced by vascular abnormalities.
These methods included the supraorbital Doppler examination and oculoplethysmography for carotid disease, ankle-brachial indices, segmental pressures, and pulse volume recordings for peripheral arterial disease, and the CW Doppler examination and impedance plethysmography for venous obstruction. In those early days, vascular testing was limited to some vascular surgery practices and physiology laboratories.
While these indirect physiologic tests were helpful for characterizing regional hemodynamics, they did not provide the detailed anatomic information on arterial lesions that vascular surgeons needed to plan treatment. It was not until direct ultrasound imaging of blood vessels became available in the late 1970s and early 1980s that interest in the non-invasive vascular laboratory increased. The “duplex concept” of combining B-mode imaging with Doppler flow detection appeared to overcome the major limitations of the indirect tests by providing two-dimensional images of an arterial lesion along with an assessment of the associated flow patterns – anatomy and hemodynamics – the best of both testing worlds.
The capabilities of duplex ultrasound were so impressive that direct duplex scanning rapidly replaced the indirect or physiologic tests for diagnosis of extracranial carotid disease and lower extremity deep venous thrombosis. But, as Thom points out, peripheral arterial disease is the only testing area in which physiologic studies are still considered as “primary” testing methods according to the IAC Standards and Guidelines for Vascular Testing Accreditation. So the challenge for the modern vascular laboratory is to determine which test to use and how to integrate physiologic testing and duplex scanning in the evaluation of patients with known or suspected peripheral arterial disease.
The clinical role of the vascular laboratory can be divided into the categories of screening, diagnosis, and follow-up. The merits of screening for peripheral arterial disease are beyond the scope of this debate, but screening tests must be safe, inexpensive, and capable of detecting the presence or absence of disease. Clearly, physiologic tests meet these requirements, and in most situations, an ankle-brachial index is all that is needed. Diagnostic testing in patients with signs and symptoms of peripheral arterial disease, including those that may be candidates for intervention, requires the specific anatomic and hemodynamic information that duplex scanning provides, but functional testing (i.e., treadmill exercise) can also be valuable in selected cases. Similarly, follow-up of peripheral arterial interventions usually requires duplex scanning to evaluate the anatomic and hemodynamic features of the treated arterial segment, while follow-up of documented but untreated peripheral arterial disease can often be accomplished primarily by physiologic tests alone.
Are lower-extremity physiologic studies no longer required in the age of modern duplex scanners? Although I am reluctant to admit it, strictly speaking, my friend, Thom, is correct when he responds with an emphatic “Wrong.” There is no reason to completely abandon the physiologic tests that have served us well since before the age of duplex scanning. A simple ankle-brachial index is easy to justify as part of almost any lower extremity arterial evaluation, and exercise treadmill testing is an excellent way to assess the functional status of a patient with peripheral arterial disease. However, vascular laboratories should consider how best to combine the use of physiologic testing and direct duplex imaging for peripheral arterial disease in order to avoid unnecessary or inappropriate testing. For most vascular laboratories, this means that initial screening should be done with physiologic tests, but the primary testing method for diagnosis and follow-up will be duplex scanning, supplemented by selective use of physiologic testing.
Dr. Zierler is professor of surgery at the University of Washington and medical director of the D.E. Strandness Jr. Vascular Laboratory at the University of Washington Medical Center and Harborview Medical Center, Seattle. He has no relevant conflicts.
Lower-Extremity Physiologic Studies Are No Longer Required – Wrong!
Okay Gene, consider an example from the cardiology world.
You walk up a flight of stairs and develop chest pain. Is it angina? If subsequent coronary catheterization reveals a 60% stenosis in your proximal right coronary artery, then you have an anatomic coronary lesion. But is this lesion causing angina? Maybe. If the catheter is pushed across the stenosis and a 12mm Hg resting pressure gradient is identified, the lesion also has hemodynamic significance. But is it causing angina? Possibly.
To clarify things, a functional study is necessary; specifically, you need to stress the heart (using treadmill exercise, biking, infusion of a catecholamine, etc.) and determine the subsequent cardiac response (pain, ECG changes, wall motion abnormalities, sestamibi/thallium uptake, etc.). If stress produces an abnormal cardiac response, does that mean you have angina? Probably.
Yes, it’s so simple a cardiologist can get it. Yet many vascular specialists seem to struggle with the concept that anatomic, hemodynamic, and functional tests provide different types of information about the arterial system. These tests are complimentary; when used properly they combine to yield information that no single test can provide. But the siren song of an image is hard to resist – after all, everyone loves pictures, and Duplex provides nice ones. With imaging, you can scan for aneurysms. You can see what plaque looks like. In some instances you can even perform operations or procedures solely on the basis of Duplex scanning. What more do you need? (Insert my sarcastic sneer here, Gene.)
Duplex even provides basic hemodynamic information about specific lesions (based on blood flow velocity changes across the lesion). However, it’s a mistake to think that hemodynamic changes across a particular lesion tell us much about the overall “hemodynamics” in a limb. Major arteries may be narrowed (or even completely occluded), but if the collateral vessels are good, the resulting hemodynamic compromise may be minimal. Unfortunately, Duplex scanning is relatively poor at identifying and assessing collateral flow. Tests like segmental pressures (and the ankle-brachial index, or ABI) often provide a better assessment of overall arterial status because the adequacy of collateral flow influences (and is reflected in) distal blood pressure measurements. It’s also naive to think that Duplex is the only means for noninvasively obtaining anatomic information about arterial disease; nonimaging tests using segmental pressures, multilevel pulse-volume recordings (PVRs), continuous-wave (CW) Doppler, etc. may not produce pretty pictures, but they can still localize arterial lesions to a particular anatomic level (aortoiliac vs. femoral-popliteal vs. infrapopliteal, etc.).
The combination of hemodynamic and functional testing is typically referred to as “physiologic” testing, but there’s much more to this than just CW Dopplerand pneumatic cuffs. Specific tests using Laser-Doppler to measure skin blood flow or microvascular perfusion pressure make it possible to (physiologically) assess the microcirculation. Transcutaneous Oxygen (TcPO2) measurement is available in many labs; this test provides valuable physiological information about skin viability and healing potential.
Where Duplex scanning meets its biggest challenge is in the area of functional testing. Yes, it’s possible to exercise the legs and identify subsequent flow (velocity) changes across certain lesions or vessels, but any seasoned sonographer knows that these studies are extremely difficult and time consuming. And because they typically can’t assess/account for collateral flow, they are often of questionable value for determining the adverse impact of arterial disease on the patient. In contrast, functional (exercise) studies performed with CW Doppler and air cuffs are simple, inexpensive, reliable, reproducible, and widely applicable.
Bottom line – physiologic studies are an indispensable part of noninvasive arterial testing and will certainly remain so. For any given patient, they are essential for identifying and quantifying the scope and impact of arterial disease. For populations, they are the test of choice for arterial screening. And, from a practical standpoint, they are required for IAC [intersocietal accreditation commission] laboratory accreditation in peripheral arterial testing (as you know, Duplex scanning is optional for arterial accreditation – but physiologic studies are mandatory!)
Finally, the same characteristics that make physiologic tests important for assessing arterial disease are equally – or perhaps even more – relevant for the assessment of venous disease, but the application of this approach remains in its infancy. It’s a good topic for another discussion.
Dr. Rooke is the Krehbiel Professor of Vascular Medicine at the Mayo Clinic, Rochester, Minn. He has no relevant conflicts.
Lower-Extremity Physiologic Studies Are No Longer Required – Yes ... Maybe!
My friend, Thom, makes a strong case for the value of physiologic studies in patients with lower extremity arterial disease – so strong, in fact, that one might wonder if there is any truly cogent “counterpoint” at all. To provide some perspective on this issue, let’s take a look back to see how this field started and consider why someone would even dare make the statement that is being debated here.
When clinical vascular laboratories appeared in the late 1960s, the concept of “nondestructive” or “noninvasive” testing was a novelty that was not widely accepted. The first vascular laboratory tests for the assessment of carotid, peripheral arterial, and venous disease were described as “indirect,” because they relied on detection of the physiologic alterations produced by vascular abnormalities.
These methods included the supraorbital Doppler examination and oculoplethysmography for carotid disease, ankle-brachial indices, segmental pressures, and pulse volume recordings for peripheral arterial disease, and the CW Doppler examination and impedance plethysmography for venous obstruction. In those early days, vascular testing was limited to some vascular surgery practices and physiology laboratories.
While these indirect physiologic tests were helpful for characterizing regional hemodynamics, they did not provide the detailed anatomic information on arterial lesions that vascular surgeons needed to plan treatment. It was not until direct ultrasound imaging of blood vessels became available in the late 1970s and early 1980s that interest in the non-invasive vascular laboratory increased. The “duplex concept” of combining B-mode imaging with Doppler flow detection appeared to overcome the major limitations of the indirect tests by providing two-dimensional images of an arterial lesion along with an assessment of the associated flow patterns – anatomy and hemodynamics – the best of both testing worlds.
The capabilities of duplex ultrasound were so impressive that direct duplex scanning rapidly replaced the indirect or physiologic tests for diagnosis of extracranial carotid disease and lower extremity deep venous thrombosis. But, as Thom points out, peripheral arterial disease is the only testing area in which physiologic studies are still considered as “primary” testing methods according to the IAC Standards and Guidelines for Vascular Testing Accreditation. So the challenge for the modern vascular laboratory is to determine which test to use and how to integrate physiologic testing and duplex scanning in the evaluation of patients with known or suspected peripheral arterial disease.
The clinical role of the vascular laboratory can be divided into the categories of screening, diagnosis, and follow-up. The merits of screening for peripheral arterial disease are beyond the scope of this debate, but screening tests must be safe, inexpensive, and capable of detecting the presence or absence of disease. Clearly, physiologic tests meet these requirements, and in most situations, an ankle-brachial index is all that is needed. Diagnostic testing in patients with signs and symptoms of peripheral arterial disease, including those that may be candidates for intervention, requires the specific anatomic and hemodynamic information that duplex scanning provides, but functional testing (i.e., treadmill exercise) can also be valuable in selected cases. Similarly, follow-up of peripheral arterial interventions usually requires duplex scanning to evaluate the anatomic and hemodynamic features of the treated arterial segment, while follow-up of documented but untreated peripheral arterial disease can often be accomplished primarily by physiologic tests alone.
Are lower-extremity physiologic studies no longer required in the age of modern duplex scanners? Although I am reluctant to admit it, strictly speaking, my friend, Thom, is correct when he responds with an emphatic “Wrong.” There is no reason to completely abandon the physiologic tests that have served us well since before the age of duplex scanning. A simple ankle-brachial index is easy to justify as part of almost any lower extremity arterial evaluation, and exercise treadmill testing is an excellent way to assess the functional status of a patient with peripheral arterial disease. However, vascular laboratories should consider how best to combine the use of physiologic testing and direct duplex imaging for peripheral arterial disease in order to avoid unnecessary or inappropriate testing. For most vascular laboratories, this means that initial screening should be done with physiologic tests, but the primary testing method for diagnosis and follow-up will be duplex scanning, supplemented by selective use of physiologic testing.
Dr. Zierler is professor of surgery at the University of Washington and medical director of the D.E. Strandness Jr. Vascular Laboratory at the University of Washington Medical Center and Harborview Medical Center, Seattle. He has no relevant conflicts.
Lower-Extremity Physiologic Studies Are No Longer Required – Wrong!
Okay Gene, consider an example from the cardiology world.
You walk up a flight of stairs and develop chest pain. Is it angina? If subsequent coronary catheterization reveals a 60% stenosis in your proximal right coronary artery, then you have an anatomic coronary lesion. But is this lesion causing angina? Maybe. If the catheter is pushed across the stenosis and a 12mm Hg resting pressure gradient is identified, the lesion also has hemodynamic significance. But is it causing angina? Possibly.
To clarify things, a functional study is necessary; specifically, you need to stress the heart (using treadmill exercise, biking, infusion of a catecholamine, etc.) and determine the subsequent cardiac response (pain, ECG changes, wall motion abnormalities, sestamibi/thallium uptake, etc.). If stress produces an abnormal cardiac response, does that mean you have angina? Probably.
Yes, it’s so simple a cardiologist can get it. Yet many vascular specialists seem to struggle with the concept that anatomic, hemodynamic, and functional tests provide different types of information about the arterial system. These tests are complimentary; when used properly they combine to yield information that no single test can provide. But the siren song of an image is hard to resist – after all, everyone loves pictures, and Duplex provides nice ones. With imaging, you can scan for aneurysms. You can see what plaque looks like. In some instances you can even perform operations or procedures solely on the basis of Duplex scanning. What more do you need? (Insert my sarcastic sneer here, Gene.)
Duplex even provides basic hemodynamic information about specific lesions (based on blood flow velocity changes across the lesion). However, it’s a mistake to think that hemodynamic changes across a particular lesion tell us much about the overall “hemodynamics” in a limb. Major arteries may be narrowed (or even completely occluded), but if the collateral vessels are good, the resulting hemodynamic compromise may be minimal. Unfortunately, Duplex scanning is relatively poor at identifying and assessing collateral flow. Tests like segmental pressures (and the ankle-brachial index, or ABI) often provide a better assessment of overall arterial status because the adequacy of collateral flow influences (and is reflected in) distal blood pressure measurements. It’s also naive to think that Duplex is the only means for noninvasively obtaining anatomic information about arterial disease; nonimaging tests using segmental pressures, multilevel pulse-volume recordings (PVRs), continuous-wave (CW) Doppler, etc. may not produce pretty pictures, but they can still localize arterial lesions to a particular anatomic level (aortoiliac vs. femoral-popliteal vs. infrapopliteal, etc.).
The combination of hemodynamic and functional testing is typically referred to as “physiologic” testing, but there’s much more to this than just CW Dopplerand pneumatic cuffs. Specific tests using Laser-Doppler to measure skin blood flow or microvascular perfusion pressure make it possible to (physiologically) assess the microcirculation. Transcutaneous Oxygen (TcPO2) measurement is available in many labs; this test provides valuable physiological information about skin viability and healing potential.
Where Duplex scanning meets its biggest challenge is in the area of functional testing. Yes, it’s possible to exercise the legs and identify subsequent flow (velocity) changes across certain lesions or vessels, but any seasoned sonographer knows that these studies are extremely difficult and time consuming. And because they typically can’t assess/account for collateral flow, they are often of questionable value for determining the adverse impact of arterial disease on the patient. In contrast, functional (exercise) studies performed with CW Doppler and air cuffs are simple, inexpensive, reliable, reproducible, and widely applicable.
Bottom line – physiologic studies are an indispensable part of noninvasive arterial testing and will certainly remain so. For any given patient, they are essential for identifying and quantifying the scope and impact of arterial disease. For populations, they are the test of choice for arterial screening. And, from a practical standpoint, they are required for IAC [intersocietal accreditation commission] laboratory accreditation in peripheral arterial testing (as you know, Duplex scanning is optional for arterial accreditation – but physiologic studies are mandatory!)
Finally, the same characteristics that make physiologic tests important for assessing arterial disease are equally – or perhaps even more – relevant for the assessment of venous disease, but the application of this approach remains in its infancy. It’s a good topic for another discussion.
Dr. Rooke is the Krehbiel Professor of Vascular Medicine at the Mayo Clinic, Rochester, Minn. He has no relevant conflicts.
Lower-Extremity Physiologic Studies Are No Longer Required – Yes ... Maybe!
My friend, Thom, makes a strong case for the value of physiologic studies in patients with lower extremity arterial disease – so strong, in fact, that one might wonder if there is any truly cogent “counterpoint” at all. To provide some perspective on this issue, let’s take a look back to see how this field started and consider why someone would even dare make the statement that is being debated here.
When clinical vascular laboratories appeared in the late 1960s, the concept of “nondestructive” or “noninvasive” testing was a novelty that was not widely accepted. The first vascular laboratory tests for the assessment of carotid, peripheral arterial, and venous disease were described as “indirect,” because they relied on detection of the physiologic alterations produced by vascular abnormalities.
These methods included the supraorbital Doppler examination and oculoplethysmography for carotid disease, ankle-brachial indices, segmental pressures, and pulse volume recordings for peripheral arterial disease, and the CW Doppler examination and impedance plethysmography for venous obstruction. In those early days, vascular testing was limited to some vascular surgery practices and physiology laboratories.
While these indirect physiologic tests were helpful for characterizing regional hemodynamics, they did not provide the detailed anatomic information on arterial lesions that vascular surgeons needed to plan treatment. It was not until direct ultrasound imaging of blood vessels became available in the late 1970s and early 1980s that interest in the non-invasive vascular laboratory increased. The “duplex concept” of combining B-mode imaging with Doppler flow detection appeared to overcome the major limitations of the indirect tests by providing two-dimensional images of an arterial lesion along with an assessment of the associated flow patterns – anatomy and hemodynamics – the best of both testing worlds.
The capabilities of duplex ultrasound were so impressive that direct duplex scanning rapidly replaced the indirect or physiologic tests for diagnosis of extracranial carotid disease and lower extremity deep venous thrombosis. But, as Thom points out, peripheral arterial disease is the only testing area in which physiologic studies are still considered as “primary” testing methods according to the IAC Standards and Guidelines for Vascular Testing Accreditation. So the challenge for the modern vascular laboratory is to determine which test to use and how to integrate physiologic testing and duplex scanning in the evaluation of patients with known or suspected peripheral arterial disease.
The clinical role of the vascular laboratory can be divided into the categories of screening, diagnosis, and follow-up. The merits of screening for peripheral arterial disease are beyond the scope of this debate, but screening tests must be safe, inexpensive, and capable of detecting the presence or absence of disease. Clearly, physiologic tests meet these requirements, and in most situations, an ankle-brachial index is all that is needed. Diagnostic testing in patients with signs and symptoms of peripheral arterial disease, including those that may be candidates for intervention, requires the specific anatomic and hemodynamic information that duplex scanning provides, but functional testing (i.e., treadmill exercise) can also be valuable in selected cases. Similarly, follow-up of peripheral arterial interventions usually requires duplex scanning to evaluate the anatomic and hemodynamic features of the treated arterial segment, while follow-up of documented but untreated peripheral arterial disease can often be accomplished primarily by physiologic tests alone.
Are lower-extremity physiologic studies no longer required in the age of modern duplex scanners? Although I am reluctant to admit it, strictly speaking, my friend, Thom, is correct when he responds with an emphatic “Wrong.” There is no reason to completely abandon the physiologic tests that have served us well since before the age of duplex scanning. A simple ankle-brachial index is easy to justify as part of almost any lower extremity arterial evaluation, and exercise treadmill testing is an excellent way to assess the functional status of a patient with peripheral arterial disease. However, vascular laboratories should consider how best to combine the use of physiologic testing and direct duplex imaging for peripheral arterial disease in order to avoid unnecessary or inappropriate testing. For most vascular laboratories, this means that initial screening should be done with physiologic tests, but the primary testing method for diagnosis and follow-up will be duplex scanning, supplemented by selective use of physiologic testing.
Dr. Zierler is professor of surgery at the University of Washington and medical director of the D.E. Strandness Jr. Vascular Laboratory at the University of Washington Medical Center and Harborview Medical Center, Seattle. He has no relevant conflicts.
The pros and cons of novel anticoagulants
Novel anticoagulants will likely replace need for vitamin K antagonists
BY MADHUKAR S. PATEL, M.D., AND ELLIOT L. CHAIKOF, M.D.
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation, that Campbell & Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs), began.
Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice.
Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared to VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs.
NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low-molecular-weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities that may influence drug effect and clearance. Lastly, it should be mentioned that the pharmacologic benefits of NOACs apply not only from a patient perspective, but also from a health care systems standpoint, as their use may provide an opportunity to deliver more cost-effective care.
Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared to warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs to VKAs or placebos for the management of nonvalvular atrial fibrillation and venous thromboembolism, and as adjunctive therapy for patients with acute coronary syndrome.6
Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation, where NOACs yield significant reductions in stroke, intracranial hemorrhage, and all-cause mortality compared to warfarin, while displaying variable effects with regard to gastrointestinal bleeding.6,7 In patients with VTE, NOACs have been found to have efficacy similar to that of VKAs with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6
Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon which class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life threatening bleeding complications.
Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monoclonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently under phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9
Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable compared to VKAs and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusion
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism, where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the healthcare system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Dr. Patel is a research fellow and Dr. Chaikof is surgeon-in-chief, both at the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported no conflicts of interest.
