User login
A Veteran Presenting for Low Testosterone and Lower Urinary Tract Symptoms
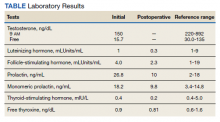
►Anish Bhatnagar, MD, Chief Medical Resident, Veterans Affairs Boston Healthcare System (VABHS) and Beth Israel Deaconess Medical Center (BIDMC): The patient noted erectile dysfunction starting 4 years ago, with accompanied decreased libido. However, until recently, he was able to achieve acceptable erectile capacity with medications. As part of his previous evaluations for erectile dysfunction, the patient had 2 total testosterone levels checked 6 months apart, both low at 150 ng/dL and 38.3 ng/dL (reference range, 220-892). The results of additional hormone studies are shown in the Table. Dr. Ananthakrishnan, can you help us interpret these laboratory results and tell us what tests you might order next?
►Sonia Ananthakrishnan, MD, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center (BMC) and Assistant Professor of Medicine, Boston University School of Medicine (BUSM): When patients present with signs of hypogonadism and an initial low morning testosterone levels, the next test should be a confirmatory repeat morning testosterone level as was done in this case. If this level is also low (for most assays < 300 ng/dL), further evaluation for primary vs secondary hypogonadism should be pursued with measurement of luteinizing hormone and follicle-stimulating hormone levels. Secondary hypogonadism should be suspected when these levels are low or inappropriately normal in the setting of a low testosterone level as in this patient. This patient does not appear to be on any medication or have reversible illnesses that we traditionally think of as possibly causing these hormone irregularities. Key examples include medications such as gonadotropin-releasing hormone analogs, glucocorticoids, and opioids, as well as conditions such as hyperprolactinemia, sleep apnea, diabetes mellitus, anorexia nervosa, or other chronic systemic illnesses, including cirrhosis or lung disease. In this setting, further evaluation of the patient’s anterior pituitary function should be undertaken. Initial screening tests showed mildly elevated prolactin and low normal thyroid-stimulating hormone levels, with a relatively normal free thyroxine. Given these abnormalities in the context of the patient’s total testosterone level < 150 ng/dL, magnetic resonance imaging (MRI) of the anterior pituitary is indicated, and what I would recommend next for evaluation of pituitary and/or hypothalamic tumor or infiltrative disease.1
►Dr. Bhatnagar: An MRI of the brain showed a large 2.7-cm sellar mass, with suprasellar extension and mass effect on the optic chiasm and pituitary infundibulum, partial extension into the right sphenoid sinus, and invasion into the right cavernous sinus. These findings were consistent with a pituitary macroadenoma. The patient was subsequently evaluated by a neurosurgeon who felt that because of the extension and compression of the mass, the patient would benefit from surgical resection.
Given his lower urinary tract symptoms, a prostate-specific antigen level was checked and returned elevated at 11.5 ng/mL. In the setting of these abnormalities, the patient underwent MRI of the abdomen, which noted a new 5.6-cm enhancing mass in the upper pole of his solitary right kidney, highly concerning for new RCC. After a multidisciplinary discussion, urology scheduled the patient for partial right nephrectomy first, with plans for pituitary resection only if the patient had adequate recovery following the urologic procedure.
Dr. Rifkin, this patient went straight from imaging to presumed diagnosis to planned surgical intervention without a confirmatory biopsy. In a patient who already has chronic kidney disease stage 4, why would we not want to pursue biopsy prior to this invasive procedure on his solitary kidney? In addition, given his baseline advanced renal disease, why pursue partial nephrectomy to delay initiation of hemodialysis instead of total nephrectomy and beginning hemodialysis?
►Ian Rifkin, MBBCh, PhD, MSc, Chief, Renal Section, VABHS, Section of Nephrology, BMC, and Associate Professor of Medicine, BUSM: In most cases, imaging alone is used to make a presumptive diagnosis of benign vs malignant renal masses. In one study, RCC was identified by MRI with 85% sensitivity and 76% specificity.2 However, as imaging and biopsy techniques have advanced, there are progressing discussions regarding the utility of biopsy. That being said, there are a number of situations in which patients currently undergo biopsy, particularly when there is diagnostic uncertainty.3 In this patient, with a history of RCC and imaging findings concerning for RCC, biopsy is unnecessary given the high clinical suspicion.
Regarding the choice of partial vs total nephrectomy, there are 2 important distinctions to be made. The first is that though it was previously felt that early initiation of dialysis improves survival, newer studies suggest that early initiation based off of glomerular filtration rate (GFR) offers no survival benefits compared to delayed initiation.4 Second, though there is less clinical data to support this, there is a signal toward the use of partial nephrectomy decreasing mortality compared to radical nephrectomy in management of RCC.5 In this patient, partial nephrectomy may not only increase rates of survival, but also delay initiation of dialysis.
►Dr. Bhatnagar: Prior to undergoing partial right nephrectomy, a morning cortisol level was found to be 5.8 μg/dL with an associated corticotropin (ACTH) level of 26 pg/mL. Dr. Ananthakrishnan, how would you interpret these laboratory results and what might you recommend prior to surgery?
►Dr. Ananthakrishnan: In a healthy patient, surgery often results in a several-fold increase in the secretion of cortisol to balance the unique stressors surgery places on the body.6 This patient is at increased risk for complete or partial adrenal insufficiency in the setting of both his pituitary macroadenoma as well as his previous left nephrectomy, which could have affected his left adrenal gland as well. Thus, this patient may not be able to mount the appropriate cortisol response needed to counter the stresses of surgery. His cortisol level is abnormally low for a morning value, with a relatively normal ACTH reference range of 6 to 50 pg/mL. He may have some degree of adrenal insufficiency, and thus will benefit from perioperative steroids.
►Dr. Bhatnagar: The patient was started on hydrocortisone and underwent a successful laparoscopic partial right nephrectomy. During the procedure, an estimated 2.5 L of blood was lost, with transfusion of 3 units of packed red blood cells. A surgical drain was left in the peritoneum. Postoperatively, he developed hypotension, requiring vasopressors and prolonged continuation of stress dosing of hydrocortisone. Over the next 4 days, the patient was weaned off vasopressors, and his creatinine level was noted to increase from a baseline of 1.8 mg/dL to 4.4 mg/dL.
Dr. Rifkin, how do you think about renal recovery in the patient postnephrectomy, and should we be concerned with the dramatic rise in his creatinine level?
►Dr. Rifkin: Removal of renal mass will result in an initial reduction of GFR proportional to the amount of functional renal tissue removed. However, in as early as 1 week, the residual nephrons begin to compensate through various mechanisms, such as modulation of efferent and afferent arterioles and renal tissue growth by hypertrophy and hyperplasia.7 In the acute setting, it may be difficult to distinguish an acute renal injury vs physiological GFR reduction postnephron loss, but often the initially elevated creatinine level may normalize/stabilize over time. Other markers of kidney function should concomitantly be monitored, including urine output, electrolyte/acid-base status, and urine sediment examination. In this patient, although his creatinine level may be elevated over the first few days, if his urine output remains robust and the urine sediment examination is normal, my concern for permanent kidney injury would be lessened.
►Dr. Bhatnagar: During the first 4 postoperative days the patient produced approximately 1 L of urine per day with a stable creatinine level. It is over this same time that the hydrocortisone was discontinued given improving hemodynamics. However, throughout postoperative day 5, the patient’s creatinine level acutely rose to a peak of 5.8 mg/dL. In addition, his urine output dramatically dropped to < 5 mL per hour, with blood clots noted in his Foley catheter. Dr. Rifkin, what is your differential for causing this acute change in both his creatinine level and urine output this far out from his procedure, and what might you do to help further evaluate?
►Dr. Rifkin: The most common cause of acute kidney injury in hospitalized patients is acute tubular necrosis (ATN).8 However, in this patient, who was recovering well postoperatively, was hemodynamically stable with a robust urine output, and in whom no apparent cause for ATN could be identified, other diagnoses were more likely. Considering the abrupt onset of oligo-anuria, the most likely diagnosis was urinary tract obstruction, particularly given the frank blood and blood clots that were present in the urine. Additional possibilities might be a late surgical complication or infection. Surgical complications could range from direct damage to the renal parenchyma to urinary leakage into the peritoneum from the site of anastomosis or tissue injury. Infections introduced either intraoperatively or developed postoperatively could also cause this sudden drop in urine output, though one would expect more systemic symptoms with this. Given that this patient has a surgical drain in place in the peritoneum, I would recommend testing the creatinine level in the peritoneal fluid drainage. If it is comparable to serum levels, this would argue against a urine leak, as we would expect the level to be significantly elevated in a leak. In addition, he should have imaging of the urinary tract followed by procedures to decompress the presumed obstructed urinary tract. These procedures might include either cystoscopy with ureteral stent placement or percutaneous nephrostomy, depending on the result of the imaging.
►Dr. Bhatnagar: The creatinine level obtained from the surgical drain was roughly equivalent to the serum creatinine, decreasing suspicion for a urine leak as the cause of his findings. Cystoscopy with ureteral stent placement was performed with subsequent increase in both urine output and concomitant decrease in serum creatinine.
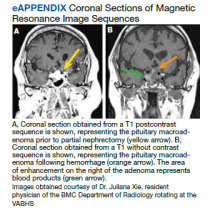
Around this time, the patient also began to note blurry vision. Evaluation revealed difficulty with visual field confrontation in the right lower quadrant, right eye ptosis, right eye impaired adduction, absent abduction and impaired upgaze, but intact downgaze. Diplopia was present with gaze in all directions. His constellation of physical examination findings were concerning for a pathologic lesion partially involving cranial nerves II and III, with definitive involvement of cranial nerve VI, but sparing of cranial nerve IV. Repeat MRI of the brain showed hemorrhage into the sellar mass, with ongoing mass effect on the optic chiasm and extension into the sinuses (eAppendix). These findings were consistent with pituitary apoplexy. Dr. Ananthakrishnan, can you tell us more about pituitary apoplexy?
►Dr. Ananthakrishnan: Pituitary apoplexy is a clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland. It typically occurs in patients with preexisting pituitary adenomas and is characterized by the onset of headache, fever, vomiting, meningismus, decreased consciousness, and sometimes death. In addition, given the location of the pituitary gland within the sella, rapid changes in size can result in compression of cranial nerves III, IV, and VI, as well as the optic chiasm, resulting in ophthalmoplegia and visual disturbances as seen in this patient.9
There are a multitude of causes of pituitary apoplexy, including alterations in coagulopathy, pituitary stimulation (eg, dynamic pituitary hormone testing), and both acute increases and decreases in blood flow.10 This patient likely had an ischemic event due to changes in vascular perfusion, spurred by both his blood loss intraoperatively and ongoing hematuria. Management of pituitary apoplexy is dependent on the patient’s hemodynamics, mass effect symptoms, electrolyte balances, and hormone dysfunction. The decision for conservative management vs surgical intervention should be made in consultation with both neurosurgery and endocrinology. Once the patient is hemodynamically stable, the next step in evaluating this patient should be repeating his hormone studies.
►Dr. Bhatnagar: An assessment of pituitary function was consistent with values obtained preoperatively. After multidisciplinary discussions, surgery was deferred, and hydrocortisone was reinitiated to reduce inflammation caused by bleeding into the mass. As the ophthalmoplegia improved, this was transitioned to dexamethasone.
Twelve days after admission, he was discharged to a subacute rehabilitation center, with improvement in his ophthalmoplegia and stabilization of his creatinine level and urine output.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543-553. doi:10.1148/radiol.2018171557
3. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44-55. doi:10.1102/1470-7330.2009.0005
4. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. doi:10.1056/NEJMoa1000552
5. Kunath F, Schmidt S, Krabbe L-M, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017;5(5):CD012045. doi:10.1002/14651858.CD012045.pub2
6. Kehlet H, Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043-1048. doi:10.1093/bja/45.10.1043
7. Chapman D, Moore R, Klarenbach S, Braam B. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J. 2010;4(5):337-343. doi:10.5489/cuaj.909
8. Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
9. Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011;15(suppl 3):S188-S196. doi:10.4103/2230-8210.84862
10. Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015;59(3):259-264. doi:10.1590/2359-3997000000047
►Anish Bhatnagar, MD, Chief Medical Resident, Veterans Affairs Boston Healthcare System (VABHS) and Beth Israel Deaconess Medical Center (BIDMC): The patient noted erectile dysfunction starting 4 years ago, with accompanied decreased libido. However, until recently, he was able to achieve acceptable erectile capacity with medications. As part of his previous evaluations for erectile dysfunction, the patient had 2 total testosterone levels checked 6 months apart, both low at 150 ng/dL and 38.3 ng/dL (reference range, 220-892). The results of additional hormone studies are shown in the Table. Dr. Ananthakrishnan, can you help us interpret these laboratory results and tell us what tests you might order next?
►Sonia Ananthakrishnan, MD, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center (BMC) and Assistant Professor of Medicine, Boston University School of Medicine (BUSM): When patients present with signs of hypogonadism and an initial low morning testosterone levels, the next test should be a confirmatory repeat morning testosterone level as was done in this case. If this level is also low (for most assays < 300 ng/dL), further evaluation for primary vs secondary hypogonadism should be pursued with measurement of luteinizing hormone and follicle-stimulating hormone levels. Secondary hypogonadism should be suspected when these levels are low or inappropriately normal in the setting of a low testosterone level as in this patient. This patient does not appear to be on any medication or have reversible illnesses that we traditionally think of as possibly causing these hormone irregularities. Key examples include medications such as gonadotropin-releasing hormone analogs, glucocorticoids, and opioids, as well as conditions such as hyperprolactinemia, sleep apnea, diabetes mellitus, anorexia nervosa, or other chronic systemic illnesses, including cirrhosis or lung disease. In this setting, further evaluation of the patient’s anterior pituitary function should be undertaken. Initial screening tests showed mildly elevated prolactin and low normal thyroid-stimulating hormone levels, with a relatively normal free thyroxine. Given these abnormalities in the context of the patient’s total testosterone level < 150 ng/dL, magnetic resonance imaging (MRI) of the anterior pituitary is indicated, and what I would recommend next for evaluation of pituitary and/or hypothalamic tumor or infiltrative disease.1
►Dr. Bhatnagar: An MRI of the brain showed a large 2.7-cm sellar mass, with suprasellar extension and mass effect on the optic chiasm and pituitary infundibulum, partial extension into the right sphenoid sinus, and invasion into the right cavernous sinus. These findings were consistent with a pituitary macroadenoma. The patient was subsequently evaluated by a neurosurgeon who felt that because of the extension and compression of the mass, the patient would benefit from surgical resection.
Given his lower urinary tract symptoms, a prostate-specific antigen level was checked and returned elevated at 11.5 ng/mL. In the setting of these abnormalities, the patient underwent MRI of the abdomen, which noted a new 5.6-cm enhancing mass in the upper pole of his solitary right kidney, highly concerning for new RCC. After a multidisciplinary discussion, urology scheduled the patient for partial right nephrectomy first, with plans for pituitary resection only if the patient had adequate recovery following the urologic procedure.
Dr. Rifkin, this patient went straight from imaging to presumed diagnosis to planned surgical intervention without a confirmatory biopsy. In a patient who already has chronic kidney disease stage 4, why would we not want to pursue biopsy prior to this invasive procedure on his solitary kidney? In addition, given his baseline advanced renal disease, why pursue partial nephrectomy to delay initiation of hemodialysis instead of total nephrectomy and beginning hemodialysis?
►Ian Rifkin, MBBCh, PhD, MSc, Chief, Renal Section, VABHS, Section of Nephrology, BMC, and Associate Professor of Medicine, BUSM: In most cases, imaging alone is used to make a presumptive diagnosis of benign vs malignant renal masses. In one study, RCC was identified by MRI with 85% sensitivity and 76% specificity.2 However, as imaging and biopsy techniques have advanced, there are progressing discussions regarding the utility of biopsy. That being said, there are a number of situations in which patients currently undergo biopsy, particularly when there is diagnostic uncertainty.3 In this patient, with a history of RCC and imaging findings concerning for RCC, biopsy is unnecessary given the high clinical suspicion.
Regarding the choice of partial vs total nephrectomy, there are 2 important distinctions to be made. The first is that though it was previously felt that early initiation of dialysis improves survival, newer studies suggest that early initiation based off of glomerular filtration rate (GFR) offers no survival benefits compared to delayed initiation.4 Second, though there is less clinical data to support this, there is a signal toward the use of partial nephrectomy decreasing mortality compared to radical nephrectomy in management of RCC.5 In this patient, partial nephrectomy may not only increase rates of survival, but also delay initiation of dialysis.
►Dr. Bhatnagar: Prior to undergoing partial right nephrectomy, a morning cortisol level was found to be 5.8 μg/dL with an associated corticotropin (ACTH) level of 26 pg/mL. Dr. Ananthakrishnan, how would you interpret these laboratory results and what might you recommend prior to surgery?
►Dr. Ananthakrishnan: In a healthy patient, surgery often results in a several-fold increase in the secretion of cortisol to balance the unique stressors surgery places on the body.6 This patient is at increased risk for complete or partial adrenal insufficiency in the setting of both his pituitary macroadenoma as well as his previous left nephrectomy, which could have affected his left adrenal gland as well. Thus, this patient may not be able to mount the appropriate cortisol response needed to counter the stresses of surgery. His cortisol level is abnormally low for a morning value, with a relatively normal ACTH reference range of 6 to 50 pg/mL. He may have some degree of adrenal insufficiency, and thus will benefit from perioperative steroids.
►Dr. Bhatnagar: The patient was started on hydrocortisone and underwent a successful laparoscopic partial right nephrectomy. During the procedure, an estimated 2.5 L of blood was lost, with transfusion of 3 units of packed red blood cells. A surgical drain was left in the peritoneum. Postoperatively, he developed hypotension, requiring vasopressors and prolonged continuation of stress dosing of hydrocortisone. Over the next 4 days, the patient was weaned off vasopressors, and his creatinine level was noted to increase from a baseline of 1.8 mg/dL to 4.4 mg/dL.
