User login
Pruritic erythematous maculopapular rash
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 35-year-old man came into our clinic with a rash that had developed a week earlier after a trip to a North Carolina beach. The rash started on his upper inner arms (not including his axilla) and then developed in his groin, thighs, buttocks, and the tops of his feet. There was no rash on his back, head, or neck. The rash was a maculopapular eruption with some confluence, and it had a discrete distribution in his bathing suit area.
The patient said the rash was very itchy, although it had improved over the past couple of days. He did not have any systemic symptoms and hadn’t used any new soaps or detergents, nor had he recently worn any new clothes. He did note, however, that he’d experienced a similar rash in the past after trips to the beach, although the previous rashes were not as severe.
None of the other family members who’d accompanied him to the beach had developed the rash.
FIGURE
A discrete maculopapular eruption in the bathing suit area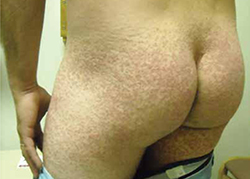
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Seabather’s eruption
The patient was given a diagnosis of seabather’s eruption (SBE), also called seabather’s dermatitis or sea lice. SBE is an intensely itchy papular-erythematous dermatitis that can develop after an individual has been swimming in the ocean.1
Planula larvae of the scyphomedusae Linuche unguiculata—commonly known as the thimble jellyfish—are to blame for this form of dermatitis.2L unguiculata are most frequently found in the waters of the Caribbean, Gulf of Mexico, southern United States, and South America.1 Cases of SBE are most common in the spring and summer months, peaking in May.3 Those at highest risk include children, people with a history of SBE, and water sports enthusiasts (eg, surfers).4
L unguiculata larvae are small enough that they can make their way through the mesh of swimwear. As the bather gets out of the water, the suit acts as a sieve, with the water draining out and many of the larvae staying behind.1 Once the jellyfish are pressed against the skin, a defense mechanism is triggered and envenomation occurs.1,5
As a result, patients will develop rashes not only in areas beneath their swimsuits, but also in the skin folds, such as the axilla, and between the upper thighs. For surfers, the trouble spots are the chest and abdomen—places where the body rubs up against the surfboard.3,6
Onset does not occur immediately. Rather, it takes several hours for the lesions to develop, and new ones may continue to develop for days.5 Immediate stinging sensations are associated with prior cases of SBE and suggest a sensitization to the antigen.3
Not all reactions are the same. Some people will have a severe response, while others appear to be immune.2 More extreme systemic symptoms, such as fever, chills, nausea, malaise, sneezing, dyspnea, vomiting, headache, abdominal pain, and diarrhea have been seen in children and in cases of extensive envenomation.4,6
“Swimmer’s itch” is included in the differential
Other possible causes of pruritic rashes like the one our patient had (TABLE) include:
Cercarial dermatitis, also known as swimmer’s itch, is a maculopapular inflammation characterized by pain, prickling, and pruritus. It develops several hours after bathing in freshwater and is limited to exposed areas of the body. The cause of the dermatitis? The larval trematodes of Shistosoma and Trichobilharzia.5
Phytophotodermatitis is an erythematous pruritic inflammation of the skin with vesicles and bullae. The eruption, which is often hyperpigmented, occurs when an individual spends time in the sun after coming into contact with light-sensitive botanicals, such as limes.7
Infectious folliculitis is an infection of the hair follicle resulting in the formation of multiple pustules. Pseudomonas aeruginosa folliculitis, often called hot tub folliculitis, may be pruritic and tender.7
Grover’s disease is also known as transient acantholytic dermatosis and generally affects middle-aged men. It is a pruritic dermatosis of scaling papules that are distributed along the trunk and can show confluence. Although the cause is unknown, it has been linked with cases of high fever, intense exercise, and significant sun exposure.7
Table
The differential for a pruritic, erythematous maculopapular rash5,7
| Condition | Characteristics |
|---|---|
| Cercarial dermatitis | A maculopapular inflammation characterized by pain, prickling, and pruritus that develops several hours after bathing in freshwater and is limited to exposed areas of the body. |
| Phytophotodermatitis | An erythematous pruritic inflammation of the skin, with vesicles and bullae appearing with hyperpigmented streaks along the body. It occurs when an individual spends time in the sun after coming into contact with light-sensitive botanicals. |
| Infectious folliculitis | An infection of the hair follicle resulting in the formation of multiple pustules. Pseudomonas aeruginosa folliculitis is associated with hot tub use. |
| Seabather’s eruption | An erythematous pruritic papular dermatitis that develops several hours after exposure to ocean water. It is limited to areas of high friction and those covered by swimwear. |
| Grover’s disease | A pruritic dermatosis of scaling papules distributed along the trunk that mainly affects middle-aged men. Onset is associated with high fever, intense exercise, and significant sun exposure. |
Treatment usually isn’t needed
SBE usually resolves spontaneously within a week or 2.1 If treatment is necessary, start with topical corticosteroids and oral antihistamines. If this proves insufficient, move on to oral corticosteroids1 (strength of recommendation [SOR]: C). To minimize risk, swimmers should remove their bathing suits and shower as soon as possible after leaving the water4,6 (SOR: C).
