User login
Are Text Pages an Effective Nudge to Increase Attendance at Internal Medicine Morning Report Conferences? A Cluster Randomized Controlled Trial
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
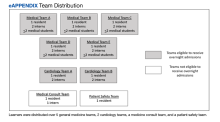
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
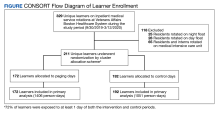
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
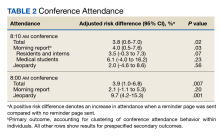
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
A Veteran Presenting for Low Testosterone and Lower Urinary Tract Symptoms
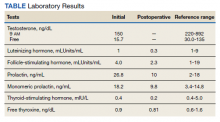
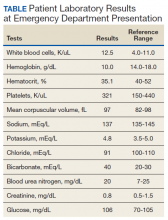
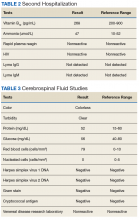
►Anish Bhatnagar, MD, Chief Medical Resident, Veterans Affairs Boston Healthcare System (VABHS) and Beth Israel Deaconess Medical Center (BIDMC): The patient noted erectile dysfunction starting 4 years ago, with accompanied decreased libido. However, until recently, he was able to achieve acceptable erectile capacity with medications. As part of his previous evaluations for erectile dysfunction, the patient had 2 total testosterone levels checked 6 months apart, both low at 150 ng/dL and 38.3 ng/dL (reference range, 220-892). The results of additional hormone studies are shown in the Table. Dr. Ananthakrishnan, can you help us interpret these laboratory results and tell us what tests you might order next?
►Sonia Ananthakrishnan, MD, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center (BMC) and Assistant Professor of Medicine, Boston University School of Medicine (BUSM): When patients present with signs of hypogonadism and an initial low morning testosterone levels, the next test should be a confirmatory repeat morning testosterone level as was done in this case. If this level is also low (for most assays < 300 ng/dL), further evaluation for primary vs secondary hypogonadism should be pursued with measurement of luteinizing hormone and follicle-stimulating hormone levels. Secondary hypogonadism should be suspected when these levels are low or inappropriately normal in the setting of a low testosterone level as in this patient. This patient does not appear to be on any medication or have reversible illnesses that we traditionally think of as possibly causing these hormone irregularities. Key examples include medications such as gonadotropin-releasing hormone analogs, glucocorticoids, and opioids, as well as conditions such as hyperprolactinemia, sleep apnea, diabetes mellitus, anorexia nervosa, or other chronic systemic illnesses, including cirrhosis or lung disease. In this setting, further evaluation of the patient’s anterior pituitary function should be undertaken. Initial screening tests showed mildly elevated prolactin and low normal thyroid-stimulating hormone levels, with a relatively normal free thyroxine. Given these abnormalities in the context of the patient’s total testosterone level < 150 ng/dL, magnetic resonance imaging (MRI) of the anterior pituitary is indicated, and what I would recommend next for evaluation of pituitary and/or hypothalamic tumor or infiltrative disease.1
►Dr. Bhatnagar: An MRI of the brain showed a large 2.7-cm sellar mass, with suprasellar extension and mass effect on the optic chiasm and pituitary infundibulum, partial extension into the right sphenoid sinus, and invasion into the right cavernous sinus. These findings were consistent with a pituitary macroadenoma. The patient was subsequently evaluated by a neurosurgeon who felt that because of the extension and compression of the mass, the patient would benefit from surgical resection.
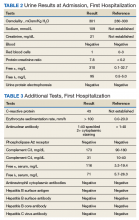
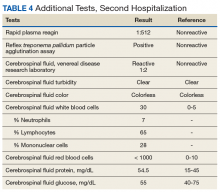
Given his lower urinary tract symptoms, a prostate-specific antigen level was checked and returned elevated at 11.5 ng/mL. In the setting of these abnormalities, the patient underwent MRI of the abdomen, which noted a new 5.6-cm enhancing mass in the upper pole of his solitary right kidney, highly concerning for new RCC. After a multidisciplinary discussion, urology scheduled the patient for partial right nephrectomy first, with plans for pituitary resection only if the patient had adequate recovery following the urologic procedure.
Dr. Rifkin, this patient went straight from imaging to presumed diagnosis to planned surgical intervention without a confirmatory biopsy. In a patient who already has chronic kidney disease stage 4, why would we not want to pursue biopsy prior to this invasive procedure on his solitary kidney? In addition, given his baseline advanced renal disease, why pursue partial nephrectomy to delay initiation of hemodialysis instead of total nephrectomy and beginning hemodialysis?
►Ian Rifkin, MBBCh, PhD, MSc, Chief, Renal Section, VABHS, Section of Nephrology, BMC, and Associate Professor of Medicine, BUSM: In most cases, imaging alone is used to make a presumptive diagnosis of benign vs malignant renal masses. In one study, RCC was identified by MRI with 85% sensitivity and 76% specificity.2 However, as imaging and biopsy techniques have advanced, there are progressing discussions regarding the utility of biopsy. That being said, there are a number of situations in which patients currently undergo biopsy, particularly when there is diagnostic uncertainty.3 In this patient, with a history of RCC and imaging findings concerning for RCC, biopsy is unnecessary given the high clinical suspicion.
Regarding the choice of partial vs total nephrectomy, there are 2 important distinctions to be made. The first is that though it was previously felt that early initiation of dialysis improves survival, newer studies suggest that early initiation based off of glomerular filtration rate (GFR) offers no survival benefits compared to delayed initiation.4 Second, though there is less clinical data to support this, there is a signal toward the use of partial nephrectomy decreasing mortality compared to radical nephrectomy in management of RCC.5 In this patient, partial nephrectomy may not only increase rates of survival, but also delay initiation of dialysis.
►Dr. Bhatnagar: Prior to undergoing partial right nephrectomy, a morning cortisol level was found to be 5.8 μg/dL with an associated corticotropin (ACTH) level of 26 pg/mL. Dr. Ananthakrishnan, how would you interpret these laboratory results and what might you recommend prior to surgery?
►Dr. Ananthakrishnan: In a healthy patient, surgery often results in a several-fold increase in the secretion of cortisol to balance the unique stressors surgery places on the body.6 This patient is at increased risk for complete or partial adrenal insufficiency in the setting of both his pituitary macroadenoma as well as his previous left nephrectomy, which could have affected his left adrenal gland as well. Thus, this patient may not be able to mount the appropriate cortisol response needed to counter the stresses of surgery. His cortisol level is abnormally low for a morning value, with a relatively normal ACTH reference range of 6 to 50 pg/mL. He may have some degree of adrenal insufficiency, and thus will benefit from perioperative steroids.
►Dr. Bhatnagar: The patient was started on hydrocortisone and underwent a successful laparoscopic partial right nephrectomy. During the procedure, an estimated 2.5 L of blood was lost, with transfusion of 3 units of packed red blood cells. A surgical drain was left in the peritoneum. Postoperatively, he developed hypotension, requiring vasopressors and prolonged continuation of stress dosing of hydrocortisone. Over the next 4 days, the patient was weaned off vasopressors, and his creatinine level was noted to increase from a baseline of 1.8 mg/dL to 4.4 mg/dL.
Dr. Rifkin, how do you think about renal recovery in the patient postnephrectomy, and should we be concerned with the dramatic rise in his creatinine level?
►Dr. Rifkin: Removal of renal mass will result in an initial reduction of GFR proportional to the amount of functional renal tissue removed. However, in as early as 1 week, the residual nephrons begin to compensate through various mechanisms, such as modulation of efferent and afferent arterioles and renal tissue growth by hypertrophy and hyperplasia.7 In the acute setting, it may be difficult to distinguish an acute renal injury vs physiological GFR reduction postnephron loss, but often the initially elevated creatinine level may normalize/stabilize over time. Other markers of kidney function should concomitantly be monitored, including urine output, electrolyte/acid-base status, and urine sediment examination. In this patient, although his creatinine level may be elevated over the first few days, if his urine output remains robust and the urine sediment examination is normal, my concern for permanent kidney injury would be lessened.
►Dr. Bhatnagar: During the first 4 postoperative days the patient produced approximately 1 L of urine per day with a stable creatinine level. It is over this same time that the hydrocortisone was discontinued given improving hemodynamics. However, throughout postoperative day 5, the patient’s creatinine level acutely rose to a peak of 5.8 mg/dL. In addition, his urine output dramatically dropped to < 5 mL per hour, with blood clots noted in his Foley catheter. Dr. Rifkin, what is your differential for causing this acute change in both his creatinine level and urine output this far out from his procedure, and what might you do to help further evaluate?
►Dr. Rifkin: The most common cause of acute kidney injury in hospitalized patients is acute tubular necrosis (ATN).8 However, in this patient, who was recovering well postoperatively, was hemodynamically stable with a robust urine output, and in whom no apparent cause for ATN could be identified, other diagnoses were more likely. Considering the abrupt onset of oligo-anuria, the most likely diagnosis was urinary tract obstruction, particularly given the frank blood and blood clots that were present in the urine. Additional possibilities might be a late surgical complication or infection. Surgical complications could range from direct damage to the renal parenchyma to urinary leakage into the peritoneum from the site of anastomosis or tissue injury. Infections introduced either intraoperatively or developed postoperatively could also cause this sudden drop in urine output, though one would expect more systemic symptoms with this. Given that this patient has a surgical drain in place in the peritoneum, I would recommend testing the creatinine level in the peritoneal fluid drainage. If it is comparable to serum levels, this would argue against a urine leak, as we would expect the level to be significantly elevated in a leak. In addition, he should have imaging of the urinary tract followed by procedures to decompress the presumed obstructed urinary tract. These procedures might include either cystoscopy with ureteral stent placement or percutaneous nephrostomy, depending on the result of the imaging.
►Dr. Bhatnagar: The creatinine level obtained from the surgical drain was roughly equivalent to the serum creatinine, decreasing suspicion for a urine leak as the cause of his findings. Cystoscopy with ureteral stent placement was performed with subsequent increase in both urine output and concomitant decrease in serum creatinine.
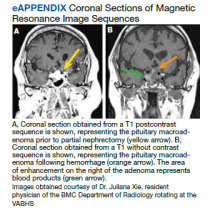
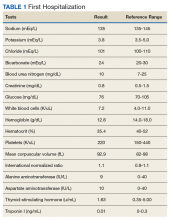
Around this time, the patient also began to note blurry vision. Evaluation revealed difficulty with visual field confrontation in the right lower quadrant, right eye ptosis, right eye impaired adduction, absent abduction and impaired upgaze, but intact downgaze. Diplopia was present with gaze in all directions. His constellation of physical examination findings were concerning for a pathologic lesion partially involving cranial nerves II and III, with definitive involvement of cranial nerve VI, but sparing of cranial nerve IV. Repeat MRI of the brain showed hemorrhage into the sellar mass, with ongoing mass effect on the optic chiasm and extension into the sinuses (eAppendix). These findings were consistent with pituitary apoplexy. Dr. Ananthakrishnan, can you tell us more about pituitary apoplexy?
►Dr. Ananthakrishnan: Pituitary apoplexy is a clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland. It typically occurs in patients with preexisting pituitary adenomas and is characterized by the onset of headache, fever, vomiting, meningismus, decreased consciousness, and sometimes death. In addition, given the location of the pituitary gland within the sella, rapid changes in size can result in compression of cranial nerves III, IV, and VI, as well as the optic chiasm, resulting in ophthalmoplegia and visual disturbances as seen in this patient.9
There are a multitude of causes of pituitary apoplexy, including alterations in coagulopathy, pituitary stimulation (eg, dynamic pituitary hormone testing), and both acute increases and decreases in blood flow.10 This patient likely had an ischemic event due to changes in vascular perfusion, spurred by both his blood loss intraoperatively and ongoing hematuria. Management of pituitary apoplexy is dependent on the patient’s hemodynamics, mass effect symptoms, electrolyte balances, and hormone dysfunction. The decision for conservative management vs surgical intervention should be made in consultation with both neurosurgery and endocrinology. Once the patient is hemodynamically stable, the next step in evaluating this patient should be repeating his hormone studies.
►Dr. Bhatnagar: An assessment of pituitary function was consistent with values obtained preoperatively. After multidisciplinary discussions, surgery was deferred, and hydrocortisone was reinitiated to reduce inflammation caused by bleeding into the mass. As the ophthalmoplegia improved, this was transitioned to dexamethasone.
Twelve days after admission, he was discharged to a subacute rehabilitation center, with improvement in his ophthalmoplegia and stabilization of his creatinine level and urine output.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543-553. doi:10.1148/radiol.2018171557
3. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44-55. doi:10.1102/1470-7330.2009.0005
4. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. doi:10.1056/NEJMoa1000552
5. Kunath F, Schmidt S, Krabbe L-M, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017;5(5):CD012045. doi:10.1002/14651858.CD012045.pub2
6. Kehlet H, Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043-1048. doi:10.1093/bja/45.10.1043
7. Chapman D, Moore R, Klarenbach S, Braam B. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J. 2010;4(5):337-343. doi:10.5489/cuaj.909
8. Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
9. Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011;15(suppl 3):S188-S196. doi:10.4103/2230-8210.84862
10. Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015;59(3):259-264. doi:10.1590/2359-3997000000047
►Anish Bhatnagar, MD, Chief Medical Resident, Veterans Affairs Boston Healthcare System (VABHS) and Beth Israel Deaconess Medical Center (BIDMC): The patient noted erectile dysfunction starting 4 years ago, with accompanied decreased libido. However, until recently, he was able to achieve acceptable erectile capacity with medications. As part of his previous evaluations for erectile dysfunction, the patient had 2 total testosterone levels checked 6 months apart, both low at 150 ng/dL and 38.3 ng/dL (reference range, 220-892). The results of additional hormone studies are shown in the Table. Dr. Ananthakrishnan, can you help us interpret these laboratory results and tell us what tests you might order next?
►Sonia Ananthakrishnan, MD, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center (BMC) and Assistant Professor of Medicine, Boston University School of Medicine (BUSM): When patients present with signs of hypogonadism and an initial low morning testosterone levels, the next test should be a confirmatory repeat morning testosterone level as was done in this case. If this level is also low (for most assays < 300 ng/dL), further evaluation for primary vs secondary hypogonadism should be pursued with measurement of luteinizing hormone and follicle-stimulating hormone levels. Secondary hypogonadism should be suspected when these levels are low or inappropriately normal in the setting of a low testosterone level as in this patient. This patient does not appear to be on any medication or have reversible illnesses that we traditionally think of as possibly causing these hormone irregularities. Key examples include medications such as gonadotropin-releasing hormone analogs, glucocorticoids, and opioids, as well as conditions such as hyperprolactinemia, sleep apnea, diabetes mellitus, anorexia nervosa, or other chronic systemic illnesses, including cirrhosis or lung disease. In this setting, further evaluation of the patient’s anterior pituitary function should be undertaken. Initial screening tests showed mildly elevated prolactin and low normal thyroid-stimulating hormone levels, with a relatively normal free thyroxine. Given these abnormalities in the context of the patient’s total testosterone level < 150 ng/dL, magnetic resonance imaging (MRI) of the anterior pituitary is indicated, and what I would recommend next for evaluation of pituitary and/or hypothalamic tumor or infiltrative disease.1
►Dr. Bhatnagar: An MRI of the brain showed a large 2.7-cm sellar mass, with suprasellar extension and mass effect on the optic chiasm and pituitary infundibulum, partial extension into the right sphenoid sinus, and invasion into the right cavernous sinus. These findings were consistent with a pituitary macroadenoma. The patient was subsequently evaluated by a neurosurgeon who felt that because of the extension and compression of the mass, the patient would benefit from surgical resection.
Given his lower urinary tract symptoms, a prostate-specific antigen level was checked and returned elevated at 11.5 ng/mL. In the setting of these abnormalities, the patient underwent MRI of the abdomen, which noted a new 5.6-cm enhancing mass in the upper pole of his solitary right kidney, highly concerning for new RCC. After a multidisciplinary discussion, urology scheduled the patient for partial right nephrectomy first, with plans for pituitary resection only if the patient had adequate recovery following the urologic procedure.
Dr. Rifkin, this patient went straight from imaging to presumed diagnosis to planned surgical intervention without a confirmatory biopsy. In a patient who already has chronic kidney disease stage 4, why would we not want to pursue biopsy prior to this invasive procedure on his solitary kidney? In addition, given his baseline advanced renal disease, why pursue partial nephrectomy to delay initiation of hemodialysis instead of total nephrectomy and beginning hemodialysis?
►Ian Rifkin, MBBCh, PhD, MSc, Chief, Renal Section, VABHS, Section of Nephrology, BMC, and Associate Professor of Medicine, BUSM: In most cases, imaging alone is used to make a presumptive diagnosis of benign vs malignant renal masses. In one study, RCC was identified by MRI with 85% sensitivity and 76% specificity.2 However, as imaging and biopsy techniques have advanced, there are progressing discussions regarding the utility of biopsy. That being said, there are a number of situations in which patients currently undergo biopsy, particularly when there is diagnostic uncertainty.3 In this patient, with a history of RCC and imaging findings concerning for RCC, biopsy is unnecessary given the high clinical suspicion.
Regarding the choice of partial vs total nephrectomy, there are 2 important distinctions to be made. The first is that though it was previously felt that early initiation of dialysis improves survival, newer studies suggest that early initiation based off of glomerular filtration rate (GFR) offers no survival benefits compared to delayed initiation.4 Second, though there is less clinical data to support this, there is a signal toward the use of partial nephrectomy decreasing mortality compared to radical nephrectomy in management of RCC.5 In this patient, partial nephrectomy may not only increase rates of survival, but also delay initiation of dialysis.
►Dr. Bhatnagar: Prior to undergoing partial right nephrectomy, a morning cortisol level was found to be 5.8 μg/dL with an associated corticotropin (ACTH) level of 26 pg/mL. Dr. Ananthakrishnan, how would you interpret these laboratory results and what might you recommend prior to surgery?
►Dr. Ananthakrishnan: In a healthy patient, surgery often results in a several-fold increase in the secretion of cortisol to balance the unique stressors surgery places on the body.6 This patient is at increased risk for complete or partial adrenal insufficiency in the setting of both his pituitary macroadenoma as well as his previous left nephrectomy, which could have affected his left adrenal gland as well. Thus, this patient may not be able to mount the appropriate cortisol response needed to counter the stresses of surgery. His cortisol level is abnormally low for a morning value, with a relatively normal ACTH reference range of 6 to 50 pg/mL. He may have some degree of adrenal insufficiency, and thus will benefit from perioperative steroids.
►Dr. Bhatnagar: The patient was started on hydrocortisone and underwent a successful laparoscopic partial right nephrectomy. During the procedure, an estimated 2.5 L of blood was lost, with transfusion of 3 units of packed red blood cells. A surgical drain was left in the peritoneum. Postoperatively, he developed hypotension, requiring vasopressors and prolonged continuation of stress dosing of hydrocortisone. Over the next 4 days, the patient was weaned off vasopressors, and his creatinine level was noted to increase from a baseline of 1.8 mg/dL to 4.4 mg/dL.
Dr. Rifkin, how do you think about renal recovery in the patient postnephrectomy, and should we be concerned with the dramatic rise in his creatinine level?
►Dr. Rifkin: Removal of renal mass will result in an initial reduction of GFR proportional to the amount of functional renal tissue removed. However, in as early as 1 week, the residual nephrons begin to compensate through various mechanisms, such as modulation of efferent and afferent arterioles and renal tissue growth by hypertrophy and hyperplasia.7 In the acute setting, it may be difficult to distinguish an acute renal injury vs physiological GFR reduction postnephron loss, but often the initially elevated creatinine level may normalize/stabilize over time. Other markers of kidney function should concomitantly be monitored, including urine output, electrolyte/acid-base status, and urine sediment examination. In this patient, although his creatinine level may be elevated over the first few days, if his urine output remains robust and the urine sediment examination is normal, my concern for permanent kidney injury would be lessened.
►Dr. Bhatnagar: During the first 4 postoperative days the patient produced approximately 1 L of urine per day with a stable creatinine level. It is over this same time that the hydrocortisone was discontinued given improving hemodynamics. However, throughout postoperative day 5, the patient’s creatinine level acutely rose to a peak of 5.8 mg/dL. In addition, his urine output dramatically dropped to < 5 mL per hour, with blood clots noted in his Foley catheter. Dr. Rifkin, what is your differential for causing this acute change in both his creatinine level and urine output this far out from his procedure, and what might you do to help further evaluate?
►Dr. Rifkin: The most common cause of acute kidney injury in hospitalized patients is acute tubular necrosis (ATN).8 However, in this patient, who was recovering well postoperatively, was hemodynamically stable with a robust urine output, and in whom no apparent cause for ATN could be identified, other diagnoses were more likely. Considering the abrupt onset of oligo-anuria, the most likely diagnosis was urinary tract obstruction, particularly given the frank blood and blood clots that were present in the urine. Additional possibilities might be a late surgical complication or infection. Surgical complications could range from direct damage to the renal parenchyma to urinary leakage into the peritoneum from the site of anastomosis or tissue injury. Infections introduced either intraoperatively or developed postoperatively could also cause this sudden drop in urine output, though one would expect more systemic symptoms with this. Given that this patient has a surgical drain in place in the peritoneum, I would recommend testing the creatinine level in the peritoneal fluid drainage. If it is comparable to serum levels, this would argue against a urine leak, as we would expect the level to be significantly elevated in a leak. In addition, he should have imaging of the urinary tract followed by procedures to decompress the presumed obstructed urinary tract. These procedures might include either cystoscopy with ureteral stent placement or percutaneous nephrostomy, depending on the result of the imaging.
►Dr. Bhatnagar: The creatinine level obtained from the surgical drain was roughly equivalent to the serum creatinine, decreasing suspicion for a urine leak as the cause of his findings. Cystoscopy with ureteral stent placement was performed with subsequent increase in both urine output and concomitant decrease in serum creatinine.
Around this time, the patient also began to note blurry vision. Evaluation revealed difficulty with visual field confrontation in the right lower quadrant, right eye ptosis, right eye impaired adduction, absent abduction and impaired upgaze, but intact downgaze. Diplopia was present with gaze in all directions. His constellation of physical examination findings were concerning for a pathologic lesion partially involving cranial nerves II and III, with definitive involvement of cranial nerve VI, but sparing of cranial nerve IV. Repeat MRI of the brain showed hemorrhage into the sellar mass, with ongoing mass effect on the optic chiasm and extension into the sinuses (eAppendix). These findings were consistent with pituitary apoplexy. Dr. Ananthakrishnan, can you tell us more about pituitary apoplexy?
►Dr. Ananthakrishnan: Pituitary apoplexy is a clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland. It typically occurs in patients with preexisting pituitary adenomas and is characterized by the onset of headache, fever, vomiting, meningismus, decreased consciousness, and sometimes death. In addition, given the location of the pituitary gland within the sella, rapid changes in size can result in compression of cranial nerves III, IV, and VI, as well as the optic chiasm, resulting in ophthalmoplegia and visual disturbances as seen in this patient.9
There are a multitude of causes of pituitary apoplexy, including alterations in coagulopathy, pituitary stimulation (eg, dynamic pituitary hormone testing), and both acute increases and decreases in blood flow.10 This patient likely had an ischemic event due to changes in vascular perfusion, spurred by both his blood loss intraoperatively and ongoing hematuria. Management of pituitary apoplexy is dependent on the patient’s hemodynamics, mass effect symptoms, electrolyte balances, and hormone dysfunction. The decision for conservative management vs surgical intervention should be made in consultation with both neurosurgery and endocrinology. Once the patient is hemodynamically stable, the next step in evaluating this patient should be repeating his hormone studies.
►Dr. Bhatnagar: An assessment of pituitary function was consistent with values obtained preoperatively. After multidisciplinary discussions, surgery was deferred, and hydrocortisone was reinitiated to reduce inflammation caused by bleeding into the mass. As the ophthalmoplegia improved, this was transitioned to dexamethasone.
Twelve days after admission, he was discharged to a subacute rehabilitation center, with improvement in his ophthalmoplegia and stabilization of his creatinine level and urine output.
►Anish Bhatnagar, MD, Chief Medical Resident, Veterans Affairs Boston Healthcare System (VABHS) and Beth Israel Deaconess Medical Center (BIDMC): The patient noted erectile dysfunction starting 4 years ago, with accompanied decreased libido. However, until recently, he was able to achieve acceptable erectile capacity with medications. As part of his previous evaluations for erectile dysfunction, the patient had 2 total testosterone levels checked 6 months apart, both low at 150 ng/dL and 38.3 ng/dL (reference range, 220-892). The results of additional hormone studies are shown in the Table. Dr. Ananthakrishnan, can you help us interpret these laboratory results and tell us what tests you might order next?
►Sonia Ananthakrishnan, MD, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center (BMC) and Assistant Professor of Medicine, Boston University School of Medicine (BUSM): When patients present with signs of hypogonadism and an initial low morning testosterone levels, the next test should be a confirmatory repeat morning testosterone level as was done in this case. If this level is also low (for most assays < 300 ng/dL), further evaluation for primary vs secondary hypogonadism should be pursued with measurement of luteinizing hormone and follicle-stimulating hormone levels. Secondary hypogonadism should be suspected when these levels are low or inappropriately normal in the setting of a low testosterone level as in this patient. This patient does not appear to be on any medication or have reversible illnesses that we traditionally think of as possibly causing these hormone irregularities. Key examples include medications such as gonadotropin-releasing hormone analogs, glucocorticoids, and opioids, as well as conditions such as hyperprolactinemia, sleep apnea, diabetes mellitus, anorexia nervosa, or other chronic systemic illnesses, including cirrhosis or lung disease. In this setting, further evaluation of the patient’s anterior pituitary function should be undertaken. Initial screening tests showed mildly elevated prolactin and low normal thyroid-stimulating hormone levels, with a relatively normal free thyroxine. Given these abnormalities in the context of the patient’s total testosterone level < 150 ng/dL, magnetic resonance imaging (MRI) of the anterior pituitary is indicated, and what I would recommend next for evaluation of pituitary and/or hypothalamic tumor or infiltrative disease.1
►Dr. Bhatnagar: An MRI of the brain showed a large 2.7-cm sellar mass, with suprasellar extension and mass effect on the optic chiasm and pituitary infundibulum, partial extension into the right sphenoid sinus, and invasion into the right cavernous sinus. These findings were consistent with a pituitary macroadenoma. The patient was subsequently evaluated by a neurosurgeon who felt that because of the extension and compression of the mass, the patient would benefit from surgical resection.
Given his lower urinary tract symptoms, a prostate-specific antigen level was checked and returned elevated at 11.5 ng/mL. In the setting of these abnormalities, the patient underwent MRI of the abdomen, which noted a new 5.6-cm enhancing mass in the upper pole of his solitary right kidney, highly concerning for new RCC. After a multidisciplinary discussion, urology scheduled the patient for partial right nephrectomy first, with plans for pituitary resection only if the patient had adequate recovery following the urologic procedure.
Dr. Rifkin, this patient went straight from imaging to presumed diagnosis to planned surgical intervention without a confirmatory biopsy. In a patient who already has chronic kidney disease stage 4, why would we not want to pursue biopsy prior to this invasive procedure on his solitary kidney? In addition, given his baseline advanced renal disease, why pursue partial nephrectomy to delay initiation of hemodialysis instead of total nephrectomy and beginning hemodialysis?
►Ian Rifkin, MBBCh, PhD, MSc, Chief, Renal Section, VABHS, Section of Nephrology, BMC, and Associate Professor of Medicine, BUSM: In most cases, imaging alone is used to make a presumptive diagnosis of benign vs malignant renal masses. In one study, RCC was identified by MRI with 85% sensitivity and 76% specificity.2 However, as imaging and biopsy techniques have advanced, there are progressing discussions regarding the utility of biopsy. That being said, there are a number of situations in which patients currently undergo biopsy, particularly when there is diagnostic uncertainty.3 In this patient, with a history of RCC and imaging findings concerning for RCC, biopsy is unnecessary given the high clinical suspicion.
Regarding the choice of partial vs total nephrectomy, there are 2 important distinctions to be made. The first is that though it was previously felt that early initiation of dialysis improves survival, newer studies suggest that early initiation based off of glomerular filtration rate (GFR) offers no survival benefits compared to delayed initiation.4 Second, though there is less clinical data to support this, there is a signal toward the use of partial nephrectomy decreasing mortality compared to radical nephrectomy in management of RCC.5 In this patient, partial nephrectomy may not only increase rates of survival, but also delay initiation of dialysis.
►Dr. Bhatnagar: Prior to undergoing partial right nephrectomy, a morning cortisol level was found to be 5.8 μg/dL with an associated corticotropin (ACTH) level of 26 pg/mL. Dr. Ananthakrishnan, how would you interpret these laboratory results and what might you recommend prior to surgery?
►Dr. Ananthakrishnan: In a healthy patient, surgery often results in a several-fold increase in the secretion of cortisol to balance the unique stressors surgery places on the body.6 This patient is at increased risk for complete or partial adrenal insufficiency in the setting of both his pituitary macroadenoma as well as his previous left nephrectomy, which could have affected his left adrenal gland as well. Thus, this patient may not be able to mount the appropriate cortisol response needed to counter the stresses of surgery. His cortisol level is abnormally low for a morning value, with a relatively normal ACTH reference range of 6 to 50 pg/mL. He may have some degree of adrenal insufficiency, and thus will benefit from perioperative steroids.
►Dr. Bhatnagar: The patient was started on hydrocortisone and underwent a successful laparoscopic partial right nephrectomy. During the procedure, an estimated 2.5 L of blood was lost, with transfusion of 3 units of packed red blood cells. A surgical drain was left in the peritoneum. Postoperatively, he developed hypotension, requiring vasopressors and prolonged continuation of stress dosing of hydrocortisone. Over the next 4 days, the patient was weaned off vasopressors, and his creatinine level was noted to increase from a baseline of 1.8 mg/dL to 4.4 mg/dL.
Dr. Rifkin, how do you think about renal recovery in the patient postnephrectomy, and should we be concerned with the dramatic rise in his creatinine level?
►Dr. Rifkin: Removal of renal mass will result in an initial reduction of GFR proportional to the amount of functional renal tissue removed. However, in as early as 1 week, the residual nephrons begin to compensate through various mechanisms, such as modulation of efferent and afferent arterioles and renal tissue growth by hypertrophy and hyperplasia.7 In the acute setting, it may be difficult to distinguish an acute renal injury vs physiological GFR reduction postnephron loss, but often the initially elevated creatinine level may normalize/stabilize over time. Other markers of kidney function should concomitantly be monitored, including urine output, electrolyte/acid-base status, and urine sediment examination. In this patient, although his creatinine level may be elevated over the first few days, if his urine output remains robust and the urine sediment examination is normal, my concern for permanent kidney injury would be lessened.
►Dr. Bhatnagar: During the first 4 postoperative days the patient produced approximately 1 L of urine per day with a stable creatinine level. It is over this same time that the hydrocortisone was discontinued given improving hemodynamics. However, throughout postoperative day 5, the patient’s creatinine level acutely rose to a peak of 5.8 mg/dL. In addition, his urine output dramatically dropped to < 5 mL per hour, with blood clots noted in his Foley catheter. Dr. Rifkin, what is your differential for causing this acute change in both his creatinine level and urine output this far out from his procedure, and what might you do to help further evaluate?
►Dr. Rifkin: The most common cause of acute kidney injury in hospitalized patients is acute tubular necrosis (ATN).8 However, in this patient, who was recovering well postoperatively, was hemodynamically stable with a robust urine output, and in whom no apparent cause for ATN could be identified, other diagnoses were more likely. Considering the abrupt onset of oligo-anuria, the most likely diagnosis was urinary tract obstruction, particularly given the frank blood and blood clots that were present in the urine. Additional possibilities might be a late surgical complication or infection. Surgical complications could range from direct damage to the renal parenchyma to urinary leakage into the peritoneum from the site of anastomosis or tissue injury. Infections introduced either intraoperatively or developed postoperatively could also cause this sudden drop in urine output, though one would expect more systemic symptoms with this. Given that this patient has a surgical drain in place in the peritoneum, I would recommend testing the creatinine level in the peritoneal fluid drainage. If it is comparable to serum levels, this would argue against a urine leak, as we would expect the level to be significantly elevated in a leak. In addition, he should have imaging of the urinary tract followed by procedures to decompress the presumed obstructed urinary tract. These procedures might include either cystoscopy with ureteral stent placement or percutaneous nephrostomy, depending on the result of the imaging.
►Dr. Bhatnagar: The creatinine level obtained from the surgical drain was roughly equivalent to the serum creatinine, decreasing suspicion for a urine leak as the cause of his findings. Cystoscopy with ureteral stent placement was performed with subsequent increase in both urine output and concomitant decrease in serum creatinine.
Around this time, the patient also began to note blurry vision. Evaluation revealed difficulty with visual field confrontation in the right lower quadrant, right eye ptosis, right eye impaired adduction, absent abduction and impaired upgaze, but intact downgaze. Diplopia was present with gaze in all directions. His constellation of physical examination findings were concerning for a pathologic lesion partially involving cranial nerves II and III, with definitive involvement of cranial nerve VI, but sparing of cranial nerve IV. Repeat MRI of the brain showed hemorrhage into the sellar mass, with ongoing mass effect on the optic chiasm and extension into the sinuses (eAppendix). These findings were consistent with pituitary apoplexy. Dr. Ananthakrishnan, can you tell us more about pituitary apoplexy?
►Dr. Ananthakrishnan: Pituitary apoplexy is a clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland. It typically occurs in patients with preexisting pituitary adenomas and is characterized by the onset of headache, fever, vomiting, meningismus, decreased consciousness, and sometimes death. In addition, given the location of the pituitary gland within the sella, rapid changes in size can result in compression of cranial nerves III, IV, and VI, as well as the optic chiasm, resulting in ophthalmoplegia and visual disturbances as seen in this patient.9
There are a multitude of causes of pituitary apoplexy, including alterations in coagulopathy, pituitary stimulation (eg, dynamic pituitary hormone testing), and both acute increases and decreases in blood flow.10 This patient likely had an ischemic event due to changes in vascular perfusion, spurred by both his blood loss intraoperatively and ongoing hematuria. Management of pituitary apoplexy is dependent on the patient’s hemodynamics, mass effect symptoms, electrolyte balances, and hormone dysfunction. The decision for conservative management vs surgical intervention should be made in consultation with both neurosurgery and endocrinology. Once the patient is hemodynamically stable, the next step in evaluating this patient should be repeating his hormone studies.
►Dr. Bhatnagar: An assessment of pituitary function was consistent with values obtained preoperatively. After multidisciplinary discussions, surgery was deferred, and hydrocortisone was reinitiated to reduce inflammation caused by bleeding into the mass. As the ophthalmoplegia improved, this was transitioned to dexamethasone.
Twelve days after admission, he was discharged to a subacute rehabilitation center, with improvement in his ophthalmoplegia and stabilization of his creatinine level and urine output.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543-553. doi:10.1148/radiol.2018171557
3. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44-55. doi:10.1102/1470-7330.2009.0005
4. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. doi:10.1056/NEJMoa1000552
5. Kunath F, Schmidt S, Krabbe L-M, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017;5(5):CD012045. doi:10.1002/14651858.CD012045.pub2
6. Kehlet H, Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043-1048. doi:10.1093/bja/45.10.1043
7. Chapman D, Moore R, Klarenbach S, Braam B. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J. 2010;4(5):337-343. doi:10.5489/cuaj.909
8. Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
9. Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011;15(suppl 3):S188-S196. doi:10.4103/2230-8210.84862
10. Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015;59(3):259-264. doi:10.1590/2359-3997000000047
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543-553. doi:10.1148/radiol.2018171557
3. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44-55. doi:10.1102/1470-7330.2009.0005
4. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. doi:10.1056/NEJMoa1000552
5. Kunath F, Schmidt S, Krabbe L-M, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017;5(5):CD012045. doi:10.1002/14651858.CD012045.pub2
6. Kehlet H, Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043-1048. doi:10.1093/bja/45.10.1043
7. Chapman D, Moore R, Klarenbach S, Braam B. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J. 2010;4(5):337-343. doi:10.5489/cuaj.909
8. Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
9. Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011;15(suppl 3):S188-S196. doi:10.4103/2230-8210.84862
10. Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015;59(3):259-264. doi:10.1590/2359-3997000000047
A Veteran With Recurrent, Painful Knee Effusion
Case Presentation: A 39-year-old Air Force veteran was admitted to the US Department of Veterans Affairs Boston Healthcare System (VABHS) for evaluation of recurrent, painful right knee effusions. On presentation, his vital signs were stable, and the examination was significant for a right knee with a large effusion and tenderness to palpation without erythema or warmth. His white blood cell count was 12.0 cells/L with an erythrocyte sedimentation rate of 23 mm/h and C-reactive protein of 11.87 mg/L. He was in remission from alcohol use but had relapsed on alcohol in the past day to treat the pain. He had a history of IV drug use but was in remission. He was previously active and enjoyed long hikes. Nine months prior to presentation, he developed his first large right knee effusion associated with pain. He reported no antecedent trauma. At that time, he presented to another hospital and underwent arthrocentesis with orthopedic surgery, but this did not lead to a diagnosis, and the effusion reaccumulated within 24 hours. Four months later, he received a corticosteroid injection that provided only minor, temporary relief. He received 5 additional arthrocenteses over 9 months, all without definitive diagnosis and with rapid reaccumulation of the fluid. His most recent arthrocentesis was 3 weeks before admission.
►Lauren E. Merz, MD, MSc, Chief Medical Resident, VABHS: Dr. Jindal, what is your approach and differential diagnosis for joint effusions in hospitalized patients?
►Shivani Jindal, MD, MPH, Hospitalist, VABHS, Instructor in Medicine, Boston University School of Medicine (BUSM): A thorough history and physical examination are important. I specifically ask about chronicity, pain, and trauma. A medical history of potential infectious exposures and the history of the present illness are also important, such as the risk of sexually transmitted infections, exposure to Lyme disease or other viral illnesses. Gonococcal arthritis is one of the most common causes of nontraumatic monoarthritis in young adults but can also present as a migratory polyarthritis.1
It sounds like he was quite active and liked to hike so a history of tick exposure is important to ascertain. I would also ask about eye inflammation and back pain to assess possible ankylosing spondyarthritis. Other inflammatory etiologies, such as gout are common, but it would be surprising to miss this diagnosis on repeated arthocenteses. A physical examination can confirm monoarthritis over polyarthritis and assess for signs of inflammatory arthritis (eg, warmth and erythema). The most important etiology to assess for and rule out in a person admitted to the hospital is septic arthritis. The severe pain, mild leukocytosis, and mildly elevated inflammatory markers could be consistent with this diagnosis but are nonspecific. However, the chronicity of this patient’s presentation and hemodynamic stability make septic arthritis less likely overall and a more indolent infection or other inflammatory process more likely.
►Dr. Merz: The patient’s medical history included posttraumatic stress disorder (PTSD) and antisocial personality disorder with multiple prior suicide attempts. He also had a history of opioid use disorder (OUD) with prior overdose and alcohol use disorder (AUD). Given his stated preference to avoid opioids and normal liver function and liver chemistry testing, the initial treatment was with acetaminophen. After this failed to provide satisfactory pain control, IV hydromorphone was added.
Dr. Jindal, how do you approach pain control in the hospital for musculoskeletal issues like this?
►Dr. Jindal: Typically, nonsteroidal anti-inflammatory medications (NSAIDs) are most effective for musculoskeletal pain, often in the form of ketorolac or ibuprofen. However, we are often limited in our NSAID use by kidney disease, gastritis, or cardiovascular disease.
►Dr. Merz: On hospital day 1, the patient asked to leave to consume alcohol to ease unremitting pain. He also expressed suicidal ideation and discharge was therefore felt to be unsafe. He was reluctant to engage with psychiatry and became physically combative while attempting to leave the hospital, necessitating the use of sedating medications and physical restraints.
Dr. Shahal, what factors led to the decision to place an involuntary hold, and how do you balance patient autonomy and patient safety?
►Dr. Talya Shahal, MD, Consult-Liaison Psychiatry Service, VABHS, Instructor in Psychiatry, Harvard Medical School: This is a delicate balance that requires constant reassessment. The patient initially presented to the emergency department with suicidal ideation, stating he was not able to tolerate the pain and thus resumed alcohol consumption after a period of nonuse. He had multiple risk factors for suicide, including 9 prior suicide attempts with the latest less than a year before presentation, active substance use with alcohol and other recreational drugs, PTSD, pain, veteran status, male sex, single status, and a history of trauma.3,4 He was also displaying impulsivity and limited insight, did not engage in his psychiatric assessment, and attempted to assault staff. As such, his suicide risk was assessed to be high at the time of the evaluation, which led to the decision to place an involuntary hold. However, we reevaluate this decision at least daily in order to reassess the risk and ensure that the balance between patient safety and autonomy are maintained.
►Dr. Merz: The involuntary hold was removed within 48 hours as the patient remained calm and engaged with the primary and consulting teams. He requested escalating doses of opioids as he felt the short-acting IV medications were not providing sustained relief. However, he was also noted to be walking outside of the hospital without assistance, and he repeatedly declined nonopioid pain modalities as well as buprenorphine/naloxone. The chronic pain service was consulted but was unable to see the patient as he was frequently outside of his room.
Dr. Shahal, how do you address OUD, pain, and stigma in the hospital?
►Dr. Shahal: It is important to remember that patients with substance use disorder (SUD) and mental illness frequently have physical causes for pain and are often undertreated.5 Patients with SUD may also have higher tolerance for opioids and may need higher doses to treat the pain.5 Modalities like buprenorphine/naloxone can be effective to treat OUD and pain, but these usually cannot be initiated while the patient is on short-acting opioids as this would precipitate withdrawal.6 However, withdrawal can be managed while inpatient, and this can be a good time to start these medications as practitioners can aggressively help with symptom control. Proactively addressing mental health concerns, particularly anxiety, AUD, insomnia, PTSD, and depression, can also have a direct impact on the perception of pain and assist with better control.2 In addition, nonpharmacologic options, such as meditation, deep breathing, and even acupuncture and Reiki can be helpful and of course less harmful to treat pain.2
► Dr. Merz: An X-ray of the knee showed no acute fracture or joint space narrowing. Magnetic resonance imaging confirmed a large knee effusion with no evidence of ligament injury. Synovial fluid showed turbid, yellow fluid with 14,110 nucleated cells (84% segmented cells and 4000 RBCs). Gram stain was negative, culture had no growth, and there were no crystals. Anticyclic citrullinated peptide (anti-CCP), rheumatoid factor, HIV testing, and HLA-B27 were negative.
Dr. Serrao, what do these studies tell us about the joint effusion and the possible diagnoses?
