User login
Should napping be recommended as a health behavior?
I was invited to a cardiology conference to talk about sleep, specifically the benefits of napping for health and cognition. After the talk, along with the usual questions related to my research, the cardiac surgeons in the room shifted the conversation to better resemble a group therapy session, sharing their harrowing personal tales of coping with sleep loss on the job. The most dramatic story involved a resident in a military hospital who, unable to avoid the effects of her mounting sleep loss, did a face plant into the open chest of the patient on the surgery table.
Epidemiology studies have associated insufficient sleep with increased disease risk, including cardiovascular and metabolic disease, diabetes, cancer, Alzheimer’s disease and related dementias, as well as early mortality. Laboratory studies that experimentally restrict sleep show deficits across many cognitive domains, including executive functions, long-term memory, as well as emotional processing and regulation. Insufficient sleep in adolescents can longitudinally predict depression, thought problems, and lower crystallized intelligence, as well as structural brain properties. In older adults, it can predict the onset of chronic disease, including Alzheimer’s disease. Repeated nights of insufficient sleep (eg, three to four nights of four to six hours of sleep) have been shown to dysregulate hormone release, elevate body temperature and heart rate, stimulate appetite, and create an imbalance between the two branches of the autonomic nervous system by prolonging sympathetic activity and reducing parasympathetic restorative activity.
Given this ever-increasing list of ill effects of poor sleep, the quest for an effective, inexpensive, and manageable intervention for sleep loss often leads to the question: What about naps? A nap is typically defined as a period of sleep between five minutes to three hours, although naps can occur at any hour, they are usually daytime sleep behaviors. Between 40% and 60% of adults nap regularly, at least once a week, and, excluding novelty nap boutiques, they are free of charge and require little management or oversight. Yet, for all their apparent positive aspects, the jury is still out on whether naps should be recommended as a sleep loss countermeasure due to the lack of agreement across studies as to their effects on health.
Naps are studied in primarily two scientific contexts: laboratory experimental studies and epidemiological studies. Laboratory experimental studies measure the effect of short bouts of sleep as a fatigue countermeasure or cognitive enhancer under total sleep deprivation, sleep restriction (four to six hours of nighttime sleep), or well-rested conditions. These experiments are usually conducted in small (20 to 30 participants) convenience samples of young adults without medical and mental health problems. Performance on computer-based cognitive tasks is tested before and after naps of varying durations. By varying nap durations, researchers can test the impact of specific sleep stages on performance improvement. For example, in well-rested, intermediate chronotype individuals, a 30-minute nap between 13:00 and 15:00 will contain mostly stage 2 sleep, whereas a nap of up to 60 minutes will include slow wave sleep, and a 90-minute nap will end on a bout of rapid eye movement sleep. Studies that vary nap duration and therefore sleep quality have demonstrated an important principle of sleep’s effect on the brain and cognitive processing, namely that each sleep stage uniquely contributes to different aspects of cognitive and emotional processing. And that when naps are inserted into a person’s day, even in well-rested conditions, they tend to perform better after the nap than if they had stayed awake. Napping leads to greater vigilance, attention, memory, motor performance, and creativity, among others, compared with equivalent wake periods.1,2 Compared with the common fatigue countermeasure—caffeine—naps enhance explicit memory performance to a greater extent.
In the second context, epidemiological studies examining the impact of napping on health outcomes are typically conducted in older, less healthy, less active populations who tend to have poorer eating habits, multiple comorbidities, psychological problems, and a wide range of socioeconomic status. The strength of this approach is the sample size, which allows for correlations between factors on a large scale while providing enough data to hopefully control for possible confounds (eg, demographics, SES, exercise and eating habits, comorbidities). However, as the data were usually collected by a different group with different goals than the current epidemiologist exploring the data, there can be a disconnect between the current study goals and the variables that were initially collected by the original research team. As such, the current researcher is left with a patchwork of dissimilar variables that they must find a way to organize to answer the current question.3
When applied to the question of health effects of napping, epidemiology researchers typically divide the population into two groups, either based on a yes or no response to a napping question, or a frequency score where those who indicate napping more than one, two, or three times a week are distinguished as nappers compared to non-nappers who don’t meet these criteria. As the field lacks standard definitions for categorizing nap behavior, it is left to the discretion of the researcher to make these decisions. Furthermore, there is usually little other information collected about napping habits that could be used to better characterize napping behavior, such as lifetime nap habits, intentional vs accidental napping, and specific motivations for napping. These secondary factors have been shown to significantly moderate the effects of napping in experimental studies.
Considering the challenges, it is not surprising that there is wide disagreement across studies as to the health effects of napping.4 On the negative side, some studies have demonstrated that napping leads to increased risk of cardiovascular disease, dementia, and mortality.5-7 On the positive side, large cohort studies that control for some of these limitations report that habitual napping can predict better health outcomes, including lower mortality risk, reduced cardiovascular disease, and increased brain volume.8,9 Furthermore, age complicates matters as recent studies in older adults report that more frequent napping may be associated with reduced propensity for sleep during morning hours, and late afternoon naps were associated with earlier melatonin onset and increased evening activity, suggesting greater circadian misalignment in nappers and strategic use of napping as an evening fatigue countermeasure. More frequent napping in older adults was also correlated with lower cognitive performance in one of three cognitive domains. These results implicate more frequent and later-in-the-day napping habits in older adults may indicate altered circadian rhythms and reduced early morning sleep, with a potential functional impact on memory function. However, the same cautionary note applies to these studies, as few nap characteristics were reported that would help interpret the study outcomes and guide recommendations.10 Thus, the important and timely question of whether napping should be recommended does not, as of yet, have an answer. For clinicians weighing the multidimensional factors associated with napping in efforts to give a considered response to their patients, I can offer a set of questions that may help with tailoring responses to each individual. A lifetime history of napping can be an indicator of a health-promoting behavior, whereas a relatively recent desire to nap may reflect an underlying comorbidity that increases fatigue, sleepiness, and unintentional daytime sleep. Motivation for napping can also be revealing, as the desire to nap may be masking symptoms of depression and anxiety.11 Nighttime sleep disturbance may promote napping or, in some cases, arise from too much napping and should always be considered as a primary health measurement. In conclusion, it’s important to recognize the significance of addressing nighttime sleep disturbance and the potential impact of napping on overall health. For many, napping can be an essential and potent habit that can be encouraged throughout the lifespan for its salutary influences.
References
1. Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003 Jul;6(7):697-8. doi: 10.1038/nn1078. PMID: 12819785.
2. Jones BJ, Spencer RMC. Role of Napping for Learning across the Lifespan. Curr Sleep Med Rep. 2020 Dec;6(4):290-297. Doi: 10.1007/s40675-020-00193-9. Epub 2020 Nov 12. PMID: 33816064; PMCID: PMC8011550.
3. Dunietz GL, Jansen EC, Hershner S, O’Brien LM, Peterson KE, Baylin A. Parallel Assessment Challenges in Nutritional and Sleep Epidemiology. Am J Epidemiol. 2021 Jun 1;190(6):954-961. doi: 10.1093/aje/kwaa230. PMID: 33089309; PMCID: PMC8168107.
4. Stang A. Daytime napping and health consequences: much epidemiologic work to do. Sleep Med. 2015 Jul;16(7):809-10. doi: 10.1016/j.sleep.2015.02.522. Epub 2015 Feb 14. PMID: 25772544.
5. Li, P., Gao, L., Yu, L., Zheng, X., Ulsa, M. C., Yang, H.-W., Gaba, A., Yaffe, K., Bennett, D. A., Buchman, A. S., Hu, K., & Leng, Y. (2022). Daytime napping and Alzheimer’s dementia: A potential bidirectional relationship. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association. https://doi.org/10.1002/alz.12636
6. Stang A, Dragano N., Moebus S, et al. Midday naps and the risk of coronary artery disease: results of the Heinz Nixdorf Recall Study Sleep, 35 (12) (2012), pp. 1705-1712
7. Wang K, Hu L, Wang L, Shu HN, Wang YT, Yuan Y, Cheng HP, Zhang YQ. Midday Napping, Nighttime Sleep, and Mortality: Prospective Cohort Evidence in China. Biomed Environ Sci. 2023 Aug 20;36(8):702-714. doi: 10.3967/bes2023.073. PMID: 37711082.
8. Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007 Feb 12;167(3):296-301. Doi: 10.1001/archinte.167.3.296. PMID: 17296887.
9. Paz V, Dashti HS, Garfield V. Is there an association between daytime napping, cognitive function, and brain volume? A Mendelian randomization study in the UK Biobank. Sleep Health. 2023 Oct;9(5):786-793. Doi: 10.1016/j.sleh.2023.05.002. Epub 2023 Jun 20. PMID: 37344293.
10. Mednick SC. Is napping in older adults problematic or productive? The answer may lie in the reason they nap. Sleep. 2024 May 10;47(5):zsae056. doi: 10.1093/sleep/zsae056. PMID: 38421680; PMCID: PMC11082470.
11. Duggan KA, McDevitt EA, Whitehurst LN, Mednick SC. To Nap, Perchance to DREAM: A Factor Analysis of College Students’ Self-Reported Reasons for Napping. Behav Sleep Med. 2018 Mar-Apr;16(2):135-153. doi: 10.1080/15402002.2016.1178115. Epub 2016 Jun 27. PMID: 27347727; PMCID: PMC5374038.
I was invited to a cardiology conference to talk about sleep, specifically the benefits of napping for health and cognition. After the talk, along with the usual questions related to my research, the cardiac surgeons in the room shifted the conversation to better resemble a group therapy session, sharing their harrowing personal tales of coping with sleep loss on the job. The most dramatic story involved a resident in a military hospital who, unable to avoid the effects of her mounting sleep loss, did a face plant into the open chest of the patient on the surgery table.
Epidemiology studies have associated insufficient sleep with increased disease risk, including cardiovascular and metabolic disease, diabetes, cancer, Alzheimer’s disease and related dementias, as well as early mortality. Laboratory studies that experimentally restrict sleep show deficits across many cognitive domains, including executive functions, long-term memory, as well as emotional processing and regulation. Insufficient sleep in adolescents can longitudinally predict depression, thought problems, and lower crystallized intelligence, as well as structural brain properties. In older adults, it can predict the onset of chronic disease, including Alzheimer’s disease. Repeated nights of insufficient sleep (eg, three to four nights of four to six hours of sleep) have been shown to dysregulate hormone release, elevate body temperature and heart rate, stimulate appetite, and create an imbalance between the two branches of the autonomic nervous system by prolonging sympathetic activity and reducing parasympathetic restorative activity.
Given this ever-increasing list of ill effects of poor sleep, the quest for an effective, inexpensive, and manageable intervention for sleep loss often leads to the question: What about naps? A nap is typically defined as a period of sleep between five minutes to three hours, although naps can occur at any hour, they are usually daytime sleep behaviors. Between 40% and 60% of adults nap regularly, at least once a week, and, excluding novelty nap boutiques, they are free of charge and require little management or oversight. Yet, for all their apparent positive aspects, the jury is still out on whether naps should be recommended as a sleep loss countermeasure due to the lack of agreement across studies as to their effects on health.
Naps are studied in primarily two scientific contexts: laboratory experimental studies and epidemiological studies. Laboratory experimental studies measure the effect of short bouts of sleep as a fatigue countermeasure or cognitive enhancer under total sleep deprivation, sleep restriction (four to six hours of nighttime sleep), or well-rested conditions. These experiments are usually conducted in small (20 to 30 participants) convenience samples of young adults without medical and mental health problems. Performance on computer-based cognitive tasks is tested before and after naps of varying durations. By varying nap durations, researchers can test the impact of specific sleep stages on performance improvement. For example, in well-rested, intermediate chronotype individuals, a 30-minute nap between 13:00 and 15:00 will contain mostly stage 2 sleep, whereas a nap of up to 60 minutes will include slow wave sleep, and a 90-minute nap will end on a bout of rapid eye movement sleep. Studies that vary nap duration and therefore sleep quality have demonstrated an important principle of sleep’s effect on the brain and cognitive processing, namely that each sleep stage uniquely contributes to different aspects of cognitive and emotional processing. And that when naps are inserted into a person’s day, even in well-rested conditions, they tend to perform better after the nap than if they had stayed awake. Napping leads to greater vigilance, attention, memory, motor performance, and creativity, among others, compared with equivalent wake periods.1,2 Compared with the common fatigue countermeasure—caffeine—naps enhance explicit memory performance to a greater extent.
In the second context, epidemiological studies examining the impact of napping on health outcomes are typically conducted in older, less healthy, less active populations who tend to have poorer eating habits, multiple comorbidities, psychological problems, and a wide range of socioeconomic status. The strength of this approach is the sample size, which allows for correlations between factors on a large scale while providing enough data to hopefully control for possible confounds (eg, demographics, SES, exercise and eating habits, comorbidities). However, as the data were usually collected by a different group with different goals than the current epidemiologist exploring the data, there can be a disconnect between the current study goals and the variables that were initially collected by the original research team. As such, the current researcher is left with a patchwork of dissimilar variables that they must find a way to organize to answer the current question.3
When applied to the question of health effects of napping, epidemiology researchers typically divide the population into two groups, either based on a yes or no response to a napping question, or a frequency score where those who indicate napping more than one, two, or three times a week are distinguished as nappers compared to non-nappers who don’t meet these criteria. As the field lacks standard definitions for categorizing nap behavior, it is left to the discretion of the researcher to make these decisions. Furthermore, there is usually little other information collected about napping habits that could be used to better characterize napping behavior, such as lifetime nap habits, intentional vs accidental napping, and specific motivations for napping. These secondary factors have been shown to significantly moderate the effects of napping in experimental studies.
Considering the challenges, it is not surprising that there is wide disagreement across studies as to the health effects of napping.4 On the negative side, some studies have demonstrated that napping leads to increased risk of cardiovascular disease, dementia, and mortality.5-7 On the positive side, large cohort studies that control for some of these limitations report that habitual napping can predict better health outcomes, including lower mortality risk, reduced cardiovascular disease, and increased brain volume.8,9 Furthermore, age complicates matters as recent studies in older adults report that more frequent napping may be associated with reduced propensity for sleep during morning hours, and late afternoon naps were associated with earlier melatonin onset and increased evening activity, suggesting greater circadian misalignment in nappers and strategic use of napping as an evening fatigue countermeasure. More frequent napping in older adults was also correlated with lower cognitive performance in one of three cognitive domains. These results implicate more frequent and later-in-the-day napping habits in older adults may indicate altered circadian rhythms and reduced early morning sleep, with a potential functional impact on memory function. However, the same cautionary note applies to these studies, as few nap characteristics were reported that would help interpret the study outcomes and guide recommendations.10 Thus, the important and timely question of whether napping should be recommended does not, as of yet, have an answer. For clinicians weighing the multidimensional factors associated with napping in efforts to give a considered response to their patients, I can offer a set of questions that may help with tailoring responses to each individual. A lifetime history of napping can be an indicator of a health-promoting behavior, whereas a relatively recent desire to nap may reflect an underlying comorbidity that increases fatigue, sleepiness, and unintentional daytime sleep. Motivation for napping can also be revealing, as the desire to nap may be masking symptoms of depression and anxiety.11 Nighttime sleep disturbance may promote napping or, in some cases, arise from too much napping and should always be considered as a primary health measurement. In conclusion, it’s important to recognize the significance of addressing nighttime sleep disturbance and the potential impact of napping on overall health. For many, napping can be an essential and potent habit that can be encouraged throughout the lifespan for its salutary influences.
References
1. Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003 Jul;6(7):697-8. doi: 10.1038/nn1078. PMID: 12819785.
2. Jones BJ, Spencer RMC. Role of Napping for Learning across the Lifespan. Curr Sleep Med Rep. 2020 Dec;6(4):290-297. Doi: 10.1007/s40675-020-00193-9. Epub 2020 Nov 12. PMID: 33816064; PMCID: PMC8011550.
3. Dunietz GL, Jansen EC, Hershner S, O’Brien LM, Peterson KE, Baylin A. Parallel Assessment Challenges in Nutritional and Sleep Epidemiology. Am J Epidemiol. 2021 Jun 1;190(6):954-961. doi: 10.1093/aje/kwaa230. PMID: 33089309; PMCID: PMC8168107.
4. Stang A. Daytime napping and health consequences: much epidemiologic work to do. Sleep Med. 2015 Jul;16(7):809-10. doi: 10.1016/j.sleep.2015.02.522. Epub 2015 Feb 14. PMID: 25772544.
5. Li, P., Gao, L., Yu, L., Zheng, X., Ulsa, M. C., Yang, H.-W., Gaba, A., Yaffe, K., Bennett, D. A., Buchman, A. S., Hu, K., & Leng, Y. (2022). Daytime napping and Alzheimer’s dementia: A potential bidirectional relationship. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association. https://doi.org/10.1002/alz.12636
6. Stang A, Dragano N., Moebus S, et al. Midday naps and the risk of coronary artery disease: results of the Heinz Nixdorf Recall Study Sleep, 35 (12) (2012), pp. 1705-1712
7. Wang K, Hu L, Wang L, Shu HN, Wang YT, Yuan Y, Cheng HP, Zhang YQ. Midday Napping, Nighttime Sleep, and Mortality: Prospective Cohort Evidence in China. Biomed Environ Sci. 2023 Aug 20;36(8):702-714. doi: 10.3967/bes2023.073. PMID: 37711082.
8. Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007 Feb 12;167(3):296-301. Doi: 10.1001/archinte.167.3.296. PMID: 17296887.
9. Paz V, Dashti HS, Garfield V. Is there an association between daytime napping, cognitive function, and brain volume? A Mendelian randomization study in the UK Biobank. Sleep Health. 2023 Oct;9(5):786-793. Doi: 10.1016/j.sleh.2023.05.002. Epub 2023 Jun 20. PMID: 37344293.
10. Mednick SC. Is napping in older adults problematic or productive? The answer may lie in the reason they nap. Sleep. 2024 May 10;47(5):zsae056. doi: 10.1093/sleep/zsae056. PMID: 38421680; PMCID: PMC11082470.
