User login
Optimizing diagnostic testing for venous thromboembolism
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
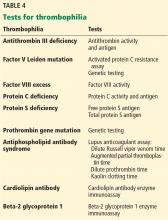
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
KEY POINTS
- A pretest clinical prediction tool such as the Wells score can help in deciding whether a patient with suspected venous thromboembolism warrants further workup.
- A clinical prediction tool should be used in concert with additional laboratory testing (eg, D-dimer) and imaging in patients at risk.
- In many cases, screening for thrombophilia to determine the cause of a venous thromboembolic event may be unwarranted.
- Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked.