User login
Prostate Cancer in Male Seniors Part 2: Treatment
This article (part 2 of 2) focuses on the treatment of prostate cancer in seniors. Part 1 provided an overview of prostate cancer epidemiology, pathology, and screening in senior patients.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Recently, the International Society of Geriatric Oncology assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
Patient Evaluation
The practice guidelines of the National Comprehensive Cancer Network (NCCN) recommend using life expectancy in determining treatment.1 Prostate cancer is considered to be an indolent disease, and active therapy may be more harmful than beneficial to older patients whose life expectancy is limited because of treatment-related sequelae. Therefore, an accurate estimation of life expectancy is important in devising treatment strategies for older patients.
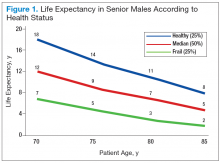
Age should not be the only factor in determining the life expectancy of patients, because life expectancy varies widely based on the patient’s health status, including preexisting comorbidities. Chronologic life expectancy can be found in the Social Security Administration’s life tables. Individual life expectancy is then projected by adding 50% to or deducting 50% from the chronologic life expectancy for men in the highest and lowest quartile of health, respectively. The life expectancy from the life tables can be applied with no addition or subtraction for men in the middle 2 quartiles of health status (Figure 1).2
Geriatric Assessment
Despite the increasing incidence of prostate cancer in older adults, no particular guidelines for its management exist. Compared with younger patients, older
patients with prostate cancer need to weigh the benefits of treatment vs the risks to avoid any potential adverse treatment-related quality of life (QOL) decreases. Clearly, for some patients there are no significant benefits from treatment (eg, improved survival).
The Comprehensive Geriatric Assessment has been created to properly assess aging in correlation to individual and patient-centered biologic and clinical metrics.
Following extensive literature reviews, the International Society of Geriatric Oncology (SIOG) Prostate Cancer Working Group recognized that the most important prognostic factors in evaluating health status in elderly patients with prostate cancer include comorbidities, functional dependence, and nutrition status.3 An important prognosticator of survival in prostate cancer is preexisting comorbidities. The Cumulative Illness Rating Scale-Geriatrics (CIRS-G) is considered the best metric currently available in assessing a patient’s death risk unrelated to cancer.
Another important factor influencing survival of older patients with prostate cancer is the patient’s level of independent activity. Independent functioning is evaluated
using (1) activities of daily living (ADL); and (2) the instrumental activities of daily living (IADL).4-6
Health Status Subgroups
The SIOG recommendation for prostate cancer treatment in older patients is based on a complete assessment of existing comorbidities of patients using the CIRS-G,
IADL, and ADL scales as well as the nutritional status of each patient.3,7-9 Based on these prognostic tools, the SIOG classifies the health status of elderly patients with prostate cancer into 4 prognostic health status categories: healthy, vulnerable, frail, and terminal.10,11
Patient Characteristics
Older patients generally present with highrisk prostate cancer at diagnosis.12,13 However, older patients are less likely to be treated with curative intent, resulting in
lower overall and disease-specific survivals. Nearly 40% of deaths due to prostate cancer occur in patients aged ≥ 75 years and 31% in the group aged ≥ 85 years.12,14 Recent reports have demonstrated that curative radiotherapy or surgery improved survival outcomes as well as QOL in the elderly, comparable with those seen in younger patients.
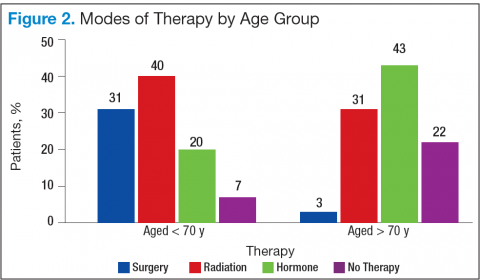
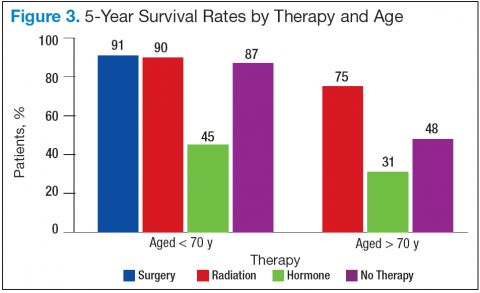
Bechis and colleagues analyzed the relationship between survival of older patients with high-risk cancer and curative local therapy.15 Treatment modalities included radical prostatectomy, external-beam radiation therapy (EBRT), watchful waiting/active surveillance, and other modalities, including primary androgen deprivation therapy (ADT). The findings were: (1) older patients more frequently presented with high-risk disease as age increased; (2) therapeutic approaches varied but were based mainly on age at diagnosis rather than on cancer risk factors (Figures 2 and 3); and (3) ADT was used more frequently in older patients compared with its use in younger patients, irrespective of the risk score, including patients with high-risk disease.
Older patients were less likely to receive radical therapy, especially surgical treatment, regardless of risk category. Forty-four percent of patients aged > 70 years with high-risk disease died of any cause at a median 5.7 years, and 21% died of prostate cancer; whereas 47% of patients aged > 75 years with high-risk disease died at a median of 5.3 years, and 20% of those died of prostate cancer.
When older patients with high-risk disease received curative local therapy, however, the mortality rate decreased.
In a study by Sun and colleagues, 4,561 senior patients who received radical prostatectomy therapy were classified into 3 age groups (aged < 60 years, aged 60 to 70 years, and aged > 70 years) based on the year of surgery (before or after 2000). Therapy outcomes were compared among the 3 groups.16 The researchers found that seniors aged > 70 years who presented with high-risk disease had poorer therapeutic outcomes. A diagnosis of advancedstage cancer and a Gleason score > 7 were more often made in patients aged > 70 years vs that of their younger counterparts. They also found greater risk of failures for these patients in biochemical recurrence, distant metastasis, and disease-specific survivals.
Most clinicians typically ruled out active treatment based on chronologic age alone, without considering existing comorbidities and overall life expectancy. According
to a study by Daskivich and colleagues, only 16% of patients aged > 75 years were aggressively treated, whereas 84% of patients aged < 55 years received aggressive curative therapy, using radical prostatectomy, radiation therapy, or brachytherapy.17
Therapeutic Approaches
Current NCCN guidelines recommend active surveillance as an option for men with low- and intermediate-risk disease with a < 10-year life expectancy and the only option for men with a < 20-year life expectancy and a very low-risk of prostate cancer (stage T1c, Gleason ≤ 6, prostate specific antigen [PSA] < 10 ng/mL, < 3 positive cores, < 50% core involvement, and PSA density < 0.15 ng/mL2). Patients who are older and have significant comorbidities should be managed with active surveillance rather than with active treatment. In the practice setting, however, studies indicated that a substantial number of older men with limited life expectancy still received aggressive treatment for low-risk cancer. Active treatment tended to decrease with age but was still common among men aged > 80 years: 25% received active local therapy, 36% received primary ADT, and only 39% received no active treatment.18
Surgery
Surgical treatment is an active therapeutic option for some patients with localized disease. Mortalities were reduced using prostatectomy vs watchful waiting, including disease-specific mortality and rates of metastasis. As newer techniques develop, laparoscopic prostatectomy may be able to provide excellent therapeutic
outcomes with quick surgical recovery times and possibly less postoperative nerve damage. Compared with younger patients, older patients experienced comparable
outcomes after surgical therapy.19-21 Despite encouraging surgical outcomes, however, surgery is not generally offered to patients aged > 70 years because
of the presumed high risks related to possible surgical complications.
Radiation Therapies
External-beam radiation therapy has been a well-established, standard mode of radiotherapy for the past several decades, among various radiation modalities, including brachytherapy (high- and low-dose radioactive seed implant therapy), cyber-knife therapy, and proton therapy. If indicated, EBRT rather than surgery is generally suggested as an active treatment for patients with localized prostate cancer. In general, EBRT and radical prostatectomy are comparable in survival
outcomes, but EBRT is preferred for older patients because it is noninvasive.21,22 Conventional EBRT technique has gradually progressed over the past several decades, advancing to 3D conformal radiotherapy, intensity-modulated radiation therapy (IMRT), image-guided radiotherapy, and then most recently to RapidArc radiation therapy.
RapidArc radiotherapy is an advanced form of IMRT that increases dose conformity and significantly shortens daily treatment times. In contrast to the static conventional IMRT technique (requiring repeated stops to deliver radiation through a 360° rotation of the therapy machine around the patient), RapidArc radiotherapy continues to deliver radiation therapy to the targeted tumor lesion with no interruption while the therapy machine is rotating around the patient. Accordingly, radiation therapy time is much shorter (up to 8 times faster) compared with conventional IMRT radiotherapy.
Systemic Therapy
Androgen-deprivation therapy can slow cancer growth, as it inhibits androgen production, blocks androgen action, or both. For localized prostate cancers with intermittent- and high-risk for recurrence, radiation therapy combined with ADT (eg, leuprolide, goserelin, triptorelin) reduces mortality of patients compared with ADT alone. In addition, hormone therapy is used for advanced, recurrent, or metastatic prostate cancers.
Most advanced and roughly one-fifth of biologically recurrent cancers ultimately convert to castrationresistant prostate cancer and may potentially benefit from nonhormonal systemic chemotherapy. Docetaxel with or without prednisone is the agent of choice for castration-resistant symptomatic metastatic prostate cancer. Cabazitaxel is a secondgeneration taxane and approved for castration-resistantmetastatic prostate cancer. Other systemic drugs (hormonal) for chemotherapy-naïve, metastatic castration-resistant prostate cancer are abiraterone (androgen synthesis inhibitor) and enzalutamide (anti-androgen).
Low-Risk Prostate Cancers
Active surveillance would be a reasonable management option for older patients with low-risk, localized prostate cancer and limited life expectancy of < 10 years. Albertsen and colleagues reported that patients with welldifferentiated prostate cancer and limited life expectancy have little chance of death due to prostate cancer but are more likely die of other causes, such as preexisting comorbidities.23 Bill-Axelson and colleagues reported a very similar cancer-specific mortality rate of only 2.5% for patients with well-differentiated prostate cancer who are receiving either active therapy or active surveillance.24 In another study, Krakowsky and colleagues reported a 97% 10-year cancer-specific survival rate in 450 patients with a median age of 70 years, and in a randomized study, Holmberg and colleagues reported no differences in overall survival for patients aged > 65 years who were randomized to surgery or watchful waiting for early-stage prostate cancer.25,26
The literature consistently reports cancer-specific survival rates approaching 100% for patients with low-risk prostate cancer. The main concern regarding aggressive
therapy for older patients with low-risk cancer and significant comorbidities, as well as limited life expectancy, is the real possibility of overtreatment and the resultant high risk of treatment-related complications and loss in QOL. For example, surgery can lead to varying degrees of incontinence, and radiation can lead to rectal bleeding from proctitis, both severely impacting patients’ QOL.