Benadryl does the trick
We advised our patient to take diphenhydramine (Benadryl) and the itching went away. We also encouraged him to remove his bathing suit and shower as soon as possible after going in the ocean.
CORRESPONDENCE Blake Fagan, MD, MAHEC Family Medicine Residency Program, 118 W.T. Weaver Boulevard, Asheville, NC 28804; blake.fagan@mahec.net
1. Rossetto AL, Dellatorre G, Silveira FL, et al. Seabather’s eruption: a clinical and epidemiological study of 38 cases in Santa Catarina State, Brazil. Rev Inst Med Trop Sao Paulo. 2009;51:169-175.
2. Black NA, Szmant AM, Tomchik RS. Planule of the scyphomedusa Linuche unguiculata as a possible cause of seabather’s eruption. Bulletin of Marine Science. 1994;54:955-960.
3. Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice.’ JAMA. 1993;269:1669-1672.
4. Kumar S, Hlady WG, Malecki JM. Risk factors for seabather’s eruption: a prospective cohort study. Public Health Rep. 1997;112:59-62.
5. Haddad V, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
6. Wong DE, Meinking TL, Rosen LB, et al. Seabather’s eruption: clinical, histologic and immunologic features. J Am Acad Dermatol. 1994;30:399-406.
7. Wolff K, Johnson RA. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill; 2009. Available at: http://www.accessmedicine.com/resourceTOC.aspx?resourceID=45. Accessed August 6, 2010.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 35-year-old man came into our clinic with a rash that had developed a week earlier after a trip to a North Carolina beach. The rash started on his upper inner arms (not including his axilla) and then developed in his groin, thighs, buttocks, and the tops of his feet. There was no rash on his back, head, or neck. The rash was a maculopapular eruption with some confluence, and it had a discrete distribution in his bathing suit area.
The patient said the rash was very itchy, although it had improved over the past couple of days. He did not have any systemic symptoms and hadn’t used any new soaps or detergents, nor had he recently worn any new clothes. He did note, however, that he’d experienced a similar rash in the past after trips to the beach, although the previous rashes were not as severe.
None of the other family members who’d accompanied him to the beach had developed the rash.
FIGURE
A discrete maculopapular eruption in the bathing suit area
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Seabather’s eruption
The patient was given a diagnosis of seabather’s eruption (SBE), also called seabather’s dermatitis or sea lice. SBE is an intensely itchy papular-erythematous dermatitis that can develop after an individual has been swimming in the ocean.1
Planula larvae of the scyphomedusae Linuche unguiculata—commonly known as the thimble jellyfish—are to blame for this form of dermatitis.2L unguiculata are most frequently found in the waters of the Caribbean, Gulf of Mexico, southern United States, and South America.1 Cases of SBE are most common in the spring and summer months, peaking in May.3 Those at highest risk include children, people with a history of SBE, and water sports enthusiasts (eg, surfers).4
L unguiculata larvae are small enough that they can make their way through the mesh of swimwear. As the bather gets out of the water, the suit acts as a sieve, with the water draining out and many of the larvae staying behind.1 Once the jellyfish are pressed against the skin, a defense mechanism is triggered and envenomation occurs.1,5
As a result, patients will develop rashes not only in areas beneath their swimsuits, but also in the skin folds, such as the axilla, and between the upper thighs. For surfers, the trouble spots are the chest and abdomen—places where the body rubs up against the surfboard.3,6
Onset does not occur immediately. Rather, it takes several hours for the lesions to develop, and new ones may continue to develop for days.5 Immediate stinging sensations are associated with prior cases of SBE and suggest a sensitization to the antigen.3
Not all reactions are the same. Some people will have a severe response, while others appear to be immune.2 More extreme systemic symptoms, such as fever, chills, nausea, malaise, sneezing, dyspnea, vomiting, headache, abdominal pain, and diarrhea have been seen in children and in cases of extensive envenomation.4,6
