User login
Thoracic Outlet Syndrome: Current Concepts, Imaging Features, and Therapeutic Strategies
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
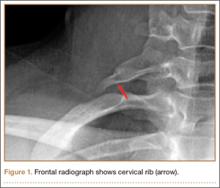
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
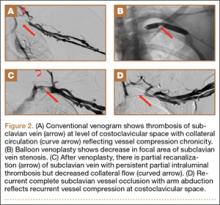
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
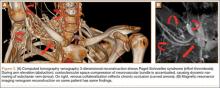
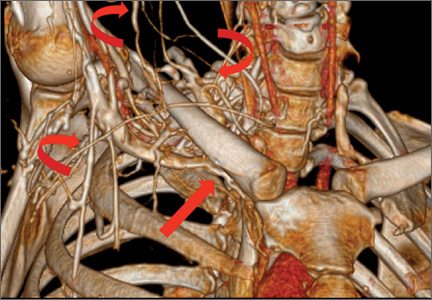
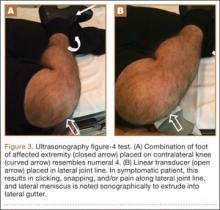
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
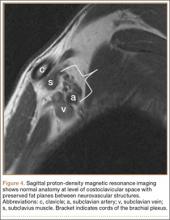
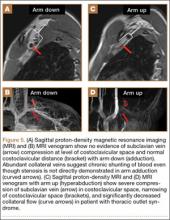
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
Wrisberg-Variant Discoid Lateral Meniscus: Current Concepts, Treatment Options, and Imaging Features With Emphasis on Dynamic Ultrasonography
First described by Young1 in 1889, discoid lateral meniscus covers a spectrum of meniscal disorders of varying morphology and stability. Determining the true incidence of discoid lateral menisci is difficult because of the large number of asymptomatic cases, though published estimates range from 1% to 17%2-4 of the population, with bilaterality occurring in up to 20%.5 The most commonly used classification system for discoid lateral menisci—reported by Watanabe and colleagues6—describes 3 types of meniscal pathology based on stability to probing and arthroscopic appearance. Type I is stable to probing, has normal tibial attachments, and is “block-shaped,” with increased thickness spanning the entire lateral tibial plateau. Type II is stable to probing and has normal tibial attachments as well, but covers less than 80% of the lateral tibial plateau. Type III (the Wrisberg variant) is unstable because it lacks a posterior meniscotibial (coronary) ligament and has only 1 posterior attachment, the posterior meniscofemoral ligament, or Wrisberg ligament. Wrisberg-variant discoid lateral menisci are rare; estimated incidence is 0.2%.7
Pathophysiology
The normal lateral meniscus, with its flat tibial and concave femoral surfaces, is crucial to load transmission across the knee joint.8 Embryologically differentiating from mesenchymal tissue within the limb bud during fetal development, a normal lateral meniscus never has a discoid shape.8-10 The implication, that discoid lateral menisci represent a congenital anomaly, is further supported by ultrastructural studies involving transmission electron microscopy. These studies have demonstrated that discoid menisci have fewer collagen fibers with a more disorganized course compared with normal menisci.11
With considerable variability, the average normal lateral meniscus is 12 mm wide and 4 mm thick.2 The blood supply to the lateral meniscus recedes during growth, with only the peripheral third remaining in adulthood8 and the inner two-thirds receiving nutrients by diffusion from the intra-articular fluid.5 In comparison, discoid lateral menisci often have poorer vascularity than normal menisci and therefore are more susceptible to tears.8,12,13
Ligamentous attachments to the lateral meniscus include the lateral meniscocapsular ligament, which attaches to the lateral joint capsule. In addition, 70% to 100% of people have accessory meniscofemoral ligaments, which insert anterior (ligament of Humphrey) or posterior (ligament of Wrisberg) to the posterior cruciate ligament.14 There are no ligamentous attachments at the popliteus hiatus or lateral collateral ligament, allowing for 9- to 11-mm excursion of the lateral meniscus during knee flexion and extension.3 Morphologically, the lack of a meniscotibial (coronary) ligament in the setting of a discoid lateral meniscus (Wrisberg variant) results in meniscal hypermobility. During knee range of motion, compressive forces between the femoral condyle and the tibial plateau spread through the peripheral portion of the meniscus and, without ligamentous attachments, allow it to displace anteriorly into the femoral intercondylar notch. This displacement results in impingement between the femur and the tibia15-18 and leads to the characteristic symptoms of “snapping knee syndrome.”10
Clinical Features
Snapping knee syndrome was first described by Kroiss19 in 1910.5 Multiple authors have described patients’ primary complaints as pain, swelling, locking, and a palpable or visible snap at terminal extension. Sudden movement of a soft-tissue structure across a bony prominence during a provocative maneuver is the source of the snapping. The syndrome has many etiologies. Extra-articular causes of lateral snapping knee syndrome include iliotibial band friction syndrome, soft-tissue tumors, hypermobile popliteus tendons, and abnormal anterior insertions of the biceps femoris tendons.20,21 Common intra-articular etiologies include ganglion, synovial, and parameniscal cysts; intra-articular loose bodies; lateral meniscal tears; and discoid lateral menisci.22 Patients with discoid lateral menisci often present with knee pain, popping, range-of-motion limitations, and snapping.23,24 However, the symptoms are quite variable and depend on type of discoid meniscus, presence of a tear, and stability of the rim.2,7,18
Obtaining a thorough history is essential in evaluating patients with suspected discoid lateral menisci. Physical examination should include evaluation of the lateral joint line for bulges, effusion, and tenderness. Patients may experience knee pain with flexion to 30° to 40° when varus or valgus stress (modified McMurray maneuver) is applied.10 In addition, a clunk may be appreciated with McMurray testing as a result of subluxation of the unstable lateral meniscus.10 The contralateral knee should be carefully evaluated, given the frequency of bilateral discoid menisci.10
The figure-4 test, a maneuver developed by LaPrade and Konowalchuk25 to detect peripheral meniscal tears or tears of the popliteomeniscal fascicles, is performed with the patient in the supine position, with the foot of the affected extremity placed on the contralateral knee. Normally, the popliteus tendon pulls the meniscus out of the joint when the knee is brought into the figure-4 position. However, without popliteomeniscal fascicles, the meniscus subluxes into the joint and becomes impinged. With the patient in the figure-4 position, reproduction of symptoms over the lateral joint line is a positive test and suggests peripheral meniscal tears and/or tears or absence of the popliteomeniscal fascicles.25
In the series reported by LaPrade and Konowalchuk,25 all of the patients who experienced symptoms during figure-4 testing were found, on arthroscopic examination, to have lateral meniscal hypermobility caused by tears of the popliteomeniscal fascicles. Despite the success of those authors in using the figure-4 technique for diagnosis, others have reported that the accuracy of the clinical examination (vs arthroscopy) in diagnosing Wrisberg-variant discoid lateral menisci ranges from 29% to 93%.5,26,27 This emphasizes the importance of diagnostic imaging in the work-up of patients with suspected Wrisberg-variant discoid lateral menisci.
