User login
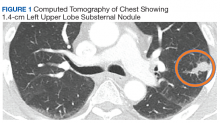
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
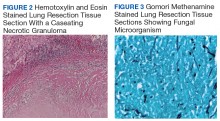
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.