“Swimmer’s itch” is included in the differential
Other possible causes of pruritic rashes like the one our patient had (TABLE) include:
Cercarial dermatitis, also known as swimmer’s itch, is a maculopapular inflammation characterized by pain, prickling, and pruritus. It develops several hours after bathing in freshwater and is limited to exposed areas of the body. The cause of the dermatitis? The larval trematodes of Shistosoma and Trichobilharzia.5
Phytophotodermatitis is an erythematous pruritic inflammation of the skin with vesicles and bullae. The eruption, which is often hyperpigmented, occurs when an individual spends time in the sun after coming into contact with light-sensitive botanicals, such as limes.7
Infectious folliculitis is an infection of the hair follicle resulting in the formation of multiple pustules. Pseudomonas aeruginosa folliculitis, often called hot tub folliculitis, may be pruritic and tender.7
Grover’s disease is also known as transient acantholytic dermatosis and generally affects middle-aged men. It is a pruritic dermatosis of scaling papules that are distributed along the trunk and can show confluence. Although the cause is unknown, it has been linked with cases of high fever, intense exercise, and significant sun exposure.7
Table
The differential for a pruritic, erythematous maculopapular rash5,7
| Condition | Characteristics |
|---|---|
| Cercarial dermatitis | A maculopapular inflammation characterized by pain, prickling, and pruritus that develops several hours after bathing in freshwater and is limited to exposed areas of the body. |
| Phytophotodermatitis | An erythematous pruritic inflammation of the skin, with vesicles and bullae appearing with hyperpigmented streaks along the body. It occurs when an individual spends time in the sun after coming into contact with light-sensitive botanicals. |
| Infectious folliculitis | An infection of the hair follicle resulting in the formation of multiple pustules. Pseudomonas aeruginosa folliculitis is associated with hot tub use. |
| Seabather’s eruption | An erythematous pruritic papular dermatitis that develops several hours after exposure to ocean water. It is limited to areas of high friction and those covered by swimwear. |
| Grover’s disease | A pruritic dermatosis of scaling papules distributed along the trunk that mainly affects middle-aged men. Onset is associated with high fever, intense exercise, and significant sun exposure. |
Treatment usually isn’t needed
SBE usually resolves spontaneously within a week or 2.1 If treatment is necessary, start with topical corticosteroids and oral antihistamines. If this proves insufficient, move on to oral corticosteroids1 (strength of recommendation [SOR]: C). To minimize risk, swimmers should remove their bathing suits and shower as soon as possible after leaving the water4,6 (SOR: C).
Benadryl does the trick
We advised our patient to take diphenhydramine (Benadryl) and the itching went away. We also encouraged him to remove his bathing suit and shower as soon as possible after going in the ocean.
CORRESPONDENCE Blake Fagan, MD, MAHEC Family Medicine Residency Program, 118 W.T. Weaver Boulevard, Asheville, NC 28804; blake.fagan@mahec.net
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 35-year-old man came into our clinic with a rash that had developed a week earlier after a trip to a North Carolina beach. The rash started on his upper inner arms (not including his axilla) and then developed in his groin, thighs, buttocks, and the tops of his feet. There was no rash on his back, head, or neck. The rash was a maculopapular eruption with some confluence, and it had a discrete distribution in his bathing suit area.
The patient said the rash was very itchy, although it had improved over the past couple of days. He did not have any systemic symptoms and hadn’t used any new soaps or detergents, nor had he recently worn any new clothes. He did note, however, that he’d experienced a similar rash in the past after trips to the beach, although the previous rashes were not as severe.
None of the other family members who’d accompanied him to the beach had developed the rash.