► Dr. Richard Serrao, MD, Infectious Disease, VABHS, Clinical Associate Professor in Medicine, BUSM: I would expect the white blood cell (WBC) count to be > 50,000 cells with > 75% polymorphonuclear cells and a positive Gram stain if this was a bacterial infection resulting in septic arthritis.7 This patient’s studies are not consistent with this diagnosis nor is the chronicity of his presentation. There are 2 important bacteria that can present with inflammatory arthritis and less pronounced findings on arthrocentesis: Borrelia burgdorferi (the bacteria causing Lyme arthritis) and Neisseria gonorrhea. Lyme arthritis could be consistent with this relapsing remitting presentation as you expect a WBC count between 3000 and 100,000 cells with a mean value between 10,000 and 25,000 cells, > 50% polymorphonuclear leukocytes, and negative Gram stains.8 Gonococcal infections often do not have marked elevations in the WBC count and the Gram stain can be variable, but you still expect the WBC count to be > 30,000 cells.7 Inflammatory causes such as gout or autoimmune conditions such as lupus often have a WBC count between 2000 and 100,000 with a negative Gram stain, which could be consistent with this patient’s presentation.7 However, the lack of crystals rules out gout and the negative anti-CCP, rheumatoid factor, and HLA-B27 make rheumatologic diseases less likely.
►Dr. Merz: The patient received a phone call from another hospital where an arthrocentesis had been performed 3 weeks before. The results included a positive polymerase chain reaction (PCR) test for Lyme disease in the synovial fluid. A subsequent serum Lyme screen was positive for 1 of 3 immunoglobulin (Ig) M bands and 10 of 10 IgG bands.
Dr. Serrao, how does Lyme arthritis typically present, and are there aspects of this case that make you suspect the diagnosis? Does the serum Lyme test give us any additional information?
►Dr. Serrao: Lyme arthritis is a late manifestation of Lyme disease. Patients typically have persistent or intermittent arthritis, and large joints are more commonly impacted than small joints. Monoarthritis of the knee is the most common, but oligoarthritis is possible as well. The swelling usually begins abruptly, lasts for weeks to months, and effusions typically recur quickly after aspiration. These findings are consistent with the patient’s clinical history.
For diagnostics, the IgG Western blot is positive if 5 of the 10 bands are positive.9 This patient far exceeds the IgG band number to diagnose Lyme disease. All patients with Lyme arthritis will have positive IgG serologies since Lyme arthritis is a late manifestation of the infection. IgM reactivity may be present, but are not necessary to diagnose Lyme arthritis.10 Synovial fluid is often not analyzed for antibody responses as they are susceptible to false positive results, but synovial PCR testing like this patient had detects approximately 70% of patients with untreated Lyme arthritis.11 However, PCR positivity does not necessarily equate with active infection. Serologic testing for Lyme disease by enzyme-linked immunosorbent assay and Western blot as well as careful history and the exclusion of other diagnoses are usually sufficient to make the diagnosis.
► Dr. Merz: On further history the patient reported that 5 years prior he found a tick on his skin with a bull’s-eye rash. He was treated with 28 days of doxycycline at that time. He did not recall any tick bites or rashes in the years since.
Dr. Serrao, is it surprising that he developed Lyme arthritis 5 years after exposure and after being treated appropriately? What is the typical treatment approach for a patient like this?
►Dr. Serrao: It is atypical to develop Lyme arthritis 5 years after reported treatment of what appeared to be early localized disease, namely, erythema migrans. This stage is usually cured with 10 days of treatment alone (he received 28 days) and is generally abortive of subsequent stages, including Lyme arthritis. Furthermore, the patient reported no symptoms of arthritis until recently since that time. Therefore, one can argue that the excessively long span of time from treatment to these first episodes of arthritis suggests the patient could have been reinfected. When available, comparing the types and number of Western blot bands (eg, new and/or more bands on subsequent serologic testing) can support a reinfection diagnosis. A delayed postinfectious inflammatory process from excessive proinflammatory immune responses that block wound repair resulting in proliferative synovitis is also possible.12 This is defined as the postinfectious, postantibiotic, or antibiotic-refractory Lyme arthritis, a diagnosis of exclusion more apparent only after patients receive appropriate antibiotic courses for the possibility of untreated Lyme as an active infection.12
Given the inherent diagnostic uncertainty between an active infection and posttreatment Lyme arthritis syndromes, it is best to approach most cases of Lyme arthritis as an active infection first especially if not yet treated with antibiotics. Diagnosis of postinflammatory processes should be considered if symptoms persist after appropriate antibiotics, and then short-term use of disease-modifying antirheumatic drugs, rather than further courses of antibiotics, is recommended.
► Dr. Merz: The patient was initiated on doxycycline with the plan to transition to ceftriaxone if there was no response. One day after diagnosis and treatment initiation and in the setting of continued pain, the patient again asked to leave the hospital to drink alcohol. After eloping and becoming intoxicated with alcohol, he returned to his room. He remained concerned about his continued pain and lack of adequate pain control. At the time, he was receiving hydromorphone, ketorolac, lorazepam, gabapentin, and quetiapine.
Dr. Serrao, do you expect this degree of pain from Lyme arthritis?
► Dr. Serrao: Lyme arthritis is typically less painful than other forms of infectious or inflammatory arthritis. Pain is usually caused by the pressure from the acute accumulation and reaccumulation of fluid. In this case, the rapid accumulation of fluid that this patient experienced as well as relief with arthrocentesis suggests that the size and acuity of the effusion was causing great discomfort. Repeated arthrocentesis can prove to be a preventative strategy to minimize synovial herniation.
►Dr. Merz: Dr. Shahal, how do you balance the patient subjectively telling you that they are in pain with objective signs that they may be tolerating the pain like walking around unassisted? Is there anything else that could have been done to prevent this adverse outcome?
►Dr. Shahal: This is one of the hardest pieces of pain management. We want to practice beneficence by believing our patients and addressing their discomfort, but we also want to practice nonmaleficence by avoiding inappropriate long-term pain treatments like opioids that have significant harm as well as avoiding exacerbating this patient’s underlying SUD. An agent like buprenorphine/naloxone could have been an excellent fit to treat pain and SUD, but the patient’s lack of interest and the frequent use of short-acting opioids were major barriers. A chronic pain consult early on is helpful in cases like this as well, but they were unable to see him since he was often out of his room. Repeated arthrocentesis may also have helped the pain. Treatment of anxiety and insomnia with medications like hydroxyzine, trazodone, melatonin, gabapentin, or buspirone as well as interventions like sleep hygiene protocols or spiritual care may have helped somewhat as well.
We know that there is a vicious cycle between pain and poorly controlled mood symptoms. Many of our veterans have PTSD, anxiety, and SUD that are exacerbated by hospitalization and pain. Maintaining optimal communication between the patient and the practitioners, using trauma-informed care, understanding the patient’s goals of care, setting expectations and limits, and attempting to address the patient’s needs while attempting to minimize stigma might be helpful. However, despite optimal care, sometimes these events cannot be avoided.
►Dr. Merz: The patient was ultimately transferred to an inpatient psychiatric unit where a taper plan for the short-acting opioids was implemented. He was psychiatrically stabilized and discharged a few days later off opioids and on doxycycline. On follow-up a few weeks later, his pain had markedly improved, and the effusion was significantly reduced in size. His mood and impulsivity had stabilized. He continues to follow-up in the infectious disease clinic.

1. Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68(1):83-90.
2. Qaseem A, McLean RM, O’Gurek D, et al. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: a clinical guideline from the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748. doi:10.7326/M19-3602
3. Silverman MM, Berman AL. Suicide risk assessment and risk formulation part I: a focus on suicide ideation in assessing suicide risk. Suicide Life Threat Behav. 2014;44(4):420-431. doi:10.1111/sltb.12065
4. Berman AL, Silverman MM. Suicide risk assessment and risk formulation part II: Suicide risk formulation and the determination of levels of risk. Suicide Life Threat Behav. 2014;44(4):432-443. doi:10.1111/sltb.12067
5. Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017;2(4):e611. Published 2017 Jul 27. doi:10.1097/PR9.0000000000000611
6. Chou R, Wagner J, Ahmed AY, et al. Treatments for Acute Pain: A Systematic Review. Agency for Healthcare Research and Quality; 2020. https://www.ncbi.nlm.nih.gov/books/NBK566506/
7. Seidman AJ, Limaiem F. Synovial fluid analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Updated May 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537114
8. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280. doi:10.1016/j.idc.2015.02.004
9. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA. 1995;274(12):937.
10. Craft JE, Grodzicki RL, Steere AC. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149(5):789-795. doi:10.1093/infdis/149.5.789
11. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229-234. doi:10.1056/NEJM199401273300401
12. Steere AC. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130(5):2148-2151. doi:10.1172/JCI138062
Case Presentation: A 39-year-old Air Force veteran was admitted to the US Department of Veterans Affairs Boston Healthcare System (VABHS) for evaluation of recurrent, painful right knee effusions. On presentation, his vital signs were stable, and the examination was significant for a right knee with a large effusion and tenderness to palpation without erythema or warmth. His white blood cell count was 12.0 cells/L with an erythrocyte sedimentation rate of 23 mm/h and C-reactive protein of 11.87 mg/L. He was in remission from alcohol use but had relapsed on alcohol in the past day to treat the pain. He had a history of IV drug use but was in remission. He was previously active and enjoyed long hikes. Nine months prior to presentation, he developed his first large right knee effusion associated with pain. He reported no antecedent trauma. At that time, he presented to another hospital and underwent arthrocentesis with orthopedic surgery, but this did not lead to a diagnosis, and the effusion reaccumulated within 24 hours. Four months later, he received a corticosteroid injection that provided only minor, temporary relief. He received 5 additional arthrocenteses over 9 months, all without definitive diagnosis and with rapid reaccumulation of the fluid. His most recent arthrocentesis was 3 weeks before admission.
►Lauren E. Merz, MD, MSc, Chief Medical Resident, VABHS: Dr. Jindal, what is your approach and differential diagnosis for joint effusions in hospitalized patients?
►Shivani Jindal, MD, MPH, Hospitalist, VABHS, Instructor in Medicine, Boston University School of Medicine (BUSM): A thorough history and physical examination are important. I specifically ask about chronicity, pain, and trauma. A medical history of potential infectious exposures and the history of the present illness are also important, such as the risk of sexually transmitted infections, exposure to Lyme disease or other viral illnesses. Gonococcal arthritis is one of the most common causes of nontraumatic monoarthritis in young adults but can also present as a migratory polyarthritis.1
It sounds like he was quite active and liked to hike so a history of tick exposure is important to ascertain. I would also ask about eye inflammation and back pain to assess possible ankylosing spondyarthritis. Other inflammatory etiologies, such as gout are common, but it would be surprising to miss this diagnosis on repeated arthocenteses. A physical examination can confirm monoarthritis over polyarthritis and assess for signs of inflammatory arthritis (eg, warmth and erythema). The most important etiology to assess for and rule out in a person admitted to the hospital is septic arthritis. The severe pain, mild leukocytosis, and mildly elevated inflammatory markers could be consistent with this diagnosis but are nonspecific. However, the chronicity of this patient’s presentation and hemodynamic stability make septic arthritis less likely overall and a more indolent infection or other inflammatory process more likely.
►Dr. Merz: The patient’s medical history included posttraumatic stress disorder (PTSD) and antisocial personality disorder with multiple prior suicide attempts. He also had a history of opioid use disorder (OUD) with prior overdose and alcohol use disorder (AUD). Given his stated preference to avoid opioids and normal liver function and liver chemistry testing, the initial treatment was with acetaminophen. After this failed to provide satisfactory pain control, IV hydromorphone was added.
Dr. Jindal, how do you approach pain control in the hospital for musculoskeletal issues like this?
►Dr. Jindal: Typically, nonsteroidal anti-inflammatory medications (NSAIDs) are most effective for musculoskeletal pain, often in the form of ketorolac or ibuprofen. However, we are often limited in our NSAID use by kidney disease, gastritis, or cardiovascular disease.
►Dr. Merz: On hospital day 1, the patient asked to leave to consume alcohol to ease unremitting pain. He also expressed suicidal ideation and discharge was therefore felt to be unsafe. He was reluctant to engage with psychiatry and became physically combative while attempting to leave the hospital, necessitating the use of sedating medications and physical restraints.
Dr. Shahal, what factors led to the decision to place an involuntary hold, and how do you balance patient autonomy and patient safety?
►Dr. Talya Shahal, MD, Consult-Liaison Psychiatry Service, VABHS, Instructor in Psychiatry, Harvard Medical School: This is a delicate balance that requires constant reassessment. The patient initially presented to the emergency department with suicidal ideation, stating he was not able to tolerate the pain and thus resumed alcohol consumption after a period of nonuse. He had multiple risk factors for suicide, including 9 prior suicide attempts with the latest less than a year before presentation, active substance use with alcohol and other recreational drugs, PTSD, pain, veteran status, male sex, single status, and a history of trauma.3,4 He was also displaying impulsivity and limited insight, did not engage in his psychiatric assessment, and attempted to assault staff. As such, his suicide risk was assessed to be high at the time of the evaluation, which led to the decision to place an involuntary hold. However, we reevaluate this decision at least daily in order to reassess the risk and ensure that the balance between patient safety and autonomy are maintained.
►Dr. Merz: The involuntary hold was removed within 48 hours as the patient remained calm and engaged with the primary and consulting teams. He requested escalating doses of opioids as he felt the short-acting IV medications were not providing sustained relief. However, he was also noted to be walking outside of the hospital without assistance, and he repeatedly declined nonopioid pain modalities as well as buprenorphine/naloxone. The chronic pain service was consulted but was unable to see the patient as he was frequently outside of his room.
Dr. Shahal, how do you address OUD, pain, and stigma in the hospital?
►Dr. Shahal: It is important to remember that patients with substance use disorder (SUD) and mental illness frequently have physical causes for pain and are often undertreated.5 Patients with SUD may also have higher tolerance for opioids and may need higher doses to treat the pain.5 Modalities like buprenorphine/naloxone can be effective to treat OUD and pain, but these usually cannot be initiated while the patient is on short-acting opioids as this would precipitate withdrawal.6 However, withdrawal can be managed while inpatient, and this can be a good time to start these medications as practitioners can aggressively help with symptom control. Proactively addressing mental health concerns, particularly anxiety, AUD, insomnia, PTSD, and depression, can also have a direct impact on the perception of pain and assist with better control.2 In addition, nonpharmacologic options, such as meditation, deep breathing, and even acupuncture and Reiki can be helpful and of course less harmful to treat pain.2
► Dr. Merz: An X-ray of the knee showed no acute fracture or joint space narrowing. Magnetic resonance imaging confirmed a large knee effusion with no evidence of ligament injury. Synovial fluid showed turbid, yellow fluid with 14,110 nucleated cells (84% segmented cells and 4000 RBCs). Gram stain was negative, culture had no growth, and there were no crystals. Anticyclic citrullinated peptide (anti-CCP), rheumatoid factor, HIV testing, and HLA-B27 were negative.
Dr. Serrao, what do these studies tell us about the joint effusion and the possible diagnoses?
► Dr. Richard Serrao, MD, Infectious Disease, VABHS, Clinical Associate Professor in Medicine, BUSM: I would expect the white blood cell (WBC) count to be > 50,000 cells with > 75% polymorphonuclear cells and a positive Gram stain if this was a bacterial infection resulting in septic arthritis.7 This patient’s studies are not consistent with this diagnosis nor is the chronicity of his presentation. There are 2 important bacteria that can present with inflammatory arthritis and less pronounced findings on arthrocentesis: Borrelia burgdorferi (the bacteria causing Lyme arthritis) and Neisseria gonorrhea. Lyme arthritis could be consistent with this relapsing remitting presentation as you expect a WBC count between 3000 and 100,000 cells with a mean value between 10,000 and 25,000 cells, > 50% polymorphonuclear leukocytes, and negative Gram stains.8 Gonococcal infections often do not have marked elevations in the WBC count and the Gram stain can be variable, but you still expect the WBC count to be > 30,000 cells.7 Inflammatory causes such as gout or autoimmune conditions such as lupus often have a WBC count between 2000 and 100,000 with a negative Gram stain, which could be consistent with this patient’s presentation.7 However, the lack of crystals rules out gout and the negative anti-CCP, rheumatoid factor, and HLA-B27 make rheumatologic diseases less likely.
►Dr. Merz: The patient received a phone call from another hospital where an arthrocentesis had been performed 3 weeks before. The results included a positive polymerase chain reaction (PCR) test for Lyme disease in the synovial fluid. A subsequent serum Lyme screen was positive for 1 of 3 immunoglobulin (Ig) M bands and 10 of 10 IgG bands.
Dr. Serrao, how does Lyme arthritis typically present, and are there aspects of this case that make you suspect the diagnosis? Does the serum Lyme test give us any additional information?
►Dr. Serrao: Lyme arthritis is a late manifestation of Lyme disease. Patients typically have persistent or intermittent arthritis, and large joints are more commonly impacted than small joints. Monoarthritis of the knee is the most common, but oligoarthritis is possible as well. The swelling usually begins abruptly, lasts for weeks to months, and effusions typically recur quickly after aspiration. These findings are consistent with the patient’s clinical history.
For diagnostics, the IgG Western blot is positive if 5 of the 10 bands are positive.9 This patient far exceeds the IgG band number to diagnose Lyme disease. All patients with Lyme arthritis will have positive IgG serologies since Lyme arthritis is a late manifestation of the infection. IgM reactivity may be present, but are not necessary to diagnose Lyme arthritis.10 Synovial fluid is often not analyzed for antibody responses as they are susceptible to false positive results, but synovial PCR testing like this patient had detects approximately 70% of patients with untreated Lyme arthritis.11 However, PCR positivity does not necessarily equate with active infection. Serologic testing for Lyme disease by enzyme-linked immunosorbent assay and Western blot as well as careful history and the exclusion of other diagnoses are usually sufficient to make the diagnosis.
► Dr. Merz: On further history the patient reported that 5 years prior he found a tick on his skin with a bull’s-eye rash. He was treated with 28 days of doxycycline at that time. He did not recall any tick bites or rashes in the years since.
Dr. Serrao, is it surprising that he developed Lyme arthritis 5 years after exposure and after being treated appropriately? What is the typical treatment approach for a patient like this?
►Dr. Serrao: It is atypical to develop Lyme arthritis 5 years after reported treatment of what appeared to be early localized disease, namely, erythema migrans. This stage is usually cured with 10 days of treatment alone (he received 28 days) and is generally abortive of subsequent stages, including Lyme arthritis. Furthermore, the patient reported no symptoms of arthritis until recently since that time. Therefore, one can argue that the excessively long span of time from treatment to these first episodes of arthritis suggests the patient could have been reinfected. When available, comparing the types and number of Western blot bands (eg, new and/or more bands on subsequent serologic testing) can support a reinfection diagnosis. A delayed postinfectious inflammatory process from excessive proinflammatory immune responses that block wound repair resulting in proliferative synovitis is also possible.12 This is defined as the postinfectious, postantibiotic, or antibiotic-refractory Lyme arthritis, a diagnosis of exclusion more apparent only after patients receive appropriate antibiotic courses for the possibility of untreated Lyme as an active infection.12
Given the inherent diagnostic uncertainty between an active infection and posttreatment Lyme arthritis syndromes, it is best to approach most cases of Lyme arthritis as an active infection first especially if not yet treated with antibiotics. Diagnosis of postinflammatory processes should be considered if symptoms persist after appropriate antibiotics, and then short-term use of disease-modifying antirheumatic drugs, rather than further courses of antibiotics, is recommended.
► Dr. Merz: The patient was initiated on doxycycline with the plan to transition to ceftriaxone if there was no response. One day after diagnosis and treatment initiation and in the setting of continued pain, the patient again asked to leave the hospital to drink alcohol. After eloping and becoming intoxicated with alcohol, he returned to his room. He remained concerned about his continued pain and lack of adequate pain control. At the time, he was receiving hydromorphone, ketorolac, lorazepam, gabapentin, and quetiapine.
Dr. Serrao, do you expect this degree of pain from Lyme arthritis?
► Dr. Serrao: Lyme arthritis is typically less painful than other forms of infectious or inflammatory arthritis. Pain is usually caused by the pressure from the acute accumulation and reaccumulation of fluid. In this case, the rapid accumulation of fluid that this patient experienced as well as relief with arthrocentesis suggests that the size and acuity of the effusion was causing great discomfort. Repeated arthrocentesis can prove to be a preventative strategy to minimize synovial herniation.
►Dr. Merz: Dr. Shahal, how do you balance the patient subjectively telling you that they are in pain with objective signs that they may be tolerating the pain like walking around unassisted? Is there anything else that could have been done to prevent this adverse outcome?
►Dr. Shahal: This is one of the hardest pieces of pain management. We want to practice beneficence by believing our patients and addressing their discomfort, but we also want to practice nonmaleficence by avoiding inappropriate long-term pain treatments like opioids that have significant harm as well as avoiding exacerbating this patient’s underlying SUD. An agent like buprenorphine/naloxone could have been an excellent fit to treat pain and SUD, but the patient’s lack of interest and the frequent use of short-acting opioids were major barriers. A chronic pain consult early on is helpful in cases like this as well, but they were unable to see him since he was often out of his room. Repeated arthrocentesis may also have helped the pain. Treatment of anxiety and insomnia with medications like hydroxyzine, trazodone, melatonin, gabapentin, or buspirone as well as interventions like sleep hygiene protocols or spiritual care may have helped somewhat as well.
We know that there is a vicious cycle between pain and poorly controlled mood symptoms. Many of our veterans have PTSD, anxiety, and SUD that are exacerbated by hospitalization and pain. Maintaining optimal communication between the patient and the practitioners, using trauma-informed care, understanding the patient’s goals of care, setting expectations and limits, and attempting to address the patient’s needs while attempting to minimize stigma might be helpful. However, despite optimal care, sometimes these events cannot be avoided.
►Dr. Merz: The patient was ultimately transferred to an inpatient psychiatric unit where a taper plan for the short-acting opioids was implemented. He was psychiatrically stabilized and discharged a few days later off opioids and on doxycycline. On follow-up a few weeks later, his pain had markedly improved, and the effusion was significantly reduced in size. His mood and impulsivity had stabilized. He continues to follow-up in the infectious disease clinic.

Case Presentation: A 39-year-old Air Force veteran was admitted to the US Department of Veterans Affairs Boston Healthcare System (VABHS) for evaluation of recurrent, painful right knee effusions. On presentation, his vital signs were stable, and the examination was significant for a right knee with a large effusion and tenderness to palpation without erythema or warmth. His white blood cell count was 12.0 cells/L with an erythrocyte sedimentation rate of 23 mm/h and C-reactive protein of 11.87 mg/L. He was in remission from alcohol use but had relapsed on alcohol in the past day to treat the pain. He had a history of IV drug use but was in remission. He was previously active and enjoyed long hikes. Nine months prior to presentation, he developed his first large right knee effusion associated with pain. He reported no antecedent trauma. At that time, he presented to another hospital and underwent arthrocentesis with orthopedic surgery, but this did not lead to a diagnosis, and the effusion reaccumulated within 24 hours. Four months later, he received a corticosteroid injection that provided only minor, temporary relief. He received 5 additional arthrocenteses over 9 months, all without definitive diagnosis and with rapid reaccumulation of the fluid. His most recent arthrocentesis was 3 weeks before admission.
►Lauren E. Merz, MD, MSc, Chief Medical Resident, VABHS: Dr. Jindal, what is your approach and differential diagnosis for joint effusions in hospitalized patients?
►Shivani Jindal, MD, MPH, Hospitalist, VABHS, Instructor in Medicine, Boston University School of Medicine (BUSM): A thorough history and physical examination are important. I specifically ask about chronicity, pain, and trauma. A medical history of potential infectious exposures and the history of the present illness are also important, such as the risk of sexually transmitted infections, exposure to Lyme disease or other viral illnesses. Gonococcal arthritis is one of the most common causes of nontraumatic monoarthritis in young adults but can also present as a migratory polyarthritis.1
It sounds like he was quite active and liked to hike so a history of tick exposure is important to ascertain. I would also ask about eye inflammation and back pain to assess possible ankylosing spondyarthritis. Other inflammatory etiologies, such as gout are common, but it would be surprising to miss this diagnosis on repeated arthocenteses. A physical examination can confirm monoarthritis over polyarthritis and assess for signs of inflammatory arthritis (eg, warmth and erythema). The most important etiology to assess for and rule out in a person admitted to the hospital is septic arthritis. The severe pain, mild leukocytosis, and mildly elevated inflammatory markers could be consistent with this diagnosis but are nonspecific. However, the chronicity of this patient’s presentation and hemodynamic stability make septic arthritis less likely overall and a more indolent infection or other inflammatory process more likely.
►Dr. Merz: The patient’s medical history included posttraumatic stress disorder (PTSD) and antisocial personality disorder with multiple prior suicide attempts. He also had a history of opioid use disorder (OUD) with prior overdose and alcohol use disorder (AUD). Given his stated preference to avoid opioids and normal liver function and liver chemistry testing, the initial treatment was with acetaminophen. After this failed to provide satisfactory pain control, IV hydromorphone was added.
Dr. Jindal, how do you approach pain control in the hospital for musculoskeletal issues like this?
►Dr. Jindal: Typically, nonsteroidal anti-inflammatory medications (NSAIDs) are most effective for musculoskeletal pain, often in the form of ketorolac or ibuprofen. However, we are often limited in our NSAID use by kidney disease, gastritis, or cardiovascular disease.
►Dr. Merz: On hospital day 1, the patient asked to leave to consume alcohol to ease unremitting pain. He also expressed suicidal ideation and discharge was therefore felt to be unsafe. He was reluctant to engage with psychiatry and became physically combative while attempting to leave the hospital, necessitating the use of sedating medications and physical restraints.
Dr. Shahal, what factors led to the decision to place an involuntary hold, and how do you balance patient autonomy and patient safety?
►Dr. Talya Shahal, MD, Consult-Liaison Psychiatry Service, VABHS, Instructor in Psychiatry, Harvard Medical School: This is a delicate balance that requires constant reassessment. The patient initially presented to the emergency department with suicidal ideation, stating he was not able to tolerate the pain and thus resumed alcohol consumption after a period of nonuse. He had multiple risk factors for suicide, including 9 prior suicide attempts with the latest less than a year before presentation, active substance use with alcohol and other recreational drugs, PTSD, pain, veteran status, male sex, single status, and a history of trauma.3,4 He was also displaying impulsivity and limited insight, did not engage in his psychiatric assessment, and attempted to assault staff. As such, his suicide risk was assessed to be high at the time of the evaluation, which led to the decision to place an involuntary hold. However, we reevaluate this decision at least daily in order to reassess the risk and ensure that the balance between patient safety and autonomy are maintained.
►Dr. Merz: The involuntary hold was removed within 48 hours as the patient remained calm and engaged with the primary and consulting teams. He requested escalating doses of opioids as he felt the short-acting IV medications were not providing sustained relief. However, he was also noted to be walking outside of the hospital without assistance, and he repeatedly declined nonopioid pain modalities as well as buprenorphine/naloxone. The chronic pain service was consulted but was unable to see the patient as he was frequently outside of his room.
Dr. Shahal, how do you address OUD, pain, and stigma in the hospital?
►Dr. Shahal: It is important to remember that patients with substance use disorder (SUD) and mental illness frequently have physical causes for pain and are often undertreated.5 Patients with SUD may also have higher tolerance for opioids and may need higher doses to treat the pain.5 Modalities like buprenorphine/naloxone can be effective to treat OUD and pain, but these usually cannot be initiated while the patient is on short-acting opioids as this would precipitate withdrawal.6 However, withdrawal can be managed while inpatient, and this can be a good time to start these medications as practitioners can aggressively help with symptom control. Proactively addressing mental health concerns, particularly anxiety, AUD, insomnia, PTSD, and depression, can also have a direct impact on the perception of pain and assist with better control.2 In addition, nonpharmacologic options, such as meditation, deep breathing, and even acupuncture and Reiki can be helpful and of course less harmful to treat pain.2
► Dr. Merz: An X-ray of the knee showed no acute fracture or joint space narrowing. Magnetic resonance imaging confirmed a large knee effusion with no evidence of ligament injury. Synovial fluid showed turbid, yellow fluid with 14,110 nucleated cells (84% segmented cells and 4000 RBCs). Gram stain was negative, culture had no growth, and there were no crystals. Anticyclic citrullinated peptide (anti-CCP), rheumatoid factor, HIV testing, and HLA-B27 were negative.
Dr. Serrao, what do these studies tell us about the joint effusion and the possible diagnoses?
► Dr. Richard Serrao, MD, Infectious Disease, VABHS, Clinical Associate Professor in Medicine, BUSM: I would expect the white blood cell (WBC) count to be > 50,000 cells with > 75% polymorphonuclear cells and a positive Gram stain if this was a bacterial infection resulting in septic arthritis.7 This patient’s studies are not consistent with this diagnosis nor is the chronicity of his presentation. There are 2 important bacteria that can present with inflammatory arthritis and less pronounced findings on arthrocentesis: Borrelia burgdorferi (the bacteria causing Lyme arthritis) and Neisseria gonorrhea. Lyme arthritis could be consistent with this relapsing remitting presentation as you expect a WBC count between 3000 and 100,000 cells with a mean value between 10,000 and 25,000 cells, > 50% polymorphonuclear leukocytes, and negative Gram stains.8 Gonococcal infections often do not have marked elevations in the WBC count and the Gram stain can be variable, but you still expect the WBC count to be > 30,000 cells.7 Inflammatory causes such as gout or autoimmune conditions such as lupus often have a WBC count between 2000 and 100,000 with a negative Gram stain, which could be consistent with this patient’s presentation.7 However, the lack of crystals rules out gout and the negative anti-CCP, rheumatoid factor, and HLA-B27 make rheumatologic diseases less likely.
►Dr. Merz: The patient received a phone call from another hospital where an arthrocentesis had been performed 3 weeks before. The results included a positive polymerase chain reaction (PCR) test for Lyme disease in the synovial fluid. A subsequent serum Lyme screen was positive for 1 of 3 immunoglobulin (Ig) M bands and 10 of 10 IgG bands.
Dr. Serrao, how does Lyme arthritis typically present, and are there aspects of this case that make you suspect the diagnosis? Does the serum Lyme test give us any additional information?
►Dr. Serrao: Lyme arthritis is a late manifestation of Lyme disease. Patients typically have persistent or intermittent arthritis, and large joints are more commonly impacted than small joints. Monoarthritis of the knee is the most common, but oligoarthritis is possible as well. The swelling usually begins abruptly, lasts for weeks to months, and effusions typically recur quickly after aspiration. These findings are consistent with the patient’s clinical history.
For diagnostics, the IgG Western blot is positive if 5 of the 10 bands are positive.9 This patient far exceeds the IgG band number to diagnose Lyme disease. All patients with Lyme arthritis will have positive IgG serologies since Lyme arthritis is a late manifestation of the infection. IgM reactivity may be present, but are not necessary to diagnose Lyme arthritis.10 Synovial fluid is often not analyzed for antibody responses as they are susceptible to false positive results, but synovial PCR testing like this patient had detects approximately 70% of patients with untreated Lyme arthritis.11 However, PCR positivity does not necessarily equate with active infection. Serologic testing for Lyme disease by enzyme-linked immunosorbent assay and Western blot as well as careful history and the exclusion of other diagnoses are usually sufficient to make the diagnosis.
► Dr. Merz: On further history the patient reported that 5 years prior he found a tick on his skin with a bull’s-eye rash. He was treated with 28 days of doxycycline at that time. He did not recall any tick bites or rashes in the years since.
Dr. Serrao, is it surprising that he developed Lyme arthritis 5 years after exposure and after being treated appropriately? What is the typical treatment approach for a patient like this?
►Dr. Serrao: It is atypical to develop Lyme arthritis 5 years after reported treatment of what appeared to be early localized disease, namely, erythema migrans. This stage is usually cured with 10 days of treatment alone (he received 28 days) and is generally abortive of subsequent stages, including Lyme arthritis. Furthermore, the patient reported no symptoms of arthritis until recently since that time. Therefore, one can argue that the excessively long span of time from treatment to these first episodes of arthritis suggests the patient could have been reinfected. When available, comparing the types and number of Western blot bands (eg, new and/or more bands on subsequent serologic testing) can support a reinfection diagnosis. A delayed postinfectious inflammatory process from excessive proinflammatory immune responses that block wound repair resulting in proliferative synovitis is also possible.12 This is defined as the postinfectious, postantibiotic, or antibiotic-refractory Lyme arthritis, a diagnosis of exclusion more apparent only after patients receive appropriate antibiotic courses for the possibility of untreated Lyme as an active infection.12
Given the inherent diagnostic uncertainty between an active infection and posttreatment Lyme arthritis syndromes, it is best to approach most cases of Lyme arthritis as an active infection first especially if not yet treated with antibiotics. Diagnosis of postinflammatory processes should be considered if symptoms persist after appropriate antibiotics, and then short-term use of disease-modifying antirheumatic drugs, rather than further courses of antibiotics, is recommended.
► Dr. Merz: The patient was initiated on doxycycline with the plan to transition to ceftriaxone if there was no response. One day after diagnosis and treatment initiation and in the setting of continued pain, the patient again asked to leave the hospital to drink alcohol. After eloping and becoming intoxicated with alcohol, he returned to his room. He remained concerned about his continued pain and lack of adequate pain control. At the time, he was receiving hydromorphone, ketorolac, lorazepam, gabapentin, and quetiapine.
Dr. Serrao, do you expect this degree of pain from Lyme arthritis?
► Dr. Serrao: Lyme arthritis is typically less painful than other forms of infectious or inflammatory arthritis. Pain is usually caused by the pressure from the acute accumulation and reaccumulation of fluid. In this case, the rapid accumulation of fluid that this patient experienced as well as relief with arthrocentesis suggests that the size and acuity of the effusion was causing great discomfort. Repeated arthrocentesis can prove to be a preventative strategy to minimize synovial herniation.
►Dr. Merz: Dr. Shahal, how do you balance the patient subjectively telling you that they are in pain with objective signs that they may be tolerating the pain like walking around unassisted? Is there anything else that could have been done to prevent this adverse outcome?
►Dr. Shahal: This is one of the hardest pieces of pain management. We want to practice beneficence by believing our patients and addressing their discomfort, but we also want to practice nonmaleficence by avoiding inappropriate long-term pain treatments like opioids that have significant harm as well as avoiding exacerbating this patient’s underlying SUD. An agent like buprenorphine/naloxone could have been an excellent fit to treat pain and SUD, but the patient’s lack of interest and the frequent use of short-acting opioids were major barriers. A chronic pain consult early on is helpful in cases like this as well, but they were unable to see him since he was often out of his room. Repeated arthrocentesis may also have helped the pain. Treatment of anxiety and insomnia with medications like hydroxyzine, trazodone, melatonin, gabapentin, or buspirone as well as interventions like sleep hygiene protocols or spiritual care may have helped somewhat as well.
We know that there is a vicious cycle between pain and poorly controlled mood symptoms. Many of our veterans have PTSD, anxiety, and SUD that are exacerbated by hospitalization and pain. Maintaining optimal communication between the patient and the practitioners, using trauma-informed care, understanding the patient’s goals of care, setting expectations and limits, and attempting to address the patient’s needs while attempting to minimize stigma might be helpful. However, despite optimal care, sometimes these events cannot be avoided.
►Dr. Merz: The patient was ultimately transferred to an inpatient psychiatric unit where a taper plan for the short-acting opioids was implemented. He was psychiatrically stabilized and discharged a few days later off opioids and on doxycycline. On follow-up a few weeks later, his pain had markedly improved, and the effusion was significantly reduced in size. His mood and impulsivity had stabilized. He continues to follow-up in the infectious disease clinic.

1. Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68(1):83-90.
2. Qaseem A, McLean RM, O’Gurek D, et al. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: a clinical guideline from the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748. doi:10.7326/M19-3602
3. Silverman MM, Berman AL. Suicide risk assessment and risk formulation part I: a focus on suicide ideation in assessing suicide risk. Suicide Life Threat Behav. 2014;44(4):420-431. doi:10.1111/sltb.12065
4. Berman AL, Silverman MM. Suicide risk assessment and risk formulation part II: Suicide risk formulation and the determination of levels of risk. Suicide Life Threat Behav. 2014;44(4):432-443. doi:10.1111/sltb.12067
5. Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017;2(4):e611. Published 2017 Jul 27. doi:10.1097/PR9.0000000000000611
6. Chou R, Wagner J, Ahmed AY, et al. Treatments for Acute Pain: A Systematic Review. Agency for Healthcare Research and Quality; 2020. https://www.ncbi.nlm.nih.gov/books/NBK566506/
7. Seidman AJ, Limaiem F. Synovial fluid analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Updated May 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537114
8. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280. doi:10.1016/j.idc.2015.02.004
9. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA. 1995;274(12):937.
10. Craft JE, Grodzicki RL, Steere AC. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149(5):789-795. doi:10.1093/infdis/149.5.789
11. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229-234. doi:10.1056/NEJM199401273300401
12. Steere AC. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130(5):2148-2151. doi:10.1172/JCI138062
1. Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68(1):83-90.
2. Qaseem A, McLean RM, O’Gurek D, et al. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: a clinical guideline from the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748. doi:10.7326/M19-3602
3. Silverman MM, Berman AL. Suicide risk assessment and risk formulation part I: a focus on suicide ideation in assessing suicide risk. Suicide Life Threat Behav. 2014;44(4):420-431. doi:10.1111/sltb.12065
4. Berman AL, Silverman MM. Suicide risk assessment and risk formulation part II: Suicide risk formulation and the determination of levels of risk. Suicide Life Threat Behav. 2014;44(4):432-443. doi:10.1111/sltb.12067
5. Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017;2(4):e611. Published 2017 Jul 27. doi:10.1097/PR9.0000000000000611
6. Chou R, Wagner J, Ahmed AY, et al. Treatments for Acute Pain: A Systematic Review. Agency for Healthcare Research and Quality; 2020. https://www.ncbi.nlm.nih.gov/books/NBK566506/
7. Seidman AJ, Limaiem F. Synovial fluid analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Updated May 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537114
8. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280. doi:10.1016/j.idc.2015.02.004
9. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA. 1995;274(12):937.
10. Craft JE, Grodzicki RL, Steere AC. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149(5):789-795. doi:10.1093/infdis/149.5.789
11. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229-234. doi:10.1056/NEJM199401273300401
12. Steere AC. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130(5):2148-2151. doi:10.1172/JCI138062
Things We Do for No Reason™: Universal Venous Thromboembolism Chemoprophylaxis in Low-Risk Hospitalized Medical Patients

Inspired by the ABIM Foundation’s Choosing Wisel y ® campaign, the “Things We Do for No Reason ™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 68-year-old woman for community-acquired pneumonia with a past medical history of hypertension, gastroesophageal reflux disease, and osteoarthritis. Her hospitalist consults physical therapy to maximize mobility; continues her home medications including pantoprazole, hydrochlorothiazide, and acetaminophen; and initiates antimicrobial therapy with ceftriaxone and azithromycin. The hospital admission order set requires administration of subcutaneous unfractionated heparin for venous thromboembolism chemoprophylaxis.
WHY YOU MIGHT THINK UNIVERSAL CHEMOPROPHYLAXIS IS NECESSARY
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), ranks among the leading preventable causes of morbidity and mortality in hospitalized patients.1 DVTs can rapidly progress to a PE, which account for 5% to 10% of in-hospital deaths.1 The negative sequelae of in-hospital VTE, including prolonged hospital stay, increased healthcare costs, and greater risks associated with pharmacologic treatment, add $9 to $18.2 billion in US healthcare expenditures each year.2 Various risk-assessment models (RAMs) identify medical patients at high risk for developing VTE based on the presence of risk factors including acute heart failure, prior history of VTE, and reduced mobility.3 Since hospitalization may itself increase the risk for VTE, medical patients often receive universal chemoprophylaxis with anticoagulants such as unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux.3 A meta-analysis of randomized controlled trials (RCTs) published by Wein et al supports the use of VTE chemoprophylaxis in high-risk patients.4 It showed statistically significant reductions in rates of PE in high-risk hospitalized medical patients with UFH (risk ratio [RR], 0.64; 95% CI, 0.50-0.82) or LMWH chemoprophylaxis (RR, 0.37; 95% CI, 0.21-0.64), compared with controls.
In recognition of the magnitude of the problem, national organizations have emphasized routine chemoprophylaxis for prevention of in-hospital VTE as a top-priority measure for patient safety.5,6 The Joint Commission includes chemoprophylaxis as a quality core metric and failure to adhere to such standards compromises hospital accreditation.5 Since 2008, the Centers for Medicare & Medicaid Services no longer reimburses hospitals for preventable VTE and requires institutions to document the rationale for omitting chemoprophylaxis if not commenced on hospital admission.6
WHY CHEMOPROPHYLAXIS FOR LOW-RISK MEDICAL PATIENTS IS UNNECESSARY
In order to understand why chemoprophylaxis fails to benefit low-risk medical patients, it is necessary to critically examine the benefits identified in trials of high-risk patients. Although RCTs and meta-analysis of chemoprophylaxis have consistently demonstrated a reduction in VTE, prevention of asymptomatic VTE identified on screening with ultrasound or venography accounts for more than 90% of the composite outcome in the three key trials.7-9 Hospitalists do not routinely screen for asymptomatic VTE, and incorporation of these events into composite VTE outcomes inflates the magnitude of benefit gained by chemoprophylaxis. Importantly, the standard of care does not include screening for asymptomatic DVTs, and studies have estimated that only 10% to 15% of asymptomatic DVTs progress to a symptomatic VTE.10
A meta-analysis of trials evaluating unselected general medical patients (ie, not those with specific high-risk conditions such as acute myocardial infarction) did not show a reduction in symptomatic VTE with chemoprophylaxis (odds ratio [OR], 0.59; 95% CI, 0.29-1.23).11 In the meta-analysis by Wein et al, which did include patients with specific high-risk conditions, chemoprophylaxis produced a small absolute risk reduction, resulting in a number needed to treat (NNT) of 345 to prevent one PE.4 This demonstrates that, even in high-risk patients, the magnitude of benefit is small. Population-level data also question the benefit of chemoprophylaxis. Flanders et al stratified 35 Michigan hospitals into high-, moderate-, and low-performance tertiles, with performance based on the rate of chemoprophylaxis use on admission for general medical patients at high-risk for VTE. The authors found no significant difference in the rate of VTE at 90 days among tertiles.12 These findings question the usefulness of universal chemoprophylaxis when applied in a real-world setting.
The high rates of VTE in the absence of chemoprophylaxis reported in historic trials may overestimate the contemporary risk. A 2019 multicenter, observational study examined the rate of hospital-acquired DVT for 1,170 low- and high-risk patients with acute medical illness admitted to the internal medicine ward.13 Of them, 250 (21%) underwent prophylaxis with parenteral anticoagulants (mean Padua Prediction Score, 4.5). The remaining 920 (79%) were not treated with prophylaxis (mean Padua Prediction Score, 2.5). All patients underwent ultrasound at admission and discharge. The average length of stay was 13 days, and just three patients (0.3%) experienced in-hospital DVT, two of whom were receiving chemoprophylaxis. Only one (0.09%) DVT was symptomatic.