High-Risk Prostate Cancers
Older patients with high-risk prostate cancer generally do not receive curative therapy. Bechis and colleagues examined the influence of age on disease-specific mortality.15 They found that patients aged > 75 years were more likely to be diagnosed with high-risk prostate cancer and treated with conservative therapy, such as ADT or watchful waiting, often resulting in death. They also found that the choice of therapy in older patients was based primarily on age rather than on comorbidities or other disease factors. Trends for such undertreatment were most evident in healthy seniors with high-risk cancer. The undertreatment of older patients with lower comorbidities contributes to the higher disease-specific mortality seen in the elderly population. Such healthy older patients were often overlooked solely because of their age and might have been denied the opportunity to receive curative and life-saving therapy early.
Summary
Most prostate cancers develop in older patients, and nearly one-fourth of prostate cancers are diagnosed in patients who are aged > 75 years. In addition, older patients show a higher tendency to present with highrisk prostate cancer. Furthermore, older patients have a higher risk of death compared with that of younger
patients, although many of them still die of causes other than prostate cancer. The most important prognostic factors in older patients, as recognized by the SIOG Prostate Cancer Working Group, included comorbidities, dependence status, and nutrition status. Management decisions for older patients with prostate cancer should be individualized and formulated based on remaining life expectancy, the patient’s functional performance and health status, as well as coexisting comorbidities and patient-specific prognostic characteristics of the prostate cancer, such as stage, Gleason score, and PSA values.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations— including indications, contraindications, warnings, and adverse effects—before administering pharmacologic
therapy to patients.
Click here to read the digital edition.
1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network Website http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated October 24, 2014. Accessed June 14, 2015.
2. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
3. Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73(1):68-91.
4. Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453-471.
5. Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171(4):1513-1519.
6. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622-626.
7. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914-919.
8. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205-206.
9. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186.
10. Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106(4):462-469.
11. Fitzpatrick JM, Graefen M, Payne HA, Scotté F, Aapro MS. A Comment on the International Society of Geriatric Oncology guidelines: evidence-based advice for the clinical setting. Oncologist. 2012;17(suppl 1):31-35.
12. Hoffman KE. Management of older men with clinically localized prostate cancer: the significance of advanced age and comorbidity. Sem Radiat Oncol. 2012;22(4):284-294.
13. Howlader N, Noon AM, Krapcho M, et al. SEER cancer statistics review 1975-2008. SEER Website. http://seer.cancer.gov/archive/csr/1975_2008. Updated November 10, 2011. Accessed June 10, 2015.
14. Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280-1283.
15. Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29(2):235-241.
16. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
17. Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058-2066.
18. Cooperburg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(1):2141-2149.
19. Richstone L, Bianco FJ, Shah HH, et al. Radical prostatectomy in men aged > or = 70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101(5):541-546.
20. Siddiqui SA, Sengupta S, Slezak JM, et al. Impact of patient age at treatment on outcome following radical retropubic prostatectomy for prostate cancer. J Urol. 2006;175(3 pt 1):952-957.
21. Bian SX, Hoffman KE. Management of prostate cancer in elderly men. Semin Radiat Oncol. 2013;23(3):198-205.
22. Payne HA, Hughes S. Radical radiotherapy for high-risk prostate cancer in older men. Oncologist. 2012;17(suppl 1):9-15.
23. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095-2101.
24. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
25. Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184(1):131-135.
26. Holmberg L, Bill-Axelson A, Helgesen F, et al; Scandinavian Prostatic Cancer Group Study Number 4. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. New Engl J Med. 2002;347(11):781-789.
This article (part 2 of 2) focuses on the treatment of prostate cancer in seniors. Part 1 provided an overview of prostate cancer epidemiology, pathology, and screening in senior patients.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Recently, the International Society of Geriatric Oncology assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
Patient Evaluation
The practice guidelines of the National Comprehensive Cancer Network (NCCN) recommend using life expectancy in determining treatment.1 Prostate cancer is considered to be an indolent disease, and active therapy may be more harmful than beneficial to older patients whose life expectancy is limited because of treatment-related sequelae. Therefore, an accurate estimation of life expectancy is important in devising treatment strategies for older patients.
Age should not be the only factor in determining the life expectancy of patients, because life expectancy varies widely based on the patient’s health status, including preexisting comorbidities. Chronologic life expectancy can be found in the Social Security Administration’s life tables. Individual life expectancy is then projected by adding 50% to or deducting 50% from the chronologic life expectancy for men in the highest and lowest quartile of health, respectively. The life expectancy from the life tables can be applied with no addition or subtraction for men in the middle 2 quartiles of health status (Figure 1).2
Geriatric Assessment
Despite the increasing incidence of prostate cancer in older adults, no particular guidelines for its management exist. Compared with younger patients, older
patients with prostate cancer need to weigh the benefits of treatment vs the risks to avoid any potential adverse treatment-related quality of life (QOL) decreases. Clearly, for some patients there are no significant benefits from treatment (eg, improved survival).
