User login
Center of Excellence site
FDA panel backs atezolizumab for mTNBC – at least for now
On the first day of a historic 3-day meeting about drugs that were granted an accelerated approval by the Food and Drug Administration for cancer indications, the first approval to come under discussion is staying in place, at least for now.
Members of the FDA’s Oncologic Drugs Advisory Committee voted 7-2 in favor of keeping in place the indication for atezolizumab (Tecentriq) for use in a certain form of breast cancer. At the same time, the committee urged the manufacturer, Genentech, to do the research needed to prove the medicine works for these patients.
The specific indication is for atezolizumab as part of a combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors are PD-L1 positive.
The FDA granted accelerated approval in 2019 for this use of atezolizumab, expecting Genentech to produce more extensive evidence of this benefit. But so far, Genentech has not produced the data proving to the FDA that atezolizumab provides the expected benefit.
The drug was already available for use in bladder cancer, having been granted a full approval for this indication in 2016.
Other accelerated approvals withdrawn
This week’s 3-day ODAC meeting is part of the FDA’s broader reconsideration of what it has described as “dangling accelerated approvals.”
Earlier discussions between the FDA and drugmakers have already triggered four voluntary withdrawals of cancer indications with these accelerated approvals, noted Julia A. Beaver, MD, and Richard Pazdur, MD, two of the FDA’s top regulators of oncology medicine, in an April 21 perspective article in the New England Journal of Medicine.
“The small percentage of drugs whose clinical benefit is ultimately not confirmed should be viewed not as a failure of accelerated approval but rather as an expected trade-off in expediting drug development that benefits patients with severe or life-threatening diseases,” Dr. Beaver and Dr. Pazdur wrote.
But making these calls can be tough. On the first day of the meeting, even ODAC panelists who backed Genentech’s bid to maintain an mTNBC indication for atezolizumab expressed discomfort with this choice.
The FDA granted the accelerated approval for use of this drug in March 2019 based on improved progression-free survival from the IMpassion130 trial. But the drug fell short in subsequent efforts to confirm the results seen in that study. The confirmatory IMpassion131 trial failed to meet the primary endpoint, the FDA staff noted in briefing materials for the ODAC meeting.
ODAC panelist Stan Lipkowitz, MD, PhD, of the National Cancer Institute, said he expected this vote had been a tough one for all members serving on ODAC that day.
“In some ways, the purist in me said I should have voted no. But when I looked at the data, there are a couple of things that struck me,” said Dr. Lipkowitz, who is the chief of the Women’s Malignancies Branch at NCI’s Center for Cancer Research. “First of all, the landscape hasn’t changed. There’s really no therapy in the first line for triple-negative metastatic that is shown to improve survival.”
Dr. Lipkowitz emphasized that Genentech needs to continue to try to prove atezolizumab works in this setting.
“There needs to be confirmatory study,” Dr. Lipkowitz concluded.
ODAC panelist Matthew Ellis, MD, PhD, of Baylor College of Medicine, Houston, said he also understood the difficult outlook for women fighting this cancer, but he voted against maintaining the approval.
“It’s not that I don’t feel the tragedy of these women,” said Dr. Ellis, citing his own decades of clinical experience.
“I just think that the data are the data,” Dr. Ellis said, adding that, in his view, “the only correct interpretation” of the evidence supported a vote against allowing the indication to stay.
The FDA considers the recommendations of its advisory committees but is not bound by them.
In a statement issued after the vote, Genentech said it intends to work with the FDA to determine the next steps for this indication of atezolizumab because “the clinically meaningful benefit demonstrated in the IMpassion130 study remains.”
The ODAC meeting continues for 2 more days, and will consider five more cancer indications that have been granted an accelerated approval.
A version of this article first appeared on Medscape.com.
On the first day of a historic 3-day meeting about drugs that were granted an accelerated approval by the Food and Drug Administration for cancer indications, the first approval to come under discussion is staying in place, at least for now.
Members of the FDA’s Oncologic Drugs Advisory Committee voted 7-2 in favor of keeping in place the indication for atezolizumab (Tecentriq) for use in a certain form of breast cancer. At the same time, the committee urged the manufacturer, Genentech, to do the research needed to prove the medicine works for these patients.
The specific indication is for atezolizumab as part of a combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors are PD-L1 positive.
The FDA granted accelerated approval in 2019 for this use of atezolizumab, expecting Genentech to produce more extensive evidence of this benefit. But so far, Genentech has not produced the data proving to the FDA that atezolizumab provides the expected benefit.
The drug was already available for use in bladder cancer, having been granted a full approval for this indication in 2016.
Other accelerated approvals withdrawn
This week’s 3-day ODAC meeting is part of the FDA’s broader reconsideration of what it has described as “dangling accelerated approvals.”
Earlier discussions between the FDA and drugmakers have already triggered four voluntary withdrawals of cancer indications with these accelerated approvals, noted Julia A. Beaver, MD, and Richard Pazdur, MD, two of the FDA’s top regulators of oncology medicine, in an April 21 perspective article in the New England Journal of Medicine.
“The small percentage of drugs whose clinical benefit is ultimately not confirmed should be viewed not as a failure of accelerated approval but rather as an expected trade-off in expediting drug development that benefits patients with severe or life-threatening diseases,” Dr. Beaver and Dr. Pazdur wrote.
But making these calls can be tough. On the first day of the meeting, even ODAC panelists who backed Genentech’s bid to maintain an mTNBC indication for atezolizumab expressed discomfort with this choice.
The FDA granted the accelerated approval for use of this drug in March 2019 based on improved progression-free survival from the IMpassion130 trial. But the drug fell short in subsequent efforts to confirm the results seen in that study. The confirmatory IMpassion131 trial failed to meet the primary endpoint, the FDA staff noted in briefing materials for the ODAC meeting.
ODAC panelist Stan Lipkowitz, MD, PhD, of the National Cancer Institute, said he expected this vote had been a tough one for all members serving on ODAC that day.
“In some ways, the purist in me said I should have voted no. But when I looked at the data, there are a couple of things that struck me,” said Dr. Lipkowitz, who is the chief of the Women’s Malignancies Branch at NCI’s Center for Cancer Research. “First of all, the landscape hasn’t changed. There’s really no therapy in the first line for triple-negative metastatic that is shown to improve survival.”
Dr. Lipkowitz emphasized that Genentech needs to continue to try to prove atezolizumab works in this setting.
“There needs to be confirmatory study,” Dr. Lipkowitz concluded.
ODAC panelist Matthew Ellis, MD, PhD, of Baylor College of Medicine, Houston, said he also understood the difficult outlook for women fighting this cancer, but he voted against maintaining the approval.
“It’s not that I don’t feel the tragedy of these women,” said Dr. Ellis, citing his own decades of clinical experience.
“I just think that the data are the data,” Dr. Ellis said, adding that, in his view, “the only correct interpretation” of the evidence supported a vote against allowing the indication to stay.
The FDA considers the recommendations of its advisory committees but is not bound by them.
In a statement issued after the vote, Genentech said it intends to work with the FDA to determine the next steps for this indication of atezolizumab because “the clinically meaningful benefit demonstrated in the IMpassion130 study remains.”
The ODAC meeting continues for 2 more days, and will consider five more cancer indications that have been granted an accelerated approval.
A version of this article first appeared on Medscape.com.
On the first day of a historic 3-day meeting about drugs that were granted an accelerated approval by the Food and Drug Administration for cancer indications, the first approval to come under discussion is staying in place, at least for now.
Members of the FDA’s Oncologic Drugs Advisory Committee voted 7-2 in favor of keeping in place the indication for atezolizumab (Tecentriq) for use in a certain form of breast cancer. At the same time, the committee urged the manufacturer, Genentech, to do the research needed to prove the medicine works for these patients.
The specific indication is for atezolizumab as part of a combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors are PD-L1 positive.
The FDA granted accelerated approval in 2019 for this use of atezolizumab, expecting Genentech to produce more extensive evidence of this benefit. But so far, Genentech has not produced the data proving to the FDA that atezolizumab provides the expected benefit.
The drug was already available for use in bladder cancer, having been granted a full approval for this indication in 2016.
Other accelerated approvals withdrawn
This week’s 3-day ODAC meeting is part of the FDA’s broader reconsideration of what it has described as “dangling accelerated approvals.”
Earlier discussions between the FDA and drugmakers have already triggered four voluntary withdrawals of cancer indications with these accelerated approvals, noted Julia A. Beaver, MD, and Richard Pazdur, MD, two of the FDA’s top regulators of oncology medicine, in an April 21 perspective article in the New England Journal of Medicine.
“The small percentage of drugs whose clinical benefit is ultimately not confirmed should be viewed not as a failure of accelerated approval but rather as an expected trade-off in expediting drug development that benefits patients with severe or life-threatening diseases,” Dr. Beaver and Dr. Pazdur wrote.
But making these calls can be tough. On the first day of the meeting, even ODAC panelists who backed Genentech’s bid to maintain an mTNBC indication for atezolizumab expressed discomfort with this choice.
The FDA granted the accelerated approval for use of this drug in March 2019 based on improved progression-free survival from the IMpassion130 trial. But the drug fell short in subsequent efforts to confirm the results seen in that study. The confirmatory IMpassion131 trial failed to meet the primary endpoint, the FDA staff noted in briefing materials for the ODAC meeting.
ODAC panelist Stan Lipkowitz, MD, PhD, of the National Cancer Institute, said he expected this vote had been a tough one for all members serving on ODAC that day.
“In some ways, the purist in me said I should have voted no. But when I looked at the data, there are a couple of things that struck me,” said Dr. Lipkowitz, who is the chief of the Women’s Malignancies Branch at NCI’s Center for Cancer Research. “First of all, the landscape hasn’t changed. There’s really no therapy in the first line for triple-negative metastatic that is shown to improve survival.”
Dr. Lipkowitz emphasized that Genentech needs to continue to try to prove atezolizumab works in this setting.
“There needs to be confirmatory study,” Dr. Lipkowitz concluded.
ODAC panelist Matthew Ellis, MD, PhD, of Baylor College of Medicine, Houston, said he also understood the difficult outlook for women fighting this cancer, but he voted against maintaining the approval.
“It’s not that I don’t feel the tragedy of these women,” said Dr. Ellis, citing his own decades of clinical experience.
“I just think that the data are the data,” Dr. Ellis said, adding that, in his view, “the only correct interpretation” of the evidence supported a vote against allowing the indication to stay.
The FDA considers the recommendations of its advisory committees but is not bound by them.
In a statement issued after the vote, Genentech said it intends to work with the FDA to determine the next steps for this indication of atezolizumab because “the clinically meaningful benefit demonstrated in the IMpassion130 study remains.”
The ODAC meeting continues for 2 more days, and will consider five more cancer indications that have been granted an accelerated approval.
A version of this article first appeared on Medscape.com.
Pleural Effusion and an Axillary Mass in a Woman With Hypertension
Editor's Note:
The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us .
Background
A 58-year-old woman seeks medical attention after she discovered a new mass in her left axilla during a routine monthly breast self-examination while showering. She has not noted any changes in either of her breasts. The mass in her left axilla is not tender, and she has not felt any other abnormal masses, including in her right axilla. She reports no other symptoms and specifically has no pain anywhere in her body. She also does not have shortness of breath, fever, night sweats, fatigue, rash, or abdominal discomfort or bloating.
Fifteen years earlier, the patient was diagnosed with high-grade, stage 1 cervical cancer and underwent surgery and chemoradiation. She has been closely monitored since that time with physical examinations and abdominal CT, with no evidence of recurrent disease. The patient has not had any other surgical procedure, except for removal of two basal cell carcinomas on her neck 4 years ago. She has had yearly routine mammograms for at least the past 15 years.
The patient has hypertension, which has been well controlled with the same medications for the past 10 years. She also has a 25-year history of type 2 diabetes mellitus, which is currently managed with diet alone. She had a "silent myocardial infarction" sometime within the past 5 years but has had no cardiac symptoms and is not taking any cardiac medications. She smoked approximately one pack of cigarettes a day for less than 2 years when she was "in her teens" but has not had any tobacco products since that time.
Pancreatic cancer was diagnosed in the patient's father at age 49 years, and breast cancer was diagnosed in her aunt on her father's side at age 67 years. Her paternal grandmother is reported to have died in her 60s after diagnosis of a "cancer in her stomach." No further information is available regarding either the actual diagnosis or the medical care provided to this individual.
To the best of the patient's knowledge, her mother's side of the family and her two brothers have no history of cancer. She has no sisters. Her mother is in her 80s and has mild dementia. The patient is not aware of any member of her family having undergone genetic testing.
Physical Examination and Workup
The patient appears well and is in no acute distress. The patient is afebrile, with a blood pressure of 135/85 mm Hg, a respiratory rate of 16 breaths/min, and a pulse of 72 beats/min. Her weight is 148 lb (67 kg), and she has no reported recent weight loss.
Examination of the skin reveals no suspicious lesions. Scars from the previous removal of the basal cell carcinomas are noted, but no evidence suggests recurrence.
Results of the head and neck examination are unremarkable; specifically, no abnormal cervical lymphadenopathy is detected. The cardiac and chest examination results are normal. The lungs are clear to percussion and auscultation. The breast examination reveals no abnormal masses. The right axilla is unremarkable; however, a single 3 × 2 cm, nontender, firm, movable but partially fixed mass is noted in the left axilla.
The abdomen appears normal, with no ascites or enlargement of the liver. The pelvic examination reveals evidence of previous surgery and local radiation but no signs of recurrence of cervical cancer. The lymph nodes appear normal, except for the findings noted above. Results of the neurologic examination are unremarkable.
Complete blood cell count, serum electrolyte levels, renal function tests, and urinalysis are all normal. Liver function tests are normal except for a mildly elevated serum alkaline phosphatase level. The fecal occult blood test result is negative.
Chest radiography reveals a suspicious small left-sided pleural effusion. No other abnormalities are observed, and no prior chest radiographs are available to compare with the current findings.
Chest CT confirms the presence of a possible small pleural effusion, with no other abnormalities noted. The radiologist suggests it will not be possible to obtain fluid safely through an interventional procedure, owing to the limited (if any) amount of fluid present. Furthermore, the radiologist recommends PET/CT to look for other evidence of metastatic cancer in the lungs or elsewhere.
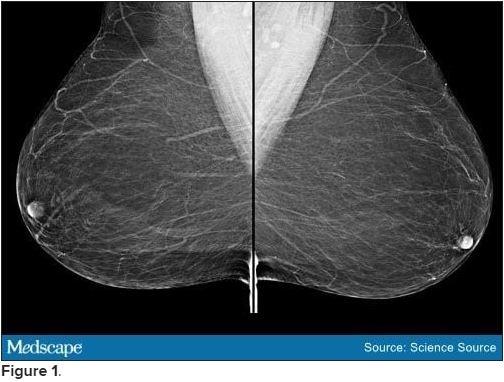
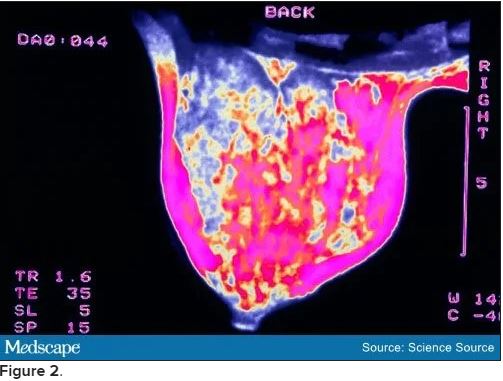
Bilateral mammograms reveal no suspicious abnormalities, and the results are unchanged from a previous examination 11 months earlier. Figure 1 shows a similar bilateral mammogram in another patient. Breast MRI shows no evidence of cancer. Figure 2 shows similar breast MRI findings in another patient.
CT of the abdomen and pelvis reveals no changes compared with a scan obtained 2 years earlier for follow-up of the previous diagnosis of cervical cancer. Specifically, no evidence suggests ascites or any pelvic masses.
An incisional biopsy sample is obtained from the left axillary mass. Light microscopy reveals a moderately well-differentiated adenocarcinoma. Immunostaining shows the cancer to be cytokeratin (CK) 7 positive and CK 20 negative (CK 7+/CK 20-, thyroid transcription factor 1 (TTF-1) negative, thyroglobulin negative, napsin A negative, and mammaglobin positive. The tumor is estrogen receptor positive (2% staining), progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2) negative.
[polldaddy:10837180]
Discussion
The correct answer: Breast.
This case is a classic example of cancer of unknown primary site or origin (CUP). CUP represents approximately 5% of cancers diagnosed in the United States (50,000 to 60,000 cases each year), with various series reporting that the site of origin is not diagnosed in between 2% and 6% of all cancer cases.[1] Worldwide, the incidence of CUP is even higher, resulting from the limited availability of sophisticated (and expensive) diagnostic technology in many regions. The median age at diagnosis of CUP is 60 years, and men and women are equally likely to be affected.
A cancer is considered a CUP if, after routine clinical assessment, physical and laboratory examination, standard imaging studies, and routine pathologic evaluation (biopsy or surgical removal of a metastatic mass lesion), a site of origin cannot be defined. With the availability of more sophisticated imaging technologies (eg, MRI), the overall percentage of cancers that are defined as a CUP has been reduced. However, even at autopsy, the site of origin of such cancer is often unable to be determined if the location was unknown before the patient's death.
Several theories have been proposed for why a metastatic lesion becomes clinically evident despite the site of origin of the cancer remaining obscure. These include (1) very slow growth of the primary cancer compared with that of the metastasis; (2) spontaneous regression of the primary cancer; (3) a prominent vascular component of the cancer, which enhances the rate of spread; and (4) unique molecular events associated with the cancer, which result in rapid progression and the growth of metastatic lesions.
Approximately 60% of CUPs are adenocarcinomas (well or moderately well differentiated); 25%-30% are poorly differentiated (including poorly differentiated adenocarcinomas); 5% are completely undifferentiated, with no defining histologic features; 5% are squamous cell cancers; and approximately 1% are carcinomas, with evidence of neuroendocrine differentiation.[1]
Immunohistochemical staining of biopsy material can be helpful in narrowing the possible anatomical sites of origin. The results are particularly relevant in the selection of therapeutic strategies and in ensuring that a rare, potentially highly curable cancer is not missed (eg, lymphoma, germ cell tumor).[2]
A critical initial test is examination of several CK subtypes that are more likely to be expressed in certain carcinomas than in others. For example, the CK 7+/CK 20- staining seen in this patient is characteristic of breast and lung cancers (among others), whereas CK 7+/CK 20+ staining would be expected in pancreatic, gastric, and urothelial cancers. A CK 7-/CK 20+ finding would be more suggestive of colon or mucinous ovarian cancer. Furthermore, approximately 70% of lung adenocarcinomas are TTF-1 positive and 60%-80% are napsin A positive. The negative findings in this patient's case make the diagnosis of metastatic lung cancer less likely.
Examination for the presence (or absence) of well-established biomarkers for breast cancer can potentially be helpful in suggesting the site of origin or in helping to define subsequent therapy. These markers include estrogen and progesterone receptors and HER2 overexpression. An additional biomarker, mammaglobin, has been reported to be expressed in 48% of breast cancers but is absent in cancers of the lung, gastrointestinal tract, ovary, and head and neck region.[2]
Of note, mammaglobin was found to be expressed in this patient. Although only 2% of the cells were reported to stain for the estrogen receptor, this finding is still considered positive and supports breast cancer as the correct diagnosis.
