User login
Smoking Cessation What Should You Recommend?
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
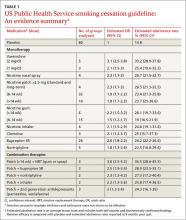
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
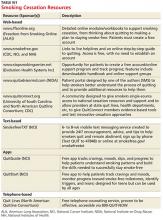
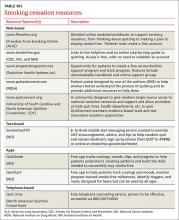
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
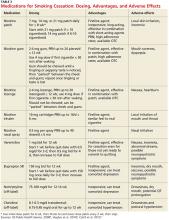
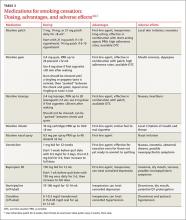
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
Smoking cessation: What should you recommend?
› Prescribe varenicline, bupropion, or nicotine replacement as first-line single pharmacotherapy for smoking cessation. A
› Provide counseling along with medication, as the combination has proven to be more effective than either option alone. A
› Refer patients to their state Quit Line—a toll-free tobacco cessation coaching service that has been shown to be an effective form of counseling. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In its 2014 report, “The Health Consequences of Smoking—50 Years of Progress,”1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risks of such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 years cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family physicians with an evidence-based update.
The guideline: Treating tobacco use and dependence
Prescribing a first-line medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (See TABLE W1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (TABLE 1).4
Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratio [OR]=1.7 [1.4-2.0], 1.3 [1.1-1.6], and 1.2 [1.1-1.4], respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR=2.5 [1.9-3.4] when 2 different clinical disciplines were involved and 2.4 [2.1-2.9] for 3 or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see TABLE W2.)
For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at motivational interviewing.org/.
In counseling patients considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (TABLE 2)4,6,7 or methods used for prior quit attempts and reasons for relapse, if appropriate; and patient preference.
Evidence update: What's best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR]=1.60; 95% CI, 1.53-1.68).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form like a lozenge with a long-acting patch was found to be more effective than either one alone (RR=1.34; 95% CI, 1.18-1.51).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR=1.18; 95% CI 0.98-1.41). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts, but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a first-line agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a first-line pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR=2.27; 95% CI, 2.02-2.55).13 It also showed varenicline to be more effective than bupropion SR (RR=1.52; 95% CI, 1.22-1.88), but about as effective as NRT (RR=1.13; 95% CI, 0.94-1.35).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR=2.7; 95% CI, 2.1-3.5).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the US Food and Drug Administration (FDA) required that black box warnings be added to the labels of both varenicline and bupropion SR based on post-marketing safety reports showing the risk of neuropsychiatric symptoms, including suicidality.15 In 2011, a large case control study by the FDA Adverse Event Reporting System also showed an increased risk of suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group vs 19% for those in the placebo group (OR=6.2; 95% CI, 2.2-19.2). Although no increased risk of adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: a meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and 2 subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline's safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a first-line treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR=1.62; 95% CI, 1.49-1.76). No increased risk of serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline, (RR=0.68; 95% CI, 0.56-0.83). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30; 95% CI, 0.93-1.82) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a first-line agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a first-line agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation vs placebo (RR=1.63; 95% CI, 1.22-2.18),7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The 6-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (OR, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks vs 47% for varenicline alone (number needed to treat [NNT]=6; 95% CI, 4-11).24
In heavy smokers—defined as those who smoke ≥20 cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT= 9; 95% CI, 4.6-71.6), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT=6).26 Consider using these combinations in patients who have high nicotine dependency levels or have been unable to quit using a single first-line agent.
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 “Vaping,” as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of 2 RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at 6 months in users of e-cigarettes containing nicotine compared with placebo e-cigarettes (9% vs 4%; RR=2.29; 95% CI, 1.05-4.96). Additionally, e-cigarette use was associated with >50% decrease in cigarette smoking vs placebo (36% vs 27%; RR=1.31; 95% CI, 1.02-1.68) or patch (61% vs 44%; RR=1.41; 95% CI, 1.20-1.67).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at 8-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, light-headedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of 4 trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation, as well. A 2013 Cochrane review of 8 trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR=1.00; 95% CI, 0.66-1.51) or as an adjunct to NRT compared with NRT alone (RR=0.95; 95% CI, 0.70-1.30).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the United States.36
A 2012 Cochrane review identified 2 recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates vs placebo (RR=3.98; 95% CI, 2.01-7.87).13
CORRESPONDENCE
Paul Bornemann, MD, 3209 Colonial Drive, Columbia, SC 29203; paul.bornemann@uscmed.sc.edu.