It should be emphasized that any evidence favoring chemoprophylaxis comes from studies of patients at high-risk of VTE. No data show benefit for low-risk patients. Therefore, any risk of chemoprophylaxis likely outweighs the benefits in low-risk patients. Importantly, the risks are underappreciated. A 2014 meta-analysis reported an increased risk of major hemorrhage (OR, 1.81; 95% CI, 1.10-2.98; P = .02) in high-risk medically ill patients on chemoprophylaxis.14 This results in a number needed to harm for major bleeding of 336, a value similar to the NNT for benefit reported by Wein et al.4 Heparin-induced thrombocytopenia, a potentially limb- and life-threatening complication of UFH or LMWH exposure, has an overall incidence of 0.3% to 0.7% in hospitalized patients on chemoprophylaxis.3 Finally, the most commonly used chemoprophylaxis medications are administered subcutaneously, resulting in injection site pain. Unsurprisingly, hospitalized patients refuse chemoprophylaxis more frequently than any other medication.15
The negative implications of inappropriate chemoprophylaxis extend beyond direct harms to patients. Poor stratification and overuse results in unnecessary healthcare costs. One single-center retrospective review demonstrated that, after integration of chemoprophylaxis into hospital order sets, 76% of patients received unnecessary administration of chemoprophylaxis, resulting in an annualized expenditure of $77,652.16 This does not take into account costs associated with major bleeds.
Unfortunately, the pendulum has shifted from an era of underprescribing chemoprophylaxis to hospitalized medical patients to one of overprescribing. Data published in 2018 suggest that providers overuse chemoprophylaxis in low-risk medical patients at more than double the rate of underusing it in high-risk patients (57% vs 21%).17
Several national societies, including the often cited American College of Chest Physicians (ACCP) and American Society of Hematology (ASH), provide guidance on the use of VTE chemoprophylaxis in acutely ill medical inpatients.3,18 The ASH guidelines conditionally recommend VTE chemoprophylaxis rather than no chemoprophylaxis.18 However, the guidelines do not provide guidance on a risk-stratified approach and disclose that this recommendation is supported by a low certainty in the evidence of the net health benefit gained.18 Guidelines from ACCP lean towards individualized care and recommend against the use of VTE chemoprophylaxis for hospitalized acutely ill, low-risk medical patients.3
WHAT YOU SHOULD DO INSTEAD
Clinicians should risk stratify using validated RAMs when making a patient-centered treatment plan on admission. The table outlines the most common RAMs with evidence for use in acute medically ill hospitalized patients. Although RAMs have limitations (eg, lack of prospective validation and complexity), the ACCP guidelines advocate for their use.3
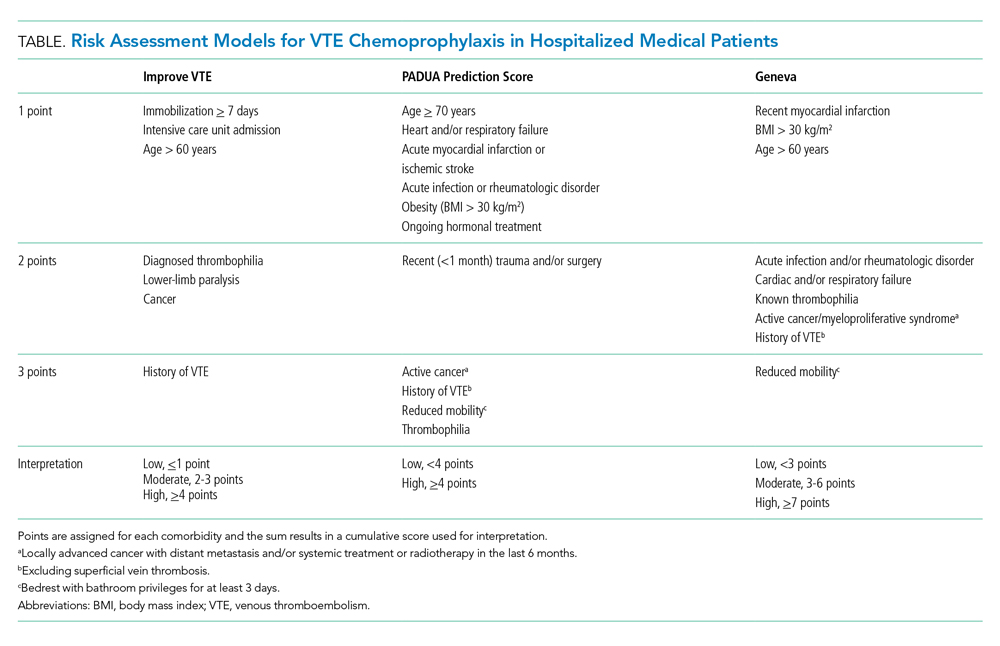
Given that immobility independently increases risk for VTE, early mobilization is a simple and cost-effective way to potentially prevent VTE in low-risk patients. In addition to this potential benefit, early mobilization shortens the length of hospital stay, improves functional status and rates of delirium in hospitalized elderly patients, and hastens postoperative recovery after major surgeries.19
RECOMMENDATIONS
- Incorporate a patient-centered, risk-stratified approach to identify low-risk patients. This can be done manually or with use of RAMS embedded in the electronic health record.
- Do not prescribe chemoprophylaxis to low-risk hospitalized medical patients.
- Emphasize the importance of early mobilization in hospitalized patients.
CONCLUSION
In regard to the case, the hospitalist should use a RAM developed for the nonsurgical, non–critically ill patient to determine her need for chemoprophylaxis. Based on the clinical data presented, the three RAMs available would classify the patient as low risk for developing an in-hospital VTE. She should not receive chemoprophylaxis given the lack of data demonstrating benefit in this population. To mitigate the potential risk of bleeding, heparin-induced thrombocytopenia, and painful injections, the hospitalist should discontinue heparin. The hospitalist should advocate for early mobilization and minimize the duration of hospital stay as appropriate.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
- Francis CW. Clinical practice. prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med. 2007;356(14):1438-1444. https://doi.org/10.1056/nejmcp067264
- Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291-302. https://doi.org/10.1160/th12-03-0162
- Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e195S-e226S. https://doi.org/10.1378/chest.11-2296
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(14):1476-1486. https://doi.org/10.1001/archinte.167.14.1476
- Performance Measurement. The Joint Commission. Updated October 26, 2020. Accessed November 8, 2019. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm
- Venous Thromboembolism Prophylaxis. Centers for Medicare & Medicaid Services. Updated May 6, 2020. Accessed November 8, 2019. https://ecqi.healthit.gov/ecqm/eh/2019/cms108v7
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325-329. https://doi.org/10.1136/bmj.38733.466748.7c
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874-879. https://doi.org/10.1161/01.cir.0000138928.83266.24
- Samama MM, Cohen AT, Darmon JY, et. al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793-800. https://doi.org/10.1056/nejm199909093411103
- Segers AE, Prins MH, Lensing AW, Buller HR. Is contrast venography a valid surrogate outcome measure in venous thromboembolism prevention studies? J Thromb Haemost. 2005;3(5):1099-1102. https://doi.org/10.1111/j.1538-7836.2005.01317.x
- Vardi M, Steinberg M, Haran M, Cohen S. Benefits versus risks of pharmacological prophylaxis to prevent symptomatic venous thromboembolism in unselected medical patients revisited. Meta-analysis of the medical literature. J Thromb Thrombolysis. 2012;34(1):11-19. https://doi.org/10.1007/s11239-012-0730-x
- Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. https://doi.org/10.1001/jamainternmed.2014.3384
- Loffredo L, Arienti V, Vidili G, et al. Low rate of intrahospital deep venous thrombosis in acutely ill medical patients: results from the AURELIO study. Mayo Clin Proc. 2019;94(1):37-43. https://doi.org/10.1016/j.mayocp.2018.07.020
- Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database Syst Rev. 2014;2014(5):CD003747. https://doi.org/10.1002/14651858.cd003747.pub4
- Popoola VO, Lau BD, Tan E, et al. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. Am J Health Syst Pharm. 2018;75(6):392-397. https://doi.org/10.2146/ajhp161057
- Chaudhary R, Damluji A, Batukbhai B, et al. Venous Thromboembolism prophylaxis: inadequate and overprophylaxis when comparing perceived versus calculated risk. Mayo Clin Proc Innov Qual Outcomes. 2017;1(3):242-247. https://doi.org/10.1016/j.mayocpiqo.2017.10.003
- Grant PJ, Conlon A, Chopra V, Flanders SA. Use of venous thromboembolism prophylaxis in hospitalized patients. JAMA Intern Med. 2018;178(8):1122-1124. https://doi.org/10.1001/jamainternmed.2018.2022
- Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. https://doi.org/10.1182/bloodadvances.2018022954
- Pashikanti L, Von Ah D. Impact of early mobilization protocol on the medical-surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87-94. https://doi.org/10.1097/nur.0b013e31824590e6

Inspired by the ABIM Foundation’s Choosing Wisel y ® campaign, the “Things We Do for No Reason ™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 68-year-old woman for community-acquired pneumonia with a past medical history of hypertension, gastroesophageal reflux disease, and osteoarthritis. Her hospitalist consults physical therapy to maximize mobility; continues her home medications including pantoprazole, hydrochlorothiazide, and acetaminophen; and initiates antimicrobial therapy with ceftriaxone and azithromycin. The hospital admission order set requires administration of subcutaneous unfractionated heparin for venous thromboembolism chemoprophylaxis.
WHY YOU MIGHT THINK UNIVERSAL CHEMOPROPHYLAXIS IS NECESSARY
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), ranks among the leading preventable causes of morbidity and mortality in hospitalized patients.1 DVTs can rapidly progress to a PE, which account for 5% to 10% of in-hospital deaths.1 The negative sequelae of in-hospital VTE, including prolonged hospital stay, increased healthcare costs, and greater risks associated with pharmacologic treatment, add $9 to $18.2 billion in US healthcare expenditures each year.2 Various risk-assessment models (RAMs) identify medical patients at high risk for developing VTE based on the presence of risk factors including acute heart failure, prior history of VTE, and reduced mobility.3 Since hospitalization may itself increase the risk for VTE, medical patients often receive universal chemoprophylaxis with anticoagulants such as unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux.3 A meta-analysis of randomized controlled trials (RCTs) published by Wein et al supports the use of VTE chemoprophylaxis in high-risk patients.4 It showed statistically significant reductions in rates of PE in high-risk hospitalized medical patients with UFH (risk ratio [RR], 0.64; 95% CI, 0.50-0.82) or LMWH chemoprophylaxis (RR, 0.37; 95% CI, 0.21-0.64), compared with controls.
In recognition of the magnitude of the problem, national organizations have emphasized routine chemoprophylaxis for prevention of in-hospital VTE as a top-priority measure for patient safety.5,6 The Joint Commission includes chemoprophylaxis as a quality core metric and failure to adhere to such standards compromises hospital accreditation.5 Since 2008, the Centers for Medicare & Medicaid Services no longer reimburses hospitals for preventable VTE and requires institutions to document the rationale for omitting chemoprophylaxis if not commenced on hospital admission.6
WHY CHEMOPROPHYLAXIS FOR LOW-RISK MEDICAL PATIENTS IS UNNECESSARY
In order to understand why chemoprophylaxis fails to benefit low-risk medical patients, it is necessary to critically examine the benefits identified in trials of high-risk patients. Although RCTs and meta-analysis of chemoprophylaxis have consistently demonstrated a reduction in VTE, prevention of asymptomatic VTE identified on screening with ultrasound or venography accounts for more than 90% of the composite outcome in the three key trials.7-9 Hospitalists do not routinely screen for asymptomatic VTE, and incorporation of these events into composite VTE outcomes inflates the magnitude of benefit gained by chemoprophylaxis. Importantly, the standard of care does not include screening for asymptomatic DVTs, and studies have estimated that only 10% to 15% of asymptomatic DVTs progress to a symptomatic VTE.10
A meta-analysis of trials evaluating unselected general medical patients (ie, not those with specific high-risk conditions such as acute myocardial infarction) did not show a reduction in symptomatic VTE with chemoprophylaxis (odds ratio [OR], 0.59; 95% CI, 0.29-1.23).11 In the meta-analysis by Wein et al, which did include patients with specific high-risk conditions, chemoprophylaxis produced a small absolute risk reduction, resulting in a number needed to treat (NNT) of 345 to prevent one PE.4 This demonstrates that, even in high-risk patients, the magnitude of benefit is small. Population-level data also question the benefit of chemoprophylaxis. Flanders et al stratified 35 Michigan hospitals into high-, moderate-, and low-performance tertiles, with performance based on the rate of chemoprophylaxis use on admission for general medical patients at high-risk for VTE. The authors found no significant difference in the rate of VTE at 90 days among tertiles.12 These findings question the usefulness of universal chemoprophylaxis when applied in a real-world setting.
The high rates of VTE in the absence of chemoprophylaxis reported in historic trials may overestimate the contemporary risk. A 2019 multicenter, observational study examined the rate of hospital-acquired DVT for 1,170 low- and high-risk patients with acute medical illness admitted to the internal medicine ward.13 Of them, 250 (21%) underwent prophylaxis with parenteral anticoagulants (mean Padua Prediction Score, 4.5). The remaining 920 (79%) were not treated with prophylaxis (mean Padua Prediction Score, 2.5). All patients underwent ultrasound at admission and discharge. The average length of stay was 13 days, and just three patients (0.3%) experienced in-hospital DVT, two of whom were receiving chemoprophylaxis. Only one (0.09%) DVT was symptomatic.
It should be emphasized that any evidence favoring chemoprophylaxis comes from studies of patients at high-risk of VTE. No data show benefit for low-risk patients. Therefore, any risk of chemoprophylaxis likely outweighs the benefits in low-risk patients. Importantly, the risks are underappreciated. A 2014 meta-analysis reported an increased risk of major hemorrhage (OR, 1.81; 95% CI, 1.10-2.98; P = .02) in high-risk medically ill patients on chemoprophylaxis.14 This results in a number needed to harm for major bleeding of 336, a value similar to the NNT for benefit reported by Wein et al.4 Heparin-induced thrombocytopenia, a potentially limb- and life-threatening complication of UFH or LMWH exposure, has an overall incidence of 0.3% to 0.7% in hospitalized patients on chemoprophylaxis.3 Finally, the most commonly used chemoprophylaxis medications are administered subcutaneously, resulting in injection site pain. Unsurprisingly, hospitalized patients refuse chemoprophylaxis more frequently than any other medication.15
The negative implications of inappropriate chemoprophylaxis extend beyond direct harms to patients. Poor stratification and overuse results in unnecessary healthcare costs. One single-center retrospective review demonstrated that, after integration of chemoprophylaxis into hospital order sets, 76% of patients received unnecessary administration of chemoprophylaxis, resulting in an annualized expenditure of $77,652.16 This does not take into account costs associated with major bleeds.
Unfortunately, the pendulum has shifted from an era of underprescribing chemoprophylaxis to hospitalized medical patients to one of overprescribing. Data published in 2018 suggest that providers overuse chemoprophylaxis in low-risk medical patients at more than double the rate of underusing it in high-risk patients (57% vs 21%).17
Several national societies, including the often cited American College of Chest Physicians (ACCP) and American Society of Hematology (ASH), provide guidance on the use of VTE chemoprophylaxis in acutely ill medical inpatients.3,18 The ASH guidelines conditionally recommend VTE chemoprophylaxis rather than no chemoprophylaxis.18 However, the guidelines do not provide guidance on a risk-stratified approach and disclose that this recommendation is supported by a low certainty in the evidence of the net health benefit gained.18 Guidelines from ACCP lean towards individualized care and recommend against the use of VTE chemoprophylaxis for hospitalized acutely ill, low-risk medical patients.3
WHAT YOU SHOULD DO INSTEAD
Clinicians should risk stratify using validated RAMs when making a patient-centered treatment plan on admission. The table outlines the most common RAMs with evidence for use in acute medically ill hospitalized patients. Although RAMs have limitations (eg, lack of prospective validation and complexity), the ACCP guidelines advocate for their use.3

Given that immobility independently increases risk for VTE, early mobilization is a simple and cost-effective way to potentially prevent VTE in low-risk patients. In addition to this potential benefit, early mobilization shortens the length of hospital stay, improves functional status and rates of delirium in hospitalized elderly patients, and hastens postoperative recovery after major surgeries.19
RECOMMENDATIONS
- Incorporate a patient-centered, risk-stratified approach to identify low-risk patients. This can be done manually or with use of RAMS embedded in the electronic health record.
- Do not prescribe chemoprophylaxis to low-risk hospitalized medical patients.
- Emphasize the importance of early mobilization in hospitalized patients.
CONCLUSION
In regard to the case, the hospitalist should use a RAM developed for the nonsurgical, non–critically ill patient to determine her need for chemoprophylaxis. Based on the clinical data presented, the three RAMs available would classify the patient as low risk for developing an in-hospital VTE. She should not receive chemoprophylaxis given the lack of data demonstrating benefit in this population. To mitigate the potential risk of bleeding, heparin-induced thrombocytopenia, and painful injections, the hospitalist should discontinue heparin. The hospitalist should advocate for early mobilization and minimize the duration of hospital stay as appropriate.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisel y ® campaign, the “Things We Do for No Reason ™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 68-year-old woman for community-acquired pneumonia with a past medical history of hypertension, gastroesophageal reflux disease, and osteoarthritis. Her hospitalist consults physical therapy to maximize mobility; continues her home medications including pantoprazole, hydrochlorothiazide, and acetaminophen; and initiates antimicrobial therapy with ceftriaxone and azithromycin. The hospital admission order set requires administration of subcutaneous unfractionated heparin for venous thromboembolism chemoprophylaxis.
WHY YOU MIGHT THINK UNIVERSAL CHEMOPROPHYLAXIS IS NECESSARY
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), ranks among the leading preventable causes of morbidity and mortality in hospitalized patients.1 DVTs can rapidly progress to a PE, which account for 5% to 10% of in-hospital deaths.1 The negative sequelae of in-hospital VTE, including prolonged hospital stay, increased healthcare costs, and greater risks associated with pharmacologic treatment, add $9 to $18.2 billion in US healthcare expenditures each year.2 Various risk-assessment models (RAMs) identify medical patients at high risk for developing VTE based on the presence of risk factors including acute heart failure, prior history of VTE, and reduced mobility.3 Since hospitalization may itself increase the risk for VTE, medical patients often receive universal chemoprophylaxis with anticoagulants such as unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux.3 A meta-analysis of randomized controlled trials (RCTs) published by Wein et al supports the use of VTE chemoprophylaxis in high-risk patients.4 It showed statistically significant reductions in rates of PE in high-risk hospitalized medical patients with UFH (risk ratio [RR], 0.64; 95% CI, 0.50-0.82) or LMWH chemoprophylaxis (RR, 0.37; 95% CI, 0.21-0.64), compared with controls.
In recognition of the magnitude of the problem, national organizations have emphasized routine chemoprophylaxis for prevention of in-hospital VTE as a top-priority measure for patient safety.5,6 The Joint Commission includes chemoprophylaxis as a quality core metric and failure to adhere to such standards compromises hospital accreditation.5 Since 2008, the Centers for Medicare & Medicaid Services no longer reimburses hospitals for preventable VTE and requires institutions to document the rationale for omitting chemoprophylaxis if not commenced on hospital admission.6
WHY CHEMOPROPHYLAXIS FOR LOW-RISK MEDICAL PATIENTS IS UNNECESSARY
In order to understand why chemoprophylaxis fails to benefit low-risk medical patients, it is necessary to critically examine the benefits identified in trials of high-risk patients. Although RCTs and meta-analysis of chemoprophylaxis have consistently demonstrated a reduction in VTE, prevention of asymptomatic VTE identified on screening with ultrasound or venography accounts for more than 90% of the composite outcome in the three key trials.7-9 Hospitalists do not routinely screen for asymptomatic VTE, and incorporation of these events into composite VTE outcomes inflates the magnitude of benefit gained by chemoprophylaxis. Importantly, the standard of care does not include screening for asymptomatic DVTs, and studies have estimated that only 10% to 15% of asymptomatic DVTs progress to a symptomatic VTE.10
A meta-analysis of trials evaluating unselected general medical patients (ie, not those with specific high-risk conditions such as acute myocardial infarction) did not show a reduction in symptomatic VTE with chemoprophylaxis (odds ratio [OR], 0.59; 95% CI, 0.29-1.23).11 In the meta-analysis by Wein et al, which did include patients with specific high-risk conditions, chemoprophylaxis produced a small absolute risk reduction, resulting in a number needed to treat (NNT) of 345 to prevent one PE.4 This demonstrates that, even in high-risk patients, the magnitude of benefit is small. Population-level data also question the benefit of chemoprophylaxis. Flanders et al stratified 35 Michigan hospitals into high-, moderate-, and low-performance tertiles, with performance based on the rate of chemoprophylaxis use on admission for general medical patients at high-risk for VTE. The authors found no significant difference in the rate of VTE at 90 days among tertiles.12 These findings question the usefulness of universal chemoprophylaxis when applied in a real-world setting.
The high rates of VTE in the absence of chemoprophylaxis reported in historic trials may overestimate the contemporary risk. A 2019 multicenter, observational study examined the rate of hospital-acquired DVT for 1,170 low- and high-risk patients with acute medical illness admitted to the internal medicine ward.13 Of them, 250 (21%) underwent prophylaxis with parenteral anticoagulants (mean Padua Prediction Score, 4.5). The remaining 920 (79%) were not treated with prophylaxis (mean Padua Prediction Score, 2.5). All patients underwent ultrasound at admission and discharge. The average length of stay was 13 days, and just three patients (0.3%) experienced in-hospital DVT, two of whom were receiving chemoprophylaxis. Only one (0.09%) DVT was symptomatic.
It should be emphasized that any evidence favoring chemoprophylaxis comes from studies of patients at high-risk of VTE. No data show benefit for low-risk patients. Therefore, any risk of chemoprophylaxis likely outweighs the benefits in low-risk patients. Importantly, the risks are underappreciated. A 2014 meta-analysis reported an increased risk of major hemorrhage (OR, 1.81; 95% CI, 1.10-2.98; P = .02) in high-risk medically ill patients on chemoprophylaxis.14 This results in a number needed to harm for major bleeding of 336, a value similar to the NNT for benefit reported by Wein et al.4 Heparin-induced thrombocytopenia, a potentially limb- and life-threatening complication of UFH or LMWH exposure, has an overall incidence of 0.3% to 0.7% in hospitalized patients on chemoprophylaxis.3 Finally, the most commonly used chemoprophylaxis medications are administered subcutaneously, resulting in injection site pain. Unsurprisingly, hospitalized patients refuse chemoprophylaxis more frequently than any other medication.15
The negative implications of inappropriate chemoprophylaxis extend beyond direct harms to patients. Poor stratification and overuse results in unnecessary healthcare costs. One single-center retrospective review demonstrated that, after integration of chemoprophylaxis into hospital order sets, 76% of patients received unnecessary administration of chemoprophylaxis, resulting in an annualized expenditure of $77,652.16 This does not take into account costs associated with major bleeds.
Unfortunately, the pendulum has shifted from an era of underprescribing chemoprophylaxis to hospitalized medical patients to one of overprescribing. Data published in 2018 suggest that providers overuse chemoprophylaxis in low-risk medical patients at more than double the rate of underusing it in high-risk patients (57% vs 21%).17
Several national societies, including the often cited American College of Chest Physicians (ACCP) and American Society of Hematology (ASH), provide guidance on the use of VTE chemoprophylaxis in acutely ill medical inpatients.3,18 The ASH guidelines conditionally recommend VTE chemoprophylaxis rather than no chemoprophylaxis.18 However, the guidelines do not provide guidance on a risk-stratified approach and disclose that this recommendation is supported by a low certainty in the evidence of the net health benefit gained.18 Guidelines from ACCP lean towards individualized care and recommend against the use of VTE chemoprophylaxis for hospitalized acutely ill, low-risk medical patients.3
WHAT YOU SHOULD DO INSTEAD
Clinicians should risk stratify using validated RAMs when making a patient-centered treatment plan on admission. The table outlines the most common RAMs with evidence for use in acute medically ill hospitalized patients. Although RAMs have limitations (eg, lack of prospective validation and complexity), the ACCP guidelines advocate for their use.3

Given that immobility independently increases risk for VTE, early mobilization is a simple and cost-effective way to potentially prevent VTE in low-risk patients. In addition to this potential benefit, early mobilization shortens the length of hospital stay, improves functional status and rates of delirium in hospitalized elderly patients, and hastens postoperative recovery after major surgeries.19
RECOMMENDATIONS
- Incorporate a patient-centered, risk-stratified approach to identify low-risk patients. This can be done manually or with use of RAMS embedded in the electronic health record.
- Do not prescribe chemoprophylaxis to low-risk hospitalized medical patients.
- Emphasize the importance of early mobilization in hospitalized patients.
CONCLUSION
In regard to the case, the hospitalist should use a RAM developed for the nonsurgical, non–critically ill patient to determine her need for chemoprophylaxis. Based on the clinical data presented, the three RAMs available would classify the patient as low risk for developing an in-hospital VTE. She should not receive chemoprophylaxis given the lack of data demonstrating benefit in this population. To mitigate the potential risk of bleeding, heparin-induced thrombocytopenia, and painful injections, the hospitalist should discontinue heparin. The hospitalist should advocate for early mobilization and minimize the duration of hospital stay as appropriate.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
- Francis CW. Clinical practice. prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med. 2007;356(14):1438-1444. https://doi.org/10.1056/nejmcp067264
- Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291-302. https://doi.org/10.1160/th12-03-0162
- Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e195S-e226S. https://doi.org/10.1378/chest.11-2296
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(14):1476-1486. https://doi.org/10.1001/archinte.167.14.1476
- Performance Measurement. The Joint Commission. Updated October 26, 2020. Accessed November 8, 2019. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm
- Venous Thromboembolism Prophylaxis. Centers for Medicare & Medicaid Services. Updated May 6, 2020. Accessed November 8, 2019. https://ecqi.healthit.gov/ecqm/eh/2019/cms108v7
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325-329. https://doi.org/10.1136/bmj.38733.466748.7c
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874-879. https://doi.org/10.1161/01.cir.0000138928.83266.24
- Samama MM, Cohen AT, Darmon JY, et. al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793-800. https://doi.org/10.1056/nejm199909093411103
- Segers AE, Prins MH, Lensing AW, Buller HR. Is contrast venography a valid surrogate outcome measure in venous thromboembolism prevention studies? J Thromb Haemost. 2005;3(5):1099-1102. https://doi.org/10.1111/j.1538-7836.2005.01317.x
- Vardi M, Steinberg M, Haran M, Cohen S. Benefits versus risks of pharmacological prophylaxis to prevent symptomatic venous thromboembolism in unselected medical patients revisited. Meta-analysis of the medical literature. J Thromb Thrombolysis. 2012;34(1):11-19. https://doi.org/10.1007/s11239-012-0730-x
- Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. https://doi.org/10.1001/jamainternmed.2014.3384
- Loffredo L, Arienti V, Vidili G, et al. Low rate of intrahospital deep venous thrombosis in acutely ill medical patients: results from the AURELIO study. Mayo Clin Proc. 2019;94(1):37-43. https://doi.org/10.1016/j.mayocp.2018.07.020
- Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database Syst Rev. 2014;2014(5):CD003747. https://doi.org/10.1002/14651858.cd003747.pub4
- Popoola VO, Lau BD, Tan E, et al. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. Am J Health Syst Pharm. 2018;75(6):392-397. https://doi.org/10.2146/ajhp161057
- Chaudhary R, Damluji A, Batukbhai B, et al. Venous Thromboembolism prophylaxis: inadequate and overprophylaxis when comparing perceived versus calculated risk. Mayo Clin Proc Innov Qual Outcomes. 2017;1(3):242-247. https://doi.org/10.1016/j.mayocpiqo.2017.10.003
- Grant PJ, Conlon A, Chopra V, Flanders SA. Use of venous thromboembolism prophylaxis in hospitalized patients. JAMA Intern Med. 2018;178(8):1122-1124. https://doi.org/10.1001/jamainternmed.2018.2022
- Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. https://doi.org/10.1182/bloodadvances.2018022954
- Pashikanti L, Von Ah D. Impact of early mobilization protocol on the medical-surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87-94. https://doi.org/10.1097/nur.0b013e31824590e6
- Francis CW. Clinical practice. prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med. 2007;356(14):1438-1444. https://doi.org/10.1056/nejmcp067264
- Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291-302. https://doi.org/10.1160/th12-03-0162
- Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e195S-e226S. https://doi.org/10.1378/chest.11-2296
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(14):1476-1486. https://doi.org/10.1001/archinte.167.14.1476
- Performance Measurement. The Joint Commission. Updated October 26, 2020. Accessed November 8, 2019. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm
- Venous Thromboembolism Prophylaxis. Centers for Medicare & Medicaid Services. Updated May 6, 2020. Accessed November 8, 2019. https://ecqi.healthit.gov/ecqm/eh/2019/cms108v7
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325-329. https://doi.org/10.1136/bmj.38733.466748.7c
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874-879. https://doi.org/10.1161/01.cir.0000138928.83266.24
- Samama MM, Cohen AT, Darmon JY, et. al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793-800. https://doi.org/10.1056/nejm199909093411103
- Segers AE, Prins MH, Lensing AW, Buller HR. Is contrast venography a valid surrogate outcome measure in venous thromboembolism prevention studies? J Thromb Haemost. 2005;3(5):1099-1102. https://doi.org/10.1111/j.1538-7836.2005.01317.x
- Vardi M, Steinberg M, Haran M, Cohen S. Benefits versus risks of pharmacological prophylaxis to prevent symptomatic venous thromboembolism in unselected medical patients revisited. Meta-analysis of the medical literature. J Thromb Thrombolysis. 2012;34(1):11-19. https://doi.org/10.1007/s11239-012-0730-x
- Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. https://doi.org/10.1001/jamainternmed.2014.3384
- Loffredo L, Arienti V, Vidili G, et al. Low rate of intrahospital deep venous thrombosis in acutely ill medical patients: results from the AURELIO study. Mayo Clin Proc. 2019;94(1):37-43. https://doi.org/10.1016/j.mayocp.2018.07.020
- Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database Syst Rev. 2014;2014(5):CD003747. https://doi.org/10.1002/14651858.cd003747.pub4
- Popoola VO, Lau BD, Tan E, et al. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. Am J Health Syst Pharm. 2018;75(6):392-397. https://doi.org/10.2146/ajhp161057
- Chaudhary R, Damluji A, Batukbhai B, et al. Venous Thromboembolism prophylaxis: inadequate and overprophylaxis when comparing perceived versus calculated risk. Mayo Clin Proc Innov Qual Outcomes. 2017;1(3):242-247. https://doi.org/10.1016/j.mayocpiqo.2017.10.003
- Grant PJ, Conlon A, Chopra V, Flanders SA. Use of venous thromboembolism prophylaxis in hospitalized patients. JAMA Intern Med. 2018;178(8):1122-1124. https://doi.org/10.1001/jamainternmed.2018.2022
- Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. https://doi.org/10.1182/bloodadvances.2018022954
- Pashikanti L, Von Ah D. Impact of early mobilization protocol on the medical-surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87-94. https://doi.org/10.1097/nur.0b013e31824590e6
© 2020 Society of Hospital Medicine
Email: blba249@uky.edu; Telephone: 267-627-4207; Twitter @theABofPharmaC.
A Veteran Presenting With Chronic Progressive Dyspnea on Exertion
Case Presentation: A 45-year-old US Coast Guard veteran with a medical history of asthma and chronic back pain was referred to the VA Boston Healthcare System (VABHS) for evaluation of progressive, unexplained dyspnea. Two years prior to presentation, the patient was an avid outdoorsman and highly active. At the time of his initial primary care physician (PCP) evaluation he reported dyspnea on exertion, and symptoms consistent with an upper respiratory tract infection (URTI) and a recent tick bite with an associated rash. He was treated with intranasal fluticasone and a course of antibiotics. His URTI symptoms and rash improved; however the dyspnea persisted and progressed over the ensuing winter and he was referred for pulmonary function testing. Additional history included a 20 pack-year history of smoking (resolved 10 years prior to the first VABHS clinical encounter) and a family history of premature coronary artery disease (CAD) in his father and 2 paternal uncles. He lived in northern New England where he previously worked as a cemetery groundskeeper.
►Kristopher Clark, MD, Chief Medical Resident, VABHS and Boston University/Boston Medical Center: Dr. Goldstein, how do you approach a patient who presents with progressive dyspnea?
►Ronald Goldstein, MD, Chief of Pulmonary and Critical Care VABHS: The evaluation of dyspnea is a common problem for pulmonary physicians. The sensation of dyspnea may originate from a wide variety of etiologies that involve pulmonary and cardiovascular disorders, neuromuscular impairment, deconditioning, or psychological issues. It is important to characterize the temporal pattern, severity, progression, relation to exertion or other triggers, the smoking history, environmental and occupational exposures to pulmonary toxins, associated symptoms, and the history of pulmonary problems.1
The physical examination may help to identify an airway or parenchymal disorder. Wheezing on chest examination would point to an obstructive defect and crackles to a possible restrictive problem, including pulmonary fibrosis. A cardiac examination should be performed to assess for evidence of heart failure, valvular heart disease, or the presence of loud P2 suggestive of pulmonary hypertension (PH). Laboratory studies, including complete blood counts are indicated.
A more complete pulmonary evaluation usually involves pulmonary function tests (PFTs), oximetry with exertion, and chest imaging. Additional cardiac testing might include electrocardiogram (ECG) and cardiac echocardiogram, followed by an exercise study, if needed. A B-natriuretic peptide determination could be considered if there is concern for congestive heart failure.2
►Dr. Clark: The initial physical examination was normal and laboratory tests were unrevealing. Given his history of asthma, he underwent spirometry testing (Table 1).
Dr. Goldstein, aside from unexplained dyspnea, what are other indications for spirometry and when should we consider ordering a full PFT, including lung volumes and diffusion capacity? Can you interpret this patient’s spirometry results?
►Dr. Goldstein: Spirometry is indicated to evaluate for a suspected obstructive defect. The test is usually performed with and without a bronchodilator to assess airway reactivity. A change in > 12% and > 200 mL suggests acute bronchodilator responsiveness. Periodic spirometry determinations are useful to assess the effect of medications or progression of disease. A reduction in forced vital capacity (FVC) may suggest a restrictive component. This possibility requires measure of lung volumes.
A full set of PFTs (ie, spirometry plus assessment of lung volumes and diffusion capacity) is required to evaluate the abnormalities associated with chronic obstructive pulmonary disease (COPD), interstitial diseases, vascular abnormalities (particularly PH), as well as for certain preoperative assessments. The single breath diffusing capacity for carbon monoxide is a measure of the overall capillary alveolar surface area of the lung. It is decreased in emphysema and interstitial disease as well as pulmonary vascular disorders. It would be particularly useful in this case as the spirometry studies were normal.
In this case, the normal FVC renders a significant restrictive disorder unlikely and his normal forced expiratory volume (FEV1) and FEV1/FVC make a significant obstructive disorder unlikely. He did not show any bronchodilator response; however, this finding does not exclude the presence of underlying asthma or reactive airway disease as patients often will not show a bronchodilator response at time of testing if they are not experiencing active bronchospasm or constriction. Further provocative testing with a methacholine challenge could be used to assess for reactive airway disease.
►Dr. Clark: The patient continued to have dyspnea when he returned to his PCP. Given his family history of premature CAD, an ECG was obtained that showed normal sinus rhythm at a rate of 70 beats per minute. A cardiology consult was placed, and he was referred for cardiac stress testing.
Dr. Maron, there are many forms of cardiac stress tests. In this case, the patient is referred for a stress test due his dyspnea. Does that symptom help you decide which test to order? How often does dyspnea present as an anginal equivalent in the absence of other cardiovascular symptoms or known cardiovascular disease?
►Bradley Maron, MD, Codirector, Pulmonary Vascular Disease Center, VABHS: In this case, stress testing should include a functional (ie, exercise) assessment if possible. Exercise capacity is a critical determinant of prognosis across the spectrum of cardiovascular disease and in a young person can be particularly informative on global health status. Furthermore, the chief complaint from this patient is dyspnea on exertion, and therefore, exercise testing is likely to be needed to reproduce or provoke the main symptom in this case. Estimates for dyspnea as a presenting symptom for ischemic heart disease vary but may be as high as 25%.3 It should be noted that cardiopulmonary exercise testing is useful for evaluating patients with unexplained dyspnea, as exercise hypoxemia, blunted decrease in VD/VT (ventilatory dead space/tidal volume), and evidence of a pulmonary mechanical limit to physical activity can inform the differential diagnosis.
►Dr. Clark: The patient underwent exercise treadmill testing and was able reach the target heart rate (> 85% age-predicted maximal heart rate) and achieve 11 metabolic equivalents. He had no chest pain or diagnostic ECG changes. The report made no mention of whether he experienced dyspnea during the test and was read as negative for exercise-induced ischemia.
He was seen by a cardiologist who noted an increased intensity S2 heart sound on examination without any other cardiopulmonary findings. It was noted that his symptoms occurred when tamping the ground or starting to walk up a hill but resolved with rest. It was also noted that his symptoms did not occur with gradual increased activity such as that performed during an exercise tolerance test. A 2-view chest X-ray was obtained and read as normal. Given the data from this evaluation thus far, the patient was told that his symptoms were most likely a result of his asthma exacerbated by dirt and dust exposure. Continued use of albuterol inhaler therapy was recommended, and no further diagnostic assessment was pursued.
Approximately 11 months later, the patient presented again to his PCP and reported progressive dyspnea. He had delayed seeking further care as he started to “feel like my symptoms were possibly in my head” given his prior negative workup. His symptoms had escalated drastically to the point where he felt short of breath with minimal exertion in addition to feeling sweaty, dizzy, fatigued, and having near-syncope when standing.
He was referred for a transthoracic echocardiogram (TTE) that revealed a left ventricular ejection fraction (LVEF) of 55 to 60% with diastolic relaxation abnormality and a normal-sized left atrium. The TTE also showed (qualitatively) a moderately dilated right ventricle with reduced systolic function, moderately severe tricuspid regurgitation, and severe elevation (> 60 mm Hg) in estimated right ventricular systolic pressure.
Dr. Maron, can you comment on how these findings may explain the patient’s symptoms? What differential diagnoses would you now consider?
►Dr. Maron: These echocardiography results exclude left ventricular systolic dysfunction or primary left-sided valvular disease at rest as a cause of the patient’s symptoms. In light of the patient’s prior normal stress test, high grade coronary disease in the absence of LV systolic dysfunction on echocardiography also seems unlikely. Estimated pulmonary artery systolic pressure > 60 mm Hg by echocardiography is highly suggestive of PH, but in and of itself does not diagnose PH nor inform pulmonary artery wedge pressure or pulmonary vascular resistance. Along with a direct measurement of pulmonary artery (PA) pressure, these data are needed to establish, classify, and prognosticate PH clinically.
►Dr. Clark: The patient was referred to a pulmonologist. His examination included bibasilar crackles and an enhanced P2 heart sound. A comprehensive pulmonary history was obtained, which noted his smoking history, possible asbestos exposure while serving in the Coast Guard, nighttime snoring without witnessed apnea events, and no personal or family history of thromboembolism or connective tissue disease.
Dr. Goldstein, is there anything in this patient’s history that could explain his symptoms and echocardiograph findings? Which tests would you order next?
►Dr. Goldstein: PH may be secondary to a wide variety of disorders including left heart disease (Group 2), advanced COPD, interstitial fibrosis, obstructive sleep apnea (OSA), or other lung diseases (Group 3), thromboembolic disorders (Group 4), and other systemic diseases such as sarcoidosis (Group 5). Group 1 is pulmonary arterial hypertension. (Table 2).
A right heart catheterization should be done to confirm the PA pressures estimated by echocardiogram. As to a cause, clinically he does not have heart failure. The limited smoking history and spirometry data do not support advanced COPD. He was noted to have crackles on physical examination suggesting an interstitial disorder. To assess the extent of interstitial disease, we would obtain a noncontrast computed tomography (CT) of the chest. The history of snoring suggesting the possibility of OSA indicating the need for overnight oximetry as significant nocturnal hypoxemia is a possible contributing cause to PH. A polysomnogram would be required to fully evaluate a sleep disturbance. The possible asbestos exposure is not likely a contributing factor as asbestosis requires significant exposure. We would obtain a ventilation/perfusion (V/Q) scan to rule out chronic thromboembolic disease. Targeted tests for causes of Group 5 disease should also be done.
►Dr. Clark: The impression from his pulmonologist was that the patient has severe PH, though the specific etiology was not yet known. Dr. Maron, can you review for us the pathophysiology behind PH and describe how the disease is classified?
►Dr. Maron: Elevated mean pulmonary artery pressure (> 20 mm Hg) diagnosed by supine right heart catheterization is the sine qua non of PH.4 However, this alone does not inform pathophysiology. As Dr. Goldstein noted, elevated PA pressure may be due to left heart disease, primary parenchymal lung disease/sleep-disordered breathing, in situ thrombotic remodeling of pulmonary arterioles following prior luminal pulmonary embolism, or in the setting of various specific predisposing conditions, such as sickle cell disease and sarcoidosis among others.5
Alternatively, pulmonary arterial hypertension (PAH) is suspected in patients with no identifiable cause of PH, pulmonary artery wedge pressure 15 mm Hg and pulmonary vascular resistance of 3.0 Wood units.6 Importantly, PAH is not synonymous with PH but is a circumspect PH disease subgroup. In turn, PAH may be idiopathic, hereditary, or associated with other select, predisposing disorders, namely systemic sclerosis. In PAH, the interplay between genetic and molecular factors results in effacement of distal pulmonary arterioles due to plexigenic, fibrotic, and/or concentric hypertrophic remodeling. Increased vascular resistance promotes early right ventricular dilation and impaired systolic function. As a result, patients with PAH are at particularly elevated risk for cor pulmonale.
►Dr. Clark: Overnight oximetry revealed baseline oxygen saturation of 94%, an oxygen nadir of 84% with a total of 7 minutes with oxygen < 90%. On a 6-minute walk test, the patient had a max heart rate of 116 and oxygen nadir of 93%. Chest CT with and without contrast showed no evidence of pulmonary emboli but noted mild emphysematous changes. A V/Q revealed no evidence of acute or chronic pulmonary thromboembolic disease. Coronary catheterization showed normal coronary anatomy without significant CAD. A right heart catheterization showed findings consistent with severe PH with normal left-sided filling pressures (Table 3).
The patient returned a normal antinuclear antibody, C-reactive protein, HIV, and liver function panel. Based on these findings, a presumptive diagnosis of group 1 PH (idiopathic PAH) was made. Given the severity of his right heart dysfunction, he was transferred to the cardiac care unit and initiated on epoprostenol.
Dr. Maron, can you review the different treatment options for idiopathic PAH and explain why epoprostenol was chosen for this patient?