11. Duggan KA, McDevitt EA, Whitehurst LN, Mednick SC. To Nap, Perchance to DREAM: A Factor Analysis of College Students’ Self-Reported Reasons for Napping. Behav Sleep Med. 2018 Mar-Apr;16(2):135-153. doi: 10.1080/15402002.2016.1178115. Epub 2016 Jun 27. PMID: 27347727; PMCID: PMC5374038.
I was invited to a cardiology conference to talk about sleep, specifically the benefits of napping for health and cognition. After the talk, along with the usual questions related to my research, the cardiac surgeons in the room shifted the conversation to better resemble a group therapy session, sharing their harrowing personal tales of coping with sleep loss on the job. The most dramatic story involved a resident in a military hospital who, unable to avoid the effects of her mounting sleep loss, did a face plant into the open chest of the patient on the surgery table.
Epidemiology studies have associated insufficient sleep with increased disease risk, including cardiovascular and metabolic disease, diabetes, cancer, Alzheimer’s disease and related dementias, as well as early mortality. Laboratory studies that experimentally restrict sleep show deficits across many cognitive domains, including executive functions, long-term memory, as well as emotional processing and regulation. Insufficient sleep in adolescents can longitudinally predict depression, thought problems, and lower crystallized intelligence, as well as structural brain properties. In older adults, it can predict the onset of chronic disease, including Alzheimer’s disease. Repeated nights of insufficient sleep (eg, three to four nights of four to six hours of sleep) have been shown to dysregulate hormone release, elevate body temperature and heart rate, stimulate appetite, and create an imbalance between the two branches of the autonomic nervous system by prolonging sympathetic activity and reducing parasympathetic restorative activity.
Given this ever-increasing list of ill effects of poor sleep, the quest for an effective, inexpensive, and manageable intervention for sleep loss often leads to the question: What about naps? A nap is typically defined as a period of sleep between five minutes to three hours, although naps can occur at any hour, they are usually daytime sleep behaviors. Between 40% and 60% of adults nap regularly, at least once a week, and, excluding novelty nap boutiques, they are free of charge and require little management or oversight. Yet, for all their apparent positive aspects, the jury is still out on whether naps should be recommended as a sleep loss countermeasure due to the lack of agreement across studies as to their effects on health.
Naps are studied in primarily two scientific contexts: laboratory experimental studies and epidemiological studies. Laboratory experimental studies measure the effect of short bouts of sleep as a fatigue countermeasure or cognitive enhancer under total sleep deprivation, sleep restriction (four to six hours of nighttime sleep), or well-rested conditions. These experiments are usually conducted in small (20 to 30 participants) convenience samples of young adults without medical and mental health problems. Performance on computer-based cognitive tasks is tested before and after naps of varying durations. By varying nap durations, researchers can test the impact of specific sleep stages on performance improvement. For example, in well-rested, intermediate chronotype individuals, a 30-minute nap between 13:00 and 15:00 will contain mostly stage 2 sleep, whereas a nap of up to 60 minutes will include slow wave sleep, and a 90-minute nap will end on a bout of rapid eye movement sleep. Studies that vary nap duration and therefore sleep quality have demonstrated an important principle of sleep’s effect on the brain and cognitive processing, namely that each sleep stage uniquely contributes to different aspects of cognitive and emotional processing. And that when naps are inserted into a person’s day, even in well-rested conditions, they tend to perform better after the nap than if they had stayed awake. Napping leads to greater vigilance, attention, memory, motor performance, and creativity, among others, compared with equivalent wake periods.1,2 Compared with the common fatigue countermeasure—caffeine—naps enhance explicit memory performance to a greater extent.
In the second context, epidemiological studies examining the impact of napping on health outcomes are typically conducted in older, less healthy, less active populations who tend to have poorer eating habits, multiple comorbidities, psychological problems, and a wide range of socioeconomic status. The strength of this approach is the sample size, which allows for correlations between factors on a large scale while providing enough data to hopefully control for possible confounds (eg, demographics, SES, exercise and eating habits, comorbidities). However, as the data were usually collected by a different group with different goals than the current epidemiologist exploring the data, there can be a disconnect between the current study goals and the variables that were initially collected by the original research team. As such, the current researcher is left with a patchwork of dissimilar variables that they must find a way to organize to answer the current question.3
When applied to the question of health effects of napping, epidemiology researchers typically divide the population into two groups, either based on a yes or no response to a napping question, or a frequency score where those who indicate napping more than one, two, or three times a week are distinguished as nappers compared to non-nappers who don’t meet these criteria. As the field lacks standard definitions for categorizing nap behavior, it is left to the discretion of the researcher to make these decisions. Furthermore, there is usually little other information collected about napping habits that could be used to better characterize napping behavior, such as lifetime nap habits, intentional vs accidental napping, and specific motivations for napping. These secondary factors have been shown to significantly moderate the effects of napping in experimental studies.
Considering the challenges, it is not surprising that there is wide disagreement across studies as to the health effects of napping.4 On the negative side, some studies have demonstrated that napping leads to increased risk of cardiovascular disease, dementia, and mortality.5-7 On the positive side, large cohort studies that control for some of these limitations report that habitual napping can predict better health outcomes, including lower mortality risk, reduced cardiovascular disease, and increased brain volume.8,9 Furthermore, age complicates matters as recent studies in older adults report that more frequent napping may be associated with reduced propensity for sleep during morning hours, and late afternoon naps were associated with earlier melatonin onset and increased evening activity, suggesting greater circadian misalignment in nappers and strategic use of napping as an evening fatigue countermeasure. More frequent napping in older adults was also correlated with lower cognitive performance in one of three cognitive domains. These results implicate more frequent and later-in-the-day napping habits in older adults may indicate altered circadian rhythms and reduced early morning sleep, with a potential functional impact on memory function. However, the same cautionary note applies to these studies, as few nap characteristics were reported that would help interpret the study outcomes and guide recommendations.10 Thus, the important and timely question of whether napping should be recommended does not, as of yet, have an answer. For clinicians weighing the multidimensional factors associated with napping in efforts to give a considered response to their patients, I can offer a set of questions that may help with tailoring responses to each individual. A lifetime history of napping can be an indicator of a health-promoting behavior, whereas a relatively recent desire to nap may reflect an underlying comorbidity that increases fatigue, sleepiness, and unintentional daytime sleep. Motivation for napping can also be revealing, as the desire to nap may be masking symptoms of depression and anxiety.11 Nighttime sleep disturbance may promote napping or, in some cases, arise from too much napping and should always be considered as a primary health measurement. In conclusion, it’s important to recognize the significance of addressing nighttime sleep disturbance and the potential impact of napping on overall health. For many, napping can be an essential and potent habit that can be encouraged throughout the lifespan for its salutary influences.
References
1. Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003 Jul;6(7):697-8. doi: 10.1038/nn1078. PMID: 12819785.
2. Jones BJ, Spencer RMC. Role of Napping for Learning across the Lifespan. Curr Sleep Med Rep. 2020 Dec;6(4):290-297. Doi: 10.1007/s40675-020-00193-9. Epub 2020 Nov 12. PMID: 33816064; PMCID: PMC8011550.
3. Dunietz GL, Jansen EC, Hershner S, O’Brien LM, Peterson KE, Baylin A. Parallel Assessment Challenges in Nutritional and Sleep Epidemiology. Am J Epidemiol. 2021 Jun 1;190(6):954-961. doi: 10.1093/aje/kwaa230. PMID: 33089309; PMCID: PMC8168107.
4. Stang A. Daytime napping and health consequences: much epidemiologic work to do. Sleep Med. 2015 Jul;16(7):809-10. doi: 10.1016/j.sleep.2015.02.522. Epub 2015 Feb 14. PMID: 25772544.
5. Li, P., Gao, L., Yu, L., Zheng, X., Ulsa, M. C., Yang, H.-W., Gaba, A., Yaffe, K., Bennett, D. A., Buchman, A. S., Hu, K., & Leng, Y. (2022). Daytime napping and Alzheimer’s dementia: A potential bidirectional relationship. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association. https://doi.org/10.1002/alz.12636
6. Stang A, Dragano N., Moebus S, et al. Midday naps and the risk of coronary artery disease: results of the Heinz Nixdorf Recall Study Sleep, 35 (12) (2012), pp. 1705-1712
7. Wang K, Hu L, Wang L, Shu HN, Wang YT, Yuan Y, Cheng HP, Zhang YQ. Midday Napping, Nighttime Sleep, and Mortality: Prospective Cohort Evidence in China. Biomed Environ Sci. 2023 Aug 20;36(8):702-714. doi: 10.3967/bes2023.073. PMID: 37711082.
8. Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007 Feb 12;167(3):296-301. Doi: 10.1001/archinte.167.3.296. PMID: 17296887.
9. Paz V, Dashti HS, Garfield V. Is there an association between daytime napping, cognitive function, and brain volume? A Mendelian randomization study in the UK Biobank. Sleep Health. 2023 Oct;9(5):786-793. Doi: 10.1016/j.sleh.2023.05.002. Epub 2023 Jun 20. PMID: 37344293.
10. Mednick SC. Is napping in older adults problematic or productive? The answer may lie in the reason they nap. Sleep. 2024 May 10;47(5):zsae056. doi: 10.1093/sleep/zsae056. PMID: 38421680; PMCID: PMC11082470.
11. Duggan KA, McDevitt EA, Whitehurst LN, Mednick SC. To Nap, Perchance to DREAM: A Factor Analysis of College Students’ Self-Reported Reasons for Napping. Behav Sleep Med. 2018 Mar-Apr;16(2):135-153. doi: 10.1080/15402002.2016.1178115. Epub 2016 Jun 27. PMID: 27347727; PMCID: PMC5374038.
OSA in pregnancy: Who to test, how to screen, and does treatment help?
The increased prevalence in pregnancy can be explained by physiologic changes impacting the upper airway such as increases in maternal blood volume and reductions in oncotic pressure, as well as increases in circulating levels of estrogen and progesterone. OSA in pregnancy is associated with adverse perinatal outcomes such as hypertensive disorders of pregnancy, gestational diabetes, severe maternal morbidity abnormalities in fetal growth, preterm birth, and congenital abnormalities in the offspring.2,3 Despite this evidence, guidelines on the screening, diagnosis, and treatment of OSA in pregnancy have only recently been published and will be reviewed here.1
The obstetric subcommittee of the Society of Anesthesia and Sleep Medicine that produced these guidelines had expertise in obstetric anesthesiology, sleep medicine and sleep research, high-risk obstetrics, and obstetric medicine. The guideline aimed to answer 3 questions: 1) Who should be screened in pregnancy for OSA, 2) how to make a diagnosis of OSA in pregnancy and the postpartum period, and 3) what is the treatment for OSA in pregnancy and the postpartum period. Although the estimated number of annual pregnancies in the US declined between 2010 to 2019, these clinical questions remain critical considering the obesity epidemic, the ability to conceive despite advanced maternal age and chronic illnesses with the use of fertility treatments, and the crisis of severe maternal morbidity and mortality. As sleep disordered breathing (SDB) has been associated with many conditions linked to maternal mortality, better management of SDB in this population is key.
Screening for OSA in the pregnant population
The guideline does not support universal screening of all people who are pregnant, but rather suggests that people who are pregnant and at high risk for OSA, such as those with a body mass index (BMI) ≥30 kg/m2 and those with hypertensive disorders of pregnancy, or diabetes, in the index pregnancy or a prior pregnancy, be screened for OSA in the first or second trimester.1 Screening for OSA in pregnancy in limited populations is recommended due to the lower yield of universal screening and its potential burden on the health care system. Furthermore, screening for OSA in early pregnancy is suggested given the practical challenges of arranging testing, initiating, and allowing time for patients to become acclimated to therapy in later stages of pregnancy. However, even when timing of diagnosis may not allow for appropriate treatment of OSA during pregnancy, knowing a person’s OSA status before delivery is beneficial, particularly for patients at risk for Cesarean delivery who may require intubation and exposure to sedative medications, as well as those receiving epidural anesthesia, as OSA is a risk factor for respiratory depression.
Although screening was thought to be beneficial in specific populations, there is insufficient evidence to recommend any one screening tool. The guideline made recommendations against the use of the Berlin questionnaire, STOP-BANG questionnaire, Epworth Sleepiness Scale, or the ASA checklist.1 These screening tools were developed and validated in nonpregnant patient populations and their pooled sensitivity and specificity to detect OSA in pregnancy is low. Individual components of these screening tools, such as prepregnancy BMI, frequency and volume of snoring, hypertension, and neck circumference ≥16 inches have, however, been associated with OSA status.
Pregnancy-specific OSA screening tools have been proposed.4,5 The guideline suggests these pregnancy-specific tools may be considered for screening for OSA in pregnancy but still require external validation, especially in high-risk populations. The committee agreed that individuals with BMI >30kg/m2, hypertension, diabetes, and those with a history of difficult intubation or Mallampati score III or IV are considered at risk for OSA in pregnancy.
Diagnosis of OSA in the pregnant population
In the general population, in-laboratory polysomnogram (PSG) is the gold standard diagnostic test. However, for patients in whom uncomplicated OSA is suspected with a moderate to high pretest probability, unattended home sleep apnea testing (HSAT) is a reasonable initial study. On the other hand, in-lab PSG is recommended in mission-critical workers and when coexisting respiratory sleep disorders, or nonrespiratory sleep disorders, are suspected. For individuals who are pregnant and suspected of having OSA, the guideline suggests that HSAT is a reasonable diagnostic tool, as many level III devices have demonstrated good agreement between the respiratory disturbance index (RDI) and apnea-hypopnea index (AHI) measured by PSG.6 Notably, most studies have examined the performance of level III devices in late pregnancy in populations with obesity; hence, the performance of these devices in early pregnancy when risk for OSA is lower, or more subtle forms of SDB may be more common, is less clear but may be an acceptable first-line test.
The guideline did not provide recommendations for next steps following an inconclusive, technically inadequate, or negative HSAT. However, recommendations to proceed with in-lab PSG in individuals with clinical suspicion for OSA and a negative HSAT is a reasonable approach, keeping in mind the time restrictions of pregnancy. The more delayed the diagnosis, the less time there will be for initiation of and acclimation to therapy to maximize potential benefits during pregnancy. HSAT is especially practical and convenient for individuals with young families. The guideline does not recommend the use of overnight oximetry for diagnostic purposes.1
The postpartum period is usually associated with weight loss and reversal of pregnancy physiology. Generally, the decision to perform a repeat sleep study following weight loss is individualized, based on factors such as improved symptoms or sustained, significant weight loss. Though data show improvement in AHI following delivery, small studies show persistent OSA in nearly half of individuals diagnosed in pregnancy. Hence, as pregnancy increases the risk for OSA, and given that the postpartum status is not always associated with resolution of OSA, the guideline recommends considering repeat diagnostic testing in the postpartum period.1 The decision to repeat testing also depends on whether OSA or OSA symptoms predated pregnancy, on the persistence of symptoms, and the degree of weight loss with delivery and the postpartum body habitus.
Treatment of OSA in the pregnant population
The guideline recommends behavior modification in OSA similarly to individuals who are not pregnant (avoidance of sedatives, smoking, and alcohol).1 However, weight loss is not recommended in pregnancy due to the potential for harm to the fetus.
The gold standard treatment for people who are pregnant and have OSA is continuous positive airway pressure (CPAP). Treatment of OSA in pregnancy is complicated by the fact that very few women are referred to sleep practices due to time restrictions and logistical reasons, and that data demonstrating improved pregnancy outcomes with CPAP are scarce, limiting the prioritization of OSA management. However, expert consensus considers a theoretical benefit in the context of lack of current evidence of harm from treatment. Hence, at this point, the guideline recommends counseling around CPAP therapy be aimed at improvement in symptoms, AHI, and quality of life, rather than pregnancy-specific outcomes.1 This recommendation was based on observations from small case series that demonstrated improved breathing parameters during sleep and symptoms, and small randomized controlled trials (RCT), limited by short-term exposure to the intervention. However, since the publication of this guideline, a large RCT that randomized pregnant women with SDB to CPAP or usual care has demonstrated significantly lower diastolic blood pressure, an altered diastolic blood pressure trajectory, and a lower rate of preeclampsia in the group treated with CPAP compared with usual care.7
This guideline provides helpful insight on who to screen and how to manage OSA in pregnancy but additional research is needed to elucidate benefits of treatment and its effects on maternal and neonatal outcomes. Multidisciplinary collaborations between obstetric and sleep teams are necessary to ensure that screening and diagnostic strategies result in management change for improved outcomes.
References
1. Dominguez JE, Cantrell S, Habib AS, et al. Society of Anesthesia and Sleep Medicine and the Society for Obstetric Anesthesia and Perinatology Consensus Guideline on the screening, diagnosis and treatment of obstructive sleep apnea in pregnancy. Obstet Gynecol. 2023;142(2):403-423.
2. Bourjeily, G, Danilack C, Bublitz M, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2000;66:233-240.
3. Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: a population-based study. Pregnancy Hypertens. 2022;30:215-220.
4. Izci-Balserak B, Zhu B, Gurubhagavatula I, Keenan BT, Pien GW. A screening algorithm for obstructive sleep apnea in pregnancy. Ann Am Thorac Soc. 2019;16(10):1286-1294.
5. Louis J, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1.e12.
6. Sharkey K, Waters K, Millman R, Moore R, Martin SM, Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnogram in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497-502.
7. Tantrakul V, Ingsathit A, Liamsombut S, et al. Treatment of obstructive sleep apnea in high-risk pregnancy: a multicenter randomized controlled trial. Respir Res. 2023;24(1):171.
