User login
Chronic Lymphocytic Leukemia and Infiltrates Seen During Excision of Nonmelanoma Skin Cancer
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
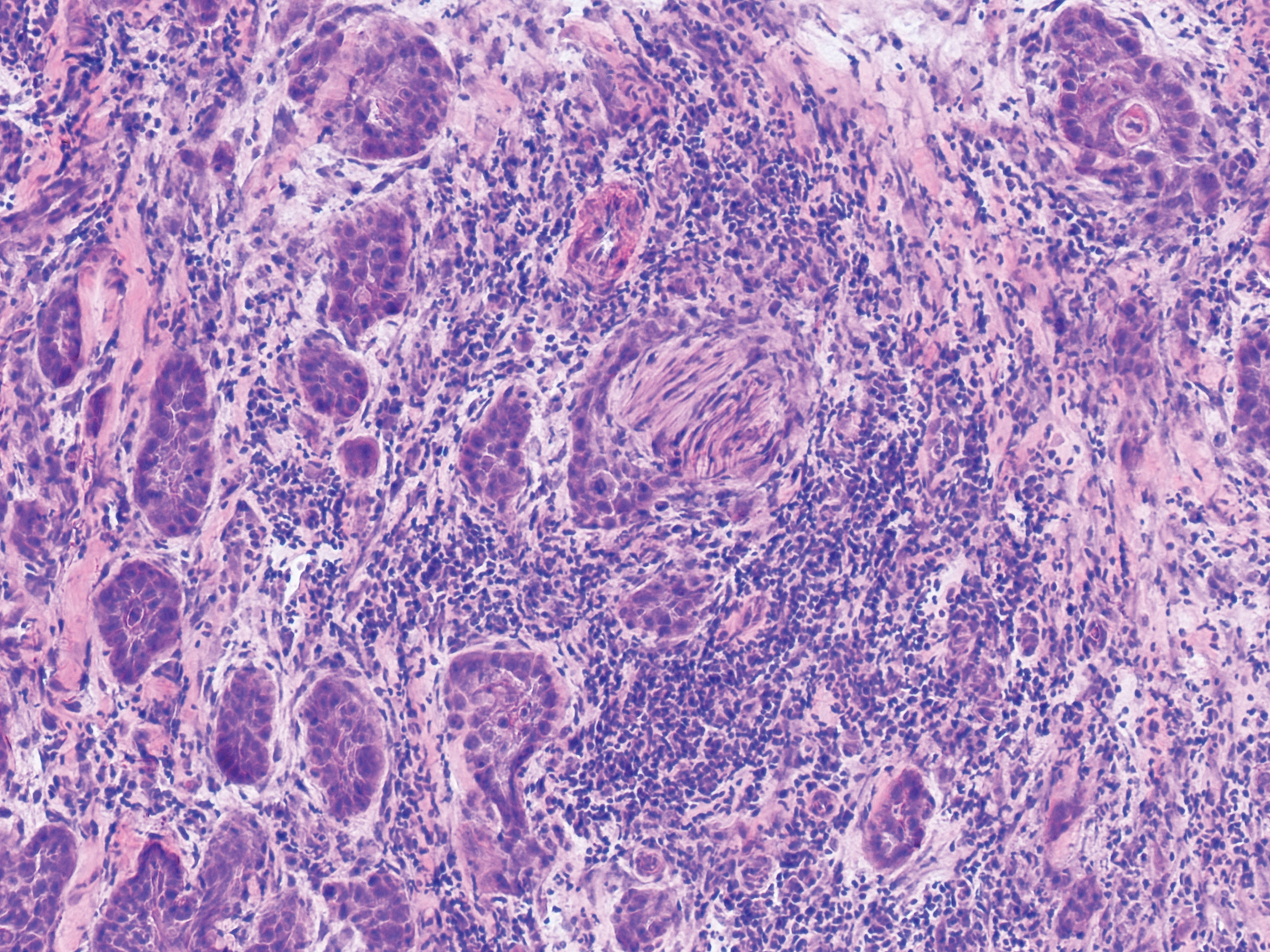
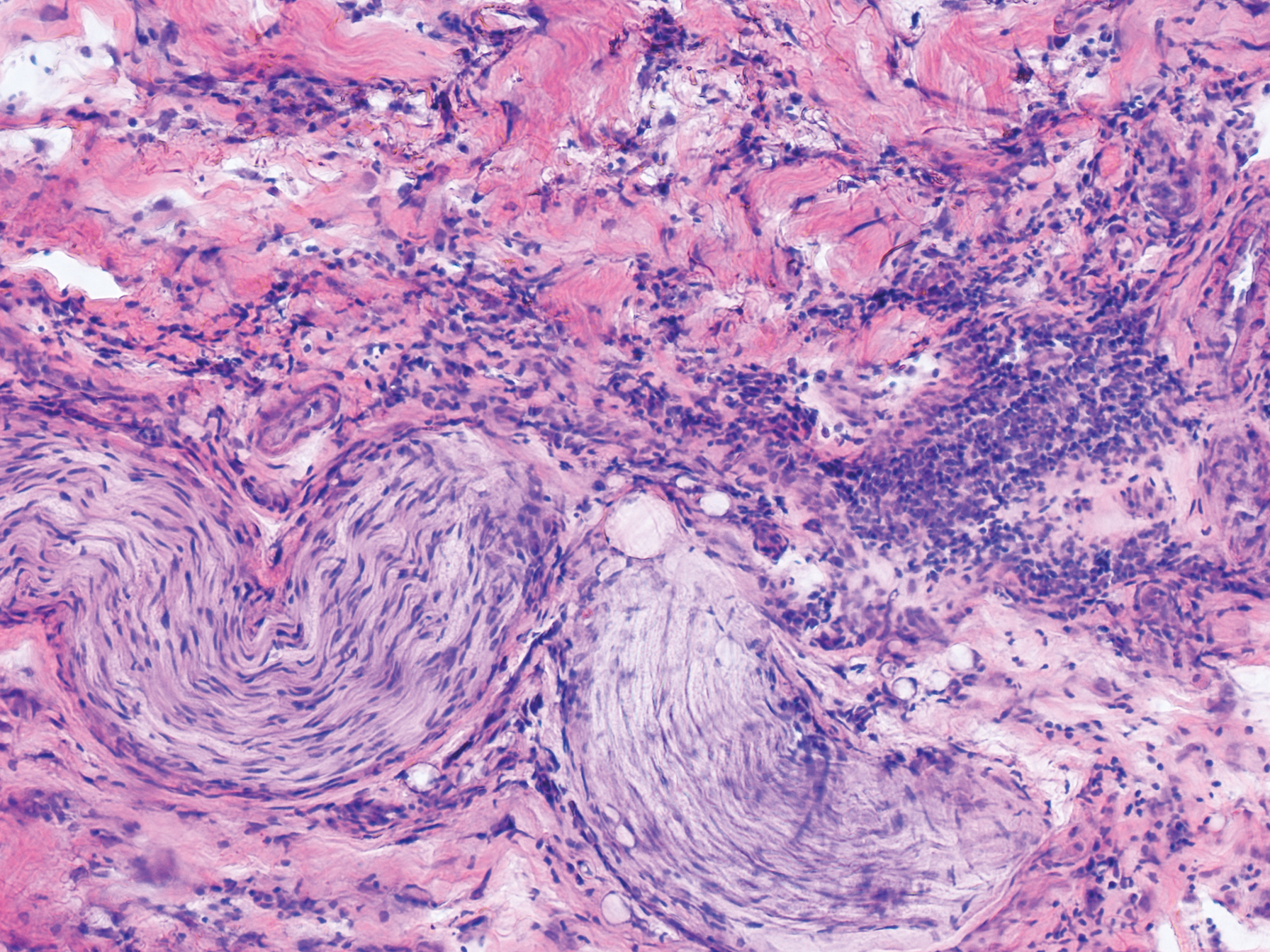
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.