►Dr. Maron: There are 14 US Food and Drug Administration-approved drug therapies for patients with PAH, which all target either nitric oxide signaling, endothelin receptors, or the prostacyclin pathway. In the current era, treatment-naïve patients with PAH are generally initiated on calcium channel antagonist therapy if there is evidence of vasoreactivity during right heart catheterization (following nitric oxide administration), dual therapy most often with an endothelin receptor antagonist and phosphodiesterase inhibitor, or parenteral prostacyclin therapy. Since < 5% of patients will demonstrate vasoreactivity, the decision at point of care in incident patients with PAH often focuses on dual oral therapy or initiation of parenteral prostacyclin therapy. In this case, the patient reported presyncope with minimal physical activity (eg, bending over or walking up stairs) and severely decreased functional status (ie, New York Heart Association Functional [NYHA] Class III – IV), and he had a cardiac index within the range of cardiogenic shock (< 2.0 L/min/m2). Collectively, this clinical profile is considered particularly high risk, therefore, a recommendation for parenteral continuous prostacyclin therapy was made.
► Dr. Clark: The patient tolerated epoprostenol and reported improvement in his symptoms. He had a tunneled line catheter placed for continuous epoprostenol infusion. He was discharged home and scheduled for outpatient follow-up in a PH clinic. At 4 months following discharge, he was reporting steady clinical and functional improvement as well as improvement in his dyspnea. A second therapy (oral phosphodiesterase type-V inhibitor) was initiated and tolerated well. Overall, he reported resolution of presyncope, NYHA Functional Class II symptoms, and the absence of important drug effects.
1.. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547-1553. doi:10.1056/NEJM199512073332307
2. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi:10.1164/rccm.201111-2042ST
3. Phibbs B, Holmes RW, Lowe CR. Transient myocardial ischemia: the significance of dyspnea. Am J Med Sci. 1968;256(4):210-221. doi:10.1097/00000441-196810000-00002
4. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. doi:10.1161/CIRCULATIONAHA.115.020207
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. Published 2019 Jan 24. doi:10.1183/13993003.01913-2018
6. Maron BA, Galiè N. Diagnosis, Treatment, and Clinical Management of Pulmonary Arterial Hypertension in the Contemporary Era: A Review. JAMA Cardiol. 2016;1(9):1056-1065. doi:10.1001/jamacardio.2016.4471
Case Presentation: A 45-year-old US Coast Guard veteran with a medical history of asthma and chronic back pain was referred to the VA Boston Healthcare System (VABHS) for evaluation of progressive, unexplained dyspnea. Two years prior to presentation, the patient was an avid outdoorsman and highly active. At the time of his initial primary care physician (PCP) evaluation he reported dyspnea on exertion, and symptoms consistent with an upper respiratory tract infection (URTI) and a recent tick bite with an associated rash. He was treated with intranasal fluticasone and a course of antibiotics. His URTI symptoms and rash improved; however the dyspnea persisted and progressed over the ensuing winter and he was referred for pulmonary function testing. Additional history included a 20 pack-year history of smoking (resolved 10 years prior to the first VABHS clinical encounter) and a family history of premature coronary artery disease (CAD) in his father and 2 paternal uncles. He lived in northern New England where he previously worked as a cemetery groundskeeper.
►Kristopher Clark, MD, Chief Medical Resident, VABHS and Boston University/Boston Medical Center: Dr. Goldstein, how do you approach a patient who presents with progressive dyspnea?
►Ronald Goldstein, MD, Chief of Pulmonary and Critical Care VABHS: The evaluation of dyspnea is a common problem for pulmonary physicians. The sensation of dyspnea may originate from a wide variety of etiologies that involve pulmonary and cardiovascular disorders, neuromuscular impairment, deconditioning, or psychological issues. It is important to characterize the temporal pattern, severity, progression, relation to exertion or other triggers, the smoking history, environmental and occupational exposures to pulmonary toxins, associated symptoms, and the history of pulmonary problems.1
The physical examination may help to identify an airway or parenchymal disorder. Wheezing on chest examination would point to an obstructive defect and crackles to a possible restrictive problem, including pulmonary fibrosis. A cardiac examination should be performed to assess for evidence of heart failure, valvular heart disease, or the presence of loud P2 suggestive of pulmonary hypertension (PH). Laboratory studies, including complete blood counts are indicated.
A more complete pulmonary evaluation usually involves pulmonary function tests (PFTs), oximetry with exertion, and chest imaging. Additional cardiac testing might include electrocardiogram (ECG) and cardiac echocardiogram, followed by an exercise study, if needed. A B-natriuretic peptide determination could be considered if there is concern for congestive heart failure.2
►Dr. Clark: The initial physical examination was normal and laboratory tests were unrevealing. Given his history of asthma, he underwent spirometry testing (Table 1).
Dr. Goldstein, aside from unexplained dyspnea, what are other indications for spirometry and when should we consider ordering a full PFT, including lung volumes and diffusion capacity? Can you interpret this patient’s spirometry results?
►Dr. Goldstein: Spirometry is indicated to evaluate for a suspected obstructive defect. The test is usually performed with and without a bronchodilator to assess airway reactivity. A change in > 12% and > 200 mL suggests acute bronchodilator responsiveness. Periodic spirometry determinations are useful to assess the effect of medications or progression of disease. A reduction in forced vital capacity (FVC) may suggest a restrictive component. This possibility requires measure of lung volumes.
A full set of PFTs (ie, spirometry plus assessment of lung volumes and diffusion capacity) is required to evaluate the abnormalities associated with chronic obstructive pulmonary disease (COPD), interstitial diseases, vascular abnormalities (particularly PH), as well as for certain preoperative assessments. The single breath diffusing capacity for carbon monoxide is a measure of the overall capillary alveolar surface area of the lung. It is decreased in emphysema and interstitial disease as well as pulmonary vascular disorders. It would be particularly useful in this case as the spirometry studies were normal.
In this case, the normal FVC renders a significant restrictive disorder unlikely and his normal forced expiratory volume (FEV1) and FEV1/FVC make a significant obstructive disorder unlikely. He did not show any bronchodilator response; however, this finding does not exclude the presence of underlying asthma or reactive airway disease as patients often will not show a bronchodilator response at time of testing if they are not experiencing active bronchospasm or constriction. Further provocative testing with a methacholine challenge could be used to assess for reactive airway disease.
►Dr. Clark: The patient continued to have dyspnea when he returned to his PCP. Given his family history of premature CAD, an ECG was obtained that showed normal sinus rhythm at a rate of 70 beats per minute. A cardiology consult was placed, and he was referred for cardiac stress testing.
Dr. Maron, there are many forms of cardiac stress tests. In this case, the patient is referred for a stress test due his dyspnea. Does that symptom help you decide which test to order? How often does dyspnea present as an anginal equivalent in the absence of other cardiovascular symptoms or known cardiovascular disease?
►Bradley Maron, MD, Codirector, Pulmonary Vascular Disease Center, VABHS: In this case, stress testing should include a functional (ie, exercise) assessment if possible. Exercise capacity is a critical determinant of prognosis across the spectrum of cardiovascular disease and in a young person can be particularly informative on global health status. Furthermore, the chief complaint from this patient is dyspnea on exertion, and therefore, exercise testing is likely to be needed to reproduce or provoke the main symptom in this case. Estimates for dyspnea as a presenting symptom for ischemic heart disease vary but may be as high as 25%.3 It should be noted that cardiopulmonary exercise testing is useful for evaluating patients with unexplained dyspnea, as exercise hypoxemia, blunted decrease in VD/VT (ventilatory dead space/tidal volume), and evidence of a pulmonary mechanical limit to physical activity can inform the differential diagnosis.
►Dr. Clark: The patient underwent exercise treadmill testing and was able reach the target heart rate (> 85% age-predicted maximal heart rate) and achieve 11 metabolic equivalents. He had no chest pain or diagnostic ECG changes. The report made no mention of whether he experienced dyspnea during the test and was read as negative for exercise-induced ischemia.
He was seen by a cardiologist who noted an increased intensity S2 heart sound on examination without any other cardiopulmonary findings. It was noted that his symptoms occurred when tamping the ground or starting to walk up a hill but resolved with rest. It was also noted that his symptoms did not occur with gradual increased activity such as that performed during an exercise tolerance test. A 2-view chest X-ray was obtained and read as normal. Given the data from this evaluation thus far, the patient was told that his symptoms were most likely a result of his asthma exacerbated by dirt and dust exposure. Continued use of albuterol inhaler therapy was recommended, and no further diagnostic assessment was pursued.
Approximately 11 months later, the patient presented again to his PCP and reported progressive dyspnea. He had delayed seeking further care as he started to “feel like my symptoms were possibly in my head” given his prior negative workup. His symptoms had escalated drastically to the point where he felt short of breath with minimal exertion in addition to feeling sweaty, dizzy, fatigued, and having near-syncope when standing.
He was referred for a transthoracic echocardiogram (TTE) that revealed a left ventricular ejection fraction (LVEF) of 55 to 60% with diastolic relaxation abnormality and a normal-sized left atrium. The TTE also showed (qualitatively) a moderately dilated right ventricle with reduced systolic function, moderately severe tricuspid regurgitation, and severe elevation (> 60 mm Hg) in estimated right ventricular systolic pressure.
Dr. Maron, can you comment on how these findings may explain the patient’s symptoms? What differential diagnoses would you now consider?
►Dr. Maron: These echocardiography results exclude left ventricular systolic dysfunction or primary left-sided valvular disease at rest as a cause of the patient’s symptoms. In light of the patient’s prior normal stress test, high grade coronary disease in the absence of LV systolic dysfunction on echocardiography also seems unlikely. Estimated pulmonary artery systolic pressure > 60 mm Hg by echocardiography is highly suggestive of PH, but in and of itself does not diagnose PH nor inform pulmonary artery wedge pressure or pulmonary vascular resistance. Along with a direct measurement of pulmonary artery (PA) pressure, these data are needed to establish, classify, and prognosticate PH clinically.
►Dr. Clark: The patient was referred to a pulmonologist. His examination included bibasilar crackles and an enhanced P2 heart sound. A comprehensive pulmonary history was obtained, which noted his smoking history, possible asbestos exposure while serving in the Coast Guard, nighttime snoring without witnessed apnea events, and no personal or family history of thromboembolism or connective tissue disease.
Dr. Goldstein, is there anything in this patient’s history that could explain his symptoms and echocardiograph findings? Which tests would you order next?
►Dr. Goldstein: PH may be secondary to a wide variety of disorders including left heart disease (Group 2), advanced COPD, interstitial fibrosis, obstructive sleep apnea (OSA), or other lung diseases (Group 3), thromboembolic disorders (Group 4), and other systemic diseases such as sarcoidosis (Group 5). Group 1 is pulmonary arterial hypertension. (Table 2).
A right heart catheterization should be done to confirm the PA pressures estimated by echocardiogram. As to a cause, clinically he does not have heart failure. The limited smoking history and spirometry data do not support advanced COPD. He was noted to have crackles on physical examination suggesting an interstitial disorder. To assess the extent of interstitial disease, we would obtain a noncontrast computed tomography (CT) of the chest. The history of snoring suggesting the possibility of OSA indicating the need for overnight oximetry as significant nocturnal hypoxemia is a possible contributing cause to PH. A polysomnogram would be required to fully evaluate a sleep disturbance. The possible asbestos exposure is not likely a contributing factor as asbestosis requires significant exposure. We would obtain a ventilation/perfusion (V/Q) scan to rule out chronic thromboembolic disease. Targeted tests for causes of Group 5 disease should also be done.
►Dr. Clark: The impression from his pulmonologist was that the patient has severe PH, though the specific etiology was not yet known. Dr. Maron, can you review for us the pathophysiology behind PH and describe how the disease is classified?
►Dr. Maron: Elevated mean pulmonary artery pressure (> 20 mm Hg) diagnosed by supine right heart catheterization is the sine qua non of PH.4 However, this alone does not inform pathophysiology. As Dr. Goldstein noted, elevated PA pressure may be due to left heart disease, primary parenchymal lung disease/sleep-disordered breathing, in situ thrombotic remodeling of pulmonary arterioles following prior luminal pulmonary embolism, or in the setting of various specific predisposing conditions, such as sickle cell disease and sarcoidosis among others.5
Alternatively, pulmonary arterial hypertension (PAH) is suspected in patients with no identifiable cause of PH, pulmonary artery wedge pressure 15 mm Hg and pulmonary vascular resistance of 3.0 Wood units.6 Importantly, PAH is not synonymous with PH but is a circumspect PH disease subgroup. In turn, PAH may be idiopathic, hereditary, or associated with other select, predisposing disorders, namely systemic sclerosis. In PAH, the interplay between genetic and molecular factors results in effacement of distal pulmonary arterioles due to plexigenic, fibrotic, and/or concentric hypertrophic remodeling. Increased vascular resistance promotes early right ventricular dilation and impaired systolic function. As a result, patients with PAH are at particularly elevated risk for cor pulmonale.
►Dr. Clark: Overnight oximetry revealed baseline oxygen saturation of 94%, an oxygen nadir of 84% with a total of 7 minutes with oxygen < 90%. On a 6-minute walk test, the patient had a max heart rate of 116 and oxygen nadir of 93%. Chest CT with and without contrast showed no evidence of pulmonary emboli but noted mild emphysematous changes. A V/Q revealed no evidence of acute or chronic pulmonary thromboembolic disease. Coronary catheterization showed normal coronary anatomy without significant CAD. A right heart catheterization showed findings consistent with severe PH with normal left-sided filling pressures (Table 3).
The patient returned a normal antinuclear antibody, C-reactive protein, HIV, and liver function panel. Based on these findings, a presumptive diagnosis of group 1 PH (idiopathic PAH) was made. Given the severity of his right heart dysfunction, he was transferred to the cardiac care unit and initiated on epoprostenol.
Dr. Maron, can you review the different treatment options for idiopathic PAH and explain why epoprostenol was chosen for this patient?
►Dr. Maron: There are 14 US Food and Drug Administration-approved drug therapies for patients with PAH, which all target either nitric oxide signaling, endothelin receptors, or the prostacyclin pathway. In the current era, treatment-naïve patients with PAH are generally initiated on calcium channel antagonist therapy if there is evidence of vasoreactivity during right heart catheterization (following nitric oxide administration), dual therapy most often with an endothelin receptor antagonist and phosphodiesterase inhibitor, or parenteral prostacyclin therapy. Since < 5% of patients will demonstrate vasoreactivity, the decision at point of care in incident patients with PAH often focuses on dual oral therapy or initiation of parenteral prostacyclin therapy. In this case, the patient reported presyncope with minimal physical activity (eg, bending over or walking up stairs) and severely decreased functional status (ie, New York Heart Association Functional [NYHA] Class III – IV), and he had a cardiac index within the range of cardiogenic shock (< 2.0 L/min/m2). Collectively, this clinical profile is considered particularly high risk, therefore, a recommendation for parenteral continuous prostacyclin therapy was made.
► Dr. Clark: The patient tolerated epoprostenol and reported improvement in his symptoms. He had a tunneled line catheter placed for continuous epoprostenol infusion. He was discharged home and scheduled for outpatient follow-up in a PH clinic. At 4 months following discharge, he was reporting steady clinical and functional improvement as well as improvement in his dyspnea. A second therapy (oral phosphodiesterase type-V inhibitor) was initiated and tolerated well. Overall, he reported resolution of presyncope, NYHA Functional Class II symptoms, and the absence of important drug effects.
Case Presentation: A 45-year-old US Coast Guard veteran with a medical history of asthma and chronic back pain was referred to the VA Boston Healthcare System (VABHS) for evaluation of progressive, unexplained dyspnea. Two years prior to presentation, the patient was an avid outdoorsman and highly active. At the time of his initial primary care physician (PCP) evaluation he reported dyspnea on exertion, and symptoms consistent with an upper respiratory tract infection (URTI) and a recent tick bite with an associated rash. He was treated with intranasal fluticasone and a course of antibiotics. His URTI symptoms and rash improved; however the dyspnea persisted and progressed over the ensuing winter and he was referred for pulmonary function testing. Additional history included a 20 pack-year history of smoking (resolved 10 years prior to the first VABHS clinical encounter) and a family history of premature coronary artery disease (CAD) in his father and 2 paternal uncles. He lived in northern New England where he previously worked as a cemetery groundskeeper.
►Kristopher Clark, MD, Chief Medical Resident, VABHS and Boston University/Boston Medical Center: Dr. Goldstein, how do you approach a patient who presents with progressive dyspnea?
►Ronald Goldstein, MD, Chief of Pulmonary and Critical Care VABHS: The evaluation of dyspnea is a common problem for pulmonary physicians. The sensation of dyspnea may originate from a wide variety of etiologies that involve pulmonary and cardiovascular disorders, neuromuscular impairment, deconditioning, or psychological issues. It is important to characterize the temporal pattern, severity, progression, relation to exertion or other triggers, the smoking history, environmental and occupational exposures to pulmonary toxins, associated symptoms, and the history of pulmonary problems.1
The physical examination may help to identify an airway or parenchymal disorder. Wheezing on chest examination would point to an obstructive defect and crackles to a possible restrictive problem, including pulmonary fibrosis. A cardiac examination should be performed to assess for evidence of heart failure, valvular heart disease, or the presence of loud P2 suggestive of pulmonary hypertension (PH). Laboratory studies, including complete blood counts are indicated.
A more complete pulmonary evaluation usually involves pulmonary function tests (PFTs), oximetry with exertion, and chest imaging. Additional cardiac testing might include electrocardiogram (ECG) and cardiac echocardiogram, followed by an exercise study, if needed. A B-natriuretic peptide determination could be considered if there is concern for congestive heart failure.2
►Dr. Clark: The initial physical examination was normal and laboratory tests were unrevealing. Given his history of asthma, he underwent spirometry testing (Table 1).
Dr. Goldstein, aside from unexplained dyspnea, what are other indications for spirometry and when should we consider ordering a full PFT, including lung volumes and diffusion capacity? Can you interpret this patient’s spirometry results?
►Dr. Goldstein: Spirometry is indicated to evaluate for a suspected obstructive defect. The test is usually performed with and without a bronchodilator to assess airway reactivity. A change in > 12% and > 200 mL suggests acute bronchodilator responsiveness. Periodic spirometry determinations are useful to assess the effect of medications or progression of disease. A reduction in forced vital capacity (FVC) may suggest a restrictive component. This possibility requires measure of lung volumes.
A full set of PFTs (ie, spirometry plus assessment of lung volumes and diffusion capacity) is required to evaluate the abnormalities associated with chronic obstructive pulmonary disease (COPD), interstitial diseases, vascular abnormalities (particularly PH), as well as for certain preoperative assessments. The single breath diffusing capacity for carbon monoxide is a measure of the overall capillary alveolar surface area of the lung. It is decreased in emphysema and interstitial disease as well as pulmonary vascular disorders. It would be particularly useful in this case as the spirometry studies were normal.
In this case, the normal FVC renders a significant restrictive disorder unlikely and his normal forced expiratory volume (FEV1) and FEV1/FVC make a significant obstructive disorder unlikely. He did not show any bronchodilator response; however, this finding does not exclude the presence of underlying asthma or reactive airway disease as patients often will not show a bronchodilator response at time of testing if they are not experiencing active bronchospasm or constriction. Further provocative testing with a methacholine challenge could be used to assess for reactive airway disease.
►Dr. Clark: The patient continued to have dyspnea when he returned to his PCP. Given his family history of premature CAD, an ECG was obtained that showed normal sinus rhythm at a rate of 70 beats per minute. A cardiology consult was placed, and he was referred for cardiac stress testing.
Dr. Maron, there are many forms of cardiac stress tests. In this case, the patient is referred for a stress test due his dyspnea. Does that symptom help you decide which test to order? How often does dyspnea present as an anginal equivalent in the absence of other cardiovascular symptoms or known cardiovascular disease?
►Bradley Maron, MD, Codirector, Pulmonary Vascular Disease Center, VABHS: In this case, stress testing should include a functional (ie, exercise) assessment if possible. Exercise capacity is a critical determinant of prognosis across the spectrum of cardiovascular disease and in a young person can be particularly informative on global health status. Furthermore, the chief complaint from this patient is dyspnea on exertion, and therefore, exercise testing is likely to be needed to reproduce or provoke the main symptom in this case. Estimates for dyspnea as a presenting symptom for ischemic heart disease vary but may be as high as 25%.3 It should be noted that cardiopulmonary exercise testing is useful for evaluating patients with unexplained dyspnea, as exercise hypoxemia, blunted decrease in VD/VT (ventilatory dead space/tidal volume), and evidence of a pulmonary mechanical limit to physical activity can inform the differential diagnosis.
►Dr. Clark: The patient underwent exercise treadmill testing and was able reach the target heart rate (> 85% age-predicted maximal heart rate) and achieve 11 metabolic equivalents. He had no chest pain or diagnostic ECG changes. The report made no mention of whether he experienced dyspnea during the test and was read as negative for exercise-induced ischemia.
He was seen by a cardiologist who noted an increased intensity S2 heart sound on examination without any other cardiopulmonary findings. It was noted that his symptoms occurred when tamping the ground or starting to walk up a hill but resolved with rest. It was also noted that his symptoms did not occur with gradual increased activity such as that performed during an exercise tolerance test. A 2-view chest X-ray was obtained and read as normal. Given the data from this evaluation thus far, the patient was told that his symptoms were most likely a result of his asthma exacerbated by dirt and dust exposure. Continued use of albuterol inhaler therapy was recommended, and no further diagnostic assessment was pursued.
Approximately 11 months later, the patient presented again to his PCP and reported progressive dyspnea. He had delayed seeking further care as he started to “feel like my symptoms were possibly in my head” given his prior negative workup. His symptoms had escalated drastically to the point where he felt short of breath with minimal exertion in addition to feeling sweaty, dizzy, fatigued, and having near-syncope when standing.
He was referred for a transthoracic echocardiogram (TTE) that revealed a left ventricular ejection fraction (LVEF) of 55 to 60% with diastolic relaxation abnormality and a normal-sized left atrium. The TTE also showed (qualitatively) a moderately dilated right ventricle with reduced systolic function, moderately severe tricuspid regurgitation, and severe elevation (> 60 mm Hg) in estimated right ventricular systolic pressure.
Dr. Maron, can you comment on how these findings may explain the patient’s symptoms? What differential diagnoses would you now consider?
►Dr. Maron: These echocardiography results exclude left ventricular systolic dysfunction or primary left-sided valvular disease at rest as a cause of the patient’s symptoms. In light of the patient’s prior normal stress test, high grade coronary disease in the absence of LV systolic dysfunction on echocardiography also seems unlikely. Estimated pulmonary artery systolic pressure > 60 mm Hg by echocardiography is highly suggestive of PH, but in and of itself does not diagnose PH nor inform pulmonary artery wedge pressure or pulmonary vascular resistance. Along with a direct measurement of pulmonary artery (PA) pressure, these data are needed to establish, classify, and prognosticate PH clinically.
►Dr. Clark: The patient was referred to a pulmonologist. His examination included bibasilar crackles and an enhanced P2 heart sound. A comprehensive pulmonary history was obtained, which noted his smoking history, possible asbestos exposure while serving in the Coast Guard, nighttime snoring without witnessed apnea events, and no personal or family history of thromboembolism or connective tissue disease.
Dr. Goldstein, is there anything in this patient’s history that could explain his symptoms and echocardiograph findings? Which tests would you order next?
►Dr. Goldstein: PH may be secondary to a wide variety of disorders including left heart disease (Group 2), advanced COPD, interstitial fibrosis, obstructive sleep apnea (OSA), or other lung diseases (Group 3), thromboembolic disorders (Group 4), and other systemic diseases such as sarcoidosis (Group 5). Group 1 is pulmonary arterial hypertension. (Table 2).
A right heart catheterization should be done to confirm the PA pressures estimated by echocardiogram. As to a cause, clinically he does not have heart failure. The limited smoking history and spirometry data do not support advanced COPD. He was noted to have crackles on physical examination suggesting an interstitial disorder. To assess the extent of interstitial disease, we would obtain a noncontrast computed tomography (CT) of the chest. The history of snoring suggesting the possibility of OSA indicating the need for overnight oximetry as significant nocturnal hypoxemia is a possible contributing cause to PH. A polysomnogram would be required to fully evaluate a sleep disturbance. The possible asbestos exposure is not likely a contributing factor as asbestosis requires significant exposure. We would obtain a ventilation/perfusion (V/Q) scan to rule out chronic thromboembolic disease. Targeted tests for causes of Group 5 disease should also be done.
►Dr. Clark: The impression from his pulmonologist was that the patient has severe PH, though the specific etiology was not yet known. Dr. Maron, can you review for us the pathophysiology behind PH and describe how the disease is classified?
►Dr. Maron: Elevated mean pulmonary artery pressure (> 20 mm Hg) diagnosed by supine right heart catheterization is the sine qua non of PH.4 However, this alone does not inform pathophysiology. As Dr. Goldstein noted, elevated PA pressure may be due to left heart disease, primary parenchymal lung disease/sleep-disordered breathing, in situ thrombotic remodeling of pulmonary arterioles following prior luminal pulmonary embolism, or in the setting of various specific predisposing conditions, such as sickle cell disease and sarcoidosis among others.5
Alternatively, pulmonary arterial hypertension (PAH) is suspected in patients with no identifiable cause of PH, pulmonary artery wedge pressure 15 mm Hg and pulmonary vascular resistance of 3.0 Wood units.6 Importantly, PAH is not synonymous with PH but is a circumspect PH disease subgroup. In turn, PAH may be idiopathic, hereditary, or associated with other select, predisposing disorders, namely systemic sclerosis. In PAH, the interplay between genetic and molecular factors results in effacement of distal pulmonary arterioles due to plexigenic, fibrotic, and/or concentric hypertrophic remodeling. Increased vascular resistance promotes early right ventricular dilation and impaired systolic function. As a result, patients with PAH are at particularly elevated risk for cor pulmonale.
►Dr. Clark: Overnight oximetry revealed baseline oxygen saturation of 94%, an oxygen nadir of 84% with a total of 7 minutes with oxygen < 90%. On a 6-minute walk test, the patient had a max heart rate of 116 and oxygen nadir of 93%. Chest CT with and without contrast showed no evidence of pulmonary emboli but noted mild emphysematous changes. A V/Q revealed no evidence of acute or chronic pulmonary thromboembolic disease. Coronary catheterization showed normal coronary anatomy without significant CAD. A right heart catheterization showed findings consistent with severe PH with normal left-sided filling pressures (Table 3).
The patient returned a normal antinuclear antibody, C-reactive protein, HIV, and liver function panel. Based on these findings, a presumptive diagnosis of group 1 PH (idiopathic PAH) was made. Given the severity of his right heart dysfunction, he was transferred to the cardiac care unit and initiated on epoprostenol.
Dr. Maron, can you review the different treatment options for idiopathic PAH and explain why epoprostenol was chosen for this patient?
►Dr. Maron: There are 14 US Food and Drug Administration-approved drug therapies for patients with PAH, which all target either nitric oxide signaling, endothelin receptors, or the prostacyclin pathway. In the current era, treatment-naïve patients with PAH are generally initiated on calcium channel antagonist therapy if there is evidence of vasoreactivity during right heart catheterization (following nitric oxide administration), dual therapy most often with an endothelin receptor antagonist and phosphodiesterase inhibitor, or parenteral prostacyclin therapy. Since < 5% of patients will demonstrate vasoreactivity, the decision at point of care in incident patients with PAH often focuses on dual oral therapy or initiation of parenteral prostacyclin therapy. In this case, the patient reported presyncope with minimal physical activity (eg, bending over or walking up stairs) and severely decreased functional status (ie, New York Heart Association Functional [NYHA] Class III – IV), and he had a cardiac index within the range of cardiogenic shock (< 2.0 L/min/m2). Collectively, this clinical profile is considered particularly high risk, therefore, a recommendation for parenteral continuous prostacyclin therapy was made.
► Dr. Clark: The patient tolerated epoprostenol and reported improvement in his symptoms. He had a tunneled line catheter placed for continuous epoprostenol infusion. He was discharged home and scheduled for outpatient follow-up in a PH clinic. At 4 months following discharge, he was reporting steady clinical and functional improvement as well as improvement in his dyspnea. A second therapy (oral phosphodiesterase type-V inhibitor) was initiated and tolerated well. Overall, he reported resolution of presyncope, NYHA Functional Class II symptoms, and the absence of important drug effects.
1.. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547-1553. doi:10.1056/NEJM199512073332307
2. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi:10.1164/rccm.201111-2042ST
3. Phibbs B, Holmes RW, Lowe CR. Transient myocardial ischemia: the significance of dyspnea. Am J Med Sci. 1968;256(4):210-221. doi:10.1097/00000441-196810000-00002
4. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. doi:10.1161/CIRCULATIONAHA.115.020207
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. Published 2019 Jan 24. doi:10.1183/13993003.01913-2018
6. Maron BA, Galiè N. Diagnosis, Treatment, and Clinical Management of Pulmonary Arterial Hypertension in the Contemporary Era: A Review. JAMA Cardiol. 2016;1(9):1056-1065. doi:10.1001/jamacardio.2016.4471
1.. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547-1553. doi:10.1056/NEJM199512073332307
2. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi:10.1164/rccm.201111-2042ST
3. Phibbs B, Holmes RW, Lowe CR. Transient myocardial ischemia: the significance of dyspnea. Am J Med Sci. 1968;256(4):210-221. doi:10.1097/00000441-196810000-00002
4. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. doi:10.1161/CIRCULATIONAHA.115.020207
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. Published 2019 Jan 24. doi:10.1183/13993003.01913-2018
6. Maron BA, Galiè N. Diagnosis, Treatment, and Clinical Management of Pulmonary Arterial Hypertension in the Contemporary Era: A Review. JAMA Cardiol. 2016;1(9):1056-1065. doi:10.1001/jamacardio.2016.4471
A Veteran Presenting With Altered Mental Status and Clonus
►Zachary Reese, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC):Dr. Weller, the differential diagnosis for altered mental status is quite broad. How does the presence of clonus change or focus your approach to altered mental status?
►Jason Weller, MD, Instructor of Neurology, Boston Medical Center (BMC) and VABHS:The presence of clonus does not significantly narrow the differential. It does, however, suggest a central component to the patient’s altered mental status. Specifically, it implies that the underlying process, whether systemic or neurologic, interferes with central nervous system (CNS) control of the neuromuscular system.1 The differential is still quite broad and includes metabolic derangements (eg, uremia, electrolyte disturbances, hypercarbia, and thyroid dysfunction), medication toxicity from olanzapine or duloxetine, and vascular processes (eg, CNS vasculitis). Infectious etiologies, both within the CNS and systemically, can cause encephalopathy, as can autoimmune processes, such as immune-mediated encephalitis. Finally, primary neurologic conditions such as myoclonic epilepsy can be considered. Given the patient’s medical history, serotonin syndrome must be considered.
►Dr. Reese: Given the concern for serotonin syndrome, the admitting medical team discontinued the patient’s duloxetine. Dr. Weller, what is the pathophysiology of serotonin syndrome, and how is it diagnosed?
►Dr. Weller: Serotonin is ubiquitous throughout the body and brain. Serotonin syndrome is caused by excess endogenous or exogenous serotonin, and this is usually caused by a variety of medications. The symptoms range from tachycardia, agitation, and diaphoresis to sustained clonus, hyperthermia, and shock.2,3 The extent of serotonin syndrome is typically thought to reflect the degree of serotonergic activity.4
Serotonin syndrome is a clinical diagnosis. While there are no tests that can confirm the diagnosis, the Hunter criteria can be used to assist with making the diagnosis.5 Per the Hunter criteria, a patient can be diagnosed with serotonin syndrome if they have taken a serotonergic agent and have at least 1 of the following: spontaneous clonus, inducible or ocular clonus with agitation or diaphoresis, tremor and hyperreflexia, or hypertonia with fever and clonus. This patient had taken duloxetine and had inducible clonus and diaphoresis, thus suggesting a diagnosis of serotonin syndrome.
►Dr. Reese: Aside from selective serotonin reuptake inhibitors (SSRIs), are there other medications that we typically prescribe that can cause serotonin syndrome?
►Dr. Weller: In addition to SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), other commonly prescribed medications that can cause serotonin syndrome are 5-HT3 antagonists (eg, ondansetron), 5-HT agonists (eg, triptans), and opioids (eg, fentanyl and tramadol). There are also case reports of atypical antipsychotics (eg, olanzapine) causing serotonin syndrome because of their antagonism of the 5-HT2 and 5-HT3 receptors.2 Additionally, linezolid is commonly overlooked as a cause of serotonin syndrome given its action as a monoamine oxidase inhibitor.4 In this patient, it would be prudent to discontinue olanzapine and duloxetine.
►Dr. Reese: Duloxetine, olanzapine, and buprenorphine/naloxone were discontinuedgiven concern for serotonin syndrome. Although there are not strong data that buprenorphine/ naloxone can cause serotonin syndrome, the team discontinued the medication in case it might be contributing to the patient’s encephalopathy, while closely monitoring the patient for withdrawal. There was a rapid improvement in the patient’s symptoms over the 24 hours after discontinuation of the 3 medications.
As part of the initial workup, the patient received a computed tomography (CT) scan of his chest to follow up pulmonary nodules identified 16 months prior. The CT scan showed interval growth of the pulmonary nodules in the right lower lobe to 2 cm with extension into the major fissure, which was concerning for malignancy. Plans were made for an outpatient positron emission tomography (PET) scan after hospital discharge.
Dr. Schlechter and Dr. Rangachari, what factors can help us determine whether or not further workup of a malignancy should occur before discharge or can be deferred to the outpatient setting?
►Benjamin Schlechter, MD, Instructor in Medicine, BIDMC; and Deepa Rangachari, MD, Assistant Professor of Medicine, BIDMC: Key considerations in this domain include rapidity of growth and any threat to critical end-organ function (ie, brain, heart, lungs, kidney, and liver). If the malignancy is bulky and/or rapidly progressing to the point that the patient has significant symptoms burden and/or end-organ dysfunction, then initiating the evaluation as an inpatient may be necessary. For suspected intrathoracic malignancies, considering whether this may be a high-grade process (ie, small cell lung cancer) is often a vital branch point. Key considerations in this regard are the following: Is it a bulky central tumor? Is there evidence of widespread metastatic disease, an obstructing mass, and/or tumor lysis? One final and critical aspect to consider is whether there are any patient- specific barriers to timely and reliable outpatient follow-up. If there is no evidence of rapid progression, bulky disease with threatened end-organ involvement, and/or issues with timely and reliable follow-up, then outpatient evaluation is often the best approach to ensure a comprehensive and well-coordinated effort on the patient’s behalf.
►Dr. Reese: Buprenorphine/naloxone was restarted without return of the symptoms. The patient was discharged home with an outpatient PET scan scheduled the following week. Unfortunately, the patient was unable to keep this appointment. Three weeks after hospital discharge, the patient presented again to the emergency department with gradually worsening altered mental status, confusion, visual hallucinations, and myoclonic jerking of the arms and legs. Medication adherence was confirmed by the patient’s wife, resulting in a low concern for serotonin syndrome. Physical examination revealed confusion, dysarthria, diffuse, arrhythmic, myoclonic jerking in all extremities, asterixis in the upper extremities, and hyperreflexia.
A CT scan of the brain did not reveal an intracranial process. A spot electroencephalograph (EEG) and magnetic resonance image (MRI) of the brain were obtained. Dr. Weller, what is the utility of spot EEG vs 24-hour EEG? When might we choose one over the other?
►Dr. Weller: If a patient is persistently altered, then a spot EEG would be sufficient to capture a seizure if that is what is causing the patient’s altered mental status. However, if the patient’s mental status is waxing and waning, then that may warrant a 24-hour EEG because the patient may need to be monitored for longer periods to capture an event that is causing intermittent alterations in mental status.6 Additionally, patients who are acutely ill may require long-term monitoring for the purpose of treatment and outcome management.
►Dr. Reese: The spot EEG showed nearly continuous generalized slowing indicative of a diffuse encephalopathy. The MRI of the brain showed scattered, nonspecific periventricular T2 hyperintense foci, suggestive of advanced chronic microvascular ischemic changes.
A PET CT was obtained and revealed mildly fluorodeoxyglucose (FDG)-avid, enlarging nodules within the right lower lobe, which was suspicious for malignancy. There were no other areas of FDG avidity on the PET scan. Valproic acid was initiated for treatment of myoclonus with transition to clonazepam when no improvement was seen. After starting clonazepam, the patient’s condition stabilized.
Dr. Weller, given the additional history, how has your differential diagnosis changed?
►Dr. Weller: Given the patient’s laboratory findings, we can be quite sure that there is not a contributing metabolic process. The findings suggestive of metastatic cancer, along with the profound neurologic changes, are most concerning for a paraneoplastic syndrome. I would suggest biopsy and consideration of a lumbar puncture. One can also send serum markers, including a paraneoplastic antibody panel.
►Dr. Reese: Biopsy of the mass in his right lower lobe revealed squamous cell lung cancer. Dr. Schlechter and Dr. Rangachari, do you have a framework for the different forms of lung cancer?
►Dr. Schlechter/Dr. Rangachari: The 2 broad categories of lung cancer are small cell and non-small cell (NSCLC). Small cell lung cancer has a tight association with tobacco exposure and is often clinically defined by rapid, bulky progression (ie, weeks to months).7,8 NSCLCs are also commonly seen in those with tobacco exposure, though not always. The main subgroups in this category are adenocarcinoma and squamous cell carcinoma. These cancers often evolve at a slower pace (ie, months to years).8 While small cell lung cancers are highgrade tumors and exquisitely sensitive to chemotherapy and radiation, NSCLCs tend to be less responsive to such therapies. The staging evaluation for either entity is the same and consists of defining localized vs metastatic disease.
►Dr. Reese: Because this patient had an MRI and PET scan that were both negative for metastatic disease, can we assume that this patient had stage I NSCLC?
►Dr. Schlechter/Dr. Rangachari: Not necessarily. While PET and MRI brain are exceptionally helpful in detecting distant metastases, they may over- or underestimate intrathoracic lymph node involvement by as much as 20%.9 As such, dedicated lymph node staging—either via bronchoscopy (endobronchial ultrasound) or surgically (mediastinoscopy) is indicated as lymph node involvement can significantly alter the stage, prognosis, and optimal therapeutic approach.10,11
►Dr. Reese: After this diagnosis was made, the teams caring for this patient attributed his altered mental status to a paraneoplastic syndrome. What is a paraneoplastic syndrome, and how does a paraneoplastic syndrome from malignancy present? Does its presence worsen a patient’s prognosis?
►Dr. Schlechter/Dr. Rangachari: A paraneoplastic syndrome is defined by an immunologic response to the cancer that ends up erroneously targeting self-antigens. Paraneoplastic syndromes are associated with a broad array of clinical findings—from endocrinopathy to encephalopathy—and certain neoplasms are more commonly associated with these syndromes than others (eg, small cell lung cancer and thymoma). Further, severity and onset of a paraneoplastic syndrome does not correlate with the burden of visible disease—and the syndrome may predate the cancer diagnosis by months to years.11 While treatment of the cancer affords the best hope of resolving the paraneoplastic syndrome, the cancer and the paraneoplastic process may have a discordant trajectory, with the paraneoplastic syndrome persisting even after the cancer is maximally treated. Although one might assume that paraneoplastic syndromes portend worse outcomes, in some cases, a presentation with the paraneoplastic syndrome may afford sooner detection of an otherwise occult/asymptomatic malignancy.
►Dr. Reese: The following week, the serum paraneoplastic antibody panel that tested for anti-Yo antibody, anti-Ri antibody,and anti-Hu antibody came back negative. Dr. Weller, what does this mean? Since we have yet to obtain a lumbar puncture, might his symptoms still be caused by a paraneoplastic syndrome?
►Dr. Weller: The negative serum test just means that he does not have antibodies to those 3 antibodies. There are now over 30 different paraneoplastic antibodies that have been discovered, and there are always more that are being discovered. So this negative test result does not exclude a paraneoplastic syndrome in the appropriate clinical context.12 Furthermore, the sensitivity and specificity for certain antibodies are different based upon source fluid, and cerebrospinal fluid testing would provide more diagnostic clarity. A negative test for paraneoplastic syndrome, by itself, would similarly not exclude a paraneoplastic syndrome. Often, empiric treatment is the best diagnostic option for paraneoplastic and autoimmune encephalopathies.
►Dr. Reese: The following week, the patient was discharged to rehabilitation with clonazepam for his symptoms and a scheduled follow-up. Given the patient’s frailty and medical comorbidities, thoracic surgery recommended consultation with radiation oncology. Dr. Schlechter and Dr. Rangachari, when do we decide to use radiation vs chemotherapy for someone with lung cancer?
►Dr. Schlechter/Dr. Rangachari: Patients with early stage, nonmetastatic NSCLC may not always be candidates for surgical resection on the basis of pulmonary function, other medical comorbidities (as in this case), anatomic considerations, and/or patient preference. In these cases, if there is lung-limited disease without lymph node involvement (ie, stage I/II NSCLC) and the patient is not felt to be an operative candidate, then alternatives to surgery include either radiation or ablation.13,14 As we care for an aging and comorbid population, evolving evidence suggests that well-selected patients with early stage disease undergoing these nonoperative approaches have roughly equivalent outcomes to those undergoing conventional surgical resection.13 In such cases, multidisciplinary consultation with a team having dedicated expertise in these various operative and nonoperative modalities is essential.
►Dr. Reese: The patient followed up with radiation oncology for consideration of radiation treatment, but his simulation CT scan showed some ground-glass opacity that were concerning for inflammation vs infection. The patient’s case was discussed at the multidisciplinary tumor board, and it was determined to treat him with antibiotics for a possible pneumonia before proceeding with radiation therapy. After he completed antibiotic treatment, he underwent 10 fractions of radiation treatment, which he tolerated well.
1. Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62.
2. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
3. Arora B, Kannikeswaran N. The serotonin syndrome-the need for physician’s awareness. Int J Emerg Med. 2010;3(4):373-377.
4. Boyer EW, Shannon M. The serotonin syndrome [published correction appears in N Engl J Med. 2007;356(23):2437 and N Engl J Med. 2009;361(17):1714]. N Engl J Med.
2005;352(11):1112-1120.
5. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM.
2003;96(9):635-642.
6. Nordli DR Jr. Usefulness of video-EEG monitoring. Epilepsia. 2006;47(suppl 1):26-30.
7. Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526-4527.
8. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
9. Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80(4):1207-1214.
10. El-Osta H, Jani P, Mansour A, Rascoe P, Jafri S. Endobronchial ultrasound for nodal staging of patients with non-smallcell lung cancer with radiologically normal mediastinum. A meta-analysis. Ann Am Thorac Soc. 2018;15(7):864-874.
11. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554.
12. McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia): 538-558.
13. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Nonsmall cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
14. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821.
►Zachary Reese, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC):Dr. Weller, the differential diagnosis for altered mental status is quite broad. How does the presence of clonus change or focus your approach to altered mental status?