The Comprehensive Geriatric Assessment has been created to properly assess aging in correlation to individual and patient-centered biologic and clinical metrics.
Following extensive literature reviews, the International Society of Geriatric Oncology (SIOG) Prostate Cancer Working Group recognized that the most important prognostic factors in evaluating health status in elderly patients with prostate cancer include comorbidities, functional dependence, and nutrition status.3 An important prognosticator of survival in prostate cancer is preexisting comorbidities. The Cumulative Illness Rating Scale-Geriatrics (CIRS-G) is considered the best metric currently available in assessing a patient’s death risk unrelated to cancer.
Another important factor influencing survival of older patients with prostate cancer is the patient’s level of independent activity. Independent functioning is evaluated
using (1) activities of daily living (ADL); and (2) the instrumental activities of daily living (IADL).4-6
Health Status Subgroups
The SIOG recommendation for prostate cancer treatment in older patients is based on a complete assessment of existing comorbidities of patients using the CIRS-G,
IADL, and ADL scales as well as the nutritional status of each patient.3,7-9 Based on these prognostic tools, the SIOG classifies the health status of elderly patients with prostate cancer into 4 prognostic health status categories: healthy, vulnerable, frail, and terminal.10,11
Patient Characteristics
Older patients generally present with highrisk prostate cancer at diagnosis.12,13 However, older patients are less likely to be treated with curative intent, resulting in
lower overall and disease-specific survivals. Nearly 40% of deaths due to prostate cancer occur in patients aged ≥ 75 years and 31% in the group aged ≥ 85 years.12,14 Recent reports have demonstrated that curative radiotherapy or surgery improved survival outcomes as well as QOL in the elderly, comparable with those seen in younger patients.
Bechis and colleagues analyzed the relationship between survival of older patients with high-risk cancer and curative local therapy.15 Treatment modalities included radical prostatectomy, external-beam radiation therapy (EBRT), watchful waiting/active surveillance, and other modalities, including primary androgen deprivation therapy (ADT). The findings were: (1) older patients more frequently presented with high-risk disease as age increased; (2) therapeutic approaches varied but were based mainly on age at diagnosis rather than on cancer risk factors (Figures 2 and 3); and (3) ADT was used more frequently in older patients compared with its use in younger patients, irrespective of the risk score, including patients with high-risk disease.
Older patients were less likely to receive radical therapy, especially surgical treatment, regardless of risk category. Forty-four percent of patients aged > 70 years with high-risk disease died of any cause at a median 5.7 years, and 21% died of prostate cancer; whereas 47% of patients aged > 75 years with high-risk disease died at a median of 5.3 years, and 20% of those died of prostate cancer.
When older patients with high-risk disease received curative local therapy, however, the mortality rate decreased.
In a study by Sun and colleagues, 4,561 senior patients who received radical prostatectomy therapy were classified into 3 age groups (aged < 60 years, aged 60 to 70 years, and aged > 70 years) based on the year of surgery (before or after 2000). Therapy outcomes were compared among the 3 groups.16 The researchers found that seniors aged > 70 years who presented with high-risk disease had poorer therapeutic outcomes. A diagnosis of advancedstage cancer and a Gleason score > 7 were more often made in patients aged > 70 years vs that of their younger counterparts. They also found greater risk of failures for these patients in biochemical recurrence, distant metastasis, and disease-specific survivals.
Most clinicians typically ruled out active treatment based on chronologic age alone, without considering existing comorbidities and overall life expectancy. According
to a study by Daskivich and colleagues, only 16% of patients aged > 75 years were aggressively treated, whereas 84% of patients aged < 55 years received aggressive curative therapy, using radical prostatectomy, radiation therapy, or brachytherapy.17
Therapeutic Approaches
Current NCCN guidelines recommend active surveillance as an option for men with low- and intermediate-risk disease with a < 10-year life expectancy and the only option for men with a < 20-year life expectancy and a very low-risk of prostate cancer (stage T1c, Gleason ≤ 6, prostate specific antigen [PSA] < 10 ng/mL, < 3 positive cores, < 50% core involvement, and PSA density < 0.15 ng/mL2). Patients who are older and have significant comorbidities should be managed with active surveillance rather than with active treatment. In the practice setting, however, studies indicated that a substantial number of older men with limited life expectancy still received aggressive treatment for low-risk cancer. Active treatment tended to decrease with age but was still common among men aged > 80 years: 25% received active local therapy, 36% received primary ADT, and only 39% received no active treatment.18
Surgery
Surgical treatment is an active therapeutic option for some patients with localized disease. Mortalities were reduced using prostatectomy vs watchful waiting, including disease-specific mortality and rates of metastasis. As newer techniques develop, laparoscopic prostatectomy may be able to provide excellent therapeutic
outcomes with quick surgical recovery times and possibly less postoperative nerve damage. Compared with younger patients, older patients experienced comparable
outcomes after surgical therapy.19-21 Despite encouraging surgical outcomes, however, surgery is not generally offered to patients aged > 70 years because
of the presumed high risks related to possible surgical complications.