Recognized relevant prognostic factors in CUP include baseline performance status, the number and location of metastatic lesions, and the response to cytotoxic chemotherapy.
Unfortunately, the overall prognosis associated with a diagnosis of CUP is poor, with median survival in various series reported to be less than 6 months. However, important exceptions to this outcome include women who present with an isolated metastatic axillary mass, as described in this case.
Previous reports of axillary adenopathy as the initial presentation of cancer in women revealed that the majority had evidence of cancer in the breast at the time of subsequent mastectomy.[3,4] As a result, in the absence of other indications found during routine workup (eg, a single pulmonary lesion suggestive of a primary lung cancer, pathologic findings inconsistent with breast cancer), an isolated adenocarcinoma in the breast (with no evidence of metastatic cancer elsewhere) should be treated as either stage II or stage III breast cancer. Note that this recommendation specifically relates to female patients. If a male patient has CUP with an isolated axillary mass, it is generally assumed that the lung is the origin of the malignancy.
In a female patient with negative mammographic findings, breast MRI can be helpful. In one series, 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram were found to have a breast abnormality on MRI.[5] Of note, and of considerable relevance to subsequent disease management, five of the 12 women with negative findings in this series underwent surgery, and in four of the cases no cancer was found. Although the number of participants in this series is limited, the absence of an MRI abnormality in the patient in this case can reasonably be considered in her future treatment plans.
Specifically, it might be suggested in this case that treatment include surgical removal of the axillary mass (if possible) followed by radiation to this area and the breast (rather than performing a mastectomy). Alternatively, treatment might begin with chemotherapy (a neoadjuvant approach) followed by surgery to remove any residual axillary mass and local/regional radiation or local/regional radiation alone. Adjuvant chemotherapy and/or hormonal therapy would then be administered.
The presence of a possible small pleural effusion is a concern because it potentially indicates more widespread metastatic disease, as does the mild elevation of the serum alkaline phosphatase level (eg, suggesting metastatic disease in bone or the liver). In the absence of other evidence of tumor spread, PET would not be unreasonable. A negative scan for evidence of metastatic disease would support a "curative" approach to the management of local disease in the axilla and presumably the breast, whereas a finding of other metastatic sites would lead to the conclusion that treatment should probably be delivered with more palliative intent.
The family history of cancer (father, paternal aunt with breast cancer, paternal grandmother with possible ovarian cancer) is intriguing and would suggest a role for genetic counseling and possibly genetic testing (eg, for BRCA mutation).
The patient in this case underwent PET. The only abnormality observed was in the left axilla. The axillary mass was subsequently resected. This was followed by curative radiation to both the axilla and left breast, adjuvant chemotherapy, and 5 years of hormonal therapy. The patient has showed no evidence of recurrence 2 years after completion of the hormonal treatment.
[polldaddy:10841207]
Discussion
The correct answer: Lung
The lungs are generally assumed to be the site of origin of the cancer in a male patient who has CUP with an isolated axillary mass. In contrast, the majority of women with axillary adenopathy as the initial presentation of cancer were found to have evidence of cancer in the breast at the time of subsequent mastectomy.[3,4]
[polldaddy:10837187]
Discussion
The correct answer: MRI
Breast MRI can be helpful in a female patient with negative mammographic findings. In one series, MRI detected a breast abnormality in 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram.[5]
Editor's Note:
The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us .
Background
A 58-year-old woman seeks medical attention after she discovered a new mass in her left axilla during a routine monthly breast self-examination while showering. She has not noted any changes in either of her breasts. The mass in her left axilla is not tender, and she has not felt any other abnormal masses, including in her right axilla. She reports no other symptoms and specifically has no pain anywhere in her body. She also does not have shortness of breath, fever, night sweats, fatigue, rash, or abdominal discomfort or bloating.
Fifteen years earlier, the patient was diagnosed with high-grade, stage 1 cervical cancer and underwent surgery and chemoradiation. She has been closely monitored since that time with physical examinations and abdominal CT, with no evidence of recurrent disease. The patient has not had any other surgical procedure, except for removal of two basal cell carcinomas on her neck 4 years ago. She has had yearly routine mammograms for at least the past 15 years.
The patient has hypertension, which has been well controlled with the same medications for the past 10 years. She also has a 25-year history of type 2 diabetes mellitus, which is currently managed with diet alone. She had a "silent myocardial infarction" sometime within the past 5 years but has had no cardiac symptoms and is not taking any cardiac medications. She smoked approximately one pack of cigarettes a day for less than 2 years when she was "in her teens" but has not had any tobacco products since that time.
Pancreatic cancer was diagnosed in the patient's father at age 49 years, and breast cancer was diagnosed in her aunt on her father's side at age 67 years. Her paternal grandmother is reported to have died in her 60s after diagnosis of a "cancer in her stomach." No further information is available regarding either the actual diagnosis or the medical care provided to this individual.
To the best of the patient's knowledge, her mother's side of the family and her two brothers have no history of cancer. She has no sisters. Her mother is in her 80s and has mild dementia. The patient is not aware of any member of her family having undergone genetic testing.
Physical Examination and Workup
The patient appears well and is in no acute distress. The patient is afebrile, with a blood pressure of 135/85 mm Hg, a respiratory rate of 16 breaths/min, and a pulse of 72 beats/min. Her weight is 148 lb (67 kg), and she has no reported recent weight loss.
Examination of the skin reveals no suspicious lesions. Scars from the previous removal of the basal cell carcinomas are noted, but no evidence suggests recurrence.
Results of the head and neck examination are unremarkable; specifically, no abnormal cervical lymphadenopathy is detected. The cardiac and chest examination results are normal. The lungs are clear to percussion and auscultation. The breast examination reveals no abnormal masses. The right axilla is unremarkable; however, a single 3 × 2 cm, nontender, firm, movable but partially fixed mass is noted in the left axilla.
The abdomen appears normal, with no ascites or enlargement of the liver. The pelvic examination reveals evidence of previous surgery and local radiation but no signs of recurrence of cervical cancer. The lymph nodes appear normal, except for the findings noted above. Results of the neurologic examination are unremarkable.
Complete blood cell count, serum electrolyte levels, renal function tests, and urinalysis are all normal. Liver function tests are normal except for a mildly elevated serum alkaline phosphatase level. The fecal occult blood test result is negative.
Chest radiography reveals a suspicious small left-sided pleural effusion. No other abnormalities are observed, and no prior chest radiographs are available to compare with the current findings.
Chest CT confirms the presence of a possible small pleural effusion, with no other abnormalities noted. The radiologist suggests it will not be possible to obtain fluid safely through an interventional procedure, owing to the limited (if any) amount of fluid present. Furthermore, the radiologist recommends PET/CT to look for other evidence of metastatic cancer in the lungs or elsewhere.
Bilateral mammograms reveal no suspicious abnormalities, and the results are unchanged from a previous examination 11 months earlier. Figure 1 shows a similar bilateral mammogram in another patient. Breast MRI shows no evidence of cancer. Figure 2 shows similar breast MRI findings in another patient.


CT of the abdomen and pelvis reveals no changes compared with a scan obtained 2 years earlier for follow-up of the previous diagnosis of cervical cancer. Specifically, no evidence suggests ascites or any pelvic masses.
An incisional biopsy sample is obtained from the left axillary mass. Light microscopy reveals a moderately well-differentiated adenocarcinoma. Immunostaining shows the cancer to be cytokeratin (CK) 7 positive and CK 20 negative (CK 7+/CK 20-, thyroid transcription factor 1 (TTF-1) negative, thyroglobulin negative, napsin A negative, and mammaglobin positive. The tumor is estrogen receptor positive (2% staining), progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2) negative.
[polldaddy:10837180]
Discussion
The correct answer: Breast.
This case is a classic example of cancer of unknown primary site or origin (CUP). CUP represents approximately 5% of cancers diagnosed in the United States (50,000 to 60,000 cases each year), with various series reporting that the site of origin is not diagnosed in between 2% and 6% of all cancer cases.[1] Worldwide, the incidence of CUP is even higher, resulting from the limited availability of sophisticated (and expensive) diagnostic technology in many regions. The median age at diagnosis of CUP is 60 years, and men and women are equally likely to be affected.
A cancer is considered a CUP if, after routine clinical assessment, physical and laboratory examination, standard imaging studies, and routine pathologic evaluation (biopsy or surgical removal of a metastatic mass lesion), a site of origin cannot be defined. With the availability of more sophisticated imaging technologies (eg, MRI), the overall percentage of cancers that are defined as a CUP has been reduced. However, even at autopsy, the site of origin of such cancer is often unable to be determined if the location was unknown before the patient's death.
Several theories have been proposed for why a metastatic lesion becomes clinically evident despite the site of origin of the cancer remaining obscure. These include (1) very slow growth of the primary cancer compared with that of the metastasis; (2) spontaneous regression of the primary cancer; (3) a prominent vascular component of the cancer, which enhances the rate of spread; and (4) unique molecular events associated with the cancer, which result in rapid progression and the growth of metastatic lesions.
Approximately 60% of CUPs are adenocarcinomas (well or moderately well differentiated); 25%-30% are poorly differentiated (including poorly differentiated adenocarcinomas); 5% are completely undifferentiated, with no defining histologic features; 5% are squamous cell cancers; and approximately 1% are carcinomas, with evidence of neuroendocrine differentiation.[1]
Immunohistochemical staining of biopsy material can be helpful in narrowing the possible anatomical sites of origin. The results are particularly relevant in the selection of therapeutic strategies and in ensuring that a rare, potentially highly curable cancer is not missed (eg, lymphoma, germ cell tumor).[2]
A critical initial test is examination of several CK subtypes that are more likely to be expressed in certain carcinomas than in others. For example, the CK 7+/CK 20- staining seen in this patient is characteristic of breast and lung cancers (among others), whereas CK 7+/CK 20+ staining would be expected in pancreatic, gastric, and urothelial cancers. A CK 7-/CK 20+ finding would be more suggestive of colon or mucinous ovarian cancer. Furthermore, approximately 70% of lung adenocarcinomas are TTF-1 positive and 60%-80% are napsin A positive. The negative findings in this patient's case make the diagnosis of metastatic lung cancer less likely.
Examination for the presence (or absence) of well-established biomarkers for breast cancer can potentially be helpful in suggesting the site of origin or in helping to define subsequent therapy. These markers include estrogen and progesterone receptors and HER2 overexpression. An additional biomarker, mammaglobin, has been reported to be expressed in 48% of breast cancers but is absent in cancers of the lung, gastrointestinal tract, ovary, and head and neck region.[2]
Of note, mammaglobin was found to be expressed in this patient. Although only 2% of the cells were reported to stain for the estrogen receptor, this finding is still considered positive and supports breast cancer as the correct diagnosis.
Recognized relevant prognostic factors in CUP include baseline performance status, the number and location of metastatic lesions, and the response to cytotoxic chemotherapy.
Unfortunately, the overall prognosis associated with a diagnosis of CUP is poor, with median survival in various series reported to be less than 6 months. However, important exceptions to this outcome include women who present with an isolated metastatic axillary mass, as described in this case.
Previous reports of axillary adenopathy as the initial presentation of cancer in women revealed that the majority had evidence of cancer in the breast at the time of subsequent mastectomy.[3,4] As a result, in the absence of other indications found during routine workup (eg, a single pulmonary lesion suggestive of a primary lung cancer, pathologic findings inconsistent with breast cancer), an isolated adenocarcinoma in the breast (with no evidence of metastatic cancer elsewhere) should be treated as either stage II or stage III breast cancer. Note that this recommendation specifically relates to female patients. If a male patient has CUP with an isolated axillary mass, it is generally assumed that the lung is the origin of the malignancy.
In a female patient with negative mammographic findings, breast MRI can be helpful. In one series, 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram were found to have a breast abnormality on MRI.[5] Of note, and of considerable relevance to subsequent disease management, five of the 12 women with negative findings in this series underwent surgery, and in four of the cases no cancer was found. Although the number of participants in this series is limited, the absence of an MRI abnormality in the patient in this case can reasonably be considered in her future treatment plans.
Specifically, it might be suggested in this case that treatment include surgical removal of the axillary mass (if possible) followed by radiation to this area and the breast (rather than performing a mastectomy). Alternatively, treatment might begin with chemotherapy (a neoadjuvant approach) followed by surgery to remove any residual axillary mass and local/regional radiation or local/regional radiation alone. Adjuvant chemotherapy and/or hormonal therapy would then be administered.
The presence of a possible small pleural effusion is a concern because it potentially indicates more widespread metastatic disease, as does the mild elevation of the serum alkaline phosphatase level (eg, suggesting metastatic disease in bone or the liver). In the absence of other evidence of tumor spread, PET would not be unreasonable. A negative scan for evidence of metastatic disease would support a "curative" approach to the management of local disease in the axilla and presumably the breast, whereas a finding of other metastatic sites would lead to the conclusion that treatment should probably be delivered with more palliative intent.
The family history of cancer (father, paternal aunt with breast cancer, paternal grandmother with possible ovarian cancer) is intriguing and would suggest a role for genetic counseling and possibly genetic testing (eg, for BRCA mutation).
The patient in this case underwent PET. The only abnormality observed was in the left axilla. The axillary mass was subsequently resected. This was followed by curative radiation to both the axilla and left breast, adjuvant chemotherapy, and 5 years of hormonal therapy. The patient has showed no evidence of recurrence 2 years after completion of the hormonal treatment.
[polldaddy:10841207]
Discussion
The correct answer: Lung
The lungs are generally assumed to be the site of origin of the cancer in a male patient who has CUP with an isolated axillary mass. In contrast, the majority of women with axillary adenopathy as the initial presentation of cancer were found to have evidence of cancer in the breast at the time of subsequent mastectomy.[3,4]
[polldaddy:10837187]
Discussion
The correct answer: MRI
Breast MRI can be helpful in a female patient with negative mammographic findings. In one series, MRI detected a breast abnormality in 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram.[5]
Editor's Note:
The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please contact us .
Background
A 58-year-old woman seeks medical attention after she discovered a new mass in her left axilla during a routine monthly breast self-examination while showering. She has not noted any changes in either of her breasts. The mass in her left axilla is not tender, and she has not felt any other abnormal masses, including in her right axilla. She reports no other symptoms and specifically has no pain anywhere in her body. She also does not have shortness of breath, fever, night sweats, fatigue, rash, or abdominal discomfort or bloating.
Fifteen years earlier, the patient was diagnosed with high-grade, stage 1 cervical cancer and underwent surgery and chemoradiation. She has been closely monitored since that time with physical examinations and abdominal CT, with no evidence of recurrent disease. The patient has not had any other surgical procedure, except for removal of two basal cell carcinomas on her neck 4 years ago. She has had yearly routine mammograms for at least the past 15 years.
The patient has hypertension, which has been well controlled with the same medications for the past 10 years. She also has a 25-year history of type 2 diabetes mellitus, which is currently managed with diet alone. She had a "silent myocardial infarction" sometime within the past 5 years but has had no cardiac symptoms and is not taking any cardiac medications. She smoked approximately one pack of cigarettes a day for less than 2 years when she was "in her teens" but has not had any tobacco products since that time.
Pancreatic cancer was diagnosed in the patient's father at age 49 years, and breast cancer was diagnosed in her aunt on her father's side at age 67 years. Her paternal grandmother is reported to have died in her 60s after diagnosis of a "cancer in her stomach." No further information is available regarding either the actual diagnosis or the medical care provided to this individual.
To the best of the patient's knowledge, her mother's side of the family and her two brothers have no history of cancer. She has no sisters. Her mother is in her 80s and has mild dementia. The patient is not aware of any member of her family having undergone genetic testing.
Physical Examination and Workup
The patient appears well and is in no acute distress. The patient is afebrile, with a blood pressure of 135/85 mm Hg, a respiratory rate of 16 breaths/min, and a pulse of 72 beats/min. Her weight is 148 lb (67 kg), and she has no reported recent weight loss.
Examination of the skin reveals no suspicious lesions. Scars from the previous removal of the basal cell carcinomas are noted, but no evidence suggests recurrence.
Results of the head and neck examination are unremarkable; specifically, no abnormal cervical lymphadenopathy is detected. The cardiac and chest examination results are normal. The lungs are clear to percussion and auscultation. The breast examination reveals no abnormal masses. The right axilla is unremarkable; however, a single 3 × 2 cm, nontender, firm, movable but partially fixed mass is noted in the left axilla.
The abdomen appears normal, with no ascites or enlargement of the liver. The pelvic examination reveals evidence of previous surgery and local radiation but no signs of recurrence of cervical cancer. The lymph nodes appear normal, except for the findings noted above. Results of the neurologic examination are unremarkable.
Complete blood cell count, serum electrolyte levels, renal function tests, and urinalysis are all normal. Liver function tests are normal except for a mildly elevated serum alkaline phosphatase level. The fecal occult blood test result is negative.
Chest radiography reveals a suspicious small left-sided pleural effusion. No other abnormalities are observed, and no prior chest radiographs are available to compare with the current findings.
Chest CT confirms the presence of a possible small pleural effusion, with no other abnormalities noted. The radiologist suggests it will not be possible to obtain fluid safely through an interventional procedure, owing to the limited (if any) amount of fluid present. Furthermore, the radiologist recommends PET/CT to look for other evidence of metastatic cancer in the lungs or elsewhere.
Bilateral mammograms reveal no suspicious abnormalities, and the results are unchanged from a previous examination 11 months earlier. Figure 1 shows a similar bilateral mammogram in another patient. Breast MRI shows no evidence of cancer. Figure 2 shows similar breast MRI findings in another patient.


CT of the abdomen and pelvis reveals no changes compared with a scan obtained 2 years earlier for follow-up of the previous diagnosis of cervical cancer. Specifically, no evidence suggests ascites or any pelvic masses.
An incisional biopsy sample is obtained from the left axillary mass. Light microscopy reveals a moderately well-differentiated adenocarcinoma. Immunostaining shows the cancer to be cytokeratin (CK) 7 positive and CK 20 negative (CK 7+/CK 20-, thyroid transcription factor 1 (TTF-1) negative, thyroglobulin negative, napsin A negative, and mammaglobin positive. The tumor is estrogen receptor positive (2% staining), progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2) negative.
[polldaddy:10837180]
Discussion
The correct answer: Breast.
This case is a classic example of cancer of unknown primary site or origin (CUP). CUP represents approximately 5% of cancers diagnosed in the United States (50,000 to 60,000 cases each year), with various series reporting that the site of origin is not diagnosed in between 2% and 6% of all cancer cases.[1] Worldwide, the incidence of CUP is even higher, resulting from the limited availability of sophisticated (and expensive) diagnostic technology in many regions. The median age at diagnosis of CUP is 60 years, and men and women are equally likely to be affected.
A cancer is considered a CUP if, after routine clinical assessment, physical and laboratory examination, standard imaging studies, and routine pathologic evaluation (biopsy or surgical removal of a metastatic mass lesion), a site of origin cannot be defined. With the availability of more sophisticated imaging technologies (eg, MRI), the overall percentage of cancers that are defined as a CUP has been reduced. However, even at autopsy, the site of origin of such cancer is often unable to be determined if the location was unknown before the patient's death.
Several theories have been proposed for why a metastatic lesion becomes clinically evident despite the site of origin of the cancer remaining obscure. These include (1) very slow growth of the primary cancer compared with that of the metastasis; (2) spontaneous regression of the primary cancer; (3) a prominent vascular component of the cancer, which enhances the rate of spread; and (4) unique molecular events associated with the cancer, which result in rapid progression and the growth of metastatic lesions.