Dr. Rifkin, how do you think about renal recovery in the patient postnephrectomy, and should we be concerned with the dramatic rise in his creatinine level?
►Dr. Rifkin: Removal of renal mass will result in an initial reduction of GFR proportional to the amount of functional renal tissue removed. However, in as early as 1 week, the residual nephrons begin to compensate through various mechanisms, such as modulation of efferent and afferent arterioles and renal tissue growth by hypertrophy and hyperplasia.7 In the acute setting, it may be difficult to distinguish an acute renal injury vs physiological GFR reduction postnephron loss, but often the initially elevated creatinine level may normalize/stabilize over time. Other markers of kidney function should concomitantly be monitored, including urine output, electrolyte/acid-base status, and urine sediment examination. In this patient, although his creatinine level may be elevated over the first few days, if his urine output remains robust and the urine sediment examination is normal, my concern for permanent kidney injury would be lessened.
►Dr. Bhatnagar: During the first 4 postoperative days the patient produced approximately 1 L of urine per day with a stable creatinine level. It is over this same time that the hydrocortisone was discontinued given improving hemodynamics. However, throughout postoperative day 5, the patient’s creatinine level acutely rose to a peak of 5.8 mg/dL. In addition, his urine output dramatically dropped to < 5 mL per hour, with blood clots noted in his Foley catheter. Dr. Rifkin, what is your differential for causing this acute change in both his creatinine level and urine output this far out from his procedure, and what might you do to help further evaluate?
►Dr. Rifkin: The most common cause of acute kidney injury in hospitalized patients is acute tubular necrosis (ATN).8 However, in this patient, who was recovering well postoperatively, was hemodynamically stable with a robust urine output, and in whom no apparent cause for ATN could be identified, other diagnoses were more likely. Considering the abrupt onset of oligo-anuria, the most likely diagnosis was urinary tract obstruction, particularly given the frank blood and blood clots that were present in the urine. Additional possibilities might be a late surgical complication or infection. Surgical complications could range from direct damage to the renal parenchyma to urinary leakage into the peritoneum from the site of anastomosis or tissue injury. Infections introduced either intraoperatively or developed postoperatively could also cause this sudden drop in urine output, though one would expect more systemic symptoms with this. Given that this patient has a surgical drain in place in the peritoneum, I would recommend testing the creatinine level in the peritoneal fluid drainage. If it is comparable to serum levels, this would argue against a urine leak, as we would expect the level to be significantly elevated in a leak. In addition, he should have imaging of the urinary tract followed by procedures to decompress the presumed obstructed urinary tract. These procedures might include either cystoscopy with ureteral stent placement or percutaneous nephrostomy, depending on the result of the imaging.
►Dr. Bhatnagar: The creatinine level obtained from the surgical drain was roughly equivalent to the serum creatinine, decreasing suspicion for a urine leak as the cause of his findings. Cystoscopy with ureteral stent placement was performed with subsequent increase in both urine output and concomitant decrease in serum creatinine.
Around this time, the patient also began to note blurry vision. Evaluation revealed difficulty with visual field confrontation in the right lower quadrant, right eye ptosis, right eye impaired adduction, absent abduction and impaired upgaze, but intact downgaze. Diplopia was present with gaze in all directions. His constellation of physical examination findings were concerning for a pathologic lesion partially involving cranial nerves II and III, with definitive involvement of cranial nerve VI, but sparing of cranial nerve IV. Repeat MRI of the brain showed hemorrhage into the sellar mass, with ongoing mass effect on the optic chiasm and extension into the sinuses (eAppendix). These findings were consistent with pituitary apoplexy. Dr. Ananthakrishnan, can you tell us more about pituitary apoplexy?
►Dr. Ananthakrishnan: Pituitary apoplexy is a clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland. It typically occurs in patients with preexisting pituitary adenomas and is characterized by the onset of headache, fever, vomiting, meningismus, decreased consciousness, and sometimes death. In addition, given the location of the pituitary gland within the sella, rapid changes in size can result in compression of cranial nerves III, IV, and VI, as well as the optic chiasm, resulting in ophthalmoplegia and visual disturbances as seen in this patient.9
There are a multitude of causes of pituitary apoplexy, including alterations in coagulopathy, pituitary stimulation (eg, dynamic pituitary hormone testing), and both acute increases and decreases in blood flow.10 This patient likely had an ischemic event due to changes in vascular perfusion, spurred by both his blood loss intraoperatively and ongoing hematuria. Management of pituitary apoplexy is dependent on the patient’s hemodynamics, mass effect symptoms, electrolyte balances, and hormone dysfunction. The decision for conservative management vs surgical intervention should be made in consultation with both neurosurgery and endocrinology. Once the patient is hemodynamically stable, the next step in evaluating this patient should be repeating his hormone studies.