Radiation Therapies
External-beam radiation therapy has been a well-established, standard mode of radiotherapy for the past several decades, among various radiation modalities, including brachytherapy (high- and low-dose radioactive seed implant therapy), cyber-knife therapy, and proton therapy. If indicated, EBRT rather than surgery is generally suggested as an active treatment for patients with localized prostate cancer. In general, EBRT and radical prostatectomy are comparable in survival
outcomes, but EBRT is preferred for older patients because it is noninvasive.21,22 Conventional EBRT technique has gradually progressed over the past several decades, advancing to 3D conformal radiotherapy, intensity-modulated radiation therapy (IMRT), image-guided radiotherapy, and then most recently to RapidArc radiation therapy.
RapidArc radiotherapy is an advanced form of IMRT that increases dose conformity and significantly shortens daily treatment times. In contrast to the static conventional IMRT technique (requiring repeated stops to deliver radiation through a 360° rotation of the therapy machine around the patient), RapidArc radiotherapy continues to deliver radiation therapy to the targeted tumor lesion with no interruption while the therapy machine is rotating around the patient. Accordingly, radiation therapy time is much shorter (up to 8 times faster) compared with conventional IMRT radiotherapy.
Systemic Therapy
Androgen-deprivation therapy can slow cancer growth, as it inhibits androgen production, blocks androgen action, or both. For localized prostate cancers with intermittent- and high-risk for recurrence, radiation therapy combined with ADT (eg, leuprolide, goserelin, triptorelin) reduces mortality of patients compared with ADT alone. In addition, hormone therapy is used for advanced, recurrent, or metastatic prostate cancers.
Most advanced and roughly one-fifth of biologically recurrent cancers ultimately convert to castrationresistant prostate cancer and may potentially benefit from nonhormonal systemic chemotherapy. Docetaxel with or without prednisone is the agent of choice for castration-resistant symptomatic metastatic prostate cancer. Cabazitaxel is a secondgeneration taxane and approved for castration-resistantmetastatic prostate cancer. Other systemic drugs (hormonal) for chemotherapy-naïve, metastatic castration-resistant prostate cancer are abiraterone (androgen synthesis inhibitor) and enzalutamide (anti-androgen).
Low-Risk Prostate Cancers
Active surveillance would be a reasonable management option for older patients with low-risk, localized prostate cancer and limited life expectancy of < 10 years. Albertsen and colleagues reported that patients with welldifferentiated prostate cancer and limited life expectancy have little chance of death due to prostate cancer but are more likely die of other causes, such as preexisting comorbidities.23 Bill-Axelson and colleagues reported a very similar cancer-specific mortality rate of only 2.5% for patients with well-differentiated prostate cancer who are receiving either active therapy or active surveillance.24 In another study, Krakowsky and colleagues reported a 97% 10-year cancer-specific survival rate in 450 patients with a median age of 70 years, and in a randomized study, Holmberg and colleagues reported no differences in overall survival for patients aged > 65 years who were randomized to surgery or watchful waiting for early-stage prostate cancer.25,26
The literature consistently reports cancer-specific survival rates approaching 100% for patients with low-risk prostate cancer. The main concern regarding aggressive
therapy for older patients with low-risk cancer and significant comorbidities, as well as limited life expectancy, is the real possibility of overtreatment and the resultant high risk of treatment-related complications and loss in QOL. For example, surgery can lead to varying degrees of incontinence, and radiation can lead to rectal bleeding from proctitis, both severely impacting patients’ QOL.
High-Risk Prostate Cancers
Older patients with high-risk prostate cancer generally do not receive curative therapy. Bechis and colleagues examined the influence of age on disease-specific mortality.15 They found that patients aged > 75 years were more likely to be diagnosed with high-risk prostate cancer and treated with conservative therapy, such as ADT or watchful waiting, often resulting in death. They also found that the choice of therapy in older patients was based primarily on age rather than on comorbidities or other disease factors. Trends for such undertreatment were most evident in healthy seniors with high-risk cancer. The undertreatment of older patients with lower comorbidities contributes to the higher disease-specific mortality seen in the elderly population. Such healthy older patients were often overlooked solely because of their age and might have been denied the opportunity to receive curative and life-saving therapy early.
Summary
Most prostate cancers develop in older patients, and nearly one-fourth of prostate cancers are diagnosed in patients who are aged > 75 years. In addition, older patients show a higher tendency to present with highrisk prostate cancer. Furthermore, older patients have a higher risk of death compared with that of younger
patients, although many of them still die of causes other than prostate cancer. The most important prognostic factors in older patients, as recognized by the SIOG Prostate Cancer Working Group, included comorbidities, dependence status, and nutrition status. Management decisions for older patients with prostate cancer should be individualized and formulated based on remaining life expectancy, the patient’s functional performance and health status, as well as coexisting comorbidities and patient-specific prognostic characteristics of the prostate cancer, such as stage, Gleason score, and PSA values.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations— including indications, contraindications, warnings, and adverse effects—before administering pharmacologic
therapy to patients.
Click here to read the digital edition.