References
1. J Am Vet Med Assoc. 1924;64:553-75 (See Br J Haematol 2008 Mar 18;141[6]:757-63).
2. J Biol Chem. 1941;138:21-33 (See Nutr Rev. 1974 Aug;32[8]:244-6).
3. Am Soc Hematol Educ Program. 2013;2013:464-70.
4. Eur Heart J. 2013 Jul;34(27):2094-2106.
5. Stroke. 2013 Jun;44(6):1676-81.
6. Nat Rev Cardiol. 2014 Dec;11(12):693-703.
7. Lancet. 2014 Mar 15;383(9921):955-62.
8. N Engl J Med. 2015;373(6):511-20.
9. N Engl J Med. 2014;371(22):2141-2.
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
BY THOMAS WAKEFIELD, M.D., ANDREA OBI, M.D., AND DAWN COLEMAN, M.D.
Recently, several new oral anticoagulants have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once- or twice-daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute venous thromboembolism (VTE) and atrial fibrillation (AF).
Dabigatran and edoxaban
As with warfarin, dabigatran and edoxaban require the use of a low-molecular-weight heparin (LMWH) or unfractionated heparin “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent that can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique among the NOACs, have been tested for extended therapy of acute DVT after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding compared to placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding compared to warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels and to reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects.
With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published a recommendation7 that lists these scenarios:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL/min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.
Currently, there exists no commercially available reversal agent for any of the NOACs and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials is lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however, drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
There are no national guidelines nor large scale studies to guide bridging NOACs for procedures. The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
Conclusion
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that for all the benefits, they each carry a risk of bleeding as they all target portions of the coagulation mechanism. We believe, that as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Dr. Wakefield is director of the Samuel and Jean Frankel Cardiovascular Center, Dr. Obi is a vascular surgery fellow, and Dr. Coleman is program director, section of vascular surgery, at the University of Michigan, Ann Arbor. They reported no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-52.
2. J Vasc Surg: Venous Lymphat Disord. 2013;1:418-26.
3. N Engl J Med. 2013;369:1406-15.
4. N Engl J Med. 2010;363:2499-2510.
5. N Engl J Med. 2013;368:699-708.
6. Arterioscler Thromb Vasc Biol. 2015;35:1056-65.
7. J Thromb Haemost. 2013;11:756-60.
Novel anticoagulants will likely replace need for vitamin K antagonists
BY MADHUKAR S. PATEL, M.D., AND ELLIOT L. CHAIKOF, M.D.
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation, that Campbell & Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs), began.
Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice.
Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared to VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs.
NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low-molecular-weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities that may influence drug effect and clearance. Lastly, it should be mentioned that the pharmacologic benefits of NOACs apply not only from a patient perspective, but also from a health care systems standpoint, as their use may provide an opportunity to deliver more cost-effective care.
Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared to warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs to VKAs or placebos for the management of nonvalvular atrial fibrillation and venous thromboembolism, and as adjunctive therapy for patients with acute coronary syndrome.6
Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation, where NOACs yield significant reductions in stroke, intracranial hemorrhage, and all-cause mortality compared to warfarin, while displaying variable effects with regard to gastrointestinal bleeding.6,7 In patients with VTE, NOACs have been found to have efficacy similar to that of VKAs with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6
Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon which class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life threatening bleeding complications.
Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monoclonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently under phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9
Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable compared to VKAs and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusion
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism, where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the healthcare system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Dr. Patel is a research fellow and Dr. Chaikof is surgeon-in-chief, both at the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported no conflicts of interest.
References
1. J Am Vet Med Assoc. 1924;64:553-75 (See Br J Haematol 2008 Mar 18;141[6]:757-63).
2. J Biol Chem. 1941;138:21-33 (See Nutr Rev. 1974 Aug;32[8]:244-6).
3. Am Soc Hematol Educ Program. 2013;2013:464-70.
4. Eur Heart J. 2013 Jul;34(27):2094-2106.
5. Stroke. 2013 Jun;44(6):1676-81.
6. Nat Rev Cardiol. 2014 Dec;11(12):693-703.
7. Lancet. 2014 Mar 15;383(9921):955-62.
8. N Engl J Med. 2015;373(6):511-20.
9. N Engl J Med. 2014;371(22):2141-2.
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
BY THOMAS WAKEFIELD, M.D., ANDREA OBI, M.D., AND DAWN COLEMAN, M.D.
Recently, several new oral anticoagulants have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once- or twice-daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute venous thromboembolism (VTE) and atrial fibrillation (AF).
Dabigatran and edoxaban
As with warfarin, dabigatran and edoxaban require the use of a low-molecular-weight heparin (LMWH) or unfractionated heparin “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent that can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique among the NOACs, have been tested for extended therapy of acute DVT after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding compared to placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding compared to warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels and to reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects.
With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published a recommendation7 that lists these scenarios:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL/min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.
Currently, there exists no commercially available reversal agent for any of the NOACs and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials is lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however, drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
There are no national guidelines nor large scale studies to guide bridging NOACs for procedures. The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
Conclusion
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that for all the benefits, they each carry a risk of bleeding as they all target portions of the coagulation mechanism. We believe, that as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Dr. Wakefield is director of the Samuel and Jean Frankel Cardiovascular Center, Dr. Obi is a vascular surgery fellow, and Dr. Coleman is program director, section of vascular surgery, at the University of Michigan, Ann Arbor. They reported no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-52.
2. J Vasc Surg: Venous Lymphat Disord. 2013;1:418-26.
3. N Engl J Med. 2013;369:1406-15.
4. N Engl J Med. 2010;363:2499-2510.
5. N Engl J Med. 2013;368:699-708.
6. Arterioscler Thromb Vasc Biol. 2015;35:1056-65.
7. J Thromb Haemost. 2013;11:756-60.
Novel anticoagulants will likely replace need for vitamin K antagonists
BY MADHUKAR S. PATEL, M.D., AND ELLIOT L. CHAIKOF, M.D.
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation, that Campbell & Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs), began.
Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice.
Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared to VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs.
NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low-molecular-weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities that may influence drug effect and clearance. Lastly, it should be mentioned that the pharmacologic benefits of NOACs apply not only from a patient perspective, but also from a health care systems standpoint, as their use may provide an opportunity to deliver more cost-effective care.
Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared to warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs to VKAs or placebos for the management of nonvalvular atrial fibrillation and venous thromboembolism, and as adjunctive therapy for patients with acute coronary syndrome.6
Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation, where NOACs yield significant reductions in stroke, intracranial hemorrhage, and all-cause mortality compared to warfarin, while displaying variable effects with regard to gastrointestinal bleeding.6,7 In patients with VTE, NOACs have been found to have efficacy similar to that of VKAs with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6
Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon which class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life threatening bleeding complications.
Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monoclonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently under phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9
Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable compared to VKAs and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusion
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism, where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the healthcare system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Dr. Patel is a research fellow and Dr. Chaikof is surgeon-in-chief, both at the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported no conflicts of interest.
References
1. J Am Vet Med Assoc. 1924;64:553-75 (See Br J Haematol 2008 Mar 18;141[6]:757-63).
2. J Biol Chem. 1941;138:21-33 (See Nutr Rev. 1974 Aug;32[8]:244-6).
3. Am Soc Hematol Educ Program. 2013;2013:464-70.
4. Eur Heart J. 2013 Jul;34(27):2094-2106.
5. Stroke. 2013 Jun;44(6):1676-81.
6. Nat Rev Cardiol. 2014 Dec;11(12):693-703.
7. Lancet. 2014 Mar 15;383(9921):955-62.
8. N Engl J Med. 2015;373(6):511-20.
9. N Engl J Med. 2014;371(22):2141-2.
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
BY THOMAS WAKEFIELD, M.D., ANDREA OBI, M.D., AND DAWN COLEMAN, M.D.
Recently, several new oral anticoagulants have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once- or twice-daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute venous thromboembolism (VTE) and atrial fibrillation (AF).
Dabigatran and edoxaban
As with warfarin, dabigatran and edoxaban require the use of a low-molecular-weight heparin (LMWH) or unfractionated heparin “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent that can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique among the NOACs, have been tested for extended therapy of acute DVT after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding compared to placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding compared to warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels and to reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects.
With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published a recommendation7 that lists these scenarios:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL/min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.
Currently, there exists no commercially available reversal agent for any of the NOACs and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials is lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however, drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
There are no national guidelines nor large scale studies to guide bridging NOACs for procedures. The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
Conclusion
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that for all the benefits, they each carry a risk of bleeding as they all target portions of the coagulation mechanism. We believe, that as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Dr. Wakefield is director of the Samuel and Jean Frankel Cardiovascular Center, Dr. Obi is a vascular surgery fellow, and Dr. Coleman is program director, section of vascular surgery, at the University of Michigan, Ann Arbor. They reported no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-52.
2. J Vasc Surg: Venous Lymphat Disord. 2013;1:418-26.
3. N Engl J Med. 2013;369:1406-15.
4. N Engl J Med. 2010;363:2499-2510.
5. N Engl J Med. 2013;368:699-708.
6. Arterioscler Thromb Vasc Biol. 2015;35:1056-65.
7. J Thromb Haemost. 2013;11:756-60.
Point/Counterpoint: Covered stent grafts vs. drug-eluting stents for treating long superficial femoral artery occlusions
Head-to-head comparisons are lacking, but similar results have been reported
BY MICHAEL D. DAKE, M.D.
Well, at least one thing is for sure – we would not have been having this discussion a mere 10 years ago.
I remained sheepishly silent for most of my early career as well-intentioned invasive and noninvasive specialists criticized the state of evidence supporting the legitimacy of endovascular interventions as a competitive strategy to manage infrainguinal peripheral arterial disease. Good data from well-controlled randomized clinical trials were not available to make a case for endovascular therapies.
Over the recent decade and a half, however, a number of contributing factors have influenced thinking and what we now consider standard of care for symptomatic disease of the superficial femoral artery (SFA). The proposal of an “endovascular first” interventional approach has evolved to a consensually agreed upon management strategy by all interested disciplines.
This did not occur on a whim. Rather, out of the shadows of relative ignorance there slowly emerged a welcomed accumulation of a large number of publications that detail the outcomes of a wide variety of randomized trials with a range of endovascular devices. This has allowed us to enter an era where valid comparisons between interventional therapies is not only possible, but allows us to more appropriately offer care to vascular patients with more nuanced strategies. These are strategies that recognize subtleties between subgroups of individuals stratified on the basis of patient demographics and lesion characteristics in a way not appreciated prior to the recent spate of endovascular device studies.
Thus, thanks to the dedication and hard work of many, we are now at a stage where we can have meaningful dialogues on a variety of endovascular topics, such as the one at hand, and proponents can argue their perspectives armed with objective evidence to support their positions. In this discussion regarding covered stent grafts and drug-eluting stents, we wish we had even more data.
Specifically, we are missing direct head-to-head comparisons between the two devices in patients with long SFA lesions. So, what do we know?
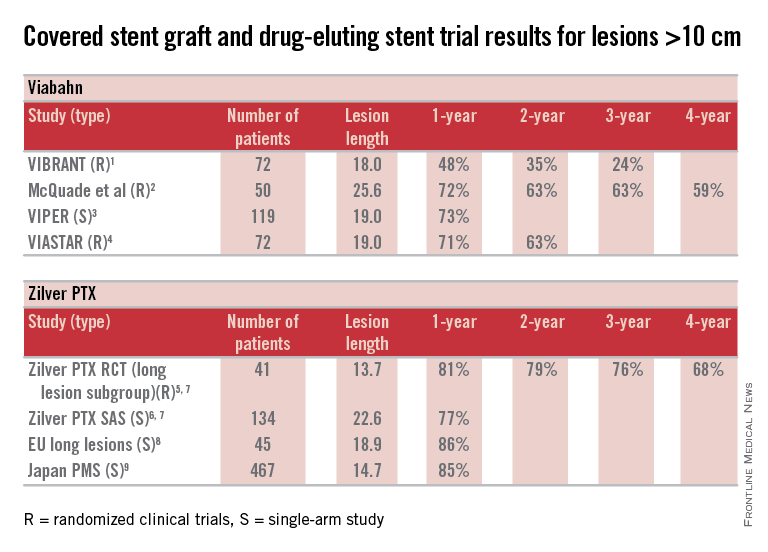
Here are some fundamental facts: The most commonly used covered stent graft for management of femoropopliteal occlusive disease is the Viabahn endoprosthesis (W. L. Gore and Associates, Flagstaff, Ariz.). The prosthesis is composed of a self-expanding nitinol stent framework and expanded polytetrafluoroethylene (ePTFE) graft with its surface lined with a coating of covalently bound heparin (Propaten bioactive surface).The only approved drug-eluting stent with significant safety and effectiveness data available is the Zilver PTX paclitaxel-eluting, self-expanding nitinol stent (Cook Medical Inc., Bloomington, Ind.).
Now in terms of the proposition, we need to discuss the meaning of the word “long” with reference to the SFA. Just what do we consider a long SFA lesion? I think all of us could agree that an arterial stenosis or occlusion of 6 cm or less is short. Lesions between 5 cm or 6 cm to 10 cm or 12 cm in length are moderately long, and disease greater than 10 cm or 12 cm is commonly characterized as long. Segments of disease greater than 20 cm long are typically considered very long or extremely long lesions from an endovascular interventional perspective.
So, how can currently available trial outcomes help us? Below, I have compiled a table that includes most of the recent clinical trial data for Viabahn and Zilver PTX in patients with long SFA occlusive disease.
OK, what can we honestly say about these data besides recognizing that we are at risk when we make any conclusions based upon cross-trial comparisons? Such an accounting of results is fraught with problems, but what we can say is that the table grossly confirms the current consensus that both devices enhance the standard of care for long lesions over traditional balloon angioplasty (PTA) and bare metal stent technologies.
Beyond this, however, it is accepted that patency results with Viabahn are lesion-length immune – that is, outcomes in long and extremely long segments of disease are not very different from the patency achieved in short lesions. This is clearly different than what is traditionally found for interventions with PTA or bare metal stents. There is not enough controlled data for extremely long lesions to reach a conclusion on drug-eluting stents; however, there is an initial suggestion that they behave in a manner more similar to stent grafts than traditional devices.
Grossly, the table suggests that the midterm and available greater than 1-year patency results with Viabahn and Zilver PTX are relatively comparable. What about the price of the device? What role does it play in our selection of the current most cost-effective endovascular strategy for long SFA lesions?
In my institution Viabahn is more expensive than Zilver PTX with a relative cost premium of about 30%-50% depending on the treatment length. Of course, when treating long TASC C and D lesions any up-front difference in the costs of the devices used initially is more than made up for by any relative reduction in subsequent reinterventions.
So, there you have it. Look at the table as simply a current snapshot. In the future, we will benefit from additional trials and comparisons, not to mention better endovascular technologies to address symptomatic long SFA lesions.
Dr. Dake is the Thelma and Henry Doelger Professor of Cardiovascular Surgery at the Stanford (Calif.) School of Medicine. He disclosed that he is a member of the Peripheral Scientific Advisory Board: Abbott Vascular Member, is on the Aortic Medical Advisory Board: W. L. Gore, is a consultant for Cook Medical, Medtronic, and Surmodics Research, and receives grants/clinical trial support from W. L. Gore, Medtronic, and Novate.
References for table
1. J. Vasc. Surg. 2013;58:386-95.
2. J. Vasc. Surg. 2010;52:584-90.
3. J. Vasc. Interv. Radiol. 2013;24:165-73.
4. Cardiovasc. Interv. Radiol. 2015;38:25-32.
5. Circ. Cardiovasc. Interv. 2011;4:495-504.
6. J. Am. Coll. Cardiol. 2013;61:2417-27.
7. J. Endovasc. Ther. 2011;18:613-23.
8. Zeller T. Oral presentations. 2014.
9. Yokoi H. Oral presentations. 2014.
Covered stent grafts in the SFA are still the endovascular champion in long lesions
BY DENNIS GABLE, M.D.
There remains a continued debate among investigators as to the best modality for treatment of stenosis/occlusion of the SFA especially with the recent advent of drug-eluting technology. However, I suggest that for long lesions over 15 cm only covered stent grafts continue to outperform the competition.
If we review the numerous studies available on treatment of SFA disease, the Viabahn-covered stent device (W. L. Gore & Associates, Flagstaff, Ariz.) is by far the most studied modality. There are currently 22 independent studies available providing data on 1,473 limbs. Several of these reports are multicenter studies and many of them are prospective randomized trials. Two of the most recent are the VIPER (J. Vasc. Intervent. Radiol. 2013;24:165-73) and VIASTAR study (JACC 2013;62:1320-27).
The VIPER study prospectively enrolled 119 patients (72 with TASC II C/D disease; mean lesion length 190 mm). Primary patency was reported at 73% at 1 year but in patients with less than 20% oversizing, as is recommended by the IFU, patency as high as 88% was noted. Additionally, there was no difference in patency in smaller-diameter vessels (5 mm) versus larger-diameter vessels (6-7 mm).
In a head-to-head randomized controlled trial of Viabahn to bare metal stents (BMS), the VIASTAR study enrolled 141 patients (72 in covered stent arm; mean lesion length 190 mm). On a per protocol evaluation, the patency at 1 year was 78% and 71% on an intention-to-treat evaluation with a patency of 70% and 63% respectively at 2 years. There was no statistical difference between the two evaluations on intention to treat vs. per protocol but there was clear superiority demonstrated against BMS.
Furthermore, in a prospective, randomized, head-to-head comparison of Viabahn to prosthetic above knee femoral popliteal bypass, it was shown that there was no difference in primary or secondary patency between the two groups out to 4 years follow-up (J. Vasc. Surg. 2010;52:584-91). This included an average lesion length of 25.6 cm with a primary and secondary patency of 59% and 74% in the Viabahn group and 58% and 71% in the surgery group. When compared to a large meta-analysis for femoral popliteal bypass outcomes reported on by Bates and AbuRahma in 2004 (J. Endovasc. Ther. 2004;11[suppl. II]:II-107–27), the patency for the surgical arm with prosthetic bypass in the above Viabahn study was similar at 4 years to the 38 peer-reviewed articles Bates et al. reviewed with over 4,000 limbs. The reported primary and secondary patency at 4 years for prosthetic femoral above knee popliteal bypass was 51% and 61%, respectively in his review. Although the above Viabahn study was not powered to formally demonstrate noninferiority to surgical bypass with prosthetic, it did strongly suggest and show just that.
How do we put these data together with the Zilver data and how do we decide what is best for our patients? Some operators have expressed concern over a perceived risk for a “higher rate of amputation” or “a worse Rutherford level of ischemia on presentation” if patients with the Viabahn stent graft occlude post procedure. Commonly, this results from extrapolation of prior studies looking at results of occlusion with an ePTFE bypass. In fact, review of peer-reviewed data reveal none of the prospective studies outlined above, or those currently available, demonstrate that either of these perceptions are true and there are no published prospective data that support these fears either. In the studies listed above as well as all current prospective studies available evaluating Viabahn usage, the highest rate of amputation reported was 5% by Fisher in 2006 with all of the remaining studies reporting an amputation rate of 2% or less (when reported). Moreover, it has not been demonstrated that patients with this device present with an increased level of ischemia secondary to sudden occlusion.
There is one report used to argue against the use of the Viabahn stent graft (J. Vasc. Surg. 2008;47:967-74). This study evaluated prospectively 109 patients (71 for claudication; 38 for critical limb ischemia) treated for SFA occlusive disease (mean lesion length 15.7 cm). Only 19 of the 109 patients (17%) were treated with Viabahn (17 for claudication; 2 for critical limb ischemia). The remaining limbs were treated with various other BMS devices (n=10). The authors concluded that patients initially treated with Viabahn who presented back with occlusion had a higher chance of presenting with acute symptoms (i.e., a worse Rutherford score). The lesion length treated in the Viabahn group, however, was nearly twice as long as all the other stent platforms combined (25.4 cm vs. 13.7 cm) and there was a higher level of tibial artery deterioration with thrombosis of the BMS group, compared with the Viabahn group (7.7% vs. 5.3%). The number of Viabahn patients presenting with acute thrombosis was not defined. With the small number of limbs treated in the Viabahn group, the conclusions expressed cannot be statistically supported.
What about the in-vogue DES device?
Dr. Dake and his colleagues recently presented 5-year data on the Zilver DES platform at VIVA 2014. He reported a primary patency at 5 years of 66.4% showing superiority to angioplasty alone as well as angioplasty with provisional stenting. This study enrolled 479 patients into the randomization arm and also had a registry arm that although often included in reporting of patency, does not stand up to the scrutiny of peer review. Even though there were some patients with longer lesions, the randomized arm mean lesion length was only 66 mm, which does not compare to the published longer mean lesion length of the Viabahn device. Bosiers et al. (J. Cardiovasc. Surg. 2013 54:115-222) reviewed 135 patients treated with the Zilver device (a subgroup derived from the 787 patients enrolled in the registry data of the Zilver trial) with a mean lesion length of 226 mm. They reported 77.6% primary patency but only at 1 year. Again, however, this is registry derived data and does not have the scientific validity of a randomized trial.
