User login
First EDition: News for and about the practice of Emergency Medicine
Diagnostic errors top malpractice claims against emergency physicians
BY ALICIA GALLEGOS
FRONTLINE MEDICAL NEWS
More than half of medical malpractice lawsuits against emergency physicians involve allegations of diagnostic errors, according to a study by national medical liability insurer The Doctors Company.
The study analyzed 332 emergency medicine claims in the insurer’s database that were closed from 2007 to 2013. The findings show the importance of strong communication in the emergency department (ED), and how communication breakdowns can be catalysts for legal claims, Darrell Ranum, vice president for patient safety and risk management for The Doctors Company, said.
Mr. Ranum pointed out one claim in which a patient’s vital signs had changed during the course of a visit. The changes were not communicated to the emergency physician, and he inappropriately discharged the patient. In another case, a patient’s history of overdoses was not conveyed during a patient hand-off, which resulted in an overdose that was not treated in a timely manner.
“Sometimes, we find that patients don’t always understand instructions,” Mr. Ranum said. “If patients don’t understand the communication from their providers or if a patient receives inadequate information, such as in situations where you have a language barrier, you can have problems there as well.”
Another key lesson from the study is the importance of a thorough differential diagnosis, Dr David B. Troxel, medical director for The Doctors Company said in an interview.
Of the 332 claims, 57% were diagnosis related; they included allegations of failure to establish a differential diagnosis, failure to order diagnostic tests, failure to address abnormal findings, and failure to consider available clinical information.
View on the News Workflow bottlenecks contribute Dr Roneet Lev is director of ED operations for Scripps Mercy Hospital in San Diego. She made her remarks in an interview. |
“Our hope is that as physicians review the findings of this study, they will scrutinize their own systems and processes and determine whether the weaknesses identified in the study exist in their organization,” Dr Troxel said.
Among the other claims, 13% related to improper management or treatment, 5% claimed improper performance of treatment, and 3% alleged failure to order medication.
Claims could have more than one contributing factor. Problems with patient assessments, such as failure to address abnormal findings, contributed to 52% of claims. Patient factors, such as physical characteristics and noncompliance, were the second-most-frequent contributor at 21%. Other factors were lack of communication among physicians (17%), poor communication between doctors and patients (14%), insufficient or lack of documentation (13%), and workflow/workload issues (12%).
On Twitter @legal_med
ED-initiated buprenorphine ups treatment rates for opioid addiction
BY BIANCA NOGRADY
FROM JAMA
Vitals Key clinical point: Emergency department–initiated buprenorphine treatment increases the likelihood that opioid-dependent patients will seek addiction treatment. Major finding: Significantly more patients in the buprenorphine group sought addiction treatment in the 30 days after randomization, compared to the referral or intervention arms. Data source: Randomized controlled trial of 104 opioid-dependent patients. Disclosures: The National Institute on Drug Abuse funded the study. Reckitt-Benckiser Pharmaceuticals provided the drug. One author reported honoraria from private industry. |
Initiating buprenorphine treatment during an emergency department visit can significantly increase the likelihood that opioid-dependent patients will seek addiction treatment, according to results of a randomized clinical trial published in April 28 in JAMA.
Researchers randomized 104 opioid-dependent patients who had presented to the ED to screening and treatment referral, screening and a brief intervention with referral to a community-based treatment service, or screening with a brief intervention and ED-initiated treatment with buprenorphine/naloxone and referral to primary care.
Significantly more patients in the buprenorphine group sought addiction treatment in the 30 days after randomization compared to the referral or intervention arms (78% vs. 37% vs. 45%, P < .001), and they showed significant reductions in the number of days of illicit opioid use per week.
“Expanded use of ED-initiated buprenorphine with community follow-up should help increase access to treatment options for this chronic and relapsing medical condition that has substantial morbidity and mortality and that affects health care use and costs,” wrote Dr Gail D’Onofrio of Yale School of Medicine, New Haven, Ct., and coauthors (JAMA 2015; 313:1636-1644 [doi:10.1001/jama.2015.3474]).
More than 75% with sickle cell crises don’t get hydroxyurea
BY MARY ANN MOON
FROM JAMA
Vitals Key clinical point: More than 75% of adults with sickle cell anemia who have frequent pain crises fail to get hydroxyurea therapy as recommended. Major finding: Only 15.1% of adults with sickle cell anemia received hydroxyurea within 3 months of their third pain crisis, only 18.2% received the agent within 6 months, and only 22.7% received it within 12 months. Data source: An analysis of information in a large nationwide insurance claims database involving 570 adults with frequent hospitalizations for sickle cell pain crises. Disclosures: This study was funded by the Lewin Group, a health care consulting firm. Dr Stettler and his associates reported having no relevant financial disclosures. |
More than 75% of adults with sickle cell anemia who have frequent pain crises fail to receive hydroxyurea therapy as strongly recommended in National Heart, Lung, and Blood Institute clinical guidelines, according to a Research Letter to the Editor published online April 28 in JAMA.
Despite proven benefits in decreasing pain crises, hospitalizations, blood transfusions, and possibly mortality, hydroxyurea, a “safe and inexpensive drug,” is thought to be underused. To document the actual use of the drug when indicated, investigators analyzed information in a nationwide insurance claims database covering nearly 27 million patients per year. They focused on the records of 570 adults hospitalized or treated in an emergency department for a sickle cell pain crisis at least three times during a 1-year period, said Dr Nicolas Stettler of the Lewin Group, a health care consulting firm in Falls Church, Va., and his associates.
Only 15.1% of these patients received hydroxyurea within 3 months of their third crisis, only 18.2% received the agent within 6 months, and only 22.7% received it within 12 months. These figures likely represent a conservative estimate of the hydroxyurea treatment gap, since the study didn’t include the large uninsured and publicly insured populations who have more limited access to health care, Dr Stettler and his associates noted (JAMA 2015;313:1671-2).
Several barriers to this treatment have been identified in previous research, including fear of adverse events, lack of clinician training, and failure to use shared decision making. “To address this gap, it may be necessary to enhance patient outreach and clinician training and develop health care quality measures aimed at increasing the use of hydroxyurea for all patients who would benefit,” they added.
Recognizing human trafficking victims
BY ALICIA GALLEGOS
AT THE AMWA ANNUAL MEETING
CHICAGO – Emergency physicians encounter nearly 40% of human trafficking victims who come in contact with health providers, according to Dr Holly G. Atkinson.
In a 2014 survey of domestic sex-trafficking victims, 88% said they encountered one or more health professions during the period in which they were being trafficked, yet none was identified as a victim by physicians during the visits. In another 2014 survey of survivors, 39% of victims reported having contact with emergency departments; 29%, with primary care physicians; 17%, with ob.gyns.; 17%, with dentists; and 3%, with pediatricians.
Key indicators for potential victimization include discrepancies between history and clinical presentation, multiple sexually transmitted diseases, and the accompaniment of a controlling third-party who is not a guardian, said Dr Atkinson, director of the human rights program at Arnhold Global Health Institute at the Icahn School of Medicine at Mount Sinai in New York City, said at the annual meeting of the American Medical Women’s Association.
In 2014, the National Human Trafficking Resource Center, operated by the antislavery organization Polaris, received 3,598 reports of sex-trafficking cases inside the United States. The National Center for Missing & Exploited Children estimates that between 100,000 and 300,000 U.S. children are at risk of being sexually trafficked each year.
Physical signs that could denote the possibility of patients being trafficked include visible tattoos with “daddy,” “property of,” or a trafficker’s street name. Perpetrators often brand their victims so that they are easily recognizable and can be returned if they escape, Dr Atkinson explained.
Dehydration, malnutrition, multiple sexually transmitted infections, and multiple pregnancies or abortions could also be clues. The inability to provide an address, confusion about their current location, an appearance younger than the stated age, avoidance of eye contact, and answers that sound scripted are potential signs of human trafficking, as is the presence of a controlling third party who does not let the patient answer questions or who interrupts or corrects the patient.
The Via Christi Health system in Wichita, Kan., recently published guidance for clinicians on how to proceed if they suspect a patient is a victim of human trafficking. Steps include following child abuse or domestic violence protocols; separating the patient from the controlling third party; providing the patient a comfortable, safe area; and ensuring a patient interview is performed by a trauma-informed social worker or nurse.
Some questions physicians may want to ask patients include: Have you ever exchanged sex for money, food, or shelter? Have you been forced to have sex against your will? Have you been asked to have sex with multiple partners? If the patient answers yes, physicians should follow child abuse protocols and mandatory reporting requirements. If the patient is aged 18 or older, doctors should obtain the patient’s permission to call law enforcement or assist the patient in calling 911.
AMWA recently launched Physicians Against the Trafficking of Humans (PATH). The PATH website includes resources for physicians and an online video about trafficking that doctors can share with their practices and colleagues.
On Twitter @legal_med
Indiana HIV outbreak prompts national advisory
BY SHARON WORCESTER
FRONTLINE MEDICAL NEWS
As health officials continue to battle an intravenous drug use–related HIV outbreak in a rural county in Indiana, the Centers for Disease Control and Prevention has issued an official health advisory stressing the need for vigilance at the state and local levels with respect to detecting and controlling similar outbreaks across the United States.
The Indiana outbreak began in November, with 11 new HIV infections diagnosed by January in Scott County, where fewer than 5 infections per year had been identified previously; since January, an unprecedented 135 infections have been confirmed, and 6 others are under investigation, according to Dr Jerome M. Adams, Indiana State Health Commissioner.
“To put this in further perspective, from 2009 through 2013, the county only reported three new cases of HIV,” Dr Adams said in a joint Indiana State Department of Health/CDC teleconference.
The affected community includes only 4,200 people, and 84% of those diagnosed with HIV also tested positive for hepatitis C virus (HCV) infection, Dr Joan Duwve, chief medical consultant, Indiana State Department of Health, said during the teleconference.
According to the CDC advisory, 96% of 108 infected individuals who were interviewed reported injecting dissolved prescription-type oxymorphone, as well as sharing syringes.
“The United States is facing an epidemic of prescription opioid abuse that must be addressed. Opioid poisoning deaths have nearly quadrupled from 1999 through 2011. This epidemic of opioid abuse has already contributed to a severe and growing epidemic of viral hepatitis among people who inject drugs,” said Dr Jonathan Mermin, director of the National Center for HIV/AIDS Viral Hepatitis, STD, and TB Prevention, who also participated in the teleconference.
New CDC data on HCV show a 150% increase in reports of acute HCV infections nationwide between 2010 to 2013.
“The majority of these infections are believed to be attributable to injection drug use, so we must act now to reverse this trend and to prevent this from undoing progress in HIV prevention to date,” he said.
Among the recommendations were routine opt-out of HIV testing as well as HCV testing per current recommendations. Further, health care professionals should report suspected clusters to their local or state health department, ensure that HCV-infected patients are also tested for HIV and vice versa, and ensure that those receiving treatment for either HIV or HCV are adhering to prescribed therapy and are engaged in ongoing care.
Syringe-sharing and sexual partners of those diagnosed with HIV or HCV should be encouraged to undergo testing, and all persons with substance abuse problems should be referred for medication-assisted treatment and counseling. In Indiana, where a public health emergency was declared on March 26 and has been extended until May 24, care includes HIV and HCV treatment, as well as substance abuse counseling and treatment, according to a “Notes From the Field Report” in the Morbidity and Mortality Weekly Report (MMWR 2015 April 24;64:1-2).
sworcester@frontlinemedcom.com
Most accidental genital trauma cases manageable in ED
BY SHARON WORCESTER
AT THE NASPAG ANNUAL MEETING
Vitals Key clinical point: With adequate sedation, most girls with AGT requiring treatment can undergo evaluation and repair in the ED. Major finding: 82% of cases reviewed were minor and managed expectantly. Data source: A retrospective medical records review of 359 cases of accidental genital trauma. Disclosures: Dr Dowlut-McElroy reported having no disclosures. One of her coauthors, Dr Julie Strickland, is a Nexplanon trainer for Merck. |
ORLANDO – Most cases of accidental genital trauma in girls are caused by straddle injuries and are isolated to the labia, and most can be managed expectantly or treated in the emergency department, according to findings from a retrospective cohort study.
Penetrating injuries, however, should be considered an indication for management in the operating room, Dr Tazim Dowlut-McElroy reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
A review of 359 cases showed that the vast majority – 82% – were minor and managed expectantly.
“Only 18% required surgical management. Of those, 37% required general anesthesia in the OR, but 63% were adequately evaluated and treated in the ED,” said Dr Dowlut-McElroy of Children’s Mercy Hospitals and Clinics, Kansas City.
About 64% of the patients presented during the day, and 36% presented at night. The most common presenting complaint was bleeding and pain (89% of cases), followed by voiding issues in 8% of cases. No presenting complaint was recorded in the remaining patient charts.
The most common mechanism of injury was straddle injury (73% of cases), followed by non-straddle blunt trauma in 15% of cases, and penetrating injury in the remaining cases, Dr Dowlut-McElroy said.
Injuries included lacerations in 86% of cases, abrasions or contusions in 7%, and hematomas in 3%.
Pediatric genital injuries comprised 0.2%-8% of reported childhood trauma, and despite public health efforts to reduce injuries, the number of such pediatric injuries continues to rise, Dr Dowlut-McElroy said.
Patients included in the current review were identified by a medical database query from January 2000 to July 2014. They were aged 0-18 years and had been treated in the ED for genital trauma; those with obstetrical injuries were excluded.
sworcester@frontlinemedcom.com
EDs pump up pediatric preparedness
BY MARY ANN MOON
FROM JAMA PEDIATRICS
Vitals Key clinical point: Nationally, emergency departments have improved their compliance with guidelines regarding emergency health care for children. Major finding: The overall median weighted pediatric readiness score (WPRS) was 68.9, a solid improvement over the previously reported median WPRS of 55. Data source: A web-based self-reported assessment of pediatric readiness at 4,149 EDs across the country that have approximately 24 million pediatric visits annually. Disclosures: This project was supported by the Emergency Medical Services for Children network and the EMS for Children National Resource Center under the U.S. Department of Health & Human Services. Dr Gausche-Hill and her associates reported having no financial disclosures. |
Pediatric readiness has improved in emergency departments across the country, as measured by EDs’ compliance with 2009 guidelines for emergency care in children, according to a report published online April 13 in JAMA Pediatrics.
In what they described as “the most comprehensive evaluation of pediatric readiness of our nation’s EDs to date,” investigators performed a web-based assessment of 4,137 hospitals’ self-reported compliance with guidelines addressing child-specific emergency care. The overall median weighted pediatric readiness score (WPRS) was 68.9, a solid improvement over the previously reported median WPRS of 55, said Dr Marianne Gausche-Hill of Harbor-UCLA Medical Center, Torrance, Calif., and her associates.