The increased prevalence in pregnancy can be explained by physiologic changes impacting the upper airway such as increases in maternal blood volume and reductions in oncotic pressure, as well as increases in circulating levels of estrogen and progesterone. OSA in pregnancy is associated with adverse perinatal outcomes such as hypertensive disorders of pregnancy, gestational diabetes, severe maternal morbidity abnormalities in fetal growth, preterm birth, and congenital abnormalities in the offspring.2,3 Despite this evidence, guidelines on the screening, diagnosis, and treatment of OSA in pregnancy have only recently been published and will be reviewed here.1
The obstetric subcommittee of the Society of Anesthesia and Sleep Medicine that produced these guidelines had expertise in obstetric anesthesiology, sleep medicine and sleep research, high-risk obstetrics, and obstetric medicine. The guideline aimed to answer 3 questions: 1) Who should be screened in pregnancy for OSA, 2) how to make a diagnosis of OSA in pregnancy and the postpartum period, and 3) what is the treatment for OSA in pregnancy and the postpartum period. Although the estimated number of annual pregnancies in the US declined between 2010 to 2019, these clinical questions remain critical considering the obesity epidemic, the ability to conceive despite advanced maternal age and chronic illnesses with the use of fertility treatments, and the crisis of severe maternal morbidity and mortality. As sleep disordered breathing (SDB) has been associated with many conditions linked to maternal mortality, better management of SDB in this population is key.
Screening for OSA in the pregnant population
The guideline does not support universal screening of all people who are pregnant, but rather suggests that people who are pregnant and at high risk for OSA, such as those with a body mass index (BMI) ≥30 kg/m2 and those with hypertensive disorders of pregnancy, or diabetes, in the index pregnancy or a prior pregnancy, be screened for OSA in the first or second trimester.1 Screening for OSA in pregnancy in limited populations is recommended due to the lower yield of universal screening and its potential burden on the health care system. Furthermore, screening for OSA in early pregnancy is suggested given the practical challenges of arranging testing, initiating, and allowing time for patients to become acclimated to therapy in later stages of pregnancy. However, even when timing of diagnosis may not allow for appropriate treatment of OSA during pregnancy, knowing a person’s OSA status before delivery is beneficial, particularly for patients at risk for Cesarean delivery who may require intubation and exposure to sedative medications, as well as those receiving epidural anesthesia, as OSA is a risk factor for respiratory depression.
Although screening was thought to be beneficial in specific populations, there is insufficient evidence to recommend any one screening tool. The guideline made recommendations against the use of the Berlin questionnaire, STOP-BANG questionnaire, Epworth Sleepiness Scale, or the ASA checklist.1 These screening tools were developed and validated in nonpregnant patient populations and their pooled sensitivity and specificity to detect OSA in pregnancy is low. Individual components of these screening tools, such as prepregnancy BMI, frequency and volume of snoring, hypertension, and neck circumference ≥16 inches have, however, been associated with OSA status.
Pregnancy-specific OSA screening tools have been proposed.4,5 The guideline suggests these pregnancy-specific tools may be considered for screening for OSA in pregnancy but still require external validation, especially in high-risk populations. The committee agreed that individuals with BMI >30kg/m2, hypertension, diabetes, and those with a history of difficult intubation or Mallampati score III or IV are considered at risk for OSA in pregnancy.
Diagnosis of OSA in the pregnant population
In the general population, in-laboratory polysomnogram (PSG) is the gold standard diagnostic test. However, for patients in whom uncomplicated OSA is suspected with a moderate to high pretest probability, unattended home sleep apnea testing (HSAT) is a reasonable initial study. On the other hand, in-lab PSG is recommended in mission-critical workers and when coexisting respiratory sleep disorders, or nonrespiratory sleep disorders, are suspected. For individuals who are pregnant and suspected of having OSA, the guideline suggests that HSAT is a reasonable diagnostic tool, as many level III devices have demonstrated good agreement between the respiratory disturbance index (RDI) and apnea-hypopnea index (AHI) measured by PSG.6 Notably, most studies have examined the performance of level III devices in late pregnancy in populations with obesity; hence, the performance of these devices in early pregnancy when risk for OSA is lower, or more subtle forms of SDB may be more common, is less clear but may be an acceptable first-line test.
The guideline did not provide recommendations for next steps following an inconclusive, technically inadequate, or negative HSAT. However, recommendations to proceed with in-lab PSG in individuals with clinical suspicion for OSA and a negative HSAT is a reasonable approach, keeping in mind the time restrictions of pregnancy. The more delayed the diagnosis, the less time there will be for initiation of and acclimation to therapy to maximize potential benefits during pregnancy. HSAT is especially practical and convenient for individuals with young families. The guideline does not recommend the use of overnight oximetry for diagnostic purposes.1
The postpartum period is usually associated with weight loss and reversal of pregnancy physiology. Generally, the decision to perform a repeat sleep study following weight loss is individualized, based on factors such as improved symptoms or sustained, significant weight loss. Though data show improvement in AHI following delivery, small studies show persistent OSA in nearly half of individuals diagnosed in pregnancy. Hence, as pregnancy increases the risk for OSA, and given that the postpartum status is not always associated with resolution of OSA, the guideline recommends considering repeat diagnostic testing in the postpartum period.1 The decision to repeat testing also depends on whether OSA or OSA symptoms predated pregnancy, on the persistence of symptoms, and the degree of weight loss with delivery and the postpartum body habitus.
Treatment of OSA in the pregnant population
The guideline recommends behavior modification in OSA similarly to individuals who are not pregnant (avoidance of sedatives, smoking, and alcohol).1 However, weight loss is not recommended in pregnancy due to the potential for harm to the fetus.
The gold standard treatment for people who are pregnant and have OSA is continuous positive airway pressure (CPAP). Treatment of OSA in pregnancy is complicated by the fact that very few women are referred to sleep practices due to time restrictions and logistical reasons, and that data demonstrating improved pregnancy outcomes with CPAP are scarce, limiting the prioritization of OSA management. However, expert consensus considers a theoretical benefit in the context of lack of current evidence of harm from treatment. Hence, at this point, the guideline recommends counseling around CPAP therapy be aimed at improvement in symptoms, AHI, and quality of life, rather than pregnancy-specific outcomes.1 This recommendation was based on observations from small case series that demonstrated improved breathing parameters during sleep and symptoms, and small randomized controlled trials (RCT), limited by short-term exposure to the intervention. However, since the publication of this guideline, a large RCT that randomized pregnant women with SDB to CPAP or usual care has demonstrated significantly lower diastolic blood pressure, an altered diastolic blood pressure trajectory, and a lower rate of preeclampsia in the group treated with CPAP compared with usual care.7
This guideline provides helpful insight on who to screen and how to manage OSA in pregnancy but additional research is needed to elucidate benefits of treatment and its effects on maternal and neonatal outcomes. Multidisciplinary collaborations between obstetric and sleep teams are necessary to ensure that screening and diagnostic strategies result in management change for improved outcomes.
References
1. Dominguez JE, Cantrell S, Habib AS, et al. Society of Anesthesia and Sleep Medicine and the Society for Obstetric Anesthesia and Perinatology Consensus Guideline on the screening, diagnosis and treatment of obstructive sleep apnea in pregnancy. Obstet Gynecol. 2023;142(2):403-423.
2. Bourjeily, G, Danilack C, Bublitz M, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2000;66:233-240.
3. Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: a population-based study. Pregnancy Hypertens. 2022;30:215-220.
4. Izci-Balserak B, Zhu B, Gurubhagavatula I, Keenan BT, Pien GW. A screening algorithm for obstructive sleep apnea in pregnancy. Ann Am Thorac Soc. 2019;16(10):1286-1294.
5. Louis J, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1.e12.
6. Sharkey K, Waters K, Millman R, Moore R, Martin SM, Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnogram in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497-502.
7. Tantrakul V, Ingsathit A, Liamsombut S, et al. Treatment of obstructive sleep apnea in high-risk pregnancy: a multicenter randomized controlled trial. Respir Res. 2023;24(1):171.
The increased prevalence in pregnancy can be explained by physiologic changes impacting the upper airway such as increases in maternal blood volume and reductions in oncotic pressure, as well as increases in circulating levels of estrogen and progesterone. OSA in pregnancy is associated with adverse perinatal outcomes such as hypertensive disorders of pregnancy, gestational diabetes, severe maternal morbidity abnormalities in fetal growth, preterm birth, and congenital abnormalities in the offspring.2,3 Despite this evidence, guidelines on the screening, diagnosis, and treatment of OSA in pregnancy have only recently been published and will be reviewed here.1
The obstetric subcommittee of the Society of Anesthesia and Sleep Medicine that produced these guidelines had expertise in obstetric anesthesiology, sleep medicine and sleep research, high-risk obstetrics, and obstetric medicine. The guideline aimed to answer 3 questions: 1) Who should be screened in pregnancy for OSA, 2) how to make a diagnosis of OSA in pregnancy and the postpartum period, and 3) what is the treatment for OSA in pregnancy and the postpartum period. Although the estimated number of annual pregnancies in the US declined between 2010 to 2019, these clinical questions remain critical considering the obesity epidemic, the ability to conceive despite advanced maternal age and chronic illnesses with the use of fertility treatments, and the crisis of severe maternal morbidity and mortality. As sleep disordered breathing (SDB) has been associated with many conditions linked to maternal mortality, better management of SDB in this population is key.
Screening for OSA in the pregnant population
The guideline does not support universal screening of all people who are pregnant, but rather suggests that people who are pregnant and at high risk for OSA, such as those with a body mass index (BMI) ≥30 kg/m2 and those with hypertensive disorders of pregnancy, or diabetes, in the index pregnancy or a prior pregnancy, be screened for OSA in the first or second trimester.1 Screening for OSA in pregnancy in limited populations is recommended due to the lower yield of universal screening and its potential burden on the health care system. Furthermore, screening for OSA in early pregnancy is suggested given the practical challenges of arranging testing, initiating, and allowing time for patients to become acclimated to therapy in later stages of pregnancy. However, even when timing of diagnosis may not allow for appropriate treatment of OSA during pregnancy, knowing a person’s OSA status before delivery is beneficial, particularly for patients at risk for Cesarean delivery who may require intubation and exposure to sedative medications, as well as those receiving epidural anesthesia, as OSA is a risk factor for respiratory depression.
Although screening was thought to be beneficial in specific populations, there is insufficient evidence to recommend any one screening tool. The guideline made recommendations against the use of the Berlin questionnaire, STOP-BANG questionnaire, Epworth Sleepiness Scale, or the ASA checklist.1 These screening tools were developed and validated in nonpregnant patient populations and their pooled sensitivity and specificity to detect OSA in pregnancy is low. Individual components of these screening tools, such as prepregnancy BMI, frequency and volume of snoring, hypertension, and neck circumference ≥16 inches have, however, been associated with OSA status.
Pregnancy-specific OSA screening tools have been proposed.4,5 The guideline suggests these pregnancy-specific tools may be considered for screening for OSA in pregnancy but still require external validation, especially in high-risk populations. The committee agreed that individuals with BMI >30kg/m2, hypertension, diabetes, and those with a history of difficult intubation or Mallampati score III or IV are considered at risk for OSA in pregnancy.
Diagnosis of OSA in the pregnant population
In the general population, in-laboratory polysomnogram (PSG) is the gold standard diagnostic test. However, for patients in whom uncomplicated OSA is suspected with a moderate to high pretest probability, unattended home sleep apnea testing (HSAT) is a reasonable initial study. On the other hand, in-lab PSG is recommended in mission-critical workers and when coexisting respiratory sleep disorders, or nonrespiratory sleep disorders, are suspected. For individuals who are pregnant and suspected of having OSA, the guideline suggests that HSAT is a reasonable diagnostic tool, as many level III devices have demonstrated good agreement between the respiratory disturbance index (RDI) and apnea-hypopnea index (AHI) measured by PSG.6 Notably, most studies have examined the performance of level III devices in late pregnancy in populations with obesity; hence, the performance of these devices in early pregnancy when risk for OSA is lower, or more subtle forms of SDB may be more common, is less clear but may be an acceptable first-line test.
The guideline did not provide recommendations for next steps following an inconclusive, technically inadequate, or negative HSAT. However, recommendations to proceed with in-lab PSG in individuals with clinical suspicion for OSA and a negative HSAT is a reasonable approach, keeping in mind the time restrictions of pregnancy. The more delayed the diagnosis, the less time there will be for initiation of and acclimation to therapy to maximize potential benefits during pregnancy. HSAT is especially practical and convenient for individuals with young families. The guideline does not recommend the use of overnight oximetry for diagnostic purposes.1
The postpartum period is usually associated with weight loss and reversal of pregnancy physiology. Generally, the decision to perform a repeat sleep study following weight loss is individualized, based on factors such as improved symptoms or sustained, significant weight loss. Though data show improvement in AHI following delivery, small studies show persistent OSA in nearly half of individuals diagnosed in pregnancy. Hence, as pregnancy increases the risk for OSA, and given that the postpartum status is not always associated with resolution of OSA, the guideline recommends considering repeat diagnostic testing in the postpartum period.1 The decision to repeat testing also depends on whether OSA or OSA symptoms predated pregnancy, on the persistence of symptoms, and the degree of weight loss with delivery and the postpartum body habitus.
Treatment of OSA in the pregnant population
The guideline recommends behavior modification in OSA similarly to individuals who are not pregnant (avoidance of sedatives, smoking, and alcohol).1 However, weight loss is not recommended in pregnancy due to the potential for harm to the fetus.
The gold standard treatment for people who are pregnant and have OSA is continuous positive airway pressure (CPAP). Treatment of OSA in pregnancy is complicated by the fact that very few women are referred to sleep practices due to time restrictions and logistical reasons, and that data demonstrating improved pregnancy outcomes with CPAP are scarce, limiting the prioritization of OSA management. However, expert consensus considers a theoretical benefit in the context of lack of current evidence of harm from treatment. Hence, at this point, the guideline recommends counseling around CPAP therapy be aimed at improvement in symptoms, AHI, and quality of life, rather than pregnancy-specific outcomes.1 This recommendation was based on observations from small case series that demonstrated improved breathing parameters during sleep and symptoms, and small randomized controlled trials (RCT), limited by short-term exposure to the intervention. However, since the publication of this guideline, a large RCT that randomized pregnant women with SDB to CPAP or usual care has demonstrated significantly lower diastolic blood pressure, an altered diastolic blood pressure trajectory, and a lower rate of preeclampsia in the group treated with CPAP compared with usual care.7
This guideline provides helpful insight on who to screen and how to manage OSA in pregnancy but additional research is needed to elucidate benefits of treatment and its effects on maternal and neonatal outcomes. Multidisciplinary collaborations between obstetric and sleep teams are necessary to ensure that screening and diagnostic strategies result in management change for improved outcomes.
References
1. Dominguez JE, Cantrell S, Habib AS, et al. Society of Anesthesia and Sleep Medicine and the Society for Obstetric Anesthesia and Perinatology Consensus Guideline on the screening, diagnosis and treatment of obstructive sleep apnea in pregnancy. Obstet Gynecol. 2023;142(2):403-423.
2. Bourjeily, G, Danilack C, Bublitz M, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2000;66:233-240.
3. Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: a population-based study. Pregnancy Hypertens. 2022;30:215-220.
4. Izci-Balserak B, Zhu B, Gurubhagavatula I, Keenan BT, Pien GW. A screening algorithm for obstructive sleep apnea in pregnancy. Ann Am Thorac Soc. 2019;16(10):1286-1294.
5. Louis J, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1.e12.
6. Sharkey K, Waters K, Millman R, Moore R, Martin SM, Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnogram in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497-502.
7. Tantrakul V, Ingsathit A, Liamsombut S, et al. Treatment of obstructive sleep apnea in high-risk pregnancy: a multicenter randomized controlled trial. Respir Res. 2023;24(1):171.
On 5 Ps: PSG, PM, PPG, PulseOx, and PAT
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
Post–intensive care syndrome and insomnia
SLEEP MEDICINE NETWORK
Nonrespiratory Sleep Section
There has been a recent interest in post–intensive care syndrome (PICS), as an increasing number of patients are surviving critical illness. PICS is defined as “new onset or worsening of impairments in physical, cognitive, and/or mental health that arises after an ICU stay and persists beyond hospital discharge.1 We know that poor sleep is a common occurrence in the ICU, which can contribute to cognitive impairment and could be due to various risk factors, including age, individual comorbidities, reason for admission, and ICU interventions.2 Sleep impairment after hospital discharge is highly prevalent for up to 1 year after hospitalization.
The most common sleep impairment described after hospital discharge from the ICU is insomnia, which coexists with anxiety, depression, and posttraumatic stress disorder.3 When patients are seen in a post-ICU clinic, a multimodal strategy is needed for the treatment of insomnia, which includes practicing good sleep hygiene, cognitive behavioral therapy for insomnia (CBT-I), and pharmacotherapy if indicated.
Since the American Academy of Sleep Medicine (AASM) 2021 clinical practice guideline on behavioral and psychological treatments for chronic insomnia, which made a strong recommendation for CBT-I, we continue to face barriers to incorporating CBT-I into our own clinical practice.4 This is due to limited access to CBT-I psychotherapists and patients’ lack of knowledge or treatment beliefs, among other reasons. However, there are numerous digital CBT-I platforms that patients can freely access from their mobile phone and are listed in the AASM article, “Digital cognitive behavioral therapy for insomnia: Platforms and characteristics,” which can help with treatment of insomnia.
For patients who are seen in post-ICU clinics, the first step in treating insomnia is discussing good sleep hygiene, providing resources for CBT-I (digital or in person), and treating coexistent psychiatric conditions.
References
1. Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. 2017;5(2):90-92.
2. Zampieri FG, et al. Ann Am Thorac Soc. 2023;20(11):1558-1560.
3. Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457-1468.
4. Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255-262.
SLEEP MEDICINE NETWORK
Nonrespiratory Sleep Section
There has been a recent interest in post–intensive care syndrome (PICS), as an increasing number of patients are surviving critical illness. PICS is defined as “new onset or worsening of impairments in physical, cognitive, and/or mental health that arises after an ICU stay and persists beyond hospital discharge.1 We know that poor sleep is a common occurrence in the ICU, which can contribute to cognitive impairment and could be due to various risk factors, including age, individual comorbidities, reason for admission, and ICU interventions.2 Sleep impairment after hospital discharge is highly prevalent for up to 1 year after hospitalization.
The most common sleep impairment described after hospital discharge from the ICU is insomnia, which coexists with anxiety, depression, and posttraumatic stress disorder.3 When patients are seen in a post-ICU clinic, a multimodal strategy is needed for the treatment of insomnia, which includes practicing good sleep hygiene, cognitive behavioral therapy for insomnia (CBT-I), and pharmacotherapy if indicated.