FIGURE
A discrete maculopapular eruption in the bathing suit area
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Seabather’s eruption
The patient was given a diagnosis of seabather’s eruption (SBE), also called seabather’s dermatitis or sea lice. SBE is an intensely itchy papular-erythematous dermatitis that can develop after an individual has been swimming in the ocean.1
Planula larvae of the scyphomedusae Linuche unguiculata—commonly known as the thimble jellyfish—are to blame for this form of dermatitis.2L unguiculata are most frequently found in the waters of the Caribbean, Gulf of Mexico, southern United States, and South America.1 Cases of SBE are most common in the spring and summer months, peaking in May.3 Those at highest risk include children, people with a history of SBE, and water sports enthusiasts (eg, surfers).4
L unguiculata larvae are small enough that they can make their way through the mesh of swimwear. As the bather gets out of the water, the suit acts as a sieve, with the water draining out and many of the larvae staying behind.1 Once the jellyfish are pressed against the skin, a defense mechanism is triggered and envenomation occurs.1,5
As a result, patients will develop rashes not only in areas beneath their swimsuits, but also in the skin folds, such as the axilla, and between the upper thighs. For surfers, the trouble spots are the chest and abdomen—places where the body rubs up against the surfboard.3,6
Onset does not occur immediately. Rather, it takes several hours for the lesions to develop, and new ones may continue to develop for days.5 Immediate stinging sensations are associated with prior cases of SBE and suggest a sensitization to the antigen.3
Not all reactions are the same. Some people will have a severe response, while others appear to be immune.2 More extreme systemic symptoms, such as fever, chills, nausea, malaise, sneezing, dyspnea, vomiting, headache, abdominal pain, and diarrhea have been seen in children and in cases of extensive envenomation.4,6
“Swimmer’s itch” is included in the differential
Other possible causes of pruritic rashes like the one our patient had (TABLE) include:
Cercarial dermatitis, also known as swimmer’s itch, is a maculopapular inflammation characterized by pain, prickling, and pruritus. It develops several hours after bathing in freshwater and is limited to exposed areas of the body. The cause of the dermatitis? The larval trematodes of Shistosoma and Trichobilharzia.5
Phytophotodermatitis is an erythematous pruritic inflammation of the skin with vesicles and bullae. The eruption, which is often hyperpigmented, occurs when an individual spends time in the sun after coming into contact with light-sensitive botanicals, such as limes.7
Infectious folliculitis is an infection of the hair follicle resulting in the formation of multiple pustules. Pseudomonas aeruginosa folliculitis, often called hot tub folliculitis, may be pruritic and tender.7
Grover’s disease is also known as transient acantholytic dermatosis and generally affects middle-aged men. It is a pruritic dermatosis of scaling papules that are distributed along the trunk and can show confluence. Although the cause is unknown, it has been linked with cases of high fever, intense exercise, and significant sun exposure.7
Table
The differential for a pruritic, erythematous maculopapular rash5,7
| Condition | Characteristics |
|---|---|
| Cercarial dermatitis | A maculopapular inflammation characterized by pain, prickling, and pruritus that develops several hours after bathing in freshwater and is limited to exposed areas of the body. |
| Phytophotodermatitis | An erythematous pruritic inflammation of the skin, with vesicles and bullae appearing with hyperpigmented streaks along the body. It occurs when an individual spends time in the sun after coming into contact with light-sensitive botanicals. |
| Infectious folliculitis | An infection of the hair follicle resulting in the formation of multiple pustules. Pseudomonas aeruginosa folliculitis is associated with hot tub use. |
| Seabather’s eruption | An erythematous pruritic papular dermatitis that develops several hours after exposure to ocean water. It is limited to areas of high friction and those covered by swimwear. |
| Grover’s disease | A pruritic dermatosis of scaling papules distributed along the trunk that mainly affects middle-aged men. Onset is associated with high fever, intense exercise, and significant sun exposure. |
Treatment usually isn’t needed
SBE usually resolves spontaneously within a week or 2.1 If treatment is necessary, start with topical corticosteroids and oral antihistamines. If this proves insufficient, move on to oral corticosteroids1 (strength of recommendation [SOR]: C). To minimize risk, swimmers should remove their bathing suits and shower as soon as possible after leaving the water4,6 (SOR: C).
Benadryl does the trick
We advised our patient to take diphenhydramine (Benadryl) and the itching went away. We also encouraged him to remove his bathing suit and shower as soon as possible after going in the ocean.
CORRESPONDENCE Blake Fagan, MD, MAHEC Family Medicine Residency Program, 118 W.T. Weaver Boulevard, Asheville, NC 28804; blake.fagan@mahec.net
1. Rossetto AL, Dellatorre G, Silveira FL, et al. Seabather’s eruption: a clinical and epidemiological study of 38 cases in Santa Catarina State, Brazil. Rev Inst Med Trop Sao Paulo. 2009;51:169-175.
2. Black NA, Szmant AM, Tomchik RS. Planule of the scyphomedusa Linuche unguiculata as a possible cause of seabather’s eruption. Bulletin of Marine Science. 1994;54:955-960.
3. Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice.’ JAMA. 1993;269:1669-1672.
4. Kumar S, Hlady WG, Malecki JM. Risk factors for seabather’s eruption: a prospective cohort study. Public Health Rep. 1997;112:59-62.
5. Haddad V, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
6. Wong DE, Meinking TL, Rosen LB, et al. Seabather’s eruption: clinical, histologic and immunologic features. J Am Acad Dermatol. 1994;30:399-406.
7. Wolff K, Johnson RA. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill; 2009. Available at: http://www.accessmedicine.com/resourceTOC.aspx?resourceID=45. Accessed August 6, 2010.