The single most important factor found to enhance pediatric readiness was to designate two people, one of whom is a physician or nurse, as the hospital’s pediatric emergency care coordinator, as recommended by the Institute of Medicine, the researchers wrote. Nearly half (47.5%) of EDs now have a physician coordinator, and 59.3% now have a nurse coordinator, compared with 18% and 12%, respectively, in a previous report. The presence of coordinators quadrupled the chances that a hospital would put important quality-improvement plans in place to address children’s care needs, the investigators said (JAMA Pediatr. 2015 April 13 [doi:10.1001/jamapediatrics.2015.138]).
Other findings included the following:
- Most physicians providing emergency care for children were specifically trained in emergency medicine (88.6%) or pediatric emergency medicine (55.4%) at high-volume hospitals; low-volume hospitals were more likely to have family-medicine-trained physicians doing so (78.9%).
- Mandatory competency evaluations for providing pediatric emergency care were relatively common for nurses (66.6%) but less so for physicians (38.7%) and midlevel staff (18.1%).
- 99.5% of EDs said all staff are trained on the location of pediatric equipment in the ED, including tools to ensure correct sizing of resuscitation equipment and appropriate dosing of medications.
- 83.1% of EDs said they verified the proper location and function of pediatric equipment daily.
- EDs routinely stocked 91% of recommended pediatric equipment. Equipment that was missing in more than 15% of EDs included laryngeal mask airways, umbilical vein catheters, central venous catheters, tracheostomy tubes, size 00 laryngoscope blades, continuous end-tidal carbon dioxide monitoring equipment, pediatric Magill forceps, and infant and child nasopharyngeal airways.
- Only 46.8% of EDs had disaster plans that specifically addressed children, as recommended. Even among high-volume hospitals, which were the most compliant with guidelines, only 67.4% had such disaster plans.
- Approximately one-third of EDs said their providers failed to weigh children and record the weight in kilograms only, as recommended. This safety measure is crucial to preventing drug-dosing errors.
- The most frequently cited barriers to complying with guidelines were the cost of child-specific training (reported by 54.4% of EDs) and a lack of educational resources (reported by 49.0%). Few EDs reported that a lack of interest was a barrier to implementing the guidelines.
Simplified PESI identified low-risk pulmonary embolism
BY AMY KARON
FROM ACADEMIC EMERGENCY MEDICINE
Vitals Key clinical point: The simplified version of the PESI identified low-risk pulmonary embolism patients. Major finding: Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days, regardless of which treatment they received. Data source: Post hoc analysis of simplified PESI scores and outcomes among 4,831 patients with acute pulmonary embolism who received either rivaroxaban or an enoxaparin–vitamin K antagonist combination. Disclosures:Bayer HealthCare Pharmaceuticals and Janssen Research & Development funded the study. Dr Fermann reported an advisory relationship with Janssen and research funding from Cardiorentis, Trevena, Novartis, Siemens, and Pfizer. Two coauthors reported employment with Bayer, and two other coauthors reported relationships with several other pharmaceutical companies. |
A simplified version of the Pulmonary Embolism Severity Index identified patients with acute pulmonary embolism who were at low risk of adverse events and might be suitable for outpatient care.
“Although guidelines, such as those from the American College of Chest Physicians, recommend outpatient treatment for selected PE patients at low risk of recurrence, existing evidence for the outpatient management of patients with PE is derived from small cohorts of patients from outside the United States,” said Dr Gregory J. Fermann of the University of Cincinnati department of emergency medicine and his associates. “Risk stratification of PE patients may allow a cohort of low-risk patients to be treated in a clinical decision unit or by a closely monitored outpatient strategy. Such an approach might relieve some of the burden placed on the emergency department,” they wrote (Acad. Emerg. Med. 2015;22:299-307).
The simplified PESI includes 6 of the 11 variables measured in PESI, which has been shown to identify patients at increased risk of death and adverse outcome events after acute PE. The six features of the simplified PESI are age greater than 80 years, history of cancer, history of chronic cardiopulmonary disease, heart rate of at least 110 beats per minutes, systolic blood pressure less than 100 mm Hg, and oxygen saturation less than 90%. Each factor is assigned a score of 1.
They carried out a post hoc analysis of simplified PESI scores and outcomes among 4,831 acute PE patients from the EINSTEIN-PE study of rivaroxaban and an enoxaparin–vitamin K antagonist combination (N. Engl. J. Med. 2012;366:1287-97).
Roughly half (53.6%) of the patients had a score of 0, one-third (36.7%) had a score of 1, and 9.7% had a score of 2 or 3, the researchers reported. Higher simplified PESI scores were associated with increased risk of almost all adverse outcomes measured, including recurrent VTE, fatal PE, all-cause mortality, and major bleeding. Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days of treatment, regardless of which protocol they received. Scores of 2 or 3 were associated with greater risk of recurrent VTE, fatal PE, all-cause mortality, and major bleeding in both treatment groups.
Diagnostic errors top malpractice claims against emergency physicians
BY ALICIA GALLEGOS
FRONTLINE MEDICAL NEWS
More than half of medical malpractice lawsuits against emergency physicians involve allegations of diagnostic errors, according to a study by national medical liability insurer The Doctors Company.
The study analyzed 332 emergency medicine claims in the insurer’s database that were closed from 2007 to 2013. The findings show the importance of strong communication in the emergency department (ED), and how communication breakdowns can be catalysts for legal claims, Darrell Ranum, vice president for patient safety and risk management for The Doctors Company, said.
Mr. Ranum pointed out one claim in which a patient’s vital signs had changed during the course of a visit. The changes were not communicated to the emergency physician, and he inappropriately discharged the patient. In another case, a patient’s history of overdoses was not conveyed during a patient hand-off, which resulted in an overdose that was not treated in a timely manner.
“Sometimes, we find that patients don’t always understand instructions,” Mr. Ranum said. “If patients don’t understand the communication from their providers or if a patient receives inadequate information, such as in situations where you have a language barrier, you can have problems there as well.”
Another key lesson from the study is the importance of a thorough differential diagnosis, Dr David B. Troxel, medical director for The Doctors Company said in an interview.
Of the 332 claims, 57% were diagnosis related; they included allegations of failure to establish a differential diagnosis, failure to order diagnostic tests, failure to address abnormal findings, and failure to consider available clinical information.
View on the News Workflow bottlenecks contribute Dr Roneet Lev is director of ED operations for Scripps Mercy Hospital in San Diego. She made her remarks in an interview. |
“Our hope is that as physicians review the findings of this study, they will scrutinize their own systems and processes and determine whether the weaknesses identified in the study exist in their organization,” Dr Troxel said.
Among the other claims, 13% related to improper management or treatment, 5% claimed improper performance of treatment, and 3% alleged failure to order medication.
Claims could have more than one contributing factor. Problems with patient assessments, such as failure to address abnormal findings, contributed to 52% of claims. Patient factors, such as physical characteristics and noncompliance, were the second-most-frequent contributor at 21%. Other factors were lack of communication among physicians (17%), poor communication between doctors and patients (14%), insufficient or lack of documentation (13%), and workflow/workload issues (12%).
On Twitter @legal_med
ED-initiated buprenorphine ups treatment rates for opioid addiction
BY BIANCA NOGRADY
FROM JAMA
Vitals Key clinical point: Emergency department–initiated buprenorphine treatment increases the likelihood that opioid-dependent patients will seek addiction treatment. Major finding: Significantly more patients in the buprenorphine group sought addiction treatment in the 30 days after randomization, compared to the referral or intervention arms. Data source: Randomized controlled trial of 104 opioid-dependent patients. Disclosures: The National Institute on Drug Abuse funded the study. Reckitt-Benckiser Pharmaceuticals provided the drug. One author reported honoraria from private industry. |
Initiating buprenorphine treatment during an emergency department visit can significantly increase the likelihood that opioid-dependent patients will seek addiction treatment, according to results of a randomized clinical trial published in April 28 in JAMA.
Researchers randomized 104 opioid-dependent patients who had presented to the ED to screening and treatment referral, screening and a brief intervention with referral to a community-based treatment service, or screening with a brief intervention and ED-initiated treatment with buprenorphine/naloxone and referral to primary care.
Significantly more patients in the buprenorphine group sought addiction treatment in the 30 days after randomization compared to the referral or intervention arms (78% vs. 37% vs. 45%, P < .001), and they showed significant reductions in the number of days of illicit opioid use per week.
“Expanded use of ED-initiated buprenorphine with community follow-up should help increase access to treatment options for this chronic and relapsing medical condition that has substantial morbidity and mortality and that affects health care use and costs,” wrote Dr Gail D’Onofrio of Yale School of Medicine, New Haven, Ct., and coauthors (JAMA 2015; 313:1636-1644 [doi:10.1001/jama.2015.3474]).
More than 75% with sickle cell crises don’t get hydroxyurea
BY MARY ANN MOON
FROM JAMA
Vitals Key clinical point: More than 75% of adults with sickle cell anemia who have frequent pain crises fail to get hydroxyurea therapy as recommended. Major finding: Only 15.1% of adults with sickle cell anemia received hydroxyurea within 3 months of their third pain crisis, only 18.2% received the agent within 6 months, and only 22.7% received it within 12 months. Data source: An analysis of information in a large nationwide insurance claims database involving 570 adults with frequent hospitalizations for sickle cell pain crises. Disclosures: This study was funded by the Lewin Group, a health care consulting firm. Dr Stettler and his associates reported having no relevant financial disclosures. |
More than 75% of adults with sickle cell anemia who have frequent pain crises fail to receive hydroxyurea therapy as strongly recommended in National Heart, Lung, and Blood Institute clinical guidelines, according to a Research Letter to the Editor published online April 28 in JAMA.
Despite proven benefits in decreasing pain crises, hospitalizations, blood transfusions, and possibly mortality, hydroxyurea, a “safe and inexpensive drug,” is thought to be underused. To document the actual use of the drug when indicated, investigators analyzed information in a nationwide insurance claims database covering nearly 27 million patients per year. They focused on the records of 570 adults hospitalized or treated in an emergency department for a sickle cell pain crisis at least three times during a 1-year period, said Dr Nicolas Stettler of the Lewin Group, a health care consulting firm in Falls Church, Va., and his associates.
Only 15.1% of these patients received hydroxyurea within 3 months of their third crisis, only 18.2% received the agent within 6 months, and only 22.7% received it within 12 months. These figures likely represent a conservative estimate of the hydroxyurea treatment gap, since the study didn’t include the large uninsured and publicly insured populations who have more limited access to health care, Dr Stettler and his associates noted (JAMA 2015;313:1671-2).
Several barriers to this treatment have been identified in previous research, including fear of adverse events, lack of clinician training, and failure to use shared decision making. “To address this gap, it may be necessary to enhance patient outreach and clinician training and develop health care quality measures aimed at increasing the use of hydroxyurea for all patients who would benefit,” they added.
Recognizing human trafficking victims
BY ALICIA GALLEGOS
AT THE AMWA ANNUAL MEETING
CHICAGO – Emergency physicians encounter nearly 40% of human trafficking victims who come in contact with health providers, according to Dr Holly G. Atkinson.
In a 2014 survey of domestic sex-trafficking victims, 88% said they encountered one or more health professions during the period in which they were being trafficked, yet none was identified as a victim by physicians during the visits. In another 2014 survey of survivors, 39% of victims reported having contact with emergency departments; 29%, with primary care physicians; 17%, with ob.gyns.; 17%, with dentists; and 3%, with pediatricians.
Key indicators for potential victimization include discrepancies between history and clinical presentation, multiple sexually transmitted diseases, and the accompaniment of a controlling third-party who is not a guardian, said Dr Atkinson, director of the human rights program at Arnhold Global Health Institute at the Icahn School of Medicine at Mount Sinai in New York City, said at the annual meeting of the American Medical Women’s Association.
In 2014, the National Human Trafficking Resource Center, operated by the antislavery organization Polaris, received 3,598 reports of sex-trafficking cases inside the United States. The National Center for Missing & Exploited Children estimates that between 100,000 and 300,000 U.S. children are at risk of being sexually trafficked each year.
Physical signs that could denote the possibility of patients being trafficked include visible tattoos with “daddy,” “property of,” or a trafficker’s street name. Perpetrators often brand their victims so that they are easily recognizable and can be returned if they escape, Dr Atkinson explained.
Dehydration, malnutrition, multiple sexually transmitted infections, and multiple pregnancies or abortions could also be clues. The inability to provide an address, confusion about their current location, an appearance younger than the stated age, avoidance of eye contact, and answers that sound scripted are potential signs of human trafficking, as is the presence of a controlling third party who does not let the patient answer questions or who interrupts or corrects the patient.
The Via Christi Health system in Wichita, Kan., recently published guidance for clinicians on how to proceed if they suspect a patient is a victim of human trafficking. Steps include following child abuse or domestic violence protocols; separating the patient from the controlling third party; providing the patient a comfortable, safe area; and ensuring a patient interview is performed by a trauma-informed social worker or nurse.
Some questions physicians may want to ask patients include: Have you ever exchanged sex for money, food, or shelter? Have you been forced to have sex against your will? Have you been asked to have sex with multiple partners? If the patient answers yes, physicians should follow child abuse protocols and mandatory reporting requirements. If the patient is aged 18 or older, doctors should obtain the patient’s permission to call law enforcement or assist the patient in calling 911.
AMWA recently launched Physicians Against the Trafficking of Humans (PATH). The PATH website includes resources for physicians and an online video about trafficking that doctors can share with their practices and colleagues.
On Twitter @legal_med
Indiana HIV outbreak prompts national advisory
BY SHARON WORCESTER
FRONTLINE MEDICAL NEWS
As health officials continue to battle an intravenous drug use–related HIV outbreak in a rural county in Indiana, the Centers for Disease Control and Prevention has issued an official health advisory stressing the need for vigilance at the state and local levels with respect to detecting and controlling similar outbreaks across the United States.
The Indiana outbreak began in November, with 11 new HIV infections diagnosed by January in Scott County, where fewer than 5 infections per year had been identified previously; since January, an unprecedented 135 infections have been confirmed, and 6 others are under investigation, according to Dr Jerome M. Adams, Indiana State Health Commissioner.
“To put this in further perspective, from 2009 through 2013, the county only reported three new cases of HIV,” Dr Adams said in a joint Indiana State Department of Health/CDC teleconference.
The affected community includes only 4,200 people, and 84% of those diagnosed with HIV also tested positive for hepatitis C virus (HCV) infection, Dr Joan Duwve, chief medical consultant, Indiana State Department of Health, said during the teleconference.
According to the CDC advisory, 96% of 108 infected individuals who were interviewed reported injecting dissolved prescription-type oxymorphone, as well as sharing syringes.
“The United States is facing an epidemic of prescription opioid abuse that must be addressed. Opioid poisoning deaths have nearly quadrupled from 1999 through 2011. This epidemic of opioid abuse has already contributed to a severe and growing epidemic of viral hepatitis among people who inject drugs,” said Dr Jonathan Mermin, director of the National Center for HIV/AIDS Viral Hepatitis, STD, and TB Prevention, who also participated in the teleconference.