Since the American Academy of Sleep Medicine (AASM) 2021 clinical practice guideline on behavioral and psychological treatments for chronic insomnia, which made a strong recommendation for CBT-I, we continue to face barriers to incorporating CBT-I into our own clinical practice.4 This is due to limited access to CBT-I psychotherapists and patients’ lack of knowledge or treatment beliefs, among other reasons. However, there are numerous digital CBT-I platforms that patients can freely access from their mobile phone and are listed in the AASM article, “Digital cognitive behavioral therapy for insomnia: Platforms and characteristics,” which can help with treatment of insomnia.
For patients who are seen in post-ICU clinics, the first step in treating insomnia is discussing good sleep hygiene, providing resources for CBT-I (digital or in person), and treating coexistent psychiatric conditions.
References
1. Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. 2017;5(2):90-92.
2. Zampieri FG, et al. Ann Am Thorac Soc. 2023;20(11):1558-1560.
3. Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457-1468.
4. Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255-262.
SLEEP MEDICINE NETWORK
Nonrespiratory Sleep Section
There has been a recent interest in post–intensive care syndrome (PICS), as an increasing number of patients are surviving critical illness. PICS is defined as “new onset or worsening of impairments in physical, cognitive, and/or mental health that arises after an ICU stay and persists beyond hospital discharge.1 We know that poor sleep is a common occurrence in the ICU, which can contribute to cognitive impairment and could be due to various risk factors, including age, individual comorbidities, reason for admission, and ICU interventions.2 Sleep impairment after hospital discharge is highly prevalent for up to 1 year after hospitalization.
The most common sleep impairment described after hospital discharge from the ICU is insomnia, which coexists with anxiety, depression, and posttraumatic stress disorder.3 When patients are seen in a post-ICU clinic, a multimodal strategy is needed for the treatment of insomnia, which includes practicing good sleep hygiene, cognitive behavioral therapy for insomnia (CBT-I), and pharmacotherapy if indicated.
Since the American Academy of Sleep Medicine (AASM) 2021 clinical practice guideline on behavioral and psychological treatments for chronic insomnia, which made a strong recommendation for CBT-I, we continue to face barriers to incorporating CBT-I into our own clinical practice.4 This is due to limited access to CBT-I psychotherapists and patients’ lack of knowledge or treatment beliefs, among other reasons. However, there are numerous digital CBT-I platforms that patients can freely access from their mobile phone and are listed in the AASM article, “Digital cognitive behavioral therapy for insomnia: Platforms and characteristics,” which can help with treatment of insomnia.
For patients who are seen in post-ICU clinics, the first step in treating insomnia is discussing good sleep hygiene, providing resources for CBT-I (digital or in person), and treating coexistent psychiatric conditions.
References
1. Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. 2017;5(2):90-92.
2. Zampieri FG, et al. Ann Am Thorac Soc. 2023;20(11):1558-1560.
3. Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457-1468.
4. Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255-262.
Home ventilation consult
Specialist input on a sudden shift in device availability
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.
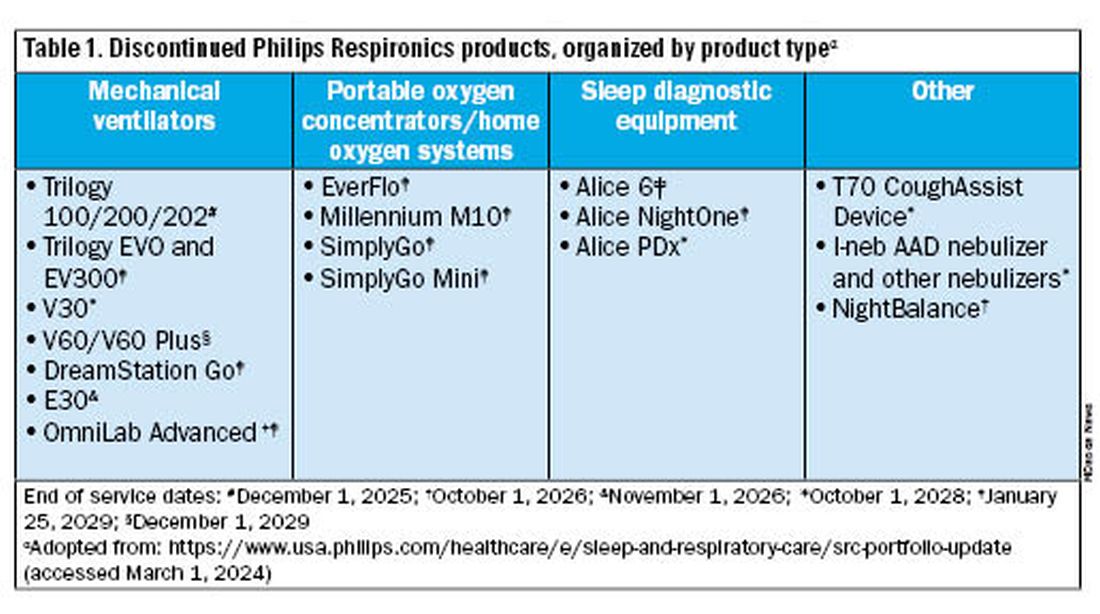
What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.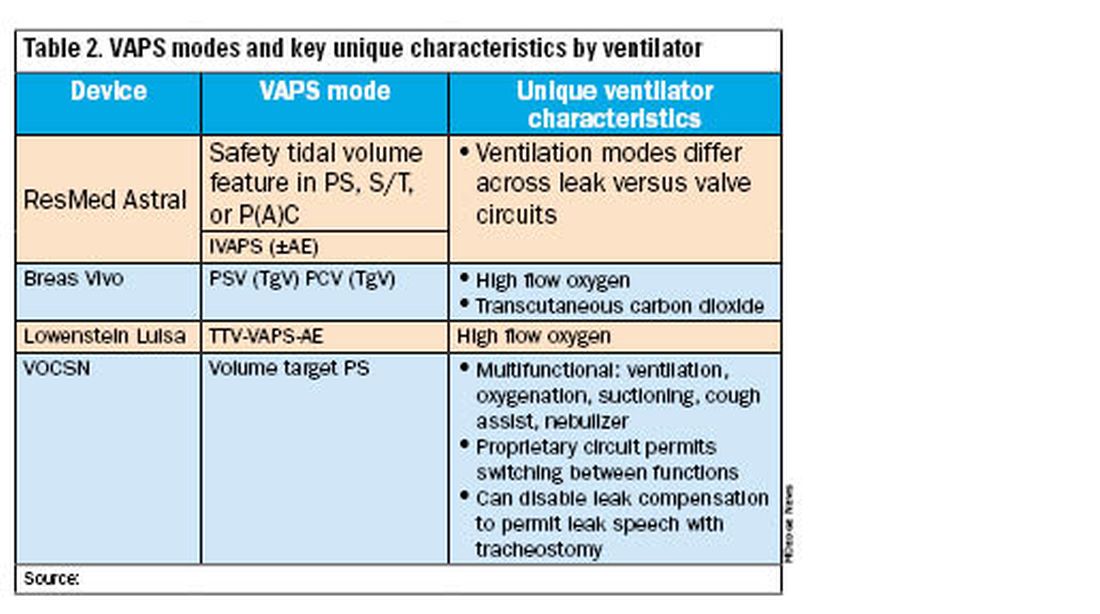
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Specialist input on a sudden shift in device availability
Specialist input on a sudden shift in device availability
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.

What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.

What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Daylight Saving Time: Saving light but endangering health
The American Academy of Sleep Medicine recently published its position statement reaffirming its support of utilizing permanent Standard Time (ST) as opposed to Daylight Saving Time (DST).1 DST usually occurs on the second Sunday in March when we “spring forward” by advancing the clock by 1 hour. The analogous “fall back” on the first Sunday in November refers to reversion back to the original ST, which is more synchronous with the sun’s natural pattern of rise and fall.
The earliest argument for DST practice dates back to the 1700s when Benjamin Franklin wrote a satirical piece in the Journal of Paris suggesting that advancing the clock to rise earlier in the summer would lead to economization in candle usage and save significant resources for Parisians. The modern version of this assertion infers that increased daylight in the evening will lead to increased consumer activity and work productivity with consequent economic benefits. Interestingly, the adoption of DST has demonstrated the opposite—a reduction in work productivity and economic losses.2 Another often-cited claim is that increased daylight in the evening could lead to fewer motor vehicle accidents. However, the reality is that DST is associated with more frequent car accidents in the morning.
The greatest drawback of DST is that, initially, it leads to sleep deprivation and chronically drives asynchronization between the circadian clock and the social clock. Humans synchronize their internal clock based on several factors, including light, temperature, feeding, and social habits. However, light is the strongest exogenous factor that regulates the internal clock. Light inhibits secretion of melatonin, an endogenous hormone that promotes sleep onset. While there is some individual variation in circadian patterns, exposure to bright light in the morning leads to increased physical, mental, and goal-directed activity.
Conversely, darkness or reduced light exposure in the evening hours promotes decreased activity and sleep onset via melatonin release. DST disrupts this natural process by promoting increased light exposure in the evening. This desynchronizes solar light from our internal clocks, causing a relative phase delay. Acutely, patients experience a form of imposed social jet lag. They lose an hour of sleep due to diminished sleep opportunity, as work and social obligations are typically not altered to allow for a later awakening. With recurrent delays, this lends to a pattern of chronic sleep deprivation which has significant health consequences.
Losing an hour of sleep opportunity as the clock advances in spring has dire consequences. The transition to DST is associated with increased cardiovascular events, including myocardial infarction, stroke, and admissions for acute atrial fibrillation.3 4 5 A large body of work has shown that acute reduction of sleep is associated with higher sympathetic tone, compromised immunity, and increased inflammation. Further, cognitive consequences can ensue in the form of altered situational awareness, increased risky behavior, and worse reaction time—which manifest as increased motor vehicle accidents, injuries, and fatalities.6 Emergency room visits and bounce-back admissions, medical errors and injuries, and missed appointments increase following the switch to DST.7 Psychiatric outcomes, including deaths due to suicide and overdose, are worse with the spring transition.8
Is the problem with DST merely limited to springtime, when we lose an hour of sleep? Not quite. During the “fall back” period, despite theoretically gaining an hour of sleep opportunity, people exhibit evidence of sleep disruption, psychiatric issues, traffic accidents, and inflammatory bowel disease exacerbations. These consequences likely stem from a discordance between circadian and social time, which leads to an earlier awakening based on circadian physiology as opposed to the clock time.
The acute impact of changing our sleep patterns during transitions in clock time may be appreciated more readily, but the damage is much more insidious. Chronic exposure to light in the late evening creates a state of enhanced arousal when the body should be winding down. The chronic incongruency between clock and solar time leads to dyssynchrony in our usual functions, such as food intake, social and physical activity, and basal temperature. Consequently, there is an impetus to fall asleep later. This leads to an accumulation of sleep debt and its associated negative consequences in the general, already chronically sleep-deprived population. This is especially impactful to adolescents and young adults who tend to have a delay in their sleep and wake patterns and, yet, are socially bound to early morning awakenings for school or work.
The scientific evidence behind the health risk and benefit profile of DST and ST is incontrovertible and in favor of ST. The hallmark of appropriate sleep habits involves consistency and appropriate duration. Changing timing forward or backward increases the likelihood of an alteration of the baseline established sleep and circadian consistency.
Unfortunately, despite multiple polls demonstrating the populace’s dislike of DST, repeat attempts to codify DST and negate ST persist. The latest initiative, the Sunshine Protection Act, which promised permanent DST, was passed by the US Senate but was thankfully foiled by Congress in 2022. The act of setting a time is not one that should be taken lightly or in isolation because there are significant, long-lasting health, safety, and socioeconomic consequences of this decision. Practically, this entails a concerted effort from all major economies since consistency is essential for trade and geopolitical relations. China, Japan, and India don’t practice DST. The European Parliament voted successfully to abolish DST in the European Union in 2019 with a plan to implement ST in 2021. Implementation has yet to be successful due to interruptions from the COVID-19 pandemic as well as the current economic and political climate in Europe. Political or theoretical political victories should not supersede the health and safety of an elected official’s constituents. As a medical community, we should continue to use our collective voice to encourage our representatives to vote in ways that positively affect our patients’ health outcomes.
References:
1. Rishi MA, et al. JCSM. 2024;20(1):121.
2. Gibson M, et al. Rev Econ Stat. 2018;100(5):783.
3. Jansky I, et al. N Engl J Med. 2008;359(18):1966.
4. Sipilia J, et al. Sleep Med. 2016;27-28:20.
5. Chudow JJ, et al. Sleep Med. 2020;69:155.
6. Fritz J, et al. Curr Biol. 2020;30(4):729.
7. Ferrazzi E, et al. J Biol Rhythms. 2018;33(5):555-564.
8. Berk M, et al. Sleep Biol Rhythms. 2008;6(1):22.
The American Academy of Sleep Medicine recently published its position statement reaffirming its support of utilizing permanent Standard Time (ST) as opposed to Daylight Saving Time (DST).1 DST usually occurs on the second Sunday in March when we “spring forward” by advancing the clock by 1 hour. The analogous “fall back” on the first Sunday in November refers to reversion back to the original ST, which is more synchronous with the sun’s natural pattern of rise and fall.
The earliest argument for DST practice dates back to the 1700s when Benjamin Franklin wrote a satirical piece in the Journal of Paris suggesting that advancing the clock to rise earlier in the summer would lead to economization in candle usage and save significant resources for Parisians. The modern version of this assertion infers that increased daylight in the evening will lead to increased consumer activity and work productivity with consequent economic benefits. Interestingly, the adoption of DST has demonstrated the opposite—a reduction in work productivity and economic losses.2 Another often-cited claim is that increased daylight in the evening could lead to fewer motor vehicle accidents. However, the reality is that DST is associated with more frequent car accidents in the morning.
The greatest drawback of DST is that, initially, it leads to sleep deprivation and chronically drives asynchronization between the circadian clock and the social clock. Humans synchronize their internal clock based on several factors, including light, temperature, feeding, and social habits. However, light is the strongest exogenous factor that regulates the internal clock. Light inhibits secretion of melatonin, an endogenous hormone that promotes sleep onset. While there is some individual variation in circadian patterns, exposure to bright light in the morning leads to increased physical, mental, and goal-directed activity.
Conversely, darkness or reduced light exposure in the evening hours promotes decreased activity and sleep onset via melatonin release. DST disrupts this natural process by promoting increased light exposure in the evening. This desynchronizes solar light from our internal clocks, causing a relative phase delay. Acutely, patients experience a form of imposed social jet lag. They lose an hour of sleep due to diminished sleep opportunity, as work and social obligations are typically not altered to allow for a later awakening. With recurrent delays, this lends to a pattern of chronic sleep deprivation which has significant health consequences.
Losing an hour of sleep opportunity as the clock advances in spring has dire consequences. The transition to DST is associated with increased cardiovascular events, including myocardial infarction, stroke, and admissions for acute atrial fibrillation.3 4 5 A large body of work has shown that acute reduction of sleep is associated with higher sympathetic tone, compromised immunity, and increased inflammation. Further, cognitive consequences can ensue in the form of altered situational awareness, increased risky behavior, and worse reaction time—which manifest as increased motor vehicle accidents, injuries, and fatalities.6 Emergency room visits and bounce-back admissions, medical errors and injuries, and missed appointments increase following the switch to DST.7 Psychiatric outcomes, including deaths due to suicide and overdose, are worse with the spring transition.8
Is the problem with DST merely limited to springtime, when we lose an hour of sleep? Not quite. During the “fall back” period, despite theoretically gaining an hour of sleep opportunity, people exhibit evidence of sleep disruption, psychiatric issues, traffic accidents, and inflammatory bowel disease exacerbations. These consequences likely stem from a discordance between circadian and social time, which leads to an earlier awakening based on circadian physiology as opposed to the clock time.
The acute impact of changing our sleep patterns during transitions in clock time may be appreciated more readily, but the damage is much more insidious. Chronic exposure to light in the late evening creates a state of enhanced arousal when the body should be winding down. The chronic incongruency between clock and solar time leads to dyssynchrony in our usual functions, such as food intake, social and physical activity, and basal temperature. Consequently, there is an impetus to fall asleep later. This leads to an accumulation of sleep debt and its associated negative consequences in the general, already chronically sleep-deprived population. This is especially impactful to adolescents and young adults who tend to have a delay in their sleep and wake patterns and, yet, are socially bound to early morning awakenings for school or work.
The scientific evidence behind the health risk and benefit profile of DST and ST is incontrovertible and in favor of ST. The hallmark of appropriate sleep habits involves consistency and appropriate duration. Changing timing forward or backward increases the likelihood of an alteration of the baseline established sleep and circadian consistency.
Unfortunately, despite multiple polls demonstrating the populace’s dislike of DST, repeat attempts to codify DST and negate ST persist. The latest initiative, the Sunshine Protection Act, which promised permanent DST, was passed by the US Senate but was thankfully foiled by Congress in 2022. The act of setting a time is not one that should be taken lightly or in isolation because there are significant, long-lasting health, safety, and socioeconomic consequences of this decision. Practically, this entails a concerted effort from all major economies since consistency is essential for trade and geopolitical relations. China, Japan, and India don’t practice DST. The European Parliament voted successfully to abolish DST in the European Union in 2019 with a plan to implement ST in 2021. Implementation has yet to be successful due to interruptions from the COVID-19 pandemic as well as the current economic and political climate in Europe. Political or theoretical political victories should not supersede the health and safety of an elected official’s constituents. As a medical community, we should continue to use our collective voice to encourage our representatives to vote in ways that positively affect our patients’ health outcomes.