So what can I conclude from these experiences? We know today that covered stent grafts have been widely used and reported on, including by Dr. Dake himself (Radiology 2000 October;217:95-104) and all appear to have had similar conclusions.
The mean lesion length treated in these studies of Viabahn is often longer than 15 cm and nearly all studies report primary patency outcomes. Zilver supporters on the other hand are prone to quote TLR which is an inferior endpoint (as recently noted in an editorial by Dr. Russell Samson (Vasc. Spec. 2015;11:2). Costs of both devices are an issue but may vary by region and institution. However, Viabahn does have the advantage of longer devices, compared with the Zilver (15 and 25 cm vs. 10 cm) so fewer devices may be required to treat long lesions. Although short lesions may be better addressed with BMS or DES, for longer SFA lesions over 12-15 cm there are very few truly comparable data that argue against the use of Viabahn.
Dr. Gable is chief of vascular and endovascular surgery at The Heart Hospital Baylor Plano (Tex.). He is also an associate medical editor for Vascular Specialist. He disclosed that he is a consultant, speaker, and receives research support from W. L. Gore and Medtronic.
Head-to-head comparisons are lacking, but similar results have been reported
BY MICHAEL D. DAKE, M.D.
Well, at least one thing is for sure – we would not have been having this discussion a mere 10 years ago.
I remained sheepishly silent for most of my early career as well-intentioned invasive and noninvasive specialists criticized the state of evidence supporting the legitimacy of endovascular interventions as a competitive strategy to manage infrainguinal peripheral arterial disease. Good data from well-controlled randomized clinical trials were not available to make a case for endovascular therapies.
Over the recent decade and a half, however, a number of contributing factors have influenced thinking and what we now consider standard of care for symptomatic disease of the superficial femoral artery (SFA). The proposal of an “endovascular first” interventional approach has evolved to a consensually agreed upon management strategy by all interested disciplines.
This did not occur on a whim. Rather, out of the shadows of relative ignorance there slowly emerged a welcomed accumulation of a large number of publications that detail the outcomes of a wide variety of randomized trials with a range of endovascular devices. This has allowed us to enter an era where valid comparisons between interventional therapies is not only possible, but allows us to more appropriately offer care to vascular patients with more nuanced strategies. These are strategies that recognize subtleties between subgroups of individuals stratified on the basis of patient demographics and lesion characteristics in a way not appreciated prior to the recent spate of endovascular device studies.
Thus, thanks to the dedication and hard work of many, we are now at a stage where we can have meaningful dialogues on a variety of endovascular topics, such as the one at hand, and proponents can argue their perspectives armed with objective evidence to support their positions. In this discussion regarding covered stent grafts and drug-eluting stents, we wish we had even more data.
Specifically, we are missing direct head-to-head comparisons between the two devices in patients with long SFA lesions. So, what do we know?

Here are some fundamental facts: The most commonly used covered stent graft for management of femoropopliteal occlusive disease is the Viabahn endoprosthesis (W. L. Gore and Associates, Flagstaff, Ariz.). The prosthesis is composed of a self-expanding nitinol stent framework and expanded polytetrafluoroethylene (ePTFE) graft with its surface lined with a coating of covalently bound heparin (Propaten bioactive surface).The only approved drug-eluting stent with significant safety and effectiveness data available is the Zilver PTX paclitaxel-eluting, self-expanding nitinol stent (Cook Medical Inc., Bloomington, Ind.).
Now in terms of the proposition, we need to discuss the meaning of the word “long” with reference to the SFA. Just what do we consider a long SFA lesion? I think all of us could agree that an arterial stenosis or occlusion of 6 cm or less is short. Lesions between 5 cm or 6 cm to 10 cm or 12 cm in length are moderately long, and disease greater than 10 cm or 12 cm is commonly characterized as long. Segments of disease greater than 20 cm long are typically considered very long or extremely long lesions from an endovascular interventional perspective.
So, how can currently available trial outcomes help us? Below, I have compiled a table that includes most of the recent clinical trial data for Viabahn and Zilver PTX in patients with long SFA occlusive disease.
OK, what can we honestly say about these data besides recognizing that we are at risk when we make any conclusions based upon cross-trial comparisons? Such an accounting of results is fraught with problems, but what we can say is that the table grossly confirms the current consensus that both devices enhance the standard of care for long lesions over traditional balloon angioplasty (PTA) and bare metal stent technologies.
Beyond this, however, it is accepted that patency results with Viabahn are lesion-length immune – that is, outcomes in long and extremely long segments of disease are not very different from the patency achieved in short lesions. This is clearly different than what is traditionally found for interventions with PTA or bare metal stents. There is not enough controlled data for extremely long lesions to reach a conclusion on drug-eluting stents; however, there is an initial suggestion that they behave in a manner more similar to stent grafts than traditional devices.
Grossly, the table suggests that the midterm and available greater than 1-year patency results with Viabahn and Zilver PTX are relatively comparable. What about the price of the device? What role does it play in our selection of the current most cost-effective endovascular strategy for long SFA lesions?
In my institution Viabahn is more expensive than Zilver PTX with a relative cost premium of about 30%-50% depending on the treatment length. Of course, when treating long TASC C and D lesions any up-front difference in the costs of the devices used initially is more than made up for by any relative reduction in subsequent reinterventions.
So, there you have it. Look at the table as simply a current snapshot. In the future, we will benefit from additional trials and comparisons, not to mention better endovascular technologies to address symptomatic long SFA lesions.
Dr. Dake is the Thelma and Henry Doelger Professor of Cardiovascular Surgery at the Stanford (Calif.) School of Medicine. He disclosed that he is a member of the Peripheral Scientific Advisory Board: Abbott Vascular Member, is on the Aortic Medical Advisory Board: W. L. Gore, is a consultant for Cook Medical, Medtronic, and Surmodics Research, and receives grants/clinical trial support from W. L. Gore, Medtronic, and Novate.
References for table
1. J. Vasc. Surg. 2013;58:386-95.
2. J. Vasc. Surg. 2010;52:584-90.
3. J. Vasc. Interv. Radiol. 2013;24:165-73.
4. Cardiovasc. Interv. Radiol. 2015;38:25-32.
5. Circ. Cardiovasc. Interv. 2011;4:495-504.
6. J. Am. Coll. Cardiol. 2013;61:2417-27.
7. J. Endovasc. Ther. 2011;18:613-23.
8. Zeller T. Oral presentations. 2014.
9. Yokoi H. Oral presentations. 2014.
Covered stent grafts in the SFA are still the endovascular champion in long lesions
BY DENNIS GABLE, M.D.
There remains a continued debate among investigators as to the best modality for treatment of stenosis/occlusion of the SFA especially with the recent advent of drug-eluting technology. However, I suggest that for long lesions over 15 cm only covered stent grafts continue to outperform the competition.
If we review the numerous studies available on treatment of SFA disease, the Viabahn-covered stent device (W. L. Gore & Associates, Flagstaff, Ariz.) is by far the most studied modality. There are currently 22 independent studies available providing data on 1,473 limbs. Several of these reports are multicenter studies and many of them are prospective randomized trials. Two of the most recent are the VIPER (J. Vasc. Intervent. Radiol. 2013;24:165-73) and VIASTAR study (JACC 2013;62:1320-27).
The VIPER study prospectively enrolled 119 patients (72 with TASC II C/D disease; mean lesion length 190 mm). Primary patency was reported at 73% at 1 year but in patients with less than 20% oversizing, as is recommended by the IFU, patency as high as 88% was noted. Additionally, there was no difference in patency in smaller-diameter vessels (5 mm) versus larger-diameter vessels (6-7 mm).
In a head-to-head randomized controlled trial of Viabahn to bare metal stents (BMS), the VIASTAR study enrolled 141 patients (72 in covered stent arm; mean lesion length 190 mm). On a per protocol evaluation, the patency at 1 year was 78% and 71% on an intention-to-treat evaluation with a patency of 70% and 63% respectively at 2 years. There was no statistical difference between the two evaluations on intention to treat vs. per protocol but there was clear superiority demonstrated against BMS.
Furthermore, in a prospective, randomized, head-to-head comparison of Viabahn to prosthetic above knee femoral popliteal bypass, it was shown that there was no difference in primary or secondary patency between the two groups out to 4 years follow-up (J. Vasc. Surg. 2010;52:584-91). This included an average lesion length of 25.6 cm with a primary and secondary patency of 59% and 74% in the Viabahn group and 58% and 71% in the surgery group. When compared to a large meta-analysis for femoral popliteal bypass outcomes reported on by Bates and AbuRahma in 2004 (J. Endovasc. Ther. 2004;11[suppl. II]:II-107–27), the patency for the surgical arm with prosthetic bypass in the above Viabahn study was similar at 4 years to the 38 peer-reviewed articles Bates et al. reviewed with over 4,000 limbs. The reported primary and secondary patency at 4 years for prosthetic femoral above knee popliteal bypass was 51% and 61%, respectively in his review. Although the above Viabahn study was not powered to formally demonstrate noninferiority to surgical bypass with prosthetic, it did strongly suggest and show just that.
How do we put these data together with the Zilver data and how do we decide what is best for our patients? Some operators have expressed concern over a perceived risk for a “higher rate of amputation” or “a worse Rutherford level of ischemia on presentation” if patients with the Viabahn stent graft occlude post procedure. Commonly, this results from extrapolation of prior studies looking at results of occlusion with an ePTFE bypass. In fact, review of peer-reviewed data reveal none of the prospective studies outlined above, or those currently available, demonstrate that either of these perceptions are true and there are no published prospective data that support these fears either. In the studies listed above as well as all current prospective studies available evaluating Viabahn usage, the highest rate of amputation reported was 5% by Fisher in 2006 with all of the remaining studies reporting an amputation rate of 2% or less (when reported). Moreover, it has not been demonstrated that patients with this device present with an increased level of ischemia secondary to sudden occlusion.
There is one report used to argue against the use of the Viabahn stent graft (J. Vasc. Surg. 2008;47:967-74). This study evaluated prospectively 109 patients (71 for claudication; 38 for critical limb ischemia) treated for SFA occlusive disease (mean lesion length 15.7 cm). Only 19 of the 109 patients (17%) were treated with Viabahn (17 for claudication; 2 for critical limb ischemia). The remaining limbs were treated with various other BMS devices (n=10). The authors concluded that patients initially treated with Viabahn who presented back with occlusion had a higher chance of presenting with acute symptoms (i.e., a worse Rutherford score). The lesion length treated in the Viabahn group, however, was nearly twice as long as all the other stent platforms combined (25.4 cm vs. 13.7 cm) and there was a higher level of tibial artery deterioration with thrombosis of the BMS group, compared with the Viabahn group (7.7% vs. 5.3%). The number of Viabahn patients presenting with acute thrombosis was not defined. With the small number of limbs treated in the Viabahn group, the conclusions expressed cannot be statistically supported.
What about the in-vogue DES device?
Dr. Dake and his colleagues recently presented 5-year data on the Zilver DES platform at VIVA 2014. He reported a primary patency at 5 years of 66.4% showing superiority to angioplasty alone as well as angioplasty with provisional stenting. This study enrolled 479 patients into the randomization arm and also had a registry arm that although often included in reporting of patency, does not stand up to the scrutiny of peer review. Even though there were some patients with longer lesions, the randomized arm mean lesion length was only 66 mm, which does not compare to the published longer mean lesion length of the Viabahn device. Bosiers et al. (J. Cardiovasc. Surg. 2013 54:115-222) reviewed 135 patients treated with the Zilver device (a subgroup derived from the 787 patients enrolled in the registry data of the Zilver trial) with a mean lesion length of 226 mm. They reported 77.6% primary patency but only at 1 year. Again, however, this is registry derived data and does not have the scientific validity of a randomized trial.
So what can I conclude from these experiences? We know today that covered stent grafts have been widely used and reported on, including by Dr. Dake himself (Radiology 2000 October;217:95-104) and all appear to have had similar conclusions.
The mean lesion length treated in these studies of Viabahn is often longer than 15 cm and nearly all studies report primary patency outcomes. Zilver supporters on the other hand are prone to quote TLR which is an inferior endpoint (as recently noted in an editorial by Dr. Russell Samson (Vasc. Spec. 2015;11:2). Costs of both devices are an issue but may vary by region and institution. However, Viabahn does have the advantage of longer devices, compared with the Zilver (15 and 25 cm vs. 10 cm) so fewer devices may be required to treat long lesions. Although short lesions may be better addressed with BMS or DES, for longer SFA lesions over 12-15 cm there are very few truly comparable data that argue against the use of Viabahn.
Dr. Gable is chief of vascular and endovascular surgery at The Heart Hospital Baylor Plano (Tex.). He is also an associate medical editor for Vascular Specialist. He disclosed that he is a consultant, speaker, and receives research support from W. L. Gore and Medtronic.
Head-to-head comparisons are lacking, but similar results have been reported
BY MICHAEL D. DAKE, M.D.
Well, at least one thing is for sure – we would not have been having this discussion a mere 10 years ago.
I remained sheepishly silent for most of my early career as well-intentioned invasive and noninvasive specialists criticized the state of evidence supporting the legitimacy of endovascular interventions as a competitive strategy to manage infrainguinal peripheral arterial disease. Good data from well-controlled randomized clinical trials were not available to make a case for endovascular therapies.
Over the recent decade and a half, however, a number of contributing factors have influenced thinking and what we now consider standard of care for symptomatic disease of the superficial femoral artery (SFA). The proposal of an “endovascular first” interventional approach has evolved to a consensually agreed upon management strategy by all interested disciplines.
This did not occur on a whim. Rather, out of the shadows of relative ignorance there slowly emerged a welcomed accumulation of a large number of publications that detail the outcomes of a wide variety of randomized trials with a range of endovascular devices. This has allowed us to enter an era where valid comparisons between interventional therapies is not only possible, but allows us to more appropriately offer care to vascular patients with more nuanced strategies. These are strategies that recognize subtleties between subgroups of individuals stratified on the basis of patient demographics and lesion characteristics in a way not appreciated prior to the recent spate of endovascular device studies.
Thus, thanks to the dedication and hard work of many, we are now at a stage where we can have meaningful dialogues on a variety of endovascular topics, such as the one at hand, and proponents can argue their perspectives armed with objective evidence to support their positions. In this discussion regarding covered stent grafts and drug-eluting stents, we wish we had even more data.
Specifically, we are missing direct head-to-head comparisons between the two devices in patients with long SFA lesions. So, what do we know?

Here are some fundamental facts: The most commonly used covered stent graft for management of femoropopliteal occlusive disease is the Viabahn endoprosthesis (W. L. Gore and Associates, Flagstaff, Ariz.). The prosthesis is composed of a self-expanding nitinol stent framework and expanded polytetrafluoroethylene (ePTFE) graft with its surface lined with a coating of covalently bound heparin (Propaten bioactive surface).The only approved drug-eluting stent with significant safety and effectiveness data available is the Zilver PTX paclitaxel-eluting, self-expanding nitinol stent (Cook Medical Inc., Bloomington, Ind.).
Now in terms of the proposition, we need to discuss the meaning of the word “long” with reference to the SFA. Just what do we consider a long SFA lesion? I think all of us could agree that an arterial stenosis or occlusion of 6 cm or less is short. Lesions between 5 cm or 6 cm to 10 cm or 12 cm in length are moderately long, and disease greater than 10 cm or 12 cm is commonly characterized as long. Segments of disease greater than 20 cm long are typically considered very long or extremely long lesions from an endovascular interventional perspective.
So, how can currently available trial outcomes help us? Below, I have compiled a table that includes most of the recent clinical trial data for Viabahn and Zilver PTX in patients with long SFA occlusive disease.
OK, what can we honestly say about these data besides recognizing that we are at risk when we make any conclusions based upon cross-trial comparisons? Such an accounting of results is fraught with problems, but what we can say is that the table grossly confirms the current consensus that both devices enhance the standard of care for long lesions over traditional balloon angioplasty (PTA) and bare metal stent technologies.
Beyond this, however, it is accepted that patency results with Viabahn are lesion-length immune – that is, outcomes in long and extremely long segments of disease are not very different from the patency achieved in short lesions. This is clearly different than what is traditionally found for interventions with PTA or bare metal stents. There is not enough controlled data for extremely long lesions to reach a conclusion on drug-eluting stents; however, there is an initial suggestion that they behave in a manner more similar to stent grafts than traditional devices.
Grossly, the table suggests that the midterm and available greater than 1-year patency results with Viabahn and Zilver PTX are relatively comparable. What about the price of the device? What role does it play in our selection of the current most cost-effective endovascular strategy for long SFA lesions?
In my institution Viabahn is more expensive than Zilver PTX with a relative cost premium of about 30%-50% depending on the treatment length. Of course, when treating long TASC C and D lesions any up-front difference in the costs of the devices used initially is more than made up for by any relative reduction in subsequent reinterventions.
So, there you have it. Look at the table as simply a current snapshot. In the future, we will benefit from additional trials and comparisons, not to mention better endovascular technologies to address symptomatic long SFA lesions.
Dr. Dake is the Thelma and Henry Doelger Professor of Cardiovascular Surgery at the Stanford (Calif.) School of Medicine. He disclosed that he is a member of the Peripheral Scientific Advisory Board: Abbott Vascular Member, is on the Aortic Medical Advisory Board: W. L. Gore, is a consultant for Cook Medical, Medtronic, and Surmodics Research, and receives grants/clinical trial support from W. L. Gore, Medtronic, and Novate.
References for table
1. J. Vasc. Surg. 2013;58:386-95.
2. J. Vasc. Surg. 2010;52:584-90.
3. J. Vasc. Interv. Radiol. 2013;24:165-73.
4. Cardiovasc. Interv. Radiol. 2015;38:25-32.
5. Circ. Cardiovasc. Interv. 2011;4:495-504.
6. J. Am. Coll. Cardiol. 2013;61:2417-27.
7. J. Endovasc. Ther. 2011;18:613-23.
8. Zeller T. Oral presentations. 2014.
9. Yokoi H. Oral presentations. 2014.
Covered stent grafts in the SFA are still the endovascular champion in long lesions
BY DENNIS GABLE, M.D.
There remains a continued debate among investigators as to the best modality for treatment of stenosis/occlusion of the SFA especially with the recent advent of drug-eluting technology. However, I suggest that for long lesions over 15 cm only covered stent grafts continue to outperform the competition.
If we review the numerous studies available on treatment of SFA disease, the Viabahn-covered stent device (W. L. Gore & Associates, Flagstaff, Ariz.) is by far the most studied modality. There are currently 22 independent studies available providing data on 1,473 limbs. Several of these reports are multicenter studies and many of them are prospective randomized trials. Two of the most recent are the VIPER (J. Vasc. Intervent. Radiol. 2013;24:165-73) and VIASTAR study (JACC 2013;62:1320-27).
The VIPER study prospectively enrolled 119 patients (72 with TASC II C/D disease; mean lesion length 190 mm). Primary patency was reported at 73% at 1 year but in patients with less than 20% oversizing, as is recommended by the IFU, patency as high as 88% was noted. Additionally, there was no difference in patency in smaller-diameter vessels (5 mm) versus larger-diameter vessels (6-7 mm).
In a head-to-head randomized controlled trial of Viabahn to bare metal stents (BMS), the VIASTAR study enrolled 141 patients (72 in covered stent arm; mean lesion length 190 mm). On a per protocol evaluation, the patency at 1 year was 78% and 71% on an intention-to-treat evaluation with a patency of 70% and 63% respectively at 2 years. There was no statistical difference between the two evaluations on intention to treat vs. per protocol but there was clear superiority demonstrated against BMS.
Furthermore, in a prospective, randomized, head-to-head comparison of Viabahn to prosthetic above knee femoral popliteal bypass, it was shown that there was no difference in primary or secondary patency between the two groups out to 4 years follow-up (J. Vasc. Surg. 2010;52:584-91). This included an average lesion length of 25.6 cm with a primary and secondary patency of 59% and 74% in the Viabahn group and 58% and 71% in the surgery group. When compared to a large meta-analysis for femoral popliteal bypass outcomes reported on by Bates and AbuRahma in 2004 (J. Endovasc. Ther. 2004;11[suppl. II]:II-107–27), the patency for the surgical arm with prosthetic bypass in the above Viabahn study was similar at 4 years to the 38 peer-reviewed articles Bates et al. reviewed with over 4,000 limbs. The reported primary and secondary patency at 4 years for prosthetic femoral above knee popliteal bypass was 51% and 61%, respectively in his review. Although the above Viabahn study was not powered to formally demonstrate noninferiority to surgical bypass with prosthetic, it did strongly suggest and show just that.