New CDC data on HCV show a 150% increase in reports of acute HCV infections nationwide between 2010 to 2013.
“The majority of these infections are believed to be attributable to injection drug use, so we must act now to reverse this trend and to prevent this from undoing progress in HIV prevention to date,” he said.
Among the recommendations were routine opt-out of HIV testing as well as HCV testing per current recommendations. Further, health care professionals should report suspected clusters to their local or state health department, ensure that HCV-infected patients are also tested for HIV and vice versa, and ensure that those receiving treatment for either HIV or HCV are adhering to prescribed therapy and are engaged in ongoing care.
Syringe-sharing and sexual partners of those diagnosed with HIV or HCV should be encouraged to undergo testing, and all persons with substance abuse problems should be referred for medication-assisted treatment and counseling. In Indiana, where a public health emergency was declared on March 26 and has been extended until May 24, care includes HIV and HCV treatment, as well as substance abuse counseling and treatment, according to a “Notes From the Field Report” in the Morbidity and Mortality Weekly Report (MMWR 2015 April 24;64:1-2).
sworcester@frontlinemedcom.com
Most accidental genital trauma cases manageable in ED
BY SHARON WORCESTER
AT THE NASPAG ANNUAL MEETING
Vitals Key clinical point: With adequate sedation, most girls with AGT requiring treatment can undergo evaluation and repair in the ED. Major finding: 82% of cases reviewed were minor and managed expectantly. Data source: A retrospective medical records review of 359 cases of accidental genital trauma. Disclosures: Dr Dowlut-McElroy reported having no disclosures. One of her coauthors, Dr Julie Strickland, is a Nexplanon trainer for Merck. |
ORLANDO – Most cases of accidental genital trauma in girls are caused by straddle injuries and are isolated to the labia, and most can be managed expectantly or treated in the emergency department, according to findings from a retrospective cohort study.
Penetrating injuries, however, should be considered an indication for management in the operating room, Dr Tazim Dowlut-McElroy reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
A review of 359 cases showed that the vast majority – 82% – were minor and managed expectantly.
“Only 18% required surgical management. Of those, 37% required general anesthesia in the OR, but 63% were adequately evaluated and treated in the ED,” said Dr Dowlut-McElroy of Children’s Mercy Hospitals and Clinics, Kansas City.
About 64% of the patients presented during the day, and 36% presented at night. The most common presenting complaint was bleeding and pain (89% of cases), followed by voiding issues in 8% of cases. No presenting complaint was recorded in the remaining patient charts.
The most common mechanism of injury was straddle injury (73% of cases), followed by non-straddle blunt trauma in 15% of cases, and penetrating injury in the remaining cases, Dr Dowlut-McElroy said.
Injuries included lacerations in 86% of cases, abrasions or contusions in 7%, and hematomas in 3%.
Pediatric genital injuries comprised 0.2%-8% of reported childhood trauma, and despite public health efforts to reduce injuries, the number of such pediatric injuries continues to rise, Dr Dowlut-McElroy said.
Patients included in the current review were identified by a medical database query from January 2000 to July 2014. They were aged 0-18 years and had been treated in the ED for genital trauma; those with obstetrical injuries were excluded.
sworcester@frontlinemedcom.com
EDs pump up pediatric preparedness
BY MARY ANN MOON
FROM JAMA PEDIATRICS
Vitals Key clinical point: Nationally, emergency departments have improved their compliance with guidelines regarding emergency health care for children. Major finding: The overall median weighted pediatric readiness score (WPRS) was 68.9, a solid improvement over the previously reported median WPRS of 55. Data source: A web-based self-reported assessment of pediatric readiness at 4,149 EDs across the country that have approximately 24 million pediatric visits annually. Disclosures: This project was supported by the Emergency Medical Services for Children network and the EMS for Children National Resource Center under the U.S. Department of Health & Human Services. Dr Gausche-Hill and her associates reported having no financial disclosures. |
Pediatric readiness has improved in emergency departments across the country, as measured by EDs’ compliance with 2009 guidelines for emergency care in children, according to a report published online April 13 in JAMA Pediatrics.
In what they described as “the most comprehensive evaluation of pediatric readiness of our nation’s EDs to date,” investigators performed a web-based assessment of 4,137 hospitals’ self-reported compliance with guidelines addressing child-specific emergency care. The overall median weighted pediatric readiness score (WPRS) was 68.9, a solid improvement over the previously reported median WPRS of 55, said Dr Marianne Gausche-Hill of Harbor-UCLA Medical Center, Torrance, Calif., and her associates.
The single most important factor found to enhance pediatric readiness was to designate two people, one of whom is a physician or nurse, as the hospital’s pediatric emergency care coordinator, as recommended by the Institute of Medicine, the researchers wrote. Nearly half (47.5%) of EDs now have a physician coordinator, and 59.3% now have a nurse coordinator, compared with 18% and 12%, respectively, in a previous report. The presence of coordinators quadrupled the chances that a hospital would put important quality-improvement plans in place to address children’s care needs, the investigators said (JAMA Pediatr. 2015 April 13 [doi:10.1001/jamapediatrics.2015.138]).
Other findings included the following:
- Most physicians providing emergency care for children were specifically trained in emergency medicine (88.6%) or pediatric emergency medicine (55.4%) at high-volume hospitals; low-volume hospitals were more likely to have family-medicine-trained physicians doing so (78.9%).
- Mandatory competency evaluations for providing pediatric emergency care were relatively common for nurses (66.6%) but less so for physicians (38.7%) and midlevel staff (18.1%).
- 99.5% of EDs said all staff are trained on the location of pediatric equipment in the ED, including tools to ensure correct sizing of resuscitation equipment and appropriate dosing of medications.
- 83.1% of EDs said they verified the proper location and function of pediatric equipment daily.
- EDs routinely stocked 91% of recommended pediatric equipment. Equipment that was missing in more than 15% of EDs included laryngeal mask airways, umbilical vein catheters, central venous catheters, tracheostomy tubes, size 00 laryngoscope blades, continuous end-tidal carbon dioxide monitoring equipment, pediatric Magill forceps, and infant and child nasopharyngeal airways.
- Only 46.8% of EDs had disaster plans that specifically addressed children, as recommended. Even among high-volume hospitals, which were the most compliant with guidelines, only 67.4% had such disaster plans.
- Approximately one-third of EDs said their providers failed to weigh children and record the weight in kilograms only, as recommended. This safety measure is crucial to preventing drug-dosing errors.
- The most frequently cited barriers to complying with guidelines were the cost of child-specific training (reported by 54.4% of EDs) and a lack of educational resources (reported by 49.0%). Few EDs reported that a lack of interest was a barrier to implementing the guidelines.
Simplified PESI identified low-risk pulmonary embolism
BY AMY KARON
FROM ACADEMIC EMERGENCY MEDICINE
Vitals Key clinical point: The simplified version of the PESI identified low-risk pulmonary embolism patients. Major finding: Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days, regardless of which treatment they received. Data source: Post hoc analysis of simplified PESI scores and outcomes among 4,831 patients with acute pulmonary embolism who received either rivaroxaban or an enoxaparin–vitamin K antagonist combination. Disclosures:Bayer HealthCare Pharmaceuticals and Janssen Research & Development funded the study. Dr Fermann reported an advisory relationship with Janssen and research funding from Cardiorentis, Trevena, Novartis, Siemens, and Pfizer. Two coauthors reported employment with Bayer, and two other coauthors reported relationships with several other pharmaceutical companies. |
A simplified version of the Pulmonary Embolism Severity Index identified patients with acute pulmonary embolism who were at low risk of adverse events and might be suitable for outpatient care.
“Although guidelines, such as those from the American College of Chest Physicians, recommend outpatient treatment for selected PE patients at low risk of recurrence, existing evidence for the outpatient management of patients with PE is derived from small cohorts of patients from outside the United States,” said Dr Gregory J. Fermann of the University of Cincinnati department of emergency medicine and his associates. “Risk stratification of PE patients may allow a cohort of low-risk patients to be treated in a clinical decision unit or by a closely monitored outpatient strategy. Such an approach might relieve some of the burden placed on the emergency department,” they wrote (Acad. Emerg. Med. 2015;22:299-307).
The simplified PESI includes 6 of the 11 variables measured in PESI, which has been shown to identify patients at increased risk of death and adverse outcome events after acute PE. The six features of the simplified PESI are age greater than 80 years, history of cancer, history of chronic cardiopulmonary disease, heart rate of at least 110 beats per minutes, systolic blood pressure less than 100 mm Hg, and oxygen saturation less than 90%. Each factor is assigned a score of 1.
They carried out a post hoc analysis of simplified PESI scores and outcomes among 4,831 acute PE patients from the EINSTEIN-PE study of rivaroxaban and an enoxaparin–vitamin K antagonist combination (N. Engl. J. Med. 2012;366:1287-97).
Roughly half (53.6%) of the patients had a score of 0, one-third (36.7%) had a score of 1, and 9.7% had a score of 2 or 3, the researchers reported. Higher simplified PESI scores were associated with increased risk of almost all adverse outcomes measured, including recurrent VTE, fatal PE, all-cause mortality, and major bleeding. Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days of treatment, regardless of which protocol they received. Scores of 2 or 3 were associated with greater risk of recurrent VTE, fatal PE, all-cause mortality, and major bleeding in both treatment groups.
Diagnostic errors top malpractice claims against emergency physicians
BY ALICIA GALLEGOS
FRONTLINE MEDICAL NEWS
More than half of medical malpractice lawsuits against emergency physicians involve allegations of diagnostic errors, according to a study by national medical liability insurer The Doctors Company.
The study analyzed 332 emergency medicine claims in the insurer’s database that were closed from 2007 to 2013. The findings show the importance of strong communication in the emergency department (ED), and how communication breakdowns can be catalysts for legal claims, Darrell Ranum, vice president for patient safety and risk management for The Doctors Company, said.
Mr. Ranum pointed out one claim in which a patient’s vital signs had changed during the course of a visit. The changes were not communicated to the emergency physician, and he inappropriately discharged the patient. In another case, a patient’s history of overdoses was not conveyed during a patient hand-off, which resulted in an overdose that was not treated in a timely manner.
“Sometimes, we find that patients don’t always understand instructions,” Mr. Ranum said. “If patients don’t understand the communication from their providers or if a patient receives inadequate information, such as in situations where you have a language barrier, you can have problems there as well.”
Another key lesson from the study is the importance of a thorough differential diagnosis, Dr David B. Troxel, medical director for The Doctors Company said in an interview.
Of the 332 claims, 57% were diagnosis related; they included allegations of failure to establish a differential diagnosis, failure to order diagnostic tests, failure to address abnormal findings, and failure to consider available clinical information.
View on the News Workflow bottlenecks contribute Dr Roneet Lev is director of ED operations for Scripps Mercy Hospital in San Diego. She made her remarks in an interview. |
“Our hope is that as physicians review the findings of this study, they will scrutinize their own systems and processes and determine whether the weaknesses identified in the study exist in their organization,” Dr Troxel said.
Among the other claims, 13% related to improper management or treatment, 5% claimed improper performance of treatment, and 3% alleged failure to order medication.
Claims could have more than one contributing factor. Problems with patient assessments, such as failure to address abnormal findings, contributed to 52% of claims. Patient factors, such as physical characteristics and noncompliance, were the second-most-frequent contributor at 21%. Other factors were lack of communication among physicians (17%), poor communication between doctors and patients (14%), insufficient or lack of documentation (13%), and workflow/workload issues (12%).
On Twitter @legal_med
ED-initiated buprenorphine ups treatment rates for opioid addiction
BY BIANCA NOGRADY
FROM JAMA
Vitals Key clinical point: Emergency department–initiated buprenorphine treatment increases the likelihood that opioid-dependent patients will seek addiction treatment. Major finding: Significantly more patients in the buprenorphine group sought addiction treatment in the 30 days after randomization, compared to the referral or intervention arms. Data source: Randomized controlled trial of 104 opioid-dependent patients. Disclosures: The National Institute on Drug Abuse funded the study. Reckitt-Benckiser Pharmaceuticals provided the drug. One author reported honoraria from private industry. |
Initiating buprenorphine treatment during an emergency department visit can significantly increase the likelihood that opioid-dependent patients will seek addiction treatment, according to results of a randomized clinical trial published in April 28 in JAMA.
Researchers randomized 104 opioid-dependent patients who had presented to the ED to screening and treatment referral, screening and a brief intervention with referral to a community-based treatment service, or screening with a brief intervention and ED-initiated treatment with buprenorphine/naloxone and referral to primary care.
Significantly more patients in the buprenorphine group sought addiction treatment in the 30 days after randomization compared to the referral or intervention arms (78% vs. 37% vs. 45%, P < .001), and they showed significant reductions in the number of days of illicit opioid use per week.
“Expanded use of ED-initiated buprenorphine with community follow-up should help increase access to treatment options for this chronic and relapsing medical condition that has substantial morbidity and mortality and that affects health care use and costs,” wrote Dr Gail D’Onofrio of Yale School of Medicine, New Haven, Ct., and coauthors (JAMA 2015; 313:1636-1644 [doi:10.1001/jama.2015.3474]).
More than 75% with sickle cell crises don’t get hydroxyurea
BY MARY ANN MOON
FROM JAMA
Vitals Key clinical point: More than 75% of adults with sickle cell anemia who have frequent pain crises fail to get hydroxyurea therapy as recommended. Major finding: Only 15.1% of adults with sickle cell anemia received hydroxyurea within 3 months of their third pain crisis, only 18.2% received the agent within 6 months, and only 22.7% received it within 12 months. Data source: An analysis of information in a large nationwide insurance claims database involving 570 adults with frequent hospitalizations for sickle cell pain crises. Disclosures: This study was funded by the Lewin Group, a health care consulting firm. Dr Stettler and his associates reported having no relevant financial disclosures. |
More than 75% of adults with sickle cell anemia who have frequent pain crises fail to receive hydroxyurea therapy as strongly recommended in National Heart, Lung, and Blood Institute clinical guidelines, according to a Research Letter to the Editor published online April 28 in JAMA.
Despite proven benefits in decreasing pain crises, hospitalizations, blood transfusions, and possibly mortality, hydroxyurea, a “safe and inexpensive drug,” is thought to be underused. To document the actual use of the drug when indicated, investigators analyzed information in a nationwide insurance claims database covering nearly 27 million patients per year. They focused on the records of 570 adults hospitalized or treated in an emergency department for a sickle cell pain crisis at least three times during a 1-year period, said Dr Nicolas Stettler of the Lewin Group, a health care consulting firm in Falls Church, Va., and his associates.
Only 15.1% of these patients received hydroxyurea within 3 months of their third crisis, only 18.2% received the agent within 6 months, and only 22.7% received it within 12 months. These figures likely represent a conservative estimate of the hydroxyurea treatment gap, since the study didn’t include the large uninsured and publicly insured populations who have more limited access to health care, Dr Stettler and his associates noted (JAMA 2015;313:1671-2).