►Jason Weller, MD, Instructor of Neurology, Boston Medical Center (BMC) and VABHS:The presence of clonus does not significantly narrow the differential. It does, however, suggest a central component to the patient’s altered mental status. Specifically, it implies that the underlying process, whether systemic or neurologic, interferes with central nervous system (CNS) control of the neuromuscular system.1 The differential is still quite broad and includes metabolic derangements (eg, uremia, electrolyte disturbances, hypercarbia, and thyroid dysfunction), medication toxicity from olanzapine or duloxetine, and vascular processes (eg, CNS vasculitis). Infectious etiologies, both within the CNS and systemically, can cause encephalopathy, as can autoimmune processes, such as immune-mediated encephalitis. Finally, primary neurologic conditions such as myoclonic epilepsy can be considered. Given the patient’s medical history, serotonin syndrome must be considered.
►Dr. Reese: Given the concern for serotonin syndrome, the admitting medical team discontinued the patient’s duloxetine. Dr. Weller, what is the pathophysiology of serotonin syndrome, and how is it diagnosed?
►Dr. Weller: Serotonin is ubiquitous throughout the body and brain. Serotonin syndrome is caused by excess endogenous or exogenous serotonin, and this is usually caused by a variety of medications. The symptoms range from tachycardia, agitation, and diaphoresis to sustained clonus, hyperthermia, and shock.2,3 The extent of serotonin syndrome is typically thought to reflect the degree of serotonergic activity.4
Serotonin syndrome is a clinical diagnosis. While there are no tests that can confirm the diagnosis, the Hunter criteria can be used to assist with making the diagnosis.5 Per the Hunter criteria, a patient can be diagnosed with serotonin syndrome if they have taken a serotonergic agent and have at least 1 of the following: spontaneous clonus, inducible or ocular clonus with agitation or diaphoresis, tremor and hyperreflexia, or hypertonia with fever and clonus. This patient had taken duloxetine and had inducible clonus and diaphoresis, thus suggesting a diagnosis of serotonin syndrome.
►Dr. Reese: Aside from selective serotonin reuptake inhibitors (SSRIs), are there other medications that we typically prescribe that can cause serotonin syndrome?
►Dr. Weller: In addition to SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), other commonly prescribed medications that can cause serotonin syndrome are 5-HT3 antagonists (eg, ondansetron), 5-HT agonists (eg, triptans), and opioids (eg, fentanyl and tramadol). There are also case reports of atypical antipsychotics (eg, olanzapine) causing serotonin syndrome because of their antagonism of the 5-HT2 and 5-HT3 receptors.2 Additionally, linezolid is commonly overlooked as a cause of serotonin syndrome given its action as a monoamine oxidase inhibitor.4 In this patient, it would be prudent to discontinue olanzapine and duloxetine.
►Dr. Reese: Duloxetine, olanzapine, and buprenorphine/naloxone were discontinuedgiven concern for serotonin syndrome. Although there are not strong data that buprenorphine/ naloxone can cause serotonin syndrome, the team discontinued the medication in case it might be contributing to the patient’s encephalopathy, while closely monitoring the patient for withdrawal. There was a rapid improvement in the patient’s symptoms over the 24 hours after discontinuation of the 3 medications.
As part of the initial workup, the patient received a computed tomography (CT) scan of his chest to follow up pulmonary nodules identified 16 months prior. The CT scan showed interval growth of the pulmonary nodules in the right lower lobe to 2 cm with extension into the major fissure, which was concerning for malignancy. Plans were made for an outpatient positron emission tomography (PET) scan after hospital discharge.
Dr. Schlechter and Dr. Rangachari, what factors can help us determine whether or not further workup of a malignancy should occur before discharge or can be deferred to the outpatient setting?
►Benjamin Schlechter, MD, Instructor in Medicine, BIDMC; and Deepa Rangachari, MD, Assistant Professor of Medicine, BIDMC: Key considerations in this domain include rapidity of growth and any threat to critical end-organ function (ie, brain, heart, lungs, kidney, and liver). If the malignancy is bulky and/or rapidly progressing to the point that the patient has significant symptoms burden and/or end-organ dysfunction, then initiating the evaluation as an inpatient may be necessary. For suspected intrathoracic malignancies, considering whether this may be a high-grade process (ie, small cell lung cancer) is often a vital branch point. Key considerations in this regard are the following: Is it a bulky central tumor? Is there evidence of widespread metastatic disease, an obstructing mass, and/or tumor lysis? One final and critical aspect to consider is whether there are any patient- specific barriers to timely and reliable outpatient follow-up. If there is no evidence of rapid progression, bulky disease with threatened end-organ involvement, and/or issues with timely and reliable follow-up, then outpatient evaluation is often the best approach to ensure a comprehensive and well-coordinated effort on the patient’s behalf.
►Dr. Reese: Buprenorphine/naloxone was restarted without return of the symptoms. The patient was discharged home with an outpatient PET scan scheduled the following week. Unfortunately, the patient was unable to keep this appointment. Three weeks after hospital discharge, the patient presented again to the emergency department with gradually worsening altered mental status, confusion, visual hallucinations, and myoclonic jerking of the arms and legs. Medication adherence was confirmed by the patient’s wife, resulting in a low concern for serotonin syndrome. Physical examination revealed confusion, dysarthria, diffuse, arrhythmic, myoclonic jerking in all extremities, asterixis in the upper extremities, and hyperreflexia.
A CT scan of the brain did not reveal an intracranial process. A spot electroencephalograph (EEG) and magnetic resonance image (MRI) of the brain were obtained. Dr. Weller, what is the utility of spot EEG vs 24-hour EEG? When might we choose one over the other?
►Dr. Weller: If a patient is persistently altered, then a spot EEG would be sufficient to capture a seizure if that is what is causing the patient’s altered mental status. However, if the patient’s mental status is waxing and waning, then that may warrant a 24-hour EEG because the patient may need to be monitored for longer periods to capture an event that is causing intermittent alterations in mental status.6 Additionally, patients who are acutely ill may require long-term monitoring for the purpose of treatment and outcome management.
►Dr. Reese: The spot EEG showed nearly continuous generalized slowing indicative of a diffuse encephalopathy. The MRI of the brain showed scattered, nonspecific periventricular T2 hyperintense foci, suggestive of advanced chronic microvascular ischemic changes.
A PET CT was obtained and revealed mildly fluorodeoxyglucose (FDG)-avid, enlarging nodules within the right lower lobe, which was suspicious for malignancy. There were no other areas of FDG avidity on the PET scan. Valproic acid was initiated for treatment of myoclonus with transition to clonazepam when no improvement was seen. After starting clonazepam, the patient’s condition stabilized.
Dr. Weller, given the additional history, how has your differential diagnosis changed?
►Dr. Weller: Given the patient’s laboratory findings, we can be quite sure that there is not a contributing metabolic process. The findings suggestive of metastatic cancer, along with the profound neurologic changes, are most concerning for a paraneoplastic syndrome. I would suggest biopsy and consideration of a lumbar puncture. One can also send serum markers, including a paraneoplastic antibody panel.
►Dr. Reese: Biopsy of the mass in his right lower lobe revealed squamous cell lung cancer. Dr. Schlechter and Dr. Rangachari, do you have a framework for the different forms of lung cancer?
►Dr. Schlechter/Dr. Rangachari: The 2 broad categories of lung cancer are small cell and non-small cell (NSCLC). Small cell lung cancer has a tight association with tobacco exposure and is often clinically defined by rapid, bulky progression (ie, weeks to months).7,8 NSCLCs are also commonly seen in those with tobacco exposure, though not always. The main subgroups in this category are adenocarcinoma and squamous cell carcinoma. These cancers often evolve at a slower pace (ie, months to years).8 While small cell lung cancers are highgrade tumors and exquisitely sensitive to chemotherapy and radiation, NSCLCs tend to be less responsive to such therapies. The staging evaluation for either entity is the same and consists of defining localized vs metastatic disease.
►Dr. Reese: Because this patient had an MRI and PET scan that were both negative for metastatic disease, can we assume that this patient had stage I NSCLC?
►Dr. Schlechter/Dr. Rangachari: Not necessarily. While PET and MRI brain are exceptionally helpful in detecting distant metastases, they may over- or underestimate intrathoracic lymph node involvement by as much as 20%.9 As such, dedicated lymph node staging—either via bronchoscopy (endobronchial ultrasound) or surgically (mediastinoscopy) is indicated as lymph node involvement can significantly alter the stage, prognosis, and optimal therapeutic approach.10,11
►Dr. Reese: After this diagnosis was made, the teams caring for this patient attributed his altered mental status to a paraneoplastic syndrome. What is a paraneoplastic syndrome, and how does a paraneoplastic syndrome from malignancy present? Does its presence worsen a patient’s prognosis?
►Dr. Schlechter/Dr. Rangachari: A paraneoplastic syndrome is defined by an immunologic response to the cancer that ends up erroneously targeting self-antigens. Paraneoplastic syndromes are associated with a broad array of clinical findings—from endocrinopathy to encephalopathy—and certain neoplasms are more commonly associated with these syndromes than others (eg, small cell lung cancer and thymoma). Further, severity and onset of a paraneoplastic syndrome does not correlate with the burden of visible disease—and the syndrome may predate the cancer diagnosis by months to years.11 While treatment of the cancer affords the best hope of resolving the paraneoplastic syndrome, the cancer and the paraneoplastic process may have a discordant trajectory, with the paraneoplastic syndrome persisting even after the cancer is maximally treated. Although one might assume that paraneoplastic syndromes portend worse outcomes, in some cases, a presentation with the paraneoplastic syndrome may afford sooner detection of an otherwise occult/asymptomatic malignancy.
►Dr. Reese: The following week, the serum paraneoplastic antibody panel that tested for anti-Yo antibody, anti-Ri antibody,and anti-Hu antibody came back negative. Dr. Weller, what does this mean? Since we have yet to obtain a lumbar puncture, might his symptoms still be caused by a paraneoplastic syndrome?
►Dr. Weller: The negative serum test just means that he does not have antibodies to those 3 antibodies. There are now over 30 different paraneoplastic antibodies that have been discovered, and there are always more that are being discovered. So this negative test result does not exclude a paraneoplastic syndrome in the appropriate clinical context.12 Furthermore, the sensitivity and specificity for certain antibodies are different based upon source fluid, and cerebrospinal fluid testing would provide more diagnostic clarity. A negative test for paraneoplastic syndrome, by itself, would similarly not exclude a paraneoplastic syndrome. Often, empiric treatment is the best diagnostic option for paraneoplastic and autoimmune encephalopathies.
►Dr. Reese: The following week, the patient was discharged to rehabilitation with clonazepam for his symptoms and a scheduled follow-up. Given the patient’s frailty and medical comorbidities, thoracic surgery recommended consultation with radiation oncology. Dr. Schlechter and Dr. Rangachari, when do we decide to use radiation vs chemotherapy for someone with lung cancer?
►Dr. Schlechter/Dr. Rangachari: Patients with early stage, nonmetastatic NSCLC may not always be candidates for surgical resection on the basis of pulmonary function, other medical comorbidities (as in this case), anatomic considerations, and/or patient preference. In these cases, if there is lung-limited disease without lymph node involvement (ie, stage I/II NSCLC) and the patient is not felt to be an operative candidate, then alternatives to surgery include either radiation or ablation.13,14 As we care for an aging and comorbid population, evolving evidence suggests that well-selected patients with early stage disease undergoing these nonoperative approaches have roughly equivalent outcomes to those undergoing conventional surgical resection.13 In such cases, multidisciplinary consultation with a team having dedicated expertise in these various operative and nonoperative modalities is essential.
►Dr. Reese: The patient followed up with radiation oncology for consideration of radiation treatment, but his simulation CT scan showed some ground-glass opacity that were concerning for inflammation vs infection. The patient’s case was discussed at the multidisciplinary tumor board, and it was determined to treat him with antibiotics for a possible pneumonia before proceeding with radiation therapy. After he completed antibiotic treatment, he underwent 10 fractions of radiation treatment, which he tolerated well.
►Zachary Reese, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC):Dr. Weller, the differential diagnosis for altered mental status is quite broad. How does the presence of clonus change or focus your approach to altered mental status?
►Jason Weller, MD, Instructor of Neurology, Boston Medical Center (BMC) and VABHS:The presence of clonus does not significantly narrow the differential. It does, however, suggest a central component to the patient’s altered mental status. Specifically, it implies that the underlying process, whether systemic or neurologic, interferes with central nervous system (CNS) control of the neuromuscular system.1 The differential is still quite broad and includes metabolic derangements (eg, uremia, electrolyte disturbances, hypercarbia, and thyroid dysfunction), medication toxicity from olanzapine or duloxetine, and vascular processes (eg, CNS vasculitis). Infectious etiologies, both within the CNS and systemically, can cause encephalopathy, as can autoimmune processes, such as immune-mediated encephalitis. Finally, primary neurologic conditions such as myoclonic epilepsy can be considered. Given the patient’s medical history, serotonin syndrome must be considered.
►Dr. Reese: Given the concern for serotonin syndrome, the admitting medical team discontinued the patient’s duloxetine. Dr. Weller, what is the pathophysiology of serotonin syndrome, and how is it diagnosed?
►Dr. Weller: Serotonin is ubiquitous throughout the body and brain. Serotonin syndrome is caused by excess endogenous or exogenous serotonin, and this is usually caused by a variety of medications. The symptoms range from tachycardia, agitation, and diaphoresis to sustained clonus, hyperthermia, and shock.2,3 The extent of serotonin syndrome is typically thought to reflect the degree of serotonergic activity.4
Serotonin syndrome is a clinical diagnosis. While there are no tests that can confirm the diagnosis, the Hunter criteria can be used to assist with making the diagnosis.5 Per the Hunter criteria, a patient can be diagnosed with serotonin syndrome if they have taken a serotonergic agent and have at least 1 of the following: spontaneous clonus, inducible or ocular clonus with agitation or diaphoresis, tremor and hyperreflexia, or hypertonia with fever and clonus. This patient had taken duloxetine and had inducible clonus and diaphoresis, thus suggesting a diagnosis of serotonin syndrome.
►Dr. Reese: Aside from selective serotonin reuptake inhibitors (SSRIs), are there other medications that we typically prescribe that can cause serotonin syndrome?
►Dr. Weller: In addition to SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), other commonly prescribed medications that can cause serotonin syndrome are 5-HT3 antagonists (eg, ondansetron), 5-HT agonists (eg, triptans), and opioids (eg, fentanyl and tramadol). There are also case reports of atypical antipsychotics (eg, olanzapine) causing serotonin syndrome because of their antagonism of the 5-HT2 and 5-HT3 receptors.2 Additionally, linezolid is commonly overlooked as a cause of serotonin syndrome given its action as a monoamine oxidase inhibitor.4 In this patient, it would be prudent to discontinue olanzapine and duloxetine.
►Dr. Reese: Duloxetine, olanzapine, and buprenorphine/naloxone were discontinuedgiven concern for serotonin syndrome. Although there are not strong data that buprenorphine/ naloxone can cause serotonin syndrome, the team discontinued the medication in case it might be contributing to the patient’s encephalopathy, while closely monitoring the patient for withdrawal. There was a rapid improvement in the patient’s symptoms over the 24 hours after discontinuation of the 3 medications.
As part of the initial workup, the patient received a computed tomography (CT) scan of his chest to follow up pulmonary nodules identified 16 months prior. The CT scan showed interval growth of the pulmonary nodules in the right lower lobe to 2 cm with extension into the major fissure, which was concerning for malignancy. Plans were made for an outpatient positron emission tomography (PET) scan after hospital discharge.
Dr. Schlechter and Dr. Rangachari, what factors can help us determine whether or not further workup of a malignancy should occur before discharge or can be deferred to the outpatient setting?
►Benjamin Schlechter, MD, Instructor in Medicine, BIDMC; and Deepa Rangachari, MD, Assistant Professor of Medicine, BIDMC: Key considerations in this domain include rapidity of growth and any threat to critical end-organ function (ie, brain, heart, lungs, kidney, and liver). If the malignancy is bulky and/or rapidly progressing to the point that the patient has significant symptoms burden and/or end-organ dysfunction, then initiating the evaluation as an inpatient may be necessary. For suspected intrathoracic malignancies, considering whether this may be a high-grade process (ie, small cell lung cancer) is often a vital branch point. Key considerations in this regard are the following: Is it a bulky central tumor? Is there evidence of widespread metastatic disease, an obstructing mass, and/or tumor lysis? One final and critical aspect to consider is whether there are any patient- specific barriers to timely and reliable outpatient follow-up. If there is no evidence of rapid progression, bulky disease with threatened end-organ involvement, and/or issues with timely and reliable follow-up, then outpatient evaluation is often the best approach to ensure a comprehensive and well-coordinated effort on the patient’s behalf.
►Dr. Reese: Buprenorphine/naloxone was restarted without return of the symptoms. The patient was discharged home with an outpatient PET scan scheduled the following week. Unfortunately, the patient was unable to keep this appointment. Three weeks after hospital discharge, the patient presented again to the emergency department with gradually worsening altered mental status, confusion, visual hallucinations, and myoclonic jerking of the arms and legs. Medication adherence was confirmed by the patient’s wife, resulting in a low concern for serotonin syndrome. Physical examination revealed confusion, dysarthria, diffuse, arrhythmic, myoclonic jerking in all extremities, asterixis in the upper extremities, and hyperreflexia.
A CT scan of the brain did not reveal an intracranial process. A spot electroencephalograph (EEG) and magnetic resonance image (MRI) of the brain were obtained. Dr. Weller, what is the utility of spot EEG vs 24-hour EEG? When might we choose one over the other?
►Dr. Weller: If a patient is persistently altered, then a spot EEG would be sufficient to capture a seizure if that is what is causing the patient’s altered mental status. However, if the patient’s mental status is waxing and waning, then that may warrant a 24-hour EEG because the patient may need to be monitored for longer periods to capture an event that is causing intermittent alterations in mental status.6 Additionally, patients who are acutely ill may require long-term monitoring for the purpose of treatment and outcome management.
►Dr. Reese: The spot EEG showed nearly continuous generalized slowing indicative of a diffuse encephalopathy. The MRI of the brain showed scattered, nonspecific periventricular T2 hyperintense foci, suggestive of advanced chronic microvascular ischemic changes.
A PET CT was obtained and revealed mildly fluorodeoxyglucose (FDG)-avid, enlarging nodules within the right lower lobe, which was suspicious for malignancy. There were no other areas of FDG avidity on the PET scan. Valproic acid was initiated for treatment of myoclonus with transition to clonazepam when no improvement was seen. After starting clonazepam, the patient’s condition stabilized.
Dr. Weller, given the additional history, how has your differential diagnosis changed?
►Dr. Weller: Given the patient’s laboratory findings, we can be quite sure that there is not a contributing metabolic process. The findings suggestive of metastatic cancer, along with the profound neurologic changes, are most concerning for a paraneoplastic syndrome. I would suggest biopsy and consideration of a lumbar puncture. One can also send serum markers, including a paraneoplastic antibody panel.
►Dr. Reese: Biopsy of the mass in his right lower lobe revealed squamous cell lung cancer. Dr. Schlechter and Dr. Rangachari, do you have a framework for the different forms of lung cancer?
►Dr. Schlechter/Dr. Rangachari: The 2 broad categories of lung cancer are small cell and non-small cell (NSCLC). Small cell lung cancer has a tight association with tobacco exposure and is often clinically defined by rapid, bulky progression (ie, weeks to months).7,8 NSCLCs are also commonly seen in those with tobacco exposure, though not always. The main subgroups in this category are adenocarcinoma and squamous cell carcinoma. These cancers often evolve at a slower pace (ie, months to years).8 While small cell lung cancers are highgrade tumors and exquisitely sensitive to chemotherapy and radiation, NSCLCs tend to be less responsive to such therapies. The staging evaluation for either entity is the same and consists of defining localized vs metastatic disease.
►Dr. Reese: Because this patient had an MRI and PET scan that were both negative for metastatic disease, can we assume that this patient had stage I NSCLC?
►Dr. Schlechter/Dr. Rangachari: Not necessarily. While PET and MRI brain are exceptionally helpful in detecting distant metastases, they may over- or underestimate intrathoracic lymph node involvement by as much as 20%.9 As such, dedicated lymph node staging—either via bronchoscopy (endobronchial ultrasound) or surgically (mediastinoscopy) is indicated as lymph node involvement can significantly alter the stage, prognosis, and optimal therapeutic approach.10,11
►Dr. Reese: After this diagnosis was made, the teams caring for this patient attributed his altered mental status to a paraneoplastic syndrome. What is a paraneoplastic syndrome, and how does a paraneoplastic syndrome from malignancy present? Does its presence worsen a patient’s prognosis?
►Dr. Schlechter/Dr. Rangachari: A paraneoplastic syndrome is defined by an immunologic response to the cancer that ends up erroneously targeting self-antigens. Paraneoplastic syndromes are associated with a broad array of clinical findings—from endocrinopathy to encephalopathy—and certain neoplasms are more commonly associated with these syndromes than others (eg, small cell lung cancer and thymoma). Further, severity and onset of a paraneoplastic syndrome does not correlate with the burden of visible disease—and the syndrome may predate the cancer diagnosis by months to years.11 While treatment of the cancer affords the best hope of resolving the paraneoplastic syndrome, the cancer and the paraneoplastic process may have a discordant trajectory, with the paraneoplastic syndrome persisting even after the cancer is maximally treated. Although one might assume that paraneoplastic syndromes portend worse outcomes, in some cases, a presentation with the paraneoplastic syndrome may afford sooner detection of an otherwise occult/asymptomatic malignancy.
►Dr. Reese: The following week, the serum paraneoplastic antibody panel that tested for anti-Yo antibody, anti-Ri antibody,and anti-Hu antibody came back negative. Dr. Weller, what does this mean? Since we have yet to obtain a lumbar puncture, might his symptoms still be caused by a paraneoplastic syndrome?
►Dr. Weller: The negative serum test just means that he does not have antibodies to those 3 antibodies. There are now over 30 different paraneoplastic antibodies that have been discovered, and there are always more that are being discovered. So this negative test result does not exclude a paraneoplastic syndrome in the appropriate clinical context.12 Furthermore, the sensitivity and specificity for certain antibodies are different based upon source fluid, and cerebrospinal fluid testing would provide more diagnostic clarity. A negative test for paraneoplastic syndrome, by itself, would similarly not exclude a paraneoplastic syndrome. Often, empiric treatment is the best diagnostic option for paraneoplastic and autoimmune encephalopathies.
►Dr. Reese: The following week, the patient was discharged to rehabilitation with clonazepam for his symptoms and a scheduled follow-up. Given the patient’s frailty and medical comorbidities, thoracic surgery recommended consultation with radiation oncology. Dr. Schlechter and Dr. Rangachari, when do we decide to use radiation vs chemotherapy for someone with lung cancer?
►Dr. Schlechter/Dr. Rangachari: Patients with early stage, nonmetastatic NSCLC may not always be candidates for surgical resection on the basis of pulmonary function, other medical comorbidities (as in this case), anatomic considerations, and/or patient preference. In these cases, if there is lung-limited disease without lymph node involvement (ie, stage I/II NSCLC) and the patient is not felt to be an operative candidate, then alternatives to surgery include either radiation or ablation.13,14 As we care for an aging and comorbid population, evolving evidence suggests that well-selected patients with early stage disease undergoing these nonoperative approaches have roughly equivalent outcomes to those undergoing conventional surgical resection.13 In such cases, multidisciplinary consultation with a team having dedicated expertise in these various operative and nonoperative modalities is essential.
►Dr. Reese: The patient followed up with radiation oncology for consideration of radiation treatment, but his simulation CT scan showed some ground-glass opacity that were concerning for inflammation vs infection. The patient’s case was discussed at the multidisciplinary tumor board, and it was determined to treat him with antibiotics for a possible pneumonia before proceeding with radiation therapy. After he completed antibiotic treatment, he underwent 10 fractions of radiation treatment, which he tolerated well.
1. Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62.
2. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
3. Arora B, Kannikeswaran N. The serotonin syndrome-the need for physician’s awareness. Int J Emerg Med. 2010;3(4):373-377.
4. Boyer EW, Shannon M. The serotonin syndrome [published correction appears in N Engl J Med. 2007;356(23):2437 and N Engl J Med. 2009;361(17):1714]. N Engl J Med.
2005;352(11):1112-1120.
5. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM.
2003;96(9):635-642.
6. Nordli DR Jr. Usefulness of video-EEG monitoring. Epilepsia. 2006;47(suppl 1):26-30.
7. Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526-4527.
8. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
9. Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80(4):1207-1214.
10. El-Osta H, Jani P, Mansour A, Rascoe P, Jafri S. Endobronchial ultrasound for nodal staging of patients with non-smallcell lung cancer with radiologically normal mediastinum. A meta-analysis. Ann Am Thorac Soc. 2018;15(7):864-874.
11. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554.
12. McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia): 538-558.
13. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Nonsmall cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
14. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821.
1. Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62.
2. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
3. Arora B, Kannikeswaran N. The serotonin syndrome-the need for physician’s awareness. Int J Emerg Med. 2010;3(4):373-377.
4. Boyer EW, Shannon M. The serotonin syndrome [published correction appears in N Engl J Med. 2007;356(23):2437 and N Engl J Med. 2009;361(17):1714]. N Engl J Med.
2005;352(11):1112-1120.
5. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM.
2003;96(9):635-642.
6. Nordli DR Jr. Usefulness of video-EEG monitoring. Epilepsia. 2006;47(suppl 1):26-30.
7. Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526-4527.
8. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
9. Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80(4):1207-1214.
10. El-Osta H, Jani P, Mansour A, Rascoe P, Jafri S. Endobronchial ultrasound for nodal staging of patients with non-smallcell lung cancer with radiologically normal mediastinum. A meta-analysis. Ann Am Thorac Soc. 2018;15(7):864-874.
11. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554.
12. McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia): 538-558.
13. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Nonsmall cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
14. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821.
A Veteran Presenting With Leg Swelling, Dyspnea, and Proteinuria
*This article has been corrected to include a missing author.
Case Presentation. A 63-year-old male with well-controlled HIV (CD4 count 757, undetectable viral load), epilepsy, and hypertension presented to the VA Boston Healthcare System (VABHS) emergency department with 1 week of bilateral leg swelling and exertional shortness of breath. He reported having no fever, cough, chest pain, pain with inspiration and orthopnea. There was no personal or family history of pulmonary embolism. He reported weight gain but was unable to quantify how much. He also reported flare up of chronic knee pain, without swelling for which he had taken up to 4 tablets of naproxen daily for several weeks. His physical examination was notable for a heart rate of 105 beats per minute and bilateral pitting edema to his knees. Laboratory testing revealed a creatinine level of 2.5 mg/dL, which was increased from a baseline of 1.0 mg/dL (Table 1), and a urine protein-to-creatinine ratio of 7.8 mg/mg (Table 2). A renal ultrasound showed normal-sized kidneys without hydronephrosis or obstructing renal calculi. The patient was admitted for further workup of his dyspnea and acute kidney injury.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. William, based on the degree of proteinuria and edema, a diagnosis of nephrotic syndrome was made. How is nephrotic syndrome defined, and how is it distinguished from glomerulonephritis?
► Jeffrey William, MD, Nephrologist, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The pathophysiology of nephrotic disease and glomerulonephritis are quite distinct, resulting in symptoms and systemic manifestations that only slightly overlap. Glomerulonephritis is characterized by inflammation of the endothelial cells of the trilayered glomerular capillary, with a resulting active urine sediment with red blood cells, white blood cells, and casts. Nephrotic syndrome mostly affects the visceral epithelial cells of the glomerular capillary, commonly referred to as podocytes, and hence, the urine sediment in nephrotic disease is often inactive. Patients with nephrotic syndrome have nephrotic-range proteinuria (excretion of > 3.5 g per 24 h or a spot urine protein-creatinine ratio > 3.5 g in the steady state) and both hypoalbuminemia (< 3 g/dL) and peripheral edema. Lipiduria and hyperlipidemia are common findings in nephrotic syndrome but are not required for a clinical diagnosis.1 In contrast, glomerulonephritis is defined by a constellation of findings that include renal insufficiency (often indicated by an elevation in blood urea nitrogen and creatinine), hypertension, hematuria, and subnephrotic range proteinuria. In practice, patients may fulfill criteria of both nephrotic and nephritic syndromes, but the preponderance of clinical evidence often points one way or the other. In this case, nephrotic syndrome was diagnosed based on the urine protein-to-creatinine ratio of 7.8 mg/mg, hypoalbuminemia, and edema.
► Dr. Li. What would be your first-line workup for evaluation of the etiology of this patient’s nephrotic syndrome?
► Dr. William. Rather than memorizing a list of etiologies of nephrotic syndrome, it is essential to consider the pathophysiology of heavy proteinuria. Though the glomerular filtration barrier is extremely complex and defects in any component can cause proteinuria, disruption of the podocyte is often involved. Common disease processes that chiefly target the podocyte include minimal change disease, primary focal and segmental glomerulosclerosis (FSGS), and membranous nephropathy, all by differing mechanisms. Minimal change disease and idiopathic/primary FSGS are increasingly thought to be at differing points on a spectrum of the same disease.2 Secondary FSGS, on the other hand, is a progressive disease, commonly resulting from longstanding hypertension, diabetes mellitus, and obesity in adults. Membranous nephropathy can also be either primary or secondary. Primary membranous nephropathy is chiefly caused by a circulating IgG4 antibody to the podocyte membrane antigen PLA2R (M-type phospholipase A2 receptor), whereas secondary membranous nephropathy can be caused by a variety of systemic etiologies, including autoimmune disease (eg, systemic lupus erythematosus), certain malignancies, chronic infections (eg, hepatitis B and C), and many medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).3-5 Paraprotein deposition diseases can also cause glomerular damage leading to nephrotic-range proteinuria.
Given these potential diagnoses, a careful history should be taken to assess exposures and recent medication use. Urine sediment evaluation is essential in the evaluation of nephrotic syndrome to determine if there is an underlying nephritic process. Select serologies may be sent to look for autoimmune disease, such as systemic lupus erythematosus and common viral exposures like hepatitis B or C. Serum and urine protein electrophoreses would be appropriate initial tests of suspected paraprotein-related diseases. Other serologies, such as antineutrophil cytoplasmic antibodies or antiglomerular basement membrane antibodies, would not necessarily be indicated here given the lack of hematuria and presence of nephrotic-range proteinuria.
► Dr. Li. The initial evaluation was notable for an erythrocyte sedimentation rate > 120 (mm/h) and a weakly positive antinuclear antibody (ANA) titer of 1:40. The remainder of his initial workup did not reveal an etiology for his nephrotic syndrome (Table 3).
Dr. William, is there a role for starting urgent empiric steroids in nephrotic syndrome while workup is ongoing? If so, do the severity of proteinuria and/or symptoms play a role or is this determination based on something else?
► Dr. William. Edema is a primary symptom of nephrotic syndrome and can often be managed with diuretics alone. If a clear medication-mediated cause is suspected, discontinuation of this agent may result in spontaneous improvement without steroid treatment. However,in cases where an etiology is unclear and there are serious thrombotic complications requiring anticoagulation, and a renal biopsy is deemed to be too risky, then empiric steroid therapy may be necessary. Children with new-onset nephrotic syndrome are presumed to have minimal change disease, given its prevalence in this patient population, and are often given empiric steroids without obtaining a renal biopsy. However, in the adult population, a renal biopsy can typically be performed quickly and safely, with pathology results interpreted within days. In this patient, since a diagnosis was unclear and there was no contraindication to renal biopsy, a biopsy should be obtained before consideration of steroids.
► Dr. Li. Steroids were deferred in anticipation of renal biopsy, which showed stage I membranous nephropathy, suggestive of membranous lupus nephritis Class V. The deposits were strongly reactive for immunoglobuline G (IgG), IgA, and complement 1q (C1q), showed co-dominant staining for IgG1, IgG2, and IgG3, and were weakly positive for the PLA2 receptor. Focal intimal arteritis in a small interlobular vessel was seen.
Dr. William, the pathology returned suggestive of lupus nephritis. Does the overall clinical picture fit with lupus nephritis?
► Dr. William. Given the history and a rather low ANA, the diagnosis of lupus nephritis seems unlikely. The lack of IgG4 and PLA2R staining in the biopsy suggests that this membranous pattern on the biopsy is likely to be secondary to a systemic etiology, but further investigation should be pursued.
► Dr. Li. The patient was discharged after the biopsy with a planned outpatient nephrology follow-up to discuss results and treatment. He was prescribed an oral diuretic, and his symptoms improved. Several days after discharge, he developed blurry vision and was evaluated in the Ophthalmology clinic. On fundoscopy, he was found to have acute papillitis, a form of optic neuritis. As part of initial evaluation of infectious etiologies of papillitis, ophthalmology recommended testing for syphilis.
Dr. Strymish, when we are considering secondary syphilis, what is the recommended approach to diagnostic testing?
► Judith Strymish, MD, Infectious Diseases, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The diagnosis of syphilis is usually made through serologic testing of blood specimens. Methods that detect the spirochete directly like dark-field smears are not readily available. Serologic tests include treponemal tests (eg, Treponema pallidum particle agglutination assay [TPPA]) and nontreponemal tests (eg, rapid plasma reagin [RPR]). One needs a confirmatory test because either test is associated with false positives. Either test can be done first. Most laboratories, including those at VABHS are now performing treponemal tests first as these have become more cost-effective.6 The TPPA treponemal test was found to have a lower false negative rate in primary syphilis compared with that of nontreponemal tests.7 Nontreponemal tests can be followed for response to therapy. If a patient has a history of treated syphilis, a nontreponemal test should be sent, since the treponemal test will remain positive for life.
If there is clinical concern for neurosyphilis, cerebrospinal fluid fluorescent (CSF) treponemal antibody needs to be sampled and sent for the nontreponemal venereal disease research laboratory (VDRL) test. The VDRL is highly specific for neurosyphilis but not as sensitive. Cerebrospinal fluid fluorescent treponemal antibody (CSF FTA) may also be sent; it is very sensitive but not very specific for neurosyphilis.
► Dr. Li. An RPR returned positive at 1:512 (was negative 14 months prior on a routine screening test), with positive reflex TPPA (Table 4). A diagnosis of secondary syphilis was made. Dr. Strymish, at this point, what additional testing and treatment is necessary?
► Dr. Strymish. With papillitis and a very high RPR, we need to assume that he has ophthalmic syphilis. This can occur in any stage of syphilis, but his eye findings and high RPR are consistent with secondary syphilis. Ophthalmic syphilis has been on the upswing, even more than is expected with recent increases in syphilis cases.8 Ophthalmic syphilis is considered a form of neurosyphilis. A lumbar puncture and treatment for neurosyphilis is recommended.9,10
► Dr. Li. A lumbar puncture was performed, and his CSF was VDRL positive. This confirmed a diagnosis of neurosyphilis (Table 4). The patient was treated for neurosyphilis with IV penicillin. The patient shared that he had episodes of unprotected oral sexual activity within the past year and approximately 1 year ago, he came in close contact (but no sexual activity) with a person who had a rash consistent with syphilis.Dr. William, syphilis would be a potential unifying diagnosis of his renal and ophthalmologic manifestations. Is syphilis known to cause membranous nephropathy?
► Dr. William. Though it is uncommon, the nephrotic syndrome is a well-described complication of secondary syphilis.11,12 Syphilis has been shown to cause nephrotic syndrome in a variety of ways. Case reports abound linking syphilis to minimal change disease and other glomerular diseases.13,14 A case report from 1993 shows a membranous pattern of glomerular disease similar to this case.15 As a form of secondary membranous nephropathy, the immunofluorescence pattern can demonstrate staining similar to the “full house” seen in lupus nephritis (IgA, IgM, and C1q, in addition to IgG and C3).16 This explains the initial interpretation of this patient’s biopsy, as lupus nephritis would be a much more common etiology of secondary membranous nephropathy than is acute syphilis with this immunofluorescence pattern. However, the data in this case are highly suggestive of a causal relationship between secondary syphilis and membranous nephropathy.
► Dr. Li. Dr. Strymish, how should this patient be screened for syphilis reinfection, and at what intervals would you recommend?
► Dr. Strymish. He will need follow-up testing to make sure that his syphilis is effectively treated. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. He will also need follow-up for normalization of his RPR. Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy according to US Centers for Disease Control and Prevention guidelines.9
His treponemal test for syphilis will likely stay positive for life. His RPR should decrease significantly with effective treatment. It makes sense to screen with RPR alone as long as he continues to have risk factors for acquiring syphilis. Routine syphilis testing is recommended for pregnant women, sexually active men who have sex with men, sexually active persons with HIV, and persons taking PrEP (pre-exposure prophylaxis) for HIV prevention. He should be screened at least yearly for syphilis.
► Dr. Li. Over the next several months, the patient’s creatinine normalized and his proteinuria resolved. His vision recovered, and he has had no further ophthalmologic complications.
Dr. William, what is his long-term renal prognosis? Do you expect that his acute episode of membranous nephropathy will have permanent effects on his renal function?
► Dr. William. His rapid response to therapy for neurosyphilis provides evidence for this etiology of his renal dysfunction and glomerulonephritis. His long-term prognosis is quite good if the syphilis is the only reason for him to have renal disease. The renal damage is often reversible in these cases. However, given his prior extensive NSAID exposure and history of hypertension, he may be at higher risk for chronic kidney disease than an otherwise healthy patient, especially after an episode of acute kidney injury. Therefore, his renal function should continue to be monitored as an outpatient.
Acknowledgments
The authors thank this veteran for sharing his story and allowing us to learn from this unusual case for the benefit of our future patients.
1. Rennke H, Denker BM. Renal Pathophysiology: The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
2. Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768-776.
3. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21.
4. Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643-656.
5. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62(5):1012-1017.
6. Pillay A. Centers for Disease Control and Prevention Syphilis Summit—Diagnostics and laboratory issues. Sex Transm Dis. 2018;45(9S)(suppl 1):S13-S16.
7. Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26(suppl A):6A-12A.
8. Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(43):1185-1188.
9. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR3):1-137. [Erratum in MMWR Recomm Rep. 2015;64(33):924.]
10. US Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Updated March 24, 2016. Accessed August 12, 2019.
11. Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643-648.1.
12. Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013:pii:bcr2012008279
13. Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: a report of nephrotic syndrome with minimal change disease. Am J Kidney Dis. 1987;9(2):176-179.
14. Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216(7):1159-1166.
15. Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351-1355.
16. Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. N Engl J Med. 1975;292(9):449-454.
*This article has been corrected to include a missing author.
Case Presentation. A 63-year-old male with well-controlled HIV (CD4 count 757, undetectable viral load), epilepsy, and hypertension presented to the VA Boston Healthcare System (VABHS) emergency department with 1 week of bilateral leg swelling and exertional shortness of breath. He reported having no fever, cough, chest pain, pain with inspiration and orthopnea. There was no personal or family history of pulmonary embolism. He reported weight gain but was unable to quantify how much. He also reported flare up of chronic knee pain, without swelling for which he had taken up to 4 tablets of naproxen daily for several weeks. His physical examination was notable for a heart rate of 105 beats per minute and bilateral pitting edema to his knees. Laboratory testing revealed a creatinine level of 2.5 mg/dL, which was increased from a baseline of 1.0 mg/dL (Table 1), and a urine protein-to-creatinine ratio of 7.8 mg/mg (Table 2). A renal ultrasound showed normal-sized kidneys without hydronephrosis or obstructing renal calculi. The patient was admitted for further workup of his dyspnea and acute kidney injury.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. William, based on the degree of proteinuria and edema, a diagnosis of nephrotic syndrome was made. How is nephrotic syndrome defined, and how is it distinguished from glomerulonephritis?
► Jeffrey William, MD, Nephrologist, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The pathophysiology of nephrotic disease and glomerulonephritis are quite distinct, resulting in symptoms and systemic manifestations that only slightly overlap. Glomerulonephritis is characterized by inflammation of the endothelial cells of the trilayered glomerular capillary, with a resulting active urine sediment with red blood cells, white blood cells, and casts. Nephrotic syndrome mostly affects the visceral epithelial cells of the glomerular capillary, commonly referred to as podocytes, and hence, the urine sediment in nephrotic disease is often inactive. Patients with nephrotic syndrome have nephrotic-range proteinuria (excretion of > 3.5 g per 24 h or a spot urine protein-creatinine ratio > 3.5 g in the steady state) and both hypoalbuminemia (< 3 g/dL) and peripheral edema. Lipiduria and hyperlipidemia are common findings in nephrotic syndrome but are not required for a clinical diagnosis.1 In contrast, glomerulonephritis is defined by a constellation of findings that include renal insufficiency (often indicated by an elevation in blood urea nitrogen and creatinine), hypertension, hematuria, and subnephrotic range proteinuria. In practice, patients may fulfill criteria of both nephrotic and nephritic syndromes, but the preponderance of clinical evidence often points one way or the other. In this case, nephrotic syndrome was diagnosed based on the urine protein-to-creatinine ratio of 7.8 mg/mg, hypoalbuminemia, and edema.
► Dr. Li. What would be your first-line workup for evaluation of the etiology of this patient’s nephrotic syndrome?
► Dr. William. Rather than memorizing a list of etiologies of nephrotic syndrome, it is essential to consider the pathophysiology of heavy proteinuria. Though the glomerular filtration barrier is extremely complex and defects in any component can cause proteinuria, disruption of the podocyte is often involved. Common disease processes that chiefly target the podocyte include minimal change disease, primary focal and segmental glomerulosclerosis (FSGS), and membranous nephropathy, all by differing mechanisms. Minimal change disease and idiopathic/primary FSGS are increasingly thought to be at differing points on a spectrum of the same disease.2 Secondary FSGS, on the other hand, is a progressive disease, commonly resulting from longstanding hypertension, diabetes mellitus, and obesity in adults. Membranous nephropathy can also be either primary or secondary. Primary membranous nephropathy is chiefly caused by a circulating IgG4 antibody to the podocyte membrane antigen PLA2R (M-type phospholipase A2 receptor), whereas secondary membranous nephropathy can be caused by a variety of systemic etiologies, including autoimmune disease (eg, systemic lupus erythematosus), certain malignancies, chronic infections (eg, hepatitis B and C), and many medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).3-5 Paraprotein deposition diseases can also cause glomerular damage leading to nephrotic-range proteinuria.
Given these potential diagnoses, a careful history should be taken to assess exposures and recent medication use. Urine sediment evaluation is essential in the evaluation of nephrotic syndrome to determine if there is an underlying nephritic process. Select serologies may be sent to look for autoimmune disease, such as systemic lupus erythematosus and common viral exposures like hepatitis B or C. Serum and urine protein electrophoreses would be appropriate initial tests of suspected paraprotein-related diseases. Other serologies, such as antineutrophil cytoplasmic antibodies or antiglomerular basement membrane antibodies, would not necessarily be indicated here given the lack of hematuria and presence of nephrotic-range proteinuria.
► Dr. Li. The initial evaluation was notable for an erythrocyte sedimentation rate > 120 (mm/h) and a weakly positive antinuclear antibody (ANA) titer of 1:40. The remainder of his initial workup did not reveal an etiology for his nephrotic syndrome (Table 3).
Dr. William, is there a role for starting urgent empiric steroids in nephrotic syndrome while workup is ongoing? If so, do the severity of proteinuria and/or symptoms play a role or is this determination based on something else?