How do we put these data together with the Zilver data and how do we decide what is best for our patients? Some operators have expressed concern over a perceived risk for a “higher rate of amputation” or “a worse Rutherford level of ischemia on presentation” if patients with the Viabahn stent graft occlude post procedure. Commonly, this results from extrapolation of prior studies looking at results of occlusion with an ePTFE bypass. In fact, review of peer-reviewed data reveal none of the prospective studies outlined above, or those currently available, demonstrate that either of these perceptions are true and there are no published prospective data that support these fears either. In the studies listed above as well as all current prospective studies available evaluating Viabahn usage, the highest rate of amputation reported was 5% by Fisher in 2006 with all of the remaining studies reporting an amputation rate of 2% or less (when reported). Moreover, it has not been demonstrated that patients with this device present with an increased level of ischemia secondary to sudden occlusion.
There is one report used to argue against the use of the Viabahn stent graft (J. Vasc. Surg. 2008;47:967-74). This study evaluated prospectively 109 patients (71 for claudication; 38 for critical limb ischemia) treated for SFA occlusive disease (mean lesion length 15.7 cm). Only 19 of the 109 patients (17%) were treated with Viabahn (17 for claudication; 2 for critical limb ischemia). The remaining limbs were treated with various other BMS devices (n=10). The authors concluded that patients initially treated with Viabahn who presented back with occlusion had a higher chance of presenting with acute symptoms (i.e., a worse Rutherford score). The lesion length treated in the Viabahn group, however, was nearly twice as long as all the other stent platforms combined (25.4 cm vs. 13.7 cm) and there was a higher level of tibial artery deterioration with thrombosis of the BMS group, compared with the Viabahn group (7.7% vs. 5.3%). The number of Viabahn patients presenting with acute thrombosis was not defined. With the small number of limbs treated in the Viabahn group, the conclusions expressed cannot be statistically supported.
What about the in-vogue DES device?
Dr. Dake and his colleagues recently presented 5-year data on the Zilver DES platform at VIVA 2014. He reported a primary patency at 5 years of 66.4% showing superiority to angioplasty alone as well as angioplasty with provisional stenting. This study enrolled 479 patients into the randomization arm and also had a registry arm that although often included in reporting of patency, does not stand up to the scrutiny of peer review. Even though there were some patients with longer lesions, the randomized arm mean lesion length was only 66 mm, which does not compare to the published longer mean lesion length of the Viabahn device. Bosiers et al. (J. Cardiovasc. Surg. 2013 54:115-222) reviewed 135 patients treated with the Zilver device (a subgroup derived from the 787 patients enrolled in the registry data of the Zilver trial) with a mean lesion length of 226 mm. They reported 77.6% primary patency but only at 1 year. Again, however, this is registry derived data and does not have the scientific validity of a randomized trial.
So what can I conclude from these experiences? We know today that covered stent grafts have been widely used and reported on, including by Dr. Dake himself (Radiology 2000 October;217:95-104) and all appear to have had similar conclusions.
The mean lesion length treated in these studies of Viabahn is often longer than 15 cm and nearly all studies report primary patency outcomes. Zilver supporters on the other hand are prone to quote TLR which is an inferior endpoint (as recently noted in an editorial by Dr. Russell Samson (Vasc. Spec. 2015;11:2). Costs of both devices are an issue but may vary by region and institution. However, Viabahn does have the advantage of longer devices, compared with the Zilver (15 and 25 cm vs. 10 cm) so fewer devices may be required to treat long lesions. Although short lesions may be better addressed with BMS or DES, for longer SFA lesions over 12-15 cm there are very few truly comparable data that argue against the use of Viabahn.
Dr. Gable is chief of vascular and endovascular surgery at The Heart Hospital Baylor Plano (Tex.). He is also an associate medical editor for Vascular Specialist. He disclosed that he is a consultant, speaker, and receives research support from W. L. Gore and Medtronic.
POINT/COUNTERPOINT: Renal artery occlusive disease – To treat or not to treat? ASTRAL and CORAL trials show no indication to treat percutaneously. There are still indications to treat renal artery occlusive disease.
Percutaneous treatment of renal artery occlusive disease is unnecessary and should be abandoned, except in pediatric cases.
BY GEORGE HAMILTON, M.D.
This position is supported by findings from both the ASTRAL trial (N. Engl. J. Med. 2009;361:1953-62) and the CORAL trial (N. Engl. J. Med. 2013 Nov. 18 [doi:10.1056/NEJMoa1310753]).
The ASTRAL trial, a prospective, randomized comparison of best medical therapy with and without stent angioplasty in more than 800 patients, was the largest trial to date when it began back in the 1990s. The well-known results showed no difference in time to first renal event, first vascular and cardiovascular events, and overall survival. Furthermore, there was no difference in these outcomes among patients with greater than 90% stenosis, with the exception of a possible difference in mortality, which trended toward improvement among those with high-grade stenosis.
We concluded that revascularization in the vast majority of patients is unlikely to improve hypertension control or renal function, and that renal artery stenosis is not pathophysiologically important. We also concluded that there is no point in screening for asymptomatic disease; this was back when every patient was getting screened, and treated primarily on the basis of finding a renal arterial stenosis.
Finally, we concluded that properly applied best medical therapy alone was an extremely good treatment.
Several flaws in the trial garnered extensive criticism, however, and the more rigidly designed CORAL trial was expected to address them. The findings confirmed those of the ASTRAL trial. In more than 900 patients from 88 centers, there was absolutely no benefit of intervention with respect to primary and secondary outcomes, including among those with high-grade stenosis.
We can now see on the basis of extensive level 1 evidence that when added to comprehensive, multifactorial medical therapy, intervention yielded no benefit.
So are there certain patient groups who might benefit more from intervention? Among listed indications are high-grade stenosis (which doesn’t apply any longer); short history of progressive failure (which is quite rare); ACE-induced renal failure (which is also quite rare); difficult-to-control hypertension (there really is no such thing now, except in a tiny percentage of patients); and – the least challenged indication – flash pulmonary edema. These remaining indications move our interventions into a very high risk group of patients.
The current debate is focused almost entirely on endovascular intervention, but a systematic review showed that there is long-term benefit in terms of renal function and hypertension with open procedures. Although overall there is increased mortality, this risk is minimized – and not significantly different from endovascular procedures – in those having only renal revascularization vs. those having concomitant aortic procedures. So open surgery remains a possible treatment option, indeed a recent level 1 study comparing stenting and open surgery, showed better long-term results with open surgery (J. Vasc. Surg. 2009;49:667-75). The authors concluded that surgical reconstruction remains the gold standard in treating renal artery stenosis. Although national data suggest an overall mortality of about 10%, it is much lower at specialist, high-volume centers with mortality rates similar to those of stent angioplasty.
Renal stenting is not a low-risk procedure. In all-comers the complication rates, serious complication rates, and mortality rates are significant with short-term equivalence between focused renal arterial surgery and percutaneous intervention.
Returning to the debate, are either methods of revascularization appropriate? Probably not.
Even in flash pulmonary edema, there is little evidence to support revascularization. Few papers exist suggesting a benefit of revascularization in reduction of flash pulmonary edema, but the patient numbers were small, and there was no benefit in terms of preservation of renal function.
The history of evolution and evaluation of the role of renal revascularization is remarkably similar to that of renal denervation, initially and with considerable conviction thought to be a cure for hypertension. However, when properly assessed by prospective randomized comparison there was found to be absolutely no benefit.
So, given the considerable objective evidence from two major trials and revisiting the basics of the pathophysiology of atherosclerotic renovascular disease, to expect benefit from treating the osteal component of renal artery occlusive disease is at best naive, in my opinion. There remains little clinical evidence of benefit for any indication, with the possible exceptions of ACE-induced renal failure and possibly flash pulmonary edema in the presence of bilateral renal arterial stenoses.
Dr. Hamilton is a professor at the Royal Free London Hospital, University College London, United Kingdom.
There are still indications to treat renal artery occlusive disease
BY MATTHEW A. CORRIERE, M.D.
Although renal artery revascularization has been grossly overutilized and is not indicated in the majority of patients with renal artery stenosis, I perform renal artery revascularization as part of my routine clinical practice and believe that there are many instances where revascularization should be considered, particularly when patients have severe symptoms despite aggressive medical therapy. While neither ASTRAL nor CORAL observed any benefit associated with revascularization, both have important limitations that should be kept in mind when interpreting the results of these trials.
These limitations can be broadly categorized as mismatch between indications for revascularization and clinical endpoints, selection biases favoring enrollment of patients with relatively mild symptoms, and inconsistencies between study protocols and contemporary decision-making strategies.
Given that ASTRAL’s primary outcome was change in renal function (defined by a 20% or greater reduction in the mean slope of the reciprocal of serum creatinine), it is important to remember that the inclusion criteria were renal artery stenosis with unexplained renal dysfunction or poorly controlled hypertension. Patients who had hypertension in the absence of significant renal dysfunction were therefore eligible, and 40% of the randomized participants had preserved baseline renal function (based on a serum creatinine of < 150 micromol/liter). Unlike patients with baseline renal dysfunction (which, in theory, might improve with revascularization), these patients with normal renal function who were treated with revascularization risked decline in renal function resulting from procedure-related adverse events without any real chance of renal function improvement. It would certainly be difficult to justify revascularization for the sake of renal function salvage in these patients, and their inclusion within a randomized trial with change in renal function as its primary outcome is problematic for the same reason.
ASTRAL also had an additional, somewhat unorthodox inclusion criterion: uncertainty on the part of the treating physician that the patient “definitely would have a worthwhile clinical benefit from revascularization.” Exclusion of patients considered likely to benefit from revascularization would seem to ensure a selection bias favoring the null hypothesis; this approach may also explain the large proportion of participants with relatively mild occlusive disease (40% had stenotic lesions that were < 70% in severity).
A high rate of both technical failure (12%) and adverse events (20%) associated with revascularization, asymmetric crossover between treatment groups (86 of the 110 patients who did not receive their randomized intervention were in the revascularization group), and lack of standardized protocol for medical therapy further limit the conclusions that can be drawn from the ASTRAL results.
Although this trial does not provide us with compelling evidence that renal revascularization should be abandoned for patients failing appropriate medical therapy, ASTRAL demonstrated that no benefit should be expected from nonselective use of revascularization, which can be associated with significant rates of both technical failure and major adverse events.
The CORAL trial overcame many of the design limitations for which ASTRAL drew criticism. CORAL’s primary endpoint (freedom from major adverse cardiovascular or renal events) allowed potential benefit for participants with either systolic hypertension or chronic kidney disease as their indication for treatment. Although participants with systolic hypertension as their inclusion criterion had to be on at least two antihypertensive medications, it is important to acknowledge the growing number of indications for these medications related to cardiovascular risk reduction in the setting of diabetes, heart disease, and other diagnoses that may be unrelated to any specific blood pressure target. Number of antihypertensive medications is therefore often a crude and potentially invalid indicator of hypertension severity or control.
In CORAL, the initial hypertension inclusion criterion of 155 mm Hg was subsequently abandoned during the trial, suggesting that hypertension in many of these patients may have been mild and/or well controlled. Although medical therapy in CORAL was standardized, it also is notable that all patients had their medical therapy adjusted prior to randomization during a roll-in phase to achieve target blood pressure goals of 130/80 in patients with CKD and/or diabetes or 140/90 otherwise. I would suggest that achievement of these blood pressure targets on the study medications (candesartan ± hydrochlorothiazide plus amlodipine-atorvastatin) might be appropriately considered success of medical therapy for patients with hypertension in the absence of renal dysfunction, making it challenging to defend proceeding with revascularization in this scenario.
The study protocol, although well designed from the perspective of attempting to isolate the effect of renal artery angioplasty and stenting, therefore did not uniformly reflect what would be considered responsible utilization of renal revascularization in a real-world environment.
Patient enrollment in CORAL was also very selective; only 947 of the 5,322 patients who were screened went on to be enrolled and randomized. It is likely that at least some of those patients who were not enrolled (especially those who declined to participate or were withdrawn by their physicians) were failing aggressive medical therapy and therefore unwilling to being excluded from angioplasty and stenting through randomization. These limitations aside, however, CORAL does provide some very useful observations that should inform treatment decisions. The results demonstrate the efficacy of contemporary medical therapy for many patients, and show that revascularization offers no additional benefit when medical therapy achieves an acceptable clinical response (defined by stable renal function and reasonable blood pressure control). Additional subgroup analyses of the CORAL data are anticipated, but will likely be underpowered to draw conclusions in the absence of identified revascularization effects.
So when should revascularization be considered for patients with atherosclerotic renal artery stenosis? In general, medical therapy is adequate for most patients and should be implemented prior to any consideration of procedural intervention. Revascularization should be considered only for patients who have failed appropriate, aggressive medical therapy; the medications used in CORAL can certainly be regarded as adequate initial therapy for symptomatic renal artery stenosis, but many providers (including myself) would argue that additional agents should be considered before proceeding with revascularization.
When decline in renal function is the indication for considering revascularization, alternative causes (such as intrinsic renal disease) should diminish enthusiasm for proceeding with angioplasty and stenting, particularly when the anatomic disease distribution does not affect the entire renal mass (as in patients with two kidneys and unilateral stenosis). Appropriate candidates for revascularization include patients with severely impaired renal function (particularly in the setting of a precipitous functional decline) or severe acute blood pressure elevation associated with hypertensive emergency (such as acute congestive heart failure, encephalopathy, acute coronary syndrome, or other signs and symptoms of target organ damage resulting from hypertension and/or volume overload). Continuation of failed medical therapy is often unacceptable to these “no-options” patients as well as their providers, both of whom presumably would be unlikely to accept randomization to ongoing medical management.
Other populations that are not represented within these trials include patients with renal artery restenosis and those with nonatherosclerotic disease; it is therefore important to exercise caution when generalizing these study results to these distinct groups of patients. Enrolling patients with severe symptoms who have failed medical therapy will likely remain challenging for future randomized studies in the absence of alternative treatment options. Although the benefits of renal angioplasty and stenting for these “no-options” patients remain to be proved, the uncertainty of response to revascularization is often easier to accept than the ongoing morbidity and mortality associated with staying the course when medical therapy has failed.
Dr. Matthew A. Corriere is a vascular surgeon at Wake Forest University School of Medicine, Winston-Salem, N.C.
This article developed from a debate held at the 2014 Vascular Annual Meeting.
Percutaneous treatment of renal artery occlusive disease is unnecessary and should be abandoned, except in pediatric cases.
BY GEORGE HAMILTON, M.D.
This position is supported by findings from both the ASTRAL trial (N. Engl. J. Med. 2009;361:1953-62) and the CORAL trial (N. Engl. J. Med. 2013 Nov. 18 [doi:10.1056/NEJMoa1310753]).
The ASTRAL trial, a prospective, randomized comparison of best medical therapy with and without stent angioplasty in more than 800 patients, was the largest trial to date when it began back in the 1990s. The well-known results showed no difference in time to first renal event, first vascular and cardiovascular events, and overall survival. Furthermore, there was no difference in these outcomes among patients with greater than 90% stenosis, with the exception of a possible difference in mortality, which trended toward improvement among those with high-grade stenosis.
We concluded that revascularization in the vast majority of patients is unlikely to improve hypertension control or renal function, and that renal artery stenosis is not pathophysiologically important. We also concluded that there is no point in screening for asymptomatic disease; this was back when every patient was getting screened, and treated primarily on the basis of finding a renal arterial stenosis.
Finally, we concluded that properly applied best medical therapy alone was an extremely good treatment.
Several flaws in the trial garnered extensive criticism, however, and the more rigidly designed CORAL trial was expected to address them. The findings confirmed those of the ASTRAL trial. In more than 900 patients from 88 centers, there was absolutely no benefit of intervention with respect to primary and secondary outcomes, including among those with high-grade stenosis.
We can now see on the basis of extensive level 1 evidence that when added to comprehensive, multifactorial medical therapy, intervention yielded no benefit.
So are there certain patient groups who might benefit more from intervention? Among listed indications are high-grade stenosis (which doesn’t apply any longer); short history of progressive failure (which is quite rare); ACE-induced renal failure (which is also quite rare); difficult-to-control hypertension (there really is no such thing now, except in a tiny percentage of patients); and – the least challenged indication – flash pulmonary edema. These remaining indications move our interventions into a very high risk group of patients.
The current debate is focused almost entirely on endovascular intervention, but a systematic review showed that there is long-term benefit in terms of renal function and hypertension with open procedures. Although overall there is increased mortality, this risk is minimized – and not significantly different from endovascular procedures – in those having only renal revascularization vs. those having concomitant aortic procedures. So open surgery remains a possible treatment option, indeed a recent level 1 study comparing stenting and open surgery, showed better long-term results with open surgery (J. Vasc. Surg. 2009;49:667-75). The authors concluded that surgical reconstruction remains the gold standard in treating renal artery stenosis. Although national data suggest an overall mortality of about 10%, it is much lower at specialist, high-volume centers with mortality rates similar to those of stent angioplasty.
Renal stenting is not a low-risk procedure. In all-comers the complication rates, serious complication rates, and mortality rates are significant with short-term equivalence between focused renal arterial surgery and percutaneous intervention.
Returning to the debate, are either methods of revascularization appropriate? Probably not.
Even in flash pulmonary edema, there is little evidence to support revascularization. Few papers exist suggesting a benefit of revascularization in reduction of flash pulmonary edema, but the patient numbers were small, and there was no benefit in terms of preservation of renal function.
The history of evolution and evaluation of the role of renal revascularization is remarkably similar to that of renal denervation, initially and with considerable conviction thought to be a cure for hypertension. However, when properly assessed by prospective randomized comparison there was found to be absolutely no benefit.
So, given the considerable objective evidence from two major trials and revisiting the basics of the pathophysiology of atherosclerotic renovascular disease, to expect benefit from treating the osteal component of renal artery occlusive disease is at best naive, in my opinion. There remains little clinical evidence of benefit for any indication, with the possible exceptions of ACE-induced renal failure and possibly flash pulmonary edema in the presence of bilateral renal arterial stenoses.
Dr. Hamilton is a professor at the Royal Free London Hospital, University College London, United Kingdom.
There are still indications to treat renal artery occlusive disease
BY MATTHEW A. CORRIERE, M.D.
Although renal artery revascularization has been grossly overutilized and is not indicated in the majority of patients with renal artery stenosis, I perform renal artery revascularization as part of my routine clinical practice and believe that there are many instances where revascularization should be considered, particularly when patients have severe symptoms despite aggressive medical therapy. While neither ASTRAL nor CORAL observed any benefit associated with revascularization, both have important limitations that should be kept in mind when interpreting the results of these trials.
These limitations can be broadly categorized as mismatch between indications for revascularization and clinical endpoints, selection biases favoring enrollment of patients with relatively mild symptoms, and inconsistencies between study protocols and contemporary decision-making strategies.
Given that ASTRAL’s primary outcome was change in renal function (defined by a 20% or greater reduction in the mean slope of the reciprocal of serum creatinine), it is important to remember that the inclusion criteria were renal artery stenosis with unexplained renal dysfunction or poorly controlled hypertension. Patients who had hypertension in the absence of significant renal dysfunction were therefore eligible, and 40% of the randomized participants had preserved baseline renal function (based on a serum creatinine of < 150 micromol/liter). Unlike patients with baseline renal dysfunction (which, in theory, might improve with revascularization), these patients with normal renal function who were treated with revascularization risked decline in renal function resulting from procedure-related adverse events without any real chance of renal function improvement. It would certainly be difficult to justify revascularization for the sake of renal function salvage in these patients, and their inclusion within a randomized trial with change in renal function as its primary outcome is problematic for the same reason.
ASTRAL also had an additional, somewhat unorthodox inclusion criterion: uncertainty on the part of the treating physician that the patient “definitely would have a worthwhile clinical benefit from revascularization.” Exclusion of patients considered likely to benefit from revascularization would seem to ensure a selection bias favoring the null hypothesis; this approach may also explain the large proportion of participants with relatively mild occlusive disease (40% had stenotic lesions that were < 70% in severity).
A high rate of both technical failure (12%) and adverse events (20%) associated with revascularization, asymmetric crossover between treatment groups (86 of the 110 patients who did not receive their randomized intervention were in the revascularization group), and lack of standardized protocol for medical therapy further limit the conclusions that can be drawn from the ASTRAL results.
Although this trial does not provide us with compelling evidence that renal revascularization should be abandoned for patients failing appropriate medical therapy, ASTRAL demonstrated that no benefit should be expected from nonselective use of revascularization, which can be associated with significant rates of both technical failure and major adverse events.