Approximately 60% of CUPs are adenocarcinomas (well or moderately well differentiated); 25%-30% are poorly differentiated (including poorly differentiated adenocarcinomas); 5% are completely undifferentiated, with no defining histologic features; 5% are squamous cell cancers; and approximately 1% are carcinomas, with evidence of neuroendocrine differentiation.[1]
Immunohistochemical staining of biopsy material can be helpful in narrowing the possible anatomical sites of origin. The results are particularly relevant in the selection of therapeutic strategies and in ensuring that a rare, potentially highly curable cancer is not missed (eg, lymphoma, germ cell tumor).[2]
A critical initial test is examination of several CK subtypes that are more likely to be expressed in certain carcinomas than in others. For example, the CK 7+/CK 20- staining seen in this patient is characteristic of breast and lung cancers (among others), whereas CK 7+/CK 20+ staining would be expected in pancreatic, gastric, and urothelial cancers. A CK 7-/CK 20+ finding would be more suggestive of colon or mucinous ovarian cancer. Furthermore, approximately 70% of lung adenocarcinomas are TTF-1 positive and 60%-80% are napsin A positive. The negative findings in this patient's case make the diagnosis of metastatic lung cancer less likely.
Examination for the presence (or absence) of well-established biomarkers for breast cancer can potentially be helpful in suggesting the site of origin or in helping to define subsequent therapy. These markers include estrogen and progesterone receptors and HER2 overexpression. An additional biomarker, mammaglobin, has been reported to be expressed in 48% of breast cancers but is absent in cancers of the lung, gastrointestinal tract, ovary, and head and neck region.[2]
Of note, mammaglobin was found to be expressed in this patient. Although only 2% of the cells were reported to stain for the estrogen receptor, this finding is still considered positive and supports breast cancer as the correct diagnosis.
Recognized relevant prognostic factors in CUP include baseline performance status, the number and location of metastatic lesions, and the response to cytotoxic chemotherapy.
Unfortunately, the overall prognosis associated with a diagnosis of CUP is poor, with median survival in various series reported to be less than 6 months. However, important exceptions to this outcome include women who present with an isolated metastatic axillary mass, as described in this case.
Previous reports of axillary adenopathy as the initial presentation of cancer in women revealed that the majority had evidence of cancer in the breast at the time of subsequent mastectomy.[3,4] As a result, in the absence of other indications found during routine workup (eg, a single pulmonary lesion suggestive of a primary lung cancer, pathologic findings inconsistent with breast cancer), an isolated adenocarcinoma in the breast (with no evidence of metastatic cancer elsewhere) should be treated as either stage II or stage III breast cancer. Note that this recommendation specifically relates to female patients. If a male patient has CUP with an isolated axillary mass, it is generally assumed that the lung is the origin of the malignancy.
In a female patient with negative mammographic findings, breast MRI can be helpful. In one series, 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram were found to have a breast abnormality on MRI.[5] Of note, and of considerable relevance to subsequent disease management, five of the 12 women with negative findings in this series underwent surgery, and in four of the cases no cancer was found. Although the number of participants in this series is limited, the absence of an MRI abnormality in the patient in this case can reasonably be considered in her future treatment plans.
Specifically, it might be suggested in this case that treatment include surgical removal of the axillary mass (if possible) followed by radiation to this area and the breast (rather than performing a mastectomy). Alternatively, treatment might begin with chemotherapy (a neoadjuvant approach) followed by surgery to remove any residual axillary mass and local/regional radiation or local/regional radiation alone. Adjuvant chemotherapy and/or hormonal therapy would then be administered.
The presence of a possible small pleural effusion is a concern because it potentially indicates more widespread metastatic disease, as does the mild elevation of the serum alkaline phosphatase level (eg, suggesting metastatic disease in bone or the liver). In the absence of other evidence of tumor spread, PET would not be unreasonable. A negative scan for evidence of metastatic disease would support a "curative" approach to the management of local disease in the axilla and presumably the breast, whereas a finding of other metastatic sites would lead to the conclusion that treatment should probably be delivered with more palliative intent.
The family history of cancer (father, paternal aunt with breast cancer, paternal grandmother with possible ovarian cancer) is intriguing and would suggest a role for genetic counseling and possibly genetic testing (eg, for BRCA mutation).
The patient in this case underwent PET. The only abnormality observed was in the left axilla. The axillary mass was subsequently resected. This was followed by curative radiation to both the axilla and left breast, adjuvant chemotherapy, and 5 years of hormonal therapy. The patient has showed no evidence of recurrence 2 years after completion of the hormonal treatment.
[polldaddy:10841207]
Discussion
The correct answer: Lung
The lungs are generally assumed to be the site of origin of the cancer in a male patient who has CUP with an isolated axillary mass. In contrast, the majority of women with axillary adenopathy as the initial presentation of cancer were found to have evidence of cancer in the breast at the time of subsequent mastectomy.[3,4]
[polldaddy:10837187]
Discussion
The correct answer: MRI
Breast MRI can be helpful in a female patient with negative mammographic findings. In one series, MRI detected a breast abnormality in 28 of 40 women (70%) with evidence of cancer in the axilla and a normal mammogram.[5]
Cell-free DNA improves response prediction in breast cancer
When the two techniques were in agreement, the accuracy of response prediction was 92.6% in the study, with a predictive value for complete response of 87.5% and a predictive value for absence of complete response of 94.7%, which was substantially better than either method alone.
“Our work identifies a new parameter that is easily combinable with MRI for a more accurate prediction of response following neoadjuvant treatment, with possible implications for current protocols for the evaluation of nodal residual disease,” researcher Francesco Ravera, MD, PhD, of the University of Genoa (Italy), said in a press release.
Dr. Ravera and colleagues presented their research in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract LB063).
Accurate response prediction is important because it guides subsequent surgical management, Dr. Ravera and colleagues noted. Pathological complete responders – generally about 25% of patients after neoadjuvant therapy – typically undergo a sentinel lymph node biopsy to ensure cancer hasn’t spread, while incomplete responders often have a complete axillary lymph node dissection.
Response is currently assessed by MRI, but accuracy is suboptimal, the researchers noted. A more accurate method might “allow the omission of sentinel lymph node biopsy in complete responders, which could be replaced by longitudinal radiologic monitoring. This would represent substantial progress in the pursuit of an effective, minimally invasive treatment,” Dr. Ravera said.
He and his colleagues turned to plasma cfDNA because it has shown potential for providing useful diagnostic, recurrence, and treatment response information in neoplastic patients.
When healthy cells die, they release similarly sized DNA fragments into the blood, but cancer cells release fragments of varying sizes. The heart of the research was using electrophoresis to assess the degree of fragmentation – called cfDNA integrity – in plasma samples from 38 patients after anthracycline/taxane-based regimens.
The researchers compared how well cfDNA, preoperative MRI, and the combination of the two methods predicted response according to surgical histology.
A total of 11 patients had pathological complete responses to neoadjuvant therapy.
The ratio of large 321-1,000 base pair sized fragments to smaller 150-220 base pair sized fragments, which the team dubbed the “cfDNA integrity index,” best predicted response. At a cutoff above 2.71, the index was 81.6% accurate in predicting pathological complete response, with a sensitivity of 81.8% and specificity of 81.5%.
The predictive power wasn’t much better than MRI, which was 77.1% accurate, with a sensitivity of 72.7% and a specificity of 81.5%.
The two techniques were concordant in their prediction in over two-thirds of patients. When the techniques agreed, accuracy was over 90%.
Prospective studies are needed to evaluate the cfDNA integrity index in combination with MRI, the researchers concluded.
The study was sponsored by the University of Genoa and others. Dr. Ravera disclosed no conflicts of interest.
When the two techniques were in agreement, the accuracy of response prediction was 92.6% in the study, with a predictive value for complete response of 87.5% and a predictive value for absence of complete response of 94.7%, which was substantially better than either method alone.
“Our work identifies a new parameter that is easily combinable with MRI for a more accurate prediction of response following neoadjuvant treatment, with possible implications for current protocols for the evaluation of nodal residual disease,” researcher Francesco Ravera, MD, PhD, of the University of Genoa (Italy), said in a press release.
Dr. Ravera and colleagues presented their research in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract LB063).
Accurate response prediction is important because it guides subsequent surgical management, Dr. Ravera and colleagues noted. Pathological complete responders – generally about 25% of patients after neoadjuvant therapy – typically undergo a sentinel lymph node biopsy to ensure cancer hasn’t spread, while incomplete responders often have a complete axillary lymph node dissection.
Response is currently assessed by MRI, but accuracy is suboptimal, the researchers noted. A more accurate method might “allow the omission of sentinel lymph node biopsy in complete responders, which could be replaced by longitudinal radiologic monitoring. This would represent substantial progress in the pursuit of an effective, minimally invasive treatment,” Dr. Ravera said.
He and his colleagues turned to plasma cfDNA because it has shown potential for providing useful diagnostic, recurrence, and treatment response information in neoplastic patients.
When healthy cells die, they release similarly sized DNA fragments into the blood, but cancer cells release fragments of varying sizes. The heart of the research was using electrophoresis to assess the degree of fragmentation – called cfDNA integrity – in plasma samples from 38 patients after anthracycline/taxane-based regimens.
The researchers compared how well cfDNA, preoperative MRI, and the combination of the two methods predicted response according to surgical histology.
A total of 11 patients had pathological complete responses to neoadjuvant therapy.
The ratio of large 321-1,000 base pair sized fragments to smaller 150-220 base pair sized fragments, which the team dubbed the “cfDNA integrity index,” best predicted response. At a cutoff above 2.71, the index was 81.6% accurate in predicting pathological complete response, with a sensitivity of 81.8% and specificity of 81.5%.
The predictive power wasn’t much better than MRI, which was 77.1% accurate, with a sensitivity of 72.7% and a specificity of 81.5%.
The two techniques were concordant in their prediction in over two-thirds of patients. When the techniques agreed, accuracy was over 90%.
Prospective studies are needed to evaluate the cfDNA integrity index in combination with MRI, the researchers concluded.
The study was sponsored by the University of Genoa and others. Dr. Ravera disclosed no conflicts of interest.
When the two techniques were in agreement, the accuracy of response prediction was 92.6% in the study, with a predictive value for complete response of 87.5% and a predictive value for absence of complete response of 94.7%, which was substantially better than either method alone.
“Our work identifies a new parameter that is easily combinable with MRI for a more accurate prediction of response following neoadjuvant treatment, with possible implications for current protocols for the evaluation of nodal residual disease,” researcher Francesco Ravera, MD, PhD, of the University of Genoa (Italy), said in a press release.
Dr. Ravera and colleagues presented their research in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract LB063).
Accurate response prediction is important because it guides subsequent surgical management, Dr. Ravera and colleagues noted. Pathological complete responders – generally about 25% of patients after neoadjuvant therapy – typically undergo a sentinel lymph node biopsy to ensure cancer hasn’t spread, while incomplete responders often have a complete axillary lymph node dissection.
Response is currently assessed by MRI, but accuracy is suboptimal, the researchers noted. A more accurate method might “allow the omission of sentinel lymph node biopsy in complete responders, which could be replaced by longitudinal radiologic monitoring. This would represent substantial progress in the pursuit of an effective, minimally invasive treatment,” Dr. Ravera said.
He and his colleagues turned to plasma cfDNA because it has shown potential for providing useful diagnostic, recurrence, and treatment response information in neoplastic patients.
When healthy cells die, they release similarly sized DNA fragments into the blood, but cancer cells release fragments of varying sizes. The heart of the research was using electrophoresis to assess the degree of fragmentation – called cfDNA integrity – in plasma samples from 38 patients after anthracycline/taxane-based regimens.
The researchers compared how well cfDNA, preoperative MRI, and the combination of the two methods predicted response according to surgical histology.
A total of 11 patients had pathological complete responses to neoadjuvant therapy.
The ratio of large 321-1,000 base pair sized fragments to smaller 150-220 base pair sized fragments, which the team dubbed the “cfDNA integrity index,” best predicted response. At a cutoff above 2.71, the index was 81.6% accurate in predicting pathological complete response, with a sensitivity of 81.8% and specificity of 81.5%.
The predictive power wasn’t much better than MRI, which was 77.1% accurate, with a sensitivity of 72.7% and a specificity of 81.5%.
The two techniques were concordant in their prediction in over two-thirds of patients. When the techniques agreed, accuracy was over 90%.
Prospective studies are needed to evaluate the cfDNA integrity index in combination with MRI, the researchers concluded.
The study was sponsored by the University of Genoa and others. Dr. Ravera disclosed no conflicts of interest.
FROM AACR 2021
Treating metastatic TNBC: Where are we now?
Treating triple-negative breast cancer (TNBC), one of the more lethal breast cancer subtypes, remains a challenge. By definition, TNBC lacks the three telltale molecular signatures known to spur tumor growth: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). A growing amount of literature shows that these frequently aggressive tumors harbor a rich array of molecular characteristics but no clear oncogenic driver.
“TNBC is incredibly heterogeneous, which makes it challenging to treat,” said Rita Nanda, MD, director of the breast oncology program and associate professor of medicine at the University of Chicago. “We have subsets of TNBC that don’t respond to currently available therapies and, as of yet, have no identifiable therapeutic targets.”
Overall, about 40% of patients with TNBC show a pathologic complete response after first-line neoadjuvant chemotherapy – typically anthracycline and taxane-based agents. But for 50% of patients, chemotherapy leaves behind substantial residual cancer tissue. These patients subsequently face a 40%-80% risk for recurrence and progression to advanced disease.
When triple-negative disease metastasizes, survival rates plummet. The most recent data from the National Cancer Institute, which tracked patients by stage of diagnosis between 2010 and 2016, showed steep declines in 5-year survival as TNBC progressed from local (91.2%) to regional (65%) to advanced-stage disease (11.5%).
Experts have started to make headway identifying and targeting different molecular features of advanced TNBC. These approaches often focus on three key areas: targeting cell surface proteins or oncogenes, stimulating an anticancer immune response, or inhibiting an overactive signaling pathway.
“For a patient with metastatic breast cancer, finding a molecular target or an oncogenic driver is essential,” said Kelly McCann, MD, PhD, a hematologist/oncologist in the department of medicine at the University of California, Los Angeles. “Because TNBC encompasses many different molecular subsets of breast cancer, the development of effective new therapeutics is going to depend on subdividing TNBC into categories with more clear targets.”
A targeted strategy
The Food and Drug Administration’s approval of sacituzumab govitecan, the first antibody-drug conjugate to treat metastatic TNBC, marked an important addition to the TNBC drug armamentarium. “Sacituzumab govitecan is one of the most exciting drugs available for the treatment of metastatic disease,” Dr. Nanda said.
Sacituzumab govitecan, approved as third-line therapy for metastatic TNBC, works by targeting the cell surface protein TROP2, expressed in about 88% of TNBC tumors but rarely in healthy cells.
In the phase 1/2 ASCENT trial, the median progression-free survival was 5.5 months and overall survival was 13.0 months in 108 patients with metastatic TNBC who had received at least two therapies prior to sacituzumab govitecan.
A subsequent phase 3 trial showed progression-free survival of 5.6 months with sacituzumab govitecan and 1.7 months with physician’s choice of chemotherapy. The median overall survival was 12.1 months and 6.7 months, respectively.
But, according to the analysis, TROP2 expression did not necessarily predict who would benefit from sacituzumab govitecan. A biomarker study revealed that although patients with moderate to high TROP2 expression exhibited the strongest treatment response, those with low TROP2 expression also survived longer when given sacituzumab govitecan, compared with chemotherapy alone.
In other words, “patients did better on sacituzumab govitecan regardless of TROP2 expression, which suggests we do not have a good biomarker for identifying who will benefit,” Dr. Nanda said.
Two other investigational antibody-drug conjugates, trastuzumab deruxtecan and ladiratuzumab vedotin, show promise in the metastatic space as well. For instance, the recent phase 2 trial evaluating trastuzumab deruxtecan in patients with HER2-positive breast cancer reported treatment response in 44% of patients with HER2-low tumors.
Given that about 36.6% of TNBC tumors exhibit low levels of HER2 expression, “trastuzumab deruxtecan represents potential in treating HER2-low TNBC,” said Yuan Yuan, MD, PhD, medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
Early results from a phase 1b study showed that trastuzumab deruxtecan produced a response rate of 37% in patients with HER2-low breast cancer.
Investigators are now recruiting for an open-label phase 3 trial to determine whether trastuzumab deruxtecan extends survival in patients with HER2-low metastatic breast cancers.
Immunotherapy advances
Immune checkpoint inhibitors represent another promising treatment avenue for metastatic TNBC. Pembrolizumab and atezolizumab, recently approved by the FDA, show moderate progression-free and overall survival benefits in patients with metastatic TNBC expressing PD-L1. Estimates of PD-L1 immune cells present in TNBC tumors vary widely, from about 20% to 65%.
Yet, data on which patients will benefit are not so clear-cut. “These drugs give us more choices and represent the fast-evolving therapeutic landscape in TNBC, but they also leave a lot of unanswered questions about PD-L1 as a biomarker,” Dr. Yuan said.
Take two recent phase 3 trials evaluating atezolizumab: IMpassion130 and IMpassion131. In IMpassion130, patients with PDL1–positive tumors exhibited significantly longer median overall survival on atezolizumab plus nab-paclitaxel (25.0 months) compared with nab-paclitaxel alone (15.5 months). As with the trend observed in the TROP2 data for sacituzumab govitecan, all patients survived longer on atezolizumab plus nab-paclitaxel regardless of PD-L1 status: 21.3 months vs. 17.6 months with nab-paclitaxel alone.
However, in IMpassion131, neither progression-free survival nor overall survival significantly improved in the PD-L1–positive group receiving atezolizumab plus paclitaxel compared with paclitaxel alone: Progression-free survival was 5.7 months vs. 6 months, respectively, and overall survival was 28.3 months vs. 22.1 months.
“It is unclear why this study failed to demonstrate a significant improvement in progression-free survival with the addition of atezolizumab to paclitaxel,” Dr. Nanda said. “Perhaps the negative finding has to do with how the trial was conducted, or perhaps the PD-L1 assay used is an unreliable biomarker of immunotherapy benefit.”
Continued efforts to understand TNBC
Given the diversity of metastatic TNBC and the absence of clear molecular targets, researchers are exploring a host of therapeutic strategies in addition to antibody-drug conjugates and immunotherapies.
On the oncogene front, researchers are investigating common mutations in TNBC. About 11% of TNBC tumors, for instance, carry germline mutations in BRCA1 and BRCA2. These tumors may be more likely to respond to platinum agents and PARP inhibitors, such as FDA-approved olaparib. In a phase 3 trial, patients with metastatic HER2-negative breast cancer and a germline BRCA mutation who received olaparib exhibited a 2.8-month longer median progression-free survival and a 42% reduced risk for disease progression or death compared with those on standard chemotherapy.
When considering signaling pathways, the PI3K/AKT/mTOR pathway has been the target of numerous clinical trials. Dysregulation of signaling through the PI3K and AKT signaling pathway occurs in 25%-30% of patients with advanced TNBC, and AKT inhibitors have been shown to extend survival in these patients. Data show, for instance, that adding capivasertib to first-line paclitaxel therapy in patients with metastatic TNBC led to longer overall survival – 19.1 months vs. 12.6 with placebo plus paclitaxel – with better survival results in patients with PIK3CA/AKT1/PTEN altered tumors.