ACKNOWLEDGEMENT
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed October 21, 2015.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service Report. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: Counseling and interventions. April 2009. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed October 21, 2015.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. US Food and Drug Administration. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: A randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla. man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. Available at: http:// www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed December 2, 2015.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. Available at: http://www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed August 3, 2015.
› Prescribe varenicline, bupropion, or nicotine replacement as first-line single pharmacotherapy for smoking cessation. A
› Provide counseling along with medication, as the combination has proven to be more effective than either option alone. A
› Refer patients to their state Quit Line—a toll-free tobacco cessation coaching service that has been shown to be an effective form of counseling. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In its 2014 report, “The Health Consequences of Smoking—50 Years of Progress,”1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risks of such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 years cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family physicians with an evidence-based update.
The guideline: Treating tobacco use and dependence
Prescribing a first-line medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (See TABLE W1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (TABLE 1).4
Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratio [OR]=1.7 [1.4-2.0], 1.3 [1.1-1.6], and 1.2 [1.1-1.4], respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR=2.5 [1.9-3.4] when 2 different clinical disciplines were involved and 2.4 [2.1-2.9] for 3 or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see TABLE W2.)
For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at motivational interviewing.org/.
In counseling patients considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (TABLE 2)4,6,7 or methods used for prior quit attempts and reasons for relapse, if appropriate; and patient preference.
Evidence update: What's best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR]=1.60; 95% CI, 1.53-1.68).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form like a lozenge with a long-acting patch was found to be more effective than either one alone (RR=1.34; 95% CI, 1.18-1.51).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR=1.18; 95% CI 0.98-1.41). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts, but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a first-line agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a first-line pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR=2.27; 95% CI, 2.02-2.55).13 It also showed varenicline to be more effective than bupropion SR (RR=1.52; 95% CI, 1.22-1.88), but about as effective as NRT (RR=1.13; 95% CI, 0.94-1.35).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR=2.7; 95% CI, 2.1-3.5).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the US Food and Drug Administration (FDA) required that black box warnings be added to the labels of both varenicline and bupropion SR based on post-marketing safety reports showing the risk of neuropsychiatric symptoms, including suicidality.15 In 2011, a large case control study by the FDA Adverse Event Reporting System also showed an increased risk of suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group vs 19% for those in the placebo group (OR=6.2; 95% CI, 2.2-19.2). Although no increased risk of adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: a meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and 2 subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline's safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a first-line treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR=1.62; 95% CI, 1.49-1.76). No increased risk of serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline, (RR=0.68; 95% CI, 0.56-0.83). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30; 95% CI, 0.93-1.82) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a first-line agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a first-line agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation vs placebo (RR=1.63; 95% CI, 1.22-2.18),7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The 6-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (OR, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks vs 47% for varenicline alone (number needed to treat [NNT]=6; 95% CI, 4-11).24
In heavy smokers—defined as those who smoke ≥20 cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT= 9; 95% CI, 4.6-71.6), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT=6).26 Consider using these combinations in patients who have high nicotine dependency levels or have been unable to quit using a single first-line agent.
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 “Vaping,” as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of 2 RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at 6 months in users of e-cigarettes containing nicotine compared with placebo e-cigarettes (9% vs 4%; RR=2.29; 95% CI, 1.05-4.96). Additionally, e-cigarette use was associated with >50% decrease in cigarette smoking vs placebo (36% vs 27%; RR=1.31; 95% CI, 1.02-1.68) or patch (61% vs 44%; RR=1.41; 95% CI, 1.20-1.67).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at 8-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, light-headedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of 4 trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation, as well. A 2013 Cochrane review of 8 trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR=1.00; 95% CI, 0.66-1.51) or as an adjunct to NRT compared with NRT alone (RR=0.95; 95% CI, 0.70-1.30).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the United States.36
A 2012 Cochrane review identified 2 recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates vs placebo (RR=3.98; 95% CI, 2.01-7.87).13
CORRESPONDENCE
Paul Bornemann, MD, 3209 Colonial Drive, Columbia, SC 29203; paul.bornemann@uscmed.sc.edu.