Several barriers to this treatment have been identified in previous research, including fear of adverse events, lack of clinician training, and failure to use shared decision making. “To address this gap, it may be necessary to enhance patient outreach and clinician training and develop health care quality measures aimed at increasing the use of hydroxyurea for all patients who would benefit,” they added.
Recognizing human trafficking victims
BY ALICIA GALLEGOS
AT THE AMWA ANNUAL MEETING
CHICAGO – Emergency physicians encounter nearly 40% of human trafficking victims who come in contact with health providers, according to Dr Holly G. Atkinson.
In a 2014 survey of domestic sex-trafficking victims, 88% said they encountered one or more health professions during the period in which they were being trafficked, yet none was identified as a victim by physicians during the visits. In another 2014 survey of survivors, 39% of victims reported having contact with emergency departments; 29%, with primary care physicians; 17%, with ob.gyns.; 17%, with dentists; and 3%, with pediatricians.
Key indicators for potential victimization include discrepancies between history and clinical presentation, multiple sexually transmitted diseases, and the accompaniment of a controlling third-party who is not a guardian, said Dr Atkinson, director of the human rights program at Arnhold Global Health Institute at the Icahn School of Medicine at Mount Sinai in New York City, said at the annual meeting of the American Medical Women’s Association.
In 2014, the National Human Trafficking Resource Center, operated by the antislavery organization Polaris, received 3,598 reports of sex-trafficking cases inside the United States. The National Center for Missing & Exploited Children estimates that between 100,000 and 300,000 U.S. children are at risk of being sexually trafficked each year.
Physical signs that could denote the possibility of patients being trafficked include visible tattoos with “daddy,” “property of,” or a trafficker’s street name. Perpetrators often brand their victims so that they are easily recognizable and can be returned if they escape, Dr Atkinson explained.
Dehydration, malnutrition, multiple sexually transmitted infections, and multiple pregnancies or abortions could also be clues. The inability to provide an address, confusion about their current location, an appearance younger than the stated age, avoidance of eye contact, and answers that sound scripted are potential signs of human trafficking, as is the presence of a controlling third party who does not let the patient answer questions or who interrupts or corrects the patient.
The Via Christi Health system in Wichita, Kan., recently published guidance for clinicians on how to proceed if they suspect a patient is a victim of human trafficking. Steps include following child abuse or domestic violence protocols; separating the patient from the controlling third party; providing the patient a comfortable, safe area; and ensuring a patient interview is performed by a trauma-informed social worker or nurse.
Some questions physicians may want to ask patients include: Have you ever exchanged sex for money, food, or shelter? Have you been forced to have sex against your will? Have you been asked to have sex with multiple partners? If the patient answers yes, physicians should follow child abuse protocols and mandatory reporting requirements. If the patient is aged 18 or older, doctors should obtain the patient’s permission to call law enforcement or assist the patient in calling 911.
AMWA recently launched Physicians Against the Trafficking of Humans (PATH). The PATH website includes resources for physicians and an online video about trafficking that doctors can share with their practices and colleagues.
On Twitter @legal_med
Indiana HIV outbreak prompts national advisory
BY SHARON WORCESTER
FRONTLINE MEDICAL NEWS
As health officials continue to battle an intravenous drug use–related HIV outbreak in a rural county in Indiana, the Centers for Disease Control and Prevention has issued an official health advisory stressing the need for vigilance at the state and local levels with respect to detecting and controlling similar outbreaks across the United States.
The Indiana outbreak began in November, with 11 new HIV infections diagnosed by January in Scott County, where fewer than 5 infections per year had been identified previously; since January, an unprecedented 135 infections have been confirmed, and 6 others are under investigation, according to Dr Jerome M. Adams, Indiana State Health Commissioner.
“To put this in further perspective, from 2009 through 2013, the county only reported three new cases of HIV,” Dr Adams said in a joint Indiana State Department of Health/CDC teleconference.
The affected community includes only 4,200 people, and 84% of those diagnosed with HIV also tested positive for hepatitis C virus (HCV) infection, Dr Joan Duwve, chief medical consultant, Indiana State Department of Health, said during the teleconference.
According to the CDC advisory, 96% of 108 infected individuals who were interviewed reported injecting dissolved prescription-type oxymorphone, as well as sharing syringes.
“The United States is facing an epidemic of prescription opioid abuse that must be addressed. Opioid poisoning deaths have nearly quadrupled from 1999 through 2011. This epidemic of opioid abuse has already contributed to a severe and growing epidemic of viral hepatitis among people who inject drugs,” said Dr Jonathan Mermin, director of the National Center for HIV/AIDS Viral Hepatitis, STD, and TB Prevention, who also participated in the teleconference.
New CDC data on HCV show a 150% increase in reports of acute HCV infections nationwide between 2010 to 2013.
“The majority of these infections are believed to be attributable to injection drug use, so we must act now to reverse this trend and to prevent this from undoing progress in HIV prevention to date,” he said.
Among the recommendations were routine opt-out of HIV testing as well as HCV testing per current recommendations. Further, health care professionals should report suspected clusters to their local or state health department, ensure that HCV-infected patients are also tested for HIV and vice versa, and ensure that those receiving treatment for either HIV or HCV are adhering to prescribed therapy and are engaged in ongoing care.
Syringe-sharing and sexual partners of those diagnosed with HIV or HCV should be encouraged to undergo testing, and all persons with substance abuse problems should be referred for medication-assisted treatment and counseling. In Indiana, where a public health emergency was declared on March 26 and has been extended until May 24, care includes HIV and HCV treatment, as well as substance abuse counseling and treatment, according to a “Notes From the Field Report” in the Morbidity and Mortality Weekly Report (MMWR 2015 April 24;64:1-2).
sworcester@frontlinemedcom.com
Most accidental genital trauma cases manageable in ED
BY SHARON WORCESTER
AT THE NASPAG ANNUAL MEETING
Vitals Key clinical point: With adequate sedation, most girls with AGT requiring treatment can undergo evaluation and repair in the ED. Major finding: 82% of cases reviewed were minor and managed expectantly. Data source: A retrospective medical records review of 359 cases of accidental genital trauma. Disclosures: Dr Dowlut-McElroy reported having no disclosures. One of her coauthors, Dr Julie Strickland, is a Nexplanon trainer for Merck. |
ORLANDO – Most cases of accidental genital trauma in girls are caused by straddle injuries and are isolated to the labia, and most can be managed expectantly or treated in the emergency department, according to findings from a retrospective cohort study.
Penetrating injuries, however, should be considered an indication for management in the operating room, Dr Tazim Dowlut-McElroy reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
A review of 359 cases showed that the vast majority – 82% – were minor and managed expectantly.
“Only 18% required surgical management. Of those, 37% required general anesthesia in the OR, but 63% were adequately evaluated and treated in the ED,” said Dr Dowlut-McElroy of Children’s Mercy Hospitals and Clinics, Kansas City.
About 64% of the patients presented during the day, and 36% presented at night. The most common presenting complaint was bleeding and pain (89% of cases), followed by voiding issues in 8% of cases. No presenting complaint was recorded in the remaining patient charts.
The most common mechanism of injury was straddle injury (73% of cases), followed by non-straddle blunt trauma in 15% of cases, and penetrating injury in the remaining cases, Dr Dowlut-McElroy said.
Injuries included lacerations in 86% of cases, abrasions or contusions in 7%, and hematomas in 3%.
Pediatric genital injuries comprised 0.2%-8% of reported childhood trauma, and despite public health efforts to reduce injuries, the number of such pediatric injuries continues to rise, Dr Dowlut-McElroy said.
Patients included in the current review were identified by a medical database query from January 2000 to July 2014. They were aged 0-18 years and had been treated in the ED for genital trauma; those with obstetrical injuries were excluded.
sworcester@frontlinemedcom.com
EDs pump up pediatric preparedness
BY MARY ANN MOON
FROM JAMA PEDIATRICS
Vitals Key clinical point: Nationally, emergency departments have improved their compliance with guidelines regarding emergency health care for children. Major finding: The overall median weighted pediatric readiness score (WPRS) was 68.9, a solid improvement over the previously reported median WPRS of 55. Data source: A web-based self-reported assessment of pediatric readiness at 4,149 EDs across the country that have approximately 24 million pediatric visits annually. Disclosures: This project was supported by the Emergency Medical Services for Children network and the EMS for Children National Resource Center under the U.S. Department of Health & Human Services. Dr Gausche-Hill and her associates reported having no financial disclosures. |
Pediatric readiness has improved in emergency departments across the country, as measured by EDs’ compliance with 2009 guidelines for emergency care in children, according to a report published online April 13 in JAMA Pediatrics.
In what they described as “the most comprehensive evaluation of pediatric readiness of our nation’s EDs to date,” investigators performed a web-based assessment of 4,137 hospitals’ self-reported compliance with guidelines addressing child-specific emergency care. The overall median weighted pediatric readiness score (WPRS) was 68.9, a solid improvement over the previously reported median WPRS of 55, said Dr Marianne Gausche-Hill of Harbor-UCLA Medical Center, Torrance, Calif., and her associates.
The single most important factor found to enhance pediatric readiness was to designate two people, one of whom is a physician or nurse, as the hospital’s pediatric emergency care coordinator, as recommended by the Institute of Medicine, the researchers wrote. Nearly half (47.5%) of EDs now have a physician coordinator, and 59.3% now have a nurse coordinator, compared with 18% and 12%, respectively, in a previous report. The presence of coordinators quadrupled the chances that a hospital would put important quality-improvement plans in place to address children’s care needs, the investigators said (JAMA Pediatr. 2015 April 13 [doi:10.1001/jamapediatrics.2015.138]).
Other findings included the following:
- Most physicians providing emergency care for children were specifically trained in emergency medicine (88.6%) or pediatric emergency medicine (55.4%) at high-volume hospitals; low-volume hospitals were more likely to have family-medicine-trained physicians doing so (78.9%).
- Mandatory competency evaluations for providing pediatric emergency care were relatively common for nurses (66.6%) but less so for physicians (38.7%) and midlevel staff (18.1%).
- 99.5% of EDs said all staff are trained on the location of pediatric equipment in the ED, including tools to ensure correct sizing of resuscitation equipment and appropriate dosing of medications.
- 83.1% of EDs said they verified the proper location and function of pediatric equipment daily.
- EDs routinely stocked 91% of recommended pediatric equipment. Equipment that was missing in more than 15% of EDs included laryngeal mask airways, umbilical vein catheters, central venous catheters, tracheostomy tubes, size 00 laryngoscope blades, continuous end-tidal carbon dioxide monitoring equipment, pediatric Magill forceps, and infant and child nasopharyngeal airways.
- Only 46.8% of EDs had disaster plans that specifically addressed children, as recommended. Even among high-volume hospitals, which were the most compliant with guidelines, only 67.4% had such disaster plans.
- Approximately one-third of EDs said their providers failed to weigh children and record the weight in kilograms only, as recommended. This safety measure is crucial to preventing drug-dosing errors.
- The most frequently cited barriers to complying with guidelines were the cost of child-specific training (reported by 54.4% of EDs) and a lack of educational resources (reported by 49.0%). Few EDs reported that a lack of interest was a barrier to implementing the guidelines.
Simplified PESI identified low-risk pulmonary embolism
BY AMY KARON
FROM ACADEMIC EMERGENCY MEDICINE
Vitals Key clinical point: The simplified version of the PESI identified low-risk pulmonary embolism patients. Major finding: Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days, regardless of which treatment they received. Data source: Post hoc analysis of simplified PESI scores and outcomes among 4,831 patients with acute pulmonary embolism who received either rivaroxaban or an enoxaparin–vitamin K antagonist combination. Disclosures:Bayer HealthCare Pharmaceuticals and Janssen Research & Development funded the study. Dr Fermann reported an advisory relationship with Janssen and research funding from Cardiorentis, Trevena, Novartis, Siemens, and Pfizer. Two coauthors reported employment with Bayer, and two other coauthors reported relationships with several other pharmaceutical companies. |
A simplified version of the Pulmonary Embolism Severity Index identified patients with acute pulmonary embolism who were at low risk of adverse events and might be suitable for outpatient care.
“Although guidelines, such as those from the American College of Chest Physicians, recommend outpatient treatment for selected PE patients at low risk of recurrence, existing evidence for the outpatient management of patients with PE is derived from small cohorts of patients from outside the United States,” said Dr Gregory J. Fermann of the University of Cincinnati department of emergency medicine and his associates. “Risk stratification of PE patients may allow a cohort of low-risk patients to be treated in a clinical decision unit or by a closely monitored outpatient strategy. Such an approach might relieve some of the burden placed on the emergency department,” they wrote (Acad. Emerg. Med. 2015;22:299-307).
The simplified PESI includes 6 of the 11 variables measured in PESI, which has been shown to identify patients at increased risk of death and adverse outcome events after acute PE. The six features of the simplified PESI are age greater than 80 years, history of cancer, history of chronic cardiopulmonary disease, heart rate of at least 110 beats per minutes, systolic blood pressure less than 100 mm Hg, and oxygen saturation less than 90%. Each factor is assigned a score of 1.
They carried out a post hoc analysis of simplified PESI scores and outcomes among 4,831 acute PE patients from the EINSTEIN-PE study of rivaroxaban and an enoxaparin–vitamin K antagonist combination (N. Engl. J. Med. 2012;366:1287-97).
Roughly half (53.6%) of the patients had a score of 0, one-third (36.7%) had a score of 1, and 9.7% had a score of 2 or 3, the researchers reported. Higher simplified PESI scores were associated with increased risk of almost all adverse outcomes measured, including recurrent VTE, fatal PE, all-cause mortality, and major bleeding. Patients with scores of 0 or 1 had low rates of major adverse events during the first 30 days of treatment, regardless of which protocol they received. Scores of 2 or 3 were associated with greater risk of recurrent VTE, fatal PE, all-cause mortality, and major bleeding in both treatment groups.
Liraglutide for obesity: New indication
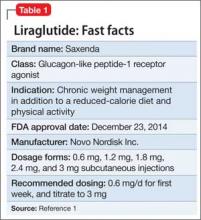
Liraglutide (rDNA origin) injection, approved by the FDA in 2010 for managing type 2 diabetes mellitus (T2DM), has a new formulation and indication for treating obesity in adults as an adjunct to a reduced-calorie diet and increased physical activity (Table 1).1
Liraglutide, recommended dosage 3 mg/d (under the brand name Saxenda), is approved for adults with a body mass index (BMI) ≥30, or those with a BMI of ≥27 and a weight-related condition such as hypertension, T2DM, or high cholesterol.1 (A 1.8-mg formulation, under the brand name Victoza, is FDA-approved for managing T2DM, but is not indicated for weight management.)