The CORAL trial overcame many of the design limitations for which ASTRAL drew criticism. CORAL’s primary endpoint (freedom from major adverse cardiovascular or renal events) allowed potential benefit for participants with either systolic hypertension or chronic kidney disease as their indication for treatment. Although participants with systolic hypertension as their inclusion criterion had to be on at least two antihypertensive medications, it is important to acknowledge the growing number of indications for these medications related to cardiovascular risk reduction in the setting of diabetes, heart disease, and other diagnoses that may be unrelated to any specific blood pressure target. Number of antihypertensive medications is therefore often a crude and potentially invalid indicator of hypertension severity or control.
In CORAL, the initial hypertension inclusion criterion of 155 mm Hg was subsequently abandoned during the trial, suggesting that hypertension in many of these patients may have been mild and/or well controlled. Although medical therapy in CORAL was standardized, it also is notable that all patients had their medical therapy adjusted prior to randomization during a roll-in phase to achieve target blood pressure goals of 130/80 in patients with CKD and/or diabetes or 140/90 otherwise. I would suggest that achievement of these blood pressure targets on the study medications (candesartan ± hydrochlorothiazide plus amlodipine-atorvastatin) might be appropriately considered success of medical therapy for patients with hypertension in the absence of renal dysfunction, making it challenging to defend proceeding with revascularization in this scenario.
The study protocol, although well designed from the perspective of attempting to isolate the effect of renal artery angioplasty and stenting, therefore did not uniformly reflect what would be considered responsible utilization of renal revascularization in a real-world environment.
Patient enrollment in CORAL was also very selective; only 947 of the 5,322 patients who were screened went on to be enrolled and randomized. It is likely that at least some of those patients who were not enrolled (especially those who declined to participate or were withdrawn by their physicians) were failing aggressive medical therapy and therefore unwilling to being excluded from angioplasty and stenting through randomization. These limitations aside, however, CORAL does provide some very useful observations that should inform treatment decisions. The results demonstrate the efficacy of contemporary medical therapy for many patients, and show that revascularization offers no additional benefit when medical therapy achieves an acceptable clinical response (defined by stable renal function and reasonable blood pressure control). Additional subgroup analyses of the CORAL data are anticipated, but will likely be underpowered to draw conclusions in the absence of identified revascularization effects.
So when should revascularization be considered for patients with atherosclerotic renal artery stenosis? In general, medical therapy is adequate for most patients and should be implemented prior to any consideration of procedural intervention. Revascularization should be considered only for patients who have failed appropriate, aggressive medical therapy; the medications used in CORAL can certainly be regarded as adequate initial therapy for symptomatic renal artery stenosis, but many providers (including myself) would argue that additional agents should be considered before proceeding with revascularization.
When decline in renal function is the indication for considering revascularization, alternative causes (such as intrinsic renal disease) should diminish enthusiasm for proceeding with angioplasty and stenting, particularly when the anatomic disease distribution does not affect the entire renal mass (as in patients with two kidneys and unilateral stenosis). Appropriate candidates for revascularization include patients with severely impaired renal function (particularly in the setting of a precipitous functional decline) or severe acute blood pressure elevation associated with hypertensive emergency (such as acute congestive heart failure, encephalopathy, acute coronary syndrome, or other signs and symptoms of target organ damage resulting from hypertension and/or volume overload). Continuation of failed medical therapy is often unacceptable to these “no-options” patients as well as their providers, both of whom presumably would be unlikely to accept randomization to ongoing medical management.
Other populations that are not represented within these trials include patients with renal artery restenosis and those with nonatherosclerotic disease; it is therefore important to exercise caution when generalizing these study results to these distinct groups of patients. Enrolling patients with severe symptoms who have failed medical therapy will likely remain challenging for future randomized studies in the absence of alternative treatment options. Although the benefits of renal angioplasty and stenting for these “no-options” patients remain to be proved, the uncertainty of response to revascularization is often easier to accept than the ongoing morbidity and mortality associated with staying the course when medical therapy has failed.
Dr. Matthew A. Corriere is a vascular surgeon at Wake Forest University School of Medicine, Winston-Salem, N.C.
This article developed from a debate held at the 2014 Vascular Annual Meeting.
Percutaneous treatment of renal artery occlusive disease is unnecessary and should be abandoned, except in pediatric cases.
BY GEORGE HAMILTON, M.D.
This position is supported by findings from both the ASTRAL trial (N. Engl. J. Med. 2009;361:1953-62) and the CORAL trial (N. Engl. J. Med. 2013 Nov. 18 [doi:10.1056/NEJMoa1310753]).
The ASTRAL trial, a prospective, randomized comparison of best medical therapy with and without stent angioplasty in more than 800 patients, was the largest trial to date when it began back in the 1990s. The well-known results showed no difference in time to first renal event, first vascular and cardiovascular events, and overall survival. Furthermore, there was no difference in these outcomes among patients with greater than 90% stenosis, with the exception of a possible difference in mortality, which trended toward improvement among those with high-grade stenosis.
We concluded that revascularization in the vast majority of patients is unlikely to improve hypertension control or renal function, and that renal artery stenosis is not pathophysiologically important. We also concluded that there is no point in screening for asymptomatic disease; this was back when every patient was getting screened, and treated primarily on the basis of finding a renal arterial stenosis.
Finally, we concluded that properly applied best medical therapy alone was an extremely good treatment.
Several flaws in the trial garnered extensive criticism, however, and the more rigidly designed CORAL trial was expected to address them. The findings confirmed those of the ASTRAL trial. In more than 900 patients from 88 centers, there was absolutely no benefit of intervention with respect to primary and secondary outcomes, including among those with high-grade stenosis.
We can now see on the basis of extensive level 1 evidence that when added to comprehensive, multifactorial medical therapy, intervention yielded no benefit.
So are there certain patient groups who might benefit more from intervention? Among listed indications are high-grade stenosis (which doesn’t apply any longer); short history of progressive failure (which is quite rare); ACE-induced renal failure (which is also quite rare); difficult-to-control hypertension (there really is no such thing now, except in a tiny percentage of patients); and – the least challenged indication – flash pulmonary edema. These remaining indications move our interventions into a very high risk group of patients.
The current debate is focused almost entirely on endovascular intervention, but a systematic review showed that there is long-term benefit in terms of renal function and hypertension with open procedures. Although overall there is increased mortality, this risk is minimized – and not significantly different from endovascular procedures – in those having only renal revascularization vs. those having concomitant aortic procedures. So open surgery remains a possible treatment option, indeed a recent level 1 study comparing stenting and open surgery, showed better long-term results with open surgery (J. Vasc. Surg. 2009;49:667-75). The authors concluded that surgical reconstruction remains the gold standard in treating renal artery stenosis. Although national data suggest an overall mortality of about 10%, it is much lower at specialist, high-volume centers with mortality rates similar to those of stent angioplasty.
Renal stenting is not a low-risk procedure. In all-comers the complication rates, serious complication rates, and mortality rates are significant with short-term equivalence between focused renal arterial surgery and percutaneous intervention.
Returning to the debate, are either methods of revascularization appropriate? Probably not.
Even in flash pulmonary edema, there is little evidence to support revascularization. Few papers exist suggesting a benefit of revascularization in reduction of flash pulmonary edema, but the patient numbers were small, and there was no benefit in terms of preservation of renal function.
The history of evolution and evaluation of the role of renal revascularization is remarkably similar to that of renal denervation, initially and with considerable conviction thought to be a cure for hypertension. However, when properly assessed by prospective randomized comparison there was found to be absolutely no benefit.
So, given the considerable objective evidence from two major trials and revisiting the basics of the pathophysiology of atherosclerotic renovascular disease, to expect benefit from treating the osteal component of renal artery occlusive disease is at best naive, in my opinion. There remains little clinical evidence of benefit for any indication, with the possible exceptions of ACE-induced renal failure and possibly flash pulmonary edema in the presence of bilateral renal arterial stenoses.
Dr. Hamilton is a professor at the Royal Free London Hospital, University College London, United Kingdom.
There are still indications to treat renal artery occlusive disease
BY MATTHEW A. CORRIERE, M.D.
Although renal artery revascularization has been grossly overutilized and is not indicated in the majority of patients with renal artery stenosis, I perform renal artery revascularization as part of my routine clinical practice and believe that there are many instances where revascularization should be considered, particularly when patients have severe symptoms despite aggressive medical therapy. While neither ASTRAL nor CORAL observed any benefit associated with revascularization, both have important limitations that should be kept in mind when interpreting the results of these trials.
These limitations can be broadly categorized as mismatch between indications for revascularization and clinical endpoints, selection biases favoring enrollment of patients with relatively mild symptoms, and inconsistencies between study protocols and contemporary decision-making strategies.
Given that ASTRAL’s primary outcome was change in renal function (defined by a 20% or greater reduction in the mean slope of the reciprocal of serum creatinine), it is important to remember that the inclusion criteria were renal artery stenosis with unexplained renal dysfunction or poorly controlled hypertension. Patients who had hypertension in the absence of significant renal dysfunction were therefore eligible, and 40% of the randomized participants had preserved baseline renal function (based on a serum creatinine of < 150 micromol/liter). Unlike patients with baseline renal dysfunction (which, in theory, might improve with revascularization), these patients with normal renal function who were treated with revascularization risked decline in renal function resulting from procedure-related adverse events without any real chance of renal function improvement. It would certainly be difficult to justify revascularization for the sake of renal function salvage in these patients, and their inclusion within a randomized trial with change in renal function as its primary outcome is problematic for the same reason.
ASTRAL also had an additional, somewhat unorthodox inclusion criterion: uncertainty on the part of the treating physician that the patient “definitely would have a worthwhile clinical benefit from revascularization.” Exclusion of patients considered likely to benefit from revascularization would seem to ensure a selection bias favoring the null hypothesis; this approach may also explain the large proportion of participants with relatively mild occlusive disease (40% had stenotic lesions that were < 70% in severity).
A high rate of both technical failure (12%) and adverse events (20%) associated with revascularization, asymmetric crossover between treatment groups (86 of the 110 patients who did not receive their randomized intervention were in the revascularization group), and lack of standardized protocol for medical therapy further limit the conclusions that can be drawn from the ASTRAL results.
Although this trial does not provide us with compelling evidence that renal revascularization should be abandoned for patients failing appropriate medical therapy, ASTRAL demonstrated that no benefit should be expected from nonselective use of revascularization, which can be associated with significant rates of both technical failure and major adverse events.
The CORAL trial overcame many of the design limitations for which ASTRAL drew criticism. CORAL’s primary endpoint (freedom from major adverse cardiovascular or renal events) allowed potential benefit for participants with either systolic hypertension or chronic kidney disease as their indication for treatment. Although participants with systolic hypertension as their inclusion criterion had to be on at least two antihypertensive medications, it is important to acknowledge the growing number of indications for these medications related to cardiovascular risk reduction in the setting of diabetes, heart disease, and other diagnoses that may be unrelated to any specific blood pressure target. Number of antihypertensive medications is therefore often a crude and potentially invalid indicator of hypertension severity or control.
In CORAL, the initial hypertension inclusion criterion of 155 mm Hg was subsequently abandoned during the trial, suggesting that hypertension in many of these patients may have been mild and/or well controlled. Although medical therapy in CORAL was standardized, it also is notable that all patients had their medical therapy adjusted prior to randomization during a roll-in phase to achieve target blood pressure goals of 130/80 in patients with CKD and/or diabetes or 140/90 otherwise. I would suggest that achievement of these blood pressure targets on the study medications (candesartan ± hydrochlorothiazide plus amlodipine-atorvastatin) might be appropriately considered success of medical therapy for patients with hypertension in the absence of renal dysfunction, making it challenging to defend proceeding with revascularization in this scenario.
The study protocol, although well designed from the perspective of attempting to isolate the effect of renal artery angioplasty and stenting, therefore did not uniformly reflect what would be considered responsible utilization of renal revascularization in a real-world environment.
Patient enrollment in CORAL was also very selective; only 947 of the 5,322 patients who were screened went on to be enrolled and randomized. It is likely that at least some of those patients who were not enrolled (especially those who declined to participate or were withdrawn by their physicians) were failing aggressive medical therapy and therefore unwilling to being excluded from angioplasty and stenting through randomization. These limitations aside, however, CORAL does provide some very useful observations that should inform treatment decisions. The results demonstrate the efficacy of contemporary medical therapy for many patients, and show that revascularization offers no additional benefit when medical therapy achieves an acceptable clinical response (defined by stable renal function and reasonable blood pressure control). Additional subgroup analyses of the CORAL data are anticipated, but will likely be underpowered to draw conclusions in the absence of identified revascularization effects.
So when should revascularization be considered for patients with atherosclerotic renal artery stenosis? In general, medical therapy is adequate for most patients and should be implemented prior to any consideration of procedural intervention. Revascularization should be considered only for patients who have failed appropriate, aggressive medical therapy; the medications used in CORAL can certainly be regarded as adequate initial therapy for symptomatic renal artery stenosis, but many providers (including myself) would argue that additional agents should be considered before proceeding with revascularization.
When decline in renal function is the indication for considering revascularization, alternative causes (such as intrinsic renal disease) should diminish enthusiasm for proceeding with angioplasty and stenting, particularly when the anatomic disease distribution does not affect the entire renal mass (as in patients with two kidneys and unilateral stenosis). Appropriate candidates for revascularization include patients with severely impaired renal function (particularly in the setting of a precipitous functional decline) or severe acute blood pressure elevation associated with hypertensive emergency (such as acute congestive heart failure, encephalopathy, acute coronary syndrome, or other signs and symptoms of target organ damage resulting from hypertension and/or volume overload). Continuation of failed medical therapy is often unacceptable to these “no-options” patients as well as their providers, both of whom presumably would be unlikely to accept randomization to ongoing medical management.
Other populations that are not represented within these trials include patients with renal artery restenosis and those with nonatherosclerotic disease; it is therefore important to exercise caution when generalizing these study results to these distinct groups of patients. Enrolling patients with severe symptoms who have failed medical therapy will likely remain challenging for future randomized studies in the absence of alternative treatment options. Although the benefits of renal angioplasty and stenting for these “no-options” patients remain to be proved, the uncertainty of response to revascularization is often easier to accept than the ongoing morbidity and mortality associated with staying the course when medical therapy has failed.
Dr. Matthew A. Corriere is a vascular surgeon at Wake Forest University School of Medicine, Winston-Salem, N.C.
This article developed from a debate held at the 2014 Vascular Annual Meeting.
POINT/COUNTERPOINT: Asymptomatic carotid stenosis: medical treatment, CEA, or CAS?
POINT: Medical treatment ends need for CEA or CAS.
By Anne L. Abbott, M.D.
The medical profession and the wider community must be congratulated upon their sustained efforts over recent decades that have seen an 80% fall in the average annual risk of stroke associated with moderate and severe (50%-99%) asymptomatic carotid stenosis (ACS).1-6 This has been achieved by better medical treatment which consists of encouraging healthy lifestyle habits and appropriate use of medication. This major impact results from the combined effect of addressing all vascular risk factors in individual patients and efforts to use the best medical treatment available at the time. Rates are now so low (around 0.5% per year for ipsilateral stroke) that procedures, such as carotid endarterectomy (CEA), are now more likely to harm than help patients.7
Even if procedures were always completely risk-free, improved medical treatment may mean we have now reached the point where carotid procedures for ACS are safe but essentially ineffective for reducing stroke risk. The latest measurements of stroke risk using medical treatment alone indicate that only about 2.5% of patients with 50%-99% ACS will have a an ipsilateral stroke due to the carotid lesion during their remaining lifetime if they are receiving pretty good quality, current medical treatment alone. This is because the average age of identifying patients with ACS in past studies was about 70 years and the average survival following diagnosis was 10 years.1 Further, only about half the strokes occurring in the distribution of an internal carotid artery with >60% proximal stenosis are due to the carotid lesion.8
Guidelines recommendations for CEA for 50%-99% or 60%-99% ACS rely on marginal, 20- to 30-year-old differences in stroke rates between patients given medical treatment alone versus those given additional CEA in best practice settings.9-11 Such recommendations are not relevant to current clinical practice largely because the medical treatment used in these studies is obsolete. Multiple independent observations regarding the improved stroke prevention efficacy of medical treatment,1-6,12 and the additional observations below, provide ample evidence that current medical treatment alone is the only routine-practice (nontrial) approach we should use for patients with 50%-99% ACS and any future role of carotid procedures in these patients could only apply to very small minority subgroups:
i. Current optimal medical treatment for patients with ACS has not been defined nor its impact measured. This means that it is likely we can lower the risk of stroke and other vascular complications in patients with ACS stenosis even further than has been achieved in the most recent studies. The definition of current optimal medical treatment will vary from patient to patient depending on which vascular risk factors they have and what has been shown effective in modifying these to reduce the risk of any complications of vascular disease.
ii. The 30-day peri-operative risk of stroke or death (and other significant complications) remains above 0% in the most recent results of trials and registries13-15 and is usually not measured in routine practice. Latest measurements of average annual ipsilateral stroke risk with medical treatment alone are about 2-3 times lower than for patients who had CEA or CAS in the Asymp tomatic Carotid Atherosclerosis Study (ACAS)10 or the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).7, 13
iii. Patients with 50%-99% ACS receiving current optimal medical treatment and with a sufficiently high average annual risk of ipsilateral stroke, indicating they may benefit from CEA, have not been identified. This rate would need to be in excess of at least 2.5%-3.0%, using results from ACAS,10 to expect any surgical benefit in routine practice. Studies of baseline degree of ACS within the 50%-99% range,10,11,16 plaque echolucency17 and most studies of detecting asymptomatic stenosis progression18-21 show that these parameters (used separately) confer a relative risk of stroke of only about 2.0-2.5. Therefore, a higher degree of baseline stenosis within the 50%-99% range, the detection of predominantly echolucent carotid plaques or asymptomatic progression are too weak on their own to identity patients likely to benefit from an additional carotid procedure. Combinations of risk markers are required for sufficient risk discrimination. For instance, results from the Asymptomatic Carotid Stenosis and Risk of Stroke Study (largest study so far of medically managed patients with moderate or severe ACS) showed that a combination of clinical features, baseline degree of stenosis and standardized ultrasonic plaque characteristics can achieve average annual ipsilateral stroke risk stratification ranging from <1.0% to 10%.16 However, like all stroke risk stratification studies performed so far, this study was performed before the era of current medical treatment and the results have not been independently tested.
iv. Even if patients with sufficiently higher than average annual risk of ipsilateral stroke are one day reliably identified, randomized trials of an additional carotid procedure will be required to determine if, and to what extent, that procedure is likely to reduce ipsilateral stroke risk in routine practice.
v. The available evidence from randomized trials and registries indicates that CAS causes about twice as many strokes or deaths as surgery (just like it does for symptomatic carotid stenosis). Therefore, currently CAS cannot be recommended.7 In conclusion, the available evidence clearly indicates that current medical treatment alone now offers the best chance of reducing the risk of ipsilateral stroke in patients with 50%-99% ACS. There is no current evidence of benefit from CEA or CAS in these patients overall, or in any particular subgroups. However, there is much evidence regarding procedural risk and unaffordable cost. Risk of ipsilateral stroke is now so low without carotid procedures it is time to shift from the historic approach of identifying ACS primarily to administer CEA. Rather, it is time to properly recognize that carotid stenosis is a risk factor of all complications of vascular disease, more than it is for ipsilateral stroke.22
The priority is to define current optimal medical treatment as best we can, recognising that patients with ACS are a risk-heterogenous population. Then we need quality independent measurements of its impact on risk of all vascular disease complications using quality prospective cohort studies. Risk stratification models should be used to identify those who may benefit from trials of more intensive medical treatment, motivational strategies, plus/minus the safest carotid procedures. If identifying patients with ACS for CEA in routine practice is to be feasible, this needs to be done within well organized environments that support patients with a wide range of stenosis severity with the primary aim of implementing current optimal medical treatment to prevent all vascular complications. Finally, it must be accepted that as medical treatment and its implementation continue to improve, the added value of carotid procedures, including for symptomatic carotid stenosis, will continue to recede until we can say, ‘good job - it is finally fixed and it is time to move on to other major health issues.’
Dr. Abbott is a neurologist and an associate professor at Monash University, Melbourne, Australia.
References
1. Stroke. 2009;40:e573-583
2. Eur J Vasc Endovasc Surg. 2009;37:625-632
3. Nat Rev Cardiol. 2011;9:116-124
4. Stroke. 2013;44
5. Management of asymptomatic carotid stenosis: Technology assessment report. 2012:83
6. Annals of Internal Medicine. 2013;158:676-685
7. Stroke. 2013;44:1186-1190
8. N Engl J Med. 2000;342:1693-1700
9. N Engl J Med. 1993;328:221-227.
10.JAMA. 1995;273:1421-1428
11. Lancet. 2004;363:1491-1502
12. Arch Neurol. 2010;67:180-186
13. N Engl J Med. 2010;363:11-23
14. J Vasc Surg. 2009;49:71-79
15. J Vasc Surg. 2011;53:307-315
16. J Vasc Surg. 2010;52:1486-1496 e1481-1485
17. Gupta A, Kesavabhotla K, Barbadaran H, Kamel H, Panda A, Giambrone A, et al. Plaque echolucency and stroke risk in asymptoamtioc carotid stenosis: A systematic review and meta-analysis. Stroke. 2014 in press.