► Dr. William. Edema is a primary symptom of nephrotic syndrome and can often be managed with diuretics alone. If a clear medication-mediated cause is suspected, discontinuation of this agent may result in spontaneous improvement without steroid treatment. However,in cases where an etiology is unclear and there are serious thrombotic complications requiring anticoagulation, and a renal biopsy is deemed to be too risky, then empiric steroid therapy may be necessary. Children with new-onset nephrotic syndrome are presumed to have minimal change disease, given its prevalence in this patient population, and are often given empiric steroids without obtaining a renal biopsy. However, in the adult population, a renal biopsy can typically be performed quickly and safely, with pathology results interpreted within days. In this patient, since a diagnosis was unclear and there was no contraindication to renal biopsy, a biopsy should be obtained before consideration of steroids.
► Dr. Li. Steroids were deferred in anticipation of renal biopsy, which showed stage I membranous nephropathy, suggestive of membranous lupus nephritis Class V. The deposits were strongly reactive for immunoglobuline G (IgG), IgA, and complement 1q (C1q), showed co-dominant staining for IgG1, IgG2, and IgG3, and were weakly positive for the PLA2 receptor. Focal intimal arteritis in a small interlobular vessel was seen.
Dr. William, the pathology returned suggestive of lupus nephritis. Does the overall clinical picture fit with lupus nephritis?
► Dr. William. Given the history and a rather low ANA, the diagnosis of lupus nephritis seems unlikely. The lack of IgG4 and PLA2R staining in the biopsy suggests that this membranous pattern on the biopsy is likely to be secondary to a systemic etiology, but further investigation should be pursued.
► Dr. Li. The patient was discharged after the biopsy with a planned outpatient nephrology follow-up to discuss results and treatment. He was prescribed an oral diuretic, and his symptoms improved. Several days after discharge, he developed blurry vision and was evaluated in the Ophthalmology clinic. On fundoscopy, he was found to have acute papillitis, a form of optic neuritis. As part of initial evaluation of infectious etiologies of papillitis, ophthalmology recommended testing for syphilis.
Dr. Strymish, when we are considering secondary syphilis, what is the recommended approach to diagnostic testing?
► Judith Strymish, MD, Infectious Diseases, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The diagnosis of syphilis is usually made through serologic testing of blood specimens. Methods that detect the spirochete directly like dark-field smears are not readily available. Serologic tests include treponemal tests (eg, Treponema pallidum particle agglutination assay [TPPA]) and nontreponemal tests (eg, rapid plasma reagin [RPR]). One needs a confirmatory test because either test is associated with false positives. Either test can be done first. Most laboratories, including those at VABHS are now performing treponemal tests first as these have become more cost-effective.6 The TPPA treponemal test was found to have a lower false negative rate in primary syphilis compared with that of nontreponemal tests.7 Nontreponemal tests can be followed for response to therapy. If a patient has a history of treated syphilis, a nontreponemal test should be sent, since the treponemal test will remain positive for life.
If there is clinical concern for neurosyphilis, cerebrospinal fluid fluorescent (CSF) treponemal antibody needs to be sampled and sent for the nontreponemal venereal disease research laboratory (VDRL) test. The VDRL is highly specific for neurosyphilis but not as sensitive. Cerebrospinal fluid fluorescent treponemal antibody (CSF FTA) may also be sent; it is very sensitive but not very specific for neurosyphilis.
► Dr. Li. An RPR returned positive at 1:512 (was negative 14 months prior on a routine screening test), with positive reflex TPPA (Table 4). A diagnosis of secondary syphilis was made. Dr. Strymish, at this point, what additional testing and treatment is necessary?
► Dr. Strymish. With papillitis and a very high RPR, we need to assume that he has ophthalmic syphilis. This can occur in any stage of syphilis, but his eye findings and high RPR are consistent with secondary syphilis. Ophthalmic syphilis has been on the upswing, even more than is expected with recent increases in syphilis cases.8 Ophthalmic syphilis is considered a form of neurosyphilis. A lumbar puncture and treatment for neurosyphilis is recommended.9,10
► Dr. Li. A lumbar puncture was performed, and his CSF was VDRL positive. This confirmed a diagnosis of neurosyphilis (Table 4). The patient was treated for neurosyphilis with IV penicillin. The patient shared that he had episodes of unprotected oral sexual activity within the past year and approximately 1 year ago, he came in close contact (but no sexual activity) with a person who had a rash consistent with syphilis.Dr. William, syphilis would be a potential unifying diagnosis of his renal and ophthalmologic manifestations. Is syphilis known to cause membranous nephropathy?
► Dr. William. Though it is uncommon, the nephrotic syndrome is a well-described complication of secondary syphilis.11,12 Syphilis has been shown to cause nephrotic syndrome in a variety of ways. Case reports abound linking syphilis to minimal change disease and other glomerular diseases.13,14 A case report from 1993 shows a membranous pattern of glomerular disease similar to this case.15 As a form of secondary membranous nephropathy, the immunofluorescence pattern can demonstrate staining similar to the “full house” seen in lupus nephritis (IgA, IgM, and C1q, in addition to IgG and C3).16 This explains the initial interpretation of this patient’s biopsy, as lupus nephritis would be a much more common etiology of secondary membranous nephropathy than is acute syphilis with this immunofluorescence pattern. However, the data in this case are highly suggestive of a causal relationship between secondary syphilis and membranous nephropathy.
► Dr. Li. Dr. Strymish, how should this patient be screened for syphilis reinfection, and at what intervals would you recommend?
► Dr. Strymish. He will need follow-up testing to make sure that his syphilis is effectively treated. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. He will also need follow-up for normalization of his RPR. Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy according to US Centers for Disease Control and Prevention guidelines.9
His treponemal test for syphilis will likely stay positive for life. His RPR should decrease significantly with effective treatment. It makes sense to screen with RPR alone as long as he continues to have risk factors for acquiring syphilis. Routine syphilis testing is recommended for pregnant women, sexually active men who have sex with men, sexually active persons with HIV, and persons taking PrEP (pre-exposure prophylaxis) for HIV prevention. He should be screened at least yearly for syphilis.
► Dr. Li. Over the next several months, the patient’s creatinine normalized and his proteinuria resolved. His vision recovered, and he has had no further ophthalmologic complications.
Dr. William, what is his long-term renal prognosis? Do you expect that his acute episode of membranous nephropathy will have permanent effects on his renal function?
► Dr. William. His rapid response to therapy for neurosyphilis provides evidence for this etiology of his renal dysfunction and glomerulonephritis. His long-term prognosis is quite good if the syphilis is the only reason for him to have renal disease. The renal damage is often reversible in these cases. However, given his prior extensive NSAID exposure and history of hypertension, he may be at higher risk for chronic kidney disease than an otherwise healthy patient, especially after an episode of acute kidney injury. Therefore, his renal function should continue to be monitored as an outpatient.
Acknowledgments
The authors thank this veteran for sharing his story and allowing us to learn from this unusual case for the benefit of our future patients.
*This article has been corrected to include a missing author.
Case Presentation. A 63-year-old male with well-controlled HIV (CD4 count 757, undetectable viral load), epilepsy, and hypertension presented to the VA Boston Healthcare System (VABHS) emergency department with 1 week of bilateral leg swelling and exertional shortness of breath. He reported having no fever, cough, chest pain, pain with inspiration and orthopnea. There was no personal or family history of pulmonary embolism. He reported weight gain but was unable to quantify how much. He also reported flare up of chronic knee pain, without swelling for which he had taken up to 4 tablets of naproxen daily for several weeks. His physical examination was notable for a heart rate of 105 beats per minute and bilateral pitting edema to his knees. Laboratory testing revealed a creatinine level of 2.5 mg/dL, which was increased from a baseline of 1.0 mg/dL (Table 1), and a urine protein-to-creatinine ratio of 7.8 mg/mg (Table 2). A renal ultrasound showed normal-sized kidneys without hydronephrosis or obstructing renal calculi. The patient was admitted for further workup of his dyspnea and acute kidney injury.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. William, based on the degree of proteinuria and edema, a diagnosis of nephrotic syndrome was made. How is nephrotic syndrome defined, and how is it distinguished from glomerulonephritis?
► Jeffrey William, MD, Nephrologist, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The pathophysiology of nephrotic disease and glomerulonephritis are quite distinct, resulting in symptoms and systemic manifestations that only slightly overlap. Glomerulonephritis is characterized by inflammation of the endothelial cells of the trilayered glomerular capillary, with a resulting active urine sediment with red blood cells, white blood cells, and casts. Nephrotic syndrome mostly affects the visceral epithelial cells of the glomerular capillary, commonly referred to as podocytes, and hence, the urine sediment in nephrotic disease is often inactive. Patients with nephrotic syndrome have nephrotic-range proteinuria (excretion of > 3.5 g per 24 h or a spot urine protein-creatinine ratio > 3.5 g in the steady state) and both hypoalbuminemia (< 3 g/dL) and peripheral edema. Lipiduria and hyperlipidemia are common findings in nephrotic syndrome but are not required for a clinical diagnosis.1 In contrast, glomerulonephritis is defined by a constellation of findings that include renal insufficiency (often indicated by an elevation in blood urea nitrogen and creatinine), hypertension, hematuria, and subnephrotic range proteinuria. In practice, patients may fulfill criteria of both nephrotic and nephritic syndromes, but the preponderance of clinical evidence often points one way or the other. In this case, nephrotic syndrome was diagnosed based on the urine protein-to-creatinine ratio of 7.8 mg/mg, hypoalbuminemia, and edema.
► Dr. Li. What would be your first-line workup for evaluation of the etiology of this patient’s nephrotic syndrome?
► Dr. William. Rather than memorizing a list of etiologies of nephrotic syndrome, it is essential to consider the pathophysiology of heavy proteinuria. Though the glomerular filtration barrier is extremely complex and defects in any component can cause proteinuria, disruption of the podocyte is often involved. Common disease processes that chiefly target the podocyte include minimal change disease, primary focal and segmental glomerulosclerosis (FSGS), and membranous nephropathy, all by differing mechanisms. Minimal change disease and idiopathic/primary FSGS are increasingly thought to be at differing points on a spectrum of the same disease.2 Secondary FSGS, on the other hand, is a progressive disease, commonly resulting from longstanding hypertension, diabetes mellitus, and obesity in adults. Membranous nephropathy can also be either primary or secondary. Primary membranous nephropathy is chiefly caused by a circulating IgG4 antibody to the podocyte membrane antigen PLA2R (M-type phospholipase A2 receptor), whereas secondary membranous nephropathy can be caused by a variety of systemic etiologies, including autoimmune disease (eg, systemic lupus erythematosus), certain malignancies, chronic infections (eg, hepatitis B and C), and many medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).3-5 Paraprotein deposition diseases can also cause glomerular damage leading to nephrotic-range proteinuria.
Given these potential diagnoses, a careful history should be taken to assess exposures and recent medication use. Urine sediment evaluation is essential in the evaluation of nephrotic syndrome to determine if there is an underlying nephritic process. Select serologies may be sent to look for autoimmune disease, such as systemic lupus erythematosus and common viral exposures like hepatitis B or C. Serum and urine protein electrophoreses would be appropriate initial tests of suspected paraprotein-related diseases. Other serologies, such as antineutrophil cytoplasmic antibodies or antiglomerular basement membrane antibodies, would not necessarily be indicated here given the lack of hematuria and presence of nephrotic-range proteinuria.
► Dr. Li. The initial evaluation was notable for an erythrocyte sedimentation rate > 120 (mm/h) and a weakly positive antinuclear antibody (ANA) titer of 1:40. The remainder of his initial workup did not reveal an etiology for his nephrotic syndrome (Table 3).
Dr. William, is there a role for starting urgent empiric steroids in nephrotic syndrome while workup is ongoing? If so, do the severity of proteinuria and/or symptoms play a role or is this determination based on something else?
► Dr. William. Edema is a primary symptom of nephrotic syndrome and can often be managed with diuretics alone. If a clear medication-mediated cause is suspected, discontinuation of this agent may result in spontaneous improvement without steroid treatment. However,in cases where an etiology is unclear and there are serious thrombotic complications requiring anticoagulation, and a renal biopsy is deemed to be too risky, then empiric steroid therapy may be necessary. Children with new-onset nephrotic syndrome are presumed to have minimal change disease, given its prevalence in this patient population, and are often given empiric steroids without obtaining a renal biopsy. However, in the adult population, a renal biopsy can typically be performed quickly and safely, with pathology results interpreted within days. In this patient, since a diagnosis was unclear and there was no contraindication to renal biopsy, a biopsy should be obtained before consideration of steroids.
► Dr. Li. Steroids were deferred in anticipation of renal biopsy, which showed stage I membranous nephropathy, suggestive of membranous lupus nephritis Class V. The deposits were strongly reactive for immunoglobuline G (IgG), IgA, and complement 1q (C1q), showed co-dominant staining for IgG1, IgG2, and IgG3, and were weakly positive for the PLA2 receptor. Focal intimal arteritis in a small interlobular vessel was seen.
Dr. William, the pathology returned suggestive of lupus nephritis. Does the overall clinical picture fit with lupus nephritis?
► Dr. William. Given the history and a rather low ANA, the diagnosis of lupus nephritis seems unlikely. The lack of IgG4 and PLA2R staining in the biopsy suggests that this membranous pattern on the biopsy is likely to be secondary to a systemic etiology, but further investigation should be pursued.
► Dr. Li. The patient was discharged after the biopsy with a planned outpatient nephrology follow-up to discuss results and treatment. He was prescribed an oral diuretic, and his symptoms improved. Several days after discharge, he developed blurry vision and was evaluated in the Ophthalmology clinic. On fundoscopy, he was found to have acute papillitis, a form of optic neuritis. As part of initial evaluation of infectious etiologies of papillitis, ophthalmology recommended testing for syphilis.
Dr. Strymish, when we are considering secondary syphilis, what is the recommended approach to diagnostic testing?
► Judith Strymish, MD, Infectious Diseases, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The diagnosis of syphilis is usually made through serologic testing of blood specimens. Methods that detect the spirochete directly like dark-field smears are not readily available. Serologic tests include treponemal tests (eg, Treponema pallidum particle agglutination assay [TPPA]) and nontreponemal tests (eg, rapid plasma reagin [RPR]). One needs a confirmatory test because either test is associated with false positives. Either test can be done first. Most laboratories, including those at VABHS are now performing treponemal tests first as these have become more cost-effective.6 The TPPA treponemal test was found to have a lower false negative rate in primary syphilis compared with that of nontreponemal tests.7 Nontreponemal tests can be followed for response to therapy. If a patient has a history of treated syphilis, a nontreponemal test should be sent, since the treponemal test will remain positive for life.
If there is clinical concern for neurosyphilis, cerebrospinal fluid fluorescent (CSF) treponemal antibody needs to be sampled and sent for the nontreponemal venereal disease research laboratory (VDRL) test. The VDRL is highly specific for neurosyphilis but not as sensitive. Cerebrospinal fluid fluorescent treponemal antibody (CSF FTA) may also be sent; it is very sensitive but not very specific for neurosyphilis.
► Dr. Li. An RPR returned positive at 1:512 (was negative 14 months prior on a routine screening test), with positive reflex TPPA (Table 4). A diagnosis of secondary syphilis was made. Dr. Strymish, at this point, what additional testing and treatment is necessary?
► Dr. Strymish. With papillitis and a very high RPR, we need to assume that he has ophthalmic syphilis. This can occur in any stage of syphilis, but his eye findings and high RPR are consistent with secondary syphilis. Ophthalmic syphilis has been on the upswing, even more than is expected with recent increases in syphilis cases.8 Ophthalmic syphilis is considered a form of neurosyphilis. A lumbar puncture and treatment for neurosyphilis is recommended.9,10
► Dr. Li. A lumbar puncture was performed, and his CSF was VDRL positive. This confirmed a diagnosis of neurosyphilis (Table 4). The patient was treated for neurosyphilis with IV penicillin. The patient shared that he had episodes of unprotected oral sexual activity within the past year and approximately 1 year ago, he came in close contact (but no sexual activity) with a person who had a rash consistent with syphilis.Dr. William, syphilis would be a potential unifying diagnosis of his renal and ophthalmologic manifestations. Is syphilis known to cause membranous nephropathy?
► Dr. William. Though it is uncommon, the nephrotic syndrome is a well-described complication of secondary syphilis.11,12 Syphilis has been shown to cause nephrotic syndrome in a variety of ways. Case reports abound linking syphilis to minimal change disease and other glomerular diseases.13,14 A case report from 1993 shows a membranous pattern of glomerular disease similar to this case.15 As a form of secondary membranous nephropathy, the immunofluorescence pattern can demonstrate staining similar to the “full house” seen in lupus nephritis (IgA, IgM, and C1q, in addition to IgG and C3).16 This explains the initial interpretation of this patient’s biopsy, as lupus nephritis would be a much more common etiology of secondary membranous nephropathy than is acute syphilis with this immunofluorescence pattern. However, the data in this case are highly suggestive of a causal relationship between secondary syphilis and membranous nephropathy.
► Dr. Li. Dr. Strymish, how should this patient be screened for syphilis reinfection, and at what intervals would you recommend?
► Dr. Strymish. He will need follow-up testing to make sure that his syphilis is effectively treated. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. He will also need follow-up for normalization of his RPR. Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy according to US Centers for Disease Control and Prevention guidelines.9
His treponemal test for syphilis will likely stay positive for life. His RPR should decrease significantly with effective treatment. It makes sense to screen with RPR alone as long as he continues to have risk factors for acquiring syphilis. Routine syphilis testing is recommended for pregnant women, sexually active men who have sex with men, sexually active persons with HIV, and persons taking PrEP (pre-exposure prophylaxis) for HIV prevention. He should be screened at least yearly for syphilis.
► Dr. Li. Over the next several months, the patient’s creatinine normalized and his proteinuria resolved. His vision recovered, and he has had no further ophthalmologic complications.
Dr. William, what is his long-term renal prognosis? Do you expect that his acute episode of membranous nephropathy will have permanent effects on his renal function?
► Dr. William. His rapid response to therapy for neurosyphilis provides evidence for this etiology of his renal dysfunction and glomerulonephritis. His long-term prognosis is quite good if the syphilis is the only reason for him to have renal disease. The renal damage is often reversible in these cases. However, given his prior extensive NSAID exposure and history of hypertension, he may be at higher risk for chronic kidney disease than an otherwise healthy patient, especially after an episode of acute kidney injury. Therefore, his renal function should continue to be monitored as an outpatient.
Acknowledgments
The authors thank this veteran for sharing his story and allowing us to learn from this unusual case for the benefit of our future patients.
1. Rennke H, Denker BM. Renal Pathophysiology: The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
2. Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768-776.
3. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21.
4. Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643-656.
5. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62(5):1012-1017.
6. Pillay A. Centers for Disease Control and Prevention Syphilis Summit—Diagnostics and laboratory issues. Sex Transm Dis. 2018;45(9S)(suppl 1):S13-S16.
7. Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26(suppl A):6A-12A.
8. Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(43):1185-1188.
9. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR3):1-137. [Erratum in MMWR Recomm Rep. 2015;64(33):924.]
10. US Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Updated March 24, 2016. Accessed August 12, 2019.
11. Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643-648.1.
12. Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013:pii:bcr2012008279
13. Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: a report of nephrotic syndrome with minimal change disease. Am J Kidney Dis. 1987;9(2):176-179.
14. Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216(7):1159-1166.
15. Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351-1355.
16. Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. N Engl J Med. 1975;292(9):449-454.
1. Rennke H, Denker BM. Renal Pathophysiology: The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
2. Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768-776.
3. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21.
4. Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643-656.
5. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62(5):1012-1017.
6. Pillay A. Centers for Disease Control and Prevention Syphilis Summit—Diagnostics and laboratory issues. Sex Transm Dis. 2018;45(9S)(suppl 1):S13-S16.
7. Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26(suppl A):6A-12A.
8. Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(43):1185-1188.
9. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR3):1-137. [Erratum in MMWR Recomm Rep. 2015;64(33):924.]
10. US Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Updated March 24, 2016. Accessed August 12, 2019.
11. Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643-648.1.
12. Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013:pii:bcr2012008279
13. Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: a report of nephrotic syndrome with minimal change disease. Am J Kidney Dis. 1987;9(2):176-179.
14. Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216(7):1159-1166.
15. Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351-1355.
16. Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. N Engl J Med. 1975;292(9):449-454.
A Veteran With Acute Progressive Encephalopathy of Unknown Etiology
Case Presentation. A 70-year-old US Marine Corps veteran of the Vietnam War with no significant past medical history was brought by ambulance to VA Boston Healthcare System (VABHS) after being found on the floor at home by his wife, awake, but with minimally coherent speech. He was moving all extremities, and there was no loss of bowel or bladder continence. He had last been seen well by his wife 30 minutes prior. When emergency medical services arrived, his finger stick blood glucose and vital signs were within normal range. In the emergency department, he was able to state his first name but then continuously repeated “7/11” to other questions. A neurologic examination revealed intact cranial nerves, full strength in all extremities, and normal reflexes. A National Institute of Health Stroke Scale (NIHSS) was 3, and a code stroke was activated. At the time of presentation, the patient was an active smoker of 15 cigarettes per day for 50 years and did not use alcohol or recreational drugs.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. Fehnel, the patient’s medical team was most worried about a transient ischemic attack (TIA) or cerebrovascular accident (CVA). Is his presentation consistent with these diagnoses, and what else is on your differential diagnosis?
►Corey R. Fehnel, MD, Neuro-Intensivist, BIDMC, and Assistant Professor of Neurology, Harvard Medical School. This patient is presenting with what appears to be an acute encephalopathy—a sudden onset of global alteration in mental status. The most worrisome underlying etiology for this presentation would be acute stroke, but this is an uncommon cause of acute encephalopathy. The differential diagnosis at this stage remains broad, but a careful neurologic examination can help narrow the possibilities. In particular, I would aim to differentiate an apparent language deficit (ie, aphasia) from a deficit of attention. A key finding that may help is the ability to name high- or low-frequency objects. If the patient can successfully name objects, aphasia is less likely. Based on the limited examination at present, the patient produces some normal speech, but perseverates; therefore, the finding remains nonspecific. My leading diagnoses are complex partial seizure and toxic/metabolic encephalopathy.
►Dr. Li. This patient’s NIHSS score is 3. How do you use this score in your management decisions for the patient?
►Dr. Fehnel. The NIHSS is a useful tool for gauging severity of ischemic and hemorrhagic stroke. However the score is not specific for establishing the diagnosis of stroke. Many common and chronic neurologic problems will score on the NIHSS, so it can never be interpreted in isolation. If the clinical history and complete neurologic examination support the diagnosis of stroke, then the NIHSS can be used with the understanding that it is biased toward anterior circulation strokes, and posterior circulation strokes will score lower even though they are potentially more life threatening.1 In this case, even though a complex partial seizure appears more likely, it is difficult to rule out the possibility of an acute stroke affecting the thalamus or, less likely, a distal middle cerebral artery occlusion. I would consider IV thrombolysis pending further history and neuroimaging results.
►Dr. Li. Initial laboratory data include a hemoglobin of 12.8 mg/dL. The white cell count, platelet count, chemistry panel, liver function tests, thyroid-stimulating hormone, and troponin were within normal range (Table 1).
Do you agree with inpatient workup for this patient whose mental status has now returned to baseline? If so, what workup would you pursue next?
►Dr. Fehnel. This patient requires inpatient admission to further evaluate the underlying etiology for his acute change in mental status. The improvement of his presenting deficit and largely normal neurovascular imaging make a neurovascular etiology less likely, but a careful risk factor evaluation for CVA/TIA should be performed, including continuous cardiac telemetry to detect atrial fibrillation. Magnetic resonance imaging (MRI) of the brain should be performed to rule out occult stroke and evaluate for a structural etiology given the more likely diagnosis of complex partial seizure. An electroencephalogram (EEG), preferably 24-hour continuous recording, should be performed. Without a clear toxic or metabolic etiology thus far to explain his acute global waxing-waning alteration in mental status and likely new-onset complex partial seizures, I would also pursue lumbar puncture for cerebrospinal fluid examination.
►Dr. Li. The hospital course was notable for episodes of acute combativeness and confusion. An MRI of the brain was deferred due to reports from the patient’s family of retained shrapnel in the lumbar spine. Routine EEG showed no seizure activity. This was followed by continuous video EEG monitoring, which showed subclinical seizure activity with a right temporal focus. He was started on valproic acid with improvement in his agitation, though confusion continued. He was discharged to an inpatient geriatric psychiatry nursing home with diagnosis of seizures and acute delirium.
Dr. Fehnel, seizures are often part of the workup for unexplained encephalopathy. In this case, the routine EEG was unrevealing, while the continuous video EEG proved valuable. In what situations would you pursue a continuous video EEG in addition to a routine EEG?
►Dr. Fehnel. EEG monitoring is only as good as the window of time during which the study is performed. If the suspicious clinical event is captured during a routine recording or an area of focal slowing is detected, a shorter study may be entirely sufficient. However, in cases where there is no clear alternative explanation, a patient’s mental status does not return to normal, or in the setting of mental status fluctuations without explanation, continuous video-EEG monitoring for at least 24 hours is indicated. While the prolonged study raises sensitivity, the exact duration of EEG recording required outside of the intensive care unit setting remains debated.2
►Dr. Li. If his encephalopathy were due to seizures alone, I would expect improvement in his mental status during interictal periods, which does not appear to be the case here. Do you feel the seizures alone can explain his encephalopathy?
►Dr. Fehnel. Complex partial seizures and the medications used to treat them can confound the examination of patients during the interictal period. We commonly debate postictal encephalopathy vs residual effect of benzodiazepines and rapid dose escalation of antiepileptic drugs as culprit in a patient’s prolonged alteration in mental status. Serial clinical examinations, continuous EEG monitoring to rule out ongoing subclinical seizures when appropriate, and judicious use of potentially sedating medications is the most helpful approach. The key issue here is the bimodal distribution of new-onset seizures. Among children there is a higher incidence of genetically related seizure disorders; whereas among adults, “acquired” and structural etiologies are more common. For this case, a more careful evaluation of acquired/structural etiologies for new-onset seizures is indicated.
►Dr. Li. At the geriatric psychiatry nursing home, the patient continued to be combative and refused medications. He was readmitted to the VABHS with encephalopathy of unclear etiology. An expanded encephalopathy workup was unrevealing (Table 2).
Dr. Fehnel, this patient’s initial cerebrospinal fluid (CSF) cell count and chemistries were completely normal. Is this sufficient to rule out encephalitis? If not, what other diagnostic tests would you send?
►Dr. Fehnel. A fully normal CSF profile reduces the likelihood of a broad range of neuro-infectious etiologies but does not completely rule those out. For example, there are reports of herpes simplex virus (HSV) encephalitis producing relatively normal profiles and even negative polymerase chain reaction assays for antibodies to HSV if the specimen is obtained very early in the course of the disease.3,4 That was not the case here as the CSF was obtained several days after his initial presentation. Given this patient’s clinical syndrome, normal CSF findings, and long smoking history without regular screening examinations, I would send a CSF specimen screening for paraneoplastic and autoimmune encephalitis. Most autoimmune encephalitis syndromes are associated with CSF lymphocytic pleocytosis or slight elevation in CSF protein levels. This patient’s diagnosis is most likely an anti-Hu paraneoplastic syndrome, which can be distinguished from other autoimmune and paraneoplastic processes by the characteristically normal CSF profile. Anti-Hu antibodies are strongly associated with non-small cell lung cancer (NSCLC). I would, therefore, also obtain more advanced chest imaging.
►Dr. Li. An autoimmune and paraneoplastic encephalitis panel was sent. While this send-out panel was pending, a CT torso was obtained to evaluate for occult malignancy in light of his significant smoking history. This showed a 3-cm spiculated mass originating from the left hilum. Bronchoalviolar lavage washings returned positive for small cell lung cancer.
Dr. Fehnel, can you explain the mechanism by which certain neoplasms can cause encephalitis?
►Dr. Fehnel. Onconeuronal antibodies Hu (NSCLC) and Ma2 (testicular seminoma), when identified, are strongly associated with the presence of an underlying malignancy. The work of Dr. Josep Dalmau and others in this area has dramatically improved our understanding of these syndromes over the past 25 years.5 The exact mechanism is not fully understood but is thought to be mediated by cytotoxic T-cell response directed at the malignancy itself with homology to intraneuronal structures, which are readily absorbed and result in neuronal cell death.6
►Dr. Li. Is there a specific treatment for paraneoplastic encephalitis, other than treating the underlying malignancy?
►Dr. Fehnel. Early treatment is associated with improved outcome and should not be delayed while waiting for laboratory confirmation in cases of high clinical suspicion. Treatment directed at the underlying tumor is the mainstay along with less specific immunosuppressive agents. Unfortunately Anti-Hu (as well as Ma2) antibodies are intraneuronal and less responsive to standard treatments relative to other paraneoplastic auto-antibodies identified on the cell surface. Immunosuppressive agents typically used in this setting include high-dose IV methylprednisolone, IV immune globulin (IVIG), rituximab, and cyclophosphamide.7
►Dr. Li. The patient was started on IVIG, methylprednisolone, cisplatin, and etoposide. His course was complicated by aspiration pneumonia, autonomic dysfunction causing tachy- and brady-arrhythmias, urosepsis, worsening somnolence, chemotherapy-induced neutropenic fevers, and ultimately septic shock. The palliative care team was closely involved throughout the final stages of his hospital course. After multiple family meetings, the patient was transitioned to comfort-focused care per family discussion and died 6 weeks after his initial presentation.
This patient had a very atypical initial presentation of small cell lung cancer. Despite the fact that a diagnosis eluded his doctors, they persisted in a thoughtful and exhaustive workup and through this perseverance were able to make the final diagnosis, which serves as an important learning case for us all.
Acknowledgments
We thank the family of this veteran for sharing his story and allowing us to learn from this case for the benefit of our future patients. We also thank Dr. Michelle Hankins, who provided oncologic expertise.
1. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157.
2. Herman ST, Abend NS, Bleck TP, et al; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95.
3. DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-S11.
4. Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015:pii:bcr201521052.
5. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
6. Greenlee JE, Clawson SA, Hill KE, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
7. Bradshaw MJ, Linnoila JJ. An overview of autoimmune and paraneoplastic encephalitides. Semin Neurol. 2018;38(3):330-343.
Case Presentation. A 70-year-old US Marine Corps veteran of the Vietnam War with no significant past medical history was brought by ambulance to VA Boston Healthcare System (VABHS) after being found on the floor at home by his wife, awake, but with minimally coherent speech. He was moving all extremities, and there was no loss of bowel or bladder continence. He had last been seen well by his wife 30 minutes prior. When emergency medical services arrived, his finger stick blood glucose and vital signs were within normal range. In the emergency department, he was able to state his first name but then continuously repeated “7/11” to other questions. A neurologic examination revealed intact cranial nerves, full strength in all extremities, and normal reflexes. A National Institute of Health Stroke Scale (NIHSS) was 3, and a code stroke was activated. At the time of presentation, the patient was an active smoker of 15 cigarettes per day for 50 years and did not use alcohol or recreational drugs.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. Fehnel, the patient’s medical team was most worried about a transient ischemic attack (TIA) or cerebrovascular accident (CVA). Is his presentation consistent with these diagnoses, and what else is on your differential diagnosis?
►Corey R. Fehnel, MD, Neuro-Intensivist, BIDMC, and Assistant Professor of Neurology, Harvard Medical School. This patient is presenting with what appears to be an acute encephalopathy—a sudden onset of global alteration in mental status. The most worrisome underlying etiology for this presentation would be acute stroke, but this is an uncommon cause of acute encephalopathy. The differential diagnosis at this stage remains broad, but a careful neurologic examination can help narrow the possibilities. In particular, I would aim to differentiate an apparent language deficit (ie, aphasia) from a deficit of attention. A key finding that may help is the ability to name high- or low-frequency objects. If the patient can successfully name objects, aphasia is less likely. Based on the limited examination at present, the patient produces some normal speech, but perseverates; therefore, the finding remains nonspecific. My leading diagnoses are complex partial seizure and toxic/metabolic encephalopathy.
►Dr. Li. This patient’s NIHSS score is 3. How do you use this score in your management decisions for the patient?
►Dr. Fehnel. The NIHSS is a useful tool for gauging severity of ischemic and hemorrhagic stroke. However the score is not specific for establishing the diagnosis of stroke. Many common and chronic neurologic problems will score on the NIHSS, so it can never be interpreted in isolation. If the clinical history and complete neurologic examination support the diagnosis of stroke, then the NIHSS can be used with the understanding that it is biased toward anterior circulation strokes, and posterior circulation strokes will score lower even though they are potentially more life threatening.1 In this case, even though a complex partial seizure appears more likely, it is difficult to rule out the possibility of an acute stroke affecting the thalamus or, less likely, a distal middle cerebral artery occlusion. I would consider IV thrombolysis pending further history and neuroimaging results.
►Dr. Li. Initial laboratory data include a hemoglobin of 12.8 mg/dL. The white cell count, platelet count, chemistry panel, liver function tests, thyroid-stimulating hormone, and troponin were within normal range (Table 1).
Do you agree with inpatient workup for this patient whose mental status has now returned to baseline? If so, what workup would you pursue next?
►Dr. Fehnel. This patient requires inpatient admission to further evaluate the underlying etiology for his acute change in mental status. The improvement of his presenting deficit and largely normal neurovascular imaging make a neurovascular etiology less likely, but a careful risk factor evaluation for CVA/TIA should be performed, including continuous cardiac telemetry to detect atrial fibrillation. Magnetic resonance imaging (MRI) of the brain should be performed to rule out occult stroke and evaluate for a structural etiology given the more likely diagnosis of complex partial seizure. An electroencephalogram (EEG), preferably 24-hour continuous recording, should be performed. Without a clear toxic or metabolic etiology thus far to explain his acute global waxing-waning alteration in mental status and likely new-onset complex partial seizures, I would also pursue lumbar puncture for cerebrospinal fluid examination.
►Dr. Li. The hospital course was notable for episodes of acute combativeness and confusion. An MRI of the brain was deferred due to reports from the patient’s family of retained shrapnel in the lumbar spine. Routine EEG showed no seizure activity. This was followed by continuous video EEG monitoring, which showed subclinical seizure activity with a right temporal focus. He was started on valproic acid with improvement in his agitation, though confusion continued. He was discharged to an inpatient geriatric psychiatry nursing home with diagnosis of seizures and acute delirium.
Dr. Fehnel, seizures are often part of the workup for unexplained encephalopathy. In this case, the routine EEG was unrevealing, while the continuous video EEG proved valuable. In what situations would you pursue a continuous video EEG in addition to a routine EEG?
►Dr. Fehnel. EEG monitoring is only as good as the window of time during which the study is performed. If the suspicious clinical event is captured during a routine recording or an area of focal slowing is detected, a shorter study may be entirely sufficient. However, in cases where there is no clear alternative explanation, a patient’s mental status does not return to normal, or in the setting of mental status fluctuations without explanation, continuous video-EEG monitoring for at least 24 hours is indicated. While the prolonged study raises sensitivity, the exact duration of EEG recording required outside of the intensive care unit setting remains debated.2
►Dr. Li. If his encephalopathy were due to seizures alone, I would expect improvement in his mental status during interictal periods, which does not appear to be the case here. Do you feel the seizures alone can explain his encephalopathy?
►Dr. Fehnel. Complex partial seizures and the medications used to treat them can confound the examination of patients during the interictal period. We commonly debate postictal encephalopathy vs residual effect of benzodiazepines and rapid dose escalation of antiepileptic drugs as culprit in a patient’s prolonged alteration in mental status. Serial clinical examinations, continuous EEG monitoring to rule out ongoing subclinical seizures when appropriate, and judicious use of potentially sedating medications is the most helpful approach. The key issue here is the bimodal distribution of new-onset seizures. Among children there is a higher incidence of genetically related seizure disorders; whereas among adults, “acquired” and structural etiologies are more common. For this case, a more careful evaluation of acquired/structural etiologies for new-onset seizures is indicated.
►Dr. Li. At the geriatric psychiatry nursing home, the patient continued to be combative and refused medications. He was readmitted to the VABHS with encephalopathy of unclear etiology. An expanded encephalopathy workup was unrevealing (Table 2).
Dr. Fehnel, this patient’s initial cerebrospinal fluid (CSF) cell count and chemistries were completely normal. Is this sufficient to rule out encephalitis? If not, what other diagnostic tests would you send?
►Dr. Fehnel. A fully normal CSF profile reduces the likelihood of a broad range of neuro-infectious etiologies but does not completely rule those out. For example, there are reports of herpes simplex virus (HSV) encephalitis producing relatively normal profiles and even negative polymerase chain reaction assays for antibodies to HSV if the specimen is obtained very early in the course of the disease.3,4 That was not the case here as the CSF was obtained several days after his initial presentation. Given this patient’s clinical syndrome, normal CSF findings, and long smoking history without regular screening examinations, I would send a CSF specimen screening for paraneoplastic and autoimmune encephalitis. Most autoimmune encephalitis syndromes are associated with CSF lymphocytic pleocytosis or slight elevation in CSF protein levels. This patient’s diagnosis is most likely an anti-Hu paraneoplastic syndrome, which can be distinguished from other autoimmune and paraneoplastic processes by the characteristically normal CSF profile. Anti-Hu antibodies are strongly associated with non-small cell lung cancer (NSCLC). I would, therefore, also obtain more advanced chest imaging.
►Dr. Li. An autoimmune and paraneoplastic encephalitis panel was sent. While this send-out panel was pending, a CT torso was obtained to evaluate for occult malignancy in light of his significant smoking history. This showed a 3-cm spiculated mass originating from the left hilum. Bronchoalviolar lavage washings returned positive for small cell lung cancer.
Dr. Fehnel, can you explain the mechanism by which certain neoplasms can cause encephalitis?
►Dr. Fehnel. Onconeuronal antibodies Hu (NSCLC) and Ma2 (testicular seminoma), when identified, are strongly associated with the presence of an underlying malignancy. The work of Dr. Josep Dalmau and others in this area has dramatically improved our understanding of these syndromes over the past 25 years.5 The exact mechanism is not fully understood but is thought to be mediated by cytotoxic T-cell response directed at the malignancy itself with homology to intraneuronal structures, which are readily absorbed and result in neuronal cell death.6
►Dr. Li. Is there a specific treatment for paraneoplastic encephalitis, other than treating the underlying malignancy?
►Dr. Fehnel. Early treatment is associated with improved outcome and should not be delayed while waiting for laboratory confirmation in cases of high clinical suspicion. Treatment directed at the underlying tumor is the mainstay along with less specific immunosuppressive agents. Unfortunately Anti-Hu (as well as Ma2) antibodies are intraneuronal and less responsive to standard treatments relative to other paraneoplastic auto-antibodies identified on the cell surface. Immunosuppressive agents typically used in this setting include high-dose IV methylprednisolone, IV immune globulin (IVIG), rituximab, and cyclophosphamide.7
►Dr. Li. The patient was started on IVIG, methylprednisolone, cisplatin, and etoposide. His course was complicated by aspiration pneumonia, autonomic dysfunction causing tachy- and brady-arrhythmias, urosepsis, worsening somnolence, chemotherapy-induced neutropenic fevers, and ultimately septic shock. The palliative care team was closely involved throughout the final stages of his hospital course. After multiple family meetings, the patient was transitioned to comfort-focused care per family discussion and died 6 weeks after his initial presentation.
This patient had a very atypical initial presentation of small cell lung cancer. Despite the fact that a diagnosis eluded his doctors, they persisted in a thoughtful and exhaustive workup and through this perseverance were able to make the final diagnosis, which serves as an important learning case for us all.
Acknowledgments
We thank the family of this veteran for sharing his story and allowing us to learn from this case for the benefit of our future patients. We also thank Dr. Michelle Hankins, who provided oncologic expertise.
Case Presentation. A 70-year-old US Marine Corps veteran of the Vietnam War with no significant past medical history was brought by ambulance to VA Boston Healthcare System (VABHS) after being found on the floor at home by his wife, awake, but with minimally coherent speech. He was moving all extremities, and there was no loss of bowel or bladder continence. He had last been seen well by his wife 30 minutes prior. When emergency medical services arrived, his finger stick blood glucose and vital signs were within normal range. In the emergency department, he was able to state his first name but then continuously repeated “7/11” to other questions. A neurologic examination revealed intact cranial nerves, full strength in all extremities, and normal reflexes. A National Institute of Health Stroke Scale (NIHSS) was 3, and a code stroke was activated. At the time of presentation, the patient was an active smoker of 15 cigarettes per day for 50 years and did not use alcohol or recreational drugs.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. Fehnel, the patient’s medical team was most worried about a transient ischemic attack (TIA) or cerebrovascular accident (CVA). Is his presentation consistent with these diagnoses, and what else is on your differential diagnosis?
►Corey R. Fehnel, MD, Neuro-Intensivist, BIDMC, and Assistant Professor of Neurology, Harvard Medical School. This patient is presenting with what appears to be an acute encephalopathy—a sudden onset of global alteration in mental status. The most worrisome underlying etiology for this presentation would be acute stroke, but this is an uncommon cause of acute encephalopathy. The differential diagnosis at this stage remains broad, but a careful neurologic examination can help narrow the possibilities. In particular, I would aim to differentiate an apparent language deficit (ie, aphasia) from a deficit of attention. A key finding that may help is the ability to name high- or low-frequency objects. If the patient can successfully name objects, aphasia is less likely. Based on the limited examination at present, the patient produces some normal speech, but perseverates; therefore, the finding remains nonspecific. My leading diagnoses are complex partial seizure and toxic/metabolic encephalopathy.
►Dr. Li. This patient’s NIHSS score is 3. How do you use this score in your management decisions for the patient?
►Dr. Fehnel. The NIHSS is a useful tool for gauging severity of ischemic and hemorrhagic stroke. However the score is not specific for establishing the diagnosis of stroke. Many common and chronic neurologic problems will score on the NIHSS, so it can never be interpreted in isolation. If the clinical history and complete neurologic examination support the diagnosis of stroke, then the NIHSS can be used with the understanding that it is biased toward anterior circulation strokes, and posterior circulation strokes will score lower even though they are potentially more life threatening.1 In this case, even though a complex partial seizure appears more likely, it is difficult to rule out the possibility of an acute stroke affecting the thalamus or, less likely, a distal middle cerebral artery occlusion. I would consider IV thrombolysis pending further history and neuroimaging results.
►Dr. Li. Initial laboratory data include a hemoglobin of 12.8 mg/dL. The white cell count, platelet count, chemistry panel, liver function tests, thyroid-stimulating hormone, and troponin were within normal range (Table 1).
Do you agree with inpatient workup for this patient whose mental status has now returned to baseline? If so, what workup would you pursue next?
►Dr. Fehnel. This patient requires inpatient admission to further evaluate the underlying etiology for his acute change in mental status. The improvement of his presenting deficit and largely normal neurovascular imaging make a neurovascular etiology less likely, but a careful risk factor evaluation for CVA/TIA should be performed, including continuous cardiac telemetry to detect atrial fibrillation. Magnetic resonance imaging (MRI) of the brain should be performed to rule out occult stroke and evaluate for a structural etiology given the more likely diagnosis of complex partial seizure. An electroencephalogram (EEG), preferably 24-hour continuous recording, should be performed. Without a clear toxic or metabolic etiology thus far to explain his acute global waxing-waning alteration in mental status and likely new-onset complex partial seizures, I would also pursue lumbar puncture for cerebrospinal fluid examination.