How it works
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1, which regulates appetite and calorie intake, is found in several regions of the brain that are involved in regulating appetite. Patients taking liraglutide lose weight because of decreased calorie intake, not increased energy expenditure.
Liraglutide is endogenously metabolized without a specific organ as a major route of elimination.1
Dosage and administration
Liraglutide is administered using a prefilled, multi-dose pen that can be injected in the abdomen, thigh, or upper arm. Recommended dosage is 3 mg/d, administered any time of day. Initiate dosage at 0.6 mg/d the first week, then titrate by 0.6 mg a week—to reduce the likelihood of adverse gastrointestinal symptoms—until 3 mg/d is reached.
Discontinue liraglutide if a patient has not lost at least 4% of body weight after 16 weeks of treatment, because it is unlikely the patient will achieve and sustain weight loss.
Efficacy
Liraglutide was studied in 3 clinical trials of obese and overweight participants who had a weight-related condition. Patients who had a history of major depressive disorder or suicide attempt were excluded from the studies. All participants in Studies 1 and 2 received instruction about following a reduced-calorie diet and increasing physical activity. In Study 3, patients were randomized to treatment after losing >5% of their body weight through reduced calorie intake and exercise; those who did not meet the required weight loss were excluded from the study. In these 56-week clinical studies:
• of 3,731 participants without diabetes or a weight-related comorbidity, such as high blood pressure or high cholesterol, 62% of patients (n = 2,313) who took liraglutide lost ≥5% of their body weight from baseline, compared with 34% of participants who received placebo
• of 635 participants with T2DM, 49% of patients (n = 311) treated with liraglutide lost ≥5% of their body weight compared with 16% placebo patients
• of 422 participants with a weight-related comorbidity, 42% of patients (n = 177) lost ≥5% of their body weight compared with 21.7% of placebo patients.
Improvements in some cardiovascular disease risk factors were observed. Long-term follow up was not studied.
Contraindictations
Liraglutide is contraindicated in patients who have a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. In a 104-week study, malignant thyroid C-cell carcinomas were detected in rats and mice given liraglutide, 1 and 3 mg/kg/d; however, it was not detected in groups given 0.03 and 0.2 mg/kg/d. It isn’t known whether liraglutide can cause thyroid C-cell tumors in humans.
Patients should not take liraglutide if they have hypersensitivity to liraglutide or any product components, are using insulin, are taking any other GLP-1 receptor agonist, or are pregnant.
Adverse effects
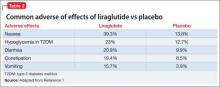
The most common reported adverse effects are nausea (39.3%), hypoglycemia in patients with T2DM (23%), diarrhea (20.9%), constipation (19.4%), and vomiting (15.7%) (Table 2). In clinical trials, 9.8% of patients discontinued treatment because of adverse effects, compared with 4.3% of those receiving placebo.
Liraglutide has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 and plasma protein binding. For a full list of drug-drug interactions, see the full prescribing information.1
Reference
1. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk A/S; 2015.
Liraglutide (rDNA origin) injection, approved by the FDA in 2010 for managing type 2 diabetes mellitus (T2DM), has a new formulation and indication for treating obesity in adults as an adjunct to a reduced-calorie diet and increased physical activity (Table 1).1
Liraglutide, recommended dosage 3 mg/d (under the brand name Saxenda), is approved for adults with a body mass index (BMI) ≥30, or those with a BMI of ≥27 and a weight-related condition such as hypertension, T2DM, or high cholesterol.1 (A 1.8-mg formulation, under the brand name Victoza, is FDA-approved for managing T2DM, but is not indicated for weight management.)
How it works
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1, which regulates appetite and calorie intake, is found in several regions of the brain that are involved in regulating appetite. Patients taking liraglutide lose weight because of decreased calorie intake, not increased energy expenditure.
Liraglutide is endogenously metabolized without a specific organ as a major route of elimination.1
Dosage and administration
Liraglutide is administered using a prefilled, multi-dose pen that can be injected in the abdomen, thigh, or upper arm. Recommended dosage is 3 mg/d, administered any time of day. Initiate dosage at 0.6 mg/d the first week, then titrate by 0.6 mg a week—to reduce the likelihood of adverse gastrointestinal symptoms—until 3 mg/d is reached.
Discontinue liraglutide if a patient has not lost at least 4% of body weight after 16 weeks of treatment, because it is unlikely the patient will achieve and sustain weight loss.
Efficacy
Liraglutide was studied in 3 clinical trials of obese and overweight participants who had a weight-related condition. Patients who had a history of major depressive disorder or suicide attempt were excluded from the studies. All participants in Studies 1 and 2 received instruction about following a reduced-calorie diet and increasing physical activity. In Study 3, patients were randomized to treatment after losing >5% of their body weight through reduced calorie intake and exercise; those who did not meet the required weight loss were excluded from the study. In these 56-week clinical studies:
• of 3,731 participants without diabetes or a weight-related comorbidity, such as high blood pressure or high cholesterol, 62% of patients (n = 2,313) who took liraglutide lost ≥5% of their body weight from baseline, compared with 34% of participants who received placebo
• of 635 participants with T2DM, 49% of patients (n = 311) treated with liraglutide lost ≥5% of their body weight compared with 16% placebo patients
• of 422 participants with a weight-related comorbidity, 42% of patients (n = 177) lost ≥5% of their body weight compared with 21.7% of placebo patients.
Improvements in some cardiovascular disease risk factors were observed. Long-term follow up was not studied.
Contraindictations
Liraglutide is contraindicated in patients who have a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. In a 104-week study, malignant thyroid C-cell carcinomas were detected in rats and mice given liraglutide, 1 and 3 mg/kg/d; however, it was not detected in groups given 0.03 and 0.2 mg/kg/d. It isn’t known whether liraglutide can cause thyroid C-cell tumors in humans.
Patients should not take liraglutide if they have hypersensitivity to liraglutide or any product components, are using insulin, are taking any other GLP-1 receptor agonist, or are pregnant.
Adverse effects
The most common reported adverse effects are nausea (39.3%), hypoglycemia in patients with T2DM (23%), diarrhea (20.9%), constipation (19.4%), and vomiting (15.7%) (Table 2). In clinical trials, 9.8% of patients discontinued treatment because of adverse effects, compared with 4.3% of those receiving placebo.
Liraglutide has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 and plasma protein binding. For a full list of drug-drug interactions, see the full prescribing information.1
Liraglutide (rDNA origin) injection, approved by the FDA in 2010 for managing type 2 diabetes mellitus (T2DM), has a new formulation and indication for treating obesity in adults as an adjunct to a reduced-calorie diet and increased physical activity (Table 1).1
Liraglutide, recommended dosage 3 mg/d (under the brand name Saxenda), is approved for adults with a body mass index (BMI) ≥30, or those with a BMI of ≥27 and a weight-related condition such as hypertension, T2DM, or high cholesterol.1 (A 1.8-mg formulation, under the brand name Victoza, is FDA-approved for managing T2DM, but is not indicated for weight management.)
How it works
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1, which regulates appetite and calorie intake, is found in several regions of the brain that are involved in regulating appetite. Patients taking liraglutide lose weight because of decreased calorie intake, not increased energy expenditure.
Liraglutide is endogenously metabolized without a specific organ as a major route of elimination.1
Dosage and administration
Liraglutide is administered using a prefilled, multi-dose pen that can be injected in the abdomen, thigh, or upper arm. Recommended dosage is 3 mg/d, administered any time of day. Initiate dosage at 0.6 mg/d the first week, then titrate by 0.6 mg a week—to reduce the likelihood of adverse gastrointestinal symptoms—until 3 mg/d is reached.
Discontinue liraglutide if a patient has not lost at least 4% of body weight after 16 weeks of treatment, because it is unlikely the patient will achieve and sustain weight loss.
Efficacy
Liraglutide was studied in 3 clinical trials of obese and overweight participants who had a weight-related condition. Patients who had a history of major depressive disorder or suicide attempt were excluded from the studies. All participants in Studies 1 and 2 received instruction about following a reduced-calorie diet and increasing physical activity. In Study 3, patients were randomized to treatment after losing >5% of their body weight through reduced calorie intake and exercise; those who did not meet the required weight loss were excluded from the study. In these 56-week clinical studies:
• of 3,731 participants without diabetes or a weight-related comorbidity, such as high blood pressure or high cholesterol, 62% of patients (n = 2,313) who took liraglutide lost ≥5% of their body weight from baseline, compared with 34% of participants who received placebo
• of 635 participants with T2DM, 49% of patients (n = 311) treated with liraglutide lost ≥5% of their body weight compared with 16% placebo patients
• of 422 participants with a weight-related comorbidity, 42% of patients (n = 177) lost ≥5% of their body weight compared with 21.7% of placebo patients.
Improvements in some cardiovascular disease risk factors were observed. Long-term follow up was not studied.
Contraindictations
Liraglutide is contraindicated in patients who have a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. In a 104-week study, malignant thyroid C-cell carcinomas were detected in rats and mice given liraglutide, 1 and 3 mg/kg/d; however, it was not detected in groups given 0.03 and 0.2 mg/kg/d. It isn’t known whether liraglutide can cause thyroid C-cell tumors in humans.
Patients should not take liraglutide if they have hypersensitivity to liraglutide or any product components, are using insulin, are taking any other GLP-1 receptor agonist, or are pregnant.
Adverse effects
The most common reported adverse effects are nausea (39.3%), hypoglycemia in patients with T2DM (23%), diarrhea (20.9%), constipation (19.4%), and vomiting (15.7%) (Table 2). In clinical trials, 9.8% of patients discontinued treatment because of adverse effects, compared with 4.3% of those receiving placebo.
Liraglutide has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 and plasma protein binding. For a full list of drug-drug interactions, see the full prescribing information.1
Reference
1. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk A/S; 2015.
Reference
1. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk A/S; 2015.
First EDition: News for and about the practice of Emergency Medicine
Drug-resistant shigellosis outbreak
BY DEEPAK CHITNIS
FROM MMWR
The United States is currently experiencing an outbreak of shigellosis caused by a strain of the Shigella sonnei bacteria that is resistant to ciprofloxacin, the most commonly prescribed antimicrobial treatment for shigellosis.
In its Morbidity and Mortality Weekly Report, the CDC revealed that 243 individuals in 32 states and the territory of Puerto Rico have come down with shigellosis between May 2014 and February 2015. Of those 243 cases, 126 isolates were tested and 109 (87%) of those were found to be nonsusceptible to ciprofloxacin. The largest clusters of the disease were found in Massachusetts (45 cases), California (25 cases), and Pennsylvania (18 cases).
Ninety-five of the cases associated with the current outbreak were traced back to the homeless population of San Francisco; about half of the remaining cases were attributed to international travelers – specifically, those visiting the Dominican Republic and India – who contracted the bacteria while abroad and unknowingly brought it to the United States. The disease is known to spread quickly in populations of children who attend child care facilities, homeless individuals, and men who have sex with men.
“These outbreaks show a troubling trend in Shigella infections in the United States,” Dr. Thomas Frieden, CDC director, said in a statement. “Drug-resistant infections are harder to treat and because Shigella spreads so easily between people, the potential for more – and larger – outbreaks is a real concern. We’re moving quickly to implement a national strategy to curb antibiotic resistance because we can’t take for granted that we’ll always have the drugs we need to fight common infections.”
Shigellosis causes an estimated 500,000 cases of diarrhea in the United States each year. To help curb the growing number of shigellosis cases, the CDC recommends that international travelers wash their hands meticulously while abroad, and follow strict dietary precautions, such as eating hot foods and drinking beverages only from sealed containers, especially when consuming water.
Otherwise healthy patients over 80 benefit from aggressive treatment for MI
BY PATRICE WENDLING
AT ACC/CRF I2 SUMMIT
Vitals Key clinical point: An early invasive treatment strategy improved most outcomes in patients aged 80 years and older with acute coronary syndromes. Major finding: Myocardial infarction, need for urgent revascularization, stroke, and death were significantly lower with invasive vs. conservative care (41% vs. 61%; risk ratio, 0.48; P < .00001). Data source: Randomized study in 457 patients aged 80 years or older with non-STEMI or unstable angina. Disclosures: The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandzari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic. |
SAN DIEGO – Patients aged 80 years and older benefit from more invasive early treatment after non-ST-elevation myocardial infarction or unstable angina, the After Eighty Trial showed.
After a median follow-up of 1.5 years, an invasive strategy that included coronary angiography significantly reduced the primary endpoint of myocardial infarction (MI), need for urgent revascularization, stroke, and death from 61% with optimal medical treatment to 41%.
That drop was driven primarily by significantly fewer MIs (17% vs. 30%) and urgent revascularizations (2% vs. 11%), lead author Dr. Nicolai Tegn reported at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
There were no significant differences between the invasive and conservative strategy groups in rates of stroke or all-cause death.
The composite of death and MI, however, significantly favored the invasive group (35% vs. 48%), he reported.
After Eighty randomly assigned 457 patients, aged 80 years or older, to either optimal medical therapy with no invasive treatments or coronary angiography at a percutaneous coronary intervention (PCI) center the day after inclusion, plus optimal medical therapy after about 4-5 hours if PCI was not performed or about 6-18 hours if it was. Of the 225 patients receiving angiography, 48% went on to balloon angioplasty and/or coronary stenting, and 3% had bypass surgery. The patients were selected from nearly 4,200 elderly patients at 17 community hospitals in Norway; over 3,700 of the patients were ineligible for the study because their life expectancy was less than 12 months because of a serious comorbidity; ongoing or recent bleeding; inability to comply with protocol; clinically unstable including ongoing ischemia; refusal to participate; logistic reasons; or other reasons.
After Eighty is a welcome study because of the under-representation of the elderly in clinical trials, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said during a press briefing at the meeting. But it raises the challenge of identifying patients in clinical practice with the same qualifying characteristics, he added, given that the study population represents only 10% of the entire screened population, “The coronary anatomy does not know the age of the patient, meaning that the findings of a benefit of an early invasive strategy seem consistent with previous studies we know across the management of patients with acute coronary syndromes,” Dr. Kandzari said.
FDA approves Anthrasil to treat inhalational anthrax
BY DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
The Food and Drug Administration has approved Anthrasil, Anthrax Immune Globulin Intravenous (Human), for treatment of inhalational anthrax when used with appropriate antibacterial drugs.
Inhalational anthrax is caused by breathing in Bacillus anthracis spores, which can occur after exposure to infected animals or contaminated animal products, or as a result of an intentional release of spores. In a statement, Dr. Karen Midthun – director of the FDA’s Center for Biologics Evaluation and Research – explained that Anthrasil “will be stored in U.S. Strategic National Stockpile to facilitate its availability in response to an anthrax emergency.”
Anthrasil was purchased by the U.S. Department of Health & Human Services’ Biomedical Advanced Research and Development Authority (BARDA) in 2011, but because it was not approved, its use prior to FDA approval would have required an emergency use authorization from the FDA.
