User login
Enhanced Radiation Dermatitis Associated With Concurrent Palliative Radiation and Vemurafenib Therapy
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
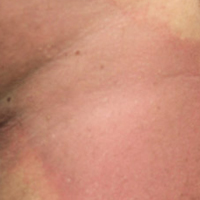
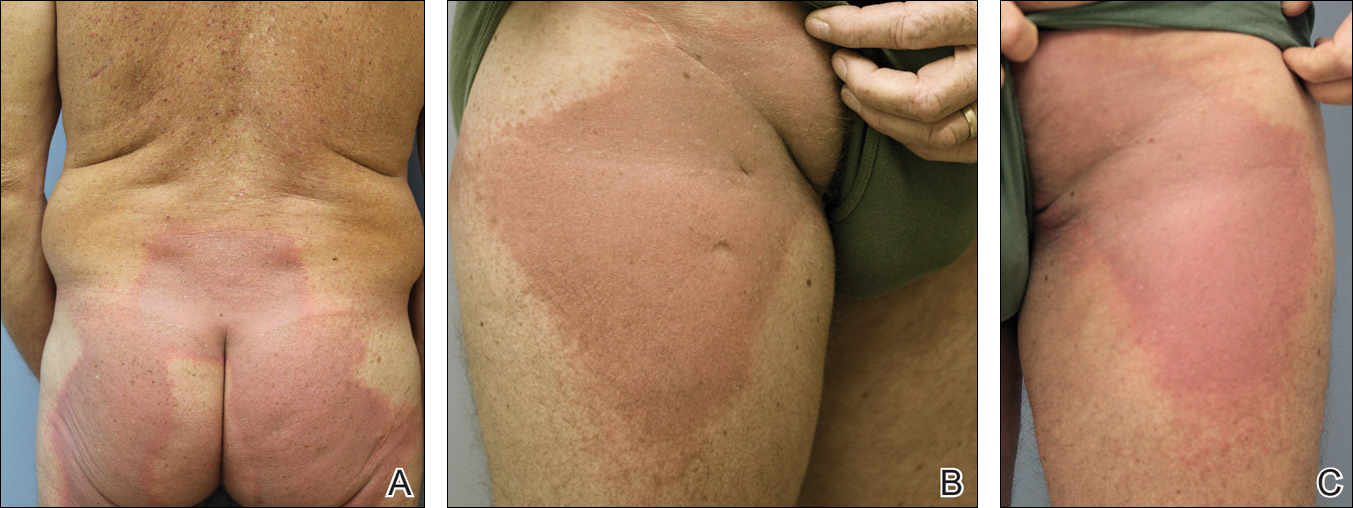
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.
The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
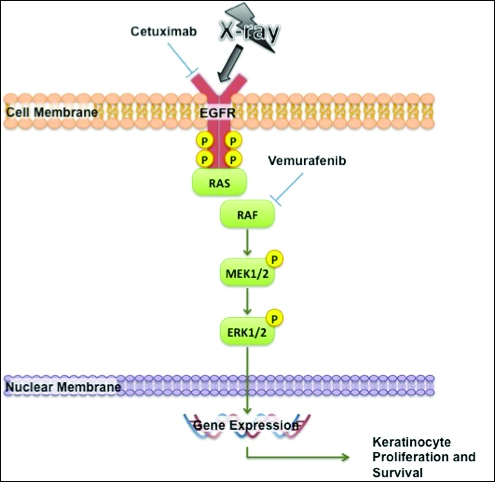
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.
We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.

The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.

We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.

The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.

We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
Practice Points
- Given the increased frequency of palliative and adjuvant radiation therapy in patients with metastatic melanoma, it is critical to understand the potential cutaneous side effects of vemurafenib when used in conjunction with radiotherapy.
- Clinicians should be aware of the increased risk for severe radiation dermatitis in patients on vemurafenib who are receiving concurrent palliative radiation therapy.