References:
1. Rishi MA, et al. JCSM. 2024;20(1):121.
2. Gibson M, et al. Rev Econ Stat. 2018;100(5):783.
3. Jansky I, et al. N Engl J Med. 2008;359(18):1966.
4. Sipilia J, et al. Sleep Med. 2016;27-28:20.
5. Chudow JJ, et al. Sleep Med. 2020;69:155.
6. Fritz J, et al. Curr Biol. 2020;30(4):729.
7. Ferrazzi E, et al. J Biol Rhythms. 2018;33(5):555-564.
8. Berk M, et al. Sleep Biol Rhythms. 2008;6(1):22.
The American Academy of Sleep Medicine recently published its position statement reaffirming its support of utilizing permanent Standard Time (ST) as opposed to Daylight Saving Time (DST).1 DST usually occurs on the second Sunday in March when we “spring forward” by advancing the clock by 1 hour. The analogous “fall back” on the first Sunday in November refers to reversion back to the original ST, which is more synchronous with the sun’s natural pattern of rise and fall.
The earliest argument for DST practice dates back to the 1700s when Benjamin Franklin wrote a satirical piece in the Journal of Paris suggesting that advancing the clock to rise earlier in the summer would lead to economization in candle usage and save significant resources for Parisians. The modern version of this assertion infers that increased daylight in the evening will lead to increased consumer activity and work productivity with consequent economic benefits. Interestingly, the adoption of DST has demonstrated the opposite—a reduction in work productivity and economic losses.2 Another often-cited claim is that increased daylight in the evening could lead to fewer motor vehicle accidents. However, the reality is that DST is associated with more frequent car accidents in the morning.
The greatest drawback of DST is that, initially, it leads to sleep deprivation and chronically drives asynchronization between the circadian clock and the social clock. Humans synchronize their internal clock based on several factors, including light, temperature, feeding, and social habits. However, light is the strongest exogenous factor that regulates the internal clock. Light inhibits secretion of melatonin, an endogenous hormone that promotes sleep onset. While there is some individual variation in circadian patterns, exposure to bright light in the morning leads to increased physical, mental, and goal-directed activity.
Conversely, darkness or reduced light exposure in the evening hours promotes decreased activity and sleep onset via melatonin release. DST disrupts this natural process by promoting increased light exposure in the evening. This desynchronizes solar light from our internal clocks, causing a relative phase delay. Acutely, patients experience a form of imposed social jet lag. They lose an hour of sleep due to diminished sleep opportunity, as work and social obligations are typically not altered to allow for a later awakening. With recurrent delays, this lends to a pattern of chronic sleep deprivation which has significant health consequences.
Losing an hour of sleep opportunity as the clock advances in spring has dire consequences. The transition to DST is associated with increased cardiovascular events, including myocardial infarction, stroke, and admissions for acute atrial fibrillation.3 4 5 A large body of work has shown that acute reduction of sleep is associated with higher sympathetic tone, compromised immunity, and increased inflammation. Further, cognitive consequences can ensue in the form of altered situational awareness, increased risky behavior, and worse reaction time—which manifest as increased motor vehicle accidents, injuries, and fatalities.6 Emergency room visits and bounce-back admissions, medical errors and injuries, and missed appointments increase following the switch to DST.7 Psychiatric outcomes, including deaths due to suicide and overdose, are worse with the spring transition.8
Is the problem with DST merely limited to springtime, when we lose an hour of sleep? Not quite. During the “fall back” period, despite theoretically gaining an hour of sleep opportunity, people exhibit evidence of sleep disruption, psychiatric issues, traffic accidents, and inflammatory bowel disease exacerbations. These consequences likely stem from a discordance between circadian and social time, which leads to an earlier awakening based on circadian physiology as opposed to the clock time.
The acute impact of changing our sleep patterns during transitions in clock time may be appreciated more readily, but the damage is much more insidious. Chronic exposure to light in the late evening creates a state of enhanced arousal when the body should be winding down. The chronic incongruency between clock and solar time leads to dyssynchrony in our usual functions, such as food intake, social and physical activity, and basal temperature. Consequently, there is an impetus to fall asleep later. This leads to an accumulation of sleep debt and its associated negative consequences in the general, already chronically sleep-deprived population. This is especially impactful to adolescents and young adults who tend to have a delay in their sleep and wake patterns and, yet, are socially bound to early morning awakenings for school or work.
The scientific evidence behind the health risk and benefit profile of DST and ST is incontrovertible and in favor of ST. The hallmark of appropriate sleep habits involves consistency and appropriate duration. Changing timing forward or backward increases the likelihood of an alteration of the baseline established sleep and circadian consistency.
Unfortunately, despite multiple polls demonstrating the populace’s dislike of DST, repeat attempts to codify DST and negate ST persist. The latest initiative, the Sunshine Protection Act, which promised permanent DST, was passed by the US Senate but was thankfully foiled by Congress in 2022. The act of setting a time is not one that should be taken lightly or in isolation because there are significant, long-lasting health, safety, and socioeconomic consequences of this decision. Practically, this entails a concerted effort from all major economies since consistency is essential for trade and geopolitical relations. China, Japan, and India don’t practice DST. The European Parliament voted successfully to abolish DST in the European Union in 2019 with a plan to implement ST in 2021. Implementation has yet to be successful due to interruptions from the COVID-19 pandemic as well as the current economic and political climate in Europe. Political or theoretical political victories should not supersede the health and safety of an elected official’s constituents. As a medical community, we should continue to use our collective voice to encourage our representatives to vote in ways that positively affect our patients’ health outcomes.
References:
1. Rishi MA, et al. JCSM. 2024;20(1):121.
2. Gibson M, et al. Rev Econ Stat. 2018;100(5):783.
3. Jansky I, et al. N Engl J Med. 2008;359(18):1966.
4. Sipilia J, et al. Sleep Med. 2016;27-28:20.
5. Chudow JJ, et al. Sleep Med. 2020;69:155.
6. Fritz J, et al. Curr Biol. 2020;30(4):729.
7. Ferrazzi E, et al. J Biol Rhythms. 2018;33(5):555-564.
8. Berk M, et al. Sleep Biol Rhythms. 2008;6(1):22.
New pharmacological interventions for residual excessive daytime sleepiness in OSA
Residual excessive daytime sleepiness (REDS) is defined as the urge to sleep during the day despite an intention to remain alert after optimal treatment of obstructive sleep apnea (OSA). This is a distressing outcome with an estimated prevalence of 9% to 22% among patients with OSA (Pépin JL, et al. Eur Respir J. 2009;33[5]:1062). The pathophysiology of the condition is complex, and experimental studies conducted on animal models have demonstrated that chronic sleep fragmentation and chronic intermittent hypoxia can result in detrimental effects on wake-promoting neurons. Additionally, there is evidence of heightened oxidative stress and alterations in melatonin secretion, with the severity and duration of the disease playing a significant role in the manifestation of these effects (Javaheri S, et al. Chest. 2020;158[2]:776). It is considered a diagnosis of exclusion, with the assessment being mostly subjective. Prior to diagnosing REDS, it is crucial to optimize positive airway pressure (PAP) therapy and nocturnal ventilation, ensure sufficient adherence to sleep hygiene practices, and exclude the presence of other sleep disorders. The Epworth Sleepiness Scale (ESS) score is widely utilized as a primary clinical tool in the assessment of sleepiness. To enhance the precision of this score, it is advantageous to take input from both family members and friends. Additional objective assessments that could be considered include the utilization of the Multiple Sleep Latency Test (MSLT) or the Maintenance of Wakefulness Test (MWT).
Off-label use of traditional central nervous system stimulants, like amphetamine or methylphenidate, in these patients is almost extinct. The potential for abuse and negative consequences outweighs the potential benefits. FDA-approved medications for treatment of REDS in OSA include modafinil, armodafinil, and solriamfetol in the United States.
Historically, modafinil and armodafinil are the first-line and most commonly used wake-promoting agents. Both agents bind to the dopamine transporter and inhibit dopamine reuptake. They have demonstrated efficacy in reducing EDS and improving wakefulness in patients with OSA treated with CPAP. A meta-analysis of 10 randomized, placebo-controlled trials of modafinil and armodafinil found that they were better than placebo by 2.2 points on the ESS score and 3 minutes on the MWT (Maintenance of Wakefulness Test) (Chapman JL, et al. Eur Respir J. 2016;47[5]:1420). Both drugs have common adverse effects of headache, nausea, nervousness, insomnia, dizziness, rhinitis, and diarrhea. Drug interaction with CYP3A4/5 substrates and oral contraceptives is a concern with these medications. In 2010, the European Medicines Agency restricted the use of modafinil only to patients with narcolepsy, considering its cardiovascular and neuropsychiatric risks (European Medicines Agency website; press release, July 22, 2010).
Solriamfetol is the newest medication being utilized for EDS in OSA and is approved in both the United States and Europe for this indication. It is a dopamine and norepinephrine reuptake inhibitor with a simultaneous effect on both transporters. It has been effective in improving wakefulness and reducing sleepiness in patients with residual OSA. In the landmark trial TONES 3, dose-dependent (37.5, 75, 150, and 300 mg/day) effects were observed, with improvements in ESS scores of –1.9 to –4.7 points and sleep latency in MWT by 4.5 to 12.8 minutes (Schweitzer PK, et al. Am J Respir Crit Care Med. 2019;199[11]:1421). The current recommended dosing for REDS in OSA is to start with the lowest dose of 37.5 mg/day and increase to the maximum dose of 150 mg/day by titrating up every 3 days if needed. A recent meta-analysis showed an indirect treatment comparison between efficacy and safety among the medications solriamfetol, modafinil, and armodafinil (Ronnebaum S, et al. J Clin Sleep Med. 2021;17[12]:2543). Six parallel-arm, placebo-controlled, randomized, controlled trials were looked at. The ESS score, MWT20 sleep latency, and CGI-C (Clinical Global Impression of Change) all got better in comparison to the placebo. Relative to the comparators and placebo at 12 weeks, solriamfetol at 150 mg and 300 mg had the highest degree of improvement in all the outcomes studied. Common adverse effects of solriamfetol include headache, nausea, decreased appetite, insomnia, dry mouth, anxiety, and minimal increase in blood pressure and heart rate. The adverse effects in terms of blood pressure and heart rate change have a dose-dependent relationship, and serial vitals monitoring is recommended for patients every 6 months to a year. This medication is contraindicated in patients receiving concomitant monoamine oxidase inhibitors (MAOIs) or within 14 days following discontinuation of an MAOI because of the risk of hypertensive reactions. Solriamfetol is renally excreted, so dose adjustment is needed in patients with moderate to severe renal impairment. It is not recommended for use in end-stage renal disease (eGFR <15 mL/min/1.73 m2) (SUNOSI. Full prescribing information. Axsome; revised 06/2023. https://www.sunosihcp.com/assets/files/sunosi.en.uspi.pdf. Accessed: Sept 24, 2023). Solriamfetol demonstrates a comparatively shorter half-life when compared with traditional pharmaceuticals like modafinil and armodafinil, implying the possibility of a decreased duration of its effects. The effect in question may exhibit interpersonal diversity in its impact on quality of life when applied in a therapeutic setting.
Pitolisant is another potential medication to treat REDS in patients with OSA. While only approved for treating EDS and cataplexy in adult US patients with narcolepsy, it is currently approved for REDS in OSA in Europe (Ozawade. European Medicines Agency. Last updated 12/05/2022. https://www.ema.europa.eu/en/medicines/human/EPAR/ozawade#product-information-section. Accessed: Oct 2, 2023). It is a selective histamine H3 receptor antagonist and an inverse agonist of the presynaptic H3 receptor. The fact that this medication is not scheduled and has a negligible or nonexistent risk of abuse is one of its advantages. It is dosed once daily, and the most frequent adverse effects include headaches and insomnia. A prolonged QT interval was observed in a few patients; caution is needed with concomitant use of other medications with known similar effects. Dosage modification is recommended in patients with moderate hepatic impairment and moderate to severe renal impairment. Drug interactions are also observed with the concomitant use of CYP2D6 inhibitors and CYP3A4 inducers. Pitolisant may reduce the efficacy of hormonal contraception, including up to 21 days after its discontinuation (WAKIX. Full prescribing information. Harmony biosciences; revised 12/2022.https://wakixhcp.com/pdf/wakix-tablets-pi.pdf. Accessed: Sept 24, 2023).
Dr. Mechineni is Sleep Attending Physician, Ascension Illinois, Alexian Brothers Medical Center, Chicago. Dr. Sahni is Assistant Professor of Clinical Medicine, Associate Program Director, Sleep Medicine Fellowship; Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago.
Residual excessive daytime sleepiness (REDS) is defined as the urge to sleep during the day despite an intention to remain alert after optimal treatment of obstructive sleep apnea (OSA). This is a distressing outcome with an estimated prevalence of 9% to 22% among patients with OSA (Pépin JL, et al. Eur Respir J. 2009;33[5]:1062). The pathophysiology of the condition is complex, and experimental studies conducted on animal models have demonstrated that chronic sleep fragmentation and chronic intermittent hypoxia can result in detrimental effects on wake-promoting neurons. Additionally, there is evidence of heightened oxidative stress and alterations in melatonin secretion, with the severity and duration of the disease playing a significant role in the manifestation of these effects (Javaheri S, et al. Chest. 2020;158[2]:776). It is considered a diagnosis of exclusion, with the assessment being mostly subjective. Prior to diagnosing REDS, it is crucial to optimize positive airway pressure (PAP) therapy and nocturnal ventilation, ensure sufficient adherence to sleep hygiene practices, and exclude the presence of other sleep disorders. The Epworth Sleepiness Scale (ESS) score is widely utilized as a primary clinical tool in the assessment of sleepiness. To enhance the precision of this score, it is advantageous to take input from both family members and friends. Additional objective assessments that could be considered include the utilization of the Multiple Sleep Latency Test (MSLT) or the Maintenance of Wakefulness Test (MWT).
Off-label use of traditional central nervous system stimulants, like amphetamine or methylphenidate, in these patients is almost extinct. The potential for abuse and negative consequences outweighs the potential benefits. FDA-approved medications for treatment of REDS in OSA include modafinil, armodafinil, and solriamfetol in the United States.
Historically, modafinil and armodafinil are the first-line and most commonly used wake-promoting agents. Both agents bind to the dopamine transporter and inhibit dopamine reuptake. They have demonstrated efficacy in reducing EDS and improving wakefulness in patients with OSA treated with CPAP. A meta-analysis of 10 randomized, placebo-controlled trials of modafinil and armodafinil found that they were better than placebo by 2.2 points on the ESS score and 3 minutes on the MWT (Maintenance of Wakefulness Test) (Chapman JL, et al. Eur Respir J. 2016;47[5]:1420). Both drugs have common adverse effects of headache, nausea, nervousness, insomnia, dizziness, rhinitis, and diarrhea. Drug interaction with CYP3A4/5 substrates and oral contraceptives is a concern with these medications. In 2010, the European Medicines Agency restricted the use of modafinil only to patients with narcolepsy, considering its cardiovascular and neuropsychiatric risks (European Medicines Agency website; press release, July 22, 2010).
Solriamfetol is the newest medication being utilized for EDS in OSA and is approved in both the United States and Europe for this indication. It is a dopamine and norepinephrine reuptake inhibitor with a simultaneous effect on both transporters. It has been effective in improving wakefulness and reducing sleepiness in patients with residual OSA. In the landmark trial TONES 3, dose-dependent (37.5, 75, 150, and 300 mg/day) effects were observed, with improvements in ESS scores of –1.9 to –4.7 points and sleep latency in MWT by 4.5 to 12.8 minutes (Schweitzer PK, et al. Am J Respir Crit Care Med. 2019;199[11]:1421). The current recommended dosing for REDS in OSA is to start with the lowest dose of 37.5 mg/day and increase to the maximum dose of 150 mg/day by titrating up every 3 days if needed. A recent meta-analysis showed an indirect treatment comparison between efficacy and safety among the medications solriamfetol, modafinil, and armodafinil (Ronnebaum S, et al. J Clin Sleep Med. 2021;17[12]:2543). Six parallel-arm, placebo-controlled, randomized, controlled trials were looked at. The ESS score, MWT20 sleep latency, and CGI-C (Clinical Global Impression of Change) all got better in comparison to the placebo. Relative to the comparators and placebo at 12 weeks, solriamfetol at 150 mg and 300 mg had the highest degree of improvement in all the outcomes studied. Common adverse effects of solriamfetol include headache, nausea, decreased appetite, insomnia, dry mouth, anxiety, and minimal increase in blood pressure and heart rate. The adverse effects in terms of blood pressure and heart rate change have a dose-dependent relationship, and serial vitals monitoring is recommended for patients every 6 months to a year. This medication is contraindicated in patients receiving concomitant monoamine oxidase inhibitors (MAOIs) or within 14 days following discontinuation of an MAOI because of the risk of hypertensive reactions. Solriamfetol is renally excreted, so dose adjustment is needed in patients with moderate to severe renal impairment. It is not recommended for use in end-stage renal disease (eGFR <15 mL/min/1.73 m2) (SUNOSI. Full prescribing information. Axsome; revised 06/2023. https://www.sunosihcp.com/assets/files/sunosi.en.uspi.pdf. Accessed: Sept 24, 2023). Solriamfetol demonstrates a comparatively shorter half-life when compared with traditional pharmaceuticals like modafinil and armodafinil, implying the possibility of a decreased duration of its effects. The effect in question may exhibit interpersonal diversity in its impact on quality of life when applied in a therapeutic setting.
Pitolisant is another potential medication to treat REDS in patients with OSA. While only approved for treating EDS and cataplexy in adult US patients with narcolepsy, it is currently approved for REDS in OSA in Europe (Ozawade. European Medicines Agency. Last updated 12/05/2022. https://www.ema.europa.eu/en/medicines/human/EPAR/ozawade#product-information-section. Accessed: Oct 2, 2023). It is a selective histamine H3 receptor antagonist and an inverse agonist of the presynaptic H3 receptor. The fact that this medication is not scheduled and has a negligible or nonexistent risk of abuse is one of its advantages. It is dosed once daily, and the most frequent adverse effects include headaches and insomnia. A prolonged QT interval was observed in a few patients; caution is needed with concomitant use of other medications with known similar effects. Dosage modification is recommended in patients with moderate hepatic impairment and moderate to severe renal impairment. Drug interactions are also observed with the concomitant use of CYP2D6 inhibitors and CYP3A4 inducers. Pitolisant may reduce the efficacy of hormonal contraception, including up to 21 days after its discontinuation (WAKIX. Full prescribing information. Harmony biosciences; revised 12/2022.https://wakixhcp.com/pdf/wakix-tablets-pi.pdf. Accessed: Sept 24, 2023).