Imaging Features
Radiography
In 1964, Picard and Constantin28 recommended that patients with suspected discoid lateral menisci undergo standard anteroposterior, lateral, tunnel, and skyline radiographs as part of the diagnostic work-up. In patients with discoid lateral menisci, plain film radiographs are often normal10 but may demonstrate lateral femoral condyle squaring, widening of the lateral joint line, lateral tibial plateau cupping, tibial eminence hypoplasia, and fibular head elevation.5,29 Plain radiography is unreliable, however, and patients often require advanced imaging, such as knee magnetic resonance imaging (MRI).10
Magnetic Resonance Imaging
Because it clearly depicts soft-tissue structures, MRI is widely used to diagnose musculoskeletal pathology in and around the knee. Criteria for the diagnosis of discoid menisci include meniscal width of 15 mm or more, ratio of minimum meniscal width to maximum tibial width on coronal slice of more than 20%, ratio of sum of width of both lateral horns to meniscal diameter (on sagittal slice showing maximum meniscal diameter) of more than 75%, and continuity of anterior and posterior horns on at least 3 consecutive sagittal slices (bow tie sign).5,30,31 Even in the presence of a tear, the described ratios have sensitivity and specificity of 95% and 97% in detecting discoid lateral menisci.30
However, the Wrisberg variant, which may consist of only a thickened portion of the posterior horn, is often more difficult to diagnose using these criteria and can even appear normal on MRI.26,32 In a series by Neuschwander and colleagues,7 none of the Wrisberg-variant menisci had a true discoid shape, suggesting that the size of the lateral meniscus may appear normal in affected patients. Appropriate positioning during MRI evaluation of patients suspected of having the Wrisberg variant was emphasized by Moser and colleagues,33 who described a case of discoid lateral meniscus not observable on initial MRI but visible on MRI performed with the affected knee extended in the locked position.
The unstable lateral meniscus may be seen subluxed anteriorly or laterally because of lack of posterior attachments. A deficiency of normal popliteomeniscal fascicles and coronary ligaments is represented by a high T2 signal interposed between the discoid lateral meniscus and the posterior joint capsule, simulating a vertical peripheral tear and suggesting presence of the Wrisberg variant (Figures 1A–1C). In addition, the posterior horn of the enlarged discoid lateral meniscus may connect to a prominent and thickened meniscofemoral ligament of Wrisberg. Despite these characteristic imaging features, some studies have found low sensitivity of MRI in the diagnosis of Wrisberg-variant discoid lateral menisci.26
Ultrasonography
There is a growing interest in using ultrasonography in the diagnosis of Wrisberg-variant discoid lateral menisci because of its availability, multiplanar capability, and lower cost compared with MRI. Ultrasonographic criteria for the diagnosis of discoid menisci include absence of normal triangular shape, presence of abnormally elongated and thickened meniscal tissue, and demonstration of a heterogeneous central pattern.5 Through use of a high-resolution probe, which better fits the anatomical concavity of the popliteal fossa, a positive predictive value of 95% and a negative predictive value of 100% have been reported for ultrasonography in the diagnosis of meniscal tears.34
Perhaps the main advantage of ultrasonography is the possibility of performing a dynamic study to evaluate the extrusion of the meniscus into the lateral gutter and to correlate this with knee snapping (Figures 2A, 2B).35 One technique for sonographic evaluation of a hypermobile lateral meniscus involves placing the patient supine with the high-resolution (9 or 12 MHz) linear transducer along the lateral knee joint line. The patient is then asked to place the foot of the affected extremity on the contralateral knee; the combination resembles the numeral 4 (figure-4 test) (Figures 3A, 3B). In a symptomatic patient, this results in clicking, snapping, and/or pain along the lateral joint line, and the lateral meniscus is noted sonographically to extrude into the lateral gutter (Figure 2B), either the result of torn popliteomeniscal fascicles or the increased meniscal mobility of Wrisberg variants.
The main drawback of ultrasonography is operator dependence. As clinicians become more familiar with ultrasonography, dynamic ultrasonography should be used for what is often a difficult diagnosis both clinically and with nondynamic imaging.
Management
The historical treatment for symptomatic discoid lateral menisci, open total meniscectomy,5,7,15,36 is no longer performed, as studies have shown it increases contact stresses proportional to the amount of meniscus removed, with up to a 235% increase after total meniscectomy,37 predisposing patients to early degenerative changes and osteoarthritis.38-41
With an appreciation of the role of menisci as load distributors and joint stabilizers in cartilage nutrition, current treatments aim to preserve as much stable meniscal tissue as possible.5 Surgical management of Wrisberg-variant discoid lateral menisci involves posterior stabilization with or without saucerization.7,33,42 The goal of arthroscopic saucerization is to preserve healthy tissue and create a stable remaining meniscus (6-8 mm in width)2,7,43,44 that provides adequate shock absorption without retearing.10 Wrisberg-variant discoid menisci can be stabilized with use of all-inside sutures from the meniscus to the joint capsule (Figures 4A–4F) when there is sufficient residual meniscus to allow for suture fixation to the posterior capsule after débridement. In contrast, some prefer an inside-out technique, as described by Neuschwander and colleagues,7 with inclusion of a mini-open approach. Any meniscal tears are addressed at time of surgery, either by partial meniscectomy or repair. Relative indications for meniscal repair include longitudinal, vertical, nondegenerative tears that are within 3 mm of the periphery (vascular zone) and are less than 3 cm in length.45 However, the majority of tears in adults are degenerative cleavage tears outside the vascular zone and therefore not amenable to repair.45,46 Before surgery, patients treated with stabilization with or without saucerization are prescribed partial weight-bearing in a hinged knee brace with gradual range of motion to 90° by 6 weeks and return to sports in 3 to 4 months.
Clinical Results
As has been consistently demonstrated, the long-term outcomes of total meniscectomy are poor function39,40,47 and radiographic evidence of lateral compartment arthritis.48 Patients who previously underwent total meniscectomy should be offered meniscal allograft transplantation, as it may offset the increased peak local contact pressures in the lateral compartment10 and improve function.49
With an appreciation for the importance of meniscus preservation, more recent studies have found encouraging results for arthroscopic saucerization and stabilization of Wrisberg-variant discoid lateral menisci. For example, Woods and Whelan44 reported excellent results in 75% of patients at 37.5-month follow-up after open repair of discoid lateral menisci lacking posterior attachments. In another study, by Neuschwander and colleagues,7 4 of 6 patients who underwent arthroscopic repair of unstable discoid lateral menisci without posterior coronary ligaments had excellent outcomes. Although these studies demonstrated symptom resolution and lack of radiographic evidence of degenerative changes at midterm follow-up,50 additional long-term studies should be performed to determine whether saucerization and stabilization prevent the onset of lateral compartment osteoarthritis.
Conclusion
Abnormally mobile discoid lateral menisci can result in painful lateral snapping knee syndromes but are often challenging to diagnose clinically and with traditional static imaging. Dynamic ultrasonography with provocative maneuvers can reveal lateral meniscal subluxation, which often cannot be appreciated on MRI, allowing for timely stabilization and symptom resolution.