The efficacy of Anthrasil was studied in animals because it was not feasible or ethical to conduct adequately controlled efficacy studies in humans, the FDA said. Monkeys and rabbits were exposed to Bacillus anthracis spores, and subsequently given either Anthrasil or a placebo. The survival rate for monkeys given Anthrasil was between 36% and 70%, with a trend toward increased survival at higher doses of Anthrasil. None of the monkeys given placebo survived. Rabbits had a 26% survival rate when given the drug, compared to 2% of those given placebo. A separate study exposed rabbits to Bacillus anthracis and treated them with either antibiotics or a combination of antibiotics and Anthrasil; survival rates were 71% for those treated with the combination and 25% for those treated with antibiotics only.
Safety was tested in 74 healthy human volunteers and the most commonly reported side effects were headache, back pain, nausea, and pain and swelling at the infusion site.
Anthrasil is manufactured by Cangene Corporation, based in Winnipeg, Canada, which developed the drug in collaboration with BARDA.
FDA: Amiodarone plus some hepatitis C antivirals may result in bradycardia
BY ELIZABETH MECHCATIE
Frontline Medical News
Taking the antiarrythmic drug amiodarone with the hepatitis C antiviral drugs ledipasvir and sofosbuvir, or with sofosbuvir plus another direct-acting antiviral drug, has been associated with cases of symptomatic bradycardia – including a fatal cardiac arrest – according to the Food and Drug Administration.
Because of the reports, the antiviral drugs’ labels now recommend against using amiodarone with those hepatitis C drugs.
An FDA statement described the bradycardia cases as “serious and life-threatening.” Gilead Sciences markets the ledipasvir and sofosbuvir combination as Harvoni and markets sofosbuvir as Sovaldi to treat chronic hepatitis C virus (HCV) infection.
Gilead issued a “Dear Health Care Provider” letter that provides further details of the cases. There have been nine postmarketing reports of symptomatic bradycardia in patients who were taking amiodarone with Harvoni; amiodarone with Sovaldi plus another hepatitis C antiviral drug, simeprevir (Olysio); or amiodarone with an investigational hepatitis C antiviral drug, daclatasvir.
Of those cases, six occurred with in the first 24 hours of starting treatment with the antivirals, and three cases occurred within the first 2-12 days after antiviral therapy was started. A pacemaker was needed in three cases, and one case was a fatal cardiac arrest.
In three cases, a “rechallenge with HCV treatment in the setting of continued amiodarone therapy resulted in recurrence of symptomatic bradycardia,” according to the Gilead letter.
The effect of coadministration on the blood levels of the antiviral drugs is not known, nor is the mechanism behind the cardiac effect.
emechcatie@frontlinemedcom.com
Clindamycin, TMP-SMX are equally effective for skin infections
BY MARY ANN MOON
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Vitals Key clinical point: Clindamycin and TMP-SMX had similar efficacy and side-effect profiles for treating uncomplicated skin infections, including both abscesses and cellulitis. Major finding: At 7-10 days after completing therapy, the rates of cure in the evaluable population were 90% with clindamycin and 88% with TMP-SMX. Data source: A prospective, multicenter, randomized, double-blind clinical trial involving 524 adults and children followed for 1 month after treatment. Disclosures: This trial was supported by the National Institutes of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences (NCT00730028). Dr. Miller reported receiving consulting fees from Cubist, Durata, and Pfizer; his associates reported ties to Cubist, Pfizer, EMMES, Theravance, AstraZeneca, Trius, Merck, and Cerexa. |
Clindamycin and trimethoprim-sulfamethoxazole are similarly safe and effective for treating uncomplicated skin infections, including both cellulitis and abscesses, in ambulatory settings in regions where MRSA is endemic.
The data comparing these two agents in an ambulatory setting are limited, though both are commonly recommended as empirical therapy for skin infections in patients who present to clinics and emergency departments and have only minor or no coexisting conditions, said Dr. Loren G. Miller of the Los Angeles Biomedical Research Institute and the division of infectious diseases at Harbor-UCLA Medical Center, and his associates.
They performed a prospective double-blind randomized trial comparing clindamycin against TMP-SMX in 524 ethnically diverse adults and children who presented as outpatients with uncomplicated skin infections during a 2-year period in Chicago, San Francisco, Los Angeles, and Nashville – areas in which community-associated MRSA is endemic. The mean patient age was 27 years, and approximately 30% were pediatric patients. All the participants had cellulitis without abscesses (including erysipelas), one or more abscesses larger than 5 cm in diameter, or both conditions. A total of 264 were randomized to clindamycin and 260 to TMP-SMX daily for 10 days.
Cure rates did not differ significantly between the two study groups. At 7-10 days after completing therapy, the rates of cure in the intention-to-treat population were 80% for clindamycin and 78% for TMP-SMX, and in the evaluable population the rates were nearly 90% and 88%, respectively.
At 1 month follow-up, the cure rates in the evaluable population were 84% for clindamycin and 78% for TMP-SMX, the investigators said (N. Engl. J. Med. 2015;372:1093-103 [doi:10.1056/NEJMoa1403789]). Rates of adverse events were nearly identical between the two study groups at about 19%, and most were mild and resolved without sequelae. There were no treatment-associated serious adverse events, and the rates of treatment discontinuation were similar at nearly 9% between patients receiving clindamycin and those receiving TMP-SMX.
Stroke ambulances speed treatment to U.S. patients
BY MITCHEL L. ZOLER
AT THE INTERNATIONAL STROKE CONFERENCE
Vitals Key clinical point: Dedicated stroke ambulances that bring a CT scanner and thrombolytic treatment to patients in the field speed thrombolytic therapy. Major finding: In Cleveland, stroke patients received thrombolysis an average of 38 minutes sooner from the CT-equipped ambulance, compared with standard protocols. Data source: Prospectively collected data on time-to-treatment from case series in Houston and in Cleveland. Disclosures: Dr. Hussain and Ms. Parker had no disclosures. |
NASHVILLE, TENN. – Bringing a CT scanner and thrombolytic treatment directly to stroke patients in the field sped the time to thrombolysis, compared with waiting for the patient to arrive at the hospital.
Some U.S. stroke centers now send out a team that can immediately assess and start treating stroke patients in the community. In 2014, the first two U.S. mobile stroke-treatment units began operating, one in Houston and the second in Cleveland.
Initial reports show both programs were successful in cutting the time to deliver thrombolytic treatment with intravenous tissue plasminogen activator (TPA) to appropriate patients.
In Houston, the active phase of the program started in May 2014, and by October 2014, 47 acute ischemic stroke patients had been treated with TPA. The mobile-unit crews started 43% of eligible patients on thrombolysis within 60 minutes of their symptom onset and another 31% were treated starting 61-80 minutes after symptom onset, said Stephanie A. Parker at the International Stroke Conference.
The unit also treats patients diagnosed with hemorrhagic stroke with intravenous nicardipine for rapid blood pressure reduction, said Ms. Parker, a critical care and emergency medicine–trained registered nurse who is project manager for the Houston mobile unit.
The Cleveland program began in July 2014; of the first 100 stroke patients seen by the mobile unit 16 of 19 eligible patients received tPA, with an average time of 56 minutes from symptom onset to treatment. This compared with an average 94 minutes to tPA onset in patients brought conventionally last year to a Cleveland-area hospital, Dr. M. Shazam Hussain said in a report at the meeting, sponsored by the American Heart Association.
The clinical impact and cost effectiveness of the pilot programs using the mobile units have not yet been assessed from the data, Dr. Hussain and Ms. Parker emphasized. Funding for the Cleveland and Houston vehicles came from local donors; the Houston program also received equipment donations from manufacturers.
The two mobile units are standard 12-foot, box-shaped ambulances outfitted with a CT scanner, a point-of-care lab, and telemedicine components as well as more standard emergency-vehicle equipment. The Houston vehicle contains “all the diagnostic equipment that is in our emergency room,” Ms. Parker said.
The concept behind both the Cleveland unit, operated by the Cleveland Clinic, and the Houston unit, operated by the University of Texas, Houston, is that the mobile stroke unit arrives to a patient with a suspected stroke, the unit is stationary while a CT scan and other diagnostic tests are run, diagnosis occurs with telemedicine assistance. If the patient is cleared for TPA treatment, the infusion starts and the vehicle carries the patient to an appropriate stroke center.
Currently, the Houston unit goes out with a vascular neurologist and a telemedicine physician on board, but plans are in place to test the feasibility of relying entirely on telemedicine when making diagnostic and treatment decisions. The Cleveland mobile unit already operates in this fashion, with no physician on board, and was the first mobile stroke unit in the world to depend completely on telemedicine, according to Dr. Hussain, a neurologist and head of the stroke program at the Cleveland Clinic.
The world’s first mobile stroke unit began operating in Saarland, Germany, in 2008 (Lancet Neurology 2012;11:397-404), and a second unit began running in Berlin after that, Dr. Hussain noted. Because of limited funding, the service he directs in Cleveland has been operating from 8 a.m.-8 p.m., 7 days a week. The program plans to expand to 24-hour coverage. The Houston mobile unit operates 24/7; it averages two runs per day and administers TPA on 1 of every 10 runs, Ms. Parker said. Both the Houston and Cleveland units tie into the local 911 emergency activation systems for their respective regions.
Consider cephalosporin a safe alternative for patients with penicillin allergy
BY DEEPAK CHITNIS
AT THE 2015 AAAAI ANNUAL MEETING
Vitals Key clinical point: Given the low risk of adverse drug reactions with cephalosporins, patients with a history of penicillin allergy can safely take cephalosporins. Major finding: The most frequent ADRs in patients taking either oral or parenteral cephalosporins were Clostridium difficile infection within 90 days (0.91%), nephropathy within 30 days (0.15%), and all-cause death within 1 day (0.10%). Data source: Retrospective, population-based analysis of 949,323 Kaiser Permanente Southern California health plan members from 2010 to 2012. Disclosures: Dr. Macy disclosed receiving research support from ALK and BioMarin. |
HOUSTON – Given the low incidence of adverse drug reactions to cephalosporin antibiotics among nearly a million California health plan patients, patients with a history of penicillin allergy can safely be given cephalosporins, according to Dr. Eric M. Macy.
The recommendation is based on the findings of a retrospective, population-based analysis of the records of 949,323 Kaiser Permanente Southern California health plan members, which was presented by Dr. Macy, of the Kaiser Permanente Medical Center in San Diego, at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Dr. Macy and his colleagues examined the records of 622,456 health plan members who were given 901,908 regimens of oral cephalosporins and 326,867 members given 487,630 parenteral cephalosporin regimens between Jan. 1, 2010, and Dec. 31, 2012.
Clostridium difficile infection within 90 days, nephropathy within 30 days, and all-cause death within 1 day were the most common cephalosporin-associated adverse drug reactions (ADRs) reported by physicians, with rates of 0.91%, 0.15%, and 0.10%, respectively. Cephalosporin-associated anaphylaxis was documented by physicians a total of five times in oral patients and eight times in parenteral patients (P = .0761), while only three serious cutaneous adverse reactions (SCARs) were recorded. All of those SCARS were attributable to other antibiotics taken at the same time as the cephalosporins, according to Dr. Macy.
Patients who reported developing new cephalosporin allergies over the course of the study period were significantly more likely to be female than male: 0.56% vs. 0.43% (P < .0001). And patients with a history of penicillin allergy were more likely to report a new cephalosporin allergy within 30 days than patients with no drug allergy, another cephalosporin allergy, or a non–beta-lactam allergy.
Drug-resistant shigellosis outbreak
BY DEEPAK CHITNIS
FROM MMWR
The United States is currently experiencing an outbreak of shigellosis caused by a strain of the Shigella sonnei bacteria that is resistant to ciprofloxacin, the most commonly prescribed antimicrobial treatment for shigellosis.
In its Morbidity and Mortality Weekly Report, the CDC revealed that 243 individuals in 32 states and the territory of Puerto Rico have come down with shigellosis between May 2014 and February 2015. Of those 243 cases, 126 isolates were tested and 109 (87%) of those were found to be nonsusceptible to ciprofloxacin. The largest clusters of the disease were found in Massachusetts (45 cases), California (25 cases), and Pennsylvania (18 cases).
Ninety-five of the cases associated with the current outbreak were traced back to the homeless population of San Francisco; about half of the remaining cases were attributed to international travelers – specifically, those visiting the Dominican Republic and India – who contracted the bacteria while abroad and unknowingly brought it to the United States. The disease is known to spread quickly in populations of children who attend child care facilities, homeless individuals, and men who have sex with men.
“These outbreaks show a troubling trend in Shigella infections in the United States,” Dr. Thomas Frieden, CDC director, said in a statement. “Drug-resistant infections are harder to treat and because Shigella spreads so easily between people, the potential for more – and larger – outbreaks is a real concern. We’re moving quickly to implement a national strategy to curb antibiotic resistance because we can’t take for granted that we’ll always have the drugs we need to fight common infections.”
Shigellosis causes an estimated 500,000 cases of diarrhea in the United States each year. To help curb the growing number of shigellosis cases, the CDC recommends that international travelers wash their hands meticulously while abroad, and follow strict dietary precautions, such as eating hot foods and drinking beverages only from sealed containers, especially when consuming water.
Otherwise healthy patients over 80 benefit from aggressive treatment for MI
BY PATRICE WENDLING
AT ACC/CRF I2 SUMMIT
Vitals Key clinical point: An early invasive treatment strategy improved most outcomes in patients aged 80 years and older with acute coronary syndromes. Major finding: Myocardial infarction, need for urgent revascularization, stroke, and death were significantly lower with invasive vs. conservative care (41% vs. 61%; risk ratio, 0.48; P < .00001). Data source: Randomized study in 457 patients aged 80 years or older with non-STEMI or unstable angina. Disclosures: The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandzari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic. |
SAN DIEGO – Patients aged 80 years and older benefit from more invasive early treatment after non-ST-elevation myocardial infarction or unstable angina, the After Eighty Trial showed.
After a median follow-up of 1.5 years, an invasive strategy that included coronary angiography significantly reduced the primary endpoint of myocardial infarction (MI), need for urgent revascularization, stroke, and death from 61% with optimal medical treatment to 41%.
That drop was driven primarily by significantly fewer MIs (17% vs. 30%) and urgent revascularizations (2% vs. 11%), lead author Dr. Nicolai Tegn reported at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
There were no significant differences between the invasive and conservative strategy groups in rates of stroke or all-cause death.
The composite of death and MI, however, significantly favored the invasive group (35% vs. 48%), he reported.