1. Rossetto AL, Dellatorre G, Silveira FL, et al. Seabather’s eruption: a clinical and epidemiological study of 38 cases in Santa Catarina State, Brazil. Rev Inst Med Trop Sao Paulo. 2009;51:169-175.
2. Black NA, Szmant AM, Tomchik RS. Planule of the scyphomedusa Linuche unguiculata as a possible cause of seabather’s eruption. Bulletin of Marine Science. 1994;54:955-960.
3. Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice.’ JAMA. 1993;269:1669-1672.
4. Kumar S, Hlady WG, Malecki JM. Risk factors for seabather’s eruption: a prospective cohort study. Public Health Rep. 1997;112:59-62.
5. Haddad V, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
6. Wong DE, Meinking TL, Rosen LB, et al. Seabather’s eruption: clinical, histologic and immunologic features. J Am Acad Dermatol. 1994;30:399-406.
7. Wolff K, Johnson RA. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill; 2009. Available at: http://www.accessmedicine.com/resourceTOC.aspx?resourceID=45. Accessed August 6, 2010.
Do preparticipation clinical exams reduce morbidity and mortality for athletes?
Though clinical preparticipation exams (PPE) are recommended by experts and required in most states, we found no medium- or better-quality evidence that demonstrates they reduce mortality or morbidity. PPEs detect only a very small percentage of cardiac abnormalities among athletes who subsequently die suddenly (strength of recommendation [SOR]: C, case series study). PPEs are also unable to accurately identify athletes with exercise-induced bronchospasm (SOR: C, small cross-sectional study) and are poorly predictive of which athletes are at increased risk of orthopedic injuries (SOR: C, cross-sectional study).
Evidence summary
A systematic review of the literature on PPE identified 310 studies of athletes age <36 years. The authors searched multiple electronic databases and reviewed the bibliographies of retrieved articles but did not perform hand searches of journals or contact authors directly. The review did not find any prospective cohort or randomized trials addressing the effectiveness of clinical PPE. The 5 studies that assessed the format of the PPE concluded that it is not adequately standardized, does not consistently address the American Heart Association (AHA) recommendations for cardiovascular screening and exam, and is administered by a variety of health care professionals, some without proper training.1
Sudden cardiac death is defined as a nontraumatic, nonviolent, unexpected event resulting from sudden cardiac arrest within 6 hours of a previously witnessed state of normal health.2 Such events occur in about 1 in 200,000 high school athletes per academic year (about 16 deaths in the US annually). Detection of cardiovascular abnormalities that may cause morbidity or mortality is difficult. A case series reviewed 158 sudden deaths that occurred in trained athletes in the US from 1985 to 1995. The athletes were identified from news accounts, the National Center for Catastrophic Sports Injury Registry, and informal communications and reports. The authors interviewed families, witnesses, and coaches, and they analyzed postmortem information. Of the 115 athletes who had a standard preparticipation medical evaluation, only 4 (3%) were suspected of having cardiovascular disease. The cardiovascular abnormality responsible for sudden death was prospectively identified in only 1 athlete.3
PPE does not accurately identify student athletes with exercise-induced bronchospasm (EIB). Of the studies on EIB, the best was a prospective cross-sectional study of 352 adolescents from 3 suburban Washington state schools. The students completed a 14-item EIB questionnaire, had a physical exam, and underwent a 7-minute exercise challenge spirometry. Complete data were available for 256 of the students. EIB was diagnosed by spirometry in 9.4% of the athletes. No student had EIB detected solely by physical exam. Using a cutoff of 2 positive questions, the questionnaire had a sensitivity of 71% and a specificity of 47%, with a negative and positive predictive value of 6% and 12%, respectively. This study concluded that EIB occurs frequently in adolescent athletes, but screening by physical exam and medical history does not accurately detect it.4
PPEs are not able to predict which student athletes will experience an orthopedic injury, and no controlled studies have been done to determine whether PPE prevents or reduces the severity of orthopedic injuries. A study surveyed 1204 student athletes (aged 13–20 years) from Richmond County, Georgia, who had a standardized PPE before participating in sports. The questionnaire was administered via mail or telephone and inquired about injuries sustained after the PPE. The response rate to the survey was 56%. The study found that a history of knee or ankle injury and abnormal findings on exam in male athletes slightly increased the likelihood of repeated injury of the same joint. However, the sensitivities of history or physical exam for ankle or knee injuries were all <25%.5
Recommendations from others
The AHA recommends a national standard for PPE and that screening should be mandatory for all high school and college athletes before participation in organized sports, with screening repeated every 2 years, and an interim history obtained during the intervening years. Specific items are given in the TABLE.6
In 2004, the American Academy of Family Physicians, along with the American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, and the American Osteopathic Academy of Sports Medicine, published recommendations for PPEs. They suggested a detailed history (consisting of a 16-point questionnaire incorporating AHA recommendations for cardiovascular screening), limited medical exam, and a detailed musculoskeletal exam evaluating strength, flexibility, and stability of major joints.7
TABLE
AHA recommendations for preparticipation exams
| CARDIOVASCULAR SCREENING QUESTIONS |
|
| CARDIOVASCULAR SCREENING EXAM |
|
| CARDIAC FINDINGS REQUIRING FURTHER EVALUATION |
|
The PPE provides us an opportunity to address preventive health issues
Beth Anne Fox, MD, MPH
East Tennessee State University, Kingsport Family Medicine Residency, Kingsport, Tennessee
Most physicians involved in screening athletes recognize the limitations of PPEs in detecting those at risk for sports-related morbidity and mortality. The history is the most important part of the examination for identifying athletes who might be at risk and should be thorough. Prepared PPE forms such as those endorsed by the AAFP and ACSM can assist in obtaining this history. Because this may be the only occasion for the athlete to see a physician, the examination is best performed by a primary care provider who can use the opportunity to address preventive health issues such as tobacco, alcohol, and drug use, depression and suicidality, sexuality, nutrition, and accident prevention. This kind of counseling is difficult to do in a group format.