1. Young RB. The external semilunar cartilage as a complete disc. In: Cleland J, Mackey JY, Young RB, eds. Memoirs and Memoranda in Anatomy. London, England: Williams & Norgate; 1889:179.
2. Jordan MR. Lateral meniscal variants: evaluation and treatment. J Am Acad Orthop Surg. 1996;4(4):191-200.
3. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176.
4. Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop. 1982;(167):19-28.
5. Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop. 2007;1(2):89-96.
6. Watanabe M, Takeda S, Ikeuchi H. Atlas of Arthroscopy. Tokyo, Japan: Igaku-Shoin; 1978.
7. Neuschwander DC, Drez D Jr, Finney TP. Lateral meniscal variant with absence of the posterior coronary ligament. J Bone Joint Surg Am. 1992;74(8):1186-1190.
8. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65(4):538-547.
9. Kaplan EB. Discoid lateral meniscus of the knee joint; nature, mechanism, and operative treatment. J Bone Joint Surg Am. 1957;39(1):77-87.
10. Kramer DE, Micheli LJ. Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg. 2009;17(11):698-707.
11. Atay OA, Pekmezci M, Doral MN, Sargon MF, Ayvaz M, Johnson DL. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35(3):475-478.
12. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
13. Good CR, Green DW, Griffith MH, Valen AW, Widmann RF, Rodeo SA. Arthroscopic treatment of symptomatic discoid meniscus in children: classification, technique, and results. Arthroscopy. 2007;23(2):157-163.
14. Harner CD, Xerogeanes JW, Livesay GA, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736-745.
15. Smillie IS. The congenital discoid meniscus. J Bone Joint Surg Br. 1948;30(4):671-682.
16. Yoo WJ, Choi IH, Chung CY, et al. Discoid lateral meniscus in children: limited knee extension and meniscal instability in the posterior segment. J Pediatr Orthop. 2008;28(5):544-548.
17. Simonian PT, Sussmann PS, Wickiewicz TL, et al. Popliteomeniscal fasciculi and the unstable lateral meniscus: clinical correlation and magnetic resonance diagnosis. Arthroscopy. 1997;13(5):590-596.
18. Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am. 1982;64(7):1068-1073.
19. Kroiss F. Die Verletzungen der Kniegelenkoszwischenknorpel und ihrer Verbindungen. Beitr Klin Chir. 1910;66:598-801.
20. Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop. 1992;(283):205-206.
21. Cooper DE. Snapping popliteus tendon syndrome. A cause of mechanical knee popping in athletes. Am J Sports Med. 1999;27(5):671-674.
22. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007;14(2):167-168.
23. Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5(1):52-56.
24. Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am. 1995;77(9):1357-1361.
25. LaPrade RF, Konowalchuk BK. Popliteomeniscal fascicle tears causing symptomatic lateral compartment knee pain: diagnosis by the figure-4 test and treatment by open repair. Am J Sports Med. 2005;33(8):1231-1236.
26. Kocher MS, DiCanzio J, Zurakowski D, Micheli LJ. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29(3):292-296.
27. Stanitski CL. Correlation of arthroscopic and clinical examinations with magnetic resonance imaging findings of injured knees in children and adolescents. Am J Sports Med. 1998;26(1):2-6.
28. Picard JJ, Constantin L. Radiological aspects of the discoid meniscus [in French]. J Radiol Electrol Med Nucl. 1964;45:839-841.
29. Kerr R. Radiologic case study. Discoid lateral meniscus. Orthopedics. 1986;9(8):1142, 1145-1147.
30. Samoto N, Kozuma M, Tokuhisa T, Kobayashi K. Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging. 2002;20(1):59-64.
31. Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology. 1989;173(2):351-354.
32. Singh K, Helms CA, Jacobs MT, Higgins LD. MRI appearance of Wrisberg variant of discoid lateral meniscus. AJR Am J Roentgenol. 2006;187(2):384-387.
33. Moser MW, Dugas J, Hartzell J, Thornton DD. A hypermobile Wrisberg variant lateral discoid meniscus seen on MRI. Clin Orthop. 2007;(456):264-267.
34. Najafi J, Bagheri S, Lahiji FA. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J Ultrasound Med. 2006;25(5):593-597.
35. Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142-150.
36. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
37. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270-275.
38. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
39. Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11(3):111-115.
40. Wroble RR, Henderson RC, Campion ER, el-Khoury GY, Albright JP. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop. 1992;(279):180-189.
41. Abdon P, Turner MS, Pettersson H, Lindstrand A, Stenstrom A, Swanson AJ. A long-term follow-up study of total meniscectomy in children. Clin Orthop. 1990;(257):166-170.
42. Rosenberg TD, Paulos LE, Parker RD, Harner CD, Gurley WD. Discoid lateral meniscus: case report of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy. 1987;3(4):277-282.
43. Fleissner PR, Eilert RE. Discoid lateral meniscus. Am J Knee Surg. 1999;12(2):125-131.
44. Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9(3):695-706.
45. Yue BW, Gupta AK, Moorman CT 3rd, Garrett WE, Helms CA. Wrisberg variant of the discoid lateral meniscus with flipped meniscal fragments simulating bucket-handle tear: MRI and arthroscopic correlation. Skeletal Radiol. 2011;40(8):1089-1094.
46. Weiss CB, Lundberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71(6):811-822.
47. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
48. Kim SJ, Chun YM, Jeong JH, Ryu SW, Oh KS, Lubis AM. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1315-1320.
49. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22(12):1344-1350.e1.
50. Ogut T, Kesmezacar H, Akgun I, Cansu E. Arthroscopic meniscectomy for discoid lateral meniscus in children and adolescents: 4.5 year follow-up. J Pediatr Orthop B. 2003;12(6):390-397.