18. J Vasc Surg. 1999;29:208-214; discussion 214-206
19. J Vasc Surg. 2013;58:128-135 e121
20 Stroke. 2013;44:792-794
21. J Vasc Surg. 2014;59:956-967
22. N Engl J Med. 1986;315:860-865.
COUNTERPOINT: Medical therapy alone is not always enough.
By Mark F. Conrad, M.D., M.MSc., and Richard P. Cambria M.D.
Stroke remains the 3rd leading cause of death in the United States.1 It is estimated that 10% to 20% of ischemic strokes can be attributed to an ipsilateral, typically high-grade carotid stenosis, thus, asymptomatic carotid bifurcation stenosis remains a potentially significant public health problem.2 Epidemiologic studies indicate that 5% to 6% of the population >65 years of age will harbor an asymptomatic and potentially surgically significant carotid stenosis.1 The modern literature linking the degree of stenosis of the internal carotid artery and risk of ipsilateral stroke dates to the natural history studies of Chambers and Norris published in the New England Journal of Medicine in 1986.3 These investigators demonstrated a significant correlation of stroke risk with a >70% ipsilateral carotid artery stenosis and progression under observation.
Carotid endarterectomy (CEA) has been the standard of care for the prevention of stroke in patients with severe (>70%) asymptomatic carotid artery stenosis (ACS) for five decades. This is supported by level 1 evidence from multiple randomized trials4, 5 and consensus guideline recommendations.2, 6 Yet the use of CEA in patients with ACS has been recently challenged by a widely publicized review article7 whose author has embarked upon an anti-CEA crusade with the zealous fervor of one who worships at the altar of the statin. In this review, 11 prospective studies of medical therapy of patients with ACS (defined by the author as >50% stenosis) were stratified by date of publication such that the four series published from 2000-2007 had a lower raw data stroke rate with medical management (.6- 1.3%) than the 1.5% stroke rate reported after CEA in the ACAS study.4,7 The authors concluded that medical management alone is “at least 3-8” times more cost effective than CEA despite a complete lack of cost data in the cited articles. In an effort to debunk this revisionist history, we will begin by addressing the four aforementioned trials and finish with a discussion of the recent literature including an observational study from our own institution.
The first study of the four followed the asymptomatic contralateral carotid artery of all patients enrolled in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) for 5 years to determine the risk of stroke in medically managed patients.8 There were 2,377 patients of whom 216 (9%) had a stenosis >60% but only 113 (4.7%) had a stenosis of 75-99%. The 5-year risk of ipsilateral stroke in the 75-99% asymptomatic stenosis cohort was 18.5% or 3.7% per year (this did not include other neurologic events such as TIA or amaurosis fugax).8 However, in the meta-analysis, no stroke rate was included in the raw data column (which was the basis of the final calculated overall stroke risk) and the final estimate quoted used the 60%-99% cohort such that the annual stroke rate was reported as 3.2%.7
The Asymptomatic Carotid Stenosis and Risk of Ipsilateral Hemispheric Ischemic Events Study (ACSRS) was a multicenter study that followed 1,115 patients with ACS >50% by duplex scanning for 6-84 months (mean 37.1) with the goal of stratifying patients into cohorts of high and low risk for future neurologic events.9 They concluded that the annual stroke rate in high-risk patients was 4.3% versus 0.7% in low-risk patients. There were 453 patients with 70%-99% stenosis by NASCET criteria with a raw stroke rate of 5.7% (1.9%/ year over an average 3-year follow-up) and a 5-year ipsilateral event rate of about 18%.9 However, when the patients with 50-69% were added, the raw stroke rate decreased to 1.3%.7
The Asymptomatic Stenosis Embolus Detection (ASED) study was a prospective trial that tested the theory that transcranial Doppler embolic signal detection would identify increased risk of ipsilateral neurologic events in patients with >60% ACS.10 Of the 240 arteries studied, 115 (48%) had a stenosis of 70%-99% but 10 of these patients were censored because they underwent CEA during the follow-up period. Their outcomes were not further stratified by degree of stenosis but the average ipsilateral carotid event rate was 3.1% per year with a 1% stroke risk per year.7, 10
The final, and most damning study in the meta-analysis, included a cohort of patients from the Second Manifestations of Arterial disease (SMART) study which is a registry of patients from the Netherlands with risk factors for, or symptoms of, arterial disease.
This study attempted to determine the risk of new vascular event in patients with ACS but 996 (27% of the registry) patients with a history of cerebrovascular disease (undefined) were excluded from analysis.11 They identified 221 patients (8% of 2684 eligible patients) with ACS >50% and reported an ipsilateral stroke event rate of .6%/year.7
However, in the 147 patients with a 70-99% stenosis, the hazard ratio for ischemic stroke was 1.7 but it was not possible to separate the patients with a moderate (50-69%) stenosis from the outcomes of those with more severe (>70%) disease.11
The major flaw with this study is that the authors excluded the 996 patients with a history of cerebrovascular disease who were at highest risk of having an ACS and subsequently suffering a stroke.
The flaw with combining the results of the above studies to conclude that medical therapy is the best way to prevent stroke in patients with severe ACS is that only half (828/1,754, 47%) of the patients included had a severe (70-99%) stenosis that would warrant CEA in the United States.6, 7
The majority of the patients studied would have been treated with best medical therapy and serial Duplex scanning. Indeed, when the moderate patients were excluded, the yearly stroke rates for patients with actual severe stenosis ranged from 2.0%-3.7%; substantially higher than the 1.5% stroke rate associated with CEA.8, 9
Randomized prospective trials such as the asymptomatic carotid artery stenosis (ACAS) study published in 1995 and the more recently updated asymptomatic carotid surgery trial (ACST) indicated a quite similar annual stroke risk in the 2% range for patients treated with medical therapy as opposed to those randomized to carotid endarterectomy (this study was excluded from the meta-analysis because patients with a remote (>6 months before entry) history of neurologic events were included).4,12 In addition, the 10-year follow-up data in the ACST trial demonstrated a sustained benefit for endarterectomy over optimal medical therapy.5 It is important to emphasize that in this trial some 80% of patients were on optimal medical therapy (aspirin plus statin agents) in the later years of the trial.
These long-term data indicate that while the protective effect of endarterectomy was more pronounced in those not on appropriate lipid-lowering therapy (the 10-year advantage of endarterectomy over medical therapy in the prevention of stroke was 5.8% in patients taking a statin and 6.2% in those who were not) the protective effect of endarterectomy over optimal medical therapy was statistically significant (P=0.002).5
This is of course relevant because clinicians in the modern era have typically used data from ACST to counsel patients about the stroke risk of asymptomatic high-grade carotid stenosis.
Finally, we followed at a cohort of 115 patients with severe (>70%) ACS who did not undergo CEA for a variety of reasons.
The average follow-up was 27 months and 86% of patients were on statin therapy.
The 5-year ipsilateral ischemic event rate was 30%, and 48% of these were strokes. When stratified by degree of stenosis, the patients with a 90%-99% stenosis had an ipsilateral neurologic event rate of 55%. This is a single center experience but the stroke rate of 3% per year is consistent with contemporary series and reiterates the reality of this risk for medically treated patients.
The question remains. When the efficacy of a CEA for the prevention of stroke in patients with ACS is supported by level 1 evidence from multiple prospective, randomized trials, why would anyone who treats patients with carotid disease allow a meta-analysis of natural history studies (that redefines a severe stenosis as >50%) convince them otherwise?
We believe that the answer lies in the unsubstantiated conclusion that “current medical intervention was estimated at least 3 to 8 times more cost effective” than CEA.7
As health care in the United States continues to evolve, we are faced with the issue of how best to spend limited resource dollars.
Is it practical or cost efficient to perform preventative surgery?
In England, an annual stroke rate of 2%-3% in a small subset of the population may be acceptable but we are not ready to concede that in the United States.
For now, we will follow the SVS practice guidelines for the management of ACS as they are evidenced based and definitive.
CEA in conjunction with medical therapy remains the best way to prevent stroke in patients with severe (>70%) ACS.
Dr. Conrad is an assistant professor of surgery, Harvard Medical School, Boston. Dr. Cambria is The Robert R. Linton MD Professor of Vascular and Endovascular Surgery, Harvard Medical School, Boston.
References
1.Neurology. 2006;67:1390-1395
2.Circulation. 2012;125:188-197
3.N Engl J of Med. 1986;315:860-865
4.JAMA. 1995;273:1421-1428
5.Lancet. 2010;376:1074-1084
6.Journal of Vascular Surgery. 2011;54:e1-31
7.Stroke. 2009;40:e573-583
8.N Engl J Med. 2000;342:1693-1700
9. Eur J Vasc Endovasc Surg. 2005;30:275-284
10.Stroke. 2005;36:1128-1133
11. Stroke. 2007;38:1470-1475
12. Lancet. 2004;363:1491-1502
POINT: Medical treatment ends need for CEA or CAS.
By Anne L. Abbott, M.D.
The medical profession and the wider community must be congratulated upon their sustained efforts over recent decades that have seen an 80% fall in the average annual risk of stroke associated with moderate and severe (50%-99%) asymptomatic carotid stenosis (ACS).1-6 This has been achieved by better medical treatment which consists of encouraging healthy lifestyle habits and appropriate use of medication. This major impact results from the combined effect of addressing all vascular risk factors in individual patients and efforts to use the best medical treatment available at the time. Rates are now so low (around 0.5% per year for ipsilateral stroke) that procedures, such as carotid endarterectomy (CEA), are now more likely to harm than help patients.7
Even if procedures were always completely risk-free, improved medical treatment may mean we have now reached the point where carotid procedures for ACS are safe but essentially ineffective for reducing stroke risk. The latest measurements of stroke risk using medical treatment alone indicate that only about 2.5% of patients with 50%-99% ACS will have a an ipsilateral stroke due to the carotid lesion during their remaining lifetime if they are receiving pretty good quality, current medical treatment alone. This is because the average age of identifying patients with ACS in past studies was about 70 years and the average survival following diagnosis was 10 years.1 Further, only about half the strokes occurring in the distribution of an internal carotid artery with >60% proximal stenosis are due to the carotid lesion.8
Guidelines recommendations for CEA for 50%-99% or 60%-99% ACS rely on marginal, 20- to 30-year-old differences in stroke rates between patients given medical treatment alone versus those given additional CEA in best practice settings.9-11 Such recommendations are not relevant to current clinical practice largely because the medical treatment used in these studies is obsolete. Multiple independent observations regarding the improved stroke prevention efficacy of medical treatment,1-6,12 and the additional observations below, provide ample evidence that current medical treatment alone is the only routine-practice (nontrial) approach we should use for patients with 50%-99% ACS and any future role of carotid procedures in these patients could only apply to very small minority subgroups:
i. Current optimal medical treatment for patients with ACS has not been defined nor its impact measured. This means that it is likely we can lower the risk of stroke and other vascular complications in patients with ACS stenosis even further than has been achieved in the most recent studies. The definition of current optimal medical treatment will vary from patient to patient depending on which vascular risk factors they have and what has been shown effective in modifying these to reduce the risk of any complications of vascular disease.
ii. The 30-day peri-operative risk of stroke or death (and other significant complications) remains above 0% in the most recent results of trials and registries13-15 and is usually not measured in routine practice. Latest measurements of average annual ipsilateral stroke risk with medical treatment alone are about 2-3 times lower than for patients who had CEA or CAS in the Asymp tomatic Carotid Atherosclerosis Study (ACAS)10 or the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).7, 13
iii. Patients with 50%-99% ACS receiving current optimal medical treatment and with a sufficiently high average annual risk of ipsilateral stroke, indicating they may benefit from CEA, have not been identified. This rate would need to be in excess of at least 2.5%-3.0%, using results from ACAS,10 to expect any surgical benefit in routine practice. Studies of baseline degree of ACS within the 50%-99% range,10,11,16 plaque echolucency17 and most studies of detecting asymptomatic stenosis progression18-21 show that these parameters (used separately) confer a relative risk of stroke of only about 2.0-2.5. Therefore, a higher degree of baseline stenosis within the 50%-99% range, the detection of predominantly echolucent carotid plaques or asymptomatic progression are too weak on their own to identity patients likely to benefit from an additional carotid procedure. Combinations of risk markers are required for sufficient risk discrimination. For instance, results from the Asymptomatic Carotid Stenosis and Risk of Stroke Study (largest study so far of medically managed patients with moderate or severe ACS) showed that a combination of clinical features, baseline degree of stenosis and standardized ultrasonic plaque characteristics can achieve average annual ipsilateral stroke risk stratification ranging from <1.0% to 10%.16 However, like all stroke risk stratification studies performed so far, this study was performed before the era of current medical treatment and the results have not been independently tested.
iv. Even if patients with sufficiently higher than average annual risk of ipsilateral stroke are one day reliably identified, randomized trials of an additional carotid procedure will be required to determine if, and to what extent, that procedure is likely to reduce ipsilateral stroke risk in routine practice.
v. The available evidence from randomized trials and registries indicates that CAS causes about twice as many strokes or deaths as surgery (just like it does for symptomatic carotid stenosis). Therefore, currently CAS cannot be recommended.7 In conclusion, the available evidence clearly indicates that current medical treatment alone now offers the best chance of reducing the risk of ipsilateral stroke in patients with 50%-99% ACS. There is no current evidence of benefit from CEA or CAS in these patients overall, or in any particular subgroups. However, there is much evidence regarding procedural risk and unaffordable cost. Risk of ipsilateral stroke is now so low without carotid procedures it is time to shift from the historic approach of identifying ACS primarily to administer CEA. Rather, it is time to properly recognize that carotid stenosis is a risk factor of all complications of vascular disease, more than it is for ipsilateral stroke.22
The priority is to define current optimal medical treatment as best we can, recognising that patients with ACS are a risk-heterogenous population. Then we need quality independent measurements of its impact on risk of all vascular disease complications using quality prospective cohort studies. Risk stratification models should be used to identify those who may benefit from trials of more intensive medical treatment, motivational strategies, plus/minus the safest carotid procedures. If identifying patients with ACS for CEA in routine practice is to be feasible, this needs to be done within well organized environments that support patients with a wide range of stenosis severity with the primary aim of implementing current optimal medical treatment to prevent all vascular complications. Finally, it must be accepted that as medical treatment and its implementation continue to improve, the added value of carotid procedures, including for symptomatic carotid stenosis, will continue to recede until we can say, ‘good job - it is finally fixed and it is time to move on to other major health issues.’
Dr. Abbott is a neurologist and an associate professor at Monash University, Melbourne, Australia.
References
1. Stroke. 2009;40:e573-583
2. Eur J Vasc Endovasc Surg. 2009;37:625-632
3. Nat Rev Cardiol. 2011;9:116-124
4. Stroke. 2013;44
5. Management of asymptomatic carotid stenosis: Technology assessment report. 2012:83
6. Annals of Internal Medicine. 2013;158:676-685
7. Stroke. 2013;44:1186-1190
8. N Engl J Med. 2000;342:1693-1700
9. N Engl J Med. 1993;328:221-227.
10.JAMA. 1995;273:1421-1428
11. Lancet. 2004;363:1491-1502
12. Arch Neurol. 2010;67:180-186
13. N Engl J Med. 2010;363:11-23
14. J Vasc Surg. 2009;49:71-79
15. J Vasc Surg. 2011;53:307-315
16. J Vasc Surg. 2010;52:1486-1496 e1481-1485
17. Gupta A, Kesavabhotla K, Barbadaran H, Kamel H, Panda A, Giambrone A, et al. Plaque echolucency and stroke risk in asymptoamtioc carotid stenosis: A systematic review and meta-analysis. Stroke. 2014 in press.
18. J Vasc Surg. 1999;29:208-214; discussion 214-206
19. J Vasc Surg. 2013;58:128-135 e121
20 Stroke. 2013;44:792-794
21. J Vasc Surg. 2014;59:956-967
22. N Engl J Med. 1986;315:860-865.
COUNTERPOINT: Medical therapy alone is not always enough.
By Mark F. Conrad, M.D., M.MSc., and Richard P. Cambria M.D.
Stroke remains the 3rd leading cause of death in the United States.1 It is estimated that 10% to 20% of ischemic strokes can be attributed to an ipsilateral, typically high-grade carotid stenosis, thus, asymptomatic carotid bifurcation stenosis remains a potentially significant public health problem.2 Epidemiologic studies indicate that 5% to 6% of the population >65 years of age will harbor an asymptomatic and potentially surgically significant carotid stenosis.1 The modern literature linking the degree of stenosis of the internal carotid artery and risk of ipsilateral stroke dates to the natural history studies of Chambers and Norris published in the New England Journal of Medicine in 1986.3 These investigators demonstrated a significant correlation of stroke risk with a >70% ipsilateral carotid artery stenosis and progression under observation.
Carotid endarterectomy (CEA) has been the standard of care for the prevention of stroke in patients with severe (>70%) asymptomatic carotid artery stenosis (ACS) for five decades. This is supported by level 1 evidence from multiple randomized trials4, 5 and consensus guideline recommendations.2, 6 Yet the use of CEA in patients with ACS has been recently challenged by a widely publicized review article7 whose author has embarked upon an anti-CEA crusade with the zealous fervor of one who worships at the altar of the statin. In this review, 11 prospective studies of medical therapy of patients with ACS (defined by the author as >50% stenosis) were stratified by date of publication such that the four series published from 2000-2007 had a lower raw data stroke rate with medical management (.6- 1.3%) than the 1.5% stroke rate reported after CEA in the ACAS study.4,7 The authors concluded that medical management alone is “at least 3-8” times more cost effective than CEA despite a complete lack of cost data in the cited articles. In an effort to debunk this revisionist history, we will begin by addressing the four aforementioned trials and finish with a discussion of the recent literature including an observational study from our own institution.
The first study of the four followed the asymptomatic contralateral carotid artery of all patients enrolled in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) for 5 years to determine the risk of stroke in medically managed patients.8 There were 2,377 patients of whom 216 (9%) had a stenosis >60% but only 113 (4.7%) had a stenosis of 75-99%. The 5-year risk of ipsilateral stroke in the 75-99% asymptomatic stenosis cohort was 18.5% or 3.7% per year (this did not include other neurologic events such as TIA or amaurosis fugax).8 However, in the meta-analysis, no stroke rate was included in the raw data column (which was the basis of the final calculated overall stroke risk) and the final estimate quoted used the 60%-99% cohort such that the annual stroke rate was reported as 3.2%.7
The Asymptomatic Carotid Stenosis and Risk of Ipsilateral Hemispheric Ischemic Events Study (ACSRS) was a multicenter study that followed 1,115 patients with ACS >50% by duplex scanning for 6-84 months (mean 37.1) with the goal of stratifying patients into cohorts of high and low risk for future neurologic events.9 They concluded that the annual stroke rate in high-risk patients was 4.3% versus 0.7% in low-risk patients. There were 453 patients with 70%-99% stenosis by NASCET criteria with a raw stroke rate of 5.7% (1.9%/ year over an average 3-year follow-up) and a 5-year ipsilateral event rate of about 18%.9 However, when the patients with 50-69% were added, the raw stroke rate decreased to 1.3%.7
The Asymptomatic Stenosis Embolus Detection (ASED) study was a prospective trial that tested the theory that transcranial Doppler embolic signal detection would identify increased risk of ipsilateral neurologic events in patients with >60% ACS.10 Of the 240 arteries studied, 115 (48%) had a stenosis of 70%-99% but 10 of these patients were censored because they underwent CEA during the follow-up period. Their outcomes were not further stratified by degree of stenosis but the average ipsilateral carotid event rate was 3.1% per year with a 1% stroke risk per year.7, 10
The final, and most damning study in the meta-analysis, included a cohort of patients from the Second Manifestations of Arterial disease (SMART) study which is a registry of patients from the Netherlands with risk factors for, or symptoms of, arterial disease.
This study attempted to determine the risk of new vascular event in patients with ACS but 996 (27% of the registry) patients with a history of cerebrovascular disease (undefined) were excluded from analysis.11 They identified 221 patients (8% of 2684 eligible patients) with ACS >50% and reported an ipsilateral stroke event rate of .6%/year.7
However, in the 147 patients with a 70-99% stenosis, the hazard ratio for ischemic stroke was 1.7 but it was not possible to separate the patients with a moderate (50-69%) stenosis from the outcomes of those with more severe (>70%) disease.11
The major flaw with this study is that the authors excluded the 996 patients with a history of cerebrovascular disease who were at highest risk of having an ACS and subsequently suffering a stroke.