Dr. Mechineni is Sleep Attending Physician, Ascension Illinois, Alexian Brothers Medical Center, Chicago. Dr. Sahni is Assistant Professor of Clinical Medicine, Associate Program Director, Sleep Medicine Fellowship; Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago.
Residual excessive daytime sleepiness (REDS) is defined as the urge to sleep during the day despite an intention to remain alert after optimal treatment of obstructive sleep apnea (OSA). This is a distressing outcome with an estimated prevalence of 9% to 22% among patients with OSA (Pépin JL, et al. Eur Respir J. 2009;33[5]:1062). The pathophysiology of the condition is complex, and experimental studies conducted on animal models have demonstrated that chronic sleep fragmentation and chronic intermittent hypoxia can result in detrimental effects on wake-promoting neurons. Additionally, there is evidence of heightened oxidative stress and alterations in melatonin secretion, with the severity and duration of the disease playing a significant role in the manifestation of these effects (Javaheri S, et al. Chest. 2020;158[2]:776). It is considered a diagnosis of exclusion, with the assessment being mostly subjective. Prior to diagnosing REDS, it is crucial to optimize positive airway pressure (PAP) therapy and nocturnal ventilation, ensure sufficient adherence to sleep hygiene practices, and exclude the presence of other sleep disorders. The Epworth Sleepiness Scale (ESS) score is widely utilized as a primary clinical tool in the assessment of sleepiness. To enhance the precision of this score, it is advantageous to take input from both family members and friends. Additional objective assessments that could be considered include the utilization of the Multiple Sleep Latency Test (MSLT) or the Maintenance of Wakefulness Test (MWT).
Off-label use of traditional central nervous system stimulants, like amphetamine or methylphenidate, in these patients is almost extinct. The potential for abuse and negative consequences outweighs the potential benefits. FDA-approved medications for treatment of REDS in OSA include modafinil, armodafinil, and solriamfetol in the United States.
Historically, modafinil and armodafinil are the first-line and most commonly used wake-promoting agents. Both agents bind to the dopamine transporter and inhibit dopamine reuptake. They have demonstrated efficacy in reducing EDS and improving wakefulness in patients with OSA treated with CPAP. A meta-analysis of 10 randomized, placebo-controlled trials of modafinil and armodafinil found that they were better than placebo by 2.2 points on the ESS score and 3 minutes on the MWT (Maintenance of Wakefulness Test) (Chapman JL, et al. Eur Respir J. 2016;47[5]:1420). Both drugs have common adverse effects of headache, nausea, nervousness, insomnia, dizziness, rhinitis, and diarrhea. Drug interaction with CYP3A4/5 substrates and oral contraceptives is a concern with these medications. In 2010, the European Medicines Agency restricted the use of modafinil only to patients with narcolepsy, considering its cardiovascular and neuropsychiatric risks (European Medicines Agency website; press release, July 22, 2010).
Solriamfetol is the newest medication being utilized for EDS in OSA and is approved in both the United States and Europe for this indication. It is a dopamine and norepinephrine reuptake inhibitor with a simultaneous effect on both transporters. It has been effective in improving wakefulness and reducing sleepiness in patients with residual OSA. In the landmark trial TONES 3, dose-dependent (37.5, 75, 150, and 300 mg/day) effects were observed, with improvements in ESS scores of –1.9 to –4.7 points and sleep latency in MWT by 4.5 to 12.8 minutes (Schweitzer PK, et al. Am J Respir Crit Care Med. 2019;199[11]:1421). The current recommended dosing for REDS in OSA is to start with the lowest dose of 37.5 mg/day and increase to the maximum dose of 150 mg/day by titrating up every 3 days if needed. A recent meta-analysis showed an indirect treatment comparison between efficacy and safety among the medications solriamfetol, modafinil, and armodafinil (Ronnebaum S, et al. J Clin Sleep Med. 2021;17[12]:2543). Six parallel-arm, placebo-controlled, randomized, controlled trials were looked at. The ESS score, MWT20 sleep latency, and CGI-C (Clinical Global Impression of Change) all got better in comparison to the placebo. Relative to the comparators and placebo at 12 weeks, solriamfetol at 150 mg and 300 mg had the highest degree of improvement in all the outcomes studied. Common adverse effects of solriamfetol include headache, nausea, decreased appetite, insomnia, dry mouth, anxiety, and minimal increase in blood pressure and heart rate. The adverse effects in terms of blood pressure and heart rate change have a dose-dependent relationship, and serial vitals monitoring is recommended for patients every 6 months to a year. This medication is contraindicated in patients receiving concomitant monoamine oxidase inhibitors (MAOIs) or within 14 days following discontinuation of an MAOI because of the risk of hypertensive reactions. Solriamfetol is renally excreted, so dose adjustment is needed in patients with moderate to severe renal impairment. It is not recommended for use in end-stage renal disease (eGFR <15 mL/min/1.73 m2) (SUNOSI. Full prescribing information. Axsome; revised 06/2023. https://www.sunosihcp.com/assets/files/sunosi.en.uspi.pdf. Accessed: Sept 24, 2023). Solriamfetol demonstrates a comparatively shorter half-life when compared with traditional pharmaceuticals like modafinil and armodafinil, implying the possibility of a decreased duration of its effects. The effect in question may exhibit interpersonal diversity in its impact on quality of life when applied in a therapeutic setting.
Pitolisant is another potential medication to treat REDS in patients with OSA. While only approved for treating EDS and cataplexy in adult US patients with narcolepsy, it is currently approved for REDS in OSA in Europe (Ozawade. European Medicines Agency. Last updated 12/05/2022. https://www.ema.europa.eu/en/medicines/human/EPAR/ozawade#product-information-section. Accessed: Oct 2, 2023). It is a selective histamine H3 receptor antagonist and an inverse agonist of the presynaptic H3 receptor. The fact that this medication is not scheduled and has a negligible or nonexistent risk of abuse is one of its advantages. It is dosed once daily, and the most frequent adverse effects include headaches and insomnia. A prolonged QT interval was observed in a few patients; caution is needed with concomitant use of other medications with known similar effects. Dosage modification is recommended in patients with moderate hepatic impairment and moderate to severe renal impairment. Drug interactions are also observed with the concomitant use of CYP2D6 inhibitors and CYP3A4 inducers. Pitolisant may reduce the efficacy of hormonal contraception, including up to 21 days after its discontinuation (WAKIX. Full prescribing information. Harmony biosciences; revised 12/2022.https://wakixhcp.com/pdf/wakix-tablets-pi.pdf. Accessed: Sept 24, 2023).
Dr. Mechineni is Sleep Attending Physician, Ascension Illinois, Alexian Brothers Medical Center, Chicago. Dr. Sahni is Assistant Professor of Clinical Medicine, Associate Program Director, Sleep Medicine Fellowship; Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago.
CPAP in overlap syndrome: Unveiling the evidence
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
The overlap syndrome (OS), which refers to the co-occurrence of OSA and COPD, was first described by Flenley in 1985 (Flenley DC. Clin Chest Med. 1985;6[4]:651). Over the years, numerous studies have demonstrated an increased risk of hospitalization and mortality in patients with OS (Brennan M, et al. 2022;1-10). Despite these findings, limited evidence exists regarding the optimal treatment approach for individuals with OS.
CPAP therapy has demonstrated various physiologic advantages for patients with OS (Srivali N, et al. Sleep Med. 2023;108:55-60), which contribute to diminished dyspnea symptoms, lowered pro-inflammatory markers, improved arterial blood gases, increased 6-minute walk distance, enhanced FEV1, and decreased mean pulmonary artery pressure (Suri TM, et al. FASEB BioAdv. 2021;3[9]:683-93). CPAP therapy in patients with OS has been linked to a reduction in COPD exacerbations (Voulgaris A, et al. Clin Respir Jour. 2023; 17[3]:165), fewer COPD-related hospitalizations (Marin JM, et al. Am J Respir Crit Care Med. 2010;182[3]:325-31), decreased cardiovascular events (Kendzerska T, et al. Ann ATS. 2019;16[1]:71), and an overall decline in mortality rates (Machado ML, et al. Eur Respir J. 2010;35[1]:132-7).
It is important to acknowledge that, as of now, no randomized clinical trial has specifically addressed the treatment of OS, leaving recommendations largely reliant on observational studies. Conversely, recent guidelines have proposed the utilization of high-intensity noninvasive ventilation (NIV) for hypercapnic patients with COPD. Thus, extensive research is warranted to characterize distinct sleep-related breathing disorders within the OS population and to investigate the effects of CPAP in comparison to other NIV modalities on patients with overlap syndrome.
Solmaz Ehteshami-Afshar, MD
Kirat Gill, MD, Section Member-at-Large
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
The overlap syndrome (OS), which refers to the co-occurrence of OSA and COPD, was first described by Flenley in 1985 (Flenley DC. Clin Chest Med. 1985;6[4]:651). Over the years, numerous studies have demonstrated an increased risk of hospitalization and mortality in patients with OS (Brennan M, et al. 2022;1-10). Despite these findings, limited evidence exists regarding the optimal treatment approach for individuals with OS.
CPAP therapy has demonstrated various physiologic advantages for patients with OS (Srivali N, et al. Sleep Med. 2023;108:55-60), which contribute to diminished dyspnea symptoms, lowered pro-inflammatory markers, improved arterial blood gases, increased 6-minute walk distance, enhanced FEV1, and decreased mean pulmonary artery pressure (Suri TM, et al. FASEB BioAdv. 2021;3[9]:683-93). CPAP therapy in patients with OS has been linked to a reduction in COPD exacerbations (Voulgaris A, et al. Clin Respir Jour. 2023; 17[3]:165), fewer COPD-related hospitalizations (Marin JM, et al. Am J Respir Crit Care Med. 2010;182[3]:325-31), decreased cardiovascular events (Kendzerska T, et al. Ann ATS. 2019;16[1]:71), and an overall decline in mortality rates (Machado ML, et al. Eur Respir J. 2010;35[1]:132-7).
It is important to acknowledge that, as of now, no randomized clinical trial has specifically addressed the treatment of OS, leaving recommendations largely reliant on observational studies. Conversely, recent guidelines have proposed the utilization of high-intensity noninvasive ventilation (NIV) for hypercapnic patients with COPD. Thus, extensive research is warranted to characterize distinct sleep-related breathing disorders within the OS population and to investigate the effects of CPAP in comparison to other NIV modalities on patients with overlap syndrome.
Solmaz Ehteshami-Afshar, MD
Kirat Gill, MD, Section Member-at-Large
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
The overlap syndrome (OS), which refers to the co-occurrence of OSA and COPD, was first described by Flenley in 1985 (Flenley DC. Clin Chest Med. 1985;6[4]:651). Over the years, numerous studies have demonstrated an increased risk of hospitalization and mortality in patients with OS (Brennan M, et al. 2022;1-10). Despite these findings, limited evidence exists regarding the optimal treatment approach for individuals with OS.
CPAP therapy has demonstrated various physiologic advantages for patients with OS (Srivali N, et al. Sleep Med. 2023;108:55-60), which contribute to diminished dyspnea symptoms, lowered pro-inflammatory markers, improved arterial blood gases, increased 6-minute walk distance, enhanced FEV1, and decreased mean pulmonary artery pressure (Suri TM, et al. FASEB BioAdv. 2021;3[9]:683-93). CPAP therapy in patients with OS has been linked to a reduction in COPD exacerbations (Voulgaris A, et al. Clin Respir Jour. 2023; 17[3]:165), fewer COPD-related hospitalizations (Marin JM, et al. Am J Respir Crit Care Med. 2010;182[3]:325-31), decreased cardiovascular events (Kendzerska T, et al. Ann ATS. 2019;16[1]:71), and an overall decline in mortality rates (Machado ML, et al. Eur Respir J. 2010;35[1]:132-7).
It is important to acknowledge that, as of now, no randomized clinical trial has specifically addressed the treatment of OS, leaving recommendations largely reliant on observational studies. Conversely, recent guidelines have proposed the utilization of high-intensity noninvasive ventilation (NIV) for hypercapnic patients with COPD. Thus, extensive research is warranted to characterize distinct sleep-related breathing disorders within the OS population and to investigate the effects of CPAP in comparison to other NIV modalities on patients with overlap syndrome.
Solmaz Ehteshami-Afshar, MD
Kirat Gill, MD, Section Member-at-Large
Implementing a hypoglossal nerve stimulation program in your sleep practice
It is estimated that almost one billion people globally are affected by obstructive sleep apnea (OSA) (Benjafield A, et al. Lancet Respir Med. 2019;7[8]:687-98). Despite such high prevalence, the treatment options for OSA are somewhat limited. As per certain estimates, nearly 50% of CPAP users discontinue treatment by the fifth year (Schoch O, et al. Respiration. 2014;87[2]:121-8). Furthermore, alternative options such as mandibular advancement devices, positional therapy, weight loss, and maxillofacial or palate surgery, also have unique challenges and limitations.
First described in 2001, hypoglossal nerve stimulation (HGNS) is a relatively new and emerging technology for the treatment of OSA (Schwartz A, et al. Arch Otolaryngol Head Neck Surg. 2001 Oct;127[10]:1216-23). HGNS therapy was approved by the Food and Drug Administration in 2014 for the treatment of moderate to severe OSA. The therapy involves surgical implantation of the HGNS device, optimization of device settings, and evaluation for treatment response. A physician-led multidisciplinary Hypoglossal Nerve Stimulation Clinic involves collaboration from essential stakeholders, most importantly sleep medicine providers, clinic staff, sleep technologists, and ENT sleep surgeons. Goals of the multidisciplinary program are to ensure timely follow-up, optimization of device settings, and maximizing treatment efficacy. This review describes steps involved in developing a successful multidisciplinary HGNS program within a sleep medicine practice.
Patient selection and evaluation
There is growing interest in HGNS relative to conventional CPAP therapy, with many patients presenting to clinic to inquire about this therapy. However, not all patients are candidates for HGNS therapy. Prioritizing appropriate patient selection and education are key first steps. The initial assessments usually occur with a sleep medicine specialist. It begins with confirmation of the diagnosis of OSA in all patients and a concerted effort to troubleshoot and address any barriers to CPAP use before consideration of surgery. Patients who are unwilling to use or unable to tolerate CPAP therapy undergo further evaluation for HGNS therapy. It is important to ensure that patients are also screened for other sleep disorders, such as insomnia or restless leg syndrome, to rule out its contribution to daytime (or nighttime) symptoms.
Other salient inclusion criteria include an apnea-hypopnea index (AHI) between 15-100 events per hour (previously 65), at least 18 years of age, and a body mass index (BMI) less than 40 kg/m2 (previously 32). Qualifying patients undergo an updated polysomnography if a recent study is not available. If the polysomnography reveals central and mixed apneas comprising less than 25 percent of the total AHI, patients are referred to ENT Sleep Surgery, and drug-induced sleep endoscopy is offered to examine upper airway anatomy. When a complete concentric collapse of the soft palate is seen on drug-induced sleep endoscopy, surgery is contraindicated. Prior palate surgery or maxillomandibular advancement (MMA) are not contraindications to HGNS therapy.
The patients receive comprehensive information on the nature of the surgery, expected recovery course, and device activation timeline. Perhaps most importantly, the patients receive structured education on HGNS therapy and potential outcomes to set realistic expectations. In the STAR trial, patients experienced a reduction in the AHI of approximately 70% (Strollo P, et al. N Engl J Med. 2014;370[2]:139-49). It is important to note that a response to therapy was defined as a reduction in the AHI by at least 50% and a value less than 20 events/hour (Strollo P, et al. Sleep. 2015;38[10]:1593-8). Therefore, patients who are expecting complete resolution of snoring and/or OSA may not be ideal candidates for surgery. Furthermore, patients who continue to experience fatigue and sleepiness on CPAP despite control of OSA may not experience amelioration of these symptoms with HGNS therapy.
Surgery and device management
The surgery, performed under general anesthesia, lasts approximately 3 hours, and may be followed by an overnight hospital stay depending on patient’s comorbidities. The device implantation involves placement of an implantable pulse generator (IPG) in the chest wall and leads to the hypoglossal nerve. The IPG is similar to a pacemaker and functions to stimulate the ipsilateral hypoglossal nerve innervating the tongue during sleep. The most common postoperative complications noted in the STAR trial data include incision site pain and swelling as well as temporary tongue weakness or paresthesia. Postoperative restrictions are minimal and include no heavy lifting for one month after surgery.
One week postsurgery, patients return to the ENT Sleep Surgery Clinic for follow-up, at which time the incisions as well as tongue strength and sensation are evaluated. In a subsequent visit between 4 and 6 weeks postsurgery, patients are evaluated in a joint Sleep Medicine and ENT clinic. They undergo device education and activation of the IPG using a dedicated programmer obtained from the device manufacturer. Device comfort features such as start delay and pause time are also programmed. Furthermore, appropriate tongue movement, lead placement, and voltage range settings are assessed during the visit. The ENT surgery team reevaluates the incision sites and assesses for tongue function and sensation. Patients are instructed to increase the voltage incrementally every week as tolerated with the goal of using the device nightly for the entirety of sleep. If patients tolerate the therapy well during the 2- to 3-month follow-up, a sleep study is scheduled to evaluate treatment effectiveness at the peak tolerable voltage. For those struggling with the therapy, adjustments to electrode configurations should be considered for pulse width, and rate. Occasionally, patients may require awake endoscopy and/or an advanced HGNS titration while asleep to determine the most appropriate settings to optimally control sleep apnea.
Until recently, patients implanted with an early version of the HGNS were limited to magnetic resonance imaging (MRI) scans of the head, neck, and extremities only. However, patients with the latest model IPGs can now undergo full-body MRI scans. It is important to note that the MRI’s Tesla cannot exceed 1.5T, necessitating specific imaging centers. Other constraints include the inability to adjust device settings remotely, which could mean long travel for minor setting adjustments such as altering start delay or pause times. Furthermore, provider education on operating and managing the device can be time consuming and may also be a barrier to implementation in a clinic. Also challenging may be the availability of an ENT surgery, which plays a critical role in the implantation of the devices and follow-up.