First described by Young1 in 1889, discoid lateral meniscus covers a spectrum of meniscal disorders of varying morphology and stability. Determining the true incidence of discoid lateral menisci is difficult because of the large number of asymptomatic cases, though published estimates range from 1% to 17%2-4 of the population, with bilaterality occurring in up to 20%.5 The most commonly used classification system for discoid lateral menisci—reported by Watanabe and colleagues6—describes 3 types of meniscal pathology based on stability to probing and arthroscopic appearance. Type I is stable to probing, has normal tibial attachments, and is “block-shaped,” with increased thickness spanning the entire lateral tibial plateau. Type II is stable to probing and has normal tibial attachments as well, but covers less than 80% of the lateral tibial plateau. Type III (the Wrisberg variant) is unstable because it lacks a posterior meniscotibial (coronary) ligament and has only 1 posterior attachment, the posterior meniscofemoral ligament, or Wrisberg ligament. Wrisberg-variant discoid lateral menisci are rare; estimated incidence is 0.2%.7
Pathophysiology
The normal lateral meniscus, with its flat tibial and concave femoral surfaces, is crucial to load transmission across the knee joint.8 Embryologically differentiating from mesenchymal tissue within the limb bud during fetal development, a normal lateral meniscus never has a discoid shape.8-10 The implication, that discoid lateral menisci represent a congenital anomaly, is further supported by ultrastructural studies involving transmission electron microscopy. These studies have demonstrated that discoid menisci have fewer collagen fibers with a more disorganized course compared with normal menisci.11
With considerable variability, the average normal lateral meniscus is 12 mm wide and 4 mm thick.2 The blood supply to the lateral meniscus recedes during growth, with only the peripheral third remaining in adulthood8 and the inner two-thirds receiving nutrients by diffusion from the intra-articular fluid.5 In comparison, discoid lateral menisci often have poorer vascularity than normal menisci and therefore are more susceptible to tears.8,12,13
Ligamentous attachments to the lateral meniscus include the lateral meniscocapsular ligament, which attaches to the lateral joint capsule. In addition, 70% to 100% of people have accessory meniscofemoral ligaments, which insert anterior (ligament of Humphrey) or posterior (ligament of Wrisberg) to the posterior cruciate ligament.14 There are no ligamentous attachments at the popliteus hiatus or lateral collateral ligament, allowing for 9- to 11-mm excursion of the lateral meniscus during knee flexion and extension.3 Morphologically, the lack of a meniscotibial (coronary) ligament in the setting of a discoid lateral meniscus (Wrisberg variant) results in meniscal hypermobility. During knee range of motion, compressive forces between the femoral condyle and the tibial plateau spread through the peripheral portion of the meniscus and, without ligamentous attachments, allow it to displace anteriorly into the femoral intercondylar notch. This displacement results in impingement between the femur and the tibia15-18 and leads to the characteristic symptoms of “snapping knee syndrome.”10
Clinical Features
Snapping knee syndrome was first described by Kroiss19 in 1910.5 Multiple authors have described patients’ primary complaints as pain, swelling, locking, and a palpable or visible snap at terminal extension. Sudden movement of a soft-tissue structure across a bony prominence during a provocative maneuver is the source of the snapping. The syndrome has many etiologies. Extra-articular causes of lateral snapping knee syndrome include iliotibial band friction syndrome, soft-tissue tumors, hypermobile popliteus tendons, and abnormal anterior insertions of the biceps femoris tendons.20,21 Common intra-articular etiologies include ganglion, synovial, and parameniscal cysts; intra-articular loose bodies; lateral meniscal tears; and discoid lateral menisci.22 Patients with discoid lateral menisci often present with knee pain, popping, range-of-motion limitations, and snapping.23,24 However, the symptoms are quite variable and depend on type of discoid meniscus, presence of a tear, and stability of the rim.2,7,18
Obtaining a thorough history is essential in evaluating patients with suspected discoid lateral menisci. Physical examination should include evaluation of the lateral joint line for bulges, effusion, and tenderness. Patients may experience knee pain with flexion to 30° to 40° when varus or valgus stress (modified McMurray maneuver) is applied.10 In addition, a clunk may be appreciated with McMurray testing as a result of subluxation of the unstable lateral meniscus.10 The contralateral knee should be carefully evaluated, given the frequency of bilateral discoid menisci.10
The figure-4 test, a maneuver developed by LaPrade and Konowalchuk25 to detect peripheral meniscal tears or tears of the popliteomeniscal fascicles, is performed with the patient in the supine position, with the foot of the affected extremity placed on the contralateral knee. Normally, the popliteus tendon pulls the meniscus out of the joint when the knee is brought into the figure-4 position. However, without popliteomeniscal fascicles, the meniscus subluxes into the joint and becomes impinged. With the patient in the figure-4 position, reproduction of symptoms over the lateral joint line is a positive test and suggests peripheral meniscal tears and/or tears or absence of the popliteomeniscal fascicles.25
In the series reported by LaPrade and Konowalchuk,25 all of the patients who experienced symptoms during figure-4 testing were found, on arthroscopic examination, to have lateral meniscal hypermobility caused by tears of the popliteomeniscal fascicles. Despite the success of those authors in using the figure-4 technique for diagnosis, others have reported that the accuracy of the clinical examination (vs arthroscopy) in diagnosing Wrisberg-variant discoid lateral menisci ranges from 29% to 93%.5,26,27 This emphasizes the importance of diagnostic imaging in the work-up of patients with suspected Wrisberg-variant discoid lateral menisci.
Imaging Features
Radiography
In 1964, Picard and Constantin28 recommended that patients with suspected discoid lateral menisci undergo standard anteroposterior, lateral, tunnel, and skyline radiographs as part of the diagnostic work-up. In patients with discoid lateral menisci, plain film radiographs are often normal10 but may demonstrate lateral femoral condyle squaring, widening of the lateral joint line, lateral tibial plateau cupping, tibial eminence hypoplasia, and fibular head elevation.5,29 Plain radiography is unreliable, however, and patients often require advanced imaging, such as knee magnetic resonance imaging (MRI).10
Magnetic Resonance Imaging
Because it clearly depicts soft-tissue structures, MRI is widely used to diagnose musculoskeletal pathology in and around the knee. Criteria for the diagnosis of discoid menisci include meniscal width of 15 mm or more, ratio of minimum meniscal width to maximum tibial width on coronal slice of more than 20%, ratio of sum of width of both lateral horns to meniscal diameter (on sagittal slice showing maximum meniscal diameter) of more than 75%, and continuity of anterior and posterior horns on at least 3 consecutive sagittal slices (bow tie sign).5,30,31 Even in the presence of a tear, the described ratios have sensitivity and specificity of 95% and 97% in detecting discoid lateral menisci.30
However, the Wrisberg variant, which may consist of only a thickened portion of the posterior horn, is often more difficult to diagnose using these criteria and can even appear normal on MRI.26,32 In a series by Neuschwander and colleagues,7 none of the Wrisberg-variant menisci had a true discoid shape, suggesting that the size of the lateral meniscus may appear normal in affected patients. Appropriate positioning during MRI evaluation of patients suspected of having the Wrisberg variant was emphasized by Moser and colleagues,33 who described a case of discoid lateral meniscus not observable on initial MRI but visible on MRI performed with the affected knee extended in the locked position.
The unstable lateral meniscus may be seen subluxed anteriorly or laterally because of lack of posterior attachments. A deficiency of normal popliteomeniscal fascicles and coronary ligaments is represented by a high T2 signal interposed between the discoid lateral meniscus and the posterior joint capsule, simulating a vertical peripheral tear and suggesting presence of the Wrisberg variant (Figures 1A–1C). In addition, the posterior horn of the enlarged discoid lateral meniscus may connect to a prominent and thickened meniscofemoral ligament of Wrisberg. Despite these characteristic imaging features, some studies have found low sensitivity of MRI in the diagnosis of Wrisberg-variant discoid lateral menisci.26
Ultrasonography
There is a growing interest in using ultrasonography in the diagnosis of Wrisberg-variant discoid lateral menisci because of its availability, multiplanar capability, and lower cost compared with MRI. Ultrasonographic criteria for the diagnosis of discoid menisci include absence of normal triangular shape, presence of abnormally elongated and thickened meniscal tissue, and demonstration of a heterogeneous central pattern.5 Through use of a high-resolution probe, which better fits the anatomical concavity of the popliteal fossa, a positive predictive value of 95% and a negative predictive value of 100% have been reported for ultrasonography in the diagnosis of meniscal tears.34
Perhaps the main advantage of ultrasonography is the possibility of performing a dynamic study to evaluate the extrusion of the meniscus into the lateral gutter and to correlate this with knee snapping (Figures 2A, 2B).35 One technique for sonographic evaluation of a hypermobile lateral meniscus involves placing the patient supine with the high-resolution (9 or 12 MHz) linear transducer along the lateral knee joint line. The patient is then asked to place the foot of the affected extremity on the contralateral knee; the combination resembles the numeral 4 (figure-4 test) (Figures 3A, 3B). In a symptomatic patient, this results in clicking, snapping, and/or pain along the lateral joint line, and the lateral meniscus is noted sonographically to extrude into the lateral gutter (Figure 2B), either the result of torn popliteomeniscal fascicles or the increased meniscal mobility of Wrisberg variants.