The flaw with combining the results of the above studies to conclude that medical therapy is the best way to prevent stroke in patients with severe ACS is that only half (828/1,754, 47%) of the patients included had a severe (70-99%) stenosis that would warrant CEA in the United States.6, 7
The majority of the patients studied would have been treated with best medical therapy and serial Duplex scanning. Indeed, when the moderate patients were excluded, the yearly stroke rates for patients with actual severe stenosis ranged from 2.0%-3.7%; substantially higher than the 1.5% stroke rate associated with CEA.8, 9
Randomized prospective trials such as the asymptomatic carotid artery stenosis (ACAS) study published in 1995 and the more recently updated asymptomatic carotid surgery trial (ACST) indicated a quite similar annual stroke risk in the 2% range for patients treated with medical therapy as opposed to those randomized to carotid endarterectomy (this study was excluded from the meta-analysis because patients with a remote (>6 months before entry) history of neurologic events were included).4,12 In addition, the 10-year follow-up data in the ACST trial demonstrated a sustained benefit for endarterectomy over optimal medical therapy.5 It is important to emphasize that in this trial some 80% of patients were on optimal medical therapy (aspirin plus statin agents) in the later years of the trial.
These long-term data indicate that while the protective effect of endarterectomy was more pronounced in those not on appropriate lipid-lowering therapy (the 10-year advantage of endarterectomy over medical therapy in the prevention of stroke was 5.8% in patients taking a statin and 6.2% in those who were not) the protective effect of endarterectomy over optimal medical therapy was statistically significant (P=0.002).5
This is of course relevant because clinicians in the modern era have typically used data from ACST to counsel patients about the stroke risk of asymptomatic high-grade carotid stenosis.
Finally, we followed at a cohort of 115 patients with severe (>70%) ACS who did not undergo CEA for a variety of reasons.
The average follow-up was 27 months and 86% of patients were on statin therapy.
The 5-year ipsilateral ischemic event rate was 30%, and 48% of these were strokes. When stratified by degree of stenosis, the patients with a 90%-99% stenosis had an ipsilateral neurologic event rate of 55%. This is a single center experience but the stroke rate of 3% per year is consistent with contemporary series and reiterates the reality of this risk for medically treated patients.
The question remains. When the efficacy of a CEA for the prevention of stroke in patients with ACS is supported by level 1 evidence from multiple prospective, randomized trials, why would anyone who treats patients with carotid disease allow a meta-analysis of natural history studies (that redefines a severe stenosis as >50%) convince them otherwise?
We believe that the answer lies in the unsubstantiated conclusion that “current medical intervention was estimated at least 3 to 8 times more cost effective” than CEA.7
As health care in the United States continues to evolve, we are faced with the issue of how best to spend limited resource dollars.
Is it practical or cost efficient to perform preventative surgery?
In England, an annual stroke rate of 2%-3% in a small subset of the population may be acceptable but we are not ready to concede that in the United States.
For now, we will follow the SVS practice guidelines for the management of ACS as they are evidenced based and definitive.
CEA in conjunction with medical therapy remains the best way to prevent stroke in patients with severe (>70%) ACS.
Dr. Conrad is an assistant professor of surgery, Harvard Medical School, Boston. Dr. Cambria is The Robert R. Linton MD Professor of Vascular and Endovascular Surgery, Harvard Medical School, Boston.
References
1.Neurology. 2006;67:1390-1395
2.Circulation. 2012;125:188-197
3.N Engl J of Med. 1986;315:860-865
4.JAMA. 1995;273:1421-1428
5.Lancet. 2010;376:1074-1084
6.Journal of Vascular Surgery. 2011;54:e1-31
7.Stroke. 2009;40:e573-583
8.N Engl J Med. 2000;342:1693-1700
9. Eur J Vasc Endovasc Surg. 2005;30:275-284
10.Stroke. 2005;36:1128-1133
11. Stroke. 2007;38:1470-1475
12. Lancet. 2004;363:1491-1502
POINT: Medical treatment ends need for CEA or CAS.
By Anne L. Abbott, M.D.
The medical profession and the wider community must be congratulated upon their sustained efforts over recent decades that have seen an 80% fall in the average annual risk of stroke associated with moderate and severe (50%-99%) asymptomatic carotid stenosis (ACS).1-6 This has been achieved by better medical treatment which consists of encouraging healthy lifestyle habits and appropriate use of medication. This major impact results from the combined effect of addressing all vascular risk factors in individual patients and efforts to use the best medical treatment available at the time. Rates are now so low (around 0.5% per year for ipsilateral stroke) that procedures, such as carotid endarterectomy (CEA), are now more likely to harm than help patients.7
Even if procedures were always completely risk-free, improved medical treatment may mean we have now reached the point where carotid procedures for ACS are safe but essentially ineffective for reducing stroke risk. The latest measurements of stroke risk using medical treatment alone indicate that only about 2.5% of patients with 50%-99% ACS will have a an ipsilateral stroke due to the carotid lesion during their remaining lifetime if they are receiving pretty good quality, current medical treatment alone. This is because the average age of identifying patients with ACS in past studies was about 70 years and the average survival following diagnosis was 10 years.1 Further, only about half the strokes occurring in the distribution of an internal carotid artery with >60% proximal stenosis are due to the carotid lesion.8
Guidelines recommendations for CEA for 50%-99% or 60%-99% ACS rely on marginal, 20- to 30-year-old differences in stroke rates between patients given medical treatment alone versus those given additional CEA in best practice settings.9-11 Such recommendations are not relevant to current clinical practice largely because the medical treatment used in these studies is obsolete. Multiple independent observations regarding the improved stroke prevention efficacy of medical treatment,1-6,12 and the additional observations below, provide ample evidence that current medical treatment alone is the only routine-practice (nontrial) approach we should use for patients with 50%-99% ACS and any future role of carotid procedures in these patients could only apply to very small minority subgroups:
i. Current optimal medical treatment for patients with ACS has not been defined nor its impact measured. This means that it is likely we can lower the risk of stroke and other vascular complications in patients with ACS stenosis even further than has been achieved in the most recent studies. The definition of current optimal medical treatment will vary from patient to patient depending on which vascular risk factors they have and what has been shown effective in modifying these to reduce the risk of any complications of vascular disease.
ii. The 30-day peri-operative risk of stroke or death (and other significant complications) remains above 0% in the most recent results of trials and registries13-15 and is usually not measured in routine practice. Latest measurements of average annual ipsilateral stroke risk with medical treatment alone are about 2-3 times lower than for patients who had CEA or CAS in the Asymp tomatic Carotid Atherosclerosis Study (ACAS)10 or the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).7, 13
iii. Patients with 50%-99% ACS receiving current optimal medical treatment and with a sufficiently high average annual risk of ipsilateral stroke, indicating they may benefit from CEA, have not been identified. This rate would need to be in excess of at least 2.5%-3.0%, using results from ACAS,10 to expect any surgical benefit in routine practice. Studies of baseline degree of ACS within the 50%-99% range,10,11,16 plaque echolucency17 and most studies of detecting asymptomatic stenosis progression18-21 show that these parameters (used separately) confer a relative risk of stroke of only about 2.0-2.5. Therefore, a higher degree of baseline stenosis within the 50%-99% range, the detection of predominantly echolucent carotid plaques or asymptomatic progression are too weak on their own to identity patients likely to benefit from an additional carotid procedure. Combinations of risk markers are required for sufficient risk discrimination. For instance, results from the Asymptomatic Carotid Stenosis and Risk of Stroke Study (largest study so far of medically managed patients with moderate or severe ACS) showed that a combination of clinical features, baseline degree of stenosis and standardized ultrasonic plaque characteristics can achieve average annual ipsilateral stroke risk stratification ranging from <1.0% to 10%.16 However, like all stroke risk stratification studies performed so far, this study was performed before the era of current medical treatment and the results have not been independently tested.
iv. Even if patients with sufficiently higher than average annual risk of ipsilateral stroke are one day reliably identified, randomized trials of an additional carotid procedure will be required to determine if, and to what extent, that procedure is likely to reduce ipsilateral stroke risk in routine practice.
v. The available evidence from randomized trials and registries indicates that CAS causes about twice as many strokes or deaths as surgery (just like it does for symptomatic carotid stenosis). Therefore, currently CAS cannot be recommended.7 In conclusion, the available evidence clearly indicates that current medical treatment alone now offers the best chance of reducing the risk of ipsilateral stroke in patients with 50%-99% ACS. There is no current evidence of benefit from CEA or CAS in these patients overall, or in any particular subgroups. However, there is much evidence regarding procedural risk and unaffordable cost. Risk of ipsilateral stroke is now so low without carotid procedures it is time to shift from the historic approach of identifying ACS primarily to administer CEA. Rather, it is time to properly recognize that carotid stenosis is a risk factor of all complications of vascular disease, more than it is for ipsilateral stroke.22
The priority is to define current optimal medical treatment as best we can, recognising that patients with ACS are a risk-heterogenous population. Then we need quality independent measurements of its impact on risk of all vascular disease complications using quality prospective cohort studies. Risk stratification models should be used to identify those who may benefit from trials of more intensive medical treatment, motivational strategies, plus/minus the safest carotid procedures. If identifying patients with ACS for CEA in routine practice is to be feasible, this needs to be done within well organized environments that support patients with a wide range of stenosis severity with the primary aim of implementing current optimal medical treatment to prevent all vascular complications. Finally, it must be accepted that as medical treatment and its implementation continue to improve, the added value of carotid procedures, including for symptomatic carotid stenosis, will continue to recede until we can say, ‘good job - it is finally fixed and it is time to move on to other major health issues.’
Dr. Abbott is a neurologist and an associate professor at Monash University, Melbourne, Australia.
References
1. Stroke. 2009;40:e573-583
2. Eur J Vasc Endovasc Surg. 2009;37:625-632
3. Nat Rev Cardiol. 2011;9:116-124
4. Stroke. 2013;44
5. Management of asymptomatic carotid stenosis: Technology assessment report. 2012:83
6. Annals of Internal Medicine. 2013;158:676-685
7. Stroke. 2013;44:1186-1190
8. N Engl J Med. 2000;342:1693-1700
9. N Engl J Med. 1993;328:221-227.
10.JAMA. 1995;273:1421-1428
11. Lancet. 2004;363:1491-1502
12. Arch Neurol. 2010;67:180-186
13. N Engl J Med. 2010;363:11-23
14. J Vasc Surg. 2009;49:71-79
15. J Vasc Surg. 2011;53:307-315
16. J Vasc Surg. 2010;52:1486-1496 e1481-1485
17. Gupta A, Kesavabhotla K, Barbadaran H, Kamel H, Panda A, Giambrone A, et al. Plaque echolucency and stroke risk in asymptoamtioc carotid stenosis: A systematic review and meta-analysis. Stroke. 2014 in press.
18. J Vasc Surg. 1999;29:208-214; discussion 214-206
19. J Vasc Surg. 2013;58:128-135 e121
20 Stroke. 2013;44:792-794
21. J Vasc Surg. 2014;59:956-967
22. N Engl J Med. 1986;315:860-865.
COUNTERPOINT: Medical therapy alone is not always enough.
By Mark F. Conrad, M.D., M.MSc., and Richard P. Cambria M.D.
Stroke remains the 3rd leading cause of death in the United States.1 It is estimated that 10% to 20% of ischemic strokes can be attributed to an ipsilateral, typically high-grade carotid stenosis, thus, asymptomatic carotid bifurcation stenosis remains a potentially significant public health problem.2 Epidemiologic studies indicate that 5% to 6% of the population >65 years of age will harbor an asymptomatic and potentially surgically significant carotid stenosis.1 The modern literature linking the degree of stenosis of the internal carotid artery and risk of ipsilateral stroke dates to the natural history studies of Chambers and Norris published in the New England Journal of Medicine in 1986.3 These investigators demonstrated a significant correlation of stroke risk with a >70% ipsilateral carotid artery stenosis and progression under observation.
Carotid endarterectomy (CEA) has been the standard of care for the prevention of stroke in patients with severe (>70%) asymptomatic carotid artery stenosis (ACS) for five decades. This is supported by level 1 evidence from multiple randomized trials4, 5 and consensus guideline recommendations.2, 6 Yet the use of CEA in patients with ACS has been recently challenged by a widely publicized review article7 whose author has embarked upon an anti-CEA crusade with the zealous fervor of one who worships at the altar of the statin. In this review, 11 prospective studies of medical therapy of patients with ACS (defined by the author as >50% stenosis) were stratified by date of publication such that the four series published from 2000-2007 had a lower raw data stroke rate with medical management (.6- 1.3%) than the 1.5% stroke rate reported after CEA in the ACAS study.4,7 The authors concluded that medical management alone is “at least 3-8” times more cost effective than CEA despite a complete lack of cost data in the cited articles. In an effort to debunk this revisionist history, we will begin by addressing the four aforementioned trials and finish with a discussion of the recent literature including an observational study from our own institution.
The first study of the four followed the asymptomatic contralateral carotid artery of all patients enrolled in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) for 5 years to determine the risk of stroke in medically managed patients.8 There were 2,377 patients of whom 216 (9%) had a stenosis >60% but only 113 (4.7%) had a stenosis of 75-99%. The 5-year risk of ipsilateral stroke in the 75-99% asymptomatic stenosis cohort was 18.5% or 3.7% per year (this did not include other neurologic events such as TIA or amaurosis fugax).8 However, in the meta-analysis, no stroke rate was included in the raw data column (which was the basis of the final calculated overall stroke risk) and the final estimate quoted used the 60%-99% cohort such that the annual stroke rate was reported as 3.2%.7
The Asymptomatic Carotid Stenosis and Risk of Ipsilateral Hemispheric Ischemic Events Study (ACSRS) was a multicenter study that followed 1,115 patients with ACS >50% by duplex scanning for 6-84 months (mean 37.1) with the goal of stratifying patients into cohorts of high and low risk for future neurologic events.9 They concluded that the annual stroke rate in high-risk patients was 4.3% versus 0.7% in low-risk patients. There were 453 patients with 70%-99% stenosis by NASCET criteria with a raw stroke rate of 5.7% (1.9%/ year over an average 3-year follow-up) and a 5-year ipsilateral event rate of about 18%.9 However, when the patients with 50-69% were added, the raw stroke rate decreased to 1.3%.7
The Asymptomatic Stenosis Embolus Detection (ASED) study was a prospective trial that tested the theory that transcranial Doppler embolic signal detection would identify increased risk of ipsilateral neurologic events in patients with >60% ACS.10 Of the 240 arteries studied, 115 (48%) had a stenosis of 70%-99% but 10 of these patients were censored because they underwent CEA during the follow-up period. Their outcomes were not further stratified by degree of stenosis but the average ipsilateral carotid event rate was 3.1% per year with a 1% stroke risk per year.7, 10
The final, and most damning study in the meta-analysis, included a cohort of patients from the Second Manifestations of Arterial disease (SMART) study which is a registry of patients from the Netherlands with risk factors for, or symptoms of, arterial disease.
This study attempted to determine the risk of new vascular event in patients with ACS but 996 (27% of the registry) patients with a history of cerebrovascular disease (undefined) were excluded from analysis.11 They identified 221 patients (8% of 2684 eligible patients) with ACS >50% and reported an ipsilateral stroke event rate of .6%/year.7
However, in the 147 patients with a 70-99% stenosis, the hazard ratio for ischemic stroke was 1.7 but it was not possible to separate the patients with a moderate (50-69%) stenosis from the outcomes of those with more severe (>70%) disease.11
The major flaw with this study is that the authors excluded the 996 patients with a history of cerebrovascular disease who were at highest risk of having an ACS and subsequently suffering a stroke.
The flaw with combining the results of the above studies to conclude that medical therapy is the best way to prevent stroke in patients with severe ACS is that only half (828/1,754, 47%) of the patients included had a severe (70-99%) stenosis that would warrant CEA in the United States.6, 7
The majority of the patients studied would have been treated with best medical therapy and serial Duplex scanning. Indeed, when the moderate patients were excluded, the yearly stroke rates for patients with actual severe stenosis ranged from 2.0%-3.7%; substantially higher than the 1.5% stroke rate associated with CEA.8, 9
Randomized prospective trials such as the asymptomatic carotid artery stenosis (ACAS) study published in 1995 and the more recently updated asymptomatic carotid surgery trial (ACST) indicated a quite similar annual stroke risk in the 2% range for patients treated with medical therapy as opposed to those randomized to carotid endarterectomy (this study was excluded from the meta-analysis because patients with a remote (>6 months before entry) history of neurologic events were included).4,12 In addition, the 10-year follow-up data in the ACST trial demonstrated a sustained benefit for endarterectomy over optimal medical therapy.5 It is important to emphasize that in this trial some 80% of patients were on optimal medical therapy (aspirin plus statin agents) in the later years of the trial.
These long-term data indicate that while the protective effect of endarterectomy was more pronounced in those not on appropriate lipid-lowering therapy (the 10-year advantage of endarterectomy over medical therapy in the prevention of stroke was 5.8% in patients taking a statin and 6.2% in those who were not) the protective effect of endarterectomy over optimal medical therapy was statistically significant (P=0.002).5
This is of course relevant because clinicians in the modern era have typically used data from ACST to counsel patients about the stroke risk of asymptomatic high-grade carotid stenosis.
Finally, we followed at a cohort of 115 patients with severe (>70%) ACS who did not undergo CEA for a variety of reasons.
The average follow-up was 27 months and 86% of patients were on statin therapy.
The 5-year ipsilateral ischemic event rate was 30%, and 48% of these were strokes. When stratified by degree of stenosis, the patients with a 90%-99% stenosis had an ipsilateral neurologic event rate of 55%. This is a single center experience but the stroke rate of 3% per year is consistent with contemporary series and reiterates the reality of this risk for medically treated patients.
The question remains. When the efficacy of a CEA for the prevention of stroke in patients with ACS is supported by level 1 evidence from multiple prospective, randomized trials, why would anyone who treats patients with carotid disease allow a meta-analysis of natural history studies (that redefines a severe stenosis as >50%) convince them otherwise?
We believe that the answer lies in the unsubstantiated conclusion that “current medical intervention was estimated at least 3 to 8 times more cost effective” than CEA.7
As health care in the United States continues to evolve, we are faced with the issue of how best to spend limited resource dollars.
Is it practical or cost efficient to perform preventative surgery?
In England, an annual stroke rate of 2%-3% in a small subset of the population may be acceptable but we are not ready to concede that in the United States.
For now, we will follow the SVS practice guidelines for the management of ACS as they are evidenced based and definitive.
CEA in conjunction with medical therapy remains the best way to prevent stroke in patients with severe (>70%) ACS.
Dr. Conrad is an assistant professor of surgery, Harvard Medical School, Boston. Dr. Cambria is The Robert R. Linton MD Professor of Vascular and Endovascular Surgery, Harvard Medical School, Boston.
References
1.Neurology. 2006;67:1390-1395
2.Circulation. 2012;125:188-197
3.N Engl J of Med. 1986;315:860-865
4.JAMA. 1995;273:1421-1428
5.Lancet. 2010;376:1074-1084
6.Journal of Vascular Surgery. 2011;54:e1-31
7.Stroke. 2009;40:e573-583
8.N Engl J Med. 2000;342:1693-1700
9. Eur J Vasc Endovasc Surg. 2005;30:275-284
10.Stroke. 2005;36:1128-1133
11. Stroke. 2007;38:1470-1475
12. Lancet. 2004;363:1491-1502
Surgical vs. endovascular repair of popliteal artery aneurysm
Surgical repair remains the optimal method to treat a popliteal artery aneurysm.
Popliteal artery aneurysm, or PAA, is the most common peripheral aneurysm, but data on this disease are nonetheless limited. A report from the VASCUNET collaboration of registries showed that 1,471 repairs were performed among a population of 58 million people in eight countries, for a rate of 9.6 per million. Most (72%) were elective, and 78% were open repairs (Eur. J. Vasc. Endovasc. Surg. 2014;47:164-71).
Although the endovascular approach has been increasingly used since 2000, outcomes have varied considerably across studies.
A review of 163 relevant studies from more than 1,600 that have been published since 1994 showed extensive heterogeneity with respect to the inclusion of symptomatic vs. asymptomatic patients, emergent vs. elective cases, poor runoff vs. good runoff, types of stents used, and types of conduits used for open repair. This renders the validity of the meta-analysis of these studies uncertain.