After Eighty randomly assigned 457 patients, aged 80 years or older, to either optimal medical therapy with no invasive treatments or coronary angiography at a percutaneous coronary intervention (PCI) center the day after inclusion, plus optimal medical therapy after about 4-5 hours if PCI was not performed or about 6-18 hours if it was. Of the 225 patients receiving angiography, 48% went on to balloon angioplasty and/or coronary stenting, and 3% had bypass surgery. The patients were selected from nearly 4,200 elderly patients at 17 community hospitals in Norway; over 3,700 of the patients were ineligible for the study because their life expectancy was less than 12 months because of a serious comorbidity; ongoing or recent bleeding; inability to comply with protocol; clinically unstable including ongoing ischemia; refusal to participate; logistic reasons; or other reasons.
After Eighty is a welcome study because of the under-representation of the elderly in clinical trials, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said during a press briefing at the meeting. But it raises the challenge of identifying patients in clinical practice with the same qualifying characteristics, he added, given that the study population represents only 10% of the entire screened population, “The coronary anatomy does not know the age of the patient, meaning that the findings of a benefit of an early invasive strategy seem consistent with previous studies we know across the management of patients with acute coronary syndromes,” Dr. Kandzari said.
FDA approves Anthrasil to treat inhalational anthrax
BY DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
The Food and Drug Administration has approved Anthrasil, Anthrax Immune Globulin Intravenous (Human), for treatment of inhalational anthrax when used with appropriate antibacterial drugs.
Inhalational anthrax is caused by breathing in Bacillus anthracis spores, which can occur after exposure to infected animals or contaminated animal products, or as a result of an intentional release of spores. In a statement, Dr. Karen Midthun – director of the FDA’s Center for Biologics Evaluation and Research – explained that Anthrasil “will be stored in U.S. Strategic National Stockpile to facilitate its availability in response to an anthrax emergency.”
Anthrasil was purchased by the U.S. Department of Health & Human Services’ Biomedical Advanced Research and Development Authority (BARDA) in 2011, but because it was not approved, its use prior to FDA approval would have required an emergency use authorization from the FDA.
The efficacy of Anthrasil was studied in animals because it was not feasible or ethical to conduct adequately controlled efficacy studies in humans, the FDA said. Monkeys and rabbits were exposed to Bacillus anthracis spores, and subsequently given either Anthrasil or a placebo. The survival rate for monkeys given Anthrasil was between 36% and 70%, with a trend toward increased survival at higher doses of Anthrasil. None of the monkeys given placebo survived. Rabbits had a 26% survival rate when given the drug, compared to 2% of those given placebo. A separate study exposed rabbits to Bacillus anthracis and treated them with either antibiotics or a combination of antibiotics and Anthrasil; survival rates were 71% for those treated with the combination and 25% for those treated with antibiotics only.
Safety was tested in 74 healthy human volunteers and the most commonly reported side effects were headache, back pain, nausea, and pain and swelling at the infusion site.
Anthrasil is manufactured by Cangene Corporation, based in Winnipeg, Canada, which developed the drug in collaboration with BARDA.
FDA: Amiodarone plus some hepatitis C antivirals may result in bradycardia
BY ELIZABETH MECHCATIE
Frontline Medical News
Taking the antiarrythmic drug amiodarone with the hepatitis C antiviral drugs ledipasvir and sofosbuvir, or with sofosbuvir plus another direct-acting antiviral drug, has been associated with cases of symptomatic bradycardia – including a fatal cardiac arrest – according to the Food and Drug Administration.
Because of the reports, the antiviral drugs’ labels now recommend against using amiodarone with those hepatitis C drugs.
An FDA statement described the bradycardia cases as “serious and life-threatening.” Gilead Sciences markets the ledipasvir and sofosbuvir combination as Harvoni and markets sofosbuvir as Sovaldi to treat chronic hepatitis C virus (HCV) infection.
Gilead issued a “Dear Health Care Provider” letter that provides further details of the cases. There have been nine postmarketing reports of symptomatic bradycardia in patients who were taking amiodarone with Harvoni; amiodarone with Sovaldi plus another hepatitis C antiviral drug, simeprevir (Olysio); or amiodarone with an investigational hepatitis C antiviral drug, daclatasvir.
Of those cases, six occurred with in the first 24 hours of starting treatment with the antivirals, and three cases occurred within the first 2-12 days after antiviral therapy was started. A pacemaker was needed in three cases, and one case was a fatal cardiac arrest.
In three cases, a “rechallenge with HCV treatment in the setting of continued amiodarone therapy resulted in recurrence of symptomatic bradycardia,” according to the Gilead letter.
The effect of coadministration on the blood levels of the antiviral drugs is not known, nor is the mechanism behind the cardiac effect.
emechcatie@frontlinemedcom.com
Clindamycin, TMP-SMX are equally effective for skin infections
BY MARY ANN MOON
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Vitals Key clinical point: Clindamycin and TMP-SMX had similar efficacy and side-effect profiles for treating uncomplicated skin infections, including both abscesses and cellulitis. Major finding: At 7-10 days after completing therapy, the rates of cure in the evaluable population were 90% with clindamycin and 88% with TMP-SMX. Data source: A prospective, multicenter, randomized, double-blind clinical trial involving 524 adults and children followed for 1 month after treatment. Disclosures: This trial was supported by the National Institutes of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences (NCT00730028). Dr. Miller reported receiving consulting fees from Cubist, Durata, and Pfizer; his associates reported ties to Cubist, Pfizer, EMMES, Theravance, AstraZeneca, Trius, Merck, and Cerexa. |
Clindamycin and trimethoprim-sulfamethoxazole are similarly safe and effective for treating uncomplicated skin infections, including both cellulitis and abscesses, in ambulatory settings in regions where MRSA is endemic.
The data comparing these two agents in an ambulatory setting are limited, though both are commonly recommended as empirical therapy for skin infections in patients who present to clinics and emergency departments and have only minor or no coexisting conditions, said Dr. Loren G. Miller of the Los Angeles Biomedical Research Institute and the division of infectious diseases at Harbor-UCLA Medical Center, and his associates.
They performed a prospective double-blind randomized trial comparing clindamycin against TMP-SMX in 524 ethnically diverse adults and children who presented as outpatients with uncomplicated skin infections during a 2-year period in Chicago, San Francisco, Los Angeles, and Nashville – areas in which community-associated MRSA is endemic. The mean patient age was 27 years, and approximately 30% were pediatric patients. All the participants had cellulitis without abscesses (including erysipelas), one or more abscesses larger than 5 cm in diameter, or both conditions. A total of 264 were randomized to clindamycin and 260 to TMP-SMX daily for 10 days.
Cure rates did not differ significantly between the two study groups. At 7-10 days after completing therapy, the rates of cure in the intention-to-treat population were 80% for clindamycin and 78% for TMP-SMX, and in the evaluable population the rates were nearly 90% and 88%, respectively.
At 1 month follow-up, the cure rates in the evaluable population were 84% for clindamycin and 78% for TMP-SMX, the investigators said (N. Engl. J. Med. 2015;372:1093-103 [doi:10.1056/NEJMoa1403789]). Rates of adverse events were nearly identical between the two study groups at about 19%, and most were mild and resolved without sequelae. There were no treatment-associated serious adverse events, and the rates of treatment discontinuation were similar at nearly 9% between patients receiving clindamycin and those receiving TMP-SMX.
Stroke ambulances speed treatment to U.S. patients
BY MITCHEL L. ZOLER
AT THE INTERNATIONAL STROKE CONFERENCE
Vitals Key clinical point: Dedicated stroke ambulances that bring a CT scanner and thrombolytic treatment to patients in the field speed thrombolytic therapy. Major finding: In Cleveland, stroke patients received thrombolysis an average of 38 minutes sooner from the CT-equipped ambulance, compared with standard protocols. Data source: Prospectively collected data on time-to-treatment from case series in Houston and in Cleveland. Disclosures: Dr. Hussain and Ms. Parker had no disclosures. |
NASHVILLE, TENN. – Bringing a CT scanner and thrombolytic treatment directly to stroke patients in the field sped the time to thrombolysis, compared with waiting for the patient to arrive at the hospital.
Some U.S. stroke centers now send out a team that can immediately assess and start treating stroke patients in the community. In 2014, the first two U.S. mobile stroke-treatment units began operating, one in Houston and the second in Cleveland.
Initial reports show both programs were successful in cutting the time to deliver thrombolytic treatment with intravenous tissue plasminogen activator (TPA) to appropriate patients.
In Houston, the active phase of the program started in May 2014, and by October 2014, 47 acute ischemic stroke patients had been treated with TPA. The mobile-unit crews started 43% of eligible patients on thrombolysis within 60 minutes of their symptom onset and another 31% were treated starting 61-80 minutes after symptom onset, said Stephanie A. Parker at the International Stroke Conference.
The unit also treats patients diagnosed with hemorrhagic stroke with intravenous nicardipine for rapid blood pressure reduction, said Ms. Parker, a critical care and emergency medicine–trained registered nurse who is project manager for the Houston mobile unit.
The Cleveland program began in July 2014; of the first 100 stroke patients seen by the mobile unit 16 of 19 eligible patients received tPA, with an average time of 56 minutes from symptom onset to treatment. This compared with an average 94 minutes to tPA onset in patients brought conventionally last year to a Cleveland-area hospital, Dr. M. Shazam Hussain said in a report at the meeting, sponsored by the American Heart Association.
The clinical impact and cost effectiveness of the pilot programs using the mobile units have not yet been assessed from the data, Dr. Hussain and Ms. Parker emphasized. Funding for the Cleveland and Houston vehicles came from local donors; the Houston program also received equipment donations from manufacturers.
The two mobile units are standard 12-foot, box-shaped ambulances outfitted with a CT scanner, a point-of-care lab, and telemedicine components as well as more standard emergency-vehicle equipment. The Houston vehicle contains “all the diagnostic equipment that is in our emergency room,” Ms. Parker said.
The concept behind both the Cleveland unit, operated by the Cleveland Clinic, and the Houston unit, operated by the University of Texas, Houston, is that the mobile stroke unit arrives to a patient with a suspected stroke, the unit is stationary while a CT scan and other diagnostic tests are run, diagnosis occurs with telemedicine assistance. If the patient is cleared for TPA treatment, the infusion starts and the vehicle carries the patient to an appropriate stroke center.
Currently, the Houston unit goes out with a vascular neurologist and a telemedicine physician on board, but plans are in place to test the feasibility of relying entirely on telemedicine when making diagnostic and treatment decisions. The Cleveland mobile unit already operates in this fashion, with no physician on board, and was the first mobile stroke unit in the world to depend completely on telemedicine, according to Dr. Hussain, a neurologist and head of the stroke program at the Cleveland Clinic.
The world’s first mobile stroke unit began operating in Saarland, Germany, in 2008 (Lancet Neurology 2012;11:397-404), and a second unit began running in Berlin after that, Dr. Hussain noted. Because of limited funding, the service he directs in Cleveland has been operating from 8 a.m.-8 p.m., 7 days a week. The program plans to expand to 24-hour coverage. The Houston mobile unit operates 24/7; it averages two runs per day and administers TPA on 1 of every 10 runs, Ms. Parker said. Both the Houston and Cleveland units tie into the local 911 emergency activation systems for their respective regions.
Consider cephalosporin a safe alternative for patients with penicillin allergy
BY DEEPAK CHITNIS
AT THE 2015 AAAAI ANNUAL MEETING
Vitals Key clinical point: Given the low risk of adverse drug reactions with cephalosporins, patients with a history of penicillin allergy can safely take cephalosporins. Major finding: The most frequent ADRs in patients taking either oral or parenteral cephalosporins were Clostridium difficile infection within 90 days (0.91%), nephropathy within 30 days (0.15%), and all-cause death within 1 day (0.10%). Data source: Retrospective, population-based analysis of 949,323 Kaiser Permanente Southern California health plan members from 2010 to 2012. Disclosures: Dr. Macy disclosed receiving research support from ALK and BioMarin. |
HOUSTON – Given the low incidence of adverse drug reactions to cephalosporin antibiotics among nearly a million California health plan patients, patients with a history of penicillin allergy can safely be given cephalosporins, according to Dr. Eric M. Macy.
The recommendation is based on the findings of a retrospective, population-based analysis of the records of 949,323 Kaiser Permanente Southern California health plan members, which was presented by Dr. Macy, of the Kaiser Permanente Medical Center in San Diego, at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Dr. Macy and his colleagues examined the records of 622,456 health plan members who were given 901,908 regimens of oral cephalosporins and 326,867 members given 487,630 parenteral cephalosporin regimens between Jan. 1, 2010, and Dec. 31, 2012.
Clostridium difficile infection within 90 days, nephropathy within 30 days, and all-cause death within 1 day were the most common cephalosporin-associated adverse drug reactions (ADRs) reported by physicians, with rates of 0.91%, 0.15%, and 0.10%, respectively. Cephalosporin-associated anaphylaxis was documented by physicians a total of five times in oral patients and eight times in parenteral patients (P = .0761), while only three serious cutaneous adverse reactions (SCARs) were recorded. All of those SCARS were attributable to other antibiotics taken at the same time as the cephalosporins, according to Dr. Macy.
Patients who reported developing new cephalosporin allergies over the course of the study period were significantly more likely to be female than male: 0.56% vs. 0.43% (P < .0001). And patients with a history of penicillin allergy were more likely to report a new cephalosporin allergy within 30 days than patients with no drug allergy, another cephalosporin allergy, or a non–beta-lactam allergy.
Drug-resistant shigellosis outbreak
BY DEEPAK CHITNIS
FROM MMWR
The United States is currently experiencing an outbreak of shigellosis caused by a strain of the Shigella sonnei bacteria that is resistant to ciprofloxacin, the most commonly prescribed antimicrobial treatment for shigellosis.
In its Morbidity and Mortality Weekly Report, the CDC revealed that 243 individuals in 32 states and the territory of Puerto Rico have come down with shigellosis between May 2014 and February 2015. Of those 243 cases, 126 isolates were tested and 109 (87%) of those were found to be nonsusceptible to ciprofloxacin. The largest clusters of the disease were found in Massachusetts (45 cases), California (25 cases), and Pennsylvania (18 cases).
Ninety-five of the cases associated with the current outbreak were traced back to the homeless population of San Francisco; about half of the remaining cases were attributed to international travelers – specifically, those visiting the Dominican Republic and India – who contracted the bacteria while abroad and unknowingly brought it to the United States. The disease is known to spread quickly in populations of children who attend child care facilities, homeless individuals, and men who have sex with men.
“These outbreaks show a troubling trend in Shigella infections in the United States,” Dr. Thomas Frieden, CDC director, said in a statement. “Drug-resistant infections are harder to treat and because Shigella spreads so easily between people, the potential for more – and larger – outbreaks is a real concern. We’re moving quickly to implement a national strategy to curb antibiotic resistance because we can’t take for granted that we’ll always have the drugs we need to fight common infections.”
Shigellosis causes an estimated 500,000 cases of diarrhea in the United States each year. To help curb the growing number of shigellosis cases, the CDC recommends that international travelers wash their hands meticulously while abroad, and follow strict dietary precautions, such as eating hot foods and drinking beverages only from sealed containers, especially when consuming water.