►Dr. Li. The hospital course was notable for episodes of acute combativeness and confusion. An MRI of the brain was deferred due to reports from the patient’s family of retained shrapnel in the lumbar spine. Routine EEG showed no seizure activity. This was followed by continuous video EEG monitoring, which showed subclinical seizure activity with a right temporal focus. He was started on valproic acid with improvement in his agitation, though confusion continued. He was discharged to an inpatient geriatric psychiatry nursing home with diagnosis of seizures and acute delirium.
Dr. Fehnel, seizures are often part of the workup for unexplained encephalopathy. In this case, the routine EEG was unrevealing, while the continuous video EEG proved valuable. In what situations would you pursue a continuous video EEG in addition to a routine EEG?
►Dr. Fehnel. EEG monitoring is only as good as the window of time during which the study is performed. If the suspicious clinical event is captured during a routine recording or an area of focal slowing is detected, a shorter study may be entirely sufficient. However, in cases where there is no clear alternative explanation, a patient’s mental status does not return to normal, or in the setting of mental status fluctuations without explanation, continuous video-EEG monitoring for at least 24 hours is indicated. While the prolonged study raises sensitivity, the exact duration of EEG recording required outside of the intensive care unit setting remains debated.2
►Dr. Li. If his encephalopathy were due to seizures alone, I would expect improvement in his mental status during interictal periods, which does not appear to be the case here. Do you feel the seizures alone can explain his encephalopathy?
►Dr. Fehnel. Complex partial seizures and the medications used to treat them can confound the examination of patients during the interictal period. We commonly debate postictal encephalopathy vs residual effect of benzodiazepines and rapid dose escalation of antiepileptic drugs as culprit in a patient’s prolonged alteration in mental status. Serial clinical examinations, continuous EEG monitoring to rule out ongoing subclinical seizures when appropriate, and judicious use of potentially sedating medications is the most helpful approach. The key issue here is the bimodal distribution of new-onset seizures. Among children there is a higher incidence of genetically related seizure disorders; whereas among adults, “acquired” and structural etiologies are more common. For this case, a more careful evaluation of acquired/structural etiologies for new-onset seizures is indicated.
►Dr. Li. At the geriatric psychiatry nursing home, the patient continued to be combative and refused medications. He was readmitted to the VABHS with encephalopathy of unclear etiology. An expanded encephalopathy workup was unrevealing (Table 2).
Dr. Fehnel, this patient’s initial cerebrospinal fluid (CSF) cell count and chemistries were completely normal. Is this sufficient to rule out encephalitis? If not, what other diagnostic tests would you send?
►Dr. Fehnel. A fully normal CSF profile reduces the likelihood of a broad range of neuro-infectious etiologies but does not completely rule those out. For example, there are reports of herpes simplex virus (HSV) encephalitis producing relatively normal profiles and even negative polymerase chain reaction assays for antibodies to HSV if the specimen is obtained very early in the course of the disease.3,4 That was not the case here as the CSF was obtained several days after his initial presentation. Given this patient’s clinical syndrome, normal CSF findings, and long smoking history without regular screening examinations, I would send a CSF specimen screening for paraneoplastic and autoimmune encephalitis. Most autoimmune encephalitis syndromes are associated with CSF lymphocytic pleocytosis or slight elevation in CSF protein levels. This patient’s diagnosis is most likely an anti-Hu paraneoplastic syndrome, which can be distinguished from other autoimmune and paraneoplastic processes by the characteristically normal CSF profile. Anti-Hu antibodies are strongly associated with non-small cell lung cancer (NSCLC). I would, therefore, also obtain more advanced chest imaging.
►Dr. Li. An autoimmune and paraneoplastic encephalitis panel was sent. While this send-out panel was pending, a CT torso was obtained to evaluate for occult malignancy in light of his significant smoking history. This showed a 3-cm spiculated mass originating from the left hilum. Bronchoalviolar lavage washings returned positive for small cell lung cancer.
Dr. Fehnel, can you explain the mechanism by which certain neoplasms can cause encephalitis?
►Dr. Fehnel. Onconeuronal antibodies Hu (NSCLC) and Ma2 (testicular seminoma), when identified, are strongly associated with the presence of an underlying malignancy. The work of Dr. Josep Dalmau and others in this area has dramatically improved our understanding of these syndromes over the past 25 years.5 The exact mechanism is not fully understood but is thought to be mediated by cytotoxic T-cell response directed at the malignancy itself with homology to intraneuronal structures, which are readily absorbed and result in neuronal cell death.6
►Dr. Li. Is there a specific treatment for paraneoplastic encephalitis, other than treating the underlying malignancy?
►Dr. Fehnel. Early treatment is associated with improved outcome and should not be delayed while waiting for laboratory confirmation in cases of high clinical suspicion. Treatment directed at the underlying tumor is the mainstay along with less specific immunosuppressive agents. Unfortunately Anti-Hu (as well as Ma2) antibodies are intraneuronal and less responsive to standard treatments relative to other paraneoplastic auto-antibodies identified on the cell surface. Immunosuppressive agents typically used in this setting include high-dose IV methylprednisolone, IV immune globulin (IVIG), rituximab, and cyclophosphamide.7
►Dr. Li. The patient was started on IVIG, methylprednisolone, cisplatin, and etoposide. His course was complicated by aspiration pneumonia, autonomic dysfunction causing tachy- and brady-arrhythmias, urosepsis, worsening somnolence, chemotherapy-induced neutropenic fevers, and ultimately septic shock. The palliative care team was closely involved throughout the final stages of his hospital course. After multiple family meetings, the patient was transitioned to comfort-focused care per family discussion and died 6 weeks after his initial presentation.
This patient had a very atypical initial presentation of small cell lung cancer. Despite the fact that a diagnosis eluded his doctors, they persisted in a thoughtful and exhaustive workup and through this perseverance were able to make the final diagnosis, which serves as an important learning case for us all.
Acknowledgments
We thank the family of this veteran for sharing his story and allowing us to learn from this case for the benefit of our future patients. We also thank Dr. Michelle Hankins, who provided oncologic expertise.
1. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157.
2. Herman ST, Abend NS, Bleck TP, et al; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95.
3. DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-S11.
4. Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015:pii:bcr201521052.
5. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
6. Greenlee JE, Clawson SA, Hill KE, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
7. Bradshaw MJ, Linnoila JJ. An overview of autoimmune and paraneoplastic encephalitides. Semin Neurol. 2018;38(3):330-343.
1. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157.
2. Herman ST, Abend NS, Bleck TP, et al; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95.
3. DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-S11.
4. Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015:pii:bcr201521052.
5. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
6. Greenlee JE, Clawson SA, Hill KE, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
7. Bradshaw MJ, Linnoila JJ. An overview of autoimmune and paraneoplastic encephalitides. Semin Neurol. 2018;38(3):330-343.
Acute Treatment of Hypertensive Urgency
The "Things We Do for No Reason" (TWDFNR) series reviews practices which have become common parts of hospital care but provide little value to our patients. Practices reviewed in the TWDFNR series do not represent "black and white" conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 67-year-old man is hospitalized with community-acquired pneumonia. He has a history of hypertension and is prescribed two antihypertensive medications (amlodipine and chlorthalidone) as an outpatient. On the evening of hospital day two, he is found to have a blood pressure of 192/95 on a scheduled vital signs check. He reports no symptoms other than cough, which is not new or worsening. The covering hospitalist reviews the documented blood pressures since admission and notes that many have been elevated despite continuation of his home regimen. The patient's nurse inquires about treating the patient with additional "as-needed" antihypertensive medications.
BACKGROUND
Hypertensive crises are common in hospitalized patients, with approximately one in seven patients experiencing an episode of hypertensive emergency and/or hypertensive urgency.1 Hypertensive emergency is typically defined as (1) a systolic blood pressure ≥180 mm Hg and/or a diastolic blood pressure ≥120 mm Hg with (2) evidence of new or worsening end-organ damage. The organs most commonly affected by severe hypertension are the brain (headache, confusion, stroke), heart (chest pain, myocardial infarction, pulmonary edema), large blood vessels (aortic dissection), and kidneys (acute hypertensive nephrosclerosis).2 With hypertensive urgency, patients experience similarly elevated blood pressure but have no symptoms or signs suggesting acute end-organ damage. Acute treatment with intravenous (IV) or immediate-acting oral medications is common; a single-center study showed that 7.4% of hospitalized patients had an order for "as needed" IV hydralazine or labetalol, with 60.3% receiving at least one dose.3 Among internal medicine and family medicine trainees in one survey, nearly half reported that they would use IV medications in a scenario where an inpatient had an asymptomatic blood pressure above 180 mm Hg.4
WHY YOU MIGHT THINK TREATING HYPERTENSIVE URGENCY IS NECESSARY
Treating patients with hypertensive urgency is based on an assumption: If one does not treat immediately, something bad (ie, end-organ damage) will occur over the next few hours. Data from the 1930s showed that patients with untreated hypertensive emergency had a one-year mortality rate >79% and a median survival of 10.4 months.5 More recent studies suggest that the in-hospital and one-year mortality for those with hypertensive emergency are 13% and 39%, respectively.6 These data demonstrate that patients with hypertensive emergency are at risk in both the short- and long-term.
Patients with hypertensive urgency are also at increased risk for long-term morbidity and mortality. The one-year mortality for those experiencing an episode of hypertensive urgency is approximately 9%.6 Given the concerns about poor outcomes, it remains a common practice in many facilities to acutely lower the blood pressure in patients with hypertensive urgency. This is highlighted by recommendations of a commonly used point-of-care medical resource, which suggests that "potential legal ramifications partially motivate lowering the blood pressure over several hours."7
WHY TREATING HYPERTENSIVE URGENCY IS UNNECESSARY AND POTENTIALLY HARMFUL
Concerns regarding overtreatment of hypertensive urgency relate to overestimated rates of hypertensive complications, the pathophysiology of hypertension itself, and the potential for adverse events related to treatment. Given that there are few trials examining hospitalized patients with hypertensive urgency, much of the data supporting a conservative approach are drawn from studies of outpatients or emergency department patients. In addition, there is little data suggesting that outcomes are different for patients presenting with a chief complaint of hypertensive urgency and those presenting with an alternate diagnosis but who are found to have blood pressures that meet the threshold for diagnosis of hypertensive urgency.
The landmark 1967 Veterans Affairs Cooperative Trial demonstrated the long-term benefits of treating patients with chronic hypertensive urgency.8 Importantly though, benefits accrued over a period of months to years, not hours. The time to the first adverse event in the placebo arm was two months, suggesting that even those with blood pressures chronically in the range of hypertensive urgency are unlikely to experience hyperacute (ie, within hours) events, even without treatment.
A more recent study, conducted by Patel et al., examined 58,836 patients seen in outpatient clinics and found to have blood pressures meeting the criteria for hypertensive urgency.9 This study included patients whose primary issue was hypertensive urgency and patients in whom the diagnosis was secondary. A total of 426 patients were referred to the hospital and only 100 (0.17%) were subsequently admitted. At seven days, the rates of the primary outcome (a composite of myocardial infarction, stroke, and/or transient ischemic attack) were 0.1% in those sent home and 0.5% in those sent to the hospital. In those patients with a systolic blood pressure ≥220 mm Hg, two out of 977 (0.2%) of those sent home and zero out of 81 of those sent to the hospital experienced the primary outcome. These data reinforce the message that, in patients with hypertensive urgency, rates of adverse events at seven days are low, even with extreme blood pressure elevation.
The human body has adapted to withstand wide variations in blood pressure.10 For example, through arteriolar constriction and reflex vasodilation, cerebral autoregulation maintains a constant cerebral blood flow within a wide range of perfusion pressures, ensuring that the brain is protected from higher mean arterial pressures.11 While this process is protective, over time the autoregulatory system becomes impaired, especially in patients with cerebrovascular disease. This places patients at risk for cerebral and/or cardiac ischemia with even slight drops in perfusion pressure.12,13 Indeed, in assessing treatment-related adverse events in a series of patients treated with intravenous nicardipine or nitroprusside for hypertensive emergency, Brooks and colleagues reported that 57% (27 of 47) of patients had overly large reductions in blood pressure (>25% reduction in mean arterial pressure) within the first 30 minutes of treatment.14 Two patients had acute ischemic events attributed to treatment with antihypertensive medications. Myocardial infarction and stroke have both been reported,12 and medication classes such as calcium channel blockers (sublingual nifedipine in particular), beta-blockers (eg, labetolol), angiotensin-converting-enzyme inhibitors (eg, captopril), and clonidine have all been implicated in treatment-related adverse events.12,15-17 Another potential issue derives from the observation that blood pressures obtained in the hospital setting are often inaccurate, owing to inappropriate patient preparation, faulty equipment, and inadequate training of staff obtaining the measurement.18
National guidelines support a cautious approach to the treatment of hypertensive urgency. The seventh Report of the Joint National Committee on Detection, Evaluation, and Treatment of Hypertension, published in 2003, noted that "patients with markedly elevated BP but without acute target-organ damage usually do not require hospitalization, but they should receive immediate combination oral antihypertensive therapy" and that "there is no evidence to suggest that failure to aggressively lower BP in the [emergency department] is associated with any increased short-term risk to the patient who presents with severe hypertension." JNC 7 also laments contemporary terminology: "Unfortunately, the term 'urgency' has led to overly aggressive management of many patients with severe, uncomplicated hypertension. Aggressive dosing with intravenous drugs or even oral agents, to rapidly lower BP is not without risk."19 The most recent JNC guideline does not comment on hypertensive urgency,20 and the 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults argues that, "¬there is no indication for referral to the emergency department, immediate reduction in BP in the emergency department, or hospitalization for [patients with hypertensive urgency]."21
WHAT CLINICIANS SHOULD DO INSTEAD
After it is confirmed that a patient has no end-organ damage (ie, the patient has hypertensive urgency, not emergency), treatable causes of hypertension should be assessed. In hospitalized patients, these include missed or held doses of outpatient medications, pain, nausea, alcohol and/or benzodiazepine withdrawal, delirium, and obstructive sleep apnea.22 If no remediable cause is identified, patients should be allowed to rest for at least 30 minutes without the administration of additional antihypertensive medications, after which time the blood pressure should be measured using the correct technique.2 Clinical trials have shown that rest is effective at lowering blood pressure in patients with hypertensive urgency.23,24 One study initially treated 549 emergency department patients with a 30-minute rest period, after which time 32% of patients had responded (defined as a SBP <180 mm Hg and DBP <110 mm Hg, with at least a 20 mm Hg reduction in baseline SBP and/or a 10 mm Hg reduction in DBP).23 Another study randomized 138 patients with hypertensive urgency to either rest or active treatment with telmisartan. Blood pressures were checked every 30 minutes for four hours. The primary endpoint (reduction of MAP of 10%-35%) was similar in both groups (68.5% in the rest group and 69.1% in the telmisartan group).24 Even if rest is ineffective, the risk-benefit ratio of acutely lowering blood pressure will typically favor withholding acute treatment in asymptomatic patients. If blood pressure remains consistently elevated, augmentation of the home regimen (eg, increasing the dose of their next scheduled antihypertensive) of oral medications may be warranted. Though not all agree with management of antihypertensives in hospitalized patients,25 acute hospitalizations afford an opportunity to modify and observe chronic hypertension.26
RECOMMENDATIONS
- Ensure that patients do not have symptoms and/or signs of end-organ damage. This can be done with a brief review of systems and a physical examination. In select cases, an electrocardiogram and a chest x-ray may be warranted.
- Search for common causes of treatable hypertension in hospitalized patients; these include pain, nausea, withdrawal syndromes, and holding of usual antihypertensive medications.
- In those patients without symptoms and/or signs of end-organ damage, allow rest, followed by reassessment.
- Do not administer intravenous or immediate-acting oral antihypertensive medications to acutely lower blood pressure. Instead, address the issues raised in Recommendation #2 and consider modifying the chronic oral antihypertensive regimen in patients who are uncontrolled as outpatients or who are not treated as outpatients. Coordinate early postdischarge follow-up for repeat blood pressure evaluation and continued modification of a patient's chronic antihypertensive regimen.
CONCLUSION
Although patients with hypertensive urgency are often treated with medications to acutely lower their blood pressure, there is no evidence to support this practice, and a strong pathophysiologic basis suggests that harm may result. The patient in the case described above should be allowed to rest for at least 30 minutes, with reevaluation of his blood pressure. If it remains elevated and no treatable secondary causes are found, the treating hospitalist should consider altering his chronic antihypertensive regimen to promote long-term blood pressure control.
Do you think this is a low-value practice? Is this truly a "Thing We Do for No Reason?" Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other "Things We Do for No Reason" topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest.
1. Shorr AF, Zilberberg MD, Sun X, et al. Severe acute hypertension among inpatients admitted from the emergency department. J Hosp Med. 2012;7(3):203-210. doi: 10.1002/jhm.969. PubMed
2. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2017. PubMed
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
4. Axon RN, Garrell R, Pfahl K, et al. Attitudes and practices of resident physicians regarding hypertension in the inpatient setting. J Clin Hypertens (Greenwich). 2010;12(9):698-705. doi: 10.1111/j.1751-7176.2010.00309.x. PubMed
5. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1974;268(6):336-345. doi: 10.1097/00000441-197412000-00004. PubMed
6. Guiga H, Decroux C, Michelet P, et al. Hospital and out-of-hospital mortality in 670 hypertensive emergencies and urgencies. J Clin Hypertens (Greenwich). 2017;19(11):1137-1142. doi: 10.1111/jch.13083. PubMed
7. Varon J, Williams EJ. Management of severe asymptomatic hypertension (hypertensive urgencies) in adults. In: Post T, ed. UpToDate, Waltham, MA. (Accessed February 13, 2018). PubMed
8. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202(11):1028-1034. soi: 10.1001/jama.1967.03130240070013 PubMed
9. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509. PubMed
10. MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58(3):785-790. doi: 10.1152/jappl.1985.58.3.785. PubMed
11. Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973;1(5852):507-510. doi: 10.1136/bmj.1.5852.507. PubMed
12. Fischberg GM, Lozano E, Rajamani K, Ameriso S, Fisher MJ. Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;19(4):339-346. doi: 10.1016/S0736-4679(00)00267-5. PubMed
13. Ross RS. Pathophysiology of coronary circulation. Br Heart J. 1971;33(2):173-184. doi: 10.1136/hrt.33.2.173. PubMed
14. Brooks TW, Finch CK, Lobo BL, Deaton PR, Varner CF. Blood pressure management in acute hypertensive emergency. Am J Health Syst Pharm. 2007;64(24):2579-2582. doi: 10.2146/ajhp070105. PubMed
15. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-1331. doi: 10.1001/jama.1996.03540160050032 PubMed
16. Hodsman GP, Isles CG, Murray GD et al. Factors related to first dose hypotensive effect of captopril: prediction and treatment. Br Med J (Clin Res Ed). 1983;286(6368):832-834. doi: 10.1136/bmj.286.6368.832. PubMed
17. Zeller KR, Von Kuhnert L, Matthews C. Rapid reduction of severe asymptomatic hypertension. A prospective, controlled trial. Arch Intern Med. 1989;149(10):2186-2189. doi: 10.1001/archinte.149.10.2186. PubMed
18. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697-716. doi: 10.1161/01.CIR.0000154900.76284.F6. PubMed
19. Chobanian AV, Bakris GL, Black HR, et al. The seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560. PubMed
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427 PubMed
21. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017. PubMed
22. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94. doi: 10.1007/s11886-015-0648-y. PubMed
23. Grassi D, O'Flaherty M, Pellizzari M, et al. Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles. J Clin Hypertens (Greenwich). 2008;10(9):662-667. doi: 10.1111/j.1751-7176.2008.00001.x. PubMed
24. Park SK, Lee DY, Kim WJ, et al. Comparing the clinical efficacy of resting and antihypertensive medication in patients of hypertensive urgency: a randomized, control trial. J Hypertens. 2017;35(7):1474-1480. doi: 10.1097/HJH.0000000000001340. PubMed
25. Steinman MA, Auerbach AD. Managing chronic disease in hospitalized patients. JAMA Intern Med. 2013;173(20):1857-1858. doi: 10.1001/jamainternmed.2013.9511. PubMed
26. Breu AC, Allen-Dicker J, Mueller S et al. Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9(5):303-309. doi: 10.1002/jhm.2137. PubMed
The "Things We Do for No Reason" (TWDFNR) series reviews practices which have become common parts of hospital care but provide little value to our patients. Practices reviewed in the TWDFNR series do not represent "black and white" conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 67-year-old man is hospitalized with community-acquired pneumonia. He has a history of hypertension and is prescribed two antihypertensive medications (amlodipine and chlorthalidone) as an outpatient. On the evening of hospital day two, he is found to have a blood pressure of 192/95 on a scheduled vital signs check. He reports no symptoms other than cough, which is not new or worsening. The covering hospitalist reviews the documented blood pressures since admission and notes that many have been elevated despite continuation of his home regimen. The patient's nurse inquires about treating the patient with additional "as-needed" antihypertensive medications.
BACKGROUND
Hypertensive crises are common in hospitalized patients, with approximately one in seven patients experiencing an episode of hypertensive emergency and/or hypertensive urgency.1 Hypertensive emergency is typically defined as (1) a systolic blood pressure ≥180 mm Hg and/or a diastolic blood pressure ≥120 mm Hg with (2) evidence of new or worsening end-organ damage. The organs most commonly affected by severe hypertension are the brain (headache, confusion, stroke), heart (chest pain, myocardial infarction, pulmonary edema), large blood vessels (aortic dissection), and kidneys (acute hypertensive nephrosclerosis).2 With hypertensive urgency, patients experience similarly elevated blood pressure but have no symptoms or signs suggesting acute end-organ damage. Acute treatment with intravenous (IV) or immediate-acting oral medications is common; a single-center study showed that 7.4% of hospitalized patients had an order for "as needed" IV hydralazine or labetalol, with 60.3% receiving at least one dose.3 Among internal medicine and family medicine trainees in one survey, nearly half reported that they would use IV medications in a scenario where an inpatient had an asymptomatic blood pressure above 180 mm Hg.4
WHY YOU MIGHT THINK TREATING HYPERTENSIVE URGENCY IS NECESSARY
Treating patients with hypertensive urgency is based on an assumption: If one does not treat immediately, something bad (ie, end-organ damage) will occur over the next few hours. Data from the 1930s showed that patients with untreated hypertensive emergency had a one-year mortality rate >79% and a median survival of 10.4 months.5 More recent studies suggest that the in-hospital and one-year mortality for those with hypertensive emergency are 13% and 39%, respectively.6 These data demonstrate that patients with hypertensive emergency are at risk in both the short- and long-term.
Patients with hypertensive urgency are also at increased risk for long-term morbidity and mortality. The one-year mortality for those experiencing an episode of hypertensive urgency is approximately 9%.6 Given the concerns about poor outcomes, it remains a common practice in many facilities to acutely lower the blood pressure in patients with hypertensive urgency. This is highlighted by recommendations of a commonly used point-of-care medical resource, which suggests that "potential legal ramifications partially motivate lowering the blood pressure over several hours."7
WHY TREATING HYPERTENSIVE URGENCY IS UNNECESSARY AND POTENTIALLY HARMFUL
Concerns regarding overtreatment of hypertensive urgency relate to overestimated rates of hypertensive complications, the pathophysiology of hypertension itself, and the potential for adverse events related to treatment. Given that there are few trials examining hospitalized patients with hypertensive urgency, much of the data supporting a conservative approach are drawn from studies of outpatients or emergency department patients. In addition, there is little data suggesting that outcomes are different for patients presenting with a chief complaint of hypertensive urgency and those presenting with an alternate diagnosis but who are found to have blood pressures that meet the threshold for diagnosis of hypertensive urgency.
The landmark 1967 Veterans Affairs Cooperative Trial demonstrated the long-term benefits of treating patients with chronic hypertensive urgency.8 Importantly though, benefits accrued over a period of months to years, not hours. The time to the first adverse event in the placebo arm was two months, suggesting that even those with blood pressures chronically in the range of hypertensive urgency are unlikely to experience hyperacute (ie, within hours) events, even without treatment.
A more recent study, conducted by Patel et al., examined 58,836 patients seen in outpatient clinics and found to have blood pressures meeting the criteria for hypertensive urgency.9 This study included patients whose primary issue was hypertensive urgency and patients in whom the diagnosis was secondary. A total of 426 patients were referred to the hospital and only 100 (0.17%) were subsequently admitted. At seven days, the rates of the primary outcome (a composite of myocardial infarction, stroke, and/or transient ischemic attack) were 0.1% in those sent home and 0.5% in those sent to the hospital. In those patients with a systolic blood pressure ≥220 mm Hg, two out of 977 (0.2%) of those sent home and zero out of 81 of those sent to the hospital experienced the primary outcome. These data reinforce the message that, in patients with hypertensive urgency, rates of adverse events at seven days are low, even with extreme blood pressure elevation.
The human body has adapted to withstand wide variations in blood pressure.10 For example, through arteriolar constriction and reflex vasodilation, cerebral autoregulation maintains a constant cerebral blood flow within a wide range of perfusion pressures, ensuring that the brain is protected from higher mean arterial pressures.11 While this process is protective, over time the autoregulatory system becomes impaired, especially in patients with cerebrovascular disease. This places patients at risk for cerebral and/or cardiac ischemia with even slight drops in perfusion pressure.12,13 Indeed, in assessing treatment-related adverse events in a series of patients treated with intravenous nicardipine or nitroprusside for hypertensive emergency, Brooks and colleagues reported that 57% (27 of 47) of patients had overly large reductions in blood pressure (>25% reduction in mean arterial pressure) within the first 30 minutes of treatment.14 Two patients had acute ischemic events attributed to treatment with antihypertensive medications. Myocardial infarction and stroke have both been reported,12 and medication classes such as calcium channel blockers (sublingual nifedipine in particular), beta-blockers (eg, labetolol), angiotensin-converting-enzyme inhibitors (eg, captopril), and clonidine have all been implicated in treatment-related adverse events.12,15-17 Another potential issue derives from the observation that blood pressures obtained in the hospital setting are often inaccurate, owing to inappropriate patient preparation, faulty equipment, and inadequate training of staff obtaining the measurement.18
National guidelines support a cautious approach to the treatment of hypertensive urgency. The seventh Report of the Joint National Committee on Detection, Evaluation, and Treatment of Hypertension, published in 2003, noted that "patients with markedly elevated BP but without acute target-organ damage usually do not require hospitalization, but they should receive immediate combination oral antihypertensive therapy" and that "there is no evidence to suggest that failure to aggressively lower BP in the [emergency department] is associated with any increased short-term risk to the patient who presents with severe hypertension." JNC 7 also laments contemporary terminology: "Unfortunately, the term 'urgency' has led to overly aggressive management of many patients with severe, uncomplicated hypertension. Aggressive dosing with intravenous drugs or even oral agents, to rapidly lower BP is not without risk."19 The most recent JNC guideline does not comment on hypertensive urgency,20 and the 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults argues that, "¬there is no indication for referral to the emergency department, immediate reduction in BP in the emergency department, or hospitalization for [patients with hypertensive urgency]."21
WHAT CLINICIANS SHOULD DO INSTEAD
After it is confirmed that a patient has no end-organ damage (ie, the patient has hypertensive urgency, not emergency), treatable causes of hypertension should be assessed. In hospitalized patients, these include missed or held doses of outpatient medications, pain, nausea, alcohol and/or benzodiazepine withdrawal, delirium, and obstructive sleep apnea.22 If no remediable cause is identified, patients should be allowed to rest for at least 30 minutes without the administration of additional antihypertensive medications, after which time the blood pressure should be measured using the correct technique.2 Clinical trials have shown that rest is effective at lowering blood pressure in patients with hypertensive urgency.23,24 One study initially treated 549 emergency department patients with a 30-minute rest period, after which time 32% of patients had responded (defined as a SBP <180 mm Hg and DBP <110 mm Hg, with at least a 20 mm Hg reduction in baseline SBP and/or a 10 mm Hg reduction in DBP).23 Another study randomized 138 patients with hypertensive urgency to either rest or active treatment with telmisartan. Blood pressures were checked every 30 minutes for four hours. The primary endpoint (reduction of MAP of 10%-35%) was similar in both groups (68.5% in the rest group and 69.1% in the telmisartan group).24 Even if rest is ineffective, the risk-benefit ratio of acutely lowering blood pressure will typically favor withholding acute treatment in asymptomatic patients. If blood pressure remains consistently elevated, augmentation of the home regimen (eg, increasing the dose of their next scheduled antihypertensive) of oral medications may be warranted. Though not all agree with management of antihypertensives in hospitalized patients,25 acute hospitalizations afford an opportunity to modify and observe chronic hypertension.26
RECOMMENDATIONS
- Ensure that patients do not have symptoms and/or signs of end-organ damage. This can be done with a brief review of systems and a physical examination. In select cases, an electrocardiogram and a chest x-ray may be warranted.
- Search for common causes of treatable hypertension in hospitalized patients; these include pain, nausea, withdrawal syndromes, and holding of usual antihypertensive medications.
- In those patients without symptoms and/or signs of end-organ damage, allow rest, followed by reassessment.
- Do not administer intravenous or immediate-acting oral antihypertensive medications to acutely lower blood pressure. Instead, address the issues raised in Recommendation #2 and consider modifying the chronic oral antihypertensive regimen in patients who are uncontrolled as outpatients or who are not treated as outpatients. Coordinate early postdischarge follow-up for repeat blood pressure evaluation and continued modification of a patient's chronic antihypertensive regimen.
CONCLUSION
Although patients with hypertensive urgency are often treated with medications to acutely lower their blood pressure, there is no evidence to support this practice, and a strong pathophysiologic basis suggests that harm may result. The patient in the case described above should be allowed to rest for at least 30 minutes, with reevaluation of his blood pressure. If it remains elevated and no treatable secondary causes are found, the treating hospitalist should consider altering his chronic antihypertensive regimen to promote long-term blood pressure control.
Do you think this is a low-value practice? Is this truly a "Thing We Do for No Reason?" Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other "Things We Do for No Reason" topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest.
The "Things We Do for No Reason" (TWDFNR) series reviews practices which have become common parts of hospital care but provide little value to our patients. Practices reviewed in the TWDFNR series do not represent "black and white" conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 67-year-old man is hospitalized with community-acquired pneumonia. He has a history of hypertension and is prescribed two antihypertensive medications (amlodipine and chlorthalidone) as an outpatient. On the evening of hospital day two, he is found to have a blood pressure of 192/95 on a scheduled vital signs check. He reports no symptoms other than cough, which is not new or worsening. The covering hospitalist reviews the documented blood pressures since admission and notes that many have been elevated despite continuation of his home regimen. The patient's nurse inquires about treating the patient with additional "as-needed" antihypertensive medications.
BACKGROUND
Hypertensive crises are common in hospitalized patients, with approximately one in seven patients experiencing an episode of hypertensive emergency and/or hypertensive urgency.1 Hypertensive emergency is typically defined as (1) a systolic blood pressure ≥180 mm Hg and/or a diastolic blood pressure ≥120 mm Hg with (2) evidence of new or worsening end-organ damage. The organs most commonly affected by severe hypertension are the brain (headache, confusion, stroke), heart (chest pain, myocardial infarction, pulmonary edema), large blood vessels (aortic dissection), and kidneys (acute hypertensive nephrosclerosis).2 With hypertensive urgency, patients experience similarly elevated blood pressure but have no symptoms or signs suggesting acute end-organ damage. Acute treatment with intravenous (IV) or immediate-acting oral medications is common; a single-center study showed that 7.4% of hospitalized patients had an order for "as needed" IV hydralazine or labetalol, with 60.3% receiving at least one dose.3 Among internal medicine and family medicine trainees in one survey, nearly half reported that they would use IV medications in a scenario where an inpatient had an asymptomatic blood pressure above 180 mm Hg.4
WHY YOU MIGHT THINK TREATING HYPERTENSIVE URGENCY IS NECESSARY
Treating patients with hypertensive urgency is based on an assumption: If one does not treat immediately, something bad (ie, end-organ damage) will occur over the next few hours. Data from the 1930s showed that patients with untreated hypertensive emergency had a one-year mortality rate >79% and a median survival of 10.4 months.5 More recent studies suggest that the in-hospital and one-year mortality for those with hypertensive emergency are 13% and 39%, respectively.6 These data demonstrate that patients with hypertensive emergency are at risk in both the short- and long-term.
Patients with hypertensive urgency are also at increased risk for long-term morbidity and mortality. The one-year mortality for those experiencing an episode of hypertensive urgency is approximately 9%.6 Given the concerns about poor outcomes, it remains a common practice in many facilities to acutely lower the blood pressure in patients with hypertensive urgency. This is highlighted by recommendations of a commonly used point-of-care medical resource, which suggests that "potential legal ramifications partially motivate lowering the blood pressure over several hours."7
WHY TREATING HYPERTENSIVE URGENCY IS UNNECESSARY AND POTENTIALLY HARMFUL
Concerns regarding overtreatment of hypertensive urgency relate to overestimated rates of hypertensive complications, the pathophysiology of hypertension itself, and the potential for adverse events related to treatment. Given that there are few trials examining hospitalized patients with hypertensive urgency, much of the data supporting a conservative approach are drawn from studies of outpatients or emergency department patients. In addition, there is little data suggesting that outcomes are different for patients presenting with a chief complaint of hypertensive urgency and those presenting with an alternate diagnosis but who are found to have blood pressures that meet the threshold for diagnosis of hypertensive urgency.
The landmark 1967 Veterans Affairs Cooperative Trial demonstrated the long-term benefits of treating patients with chronic hypertensive urgency.8 Importantly though, benefits accrued over a period of months to years, not hours. The time to the first adverse event in the placebo arm was two months, suggesting that even those with blood pressures chronically in the range of hypertensive urgency are unlikely to experience hyperacute (ie, within hours) events, even without treatment.
A more recent study, conducted by Patel et al., examined 58,836 patients seen in outpatient clinics and found to have blood pressures meeting the criteria for hypertensive urgency.9 This study included patients whose primary issue was hypertensive urgency and patients in whom the diagnosis was secondary. A total of 426 patients were referred to the hospital and only 100 (0.17%) were subsequently admitted. At seven days, the rates of the primary outcome (a composite of myocardial infarction, stroke, and/or transient ischemic attack) were 0.1% in those sent home and 0.5% in those sent to the hospital. In those patients with a systolic blood pressure ≥220 mm Hg, two out of 977 (0.2%) of those sent home and zero out of 81 of those sent to the hospital experienced the primary outcome. These data reinforce the message that, in patients with hypertensive urgency, rates of adverse events at seven days are low, even with extreme blood pressure elevation.
The human body has adapted to withstand wide variations in blood pressure.10 For example, through arteriolar constriction and reflex vasodilation, cerebral autoregulation maintains a constant cerebral blood flow within a wide range of perfusion pressures, ensuring that the brain is protected from higher mean arterial pressures.11 While this process is protective, over time the autoregulatory system becomes impaired, especially in patients with cerebrovascular disease. This places patients at risk for cerebral and/or cardiac ischemia with even slight drops in perfusion pressure.12,13 Indeed, in assessing treatment-related adverse events in a series of patients treated with intravenous nicardipine or nitroprusside for hypertensive emergency, Brooks and colleagues reported that 57% (27 of 47) of patients had overly large reductions in blood pressure (>25% reduction in mean arterial pressure) within the first 30 minutes of treatment.14 Two patients had acute ischemic events attributed to treatment with antihypertensive medications. Myocardial infarction and stroke have both been reported,12 and medication classes such as calcium channel blockers (sublingual nifedipine in particular), beta-blockers (eg, labetolol), angiotensin-converting-enzyme inhibitors (eg, captopril), and clonidine have all been implicated in treatment-related adverse events.12,15-17 Another potential issue derives from the observation that blood pressures obtained in the hospital setting are often inaccurate, owing to inappropriate patient preparation, faulty equipment, and inadequate training of staff obtaining the measurement.18
National guidelines support a cautious approach to the treatment of hypertensive urgency. The seventh Report of the Joint National Committee on Detection, Evaluation, and Treatment of Hypertension, published in 2003, noted that "patients with markedly elevated BP but without acute target-organ damage usually do not require hospitalization, but they should receive immediate combination oral antihypertensive therapy" and that "there is no evidence to suggest that failure to aggressively lower BP in the [emergency department] is associated with any increased short-term risk to the patient who presents with severe hypertension." JNC 7 also laments contemporary terminology: "Unfortunately, the term 'urgency' has led to overly aggressive management of many patients with severe, uncomplicated hypertension. Aggressive dosing with intravenous drugs or even oral agents, to rapidly lower BP is not without risk."19 The most recent JNC guideline does not comment on hypertensive urgency,20 and the 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults argues that, "¬there is no indication for referral to the emergency department, immediate reduction in BP in the emergency department, or hospitalization for [patients with hypertensive urgency]."21
WHAT CLINICIANS SHOULD DO INSTEAD
After it is confirmed that a patient has no end-organ damage (ie, the patient has hypertensive urgency, not emergency), treatable causes of hypertension should be assessed. In hospitalized patients, these include missed or held doses of outpatient medications, pain, nausea, alcohol and/or benzodiazepine withdrawal, delirium, and obstructive sleep apnea.22 If no remediable cause is identified, patients should be allowed to rest for at least 30 minutes without the administration of additional antihypertensive medications, after which time the blood pressure should be measured using the correct technique.2 Clinical trials have shown that rest is effective at lowering blood pressure in patients with hypertensive urgency.23,24 One study initially treated 549 emergency department patients with a 30-minute rest period, after which time 32% of patients had responded (defined as a SBP <180 mm Hg and DBP <110 mm Hg, with at least a 20 mm Hg reduction in baseline SBP and/or a 10 mm Hg reduction in DBP).23 Another study randomized 138 patients with hypertensive urgency to either rest or active treatment with telmisartan. Blood pressures were checked every 30 minutes for four hours. The primary endpoint (reduction of MAP of 10%-35%) was similar in both groups (68.5% in the rest group and 69.1% in the telmisartan group).24 Even if rest is ineffective, the risk-benefit ratio of acutely lowering blood pressure will typically favor withholding acute treatment in asymptomatic patients. If blood pressure remains consistently elevated, augmentation of the home regimen (eg, increasing the dose of their next scheduled antihypertensive) of oral medications may be warranted. Though not all agree with management of antihypertensives in hospitalized patients,25 acute hospitalizations afford an opportunity to modify and observe chronic hypertension.26
RECOMMENDATIONS
- Ensure that patients do not have symptoms and/or signs of end-organ damage. This can be done with a brief review of systems and a physical examination. In select cases, an electrocardiogram and a chest x-ray may be warranted.
- Search for common causes of treatable hypertension in hospitalized patients; these include pain, nausea, withdrawal syndromes, and holding of usual antihypertensive medications.
- In those patients without symptoms and/or signs of end-organ damage, allow rest, followed by reassessment.
- Do not administer intravenous or immediate-acting oral antihypertensive medications to acutely lower blood pressure. Instead, address the issues raised in Recommendation #2 and consider modifying the chronic oral antihypertensive regimen in patients who are uncontrolled as outpatients or who are not treated as outpatients. Coordinate early postdischarge follow-up for repeat blood pressure evaluation and continued modification of a patient's chronic antihypertensive regimen.
CONCLUSION
Although patients with hypertensive urgency are often treated with medications to acutely lower their blood pressure, there is no evidence to support this practice, and a strong pathophysiologic basis suggests that harm may result. The patient in the case described above should be allowed to rest for at least 30 minutes, with reevaluation of his blood pressure. If it remains elevated and no treatable secondary causes are found, the treating hospitalist should consider altering his chronic antihypertensive regimen to promote long-term blood pressure control.
Do you think this is a low-value practice? Is this truly a "Thing We Do for No Reason?" Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other "Things We Do for No Reason" topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest.