Currently, Inspire Medical Systems is the only FDA-approved hypoglossal nerve stimulation device available in the United States, and globally, more than 45,000 patients have been implanted. However, the field of neurostimulation is rapidly growing. Companies like LivaNova have secured Investigational Device Exemption for their HGNS device. The Genio system by Nyxoah is evaluating the use of bilateral hypoglossal nerve stimulation in patients with OSA and complete concentric collapse of the palate. A multidisciplinary Hypoglossal Nerve Stimulation Clinic is an important component of a comprehensive sleep medicine clinic for patient care and medical education. In the appropriate patient, this emerging technology may provide improvement in OSA severity and symptoms.
Dr. Gill is Clinical Associate Professor, Division of Sleep Medicine, Stanford (Calif.) University.
It is estimated that almost one billion people globally are affected by obstructive sleep apnea (OSA) (Benjafield A, et al. Lancet Respir Med. 2019;7[8]:687-98). Despite such high prevalence, the treatment options for OSA are somewhat limited. As per certain estimates, nearly 50% of CPAP users discontinue treatment by the fifth year (Schoch O, et al. Respiration. 2014;87[2]:121-8). Furthermore, alternative options such as mandibular advancement devices, positional therapy, weight loss, and maxillofacial or palate surgery, also have unique challenges and limitations.
First described in 2001, hypoglossal nerve stimulation (HGNS) is a relatively new and emerging technology for the treatment of OSA (Schwartz A, et al. Arch Otolaryngol Head Neck Surg. 2001 Oct;127[10]:1216-23). HGNS therapy was approved by the Food and Drug Administration in 2014 for the treatment of moderate to severe OSA. The therapy involves surgical implantation of the HGNS device, optimization of device settings, and evaluation for treatment response. A physician-led multidisciplinary Hypoglossal Nerve Stimulation Clinic involves collaboration from essential stakeholders, most importantly sleep medicine providers, clinic staff, sleep technologists, and ENT sleep surgeons. Goals of the multidisciplinary program are to ensure timely follow-up, optimization of device settings, and maximizing treatment efficacy. This review describes steps involved in developing a successful multidisciplinary HGNS program within a sleep medicine practice.
Patient selection and evaluation
There is growing interest in HGNS relative to conventional CPAP therapy, with many patients presenting to clinic to inquire about this therapy. However, not all patients are candidates for HGNS therapy. Prioritizing appropriate patient selection and education are key first steps. The initial assessments usually occur with a sleep medicine specialist. It begins with confirmation of the diagnosis of OSA in all patients and a concerted effort to troubleshoot and address any barriers to CPAP use before consideration of surgery. Patients who are unwilling to use or unable to tolerate CPAP therapy undergo further evaluation for HGNS therapy. It is important to ensure that patients are also screened for other sleep disorders, such as insomnia or restless leg syndrome, to rule out its contribution to daytime (or nighttime) symptoms.
Other salient inclusion criteria include an apnea-hypopnea index (AHI) between 15-100 events per hour (previously 65), at least 18 years of age, and a body mass index (BMI) less than 40 kg/m2 (previously 32). Qualifying patients undergo an updated polysomnography if a recent study is not available. If the polysomnography reveals central and mixed apneas comprising less than 25 percent of the total AHI, patients are referred to ENT Sleep Surgery, and drug-induced sleep endoscopy is offered to examine upper airway anatomy. When a complete concentric collapse of the soft palate is seen on drug-induced sleep endoscopy, surgery is contraindicated. Prior palate surgery or maxillomandibular advancement (MMA) are not contraindications to HGNS therapy.
The patients receive comprehensive information on the nature of the surgery, expected recovery course, and device activation timeline. Perhaps most importantly, the patients receive structured education on HGNS therapy and potential outcomes to set realistic expectations. In the STAR trial, patients experienced a reduction in the AHI of approximately 70% (Strollo P, et al. N Engl J Med. 2014;370[2]:139-49). It is important to note that a response to therapy was defined as a reduction in the AHI by at least 50% and a value less than 20 events/hour (Strollo P, et al. Sleep. 2015;38[10]:1593-8). Therefore, patients who are expecting complete resolution of snoring and/or OSA may not be ideal candidates for surgery. Furthermore, patients who continue to experience fatigue and sleepiness on CPAP despite control of OSA may not experience amelioration of these symptoms with HGNS therapy.
Surgery and device management
The surgery, performed under general anesthesia, lasts approximately 3 hours, and may be followed by an overnight hospital stay depending on patient’s comorbidities. The device implantation involves placement of an implantable pulse generator (IPG) in the chest wall and leads to the hypoglossal nerve. The IPG is similar to a pacemaker and functions to stimulate the ipsilateral hypoglossal nerve innervating the tongue during sleep. The most common postoperative complications noted in the STAR trial data include incision site pain and swelling as well as temporary tongue weakness or paresthesia. Postoperative restrictions are minimal and include no heavy lifting for one month after surgery.
One week postsurgery, patients return to the ENT Sleep Surgery Clinic for follow-up, at which time the incisions as well as tongue strength and sensation are evaluated. In a subsequent visit between 4 and 6 weeks postsurgery, patients are evaluated in a joint Sleep Medicine and ENT clinic. They undergo device education and activation of the IPG using a dedicated programmer obtained from the device manufacturer. Device comfort features such as start delay and pause time are also programmed. Furthermore, appropriate tongue movement, lead placement, and voltage range settings are assessed during the visit. The ENT surgery team reevaluates the incision sites and assesses for tongue function and sensation. Patients are instructed to increase the voltage incrementally every week as tolerated with the goal of using the device nightly for the entirety of sleep. If patients tolerate the therapy well during the 2- to 3-month follow-up, a sleep study is scheduled to evaluate treatment effectiveness at the peak tolerable voltage. For those struggling with the therapy, adjustments to electrode configurations should be considered for pulse width, and rate. Occasionally, patients may require awake endoscopy and/or an advanced HGNS titration while asleep to determine the most appropriate settings to optimally control sleep apnea.
Until recently, patients implanted with an early version of the HGNS were limited to magnetic resonance imaging (MRI) scans of the head, neck, and extremities only. However, patients with the latest model IPGs can now undergo full-body MRI scans. It is important to note that the MRI’s Tesla cannot exceed 1.5T, necessitating specific imaging centers. Other constraints include the inability to adjust device settings remotely, which could mean long travel for minor setting adjustments such as altering start delay or pause times. Furthermore, provider education on operating and managing the device can be time consuming and may also be a barrier to implementation in a clinic. Also challenging may be the availability of an ENT surgery, which plays a critical role in the implantation of the devices and follow-up.
Currently, Inspire Medical Systems is the only FDA-approved hypoglossal nerve stimulation device available in the United States, and globally, more than 45,000 patients have been implanted. However, the field of neurostimulation is rapidly growing. Companies like LivaNova have secured Investigational Device Exemption for their HGNS device. The Genio system by Nyxoah is evaluating the use of bilateral hypoglossal nerve stimulation in patients with OSA and complete concentric collapse of the palate. A multidisciplinary Hypoglossal Nerve Stimulation Clinic is an important component of a comprehensive sleep medicine clinic for patient care and medical education. In the appropriate patient, this emerging technology may provide improvement in OSA severity and symptoms.
Dr. Gill is Clinical Associate Professor, Division of Sleep Medicine, Stanford (Calif.) University.
It is estimated that almost one billion people globally are affected by obstructive sleep apnea (OSA) (Benjafield A, et al. Lancet Respir Med. 2019;7[8]:687-98). Despite such high prevalence, the treatment options for OSA are somewhat limited. As per certain estimates, nearly 50% of CPAP users discontinue treatment by the fifth year (Schoch O, et al. Respiration. 2014;87[2]:121-8). Furthermore, alternative options such as mandibular advancement devices, positional therapy, weight loss, and maxillofacial or palate surgery, also have unique challenges and limitations.
First described in 2001, hypoglossal nerve stimulation (HGNS) is a relatively new and emerging technology for the treatment of OSA (Schwartz A, et al. Arch Otolaryngol Head Neck Surg. 2001 Oct;127[10]:1216-23). HGNS therapy was approved by the Food and Drug Administration in 2014 for the treatment of moderate to severe OSA. The therapy involves surgical implantation of the HGNS device, optimization of device settings, and evaluation for treatment response. A physician-led multidisciplinary Hypoglossal Nerve Stimulation Clinic involves collaboration from essential stakeholders, most importantly sleep medicine providers, clinic staff, sleep technologists, and ENT sleep surgeons. Goals of the multidisciplinary program are to ensure timely follow-up, optimization of device settings, and maximizing treatment efficacy. This review describes steps involved in developing a successful multidisciplinary HGNS program within a sleep medicine practice.
Patient selection and evaluation
There is growing interest in HGNS relative to conventional CPAP therapy, with many patients presenting to clinic to inquire about this therapy. However, not all patients are candidates for HGNS therapy. Prioritizing appropriate patient selection and education are key first steps. The initial assessments usually occur with a sleep medicine specialist. It begins with confirmation of the diagnosis of OSA in all patients and a concerted effort to troubleshoot and address any barriers to CPAP use before consideration of surgery. Patients who are unwilling to use or unable to tolerate CPAP therapy undergo further evaluation for HGNS therapy. It is important to ensure that patients are also screened for other sleep disorders, such as insomnia or restless leg syndrome, to rule out its contribution to daytime (or nighttime) symptoms.
Other salient inclusion criteria include an apnea-hypopnea index (AHI) between 15-100 events per hour (previously 65), at least 18 years of age, and a body mass index (BMI) less than 40 kg/m2 (previously 32). Qualifying patients undergo an updated polysomnography if a recent study is not available. If the polysomnography reveals central and mixed apneas comprising less than 25 percent of the total AHI, patients are referred to ENT Sleep Surgery, and drug-induced sleep endoscopy is offered to examine upper airway anatomy. When a complete concentric collapse of the soft palate is seen on drug-induced sleep endoscopy, surgery is contraindicated. Prior palate surgery or maxillomandibular advancement (MMA) are not contraindications to HGNS therapy.
The patients receive comprehensive information on the nature of the surgery, expected recovery course, and device activation timeline. Perhaps most importantly, the patients receive structured education on HGNS therapy and potential outcomes to set realistic expectations. In the STAR trial, patients experienced a reduction in the AHI of approximately 70% (Strollo P, et al. N Engl J Med. 2014;370[2]:139-49). It is important to note that a response to therapy was defined as a reduction in the AHI by at least 50% and a value less than 20 events/hour (Strollo P, et al. Sleep. 2015;38[10]:1593-8). Therefore, patients who are expecting complete resolution of snoring and/or OSA may not be ideal candidates for surgery. Furthermore, patients who continue to experience fatigue and sleepiness on CPAP despite control of OSA may not experience amelioration of these symptoms with HGNS therapy.
Surgery and device management
The surgery, performed under general anesthesia, lasts approximately 3 hours, and may be followed by an overnight hospital stay depending on patient’s comorbidities. The device implantation involves placement of an implantable pulse generator (IPG) in the chest wall and leads to the hypoglossal nerve. The IPG is similar to a pacemaker and functions to stimulate the ipsilateral hypoglossal nerve innervating the tongue during sleep. The most common postoperative complications noted in the STAR trial data include incision site pain and swelling as well as temporary tongue weakness or paresthesia. Postoperative restrictions are minimal and include no heavy lifting for one month after surgery.
One week postsurgery, patients return to the ENT Sleep Surgery Clinic for follow-up, at which time the incisions as well as tongue strength and sensation are evaluated. In a subsequent visit between 4 and 6 weeks postsurgery, patients are evaluated in a joint Sleep Medicine and ENT clinic. They undergo device education and activation of the IPG using a dedicated programmer obtained from the device manufacturer. Device comfort features such as start delay and pause time are also programmed. Furthermore, appropriate tongue movement, lead placement, and voltage range settings are assessed during the visit. The ENT surgery team reevaluates the incision sites and assesses for tongue function and sensation. Patients are instructed to increase the voltage incrementally every week as tolerated with the goal of using the device nightly for the entirety of sleep. If patients tolerate the therapy well during the 2- to 3-month follow-up, a sleep study is scheduled to evaluate treatment effectiveness at the peak tolerable voltage. For those struggling with the therapy, adjustments to electrode configurations should be considered for pulse width, and rate. Occasionally, patients may require awake endoscopy and/or an advanced HGNS titration while asleep to determine the most appropriate settings to optimally control sleep apnea.
Until recently, patients implanted with an early version of the HGNS were limited to magnetic resonance imaging (MRI) scans of the head, neck, and extremities only. However, patients with the latest model IPGs can now undergo full-body MRI scans. It is important to note that the MRI’s Tesla cannot exceed 1.5T, necessitating specific imaging centers. Other constraints include the inability to adjust device settings remotely, which could mean long travel for minor setting adjustments such as altering start delay or pause times. Furthermore, provider education on operating and managing the device can be time consuming and may also be a barrier to implementation in a clinic. Also challenging may be the availability of an ENT surgery, which plays a critical role in the implantation of the devices and follow-up.
Currently, Inspire Medical Systems is the only FDA-approved hypoglossal nerve stimulation device available in the United States, and globally, more than 45,000 patients have been implanted. However, the field of neurostimulation is rapidly growing. Companies like LivaNova have secured Investigational Device Exemption for their HGNS device. The Genio system by Nyxoah is evaluating the use of bilateral hypoglossal nerve stimulation in patients with OSA and complete concentric collapse of the palate. A multidisciplinary Hypoglossal Nerve Stimulation Clinic is an important component of a comprehensive sleep medicine clinic for patient care and medical education. In the appropriate patient, this emerging technology may provide improvement in OSA severity and symptoms.
Dr. Gill is Clinical Associate Professor, Division of Sleep Medicine, Stanford (Calif.) University.
CPAP for OSA: What is the verdict?
Obstructive sleep apnea (OSA) affects roughly 1 billion people worldwide, according to a report by the American Academy of Sleep Medicine. Severe OSA has been associated with an elevated risk of all-cause and cardiovascular-specific mortality. Studies support an association between OSA and a host of comorbidities, including hypertension, stroke, atrial fibrillation, mood disorders, and neurocognitive outcomes. Undiagnosed and untreated OSA also has major economic and societal costs, reducing workplace productivity and increasing one’s risk of accidents both on the job and while driving.
Positive airway pressure (PAP) is widely considered the most effective treatment for OSA. The majority of patients tolerate CPAP: real-world estimates using international big data show good adherence in over 70% of patients. Robust evidence shows that PAP reduces snoring, decreases daytime sleepiness, and improves quality of life in a dose-dependent manner. Economic analyses have also found CPAP to be cost-effective (Streatfeild, et al. Sleep. 2019;42[12]:zsz181).
But what do we know about the impact of PAP on health outcomes? Perhaps the best studied outcome is cardiovascular disease. Results of observational trials have suggested that CPAP adherence was associated with survival (Pepin JL et al. Chest. 2022;161[6]:1657). However, it has been speculated that these findings may have been driven, at least in part, by the “healthy user effect.” This phenomenon refers to the tendency for people who engage in one health-promoting behavior (eg, CPAP adherence) to engage in another as well (eg, eating well, exercising, taking prescribed medications). When we observe that patients who use CPAP live longer, we must ask ourselves whether perhaps their better outcomes resulted from healthy habits in general, as opposed to their CPAP usage per se.
Randomization eliminates the potential for the healthy user effect, by assigning patients to a certain intervention as opposed to simply observing whether they choose to use it. And herein lies one of the great disappointments for our field over the past decade: multiple large-scale randomized controlled trials have failed to demonstrate that CPAP reduces cardiovascular mortality, even in patients with pre-existing CAD. The first two of these were the SAVE (Sleep Apnea Cardiovascular Endpoints) (McEvoy R, et al. N Engl J Med. 2016;375[10]:919) and RICCADSA (Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA) (Peker Y, et al. Am J Respir Crit Care Med. 2016;194[5]:613) trials evaluating the effects of PAP on a composite endpoint that included cardiovascular death and nonfatal cardiovascular events. Both trials found no difference between PAP and control groups, leading to a conclusion that PAP did not prevent cardiovascular events in patients with moderate-to-severe OSA and established cardiovascular disease. The ISAAC study (Impact of Sleep Apnea syndrome in the evolution of Acute Coronary syndrome) also failed to show a benefit of CPAP for secondary prevention of cardiovascular events in patients with moderate to severe OSA.
These negative findings were echoed in a recent report by the Agency for Healthcare Research and Quality evaluating a variety of long-term health outcomes in obstructive sleep apnea. The authors stated that “RCTs do not provide evidence that CPAP prescription affects long-term, clinically important outcomes. Specifically, with low strength of evidence, RCTs do not demonstrate that CPAP affects all-cause mortality, various CV outcomes, clinically important changes in psychosocial measures, or other clinical events” (AHRQ, Project ID: SLPT0919, 12/1/2022).
What plausible explanations have been offered for these negative results? Perhaps trials were underpowered. Perhaps patients did not use PAP for a sufficient duration to achieve benefit (usage was under 3 hours in most studies). Perhaps the patients selected for these trials were at such low-risk of adverse outcomes in the first place that treating their OSA didn’t have much impact. Many trials have excluded sleepy patients due to ethical concerns about withholding treatment from this population. But this may have effectively excluded the patients most likely to benefit; in other studies, sleepy patients seem to experience the greatest cardiovascular risk reduction with CPAP. For example, a meta-analysis showed that CPAP is most strongly associated with blood pressure reduction in patients who are sleepy, compared with those with minimally symptomatic OSA (Bratton D, et al. Thorax. 2014;69[12]:1128). And, recent work suggests that even among non-sleepy patients, it might be possible to identify a subset who could benefit from CPAP. A recent analysis suggested that non-sleepy patients who exhibit a higher change in heart rate following a respiratory event may derive greater cardiovascular benefit from CPAP therapy (Azarbarzin, et al. Am J Respir Crit Care Med. 2022;206[6]:767).