Otherwise healthy patients over 80 benefit from aggressive treatment for MI
BY PATRICE WENDLING
AT ACC/CRF I2 SUMMIT
Vitals Key clinical point: An early invasive treatment strategy improved most outcomes in patients aged 80 years and older with acute coronary syndromes. Major finding: Myocardial infarction, need for urgent revascularization, stroke, and death were significantly lower with invasive vs. conservative care (41% vs. 61%; risk ratio, 0.48; P < .00001). Data source: Randomized study in 457 patients aged 80 years or older with non-STEMI or unstable angina. Disclosures: The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandzari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic. |
SAN DIEGO – Patients aged 80 years and older benefit from more invasive early treatment after non-ST-elevation myocardial infarction or unstable angina, the After Eighty Trial showed.
After a median follow-up of 1.5 years, an invasive strategy that included coronary angiography significantly reduced the primary endpoint of myocardial infarction (MI), need for urgent revascularization, stroke, and death from 61% with optimal medical treatment to 41%.
That drop was driven primarily by significantly fewer MIs (17% vs. 30%) and urgent revascularizations (2% vs. 11%), lead author Dr. Nicolai Tegn reported at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
There were no significant differences between the invasive and conservative strategy groups in rates of stroke or all-cause death.
The composite of death and MI, however, significantly favored the invasive group (35% vs. 48%), he reported.
After Eighty randomly assigned 457 patients, aged 80 years or older, to either optimal medical therapy with no invasive treatments or coronary angiography at a percutaneous coronary intervention (PCI) center the day after inclusion, plus optimal medical therapy after about 4-5 hours if PCI was not performed or about 6-18 hours if it was. Of the 225 patients receiving angiography, 48% went on to balloon angioplasty and/or coronary stenting, and 3% had bypass surgery. The patients were selected from nearly 4,200 elderly patients at 17 community hospitals in Norway; over 3,700 of the patients were ineligible for the study because their life expectancy was less than 12 months because of a serious comorbidity; ongoing or recent bleeding; inability to comply with protocol; clinically unstable including ongoing ischemia; refusal to participate; logistic reasons; or other reasons.
After Eighty is a welcome study because of the under-representation of the elderly in clinical trials, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said during a press briefing at the meeting. But it raises the challenge of identifying patients in clinical practice with the same qualifying characteristics, he added, given that the study population represents only 10% of the entire screened population, “The coronary anatomy does not know the age of the patient, meaning that the findings of a benefit of an early invasive strategy seem consistent with previous studies we know across the management of patients with acute coronary syndromes,” Dr. Kandzari said.
FDA approves Anthrasil to treat inhalational anthrax
BY DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
The Food and Drug Administration has approved Anthrasil, Anthrax Immune Globulin Intravenous (Human), for treatment of inhalational anthrax when used with appropriate antibacterial drugs.
Inhalational anthrax is caused by breathing in Bacillus anthracis spores, which can occur after exposure to infected animals or contaminated animal products, or as a result of an intentional release of spores. In a statement, Dr. Karen Midthun – director of the FDA’s Center for Biologics Evaluation and Research – explained that Anthrasil “will be stored in U.S. Strategic National Stockpile to facilitate its availability in response to an anthrax emergency.”
Anthrasil was purchased by the U.S. Department of Health & Human Services’ Biomedical Advanced Research and Development Authority (BARDA) in 2011, but because it was not approved, its use prior to FDA approval would have required an emergency use authorization from the FDA.
The efficacy of Anthrasil was studied in animals because it was not feasible or ethical to conduct adequately controlled efficacy studies in humans, the FDA said. Monkeys and rabbits were exposed to Bacillus anthracis spores, and subsequently given either Anthrasil or a placebo. The survival rate for monkeys given Anthrasil was between 36% and 70%, with a trend toward increased survival at higher doses of Anthrasil. None of the monkeys given placebo survived. Rabbits had a 26% survival rate when given the drug, compared to 2% of those given placebo. A separate study exposed rabbits to Bacillus anthracis and treated them with either antibiotics or a combination of antibiotics and Anthrasil; survival rates were 71% for those treated with the combination and 25% for those treated with antibiotics only.
Safety was tested in 74 healthy human volunteers and the most commonly reported side effects were headache, back pain, nausea, and pain and swelling at the infusion site.
Anthrasil is manufactured by Cangene Corporation, based in Winnipeg, Canada, which developed the drug in collaboration with BARDA.
FDA: Amiodarone plus some hepatitis C antivirals may result in bradycardia
BY ELIZABETH MECHCATIE
Frontline Medical News
Taking the antiarrythmic drug amiodarone with the hepatitis C antiviral drugs ledipasvir and sofosbuvir, or with sofosbuvir plus another direct-acting antiviral drug, has been associated with cases of symptomatic bradycardia – including a fatal cardiac arrest – according to the Food and Drug Administration.
Because of the reports, the antiviral drugs’ labels now recommend against using amiodarone with those hepatitis C drugs.
An FDA statement described the bradycardia cases as “serious and life-threatening.” Gilead Sciences markets the ledipasvir and sofosbuvir combination as Harvoni and markets sofosbuvir as Sovaldi to treat chronic hepatitis C virus (HCV) infection.
Gilead issued a “Dear Health Care Provider” letter that provides further details of the cases. There have been nine postmarketing reports of symptomatic bradycardia in patients who were taking amiodarone with Harvoni; amiodarone with Sovaldi plus another hepatitis C antiviral drug, simeprevir (Olysio); or amiodarone with an investigational hepatitis C antiviral drug, daclatasvir.
Of those cases, six occurred with in the first 24 hours of starting treatment with the antivirals, and three cases occurred within the first 2-12 days after antiviral therapy was started. A pacemaker was needed in three cases, and one case was a fatal cardiac arrest.
In three cases, a “rechallenge with HCV treatment in the setting of continued amiodarone therapy resulted in recurrence of symptomatic bradycardia,” according to the Gilead letter.
The effect of coadministration on the blood levels of the antiviral drugs is not known, nor is the mechanism behind the cardiac effect.
emechcatie@frontlinemedcom.com
Clindamycin, TMP-SMX are equally effective for skin infections
BY MARY ANN MOON
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Vitals Key clinical point: Clindamycin and TMP-SMX had similar efficacy and side-effect profiles for treating uncomplicated skin infections, including both abscesses and cellulitis. Major finding: At 7-10 days after completing therapy, the rates of cure in the evaluable population were 90% with clindamycin and 88% with TMP-SMX. Data source: A prospective, multicenter, randomized, double-blind clinical trial involving 524 adults and children followed for 1 month after treatment. Disclosures: This trial was supported by the National Institutes of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences (NCT00730028). Dr. Miller reported receiving consulting fees from Cubist, Durata, and Pfizer; his associates reported ties to Cubist, Pfizer, EMMES, Theravance, AstraZeneca, Trius, Merck, and Cerexa. |
Clindamycin and trimethoprim-sulfamethoxazole are similarly safe and effective for treating uncomplicated skin infections, including both cellulitis and abscesses, in ambulatory settings in regions where MRSA is endemic.
The data comparing these two agents in an ambulatory setting are limited, though both are commonly recommended as empirical therapy for skin infections in patients who present to clinics and emergency departments and have only minor or no coexisting conditions, said Dr. Loren G. Miller of the Los Angeles Biomedical Research Institute and the division of infectious diseases at Harbor-UCLA Medical Center, and his associates.
They performed a prospective double-blind randomized trial comparing clindamycin against TMP-SMX in 524 ethnically diverse adults and children who presented as outpatients with uncomplicated skin infections during a 2-year period in Chicago, San Francisco, Los Angeles, and Nashville – areas in which community-associated MRSA is endemic. The mean patient age was 27 years, and approximately 30% were pediatric patients. All the participants had cellulitis without abscesses (including erysipelas), one or more abscesses larger than 5 cm in diameter, or both conditions. A total of 264 were randomized to clindamycin and 260 to TMP-SMX daily for 10 days.
Cure rates did not differ significantly between the two study groups. At 7-10 days after completing therapy, the rates of cure in the intention-to-treat population were 80% for clindamycin and 78% for TMP-SMX, and in the evaluable population the rates were nearly 90% and 88%, respectively.
At 1 month follow-up, the cure rates in the evaluable population were 84% for clindamycin and 78% for TMP-SMX, the investigators said (N. Engl. J. Med. 2015;372:1093-103 [doi:10.1056/NEJMoa1403789]). Rates of adverse events were nearly identical between the two study groups at about 19%, and most were mild and resolved without sequelae. There were no treatment-associated serious adverse events, and the rates of treatment discontinuation were similar at nearly 9% between patients receiving clindamycin and those receiving TMP-SMX.
Stroke ambulances speed treatment to U.S. patients
BY MITCHEL L. ZOLER
AT THE INTERNATIONAL STROKE CONFERENCE
Vitals Key clinical point: Dedicated stroke ambulances that bring a CT scanner and thrombolytic treatment to patients in the field speed thrombolytic therapy. Major finding: In Cleveland, stroke patients received thrombolysis an average of 38 minutes sooner from the CT-equipped ambulance, compared with standard protocols. Data source: Prospectively collected data on time-to-treatment from case series in Houston and in Cleveland. Disclosures: Dr. Hussain and Ms. Parker had no disclosures. |
NASHVILLE, TENN. – Bringing a CT scanner and thrombolytic treatment directly to stroke patients in the field sped the time to thrombolysis, compared with waiting for the patient to arrive at the hospital.
Some U.S. stroke centers now send out a team that can immediately assess and start treating stroke patients in the community. In 2014, the first two U.S. mobile stroke-treatment units began operating, one in Houston and the second in Cleveland.
Initial reports show both programs were successful in cutting the time to deliver thrombolytic treatment with intravenous tissue plasminogen activator (TPA) to appropriate patients.
In Houston, the active phase of the program started in May 2014, and by October 2014, 47 acute ischemic stroke patients had been treated with TPA. The mobile-unit crews started 43% of eligible patients on thrombolysis within 60 minutes of their symptom onset and another 31% were treated starting 61-80 minutes after symptom onset, said Stephanie A. Parker at the International Stroke Conference.
The unit also treats patients diagnosed with hemorrhagic stroke with intravenous nicardipine for rapid blood pressure reduction, said Ms. Parker, a critical care and emergency medicine–trained registered nurse who is project manager for the Houston mobile unit.
The Cleveland program began in July 2014; of the first 100 stroke patients seen by the mobile unit 16 of 19 eligible patients received tPA, with an average time of 56 minutes from symptom onset to treatment. This compared with an average 94 minutes to tPA onset in patients brought conventionally last year to a Cleveland-area hospital, Dr. M. Shazam Hussain said in a report at the meeting, sponsored by the American Heart Association.
The clinical impact and cost effectiveness of the pilot programs using the mobile units have not yet been assessed from the data, Dr. Hussain and Ms. Parker emphasized. Funding for the Cleveland and Houston vehicles came from local donors; the Houston program also received equipment donations from manufacturers.
The two mobile units are standard 12-foot, box-shaped ambulances outfitted with a CT scanner, a point-of-care lab, and telemedicine components as well as more standard emergency-vehicle equipment. The Houston vehicle contains “all the diagnostic equipment that is in our emergency room,” Ms. Parker said.
The concept behind both the Cleveland unit, operated by the Cleveland Clinic, and the Houston unit, operated by the University of Texas, Houston, is that the mobile stroke unit arrives to a patient with a suspected stroke, the unit is stationary while a CT scan and other diagnostic tests are run, diagnosis occurs with telemedicine assistance. If the patient is cleared for TPA treatment, the infusion starts and the vehicle carries the patient to an appropriate stroke center.
Currently, the Houston unit goes out with a vascular neurologist and a telemedicine physician on board, but plans are in place to test the feasibility of relying entirely on telemedicine when making diagnostic and treatment decisions. The Cleveland mobile unit already operates in this fashion, with no physician on board, and was the first mobile stroke unit in the world to depend completely on telemedicine, according to Dr. Hussain, a neurologist and head of the stroke program at the Cleveland Clinic.
The world’s first mobile stroke unit began operating in Saarland, Germany, in 2008 (Lancet Neurology 2012;11:397-404), and a second unit began running in Berlin after that, Dr. Hussain noted. Because of limited funding, the service he directs in Cleveland has been operating from 8 a.m.-8 p.m., 7 days a week. The program plans to expand to 24-hour coverage. The Houston mobile unit operates 24/7; it averages two runs per day and administers TPA on 1 of every 10 runs, Ms. Parker said. Both the Houston and Cleveland units tie into the local 911 emergency activation systems for their respective regions.
Consider cephalosporin a safe alternative for patients with penicillin allergy
BY DEEPAK CHITNIS
AT THE 2015 AAAAI ANNUAL MEETING
Vitals Key clinical point: Given the low risk of adverse drug reactions with cephalosporins, patients with a history of penicillin allergy can safely take cephalosporins. Major finding: The most frequent ADRs in patients taking either oral or parenteral cephalosporins were Clostridium difficile infection within 90 days (0.91%), nephropathy within 30 days (0.15%), and all-cause death within 1 day (0.10%). Data source: Retrospective, population-based analysis of 949,323 Kaiser Permanente Southern California health plan members from 2010 to 2012. Disclosures: Dr. Macy disclosed receiving research support from ALK and BioMarin. |
HOUSTON – Given the low incidence of adverse drug reactions to cephalosporin antibiotics among nearly a million California health plan patients, patients with a history of penicillin allergy can safely be given cephalosporins, according to Dr. Eric M. Macy.
The recommendation is based on the findings of a retrospective, population-based analysis of the records of 949,323 Kaiser Permanente Southern California health plan members, which was presented by Dr. Macy, of the Kaiser Permanente Medical Center in San Diego, at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Dr. Macy and his colleagues examined the records of 622,456 health plan members who were given 901,908 regimens of oral cephalosporins and 326,867 members given 487,630 parenteral cephalosporin regimens between Jan. 1, 2010, and Dec. 31, 2012.
Clostridium difficile infection within 90 days, nephropathy within 30 days, and all-cause death within 1 day were the most common cephalosporin-associated adverse drug reactions (ADRs) reported by physicians, with rates of 0.91%, 0.15%, and 0.10%, respectively. Cephalosporin-associated anaphylaxis was documented by physicians a total of five times in oral patients and eight times in parenteral patients (P = .0761), while only three serious cutaneous adverse reactions (SCARs) were recorded. All of those SCARS were attributable to other antibiotics taken at the same time as the cephalosporins, according to Dr. Macy.
Patients who reported developing new cephalosporin allergies over the course of the study period were significantly more likely to be female than male: 0.56% vs. 0.43% (P < .0001). And patients with a history of penicillin allergy were more likely to report a new cephalosporin allergy within 30 days than patients with no drug allergy, another cephalosporin allergy, or a non–beta-lactam allergy.