ACKNOWLEDGEMENT
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
› Prescribe varenicline, bupropion, or nicotine replacement as first-line single pharmacotherapy for smoking cessation. A
› Provide counseling along with medication, as the combination has proven to be more effective than either option alone. A
› Refer patients to their state Quit Line—a toll-free tobacco cessation coaching service that has been shown to be an effective form of counseling. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In its 2014 report, “The Health Consequences of Smoking—50 Years of Progress,”1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risks of such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 years cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family physicians with an evidence-based update.
The guideline: Treating tobacco use and dependence
Prescribing a first-line medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (See TABLE W1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (TABLE 1).4
Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratio [OR]=1.7 [1.4-2.0], 1.3 [1.1-1.6], and 1.2 [1.1-1.4], respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR=2.5 [1.9-3.4] when 2 different clinical disciplines were involved and 2.4 [2.1-2.9] for 3 or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see TABLE W2.)
For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at motivational interviewing.org/.
In counseling patients considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (TABLE 2)4,6,7 or methods used for prior quit attempts and reasons for relapse, if appropriate; and patient preference.
Evidence update: What's best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR]=1.60; 95% CI, 1.53-1.68).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form like a lozenge with a long-acting patch was found to be more effective than either one alone (RR=1.34; 95% CI, 1.18-1.51).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR=1.18; 95% CI 0.98-1.41). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts, but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a first-line agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a first-line pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR=2.27; 95% CI, 2.02-2.55).13 It also showed varenicline to be more effective than bupropion SR (RR=1.52; 95% CI, 1.22-1.88), but about as effective as NRT (RR=1.13; 95% CI, 0.94-1.35).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR=2.7; 95% CI, 2.1-3.5).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the US Food and Drug Administration (FDA) required that black box warnings be added to the labels of both varenicline and bupropion SR based on post-marketing safety reports showing the risk of neuropsychiatric symptoms, including suicidality.15 In 2011, a large case control study by the FDA Adverse Event Reporting System also showed an increased risk of suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group vs 19% for those in the placebo group (OR=6.2; 95% CI, 2.2-19.2). Although no increased risk of adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: a meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and 2 subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline's safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a first-line treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR=1.62; 95% CI, 1.49-1.76). No increased risk of serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline, (RR=0.68; 95% CI, 0.56-0.83). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30; 95% CI, 0.93-1.82) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a first-line agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a first-line agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation vs placebo (RR=1.63; 95% CI, 1.22-2.18),7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The 6-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (OR, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks vs 47% for varenicline alone (number needed to treat [NNT]=6; 95% CI, 4-11).24
In heavy smokers—defined as those who smoke ≥20 cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT= 9; 95% CI, 4.6-71.6), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT=6).26 Consider using these combinations in patients who have high nicotine dependency levels or have been unable to quit using a single first-line agent.
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 “Vaping,” as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of 2 RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at 6 months in users of e-cigarettes containing nicotine compared with placebo e-cigarettes (9% vs 4%; RR=2.29; 95% CI, 1.05-4.96). Additionally, e-cigarette use was associated with >50% decrease in cigarette smoking vs placebo (36% vs 27%; RR=1.31; 95% CI, 1.02-1.68) or patch (61% vs 44%; RR=1.41; 95% CI, 1.20-1.67).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at 8-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, light-headedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of 4 trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation, as well. A 2013 Cochrane review of 8 trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR=1.00; 95% CI, 0.66-1.51) or as an adjunct to NRT compared with NRT alone (RR=0.95; 95% CI, 0.70-1.30).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the United States.36
A 2012 Cochrane review identified 2 recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates vs placebo (RR=3.98; 95% CI, 2.01-7.87).13
CORRESPONDENCE
Paul Bornemann, MD, 3209 Colonial Drive, Columbia, SC 29203; paul.bornemann@uscmed.sc.edu.
ACKNOWLEDGEMENT
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed October 21, 2015.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service Report. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: Counseling and interventions. April 2009. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed October 21, 2015.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. US Food and Drug Administration. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: A randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla. man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. Available at: http:// www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed December 2, 2015.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. Available at: http://www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed August 3, 2015.
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed October 21, 2015.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service Report. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: Counseling and interventions. April 2009. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed October 21, 2015.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. US Food and Drug Administration. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: A randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla. man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. Available at: http:// www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed December 2, 2015.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. Available at: http://www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed August 3, 2015.