But there’s more to learn about treating metastatic TNBC. “Relapses tend to occur early in TNBC, and some tumors are inherently resistant to chemotherapy from the get-go,” said Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai, New York. “Understanding the causes of drug response and resistance in patients with metastatic TNBC represents the holy grail.”
Dr. Nanda agreed, noting that advancing treatments for TNBC will hinge on identifying the key factors driving metastasis. “For TNBC, we are still trying to elucidate the best molecular targets, while at the same time trying to identify robust biomarkers to predict benefit from therapies we already have available,” she said.
A version of this article first appeared on Medscape.com.
Treating triple-negative breast cancer (TNBC), one of the more lethal breast cancer subtypes, remains a challenge. By definition, TNBC lacks the three telltale molecular signatures known to spur tumor growth: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). A growing amount of literature shows that these frequently aggressive tumors harbor a rich array of molecular characteristics but no clear oncogenic driver.
“TNBC is incredibly heterogeneous, which makes it challenging to treat,” said Rita Nanda, MD, director of the breast oncology program and associate professor of medicine at the University of Chicago. “We have subsets of TNBC that don’t respond to currently available therapies and, as of yet, have no identifiable therapeutic targets.”
Overall, about 40% of patients with TNBC show a pathologic complete response after first-line neoadjuvant chemotherapy – typically anthracycline and taxane-based agents. But for 50% of patients, chemotherapy leaves behind substantial residual cancer tissue. These patients subsequently face a 40%-80% risk for recurrence and progression to advanced disease.
When triple-negative disease metastasizes, survival rates plummet. The most recent data from the National Cancer Institute, which tracked patients by stage of diagnosis between 2010 and 2016, showed steep declines in 5-year survival as TNBC progressed from local (91.2%) to regional (65%) to advanced-stage disease (11.5%).
Experts have started to make headway identifying and targeting different molecular features of advanced TNBC. These approaches often focus on three key areas: targeting cell surface proteins or oncogenes, stimulating an anticancer immune response, or inhibiting an overactive signaling pathway.
“For a patient with metastatic breast cancer, finding a molecular target or an oncogenic driver is essential,” said Kelly McCann, MD, PhD, a hematologist/oncologist in the department of medicine at the University of California, Los Angeles. “Because TNBC encompasses many different molecular subsets of breast cancer, the development of effective new therapeutics is going to depend on subdividing TNBC into categories with more clear targets.”
A targeted strategy
The Food and Drug Administration’s approval of sacituzumab govitecan, the first antibody-drug conjugate to treat metastatic TNBC, marked an important addition to the TNBC drug armamentarium. “Sacituzumab govitecan is one of the most exciting drugs available for the treatment of metastatic disease,” Dr. Nanda said.
Sacituzumab govitecan, approved as third-line therapy for metastatic TNBC, works by targeting the cell surface protein TROP2, expressed in about 88% of TNBC tumors but rarely in healthy cells.
In the phase 1/2 ASCENT trial, the median progression-free survival was 5.5 months and overall survival was 13.0 months in 108 patients with metastatic TNBC who had received at least two therapies prior to sacituzumab govitecan.
A subsequent phase 3 trial showed progression-free survival of 5.6 months with sacituzumab govitecan and 1.7 months with physician’s choice of chemotherapy. The median overall survival was 12.1 months and 6.7 months, respectively.
But, according to the analysis, TROP2 expression did not necessarily predict who would benefit from sacituzumab govitecan. A biomarker study revealed that although patients with moderate to high TROP2 expression exhibited the strongest treatment response, those with low TROP2 expression also survived longer when given sacituzumab govitecan, compared with chemotherapy alone.
In other words, “patients did better on sacituzumab govitecan regardless of TROP2 expression, which suggests we do not have a good biomarker for identifying who will benefit,” Dr. Nanda said.
Two other investigational antibody-drug conjugates, trastuzumab deruxtecan and ladiratuzumab vedotin, show promise in the metastatic space as well. For instance, the recent phase 2 trial evaluating trastuzumab deruxtecan in patients with HER2-positive breast cancer reported treatment response in 44% of patients with HER2-low tumors.
Given that about 36.6% of TNBC tumors exhibit low levels of HER2 expression, “trastuzumab deruxtecan represents potential in treating HER2-low TNBC,” said Yuan Yuan, MD, PhD, medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
Early results from a phase 1b study showed that trastuzumab deruxtecan produced a response rate of 37% in patients with HER2-low breast cancer.
Investigators are now recruiting for an open-label phase 3 trial to determine whether trastuzumab deruxtecan extends survival in patients with HER2-low metastatic breast cancers.
Immunotherapy advances
Immune checkpoint inhibitors represent another promising treatment avenue for metastatic TNBC. Pembrolizumab and atezolizumab, recently approved by the FDA, show moderate progression-free and overall survival benefits in patients with metastatic TNBC expressing PD-L1. Estimates of PD-L1 immune cells present in TNBC tumors vary widely, from about 20% to 65%.
Yet, data on which patients will benefit are not so clear-cut. “These drugs give us more choices and represent the fast-evolving therapeutic landscape in TNBC, but they also leave a lot of unanswered questions about PD-L1 as a biomarker,” Dr. Yuan said.
Take two recent phase 3 trials evaluating atezolizumab: IMpassion130 and IMpassion131. In IMpassion130, patients with PDL1–positive tumors exhibited significantly longer median overall survival on atezolizumab plus nab-paclitaxel (25.0 months) compared with nab-paclitaxel alone (15.5 months). As with the trend observed in the TROP2 data for sacituzumab govitecan, all patients survived longer on atezolizumab plus nab-paclitaxel regardless of PD-L1 status: 21.3 months vs. 17.6 months with nab-paclitaxel alone.
However, in IMpassion131, neither progression-free survival nor overall survival significantly improved in the PD-L1–positive group receiving atezolizumab plus paclitaxel compared with paclitaxel alone: Progression-free survival was 5.7 months vs. 6 months, respectively, and overall survival was 28.3 months vs. 22.1 months.
“It is unclear why this study failed to demonstrate a significant improvement in progression-free survival with the addition of atezolizumab to paclitaxel,” Dr. Nanda said. “Perhaps the negative finding has to do with how the trial was conducted, or perhaps the PD-L1 assay used is an unreliable biomarker of immunotherapy benefit.”
Continued efforts to understand TNBC
Given the diversity of metastatic TNBC and the absence of clear molecular targets, researchers are exploring a host of therapeutic strategies in addition to antibody-drug conjugates and immunotherapies.
On the oncogene front, researchers are investigating common mutations in TNBC. About 11% of TNBC tumors, for instance, carry germline mutations in BRCA1 and BRCA2. These tumors may be more likely to respond to platinum agents and PARP inhibitors, such as FDA-approved olaparib. In a phase 3 trial, patients with metastatic HER2-negative breast cancer and a germline BRCA mutation who received olaparib exhibited a 2.8-month longer median progression-free survival and a 42% reduced risk for disease progression or death compared with those on standard chemotherapy.
When considering signaling pathways, the PI3K/AKT/mTOR pathway has been the target of numerous clinical trials. Dysregulation of signaling through the PI3K and AKT signaling pathway occurs in 25%-30% of patients with advanced TNBC, and AKT inhibitors have been shown to extend survival in these patients. Data show, for instance, that adding capivasertib to first-line paclitaxel therapy in patients with metastatic TNBC led to longer overall survival – 19.1 months vs. 12.6 with placebo plus paclitaxel – with better survival results in patients with PIK3CA/AKT1/PTEN altered tumors.
But there’s more to learn about treating metastatic TNBC. “Relapses tend to occur early in TNBC, and some tumors are inherently resistant to chemotherapy from the get-go,” said Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai, New York. “Understanding the causes of drug response and resistance in patients with metastatic TNBC represents the holy grail.”
Dr. Nanda agreed, noting that advancing treatments for TNBC will hinge on identifying the key factors driving metastasis. “For TNBC, we are still trying to elucidate the best molecular targets, while at the same time trying to identify robust biomarkers to predict benefit from therapies we already have available,” she said.
A version of this article first appeared on Medscape.com.
Treating triple-negative breast cancer (TNBC), one of the more lethal breast cancer subtypes, remains a challenge. By definition, TNBC lacks the three telltale molecular signatures known to spur tumor growth: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). A growing amount of literature shows that these frequently aggressive tumors harbor a rich array of molecular characteristics but no clear oncogenic driver.
“TNBC is incredibly heterogeneous, which makes it challenging to treat,” said Rita Nanda, MD, director of the breast oncology program and associate professor of medicine at the University of Chicago. “We have subsets of TNBC that don’t respond to currently available therapies and, as of yet, have no identifiable therapeutic targets.”
Overall, about 40% of patients with TNBC show a pathologic complete response after first-line neoadjuvant chemotherapy – typically anthracycline and taxane-based agents. But for 50% of patients, chemotherapy leaves behind substantial residual cancer tissue. These patients subsequently face a 40%-80% risk for recurrence and progression to advanced disease.
When triple-negative disease metastasizes, survival rates plummet. The most recent data from the National Cancer Institute, which tracked patients by stage of diagnosis between 2010 and 2016, showed steep declines in 5-year survival as TNBC progressed from local (91.2%) to regional (65%) to advanced-stage disease (11.5%).
Experts have started to make headway identifying and targeting different molecular features of advanced TNBC. These approaches often focus on three key areas: targeting cell surface proteins or oncogenes, stimulating an anticancer immune response, or inhibiting an overactive signaling pathway.
“For a patient with metastatic breast cancer, finding a molecular target or an oncogenic driver is essential,” said Kelly McCann, MD, PhD, a hematologist/oncologist in the department of medicine at the University of California, Los Angeles. “Because TNBC encompasses many different molecular subsets of breast cancer, the development of effective new therapeutics is going to depend on subdividing TNBC into categories with more clear targets.”
A targeted strategy
The Food and Drug Administration’s approval of sacituzumab govitecan, the first antibody-drug conjugate to treat metastatic TNBC, marked an important addition to the TNBC drug armamentarium. “Sacituzumab govitecan is one of the most exciting drugs available for the treatment of metastatic disease,” Dr. Nanda said.
Sacituzumab govitecan, approved as third-line therapy for metastatic TNBC, works by targeting the cell surface protein TROP2, expressed in about 88% of TNBC tumors but rarely in healthy cells.
In the phase 1/2 ASCENT trial, the median progression-free survival was 5.5 months and overall survival was 13.0 months in 108 patients with metastatic TNBC who had received at least two therapies prior to sacituzumab govitecan.
A subsequent phase 3 trial showed progression-free survival of 5.6 months with sacituzumab govitecan and 1.7 months with physician’s choice of chemotherapy. The median overall survival was 12.1 months and 6.7 months, respectively.
But, according to the analysis, TROP2 expression did not necessarily predict who would benefit from sacituzumab govitecan. A biomarker study revealed that although patients with moderate to high TROP2 expression exhibited the strongest treatment response, those with low TROP2 expression also survived longer when given sacituzumab govitecan, compared with chemotherapy alone.
In other words, “patients did better on sacituzumab govitecan regardless of TROP2 expression, which suggests we do not have a good biomarker for identifying who will benefit,” Dr. Nanda said.
Two other investigational antibody-drug conjugates, trastuzumab deruxtecan and ladiratuzumab vedotin, show promise in the metastatic space as well. For instance, the recent phase 2 trial evaluating trastuzumab deruxtecan in patients with HER2-positive breast cancer reported treatment response in 44% of patients with HER2-low tumors.
Given that about 36.6% of TNBC tumors exhibit low levels of HER2 expression, “trastuzumab deruxtecan represents potential in treating HER2-low TNBC,” said Yuan Yuan, MD, PhD, medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
Early results from a phase 1b study showed that trastuzumab deruxtecan produced a response rate of 37% in patients with HER2-low breast cancer.
Investigators are now recruiting for an open-label phase 3 trial to determine whether trastuzumab deruxtecan extends survival in patients with HER2-low metastatic breast cancers.
Immunotherapy advances
Immune checkpoint inhibitors represent another promising treatment avenue for metastatic TNBC. Pembrolizumab and atezolizumab, recently approved by the FDA, show moderate progression-free and overall survival benefits in patients with metastatic TNBC expressing PD-L1. Estimates of PD-L1 immune cells present in TNBC tumors vary widely, from about 20% to 65%.
Yet, data on which patients will benefit are not so clear-cut. “These drugs give us more choices and represent the fast-evolving therapeutic landscape in TNBC, but they also leave a lot of unanswered questions about PD-L1 as a biomarker,” Dr. Yuan said.
Take two recent phase 3 trials evaluating atezolizumab: IMpassion130 and IMpassion131. In IMpassion130, patients with PDL1–positive tumors exhibited significantly longer median overall survival on atezolizumab plus nab-paclitaxel (25.0 months) compared with nab-paclitaxel alone (15.5 months). As with the trend observed in the TROP2 data for sacituzumab govitecan, all patients survived longer on atezolizumab plus nab-paclitaxel regardless of PD-L1 status: 21.3 months vs. 17.6 months with nab-paclitaxel alone.
However, in IMpassion131, neither progression-free survival nor overall survival significantly improved in the PD-L1–positive group receiving atezolizumab plus paclitaxel compared with paclitaxel alone: Progression-free survival was 5.7 months vs. 6 months, respectively, and overall survival was 28.3 months vs. 22.1 months.
“It is unclear why this study failed to demonstrate a significant improvement in progression-free survival with the addition of atezolizumab to paclitaxel,” Dr. Nanda said. “Perhaps the negative finding has to do with how the trial was conducted, or perhaps the PD-L1 assay used is an unreliable biomarker of immunotherapy benefit.”
Continued efforts to understand TNBC
Given the diversity of metastatic TNBC and the absence of clear molecular targets, researchers are exploring a host of therapeutic strategies in addition to antibody-drug conjugates and immunotherapies.
On the oncogene front, researchers are investigating common mutations in TNBC. About 11% of TNBC tumors, for instance, carry germline mutations in BRCA1 and BRCA2. These tumors may be more likely to respond to platinum agents and PARP inhibitors, such as FDA-approved olaparib. In a phase 3 trial, patients with metastatic HER2-negative breast cancer and a germline BRCA mutation who received olaparib exhibited a 2.8-month longer median progression-free survival and a 42% reduced risk for disease progression or death compared with those on standard chemotherapy.
When considering signaling pathways, the PI3K/AKT/mTOR pathway has been the target of numerous clinical trials. Dysregulation of signaling through the PI3K and AKT signaling pathway occurs in 25%-30% of patients with advanced TNBC, and AKT inhibitors have been shown to extend survival in these patients. Data show, for instance, that adding capivasertib to first-line paclitaxel therapy in patients with metastatic TNBC led to longer overall survival – 19.1 months vs. 12.6 with placebo plus paclitaxel – with better survival results in patients with PIK3CA/AKT1/PTEN altered tumors.
But there’s more to learn about treating metastatic TNBC. “Relapses tend to occur early in TNBC, and some tumors are inherently resistant to chemotherapy from the get-go,” said Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai, New York. “Understanding the causes of drug response and resistance in patients with metastatic TNBC represents the holy grail.”
Dr. Nanda agreed, noting that advancing treatments for TNBC will hinge on identifying the key factors driving metastasis. “For TNBC, we are still trying to elucidate the best molecular targets, while at the same time trying to identify robust biomarkers to predict benefit from therapies we already have available,” she said.
A version of this article first appeared on Medscape.com.
How has COVID-19 affected metastatic breast cancer treatment decisions?
Patients with metastatic breast cancer (MBC) face an elevated risk of severe illness or dying from COVID-19. Given the slow rollout of the Moderna and Pfizer COVID vaccines and new, more infectious viral variants circulating in the United States, oncologists will face a challenging balancing act for the foreseeable future: sustaining patients› MBC care while safeguarding them from COVID. The scale leans heavily toward continuing treatment, experts say.
“If we stop treatment for metastatic breast cancer, death will occur quickly,” Fatima F. Cardoso, MD, director of the breast unit at Champalimaud Clinical Centre in Lisbon, Portugal, stated this past August in a Medscape perspective.
Joanne Mortimer, MD, director of Women’s Cancer Programs at City of Hope, a comprehensive cancer center near Los Angeles, expressed a similar sentiment. “Having MBC is worse than getting COVID,” she told Medscape. “We can’t stop treating patients with MBC because of concerns of exposure.”
But maintaining treatment does not mean business as usual. Oncologists have had to modify their pre-pandemic practices to some degree, and that degree largely depends on local COVID conditions.
“Weighing the risk of treatment with the risk of contracting the virus means that many places have carried on treating metastatic breast cancer in a more thoughtful, careful way,” said Jill Dietz, MD, president of the American Society of Breast Surgeons. “That means focusing on high-value treatments for patients with the goal of maximizing their outcomes and quality of life while limiting in-person visits and potentially unnecessary elements of care.”
To guide this more careful approach, Dr. Dietz and colleagues from the recently formed COVID-19 Pandemic Breast Cancer Consortium published recommendations in April 2020 to account for different disease types and severities. These recommendations align closely with those from Cardoso and colleagues in Europe, also published last April.
Although issued early in the pandemic when there was greater uncertainty about viral transmission, adverse outcomes, and treatment for COVID-19, these recommendations still hold almost a year later.
“The framework has proven to be timeless in that it can help institutions where they are in the pandemic,” Dr. Dietz said.
The recommendations at play
For MBC, in particular, Dr. Dietz and colleagues focused on patients who need systemic care but whose treatment can be modified to keep them home more. The modifications include prescribing oral agents such as capecitabine, vinorelbine, and cyclophosphamide to minimize visits to the hospital or infusion suite.
To limit adverse events associated with these oral drugs, Dr. Dietz and colleagues also recommended reducing the dose when possible. For instance, research shows that lowering the dose of the CDK4/6 inhibitor palbociclib in patients with HR+/HER2-negative MBC does not diminish efficacy.
When oral agents are not an option, Dr. Dietz and colleagues suggested stretching out the intervals for chemotherapy infusions or injections. Data show that trastuzumab and pertuzumab injections for metastatic HER2-positive tumors «may reasonably be administered at longer intervals,» such as 4 weeks instead of 3 weeks.
The extent to which oncologists have applied these recommendations hinges on two factors: the local severity of COVID-19 cases and institution-specific policies.
For some oncologists, the pandemic has largely left treatment decisions untouched. “COVID-19 has only minimally impacted my practice,” said Rita Nanda, MD, director of the Breast Oncology Program and associate professor of medicine at University of Chicago Medicine.
Dr. Nanda recalled her concerns in the early days of the pandemic. As the nation watched COVID cases surge across New York City, Chicagoans prepared for the worst, fashioning the McCormick Place Convention Center into a field hospital for COVID patients.
“But fortunately, we were never overwhelmed at the University of Chicago and never needed to use the convention center,” Dr. Nanda said. “We did not have to alter or limit the type of therapy patients with MBC could receive.”
The main changes described by Dr. Nanda at the University of Chicago have centered around limiting the flow of traffic within the infusion suite or hospital by implementing prescreening checks to catch patients with COVID symptoms, keeping waiting rooms empty and infusion centers socially distanced, and having patients come in for appointments solo. The university’s home phlebotomy service also came in handy. Implemented before the pandemic, this service allowed patients with MBC to get their labs done at home before coming in for treatment.
“Overall, with social distancing, mask wearing, and limiting who comes in to the clinic, we have been able to keep patients and staff safe without altering treatment-specific decisions,” Dr. Nanda said.
Streamlining the foot traffic in the cancer center also worked well for Lisa A. Carey, MD, chief of the Division of Hematology/Oncology and deputy director of clinical sciences at University of North Carolina-Chapel Hill. “The truth is, oncological principles of care are still in place,” said Dr. Carey, the Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research. “COVID hasn’t altered those; it has only thrown a little wrench in how we deliver that care.”
Kelly McCann, MD, PhD, a hematologist/oncologist in the Department of Medicine at the David Geffen School of Medicine, University of California, Los Angeles, has had to walk a tighter rope to protect her patients with MBC, as COVID cases began to soar in LA county last fall.
To keep patients with MBC home more, Dr. McCann said many chose oral cytotoxic chemotherapies over infusional therapies. Some patients with HER2-positive MBC, for instance, opted for the oral combination neratinib + capecitabine over a trastuzumab-deruxtecan infusion, and others with triple-negative tumors chose capecitabine over a taxane.
At City of Hope, Mortimer has had a different experience of LA county’s COVID surge. Because the center only treats patients with cancer, “we have not had huge numbers of patients with COVID or had to significantly modify our practice outside of screening patients who come in and doing fewer scans compared to pre-COVID,” she said. With these precautions, “we have had no internal transmission of COVID within our institution.”
Still, patients with MBC have gotten COVID, and treating both illnesses does complicate decision-making. In some cases, Mortimer has postponed cancer treatment for patients who can delay for a few weeks while they recover from COVID. But patients who need to continue MBC treatment receive care in a separate unit, and Mortimer considers prescribing Eli Lilly’s recently approved antibody therapy bamlanivimab to treat COVID symptoms. “For each situation, we can always page our infectious disease experts to address any concerns or questions,” Dr. Mortimer said.
The MBC and COVID toll
The biggest hurdle for patients with MBC has been less about treatment decisions and more about handling the psychological toll of the pandemic, according to Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai in New York City.
“These are my personal observations, but I’ve seen how much more stressful it is to have metastatic breast cancer during the pandemic,” said Dr. Shapiro, who worries that fear of COVID may fuel or exacerbate patients’ depression and anxiety. “Patients can’t have family and friends by their side during infusions or appointments, and many feel isolated because of the risk of exposure.”
Because most patients with MBC still need in-person care such as exams, blood draws, or chemotherapy infusions, Dr. McCann has found that many of her patients “are afraid to come to a medical center and have been delaying appointments, imaging, and procedures.”
The psychological toll of treating breast cancer during the pandemic has touched oncologists as well. A recent survey found that burnout scores were significantly higher among physicians whose patients experienced delays in care, including chemotherapy or specialty consultations.
Getting patients vaccinated will improve protection and hopefully lessen fears surrounding COVID infection and transmission. Preliminary recommendations from the National Comprehensive Cancer Network›s COVID-19 Vaccination Advisory Committee state that patients with cancer «should be prioritized for vaccination.»
Dr. McCann agreed. “I’ve recommended COVID-19 vaccination to all of my patients with MBC,” she said. But because a lot of these therapies suppress the immune system to some degree, “I’ll recommend a period of time for vaccination in which the immune system is expected to have recovered, such as in the days prior to a dose of chemotherapy.”
Overall, according to Dr. Carey, institutional responses to treating MBC during the pandemic have been very similar: The key has been that “no one is keeping secrets,” she said. “Our global oncology community is sharing and adopting best practices. Our focus has been doing right by our patients.”
A version of this article first appeared on Medscape.com.
Patients with metastatic breast cancer (MBC) face an elevated risk of severe illness or dying from COVID-19. Given the slow rollout of the Moderna and Pfizer COVID vaccines and new, more infectious viral variants circulating in the United States, oncologists will face a challenging balancing act for the foreseeable future: sustaining patients› MBC care while safeguarding them from COVID. The scale leans heavily toward continuing treatment, experts say.
“If we stop treatment for metastatic breast cancer, death will occur quickly,” Fatima F. Cardoso, MD, director of the breast unit at Champalimaud Clinical Centre in Lisbon, Portugal, stated this past August in a Medscape perspective.
Joanne Mortimer, MD, director of Women’s Cancer Programs at City of Hope, a comprehensive cancer center near Los Angeles, expressed a similar sentiment. “Having MBC is worse than getting COVID,” she told Medscape. “We can’t stop treating patients with MBC because of concerns of exposure.”
But maintaining treatment does not mean business as usual. Oncologists have had to modify their pre-pandemic practices to some degree, and that degree largely depends on local COVID conditions.
“Weighing the risk of treatment with the risk of contracting the virus means that many places have carried on treating metastatic breast cancer in a more thoughtful, careful way,” said Jill Dietz, MD, president of the American Society of Breast Surgeons. “That means focusing on high-value treatments for patients with the goal of maximizing their outcomes and quality of life while limiting in-person visits and potentially unnecessary elements of care.”
To guide this more careful approach, Dr. Dietz and colleagues from the recently formed COVID-19 Pandemic Breast Cancer Consortium published recommendations in April 2020 to account for different disease types and severities. These recommendations align closely with those from Cardoso and colleagues in Europe, also published last April.
Although issued early in the pandemic when there was greater uncertainty about viral transmission, adverse outcomes, and treatment for COVID-19, these recommendations still hold almost a year later.
“The framework has proven to be timeless in that it can help institutions where they are in the pandemic,” Dr. Dietz said.
The recommendations at play
For MBC, in particular, Dr. Dietz and colleagues focused on patients who need systemic care but whose treatment can be modified to keep them home more. The modifications include prescribing oral agents such as capecitabine, vinorelbine, and cyclophosphamide to minimize visits to the hospital or infusion suite.
To limit adverse events associated with these oral drugs, Dr. Dietz and colleagues also recommended reducing the dose when possible. For instance, research shows that lowering the dose of the CDK4/6 inhibitor palbociclib in patients with HR+/HER2-negative MBC does not diminish efficacy.
When oral agents are not an option, Dr. Dietz and colleagues suggested stretching out the intervals for chemotherapy infusions or injections. Data show that trastuzumab and pertuzumab injections for metastatic HER2-positive tumors «may reasonably be administered at longer intervals,» such as 4 weeks instead of 3 weeks.
The extent to which oncologists have applied these recommendations hinges on two factors: the local severity of COVID-19 cases and institution-specific policies.
For some oncologists, the pandemic has largely left treatment decisions untouched. “COVID-19 has only minimally impacted my practice,” said Rita Nanda, MD, director of the Breast Oncology Program and associate professor of medicine at University of Chicago Medicine.
Dr. Nanda recalled her concerns in the early days of the pandemic. As the nation watched COVID cases surge across New York City, Chicagoans prepared for the worst, fashioning the McCormick Place Convention Center into a field hospital for COVID patients.
“But fortunately, we were never overwhelmed at the University of Chicago and never needed to use the convention center,” Dr. Nanda said. “We did not have to alter or limit the type of therapy patients with MBC could receive.”
The main changes described by Dr. Nanda at the University of Chicago have centered around limiting the flow of traffic within the infusion suite or hospital by implementing prescreening checks to catch patients with COVID symptoms, keeping waiting rooms empty and infusion centers socially distanced, and having patients come in for appointments solo. The university’s home phlebotomy service also came in handy. Implemented before the pandemic, this service allowed patients with MBC to get their labs done at home before coming in for treatment.
“Overall, with social distancing, mask wearing, and limiting who comes in to the clinic, we have been able to keep patients and staff safe without altering treatment-specific decisions,” Dr. Nanda said.
Streamlining the foot traffic in the cancer center also worked well for Lisa A. Carey, MD, chief of the Division of Hematology/Oncology and deputy director of clinical sciences at University of North Carolina-Chapel Hill. “The truth is, oncological principles of care are still in place,” said Dr. Carey, the Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research. “COVID hasn’t altered those; it has only thrown a little wrench in how we deliver that care.”
Kelly McCann, MD, PhD, a hematologist/oncologist in the Department of Medicine at the David Geffen School of Medicine, University of California, Los Angeles, has had to walk a tighter rope to protect her patients with MBC, as COVID cases began to soar in LA county last fall.
To keep patients with MBC home more, Dr. McCann said many chose oral cytotoxic chemotherapies over infusional therapies. Some patients with HER2-positive MBC, for instance, opted for the oral combination neratinib + capecitabine over a trastuzumab-deruxtecan infusion, and others with triple-negative tumors chose capecitabine over a taxane.
At City of Hope, Mortimer has had a different experience of LA county’s COVID surge. Because the center only treats patients with cancer, “we have not had huge numbers of patients with COVID or had to significantly modify our practice outside of screening patients who come in and doing fewer scans compared to pre-COVID,” she said. With these precautions, “we have had no internal transmission of COVID within our institution.”
Still, patients with MBC have gotten COVID, and treating both illnesses does complicate decision-making. In some cases, Mortimer has postponed cancer treatment for patients who can delay for a few weeks while they recover from COVID. But patients who need to continue MBC treatment receive care in a separate unit, and Mortimer considers prescribing Eli Lilly’s recently approved antibody therapy bamlanivimab to treat COVID symptoms. “For each situation, we can always page our infectious disease experts to address any concerns or questions,” Dr. Mortimer said.
The MBC and COVID toll
The biggest hurdle for patients with MBC has been less about treatment decisions and more about handling the psychological toll of the pandemic, according to Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai in New York City.
“These are my personal observations, but I’ve seen how much more stressful it is to have metastatic breast cancer during the pandemic,” said Dr. Shapiro, who worries that fear of COVID may fuel or exacerbate patients’ depression and anxiety. “Patients can’t have family and friends by their side during infusions or appointments, and many feel isolated because of the risk of exposure.”
Because most patients with MBC still need in-person care such as exams, blood draws, or chemotherapy infusions, Dr. McCann has found that many of her patients “are afraid to come to a medical center and have been delaying appointments, imaging, and procedures.”
The psychological toll of treating breast cancer during the pandemic has touched oncologists as well. A recent survey found that burnout scores were significantly higher among physicians whose patients experienced delays in care, including chemotherapy or specialty consultations.
Getting patients vaccinated will improve protection and hopefully lessen fears surrounding COVID infection and transmission. Preliminary recommendations from the National Comprehensive Cancer Network›s COVID-19 Vaccination Advisory Committee state that patients with cancer «should be prioritized for vaccination.»
Dr. McCann agreed. “I’ve recommended COVID-19 vaccination to all of my patients with MBC,” she said. But because a lot of these therapies suppress the immune system to some degree, “I’ll recommend a period of time for vaccination in which the immune system is expected to have recovered, such as in the days prior to a dose of chemotherapy.”
Overall, according to Dr. Carey, institutional responses to treating MBC during the pandemic have been very similar: The key has been that “no one is keeping secrets,” she said. “Our global oncology community is sharing and adopting best practices. Our focus has been doing right by our patients.”
A version of this article first appeared on Medscape.com.
Patients with metastatic breast cancer (MBC) face an elevated risk of severe illness or dying from COVID-19. Given the slow rollout of the Moderna and Pfizer COVID vaccines and new, more infectious viral variants circulating in the United States, oncologists will face a challenging balancing act for the foreseeable future: sustaining patients› MBC care while safeguarding them from COVID. The scale leans heavily toward continuing treatment, experts say.
“If we stop treatment for metastatic breast cancer, death will occur quickly,” Fatima F. Cardoso, MD, director of the breast unit at Champalimaud Clinical Centre in Lisbon, Portugal, stated this past August in a Medscape perspective.
Joanne Mortimer, MD, director of Women’s Cancer Programs at City of Hope, a comprehensive cancer center near Los Angeles, expressed a similar sentiment. “Having MBC is worse than getting COVID,” she told Medscape. “We can’t stop treating patients with MBC because of concerns of exposure.”
But maintaining treatment does not mean business as usual. Oncologists have had to modify their pre-pandemic practices to some degree, and that degree largely depends on local COVID conditions.
“Weighing the risk of treatment with the risk of contracting the virus means that many places have carried on treating metastatic breast cancer in a more thoughtful, careful way,” said Jill Dietz, MD, president of the American Society of Breast Surgeons. “That means focusing on high-value treatments for patients with the goal of maximizing their outcomes and quality of life while limiting in-person visits and potentially unnecessary elements of care.”
To guide this more careful approach, Dr. Dietz and colleagues from the recently formed COVID-19 Pandemic Breast Cancer Consortium published recommendations in April 2020 to account for different disease types and severities. These recommendations align closely with those from Cardoso and colleagues in Europe, also published last April.
Although issued early in the pandemic when there was greater uncertainty about viral transmission, adverse outcomes, and treatment for COVID-19, these recommendations still hold almost a year later.
“The framework has proven to be timeless in that it can help institutions where they are in the pandemic,” Dr. Dietz said.
The recommendations at play
For MBC, in particular, Dr. Dietz and colleagues focused on patients who need systemic care but whose treatment can be modified to keep them home more. The modifications include prescribing oral agents such as capecitabine, vinorelbine, and cyclophosphamide to minimize visits to the hospital or infusion suite.
To limit adverse events associated with these oral drugs, Dr. Dietz and colleagues also recommended reducing the dose when possible. For instance, research shows that lowering the dose of the CDK4/6 inhibitor palbociclib in patients with HR+/HER2-negative MBC does not diminish efficacy.
When oral agents are not an option, Dr. Dietz and colleagues suggested stretching out the intervals for chemotherapy infusions or injections. Data show that trastuzumab and pertuzumab injections for metastatic HER2-positive tumors «may reasonably be administered at longer intervals,» such as 4 weeks instead of 3 weeks.
The extent to which oncologists have applied these recommendations hinges on two factors: the local severity of COVID-19 cases and institution-specific policies.
For some oncologists, the pandemic has largely left treatment decisions untouched. “COVID-19 has only minimally impacted my practice,” said Rita Nanda, MD, director of the Breast Oncology Program and associate professor of medicine at University of Chicago Medicine.
Dr. Nanda recalled her concerns in the early days of the pandemic. As the nation watched COVID cases surge across New York City, Chicagoans prepared for the worst, fashioning the McCormick Place Convention Center into a field hospital for COVID patients.
“But fortunately, we were never overwhelmed at the University of Chicago and never needed to use the convention center,” Dr. Nanda said. “We did not have to alter or limit the type of therapy patients with MBC could receive.”
The main changes described by Dr. Nanda at the University of Chicago have centered around limiting the flow of traffic within the infusion suite or hospital by implementing prescreening checks to catch patients with COVID symptoms, keeping waiting rooms empty and infusion centers socially distanced, and having patients come in for appointments solo. The university’s home phlebotomy service also came in handy. Implemented before the pandemic, this service allowed patients with MBC to get their labs done at home before coming in for treatment.
“Overall, with social distancing, mask wearing, and limiting who comes in to the clinic, we have been able to keep patients and staff safe without altering treatment-specific decisions,” Dr. Nanda said.
Streamlining the foot traffic in the cancer center also worked well for Lisa A. Carey, MD, chief of the Division of Hematology/Oncology and deputy director of clinical sciences at University of North Carolina-Chapel Hill. “The truth is, oncological principles of care are still in place,” said Dr. Carey, the Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research. “COVID hasn’t altered those; it has only thrown a little wrench in how we deliver that care.”
Kelly McCann, MD, PhD, a hematologist/oncologist in the Department of Medicine at the David Geffen School of Medicine, University of California, Los Angeles, has had to walk a tighter rope to protect her patients with MBC, as COVID cases began to soar in LA county last fall.
To keep patients with MBC home more, Dr. McCann said many chose oral cytotoxic chemotherapies over infusional therapies. Some patients with HER2-positive MBC, for instance, opted for the oral combination neratinib + capecitabine over a trastuzumab-deruxtecan infusion, and others with triple-negative tumors chose capecitabine over a taxane.
At City of Hope, Mortimer has had a different experience of LA county’s COVID surge. Because the center only treats patients with cancer, “we have not had huge numbers of patients with COVID or had to significantly modify our practice outside of screening patients who come in and doing fewer scans compared to pre-COVID,” she said. With these precautions, “we have had no internal transmission of COVID within our institution.”
Still, patients with MBC have gotten COVID, and treating both illnesses does complicate decision-making. In some cases, Mortimer has postponed cancer treatment for patients who can delay for a few weeks while they recover from COVID. But patients who need to continue MBC treatment receive care in a separate unit, and Mortimer considers prescribing Eli Lilly’s recently approved antibody therapy bamlanivimab to treat COVID symptoms. “For each situation, we can always page our infectious disease experts to address any concerns or questions,” Dr. Mortimer said.
The MBC and COVID toll
The biggest hurdle for patients with MBC has been less about treatment decisions and more about handling the psychological toll of the pandemic, according to Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai in New York City.
“These are my personal observations, but I’ve seen how much more stressful it is to have metastatic breast cancer during the pandemic,” said Dr. Shapiro, who worries that fear of COVID may fuel or exacerbate patients’ depression and anxiety. “Patients can’t have family and friends by their side during infusions or appointments, and many feel isolated because of the risk of exposure.”
Because most patients with MBC still need in-person care such as exams, blood draws, or chemotherapy infusions, Dr. McCann has found that many of her patients “are afraid to come to a medical center and have been delaying appointments, imaging, and procedures.”
The psychological toll of treating breast cancer during the pandemic has touched oncologists as well. A recent survey found that burnout scores were significantly higher among physicians whose patients experienced delays in care, including chemotherapy or specialty consultations.
Getting patients vaccinated will improve protection and hopefully lessen fears surrounding COVID infection and transmission. Preliminary recommendations from the National Comprehensive Cancer Network›s COVID-19 Vaccination Advisory Committee state that patients with cancer «should be prioritized for vaccination.»
Dr. McCann agreed. “I’ve recommended COVID-19 vaccination to all of my patients with MBC,” she said. But because a lot of these therapies suppress the immune system to some degree, “I’ll recommend a period of time for vaccination in which the immune system is expected to have recovered, such as in the days prior to a dose of chemotherapy.”
Overall, according to Dr. Carey, institutional responses to treating MBC during the pandemic have been very similar: The key has been that “no one is keeping secrets,” she said. “Our global oncology community is sharing and adopting best practices. Our focus has been doing right by our patients.”
A version of this article first appeared on Medscape.com.
PET predicts response to endocrine therapy in ER+ breast cancer
Endocrine therapy is the standard of care for estrogen receptor–positive (ER+) breast cancer, but only about half of women respond. At present, there is no method for identifying the women who are likely – and also unlikely – to respond.
But a new approach looks to be useful. It involves a trial of estrogen followed by imaging that measures the function of estrogen receptors in the cancer cells.
This functional testing of estrogen receptors on breast cancer cells was perfectly accurate in predicting endocrine therapy response in 43 postmenopausal women with advanced ER+ disease, say researchers from Washington University, St. Louis, led by Farrokh Dehdashti, MD.
“There is an unmet clinical need to develop more precise predictive biomarkers. The results of this study are extremely promising,” they conclude.
The study was published online in Nature Communications.
For the study, the women were first infused with a radioactive progestin analog – 21-[18F]fluorofuranylnorprogesterone (FFNP) – that binds progesterone receptors. About 40 minutes later, they had a PET scan to assess its uptake, an indication of progesterone-receptor abundance.
The women were then given three 200-mg doses of estradiol over 24 hours.