An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
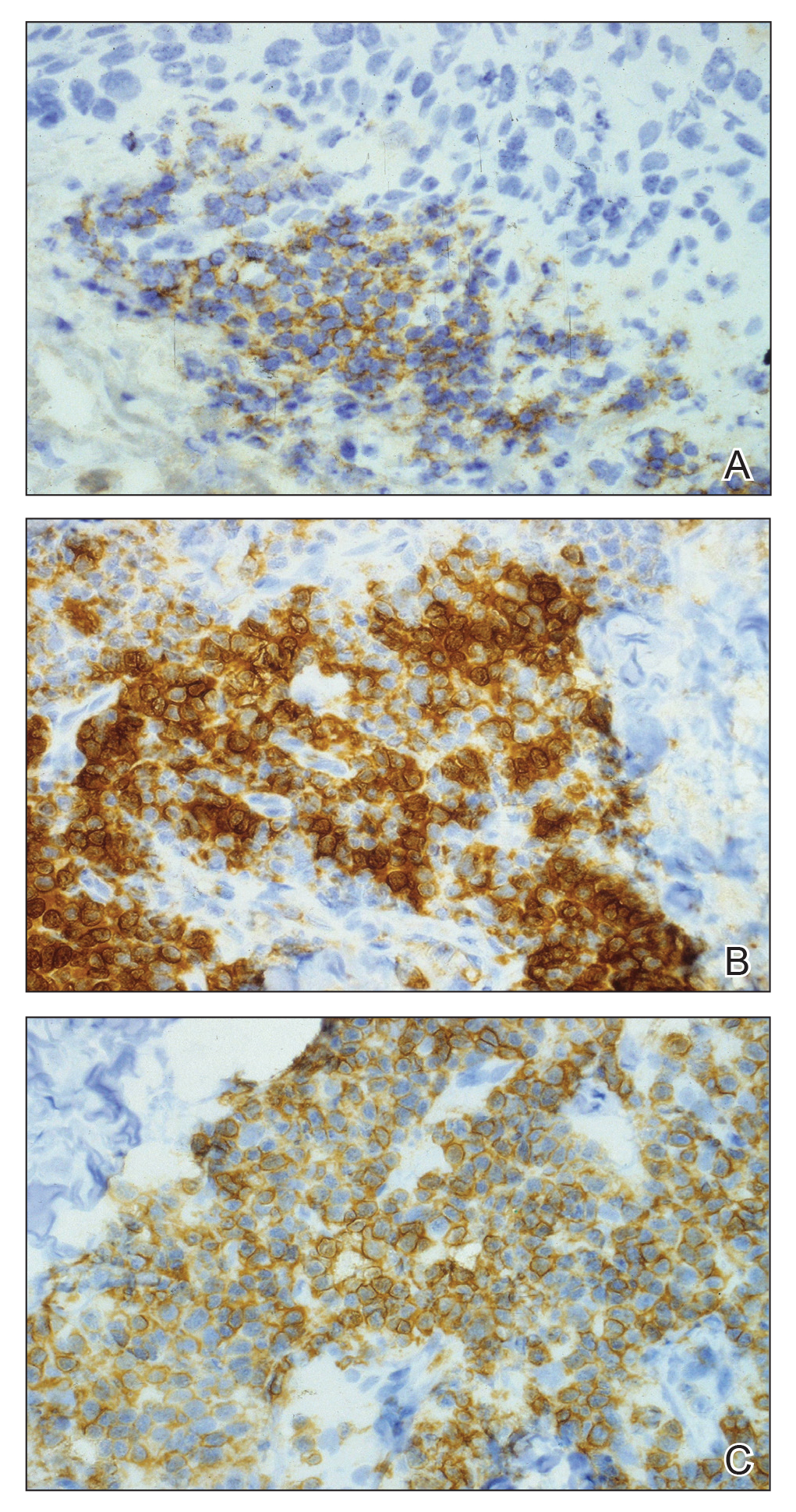
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7
The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.
An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7
The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.
An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7
The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
Practice Points
- Chronic lymphocytic leukemia (CLL) may be seen during histologic examination of specimens during Mohs micrographic surgery as a monomorphic infiltrate of small mature lymphocytes with dense nuclei. Patients may be unaware of their diagnosis, which can be the presenting feature.
- An infiltrate of CLL may mimic aggressive behavior of nonmelanoma skin cancers including perineural invasion. A leukemic infiltrate may appear more dense and monomorphic. If needed, immunohistochemical staining of leukemic cells will show CD5 and CD23 positivity.
- Anecdotally, patients with CLL may not remember this pertinent medical history. Whether due to its asymptomatic nature or lack of treatment in early stages, direct questioning about CLL may be warranted if this characteristic infiltrate is encountered.