1. Wingfield K, Matheson GO, Meeuwisse WH. Preparticipation evaluation: an evidence-based review. Clin J Sport Med 2004;14:109-122.
2. Lyznicki JM, Nielsen NH, Schneider JF. Cardiovascular screening of athletes. Am Fam Physician 2000;62:765-774.Erratum in: Am Fam Physician, 2001; 63:2332.
3. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA 1996;276:199-204.
4. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr 2002;141:343-348.
5. DuRant RH, Pendergrast RA, Seymore C, Gaillard G, Donner J. Findings from the preparticipation athletic examination and athletic injuries. Am J Dis Child 1992;146:85.-
6. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 1996;94:850-856.
7. Smith DM. American Academy of Family Physicians, Preparticipation Physical Evaluation Task Force. Preparticipation Physical Evaluation. 3rd ed. Minneapolis: McGraw-Hill Healthcare; 2004.
Though clinical preparticipation exams (PPE) are recommended by experts and required in most states, we found no medium- or better-quality evidence that demonstrates they reduce mortality or morbidity. PPEs detect only a very small percentage of cardiac abnormalities among athletes who subsequently die suddenly (strength of recommendation [SOR]: C, case series study). PPEs are also unable to accurately identify athletes with exercise-induced bronchospasm (SOR: C, small cross-sectional study) and are poorly predictive of which athletes are at increased risk of orthopedic injuries (SOR: C, cross-sectional study).
Evidence summary
A systematic review of the literature on PPE identified 310 studies of athletes age <36 years. The authors searched multiple electronic databases and reviewed the bibliographies of retrieved articles but did not perform hand searches of journals or contact authors directly. The review did not find any prospective cohort or randomized trials addressing the effectiveness of clinical PPE. The 5 studies that assessed the format of the PPE concluded that it is not adequately standardized, does not consistently address the American Heart Association (AHA) recommendations for cardiovascular screening and exam, and is administered by a variety of health care professionals, some without proper training.1
Sudden cardiac death is defined as a nontraumatic, nonviolent, unexpected event resulting from sudden cardiac arrest within 6 hours of a previously witnessed state of normal health.2 Such events occur in about 1 in 200,000 high school athletes per academic year (about 16 deaths in the US annually). Detection of cardiovascular abnormalities that may cause morbidity or mortality is difficult. A case series reviewed 158 sudden deaths that occurred in trained athletes in the US from 1985 to 1995. The athletes were identified from news accounts, the National Center for Catastrophic Sports Injury Registry, and informal communications and reports. The authors interviewed families, witnesses, and coaches, and they analyzed postmortem information. Of the 115 athletes who had a standard preparticipation medical evaluation, only 4 (3%) were suspected of having cardiovascular disease. The cardiovascular abnormality responsible for sudden death was prospectively identified in only 1 athlete.3
PPE does not accurately identify student athletes with exercise-induced bronchospasm (EIB). Of the studies on EIB, the best was a prospective cross-sectional study of 352 adolescents from 3 suburban Washington state schools. The students completed a 14-item EIB questionnaire, had a physical exam, and underwent a 7-minute exercise challenge spirometry. Complete data were available for 256 of the students. EIB was diagnosed by spirometry in 9.4% of the athletes. No student had EIB detected solely by physical exam. Using a cutoff of 2 positive questions, the questionnaire had a sensitivity of 71% and a specificity of 47%, with a negative and positive predictive value of 6% and 12%, respectively. This study concluded that EIB occurs frequently in adolescent athletes, but screening by physical exam and medical history does not accurately detect it.4
PPEs are not able to predict which student athletes will experience an orthopedic injury, and no controlled studies have been done to determine whether PPE prevents or reduces the severity of orthopedic injuries. A study surveyed 1204 student athletes (aged 13–20 years) from Richmond County, Georgia, who had a standardized PPE before participating in sports. The questionnaire was administered via mail or telephone and inquired about injuries sustained after the PPE. The response rate to the survey was 56%. The study found that a history of knee or ankle injury and abnormal findings on exam in male athletes slightly increased the likelihood of repeated injury of the same joint. However, the sensitivities of history or physical exam for ankle or knee injuries were all <25%.5