The FFNP infusion and PET scan were repeated the next day.
Estradiol will cause cancer cells with functional estrogen receptors to produce more progesterone receptors, so increased uptake of the radioactive analog indicates functional estrogen receptors that will respond to endocrine therapy. If estrogen receptors are not functional, and therefore not amenable to endocrine therapy (ET), estradiol will not upregulate progesterone receptors.
The results proved the theory. FFNP uptake increased more than 6.7% in 28 subjects and a median of 25.4%. All 28 women responded to subsequent ET, including 15 partial responses and 13 women with stable disease at 6 months.
Median survival was not reached after a median follow up of 27.1 months.
Uptake increased no more than 6.7% in 15 subjects and, in fact, fell a median of 0.7% from baseline. None of these women responded to ET. The median survival was 22.6 months.
“We observed 100% agreement between the response to estrogen challenge and the response to hormone therapy. … This method should work for any therapy that depends on a functional estrogen receptor, and it could provide valuable information to oncologists deciding how best to treat their patients,” Dr. Dehdashti said in a press release.
A larger multicenter confirmation trial is in the works.
Oncology needs “to get away from empiric therapies and make therapy more individualized” to save patients from the morbidity and expense of ineffective treatment and wasting time when other options are available, Dr. Dehdashti told this news organization.
“It would be a good thing if we could identify endocrine-resistant patients,” said Charles Shapiro, MD, a professor and director of translational breast cancer research at Mount Sinai Hospital, New York.
However, he wondered “about the exportability to less resource-intensive community settings where most oncology care occurs. This technology, assuming the results are confirmed in a larger study, [needs] a cost-effectiveness analysis” vs. the empiric approach, Dr. Shapiro said in an interview.
The women taking part in this study were a median of 60 years old, and most had metastatic disease. PET imaging extended from the base of the skull to the upper thighs, with data derived from bone, lung, breast, and other tumor sites. ET options included aromatase inhibitors, fulvestrant, and tamoxifen in combination with other agents.
Almost three-quarters of the women had prior systemic treatment, most often a hormone therapy–based regimen. Prior treatment had no effect on FFNP uptake.
There were no adverse events with the radiotracer, but the estradiol made a few women nauseous, among other transient discomforts, the team reported.
The work was funded by the National Cancer Institute and Washington University, St. Louis. Dr. Shapiro and Dr. Dehdashti have disclosed no relevant financial relationships. Several investigators reported consulting fees and/or other ties to a number of companies, including Pfizer, Merck, Avid Radiopharmaceutical, and Radius Health.
A version of this article first appeared on Medscape.com.
Endocrine therapy is the standard of care for estrogen receptor–positive (ER+) breast cancer, but only about half of women respond. At present, there is no method for identifying the women who are likely – and also unlikely – to respond.
But a new approach looks to be useful. It involves a trial of estrogen followed by imaging that measures the function of estrogen receptors in the cancer cells.
This functional testing of estrogen receptors on breast cancer cells was perfectly accurate in predicting endocrine therapy response in 43 postmenopausal women with advanced ER+ disease, say researchers from Washington University, St. Louis, led by Farrokh Dehdashti, MD.
“There is an unmet clinical need to develop more precise predictive biomarkers. The results of this study are extremely promising,” they conclude.
The study was published online in Nature Communications.
For the study, the women were first infused with a radioactive progestin analog – 21-[18F]fluorofuranylnorprogesterone (FFNP) – that binds progesterone receptors. About 40 minutes later, they had a PET scan to assess its uptake, an indication of progesterone-receptor abundance.
The women were then given three 200-mg doses of estradiol over 24 hours.
The FFNP infusion and PET scan were repeated the next day.
Estradiol will cause cancer cells with functional estrogen receptors to produce more progesterone receptors, so increased uptake of the radioactive analog indicates functional estrogen receptors that will respond to endocrine therapy. If estrogen receptors are not functional, and therefore not amenable to endocrine therapy (ET), estradiol will not upregulate progesterone receptors.
The results proved the theory. FFNP uptake increased more than 6.7% in 28 subjects and a median of 25.4%. All 28 women responded to subsequent ET, including 15 partial responses and 13 women with stable disease at 6 months.
Median survival was not reached after a median follow up of 27.1 months.
Uptake increased no more than 6.7% in 15 subjects and, in fact, fell a median of 0.7% from baseline. None of these women responded to ET. The median survival was 22.6 months.
“We observed 100% agreement between the response to estrogen challenge and the response to hormone therapy. … This method should work for any therapy that depends on a functional estrogen receptor, and it could provide valuable information to oncologists deciding how best to treat their patients,” Dr. Dehdashti said in a press release.
A larger multicenter confirmation trial is in the works.
Oncology needs “to get away from empiric therapies and make therapy more individualized” to save patients from the morbidity and expense of ineffective treatment and wasting time when other options are available, Dr. Dehdashti told this news organization.
“It would be a good thing if we could identify endocrine-resistant patients,” said Charles Shapiro, MD, a professor and director of translational breast cancer research at Mount Sinai Hospital, New York.
However, he wondered “about the exportability to less resource-intensive community settings where most oncology care occurs. This technology, assuming the results are confirmed in a larger study, [needs] a cost-effectiveness analysis” vs. the empiric approach, Dr. Shapiro said in an interview.
The women taking part in this study were a median of 60 years old, and most had metastatic disease. PET imaging extended from the base of the skull to the upper thighs, with data derived from bone, lung, breast, and other tumor sites. ET options included aromatase inhibitors, fulvestrant, and tamoxifen in combination with other agents.
Almost three-quarters of the women had prior systemic treatment, most often a hormone therapy–based regimen. Prior treatment had no effect on FFNP uptake.
There were no adverse events with the radiotracer, but the estradiol made a few women nauseous, among other transient discomforts, the team reported.
The work was funded by the National Cancer Institute and Washington University, St. Louis. Dr. Shapiro and Dr. Dehdashti have disclosed no relevant financial relationships. Several investigators reported consulting fees and/or other ties to a number of companies, including Pfizer, Merck, Avid Radiopharmaceutical, and Radius Health.
A version of this article first appeared on Medscape.com.
Endocrine therapy is the standard of care for estrogen receptor–positive (ER+) breast cancer, but only about half of women respond. At present, there is no method for identifying the women who are likely – and also unlikely – to respond.
But a new approach looks to be useful. It involves a trial of estrogen followed by imaging that measures the function of estrogen receptors in the cancer cells.
This functional testing of estrogen receptors on breast cancer cells was perfectly accurate in predicting endocrine therapy response in 43 postmenopausal women with advanced ER+ disease, say researchers from Washington University, St. Louis, led by Farrokh Dehdashti, MD.
“There is an unmet clinical need to develop more precise predictive biomarkers. The results of this study are extremely promising,” they conclude.
The study was published online in Nature Communications.
For the study, the women were first infused with a radioactive progestin analog – 21-[18F]fluorofuranylnorprogesterone (FFNP) – that binds progesterone receptors. About 40 minutes later, they had a PET scan to assess its uptake, an indication of progesterone-receptor abundance.
The women were then given three 200-mg doses of estradiol over 24 hours.
The FFNP infusion and PET scan were repeated the next day.
Estradiol will cause cancer cells with functional estrogen receptors to produce more progesterone receptors, so increased uptake of the radioactive analog indicates functional estrogen receptors that will respond to endocrine therapy. If estrogen receptors are not functional, and therefore not amenable to endocrine therapy (ET), estradiol will not upregulate progesterone receptors.
The results proved the theory. FFNP uptake increased more than 6.7% in 28 subjects and a median of 25.4%. All 28 women responded to subsequent ET, including 15 partial responses and 13 women with stable disease at 6 months.
Median survival was not reached after a median follow up of 27.1 months.
Uptake increased no more than 6.7% in 15 subjects and, in fact, fell a median of 0.7% from baseline. None of these women responded to ET. The median survival was 22.6 months.
“We observed 100% agreement between the response to estrogen challenge and the response to hormone therapy. … This method should work for any therapy that depends on a functional estrogen receptor, and it could provide valuable information to oncologists deciding how best to treat their patients,” Dr. Dehdashti said in a press release.
A larger multicenter confirmation trial is in the works.
Oncology needs “to get away from empiric therapies and make therapy more individualized” to save patients from the morbidity and expense of ineffective treatment and wasting time when other options are available, Dr. Dehdashti told this news organization.
“It would be a good thing if we could identify endocrine-resistant patients,” said Charles Shapiro, MD, a professor and director of translational breast cancer research at Mount Sinai Hospital, New York.
However, he wondered “about the exportability to less resource-intensive community settings where most oncology care occurs. This technology, assuming the results are confirmed in a larger study, [needs] a cost-effectiveness analysis” vs. the empiric approach, Dr. Shapiro said in an interview.
The women taking part in this study were a median of 60 years old, and most had metastatic disease. PET imaging extended from the base of the skull to the upper thighs, with data derived from bone, lung, breast, and other tumor sites. ET options included aromatase inhibitors, fulvestrant, and tamoxifen in combination with other agents.
Almost three-quarters of the women had prior systemic treatment, most often a hormone therapy–based regimen. Prior treatment had no effect on FFNP uptake.
There were no adverse events with the radiotracer, but the estradiol made a few women nauseous, among other transient discomforts, the team reported.
The work was funded by the National Cancer Institute and Washington University, St. Louis. Dr. Shapiro and Dr. Dehdashti have disclosed no relevant financial relationships. Several investigators reported consulting fees and/or other ties to a number of companies, including Pfizer, Merck, Avid Radiopharmaceutical, and Radius Health.
A version of this article first appeared on Medscape.com.
Obesity ‘clearly’ not tied to worse survival in metastatic breast cancer
First large cohort study
The relationship between obesity and overweight and breast cancer has some elements of mystery. But this is not one of them: in metastatic breast cancer (MBC), excess body weight does not negatively influence outcomes.
Multiple small studies have demonstrated this point, and now, for the first time, a large multicenter cohort analysis indicates the same.
Using medical records from 18 French comprehensive cancer centers, investigators reviewed body mass index (BMI) and overall survival (OS) data for nearly 13,000 women. The median OS was 47.4 months, and the median follow-up was about the same length of time. The team reports that obesity and overweight “were clearly not associated with prognosis.”
However, underweight was independently associated with worse OS (median, 33 months; hazard ratio, 1.14; 95% confidence interval, 1.02-1.27), report Khalil Saleh, MD, of Gustave Roussy in Villejuif, France, and colleagues.
In short, obesity or overweight had no effect on the primary outcome of OS, but underweight did.
“Underweight should be the subject of clinical attention at the time of diagnosis of MBC, and specific management should be implemented,” said study author Elise Deluche, MD, of CHU de Limoges, in an email to this news organization.
The study was published online Dec. 1 in The Breast.
“It’s really wonderful to have such a large cohort to look at this question,” said Jennifer Ligibel, MD, of the Dana-Farber Cancer Institute, Boston, who was asked for comment.
Is this another case of obesity paradox in cancer (as in renal cell carcinoma and melanoma, where excess weight is tied to better cancer-specific survival)?
No, said Dr. Ligibel: “There’s no hint at all [in this study] that people with obesity and overweight did better. … They just didn’t have worse outcomes.”
The study authors point out that the opposite is true in early-stage breast cancer. In this patient population, excess weight is associated with worse outcomes.
For example, in a 2014 meta-analysis of 82 follow-up studies in early-stage disease, obesity was associated with higher total mortality (relative risk, 1.41) and breast cancer–specific mortality (RR, 1.35) as compared to normal weight.
Why is there such a contrast between early- and late-stage disease?
“I don’t think we know exactly,” answered Dr. Ligibel. “It may be that, with breast cancer, as disease progresses, the pathways through which lifestyle may impact breast cancer may become less important.
“Obesity and overweight are associated with cancer risk in general,” said Dr. Ligibel, citing more than a dozen malignancies, including breast cancer.
But there is also an age element. Overweight or obesity is an independent predictor of breast cancer risk in postmenopausal women, but in premenopausal women, it appears to be protective. “Historically, there has been a lower risk of hormone receptor–positive breast cancer in women with obesity at younger ages that we don’t completely understand,” Dr. Ligibel noted.
That age-based difference is a conundrum, said Dr. Ligibel: “People have been trying to figure that out for a long time.”
Dr. Ligibel summarized as follows:
“There is a clear relationship between obesity and the risk of developing breast cancer; there is a clear relationship in early breast cancer that obesity is related to an increased risk of occurrence and mortality. What we are seeing from this study is that, by the time you get to metastatic breast cancer, body weight does not seem to play as important a role.”
More study details
The findings come from the French National Epidemiological Strategy and Medical Economics–Metastatic Breast Cancer observational cohort, which includes 22,000-plus consecutive patients who were newly diagnosed with metastatic disease between 2008 and 2016.
A total of 12,999 women for whom BMI data were available when they were diagnosed with metastatic breast cancer were selected for analysis. They were divided into four groups, according to World Health Organization classification: underweight (BMI <18.5 kg/m2), normal weight (18.5-24.9), overweight (25.0-29.9), and obese (≥30.0).
A total of 20% of women were obese, which is a much lower percentage than the 40%-50% that would be expected in a comparable American cohort, said Dr. Ligibel. Also, 5% of the French cohort was underweight.
Multivariate Cox analyses were carried out for OS and for first-line progression-free survival (PFS).
As noted above, underweight was independently associated with a worse OS. It was also tied to worse first-line PFS (HR, 1.11; 95% CI, 1.01-1.22). Overweight or obesity had no effect.
“Patients with a low BMI had more visceral metastases and a greater number of metastatic sites,” pointed out study author Dr. Deluche. “We attribute the fat loss in patients with metastatic breast cancer to aggressive tumor behavior with a higher energy requirement.”
The study authors also observe that in early-stage breast cancer, underweight is not associated with overall or breast cancer–specific survival. “Underweight at metastatic diagnosis seems to have a different significance and impact,” they write. The French team also observes that, in other cancers, underweight is also an adverse prognostic factor and has been associated with a higher risk for death.
The study authors acknowledge that BMI has limitations as a measure of body type. “BMI alone cannot estimate a woman’s muscle mass and adiposity,” they observe. The suggestion is that, among women with a similar BMI, some might be muscular, whereas others might have more body fat.
Multiple study authors report financial ties to industry, including pharmaceutical companies with drugs used in breast cancer. The database used in the study receives financial support from AstraZeneca, Daiichi Sankyo, Eisai, MSD, Pfizer, and Roche. Dr. Ligibel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First large cohort study
First large cohort study
The relationship between obesity and overweight and breast cancer has some elements of mystery. But this is not one of them: in metastatic breast cancer (MBC), excess body weight does not negatively influence outcomes.
Multiple small studies have demonstrated this point, and now, for the first time, a large multicenter cohort analysis indicates the same.
Using medical records from 18 French comprehensive cancer centers, investigators reviewed body mass index (BMI) and overall survival (OS) data for nearly 13,000 women. The median OS was 47.4 months, and the median follow-up was about the same length of time. The team reports that obesity and overweight “were clearly not associated with prognosis.”
However, underweight was independently associated with worse OS (median, 33 months; hazard ratio, 1.14; 95% confidence interval, 1.02-1.27), report Khalil Saleh, MD, of Gustave Roussy in Villejuif, France, and colleagues.
In short, obesity or overweight had no effect on the primary outcome of OS, but underweight did.
“Underweight should be the subject of clinical attention at the time of diagnosis of MBC, and specific management should be implemented,” said study author Elise Deluche, MD, of CHU de Limoges, in an email to this news organization.
The study was published online Dec. 1 in The Breast.
“It’s really wonderful to have such a large cohort to look at this question,” said Jennifer Ligibel, MD, of the Dana-Farber Cancer Institute, Boston, who was asked for comment.
Is this another case of obesity paradox in cancer (as in renal cell carcinoma and melanoma, where excess weight is tied to better cancer-specific survival)?
No, said Dr. Ligibel: “There’s no hint at all [in this study] that people with obesity and overweight did better. … They just didn’t have worse outcomes.”
The study authors point out that the opposite is true in early-stage breast cancer. In this patient population, excess weight is associated with worse outcomes.
For example, in a 2014 meta-analysis of 82 follow-up studies in early-stage disease, obesity was associated with higher total mortality (relative risk, 1.41) and breast cancer–specific mortality (RR, 1.35) as compared to normal weight.
Why is there such a contrast between early- and late-stage disease?
“I don’t think we know exactly,” answered Dr. Ligibel. “It may be that, with breast cancer, as disease progresses, the pathways through which lifestyle may impact breast cancer may become less important.
“Obesity and overweight are associated with cancer risk in general,” said Dr. Ligibel, citing more than a dozen malignancies, including breast cancer.
But there is also an age element. Overweight or obesity is an independent predictor of breast cancer risk in postmenopausal women, but in premenopausal women, it appears to be protective. “Historically, there has been a lower risk of hormone receptor–positive breast cancer in women with obesity at younger ages that we don’t completely understand,” Dr. Ligibel noted.
That age-based difference is a conundrum, said Dr. Ligibel: “People have been trying to figure that out for a long time.”
Dr. Ligibel summarized as follows:
“There is a clear relationship between obesity and the risk of developing breast cancer; there is a clear relationship in early breast cancer that obesity is related to an increased risk of occurrence and mortality. What we are seeing from this study is that, by the time you get to metastatic breast cancer, body weight does not seem to play as important a role.”
More study details
The findings come from the French National Epidemiological Strategy and Medical Economics–Metastatic Breast Cancer observational cohort, which includes 22,000-plus consecutive patients who were newly diagnosed with metastatic disease between 2008 and 2016.
A total of 12,999 women for whom BMI data were available when they were diagnosed with metastatic breast cancer were selected for analysis. They were divided into four groups, according to World Health Organization classification: underweight (BMI <18.5 kg/m2), normal weight (18.5-24.9), overweight (25.0-29.9), and obese (≥30.0).
A total of 20% of women were obese, which is a much lower percentage than the 40%-50% that would be expected in a comparable American cohort, said Dr. Ligibel. Also, 5% of the French cohort was underweight.
Multivariate Cox analyses were carried out for OS and for first-line progression-free survival (PFS).
As noted above, underweight was independently associated with a worse OS. It was also tied to worse first-line PFS (HR, 1.11; 95% CI, 1.01-1.22). Overweight or obesity had no effect.
“Patients with a low BMI had more visceral metastases and a greater number of metastatic sites,” pointed out study author Dr. Deluche. “We attribute the fat loss in patients with metastatic breast cancer to aggressive tumor behavior with a higher energy requirement.”
The study authors also observe that in early-stage breast cancer, underweight is not associated with overall or breast cancer–specific survival. “Underweight at metastatic diagnosis seems to have a different significance and impact,” they write. The French team also observes that, in other cancers, underweight is also an adverse prognostic factor and has been associated with a higher risk for death.
The study authors acknowledge that BMI has limitations as a measure of body type. “BMI alone cannot estimate a woman’s muscle mass and adiposity,” they observe. The suggestion is that, among women with a similar BMI, some might be muscular, whereas others might have more body fat.
Multiple study authors report financial ties to industry, including pharmaceutical companies with drugs used in breast cancer. The database used in the study receives financial support from AstraZeneca, Daiichi Sankyo, Eisai, MSD, Pfizer, and Roche. Dr. Ligibel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The relationship between obesity and overweight and breast cancer has some elements of mystery. But this is not one of them: in metastatic breast cancer (MBC), excess body weight does not negatively influence outcomes.
Multiple small studies have demonstrated this point, and now, for the first time, a large multicenter cohort analysis indicates the same.
Using medical records from 18 French comprehensive cancer centers, investigators reviewed body mass index (BMI) and overall survival (OS) data for nearly 13,000 women. The median OS was 47.4 months, and the median follow-up was about the same length of time. The team reports that obesity and overweight “were clearly not associated with prognosis.”
However, underweight was independently associated with worse OS (median, 33 months; hazard ratio, 1.14; 95% confidence interval, 1.02-1.27), report Khalil Saleh, MD, of Gustave Roussy in Villejuif, France, and colleagues.
In short, obesity or overweight had no effect on the primary outcome of OS, but underweight did.
“Underweight should be the subject of clinical attention at the time of diagnosis of MBC, and specific management should be implemented,” said study author Elise Deluche, MD, of CHU de Limoges, in an email to this news organization.
The study was published online Dec. 1 in The Breast.
“It’s really wonderful to have such a large cohort to look at this question,” said Jennifer Ligibel, MD, of the Dana-Farber Cancer Institute, Boston, who was asked for comment.
Is this another case of obesity paradox in cancer (as in renal cell carcinoma and melanoma, where excess weight is tied to better cancer-specific survival)?
No, said Dr. Ligibel: “There’s no hint at all [in this study] that people with obesity and overweight did better. … They just didn’t have worse outcomes.”
The study authors point out that the opposite is true in early-stage breast cancer. In this patient population, excess weight is associated with worse outcomes.
For example, in a 2014 meta-analysis of 82 follow-up studies in early-stage disease, obesity was associated with higher total mortality (relative risk, 1.41) and breast cancer–specific mortality (RR, 1.35) as compared to normal weight.
Why is there such a contrast between early- and late-stage disease?
“I don’t think we know exactly,” answered Dr. Ligibel. “It may be that, with breast cancer, as disease progresses, the pathways through which lifestyle may impact breast cancer may become less important.
“Obesity and overweight are associated with cancer risk in general,” said Dr. Ligibel, citing more than a dozen malignancies, including breast cancer.
But there is also an age element. Overweight or obesity is an independent predictor of breast cancer risk in postmenopausal women, but in premenopausal women, it appears to be protective. “Historically, there has been a lower risk of hormone receptor–positive breast cancer in women with obesity at younger ages that we don’t completely understand,” Dr. Ligibel noted.
That age-based difference is a conundrum, said Dr. Ligibel: “People have been trying to figure that out for a long time.”
Dr. Ligibel summarized as follows:
“There is a clear relationship between obesity and the risk of developing breast cancer; there is a clear relationship in early breast cancer that obesity is related to an increased risk of occurrence and mortality. What we are seeing from this study is that, by the time you get to metastatic breast cancer, body weight does not seem to play as important a role.”
More study details
The findings come from the French National Epidemiological Strategy and Medical Economics–Metastatic Breast Cancer observational cohort, which includes 22,000-plus consecutive patients who were newly diagnosed with metastatic disease between 2008 and 2016.
A total of 12,999 women for whom BMI data were available when they were diagnosed with metastatic breast cancer were selected for analysis. They were divided into four groups, according to World Health Organization classification: underweight (BMI <18.5 kg/m2), normal weight (18.5-24.9), overweight (25.0-29.9), and obese (≥30.0).
A total of 20% of women were obese, which is a much lower percentage than the 40%-50% that would be expected in a comparable American cohort, said Dr. Ligibel. Also, 5% of the French cohort was underweight.
Multivariate Cox analyses were carried out for OS and for first-line progression-free survival (PFS).
As noted above, underweight was independently associated with a worse OS. It was also tied to worse first-line PFS (HR, 1.11; 95% CI, 1.01-1.22). Overweight or obesity had no effect.
“Patients with a low BMI had more visceral metastases and a greater number of metastatic sites,” pointed out study author Dr. Deluche. “We attribute the fat loss in patients with metastatic breast cancer to aggressive tumor behavior with a higher energy requirement.”
The study authors also observe that in early-stage breast cancer, underweight is not associated with overall or breast cancer–specific survival. “Underweight at metastatic diagnosis seems to have a different significance and impact,” they write. The French team also observes that, in other cancers, underweight is also an adverse prognostic factor and has been associated with a higher risk for death.
The study authors acknowledge that BMI has limitations as a measure of body type. “BMI alone cannot estimate a woman’s muscle mass and adiposity,” they observe. The suggestion is that, among women with a similar BMI, some might be muscular, whereas others might have more body fat.
Multiple study authors report financial ties to industry, including pharmaceutical companies with drugs used in breast cancer. The database used in the study receives financial support from AstraZeneca, Daiichi Sankyo, Eisai, MSD, Pfizer, and Roche. Dr. Ligibel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Entinostat doesn’t overcome endocrine resistance in advanced breast cancer
The study showed no difference in response, progression-free survival, or overall survival whether entinostat was added to exemestane or exemestane was given with placebo.
These results were reported at the 2020 San Antonio Breast Cancer Symposium.
“Clearly, we were very disappointed with these results after so many years of work,” said study investigator Roisin M. Connolly, MD, of University College Cork (Ireland) and Cork University Hospital in Wilton, Ireland.
“I think we’ve realized again the importance of phase 3 confirmation of promising phase 2 data,” she said, referring to results of the phase 2 ENCORE 301 trial.
“I think that the results speak for themselves. In this population of endocrine-resistant patients, the HDAC inhibitors clearly do not have a role unless we find something further on additional review of the correlative analyses,” Dr. Connolly said.
Why HDAC inhibitors in advanced breast cancer?
“Despite many advances in breast cancer in recent decades, resistance to endocrine therapy remains a significant clinical problem,” Dr. Connolly said.
One suggested approach to overcoming this resistance is to block the overacetylation of histones using HDAC inhibitors. This has been shown in preclinical studies with entinostat to inhibit growth factor signaling pathways and normalize the expression of the estrogen receptor, helping to overcome resistance to aromatase inhibitors in letrozole-resistant mouse models.
Results from the phase 2 ENCORE 301 trial, published in the Journal of Clinical Oncology, also suggested this approach could be effective. There was a 2-month improvement in progression-free survival and an 8.3-month improvement in overall survival when entinostat was added to exemestane.
Phase 3 trial details and results
The E2112 study enrolled 608 women with hormone receptor–positive, HER2-negative advanced breast cancer, 85% of whom had experienced progression after taking a nonsteroidal aromatase inhibitor in the metastatic setting.
The type of endocrine resistance, such as if ESR1 mutations were present, was not determined. Tissue samples and blood samples have been archived, so this might be a question that is investigated later on.
A quarter of patients had received one prior chemotherapy regimen for metastatic disease, around 30% had been treated with fulvestrant, and about a third of patients had received a CDK4/6 inhibitor.
“I think we had representation from both patients who did receive and did not receive a prior CDK4/6 inhibitor within E2112,” Dr. Connolly said, observing that the study started in 2014 before the use of these drugs was really established.
Patients were randomized to receive entinostat (5 mg daily) plus exemestane (25 mg daily) or exemestane plus placebo (at the same dose).
The median progression-free survival was 3.3 months in the entinostat arm and 3.1 months in the placebo arm (hazard ratio, 0.87; P = .30).
The median overall survival was 23.4 months with entinostat and 21.7 months with placebo (HR, 0.99; P = .94). The overall response rates were a respective 4.6% and 4.3%.
Grade 3/4 adverse events were more frequent in the entinostat arm. The most common were neutropenia (20% with entinostat vs. <1% with placebo), hypophosphatemia (14% vs. 1%), and anemia (8% vs. 2%).
There were three treatment-related deaths (heart failure, pneumonitis, and hepatic failure) in the entinostat arm and one (MI) in the placebo arm.
Implications and next steps
“The study is completely negative, with no benefit in progression-free or overall survival,” commented Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the study.
“It is unclear that this is a good clinical approach for further trials in advanced breast cancer, as correlative studies suggest the drug did hit the target,” he added.
Dr. Burstein’s takeaway was that “HDAC inhibition is stuck in a cul-de-sac, if not a complete dead end, for breast cancer.”
When asked if using a different aromatase inhibitor than exemestane might have affected the results, Dr. Connolly said that “it’s possible, but I think it’s unlikely.”
Exemestane was used in the phase 3 trial because it had been used in the ENCORE 301 study. Preclinical work had shown that both letrozole- and exemestane-resistant models benefited from the addition of an HDAC inhibitor.
“There is ongoing investigation of HDAC inhibitors in various combinations,” Dr. Connolly said. “HDAC inhibitors have been used with chemotherapies and other targeted therapies over the years but unfortunately have not broken into the solid tumor space. I think that ongoing work will be required to see where these may fit in the future.”
The E2122 study was coordinated by the ECOG-ACRIN Cancer Research Group with funding from the National Institutes of Health. Dr. Connolly disclosed relationships with Genentech, Merck, Novartis, Puma Biotechnology, Marcogenics, and Pfizer. Dr. Burstein had no relevant disclosures.
The study showed no difference in response, progression-free survival, or overall survival whether entinostat was added to exemestane or exemestane was given with placebo.
These results were reported at the 2020 San Antonio Breast Cancer Symposium.
“Clearly, we were very disappointed with these results after so many years of work,” said study investigator Roisin M. Connolly, MD, of University College Cork (Ireland) and Cork University Hospital in Wilton, Ireland.
“I think we’ve realized again the importance of phase 3 confirmation of promising phase 2 data,” she said, referring to results of the phase 2 ENCORE 301 trial.
“I think that the results speak for themselves. In this population of endocrine-resistant patients, the HDAC inhibitors clearly do not have a role unless we find something further on additional review of the correlative analyses,” Dr. Connolly said.
Why HDAC inhibitors in advanced breast cancer?
“Despite many advances in breast cancer in recent decades, resistance to endocrine therapy remains a significant clinical problem,” Dr. Connolly said.
One suggested approach to overcoming this resistance is to block the overacetylation of histones using HDAC inhibitors. This has been shown in preclinical studies with entinostat to inhibit growth factor signaling pathways and normalize the expression of the estrogen receptor, helping to overcome resistance to aromatase inhibitors in letrozole-resistant mouse models.
Results from the phase 2 ENCORE 301 trial, published in the Journal of Clinical Oncology, also suggested this approach could be effective. There was a 2-month improvement in progression-free survival and an 8.3-month improvement in overall survival when entinostat was added to exemestane.
Phase 3 trial details and results
The E2112 study enrolled 608 women with hormone receptor–positive, HER2-negative advanced breast cancer, 85% of whom had experienced progression after taking a nonsteroidal aromatase inhibitor in the metastatic setting.
The type of endocrine resistance, such as if ESR1 mutations were present, was not determined. Tissue samples and blood samples have been archived, so this might be a question that is investigated later on.
A quarter of patients had received one prior chemotherapy regimen for metastatic disease, around 30% had been treated with fulvestrant, and about a third of patients had received a CDK4/6 inhibitor.
“I think we had representation from both patients who did receive and did not receive a prior CDK4/6 inhibitor within E2112,” Dr. Connolly said, observing that the study started in 2014 before the use of these drugs was really established.
Patients were randomized to receive entinostat (5 mg daily) plus exemestane (25 mg daily) or exemestane plus placebo (at the same dose).
The median progression-free survival was 3.3 months in the entinostat arm and 3.1 months in the placebo arm (hazard ratio, 0.87; P = .30).
The median overall survival was 23.4 months with entinostat and 21.7 months with placebo (HR, 0.99; P = .94). The overall response rates were a respective 4.6% and 4.3%.
Grade 3/4 adverse events were more frequent in the entinostat arm. The most common were neutropenia (20% with entinostat vs. <1% with placebo), hypophosphatemia (14% vs. 1%), and anemia (8% vs. 2%).
There were three treatment-related deaths (heart failure, pneumonitis, and hepatic failure) in the entinostat arm and one (MI) in the placebo arm.
Implications and next steps
“The study is completely negative, with no benefit in progression-free or overall survival,” commented Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the study.
“It is unclear that this is a good clinical approach for further trials in advanced breast cancer, as correlative studies suggest the drug did hit the target,” he added.
Dr. Burstein’s takeaway was that “HDAC inhibition is stuck in a cul-de-sac, if not a complete dead end, for breast cancer.”
When asked if using a different aromatase inhibitor than exemestane might have affected the results, Dr. Connolly said that “it’s possible, but I think it’s unlikely.”
Exemestane was used in the phase 3 trial because it had been used in the ENCORE 301 study. Preclinical work had shown that both letrozole- and exemestane-resistant models benefited from the addition of an HDAC inhibitor.
“There is ongoing investigation of HDAC inhibitors in various combinations,” Dr. Connolly said. “HDAC inhibitors have been used with chemotherapies and other targeted therapies over the years but unfortunately have not broken into the solid tumor space. I think that ongoing work will be required to see where these may fit in the future.”
The E2122 study was coordinated by the ECOG-ACRIN Cancer Research Group with funding from the National Institutes of Health. Dr. Connolly disclosed relationships with Genentech, Merck, Novartis, Puma Biotechnology, Marcogenics, and Pfizer. Dr. Burstein had no relevant disclosures.
The study showed no difference in response, progression-free survival, or overall survival whether entinostat was added to exemestane or exemestane was given with placebo.
These results were reported at the 2020 San Antonio Breast Cancer Symposium.
“Clearly, we were very disappointed with these results after so many years of work,” said study investigator Roisin M. Connolly, MD, of University College Cork (Ireland) and Cork University Hospital in Wilton, Ireland.
“I think we’ve realized again the importance of phase 3 confirmation of promising phase 2 data,” she said, referring to results of the phase 2 ENCORE 301 trial.
“I think that the results speak for themselves. In this population of endocrine-resistant patients, the HDAC inhibitors clearly do not have a role unless we find something further on additional review of the correlative analyses,” Dr. Connolly said.
Why HDAC inhibitors in advanced breast cancer?
“Despite many advances in breast cancer in recent decades, resistance to endocrine therapy remains a significant clinical problem,” Dr. Connolly said.
One suggested approach to overcoming this resistance is to block the overacetylation of histones using HDAC inhibitors. This has been shown in preclinical studies with entinostat to inhibit growth factor signaling pathways and normalize the expression of the estrogen receptor, helping to overcome resistance to aromatase inhibitors in letrozole-resistant mouse models.
Results from the phase 2 ENCORE 301 trial, published in the Journal of Clinical Oncology, also suggested this approach could be effective. There was a 2-month improvement in progression-free survival and an 8.3-month improvement in overall survival when entinostat was added to exemestane.
Phase 3 trial details and results
The E2112 study enrolled 608 women with hormone receptor–positive, HER2-negative advanced breast cancer, 85% of whom had experienced progression after taking a nonsteroidal aromatase inhibitor in the metastatic setting.
The type of endocrine resistance, such as if ESR1 mutations were present, was not determined. Tissue samples and blood samples have been archived, so this might be a question that is investigated later on.
A quarter of patients had received one prior chemotherapy regimen for metastatic disease, around 30% had been treated with fulvestrant, and about a third of patients had received a CDK4/6 inhibitor.
“I think we had representation from both patients who did receive and did not receive a prior CDK4/6 inhibitor within E2112,” Dr. Connolly said, observing that the study started in 2014 before the use of these drugs was really established.
Patients were randomized to receive entinostat (5 mg daily) plus exemestane (25 mg daily) or exemestane plus placebo (at the same dose).
The median progression-free survival was 3.3 months in the entinostat arm and 3.1 months in the placebo arm (hazard ratio, 0.87; P = .30).
The median overall survival was 23.4 months with entinostat and 21.7 months with placebo (HR, 0.99; P = .94). The overall response rates were a respective 4.6% and 4.3%.
Grade 3/4 adverse events were more frequent in the entinostat arm. The most common were neutropenia (20% with entinostat vs. <1% with placebo), hypophosphatemia (14% vs. 1%), and anemia (8% vs. 2%).
There were three treatment-related deaths (heart failure, pneumonitis, and hepatic failure) in the entinostat arm and one (MI) in the placebo arm.
Implications and next steps
“The study is completely negative, with no benefit in progression-free or overall survival,” commented Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the study.
“It is unclear that this is a good clinical approach for further trials in advanced breast cancer, as correlative studies suggest the drug did hit the target,” he added.
Dr. Burstein’s takeaway was that “HDAC inhibition is stuck in a cul-de-sac, if not a complete dead end, for breast cancer.”
When asked if using a different aromatase inhibitor than exemestane might have affected the results, Dr. Connolly said that “it’s possible, but I think it’s unlikely.”
Exemestane was used in the phase 3 trial because it had been used in the ENCORE 301 study. Preclinical work had shown that both letrozole- and exemestane-resistant models benefited from the addition of an HDAC inhibitor.
“There is ongoing investigation of HDAC inhibitors in various combinations,” Dr. Connolly said. “HDAC inhibitors have been used with chemotherapies and other targeted therapies over the years but unfortunately have not broken into the solid tumor space. I think that ongoing work will be required to see where these may fit in the future.”
The E2122 study was coordinated by the ECOG-ACRIN Cancer Research Group with funding from the National Institutes of Health. Dr. Connolly disclosed relationships with Genentech, Merck, Novartis, Puma Biotechnology, Marcogenics, and Pfizer. Dr. Burstein had no relevant disclosures.
FROM SABCS 2020
After 48 years, NCI aims to track breast cancer recurrences
Change to SEER eventually planned.
Patients with breast cancer want accurate information on the risk of their cancer recurring once they have completed treatment.
“I would like to know the true stats of how many breast cancers come back no matter what the hell we do for treatment,” comments a typical post on a breast cancer patient bulletin board.
But those statistics have not been available from a robust population-based source.
Now, there is hope that they will – at last – be collected.
A new pilot project at the National Cancer Institute is setting out to collect that information, although the researchers say it is a “long-term goal” that will take a few years.
But it has already been a long time coming. The mother lode of all U.S. cancer data, the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program, started collecting cancer data in 1973.
“When they began to capture cancer data, the focus was primarily on the incidence of cancer, the different types of cancer, and survival,” explained Esmeralda Ramirez-Pena, PhD, MPH, cancer prevention fellow at the NCI.
“Later, SEER expanded to include subgroups of various cancers and different stages at diagnosis,” she added.
But this database has never included information on cancer recurrence.
In a 2017 press statement, the NCI commented: “Collecting recurrence data has been challenging for cancer registries because recurrence can be diagnosed through diverse methods and in a variety of locations.”
New project
The NCI now has a “long-term goal” to implement additional “data elements” into SEER that will allow calculation of breast cancer recurrences, said Dr. Ramirez-Pena.
The Breast Cancer Recurrence Project, a pilot program funded via an NCI–Department of Energy collaboration, “will take a couple of years,” she said.
She presented some details of the new project as a poster at the recent San Antonio Breast Cancer Symposium 2020.
“SEER has added data elements over time,” she said, and this latest move will – at last – include information on breast cancer recurrence.
Why the change now?
“There’s been so much interest [in breast cancer recurrence]. It’s a top cause of cancer death in the United States and globally. The urgent need is evident,” she explained.
Breast cancer advocates have long been calling for SEER to count recurrence, including metastatic recurrence.
Katherine O’Brien, a breast cancer “metser” from Chicago, is credited with especially turning the heat up on the NCI.