Another, distinct reason for these negative results is that the AHI – our main metric for quantifying OSA severity for several decades – fails to capture the disorder’s heterogeneity. Identifying different phenotypes of OSA may enable more personalized approaches to prognostication as well as treatment. For example, one study identified four symptom clusters of OSA – patients with disturbed sleep, minimally symptomatic, excessively sleepy, and moderately sleepy – who may exhibit different responses to CPAP treatment. Further work is needed to discern whether these clusters reliably predict outcomes in a manner that can be useful clinically (Zinchuk A, et al. Sleep Med Rev. 2017;35:113).
So, what is the verdict for CPAP? Sleepy patients with even mild OSA warrant treatment, as is common practice, and these patients are more likely to adhere to therapy. Patients with other symptoms potentially related to untreated OSA should be offered treatment as well. But in asymptomatic patients, it is difficult to make a compelling case to start CPAP on the basis of the AHI alone. It is our hope that novel ways of classifying OSA severity and phenotype will allow better prediction of which patients will experience a protective effect from CPAP. For example, certain subsets of patients may realize greater benefits from CPAP, including those with a high hypoxic burden (Trzepizur W, et al. Am J Respir Crit Care Med. 2022;205[1]:108).
For now though, we can allow the evidence that has accumulated in recent years to guide our collaborative decision-making with patients about whether to try CPAP. Depending on how exuberantly we sang CPAP’s praises, we may need to temper our song – at least with regards to cardiovascular risk reduction. In the sleep world, patients are educated not only by sleep providers but also by respiratory therapists who help patients with initial CPAP setups. Consistent, evidence-based messaging by the entire health care team is key. We cannot say that “using CPAP prevents heart attacks” but rather “we’re still not quite sure.”
As in other areas of medicine, sleep medicine may see a shift in focus toward symptoms and patient-oriented outcomes as opposed to the presence of comorbidities. In fact, the recently revised International Classification of Sleep Disorders (ICSD-3-TR) released this year eliminated comorbidity criteria from the definition of Obstructive Sleep Apnea in adults. If adopted by Centers for Medicare & Medicaid Services and other insurers, patients with mild OSA by sleep testing (AHI≥5 but <15) who lack symptoms will no longer qualify for CPAP on the basis of having hypertension, a mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type 2 diabetes mellitus. How will this major revision impact the sleep medicine world? Practically speaking, it is likely that fewer patients who present without symptoms and are found to have only mild OSA will end up on PAP.
There will undoubtedly be frustration related to these greater restrictions on who qualifies for PAP. On the other hand, perhaps our energy is better focused on procuring PAP not for asymptomatic patients but rather promoting access and adherence in those who are symptomatic. Differential access to CPAP remains a major problem that very likely contributes to health disparities. In fact, a recent international committee acknowledged that the current CMS criteria for PAP coverage create disproportionate difficulties for non-white patients and those of low socioeconomic background to meet adherence criteria. Their specific recommendations to reduce this disparity in PAP access included eradication of requirements for repeat polysomnography and eliminating the 4-hour rule.
We are moving toward a more personalized approach to characterizing OSA, which eventually may allow for more nuanced, individualized counseling rather than a “one-size -called-CPAP-fits-all” approach. Until we are there, a patient-centered approach that elicits the presence of sleep-related symptoms and daytime impairment, as opposed to isolated comorbidities, provides the most compelling justification for CPAP.
Obstructive sleep apnea (OSA) affects roughly 1 billion people worldwide, according to a report by the American Academy of Sleep Medicine. Severe OSA has been associated with an elevated risk of all-cause and cardiovascular-specific mortality. Studies support an association between OSA and a host of comorbidities, including hypertension, stroke, atrial fibrillation, mood disorders, and neurocognitive outcomes. Undiagnosed and untreated OSA also has major economic and societal costs, reducing workplace productivity and increasing one’s risk of accidents both on the job and while driving.
Positive airway pressure (PAP) is widely considered the most effective treatment for OSA. The majority of patients tolerate CPAP: real-world estimates using international big data show good adherence in over 70% of patients. Robust evidence shows that PAP reduces snoring, decreases daytime sleepiness, and improves quality of life in a dose-dependent manner. Economic analyses have also found CPAP to be cost-effective (Streatfeild, et al. Sleep. 2019;42[12]:zsz181).
But what do we know about the impact of PAP on health outcomes? Perhaps the best studied outcome is cardiovascular disease. Results of observational trials have suggested that CPAP adherence was associated with survival (Pepin JL et al. Chest. 2022;161[6]:1657). However, it has been speculated that these findings may have been driven, at least in part, by the “healthy user effect.” This phenomenon refers to the tendency for people who engage in one health-promoting behavior (eg, CPAP adherence) to engage in another as well (eg, eating well, exercising, taking prescribed medications). When we observe that patients who use CPAP live longer, we must ask ourselves whether perhaps their better outcomes resulted from healthy habits in general, as opposed to their CPAP usage per se.
Randomization eliminates the potential for the healthy user effect, by assigning patients to a certain intervention as opposed to simply observing whether they choose to use it. And herein lies one of the great disappointments for our field over the past decade: multiple large-scale randomized controlled trials have failed to demonstrate that CPAP reduces cardiovascular mortality, even in patients with pre-existing CAD. The first two of these were the SAVE (Sleep Apnea Cardiovascular Endpoints) (McEvoy R, et al. N Engl J Med. 2016;375[10]:919) and RICCADSA (Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA) (Peker Y, et al. Am J Respir Crit Care Med. 2016;194[5]:613) trials evaluating the effects of PAP on a composite endpoint that included cardiovascular death and nonfatal cardiovascular events. Both trials found no difference between PAP and control groups, leading to a conclusion that PAP did not prevent cardiovascular events in patients with moderate-to-severe OSA and established cardiovascular disease. The ISAAC study (Impact of Sleep Apnea syndrome in the evolution of Acute Coronary syndrome) also failed to show a benefit of CPAP for secondary prevention of cardiovascular events in patients with moderate to severe OSA.
These negative findings were echoed in a recent report by the Agency for Healthcare Research and Quality evaluating a variety of long-term health outcomes in obstructive sleep apnea. The authors stated that “RCTs do not provide evidence that CPAP prescription affects long-term, clinically important outcomes. Specifically, with low strength of evidence, RCTs do not demonstrate that CPAP affects all-cause mortality, various CV outcomes, clinically important changes in psychosocial measures, or other clinical events” (AHRQ, Project ID: SLPT0919, 12/1/2022).
What plausible explanations have been offered for these negative results? Perhaps trials were underpowered. Perhaps patients did not use PAP for a sufficient duration to achieve benefit (usage was under 3 hours in most studies). Perhaps the patients selected for these trials were at such low-risk of adverse outcomes in the first place that treating their OSA didn’t have much impact. Many trials have excluded sleepy patients due to ethical concerns about withholding treatment from this population. But this may have effectively excluded the patients most likely to benefit; in other studies, sleepy patients seem to experience the greatest cardiovascular risk reduction with CPAP. For example, a meta-analysis showed that CPAP is most strongly associated with blood pressure reduction in patients who are sleepy, compared with those with minimally symptomatic OSA (Bratton D, et al. Thorax. 2014;69[12]:1128). And, recent work suggests that even among non-sleepy patients, it might be possible to identify a subset who could benefit from CPAP. A recent analysis suggested that non-sleepy patients who exhibit a higher change in heart rate following a respiratory event may derive greater cardiovascular benefit from CPAP therapy (Azarbarzin, et al. Am J Respir Crit Care Med. 2022;206[6]:767).
Another, distinct reason for these negative results is that the AHI – our main metric for quantifying OSA severity for several decades – fails to capture the disorder’s heterogeneity. Identifying different phenotypes of OSA may enable more personalized approaches to prognostication as well as treatment. For example, one study identified four symptom clusters of OSA – patients with disturbed sleep, minimally symptomatic, excessively sleepy, and moderately sleepy – who may exhibit different responses to CPAP treatment. Further work is needed to discern whether these clusters reliably predict outcomes in a manner that can be useful clinically (Zinchuk A, et al. Sleep Med Rev. 2017;35:113).
So, what is the verdict for CPAP? Sleepy patients with even mild OSA warrant treatment, as is common practice, and these patients are more likely to adhere to therapy. Patients with other symptoms potentially related to untreated OSA should be offered treatment as well. But in asymptomatic patients, it is difficult to make a compelling case to start CPAP on the basis of the AHI alone. It is our hope that novel ways of classifying OSA severity and phenotype will allow better prediction of which patients will experience a protective effect from CPAP. For example, certain subsets of patients may realize greater benefits from CPAP, including those with a high hypoxic burden (Trzepizur W, et al. Am J Respir Crit Care Med. 2022;205[1]:108).
For now though, we can allow the evidence that has accumulated in recent years to guide our collaborative decision-making with patients about whether to try CPAP. Depending on how exuberantly we sang CPAP’s praises, we may need to temper our song – at least with regards to cardiovascular risk reduction. In the sleep world, patients are educated not only by sleep providers but also by respiratory therapists who help patients with initial CPAP setups. Consistent, evidence-based messaging by the entire health care team is key. We cannot say that “using CPAP prevents heart attacks” but rather “we’re still not quite sure.”
As in other areas of medicine, sleep medicine may see a shift in focus toward symptoms and patient-oriented outcomes as opposed to the presence of comorbidities. In fact, the recently revised International Classification of Sleep Disorders (ICSD-3-TR) released this year eliminated comorbidity criteria from the definition of Obstructive Sleep Apnea in adults. If adopted by Centers for Medicare & Medicaid Services and other insurers, patients with mild OSA by sleep testing (AHI≥5 but <15) who lack symptoms will no longer qualify for CPAP on the basis of having hypertension, a mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type 2 diabetes mellitus. How will this major revision impact the sleep medicine world? Practically speaking, it is likely that fewer patients who present without symptoms and are found to have only mild OSA will end up on PAP.
There will undoubtedly be frustration related to these greater restrictions on who qualifies for PAP. On the other hand, perhaps our energy is better focused on procuring PAP not for asymptomatic patients but rather promoting access and adherence in those who are symptomatic. Differential access to CPAP remains a major problem that very likely contributes to health disparities. In fact, a recent international committee acknowledged that the current CMS criteria for PAP coverage create disproportionate difficulties for non-white patients and those of low socioeconomic background to meet adherence criteria. Their specific recommendations to reduce this disparity in PAP access included eradication of requirements for repeat polysomnography and eliminating the 4-hour rule.
We are moving toward a more personalized approach to characterizing OSA, which eventually may allow for more nuanced, individualized counseling rather than a “one-size -called-CPAP-fits-all” approach. Until we are there, a patient-centered approach that elicits the presence of sleep-related symptoms and daytime impairment, as opposed to isolated comorbidities, provides the most compelling justification for CPAP.
Obstructive sleep apnea (OSA) affects roughly 1 billion people worldwide, according to a report by the American Academy of Sleep Medicine. Severe OSA has been associated with an elevated risk of all-cause and cardiovascular-specific mortality. Studies support an association between OSA and a host of comorbidities, including hypertension, stroke, atrial fibrillation, mood disorders, and neurocognitive outcomes. Undiagnosed and untreated OSA also has major economic and societal costs, reducing workplace productivity and increasing one’s risk of accidents both on the job and while driving.
Positive airway pressure (PAP) is widely considered the most effective treatment for OSA. The majority of patients tolerate CPAP: real-world estimates using international big data show good adherence in over 70% of patients. Robust evidence shows that PAP reduces snoring, decreases daytime sleepiness, and improves quality of life in a dose-dependent manner. Economic analyses have also found CPAP to be cost-effective (Streatfeild, et al. Sleep. 2019;42[12]:zsz181).
But what do we know about the impact of PAP on health outcomes? Perhaps the best studied outcome is cardiovascular disease. Results of observational trials have suggested that CPAP adherence was associated with survival (Pepin JL et al. Chest. 2022;161[6]:1657). However, it has been speculated that these findings may have been driven, at least in part, by the “healthy user effect.” This phenomenon refers to the tendency for people who engage in one health-promoting behavior (eg, CPAP adherence) to engage in another as well (eg, eating well, exercising, taking prescribed medications). When we observe that patients who use CPAP live longer, we must ask ourselves whether perhaps their better outcomes resulted from healthy habits in general, as opposed to their CPAP usage per se.
Randomization eliminates the potential for the healthy user effect, by assigning patients to a certain intervention as opposed to simply observing whether they choose to use it. And herein lies one of the great disappointments for our field over the past decade: multiple large-scale randomized controlled trials have failed to demonstrate that CPAP reduces cardiovascular mortality, even in patients with pre-existing CAD. The first two of these were the SAVE (Sleep Apnea Cardiovascular Endpoints) (McEvoy R, et al. N Engl J Med. 2016;375[10]:919) and RICCADSA (Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA) (Peker Y, et al. Am J Respir Crit Care Med. 2016;194[5]:613) trials evaluating the effects of PAP on a composite endpoint that included cardiovascular death and nonfatal cardiovascular events. Both trials found no difference between PAP and control groups, leading to a conclusion that PAP did not prevent cardiovascular events in patients with moderate-to-severe OSA and established cardiovascular disease. The ISAAC study (Impact of Sleep Apnea syndrome in the evolution of Acute Coronary syndrome) also failed to show a benefit of CPAP for secondary prevention of cardiovascular events in patients with moderate to severe OSA.
These negative findings were echoed in a recent report by the Agency for Healthcare Research and Quality evaluating a variety of long-term health outcomes in obstructive sleep apnea. The authors stated that “RCTs do not provide evidence that CPAP prescription affects long-term, clinically important outcomes. Specifically, with low strength of evidence, RCTs do not demonstrate that CPAP affects all-cause mortality, various CV outcomes, clinically important changes in psychosocial measures, or other clinical events” (AHRQ, Project ID: SLPT0919, 12/1/2022).
What plausible explanations have been offered for these negative results? Perhaps trials were underpowered. Perhaps patients did not use PAP for a sufficient duration to achieve benefit (usage was under 3 hours in most studies). Perhaps the patients selected for these trials were at such low-risk of adverse outcomes in the first place that treating their OSA didn’t have much impact. Many trials have excluded sleepy patients due to ethical concerns about withholding treatment from this population. But this may have effectively excluded the patients most likely to benefit; in other studies, sleepy patients seem to experience the greatest cardiovascular risk reduction with CPAP. For example, a meta-analysis showed that CPAP is most strongly associated with blood pressure reduction in patients who are sleepy, compared with those with minimally symptomatic OSA (Bratton D, et al. Thorax. 2014;69[12]:1128). And, recent work suggests that even among non-sleepy patients, it might be possible to identify a subset who could benefit from CPAP. A recent analysis suggested that non-sleepy patients who exhibit a higher change in heart rate following a respiratory event may derive greater cardiovascular benefit from CPAP therapy (Azarbarzin, et al. Am J Respir Crit Care Med. 2022;206[6]:767).
Another, distinct reason for these negative results is that the AHI – our main metric for quantifying OSA severity for several decades – fails to capture the disorder’s heterogeneity. Identifying different phenotypes of OSA may enable more personalized approaches to prognostication as well as treatment. For example, one study identified four symptom clusters of OSA – patients with disturbed sleep, minimally symptomatic, excessively sleepy, and moderately sleepy – who may exhibit different responses to CPAP treatment. Further work is needed to discern whether these clusters reliably predict outcomes in a manner that can be useful clinically (Zinchuk A, et al. Sleep Med Rev. 2017;35:113).
So, what is the verdict for CPAP? Sleepy patients with even mild OSA warrant treatment, as is common practice, and these patients are more likely to adhere to therapy. Patients with other symptoms potentially related to untreated OSA should be offered treatment as well. But in asymptomatic patients, it is difficult to make a compelling case to start CPAP on the basis of the AHI alone. It is our hope that novel ways of classifying OSA severity and phenotype will allow better prediction of which patients will experience a protective effect from CPAP. For example, certain subsets of patients may realize greater benefits from CPAP, including those with a high hypoxic burden (Trzepizur W, et al. Am J Respir Crit Care Med. 2022;205[1]:108).
For now though, we can allow the evidence that has accumulated in recent years to guide our collaborative decision-making with patients about whether to try CPAP. Depending on how exuberantly we sang CPAP’s praises, we may need to temper our song – at least with regards to cardiovascular risk reduction. In the sleep world, patients are educated not only by sleep providers but also by respiratory therapists who help patients with initial CPAP setups. Consistent, evidence-based messaging by the entire health care team is key. We cannot say that “using CPAP prevents heart attacks” but rather “we’re still not quite sure.”
As in other areas of medicine, sleep medicine may see a shift in focus toward symptoms and patient-oriented outcomes as opposed to the presence of comorbidities. In fact, the recently revised International Classification of Sleep Disorders (ICSD-3-TR) released this year eliminated comorbidity criteria from the definition of Obstructive Sleep Apnea in adults. If adopted by Centers for Medicare & Medicaid Services and other insurers, patients with mild OSA by sleep testing (AHI≥5 but <15) who lack symptoms will no longer qualify for CPAP on the basis of having hypertension, a mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type 2 diabetes mellitus. How will this major revision impact the sleep medicine world? Practically speaking, it is likely that fewer patients who present without symptoms and are found to have only mild OSA will end up on PAP.
There will undoubtedly be frustration related to these greater restrictions on who qualifies for PAP. On the other hand, perhaps our energy is better focused on procuring PAP not for asymptomatic patients but rather promoting access and adherence in those who are symptomatic. Differential access to CPAP remains a major problem that very likely contributes to health disparities. In fact, a recent international committee acknowledged that the current CMS criteria for PAP coverage create disproportionate difficulties for non-white patients and those of low socioeconomic background to meet adherence criteria. Their specific recommendations to reduce this disparity in PAP access included eradication of requirements for repeat polysomnography and eliminating the 4-hour rule.
We are moving toward a more personalized approach to characterizing OSA, which eventually may allow for more nuanced, individualized counseling rather than a “one-size -called-CPAP-fits-all” approach. Until we are there, a patient-centered approach that elicits the presence of sleep-related symptoms and daytime impairment, as opposed to isolated comorbidities, provides the most compelling justification for CPAP.

