Recommendations from others
The AHA recommends a national standard for PPE and that screening should be mandatory for all high school and college athletes before participation in organized sports, with screening repeated every 2 years, and an interim history obtained during the intervening years. Specific items are given in the TABLE.6
In 2004, the American Academy of Family Physicians, along with the American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, and the American Osteopathic Academy of Sports Medicine, published recommendations for PPEs. They suggested a detailed history (consisting of a 16-point questionnaire incorporating AHA recommendations for cardiovascular screening), limited medical exam, and a detailed musculoskeletal exam evaluating strength, flexibility, and stability of major joints.7
TABLE
AHA recommendations for preparticipation exams
| CARDIOVASCULAR SCREENING QUESTIONS |
|
| CARDIOVASCULAR SCREENING EXAM |
|
| CARDIAC FINDINGS REQUIRING FURTHER EVALUATION |
|
The PPE provides us an opportunity to address preventive health issues
Beth Anne Fox, MD, MPH
East Tennessee State University, Kingsport Family Medicine Residency, Kingsport, Tennessee
Most physicians involved in screening athletes recognize the limitations of PPEs in detecting those at risk for sports-related morbidity and mortality. The history is the most important part of the examination for identifying athletes who might be at risk and should be thorough. Prepared PPE forms such as those endorsed by the AAFP and ACSM can assist in obtaining this history. Because this may be the only occasion for the athlete to see a physician, the examination is best performed by a primary care provider who can use the opportunity to address preventive health issues such as tobacco, alcohol, and drug use, depression and suicidality, sexuality, nutrition, and accident prevention. This kind of counseling is difficult to do in a group format.
Though clinical preparticipation exams (PPE) are recommended by experts and required in most states, we found no medium- or better-quality evidence that demonstrates they reduce mortality or morbidity. PPEs detect only a very small percentage of cardiac abnormalities among athletes who subsequently die suddenly (strength of recommendation [SOR]: C, case series study). PPEs are also unable to accurately identify athletes with exercise-induced bronchospasm (SOR: C, small cross-sectional study) and are poorly predictive of which athletes are at increased risk of orthopedic injuries (SOR: C, cross-sectional study).
Evidence summary
A systematic review of the literature on PPE identified 310 studies of athletes age <36 years. The authors searched multiple electronic databases and reviewed the bibliographies of retrieved articles but did not perform hand searches of journals or contact authors directly. The review did not find any prospective cohort or randomized trials addressing the effectiveness of clinical PPE. The 5 studies that assessed the format of the PPE concluded that it is not adequately standardized, does not consistently address the American Heart Association (AHA) recommendations for cardiovascular screening and exam, and is administered by a variety of health care professionals, some without proper training.1
Sudden cardiac death is defined as a nontraumatic, nonviolent, unexpected event resulting from sudden cardiac arrest within 6 hours of a previously witnessed state of normal health.2 Such events occur in about 1 in 200,000 high school athletes per academic year (about 16 deaths in the US annually). Detection of cardiovascular abnormalities that may cause morbidity or mortality is difficult. A case series reviewed 158 sudden deaths that occurred in trained athletes in the US from 1985 to 1995. The athletes were identified from news accounts, the National Center for Catastrophic Sports Injury Registry, and informal communications and reports. The authors interviewed families, witnesses, and coaches, and they analyzed postmortem information. Of the 115 athletes who had a standard preparticipation medical evaluation, only 4 (3%) were suspected of having cardiovascular disease. The cardiovascular abnormality responsible for sudden death was prospectively identified in only 1 athlete.3
PPE does not accurately identify student athletes with exercise-induced bronchospasm (EIB). Of the studies on EIB, the best was a prospective cross-sectional study of 352 adolescents from 3 suburban Washington state schools. The students completed a 14-item EIB questionnaire, had a physical exam, and underwent a 7-minute exercise challenge spirometry. Complete data were available for 256 of the students. EIB was diagnosed by spirometry in 9.4% of the athletes. No student had EIB detected solely by physical exam. Using a cutoff of 2 positive questions, the questionnaire had a sensitivity of 71% and a specificity of 47%, with a negative and positive predictive value of 6% and 12%, respectively. This study concluded that EIB occurs frequently in adolescent athletes, but screening by physical exam and medical history does not accurately detect it.4