In 2015, Ms. O’Brien spearheaded the creation of an online petition on the website change.org, calling on the NCI’s SEER, the Centers for Disease Control and Prevention, and all state cancer registries to start counting all people living with metastatic breast cancer, including those whose early-stage disease progressed. The petition, which is now closed, collected nearly 12,000 signatures.
Tracking recurrences
In the new project, cancer recurrence is defined as a cancer that was treated, reduced to undetectable levels, and later returned either locally, regionally, or distantly.
Tracking recurrence is not a simple matter because posttreatment surveillance to detect it includes clinical exams, biomarker testing, pathologic studies, molecular testing, imaging, and patient-reported symptoms and because recurrence frequency varies by subtype of breast cancer and TNM classification. Additionally, recurrence may depend on age at diagnosis, a variety of risk factors, treatment type, and access to quality of care.
“It’s likely there are many elements that influence recurrence,” said Dr. Ramirez-Pena.
To get a handle on the complexity, the NCI needs to first identify which data are needed to tally recurrence and the frequency at which they are collected, explained Dr. Ramirez-Pena. To do so, she and her coinvestigators conducted a systematic review of phase 3 clinical trials of early-stage breast cancer.
On their own, such trials are not sufficient to provide recurrence estimates at the population level because they lack diversity, represent fewer than 5% of all cancer patients, and the study period may not be long enough to capture recurrences for long-latency breast cancers, such as estrogen receptor–positive malignancies.
Nonetheless, these clinical trials provide a starting place.
The investigators identified 444 early-stage clinical trials. They stratified participants by subtype and tumor characteristics, which will enable analysis of risk-group and treatment-dependent differences in recurrence.
The changing science of breast cancer makes this work a challenge, the investigators said. For example, in clinical trials from the early 1990s through the early 2000s, receptor status and subtyping was not commonly reported, and some treatment endpoints were added during the past few years.
“Our next step will be to extract recurrence rates from these trials so we can eventually provide individualized information about recurrence risk to survivors,” Dr. Ramirez-Pena said, describing the big-picture aims.
The Breast Cancer Recurrence Project is collaborating with external agencies, such as the International Agency for Research on Cancer and Public Health England, in fine-tuning data elements, because “recurrence is not captured well globally either,” said Dr. Ramirez-Pena.
The study was supported by NCI.
A version of this article first appeared on Medscape.com.
Change to SEER eventually planned.
Change to SEER eventually planned.
Patients with breast cancer want accurate information on the risk of their cancer recurring once they have completed treatment.
“I would like to know the true stats of how many breast cancers come back no matter what the hell we do for treatment,” comments a typical post on a breast cancer patient bulletin board.
But those statistics have not been available from a robust population-based source.
Now, there is hope that they will – at last – be collected.
A new pilot project at the National Cancer Institute is setting out to collect that information, although the researchers say it is a “long-term goal” that will take a few years.
But it has already been a long time coming. The mother lode of all U.S. cancer data, the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program, started collecting cancer data in 1973.
“When they began to capture cancer data, the focus was primarily on the incidence of cancer, the different types of cancer, and survival,” explained Esmeralda Ramirez-Pena, PhD, MPH, cancer prevention fellow at the NCI.
“Later, SEER expanded to include subgroups of various cancers and different stages at diagnosis,” she added.
But this database has never included information on cancer recurrence.
In a 2017 press statement, the NCI commented: “Collecting recurrence data has been challenging for cancer registries because recurrence can be diagnosed through diverse methods and in a variety of locations.”
New project
The NCI now has a “long-term goal” to implement additional “data elements” into SEER that will allow calculation of breast cancer recurrences, said Dr. Ramirez-Pena.
The Breast Cancer Recurrence Project, a pilot program funded via an NCI–Department of Energy collaboration, “will take a couple of years,” she said.
She presented some details of the new project as a poster at the recent San Antonio Breast Cancer Symposium 2020.
“SEER has added data elements over time,” she said, and this latest move will – at last – include information on breast cancer recurrence.
Why the change now?
“There’s been so much interest [in breast cancer recurrence]. It’s a top cause of cancer death in the United States and globally. The urgent need is evident,” she explained.
Breast cancer advocates have long been calling for SEER to count recurrence, including metastatic recurrence.
Katherine O’Brien, a breast cancer “metser” from Chicago, is credited with especially turning the heat up on the NCI.
In 2015, Ms. O’Brien spearheaded the creation of an online petition on the website change.org, calling on the NCI’s SEER, the Centers for Disease Control and Prevention, and all state cancer registries to start counting all people living with metastatic breast cancer, including those whose early-stage disease progressed. The petition, which is now closed, collected nearly 12,000 signatures.
Tracking recurrences
In the new project, cancer recurrence is defined as a cancer that was treated, reduced to undetectable levels, and later returned either locally, regionally, or distantly.
Tracking recurrence is not a simple matter because posttreatment surveillance to detect it includes clinical exams, biomarker testing, pathologic studies, molecular testing, imaging, and patient-reported symptoms and because recurrence frequency varies by subtype of breast cancer and TNM classification. Additionally, recurrence may depend on age at diagnosis, a variety of risk factors, treatment type, and access to quality of care.
“It’s likely there are many elements that influence recurrence,” said Dr. Ramirez-Pena.
To get a handle on the complexity, the NCI needs to first identify which data are needed to tally recurrence and the frequency at which they are collected, explained Dr. Ramirez-Pena. To do so, she and her coinvestigators conducted a systematic review of phase 3 clinical trials of early-stage breast cancer.
On their own, such trials are not sufficient to provide recurrence estimates at the population level because they lack diversity, represent fewer than 5% of all cancer patients, and the study period may not be long enough to capture recurrences for long-latency breast cancers, such as estrogen receptor–positive malignancies.
Nonetheless, these clinical trials provide a starting place.
The investigators identified 444 early-stage clinical trials. They stratified participants by subtype and tumor characteristics, which will enable analysis of risk-group and treatment-dependent differences in recurrence.
The changing science of breast cancer makes this work a challenge, the investigators said. For example, in clinical trials from the early 1990s through the early 2000s, receptor status and subtyping was not commonly reported, and some treatment endpoints were added during the past few years.
“Our next step will be to extract recurrence rates from these trials so we can eventually provide individualized information about recurrence risk to survivors,” Dr. Ramirez-Pena said, describing the big-picture aims.
The Breast Cancer Recurrence Project is collaborating with external agencies, such as the International Agency for Research on Cancer and Public Health England, in fine-tuning data elements, because “recurrence is not captured well globally either,” said Dr. Ramirez-Pena.
The study was supported by NCI.
A version of this article first appeared on Medscape.com.
Patients with breast cancer want accurate information on the risk of their cancer recurring once they have completed treatment.
“I would like to know the true stats of how many breast cancers come back no matter what the hell we do for treatment,” comments a typical post on a breast cancer patient bulletin board.
But those statistics have not been available from a robust population-based source.
Now, there is hope that they will – at last – be collected.
A new pilot project at the National Cancer Institute is setting out to collect that information, although the researchers say it is a “long-term goal” that will take a few years.
But it has already been a long time coming. The mother lode of all U.S. cancer data, the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program, started collecting cancer data in 1973.
“When they began to capture cancer data, the focus was primarily on the incidence of cancer, the different types of cancer, and survival,” explained Esmeralda Ramirez-Pena, PhD, MPH, cancer prevention fellow at the NCI.
“Later, SEER expanded to include subgroups of various cancers and different stages at diagnosis,” she added.
But this database has never included information on cancer recurrence.
In a 2017 press statement, the NCI commented: “Collecting recurrence data has been challenging for cancer registries because recurrence can be diagnosed through diverse methods and in a variety of locations.”
New project
The NCI now has a “long-term goal” to implement additional “data elements” into SEER that will allow calculation of breast cancer recurrences, said Dr. Ramirez-Pena.
The Breast Cancer Recurrence Project, a pilot program funded via an NCI–Department of Energy collaboration, “will take a couple of years,” she said.
She presented some details of the new project as a poster at the recent San Antonio Breast Cancer Symposium 2020.
“SEER has added data elements over time,” she said, and this latest move will – at last – include information on breast cancer recurrence.
Why the change now?
“There’s been so much interest [in breast cancer recurrence]. It’s a top cause of cancer death in the United States and globally. The urgent need is evident,” she explained.
Breast cancer advocates have long been calling for SEER to count recurrence, including metastatic recurrence.
Katherine O’Brien, a breast cancer “metser” from Chicago, is credited with especially turning the heat up on the NCI.
In 2015, Ms. O’Brien spearheaded the creation of an online petition on the website change.org, calling on the NCI’s SEER, the Centers for Disease Control and Prevention, and all state cancer registries to start counting all people living with metastatic breast cancer, including those whose early-stage disease progressed. The petition, which is now closed, collected nearly 12,000 signatures.
Tracking recurrences
In the new project, cancer recurrence is defined as a cancer that was treated, reduced to undetectable levels, and later returned either locally, regionally, or distantly.
Tracking recurrence is not a simple matter because posttreatment surveillance to detect it includes clinical exams, biomarker testing, pathologic studies, molecular testing, imaging, and patient-reported symptoms and because recurrence frequency varies by subtype of breast cancer and TNM classification. Additionally, recurrence may depend on age at diagnosis, a variety of risk factors, treatment type, and access to quality of care.
“It’s likely there are many elements that influence recurrence,” said Dr. Ramirez-Pena.
To get a handle on the complexity, the NCI needs to first identify which data are needed to tally recurrence and the frequency at which they are collected, explained Dr. Ramirez-Pena. To do so, she and her coinvestigators conducted a systematic review of phase 3 clinical trials of early-stage breast cancer.
On their own, such trials are not sufficient to provide recurrence estimates at the population level because they lack diversity, represent fewer than 5% of all cancer patients, and the study period may not be long enough to capture recurrences for long-latency breast cancers, such as estrogen receptor–positive malignancies.
Nonetheless, these clinical trials provide a starting place.
The investigators identified 444 early-stage clinical trials. They stratified participants by subtype and tumor characteristics, which will enable analysis of risk-group and treatment-dependent differences in recurrence.
The changing science of breast cancer makes this work a challenge, the investigators said. For example, in clinical trials from the early 1990s through the early 2000s, receptor status and subtyping was not commonly reported, and some treatment endpoints were added during the past few years.
“Our next step will be to extract recurrence rates from these trials so we can eventually provide individualized information about recurrence risk to survivors,” Dr. Ramirez-Pena said, describing the big-picture aims.
The Breast Cancer Recurrence Project is collaborating with external agencies, such as the International Agency for Research on Cancer and Public Health England, in fine-tuning data elements, because “recurrence is not captured well globally either,” said Dr. Ramirez-Pena.
The study was supported by NCI.
A version of this article first appeared on Medscape.com.
Margetuximab approved for HER2-positive metastatic breast cancer
The new drug is indicated for use in combination with chemotherapy for the treatment of patients with metastatic HER2-positive breast cancer who have already received two or more prior anti-HER2 regimens, with at least one for metastatic disease.
Margetuximab-cmkb is also the first HER2-targeted therapy shown to improve progression-free survival (PFS) as compared with the first-ever HER2-targeted agent, trastuzumab in a head-to-head, phase 3 clinical trial (known as SOPHIA).
“Early detection and treatment have had a positive impact on the survival of patients with breast cancer, but the prognosis for people diagnosed with metastatic breast cancer remains poor, and additional treatments are needed,” said Hope S. Rugo, MD, director of breast oncology and clinical trials education, University of California, San Francisco, Diller Family Comprehensive Cancer Center, in a press release.
“As the only HER2-targeted agent to have shown a PFS improvement versus trastuzumab in a head-to-head phase 3 clinical trial, margetuximab with chemotherapy represents the newest treatment option for patients who have progressed on available HER2-directed therapies,” said Dr. Rugo, who is an investigator in the SOPHIA trial.
Like trastuzumab, margetuximab-cmkb binds HER2 with high specificity and affinity and disrupts signaling that drives cell proliferation and survival, but margetuximab binds with elevated affinity to both the lower- and higher-affinity forms of CD16A, an Fc gamma receptor important for antibody dependent cell-mediated cytotoxicity against tumor cells, according to the manufacturer, MacroGenics.
Details of the pivotal trial
The SOPHIA trial was a randomized, open-label, phase 3 clinical trial that compared margetuximab-cmkb plus chemotherapy with trastuzumab plus chemotherapy in both arms in patients with HER2-positive metastatic breast cancer who had previously been treated with anti–HER2-targeted therapies.
All patients in the cohort had previously received trastuzumab, all but one patient had previously also received pertuzumab, and most of the patients (91%) had also been treated with ado-trastuzumab emtansine, or T-DM1.
The trial randomly assigned 536 patients to receive either margetuximab-cmkb (n = 266) given intravenously at 15 mg/kg every 3 weeks or trastuzumab (n = 270) given intravenously at 6 mg/kg (or 8 mg/kg for loading dose) every 3 weeks in combination with either capecitabine, eribulin, gemcitabine, or vinorelbine, given at the standard doses.
As compared with trastuzumab, margetuximab plus chemotherapy led to a significant 24% reduction in the risk for progression or death (hazard ratio, 0.76).
The median PFS also favored margetuximab (5.8 months vs. 4.9 months), as did the overall response rate (22% vs. 16%).
The final overall survival analysis is expected in the second half of 2021.
Common adverse events associated with the margetuximab regimen included fatigue/asthenia (57%), nausea (33%), diarrhea (25%), and vomiting (21%). Infusion-related reactions occurred in 13% of patients receiving margetuximab, and almost all were grade 1 or 2, with only 1.5% at grade 3.
The product also carries a boxed warning for left ventricular dysfunction and embryo-fetal toxicity.
A version of this article first appeared on Medscape.com.
The new drug is indicated for use in combination with chemotherapy for the treatment of patients with metastatic HER2-positive breast cancer who have already received two or more prior anti-HER2 regimens, with at least one for metastatic disease.
Margetuximab-cmkb is also the first HER2-targeted therapy shown to improve progression-free survival (PFS) as compared with the first-ever HER2-targeted agent, trastuzumab in a head-to-head, phase 3 clinical trial (known as SOPHIA).
“Early detection and treatment have had a positive impact on the survival of patients with breast cancer, but the prognosis for people diagnosed with metastatic breast cancer remains poor, and additional treatments are needed,” said Hope S. Rugo, MD, director of breast oncology and clinical trials education, University of California, San Francisco, Diller Family Comprehensive Cancer Center, in a press release.
“As the only HER2-targeted agent to have shown a PFS improvement versus trastuzumab in a head-to-head phase 3 clinical trial, margetuximab with chemotherapy represents the newest treatment option for patients who have progressed on available HER2-directed therapies,” said Dr. Rugo, who is an investigator in the SOPHIA trial.
Like trastuzumab, margetuximab-cmkb binds HER2 with high specificity and affinity and disrupts signaling that drives cell proliferation and survival, but margetuximab binds with elevated affinity to both the lower- and higher-affinity forms of CD16A, an Fc gamma receptor important for antibody dependent cell-mediated cytotoxicity against tumor cells, according to the manufacturer, MacroGenics.
Details of the pivotal trial
The SOPHIA trial was a randomized, open-label, phase 3 clinical trial that compared margetuximab-cmkb plus chemotherapy with trastuzumab plus chemotherapy in both arms in patients with HER2-positive metastatic breast cancer who had previously been treated with anti–HER2-targeted therapies.
All patients in the cohort had previously received trastuzumab, all but one patient had previously also received pertuzumab, and most of the patients (91%) had also been treated with ado-trastuzumab emtansine, or T-DM1.
The trial randomly assigned 536 patients to receive either margetuximab-cmkb (n = 266) given intravenously at 15 mg/kg every 3 weeks or trastuzumab (n = 270) given intravenously at 6 mg/kg (or 8 mg/kg for loading dose) every 3 weeks in combination with either capecitabine, eribulin, gemcitabine, or vinorelbine, given at the standard doses.
As compared with trastuzumab, margetuximab plus chemotherapy led to a significant 24% reduction in the risk for progression or death (hazard ratio, 0.76).
The median PFS also favored margetuximab (5.8 months vs. 4.9 months), as did the overall response rate (22% vs. 16%).
The final overall survival analysis is expected in the second half of 2021.
Common adverse events associated with the margetuximab regimen included fatigue/asthenia (57%), nausea (33%), diarrhea (25%), and vomiting (21%). Infusion-related reactions occurred in 13% of patients receiving margetuximab, and almost all were grade 1 or 2, with only 1.5% at grade 3.
The product also carries a boxed warning for left ventricular dysfunction and embryo-fetal toxicity.
A version of this article first appeared on Medscape.com.
The new drug is indicated for use in combination with chemotherapy for the treatment of patients with metastatic HER2-positive breast cancer who have already received two or more prior anti-HER2 regimens, with at least one for metastatic disease.
Margetuximab-cmkb is also the first HER2-targeted therapy shown to improve progression-free survival (PFS) as compared with the first-ever HER2-targeted agent, trastuzumab in a head-to-head, phase 3 clinical trial (known as SOPHIA).
“Early detection and treatment have had a positive impact on the survival of patients with breast cancer, but the prognosis for people diagnosed with metastatic breast cancer remains poor, and additional treatments are needed,” said Hope S. Rugo, MD, director of breast oncology and clinical trials education, University of California, San Francisco, Diller Family Comprehensive Cancer Center, in a press release.
“As the only HER2-targeted agent to have shown a PFS improvement versus trastuzumab in a head-to-head phase 3 clinical trial, margetuximab with chemotherapy represents the newest treatment option for patients who have progressed on available HER2-directed therapies,” said Dr. Rugo, who is an investigator in the SOPHIA trial.
Like trastuzumab, margetuximab-cmkb binds HER2 with high specificity and affinity and disrupts signaling that drives cell proliferation and survival, but margetuximab binds with elevated affinity to both the lower- and higher-affinity forms of CD16A, an Fc gamma receptor important for antibody dependent cell-mediated cytotoxicity against tumor cells, according to the manufacturer, MacroGenics.
Details of the pivotal trial
The SOPHIA trial was a randomized, open-label, phase 3 clinical trial that compared margetuximab-cmkb plus chemotherapy with trastuzumab plus chemotherapy in both arms in patients with HER2-positive metastatic breast cancer who had previously been treated with anti–HER2-targeted therapies.
All patients in the cohort had previously received trastuzumab, all but one patient had previously also received pertuzumab, and most of the patients (91%) had also been treated with ado-trastuzumab emtansine, or T-DM1.
The trial randomly assigned 536 patients to receive either margetuximab-cmkb (n = 266) given intravenously at 15 mg/kg every 3 weeks or trastuzumab (n = 270) given intravenously at 6 mg/kg (or 8 mg/kg for loading dose) every 3 weeks in combination with either capecitabine, eribulin, gemcitabine, or vinorelbine, given at the standard doses.
As compared with trastuzumab, margetuximab plus chemotherapy led to a significant 24% reduction in the risk for progression or death (hazard ratio, 0.76).
The median PFS also favored margetuximab (5.8 months vs. 4.9 months), as did the overall response rate (22% vs. 16%).
The final overall survival analysis is expected in the second half of 2021.
Common adverse events associated with the margetuximab regimen included fatigue/asthenia (57%), nausea (33%), diarrhea (25%), and vomiting (21%). Infusion-related reactions occurred in 13% of patients receiving margetuximab, and almost all were grade 1 or 2, with only 1.5% at grade 3.
The product also carries a boxed warning for left ventricular dysfunction and embryo-fetal toxicity.
A version of this article first appeared on Medscape.com.