The main drawback of ultrasonography is operator dependence. As clinicians become more familiar with ultrasonography, dynamic ultrasonography should be used for what is often a difficult diagnosis both clinically and with nondynamic imaging.
Management
The historical treatment for symptomatic discoid lateral menisci, open total meniscectomy,5,7,15,36 is no longer performed, as studies have shown it increases contact stresses proportional to the amount of meniscus removed, with up to a 235% increase after total meniscectomy,37 predisposing patients to early degenerative changes and osteoarthritis.38-41
With an appreciation of the role of menisci as load distributors and joint stabilizers in cartilage nutrition, current treatments aim to preserve as much stable meniscal tissue as possible.5 Surgical management of Wrisberg-variant discoid lateral menisci involves posterior stabilization with or without saucerization.7,33,42 The goal of arthroscopic saucerization is to preserve healthy tissue and create a stable remaining meniscus (6-8 mm in width)2,7,43,44 that provides adequate shock absorption without retearing.10 Wrisberg-variant discoid menisci can be stabilized with use of all-inside sutures from the meniscus to the joint capsule (Figures 4A–4F) when there is sufficient residual meniscus to allow for suture fixation to the posterior capsule after débridement. In contrast, some prefer an inside-out technique, as described by Neuschwander and colleagues,7 with inclusion of a mini-open approach. Any meniscal tears are addressed at time of surgery, either by partial meniscectomy or repair. Relative indications for meniscal repair include longitudinal, vertical, nondegenerative tears that are within 3 mm of the periphery (vascular zone) and are less than 3 cm in length.45 However, the majority of tears in adults are degenerative cleavage tears outside the vascular zone and therefore not amenable to repair.45,46 Before surgery, patients treated with stabilization with or without saucerization are prescribed partial weight-bearing in a hinged knee brace with gradual range of motion to 90° by 6 weeks and return to sports in 3 to 4 months.
Clinical Results
As has been consistently demonstrated, the long-term outcomes of total meniscectomy are poor function39,40,47 and radiographic evidence of lateral compartment arthritis.48 Patients who previously underwent total meniscectomy should be offered meniscal allograft transplantation, as it may offset the increased peak local contact pressures in the lateral compartment10 and improve function.49
With an appreciation for the importance of meniscus preservation, more recent studies have found encouraging results for arthroscopic saucerization and stabilization of Wrisberg-variant discoid lateral menisci. For example, Woods and Whelan44 reported excellent results in 75% of patients at 37.5-month follow-up after open repair of discoid lateral menisci lacking posterior attachments. In another study, by Neuschwander and colleagues,7 4 of 6 patients who underwent arthroscopic repair of unstable discoid lateral menisci without posterior coronary ligaments had excellent outcomes. Although these studies demonstrated symptom resolution and lack of radiographic evidence of degenerative changes at midterm follow-up,50 additional long-term studies should be performed to determine whether saucerization and stabilization prevent the onset of lateral compartment osteoarthritis.
Conclusion
Abnormally mobile discoid lateral menisci can result in painful lateral snapping knee syndromes but are often challenging to diagnose clinically and with traditional static imaging. Dynamic ultrasonography with provocative maneuvers can reveal lateral meniscal subluxation, which often cannot be appreciated on MRI, allowing for timely stabilization and symptom resolution.
First described by Young1 in 1889, discoid lateral meniscus covers a spectrum of meniscal disorders of varying morphology and stability. Determining the true incidence of discoid lateral menisci is difficult because of the large number of asymptomatic cases, though published estimates range from 1% to 17%2-4 of the population, with bilaterality occurring in up to 20%.5 The most commonly used classification system for discoid lateral menisci—reported by Watanabe and colleagues6—describes 3 types of meniscal pathology based on stability to probing and arthroscopic appearance. Type I is stable to probing, has normal tibial attachments, and is “block-shaped,” with increased thickness spanning the entire lateral tibial plateau. Type II is stable to probing and has normal tibial attachments as well, but covers less than 80% of the lateral tibial plateau. Type III (the Wrisberg variant) is unstable because it lacks a posterior meniscotibial (coronary) ligament and has only 1 posterior attachment, the posterior meniscofemoral ligament, or Wrisberg ligament. Wrisberg-variant discoid lateral menisci are rare; estimated incidence is 0.2%.7
Pathophysiology
The normal lateral meniscus, with its flat tibial and concave femoral surfaces, is crucial to load transmission across the knee joint.8 Embryologically differentiating from mesenchymal tissue within the limb bud during fetal development, a normal lateral meniscus never has a discoid shape.8-10 The implication, that discoid lateral menisci represent a congenital anomaly, is further supported by ultrastructural studies involving transmission electron microscopy. These studies have demonstrated that discoid menisci have fewer collagen fibers with a more disorganized course compared with normal menisci.11
With considerable variability, the average normal lateral meniscus is 12 mm wide and 4 mm thick.2 The blood supply to the lateral meniscus recedes during growth, with only the peripheral third remaining in adulthood8 and the inner two-thirds receiving nutrients by diffusion from the intra-articular fluid.5 In comparison, discoid lateral menisci often have poorer vascularity than normal menisci and therefore are more susceptible to tears.8,12,13
Ligamentous attachments to the lateral meniscus include the lateral meniscocapsular ligament, which attaches to the lateral joint capsule. In addition, 70% to 100% of people have accessory meniscofemoral ligaments, which insert anterior (ligament of Humphrey) or posterior (ligament of Wrisberg) to the posterior cruciate ligament.14 There are no ligamentous attachments at the popliteus hiatus or lateral collateral ligament, allowing for 9- to 11-mm excursion of the lateral meniscus during knee flexion and extension.3 Morphologically, the lack of a meniscotibial (coronary) ligament in the setting of a discoid lateral meniscus (Wrisberg variant) results in meniscal hypermobility. During knee range of motion, compressive forces between the femoral condyle and the tibial plateau spread through the peripheral portion of the meniscus and, without ligamentous attachments, allow it to displace anteriorly into the femoral intercondylar notch. This displacement results in impingement between the femur and the tibia15-18 and leads to the characteristic symptoms of “snapping knee syndrome.”10
Clinical Features
Snapping knee syndrome was first described by Kroiss19 in 1910.5 Multiple authors have described patients’ primary complaints as pain, swelling, locking, and a palpable or visible snap at terminal extension. Sudden movement of a soft-tissue structure across a bony prominence during a provocative maneuver is the source of the snapping. The syndrome has many etiologies. Extra-articular causes of lateral snapping knee syndrome include iliotibial band friction syndrome, soft-tissue tumors, hypermobile popliteus tendons, and abnormal anterior insertions of the biceps femoris tendons.20,21 Common intra-articular etiologies include ganglion, synovial, and parameniscal cysts; intra-articular loose bodies; lateral meniscal tears; and discoid lateral menisci.22 Patients with discoid lateral menisci often present with knee pain, popping, range-of-motion limitations, and snapping.23,24 However, the symptoms are quite variable and depend on type of discoid meniscus, presence of a tear, and stability of the rim.2,7,18
Obtaining a thorough history is essential in evaluating patients with suspected discoid lateral menisci. Physical examination should include evaluation of the lateral joint line for bulges, effusion, and tenderness. Patients may experience knee pain with flexion to 30° to 40° when varus or valgus stress (modified McMurray maneuver) is applied.10 In addition, a clunk may be appreciated with McMurray testing as a result of subluxation of the unstable lateral meniscus.10 The contralateral knee should be carefully evaluated, given the frequency of bilateral discoid menisci.10
The figure-4 test, a maneuver developed by LaPrade and Konowalchuk25 to detect peripheral meniscal tears or tears of the popliteomeniscal fascicles, is performed with the patient in the supine position, with the foot of the affected extremity placed on the contralateral knee. Normally, the popliteus tendon pulls the meniscus out of the joint when the knee is brought into the figure-4 position. However, without popliteomeniscal fascicles, the meniscus subluxes into the joint and becomes impinged. With the patient in the figure-4 position, reproduction of symptoms over the lateral joint line is a positive test and suggests peripheral meniscal tears and/or tears or absence of the popliteomeniscal fascicles.25
In the series reported by LaPrade and Konowalchuk,25 all of the patients who experienced symptoms during figure-4 testing were found, on arthroscopic examination, to have lateral meniscal hypermobility caused by tears of the popliteomeniscal fascicles. Despite the success of those authors in using the figure-4 technique for diagnosis, others have reported that the accuracy of the clinical examination (vs arthroscopy) in diagnosing Wrisberg-variant discoid lateral menisci ranges from 29% to 93%.5,26,27 This emphasizes the importance of diagnostic imaging in the work-up of patients with suspected Wrisberg-variant discoid lateral menisci.
Imaging Features
Radiography
In 1964, Picard and Constantin28 recommended that patients with suspected discoid lateral menisci undergo standard anteroposterior, lateral, tunnel, and skyline radiographs as part of the diagnostic work-up. In patients with discoid lateral menisci, plain film radiographs are often normal10 but may demonstrate lateral femoral condyle squaring, widening of the lateral joint line, lateral tibial plateau cupping, tibial eminence hypoplasia, and fibular head elevation.5,29 Plain radiography is unreliable, however, and patients often require advanced imaging, such as knee magnetic resonance imaging (MRI).10
Magnetic Resonance Imaging
Because it clearly depicts soft-tissue structures, MRI is widely used to diagnose musculoskeletal pathology in and around the knee. Criteria for the diagnosis of discoid menisci include meniscal width of 15 mm or more, ratio of minimum meniscal width to maximum tibial width on coronal slice of more than 20%, ratio of sum of width of both lateral horns to meniscal diameter (on sagittal slice showing maximum meniscal diameter) of more than 75%, and continuity of anterior and posterior horns on at least 3 consecutive sagittal slices (bow tie sign).5,30,31 Even in the presence of a tear, the described ratios have sensitivity and specificity of 95% and 97% in detecting discoid lateral menisci.30
However, the Wrisberg variant, which may consist of only a thickened portion of the posterior horn, is often more difficult to diagnose using these criteria and can even appear normal on MRI.26,32 In a series by Neuschwander and colleagues,7 none of the Wrisberg-variant menisci had a true discoid shape, suggesting that the size of the lateral meniscus may appear normal in affected patients. Appropriate positioning during MRI evaluation of patients suspected of having the Wrisberg variant was emphasized by Moser and colleagues,33 who described a case of discoid lateral meniscus not observable on initial MRI but visible on MRI performed with the affected knee extended in the locked position.
The unstable lateral meniscus may be seen subluxed anteriorly or laterally because of lack of posterior attachments. A deficiency of normal popliteomeniscal fascicles and coronary ligaments is represented by a high T2 signal interposed between the discoid lateral meniscus and the posterior joint capsule, simulating a vertical peripheral tear and suggesting presence of the Wrisberg variant (Figures 1A–1C). In addition, the posterior horn of the enlarged discoid lateral meniscus may connect to a prominent and thickened meniscofemoral ligament of Wrisberg. Despite these characteristic imaging features, some studies have found low sensitivity of MRI in the diagnosis of Wrisberg-variant discoid lateral menisci.26
Ultrasonography
There is a growing interest in using ultrasonography in the diagnosis of Wrisberg-variant discoid lateral menisci because of its availability, multiplanar capability, and lower cost compared with MRI. Ultrasonographic criteria for the diagnosis of discoid menisci include absence of normal triangular shape, presence of abnormally elongated and thickened meniscal tissue, and demonstration of a heterogeneous central pattern.5 Through use of a high-resolution probe, which better fits the anatomical concavity of the popliteal fossa, a positive predictive value of 95% and a negative predictive value of 100% have been reported for ultrasonography in the diagnosis of meniscal tears.34
Perhaps the main advantage of ultrasonography is the possibility of performing a dynamic study to evaluate the extrusion of the meniscus into the lateral gutter and to correlate this with knee snapping (Figures 2A, 2B).35 One technique for sonographic evaluation of a hypermobile lateral meniscus involves placing the patient supine with the high-resolution (9 or 12 MHz) linear transducer along the lateral knee joint line. The patient is then asked to place the foot of the affected extremity on the contralateral knee; the combination resembles the numeral 4 (figure-4 test) (Figures 3A, 3B). In a symptomatic patient, this results in clicking, snapping, and/or pain along the lateral joint line, and the lateral meniscus is noted sonographically to extrude into the lateral gutter (Figure 2B), either the result of torn popliteomeniscal fascicles or the increased meniscal mobility of Wrisberg variants.
The main drawback of ultrasonography is operator dependence. As clinicians become more familiar with ultrasonography, dynamic ultrasonography should be used for what is often a difficult diagnosis both clinically and with nondynamic imaging.
Management
The historical treatment for symptomatic discoid lateral menisci, open total meniscectomy,5,7,15,36 is no longer performed, as studies have shown it increases contact stresses proportional to the amount of meniscus removed, with up to a 235% increase after total meniscectomy,37 predisposing patients to early degenerative changes and osteoarthritis.38-41
With an appreciation of the role of menisci as load distributors and joint stabilizers in cartilage nutrition, current treatments aim to preserve as much stable meniscal tissue as possible.5 Surgical management of Wrisberg-variant discoid lateral menisci involves posterior stabilization with or without saucerization.7,33,42 The goal of arthroscopic saucerization is to preserve healthy tissue and create a stable remaining meniscus (6-8 mm in width)2,7,43,44 that provides adequate shock absorption without retearing.10 Wrisberg-variant discoid menisci can be stabilized with use of all-inside sutures from the meniscus to the joint capsule (Figures 4A–4F) when there is sufficient residual meniscus to allow for suture fixation to the posterior capsule after débridement. In contrast, some prefer an inside-out technique, as described by Neuschwander and colleagues,7 with inclusion of a mini-open approach. Any meniscal tears are addressed at time of surgery, either by partial meniscectomy or repair. Relative indications for meniscal repair include longitudinal, vertical, nondegenerative tears that are within 3 mm of the periphery (vascular zone) and are less than 3 cm in length.45 However, the majority of tears in adults are degenerative cleavage tears outside the vascular zone and therefore not amenable to repair.45,46 Before surgery, patients treated with stabilization with or without saucerization are prescribed partial weight-bearing in a hinged knee brace with gradual range of motion to 90° by 6 weeks and return to sports in 3 to 4 months.
Clinical Results
As has been consistently demonstrated, the long-term outcomes of total meniscectomy are poor function39,40,47 and radiographic evidence of lateral compartment arthritis.48 Patients who previously underwent total meniscectomy should be offered meniscal allograft transplantation, as it may offset the increased peak local contact pressures in the lateral compartment10 and improve function.49
With an appreciation for the importance of meniscus preservation, more recent studies have found encouraging results for arthroscopic saucerization and stabilization of Wrisberg-variant discoid lateral menisci. For example, Woods and Whelan44 reported excellent results in 75% of patients at 37.5-month follow-up after open repair of discoid lateral menisci lacking posterior attachments. In another study, by Neuschwander and colleagues,7 4 of 6 patients who underwent arthroscopic repair of unstable discoid lateral menisci without posterior coronary ligaments had excellent outcomes. Although these studies demonstrated symptom resolution and lack of radiographic evidence of degenerative changes at midterm follow-up,50 additional long-term studies should be performed to determine whether saucerization and stabilization prevent the onset of lateral compartment osteoarthritis.
Conclusion
Abnormally mobile discoid lateral menisci can result in painful lateral snapping knee syndromes but are often challenging to diagnose clinically and with traditional static imaging. Dynamic ultrasonography with provocative maneuvers can reveal lateral meniscal subluxation, which often cannot be appreciated on MRI, allowing for timely stabilization and symptom resolution.
1. Young RB. The external semilunar cartilage as a complete disc. In: Cleland J, Mackey JY, Young RB, eds. Memoirs and Memoranda in Anatomy. London, England: Williams & Norgate; 1889:179.
2. Jordan MR. Lateral meniscal variants: evaluation and treatment. J Am Acad Orthop Surg. 1996;4(4):191-200.
3. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176.
4. Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop. 1982;(167):19-28.
5. Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop. 2007;1(2):89-96.
6. Watanabe M, Takeda S, Ikeuchi H. Atlas of Arthroscopy. Tokyo, Japan: Igaku-Shoin; 1978.
7. Neuschwander DC, Drez D Jr, Finney TP. Lateral meniscal variant with absence of the posterior coronary ligament. J Bone Joint Surg Am. 1992;74(8):1186-1190.
8. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65(4):538-547.
9. Kaplan EB. Discoid lateral meniscus of the knee joint; nature, mechanism, and operative treatment. J Bone Joint Surg Am. 1957;39(1):77-87.
10. Kramer DE, Micheli LJ. Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg. 2009;17(11):698-707.
11. Atay OA, Pekmezci M, Doral MN, Sargon MF, Ayvaz M, Johnson DL. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35(3):475-478.
12. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
13. Good CR, Green DW, Griffith MH, Valen AW, Widmann RF, Rodeo SA. Arthroscopic treatment of symptomatic discoid meniscus in children: classification, technique, and results. Arthroscopy. 2007;23(2):157-163.
14. Harner CD, Xerogeanes JW, Livesay GA, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736-745.
15. Smillie IS. The congenital discoid meniscus. J Bone Joint Surg Br. 1948;30(4):671-682.
16. Yoo WJ, Choi IH, Chung CY, et al. Discoid lateral meniscus in children: limited knee extension and meniscal instability in the posterior segment. J Pediatr Orthop. 2008;28(5):544-548.
17. Simonian PT, Sussmann PS, Wickiewicz TL, et al. Popliteomeniscal fasciculi and the unstable lateral meniscus: clinical correlation and magnetic resonance diagnosis. Arthroscopy. 1997;13(5):590-596.
18. Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am. 1982;64(7):1068-1073.
19. Kroiss F. Die Verletzungen der Kniegelenkoszwischenknorpel und ihrer Verbindungen. Beitr Klin Chir. 1910;66:598-801.
20. Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop. 1992;(283):205-206.
21. Cooper DE. Snapping popliteus tendon syndrome. A cause of mechanical knee popping in athletes. Am J Sports Med. 1999;27(5):671-674.
22. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007;14(2):167-168.
23. Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5(1):52-56.
24. Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am. 1995;77(9):1357-1361.
25. LaPrade RF, Konowalchuk BK. Popliteomeniscal fascicle tears causing symptomatic lateral compartment knee pain: diagnosis by the figure-4 test and treatment by open repair. Am J Sports Med. 2005;33(8):1231-1236.
26. Kocher MS, DiCanzio J, Zurakowski D, Micheli LJ. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29(3):292-296.
27. Stanitski CL. Correlation of arthroscopic and clinical examinations with magnetic resonance imaging findings of injured knees in children and adolescents. Am J Sports Med. 1998;26(1):2-6.
28. Picard JJ, Constantin L. Radiological aspects of the discoid meniscus [in French]. J Radiol Electrol Med Nucl. 1964;45:839-841.
29. Kerr R. Radiologic case study. Discoid lateral meniscus. Orthopedics. 1986;9(8):1142, 1145-1147.
30. Samoto N, Kozuma M, Tokuhisa T, Kobayashi K. Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging. 2002;20(1):59-64.
31. Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology. 1989;173(2):351-354.
32. Singh K, Helms CA, Jacobs MT, Higgins LD. MRI appearance of Wrisberg variant of discoid lateral meniscus. AJR Am J Roentgenol. 2006;187(2):384-387.
33. Moser MW, Dugas J, Hartzell J, Thornton DD. A hypermobile Wrisberg variant lateral discoid meniscus seen on MRI. Clin Orthop. 2007;(456):264-267.
34. Najafi J, Bagheri S, Lahiji FA. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J Ultrasound Med. 2006;25(5):593-597.
35. Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142-150.
36. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
37. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270-275.
38. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
39. Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11(3):111-115.
40. Wroble RR, Henderson RC, Campion ER, el-Khoury GY, Albright JP. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop. 1992;(279):180-189.
41. Abdon P, Turner MS, Pettersson H, Lindstrand A, Stenstrom A, Swanson AJ. A long-term follow-up study of total meniscectomy in children. Clin Orthop. 1990;(257):166-170.
42. Rosenberg TD, Paulos LE, Parker RD, Harner CD, Gurley WD. Discoid lateral meniscus: case report of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy. 1987;3(4):277-282.
43. Fleissner PR, Eilert RE. Discoid lateral meniscus. Am J Knee Surg. 1999;12(2):125-131.
44. Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9(3):695-706.
45. Yue BW, Gupta AK, Moorman CT 3rd, Garrett WE, Helms CA. Wrisberg variant of the discoid lateral meniscus with flipped meniscal fragments simulating bucket-handle tear: MRI and arthroscopic correlation. Skeletal Radiol. 2011;40(8):1089-1094.
46. Weiss CB, Lundberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71(6):811-822.
47. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
48. Kim SJ, Chun YM, Jeong JH, Ryu SW, Oh KS, Lubis AM. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1315-1320.
49. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22(12):1344-1350.e1.
50. Ogut T, Kesmezacar H, Akgun I, Cansu E. Arthroscopic meniscectomy for discoid lateral meniscus in children and adolescents: 4.5 year follow-up. J Pediatr Orthop B. 2003;12(6):390-397.
1. Young RB. The external semilunar cartilage as a complete disc. In: Cleland J, Mackey JY, Young RB, eds. Memoirs and Memoranda in Anatomy. London, England: Williams & Norgate; 1889:179.
2. Jordan MR. Lateral meniscal variants: evaluation and treatment. J Am Acad Orthop Surg. 1996;4(4):191-200.
3. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176.
4. Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop. 1982;(167):19-28.
5. Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop. 2007;1(2):89-96.
6. Watanabe M, Takeda S, Ikeuchi H. Atlas of Arthroscopy. Tokyo, Japan: Igaku-Shoin; 1978.
7. Neuschwander DC, Drez D Jr, Finney TP. Lateral meniscal variant with absence of the posterior coronary ligament. J Bone Joint Surg Am. 1992;74(8):1186-1190.
8. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65(4):538-547.
9. Kaplan EB. Discoid lateral meniscus of the knee joint; nature, mechanism, and operative treatment. J Bone Joint Surg Am. 1957;39(1):77-87.
10. Kramer DE, Micheli LJ. Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg. 2009;17(11):698-707.
11. Atay OA, Pekmezci M, Doral MN, Sargon MF, Ayvaz M, Johnson DL. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35(3):475-478.
12. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
13. Good CR, Green DW, Griffith MH, Valen AW, Widmann RF, Rodeo SA. Arthroscopic treatment of symptomatic discoid meniscus in children: classification, technique, and results. Arthroscopy. 2007;23(2):157-163.
14. Harner CD, Xerogeanes JW, Livesay GA, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736-745.
15. Smillie IS. The congenital discoid meniscus. J Bone Joint Surg Br. 1948;30(4):671-682.
16. Yoo WJ, Choi IH, Chung CY, et al. Discoid lateral meniscus in children: limited knee extension and meniscal instability in the posterior segment. J Pediatr Orthop. 2008;28(5):544-548.
17. Simonian PT, Sussmann PS, Wickiewicz TL, et al. Popliteomeniscal fasciculi and the unstable lateral meniscus: clinical correlation and magnetic resonance diagnosis. Arthroscopy. 1997;13(5):590-596.
18. Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am. 1982;64(7):1068-1073.
19. Kroiss F. Die Verletzungen der Kniegelenkoszwischenknorpel und ihrer Verbindungen. Beitr Klin Chir. 1910;66:598-801.
20. Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop. 1992;(283):205-206.
21. Cooper DE. Snapping popliteus tendon syndrome. A cause of mechanical knee popping in athletes. Am J Sports Med. 1999;27(5):671-674.
22. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007;14(2):167-168.
23. Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5(1):52-56.
24. Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am. 1995;77(9):1357-1361.
25. LaPrade RF, Konowalchuk BK. Popliteomeniscal fascicle tears causing symptomatic lateral compartment knee pain: diagnosis by the figure-4 test and treatment by open repair. Am J Sports Med. 2005;33(8):1231-1236.
26. Kocher MS, DiCanzio J, Zurakowski D, Micheli LJ. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29(3):292-296.
27. Stanitski CL. Correlation of arthroscopic and clinical examinations with magnetic resonance imaging findings of injured knees in children and adolescents. Am J Sports Med. 1998;26(1):2-6.
28. Picard JJ, Constantin L. Radiological aspects of the discoid meniscus [in French]. J Radiol Electrol Med Nucl. 1964;45:839-841.
29. Kerr R. Radiologic case study. Discoid lateral meniscus. Orthopedics. 1986;9(8):1142, 1145-1147.
30. Samoto N, Kozuma M, Tokuhisa T, Kobayashi K. Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging. 2002;20(1):59-64.
31. Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology. 1989;173(2):351-354.
32. Singh K, Helms CA, Jacobs MT, Higgins LD. MRI appearance of Wrisberg variant of discoid lateral meniscus. AJR Am J Roentgenol. 2006;187(2):384-387.
33. Moser MW, Dugas J, Hartzell J, Thornton DD. A hypermobile Wrisberg variant lateral discoid meniscus seen on MRI. Clin Orthop. 2007;(456):264-267.
34. Najafi J, Bagheri S, Lahiji FA. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J Ultrasound Med. 2006;25(5):593-597.
35. Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142-150.
36. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
37. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270-275.
38. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
39. Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11(3):111-115.
40. Wroble RR, Henderson RC, Campion ER, el-Khoury GY, Albright JP. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop. 1992;(279):180-189.
41. Abdon P, Turner MS, Pettersson H, Lindstrand A, Stenstrom A, Swanson AJ. A long-term follow-up study of total meniscectomy in children. Clin Orthop. 1990;(257):166-170.
42. Rosenberg TD, Paulos LE, Parker RD, Harner CD, Gurley WD. Discoid lateral meniscus: case report of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy. 1987;3(4):277-282.
43. Fleissner PR, Eilert RE. Discoid lateral meniscus. Am J Knee Surg. 1999;12(2):125-131.
44. Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9(3):695-706.
45. Yue BW, Gupta AK, Moorman CT 3rd, Garrett WE, Helms CA. Wrisberg variant of the discoid lateral meniscus with flipped meniscal fragments simulating bucket-handle tear: MRI and arthroscopic correlation. Skeletal Radiol. 2011;40(8):1089-1094.
46. Weiss CB, Lundberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71(6):811-822.
47. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
48. Kim SJ, Chun YM, Jeong JH, Ryu SW, Oh KS, Lubis AM. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1315-1320.
49. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22(12):1344-1350.e1.
50. Ogut T, Kesmezacar H, Akgun I, Cansu E. Arthroscopic meniscectomy for discoid lateral meniscus in children and adolescents: 4.5 year follow-up. J Pediatr Orthop B. 2003;12(6):390-397.