PPEs are not able to predict which student athletes will experience an orthopedic injury, and no controlled studies have been done to determine whether PPE prevents or reduces the severity of orthopedic injuries. A study surveyed 1204 student athletes (aged 13–20 years) from Richmond County, Georgia, who had a standardized PPE before participating in sports. The questionnaire was administered via mail or telephone and inquired about injuries sustained after the PPE. The response rate to the survey was 56%. The study found that a history of knee or ankle injury and abnormal findings on exam in male athletes slightly increased the likelihood of repeated injury of the same joint. However, the sensitivities of history or physical exam for ankle or knee injuries were all <25%.5
Recommendations from others
The AHA recommends a national standard for PPE and that screening should be mandatory for all high school and college athletes before participation in organized sports, with screening repeated every 2 years, and an interim history obtained during the intervening years. Specific items are given in the TABLE.6
In 2004, the American Academy of Family Physicians, along with the American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, and the American Osteopathic Academy of Sports Medicine, published recommendations for PPEs. They suggested a detailed history (consisting of a 16-point questionnaire incorporating AHA recommendations for cardiovascular screening), limited medical exam, and a detailed musculoskeletal exam evaluating strength, flexibility, and stability of major joints.7
TABLE
AHA recommendations for preparticipation exams
| CARDIOVASCULAR SCREENING QUESTIONS |
|
| CARDIOVASCULAR SCREENING EXAM |
|
| CARDIAC FINDINGS REQUIRING FURTHER EVALUATION |
|
The PPE provides us an opportunity to address preventive health issues
Beth Anne Fox, MD, MPH
East Tennessee State University, Kingsport Family Medicine Residency, Kingsport, Tennessee
Most physicians involved in screening athletes recognize the limitations of PPEs in detecting those at risk for sports-related morbidity and mortality. The history is the most important part of the examination for identifying athletes who might be at risk and should be thorough. Prepared PPE forms such as those endorsed by the AAFP and ACSM can assist in obtaining this history. Because this may be the only occasion for the athlete to see a physician, the examination is best performed by a primary care provider who can use the opportunity to address preventive health issues such as tobacco, alcohol, and drug use, depression and suicidality, sexuality, nutrition, and accident prevention. This kind of counseling is difficult to do in a group format.
1. Wingfield K, Matheson GO, Meeuwisse WH. Preparticipation evaluation: an evidence-based review. Clin J Sport Med 2004;14:109-122.
2. Lyznicki JM, Nielsen NH, Schneider JF. Cardiovascular screening of athletes. Am Fam Physician 2000;62:765-774.Erratum in: Am Fam Physician, 2001; 63:2332.
3. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA 1996;276:199-204.
4. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr 2002;141:343-348.
5. DuRant RH, Pendergrast RA, Seymore C, Gaillard G, Donner J. Findings from the preparticipation athletic examination and athletic injuries. Am J Dis Child 1992;146:85.-
6. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 1996;94:850-856.
7. Smith DM. American Academy of Family Physicians, Preparticipation Physical Evaluation Task Force. Preparticipation Physical Evaluation. 3rd ed. Minneapolis: McGraw-Hill Healthcare; 2004.
1. Wingfield K, Matheson GO, Meeuwisse WH. Preparticipation evaluation: an evidence-based review. Clin J Sport Med 2004;14:109-122.
2. Lyznicki JM, Nielsen NH, Schneider JF. Cardiovascular screening of athletes. Am Fam Physician 2000;62:765-774.Erratum in: Am Fam Physician, 2001; 63:2332.
3. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA 1996;276:199-204.
4. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr 2002;141:343-348.
5. DuRant RH, Pendergrast RA, Seymore C, Gaillard G, Donner J. Findings from the preparticipation athletic examination and athletic injuries. Am J Dis Child 1992;146:85.-
6. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 1996;94:850-856.
7. Smith DM. American Academy of Family Physicians, Preparticipation Physical Evaluation Task Force. Preparticipation Physical Evaluation. 3rd ed. Minneapolis: McGraw-Hill Healthcare; 2004.
Evidence-based answers from the Family Physicians Inquiries Network