This article (part 2 of 2) focuses on the treatment of prostate cancer in seniors. Part 1 provided an overview of prostate cancer epidemiology, pathology, and screening in senior patients.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Recently, the International Society of Geriatric Oncology assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
Patient Evaluation
The practice guidelines of the National Comprehensive Cancer Network (NCCN) recommend using life expectancy in determining treatment.1 Prostate cancer is considered to be an indolent disease, and active therapy may be more harmful than beneficial to older patients whose life expectancy is limited because of treatment-related sequelae. Therefore, an accurate estimation of life expectancy is important in devising treatment strategies for older patients.
Age should not be the only factor in determining the life expectancy of patients, because life expectancy varies widely based on the patient’s health status, including preexisting comorbidities. Chronologic life expectancy can be found in the Social Security Administration’s life tables. Individual life expectancy is then projected by adding 50% to or deducting 50% from the chronologic life expectancy for men in the highest and lowest quartile of health, respectively. The life expectancy from the life tables can be applied with no addition or subtraction for men in the middle 2 quartiles of health status (Figure 1).2
Geriatric Assessment
Despite the increasing incidence of prostate cancer in older adults, no particular guidelines for its management exist. Compared with younger patients, older
patients with prostate cancer need to weigh the benefits of treatment vs the risks to avoid any potential adverse treatment-related quality of life (QOL) decreases. Clearly, for some patients there are no significant benefits from treatment (eg, improved survival).
The Comprehensive Geriatric Assessment has been created to properly assess aging in correlation to individual and patient-centered biologic and clinical metrics.
Following extensive literature reviews, the International Society of Geriatric Oncology (SIOG) Prostate Cancer Working Group recognized that the most important prognostic factors in evaluating health status in elderly patients with prostate cancer include comorbidities, functional dependence, and nutrition status.3 An important prognosticator of survival in prostate cancer is preexisting comorbidities. The Cumulative Illness Rating Scale-Geriatrics (CIRS-G) is considered the best metric currently available in assessing a patient’s death risk unrelated to cancer.
Another important factor influencing survival of older patients with prostate cancer is the patient’s level of independent activity. Independent functioning is evaluated
using (1) activities of daily living (ADL); and (2) the instrumental activities of daily living (IADL).4-6
Health Status Subgroups
The SIOG recommendation for prostate cancer treatment in older patients is based on a complete assessment of existing comorbidities of patients using the CIRS-G,
IADL, and ADL scales as well as the nutritional status of each patient.3,7-9 Based on these prognostic tools, the SIOG classifies the health status of elderly patients with prostate cancer into 4 prognostic health status categories: healthy, vulnerable, frail, and terminal.10,11
Patient Characteristics
Older patients generally present with highrisk prostate cancer at diagnosis.12,13 However, older patients are less likely to be treated with curative intent, resulting in
lower overall and disease-specific survivals. Nearly 40% of deaths due to prostate cancer occur in patients aged ≥ 75 years and 31% in the group aged ≥ 85 years.12,14 Recent reports have demonstrated that curative radiotherapy or surgery improved survival outcomes as well as QOL in the elderly, comparable with those seen in younger patients.
Bechis and colleagues analyzed the relationship between survival of older patients with high-risk cancer and curative local therapy.15 Treatment modalities included radical prostatectomy, external-beam radiation therapy (EBRT), watchful waiting/active surveillance, and other modalities, including primary androgen deprivation therapy (ADT). The findings were: (1) older patients more frequently presented with high-risk disease as age increased; (2) therapeutic approaches varied but were based mainly on age at diagnosis rather than on cancer risk factors (Figures 2 and 3); and (3) ADT was used more frequently in older patients compared with its use in younger patients, irrespective of the risk score, including patients with high-risk disease.
Older patients were less likely to receive radical therapy, especially surgical treatment, regardless of risk category. Forty-four percent of patients aged > 70 years with high-risk disease died of any cause at a median 5.7 years, and 21% died of prostate cancer; whereas 47% of patients aged > 75 years with high-risk disease died at a median of 5.3 years, and 20% of those died of prostate cancer.
When older patients with high-risk disease received curative local therapy, however, the mortality rate decreased.
In a study by Sun and colleagues, 4,561 senior patients who received radical prostatectomy therapy were classified into 3 age groups (aged < 60 years, aged 60 to 70 years, and aged > 70 years) based on the year of surgery (before or after 2000). Therapy outcomes were compared among the 3 groups.16 The researchers found that seniors aged > 70 years who presented with high-risk disease had poorer therapeutic outcomes. A diagnosis of advancedstage cancer and a Gleason score > 7 were more often made in patients aged > 70 years vs that of their younger counterparts. They also found greater risk of failures for these patients in biochemical recurrence, distant metastasis, and disease-specific survivals.