►Dr. Bhatnagar: An assessment of pituitary function was consistent with values obtained preoperatively. After multidisciplinary discussions, surgery was deferred, and hydrocortisone was reinitiated to reduce inflammation caused by bleeding into the mass. As the ophthalmoplegia improved, this was transitioned to dexamethasone.
Twelve days after admission, he was discharged to a subacute rehabilitation center, with improvement in his ophthalmoplegia and stabilization of his creatinine level and urine output.
►Anish Bhatnagar, MD, Chief Medical Resident, Veterans Affairs Boston Healthcare System (VABHS) and Beth Israel Deaconess Medical Center (BIDMC): The patient noted erectile dysfunction starting 4 years ago, with accompanied decreased libido. However, until recently, he was able to achieve acceptable erectile capacity with medications. As part of his previous evaluations for erectile dysfunction, the patient had 2 total testosterone levels checked 6 months apart, both low at 150 ng/dL and 38.3 ng/dL (reference range, 220-892). The results of additional hormone studies are shown in the Table. Dr. Ananthakrishnan, can you help us interpret these laboratory results and tell us what tests you might order next?
►Sonia Ananthakrishnan, MD, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center (BMC) and Assistant Professor of Medicine, Boston University School of Medicine (BUSM): When patients present with signs of hypogonadism and an initial low morning testosterone levels, the next test should be a confirmatory repeat morning testosterone level as was done in this case. If this level is also low (for most assays < 300 ng/dL), further evaluation for primary vs secondary hypogonadism should be pursued with measurement of luteinizing hormone and follicle-stimulating hormone levels. Secondary hypogonadism should be suspected when these levels are low or inappropriately normal in the setting of a low testosterone level as in this patient. This patient does not appear to be on any medication or have reversible illnesses that we traditionally think of as possibly causing these hormone irregularities. Key examples include medications such as gonadotropin-releasing hormone analogs, glucocorticoids, and opioids, as well as conditions such as hyperprolactinemia, sleep apnea, diabetes mellitus, anorexia nervosa, or other chronic systemic illnesses, including cirrhosis or lung disease. In this setting, further evaluation of the patient’s anterior pituitary function should be undertaken. Initial screening tests showed mildly elevated prolactin and low normal thyroid-stimulating hormone levels, with a relatively normal free thyroxine. Given these abnormalities in the context of the patient’s total testosterone level < 150 ng/dL, magnetic resonance imaging (MRI) of the anterior pituitary is indicated, and what I would recommend next for evaluation of pituitary and/or hypothalamic tumor or infiltrative disease.1
►Dr. Bhatnagar: An MRI of the brain showed a large 2.7-cm sellar mass, with suprasellar extension and mass effect on the optic chiasm and pituitary infundibulum, partial extension into the right sphenoid sinus, and invasion into the right cavernous sinus. These findings were consistent with a pituitary macroadenoma. The patient was subsequently evaluated by a neurosurgeon who felt that because of the extension and compression of the mass, the patient would benefit from surgical resection.
Given his lower urinary tract symptoms, a prostate-specific antigen level was checked and returned elevated at 11.5 ng/mL. In the setting of these abnormalities, the patient underwent MRI of the abdomen, which noted a new 5.6-cm enhancing mass in the upper pole of his solitary right kidney, highly concerning for new RCC. After a multidisciplinary discussion, urology scheduled the patient for partial right nephrectomy first, with plans for pituitary resection only if the patient had adequate recovery following the urologic procedure.
Dr. Rifkin, this patient went straight from imaging to presumed diagnosis to planned surgical intervention without a confirmatory biopsy. In a patient who already has chronic kidney disease stage 4, why would we not want to pursue biopsy prior to this invasive procedure on his solitary kidney? In addition, given his baseline advanced renal disease, why pursue partial nephrectomy to delay initiation of hemodialysis instead of total nephrectomy and beginning hemodialysis?
