User login
Lady Windermere syndrome: Mycobacterium of sophistication
A 75-year-old woman was referred to our pulmonary clinic with a 4-year history of intermittent episodes of persistent cough, occasionally productive of sputum, and mild exertional dyspnea. She had been treated with azithromycin for presumed community-acquired pneumonia, and her symptoms had initially improved. Subsequently, she experienced discrete, recurrent episodes of “bronchitis,” with productive cough and mild exertional dyspnea. Testing for latent tuberculosis had been negative. She reported a 10-pack-year smoking history in the remote past.
Her medical history included asthma, atrial fibrillation, gastroesophageal reflux disorder, hyperlipidemia, osteopenia, hypothyroidism, and allergic rhinitis. Her current medications were metoprolol, propafenone, and warfarin.
ABNORMALITIES ON PREVIOUS IMAGING
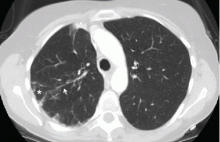
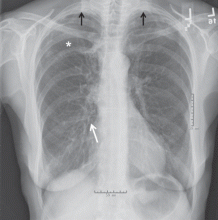
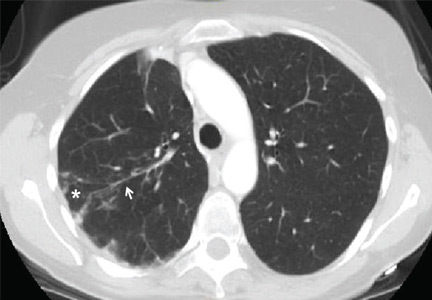
Computed tomography (CT) in April 2010 had revealed scattered linear, nodular, and “tree-in-bud” opacities involving the bilateral apices and the upper, middle, and lower lobes of the right lung, suggestive of bronchiolitis. Mild bronchiectasis had also been noted (Figure 1). Chest radiography had demonstrated signs of bronchiectasis and several scattered nodules (Figure 2). These abnormalities were still present on another CT scan in May 2013.
The patient had not undergone bronchoscopy before she was referred to our clinic.
WORKUP AT OUR CLINIC
On examination, the patient was lean, with a body mass index of 20.53 kg/m2. She appeared calm, well-groomed, and well-dressed, and had a very polite manner. When she coughed, she tried to suppress it, as if she were self-conscious about it. Her heart rhythm was irregularly irregular with a normal rate.
Expectorated sputum samples were obtained. Stains for acid-fast bacilli were negative, but three cultures were positive for acid-fast bacilli consistent with Mycobacterium avium-intracellulare. Serologic studies were negative for fungal infection and immunoglobulin deficiency.
Based on her symptoms and on the findings of imaging studies and sputum culture, we arrived at the diagnosis of nontuberculous mycobacterial lung infection, specifically, Lady Windermere syndrome.
NONTUBERCULOUS MYOCOBACTERIAL LUNG INFECTION
The diagnosis of nontuberculous mycobacterial lung infection is based on respiratory symptoms, findings on imaging (eg, nodular or cavitary opacities on radiography, or multifocal bronchiectasis and multiple small nodules on CT), and a positive culture for nontuberculous mycobacterial infection in more than two specimens of expectorated sputum or in more than one specimen from bronchoalveolar lavage. Lung biopsy with tissue culture is another way to confirm the diagnosis.
LADY WINDERMERE SYNDROME
Lady Windermere syndrome was described more than 20 years ago.1 The name derives from the lead character in Oscar Wilde’s play Lady Windermere’s Fan, which satirizes the strict morals and polite manners typical of the Victorian era in Great Britain.2
The patient with Lady Windermere syndrome is typically a thin, lean, well-mannered elderly woman who voluntarily suppresses her cough out of politeness. Suppression of the cough is thought to predispose to lung infection by allowing secretions to collect in the airways, especially in the right middle lobe, which has the longest and narrowest of the lobar bronchi.3,4
Symptoms of Lady Windermere syndrome include cough, sputum production, and fatigue similar to that of acute or chronic bronchitis. Dyspnea, fever, and hemoptysis are less common.5 The differential diagnosis for these symptoms is broad and includes asthma, chronic obstructive pulmonary disease, gastroesophageal reflux disease, pneumonia, bronchiectasis, cystic fibrosis, interstitial lung disease, postnasal drip, lung cancer, and heart failure.
A prospective cohort study by Kim et al6 yielded descriptions of typical patients with Lady Windermere syndrome. Patients were tall and lean, tended to have scoliosis, and more commonly had pectus excavatum or mitral valve prolapse; 95% were women, 91% were white, and the average age was 60. The morphologic features are thought to contribute to impaired clearance of airway secretions by altered mechanics during coughing.
HALLMARKS ON IMAGING
Kim et al6 reported that the most common findings on lung imaging in nontuberculous mycobacterial infection were bronchiectasis involving the right middle lobe (90%), nodules involving the right lower lobe (73%) and right middle lobe (71%), and, less commonly, a cavitary infiltrate involving the right upper lobe (17%) or right middle lobe (10%).
Key findings on imaging in Lady Windermere syndrome include opacities and “cylindrical bronchiectasis” predominantly involving the right middle lobe or lingula.5 Bronchiolar inflammation in response to nontuberculous mycobacterial infection may cause a nodular appearance, often progressing to a tree-in-bud appearance on CT.
Other diagnostic considerations for tree-in-bud appearance on CT include fungal, viral, or other bacterial infection, aspiration pneumonitis, inhalation of a foreign substance, cystic fibrosis, rheumatoid arthritis, SjÖgren syndrome, bronchiolitis obliterans, and neoplastic disease.
CURRENT TREATMENT OPTIONS
Treatment of nontuberculous mycobacterial lung infection, including Lady Windermere syndrome, is not necessary in every case, given the variability in clinical symptoms and in disease progression. Patients with progressive symptoms or radiographic changes should be considered candidates for treatment.
Management is directed at the underlying infection. M avium-intracellulare is ubiquitous in the environment, including in soil and water, and it has been reported as the most common pathogen in nontuberculous mycobacterial lung infection.7
Nodular-bronchiectatic nontuberculous mycobacterial lung disease typically progresses more slowly than fibrocavitary disease. For patients with nodular-bronchiectatic disease, follow-up over months or years may be needed before clinical or radiographic changes become apparent.
When treatment is indicated for nodular-bronchiectatic nontuberculous mycobacterial lung infection, it should include a macrolide antibiotic, ethambutol, and rifampin.7,8 Monotherapy with a macrolide is not recommended because of the risk of macrolide resistance. Addition of an aminoglycoside may be considered when treating fibrocavitary disease or widespread nodular bronchiectatic disease.
Management of bronchiectasis, when present, includes chest physiotherapy, pulmonary hygiene therapy, and awareness of the predisposition for nonmycobacterial lung infection. The decision to prescribe antimicrobials should take into consideration the risks and benefits for each patient.
Because treatment involves multidrug regimens, drug interactions and adverse effects need to be considered and monitored, especially in elderly patients, who may already be taking multiple medications. Treatment should be continued until a patient has negative sputum cultures for acid-fast bacilli while on therapy, for 1 year.
- Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest 1992; 101:1605–1609.
- Kasthoori JJ, Liam CK, Wastie ML. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Med J 2008; 49:e47–e49.
- Dhillon SS, Watanakunakorn C. Lady Windermere syndrome: middle lobe bronchiectasis and mycobacterium avium complex infection due to voluntary cough suppression. Clin Infect Dis 2000; 30:572–575.
- Reich JM. Pathogenesis of Lady Windermere syndrome. Scand J Infect Dis 2012; 44:1–2.
- Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Chest 2008; 133:243–251.
- Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008; 178:1066–1074.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416.
- Mason RJ, Broaddus VC, Martin T, et al, editors. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Philadelphia, PA: Saunders; 2010.
A 75-year-old woman was referred to our pulmonary clinic with a 4-year history of intermittent episodes of persistent cough, occasionally productive of sputum, and mild exertional dyspnea. She had been treated with azithromycin for presumed community-acquired pneumonia, and her symptoms had initially improved. Subsequently, she experienced discrete, recurrent episodes of “bronchitis,” with productive cough and mild exertional dyspnea. Testing for latent tuberculosis had been negative. She reported a 10-pack-year smoking history in the remote past.
Her medical history included asthma, atrial fibrillation, gastroesophageal reflux disorder, hyperlipidemia, osteopenia, hypothyroidism, and allergic rhinitis. Her current medications were metoprolol, propafenone, and warfarin.
ABNORMALITIES ON PREVIOUS IMAGING
Computed tomography (CT) in April 2010 had revealed scattered linear, nodular, and “tree-in-bud” opacities involving the bilateral apices and the upper, middle, and lower lobes of the right lung, suggestive of bronchiolitis. Mild bronchiectasis had also been noted (Figure 1). Chest radiography had demonstrated signs of bronchiectasis and several scattered nodules (Figure 2). These abnormalities were still present on another CT scan in May 2013.
The patient had not undergone bronchoscopy before she was referred to our clinic.
WORKUP AT OUR CLINIC
On examination, the patient was lean, with a body mass index of 20.53 kg/m2. She appeared calm, well-groomed, and well-dressed, and had a very polite manner. When she coughed, she tried to suppress it, as if she were self-conscious about it. Her heart rhythm was irregularly irregular with a normal rate.
Expectorated sputum samples were obtained. Stains for acid-fast bacilli were negative, but three cultures were positive for acid-fast bacilli consistent with Mycobacterium avium-intracellulare. Serologic studies were negative for fungal infection and immunoglobulin deficiency.
Based on her symptoms and on the findings of imaging studies and sputum culture, we arrived at the diagnosis of nontuberculous mycobacterial lung infection, specifically, Lady Windermere syndrome.
NONTUBERCULOUS MYOCOBACTERIAL LUNG INFECTION
The diagnosis of nontuberculous mycobacterial lung infection is based on respiratory symptoms, findings on imaging (eg, nodular or cavitary opacities on radiography, or multifocal bronchiectasis and multiple small nodules on CT), and a positive culture for nontuberculous mycobacterial infection in more than two specimens of expectorated sputum or in more than one specimen from bronchoalveolar lavage. Lung biopsy with tissue culture is another way to confirm the diagnosis.
LADY WINDERMERE SYNDROME
Lady Windermere syndrome was described more than 20 years ago.1 The name derives from the lead character in Oscar Wilde’s play Lady Windermere’s Fan, which satirizes the strict morals and polite manners typical of the Victorian era in Great Britain.2
The patient with Lady Windermere syndrome is typically a thin, lean, well-mannered elderly woman who voluntarily suppresses her cough out of politeness. Suppression of the cough is thought to predispose to lung infection by allowing secretions to collect in the airways, especially in the right middle lobe, which has the longest and narrowest of the lobar bronchi.3,4
Symptoms of Lady Windermere syndrome include cough, sputum production, and fatigue similar to that of acute or chronic bronchitis. Dyspnea, fever, and hemoptysis are less common.5 The differential diagnosis for these symptoms is broad and includes asthma, chronic obstructive pulmonary disease, gastroesophageal reflux disease, pneumonia, bronchiectasis, cystic fibrosis, interstitial lung disease, postnasal drip, lung cancer, and heart failure.
A prospective cohort study by Kim et al6 yielded descriptions of typical patients with Lady Windermere syndrome. Patients were tall and lean, tended to have scoliosis, and more commonly had pectus excavatum or mitral valve prolapse; 95% were women, 91% were white, and the average age was 60. The morphologic features are thought to contribute to impaired clearance of airway secretions by altered mechanics during coughing.
HALLMARKS ON IMAGING
Kim et al6 reported that the most common findings on lung imaging in nontuberculous mycobacterial infection were bronchiectasis involving the right middle lobe (90%), nodules involving the right lower lobe (73%) and right middle lobe (71%), and, less commonly, a cavitary infiltrate involving the right upper lobe (17%) or right middle lobe (10%).
Key findings on imaging in Lady Windermere syndrome include opacities and “cylindrical bronchiectasis” predominantly involving the right middle lobe or lingula.5 Bronchiolar inflammation in response to nontuberculous mycobacterial infection may cause a nodular appearance, often progressing to a tree-in-bud appearance on CT.
Other diagnostic considerations for tree-in-bud appearance on CT include fungal, viral, or other bacterial infection, aspiration pneumonitis, inhalation of a foreign substance, cystic fibrosis, rheumatoid arthritis, SjÖgren syndrome, bronchiolitis obliterans, and neoplastic disease.
CURRENT TREATMENT OPTIONS
Treatment of nontuberculous mycobacterial lung infection, including Lady Windermere syndrome, is not necessary in every case, given the variability in clinical symptoms and in disease progression. Patients with progressive symptoms or radiographic changes should be considered candidates for treatment.
Management is directed at the underlying infection. M avium-intracellulare is ubiquitous in the environment, including in soil and water, and it has been reported as the most common pathogen in nontuberculous mycobacterial lung infection.7
Nodular-bronchiectatic nontuberculous mycobacterial lung disease typically progresses more slowly than fibrocavitary disease. For patients with nodular-bronchiectatic disease, follow-up over months or years may be needed before clinical or radiographic changes become apparent.
When treatment is indicated for nodular-bronchiectatic nontuberculous mycobacterial lung infection, it should include a macrolide antibiotic, ethambutol, and rifampin.7,8 Monotherapy with a macrolide is not recommended because of the risk of macrolide resistance. Addition of an aminoglycoside may be considered when treating fibrocavitary disease or widespread nodular bronchiectatic disease.
Management of bronchiectasis, when present, includes chest physiotherapy, pulmonary hygiene therapy, and awareness of the predisposition for nonmycobacterial lung infection. The decision to prescribe antimicrobials should take into consideration the risks and benefits for each patient.
Because treatment involves multidrug regimens, drug interactions and adverse effects need to be considered and monitored, especially in elderly patients, who may already be taking multiple medications. Treatment should be continued until a patient has negative sputum cultures for acid-fast bacilli while on therapy, for 1 year.
A 75-year-old woman was referred to our pulmonary clinic with a 4-year history of intermittent episodes of persistent cough, occasionally productive of sputum, and mild exertional dyspnea. She had been treated with azithromycin for presumed community-acquired pneumonia, and her symptoms had initially improved. Subsequently, she experienced discrete, recurrent episodes of “bronchitis,” with productive cough and mild exertional dyspnea. Testing for latent tuberculosis had been negative. She reported a 10-pack-year smoking history in the remote past.
Her medical history included asthma, atrial fibrillation, gastroesophageal reflux disorder, hyperlipidemia, osteopenia, hypothyroidism, and allergic rhinitis. Her current medications were metoprolol, propafenone, and warfarin.
ABNORMALITIES ON PREVIOUS IMAGING
Computed tomography (CT) in April 2010 had revealed scattered linear, nodular, and “tree-in-bud” opacities involving the bilateral apices and the upper, middle, and lower lobes of the right lung, suggestive of bronchiolitis. Mild bronchiectasis had also been noted (Figure 1). Chest radiography had demonstrated signs of bronchiectasis and several scattered nodules (Figure 2). These abnormalities were still present on another CT scan in May 2013.
The patient had not undergone bronchoscopy before she was referred to our clinic.
WORKUP AT OUR CLINIC
On examination, the patient was lean, with a body mass index of 20.53 kg/m2. She appeared calm, well-groomed, and well-dressed, and had a very polite manner. When she coughed, she tried to suppress it, as if she were self-conscious about it. Her heart rhythm was irregularly irregular with a normal rate.
Expectorated sputum samples were obtained. Stains for acid-fast bacilli were negative, but three cultures were positive for acid-fast bacilli consistent with Mycobacterium avium-intracellulare. Serologic studies were negative for fungal infection and immunoglobulin deficiency.
Based on her symptoms and on the findings of imaging studies and sputum culture, we arrived at the diagnosis of nontuberculous mycobacterial lung infection, specifically, Lady Windermere syndrome.
NONTUBERCULOUS MYOCOBACTERIAL LUNG INFECTION
The diagnosis of nontuberculous mycobacterial lung infection is based on respiratory symptoms, findings on imaging (eg, nodular or cavitary opacities on radiography, or multifocal bronchiectasis and multiple small nodules on CT), and a positive culture for nontuberculous mycobacterial infection in more than two specimens of expectorated sputum or in more than one specimen from bronchoalveolar lavage. Lung biopsy with tissue culture is another way to confirm the diagnosis.
LADY WINDERMERE SYNDROME
Lady Windermere syndrome was described more than 20 years ago.1 The name derives from the lead character in Oscar Wilde’s play Lady Windermere’s Fan, which satirizes the strict morals and polite manners typical of the Victorian era in Great Britain.2
The patient with Lady Windermere syndrome is typically a thin, lean, well-mannered elderly woman who voluntarily suppresses her cough out of politeness. Suppression of the cough is thought to predispose to lung infection by allowing secretions to collect in the airways, especially in the right middle lobe, which has the longest and narrowest of the lobar bronchi.3,4
Symptoms of Lady Windermere syndrome include cough, sputum production, and fatigue similar to that of acute or chronic bronchitis. Dyspnea, fever, and hemoptysis are less common.5 The differential diagnosis for these symptoms is broad and includes asthma, chronic obstructive pulmonary disease, gastroesophageal reflux disease, pneumonia, bronchiectasis, cystic fibrosis, interstitial lung disease, postnasal drip, lung cancer, and heart failure.
A prospective cohort study by Kim et al6 yielded descriptions of typical patients with Lady Windermere syndrome. Patients were tall and lean, tended to have scoliosis, and more commonly had pectus excavatum or mitral valve prolapse; 95% were women, 91% were white, and the average age was 60. The morphologic features are thought to contribute to impaired clearance of airway secretions by altered mechanics during coughing.
HALLMARKS ON IMAGING
Kim et al6 reported that the most common findings on lung imaging in nontuberculous mycobacterial infection were bronchiectasis involving the right middle lobe (90%), nodules involving the right lower lobe (73%) and right middle lobe (71%), and, less commonly, a cavitary infiltrate involving the right upper lobe (17%) or right middle lobe (10%).
Key findings on imaging in Lady Windermere syndrome include opacities and “cylindrical bronchiectasis” predominantly involving the right middle lobe or lingula.5 Bronchiolar inflammation in response to nontuberculous mycobacterial infection may cause a nodular appearance, often progressing to a tree-in-bud appearance on CT.
Other diagnostic considerations for tree-in-bud appearance on CT include fungal, viral, or other bacterial infection, aspiration pneumonitis, inhalation of a foreign substance, cystic fibrosis, rheumatoid arthritis, SjÖgren syndrome, bronchiolitis obliterans, and neoplastic disease.
CURRENT TREATMENT OPTIONS
Treatment of nontuberculous mycobacterial lung infection, including Lady Windermere syndrome, is not necessary in every case, given the variability in clinical symptoms and in disease progression. Patients with progressive symptoms or radiographic changes should be considered candidates for treatment.
Management is directed at the underlying infection. M avium-intracellulare is ubiquitous in the environment, including in soil and water, and it has been reported as the most common pathogen in nontuberculous mycobacterial lung infection.7
Nodular-bronchiectatic nontuberculous mycobacterial lung disease typically progresses more slowly than fibrocavitary disease. For patients with nodular-bronchiectatic disease, follow-up over months or years may be needed before clinical or radiographic changes become apparent.
When treatment is indicated for nodular-bronchiectatic nontuberculous mycobacterial lung infection, it should include a macrolide antibiotic, ethambutol, and rifampin.7,8 Monotherapy with a macrolide is not recommended because of the risk of macrolide resistance. Addition of an aminoglycoside may be considered when treating fibrocavitary disease or widespread nodular bronchiectatic disease.
Management of bronchiectasis, when present, includes chest physiotherapy, pulmonary hygiene therapy, and awareness of the predisposition for nonmycobacterial lung infection. The decision to prescribe antimicrobials should take into consideration the risks and benefits for each patient.
Because treatment involves multidrug regimens, drug interactions and adverse effects need to be considered and monitored, especially in elderly patients, who may already be taking multiple medications. Treatment should be continued until a patient has negative sputum cultures for acid-fast bacilli while on therapy, for 1 year.
- Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest 1992; 101:1605–1609.
- Kasthoori JJ, Liam CK, Wastie ML. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Med J 2008; 49:e47–e49.
- Dhillon SS, Watanakunakorn C. Lady Windermere syndrome: middle lobe bronchiectasis and mycobacterium avium complex infection due to voluntary cough suppression. Clin Infect Dis 2000; 30:572–575.
- Reich JM. Pathogenesis of Lady Windermere syndrome. Scand J Infect Dis 2012; 44:1–2.
- Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Chest 2008; 133:243–251.
- Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008; 178:1066–1074.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416.
- Mason RJ, Broaddus VC, Martin T, et al, editors. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Philadelphia, PA: Saunders; 2010.
- Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest 1992; 101:1605–1609.
- Kasthoori JJ, Liam CK, Wastie ML. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Med J 2008; 49:e47–e49.
- Dhillon SS, Watanakunakorn C. Lady Windermere syndrome: middle lobe bronchiectasis and mycobacterium avium complex infection due to voluntary cough suppression. Clin Infect Dis 2000; 30:572–575.
- Reich JM. Pathogenesis of Lady Windermere syndrome. Scand J Infect Dis 2012; 44:1–2.
- Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Chest 2008; 133:243–251.
- Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008; 178:1066–1074.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416.
- Mason RJ, Broaddus VC, Martin T, et al, editors. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Philadelphia, PA: Saunders; 2010.