Most clinicians typically ruled out active treatment based on chronologic age alone, without considering existing comorbidities and overall life expectancy. According
to a study by Daskivich and colleagues, only 16% of patients aged > 75 years were aggressively treated, whereas 84% of patients aged < 55 years received aggressive curative therapy, using radical prostatectomy, radiation therapy, or brachytherapy.17
Therapeutic Approaches
Current NCCN guidelines recommend active surveillance as an option for men with low- and intermediate-risk disease with a < 10-year life expectancy and the only option for men with a < 20-year life expectancy and a very low-risk of prostate cancer (stage T1c, Gleason ≤ 6, prostate specific antigen [PSA] < 10 ng/mL, < 3 positive cores, < 50% core involvement, and PSA density < 0.15 ng/mL2). Patients who are older and have significant comorbidities should be managed with active surveillance rather than with active treatment. In the practice setting, however, studies indicated that a substantial number of older men with limited life expectancy still received aggressive treatment for low-risk cancer. Active treatment tended to decrease with age but was still common among men aged > 80 years: 25% received active local therapy, 36% received primary ADT, and only 39% received no active treatment.18
Surgery
Surgical treatment is an active therapeutic option for some patients with localized disease. Mortalities were reduced using prostatectomy vs watchful waiting, including disease-specific mortality and rates of metastasis. As newer techniques develop, laparoscopic prostatectomy may be able to provide excellent therapeutic
outcomes with quick surgical recovery times and possibly less postoperative nerve damage. Compared with younger patients, older patients experienced comparable
outcomes after surgical therapy.19-21 Despite encouraging surgical outcomes, however, surgery is not generally offered to patients aged > 70 years because
of the presumed high risks related to possible surgical complications.
Radiation Therapies
External-beam radiation therapy has been a well-established, standard mode of radiotherapy for the past several decades, among various radiation modalities, including brachytherapy (high- and low-dose radioactive seed implant therapy), cyber-knife therapy, and proton therapy. If indicated, EBRT rather than surgery is generally suggested as an active treatment for patients with localized prostate cancer. In general, EBRT and radical prostatectomy are comparable in survival
outcomes, but EBRT is preferred for older patients because it is noninvasive.21,22 Conventional EBRT technique has gradually progressed over the past several decades, advancing to 3D conformal radiotherapy, intensity-modulated radiation therapy (IMRT), image-guided radiotherapy, and then most recently to RapidArc radiation therapy.
RapidArc radiotherapy is an advanced form of IMRT that increases dose conformity and significantly shortens daily treatment times. In contrast to the static conventional IMRT technique (requiring repeated stops to deliver radiation through a 360° rotation of the therapy machine around the patient), RapidArc radiotherapy continues to deliver radiation therapy to the targeted tumor lesion with no interruption while the therapy machine is rotating around the patient. Accordingly, radiation therapy time is much shorter (up to 8 times faster) compared with conventional IMRT radiotherapy.
Systemic Therapy
Androgen-deprivation therapy can slow cancer growth, as it inhibits androgen production, blocks androgen action, or both. For localized prostate cancers with intermittent- and high-risk for recurrence, radiation therapy combined with ADT (eg, leuprolide, goserelin, triptorelin) reduces mortality of patients compared with ADT alone. In addition, hormone therapy is used for advanced, recurrent, or metastatic prostate cancers.
Most advanced and roughly one-fifth of biologically recurrent cancers ultimately convert to castrationresistant prostate cancer and may potentially benefit from nonhormonal systemic chemotherapy. Docetaxel with or without prednisone is the agent of choice for castration-resistant symptomatic metastatic prostate cancer. Cabazitaxel is a secondgeneration taxane and approved for castration-resistantmetastatic prostate cancer. Other systemic drugs (hormonal) for chemotherapy-naïve, metastatic castration-resistant prostate cancer are abiraterone (androgen synthesis inhibitor) and enzalutamide (anti-androgen).
Low-Risk Prostate Cancers
Active surveillance would be a reasonable management option for older patients with low-risk, localized prostate cancer and limited life expectancy of < 10 years. Albertsen and colleagues reported that patients with welldifferentiated prostate cancer and limited life expectancy have little chance of death due to prostate cancer but are more likely die of other causes, such as preexisting comorbidities.23 Bill-Axelson and colleagues reported a very similar cancer-specific mortality rate of only 2.5% for patients with well-differentiated prostate cancer who are receiving either active therapy or active surveillance.24 In another study, Krakowsky and colleagues reported a 97% 10-year cancer-specific survival rate in 450 patients with a median age of 70 years, and in a randomized study, Holmberg and colleagues reported no differences in overall survival for patients aged > 65 years who were randomized to surgery or watchful waiting for early-stage prostate cancer.25,26
The literature consistently reports cancer-specific survival rates approaching 100% for patients with low-risk prostate cancer. The main concern regarding aggressive
therapy for older patients with low-risk cancer and significant comorbidities, as well as limited life expectancy, is the real possibility of overtreatment and the resultant high risk of treatment-related complications and loss in QOL. For example, surgery can lead to varying degrees of incontinence, and radiation can lead to rectal bleeding from proctitis, both severely impacting patients’ QOL.
High-Risk Prostate Cancers
Older patients with high-risk prostate cancer generally do not receive curative therapy. Bechis and colleagues examined the influence of age on disease-specific mortality.15 They found that patients aged > 75 years were more likely to be diagnosed with high-risk prostate cancer and treated with conservative therapy, such as ADT or watchful waiting, often resulting in death. They also found that the choice of therapy in older patients was based primarily on age rather than on comorbidities or other disease factors. Trends for such undertreatment were most evident in healthy seniors with high-risk cancer. The undertreatment of older patients with lower comorbidities contributes to the higher disease-specific mortality seen in the elderly population. Such healthy older patients were often overlooked solely because of their age and might have been denied the opportunity to receive curative and life-saving therapy early.