►Ian Rifkin, MBBCh, PhD, MSc, Chief, Renal Section, VABHS, Section of Nephrology, BMC, and Associate Professor of Medicine, BUSM: In most cases, imaging alone is used to make a presumptive diagnosis of benign vs malignant renal masses. In one study, RCC was identified by MRI with 85% sensitivity and 76% specificity.2 However, as imaging and biopsy techniques have advanced, there are progressing discussions regarding the utility of biopsy. That being said, there are a number of situations in which patients currently undergo biopsy, particularly when there is diagnostic uncertainty.3 In this patient, with a history of RCC and imaging findings concerning for RCC, biopsy is unnecessary given the high clinical suspicion.
Regarding the choice of partial vs total nephrectomy, there are 2 important distinctions to be made. The first is that though it was previously felt that early initiation of dialysis improves survival, newer studies suggest that early initiation based off of glomerular filtration rate (GFR) offers no survival benefits compared to delayed initiation.4 Second, though there is less clinical data to support this, there is a signal toward the use of partial nephrectomy decreasing mortality compared to radical nephrectomy in management of RCC.5 In this patient, partial nephrectomy may not only increase rates of survival, but also delay initiation of dialysis.
►Dr. Bhatnagar: Prior to undergoing partial right nephrectomy, a morning cortisol level was found to be 5.8 μg/dL with an associated corticotropin (ACTH) level of 26 pg/mL. Dr. Ananthakrishnan, how would you interpret these laboratory results and what might you recommend prior to surgery?
►Dr. Ananthakrishnan: In a healthy patient, surgery often results in a several-fold increase in the secretion of cortisol to balance the unique stressors surgery places on the body.6 This patient is at increased risk for complete or partial adrenal insufficiency in the setting of both his pituitary macroadenoma as well as his previous left nephrectomy, which could have affected his left adrenal gland as well. Thus, this patient may not be able to mount the appropriate cortisol response needed to counter the stresses of surgery. His cortisol level is abnormally low for a morning value, with a relatively normal ACTH reference range of 6 to 50 pg/mL. He may have some degree of adrenal insufficiency, and thus will benefit from perioperative steroids.
►Dr. Bhatnagar: The patient was started on hydrocortisone and underwent a successful laparoscopic partial right nephrectomy. During the procedure, an estimated 2.5 L of blood was lost, with transfusion of 3 units of packed red blood cells. A surgical drain was left in the peritoneum. Postoperatively, he developed hypotension, requiring vasopressors and prolonged continuation of stress dosing of hydrocortisone. Over the next 4 days, the patient was weaned off vasopressors, and his creatinine level was noted to increase from a baseline of 1.8 mg/dL to 4.4 mg/dL.
Dr. Rifkin, how do you think about renal recovery in the patient postnephrectomy, and should we be concerned with the dramatic rise in his creatinine level?
►Dr. Rifkin: Removal of renal mass will result in an initial reduction of GFR proportional to the amount of functional renal tissue removed. However, in as early as 1 week, the residual nephrons begin to compensate through various mechanisms, such as modulation of efferent and afferent arterioles and renal tissue growth by hypertrophy and hyperplasia.7 In the acute setting, it may be difficult to distinguish an acute renal injury vs physiological GFR reduction postnephron loss, but often the initially elevated creatinine level may normalize/stabilize over time. Other markers of kidney function should concomitantly be monitored, including urine output, electrolyte/acid-base status, and urine sediment examination. In this patient, although his creatinine level may be elevated over the first few days, if his urine output remains robust and the urine sediment examination is normal, my concern for permanent kidney injury would be lessened.
►Dr. Bhatnagar: During the first 4 postoperative days the patient produced approximately 1 L of urine per day with a stable creatinine level. It is over this same time that the hydrocortisone was discontinued given improving hemodynamics. However, throughout postoperative day 5, the patient’s creatinine level acutely rose to a peak of 5.8 mg/dL. In addition, his urine output dramatically dropped to < 5 mL per hour, with blood clots noted in his Foley catheter. Dr. Rifkin, what is your differential for causing this acute change in both his creatinine level and urine output this far out from his procedure, and what might you do to help further evaluate?
►Dr. Rifkin: The most common cause of acute kidney injury in hospitalized patients is acute tubular necrosis (ATN).8 However, in this patient, who was recovering well postoperatively, was hemodynamically stable with a robust urine output, and in whom no apparent cause for ATN could be identified, other diagnoses were more likely. Considering the abrupt onset of oligo-anuria, the most likely diagnosis was urinary tract obstruction, particularly given the frank blood and blood clots that were present in the urine. Additional possibilities might be a late surgical complication or infection. Surgical complications could range from direct damage to the renal parenchyma to urinary leakage into the peritoneum from the site of anastomosis or tissue injury. Infections introduced either intraoperatively or developed postoperatively could also cause this sudden drop in urine output, though one would expect more systemic symptoms with this. Given that this patient has a surgical drain in place in the peritoneum, I would recommend testing the creatinine level in the peritoneal fluid drainage. If it is comparable to serum levels, this would argue against a urine leak, as we would expect the level to be significantly elevated in a leak. In addition, he should have imaging of the urinary tract followed by procedures to decompress the presumed obstructed urinary tract. These procedures might include either cystoscopy with ureteral stent placement or percutaneous nephrostomy, depending on the result of the imaging.