However, based on the few studies with complete data concerning mortality, major adverse events, primary and secondary patency, and limb salvage – with separate analysis for elective and emergency repairs – it appears that the availability of the great saphenous vein (GSV) is an important determinant when deciding whether to perform an open repair, that the posterior approach is preferred (except in cases of aneurysms extending to the adductor canal or trifurcation vessels), and that elective open repair is associated with significantly better outcomes than endovascular repair on a number of measures.
For example, no difference was seen in mortality in 23 selected studies, but the 3-year primary patency was significantly better with open repair (85% vs. 58%), while the 3-year rate of major adverse events, including mortality, major amputation, graft thrombosis, and reintervention was lower (20% vs. 38%).
These findings were confirmed in a study of 149 elective repairs. In that series, major adverse events were significantly more common in endovascular vs. open cases (hazard ratio, 2.1), and poor runoff was associated with a higher risk of major adverse events regardless of the technique used (J. Vasc. Surg. 2014 60:631-8.e2).
A recent decision analysis model applied to patients with asymptomatic PAA also demonstrated that elective open repair with a GSV bypass is the preferred treatment for all outcomes, with stenting recommended in high-risk patient or those without a suitable vein (J. Vasc. Surg. 2014;59:651-62).
One concern with endovascular repair is the risk of stent fracture, particularly in younger more active patients as data suggest that the more active the individual, the greater the risk of stent fracture. In one study, the frequency of stent fracture in younger patients was 17%. This suggests that stenting is probably not the best technique to be used in active individuals.
The current data suggest that the best outcomes are achieved with elective open repair using the great saphenous vein. However, patency rates above 80% at 2 years have been reported recently for elective endovascular repair associated with dual antiplatelet therapy, thus it is possible that new stent grafts and best medical therapy could improve the results of endovascular repair.
Open repair also appears to be best in most emergent cases. In one meta-analysis of 11 studies involving 223 patients, graft thrombosis occurred in 8% of open cases vs. 53% of endovascular cases, patency at 6 months was 82% vs. 68%, and reintervention rates were 25% vs. 43% in open vs. endovascular cases, respectively.
Thrombolysis was associated with a significant improvement in 1-year primary graft patency rates compared with surgery alone, but this did not affect the amputation rate, and endovascular repair does not appear to improve the severe prognosis of acute ischemia in patients with PAA.
Despite the deceiving results of endovascular repair in those with acute ischemia, this technique could, however, be very useful in other emergent situations. For example, very old patients presenting with a ruptured PAA and a good runoff could benefit from an endovascular repair.
In summary, no level 1 evidence regarding open vs. endovascular repair for popliteal artery aneurysms can be obtained; most studies are retrospective and lack important characteristics. Based on the data that do exist, however, open repair with a vein bypass appears to be the best technique for most patients with PAA. Stenting should be reserved for high-risk and elderly asymptomatic patients. As for those with acute limb ischemia, no strong recommendation can be made based on the available data.
Dr. Jean-Baptiste Ricco is professor and chief of vascular surgery at the University of Poitiers, France. He reported having no disclosures. This and the accompanying perspective by Dr. Marone were based upon their live debate at the 2014 Vascular Annual Meeting.
Endovascular repair of PAA is an effective and durable treatment.
Outcomes following endovascular repair of PAA are at least equivalent to those following open repair with respect to patency and limb salvage in elective cases.
In the Swedish Vascular Registry – the largest report of open repair, representing 717 limbs treated with a mean follow-up of 7.2 years – the primary patency rate at 1 year for cases performed with a medial approach was 90% with vein conduit, and 72% with prosthetic conduit. For cases involving a posterior approach, the rates were 85% with vein conduit, and 81% with prosthetic conduit. The amputation rate was 9.6% at 1 year and 11% at last follow-up.
Furthermore, open surgical procedures are associated with a high wound complication rate. The average across studies is 7%, and was as high as 28% in some series. Open procedures are also associated with variable graft patency, continued aneurysm expansion (which occurred in a third of limbs treated with a medial approach in one series), and a significant amputation rate.
Data regarding endovascular PAA repair are encouraging. In the only prospective randomized trial to date, no significant difference was seen at 46 months with respect to primary patency (100% with open repair vs. 93.3% with endovascular repair), or limb salvage (100% for both).
Hospital length of stay, however, was significantly shorter with endovascular repair (7.7 days vs. 4.3 days), as was operative time (155 minutes vs. 75 minutes).
An update to that 2005 study (J. Vasc. Surg. 2005;42:185-93) showed no difference in patency at 72 months.
While there is a paucity of level 1 evidence (only 15 patients were included in each arm of that study), prospective cohort studies and institutional reviews also demonstrate the value of endovascular repair. Secondary patency at 1 and 3 years were 87% and 85%, respectively, in a 2013 study (Ann. Vasc. Surg. 2013;27:259-65), and the 1- and 2-year amputation rates were 2% and 3%, respectively.
In another series, 1-year primary patency was 92.9% with endovascular repair, compared with 83.3% with open repair, and 3-year patency was 63.7% vs. 77.8%. The differences were not statistically significant.
Length of stay was 3.9 days vs. 9.5 days. Eight wound infections and 2 hematomas occurred in the open repair patients, and two patients experienced enlargement requiring decompression.
The University of Pittsburgh experience with 50 endovascular repairs and 111 open repairs performed between 2004 and 2010 showed that morbidity was 14% vs. 32% for endovascular vs. open repair, and mortality was 2% vs. 3.6%, respectively, at 29-month follow-up. Wound infection rates were 2% and 16.2%, respectively, length of stay was 1 vs. 4 days, and reintervention and thrombosis rates did not differ significantly (12.2% vs. 10.8%, and 8 vs. 12 patients).
No significant differences were seen in aneurysm growth, primary assisted patency at 3 years, secondary patency at 3 years, or amputation rates at 1 year or 3 years.
A 2013 update also showed no differences in these outcomes.
In summary, endovascular repair of PAA is acceptable, with outcomes comparable to or better than open repair in elective cases. Long-term durability has been demonstrated, limb preservation is equivalent to open repair, and thrombotic complications are rare and can be treated successfully with re-intervention.
Furthermore, endovascular repair can be performed without the need for general anesthesia, lower morbidity can be expected perioperatively, hospital length of stay is shorter, and patients have a quicker return of functional status.
Dr. Luke Marone is a vascular surgeon at the University of Pittsburgh School of Medicine. He disclosed he is a consultant for Abiomed and Abbott.
Surgical repair remains the optimal method to treat a popliteal artery aneurysm.
Popliteal artery aneurysm, or PAA, is the most common peripheral aneurysm, but data on this disease are nonetheless limited. A report from the VASCUNET collaboration of registries showed that 1,471 repairs were performed among a population of 58 million people in eight countries, for a rate of 9.6 per million. Most (72%) were elective, and 78% were open repairs (Eur. J. Vasc. Endovasc. Surg. 2014;47:164-71).
Although the endovascular approach has been increasingly used since 2000, outcomes have varied considerably across studies.
A review of 163 relevant studies from more than 1,600 that have been published since 1994 showed extensive heterogeneity with respect to the inclusion of symptomatic vs. asymptomatic patients, emergent vs. elective cases, poor runoff vs. good runoff, types of stents used, and types of conduits used for open repair. This renders the validity of the meta-analysis of these studies uncertain.
However, based on the few studies with complete data concerning mortality, major adverse events, primary and secondary patency, and limb salvage – with separate analysis for elective and emergency repairs – it appears that the availability of the great saphenous vein (GSV) is an important determinant when deciding whether to perform an open repair, that the posterior approach is preferred (except in cases of aneurysms extending to the adductor canal or trifurcation vessels), and that elective open repair is associated with significantly better outcomes than endovascular repair on a number of measures.
For example, no difference was seen in mortality in 23 selected studies, but the 3-year primary patency was significantly better with open repair (85% vs. 58%), while the 3-year rate of major adverse events, including mortality, major amputation, graft thrombosis, and reintervention was lower (20% vs. 38%).
These findings were confirmed in a study of 149 elective repairs. In that series, major adverse events were significantly more common in endovascular vs. open cases (hazard ratio, 2.1), and poor runoff was associated with a higher risk of major adverse events regardless of the technique used (J. Vasc. Surg. 2014 60:631-8.e2).
A recent decision analysis model applied to patients with asymptomatic PAA also demonstrated that elective open repair with a GSV bypass is the preferred treatment for all outcomes, with stenting recommended in high-risk patient or those without a suitable vein (J. Vasc. Surg. 2014;59:651-62).
One concern with endovascular repair is the risk of stent fracture, particularly in younger more active patients as data suggest that the more active the individual, the greater the risk of stent fracture. In one study, the frequency of stent fracture in younger patients was 17%. This suggests that stenting is probably not the best technique to be used in active individuals.
The current data suggest that the best outcomes are achieved with elective open repair using the great saphenous vein. However, patency rates above 80% at 2 years have been reported recently for elective endovascular repair associated with dual antiplatelet therapy, thus it is possible that new stent grafts and best medical therapy could improve the results of endovascular repair.
Open repair also appears to be best in most emergent cases. In one meta-analysis of 11 studies involving 223 patients, graft thrombosis occurred in 8% of open cases vs. 53% of endovascular cases, patency at 6 months was 82% vs. 68%, and reintervention rates were 25% vs. 43% in open vs. endovascular cases, respectively.
Thrombolysis was associated with a significant improvement in 1-year primary graft patency rates compared with surgery alone, but this did not affect the amputation rate, and endovascular repair does not appear to improve the severe prognosis of acute ischemia in patients with PAA.
Despite the deceiving results of endovascular repair in those with acute ischemia, this technique could, however, be very useful in other emergent situations. For example, very old patients presenting with a ruptured PAA and a good runoff could benefit from an endovascular repair.
In summary, no level 1 evidence regarding open vs. endovascular repair for popliteal artery aneurysms can be obtained; most studies are retrospective and lack important characteristics. Based on the data that do exist, however, open repair with a vein bypass appears to be the best technique for most patients with PAA. Stenting should be reserved for high-risk and elderly asymptomatic patients. As for those with acute limb ischemia, no strong recommendation can be made based on the available data.
Dr. Jean-Baptiste Ricco is professor and chief of vascular surgery at the University of Poitiers, France. He reported having no disclosures. This and the accompanying perspective by Dr. Marone were based upon their live debate at the 2014 Vascular Annual Meeting.
Endovascular repair of PAA is an effective and durable treatment.
Outcomes following endovascular repair of PAA are at least equivalent to those following open repair with respect to patency and limb salvage in elective cases.
In the Swedish Vascular Registry – the largest report of open repair, representing 717 limbs treated with a mean follow-up of 7.2 years – the primary patency rate at 1 year for cases performed with a medial approach was 90% with vein conduit, and 72% with prosthetic conduit. For cases involving a posterior approach, the rates were 85% with vein conduit, and 81% with prosthetic conduit. The amputation rate was 9.6% at 1 year and 11% at last follow-up.
Furthermore, open surgical procedures are associated with a high wound complication rate. The average across studies is 7%, and was as high as 28% in some series. Open procedures are also associated with variable graft patency, continued aneurysm expansion (which occurred in a third of limbs treated with a medial approach in one series), and a significant amputation rate.
Data regarding endovascular PAA repair are encouraging. In the only prospective randomized trial to date, no significant difference was seen at 46 months with respect to primary patency (100% with open repair vs. 93.3% with endovascular repair), or limb salvage (100% for both).
Hospital length of stay, however, was significantly shorter with endovascular repair (7.7 days vs. 4.3 days), as was operative time (155 minutes vs. 75 minutes).
An update to that 2005 study (J. Vasc. Surg. 2005;42:185-93) showed no difference in patency at 72 months.
While there is a paucity of level 1 evidence (only 15 patients were included in each arm of that study), prospective cohort studies and institutional reviews also demonstrate the value of endovascular repair. Secondary patency at 1 and 3 years were 87% and 85%, respectively, in a 2013 study (Ann. Vasc. Surg. 2013;27:259-65), and the 1- and 2-year amputation rates were 2% and 3%, respectively.
In another series, 1-year primary patency was 92.9% with endovascular repair, compared with 83.3% with open repair, and 3-year patency was 63.7% vs. 77.8%. The differences were not statistically significant.
Length of stay was 3.9 days vs. 9.5 days. Eight wound infections and 2 hematomas occurred in the open repair patients, and two patients experienced enlargement requiring decompression.
The University of Pittsburgh experience with 50 endovascular repairs and 111 open repairs performed between 2004 and 2010 showed that morbidity was 14% vs. 32% for endovascular vs. open repair, and mortality was 2% vs. 3.6%, respectively, at 29-month follow-up. Wound infection rates were 2% and 16.2%, respectively, length of stay was 1 vs. 4 days, and reintervention and thrombosis rates did not differ significantly (12.2% vs. 10.8%, and 8 vs. 12 patients).
No significant differences were seen in aneurysm growth, primary assisted patency at 3 years, secondary patency at 3 years, or amputation rates at 1 year or 3 years.
A 2013 update also showed no differences in these outcomes.
In summary, endovascular repair of PAA is acceptable, with outcomes comparable to or better than open repair in elective cases. Long-term durability has been demonstrated, limb preservation is equivalent to open repair, and thrombotic complications are rare and can be treated successfully with re-intervention.
Furthermore, endovascular repair can be performed without the need for general anesthesia, lower morbidity can be expected perioperatively, hospital length of stay is shorter, and patients have a quicker return of functional status.
Dr. Luke Marone is a vascular surgeon at the University of Pittsburgh School of Medicine. He disclosed he is a consultant for Abiomed and Abbott.
Surgical repair remains the optimal method to treat a popliteal artery aneurysm.
Popliteal artery aneurysm, or PAA, is the most common peripheral aneurysm, but data on this disease are nonetheless limited. A report from the VASCUNET collaboration of registries showed that 1,471 repairs were performed among a population of 58 million people in eight countries, for a rate of 9.6 per million. Most (72%) were elective, and 78% were open repairs (Eur. J. Vasc. Endovasc. Surg. 2014;47:164-71).
Although the endovascular approach has been increasingly used since 2000, outcomes have varied considerably across studies.
A review of 163 relevant studies from more than 1,600 that have been published since 1994 showed extensive heterogeneity with respect to the inclusion of symptomatic vs. asymptomatic patients, emergent vs. elective cases, poor runoff vs. good runoff, types of stents used, and types of conduits used for open repair. This renders the validity of the meta-analysis of these studies uncertain.
However, based on the few studies with complete data concerning mortality, major adverse events, primary and secondary patency, and limb salvage – with separate analysis for elective and emergency repairs – it appears that the availability of the great saphenous vein (GSV) is an important determinant when deciding whether to perform an open repair, that the posterior approach is preferred (except in cases of aneurysms extending to the adductor canal or trifurcation vessels), and that elective open repair is associated with significantly better outcomes than endovascular repair on a number of measures.
For example, no difference was seen in mortality in 23 selected studies, but the 3-year primary patency was significantly better with open repair (85% vs. 58%), while the 3-year rate of major adverse events, including mortality, major amputation, graft thrombosis, and reintervention was lower (20% vs. 38%).
These findings were confirmed in a study of 149 elective repairs. In that series, major adverse events were significantly more common in endovascular vs. open cases (hazard ratio, 2.1), and poor runoff was associated with a higher risk of major adverse events regardless of the technique used (J. Vasc. Surg. 2014 60:631-8.e2).
A recent decision analysis model applied to patients with asymptomatic PAA also demonstrated that elective open repair with a GSV bypass is the preferred treatment for all outcomes, with stenting recommended in high-risk patient or those without a suitable vein (J. Vasc. Surg. 2014;59:651-62).
One concern with endovascular repair is the risk of stent fracture, particularly in younger more active patients as data suggest that the more active the individual, the greater the risk of stent fracture. In one study, the frequency of stent fracture in younger patients was 17%. This suggests that stenting is probably not the best technique to be used in active individuals.
The current data suggest that the best outcomes are achieved with elective open repair using the great saphenous vein. However, patency rates above 80% at 2 years have been reported recently for elective endovascular repair associated with dual antiplatelet therapy, thus it is possible that new stent grafts and best medical therapy could improve the results of endovascular repair.
Open repair also appears to be best in most emergent cases. In one meta-analysis of 11 studies involving 223 patients, graft thrombosis occurred in 8% of open cases vs. 53% of endovascular cases, patency at 6 months was 82% vs. 68%, and reintervention rates were 25% vs. 43% in open vs. endovascular cases, respectively.
Thrombolysis was associated with a significant improvement in 1-year primary graft patency rates compared with surgery alone, but this did not affect the amputation rate, and endovascular repair does not appear to improve the severe prognosis of acute ischemia in patients with PAA.
Despite the deceiving results of endovascular repair in those with acute ischemia, this technique could, however, be very useful in other emergent situations. For example, very old patients presenting with a ruptured PAA and a good runoff could benefit from an endovascular repair.
In summary, no level 1 evidence regarding open vs. endovascular repair for popliteal artery aneurysms can be obtained; most studies are retrospective and lack important characteristics. Based on the data that do exist, however, open repair with a vein bypass appears to be the best technique for most patients with PAA. Stenting should be reserved for high-risk and elderly asymptomatic patients. As for those with acute limb ischemia, no strong recommendation can be made based on the available data.
Dr. Jean-Baptiste Ricco is professor and chief of vascular surgery at the University of Poitiers, France. He reported having no disclosures. This and the accompanying perspective by Dr. Marone were based upon their live debate at the 2014 Vascular Annual Meeting.
Endovascular repair of PAA is an effective and durable treatment.
Outcomes following endovascular repair of PAA are at least equivalent to those following open repair with respect to patency and limb salvage in elective cases.
In the Swedish Vascular Registry – the largest report of open repair, representing 717 limbs treated with a mean follow-up of 7.2 years – the primary patency rate at 1 year for cases performed with a medial approach was 90% with vein conduit, and 72% with prosthetic conduit. For cases involving a posterior approach, the rates were 85% with vein conduit, and 81% with prosthetic conduit. The amputation rate was 9.6% at 1 year and 11% at last follow-up.
Furthermore, open surgical procedures are associated with a high wound complication rate. The average across studies is 7%, and was as high as 28% in some series. Open procedures are also associated with variable graft patency, continued aneurysm expansion (which occurred in a third of limbs treated with a medial approach in one series), and a significant amputation rate.
Data regarding endovascular PAA repair are encouraging. In the only prospective randomized trial to date, no significant difference was seen at 46 months with respect to primary patency (100% with open repair vs. 93.3% with endovascular repair), or limb salvage (100% for both).
Hospital length of stay, however, was significantly shorter with endovascular repair (7.7 days vs. 4.3 days), as was operative time (155 minutes vs. 75 minutes).
An update to that 2005 study (J. Vasc. Surg. 2005;42:185-93) showed no difference in patency at 72 months.
While there is a paucity of level 1 evidence (only 15 patients were included in each arm of that study), prospective cohort studies and institutional reviews also demonstrate the value of endovascular repair. Secondary patency at 1 and 3 years were 87% and 85%, respectively, in a 2013 study (Ann. Vasc. Surg. 2013;27:259-65), and the 1- and 2-year amputation rates were 2% and 3%, respectively.
In another series, 1-year primary patency was 92.9% with endovascular repair, compared with 83.3% with open repair, and 3-year patency was 63.7% vs. 77.8%. The differences were not statistically significant.
Length of stay was 3.9 days vs. 9.5 days. Eight wound infections and 2 hematomas occurred in the open repair patients, and two patients experienced enlargement requiring decompression.
The University of Pittsburgh experience with 50 endovascular repairs and 111 open repairs performed between 2004 and 2010 showed that morbidity was 14% vs. 32% for endovascular vs. open repair, and mortality was 2% vs. 3.6%, respectively, at 29-month follow-up. Wound infection rates were 2% and 16.2%, respectively, length of stay was 1 vs. 4 days, and reintervention and thrombosis rates did not differ significantly (12.2% vs. 10.8%, and 8 vs. 12 patients).
No significant differences were seen in aneurysm growth, primary assisted patency at 3 years, secondary patency at 3 years, or amputation rates at 1 year or 3 years.
A 2013 update also showed no differences in these outcomes.
In summary, endovascular repair of PAA is acceptable, with outcomes comparable to or better than open repair in elective cases. Long-term durability has been demonstrated, limb preservation is equivalent to open repair, and thrombotic complications are rare and can be treated successfully with re-intervention.
Furthermore, endovascular repair can be performed without the need for general anesthesia, lower morbidity can be expected perioperatively, hospital length of stay is shorter, and patients have a quicker return of functional status.
Dr. Luke Marone is a vascular surgeon at the University of Pittsburgh School of Medicine. He disclosed he is a consultant for Abiomed and Abbott.

