1. Shorr AF, Zilberberg MD, Sun X, et al. Severe acute hypertension among inpatients admitted from the emergency department. J Hosp Med. 2012;7(3):203-210. doi: 10.1002/jhm.969. PubMed
2. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2017. PubMed
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
4. Axon RN, Garrell R, Pfahl K, et al. Attitudes and practices of resident physicians regarding hypertension in the inpatient setting. J Clin Hypertens (Greenwich). 2010;12(9):698-705. doi: 10.1111/j.1751-7176.2010.00309.x. PubMed
5. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1974;268(6):336-345. doi: 10.1097/00000441-197412000-00004. PubMed
6. Guiga H, Decroux C, Michelet P, et al. Hospital and out-of-hospital mortality in 670 hypertensive emergencies and urgencies. J Clin Hypertens (Greenwich). 2017;19(11):1137-1142. doi: 10.1111/jch.13083. PubMed
7. Varon J, Williams EJ. Management of severe asymptomatic hypertension (hypertensive urgencies) in adults. In: Post T, ed. UpToDate, Waltham, MA. (Accessed February 13, 2018). PubMed
8. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202(11):1028-1034. soi: 10.1001/jama.1967.03130240070013 PubMed
9. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509. PubMed
10. MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58(3):785-790. doi: 10.1152/jappl.1985.58.3.785. PubMed
11. Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973;1(5852):507-510. doi: 10.1136/bmj.1.5852.507. PubMed
12. Fischberg GM, Lozano E, Rajamani K, Ameriso S, Fisher MJ. Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;19(4):339-346. doi: 10.1016/S0736-4679(00)00267-5. PubMed
13. Ross RS. Pathophysiology of coronary circulation. Br Heart J. 1971;33(2):173-184. doi: 10.1136/hrt.33.2.173. PubMed
14. Brooks TW, Finch CK, Lobo BL, Deaton PR, Varner CF. Blood pressure management in acute hypertensive emergency. Am J Health Syst Pharm. 2007;64(24):2579-2582. doi: 10.2146/ajhp070105. PubMed
15. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-1331. doi: 10.1001/jama.1996.03540160050032 PubMed
16. Hodsman GP, Isles CG, Murray GD et al. Factors related to first dose hypotensive effect of captopril: prediction and treatment. Br Med J (Clin Res Ed). 1983;286(6368):832-834. doi: 10.1136/bmj.286.6368.832. PubMed
17. Zeller KR, Von Kuhnert L, Matthews C. Rapid reduction of severe asymptomatic hypertension. A prospective, controlled trial. Arch Intern Med. 1989;149(10):2186-2189. doi: 10.1001/archinte.149.10.2186. PubMed
18. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697-716. doi: 10.1161/01.CIR.0000154900.76284.F6. PubMed
19. Chobanian AV, Bakris GL, Black HR, et al. The seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560. PubMed
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427 PubMed
21. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017. PubMed
22. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94. doi: 10.1007/s11886-015-0648-y. PubMed
23. Grassi D, O'Flaherty M, Pellizzari M, et al. Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles. J Clin Hypertens (Greenwich). 2008;10(9):662-667. doi: 10.1111/j.1751-7176.2008.00001.x. PubMed
24. Park SK, Lee DY, Kim WJ, et al. Comparing the clinical efficacy of resting and antihypertensive medication in patients of hypertensive urgency: a randomized, control trial. J Hypertens. 2017;35(7):1474-1480. doi: 10.1097/HJH.0000000000001340. PubMed
25. Steinman MA, Auerbach AD. Managing chronic disease in hospitalized patients. JAMA Intern Med. 2013;173(20):1857-1858. doi: 10.1001/jamainternmed.2013.9511. PubMed
26. Breu AC, Allen-Dicker J, Mueller S et al. Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9(5):303-309. doi: 10.1002/jhm.2137. PubMed
1. Shorr AF, Zilberberg MD, Sun X, et al. Severe acute hypertension among inpatients admitted from the emergency department. J Hosp Med. 2012;7(3):203-210. doi: 10.1002/jhm.969. PubMed
2. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2017. PubMed
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
4. Axon RN, Garrell R, Pfahl K, et al. Attitudes and practices of resident physicians regarding hypertension in the inpatient setting. J Clin Hypertens (Greenwich). 2010;12(9):698-705. doi: 10.1111/j.1751-7176.2010.00309.x. PubMed
5. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1974;268(6):336-345. doi: 10.1097/00000441-197412000-00004. PubMed
6. Guiga H, Decroux C, Michelet P, et al. Hospital and out-of-hospital mortality in 670 hypertensive emergencies and urgencies. J Clin Hypertens (Greenwich). 2017;19(11):1137-1142. doi: 10.1111/jch.13083. PubMed
7. Varon J, Williams EJ. Management of severe asymptomatic hypertension (hypertensive urgencies) in adults. In: Post T, ed. UpToDate, Waltham, MA. (Accessed February 13, 2018). PubMed
8. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202(11):1028-1034. soi: 10.1001/jama.1967.03130240070013 PubMed
9. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509. PubMed
10. MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58(3):785-790. doi: 10.1152/jappl.1985.58.3.785. PubMed
11. Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973;1(5852):507-510. doi: 10.1136/bmj.1.5852.507. PubMed
12. Fischberg GM, Lozano E, Rajamani K, Ameriso S, Fisher MJ. Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;19(4):339-346. doi: 10.1016/S0736-4679(00)00267-5. PubMed
13. Ross RS. Pathophysiology of coronary circulation. Br Heart J. 1971;33(2):173-184. doi: 10.1136/hrt.33.2.173. PubMed
14. Brooks TW, Finch CK, Lobo BL, Deaton PR, Varner CF. Blood pressure management in acute hypertensive emergency. Am J Health Syst Pharm. 2007;64(24):2579-2582. doi: 10.2146/ajhp070105. PubMed
15. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-1331. doi: 10.1001/jama.1996.03540160050032 PubMed
16. Hodsman GP, Isles CG, Murray GD et al. Factors related to first dose hypotensive effect of captopril: prediction and treatment. Br Med J (Clin Res Ed). 1983;286(6368):832-834. doi: 10.1136/bmj.286.6368.832. PubMed
17. Zeller KR, Von Kuhnert L, Matthews C. Rapid reduction of severe asymptomatic hypertension. A prospective, controlled trial. Arch Intern Med. 1989;149(10):2186-2189. doi: 10.1001/archinte.149.10.2186. PubMed
18. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697-716. doi: 10.1161/01.CIR.0000154900.76284.F6. PubMed
19. Chobanian AV, Bakris GL, Black HR, et al. The seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560. PubMed
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427 PubMed
21. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017. PubMed
22. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94. doi: 10.1007/s11886-015-0648-y. PubMed
23. Grassi D, O'Flaherty M, Pellizzari M, et al. Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles. J Clin Hypertens (Greenwich). 2008;10(9):662-667. doi: 10.1111/j.1751-7176.2008.00001.x. PubMed
24. Park SK, Lee DY, Kim WJ, et al. Comparing the clinical efficacy of resting and antihypertensive medication in patients of hypertensive urgency: a randomized, control trial. J Hypertens. 2017;35(7):1474-1480. doi: 10.1097/HJH.0000000000001340. PubMed
25. Steinman MA, Auerbach AD. Managing chronic disease in hospitalized patients. JAMA Intern Med. 2013;173(20):1857-1858. doi: 10.1001/jamainternmed.2013.9511. PubMed
26. Breu AC, Allen-Dicker J, Mueller S et al. Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9(5):303-309. doi: 10.1002/jhm.2137. PubMed
© 2018 Society of Hospital Medicine
A Veteran With Fibromyalgia Presenting With Dyspnea
Case Presentation. A 64-year-old US Army veteran with a history of colorectal cancer, melanoma, and fibrinolytic presented with dyspnea to VA Boston Healthcare System (VABHS). Seven years prior to the current presentation, at the time of her diagnosis of colorectal cancer, the patient was found to be HIV negative but to have a positive purified protein derivative (PPD) test. She was treated with isoniazid (INH) therapy for 9 months. Sputum cultures collected prior to initiation of therapy grew Mycobacterium avium complex (MAC) in 1 of 3 samples, with these results reported several months after initiation of therapy. She was a never smoker with no known travel or exposure. At the time of the current presentation, her medications included bupropion, levothyroxine, capsaicin, cyclobenzaprine, ibuprofen, and acetaminophen.
►Lakshmana Swamy, MD, Chief Medical Resident, VABHS and Boston Medical Center. Dr. Monach, this patient is on a variety of pain medications and has a diagnosis of fibromyalgia. This diagnosis often frustrates doctors and patients alike. Can you tell us about fibromyalgia from the rheumatologist’s perspective and what you think of her current treatment regimen?
►Paul A. Monach, MD, PhD, Chief, Section of Rheumatology, VABHS and Associate Professor of Medicine, Boston University School of Medicine. Fibromyalgia is a syndrome of chronic widespread pain without known pathology in the musculoskeletal system. It is thought to be caused by chronic dysfunction of pain-processing pathways in the central nervous system (CNS). It is often accompanied by other somatic symptoms such as chronic fatigue, irritable bowel syndrome, and bladder pain. It is a common condition, affecting up to 5% of otherwise healthy women. It is particularly common in persons with chronic nonrestorative sleep or posttraumatic stress disorder from a wide range of causes. However, it also is more common in persons with autoimmune inflammatory diseases, such as lupus, Sjögren syndrome, or rheumatoid arthritis. Concern for one of these diseases is the main reason to consider referring a patient for evaluation by a rheumatologist. Often rheumatologists participate in the management of fibromyalgia. A patient should be given appropriate expectations by the referring physician.
Effectiveness of treatment varies widely among patients. Nonpharmacologic approaches such as aerobic exercise, cognitive behavioral therapy, and tai chi have support from clinical trials, and yoga and aquatherapy also are widely used.1,2 The classes of drugs used are the same as for neuropathic pain: tricyclics, including cyclobenzaprine; serotonin and norepinephrine reuptake inhibitors (SNRIs); and gabapentinoids. In contrast, nonsteroidal anti-inflammatory drugs and opioids are ineffective unless there is a superimposed mechanical or inflammatory cause in the periphery. The key point is that continuation of any treatment should be based entirely on the patient’s own assessment of benefit.
►Dr. Swamy. Seven years later, the patient returned to her primary care provider, reporting increased dyspnea on exertion as well as significant fatigue. She was referred to the pulmonary department and had repeat computed tomography (CT) scans of the chest, which indicated persistent right middle lobe (RML) bronchiectasis. She then underwent bronchoscopy with a subsequent bronchoalveolar lavage (BAL) culture growing MAC. Dr. Fine, please interpret the baseline and follow-up CT scans and help us understand the significance of the MAC on sputum and BAL cultures.
►Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine. Prior to this presentation, the patient had a pleural-based area of fibrosis with possible associated RML bronchiectasis. This appears to be a postinflammatory process without classic features of malignant or metastatic disease. She then had a sputum, which grew MAC in only 1 of 3 samples and in liquid media only. Importantly, the sputum was not smear positive. All of this suggests a low organism burden. One possibility is that this could reflect colonization with MAC; it is not uncommon for patients with underlying chronic changes in their lung to grow MAC, and it is often difficult to tell whether it is indicative of active disease. Structural lung disease, such as bronchiectasis, predisposes a patient to MAC, but chronic MAC also may cause bronchiectasis. This chicken-and-egg scenario comes up frequently. She may have a MAC infection, but as she is HIV negative and asymptomatic, there is no urgent indication to treat, especially as the burden of therapy is not insignificant.
►Dr. Swamy. Do we need to worry about Mycobacterium tuberculosis (MTB)?
►Dr. Fine. Although she was previously PPD positive, she had already completed 1 year of isoniazid (INH) therapy, making active MTB less likely. From an infection control standpoint, it is important to distinguish MAC from MTB. The former is not contagious, and there is no need for airborne isolation.
►Dr. Swamy. Dr. Fine, where does MAC come from? Does it commonly cause disease?
►Dr. Fine. In the environment, MAC is nearly ubiquitous , especially in water and soil. In one study, 20% of showerheads were positive for MAC; when patients are infected, we may suggest changing/bleaching the showerhead, but there are no definitive recommendations.3 Because MAC is so common in the environment, it is unlikely that measures to target MAC colonization will be clinically meaningful. On the other hand, the incidence of nontuberculous mycobacterial infections is increasing across the US, and it may be a common and frequently underdiagnosed cause of chronic cough, especially in postmenopausal women.
►Dr. Swamy. Four years prior to the current presentation, the patient developed a cough after an upper respiratory tract infection that persisted for more than 2 weeks. Given her history, she underwent a repeat chest CT, which noted a slight increase in nodularity and ground-glass opacity restricted to the RML. She also reported dyspnea on exertion and was referred to the pulmonary medicine department. By the time she arrived, her dyspnea had largely resolved, but she reported persistent fatigue without other systemic symptoms, such as fevers or chills. Dr. Fine, does MAC explain this patient’s dyspnea?
►Dr. Fine. As her pulmonary symptoms resolved in a short period of time with only azithromycin, it is very unlikely that her symptoms were related to her prior disease. The MAC infection is not likely to cause dyspnea on exertion and fatigue and should be worked up more broadly before attributing it to MAC. In view of this, it would not be unreasonable to follow her clinically and see her again in 6 to 8 weeks. In this context, we also should consider the untoward impact of repeated radiation exposure derived from multiple CT scans. When a patient has an abnormality on CT scan, it often leads to further scans even if the symptoms do not match the previous findings, as in this case.
►Dr. Swamy. Given her ongoing fatigue and systemic symptoms (morning stiffness of the shoulders, legs, and thighs, and leg cramps), she was referred to the rheumatology department where the physical examination revealed muscle tenderness in her proximal arms and legs with normal strength, tender points at the elbows and medial side of the bilateral knees, significant tenderness of lower legs, and no synovitis.
Dr. Monach, can you walk us through your approach to this patient? Are we seeing manifestations of fibromyalgia? What diagnoses concerns you and how would you proceed?
►Dr. Monach. The history and exam are most helpful in raising or reducing suspicion for an underlying inflammatory disease. Areas of tenderness described in her case are typical of fibromyalgia, although it can be difficult to interpret symptoms in the hip girdle and shoulder girdle because objective findings are often absent on exam in patients with inflammatory arthritis or bursitis. Similarly, tenderness at sites of tendon insertion (enthuses) without objective abnormalities is common in different forms of spondyloarthritis, so tenderness at the elbow, knee, lateral hip, and low back can be difficult to interpret. What this patient is lacking is prominent subjective or objective findings in the joints most commonly affected in rheumatoid arthritis and lupus: wrists, hands, ankles, and feet.
►Dr. Swamy. Initial laboratory data include an erythrocyte sedimentation rate of 79 with a normal C-reactive protein. A tentative diagnosis of polymyalgia rheumatic is made with consideration of a trial treatment of prednisone.
Dr. Monach, this patient has an indolent infection and is about to be given glucocorticoids. Could you describe the situations in which you feel that glucocorticoids cause a relative immunosuppression?
►Dr. Monach. Glucocorticoids are considered safe in a patient whose infection is not intrinsically dangerous or who has started appropriate antibiotics for that infection. Although all toxicities of glucocorticoids are dose dependent, the long-standing assertion that doses below 10 mg to 15 mg do not increase risk of infection is contradicted by data published in the past 10 to 15 years, with the caveat that these patients were on long-term treatment.
►Dr. Swamy. The patient was started on prednisone 15 mg per day for 15 days. She returned to the clinic after 1 week of prednisone troutment and noted “significant improvement in fatigue, morning stiffness of shoulders, thighs, leg, back is better, leg cramps resolved, shooting pain in many joints resolved.” Further laboratory results were notable for a negative rheumatoid factor, negative antinuclear antibody, and a cyclic citrullinated peptide of 60. A presumptive diagnosis of rheumatoid arthritis (RA) was made and plaquenil 200 mg twice daily was started.
Dr. Monach, can you explain why RA comes up now on serology but was not considered initially? Why does this presentation fit RA, and was her response to treatment typical? How does this fit in with her previous diagnosis of fibromyalgia? Was that just an atypical, indolent presentation of RA?
►Dr. Monach. Though her presentation is atypical for RA, in elderly patients, RA can present with symptoms resembling polymyalgia rheumatica. The question is whether she had RA all along (in which case “elderly onset” would not apply) or had fibromyalgia and developed RA more recently. The response to empiric glucocorticoid therapy is helpful, since fibromyalgia should not improve with prednisone even in a patient with RA unless treatment of RA would allow better sleep and ability to exercise. Rheumatoid arthritis typically responds very well to prednisone in the 5-mg to 15-mg range.
►Dr. Swamy. Given the new diagnosis of an inflammatory arthritis requiring immunosuppression, bronchoscopy with BAL is performed to evaluate for the presence of MAC. These cultures were positive for MAC.
Dr. Fine, does the positive BAL culture indicate an active MAC infection?
►Dr. Fine. Yes, based on these updated data, the patient has an active MAC infection. Active infection is defined as symptoms or imaging consistent with the diagnosis, supporting microbiology data (either 2 sputum or 1 BAL sample growing MAC) and the exclusion of other causes. Previously, this patient grew MAC in just one expectorated sputum; this did not meet the microbiologic criteria. Now sputum has grown in the BAL sample; along with the CT imaging, this is enough to diagnosis active MAC infection.
Treatment for MAC must consider the details of each case. First, this is not an emergency; treatment decisions should be made with the rheumatologist to consider the planned immunosuppression. For example, we must consider potential drug interactions. A specific point should be made of the use of tumor necrosis factor (TNF)-α inhibition, which data indicate can reactivate TB and may inhibit mechanisms that restrain mycobacterial disease. Serious cases of MAC infection have been reported in the literature in the setting of TNF-α inhibition.5,6 Despite these concerns, there is not a contraindication to using these therapies from the perspective of the active MAC disease. All of these decisions will impact the need to commit the patient to MAC therapy.
►Dr. Swamy. Dr. Fine, what do you consider prior to initiating MAC therapy?
► Dr. Fine. The decision to pursue MAC therapy should not be taken lightly. Therapy often entails prolonged multidrug regimens, usually spanning more than a year, with frequent adverse effects. Outside of very specific cases, such as TNF-β inhibition, MAC is rarely a life-threatening disease, so the benefit may be limited. Treatment for MAC is certainly unlikely to be fruitful without a diligent and motivated patient able to handle the high and prolonged pill burden. Of note, it is also important to keep this patient up-to-date with influenza and pneumonia vaccination given her structural lung disease.
►Dr. Swamy. The decision is made to treat MAC with azithromycin, rifampin, and ethambutol. The disease is noted to be nonfibrocavitary. The patient underwent monthly liver function test monitoring and visual acuity testing, which were unremarkable. Dr. Fine, can you describe the phenotypes of nontuberculous mycobacterial (NTM) disease?
►Dr. Fine. There are 3 main phenotypes of NTM.3 First, we see the elderly man with preexisting lung disease—usually chronic obstructive pulmonary disease—with fibrocavitary and/or reticulonodular appearance. Second, we see the slim, elderly woman often without any preexisting lung disease presenting with focal bronchiectasis and nodular lesions in right middle lobe and lingula—the Lady Windermere syndrome. This eponym is derived from Oscar Wilde’s play “Lady Windermere’s Fan, a Play About a Good Woman,” and was first associated with this disease in 1992.7 At the time, it was thought that the voluntary suppression of cough led to poorly draining lung regions, vulnerable to engraftment by atypical mycobacteria. Infection with atypical mycobacteria are associated with this population; however, it is no longer thought to be due to the voluntary suppression of cough.7,8 Third, we do occasionally see atypical presentations, such as focal masses and solitary nodules.
►Dr. Swamy. At 1-year follow-up she successfully completed MAC therapy and noted ongoing control of rheumatoid symptoms.
1. Bernardy K, Klose P, Welsch P, Häuser W. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome—a systematic review and meta-analysis of randomized controlled trials. Eur J Pain. 2018;22(2):242-260.
2. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279.
3. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108(3):417-425.
4. Aucott JN. Glucocorticoids and infection. Endocrinol Metab Clin North Am. 1994;23(3):655-670.
5. Curtis JR, Yang S, Patkar NM, et al. Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(7):990-997.
6. Lane MA, McDonald JR, Zeringue AL, et al. TNF-α antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine (Baltimore). 2011;90(2):139-145.
7. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101(6):1605-1609.
8. Kasthoori JJ, Liam CK, Wastie ML. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Med J. 2008;49(2):e47-e49.
Case Presentation. A 64-year-old US Army veteran with a history of colorectal cancer, melanoma, and fibrinolytic presented with dyspnea to VA Boston Healthcare System (VABHS). Seven years prior to the current presentation, at the time of her diagnosis of colorectal cancer, the patient was found to be HIV negative but to have a positive purified protein derivative (PPD) test. She was treated with isoniazid (INH) therapy for 9 months. Sputum cultures collected prior to initiation of therapy grew Mycobacterium avium complex (MAC) in 1 of 3 samples, with these results reported several months after initiation of therapy. She was a never smoker with no known travel or exposure. At the time of the current presentation, her medications included bupropion, levothyroxine, capsaicin, cyclobenzaprine, ibuprofen, and acetaminophen.
►Lakshmana Swamy, MD, Chief Medical Resident, VABHS and Boston Medical Center. Dr. Monach, this patient is on a variety of pain medications and has a diagnosis of fibromyalgia. This diagnosis often frustrates doctors and patients alike. Can you tell us about fibromyalgia from the rheumatologist’s perspective and what you think of her current treatment regimen?
►Paul A. Monach, MD, PhD, Chief, Section of Rheumatology, VABHS and Associate Professor of Medicine, Boston University School of Medicine. Fibromyalgia is a syndrome of chronic widespread pain without known pathology in the musculoskeletal system. It is thought to be caused by chronic dysfunction of pain-processing pathways in the central nervous system (CNS). It is often accompanied by other somatic symptoms such as chronic fatigue, irritable bowel syndrome, and bladder pain. It is a common condition, affecting up to 5% of otherwise healthy women. It is particularly common in persons with chronic nonrestorative sleep or posttraumatic stress disorder from a wide range of causes. However, it also is more common in persons with autoimmune inflammatory diseases, such as lupus, Sjögren syndrome, or rheumatoid arthritis. Concern for one of these diseases is the main reason to consider referring a patient for evaluation by a rheumatologist. Often rheumatologists participate in the management of fibromyalgia. A patient should be given appropriate expectations by the referring physician.
Effectiveness of treatment varies widely among patients. Nonpharmacologic approaches such as aerobic exercise, cognitive behavioral therapy, and tai chi have support from clinical trials, and yoga and aquatherapy also are widely used.1,2 The classes of drugs used are the same as for neuropathic pain: tricyclics, including cyclobenzaprine; serotonin and norepinephrine reuptake inhibitors (SNRIs); and gabapentinoids. In contrast, nonsteroidal anti-inflammatory drugs and opioids are ineffective unless there is a superimposed mechanical or inflammatory cause in the periphery. The key point is that continuation of any treatment should be based entirely on the patient’s own assessment of benefit.
►Dr. Swamy. Seven years later, the patient returned to her primary care provider, reporting increased dyspnea on exertion as well as significant fatigue. She was referred to the pulmonary department and had repeat computed tomography (CT) scans of the chest, which indicated persistent right middle lobe (RML) bronchiectasis. She then underwent bronchoscopy with a subsequent bronchoalveolar lavage (BAL) culture growing MAC. Dr. Fine, please interpret the baseline and follow-up CT scans and help us understand the significance of the MAC on sputum and BAL cultures.
►Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine. Prior to this presentation, the patient had a pleural-based area of fibrosis with possible associated RML bronchiectasis. This appears to be a postinflammatory process without classic features of malignant or metastatic disease. She then had a sputum, which grew MAC in only 1 of 3 samples and in liquid media only. Importantly, the sputum was not smear positive. All of this suggests a low organism burden. One possibility is that this could reflect colonization with MAC; it is not uncommon for patients with underlying chronic changes in their lung to grow MAC, and it is often difficult to tell whether it is indicative of active disease. Structural lung disease, such as bronchiectasis, predisposes a patient to MAC, but chronic MAC also may cause bronchiectasis. This chicken-and-egg scenario comes up frequently. She may have a MAC infection, but as she is HIV negative and asymptomatic, there is no urgent indication to treat, especially as the burden of therapy is not insignificant.
►Dr. Swamy. Do we need to worry about Mycobacterium tuberculosis (MTB)?
►Dr. Fine. Although she was previously PPD positive, she had already completed 1 year of isoniazid (INH) therapy, making active MTB less likely. From an infection control standpoint, it is important to distinguish MAC from MTB. The former is not contagious, and there is no need for airborne isolation.
►Dr. Swamy. Dr. Fine, where does MAC come from? Does it commonly cause disease?
►Dr. Fine. In the environment, MAC is nearly ubiquitous , especially in water and soil. In one study, 20% of showerheads were positive for MAC; when patients are infected, we may suggest changing/bleaching the showerhead, but there are no definitive recommendations.3 Because MAC is so common in the environment, it is unlikely that measures to target MAC colonization will be clinically meaningful. On the other hand, the incidence of nontuberculous mycobacterial infections is increasing across the US, and it may be a common and frequently underdiagnosed cause of chronic cough, especially in postmenopausal women.
►Dr. Swamy. Four years prior to the current presentation, the patient developed a cough after an upper respiratory tract infection that persisted for more than 2 weeks. Given her history, she underwent a repeat chest CT, which noted a slight increase in nodularity and ground-glass opacity restricted to the RML. She also reported dyspnea on exertion and was referred to the pulmonary medicine department. By the time she arrived, her dyspnea had largely resolved, but she reported persistent fatigue without other systemic symptoms, such as fevers or chills. Dr. Fine, does MAC explain this patient’s dyspnea?
►Dr. Fine. As her pulmonary symptoms resolved in a short period of time with only azithromycin, it is very unlikely that her symptoms were related to her prior disease. The MAC infection is not likely to cause dyspnea on exertion and fatigue and should be worked up more broadly before attributing it to MAC. In view of this, it would not be unreasonable to follow her clinically and see her again in 6 to 8 weeks. In this context, we also should consider the untoward impact of repeated radiation exposure derived from multiple CT scans. When a patient has an abnormality on CT scan, it often leads to further scans even if the symptoms do not match the previous findings, as in this case.
►Dr. Swamy. Given her ongoing fatigue and systemic symptoms (morning stiffness of the shoulders, legs, and thighs, and leg cramps), she was referred to the rheumatology department where the physical examination revealed muscle tenderness in her proximal arms and legs with normal strength, tender points at the elbows and medial side of the bilateral knees, significant tenderness of lower legs, and no synovitis.
Dr. Monach, can you walk us through your approach to this patient? Are we seeing manifestations of fibromyalgia? What diagnoses concerns you and how would you proceed?
►Dr. Monach. The history and exam are most helpful in raising or reducing suspicion for an underlying inflammatory disease. Areas of tenderness described in her case are typical of fibromyalgia, although it can be difficult to interpret symptoms in the hip girdle and shoulder girdle because objective findings are often absent on exam in patients with inflammatory arthritis or bursitis. Similarly, tenderness at sites of tendon insertion (enthuses) without objective abnormalities is common in different forms of spondyloarthritis, so tenderness at the elbow, knee, lateral hip, and low back can be difficult to interpret. What this patient is lacking is prominent subjective or objective findings in the joints most commonly affected in rheumatoid arthritis and lupus: wrists, hands, ankles, and feet.
►Dr. Swamy. Initial laboratory data include an erythrocyte sedimentation rate of 79 with a normal C-reactive protein. A tentative diagnosis of polymyalgia rheumatic is made with consideration of a trial treatment of prednisone.
Dr. Monach, this patient has an indolent infection and is about to be given glucocorticoids. Could you describe the situations in which you feel that glucocorticoids cause a relative immunosuppression?
►Dr. Monach. Glucocorticoids are considered safe in a patient whose infection is not intrinsically dangerous or who has started appropriate antibiotics for that infection. Although all toxicities of glucocorticoids are dose dependent, the long-standing assertion that doses below 10 mg to 15 mg do not increase risk of infection is contradicted by data published in the past 10 to 15 years, with the caveat that these patients were on long-term treatment.
►Dr. Swamy. The patient was started on prednisone 15 mg per day for 15 days. She returned to the clinic after 1 week of prednisone troutment and noted “significant improvement in fatigue, morning stiffness of shoulders, thighs, leg, back is better, leg cramps resolved, shooting pain in many joints resolved.” Further laboratory results were notable for a negative rheumatoid factor, negative antinuclear antibody, and a cyclic citrullinated peptide of 60. A presumptive diagnosis of rheumatoid arthritis (RA) was made and plaquenil 200 mg twice daily was started.
Dr. Monach, can you explain why RA comes up now on serology but was not considered initially? Why does this presentation fit RA, and was her response to treatment typical? How does this fit in with her previous diagnosis of fibromyalgia? Was that just an atypical, indolent presentation of RA?
►Dr. Monach. Though her presentation is atypical for RA, in elderly patients, RA can present with symptoms resembling polymyalgia rheumatica. The question is whether she had RA all along (in which case “elderly onset” would not apply) or had fibromyalgia and developed RA more recently. The response to empiric glucocorticoid therapy is helpful, since fibromyalgia should not improve with prednisone even in a patient with RA unless treatment of RA would allow better sleep and ability to exercise. Rheumatoid arthritis typically responds very well to prednisone in the 5-mg to 15-mg range.
►Dr. Swamy. Given the new diagnosis of an inflammatory arthritis requiring immunosuppression, bronchoscopy with BAL is performed to evaluate for the presence of MAC. These cultures were positive for MAC.
Dr. Fine, does the positive BAL culture indicate an active MAC infection?
►Dr. Fine. Yes, based on these updated data, the patient has an active MAC infection. Active infection is defined as symptoms or imaging consistent with the diagnosis, supporting microbiology data (either 2 sputum or 1 BAL sample growing MAC) and the exclusion of other causes. Previously, this patient grew MAC in just one expectorated sputum; this did not meet the microbiologic criteria. Now sputum has grown in the BAL sample; along with the CT imaging, this is enough to diagnosis active MAC infection.
Treatment for MAC must consider the details of each case. First, this is not an emergency; treatment decisions should be made with the rheumatologist to consider the planned immunosuppression. For example, we must consider potential drug interactions. A specific point should be made of the use of tumor necrosis factor (TNF)-α inhibition, which data indicate can reactivate TB and may inhibit mechanisms that restrain mycobacterial disease. Serious cases of MAC infection have been reported in the literature in the setting of TNF-α inhibition.5,6 Despite these concerns, there is not a contraindication to using these therapies from the perspective of the active MAC disease. All of these decisions will impact the need to commit the patient to MAC therapy.
►Dr. Swamy. Dr. Fine, what do you consider prior to initiating MAC therapy?
► Dr. Fine. The decision to pursue MAC therapy should not be taken lightly. Therapy often entails prolonged multidrug regimens, usually spanning more than a year, with frequent adverse effects. Outside of very specific cases, such as TNF-β inhibition, MAC is rarely a life-threatening disease, so the benefit may be limited. Treatment for MAC is certainly unlikely to be fruitful without a diligent and motivated patient able to handle the high and prolonged pill burden. Of note, it is also important to keep this patient up-to-date with influenza and pneumonia vaccination given her structural lung disease.
►Dr. Swamy. The decision is made to treat MAC with azithromycin, rifampin, and ethambutol. The disease is noted to be nonfibrocavitary. The patient underwent monthly liver function test monitoring and visual acuity testing, which were unremarkable. Dr. Fine, can you describe the phenotypes of nontuberculous mycobacterial (NTM) disease?
►Dr. Fine. There are 3 main phenotypes of NTM.3 First, we see the elderly man with preexisting lung disease—usually chronic obstructive pulmonary disease—with fibrocavitary and/or reticulonodular appearance. Second, we see the slim, elderly woman often without any preexisting lung disease presenting with focal bronchiectasis and nodular lesions in right middle lobe and lingula—the Lady Windermere syndrome. This eponym is derived from Oscar Wilde’s play “Lady Windermere’s Fan, a Play About a Good Woman,” and was first associated with this disease in 1992.7 At the time, it was thought that the voluntary suppression of cough led to poorly draining lung regions, vulnerable to engraftment by atypical mycobacteria. Infection with atypical mycobacteria are associated with this population; however, it is no longer thought to be due to the voluntary suppression of cough.7,8 Third, we do occasionally see atypical presentations, such as focal masses and solitary nodules.
►Dr. Swamy. At 1-year follow-up she successfully completed MAC therapy and noted ongoing control of rheumatoid symptoms.
Case Presentation. A 64-year-old US Army veteran with a history of colorectal cancer, melanoma, and fibrinolytic presented with dyspnea to VA Boston Healthcare System (VABHS). Seven years prior to the current presentation, at the time of her diagnosis of colorectal cancer, the patient was found to be HIV negative but to have a positive purified protein derivative (PPD) test. She was treated with isoniazid (INH) therapy for 9 months. Sputum cultures collected prior to initiation of therapy grew Mycobacterium avium complex (MAC) in 1 of 3 samples, with these results reported several months after initiation of therapy. She was a never smoker with no known travel or exposure. At the time of the current presentation, her medications included bupropion, levothyroxine, capsaicin, cyclobenzaprine, ibuprofen, and acetaminophen.
►Lakshmana Swamy, MD, Chief Medical Resident, VABHS and Boston Medical Center. Dr. Monach, this patient is on a variety of pain medications and has a diagnosis of fibromyalgia. This diagnosis often frustrates doctors and patients alike. Can you tell us about fibromyalgia from the rheumatologist’s perspective and what you think of her current treatment regimen?
►Paul A. Monach, MD, PhD, Chief, Section of Rheumatology, VABHS and Associate Professor of Medicine, Boston University School of Medicine. Fibromyalgia is a syndrome of chronic widespread pain without known pathology in the musculoskeletal system. It is thought to be caused by chronic dysfunction of pain-processing pathways in the central nervous system (CNS). It is often accompanied by other somatic symptoms such as chronic fatigue, irritable bowel syndrome, and bladder pain. It is a common condition, affecting up to 5% of otherwise healthy women. It is particularly common in persons with chronic nonrestorative sleep or posttraumatic stress disorder from a wide range of causes. However, it also is more common in persons with autoimmune inflammatory diseases, such as lupus, Sjögren syndrome, or rheumatoid arthritis. Concern for one of these diseases is the main reason to consider referring a patient for evaluation by a rheumatologist. Often rheumatologists participate in the management of fibromyalgia. A patient should be given appropriate expectations by the referring physician.
Effectiveness of treatment varies widely among patients. Nonpharmacologic approaches such as aerobic exercise, cognitive behavioral therapy, and tai chi have support from clinical trials, and yoga and aquatherapy also are widely used.1,2 The classes of drugs used are the same as for neuropathic pain: tricyclics, including cyclobenzaprine; serotonin and norepinephrine reuptake inhibitors (SNRIs); and gabapentinoids. In contrast, nonsteroidal anti-inflammatory drugs and opioids are ineffective unless there is a superimposed mechanical or inflammatory cause in the periphery. The key point is that continuation of any treatment should be based entirely on the patient’s own assessment of benefit.
►Dr. Swamy. Seven years later, the patient returned to her primary care provider, reporting increased dyspnea on exertion as well as significant fatigue. She was referred to the pulmonary department and had repeat computed tomography (CT) scans of the chest, which indicated persistent right middle lobe (RML) bronchiectasis. She then underwent bronchoscopy with a subsequent bronchoalveolar lavage (BAL) culture growing MAC. Dr. Fine, please interpret the baseline and follow-up CT scans and help us understand the significance of the MAC on sputum and BAL cultures.
►Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine. Prior to this presentation, the patient had a pleural-based area of fibrosis with possible associated RML bronchiectasis. This appears to be a postinflammatory process without classic features of malignant or metastatic disease. She then had a sputum, which grew MAC in only 1 of 3 samples and in liquid media only. Importantly, the sputum was not smear positive. All of this suggests a low organism burden. One possibility is that this could reflect colonization with MAC; it is not uncommon for patients with underlying chronic changes in their lung to grow MAC, and it is often difficult to tell whether it is indicative of active disease. Structural lung disease, such as bronchiectasis, predisposes a patient to MAC, but chronic MAC also may cause bronchiectasis. This chicken-and-egg scenario comes up frequently. She may have a MAC infection, but as she is HIV negative and asymptomatic, there is no urgent indication to treat, especially as the burden of therapy is not insignificant.
►Dr. Swamy. Do we need to worry about Mycobacterium tuberculosis (MTB)?
►Dr. Fine. Although she was previously PPD positive, she had already completed 1 year of isoniazid (INH) therapy, making active MTB less likely. From an infection control standpoint, it is important to distinguish MAC from MTB. The former is not contagious, and there is no need for airborne isolation.
►Dr. Swamy. Dr. Fine, where does MAC come from? Does it commonly cause disease?
►Dr. Fine. In the environment, MAC is nearly ubiquitous , especially in water and soil. In one study, 20% of showerheads were positive for MAC; when patients are infected, we may suggest changing/bleaching the showerhead, but there are no definitive recommendations.3 Because MAC is so common in the environment, it is unlikely that measures to target MAC colonization will be clinically meaningful. On the other hand, the incidence of nontuberculous mycobacterial infections is increasing across the US, and it may be a common and frequently underdiagnosed cause of chronic cough, especially in postmenopausal women.
►Dr. Swamy. Four years prior to the current presentation, the patient developed a cough after an upper respiratory tract infection that persisted for more than 2 weeks. Given her history, she underwent a repeat chest CT, which noted a slight increase in nodularity and ground-glass opacity restricted to the RML. She also reported dyspnea on exertion and was referred to the pulmonary medicine department. By the time she arrived, her dyspnea had largely resolved, but she reported persistent fatigue without other systemic symptoms, such as fevers or chills. Dr. Fine, does MAC explain this patient’s dyspnea?
►Dr. Fine. As her pulmonary symptoms resolved in a short period of time with only azithromycin, it is very unlikely that her symptoms were related to her prior disease. The MAC infection is not likely to cause dyspnea on exertion and fatigue and should be worked up more broadly before attributing it to MAC. In view of this, it would not be unreasonable to follow her clinically and see her again in 6 to 8 weeks. In this context, we also should consider the untoward impact of repeated radiation exposure derived from multiple CT scans. When a patient has an abnormality on CT scan, it often leads to further scans even if the symptoms do not match the previous findings, as in this case.
►Dr. Swamy. Given her ongoing fatigue and systemic symptoms (morning stiffness of the shoulders, legs, and thighs, and leg cramps), she was referred to the rheumatology department where the physical examination revealed muscle tenderness in her proximal arms and legs with normal strength, tender points at the elbows and medial side of the bilateral knees, significant tenderness of lower legs, and no synovitis.
Dr. Monach, can you walk us through your approach to this patient? Are we seeing manifestations of fibromyalgia? What diagnoses concerns you and how would you proceed?
►Dr. Monach. The history and exam are most helpful in raising or reducing suspicion for an underlying inflammatory disease. Areas of tenderness described in her case are typical of fibromyalgia, although it can be difficult to interpret symptoms in the hip girdle and shoulder girdle because objective findings are often absent on exam in patients with inflammatory arthritis or bursitis. Similarly, tenderness at sites of tendon insertion (enthuses) without objective abnormalities is common in different forms of spondyloarthritis, so tenderness at the elbow, knee, lateral hip, and low back can be difficult to interpret. What this patient is lacking is prominent subjective or objective findings in the joints most commonly affected in rheumatoid arthritis and lupus: wrists, hands, ankles, and feet.
►Dr. Swamy. Initial laboratory data include an erythrocyte sedimentation rate of 79 with a normal C-reactive protein. A tentative diagnosis of polymyalgia rheumatic is made with consideration of a trial treatment of prednisone.
Dr. Monach, this patient has an indolent infection and is about to be given glucocorticoids. Could you describe the situations in which you feel that glucocorticoids cause a relative immunosuppression?
►Dr. Monach. Glucocorticoids are considered safe in a patient whose infection is not intrinsically dangerous or who has started appropriate antibiotics for that infection. Although all toxicities of glucocorticoids are dose dependent, the long-standing assertion that doses below 10 mg to 15 mg do not increase risk of infection is contradicted by data published in the past 10 to 15 years, with the caveat that these patients were on long-term treatment.
►Dr. Swamy. The patient was started on prednisone 15 mg per day for 15 days. She returned to the clinic after 1 week of prednisone troutment and noted “significant improvement in fatigue, morning stiffness of shoulders, thighs, leg, back is better, leg cramps resolved, shooting pain in many joints resolved.” Further laboratory results were notable for a negative rheumatoid factor, negative antinuclear antibody, and a cyclic citrullinated peptide of 60. A presumptive diagnosis of rheumatoid arthritis (RA) was made and plaquenil 200 mg twice daily was started.
Dr. Monach, can you explain why RA comes up now on serology but was not considered initially? Why does this presentation fit RA, and was her response to treatment typical? How does this fit in with her previous diagnosis of fibromyalgia? Was that just an atypical, indolent presentation of RA?
►Dr. Monach. Though her presentation is atypical for RA, in elderly patients, RA can present with symptoms resembling polymyalgia rheumatica. The question is whether she had RA all along (in which case “elderly onset” would not apply) or had fibromyalgia and developed RA more recently. The response to empiric glucocorticoid therapy is helpful, since fibromyalgia should not improve with prednisone even in a patient with RA unless treatment of RA would allow better sleep and ability to exercise. Rheumatoid arthritis typically responds very well to prednisone in the 5-mg to 15-mg range.
►Dr. Swamy. Given the new diagnosis of an inflammatory arthritis requiring immunosuppression, bronchoscopy with BAL is performed to evaluate for the presence of MAC. These cultures were positive for MAC.
Dr. Fine, does the positive BAL culture indicate an active MAC infection?
►Dr. Fine. Yes, based on these updated data, the patient has an active MAC infection. Active infection is defined as symptoms or imaging consistent with the diagnosis, supporting microbiology data (either 2 sputum or 1 BAL sample growing MAC) and the exclusion of other causes. Previously, this patient grew MAC in just one expectorated sputum; this did not meet the microbiologic criteria. Now sputum has grown in the BAL sample; along with the CT imaging, this is enough to diagnosis active MAC infection.
Treatment for MAC must consider the details of each case. First, this is not an emergency; treatment decisions should be made with the rheumatologist to consider the planned immunosuppression. For example, we must consider potential drug interactions. A specific point should be made of the use of tumor necrosis factor (TNF)-α inhibition, which data indicate can reactivate TB and may inhibit mechanisms that restrain mycobacterial disease. Serious cases of MAC infection have been reported in the literature in the setting of TNF-α inhibition.5,6 Despite these concerns, there is not a contraindication to using these therapies from the perspective of the active MAC disease. All of these decisions will impact the need to commit the patient to MAC therapy.
►Dr. Swamy. Dr. Fine, what do you consider prior to initiating MAC therapy?
► Dr. Fine. The decision to pursue MAC therapy should not be taken lightly. Therapy often entails prolonged multidrug regimens, usually spanning more than a year, with frequent adverse effects. Outside of very specific cases, such as TNF-β inhibition, MAC is rarely a life-threatening disease, so the benefit may be limited. Treatment for MAC is certainly unlikely to be fruitful without a diligent and motivated patient able to handle the high and prolonged pill burden. Of note, it is also important to keep this patient up-to-date with influenza and pneumonia vaccination given her structural lung disease.
►Dr. Swamy. The decision is made to treat MAC with azithromycin, rifampin, and ethambutol. The disease is noted to be nonfibrocavitary. The patient underwent monthly liver function test monitoring and visual acuity testing, which were unremarkable. Dr. Fine, can you describe the phenotypes of nontuberculous mycobacterial (NTM) disease?
►Dr. Fine. There are 3 main phenotypes of NTM.3 First, we see the elderly man with preexisting lung disease—usually chronic obstructive pulmonary disease—with fibrocavitary and/or reticulonodular appearance. Second, we see the slim, elderly woman often without any preexisting lung disease presenting with focal bronchiectasis and nodular lesions in right middle lobe and lingula—the Lady Windermere syndrome. This eponym is derived from Oscar Wilde’s play “Lady Windermere’s Fan, a Play About a Good Woman,” and was first associated with this disease in 1992.7 At the time, it was thought that the voluntary suppression of cough led to poorly draining lung regions, vulnerable to engraftment by atypical mycobacteria. Infection with atypical mycobacteria are associated with this population; however, it is no longer thought to be due to the voluntary suppression of cough.7,8 Third, we do occasionally see atypical presentations, such as focal masses and solitary nodules.
►Dr. Swamy. At 1-year follow-up she successfully completed MAC therapy and noted ongoing control of rheumatoid symptoms.
1. Bernardy K, Klose P, Welsch P, Häuser W. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome—a systematic review and meta-analysis of randomized controlled trials. Eur J Pain. 2018;22(2):242-260.
2. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279.
3. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108(3):417-425.
4. Aucott JN. Glucocorticoids and infection. Endocrinol Metab Clin North Am. 1994;23(3):655-670.
5. Curtis JR, Yang S, Patkar NM, et al. Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(7):990-997.
6. Lane MA, McDonald JR, Zeringue AL, et al. TNF-α antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine (Baltimore). 2011;90(2):139-145.
7. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101(6):1605-1609.
8. Kasthoori JJ, Liam CK, Wastie ML. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Med J. 2008;49(2):e47-e49.
1. Bernardy K, Klose P, Welsch P, Häuser W. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome—a systematic review and meta-analysis of randomized controlled trials. Eur J Pain. 2018;22(2):242-260.
2. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279.
3. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108(3):417-425.
4. Aucott JN. Glucocorticoids and infection. Endocrinol Metab Clin North Am. 1994;23(3):655-670.
5. Curtis JR, Yang S, Patkar NM, et al. Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(7):990-997.
6. Lane MA, McDonald JR, Zeringue AL, et al. TNF-α antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine (Baltimore). 2011;90(2):139-145.
7. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101(6):1605-1609.
8. Kasthoori JJ, Liam CK, Wastie ML. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Med J. 2008;49(2):e47-e49.