►Dr. Bhatnagar: The creatinine level obtained from the surgical drain was roughly equivalent to the serum creatinine, decreasing suspicion for a urine leak as the cause of his findings. Cystoscopy with ureteral stent placement was performed with subsequent increase in both urine output and concomitant decrease in serum creatinine.
Around this time, the patient also began to note blurry vision. Evaluation revealed difficulty with visual field confrontation in the right lower quadrant, right eye ptosis, right eye impaired adduction, absent abduction and impaired upgaze, but intact downgaze. Diplopia was present with gaze in all directions. His constellation of physical examination findings were concerning for a pathologic lesion partially involving cranial nerves II and III, with definitive involvement of cranial nerve VI, but sparing of cranial nerve IV. Repeat MRI of the brain showed hemorrhage into the sellar mass, with ongoing mass effect on the optic chiasm and extension into the sinuses (eAppendix). These findings were consistent with pituitary apoplexy. Dr. Ananthakrishnan, can you tell us more about pituitary apoplexy?
►Dr. Ananthakrishnan: Pituitary apoplexy is a clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland. It typically occurs in patients with preexisting pituitary adenomas and is characterized by the onset of headache, fever, vomiting, meningismus, decreased consciousness, and sometimes death. In addition, given the location of the pituitary gland within the sella, rapid changes in size can result in compression of cranial nerves III, IV, and VI, as well as the optic chiasm, resulting in ophthalmoplegia and visual disturbances as seen in this patient.9
There are a multitude of causes of pituitary apoplexy, including alterations in coagulopathy, pituitary stimulation (eg, dynamic pituitary hormone testing), and both acute increases and decreases in blood flow.10 This patient likely had an ischemic event due to changes in vascular perfusion, spurred by both his blood loss intraoperatively and ongoing hematuria. Management of pituitary apoplexy is dependent on the patient’s hemodynamics, mass effect symptoms, electrolyte balances, and hormone dysfunction. The decision for conservative management vs surgical intervention should be made in consultation with both neurosurgery and endocrinology. Once the patient is hemodynamically stable, the next step in evaluating this patient should be repeating his hormone studies.
►Dr. Bhatnagar: An assessment of pituitary function was consistent with values obtained preoperatively. After multidisciplinary discussions, surgery was deferred, and hydrocortisone was reinitiated to reduce inflammation caused by bleeding into the mass. As the ophthalmoplegia improved, this was transitioned to dexamethasone.
Twelve days after admission, he was discharged to a subacute rehabilitation center, with improvement in his ophthalmoplegia and stabilization of his creatinine level and urine output.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543-553. doi:10.1148/radiol.2018171557
3. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44-55. doi:10.1102/1470-7330.2009.0005
4. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. doi:10.1056/NEJMoa1000552
5. Kunath F, Schmidt S, Krabbe L-M, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017;5(5):CD012045. doi:10.1002/14651858.CD012045.pub2
6. Kehlet H, Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043-1048. doi:10.1093/bja/45.10.1043
7. Chapman D, Moore R, Klarenbach S, Braam B. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J. 2010;4(5):337-343. doi:10.5489/cuaj.909
8. Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
9. Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011;15(suppl 3):S188-S196. doi:10.4103/2230-8210.84862
10. Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015;59(3):259-264. doi:10.1590/2359-3997000000047
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543-553. doi:10.1148/radiol.2018171557
3. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44-55. doi:10.1102/1470-7330.2009.0005
4. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. doi:10.1056/NEJMoa1000552
5. Kunath F, Schmidt S, Krabbe L-M, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017;5(5):CD012045. doi:10.1002/14651858.CD012045.pub2
6. Kehlet H, Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043-1048. doi:10.1093/bja/45.10.1043
7. Chapman D, Moore R, Klarenbach S, Braam B. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J. 2010;4(5):337-343. doi:10.5489/cuaj.909
8. Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
9. Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011;15(suppl 3):S188-S196. doi:10.4103/2230-8210.84862
10. Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015;59(3):259-264. doi:10.1590/2359-3997000000047