Summary
Most prostate cancers develop in older patients, and nearly one-fourth of prostate cancers are diagnosed in patients who are aged > 75 years. In addition, older patients show a higher tendency to present with highrisk prostate cancer. Furthermore, older patients have a higher risk of death compared with that of younger
patients, although many of them still die of causes other than prostate cancer. The most important prognostic factors in older patients, as recognized by the SIOG Prostate Cancer Working Group, included comorbidities, dependence status, and nutrition status. Management decisions for older patients with prostate cancer should be individualized and formulated based on remaining life expectancy, the patient’s functional performance and health status, as well as coexisting comorbidities and patient-specific prognostic characteristics of the prostate cancer, such as stage, Gleason score, and PSA values.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations— including indications, contraindications, warnings, and adverse effects—before administering pharmacologic
therapy to patients.
Click here to read the digital edition.
1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network Website http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated October 24, 2014. Accessed June 14, 2015.
2. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
3. Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73(1):68-91.
4. Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453-471.
5. Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171(4):1513-1519.
6. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622-626.
7. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914-919.
8. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205-206.
9. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186.
10. Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106(4):462-469.
11. Fitzpatrick JM, Graefen M, Payne HA, Scotté F, Aapro MS. A Comment on the International Society of Geriatric Oncology guidelines: evidence-based advice for the clinical setting. Oncologist. 2012;17(suppl 1):31-35.
12. Hoffman KE. Management of older men with clinically localized prostate cancer: the significance of advanced age and comorbidity. Sem Radiat Oncol. 2012;22(4):284-294.
13. Howlader N, Noon AM, Krapcho M, et al. SEER cancer statistics review 1975-2008. SEER Website. http://seer.cancer.gov/archive/csr/1975_2008. Updated November 10, 2011. Accessed June 10, 2015.
14. Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280-1283.
15. Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29(2):235-241.
16. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
17. Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058-2066.
18. Cooperburg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(1):2141-2149.
19. Richstone L, Bianco FJ, Shah HH, et al. Radical prostatectomy in men aged > or = 70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101(5):541-546.
20. Siddiqui SA, Sengupta S, Slezak JM, et al. Impact of patient age at treatment on outcome following radical retropubic prostatectomy for prostate cancer. J Urol. 2006;175(3 pt 1):952-957.
21. Bian SX, Hoffman KE. Management of prostate cancer in elderly men. Semin Radiat Oncol. 2013;23(3):198-205.
22. Payne HA, Hughes S. Radical radiotherapy for high-risk prostate cancer in older men. Oncologist. 2012;17(suppl 1):9-15.
23. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095-2101.
24. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
25. Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184(1):131-135.
26. Holmberg L, Bill-Axelson A, Helgesen F, et al; Scandinavian Prostatic Cancer Group Study Number 4. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. New Engl J Med. 2002;347(11):781-789.
1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network Website http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated October 24, 2014. Accessed June 14, 2015.
2. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
3. Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73(1):68-91.
4. Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453-471.
5. Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171(4):1513-1519.
6. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622-626.
7. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914-919.
8. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205-206.
9. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186.
10. Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106(4):462-469.
11. Fitzpatrick JM, Graefen M, Payne HA, Scotté F, Aapro MS. A Comment on the International Society of Geriatric Oncology guidelines: evidence-based advice for the clinical setting. Oncologist. 2012;17(suppl 1):31-35.
12. Hoffman KE. Management of older men with clinically localized prostate cancer: the significance of advanced age and comorbidity. Sem Radiat Oncol. 2012;22(4):284-294.
13. Howlader N, Noon AM, Krapcho M, et al. SEER cancer statistics review 1975-2008. SEER Website. http://seer.cancer.gov/archive/csr/1975_2008. Updated November 10, 2011. Accessed June 10, 2015.
14. Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280-1283.
15. Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29(2):235-241.
16. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
17. Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058-2066.
18. Cooperburg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(1):2141-2149.
19. Richstone L, Bianco FJ, Shah HH, et al. Radical prostatectomy in men aged > or = 70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101(5):541-546.
20. Siddiqui SA, Sengupta S, Slezak JM, et al. Impact of patient age at treatment on outcome following radical retropubic prostatectomy for prostate cancer. J Urol. 2006;175(3 pt 1):952-957.
21. Bian SX, Hoffman KE. Management of prostate cancer in elderly men. Semin Radiat Oncol. 2013;23(3):198-205.
22. Payne HA, Hughes S. Radical radiotherapy for high-risk prostate cancer in older men. Oncologist. 2012;17(suppl 1):9-15.
23. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095-2101.
24. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
25. Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184(1):131-135.
26. Holmberg L, Bill-Axelson A, Helgesen F, et al; Scandinavian Prostatic Cancer Group Study Number 4. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. New Engl J Med. 2002;347(11):781-789.
Prostate Cancer in Seniors, Part 1: Epidemiology, Pathology, and Screening
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
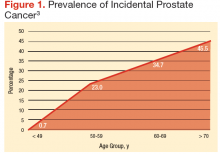
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were
designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
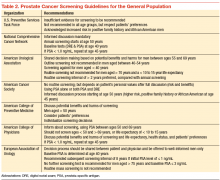
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
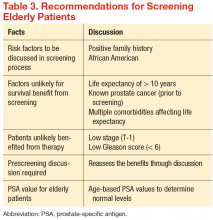
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of patients who are aged < 65 years
- Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were
designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of patients who are aged < 65 years
- Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were
designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of patients who are aged < 65 years
- Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.